Engineering MXenes for electrochemical environmental pollutant sensing
Muhammad Hussnain
Afzal
a,
Wajeeha
Pervaiz
b,
Muhammad
Asif
c,
Zhuo
Huang
d,
Jiawei
Dai
a,
You
Xu
a,
Jiannan
Zhu
a,
Tiansui
Zhang
a,
Zhuang
Rao
a,
Guangfang
Li
 a,
Zhengyun
Wang
*a and
Hongfang
Liu
a,
Zhengyun
Wang
*a and
Hongfang
Liu
 *a
*a
aHubei Key Laboratory of Material Chemistry and Service Failure, Key Laboratory of Material Chemistry for Energy Conversion and Storage (Ministry of Education), Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Rd, Wuhan, China. E-mail: wzyhustsh@163.com; liuhf@hust.edu.cn
bCollege of Food Science and Technology, Huazhong Agricultural University, 1 Shizishan Street, Wuhan, China
cSchool of Chemistry and Chemical Engineering, Shanxi University, 92 Wucheng Rd, Taiyuan, China
dChangjiang River Scientific Research Institute of Changjiang Water Resources Commission, 289 Huangpu Street, Wuhan, China
First published on 16th October 2024
Abstract
Environmental pollutant sensing is essential to the sustainable development of human health and ecosystems. As a category of two-dimensional materials consisting of nitrides and carbides, MXenes have emerged as highly attractive candidates for electrochemical sensing of environmental pollutants, including toxic gases, harmful volatile organic compounds, and biologically relevant components, owing to their strong metallic conductivity, easy customization, abundant surface functional groups and large interlayer spacings. This comprehensive review firstly assesses the environmental pollutant sensing mechanism and modular MXene electrode fabrication methods. Subsequently, the research progress on MXenes is summarized by comparing their performances in environmental pollutant detection. Furthermore, ways to improve the electrochemical stability and selectivity of MXenes using different techniques are further discussed. Finally, challenges faced in this field and prospective directions for future research are suggested by integrating emerging technologies and interdisciplinary approaches. The key objective of this review is to motivate engineers and materials scientists to consider incorporating MXenes into technologies for environmental protection, thereby providing inventive solutions to urgent global issues.
Environmental significanceGrowing environmental pollutants have harmful impacts on human health, necessitating the development of advanced electrochemical sensing technology for the real-time monitoring of pollutants. This is essential to the sustainable development of human society and ecosystems. |
1. Introduction
Environmental pollutants pose a multitude of dangers that threaten human health. Air pollution, characterized by emissions of particulate matter such as sulfur dioxide and nitrogen oxides, is a major concern, contributing to respiratory diseases and climate change.1,2 Water pollution, stemming from contaminants such as heavy metal ions (HMIs), pathogens and toxic substances, has harmful effects on aquatic ecosystems and human health.3 Soil pollution, caused by pesticides and heavy metals, can adversely affect crop growth, break the food chain and further harm biodiversity.4,5 To address these challenges and effectively manage environmental pollution, it is imperative to recognize the dangers of environmental pollution and implement innovative technologies, especially developing advanced sensing techniques for the real-time monitoring of these pollutants.6Several traditional approaches, including spectroscopy and chromatography, are available for the determination of environmental toxicants. For instance, inorganic cations and anions can be quantitatively determined using approaches such as hydride generation-atomic absorption spectrometry (HG-AAS),7 electrothermal atomic absorption spectrometry (ET-AAS),8 inductively coupled plasma-optical emission spectrometry (ICP-OES),9 surface-enhanced Raman scattering (SERS),10 inductively coupled plasma-mass spectrometry (ICP-MS),11 hydride generation-atomic fluorescence spectrometry (HG-AFS),12 spectrophotometry,13 and X-ray fluorescence (XRF).14 Furthermore, organic compounds are evaluated using gas chromatography (GC),15 gas chromatography-mass spectrometry (GC-MS),16 liquid chromatography-mass spectrometry (LC-MS),17 capillary electrophoresis,18 high-performance liquid chromatography (HPLC),19 and immunoassay techniques.20 However, these methods have a number of shortcomings such as lengthy sample preparation processes, large instrument size, and specialized staff requirements, which restrict their broader on-site environmental applications.21 In addition, some other sensing methods have been developed. Table 1 summarizes the key performance metrics, including sensitivity, selectivity, response time, stability, and fabrication complexity, using fluorescence,22,23 colorimetric,24,25 chemiluminescence,26 piezoresistive,27 surface plasmon resonance (SPR)28 and surface-enhanced Raman scattering (SERS)29 sensors. These sensing techniques face challenges with sensitivity owing to their reliance on indirect optical or mechanical signal transduction, often requiring probes to amplify signals.30 Reproducibility is also problematic, especially for methods relying on surface enhancements because of difficulties in precise fabrication and maintaining sensor performance over long-term deployments. Among these sensing techniques, electrochemical sensors are a significant subgroup of chemical sensors with necessary attributes to effectively meet the demands for on-site environmental monitoring in terms of fast response, high sensitivity, good portability, and low cost.31–33 These advancements endow electrochemical sensors with the following functions: identification of organic pollutants in groundwater,34 quantification of trace metals in natural water bodies,35,36 surveillance of carcinogens in food,37 and environmental samples.38,39
| Sensing type | Advantages | Limitations | Applications |
|---|---|---|---|
| Electrochemical | High sensitivity | Moderate selectivity | Detection of heavy metals (e.g., Pb, Cd, Hg) in water |
| Rapid response | Stability issues | Monitoring of organic pollutants (e.g., pesticides, phenols) in soil and air | |
| Portable setup | Fouling effects | Environmental monitoring of water quality and contamination | |
| Low cost | Food safety | ||
| Miniaturized design | |||
| Fluorescence | High selectivity for organic compounds | Lower sensitivity | Detection of organic pollutants (e.g., PAHs, PCBs) in water |
| Multi-analyte ability | Instrumentation needs | Monitoring of biomarkers (e.g., proteins, DNA) in biological samples | |
| Broad linear ranges | Require labeling | Water quality assessment in environmental monitoring | |
| Susceptible to interference from background fluorescence | |||
| Colorimetric | High sensitivity for heavy metals | Moderate sensitivity | Detection of heavy metals (e.g., Cu, Zn, Fe) in water screening of water pollutants (e.g., nitrate, phosphate) in environmental samples |
| Simple instrumentation | Limited quantitative analysis | On-site testing in field applications | |
| Rapid detection | Subject to color interference from sample matrix | ||
| Low-cost | |||
| Short color development time | |||
| (Electro)chemiluminescence | High sensitivity for reactive species | Complexity in instrumentation and operation | Detection of reactive species (e.g., hydrogen peroxide, ozone) in water |
| No external power | Limited stability of chemiluminescent reagents | Monitoring of organic pollutants (e.g., pesticides, VOCs) in air | |
| Low detection limit | Biomedical diagnostics for disease biomarkers | ||
| Real-time monitoring | |||
| Piezoresistive | High sensitivity for gas pollutants | Complex fabrication | Detection of gas pollutants (e.g., NO2, CO, SO2) in air |
| Multi-analyte quantification | Small analytes needed | Monitoring of volatile organic compounds (e.g., benzene, toluene) in industrial settings | |
| Label-free | Susceptible to mechanical drift | Industrial process control and leak detection | |
| Real-time monitoring | Limited to specific analytes | ||
| Compact and portable devices | Requires calibration | ||
| Surface plasmon resonance (SPR) | High selectivity for biomolecules | Expensive equipment | Detection of biomolecules (e.g., proteins, DNA) in biological samples |
| Label free detection | Bulk optics configurations | Environmental monitoring of water contaminants (e.g., pesticides, pharmaceuticals) | |
| Real-time monitoring | Specialized fabrication | Biosensing applications in medical diagnostics | |
| Limited multiplexing | |||
| Limited to certain sample types | |||
| Sensitivity to sample matrix effects | |||
| Surface-enhanced Raman scattering (SERS) | High sensitivity and specificity for trace contaminants | Limited reproducibility complexity in data analysis | Detection of trace contaminants (e.g., heavy metals, organic pollutants) in water |
| Ultra-sensitive for single molecule | Analysis of complex samples (e.g., environmental samples, biological fluids) | ||
| Molecular fingerprinting capability | Forensic applications for substance identification | ||
| Non-destructiveness | Food safety |
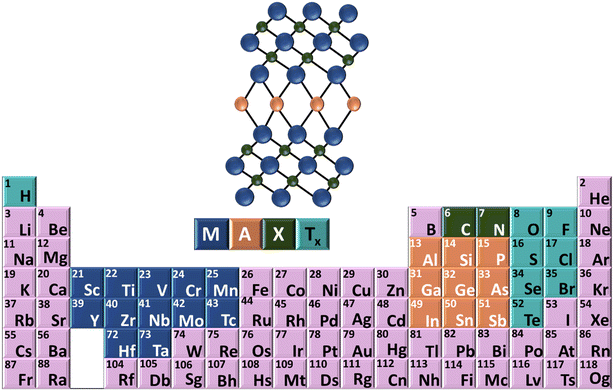
Two-dimensional (2D) materials have been demonstrated to show good promising electrochemical sensing because of mass transfer, fast electron transfer kinetics, large surface area, and outstanding electrocatalytic performance.40,41 MXenes, a distinctive group of 2D materials containing transition metal nitrides or carbides, have attracted widespread attention in environmental pollutant-related electrochemical sensing due to their exceptional metallic conductivity, abundant active catalytic sites, surface functionalization, surface area, hydrophilicity and biocompatibility.42,43 With constituents such as Ti, C, or N, along with non-toxic breakdown products (CO2 and N2), MXenes have emerged as a focal point in environmental remediation research.44 Over 30 MXene materials have been documented since 2011, including Ti3N3Tx,45 Ti3C2Tx,45,46 Nb2CTx,46 V2CTx,47 Zr3C2,48 and Lu2CTx.49 Moreover, some MXene materials predicted theoretically on the basis of MAX precursors,50 where “M” symbolizes early transition metals, “A” denotes group IIIA or IVA elements, and “X” represents elements such as carbon and nitrogen, while “Tx” signifies surface termination groups such as –O, –F, and –OH, as illustrated in Fig. 1. Notably, MXenes such as Ti3C2Tx and Ti2CTx based on titanium have been harnessed for environmental purification purposes.51 Ti3C2Tx has widespread applications in various environmental sensing applications, including the electrochemical detection of bromate, phenol, nitrite, pharmaceuticals, and heavy metal ions.52
Although some review papers have already introduced the MXene-based electrochemical sensors for pollutant sensing,30,53 it is essential to summarize the characteristics of MXene-based electrochemical sensors in detecting pollutants from the sensing behavior of MXenes and electrode fabrication methods. In this review, we first introduce the electrochemical sensing principles of MXenes, sensing mechanism and modular-based MXene electrode fabrication. Next, we summarize the progress of MXene-based materials in relation to their use in electrochemical sensing including the identification of organic agents such as pesticides and pharmaceuticals, and inorganic chemical compounds such as heavy metals and nitrites. Further, we discuss the electrochemical stability of MXenes and their impact on sensing and molecular imprinting techniques to enhance the selectivity of these sensors. Finally, a comprehensive outlook on the present challenges and potential developments in the area of electrochemical sensing with MXenes is provided.
2. Electrochemical sensing principle and modular MXene electrode fabrication
2.1 Electrochemical sensing principle
Electrochemical methods such as voltammetry, amperometry, and impedance spectroscopy offer rapid and sensitive detection, suitable for field deployment and miniaturization. Electrochemical (EC) sensing systems typically include a transducer (usually an electrode), a capture probe and a reporter probe. The transducer converts chemical reactions into measurable electrical signals, influenced by factors such as electrode material and redox reaction kinetics. The working principle and methods of MXenes involving ECs are illustrated in Fig. 2. Redox-active species undergo oxidation or reduction at the electrode surface.54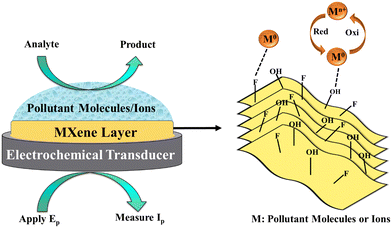 | ||
| Fig. 2 Working principle of MXene-based electrochemical sensors (Ep = applied potential, Ip = current signal). | ||
| MXene → MXene+ + e− |
| MXene+ + C6H5OH → MXene + C6H5O˙ + H+ |
| MXene + C6H5O˙ + H+ + e− → MXene + C6H5OH |
| (M2+)solution + NH2–Ti3C2Tx → M2+–NH2–Ti3C2Tx |
| M2+–NH2–Ti3C2Tx + 2e− → M0 + NH2–Ti3C2Tx |
| M0 + NH2–Ti3C2Tx → (M2+)solution + NH2–Ti3C2Tx + 2e− |
2.2 Modular MXene-based electrode fabrication
Developing large-scale and modular fabrication approach is essential to the integration of MXene-based electrodes in the electrochemical sensors.59 MXene flakes with functionalized surfaces and good dispersity facilitate modular electrode fabrication via solution-based processes. Specifically, stable colloidal dispersions of MXenes enable the use of printing or spray coating methods for electrode fabrication. The common laboratory method for producing MXene films is vacuum-assisted filtration (VAF), but scalability is crucial to ensure consistency among batches and overcome limitations in production scale. The modified VAF method can be used for the scalable production of MXene electrodes. In this context, in 2021, Wang et al.60 introduced a silt filtration-inspired method for swiftly preparing MXene films, by combining ion-induced MXene dispersion gelation with vacuum-assisted filtration, as depicted in Fig. 3a. This technique significantly reduces preparation time to seconds, making it the fastest method available. The key factors for rapid preparation include achieving proper gaps between MXene microgel particles and ensuring strong gelation and riveting effects within the microgel structure. The resulting MXene microgel film (MMF) exhibits exceptional mechanical properties, conductivity, and enhanced electrochemical properties compared to conventional MXene films. However, increased pressure notably intensified the restacking occurring between MXene flakes, and due to this, the approachability of electrolyte ions reduces and decreases the utilization rate of active sites, which reduces the performance of assembled MXene films.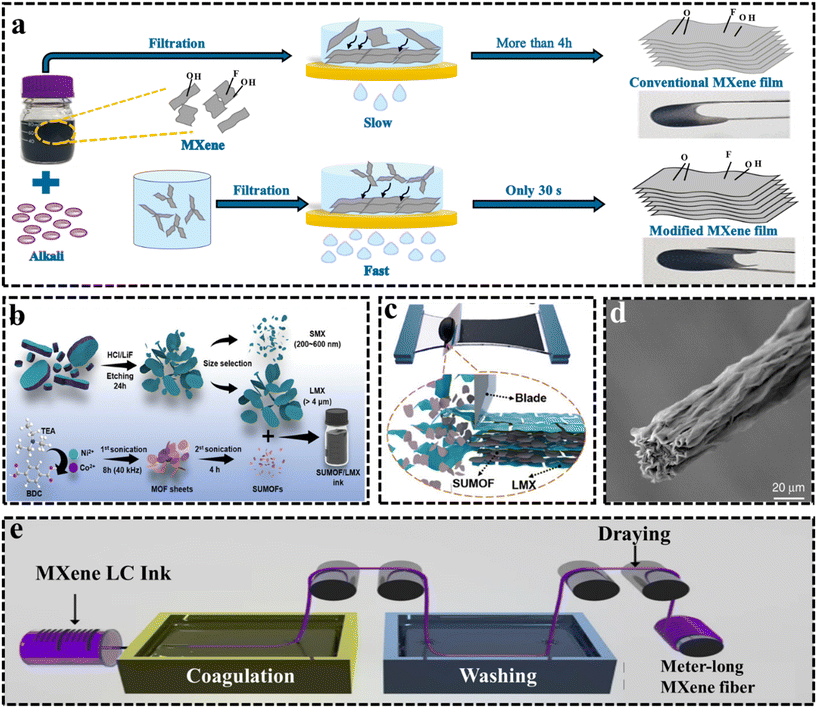 | ||
| Fig. 3 (a) Schematic of the preparation process of the modified MXene film (MMF) via a novel method combining ion-induced gelation of MXene dispersions with vacuum-assisted filtration. (b) Synthesis processes of the LMX, SMX, SUMOFs, and SUMOF/LMX composite inks.61 Reproduced from ref. 61 with permission from Wiley, Copyright 2023. (c) Schematic of the fabrication process of stretchable and bendable SUMOF/LMX composite films.61 Reproduced from ref. 61 with permission from Wiley, Copyright 2023. (d) SEM images of Ti3C2Tx MXene fibers. (e) Schematic of the wet-spinning process of the Ti3C2Tx MXene fiber.62 | ||
Another method, known as “spin coating”, is widely employed for fabricating uniform MXene-based films. This method is faster and more efficient than VAF. Initially, the MXene solution and additives undergo intense stirring or ultrasonication to form a homogeneous solution. Subsequently, the homogeneous solution is spread onto a clean substrate under centrifugal force at elevated temperatures or vacuum to allow solvent evaporation. Montazeri et al.63 developed MXene transparent contacts using a spin-coating method and applied them to Ti3C2-based MXene photodetectors. The transparent MXene film they produced reduced the trade-off between carrier transmission distance and responsiveness, leading to a four-fold increase in photodetector sensitivity compared to gold-based devices.
Another technique named “blade coating” is a scalable technique used in industrial settings, which offers a solution for producing free-standing films and coatings. In 2020, Zhang et al.64 demonstrated a method to produce highly robust and electrically conductive Ti3C2Tx MXene films using large flakes and a scalable blade coating process. They synthesized large MXene flakes by selecting appropriate MAX phase and optimizing etching conditions. The liquid crystalline MXenes and suitable dispersion properties allowed for aligned flakes during coating, resulting in films with exceptional tensile strength (∼570 MPa), Young's modulus (∼20.6 GPa), and electrical conductivity (∼15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 S cm−1 after annealing). In another study, In 2023, Jiang et al.61 used a scalable blade coating method to fabricate stretchable and bendable small-sized ultrathin/large-sized Ti3C2Tx MXene (SUMOF/LMX) composite films, as shown in Fig. 3(b and c). These films feature LMX sheets as a flexible scaffold, enabling the assembly of SUMOFs into an orderly layer structure on an elastic substrate. The resulting composite film demonstrates improved tensile strength (≈ 97 MPa). This study highlights the development of MOF-based films with excellent mechanical, electrical, and energy storage properties, offering promising prospects for commercial applications in wearable electronics.
100 S cm−1 after annealing). In another study, In 2023, Jiang et al.61 used a scalable blade coating method to fabricate stretchable and bendable small-sized ultrathin/large-sized Ti3C2Tx MXene (SUMOF/LMX) composite films, as shown in Fig. 3(b and c). These films feature LMX sheets as a flexible scaffold, enabling the assembly of SUMOFs into an orderly layer structure on an elastic substrate. The resulting composite film demonstrates improved tensile strength (≈ 97 MPa). This study highlights the development of MOF-based films with excellent mechanical, electrical, and energy storage properties, offering promising prospects for commercial applications in wearable electronics.
Furthermore, Kim et al.65 introduced a slot-die coating method for large-scale fabrication of Ti3C2Tx layers, enabling precise control over coating thickness from nanometers to micrometers. The Ti3C2Tx membranes demonstrated outstanding nanofiltration performance, with a water permeance of 190 LMH bar−1 and a molecular weight cutoff of 269 Da, surpassing previous MXene membranes and comparable to other 2D materials. These membranes also exhibited enhanced stability under harsh oxidizing aqueous conditions, maintaining stable performance over 30 days of cross-flow filtration, the longest among 2D material-based membranes. The slot-die coating's continuous, fast, scalable, and precise nature makes industrial-scale production of MXene films feasible in sensors.
In another study in 2020, Lipton et al.66 developed a method to create Ti3C2Tx MXene freestanding films by drop-casting onto hydrophobic plastic substrates. These films, due to the substrate's hydrophobic nature, can be easily separated, showcasing preferential MXene–MXene interactions over MXene–substrate interactions. The drop-casting (DC) technique allows for the production of large-area (>125 cm2), thick (23.2 mm) films with smooth surfaces (14 nm RMS roughness) and high electrical conductivity (∼7000 S cm−1). By employing a DC technique on common plastic substrates, the study improved Ti3C2Tx flake alignment through natural forces during film deposition. Moreover in 2020, Eom et al.62 developed a large-scale method for creating pure MXene fibers without additives or binders. They utilized wet-spinning assembly, where MXene dispersion was extruded through a nozzle into a coagulant solution consisting of NH4Cl (50 g), NH4OH solution (20 mL), and deionized water (DIW) (1000 mL). The MXene dispersions were extruded at a velocity of 7 mL h−1. By introducing ammonium ions during coagulation, they produced flexible, meter-long MXene fibers with high electrical conductivity (7713 S cm−1). The SEM image of the MXene fiber is presented in Fig. 3d, while the wet-spinning process of the Ti3C2Tx MXene fiber is shown in Fig. 3e. These methods are usually only effective for preparing thicker films. Concentrated MXene solutions are required for the coating process because a certain level of viscosity is necessary to maintain the shape of the applied solution on the target substrate. Moreover, any residual coating solution may lead to contamination and damage to the film. Additionally, these techniques inherently involve significant wastage, which can be critical when implemented on an industrial scale. However, despite these numerous efforts, there is still room for improvement to meet commercial standards.
3. MXene-based electrochemical sensors for organic pollutants, pesticides and pharmaceuticals
MXenes exhibit a notable capacity for detecting an extensive array of environmental organic pollutants, especially in water phenolic compounds, introduced into ecosystems through agricultural, industrial, sewage, and pharmaceutical sources, pose substantial health risks owing to their toxic and mutagenic, carcinogenic, and teratogenic properties.673.1 MXene-based electrochemical sensors for organic pollutants
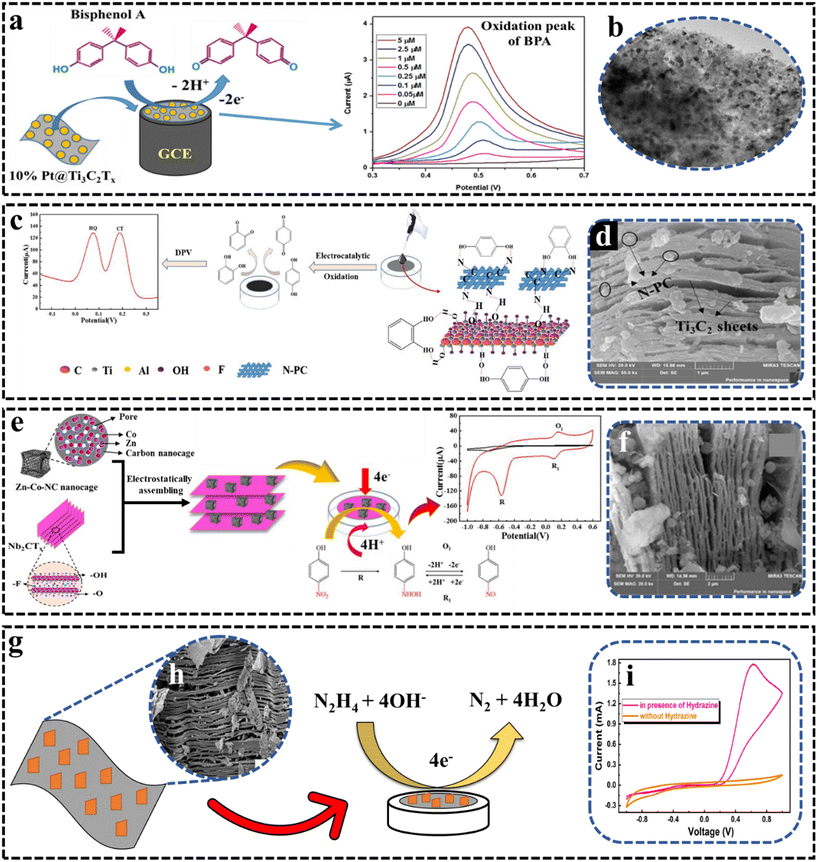 | ||
| Fig. 4 (a) Schematic representation of the sensing mechanism relying on BPA oxidation at the Pt@Ti3C2Tx-modified electrode.52 Reproduced from ref. 52 with permission from Elsevier, Copyright 2021. (b) TEM image of 10%Pt@Ti3C2Tx.52 Reproduced from ref. 52 with permission from Elsevier, Copyright 2021. (c) Schematic of the application of alk-Ti3C2/N-PC/GCE.69 Reproduced from ref. 69 with permission from Elsevier, Copyright 2020. (d) SEM image of alk-Ti3C2/N-PC. Reproduced from ref. 69 with permission from Elsevier, Copyright 2020. (e) Schematic of the preparation and application of Nb2CTx/Zn–Co-NC/GCE. Reproduced from ref. 70 with permission from Elsevier, Copyright 2021. (f) SEM image of the Nb2CTx/Zn–Co-NC composite.70 Reproduced from ref. 70 with permission from Elsevier, Copyright 2021. (g) Sensing mechanism of hydrazine. (h) SEM image of 10%Er-Nb2C. Reproduced from ref. 71 with permission from Elsevier, Copyright 2021. (i) CV curve in the absence and presence of 13.76 mM of hydrazine.71 Reproduced from ref. 71 with permission from Elsevier, Copyright 2021. | ||
Hydroquinone (HQ) and catechol (CT) are toxic phenolic compounds, which usually co-exist in samples and it is a challenge to detect because both have a nearly similar structure. In 2020, Huang et al.69 developed an electrochemical sensor using alkalized-Ti3C2/nitrogen-doped porous carbon (N-PC) to detect HQ and CT. The alkalized-Ti3C2 exhibited an expanded interlayer spacing and the capability to capture HQ and CT due to hydrogen bond interactions between the OH groups on its surface and those in HQ and CT. Additionally, the N-PC derived from MOF-5-NH2 featured a unique C–N bond, which attracted the OH groups of HQ and CT. Thus, integrating N-PC as an intercalator into the Ti3C2 sheets had a dual effect on sensing HQ and CT, enhancing the sensor's performance, as shown in Fig. 4c, while Fig. 4d presents the SEM image of alk-Ti3C2/N-PC. The XPS results indicate the presence of pyridinic-N, pyrrolic-N, and graphitic-N, with pyrrolic-N suggesting an abundance of adsorption sites for phenolic pollutants. This sensor leveraged the excellent electrical conductivity of alk-Ti3C2 and the large surface area of N-PC to detect HQ and CT based on their benzenediol/benzoquinone redox reactions. The sensor showed a wide linear range of 0.5–150 μM with low detection limits of 4.8 nM for HQ and 3.1 nM for CT. Furthermore, the sensor showed excellent selectivity, and the relative standard deviation (RSD) was less than ±6.6%.
Hydrazine is a crucial raw material, yet it is also a significant environmental pollutant and a potent carcinogen. Due to its excessive use and mishandling, substantial quantities of hydrazine are released into the environment each year. Consequently, developing a reliable and sensitive method for the detection of hydrazine is of utmost importance. In this regard, Yao et al.72 used a Ti3C2Tx MXene material as a conductive platform and combined it with ZIF-8, known for its unique electrocatalytic activity, to create a MXene/ZIF-8 nanocomposite. This composite was applied to a glassy carbon electrode (GCE) to develop a sensor for electrochemical hydrazine detection. The synergistic effect of MXene's high electrical conductivity and ZIF-8's electrocatalytic properties significantly enhanced the sensor's electrochemical performance for hydrazine detection, suggesting that the MXene/ZIF-8 composite has great potential for electrochemical sensing applications. The current response increased linearly with hydrazine additions across a wide range from 10 μmol L−1 to 7.7 mmol L−1. Beyond 7.7 mmol L−1, the response deviated from linearity, probably due to the saturation of the electroactive sites on the modified electrode. The limit of detection (LOD) was calculated to be 5.1 μmol L−1.
From the above discussion, we conclude that both Ti-based and Nb-based MXenes exhibit high sensitivity and selectivity in detecting various organic pollutants. Ti-based sensors, particularly those enhanced with noble metal nanoparticles such as Pt and Pd, show a superior catalytic activity and stability, leading to lower detection limits and broader linear ranges. Furthermore, Nb-based MXenes, especially when doped with elements such as Er, demonstrate significant improvements in sensitivity and a wide linear detection range. More work is required on other metal MXenes, beyond Ti, to enhance the sensitivity and stability of MXene-based sensors, similar to the focus initially given to Ti-based MXenes. In our opinion, noble metals show good sensing performances with Ti-based MXenes, hence it is necessary to try these metals with other MXenes and also try non-noble metals with MXenes to make them cost-efficient. Overall, MXenes represent a promising platform for developing affordable, high-performance electroanalytical techniques needed for organic pollutant detection. The performances of different MXene-based electrochemical sensors for sensing various organic pollutants are summarized in Table 2.
| Sensing material | Analyte | Detection method | LOD | Linear range | Ref. |
|---|---|---|---|---|---|
| Gr/Ti3C2Tx | BPA | DPV | 0.35 μM | 1–10 μM | 73 |
| Amperometry | 4.08 nM | 10–180 nM | |||
| Ti3C2-MWCNTs | HQ | DPV | 0.0066 μM | 2–150 μM | 74 |
| CT | 0.0039 μM | ||||
| Ti3C2F/MnMoO4 | CT | DPV | 0.0003 μM | 5–65 nM | 75 |
| HQ | 0.00026 μM | ||||
| Alk-Ti3C2/N-PC | CT | DPV | 0.0031 μM | 0.5–150 μM | 69 |
| HQ | 0.0048 μM | — | |||
| Nb2CTx/Zn–Co-NC | 4-Nitrophenol | 70 nM | 1–500 μM | 70 | |
| N–Ti3C2/PC | 4-Aminophenol | DPV | 59 nM | 1–150 μM | 76 |
| Acetaminophen | 50 nM | ||||
| MXene/ZIF-8/GCE | Hydrazine | Amperometry | 5.1 μmol L−1 | 10–7700 μmol L−1 | 72 |
| Er-doped Nb2C MXene | Hydrazine | LSV/CV | 67 μM | 71 | |
| Ti3C2/MWCNTs | Ochratoxin A | 0.028 μM | 0.09–10 μM | 77 | |
| Ti3C2Tx@AgNC/NH2-MWCNTs | CBZ | DPV | 0.0001 μM | 0.0003–10 μM | 78 |
| ERGO/MXene | CBZ | DPV | 0.67 nM | 2 nM–10 μM | 79 |
| Ti3C2Tx MXene | CBZ | DPV | 10.3 nM | 0.05–100 μM | 80 |
| β-CD-MOF/MXene/CNHs | CBZ | EIS | 0.001 μM | 0.003–10 μM | 81 |
| Mo2C@NiMn-LDH | CBZ | DPV | 0.2 nM | 0.001–232.14 μM | 82 |
| MnO2NWs@Mo2TiC2 | Fenitrothion | DPV | 1 nmol L−1 | 1–20 nmol L−1 | 83 |
| Ti3C2Tx-flakes | Methiocarb | DPV | 0.19 μg ml−1 | 1–60 μg mL−1 | 84 |
| Diethofencarb | DPV | 0.46 μg ml−1 | 1–55 μg mL−1 | ||
| MIP/d-S-Ti3C2Tx/QCM | Chlorpyrifos | CV | 0.31 pM | 10−12–10−10 M | 85 |
| Ti3C2Tx–TiO2 | Thiabendazole | DPASV | 0.1 nM | 0.3–100.0 nM | 86 |
| TFA@Nb2CTx | RIF | CV | 4.8 pM | 100 pM–1 μM | 87 |
| MXene/DNA/PtNPs/Pd | DA | Amperometry | 0.03 μM | 0.2–1000 μM | 88 |
| MXene-perylene diimide | DA | 0.24 μM | 100–1000 μM | 89 | |
| Nb4C3Tx | DA | 0.023 μM | — | 90 | |
| Ti3C2Tx/Nafion | DA | CV | 0.003 μM | 0.015–10 μM | 91 |
| Ti3C2Tx | Acetaminophen | DPV | 48 nM | 0.25–2000 μM | 92 |
| Isoniazid | 64 nM | 0.1–4.6 mM | |||
| Ti3C2/MWCNTs/Chit | Acetaminophen | DPV | 0.28 nM | 0.0042–7.1 μM | 93 |
| Ifosfamide | 0.31 nM | 0.0011–1 μM | |||
| Sumatriptan | 0.42 nM | 0.0033–61 μM | |||
| Domperidone | 0.34 nM | 0.0046–7.3 μM | |||
| Ti3C2Tx | Adrenaline | Chronoamperometry | 0.0095 μM | 0.02–10 μM | 94 |
| Ti3C2Tx/polypyrrole | Uric acid | DPV | 0.15 μM | 50–500 μM | 95 |
| DA | 0.37 μM | 12.5–125 μM | |||
| MXene/MWCNTs | Synephrine | LSC/CV | 0.167 μM | 0.5–70 μM | 96 |
| MXene/N-rGO | Adrenaline | DPV | 3 nM | 10 nM–90 μM | 97 |
| Ti3C2/BN-nanocomposite | Sulfadiazine | DPV | 3 nM | 59–186 μM | 98 |
| AuNP@Ti3C2Tx | Folic acid | CV | 6.2 nM | 0.02–3580 μM | 99 |
| Uric acid | 11.5 nM | 0.03–1520 μM | |||
| Ti3C2Tx | Uric acid | DPV | 75 nM | 0.5–1500 μM | 100 |
| DA | 60 nM | 0.5–50 μM | |||
| Ascorbic acid | 4.6 μM | 100–1000 μM | |||
| MXene/CuNPs | Piroxicam | SWV | 50 nM | 0.1–80 μM | 101 |
| Ti3C2/rGO | FZD | DPV | 0.002 μM | 0.01–111 μM | 102 |
| CoVO/Ti3C2Tx | NO2− | DPV | 0.1 μM | 0.5–2000 μM | 103 |
| Ti3C2Tx–Fe2O3 | H2O2 | CV | 7.46 nM | 10–1000 nM | 104 |
| Ti3C2Tx–Fe2O3 | H2O2 | Amperometry | 5.0 μM | 0.005–5.0 mM | 105 |
| Ti3C2Tx/Pt NP | H2O2 | Amperometry | 0.448 μM | 490 μM–53.6 mM | 106 |
| Nafion/Hb/Ti3C2 | H2O2 | Amperometry | 0.02 μM | 0.1–260 μM | 107 |
| TiO2–Ti3C2/Nafion/Hb | H2O2 | Amperometry | 0.014 μM | 0.1–380 μM | 108 |
| Ti3C2Tx | H2O2 | Chronoamperometry | 0.0007 μM | — | 109 |
| NiO/Ti3C2Tx | H2O2 | DPV | 0.34 μM | 0.01–4.5 mM | 110 |
| CuO–CeO2/Ti3C2Tx | H2O2 | Chronoamperometry | 1.67 μM | 5.0–100 μM | 111 |
| AuNPs/Ti3C2Tx | NO2− | Amperometry | 0.14 μM | 1–4581 μM | 112 |
| AuNPs/Ti3C2Tx-PDDA | NO2− | Amperometry | 59 nM | 0.1–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 μM 500 μM |
113 |
| Pd–Cu–Mo2C | NO2− | Amperometry | 0.35 nM | 5–165 nM | 114 |
| Ti3C2/Nafion/Hb | NO2− | Amperometry | 0.12 μM | 0.5–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 μM 800 μM |
115 |
| Ti3C2Tx | BrO3 | DPV | 0.041 μM | 50 nM–5 μM | 116 |
| Ti3C2Tx MXene | H2S | Amperometry | 16 nM | 0.1–300 μM | 117 |
| Cu/Cu2O/TiO2/Ti3C2 | H2O2 | CV | 0.42 μM | 0.002–28.328 mM | 118 |
| Ti3C2@N–C | Cd2+ | SWASV | 0.002 μM | 0.1–4 μM | 119 |
| Pb2+ | SWASV | 0.0011 μM | 0.05–2 μM | ||
| Alk-Ti3C2 | Pb2+ | SWASV | 41 nM | 0.1–1.5 μM | 120 |
| Cd2+ | 98 nM | ||||
| Hg2+ | 130 nM | ||||
| Cu2+ | 32 nM | ||||
| Nb4C3Tx | Pb2+ | SWASV | 0.012 | 0.025–0.5 μM | 121 |
| Fe-MOF/Ti3C2Tx | As3+ | SWASV | 0.58 ng L−1 | 1–100 ng L−1 | 122 |
| H–C3N4/Ti3C2Tx | Cd2+ | SWASV | 1 nM | 50 nM–1.5 μM | 123 |
| Pb2+ | 0.6 nM | ||||
| Ti3C2Tx/BiNPs | Pb2+ | SWASV | 0.0108 μM | 0.06–0.6 μM | 124 |
| Cd2+ | 0.0124 μM | 0.08–0.6 μM | |||
| Bi@d-Ti3C2 | Cd2+ | SWASV | 0.4 μg L−1 | 1–20 μg L−1 | 125 |
| Zn2+ | 0.5 μg L−1 | ||||
| Pb2+ | 0.2 μg L−1 | ||||
| PANI-Ti3C2 | Hg2+ | SWASV | 0.017 μg L−1 | 0.1–20 μg L−1 | 126 |
| Ti3C2Tx/MWCNTs | Cu2+ | SWASV | 0.1 ppb | 10–500 ppb | 127 |
| Zn2+ | 1.5 ppb | 200–600 ppb | |||
| NH2–Ti3C2Tx | Cd2+ | DPASV | 0.41 μg L−1 | 10–500 μg L−1 | 58 |
| Pb2+ | 0.31 μg L−1 |
3.2 MXene-based electrochemical sensors for pesticides
Pesticide residues pose serious health issues.128 Some esters derived from phosphoric acid are known as organophosphate pesticides (OPs), which are widely used across the world to boost the agricultural output. However, their overuse may contaminate ecosystems and constitute a serious risk to human health.129,130 Consequently, researchers have developed diverse sensors for real-world pesticide detection.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 684 μA mM−1 cm−2) and selectivity toward CBZ. The DPV response of sensor is depicted in Fig. 5b. The synergistic effect of AuNPs, ZIF-L, and Ti3C2Tx in the unique material structure contributed to its high-performance characteristics. The MXene-based nanocomposite has also been employed to detect the widely used pesticide Thiabendazole (TBZ), a benzimidazole fungicide that helps preserve the freshness of fruits and vegetables and control various fungal diseases in crops. In 2023, Zhong et al.86 introduced an innovative method for sensing thiabendazole (TBZ) using an indirect signal amplification strategy. They employed anodic stripping voltammetry of Cu2+ on a modified electrode made of hierarchical Ti3C2Tx–TiO2 for TBZ sensing. The integration of TiO2 particles effectively prevents the stacking of the Ti3C2Tx multilayered structure, thereby increasing the active surface area for the adsorption and loading of Cu2+. This enhancement boosts the electrochemical signal and Cu2+ quickly coordinates with TBZ, forming a non-electroactive Cu2+–TBZ complex as shown in Fig. 5c. The sensor exhibited outstanding electrochemical properties for TBZ detection, featuring a linear range of 0.3 to 100 nM and an impressively low LOD of 0.1 nM. Fig. 5d presents the SEM image of the Ti3C2Tx–TiO composite, while Fig. 5e shows the DPV response of TBZ toward the sensor. Additionally, the sensor demonstrated excellent reproducibility, anti-interference characteristics, and stability, proving its effectiveness in detecting TBZ in water and fruit samples.
684 μA mM−1 cm−2) and selectivity toward CBZ. The DPV response of sensor is depicted in Fig. 5b. The synergistic effect of AuNPs, ZIF-L, and Ti3C2Tx in the unique material structure contributed to its high-performance characteristics. The MXene-based nanocomposite has also been employed to detect the widely used pesticide Thiabendazole (TBZ), a benzimidazole fungicide that helps preserve the freshness of fruits and vegetables and control various fungal diseases in crops. In 2023, Zhong et al.86 introduced an innovative method for sensing thiabendazole (TBZ) using an indirect signal amplification strategy. They employed anodic stripping voltammetry of Cu2+ on a modified electrode made of hierarchical Ti3C2Tx–TiO2 for TBZ sensing. The integration of TiO2 particles effectively prevents the stacking of the Ti3C2Tx multilayered structure, thereby increasing the active surface area for the adsorption and loading of Cu2+. This enhancement boosts the electrochemical signal and Cu2+ quickly coordinates with TBZ, forming a non-electroactive Cu2+–TBZ complex as shown in Fig. 5c. The sensor exhibited outstanding electrochemical properties for TBZ detection, featuring a linear range of 0.3 to 100 nM and an impressively low LOD of 0.1 nM. Fig. 5d presents the SEM image of the Ti3C2Tx–TiO composite, while Fig. 5e shows the DPV response of TBZ toward the sensor. Additionally, the sensor demonstrated excellent reproducibility, anti-interference characteristics, and stability, proving its effectiveness in detecting TBZ in water and fruit samples.
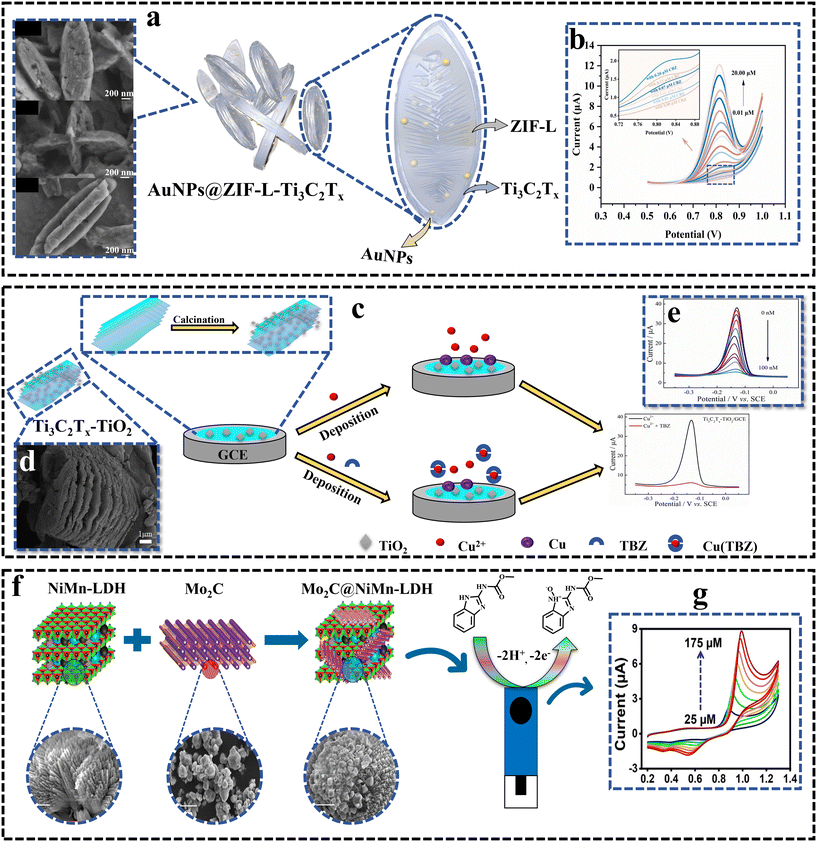 | ||
| Fig. 5 (a) SEM image of AuNPs@ZIF-L–Ti3C2Tx. Reproduced from ref. 131 with permission from Elsevier, Copyright 2024. (b) DPV AuNPs@ZIF-L–Ti3C2Tx/GCE upon the successive injection of CBZ into 0.1 M PBS/pH 7.0; the inset is 0 μM to 0.20 μM CBZ.131 Reproduced from ref. 131 with permission from Elsevier, Copyright 2024. (c) Fabrication of Ti3C2Tx–TiO2/GCE and underlying electrochemical detection principle. (d) SEM image of the Ti3C2Tx–TiO2 composite. Reproduced from ref. 86 with permission from Elsevier, Copyright 2023. (e) DPV curves of Ti3C2Tx–TiO2/GCE response to different concentrations of TBZ in 0.1 M PBS (pH 6.0).86 Reproduced from ref. 86 with permission from Elsevier, Copyright 2023. (f) Schematic of the preparation of the Mo2C@NiMn-LDH composite for the electrochemical detection of CBZ. Reproduced from ref. 82 with permission from the American Chemical Society, Copyright 2021. (g) CVs of different concentrations of CBZ at Mo2C@NiMn-LDH/SPCE.82 Reproduced from ref. 82 with permission from the American Chemical Society, Copyright 2021. | ||
The above-mentioned results reported in the literature shows that the Ti-MXene has been more extensively studied and used in pesticide detection. There is a growing body of literature exploring the electrochemical sensing potential of the Ti-MXene and its composites for various analytes including pesticides. Mo-MXenes, although less extensively studied in the context of pesticide sensing, have shown promising results and have been successfully employed in the development of sensors for OP detection. In summary, both Mo-MXenes and Ti-MXenes have demonstrated potential for pesticide sensing. Ti-MXenes have been more widely studied and integrated into various sensor designs, while Mo-MXene shows promise in terms of its material properties and performance in pesticide detection. Overall, future research in Mo-MXene-based electrochemical sensors for pesticide detection should focus on further improving the detection limits, expanding the range of detectable pesticides and enhancing the sensors' performance in complex sample matrices. Additionally, efforts should be made to explore the scalability and practicality of these sensors for real-world applications, including field testing and integration into monitoring systems. Continued advancements in MXene-based sensor technology hold great potential for addressing pesticide contamination issues and ensuring food safety.
3.3 MXene-based electrochemical sensors for pharmaceutical detection
Pharmaceutical pollutants can lead to antibiotic resistance, hormone disruption and undesired physiological effects. The rising pharmaceutical use due to population growth makes these pollutants, often overlooked by authorities, a potential major societal concern.132,133 The detection of the concentration of specific pharmaceutical contaminants in the environment is a challenge due to their trace levels.133–136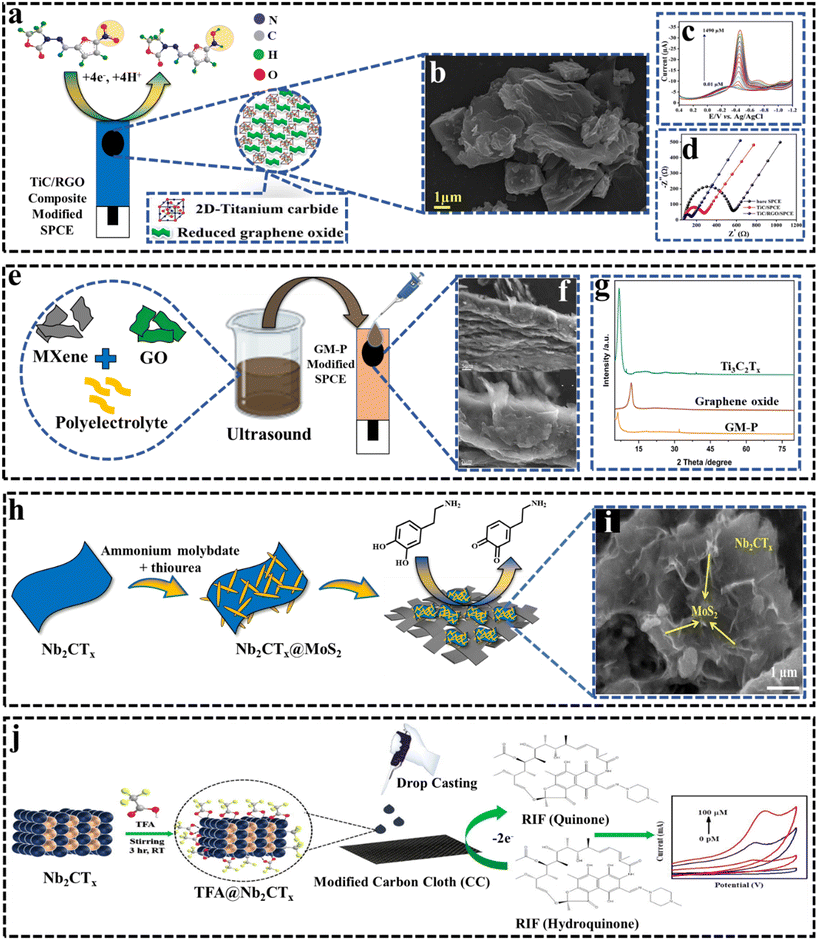 | ||
| Fig. 6 (a) Illustration of the TiC/rGO mechanism towards FZD. Reproduced from ref. 102 with permission from Elsevier, Copyright 2021. (b) FESEM image of the TiC/RGO composite. Reproduced from ref. 102 with permission from Elsevier, Copyright 2021. (c) DPV response of TiC/RGO/SPCE towards FZD detection. Reproduced from ref. 102 with permission from Elsevier, Copyright 2021. (d) Nyquist plot for bare, TiC and TiC/SPCE electrodes.102 Reproduced from ref. 102 with permission from Elsevier, Copyright 2021. (e) Schematic of the synthesis process of MXenes and graphene nanosheets self-assembled into GM-P composites under low-temperature ultrasound with the assistance of PDDA for electrochemical detection of FZD. (f) SEM images of GM-P. Reproduced from ref. 140 with permission from Elsevier, Copyright 2024. (g) XRD patterns of the MXene (Ti3C2Tx), graphene oxide, and GM-P samples.140 Reproduced from ref. 140 with permission from Elsevier, Copyright 2024. (h) Schematic of the synthesis process of Nb2CTx@MoS2. (i) SEM image of Nb2CTx@MoS2.141 Reproduced from ref. 141 with permission from Elsevier, Copyright 2023. (j) Diagram showing the electrochemical detection of the anti-tuberculosis medicine RIF using TFA-modified Nb2CTx MXenes.87 Reproduced from ref. 87 with permission from Elsevier, Copyright 2023. | ||
Given the rapidly expanding applications of MXenes, they can also be utilized to detect dopamine (DA), a widely known peripheral vasostimulant used in the treatment of low blood pressure, slow heart rate, and cardiac arrest. In this regard, Shahzad et al.91 (2019) introduced simple Ti3C2Tx/Nafion-based electrochemical sensor for the detection of DA. It is observed that the negative surface functional groups of MXenes are helpful for DA sensing, and additional Nafion promotes the interaction of DA with electrode surface, which results in a better electrocatalytic response. Sensors show good sensitivity with a low limit of detection of 3 nM and as well as good selectivity.
Acetaminophen (ACOP) and isoniazid (INZ) are pharmaceutical compounds that can act as environmental pollutants when they enter water bodies through improper disposal or wastewater discharge. Acetaminophen, a common pain reliever, and isoniazid, an antibiotic for tuberculosis, can pose risks to aquatic ecosystems and human health due to their persistence and bioactivity. Therefore, it is necessary to detect these pollutants to mitigate their environmental impact and protect public health. Therefore, in 2019 Zhang et al.92 developed a sensitive electrochemical sensor based on MXene-modified SPE. Different Surface functional groups on MXenes provide multiple binding sites for ACOP and INZ. MXene's high conductivity and large surface area facilitate electron transfer, improving the oxidation process. The use of 0.1 M H2SO4 as the electrolyte ensures optimal conditions for the electrooxidation of both drugs, as ACOP is deprotonated and repelled by the negatively charged MXene surface at higher pH levels. The detection limits for ACOP and INZ are 0.048 μM and 0.064 mM, respectively, indicating high sensitivity.
Nb-based MXenes show significant potential in sensing pharmaceutical residues. To expand their applicability, Ankitha et al.87 (2023) synthesized an exceptionally stable Nb2CTx MXene modified with trifluoroacetic acid (TFA) for the electrochemical detection of rifampicin (RIF), an anti-tuberculosis drug. An increase in the d-spacing of Nb2CTx was observed, suggesting the intercalation of TFA between the layers of Nb2CTx nanosheets. This intercalation led to increased conductivity and high electron transfer kinetics towards RIF analyte molecules. Furthermore, the oxidation of Nb2CTx is also observed. When applied to a carbon cloth (CC) substrate, as illustrated in Fig. 6j, the 0.3TFA@Nb2CTx modification demonstrated the ability to detect RIF concentrations linearly within the range of 100 pM to 1 μM, achieving an outstandingly low detection limit of 4.8 pM. This LOD enhancement is a significant advancement in comparison to the existing literature. The sensor shows excellent selectivity towards RIF even in the presence of 10 folds concentration of interferents.
The above-mentioned results reported in the literature indicate that MXene materials, especially Ti- and Nb-based MXenes, have shown great potential as electrochemical sensing platforms for detecting various pharmaceutical pollutants. Their exceptional properties such as large surface area and adjustable surface functionalization make them suitable for electroanalytical applications. Sensors based on MXene/composite materials have enabled low detection limits, wide linear ranges and high sensitivities for different pharmaceutical targets. Doping/intercalation with conductive nanomaterials or metals is an effective strategy to further improve the electrochemical performance by enhancing the active sites, charge transfer and mass transport. However, to advance this field, future research should focus on broadening the analytical applications to enable multi-class detection of diverse pharmaceuticals simultaneously. Exploring synergistic combinations of new MXene variants (e.g., V2C and Nb2C) with catalytic nanomaterials can lead to even more efficient sensors. Additionally, developing flexible and wearable sensor platforms that integrate MXenes will be crucial for real-time in situ and in vivo monitoring, paving the way for innovative applications in health and environmental monitoring.
4. MXene-based electrochemical sensors for inorganic pollutant detection
Some inorganic compounds such as noxious gases and heavy metal ions (HMIs) emanate from diverse sources.143–145 In this section, we highlight the accomplishments of electrochemical sensors using MXenes and their composites in the detection of inorganic toxins, encompassing HMI, hazardous gases, nitrates, and other forms of inorganic contaminants.4.1 MXene-based electrochemical sensors for nitrite and hydrogen peroxide detection
Nitrite is one of the inorganic environmental contaminants that might have a negative impact on the ecological environment due to its extensive adoption as a nitrogen fertilizer in agriculture and an industrial food additive. Elevated nitrite levels within the human body can interact with oxygenated hemoglobin, resulting in the formation of methemoglobin. This altered hemoglobin variant exhibits diminished capability for oxygen transportation in the bloodstream. Additionally, it can lead to the production of cancer causing N-nitrosamines in human beings, which could have negative health effects.146 According to available reports,147 it has been documented that the ingestion of nitrite in quantities ranging from 0.3 to 0.5 grams has been associated with instances of poisoning, while a higher dosage of approximately 3 grams has been linked to cases resulting in fatality.148,149 The World Health Organization's standards state that there should be no more than 3 mg L−1 of nitrite in drinking water, hence it is crucial to precisely measure the amount of nitrite present in the environment.147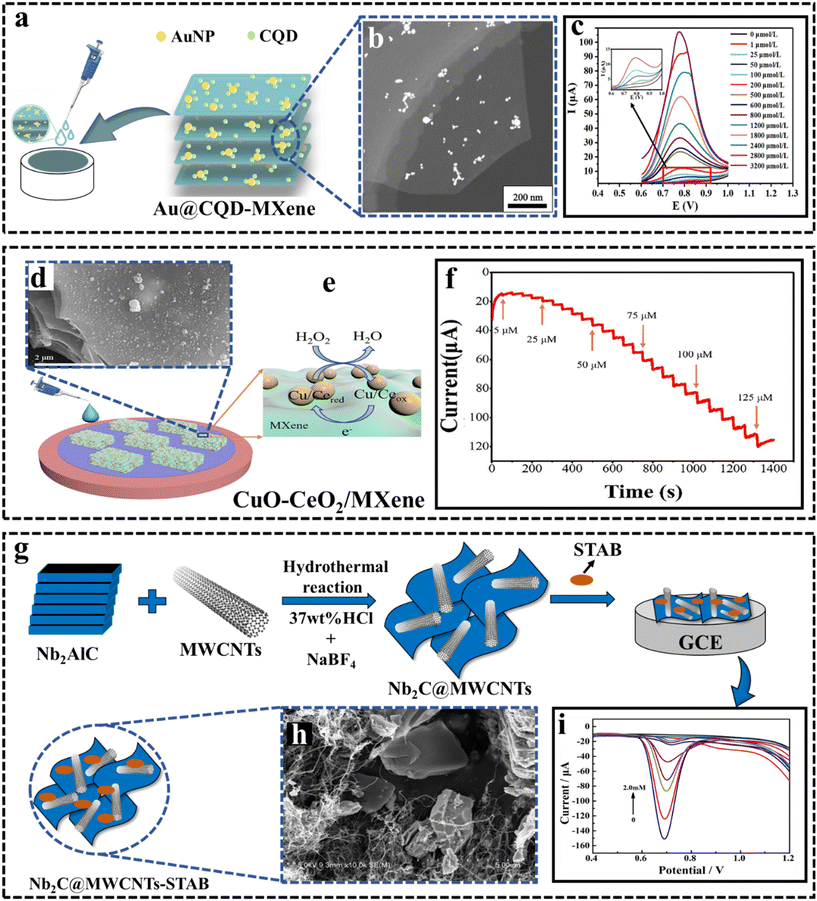 | ||
| Fig. 7 (a) Development of the nitrite detection platform using Au@CQDs–MXene/GCE. Reproduced from ref. 150 with permission from Elsevier, Copyright 2022. (b) TEM images of Au@CQDs–MXene. Reproduced from ref. 150 with permission from Elsevier, Copyright 2022. (c) DPV response of the Au@CQDs–MXene modified GCE in 0.1 M PBS (pH 7.0) with different concentrations of nitrite (0 μM–3.2 mM); the inset shows the peaks from 0 μM to 100 μM.150 Reproduced from ref. 150 with permission from Elsevier, Copyright 2022. (d) SEM images of CuO–CeO2/MXene. Reproduced from ref. 111 with permission from Elsevier, Copyright 2022. (e) Assembly of the electrochemical H2O2 sensor using CuO–CeO2. Reproduced from ref. 111 with permission from Elsevier, Copyright 2022. (f) The current responses of CuO–CeO2/MXene/GCE for different concentrations of H2O2.111 Reproduced from ref. 111 with permission from Elsevier, Copyright 2022. (g) Schematic of the construction of the Nb2C@MWCNTs-STAB/GCE sensor. (h) SEM image of Nb2C@MWCNTs-STAB. Reproduced from ref. 151 with permission from Elsevier, Copyright 2022. (i) DPV responses of the Nb2C@MWCNTs-STAB/GCE sensor in NO2− solutions of different concentrations.151 Reproduced from ref. 151 with permission from Elsevier, Copyright 2022. | ||
On another note, hydrogen peroxide (H2O2) is an extensively used oxidant in several sectors including food processing, paper manufacture, wastewater treatment, textiles, and chemicals.152 It is also employed for disinfection in the food industry, which can lead to H2O2 residues in food. Excessive H2O2 poses health risks including cancer, diabetes, and Alzheimer's disease.153 Therefore, it is essential to create credible methods for the precise detection of H2O2 in food and blood samples.154,155 In 2021, Cheng et al.155 introduced a distinctive electrochemical H2O2 sensor by fusing an ultra-thin MXene nanosheet with a three-dimensional flower-like Cu-MOF. Cu-MOF displayed high electrocatalytic activity for H2O2 reduction. The introduction of MXenes significantly enhanced the sensor's current response to H2O2 by improving the electrical conductivity and accelerating electron transfer rates. Additionally, MXene modification on the electrode surface facilitated the immobilization of Cu-MOF via electrostatic interaction, using MXene's large surface area and negative surface groups. The sensor exhibits an extensive linear range spanning from 1 mol L−1 to 6.12 mmol L−1 and an estimated limit of detection of 0.35 mol L−1 for H2O2 detection, utilizing chronoamperometry at a detection potential of 0.35 V. Following this research in 2022 Zhou et al.111 introduced an electrochemical sensor designed for continuous H2O2 sensing, relying on CuO–CeO2/Ti3C2Tx. The SEM image of CuO–CeO2/Ti3C2Tx is illustrated in Fig. 7d. The CuO–CeO2 nanocomposites synthesized by a sol–gel method served as catalysts, facilitating the process of H2O2 electrolysis on the electrode surface and amplifying the reduction current for H2O2 sensing, as shown in Fig. 7e. Electron-rich –F functional groups are present on the surface of MXenes, and CuO–CeO2 binds to the MXene surface, forming a nanoparticle layer. This layer facilitates the exposure of more reaction sites for H2O2. In essence, MXenes enhance the interaction with CuO–CeO2, promoting the efficient utilization of H2O2. It exhibited a strong linear relationship between 5 and 100 μM for H2O2 detection, and the current response is shown in Fig. 7f, boasting a low LOD 1.67 μM.
MXene materials have shown great potential as effective sensing platforms for nitrite and hydrogen peroxide due to their unique properties such as high conductivity, tunable surface chemistry and layered structure. For nitrite sensing, sensors based on Ti- and Nb-MXenes have demonstrated low detection limits, wide linear ranges and good selectivity. Functionalization strategies such as doping and composite formation have further improved the sensor performance. Regarding hydrogen peroxide sensing, noble metal–MXene and metal oxide–MXene composites have enabled sensitive detection. The synergistic effects between different components enhance active sites, electron transfer and anti-fouling ability. However, there is still room for improvement in some aspects. Future work could focus on exploring new MXene variants and their combinations with additional catalysts. The fabrication of hierarchical 3D structures and flexible devices would expand the practical applications.
4.2 MXene-based electrochemical sensors for heavy metals ion detection
Heavy metal contamination has long been recognized as one of the most widespread and important environmental issues in the natural environment. Heavy metal contamination is known for its non-biological decay and high cytotoxicity to organisms, which have a serious hazard to the living environment.157 Heavy metals such as lead (Pb), mercury (Hg), cadmium (Cd), chromium (Cr), iron (Fe), copper (Cu), arsenic (As), and manganese (Mn) are discharged from various industries and remain persistent in the environment for extended periods. After release, these metal ions enter water bodies, make their way to the surface water, and pose serious risks to aquatic life and human health. As a result, researchers and environmental scientists are very concerned with the identification and removal of these dangerous contaminants.158,159 In this context, the MXene is highly effective for the electrochemical detection of heavy metals due to their advantageous properties.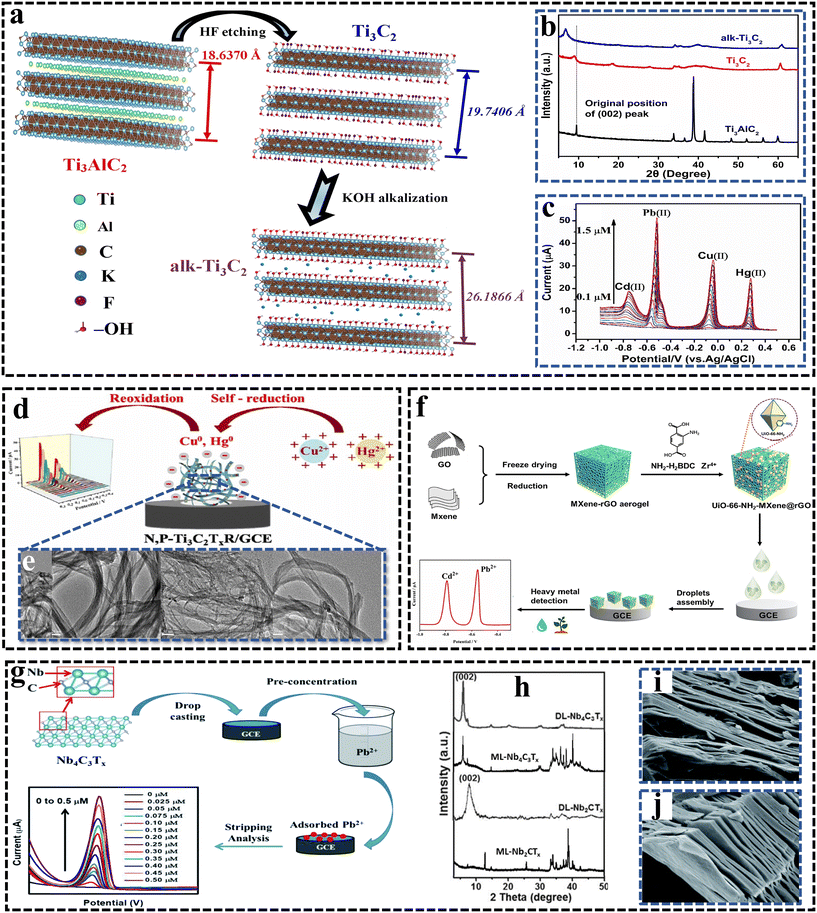 | ||
| Fig. 8 (a) Structural illustrations of the parental Ti3AlC2 MAX phase and the resulting MXene before and after alkalization. Reproduced from ref. 120 with permission from Elsevier, Copyright 2017. (b) XRD patterns of the synthesized Ti3AlC2, Ti3C2 and alk-Ti3C2. Reproduced from ref. 120 with permission from Elsevier, Copyright 2017. (c) SWASV response of the alk-Ti3C2-modified GCE for the simultaneous analysis of Cd(II), Pb(II), Cu(II) and Hg(II) over a concentration range of 0.1–1.5 μM.120 Reproduced from ref. 120 with permission from Elsevier, Copyright 2017. (d) Schematic of the synthesis of N,P-Ti3C2TxR and electrochemical sensing of Cu2+ and Hg2+. Reproduced from ref. 160 with permission from Elsevier, Copyright 2022. (e) TEM image of N,P-Ti3C2Tx.160 Reproduced from ref. 160 with permission from Elsevier, Copyright 2022. (f) Schematic of the construction process of the UiO-66-NH2-MXene@rGO/GCE sensor electrode and simultaneous detection of Cd2+ and Pb2+.161 Reproduced from ref. 161 with permission from Elsevier, Copyright 2024. (g) Schematic of the construction of the Nb4C3Tx sensor and detection towards Pb2+. (h) XRD pattern of ML-Nb2CTx, DL-Nb2CTx, ML-Nb4C3Tx, and DL-Nb4C3Tx. (i) SEM image of ML-Nb2CTx. (j) SEM image of ML-Nb4C3Tx.121 | ||
5. Electrochemical stability of MXenes
The electrochemical stability of MXenes is crucial for unlocking their full potential in electrochemical applications. MXenes exhibit inherent electrochemical instability, which arises from factors such as reactive surface terminations, residual impurities, morphology-related defects, and unfavorable electrode potentials. Reactive surface terminations, particularly –OH groups, are prone to oxidation reactions during potential cycling, leading to surface degradation. Additionally, residual etching byproducts or contaminants left on MXene surfaces can catalyze unwanted side reactions, accelerating degradation over time.165 Furthermore, structural defects, poor crystallinity, and small particle sizes increase the number of reactive sites vulnerable to attack, compromising overall stability.166 Regarding the potential window range, MXenes show redox activity within a narrow potential window of −1 to 1 V vs. Ag/AgCl in water. Thus, operating MXenes beyond their stable potential window or exposing them to harsh environments would exacerbate corrosion processes, further compromising stability. Mechanical stresses from repeated ion intercalation/deintercalation during cycling can also contribute to instability.167,168 This instability not only affects sensor sensitivity over time due to surface area and redox activity loss but also leads to poor reproducibility and a short sensor lifespan, necessitating frequent replacement. In addition, surface changes during degradation make the accurate quantification of analyte concentrations a challenge. Enhancing MXene's electrochemical stability is vital for improving the sensor performance and reliability.Some strategies can be employed to enhance MXene's electrochemical stability. Surface modification is highly effective. For example, the fluorination of –OH sites introduces stronger –F termination layers or passivates surfaces with polymers or oxides and composition tuning is achieved through alloying or doping with stable elements. Moreover, surface coatings, electrolyte engineering, potential window selection, material densification, and ambient control can all contribute to stabilizing MXene nanomaterials and ensuring reliable performance in electrochemical applications. Zhu et al.169 intercalated polyoxometalate (POM) into an MXene (Ti3C2Tx) layer for the first time by using cetyltrimethylammonium (CTA+) cations. This CTAMX-PW12 hybrid exhibited superior electrochemical stability, retaining 85% of its initial capacitance after 5000 cycles compared to other hybrids that became inactive after 500 cycles. Redox reactions in CTAMX-PW12 remained diffusion-controlled, indicating the successful intercalation of POMs. Furthermore, Hao et al.170 introduced a new method by bridging a Ti3C2Tx MXene with tannic acid (TA) molecules, which was found to significantly enhance its electrochemical stability, oxidation resistance, and structural integrity. Cycling tests indicated that the MXene@TA electrode retained 96.1% capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1, whereas pure MXenes experienced a sharp drop after just 1500 cycles. TA bridging significantly improved the electrochemical cycling stability, rate performance, and retention of MXene materials in both half-cell and full-device configurations.
000 cycles at 5 A g−1, whereas pure MXenes experienced a sharp drop after just 1500 cycles. TA bridging significantly improved the electrochemical cycling stability, rate performance, and retention of MXene materials in both half-cell and full-device configurations.
6. Molecular imprinting techniques for increasing the selectivity of MXenes
Essentially, the selectivity of MXenes is moderate because of its open adsorption sites for target molecules. Therefore, how to increase the selectivity of MXene is crucial to realize the real sensing applications of MXene-based electrodes. Molecularly imprinted polymers (MIPs), also known as “plastic antibodies”, are synthetic recognition elements designed to mimic natural receptors,171 because they can create selective binding sites for target analytes via a process similar to the lock and key principle.172 MIPs have been integrated with MXenes to enhance the electrode responses in specific electrochemical reactions.173 The formation of MXene–MIPs involves several steps. Initially, a suspension of MXenes is dropped onto the working electrode surface and allowed to dry. Subsequently, the electrode is immersed in a solution containing both the monomer and the template molecule. Following this, electrochemical parameters are adjusted, and electro-polymerization is initiated using the CV technique. This polymerization method effectively regulates the thickness and porosity of the films. In this section, some electrochemical studies employing MXene–MIP composites are discussed. For example, Wang and colleagues174 developed an electrochemical sensor for detecting ascorbic acid (AA) by modifying a glassy carbon electrode with MXenes and an imprinted poly-(o-phenylenediamine) (o-PD) polymer. They electro-polymerized o-PD onto the MXene-modified electrode to create an imprinted film that provides specific binding sites for AA. The electro-polymerization time affects the thickness of MIP films, and thicker films of MIP provide more imprinting sites to improve the selectivity. However, if polymer is too thick, the AA template molecules cannot be removed. The polymer thickness can be improved by the number of scan cycles. A recent study by Shi et al.175 has introduced a novel MXene fiber-based electrochemical sensor with MIPs. The MXene fibers provided superior electrical conductivity, while the MIP layer allowed simultaneous recognition and quantification of hydrocortisone (HC) with high selectivity. By increasing the concentration of HC, the current decreases because the HC molecules occupied the holes of the MIPs and restricted the electron transfer. Following the above-mentioned work, Ma et al.176 developed a molecular imprinting sensor using an MXene/NH2-CNTs substrate material, achieving high sensitivity and selectivity. The MIP layer's cavity network allowed simultaneous identification and quantification of fisetin, ensuring selectivity. After electro-polymerization on MXene/NH2-CNTs/GCE, a smooth and uniform ppy film was observed. The extraction of fisetin molecules resulted in increased surface porosity, indicating the successful removal of template molecules. Comparatively, the surface of NIP/MXene/NH2-CNTs/GCE appeared hazier and denser, while MIP/GCE exhibited rough surfaces, suggesting specific imprinted sites. These observations confirm the successful coating of molecularly imprinted cavities on the MXene/NH2-CNTs nanocomposite surface. In another study, Lu and co-workers177 created a new dual-signal ratiometric MIP biosensor for the electrochemical detection of CC using MXene/Fe@Ti-MOF-NH2 nanomaterials. The electro-polymerization method synthesized a conductive pTHi built-in signal film and MIP film, increasing the recognition sites and enabling dual-signal amplification.Although MIP–MXene electrochemical sensors have great potential but there are still many challenges that need to be addressed. MIPs are employed for sensing small molecules such as disease biomarkers and amino acids, but when applying on large molecules, there is a huge gap between selectivity and applicability. Thus, more intense research is needed to focus on the feasibility of MIP on large molecules. Furthermore, many factors such as the molar ratio of template to monomer, scan rate and cycles of polymerization, elution time, pH of electro-polymerization solution and incubation time have influences on MIP-based electrochemical sensor performance. These factors should also be fully considered for better sensing performances.
7. Conclusion and future perspectives
MXenes possess inherent qualities including substantial specific surface area, high electrical conductivity, notable redox capacities and important electrocatalytic and electrochemical properties, making them highly appealing for environmental pollutant sensors. When coupled with recognition elements or composited with supportive nanomaterials, MXenes have demonstrated excellent performance in identifying various toxins. For instance, in environmental monitoring, MXenes are ideal for detecting pollutants such as heavy metals and organic contaminants in water and air due to their high sensitivity and rapid response times. In industrial safety, they are useful for detecting hazardous gases and chemicals, ensuring workplace safety through continuous monitoring. However, several challenges remain yet to be addressed for their full commercialization.Certain challenges persist in the pursuit of MXene-based electrochemical sensor applications, including fabrication approaches and mechanism study. Various alternative etching agents including LiF/HCl, molten ZnCl2 and electrochemical techniques have been suggested as substitutes for HF etchants. However, the results obtained from these alternatives give rise to significant inquiries. Therefore, the pursuit of safer, ecologically sustainable and economically efficient techniques for large-scale manufacturing is of utmost importance. Innovative experimental approaches and conditions are necessary to address the issues for the synthesis of MXenes. Scale-up processes such as blade coating or slot-die printing holding appeal for industrial production need focus because the integration of MXenes into in-line sensing platforms for continuous monitoring is based on these approaches. The microfabrication approach for MXene warrants exploration. The operational mechanism of MXenes as environmental pollutant sensors, particularly the interaction between MXene-based substrates and pollutants for achieving sensor selectivity, is not fully understood. Interactions between the MXene surface and contaminant molecules are crucial for sensor selectivity. To facilitate practical applications, it is essential to comprehensively understand and accurately interpret the signal mechanism in MXene-based resistance sensors using in situ characterization technologies. Additionally, gaining a deeper insight into the structure–property relationship of MXenes will greatly contribute to advancing our understanding of their sensing mechanism. Selectivity also merits improvement, such as via molecular imprinting which displays early success but requires streamlining. Portability and miniaturization are essential for on-site applications. Advancing fabrication of flexible, freestanding MXene films and sensors integrated with microfluidics could transform deployment. It should be noted that when MXenes are subjected to air or aqueous solutions, they undergo rapid oxidation, resulting in the formation of transition metal oxides such as TiO2. This oxidative process poses a challenge for flexible sensors that depend on the inherent high conductivity of MXenes, negatively impacting their performances, while surface modifications, protective coatings, or novel synthesis techniques are crucial for improving sensor longevity and performance. It is essential to focus more on addressing this challenge to ensure the practical viability of MXene-based sensors in various applications. Moreover, exploring the synthesis of new MXene compositions and structures can lead to improved sensing capabilities. For instance, developing MXenes with different transition metal compositions (e.g., Nb, V, and Zr) can introduce unique catalytic properties and selectivity.
Cost-effectiveness is a crucial consideration in sensor technology. Our analysis reveals that MXene-based electrochemical sensors demonstrate significant cost-effectiveness compared to alternative methods, as highlighted in Table 1. With lower material costs, streamlined fabrication processes, and reduced operational expenses, MXene-based sensors emerge as economically feasible solutions for diverse sensing applications. Additionally, their inherent stability and scalability for mass production enhance long-term reliability and affordability. These findings highlights the potential of MXene-based electrochemical sensors as a cost-effective and practical choice in the field of sensor technology we also electrode recycle is another important issue. The concept of a recyclable electrode aligns with the principles of sustainability and cost-effectiveness, aiming to reduce waste and promote the efficient use of resources in the development and application of electrochemical sensors. It should be paid more attention to develop effective strategies to enhance the recyclability of MXene electrodes in electrochemical sensors. Moreover, optimizing the regeneration process and ensuring efficient restoration of the electrode electrochemical properties after each use may be an efficient approach. By integrating improved regeneration techniques and exploring eco-friendly synthesis methods, MXenes would offer immense potential for innovative advancements across various fields such as electronics, energy, environmental science, and healthcare, positioning them as transformative materials in modern technology and research landscapes.
Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors report no declarations of interest.Acknowledgements
The authors gratefully acknowledge the financial support from the National Key Research and Development Program of China Sino-Austrian intergovernmental industry-university-research cooperation project (Project No. 2022YFE0117000), the Fundamental Research Funds for the Central Universities (HUST: YCJJ202203008), National Natural Science Foundation of China (52171069 and 22005109), and the Open Project Program of Hubei Key Laboratory of Materials Chemistry and Service Failure (2020MCF02).References
- H. M. Tran, F. J. Tsai, Y. L. Lee, J. H. Chang, L. T. Chang, T. Y. Chang, K. F. Chung, H. P. Kuo, K. Y. Lee and K. J. Chuang, The impact of air pollution on respiratory diseases in an era of climate change: A review of the current evidence, Sci. Total Environ., 2023, 898, 166340 Search PubMed.
- A. Aziz, M. Asif, G. Ashraf, T. Iftikhar, W. Hussain and S. Wang, Environmental significance of wearable sensors based on MXene and graphene, Trends Environ. Anal. Chem., 2022, 36, e00180 CrossRef CAS.
- B. Pratap, S. Kumar, S. Nand, I. Azad, R. N. Bharagava, L. F. R. Ferreira and V. Dutta, Wastewater generation and treatment by various eco-friendly technologies: Possible health hazards and further reuse for environmental safety, Chemosphere, 2023, 313, 137547 Search PubMed.
- A. Alengebawy, S. T. Abdelkhalek, S. R. Qureshi and M.-Q. Wang, Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications, Toxics, 2021, 9, 42 CrossRef CAS PubMed.
- L. Järup, Hazards of heavy metal contamination, Br. Med. Bull., 2003, 68, 167–182 Search PubMed.
- L. Chen, M. Wakeel, T. U. Haq, N. S. Alharbi, C. Chen and X. Ren, Recent progress in environmental remediation, colloidal behavior and biological effects of MXene: a review, Environ. Sci.: Nano, 2022, 9, 3168–3205 RSC.
- E. M. Becker, M. B. Dessuy, W. Boschetti, M. G. R. Vale, S. L. Ferreira and B. Welz, Development of an analytical method for the determination of arsenic in gasoline samples by hydride generation–graphite furnace atomic absorption spectrometry, Spectrochim. Acta, Part B, 2012, 71, 102–106 CrossRef.
- B. T. Kildahl and W. Lund, Determination of arsenic and antimony in wine by electrothermal atomic absorption spectrometry, Fresenius' J. Anal. Chem., 1996, 354, 93–96 CrossRef CAS.
- J. F. Paula, R. E. Froes-Silva and V. S. Ciminelli, Arsenic determination in complex mining residues by ICP OES after ultrasonic extraction, Microchem. J., 2012, 104, 12–16 CrossRef CAS.
- H. Li, W. Hu, M. M. Hassan, Z. Zhang and Q. Chen, A facile and sensitive SERS-based biosensor for colormetric detection of acetamiprid in green tea based on unmodified gold nanoparticles, J. Food Meas. Charact., 2019, 13, 259–268 CrossRef.
- H. Malassa, M. Al-Qutob, M. Al-Khatib and F. Al-Rimawi, Determination of different trace heavy metals in ground water of South West Bank/Palestine by ICP/MS, J. Environ. Prot., 2013, 4, 818–827 CrossRef.
- P. Cava-Montesinos, M. L. Cervera, A. N. Pastor and M. de la Guardia, Determination of As, Sb, Se, Te and Bi in milk by slurry sampling hydride generation atomic fluorescence spectrometry, Talanta, 2004, 62, 173–182 CrossRef PubMed.
- K. Shrivas and K. S. Patel, On-site determination of arsenic in contaminated water, Anal. Lett., 2004, 37, 333–344 CrossRef CAS.
- F. M. Peinado, S. M. Ruano, M. B González and C. E. Molina, A rapid field procedure for screening trace elements in polluted soil using portable X-ray fluorescence (PXRF), Geoderma, 2011, 400, 1637–1644 Search PubMed.
- M. A. Farajzadeh, M. R. A. Mogaddam, S. R. Aghdam, N. Nouri and M. Bamorrowat, Application of elevated temperature-dispersive liquid-liquid microextraction for determination of organophosphorus pesticides residues in aqueous samples followed by gas chromatography-flame ionization detection, Food Chem., 2016, 212, 198–204 CrossRef CAS.
- C. Chang, J. Luo, M. Chen, K. Wu, T. Dong, X. He, K. Zhou, L. Wang, D. Chen and Z. Zhou, Determination of twenty organophosphorus pesticides in blood serum by gas chromatography-tandem mass spectrometry, Anal. Methods, 2016, 8, 4487–4496 RSC.
- A. Cappiello, G. Famiglini, P. Palma and F. Mangani, Trace level determination of organophosphorus pesticides in water with the new direct-electron ionization LC/MS interface, Anal. Chem., 2002, 74, 3547–3554 CrossRef CAS PubMed.
- T. Tang, J. Deng, M. Zhang, G. Shi and T. Zhou, Quantum dot-DNA aptamer conjugates coupled with capillary electrophoresis: A universal strategy for ratiometric detection of organophosphorus pesticides, Talanta, 2016, 146, 55–61 CrossRef CAS PubMed.
- L. Floridia, A. M. Pietropaolo, M. Tavazzani, F. M. Rubino and A. Colombi, High-performance liquid chromatography of methotrexate for environmental monitoring of surface contamination in hospital departments and assessment of occupational exposure, J. Chromatogr. B: Biomed. Sci. Appl., 1999, 726, 95–103 CrossRef CAS.
- Z. Song, Y. Wang, Y. Dong, K. Xu, H. Long, C. Deng, Y. Yin, S. A. Eremin, M. Meng and R. Xi, A validated chemiluminescence immunoassay for methotrexate (MTX) and its application in a pharmacokinetic study, Anal. Methods, 2016, 8, 162–170 RSC.
- S. Patel, R. Jamunkar, D. Sinha, T. K. Patle, T. Kant, K. Dewangan and K. Shrivas, Recent development in nanomaterials fabricated paper-based colorimetric and fluorescent sensors: A review, Trends Environ. Anal. Chem., 2021, 31, e00136 CrossRef CAS.
- M. L. Desai, H. Basu, R. K. Singhal, S. Saha and S. K. Kailasa, Ultra-small two dimensional MXene nanosheets for selective and sensitive fluorescence detection of Ag+ and Mn2+ ions, Colloids Surf., A, 2019, 565, 70–77 CrossRef CAS.
- C. Lin, X. Song, W. Ye, T. Liu, M. Rong and L. Niu, Recent Progress in Optical Sensors Based on MXenes Quantum Dots and MXenes Nanosheets, J. Anal. Test., 2024, 8, 95–113 CrossRef.
- Y. Li, Z. Kang, L. Kong, H. Shi, Y. Zhang, M. Cui and D.-P. Yang, MXene-Ti3C2/CuS nanocomposites: Enhanced peroxidase-like activity and sensitive colorimetric cholesterol detection, Mater. Sci. Eng., C, 2019, 104, 110000 CrossRef CAS.
- D. Zhu, B. Liu and G. Wei, Two-dimensional material-based colorimetric biosensors: A review, Biosensors, 2021, 11, 259 CrossRef CAS PubMed.
- Y. Wang, H. Wang, L. Cai, C. Liu, B. Zhang, G. Fang and S. Wang, A novel electrochemiluminescence sensor based on MXene and sodium ascorbate coordinated amplification CNNS signal strategy for ultrasensitive and selective determination of histamine, Sens. Actuators, B, 2021, 349, 130790 CrossRef CAS.
- Q. Wei, G. Chen, H. Pan, Z. Ye, C. Au, C. Chen, X. Zhao, Y. Zhou, X. Xiao and H. Tai, MXene-sponge based high-performance piezoresistive sensor for wearable biomonitoring and real-time tactile sensing, Small Methods, 2022, 6, 2101051 CrossRef CAS.
- L. Wu, Q. You, Y. Shan, S. Gan, Y. Zhao, X. Dai and Y. Xiang, Few-layer Ti3C2Tx MXene: A promising surface plasmon resonance biosensing material to enhance the sensitivity, Sens. Actuators, B, 2018, 277, 210–215 CrossRef CAS.
- Y. Peng, P. Cai, L. Yang, Y. Liu, L. Zhu, Q. Zhang, J. Liu, Z. Huang and Y. Yang, Theoretical and experimental studies of Ti3C2 MXene for surface-enhanced Raman spectroscopy-based sensing, ACS Omega, 2020, 5, 26486–26496 CrossRef CAS PubMed.
- L. Wu, X. Yuan, Y. Tang, S. Wageh, O. A. Al-Hartomy, A. G. Al-Sehemi, J. Yang, Y. Xiang, H. Zhang and Y. Qin, MXene sensors based on optical and electrical sensing signals: from biological, chemical, and physical sensing to emerging intelligent and bionic devices, PhotoniX, 2023, 4, 15 CrossRef.
- G. Hanrahan, D. G. Patil and J. Wang, Electrochemical sensors for environmental monitoring: design, development and applications, J. Environ. Monit., 2004, 6, 657–664 RSC.
- J. Zhao, Y. Kan, Z. Chen, H. Li and W. Zhang, MOFs-Modified Electrochemical Sensors and the Application in the Detection of Opioids, Biosensors, 2023, 13, 284 CrossRef CAS.
- Y. Hui, Z. Huang, M. E. E. Alahi, A. Nag, S. Feng and S. C. Mukhopadhyay, Recent advancements in electrochemical biosensors for monitoring the water quality, Biosensors, 2022, 12, 551 CrossRef CAS PubMed.
- K. G. Coronado-Apodaca, S. Rodríguez-De Luna, R. Araújo, M. A. Oyervides-Muñoz, G. M. González-Meza, L. Parra-Arroyo, J. E. Sosa-Hernandez, H. M. Iqbal and R. Parra-Saldivar, Occurrence, transport, and detection techniques of emerging pollutants in groundwater, MethodsX, 2023, 102160 CrossRef CAS.
- A. Inobeme, J. T. Mathew, E. Jatto, J. Inobeme, C. O. Adetunji, M. Muniratu, B. I. Onyeachu, M. A. Adekoya, A. I. Ajai and A. Mann, Recent advances in instrumental techniques for heavy metal quantification, Environ. Monit. Assess., 2023, 195, 452 CrossRef.
- N. H. Solangi, N. M. Mubarak, R. R. Karri, S. A. Mazari, S. K. Kailasa and A. Alfantazi, Applications of advanced MXene-based composite membranes for sustainable water desalination, Chemosphere, 2023, 314, 137643 CrossRef CAS PubMed.
- V. S. Manikandan, B. Adhikari and A. Chen, Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages, Analyst, 2018, 143, 4537–4554 RSC.
- H. Munawar, J. S. Mankar, M. D. Sharma, A. Garcia-Cruz, L. A. L. Fernandes, M. Peacock and R. J. Krupadam, Highly selective electrochemical nanofilm sensor for detection of carcinogenic PAHs in environmental samples, Talanta, 2020, 219, 121273 CrossRef CAS PubMed.
- S. K. Kailasa, D. J. Joshi, J. R. Koduru and N. I. Malek, Review on MXenes-based nanomaterials for sustainable opportunities in energy storage, sensing and electrocatalytic reactions, J. Mol. Liq., 2021, 342, 117524 CrossRef CAS.
- N. Baig, Two-dimensional nanomaterials: A critical review of recent progress, properties, applications, and future directions, Composites, Part A, 2023, 165, 107362 CrossRef CAS.
- I. Ahmad, Y. Raharjo, A. Batool, A. Zakir, H. Manzoor, A. Arooj, J. Khalid, N. Ali and K. Rasool, Surface Engineering of MXene Quantum Dots for the Designing of Optical Metal Sensors, Trends Environ. Anal. Chem., 2023, 39, e00210 CrossRef CAS.
- Y. Wu, X. Li, H. Zhao, F. Yao, J. Cao, Z. Chen, X. Huang, D. Wang and Q. Yang, Recent advances in transition metal carbides and nitrides (MXenes): Characteristics, environmental remediation and challenges, Chem. Eng. J., 2021, 418, 129296 CrossRef CAS.
- S. Li, L. Wang, J. Peng, M. Zhai and W. Shi, Efficient thorium (IV) removal by two-dimensional Ti2CTx MXene from aqueous solution, Chem. Eng. J., 2019, 366, 192–199 CrossRef CAS.
- Y. Zhang, L. Wang, N. Zhang and Z. Zhou, Adsorptive environmental applications of MXene nanomaterials: a review, RSC Adv., 2018, 8, 19895–19905 RSC.
- R. P. Pandey, K. Rasool, V. E. Madhavan, B. Aïssa, Y. Gogotsi and K. A. Mahmoud, Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2Tx) nanosheets, J. Mater. Chem. A, 2018, 6, 3522–3533 RSC.
- C. Cui, R. Guo, E. Ren, H. Xiao, X. Lai, Q. Qin, S. Jiang, H. Shen, M. Zhou and W. Qin, Facile hydrothermal synthesis of rod-like Nb2O5/Nb2CTx composites for visible-light driven photocatalytic degradation of organic pollutants, Environ. Res., 2021, 193, 110587 CrossRef CAS PubMed.
- C. Wang, X. Liu, N. K. Demir, J. P. Chen and K. Li, Applications of water stable metal–organic frameworks, Chem. Soc. Rev., 2016, 45, 5107–5134 RSC.
- J. Zhou, X. Zha, F. Y. Chen, Q. Ye, P. Eklund, S. Du and Q. Huang, A two-dimensional zirconium carbide by selective etching of Al3C3 from nanolaminated Zr3Al3C5, Angew. Chem., Int. Ed., 2016, 55, 5008–5013 CrossRef CAS.
- X. Bai, X.-H. Zha, Y. Qiao, N. Qiu, Y. Zhang, K. Luo, J. He, Q. Li, Q. Huang and J. S. Francisco, Two-dimensional semiconducting Lu2CT2 (T= F, OH) MXene with low work function and high carrier mobility, Nanoscale, 2020, 12, 3795–3802 RSC.
- Z. H. Jaffari, S. M. Abuabdou, D.-Q. Ng and M. J. Bashir, Insight into two-dimensional MXenes for environmental applications: Recent progress, challenges, and prospects, FlatChem, 2021, 28, 100256 CrossRef CAS.
- A. Shahzad, M. Nawaz, M. Moztahida, J. Jang, K. Tahir, J. Kim, Y. Lim, V. S. Vassiliadis, S. H. Woo and D. S. Lee, Ti3C2Tx MXene core-shell spheres for ultrahigh removal of mercuric ions, Chem. Eng. J., 2019, 368, 400–408 CrossRef CAS.
- P. A. Rasheed, R. P. Pandey, K. A. Jabbar and K. A. Mahmoud, Platinum nanoparticles/Ti3C2Tx (MXene) composite for the effectual electrochemical sensing of Bisphenol A in aqueous media, J. Electroanal. Chem., 2021, 880, 114934 CrossRef CAS.
- A. Rhouati, M. Berkani, Y. Vasseghian and N. Golzadeh, MXene-based electrochemical sensors for detection of environmental pollutants: A comprehensive review, Chemosphere, 2022, 291, 132921 CrossRef CAS.
- F. A. Arris, A. M. Benoudjit, F. Sanober and W. W. A. Wan Salim, Characterization of electrochemical transducers for biosensor applications, Multifaceted Protocol in Biotechnology, 2018, pp. 119–137 Search PubMed.
- X. Liu, Y. Yao, Y. Ying and J. Ping, Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection, TrAC, Trends Anal. Chem., 2019, 115, 187–202 CrossRef CAS.
- H. Yin, H. He, T. Li, M. Hu, W. Huang, Z. Wang, X. Yang, W. Yao, F. Xiao and Y. Sun, Ultra-sensitive detection of multiplexed heavy metal ions by MOF-derived carbon film encapsulating BiCu alloy nanoparticles in potable electrochemical sensing system, Anal. Chim. Acta, 2023, 1239, 340730 CrossRef CAS PubMed.
- Z. Wang, J. Shin, J. H. Park, H. Lee, D. H. Kim and H. Liu, Engineering materials for electrochemical sweat sensing, Adv. Funct. Mater., 2021, 31, 2008130 CrossRef CAS.
- Y. Chen, P. Zhao, Z. Hu, Y. Liang, H. Han, M. Yang, X. Luo, C. Hou and D. Huo, Amino-functionalized multilayer Ti3C2Tx enabled electrochemical sensor for simultaneous determination of Cd2+ and Pb2+ in food samples, Food Chem., 2023, 402, 134269 CrossRef CAS.
- Z. Otgonbayar and W.-C. Oh, Comprehensive and multi-functional MXene based sensors: An updated review, FlatChem, 2023, 100524 CrossRef CAS.
- J. Wang, H. Kang, H. Ma, Y. Liu, Z. Xie, Y. Wang and Z. Fan, Super-fast fabrication of MXene film through a combination of ion induced gelation and vacuum-assisted filtration, Eng. Sci., 2021, 15, 57–66 CAS.
- M. Jiang, D. Jiang, X. Cao, J. Wang, Y. Sun, M. Zhang and J. Liu, Scalable 2D/2D assembly of ultrathin MOF/MXene sheets for stretchable and bendable energy storage devices, Adv. Funct. Mater., 2023, 2312692 Search PubMed.
- W. Eom, H. Shin, R. B. Ambade, S. H. Lee, K. H. Lee, D. J. Kang and T. H. Han, Large-scale wet-spinning of highly electroconductive MXene fibers, Nat. Commun., 2020, 11, 2825 CrossRef CAS PubMed.
- K. Montazeri, M. Currie, L. Verger, P. Dianat, M. W. Barsoum and B. Nabet, Beyond Gold: Spin-Coated Ti3C2-Based MXene Photodetectors, Adv. Mater., 2019, 31, 1903271 CrossRef CAS.
- J. Zhang, N. Kong, S. Uzun, A. Levitt, S. Seyedin, P. A. Lynch, S. Qin, M. Han, W. Yang and J. Liu, Scalable manufacturing of free-standing, strong Ti3C2Tx MXene films with outstanding conductivity, Adv. Mater., 2020, 32, 2001093 CrossRef CAS PubMed.
- J. H. Kim, G. S. Park, Y.-J. Kim, E. Choi, J. Kang, O. Kwon, S. J. Kim, J. H. Cho and D. W. Kim, Large-Area Ti3C2Tx-MXene coating: toward industrial-scale fabrication and molecular separation, ACS Nano, 2021, 15, 8860–8869 CrossRef CAS PubMed.
- J. Lipton, J. A. Röhr, V. Dang, A. Goad, K. Maleski, F. Lavini, M. Han, E. H. Tsai, G.-M. Weng and J. Kong, Scalable, highly conductive, and micropatternable MXene films for enhanced electromagnetic interference shielding, Matter, 2020, 3, 546–557 CrossRef.
- M. Zhou, J. Zhang and C. Sun, Occurrence, ecological and human health risks, and seasonal variations of phenolic compounds in surface water and sediment of a potential polluted river basin in China, Int. J. Environ. Res. Public Health, 2017, 14, 1140 CrossRef.
- A. E. Vilian, J. N. Tiwari, M. Alhammadi, G. Bhaskaran, S.-K. Hwang, S. Kim, K. Kumar, A. S. Kumar, Y. S. Huh and Y.-K. Han, Palladium nanoparticles anchored MoS2-MXene composite modified electrode for rapid sensing of toxic bisphenol A in aqueous media, Chem. Eng. J., 2023, 469, 144017 CrossRef.
- R. Huang, D. Liao, S. Chen, J. Yu and X. Jiang, A strategy for effective electrochemical detection of hydroquinone and catechol: Decoration of alkalization-intercalated Ti3C2 with MOF-derived N-doped porous carbon, Sens. Actuators, B, 2020, 320, 128386 CrossRef CAS.
- R. Huang, D. Liao, Z. Liu, J. Yu and X. Jiang, Electrostatically assembling 2D hierarchical Nb2CTx and zifs-derivatives into Zn-Co-NC nanocage for the electrochemical detection of 4-nitrophenol, Sens. Actuators, B, 2021, 338, 129828 CrossRef CAS.
- S. Gul, M. I. Serna, S. A. Zahra, N. Arif, M. Iqbal, D. Akinwande and S. Rizwan, Un-doped and Er-adsorbed layered Nb2C MXene for efficient hydrazine sensing application, Surf. Interfaces, 2021, 24, 101074 CrossRef CAS.
- Y. Yao, X. Han, X. Yang, J. Zhao and C. Chai, Detection of Hydrazine at MXene/ZIF-8 Nanocomposite Modified Electrode, Chin. J. Chem., 2021, 39, 330–336 CrossRef CAS.
- J. Rajendran, T. S. Kannan, L. S. Dhanasekaran, P. Murugan, R. Atchudan, Z. A. ALOthman, M. Ouladsmane and A. K. Sundramoorthy, Preparation of 2D Graphene/MXene nanocomposite for the electrochemical determination of hazardous bisphenol A in plastic products, Chemosphere, 2022, 287, 132106 CrossRef CAS PubMed.
- R. Huang, S. Chen, J. Yu and X. Jiang, Self-assembled Ti3C2/MWCNTs nanocomposites modified glassy carbon electrode for electrochemical simultaneous detection of hydroquinone and catechol, Ecotoxicol. Environ. Saf., 2019, 184, 109619 CrossRef CAS.
- K. S. Ranjith, A. E. Vilian, S. M. Ghoreishian, R. Umapathi, S.-K. Hwang, C. W. Oh, Y. S. Huh and Y.-K. Han, Hybridized 1D–2D MnMoO4–MXene nanocomposites as high-performing electrochemical sensing platform for the sensitive detection of dihydroxybenzene isomers in wastewater samples, J. Hazard. Mater., 2022, 421, 126775 CrossRef CAS PubMed.
- D. Liao, Z. Liu, R. Huang, J. Yu and X. Jiang, In-situ construction of porous carbon on embedded N-doped MXene nanosheets composite for simultaneous determination of 4-aminophenol and acetaminophen, Microchem. J., 2022, 175, 107067 CrossRef CAS.
- H. Huang, D. Wang, Y. Zhou, D. Wu, X. Liao, W. Xiong, J. Du and Y. Hong, Multiwalled carbon nanotubes modified two dimensional MXene with high antifouling property for sensitive detection of ochratoxin A, Nanotechnology, 2021, 32, 455501 CrossRef CAS PubMed.
- W. Zhong, F. Gao, J. Zou, S. Liu, M. Li, Y. Gao, Y. Yu, X. Wang and L. Lu, MXene@ Ag-based ratiometric electrochemical sensing strategy for effective detection of carbendazim in vegetable samples, Food Chem., 2021, 360, 130006 CrossRef CAS PubMed.
- Y. Xie, F. Gao, X. Tu, X. Ma, Q. Xu, R. Dai, X. Huang, Y. Yu and L. Lu, Facile synthesis of MXene/electrochemically reduced graphene oxide composites and their application for electrochemical sensing of carbendazim, J. Electrochem. Soc., 2019, 166, B1673 CrossRef CAS.
- D. Wu, M. Wu, J. Yang, H. Zhang, K. Xie, C.-T. Lin, A. Yu, J. Yu and L. Fu, Delaminated Ti3C2Tx (MXene) for electrochemical carbendazim sensing, Mater. Lett., 2019, 236, 412–415 CrossRef CAS.
- X. Tu, F. Gao, X. Ma, J. Zou, Y. Yu, M. Li, F. Qu, X. Huang and L. Lu, Mxene/carbon nanohorn/β-cyclodextrin-Metal-organic frameworks as high-performance electrochemical sensing platform for sensitive detection of carbendazim pesticide, J. Hazard. Mater., 2020, 396, 122776 CrossRef CAS PubMed.
- X. B. Joseph, J. N. Baby, S.-F. Wang, B. Sriram and M. George, Interfacial superassembly of Mo2C@NiMn-LDH frameworks for electrochemical monitoring of carbendazim fungicide, ACS Sustainable Chem. Eng., 2021, 9, 14900–14910 CrossRef CAS.
- B. B. Yola, G. Kotan, O. Akyıldırım, N. Atar and M. L. Yola, Electrochemical determination of fenitrothion pesticide based on ultrathin manganese oxide nanowires/molybdenum titanium carbide MXene ionic nanocomposite and molecularly imprinting polymer, Microchim. Acta, 2024, 191, 230 CrossRef CAS.
- A. Sinha, K. Ma and H. Zhao, 2D Ti3C2Tx flakes prepared by in-situ HF etchant for simultaneous screening of carbamate pesticides, J. Colloid Interface Sci., 2021, 590, 365–374 CrossRef CAS.
- S. Kadirsoy, N. Atar and M. L. Yola, Molecularly imprinted QCM sensor based on delaminated MXene for chlorpyrifos detection and QCM sensor validation, New J. Chem., 2020, 44, 6524–6532 RSC.
- W. Zhong, J. Zou, Q. Yu, Y. Gao, F. Qu, S. Liu, H. Zhou and L. Lu, Ultrasensitive indirect electrochemical sensing of thiabendazole in fruit and water by the anodic stripping voltammetry of Cu2+ with hierarchical Ti3C2Tx-TiO2 for signal amplification, Food Chem., 2023, 402, 134379 CrossRef CAS.
- M. Ankitha, N. Shabana, P. V. Vaishag and P. A. Rasheed, A novel flexible electrode with highly stable trifluoroacetic acid modified Nb2CTx MXene for the sensitive detection of rifampicin, J. Electroanal. Chem., 2023, 928, 117088 CrossRef CAS.
- J. Zheng, B. Wang, A. Ding, B. Weng and J. Chen, Synthesis of MXene/DNA/Pd/Pt nanocomposite for sensitive detection of dopamine, J. Electroanal. Chem., 2018, 816, 189–194 CrossRef CAS.
- U. Amara, M. T. Mehran, B. Sarfaraz, K. Mahmood, A. Hayat, M. Nasir, S. Riaz and M. H. Nawaz, Perylene diimide/MXene-modified graphitic pencil electrode-based electrochemical sensor for dopamine detection, Microchim. Acta, 2021, 188, 230 CrossRef CAS.
- P. A. Rasheed, R. P. Pandey, T. Gomez, K. A. Jabbar, K. Prenger, M. Naguib, B. Aïssa and K. A. Mahmoud, Nb-based MXenes for efficient electrochemical sensing of small biomolecules in the anodic potential, Electrochem. Commun., 2020, 119, 106811 CrossRef.
- F. Shahzad, A. Iqbal, S. A. Zaidi, S.-W. Hwang and C. M. Koo, Nafion-stabilized two-dimensional transition metal carbide (Ti3C2Tx MXene) as a high-performance electrochemical sensor for neurotransmitter, J. Ind. Eng. Chem., 2019, 79, 338–344 CrossRef CAS.
- Y. Zhang, X. Jiang, J. Zhang, H. Zhang and Y. Li, Simultaneous voltammetric determination of acetaminophen and isoniazid using MXene modified screen-printed electrode, Biosens. Bioelectron., 2019, 130, 315–321 CrossRef CAS PubMed.
- P. K. Kalambate, A. Sinha, Y. Li, Y. Shen and Y. Huang, An electrochemical sensor for ifosfamide, acetaminophen, domperidone, and sumatriptan based on self-assembled MXene/MWCNT/chitosan nanocomposite thin film, Microchim. Acta, 2020, 187, 1–12 CrossRef.
- S. S. Shankar, R. M. Shereema and R. Rakhi, Electrochemical determination of adrenaline using MXene/graphite composite paste electrodes, ACS Appl. Mater. Interfaces, 2018, 10, 43343–43351 CrossRef CAS.
- Q. You, Z. Guo, R. Zhang, Z. Chang, M. Ge, Q. Mei and W.-F. Dong, Simultaneous recognition of dopamine and uric acid in the presence of ascorbic acid via an intercalated MXene/PPy nanocomposite, Sensors, 2021, 21, 3069 CrossRef CAS PubMed.
- M. Gao, Y. Xie, W. Yang and L. Lu, Fabrication of novel electrochemical sensor based on MXene/MWCNTs composite for sensitive detection of synephrine, Int. J. Electrochem. Sci., 2020, 15, 4619–4630 CrossRef CAS.
- S. Chen, M. Shi, Q. Xu, J. Xu, X. Duan, Y. Gao, L. Lu, F. Gao, X. Wang and Y. Yu, Ti3C2Tx MXene/nitrogen-doped reduced graphene oxide composite: a high-performance electrochemical sensing platform for adrenaline detection, Nanotechnology, 2021, 32, 265501 CrossRef CAS PubMed.
- T. Kokulnathan, E. Ashok Kumar and T.-J. Wang, Design and in situ synthesis of titanium carbide/boron nitride nanocomposite: investigation of electrocatalytic activity for the sulfadiazine sensor, ACS Sustainable Chem. Eng., 2020, 8, 12471–12481 CrossRef CAS.
- S. Elumalai, V. Mani, N. Jeromiyas, V. K. Ponnusamy and M. Yoshimura, A composite film prepared from titanium carbide Ti3C2Tx (MXene) and gold nanoparticles for voltammetric determination of uric acid and folic acid, Microchim. Acta, 2020, 187, 1–10 CrossRef.
- N. Murugan, R. Jerome, M. Preethika, A. Sundaramurthy and A. K. Sundramoorthy, 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid, J. Mater. Sci. Technol., 2021, 72, 122–131 CrossRef CAS.
- R. Zhang, J. Liu and Y. Li, MXene with great adsorption ability toward organic dye: an excellent material for constructing a ratiometric electrochemical sensing platform, ACS Sens., 2019, 4, 2058–2064 CrossRef CAS.
- R. Rajakumaran, J. Anupriya and S.-M. Chen, 2D-Titanium carbide MXene/RGO composite modified electrode for selective detection of carcinogenic residue furazolidone in food and biological samples, Mater. Lett., 2021, 297, 129979 CrossRef CAS.
- J. Zhuang, H. Pan and W. Feng, 3D urchin–like CoVO/MXene nanosheet composites for enhanced detection signal of nitrite, Sens. Actuators, B, 2023, 378, 133207 CrossRef CAS.
- R. D. Nagarajan, A. Sundaramurthy and A. K. Sundramoorthy, Synthesis and characterization of MXene (Ti3C2Tx)/Iron oxide composite for ultrasensitive electrochemical detection of hydrogen peroxide, Chemosphere, 2022, 286, 131478 CrossRef CAS.
- S. Neampet, N. Ruecha, J. Qin, W. Wonsawat, O. Chailapakul and N. Rodthongkum, A nanocomposite prepared from platinum particles, polyaniline and a Ti3C2 MXene for amperometric sensing of hydrogen peroxide and lactate, Microchim. Acta, 2019, 186, 1–8 CrossRef.
- L. Lorencova, T. Bertok, J. Filip, M. Jerigova, D. Velic, P. Kasak, K. A. Mahmoud and J. Tkac, Highly stable Ti3C2Tx (MXene)/Pt nanoparticles-modified glassy carbon electrode for H2O2 and small molecules sensing applications, Sens. Actuators, B, 2018, 263, 360–368 CrossRef CAS.
- F. Wang, C. Yang, C. Duan, D. Xiao, Y. Tang and J. Zhu, An organ-like titanium carbide material (MXene) with multilayer structure encapsulating hemoglobin for a mediator-free biosensor, J. Electrochem. Soc., 2014, 162, B16 CrossRef.
- F. Wang, C. Yang, M. Duan, Y. Tang and J. Zhu, TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances, Biosens. Bioelectron., 2015, 74, 1022–1028 CrossRef CAS PubMed.
- L. Lorencova, T. Bertok, E. Dosekova, A. Holazova, D. Paprckova, A. Vikartovska, V. Sasinkova, J. Filip, P. Kasak and M. Jerigova, Electrochemical performance of Ti3C2Tx MXene in aqueous media: towards ultrasensitive H2O2 sensing, Electrochim. Acta, 2017, 235, 471–479 CrossRef CAS.
- R. Ramachandran, C. Zhao, M. Rajkumar, K. Rajavel, P. Zhu, W. Xuan, Z.-X. Xu and F. Wang, Porous nickel oxide microsphere and Ti3C2Tx hybrid derived from metal-organic framework for battery-type supercapacitor electrode and non-enzymatic H2O2 sensor, Electrochim. Acta, 2019, 322, 134771 CrossRef CAS.
- K. Zhou, Y. Li, S. Zhuang, J. Ren, F. Tang, J. Mu and P. Wang, A novel electrochemical sensor based on CuO-CeO2/MXene nanocomposite for quantitative and continuous detection of H2O2, J. Electroanal. Chem., 2022, 921, 116655 CrossRef CAS.
- H. Zou, F. Zhang, H. Wang, J. Xia, L. Gao and Z. Wang, Au nanoparticles supported on functionalized two-dimensional titanium carbide for the sensitive detection of nitrite, New J. Chem., 2019, 43, 2464–2470 RSC.
- Y. Wang, Z. Zeng, J. Qiao, S. Dong, Q. Liang and S. Shao, Ultrasensitive determination of nitrite based on electrochemical platform of AuNPs deposited on PDDA-modified MXene nanosheets, Talanta, 2021, 221, 121605 CrossRef CAS.
- A. E. Vilian, R. Umapathi, S.-K. Hwang, Y. S. Huh and Y.-K. Han, Pd–Cu nanospheres supported on Mo2C for the electrochemical sensing of nitrites, J. Hazard. Mater., 2021, 408, 124914 CrossRef CAS.
- H. Liu, C. Duan, C. Yang, W. Shen, F. Wang and Z. Zhu, A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2, Sens. Actuators, B, 2015, 218, 60–66 CrossRef CAS.
- P. A. Rasheed, R. P. Pandey, K. Rasool and K. A. Mahmoud, Ultra-sensitive electrocatalytic detection of bromate in drinking water based on Nafion/Ti3C2Tx (MXene) modified glassy carbon electrode, Sens. Actuators, B, 2018, 265, 652–659 CrossRef.
- X. Liu, L. He, P. Li, X. Li and P. Zhang, A direct electrochemical H2S sensor based on Ti3C2Tx MXene, ChemElectroChem, 2021, 8, 3658–3665 CrossRef CAS.
- Q. Li, X. Wang, L. Chen, X. Liu, J. Ma, L. Wang and W. Wang, Cu/Cu2O nanoparticles modified Ti3C2 MXene with in-situ formed TiO2-X for detection of hydrogen peroxide, Ceram. Int., 2023, 49(6), 9632–9641 CrossRef CAS.
- X. Zhang, D. An, Z. Bi, W. Shan, B. Zhu, L. Zhou, L. Yu, H. Zhang, S. Xia and M. Qiu, Ti3C2-MXene@N-doped carbon heterostructure-based electrochemical sensor for simultaneous detection of heavy metals, J. Electroanal. Chem., 2022, 911, 116239 CrossRef CAS.
- X. Zhu, B. Liu, H. Hou, Z. Huang, K. M. Zeinu, L. Huang, X. Yuan, D. Guo, J. Hu and J. Yang, Alkaline intercalation of Ti3C2 MXene for simultaneous electrochemical detection of Cd (II), Pb (II), Cu (II) and Hg (II), Electrochim. Acta, 2017, 248, 46–57 CrossRef CAS.
- P. A. Rasheed, R. P. Pandey, T. Gomez, M. Naguib and K. A. Mahmoud, Large interlayer spacing Nb4C3Tx (MXene) promotes the ultrasensitive electrochemical detection of Pb2+ on glassy carbon electrodes, RSC Adv., 2020, 10, 24697–24704 RSC.
- P. Xiao, G. Zhu, X. Shang, B. Hu, B. Zhang, Z. Tang, J. Yang and J. Liu, An Fe-MOF/MXene-based ultra-sensitive electrochemical sensor for arsenic (III) measurement, J. Electroanal. Chem., 2022, 916, 116382 CrossRef CAS.
- X. Lv, F. Pei, S. Feng, Y. Wu, S.-M. Chen, Q. Hao and W. Lei, Facile synthesis of protonated carbon nitride/Ti3C2Tx nanocomposite for simultaneous detection of Pb2+ and Cd2+, J. Electrochem. Soc., 2020, 167, 067509 CrossRef CAS.
- Y. He, L. Ma, L. Zhou, G. Liu, Y. Jiang and J. Gao, Preparation and application of bismuth/MXene nano-composite as electrochemical sensor for heavy metal ions detection, Nanomaterials, 2020, 10, 866 CrossRef CAS.
- X. Zhu, B. Liu, L. Li, L. Wu, S. Chen, L. Huang, J. Yang, S. Liang, K. Xiao and J. Hu, A micromilled microgrid sensor with delaminated MXene-bismuth nanocomposite assembly for simultaneous electrochemical detection of lead (II), cadmium (II) and zinc (II), Microchim. Acta, 2019, 186, 1–7 CrossRef CAS PubMed.
- H. Cheng and J. Yang, Preparation of Ti3C2-PANI composite as sensor for electrochemical determination of mercury ions in water, Int. J. Electrochem. Sci., 2020, 15, 2295–2306 CrossRef CAS.
- X. Hui, M. Sharifuzzaman, S. Sharma, X. Xuan, S. Zhang, S. G. Ko, S. H. Yoon and J. Y. Park, High-performance flexible electrochemical heavy metal sensor based on layer-by-layer assembly of Ti3C2Tx/MWNTs nanocomposites for noninvasive detection of copper and zinc ions in human biofluids, ACS Appl. Mater. Interfaces, 2020, 12, 48928–48937 CrossRef CAS.
- A. Reyes-Calderón, S. Pérez-Uribe, A. G. Ramos-Delgado, S. Ramalingam, G. Oza, R. Parra-Saldívar, R. A. Ramirez-Mendoza, H. M. Iqbal and A. Sharma, Analytical and regulatory considerations to mitigate highly hazardous toxins from environmental matrices, J. Hazard. Mater., 2022, 423, 127031 CrossRef.
- H. Shi, G. Zhao, M. Liu, L. Fan and T. Cao, Aptamer-based colorimetric sensing of acetamiprid in soil samples: Sensitivity, selectivity and mechanism, J. Hazard. Mater., 2013, 260, 754–761 CrossRef CAS PubMed.
- D. Song, X. Jiang, Y. Li, X. Lu, S. Luan, Y. Wang, Y. Li and F. Gao, Metal–organic frameworks-derived MnO2/Mn3O4 microcuboids with hierarchically ordered nanosheets and Ti3C2 MXene/Au NPs composites for electrochemical pesticide detection, J. Hazard. Mater., 2019, 373, 367–376 CrossRef CAS PubMed.
- X. Liu, Y. Zhang, Y.-Q. Liu, K. Fu, Y. Wang and N. Wei, Topological structure of Au nanoparticles@salix leaf-like metal–organic framework-MXene for highly sensitive determination of carbendazim, Sens. Actuators, B, 2024, 404, 135251 CrossRef CAS.
- R. Abazari, A. R. Mahjoub, S. Sanati, Z. Rezvani, Z. Hou and H. Dai, Ni–Ti layered double hydroxide@ graphitic carbon nitride nanosheet: a novel nanocomposite with high and ultrafast sonophotocatalytic performance for degradation of antibiotics, Inorg. Chem., 2019, 58, 1834–1849 CrossRef CAS PubMed.
- S. Jones, A. Pramanik, R. Kanchanapally, B. P. Viraka Nellore, S. Begum, C. Sweet and P. C. Ray, Multifunctional three-dimensional chitosan/gold nanoparticle/graphene oxide architecture for separation, label-free SERS identification of pharmaceutical contaminants, and effective killing of superbugs, ACS Sustainable Chem. Eng., 2017, 5, 7175–7187 CrossRef CAS.
- P. C. Ray, S. A. Khan, A. K. Singh, D. Senapati and Z. Fan, Nanomaterials for targeted detection and photothermal killing of bacteria, Chem. Soc. Rev., 2012, 41, 3193–3209 RSC.
- T. U. Berendonk, C. M. Manaia, C. Merlin, D. Fatta-Kassinos, E. Cytryn, F. Walsh, H. Bürgmann, H. Sørum, M. Norström and M.-N. Pons, Tackling antibiotic resistance: the environmental framework, Nat. Rev. Microbiol., 2015, 13, 310–317 CrossRef CAS PubMed.
- Q.-Q. Zhang, G.-G. Ying, C.-G. Pan, Y.-S. Liu and J.-L. Zhao, Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance, Environ. Sci. Technol., 2015, 49, 6772–6782 CrossRef CAS PubMed.
- K. M. M. N. Silva, D. É. L. de Carvalho, V. M. M. Valente, J. C. C. Rubio, P. E. Faria and P. P. Silva-Caldeira, Concomitant and controlled release of furazolidone and bismuth (III) incorporated in a cross-linked sodium alginate-carboxymethyl cellulose hydrogel, Int. J. Biol. Macromol., 2019, 126, 359–366 CrossRef CAS PubMed.
- J. Lewkowski, D. Rogacz and P. Rychter, Hazardous ecotoxicological impact of two commonly used nitrofuran-derived antibacterial drugs: Furazolidone and nitrofurantoin, Chemosphere, 2019, 222, 381–390 CrossRef CAS.
- M. R. Farani, B. N. Khiarak, R. Tao, Z. Wang, S. Ahmadi, M. Hassanpour, M. Rabiee, M. R. Saeb, E. C. Lima and N. Rabiee, 2D MXene nanocomposites: electrochemical and biomedical applications, Environ. Sci.: Nano, 2022, 9, 4038–4068 RSC.
- K. Wei, K. Pan, G. Qu, J. Qin, J. Lv and Y. Liang, Self-assembly of GO-MXene hybrid composites by polyelectrolyte: As an efficient electrocatalyst for selective electrochemical detection of the antibiotic furazolidone, J. Environ. Chem. Eng., 2024, 112319 CrossRef CAS.
- M. Ankitha, N. Shabana, A. M. Arjun, P. Muhsin and P. A. Rasheed, Ultrasensitive electrochemical detection of dopamine from human serum samples by Nb2CTx-MoS2 hetero structures, Microchem. J., 2023, 187, 108424 CrossRef CAS.
- M. Ankitha, N. Shabana and P. A. Rasheed, A novel ReS2–Nb2CTx composite as a sensing platform for ultrasensitive and selective electrochemical detection of dipyramidole from human serum, Graphene 2D Materials, 2023, 8, 27–37 CrossRef.
- Z. Khanam, S. Ahmad, M. S. Tanweer, W. A. Siddiqi and M. Alam, in 2D nanomaterials for energy and environmental sustainability, Springer, 2022, pp. 149–172 Search PubMed.
- O. Kanoun, T. Lazarević-Pašti, I. Pašti, S. Nasraoui, M. Talbi, A. Brahem, A. Adiraju, E. Sheremet, R. D. Rodriguez and M. Ben Ali, A review of nanocomposite-modified electrochemical sensors for water quality monitoring, Sensors, 2021, 21, 4131 CrossRef CAS PubMed.
- S. Su, S. Chen and C. Fan, Recent advances in two-dimensional nanomaterials-based electrochemical sensors for environmental analysis, Green Energy Environ., 2018, 3, 97–106 CrossRef.
- N. J. Vickers, Animal communication: when i'm calling you, will you answer too?, Curr. Biol., 2017, 27, R713–R715 CrossRef CAS PubMed.
- T. Peng, Z. Hu, X. Yang, Y. Gao and C. Ma, Nitrite-induced acute kidney injury with secondary hyperparathyroidism: Case report and literature review, Medicine, 2018, 97(8), e9889 CrossRef.
- S. D. McCann, M. S. Tweet and M. S. Wahl, Rising incidence and high mortality in intentional sodium nitrite exposures reported to US poison centers, Clin. Toxicol., 2021, 59, 1264–1269 CrossRef.
- F. Hosseini, M. Majdi, S. Naghshi, F. Sheikhhossein, K. Djafarian and S. Shab-Bidar, Nitrate-nitrite exposure through drinking water and diet and risk of colorectal cancer: A systematic review and meta-analysis of observational studies, Clin. Nutr., 2021, 40, 3073–3081 CrossRef CAS.
- X. Feng, G. Han, J. Cai and X. Wang, Au@Carbon quantum Dots-MXene nanocomposite as an electrochemical sensor for sensitive detection of nitrite, J. Colloid Interface Sci., 2022, 607, 1313–1322 CrossRef CAS.
- Y. Chen, G. I. Waterhouse, X. Qiao, Y. Sun and Z. Xu, Sensitive analytical detection of nitrite using an electrochemical sensor with STAB-functionalized Nb2C@MWCNTs for signal amplification, Food Chem., 2022, 372, 131356 CrossRef CAS PubMed.
- R. J. Lewis and G. Hutchings, Recent advances in the direct synthesis of H2O2, ChemCatChem, 2019, 11, 298–308 CrossRef CAS.
- Q. Wu, Y. Li, Y. Li, D. Wang and B. Z. Tang, Hydrogen peroxide-responsive AIE probe for imaging-guided organelle targeting and photodynamic cancer cell ablation, Mater. Chem. Front., 2021, 5, 3489–3496 RSC.
- W. Chen, H. Fan, K. Balakrishnan, Y. Wang, H. Sun, Y. Fan, V. Gandhi, L. A. Arnold and X. Peng, Discovery and optimization of novel hydrogen peroxide activated aromatic nitrogen mustard derivatives as highly potent anticancer agents, J. Med. Chem., 2018, 61, 9132–9145 CrossRef CAS.
- D. Cheng, P. Li, X. Zhu, M. Liu, Y. Zhang and Y. Liu, Enzyme-free Electrochemical Detection of Hydrogen Peroxide Based on the Three-Dimensional Flower-like Cu-based Metal Organic Frameworks and MXene Nanosheets, Chin. J. Chem., 2021, 39, 2181–2187 CrossRef CAS.
- A. M. Arjun, N. Shabana, M. Ankitha and P. A. Rasheed, Electrochemical deposition of Prussian blue on Nb2CTx MXene modified carbon cloth for the non-enzymatic electrochemical detection of hydrogen peroxide, Microchem. J., 2023, 185, 108301 CrossRef.
- D. Gan, Q. Huang, J. Dou, H. Huang, J. Chen, M. Liu, Y. Wen, Z. Yang, X. Zhang and Y. Wei, Bioinspired functionalization of MXenes (Ti3C2TX) with amino acids for efficient removal of heavy metal ions, Appl. Surf. Sci., 2020, 504, 144603 CrossRef CAS.
- T. Rasheed, A. A. Hassan, F. Kausar, F. Sher, M. Bilal and H. M. Iqbal, Carbon nanotubes assisted analytical detection–Sensing/delivery cues for environmental and biomedical monitoring, TrAC, Trends Anal. Chem., 2020, 132, 116066 CrossRef CAS.
- T. Rasheed, M. Bilal, F. Nabeel, H. M. Iqbal, C. Li and Y. Zhou, Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals, Sci. Total Environ., 2018, 615, 476–485 CrossRef CAS PubMed.
- Y. Xia, Y. Zhao, F. Ai, Y. Yi, T. Liu, H. Lin and G. Zhu, N and P co-doped MXenes nanoribbons for electrodeposition-free stripping analysis of Cu (II) and Hg (II), J. Hazard. Mater., 2022, 425, 127974 CrossRef CAS PubMed.
- J. Dong, X. Li, L. Wen, Y. Ma, J. Xu, H. Luo, J. Hou, C. Hou and D. Huo, A novel electrochemical strategy based on MXene@rGO composite aerogel-doped UiO-66-NH2 for simultaneous detection of cadmium and lead in grain and water samples, Food Chem., 2024, 437, 137835 CrossRef CAS.
- P. A. Rasheed, R. P. Pandey, K. A. Jabbar and K. A. Mahmoud, Nb4C3Tx (MXene)/Au/DNA aptasensor for the ultraselective electrochemical detection of lead in water samples, Electroanalysis, 2022, 34, 1540–1546 CrossRef CAS.
- K. Maleski, C. E. Ren, M.-Q. Zhao, B. Anasori and Y. Gogotsi, Size-dependent physical and electrochemical properties of two-dimensional MXene flakes, ACS Appl. Mater. Interfaces, 2018, 10, 24491–24498 CrossRef CAS.
- W. Huang, L. Hu, Y. Tang, Z. Xie and H. Zhang, Recent advances in functional 2D MXene-based nanostructures for next-generation devices, Adv. Funct. Mater., 2020, 30, 2005223 CrossRef CAS.
- A. Bhat, S. Anwer, K. S. Bhat, M. I. H. Mohideen, K. Liao and A. Qurashi, Prospects challenges and stability of 2D MXenes for clean energy conversion and storage applications, npj 2D Mater. Appl., 2021, 5, 61 CrossRef CAS.
- R. A. Soomro, P. Zhang, B. Fan, Y. Wei and B. Xu, Progression in the oxidation stability of MXenes, Nano-Micro Lett., 2023, 15, 108 CrossRef CAS.
- N. Ganji, C. A. Reardon-Lochbaum, S. B. Ambade, C. M. Anastasia, P. M. Eckhert, Z. Rosenzweig, J. A. Pedersen and D. H. Fairbrother, Stability of Ti3C2Tx MXenes in engineered environments, Environ. Sci.: Nano, 2024, 11(2), 494–506 RSC.
- Z. Lin, H. Shao, K. Xu, P.-L. Taberna and P. Simon, MXenes as high-rate electrodes for energy storage, Trends Chem., 2020, 2, 654–664 CrossRef CAS.
- J.-J. Zhu and P. Gomez-Romero, Polyoxometalate intercalated MXene with enhanced electrochemical stability, Nanoscale, 2022, 14, 14921–14934 RSC.
- Z. Hao, S. Zhang, S. Yang, X. Li, Y. Gao, J. Peng, L. Li, L. Bao and X. Li, Bridged Ti3C2Tx MXene film with superior oxidation resistance and structural stability for high-performance flexible supercapacitors, ACS Appl. Energy Mater., 2022, 5, 2898–2908 CrossRef CAS.
- M. Cieplak and W. Kutner, Artificial biosensors: How can molecular imprinting mimic biorecognition?, Trends Biotechnol., 2016, 34, 922–941 CrossRef CAS.
- C. Li, B. Che and L. Deng, Electrochemical biosensors based on carbon nanomaterials for diagnosis of human respiratory diseases, Biosensors, 2022, 13, 12 CrossRef PubMed.
- A. Singhal, S. Yadav, M. A. Sadique, R. Khan, A. K. Kaushik, N. Sathish and A. K. Srivastava, MXene-modified molecularly imprinted polymers as an artificial bio-recognition platform for efficient electrochemical sensing: progress and perspectives, Phys. Chem. Chem. Phys., 2022, 24, 19164–19176 RSC.
- Q. Wang, X. Xiao, X. Hu, L. Huang, T. Li and M. Yang, Molecularly imprinted electrochemical sensor for ascorbic acid determination based on MXene modified electrode, Mater. Lett., 2021, 285, 129158 CrossRef CAS.
- Z. Shi, Z. Wang, K. Li, Y. Wang, Z. Li and Z. Zhu, MXene fibers-based molecularly imprinted disposable electrochemical sensor for sensitive and selective detection of hydrocortisone, Talanta, 2024, 266, 125100 CrossRef CAS PubMed.
- X. Ma, X. Tu, F. Gao, Y. Xie, X. Huang, C. Fernandez, F. Qu, G. Liu, L. Lu and Y. Yu, Hierarchical porous MXene/amino carbon nanotubes-based molecular imprinting sensor for highly sensitive and selective sensing of fisetin, Sens. Actuators, B, 2020, 309, 127815 CrossRef CAS.
- Z. Lu, K. Wei, H. Ma, R. Duan, M. Sun, P. Zou, J. Yin, X. Wang, Y. Wang and C. Wu, Bimetallic MOF synergy molecularly imprinted ratiometric electrochemical sensor based on MXene decorated with polythionine for ultra-sensitive sensing of catechol, Anal. Chim. Acta, 2023, 1251, 340983 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |