A review on the role of nanotechnological interventions in sequestration, mitigation and value-added product conversion of micro-/nanoplastics
Jasasmita
Das†
 ,
Emansi
Yadav†
,
Emansi
Yadav†
 and
Krishna Mohan
Poluri
and
Krishna Mohan
Poluri
 *
*
Department of Biosciences and Bioengineering, Centre for Nanotechnology, Indian Institute of Technology Roorkee, Roorkee, Uttarakhand 247667, India. E-mail: krishna.poluri@bt.iitr.ac.in; mohanpmk@gmail.com
First published on 30th October 2024
Abstract
The buildup of microplastics (MPs)/nanoplastics (NPs) in the aquatic biota has sparked concern owing to their negative consequences on human health and the environment, making it a global issue in recent years. As a result, to achieve sustainable development goals, management of MP/NP contamination is crucial. Although various studies have evaluated the harmful effects of MPs/NPs, insufficient attention has been focused on managing MPs/NPs through nanotechnological interventions. Initially, this review presents the key aspects of advanced strategies, including adsorption, membrane filtration, photocatalytic degradation, magnetic separation, and electrochemistry-driven methods, for efficiently sequestering/degrading MPs/NPs present in the aquatic environment. Subsequently, an in-depth discussion is presented on the aforementioned strategies and various nanomaterials/nanocomposites (e.g. micromotors, microswimmers, MOFs, GO, and CNTs) for the mitigation of MPs/NPs. Furthermore, the outlook section offers insights into the conversion of MPs/NPs into valuable products using nanointerventions. Finally, a brief overview of the economic aspects/cost analysis of MP/NP management, future directions, and prospects is comprehensively documented as a futuristic approach.
Environmental significanceThe ubiquitous nature of micro-/nanoplastics (MPs/NPs) has several adverse effects on human health and the aquatic biota. Therefore, the sequestration/mitigation/degradation of MPs/NPs present in the aquatic environment is the foremost solution. The introduction of nanointerventions through the utilization of advanced technologies and the use of numerous nanomaterials can be implemented for the abatement of MPs/NPs from water bodies, thereby providing an economically feasible and sustainable pathway by converting MPs/NPs into value-added products. |
1. Introduction
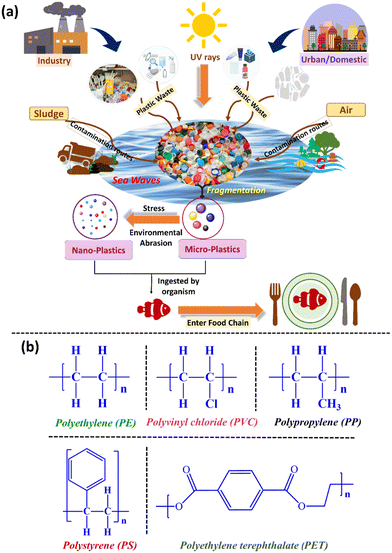
The widespread use of plastic and its derivative products has become a major environmental issue in modern society. Plastic has been widely used in all aspects of life during the last few decades because of its reasonable price, light weight, and stability.1,2 However, the improper disposal of plastic items in the surrounding environment has led to an increase in plastic debris globally to around 242 million tons.2 Current studies predict that plastic waste weighs 6.3 billion tons worldwide, which will continue to grow by around 12 million tons annually.3–5 It is also predicted that around 4.8–12 million tons of plastic debris will enter the sea.3–5 Environmental pollution with microplastics (MPs) and nanoplastics (NPs) as well as their unavoidable exposure to humans have aroused concerns around the world. Owing to their degradation, mechanical stress, and ultraviolet deterioration, these plastic debris are converted into smaller-size particles with varying grain sizes ranging from 0.1 to 5000 μm, finally forming ubiquitous MPs and NPs.3,4 Plastics degrade through a variety of processes in aquatic settings, resulting in their fragmentation into tiny particles known as “nanoplastics”.5–8 However, owing to the low-biodegradability and persistent nature of plastics even in small forms such as MPs/NPs, they are easily ingested by aquatic creatures such as whales and fish.9–11 This causes esophageal blockage and endocrine dysfunction, which lead to developmental abnormalities in diverse species.11 MPs/NPs pose a colossal threat to human health and the environment as they can easily pass through the cell membrane and may have toxicological effects on tissues, cells, and organs.10–12Micro-/nanoplastics are either manufactured as microbeads for a variety of purposes, including cosmetics, or they are formed through the breakdown of plastic debris under harsh environmental conditions.12–14 Generally, MPs/NPs found in cosmetics, personal care, and health products such as sanitizers, disinfectants, toothpaste, shampoo, and body wash are regarded as primary MPs/NPs. Primary MPs/NPs are also produced from plastic pellets and synthetic fibers used in the laundry and textile industries.15–19 Secondary MPs/NPs are formed as a result of the disintegration of pre-existing big pieces of plastic caused by natural agents such as water, wind, and sunlight.15–21 According to recent studies, the most abundant MPs/NPs are made of polystyrene (PS), polyethylene (PE), polyvinyl chloride (PVC), polyamides (PA), polyurethane (PUR), polypropylene (PP), polytetrafluoroethylene (PTFE), polyethylene terephthalate (PET), and poly(methyl methacrylate) (PMMA) (Fig. 1b).22,23 They are available in a variety of shapes and morphologies, including fibers, pieces, films, pellets, and beads, which influence their behavior and fate in nature. Plastics are introduced into the aquatic environment from many sources, and then degraded into MPs/NPs by UV-radiation or sea waves due to stress or environmental abrasion, as shown in Fig. 1a. Moreover, the small size and high surface area to volume ratio of NPs compared to MPs enable them to adsorb other highly toxic environmental pollutants such as heavy metals (Hg, Pb, Cd, and As), polyaromatic hydrocarbons, polychlorinated biphenyls and pesticides present in the aqueous environment on their surface.24–26 Therefore, the toxicity of these functionalized NPs can be enhanced, resulting in harmful effects on human health and aquatic biota.
In recent years, many physical and chemical approaches for separating contaminants from aqueous solutions have been published. The physical methods include adsorption, sedimentation, distillation, and filtration, while chemical methods such as chemical precipitation, coagulation, chemical oxidation, solvent extraction, and ion-exchange procedures are commonly utilized for the removal of pollutants from water bodies. However, these technologies are mostly suitable for the separation of bulk pollutants or large-sized pollutants, rather than micro-/nanopollutants. As a result, major efforts have been undertaken to promote technical innovation, including the development of novel nanomaterials advancing these techniques that provide additional insight into this challenge. For example, adsorption is the most common and efficient process used in wastewater treatment to remove organic (dyes, pesticides, and antibiotics) and inorganic pollutants (heavy metal ions and trace metal ions).27 Conventional adsorbents including clay, zeolites, activated carbon, and biochar-based adsorbents without modification typically have restricted efficiency owing to their lack of selectivity and low specific surface area (SSA), and therefore cannot selectively remove certain pollutants. Alternatively, nano-adsorbents such as magnetic nanomaterials, carbon nanotubes (CNTs), and metal–organic frameworks (MOFs) provide substantial advantages due to their exceptionally high SSA, abundant sorption sites, variable pore size, short intraparticle diffusion distance, and surface chemistry.24
The advent of nanotechnology is a vital enabler that can address a wide range of societal and technological challenges. Pollution remediation methods based on nanotechnology have emerged as unique and sustainable solution for a greener environment.28–30 In this direction of MP/NP sequestration and mitigation, nano-enabled technologies have promising scope for considerable improvements in the identification, detection, characterisation, and removal efficiency. For example, recently, Romphophak et al. reviewed the crucial treatment technologies utilized for removing MPs/NPs and their mechanism.31 Another review by Chellasamy et al. explored the use of bionanomaterials for the remediation of MPs, including flocculants, adsorbents, catalysts, and filter membranes.32 Recently, Sajid et al. discussed several nano-based adsorbents for the adsorptive removal of MPs.33 Similarly, a recent review considered the use of nanomaterials to remediate MPs, discussing the ways for treating MPs, including separation/capture and degradation. Moreover, it also addressed the use of nanoparticles in the adsorptive and catalytic treatment of MPs.34
Therefore, this review critically analyzes the mitigation of MPs/NPs and aims to provide a systematic literature study on the novel nano-sized/nano-based materials and nanotechnological advancements used to remove and degrade MPs/NPs from aqueous environment. Further, this review aims to update the knowledge on the intervention of different nanotechnologies developed for the mitigation of MPs/NPs, and also includes the advancements of different nanomaterials, as follows: (a) magnetic nanomaterials (b) metal–organic frameworks (MOFs), (c) carbon-based nanomaterials, (d) biocompatible nanomaterials and (e) biochar-based nanoadsorbents for the abatement of MPs/NPs. In essence, this review also discusses the degradation of MPs/NPs into some significant chemical products by using advanced nano-photocatalysts. Moreover, in addition to technological and material advancements in the removal of MPs/NPs, we also discuss the economic feasibility and conversion of MPs/NPs into valuable products. By describing the detailed mechanisms and performance of water treatment systems for eliminating MPs and NPs, this review aims to shed light on the present gaps for future research on the advancement of water treatment. This can be further used as a reference by future researchers studying the abatement of MPs/NPs in water bodies.
2. Outlook on the nano-enabled strategies for the remediation of MPs/NPs from aqueous solution
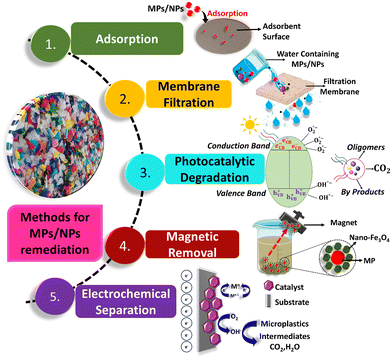
Presently, the remediation of MPs/NPs from aqueous solution is necessary to save the environment from the toxicity of MPs/NPs. In this case, verifying the sources of MPs/NPs, the underlying processes in removal strategies, and the potential health risks associated with MPs/NPs in drinking water, may also help in developing path-breaking technologies for monitoring and reducing MPs/NPs in aquatic environments. In this direction, several effective removal strategies have been explored for the mitigation of MPs/NPs in aquatic and terrestrial environments globally. However, owing to their nanoscale size, it is challenging to separate/mitigate and identify them from the water/soil bodies.35 Indeed, techniques have been developed for the identification, quantification, and separation of NPs in a matrix.36Among the various techniques, coagulation, adsorption, photocatalytic degradation, advanced oxidation processes, flocculation, filtering, electrochemical degradation, and biological degradation have demonstrated significant promise for removing MPs/NPs from urban waterways. The application of these techniques has evidenced that a key element in both the detection and mitigation of MPs/NPs is their size, although the above-mentioned methods have certain advantages and disadvantages. For example, the coagulation approach has low efficiency and is mainly found to be suitable for bulk plastics or fibrous MPs.20 Alternatively, methods such as biodegradation are time-consuming, less efficient, and susceptible to environmental conditions, which can hinder the removal of MPs/NPs in aqueous samples.37 This section presents a detailed study-by-study approach of different advanced technologies including (a) adsorption, (b) membrane filtration, (c) photocatalytic degradation, (d) magnetic separation, and (e) electrochemistry-driven separation that utilize nanomaterials for the mitigation/degradation of MPs/NPs (Fig. 2). On comparing the efficiency of several methods for the mitigation of MPs/NPs from aqueous solution, a comparative assessment of all the aforementioned methods together with their advantages and disadvantages is provided in Table 1. The salient features of these unique techniques are briefly outlined in the following subsections.
| S. no. | Method | Removal efficiency (%) | Advantages | Disadvantages |
|---|---|---|---|---|
| 1. | Adsorption | ∼26–100 | The procedure is simple, effective, and can replenish the adsorbent for future cycles | Adsorbent synthesis requires significant energy and efficacy varies based on the type of adsorbent or precursor utilized, as well as the potential procedure needed for desorption |
| 2. | Membrane filtration | ∼98 | For large-scale treatment, facile, high removal efficiency and selectivity | Cake formation and chemical washing may require cleaning the surface of the membrane |
| 3. | Magnetic separation | ∼90–100 | Modification of material is easy, feasible, and reusable | Possibility of metal leaching during separation |
| 4. | Photocatalytic degradation | ∼5–80 | Sunlight exposure can naturally produce simpler byproducts such as H2O and CO2 | The method is slow and requires a longer period to attain high removal efficiency. Furthermore, a high probability of the formation of relatively toxic by-products in the case of incomplete degradation |
| 5. | Electrochemistry-driven separation | ∼90–99 | High pollutant removal efficiency, no extra chemicals for process operation | To stop high energy consumption, a suitable current density is required, and the materials cannot be recycled |
2.1. Adsorption
Adsorption is the most prominently used method for the removal of environmental contaminants from aqueous solution. For the remediation of MPs/NPs, it is one of the most advantageous technology, owing to its simplicity, the availability of a wide range of adsorbents, low-cost, and efficiency.35–38 The adsorption processes are essentially based on principles such as π–π interactions, electrostatic interactions, complex formation, hydrogen bonding, hydrophobic interactions, and physical adhesion.36–38 Further, the adsorption capacity is substantially determined by the physicochemical features of adsorbents (e.g., nanostructure, chemical composition, and electronic property).38 A variety of nano-adsorbents, including metal-based adsorbents, metal oxides, metal hydroxides, metal-based nanoparticles, MOFs, ZIFs, biochar/modified biochar, and carbon-based adsorbents, has been reported for the removal of MPs/NPs.35–39 These adsorbents have distinguishing qualities such as high specific surface area with customized pore size, which enhances the number of surface active sites, and tunable textural properties, making them unique among their peers.36–39 Besides these physical properties, materials having different surface functional groups such as –COOH, –SH, and –NH2 are resourceful for the enhanced and selective removal of MPs/NPs. Moreover, the removal efficiency and adsorbent performance are also influenced by the experimental parameters (e.g., pH value, temperature, time, and interfering chemicals).In this regard, the use of Zr-based-MOFs, denoted as UiO-66-OH@MF-3, and Co-based-MOFs, denoted as ZIF-67, was reported by Chen et al. and Wan et al., respectively, for the removal of MPs/NPs. The maximum adsorption capacity obtained for these MOFs was about 11.6 and 34.5 mg g−1, respectively, towards the removal of MPs/NPs.40,41 Recently, Modak et al. established that Cr-based MOFs (Cr-MOF/MIL-101) can efficiently remove PS NPs from water (96% removal efficiency) with increased adsorption capacity (800 mg g−1).42 Similarly, Peng et al. created magnetic algal robots (MARs) coated with Fe3O4 magnetic nanoparticles, which were used as adsorbents for the efficient removal of MPs/NPs.43 This biohybrid microrobot exhibited a high capture and removal efficiency for MPs/NPs. These MARs, which are recyclable microrobots with environmentally favorable and low-cost properties, provide an appealing technique for the removal of MPs/NPs by electrostatic adsorption. Thus, these findings greatly encourage the development of adsorption strategies using several nano-adsorbents for the removal of MPs/NPs from water bodies.
2.2. Membrane filtration
Membrane filtration is an emerging technique for the efficient and selective remediation of contaminants from water streams. For large-scale wastewater treatment purposes, it has shown an impactful performance in the effective removal of particular pollutants. The filtration method is categorized based on the membrane pore size (microfiltration/MF: 0.1–10 μm, ultrafiltration/UF: 20–50 nm, and nanofiltration/NF: 1–10 nm). Among them, the most used methods for the selective and efficient removal are nanofiltration and ultra-filtration processes, which enable the removal of NPs in the enhanced filtration process.28 The different membrane pore sizes of these processes fractionates the varying-size NPs from aqueous solution.29,30 Owing to the small size of MPs/NPs, the properties of nano-ultrafiltration membranes are more effective in the adsorption and separation of MPs/NPs.44–46 Several mechanisms are involved in this process, including size exclusion based on pore/particle size, electrostatic attraction, electrostatic repulsion, charge separation, and ion exchange.45–47 Owing to its advantages, this method is widely used compared to its counterparts for large-scale water treatment purposes.In this direction, recently Mohana et al. reported the abatement of MPs and NPs via conventional and dynamic membrane filtration methods.45 They discussed the effectiveness of the nanofiltration method and its advantages over the conventional membrane process for the high removal efficacy of MPs and NPs. Moreover, Enfrin et al. reported an ultrafiltration membrane that could reject more than 25% of NPs within 48 h.47 Similarly, in a recent article, Pramanik et al. reported the successful removal of 96% of NPs and 91% removal of MPs using a UF membrane.46 Furthermore, the activity and efficiency of the membrane performance can be enhanced by modifying the surface of the membrane with different functional groups, which can selectively remove or trap MPs/NPs. The efficiency of UF membranes can be a viable alternative for completely removing NPs from water and wastewater.
2.3. Photocatalytic degradation of MPs/NPs
Photocatalysis is popularly used as an environmentally safe and sustainable method for the degradation of MPs/NPs, which employs direct sunlight and does not need any chemical reagents/external electrical energy.48–55 The basic principle of the process is as follows: (i) the irradiation of photon energy on the semiconductor occurs in the 1st step, (ii) when it exceeds the band gap of the photocatalyst, the electrons (e−) present in the valence band (VB) are excited and transition from the top of the VB to the bottom of the conduction band (CB). Subsequently, holes (h+) are left on the VB, resulting in the separation of electrons and holes. Further, these photo-generated carriers are transported to the surface of the semiconductor, and then undergo a sequence of reactions. The formation of h+ during this process causes the direct oxidation of polymers and some available free radicals, which are formed due to the reactivity of plastics and plastic-derived chemicals.Pioneering research with MPs has demonstrated that the photocatalytic process can be a viable option for the breakdown of MPs/NPs. In this direction, Domínguez-Jaimes et al. reported the use of anodized TiO2 (TiO2/B, TiO2/T, and TiO2/M) as a photocatalyst for the efficient elimination of polystyrene-based NPs (PS NPs) from aqueous solutions.49 When the photocatalyst absorbs light with energy E ≥ Eg, then e− present in the VB is transported to the CB, leaving behind h+. They further react with H2O, OH−, and O2 adsorbed on the photocatalyst surface and generate reactive oxygen species such as superoxide and hydroxyl radicals. Subsequently, these species start the MP/NP breakdown process, which leads to the breakage of the chain, crosslinking, branching, and eventually complete mineralization into CO2 and H2O. Similarly, Tofa et al. examined the photocatalytic degradation of LDPE MPs residue using ZnO nanorods.55 The results demonstrated that during the process, several low molecular weight compounds (unsaturated groups, peroxides, hydroperoxides, and carbonyl compounds) were produced.55 These studies demonstrate the viability of MP/NP degradation using heterogeneous nanostructured photocatalysts.
Photoreforming is another promising new approach sometimes known as photosynthesis or photo-upcycling, which has sparked widespread interest in MP/NP remediation. It allows the connection of H2 production and MP/NP degradation through the construction of excellent photocatalysts. During this process, MPs/NPs act as sacrificial electron donors, which are further oxidized by photogenerated h+. Moreover, the photoexcited electrons subsequently reduce H2O molecules, producing H2. The potential of the CB of the photocatalyst should be lower than the reduction potential of H+ to H2 (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V vs. reversible hydrogen electrode (RHE)), but the VB potential should be higher than the oxidation potential of MPs/NPs (0
V vs. reversible hydrogen electrode (RHE)), but the VB potential should be higher than the oxidation potential of MPs/NPs (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V RHE). Therefore, when exposed to light, the photocatalyst e− are stimulated to the CB, leading to the reduction of H+ and production of H2 in an anaerobic environment. The remaining h+ in the VB participates in the MP/NP degradation process, followed by direct or indirect approaches (ROS formation), which is comparable to the aforementioned photocatalytic degradation process.56
V RHE). Therefore, when exposed to light, the photocatalyst e− are stimulated to the CB, leading to the reduction of H+ and production of H2 in an anaerobic environment. The remaining h+ in the VB participates in the MP/NP degradation process, followed by direct or indirect approaches (ROS formation), which is comparable to the aforementioned photocatalytic degradation process.56
Although the research on the photoreforming of plastic dates back to the 1980s, the application of MPs/NPs is still in its early stages, with only a few studies available.57–60 MXene/ZnxCd1−xS synthesized by Cao et al., hydrophilic C3N4, and graphitic C3N4 (g-C3N4)/CuFeO2 reported by Lai et al. have been used as catalysts to improve the photoreformation efficiency.57,58 These strategies have significant economic and environmental implications for dealing with MP/NP pollution and energy challenges by combining H2 production with MP/NP degradation.
2.4. Magnetic removal of MPs/NPs
The remediation of MP/NP-contaminated water samples via the magnetic separation method is widely used as an advanced technique. Magnetic nanostructures for MP/NP cleanup have several advantages, including environmental friendliness, low cost, low toxicity, and hydrophobicity. Recently, Shi et al. reported the magnetic separation of PE MPs from aqueous solution by using magnetic sepiolite, in which a magnetic field is generated using a permanent magnet (Fig. 3).61 The removal efficiency of PE MPs achieved using this method was 98.4%. Recently, magnetic separation has been used in the assay of MPs/NPs in water bodies. Generally, the hydrophobic surface of MPs can be easily magnetized by binding with nanoparticles, and further these magnetized forms of MPs are separated in the presence of a magnetic field.62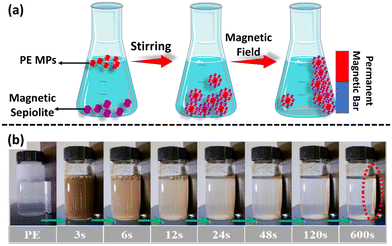 | ||
| Fig. 3 (a) Magnetic separation of MPs dispersed in water via magnetic sepiolite. (b) Images depicting the removal of MP contamination over time, adapted with permission.61 Copyright 2022, Elsevier. | ||
According to a recent study, this approach could also separate MPs/NPs by using microalgae-linked magnetized nanoparticles within a short reaction period.63 Tang et al. produced magnetic carbon nanotubes (M-CNTs) for the adsorption of MPs, followed by the separation of MPs using magnetic force from the water sample.64 In comparison, the magnetic separation method has potential to be more efficient and environmentally beneficial in removing MPs from water.65 However, MPs in the environment typically contain a variety of diverse polymer compositions and sizes.66 The real removal efficacy of magnetic technology can vary greatly, but it has not been thoroughly researched to date. Generally, in the magnetic separation method, the magnetic material is attached to the specific pollutant and separates the pollutant with the formation of bigger aggregates by binding with them. Moreover, the particular material can be further separated from the MPs/NPs by using an external magnetic field.67 This method has high efficiency and selectivity for the separation of particular pollutants, and hence can be widely used for the abatement of MPs/NPs from water streams.
2.5. Electrochemistry-driven separation of MPs/NPs
Electrochemical water treatment systems have a variety of advantages over conventional approaches, such as compact reactor vessels, high pollutant removal efficiency, and no requirement for additional hazardous chemicals. Electrochemistry-driven MP/NP identification in ground water as well as surface water is generally based on the distinction of the electronic characteristics of MPs/NPs with other particles in the presence of an electric field. The material identification and detection can be determined by evaluating the electric current/impedance of a mixture of particles (MPs/NPs).68–70 Electrochemistry-based techniques such as (i) electrocoagulation, (ii) electro-adsorption, (iii) electrokinetic separation, and (iv) electrochemical degradation are distinguished from others due to their high selectivity, high energy efficiency, ease of implementation, environmental friendliness, and versatile applicability. Numerous efforts have been devoted to improving the electrochemical performance by adjusting the electrode materials, electrolyte concentration, current density, and MP/NP characteristics.70–72 Thus, electrochemical treatment for the destruction of MPs/NPs in water is crucial to prevent their accumulation in sewage sludge and biosolids.The electrocoagulation method has emerged as a robust approach that uses a target metal as an anode to electrically create coagulants.68 To date, this method has been widely employed for the removal of MPs/NPs from urban waterways. The electrocoagulation process typically consists of three phases. Considering the Fe anode as an example, (a) in the first phase, the electro-oxidation of the Fe anode and H2O leads to the formation of Fe3+ ions, together with O2 bubbles, whereas the electro-reduction of H2O at the cathode produces molecular hydrogen and OH− ions. Further, the generated Fe3+ ions combine with OH− ions, leading to the formation of micro-coagulants, such as Fe (oxy)hydroxides and Fe oxides. (b) The suspended substances may be destabilized by charged micro-coagulants via neutralization, allowing them to adhere together. Subsequently, the suspended MPs/NPs are trapped and absorbed in the produced microflocs. (c) Finally, the size of the microflocs increases when collisions occur, and during this period, a large number of MPs/NPs is captured by physical/chemical processes.68
Electro-adsorption is defined as the polarization potential-driven adsorption of compounds on the surface of charged electrodes via an electric current.73 In the presence of an electric field, the movement and adsorption of positively charged MPs/NPs towards the cathode surface and negatively charged MPs/NPs towards the anode surface happen simultaneously. Given that most of the MPs/NPs are negatively charged under neutral conditions, the role of the anode is crucial in this process. The performance of the method is strongly dependent on the specific capacity of the electrode. This can be enhanced by adjusting the physicochemical parameters of the electrode materials, including their SSA, nanostructures, surface functional groups, and conductivity.74
The electrokinetic separation of tiny particles or ions present in aqueous media is an electric field-driven process, which is often performed through the electrophoretic motion of various particles/ions together with the electro-osmotic flow of solvents.75 Electrophoresis and electro-osmosis are the two primary types of processes involved in the separation of MPs/NPs from aqueous media. Similarly, dielectrophoresis (DEP) is the process in which the movement of dielectrically polarized particles occurs in a nonuniform electric field. This is the most extensively utilized electrokinetic technique for controlling the mobility of MPs/NPs in microfluidic channels, particularly PS MPs.76
The degradation or mineralization of organic contaminants such as MPs/NPs via the electrochemical degradation process is an effective and disruptive technology. This method has several advantages such as ease of operation, high degradation capability, and environmental compatibility, showing high removal efficiency towards the mitigation of MPs/NPs.76,77 In this direction, recent attempts have been devoted to the electrochemical oxidation of MPs/NPs. For example, Miao et al. demonstrated the degradation of PVC MPs by utilizing an electro-Fenton chemistry-based TiO2–graphite composite material. This study included the dechlorination efficiency of the composite and the effect of initial concentration with respect to time on the degradation of PVC MPs into CO2 and H2O (Fig. 4).72 In general, the electrochemical oxidation process generates powerful hydroxyl radicals (·OH) having a redox potential of 2.8 V vs. SHE, which act as the major reactive species responsible for the oxidation of MPs/NPs.77 However, the efficiency towards the removal of MPs/NPs not only depends on the methodologies but also depends on the materials used for their removal. Therefore, in the subsequent section, we present a detailed discussion on the sequestration of MPs/NPs using several advanced nanomaterials.
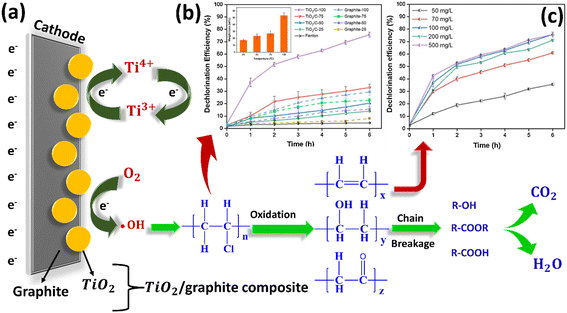 | ||
| Fig. 4 (a) Schematic of PVC MP degradation via an electro-Fenton-like system with a TiO2/graphite cathode. (b) Comparison of dechlorination efficiencies with the TiO2/C and graphite cathodes at different reaction temperatures and conventional Fenton reaction at room temperature. The inset in (b) depicts weight loss of PVC microplastics with the TiO2/C cathode. (c) Effect of initial concentration of PVC MPs on their degradation. Error bars represent the standard error of the mean. Graphs adapted with permission.72 Copyright 2020, Elsevier. | ||
3. A brief overview of nanomaterials/composites for the mitigation of MPs/NPs
Nanomaterials are widely utilized for the separation of various contaminants from aqueous solutions. They often have large specific surface areas, which increase the number of surface active sites and create high porosity as excellent features to adsorb/separate contaminants from water bodies. Furthermore, they can be customized to target specific contaminants using different functional groups present on the surface of specimens. Their high reactivity and sorption capabilities make them a viable choice for the separation of desired pollutants. However, this research topic is still in its early phases of investigation, with limited accessible research. Various classes of nanomaterials that are involved in the separation of MPs/NPs include a) magnetic nanomaterials, b) metal–organic frameworks (MOFs), c) carbon-based, d) biocompatible, and e) biochar-based nanomaterials. The following sub-sections explore the potential of the unique nanomaterials/nanocomposites in removing MPs/NPs from water bodies.3.1. Magnetic nanomaterials for separation of MPs/NPs
The abatement of ubiquitous MPs/NPs from water bodies using magnetic nanomaterials has been widely applied, owing to their ease of application and enhanced efficiency.78–85 Magnetic nanoparticles for the removal of MPs/NPs provide various advantages over traditional materials. Once the MPs/NPs are removed, the residual nanoparticles can be recovered using a strong magnetic field to eliminate potential secondary pollutants. Thus, they provide a fast, simple, and economical approach for the removal of MPs/NPs. Further, magnetic nanomaterials can remove different types of MPs with varying shapes and sizes owing to their magnetic behavior.86–96 A summary of the different magnetic nanomaterials applied for the removal of MP/NPs is provided in Table 2.| S. no. | Nanomaterial | Type of MP/NPs | Removal efficiency (%) | References |
|---|---|---|---|---|
| 1. | Magnetic sepiolite | 48 μm PE MPs | 98 | 61 |
| 2. | Nanopowder Fe | 100 μm HDPE MP | 99 | 62 |
| 3. | Hydrophobic Fe-nanoparticle | 10–20 μm: PE, PS | 92 | 81 |
| >1 μm: PE, PET, PS, PVC, PP | 93 | |||
| 4. | Fe3O4@SiO2 magnetic Janus particles | PS MPs | 92.08 | 82 |
| PE MPs (10 μm) | 60.67 | |||
| 5. | Nano-Fe3O4 | MPs-200, 500, 900 μm | 87 | |
| PE | 79–94 | |||
| PP | 80–90 | |||
| PS | 81–93 | |||
| PET | 57–72 | |||
| 6. | Magnetic artificial nano-melanin | MPs/NPs | 89–99 | 88 |
| 7. | Fe2O4–MnO2 micromotor | PE MPs | 10 | 89 |
| 8. | Magnetic Fe-nano-fish net (ac-nZVI) | 10 μm: PS | 95.71 | 90 |
| PVC | 97.64 | |||
| PMMA | 94.97 | |||
| 2 μm: PS | 97.47 | |||
| PVC | 98.58 | |||
| PMMA | 95.10 | |||
| 9. | PDMS-co-APMS-coated nano-Fe-oxide | PS NPs (100–1000 nm) | 90–93 | 91 |
| 10. | Magnetic microsubmarines | 100 μm PS MPs | 75 | 92 |
| 11. | FNP | PS NPs (100–1000 nm) | 83.1–92.9 | 93 |
| 12. | Fe3O4@C12 | PS NPs (100 nm) | 92.89 | 94 |
| 13. | Fe3O4/C | PS NPs (70 nm) | 99 | 95 |
| 14. | PEG/nano-Fe3O4 | PE MPs (13–149 μm) | — | 96 |
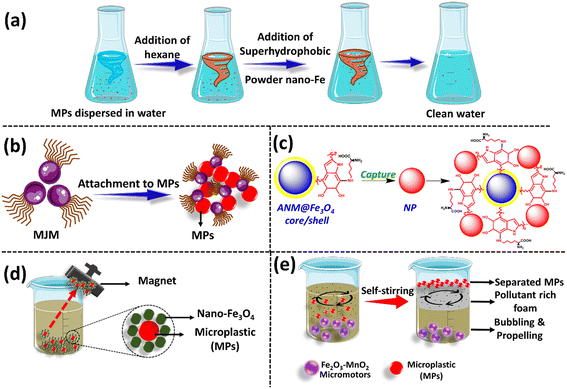
Together with magnetization, superhydrophobicity endows materials with new properties and assists in achieving greater removal efficiency of hydrophobic waste such as MPs/NPs. Magnetic materials with superhydrophobic surfaces exhibit a water contact angle of ≥150° and a sliding angle of ≤10°, which provide self-cleaning properties.79,80 Taking advantage of the opportunity provided by these super-wettable and super hydrophobic surfaces, they are considered an impactful platform in the separation of MPs and NPs. In this direction, Rius-Ayra et al. reported powdered nanostructures of iron having super wettable properties that can separate oil-in-water emulsions, and hence provide superhydrophobic materials that can capture and separate microplastic fibers (MPF).81 The nanomaterial was developed through a sustainable approach combined with the high-energy ball milling (HEBM) and liquid phase deposition (LPD) methods. The HEBM method produced the required nanostructures, while the LPD method produced superhydrophobic surfaces. In this study, different amounts of high-density polyethylene (HDPE)-MPF were suspended in deionized water, which were displaced upon the addition of hexane from the aqueous phase to the organic phase.81 At this point, the addition of the developed nanomaterial captured the MPFs owing to its superhydrophobic surface and further retrieved by applying a constant magnetic field (Fig. 5a). The water left was reported to be free of MPFs and the nanomaterial could be consecutively recycled up to 10 times, with the highest removal efficiency being close to 99%.81
Further exploring surface-functionalized iron nanomaterials, Li et al. synthesized phosphorylated magnetic Janus microparticles (MJMs) as the core material using amino-functionalized magnetic silica particles (Fe3O4@SiO2). The MJMs were used for the removal of PS and PE (10 μm) MPs from an aqueous solution.82 Janus particles are known to be chemically asymmetric particles that include functional groups with diverse characteristics. Owing to this, both hydrophilic and hydrophobic properties can be incorporated within the material.83 This multifunctionality offers several applications in water remediation,84 targeted drug delivery,85 emulsion flooding,86 and crude oil recovery. MJMs with tunable amphiphilic surfaces provide intense hydrophobic interactions with MPs, while preserving their hydrophilicity for better dispersion in water and enhancing the removal efficiency, as depicted in Fig. 5b. Fe3O4 functions as the magnetic core of these particles and enables their easy recovery in the presence of a magnetic field.82 To further enhance the hydrophobicity of the synthesized MJMs, N-octadecylphosphonic acid (PAC18) was grafted to their surface. This study investigated the MP removal efficiency of three materials, Fe3O4@SiO2, MJM (Fe3O4@SiO2 partially grafted with PAC18), and Fe3O4@SiO2–PAC18 (completely grafted with PAC18). Among them, Fe3O4@SiO2–PAC18 showed a high removal efficiency of 92.08% and 56.83% for PS MPs and 60.67% and 49.17% for PE MPs, respectively.82 This study evidenced that the functionalization of the Fe3O4@SiO2 surface with the hydrophobic PAC18 group promotes its interaction with MPs, resulting in an enhanced MP removal efficiency.
In this direction, Chen et al. developed a core–shell superparamagnetic melanin nanoparticle, which together with the presence of an external magnetic field achieved an average removal efficiency of 89.3% for NPs and MPs from water.88 Fe3O4 magnetic nanoparticles were used and their surface functionalized with dopamine (DA) and L-lysine, forming artificial nano-melanin (ANM) onto the Fe3O4 surface (Fig. 5c). The aggregation and separation of ANM were found to be pH controlled, and pH of 6.8 was noted as the optimal condition. It has been reported that 1 kg of magnetic ANM could effectively remove up to 7.88 kg of NPs and about 2.72 kg of small MPs. Here, the shell of the core–shell nanocomposite provided attractive interactions (electrostatic, hydrophobic, and Van der Waals) with the MPs/NPs present in the sample, and the Fe core assisted its removal by magnetization.88
Recently, Shi et al. applied nano-Fe3O4 with a diameter of <30 nm for the magnetic separation of various types and shapes of MPs from the aqueous environment, as shown in Fig. 5d.87 This study established that 1.3 g L−1 of nano-Fe3O4 with magnetization for 150 min provided the optimal conditions for the maximum removal of MPs from the water sample. The removal efficiency for four different types of MPs (PE, PS, PP, and PET) was studied using the developed material. The removal efficiency of PET was reported to be significantly different from that for other types of plastics. The low crystallinity and lower contact angle of PET led to poor hydrophobicity, and hence caused a low adsorption of nano-Fe3O4 and removal efficiency. The average removal efficiency of common types of plastics with a size in the range of 200–900 μm was 86.87% ± 6.92%, 85.05% ± 4.70%, 86.11% ± 6.21%, and 62.83% ± 3.4% for PE, PP, PS, and PET, respectively.87 Similarly, Ye et al. developed Fe2O3–MnO2-based core–shell magnetic micromotors, which could successfully separate up to 90% of organic contaminants and 10% of suspended MPs in just a few hours (Fig. 5e). The low cost, possibility of large-scale fabrication, and good motion behavior of these materials make them a good option for further enhancement and application in the separation of MPs/NPs from the environment.89
In a futuristic approach, Zhang et al. reported the use of naturally derived cellulose nanofibers having alkylated nanoscale zerovalent iron (ac-nZVI) as magnetic hydrophobic “nano-fishnets” for the rapid removal of MPs/NPs from seawater.90 The cellulosic nanofibers provided stability to the material, uniformly distributed the Fe-nanoparticles across the fibers, and assisted in the capture of MPs. Owing to the alkylation of the material, its adsorption mechanism is based on electrostatic and hydrophobic interactions with MPs. The separation of MPs was studied by using commercially available spherical polymer particles of various sizes of PS, PVC, and PMMA. According to the results, at the adsorbent dosage of 5.0 g L−1 ac-nZVI, the removal efficiency of the aforementioned MP/NPs was in the range of 94.97% to 98.58%.90 The working mechanism of the developed nanomaterial was studied with the help of SEM-EDS mapping. This study established that for 10 μm MPs, ac-nZVI acted like a ‘creeper’ by wrapping on the MP surface, leading to its magnetization. In contrast, for 2 μm MPs, the material served as a glue, binding the MPs into heterogenous aggregates via magnetic attraction, while for 100 nm MPs, it acted as a fishnet, leading to the formation of clusters of MPs by self-aggregation.90
3.2. Metal–organic frameworks for the abatement of MPs/NPs
For the separation of MPs/NPs from aqueous solution, another class of prominent material, metal–organic frameworks (MOFs), has been used widely.97 These materials are porous extended structures composed of metal ions and organic linkers. Thus, their porous, tunable structure and rich functionality make them potential agents for wastewater treatment.98–102 Usually, the pores in MOFs are stable during the elimination of the guest molecule and can be substituted with another molecule, making them suitable materials for wastewater treatment. However, to increase the framework stability of MOFs, the functionalization of MOFs with different functional groups can be an innovative solution. MOFs, having high porosity, ease of recycling, and high selectivity towards the targeted pollutants, show a high removal efficiency for MPs/NPs from water samples.103–107 Therefore, a comprehensive analysis of the various MOFs for the sequestration of MPs/NPs is provided in Table 3.| S. no. | MOF based nanomaterials | Type of MP/NP | Adsorbent dosage, pH | SAa (m2 g−1) | Pore volume (cm3 g−1) | REa (%) | Cycles | Reference |
|---|---|---|---|---|---|---|---|---|
| a RE (removal efficiency) and SA (surface area). | ||||||||
| 1. | UiO-66-OH@MF-3 (Zr-MOF on foam) | PVDA, PVDF (size 260–22 nm) | 2.4% loading on foam, - | 527 | 0.34 | 95.5 | 90% RE 10 cycles | 40 |
| 2. | ZIF-67 | PS MPs (size <10 μm) | 0.4 g L−1, 8 | — | — | 92.1 | — | 41 |
| 3. | Cr-MOF/MIL-101 | PS NPs (50–70 nm) | 100 ppm, 5 | 2281.8 | 0.23 | 96 | — | 42 |
| 4. | 2D MOF@C@FeO | PS MPs (1 μm) | 3000 mg L−1, 7 | 749.7 | — | ∼100 | 90% removal efficiency after 6 cycles | 101 |
| 5. | UiO-66-NH2 | PS NPs (30 nm) | 1 g L−1, 6 | 506 | 340 | ∼100 | — | 103 |
| 6. | Fe3O4@SiO2@MIL-53(Al) | PS, PVC, PP, PES MPs | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 material to MP ratio, 6 1 material to MP ratio, 6 |
— | — | 54.10 | 28–46% | 104 |
| 93.17 | 93–92% | |||||||
| 85.16 | 78–82% | |||||||
| 88.28 | 79–83% RE after 5 cycles | |||||||
| 7. | Ni-MOF | PS MPs | — | 112.462 | — | 99 | — | 105 |
| 8. | Nano-Fe@ZIF-8 | PS MPs (1 μm) | — | 1038 | — | 98 | — | 106 |
| 9. | MOF-545-oxime | PA MPs | — | 1157 | — | 32.6 | Up to 5 cycles | 107 |
| 6 | ||||||||
In this direction, Modak et al. explored the applicability of Cr-based MOFs in the removal of PS NPs at pH 5 (50–70 nm) from aqueous solutions.42 The major mechanisms for the adsorption of MPs on MOFs are electrostatic, π–π interactions and acid–base interactions. Further, it was reported that the removal efficiency of the Cr-MOF dropped from 91% ± 4.3% in the first cycle to 68% ± 15% in the fourth cycle.42 Hence Cr-MOFs also offer a viable alternative as MOFs for the removal of MPs/NPs.
Similarly, Chen et al. designed and synthesized Zr-MOF systems with various functionalities such as UiO-66-X (where X = H, NH2, OH, Br, and NO2) for the removal of NPs from water samples.40 The various functionalized Zr-MOFs were studied to obtain the maximum efficiency for the removal of different MPs/NPs, as demonstrated in Fig. 6a. The best material was reported to be UiO-66-OH@MF-3 with a removal efficiency of 95.5% ± 1.2% and it could be recycled up to 10 cycles. This study established that the uniform distribution of positively charged MOF nanoparticles assisted strong electrostatic interactions with the negatively charged MPs, which aided their removal.40 Further, various functional groups or defects on the MOF surface provided active sites for interactions including hydrogen bonding or Van der Waals interaction with MPs, which enhanced their removal. The increased water stability of the developed Zr-MOFs together with their flexibility and good strength adds to the merits of these materials. Furthermore, their interpenetrated pore structure allows the rapid passage of the solvent, ensuring sufficient interaction between the MPs and the MOF.40
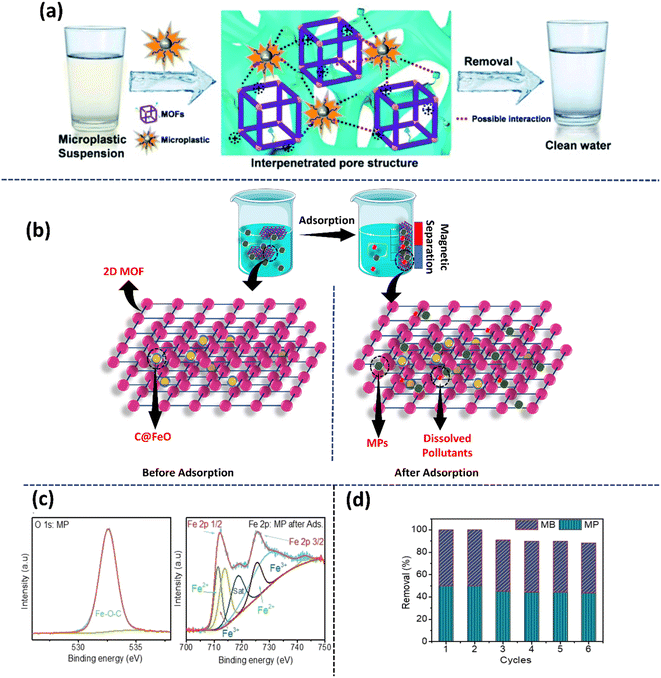 | ||
| Fig. 6 (a) Removal of microplastic impurities from water through adsorption on Zr-MOFs. Adapted with permission.42 Copyright 2023, ACS. (b) Schematic illustrating the structure of a 2D-MOF@C@FeO nanopillar and its adsorption/removal process for MPs and other dissolved impurities from an aqueous sample. (c) High-resolution O 1 s and Fe 2p XPS of a 2D-MOF@C@FeO nanopillar after the adsorption of MPs. (d) Graph showing the reusability and stability of the nanopillared structure, exhibiting 90% removal efficiency of MPs after 6 cycles. Graphs adapted with permission.101 Copyright 2023, Elsevier. | ||
Further, transforming 3D MOFs to 2D MOFs provides an opportunity to tune their surface chemistry.100 Notably, Haris et al. engineered carbon-encapsulated iron oxide (C@FeO) nanopillars, which self-assembled into the 2D MOF sheets, giving rise to a heterostructured adsorbent for MPs.101 This study examined the material for its MP removal efficiency and it was reported that 2D-MOF@C@FeO removed ∼100% of the MPs in the solution within 60 min. Interestingly, the peak fitting study showed a high iron peak intensity for 2D-MOF@C@FeO after the adsorption of MPs, which indicates the role of electrostatic interactions between the negatively charged MPs and positively charged iron ions in the effective removal of MPs by the adsorbent (Fig. 6b). Further, the magnetic property of the synthesized 2D MOF@C@FeO allowed its recovery using an external magnet and it could be recycled by washing several times with water and ethanol followed by drying at 105 °C for 4 h. This material was reported to exhibit excellent stability and renewability by effectively removing 90% of the MPs even after 6 cycles (Fig. 6c and d).101
Zeolitic imidazolate framework (ZIF)-based MOFs have been explored for the efficient removal of contaminants such as heavy metals and organic pollutants from water samples.102 These materials are easy to prepare and possess a large SSA, stable structure and large adsorption capacity. In an approach to employing a ZIF for MP/NP removal, Wan et al. used the ZIF-67 material with a central metal ion (Co2+) and 2-methylimidazole acting as the organic ligand.41 The nanomaterial with an average diameter of 92 nm was evaluated for the removal of PS MPs (size <10 μm) from water samples. It was reported that together with the electrostatic interaction between the opposite surface charges of the ZIF-67-based MOF and MP particles, π–π stacking and hydrogen bonding also assisted in the removal of MPs. The efficiency of the developed material for the removal of PS MPs was reported to be 88.3% in the pH range of 3–10.41
3.3. Carbon-based nanomaterials for the sequestration of MPs/NPs
Carbon-based materials are another prominent nanoadsorbent for the mitigation of MPs/NPs from water bodies.108–113 Due to the growing risks of MP/NP pollution, it is important to develop efficient and ecofriendly materials to eliminate NPs from the aquatic environment. Carbon-based nanomaterials offer a large SSA with a high number of adsorption active sites, and thus can act as potential adsorbents for environmental pollutants.113–117 Various carbon-based materials such as fly ash, graphitic carbons, and carbon nanotubes (CNTs) have been demonstrated for the abatement of MPs/NPs and a summary of these materials is provided in Table 4.| S. no. | Material | pH | Type of MP/NPs | REa (%) | Reusable cycles | RE after recycling (%) | Reference |
|---|---|---|---|---|---|---|---|
| a RE (removal efficiency). | |||||||
| 1. | Fe-fly ash | 5–7 | PS NPs 80 nm | 94.1 | 4 | 89.8 | 110 |
| 2. | M-CNT | — | PE, PET, PA MPs | 100 | 4 | 80 | 114 |
| 3. | G@LDO | 7–10 | PS MPs 80 nm | >95 | 5 | 80 | 117 |
| 4. | Cellulose | 6.9 | PMMA, PVC, PVAc NPs (50–137 nm) | 98 | — | — | 118 |
| 5. | Chitin–GO sponges | 6 | PS MPs 900 nm | 92.9 | 3 | 89.8 | 119 |
| 6. | ChCN sponges | 6–8 | PS MPs 900 nm | 92.1 | 3 | 90.6 | 120 |
| 7. | Oat protein sponge | 6 | PS MPs (1 μm) | 85 | 4 | 60 | 121 |
| 8. | Electrospun membrane M+ | 7.2 | PS NPs 50 nm 100 nm 500 nm | 89.9 | — | — | 122 |
| 99.3 | |||||||
| 99.4 | |||||||
| 9. | Surface-functionalised microbubbles | 7 | 5 μm PS and PMMA | 82–97 | — | — | 123 |
| 10. | PDA-MCS | 6 | PET MPs (10–20 μm) | 92 | 3 | 83.4 | 124 |
| 11. | CuNi@C | 4 | PS NPs (100 nm) | 95.55 | 4 | 90 | 125 |
Among the various adsorbents, fly ash (FA), which is produced as solid waste from burning coal, has been successfully used in water pollution control. Modified FA has a large SSA, high porosity, and high adsorption capacity.108,109 In a recent study, Zhao et al. synthesized Fe-modified FA as a novel material for the effective removal of PS NPs from aqueous samples.110 This material was reported to exhibit a high removal efficiency of 94.1%, which was reduced to 89.8% after 4 cycles, and a high adsorption capacity of 83.1 mg g−1 for NPs at room temperature. This study also established that the pH and interfering ions in the aqueous environment have a strong impact on the adsorption of NPs. Thus, loading FA with Fe ions (Fe2+/Fe3+, 0.8–35.8 wt%) tremendously improved its removal ability, given that it was seen that iron oxides have a high tendency to trap NPs in aqueous solution. The general mechanisms followed during the adsorption of NPs onto the surface of different nanomaterials are complexation, electrostatic attraction, and π–π interactions.110
CNTs are reported as excellent adsorbents for the removal of pollutants as well as hydrophobic aromatic compounds from aqueous solutions.111,112 These materials exhibit amazing qualities such as high specific surface area with tunable porosity, hollow internal structures, layered structures, and mesopores, which have been properly established. Moreover, their hydrophobicity, distinct sidewall curvature, continuous π-conjugation, tunable surface functionalities, tunable surface characteristics, light weight, and high corrosion resistance distinguish them from their peers.111 The structure of CNTs is well-defined and homogenous at the atomic level, together with a suitable pore size and hexagonally organized carbon atoms.111,112 Thus, they can be used as an excellent support for coating or depositing additional materials. CNTs can interact with several pollutants by a variety of mechanisms, including hydrophobicity, chemical interaction, electrostatic interaction, stacking, Lewis acid–base interactions, Van der Waals interactions, surface complexation, and hydrogen bonding.112,113
Further, the loading of CNTs with magnetic materials such as iron oxide can enhance their adsorption rate and assist in the easy removal of pollutants.113 In this direction, Tang et al. developed an efficient magnetic carbon nanotube (M-CNT) and studied its removal efficacy for 3 types of MPs including PE, PET, and PA (size = 48 μm).114 They reported that with an increase in the dosage of M-CNT adsorbent, the removal efficiency increased. Moreover, ∼100% removal efficiency of MPs from the sample solution was obtained at an M-CNT dosage of 5 g L−1. This study established that the working mechanism of M-CNT for the removal of PE was majorly due to strong hydrophobic interactions. However, in the case of PET, the combined contribution of π–π interactions, complexation, and electrostatic interactions played a significant role in its removal. Alternatively, hydrogen bonding on the PA surface caused its effective adsorption on the surface of M-CNT.114
Besides CNTs, graphene-like sp2 hybridized carbon materials (GCs) have also been employed in water purification. Supported by its intrinsic hydrophobicity, large surface area, and rich functionality, GCs have been utilized as adsorbents for the sequestration of organic pollutants.115 These materials exhibit a unique honey-comb structure, which provides high tolerance against harsh environmental conditions,116 making them potential materials for the removal of MPs from water bodies. In a recent study, Peng et al. combined graphene-like carbon with a layered double oxide (LDO), an alkali metal oxide formed by calcining layered double hydroxides, to form highly efficient G@LDO adsorbents for the removal of MPs in real water samples (Fig. 7a).117 The organic LDH was intercalated with SDS (lauryl sodium sulfate), forming 3D OLDH, which was calcined at 700 °C to form G@LDO700. The presence of graphitic carbon in G@LDO700 stabilized LDO under acidic and alkaline conditions. The removal efficiency of 80% and >90% for PS MP was obtained for G@LDO700 in the pH range of 3–5 and 7–10, respectively. After the adsorption of PS, G@LDO700 could be rinsed with ethanol, followed by calcination at 700 °C in an Ar atmosphere for 4 h and was reusable with a high removal efficiency of ∼ 80% even after 5 cycles (Fig. 7b and c).117
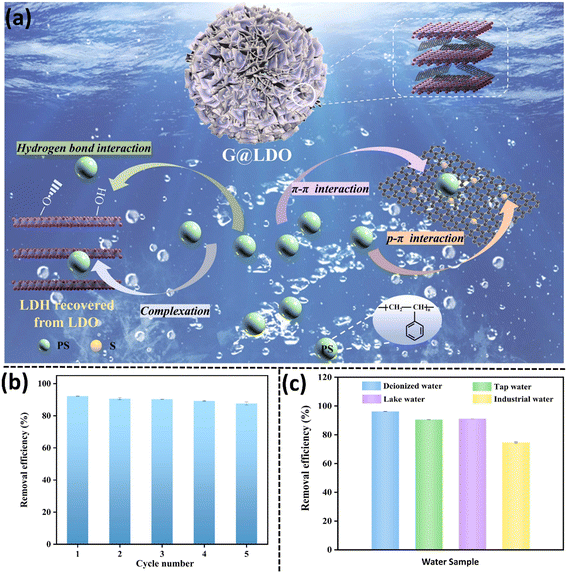 | ||
| Fig. 7 (a) Proposed mechanism and interaction involved in the adsorptive removal of PS NPs by G@LDO700. (b) Graph showing reusability of G@LDO exhibiting a high removal efficiency of ∼90% after 5 cycles. (c) Graph depicting G@LDO removal efficiency for PS NPs in real water samples. Figure and graphs were adapted with permission.117 Copyright 2022, Elsevier. | ||
3.4. Mitigation of MPs/NPs by biocompatible nanomaterials
Although numerous metal-based and magnetic materials or polymers have been developed, the metal-leaching problem remains a major concern. Several materials such as Fe-nanoparticles and MOFs are effective in removing plastic debris (MPs and NPs) from water samples. However, the leaching of metal ions into water bodies may result in secondary contamination, which can be hazardous to human health and the environment. Therefore, metal-free biocompatible nanomaterials that are environmentally friendly are becoming increasingly popular for the effective removal of MPs/NPs.118–121,126,127 Naturally generated materials from renewable sources, such as cellulose and chitin, have the potential to act as adsorbents for the removal of MPs/NPs.121–125 In this regard, a comprehensive analysis of different carbon-based and biocompatible nanomaterials is provided in Table 4.Cellulose, a renewable polymer having excellent biodegradability and biocompatibility towards the environment, plays a promising role in the adsorption of MPs/NPs from aqueous solutions. In this direction, Batool et al. synthesized polyethyleneimine-functionalized cellulose (PEI@CE) nanofibers and studied their removal efficiency against PMMA, PVC, and PVAc NPs (50–137 nm).118 It was reported that the PEI@CE fibers exhibited a high average removal efficiency of 98% for NPs with a minimum adsorbent dosage of 2 g L−1. Further, the SEM images of the fibers before and after the adsorption of NPs depicted the uniform distribution of spherical NP particles on the surface of PEI@CE.118
Similarly, Sun et al. prepared ecofriendly adsorbents using chitin and GO (chitin–GO sponges, ChGO) to remove 3 types of PS MPs, PS, PS-COOH, and PS-NH2, from water samples.119 This study demonstrated that with an increase in the concentration of GO, the removal efficiency towards various MPs increased. The highest removal efficiency was reported for ChGO-300 as 92.2% for PS, 74.9% for PS-COOH, and 90.2% for PS-NH2. This study also analysed the reusability of ChGO-300 recovered after 24 h of MP adsorption. The sponge was rinsed with ethanol and frozen at −80 °C for 4 h, and then freeze-dried to regenerate the sponge, which was effective for up to 3 cycles.119
The mechanism involved in this process for the removal of MPs is through the electrostatic attraction between negatively charged PS and positively charged adsorbent. Interestingly, it was noted that the sponges have no negative impact on algae growth, demonstrating their biocompatibility and tremendous promise in MP removal from the aquatic environment.119 Similarly, oxygen-doped carbon nitride (O-C3N4), which is structurally similar to GO, has also been employed as an efficient adsorbent for the mitigation of contaminants.127 Sun et al. studied the removal efficiency of MPs using functionalized ChGO (O-C3N4 based) sponges by obtaining five different types of chitin-based sponges such as Ch, ChCN (chitin and O-C3N4), ChGO, and ChGO-X (where X = CL (carboxymethyl cellulose), and CT (chitosan)) (Fig. 8a).120 It was reported that chitin-based sponges, ChCN, ChGO, and ChGO–CT, exhibited good removal efficiency towards PS (89.6–92.1%), PS-NH2 (83.2–87.1%), and PS-COOH (80.4–81.3%) MPs.120
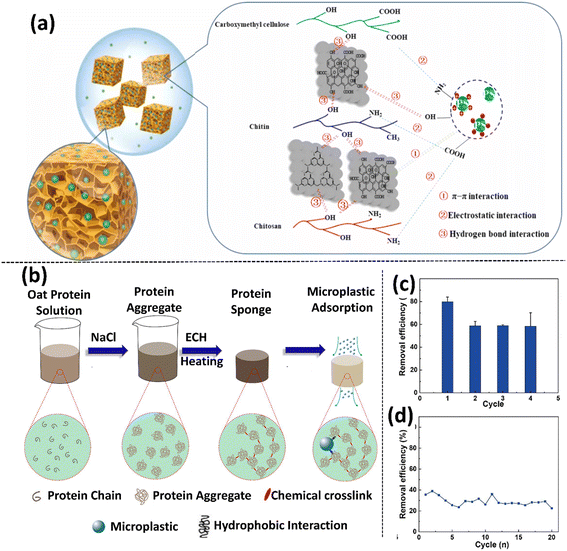 | ||
| Fig. 8 (a) Illustration of the possible interaction involved in the removal of MPs by a ChCN sponge. Adapted with permission.120 Copyright 2021, Elsevier. (b) Schematic of the fabrication of an oat protein sponge and its applicability in the adsorption of microplastics. (c) Graph depicting the removal efficiency of the OPS over 4 cycles. (d) Removal efficiency and fast adsorption of MPs on the OPS for 20 cycles. Error bars represent standard deviation (n = 3). (b–d) Adapted with permission.121 Copyright 2021, Elsevier. | ||
Although chitin and GO sponges exhibit a high MP removal efficiency, the large-scale synthesis of graphene materials for wastewater treatment is very expensive. Therefore, it is necessary to choose materials that are economically feasible together with being biodegradable and biocompatible. In this regard, Wang et al. synthesized a highly porous and novel oat protein-based sponge (OPS) and investigated its removal efficiency for 1 μm PS MPs (Fig. 8b).121 The highest removal efficiency of 85% was obtained for OPS (Fig. 8c and d). Owing to its chemical crosslinking and connected porous protein structure, OPS exhibited excellent permeability and mechanical properties towards the removal of MPs from aqueous solution. This property of OPS allows water to flow through and the MPs are trapped within it due to the hydrophobic interactions between the benzene ring of the PS MPs with the protein side chain.121 Although the removal efficiency of OPS was lower than that of other reported sponges, its low cost and biodegradability make it a viable material for the remediation of MPs/NPs.
3.5. Adsorptive removal of MPs/NPs by biochar-based nanoadsorbents
Biochar-based adsorbents provide another alternative to green materials for removing MPs/NPs from aqueous solutions.128–134 The unique characteristics of biochar, such as its high SSA, heteroatom-enriched functionalities, high porosity, and eco-friendliness, make it a promising material for wastewater treatment.135–140 A comprehensive analysis of different biochar-based nanoadsorbents is presented in Table 5.| S. no. | Source | Biochar | T (°C), pH | SAa (m2 g−1) | Type of MP/NP | REa (%) | Removal mechanism | Reusability cycles | Reference |
|---|---|---|---|---|---|---|---|---|---|
| a T (pyrolysis temperature), RE (removal efficiency), and SA (surface area). | |||||||||
| 1. | Scots pine bark | AC | 475, — | 615 | PE MPs | 100 | Pore entrapment | — | 128 |
| 2. | Sugarcane | BC-750 | 750, 3–7.5 | 540.36 | PS NPs (500 nm) | 99 | Electrostatic interaction | — | 129 |
| 3. | Prosopis juliflora (plant) | FB-850 | 850, 5.5 | 34.5 | 100 | Electrostatic interaction | ∼95% RE after 4 cycles | 130 | |
| 4. | Sawdust | MBC | 500, 7 | 363.80 | PS MPs (1 μm) | 94.81 | Electrostatic interaction, chemical bonding | ∼95% RE after 5 cycles | 131 |
| Mg-MBC | 265.47 | 98.75 | |||||||
| Zn-MBC | 329.87 | 99.46 | |||||||
| 5. | Rape straw | CMB | 800, 7 | 683.6 | PS NPs (600 nm) | 95.2 | Hydrophobic interaction, aggregation | ∼90% RE after 5 cycles | 132 |
| CPS NPs (1 μm) | 91.2 | ||||||||
| 6. | Corn cob | MCCBC | 500, 7 | — | PA MPs (aged, size <75 μm) | 97 | Surface adsorption, magnetic separation | — | 133 |
| 7. | Corncob | CB-900 | 900, 5 | 36.3 | PS NPs (50 nm) | — | Pore entrapment, hydrophobic interaction, H-bonding | — | 134 |
| ACB-900 | 60.8 | ||||||||
| 8. | Peanut shells | PSB | 500, 6 | 128 | CPS MPs 1 μm | 75.53 | Electrostatic interaction, steric hindrance | — | 135 |
| 9. | Rice straw | RB | 500, 5 | 35.45 | PS NPs 300 nm | 48.00 | Electrostatic interaction, H-bonding, π–π interaction | ∼90% RE after 5 cycles for MRB biochar | 136 |
| MRB | 327.65 | 99.96 | |||||||
| 10. | Banana peel | BPB | 650, — | 12.27 | PS MP 150 to 300 μm | 92.16 | Pore entrapment, entangled | — | 137 |
| 11. | Corn straw | C500 | 500, 8–9 | 177.5 | PS MP 10 μm | 95 | Stuck, trapped, entangled | — | 138 |
| 12. | Cellulose | BC | 400, 6 | — | PS (2 μm, 200 nm, 20 nm) | 90 | Aggregation | — | 139 |
| 13. | Jujube | BC700 | 700, 6–7 | — | PE MPs | 99 | Entrapment, entangled, electrostatic interactions | — | 140 |
Siipola et al. created a low-cost biochar adsorbent from pine and spruce bark for wastewater purification and investigated its use for the removal of MPs.128 Further in this direction, Ganie et al. used sugarcane-derived biochar for the adsorptive removal of negatively charged PS NPs (size <500 nm) from water samples.129 High temperatures cause the volatilization of volatile organic materials, resulting in enhanced porosity and surface area for the effective adsorption of specific contaminants. As a result, BC-750 (synthesized at 750 °C) was found to be very porous and had a substantially high SSA of 540.36 m2 g−1. Indeed, BC-750, which has a large specific surface area and low negative surface charge, was reported to remove more than 99% of NPs at pH levels in the range of 3–7.5.129
Singh et al. synthesized an iron biochar magnetic composite (FB) with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 1
10 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 iron loadings and studied their efficiency for the removal of NPs.130 The study focused on three different types of NPs, carboxylate-modified NP1 (1000 nm), amine-modified NP2 (1000 nm), and carboxylate-modified NP3 (30 nm). The highest reported removal efficiency was ∼100% for NP1 and NP2 using FB-550, and FB-850 at a 1
1 iron loadings and studied their efficiency for the removal of NPs.130 The study focused on three different types of NPs, carboxylate-modified NP1 (1000 nm), amine-modified NP2 (1000 nm), and carboxylate-modified NP3 (30 nm). The highest reported removal efficiency was ∼100% for NP1 and NP2 using FB-550, and FB-850 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 iron loading.130 Similarly, Wang et al. compared the removal efficiency of simple sawdust-derived biochar (BC), magnetic-biochar (MBC), and Mg/Zn-MBC for 1.0 μm PS NPs.131 The removal efficiency of PS NPs for different biochar was reported for BC (25.89%), MBC (94.81%), Mg-MBC (98.75%), and Zn-MBC (99.46%). The results showed that modification of biochar by loading Fe (10 wt%), Mg (6.85 wt%), and Zn (6.07 wt%) significantly improved its adsorption ability towards PS NPs. Furthermore, the SEM examination revealed that after loading Mg and Zn on MBC, its surface became significantly rougher, resulting in a high NP trapping efficiency and adsorption equilibrium in a shorter period.131 Furthermore, this study revealed that the degradation of MPs and renewal of the adsorbent could be done concurrently by in situ pyrolysis due to the intrinsic catalytic activity of the modified biochars, where Mg/Zn-MBC facilitated hydrogenation processes, generating small molecules. The performance of the regenerated magnetic biochar was assessed for 5 consecutive cycles, and both Mg and Zn-MBC retained ∼95% MP adsorption efficiency.131
1 iron loading.130 Similarly, Wang et al. compared the removal efficiency of simple sawdust-derived biochar (BC), magnetic-biochar (MBC), and Mg/Zn-MBC for 1.0 μm PS NPs.131 The removal efficiency of PS NPs for different biochar was reported for BC (25.89%), MBC (94.81%), Mg-MBC (98.75%), and Zn-MBC (99.46%). The results showed that modification of biochar by loading Fe (10 wt%), Mg (6.85 wt%), and Zn (6.07 wt%) significantly improved its adsorption ability towards PS NPs. Furthermore, the SEM examination revealed that after loading Mg and Zn on MBC, its surface became significantly rougher, resulting in a high NP trapping efficiency and adsorption equilibrium in a shorter period.131 Furthermore, this study revealed that the degradation of MPs and renewal of the adsorbent could be done concurrently by in situ pyrolysis due to the intrinsic catalytic activity of the modified biochars, where Mg/Zn-MBC facilitated hydrogenation processes, generating small molecules. The performance of the regenerated magnetic biochar was assessed for 5 consecutive cycles, and both Mg and Zn-MBC retained ∼95% MP adsorption efficiency.131
Shi et al. proposed the surface modification of magnetic biochar with cationic surfactants to remove NPs via aggregation.132 This study investigated the removal of PS (600 nm) and carboxylate-modified PS (CPS-1000 nm) NPs using cetyltrimethylammonium bromide-modified magnetic biochar (CMB). The highest removal efficiency of 95.2% and 91.2% for PS and CPS, respectively, was obtained at pH of 7 with the CMB adsorbent dosage of 8 mg L−1.132 After analyzing the mechanism of NP removal, it was determined that the modified CMB was made up of CTAB having a 16-C alkyl chain, which resulted in a network of hydrophobic molecular layers on the biochar surface. Therefore, the cetyl tail may capture PS/CPS NPs, causing them to aggregate and form clusters. These NP clusters could be eliminated once they get big enough and rise to the liquid surface. Consequently, it can be concluded that CMB promotes NP aggregation and instability, which results in their effective elimination.132
In this direction, recently Li et al. investigated the efficacy of magnetic biochar (MCCBC) on pristine PA MPs (27–307 μm) and aged PA MP.133 The synthesized MCCBC showed 25% removal efficiency for pristine PA (PA-0). In contrast, 97% removal efficiency was observed for PA-0.5 (0.5 days of aging time) and PA-1 (1 day of aging time). Thus, it was evident that compared to pristine PA, the biochar was far more effective for the aged PA. Furthermore, this study showed that carboxyl groups show strong affinity towards iron oxide.133 Therefore, the formation of strong complexation was observed between the acidic carboxy groups present on the surface of the aged PA and iron oxide present on the surface of MCCBC, ensuring the effective adsorption of PA MPs on the biochar.133
4. Advances in nanomaterials for degradation/mitigation of MPs/NPs
Physical separation methods for the remediation of MP/NP pollution have to be followed by degradation and/or generation of value-added products for a sustainable environment. This initiated the discovery of chemical techniques for the degradation and complete conversion/remediation of MPs/NPs. In this direction, biodegradation and advanced oxidation processes (AOPs) are two of the most recently explored approaches for microplastic removal. AOPs are comprised of several degradation mechanisms and can eradicate organic contaminants by reactive oxygen species (ROS, such as OH˙ and O2˙−) with high standard reduction potentials. These processes can randomly break the chemical bonds present in the framework of organic contaminants, which leads to chain breakage, and even the mineralization of contaminants. In most cases, semiconductor photocatalysis technology is employed to effectively destroy water pollutants, such as MPs.141–145 The use of solar or visible light allows the photocatalytic degradation of MPs. UV-Vis spectroscopy were used to investigate the absorption capacity of catalysts to better understand the components that influence their performance and degradation mechanisms.144–150 The photocatalytic degradation of MPs/NPs provides an economical approach towards the remediation of plastic debris into harmless by-products via a clean energy source (solar light).150–155 Photocatalysts such as titanium dioxide (TiO2), zinc oxide (ZnO), bismuth-based oxides, and graphitic carbon nitride (C3N4) with a wide band gap are majorly used as photocatalysts for the degradation of MPs/NPs. In this regard, a comprehensive analysis of the various photocatalysts employed for the degradation of MPs/NPs is presented in Table 6.| S. no. | Catalyst | Band gap (eV) | Type of MP/NP | pH | Source of light | Reaction time | Degradation efficiency | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | TiO2 | 3.46–3.11 | PS NPs (314 nm) | — | UV | 50 h | 23.5% NP concentration decrease | 49 |
| 2. | ZnO nanorods | — | PP MPs (154 μm) | — | Tungsten-halogen lamp 120 W | 456 h | 65% degradation of PP MPs | 141 |
| 3. | BiOCl-1 | 3.29 | PE MPs (200–250 μm) | 3 | UV (250 W Xe lamp) | 5 h | 5.38% MP mass loss | 142 |
| 4. | BiVO4–Fe3O4 microrobots | — | PP, PET, PLA, PCL MPs | — | Sunlight | 7 days | 3% MP mass loss | 146 |
| 5. | BiOI–Fe3O4 microswimmer | 1.9 | PS MPs (1 μm) | 7 | Sunlight | 120 h | 64% degradation of MPs | 147 |
| 6. | C3N4 nanosheets | 2.69 | PET MPs | 7 | Sunlight | — | — | 148 |
| 7. | TiO2/MIL-100(Fe) | 2.21–2.65 | PET NPs (111 nm) | 3 | Sunlight | — | — | 152 |
| 8. | Au@Ni@TiO2-micromotors | — | PS-COOH MPs | — | 315 mW UV | — | 67% MPs clearance | 153 |
| 9. | C3N4/WO3 | 2.70 | PET MPs | — | 300 W Xe lamp | — | — | 154 |
| 10. | CuxO | 1.6–2 | PS NPs (350 nm) | — | — | 50 h | 18–23% degradation | 155 |
| 15% mineralisation of NPs |
TiO2-based photocatalysts, which are well-known semiconductor materials, have been frequently employed for photocatalytic degradation, owing to their superior photocatalytic characteristics and their capability to produce oxidative species on their surface.48 In this direction, the photocatalytic efficiency of three distinct TiO2 photocatalysts produced via anodization was investigated by Domínguez-Jaimes et al.49 The statistical study demonstrated that utilizing TiO2-mixed (TiO2/M) and TiO2-nanotubular (TiO2/T) as photocatalysts to eliminate PS NPs was more efficient as compared to photolysis. The results demonstrated that TiO2/M, having a nanograss and nanotubular morphology with multilayer structure, outperformed the other photocatalysts. This newly created structure displayed the best-photogenerated charge carrier transfer, which is highly efficient for the photodegradation of PS NP. The interaction between the NP particles and TiO2/M structure reduced the pollutant concentration by 23.5% in the best-case scenario.49 These findings illustrate the feasibility of the degradation of PS NPs by employing nanostructured catalysts via heterogeneous photocatalysis.
ZnO has also been examined for its use in the photocatalytic degradation of MPs/NPs. A prominent example demonstrated by Uheida et al. explained the use of ZnO nanorods for the photocatalytic degradation of PP MPs (154.8 μm).141 ZnO nanorods under solar irradiation for 456 h degraded 65% of PP MPs. The presence of a carbonyl group with increased carbonyl index (CI ∼ 40) in the FTIR analysis visibly displayed the significant degradation of PP MPs.141 Further, the photocatalytic performance of ZnO nanorods could be enhanced by doping with metals such as Pt. It was observed that Pt/ZnO nanorods exhibited enhanced activity for the photocatalytic degradation of LDPE MPs under solar light.141
Similarly, bismuth-based oxides exhibit high potential as light-activated photocatalysts for environmental remediation. In this direction, Jiang et al. synthesized hydroxyl-rich ultrathin BiOCl nanostructures for the photocatalytic degradation of PE MPs (200–250 μm).142 The ultrathin structure obtained by the addition of mannitol during the synthesis of BiOCl increased the degradation by 24 times compared to regular BiOCl nanosheets. In addition, the SEM images of PE MPs over different degradation times (0 to 10 h) displayed a significant decrease in size, which were almost degraded by 10 h (Fig. 9a). The surface hydroxyl groups on the BiOCl catalyst effectively enhanced the hydroxyl radical production, thus playing a crucial role in MP degradation.142 The mechanism involves the reaction of hydroxyl radicals as active species with the PE molecule, leading to a series of radical formation reactions, which results in the formation of formaldehyde. This by-product can eventually be broken down to CO2 and H2O, resulting in the complete mineralization of PE MP.142
Initiation:
| BiOI–Fe3O4 → BiOI–Fe3O4(e−)CB + BiOI–Fe3O4(h+)VB | (1) |
| BiOI–Fe3O4(O2) + e− → O2˙− | (2) |
| BiOI–Fe3O4(H2O) + h− → BiOI–Fe3O4(OH˙) + H+ | (3) |
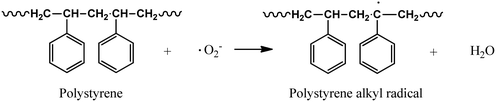 | (4) |
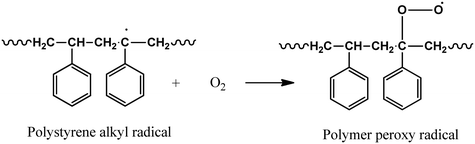 | (5) |
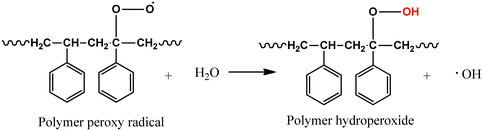 | (6) |
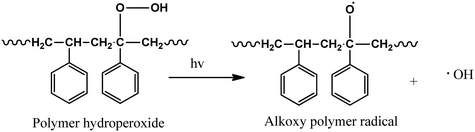 | (7) |
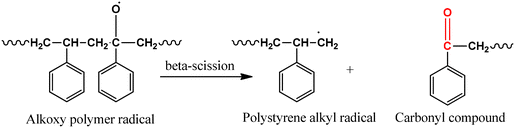 | (8) |
 | (9) |
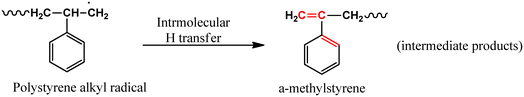
| Intermediate Products → CO2 + H2O | (10) |
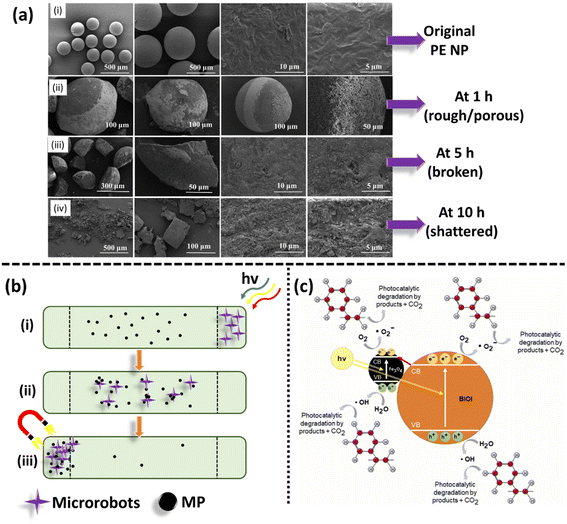 | ||
| Fig. 9 (a) SEM images (i–iv) illustrating the photocatalytic degradation of PE NP by the BiOCl catalyst at different times ranging of 0–10 h. Adapted with permission.142 Copyright 2021, Elsevier. (b) Schematic representation of the removal of MPs through an aqueous channel by BiVO4–Fe3O4 microrobots, (i) stimulating the microrobots by solar light and propelling into a chamber containing MP impurities, (ii) adsorption of microrobots on the surface of MPs, and (iii) separation of MPs attached to microrobots via magnetization. (c) Mechanism of photocatalytic degradation of PS MPs by BiOI-Fe3O4 microswimmers. Adapted with permission.147 Copyright 2022, Elsevier. | ||
In a futuristic approach, micromotors/microswimmers incorporating nanomaterials have also been explored for environmental remediation,143 cargo transportation,144 and biomedical applications.145 Furthermore, light-harvesting micromotors/microswimmers with semiconducting materials having well-defined band gaps, self-propagation properties in water and H2O2-rich environment can act as advanced catalysts for the photocatalytic degradation of MPs/NPs. Notably, few studies examining the efficiency of these micro-machines for plastic degradation have been reported. Recently, Mousavi et al. studied the degradation of MPs by light-driven photocatalytic BiVO4–Fe3O4 microbots (Fig. 9b).146 This study demonstrated the photocatalytic degradation of various MPs such as PP, PET, polycaprolactone (PCL), and polylactic acid (PLA) by BiVO4–Fe3O4 microbots. The maximum mass loss of ∼3% was obtained for PLA after 7 days degradation. Further, functionalization by nano-Fe3O4 made the microbots magnetic, allowing them to be collected after the experiment and recycled for another use.146 Similarly, Khairudin et al. examined the photocatalytic degradation of MPs by using a hybrid material (bismuth-magnetic microswimmers).147 In this study, BiOI was used as the photocatalyst owing to its small band gap (1.9 eV), which can easily absorb visible light. It was reported that this catalyst showed 56% degradation efficiency within 24 h for PS MPs.147 To understand the mechanism during photocatalysis, the detailed explanation of each step involved in the degradation of PS MPs by the BiOI photocatalyst is provided in eqn (1)–(10). The degradation begins with the initiation reaction, followed by radical formation, which acts as the photocatalyst for the degradation of PS MPs (Fig. 9c).147
Besides Ti, Zn, and Bi-based photocatalysts, polymeric carbon nitride (PCN) acts as a promising alternative owing to its well-defined band gap and fine optical performance. In a recent study, Liu et al. doped I− in a PCN photocatalyst (C3N4 nanosheets) to obtain efficient activity for the degradation of PET MPs and H2 evolution.148 The synthesized photocatalyst consisted of abundant active sites (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, –C–OH, and –OH), which can act as homogenous photocatalytic systems for efficient H2 production and PET MP degradation under visible light. Further, this study reported that the H2 evolution rate reached the highest up to 600.3 μmol g−1 h−1, and PET MPs were eventually degraded into smaller and harmless products such as glycolate, acetate, and methanol.148 Similarly, Amato et al. recently reported the photocatalytic degradation of low-density polyethylene MPs by a biowaste-based TiO2 hybrid photocatalyst.149 Its mechanism involves the formation of OH· radicals, which are responsible for the initiation of alkyl radicals (R1·), followed by a termination reaction with another alkyl radical (R2·), leading to the formation of an alkane (R3H). Alternatively, R1· may undergo a reaction with OH· to produce alcohols (ROH), which may be further oxidised to carboxylic acids (RCOOH) or esters (RCOOR).149 A similar mechanism was observed to be followed in the degradation of MPs/NPs by other hybrid photocatalysts of Ti and Si.149–151
N, –C–OH, and –OH), which can act as homogenous photocatalytic systems for efficient H2 production and PET MP degradation under visible light. Further, this study reported that the H2 evolution rate reached the highest up to 600.3 μmol g−1 h−1, and PET MPs were eventually degraded into smaller and harmless products such as glycolate, acetate, and methanol.148 Similarly, Amato et al. recently reported the photocatalytic degradation of low-density polyethylene MPs by a biowaste-based TiO2 hybrid photocatalyst.149 Its mechanism involves the formation of OH· radicals, which are responsible for the initiation of alkyl radicals (R1·), followed by a termination reaction with another alkyl radical (R2·), leading to the formation of an alkane (R3H). Alternatively, R1· may undergo a reaction with OH· to produce alcohols (ROH), which may be further oxidised to carboxylic acids (RCOOH) or esters (RCOOR).149 A similar mechanism was observed to be followed in the degradation of MPs/NPs by other hybrid photocatalysts of Ti and Si.149–151
This approach of photocatalytic degradation stands out as a promising process for the degradation of MPs/NPs due to two major reasons, as follows: (a) smaller/simpler organic byproducts are formed, which can be reused for laboratory and industrial purposes, and (b) it can be optimized to be used in tertiary recycling. According to the GS-MS results from various studies, the most common photocatalytic degradation byproducts produced from the degradation of MP/NP plastic polymers include ethenyloxy/acetyl radicals, hydroxypropyl, acetone, ethyl alcohols, acetaldehyde, and formaldehyde.141–149 The majority of these byproducts are actively employed in laboratories and have a relatively less harmful impact on human health and the aquatic environment, for example, hydroxypropyl and acetyl groups are commonly utilized in pharmaceutical substances.141 Hydroxypropyl cellulose is used to alleviate eye discomfort. In addition to being a popular laboratory solvent, acetone is utilized in the manufacture of coatings, plastics, cosmetics, and pharmaceuticals. Ethyl alcohol is widely employed as a solvent, and also the primary component of alcoholic beverages for human consumption.141 Further, the intermediate by-products formed can eventually be converted into CO2 and H2O, leading to the complete mineralization of MP/NP wastes.142 However, after extended exposure, some of the byproducts formed due to incomplete degradation may have detrimental effects on the environment and human health. For instance, prolonged exposure to formaldehyde may result in nausea, dizziness, and eye discomfort. Similarly, radical by-products may cause damage to the structure of cells. Therefore, sufficient precaution is also essential to avoid the effects of harmful byproducts.141,142 As a result, most of the degraded byproducts of micro-/nanoplastics demonstrate low toxicity towards humans and the environment compared to the precursor, thereby providing a potentially safer pathway for mitigating MP/NP pollution.
5. Prospect towards the conversion/reduction of MPs/NPs into value-added products
Efforts have been devoted to decreasing plastic waste disposal through reduction, reuse, and recycling. Plastic garbage can be used to produce fuels and chemicals, thereby contributing to sustainability in the future, i.e., “Trash to Treasure”.156,157 In this direction, four major technologies (primary, secondary, tertiary, and quaternary) for plastic disposal have been developed. In general, primary and secondary recycling is the practice of directly reusing unpolluted plastic trash to create new items and the dissolution of blended plastics in an appropriate organic solvent to produce distinct components, respectively. However, the tertiary and quaternary recycling processes are the most used technologies involved in the conversion/reduction of MPs/NPs into valuable chemicals.Tertiary recycling, also known as chemical recycling, uses chemical methods such as pyrolysis, hydrocracking, solvolysis, gasification, and photoreforming to turn MPs/NPs into valuable chemicals. The repeating hydrocarbon backbones in MPs/NPs are also cleaved in this technique to form oligomers with shorter chains. This method has received widespread attention due to its great economic advantage.156–158 Tertiary recycling refers to the process of converting plastic products into valuable petrochemical and fuel feedstock. Because primary and secondary recycling methods are expensive to perform and require significant energy, tertiary recycling can be considered an inexpensive process that recycles materials, while remaining sustainable. Tertiary recycling is defined as a chemical process that recycles MPs/NPs using a solvent.158,159 However, its major disadvantage is the necessity for additional purification of the end-products, which are heterogeneous mixtures of different products and oligomers.160 Similarly, quaternary recycling is the burning of plastics, which is typically employed for energy recovery to cope with severely contaminated plastic trash that is very difficult to dispose of through other means. By burning the complicated mixtures, heat energy is generated, and some hazardous or greenhouse gasses may be formed concurrently. Considering its simplicity and efficiency, this quaternary recycling is frequently utilized around the world. The bulk plastics or the degraded MPs/NPs can be converted into valuable products such as CO2, H2O, olefins, alkanes, wax gasoline, hydrogen, and even carbon by bond cleavage. Recently, Ren et al. reported the conversion of HDPE MP via an NiCl2 nanocatalyst into various carbon-based materials such as carbon nanosheets, core–shell carbon, and carbon composites.158
In this direction, thermochemical reduction technologies, such as pyrolysis and gasification, are seen as potential methods for converting MPs/NPs into biochar, bio-oil, and syngas.161 The gasification of MPs/NPs provides a combination of gaseous fuels, including H2, CO, CO2, CH4, and N2. In this regard, the solvolysis process shows promise for converting MPs/NPs into value-added products. The primary advantage of this process is its capacity to purify acquired monomers, allowing the re-polymerization and creation of new polymers of virgin-grade quality.162 Similarly, microwave catalysis is a promising method for efficiently converting MP/NPs into value-added fuels such as H2 and carbon-based nano-compounds such as CNTs and nanocomposite materials.161–165 Compared to the classical plastic degradation by thermo-catalysis, microwave catalysis is more preferable. This is owing to its advantages such as uniform heating throughout the vessel rather than superficial heating, quick reaction rate, and a fast heating rate. According to these findings, this method can help reduce the energy usage, while also increasing the performance quality of items derived from plastic conversion/reduction.
However, for the remediation of MPs/NPs, photocatalytic degradation and electrochemical conversion methods are well known, which were discussed in the previous sections. In this direction, Cao et al. fabricated a series of nanocomposites, MXene/ZnxCd1−xS (x = 0, 0.2, 0.4, 0.5, 0.6, 0.8, 1.0), as efficient photocatalysts for the degradation of PET MP.57 It was reported that utilizing the MXene/Zn0.6Cd0.4S nanocomposite as a catalyst in an alkaline PET MP solution resulted in the optimal H2 generation (14.17 mmol g−1 h−1).57 Further, Nabi et al. examined the solid-state breakdown of PE and PS MPs using a Triton X-100-based TiO2 nanoparticle film.59 The strong interaction was due to the large exposed surface area of MPs, which led to their direct interaction with TiO2 nanoparticle film and the end-product, resulting in a 99.9% efficiency in mineralizing 5 μm PS into CO2 within 24 h.59
In a similar direction, Kang et al. developed helical CNTs for the degradation of MPs.60 The helical CNTs were produced using a high loading of nitrogen, followed by the encapsulation of manganese carbine nanoparticles to create magnetic nanohybrid helical CNTs for the degradation of MPs derived from cosmetic products. The mineralization of MP particles was demonstrated by combining hydrothermal hydrolysis and carbocatalytic oxidation. The CNT nanohybrid removed 50 wt% of MPs with an adsorbent dosage of 0.2 g L−1 and 5 g L−1 MP concentration. The synergistic impacts of the CNT and Mn nanoparticles, combined with the highly ordered graphitic nanocarbon, secured the durability of the nanocomposite during the AOP, enhanced the performance of the degradation process, and could be recycled for three cycles.60
Similarly, Mao et al. demonstrated the conversion of MPs into a valuable product by using a novel Mn0.1Ni0.9Co2O4−δ rod-shaped fiber (RSFs) nanocatalyst.165 These RSF nanocatalysts could convert PET MPs into terephthalic acid, formate ion, and K2SO4. Initially, MP PET was hydrolyzed in a KOH solution to produce ethylene glycol and terephthalic acid. Subsequently, they were utilized as the electrolyte to generate formate ions at the anode. Meanwhile, the H2 evolution reaction occurred at the cathode to generate H2. This study found that Mn0.1Ni0.9Co2O4−δ RSF catalyst had the best Faradaic efficiency (>95%) at 1.42 V vs. RHE, with promising formate ion production.165 However, from an economic standpoint, a brief cost analysis of MP/NP conversion/remediation is offered in the following section.
6. Economic feasibility/cost analysis of conversion/remediation of MPs/NPs
To address plastic waste concerns, it is necessary to develop cost-effective, scalable, and impactful technologies and behaviors. Environmental sustainability relies heavily on the economics of plastic conversion technology.164 However, the profitability of these technologies is constrained by the capped sales value of items such as electricity and liquid fuels during the collapse of the oil market. Converting lower-valued plastic mixtures, such as thermoplastic polyolefins, to monomers (ethylene or propylene) is not always cost-effective. Waste plastics can be converted into high-value goods such as wax and BTX (benzene, toluene, and xylenes), which are more expensive than crude oil and fuels.164–170Production costs are an important consideration when using thermochemical technology in waste management.170–172 In this direction, Fivga and Dimitriou used the Aspen HYSYS software to develop a cost-effective pyrolysis unit capable of processing 100 kg of plastic waste per hour.161 The suggested rate of 1000 kg h−1 resulted in the fuel production cost of $ 1.10 per kg, which is 58% higher than the present market scenario. However, a large-scale production technique can reduce costs by 2–18.9 times, indicating possible cost-effectiveness.161 This form of techno-economic study must be strengthened soon to make the conversion process of plastic debris into valuable products more economically feasible. Given the molecular properties of PE plastic, it would be preferable to convert it to refinery products with a comparable C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H ratio.165–172 Similarly, the cost analysis and economic feasibility of different MPs/NPs have been studied by some research groups.
H ratio.165–172 Similarly, the cost analysis and economic feasibility of different MPs/NPs have been studied by some research groups.
Khairudin et al. investigated the economics of the degradation of MPs utilizing a magnetically recyclable microswimmer.147 The cost analysis was performed by utilizing 500 mg L−1 BiOI–Fe3O4 microswimmer to accomplish 70% MP removal, which was projected to be roughly $ 6.69 per h.147 The material for the microswimmer itself accounted for 45% of the total cost involved. As a result, fluctuations in raw material prices for the synthesis of the microswimmer may result in increased operational costs in the future. This was compared to the previously reported technique for PE MPs using a membrane bioreactor (MBR), which is a popular method of extracting MPs from wastewater.147 According to Vuori et al., it would cost $ 158.75 per h for the removal of PE MPs using MBR, whereas utilizing a microswimmer would cost approximately $ 6.69 per h, indicating the feasibility of this approach.170 In this direction, Peng et al. also reported a low-cost biohybrid Fe3O4 magnetic microrobot that can remove MPs/NPs from aquatic ecosystems.43 The reusable and low-cost properties of microrobots are regarded as critical factors influencing their long-term and sustainable use for environmental cleanup.43 Similarly, Mao et al. conducted a preliminary technical and economic analysis (TEA) on the conversion of MP PET into formate ions utilizing a spinel nanocatalyst (Mn0.1Ni0.9Co2O4−δ RSFs) via the electrochemical approach.166 Their investigation showed that upcycling trash PET yields around $226 per tonne at current density of 50 mA cm−2. This study not only proposed an electrocatalytic technique for upcycling PET MPs but can also guide the development of high-performance electrocatalysts.166 It is noteworthy to mention that every type of plastic has a defined limit of recyclability, and therefore introducing plastics into the circular economy should eventually preserve an end-of-life value, while still contributing to growing plastic trash.
7. Technical limitations and future perspectives of nanotechnological interventions
Nanotechnological interventions possess high potential in tackling the problem of MPs and NPs. It offers more efficient, economically feasible, and sustainable solutions to MP/NP pollution in the aqueous environment. However, to date, most methods are only effective in the laboratory-scale removal and degradation of MPs/NPs, and the processes/experimental conditions and MPs/NPs used are mainly custom-made. Hence, futuristic approaches need to be adapted under natural environmental conditions. Therefore, some much needed futuristic approaches and certain technical limitations are provided in the following section.(a) The MPs/NPs present in the natural aqueous environment are aged and have irregular surface properties, and hence it is important to conduct removal/conversion/degradation studies using advanced nanomaterials/composites on the naturally obtained MP/NP pollutant particles.
(b) Currently, most studies use synthetic water samples, which may obscure the impact of several elements in actual waste fluids (e.g., heavy metals, DOM, inorganic salts, minerals, and microbes). Future research should examine the separation/mitigation of MPs/NPs from actual urban wastewater utilizing nanocatalysts/nanocomposites. Further, the conversion of these plastic pollutants to value-added products or completely biodegradable products should be investigated.
(c) In a similar context, it can be seen from this review that the majority of the nanomaterials/nanocomposites available for the removal of MPs/NPs are adsorption based. Although adsorption offers an efficient technique for the removal of toxic waste from aqueous samples, the waste remains on the adsorbent or collected in another solvent, and thus the waste persists. Hence, the upscaling of efficient nanotechnologies for mitigation and conversion is necessary.
(d) Secondary pollution is an important aspect that needs to be further studied with respect to methods such as photocatalytic degradation of MPs/NPs. In several studies, degradation did not result in complete mineralization and may produce toxic byproducts. Thus, the toxicity of byproducts as well as the incomplete degradation of MPs/NPs must be addressed effectively.
(e) Although nanomaterials offer a diverse range of effective materials against MP/NPs, it must carefully considered that these materials are also in the nano-range, similar to plastic debris, and hence they may also act as secondary pollutants.
(f) Nano-toxicity caused by the very small size of nanomaterials is a field that still requires more studies. To prevent secondary pollution, analysis of the toxicity of the adsorbents is required in future studies. Adsorbents loaded with MPs require suitable treatment. Biodegradable adsorbents are preferred owing to their green character and less poisonous temperament.
(g) The separation or recovery of nanomaterials/nanocatalysts after the removal of MPs/NPs from an aqueous solution is one of the major issues, which needs to be thoroughly investigated in future studies. In general, their regeneration should be cost effective and environmentally friendly.
(h) More research is needed to determine the economic viability of using adsorbents/nanomaterials to remove MPs/NPs from large-scale water streams in real-world applications.
8. Conclusion
This study comprehensively investigated the use of nanointerventions to reduce microplastics (MPs) and nanoplastics (NPs) in water bodies. MPs/NPs can be removed using advanced methods such as adsorption, membrane filtering, photocatalytic degradation, magnetic separation, and electrochemical sequestration. Further, magnetic separation and electrocoagulation (EC) have demonstrated a promising performance for the removal of MPs and NPs from water streams. However, to obtain a greater performance, magnetic separation typically requires iron-based or other metal-based components, which may result in higher amounts of metals in the water and present a risk to human health. Thus, toxicity studies are essential in future investigations to better understand the effluent quality following the magnetic separation method. Indeed, membrane filtration has been proven to be an excellent alternative for treating huge volumes of MP/NP wastewater, together with photocatalytic degradation to convert MPs/NPs into value-added compounds. A thorough understanding of the usage of several nanomaterials (magnetic nanomaterials, MOFs, carbon-based nanomaterials, and biocompatible and biochar-based nanomaterials) for the absorption of MPs/NPs was provided. MOFs, microswimmers, micromotors, g-C3N4, and nano-Fe3O4 have demonstrated a strong inclination to remove MPs/NPs. However, despite the recent efforts to remove MPs and NPs, it should be remembered that there are still certain information gaps to be explored for the adequate investigation of MP/NP removal behaviors and the mechanisms in real sewage are required in future examinations. Moreover, the pertinent factors including characteristics of MPs/NPs (e.g., size, shape, surface charge, and chemical configuration), characteristics of nanomaterials (e.g., adsorbent dose, adsorbent type, and dosage time), and attributes of environmental media (e.g., solution pH, salinity, coexisting toxic pollutants, natural organic matter, temperature, and interfering ions) can alter the removal performance of MPs/NPs. The integration of nanotechnologies/nanomaterials with advanced separation/mitigation techniques will provide a synergistic performance enhancement and unique solutions for the removal of MPs/NPs. At the commercial level, these integration studies need to be conducted in the future to better execute the function of nanotechnologies for the efficient removal of MPs and NPs.Data availability
The current article is a critical review. Hence, no new data have been generated for preparing this article.Author contributions
J. Das: conceptualization, writing – original draft; E. Yadav: writing – original draft, K. M. Poluri: conceptualization, funding acquisition, writing – review & editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
EY acknowledges the receipt of UGC-JRF research fellowship (CSIR/UGC/221610038074/JUN22C01247) for PhD program. KMP acknowledges the financial support of BT/PR45943/BCE/8/1785/2023 from DBT (Department of Biotechnology), and STR/2022/000008 and CRG/2022/003028 from SERB (Science and Engineering Research Board), Govt. of India.References
- P. Li, H. Jiang, A. Barr, Z. Ren, R. Gao, H. Wang, W. Fan, M. Zhu, G. Xu and J. Li, Reusable polyacrylonitrile-sulfur extractor of heavy metal ions from wastewater, Adv. Funct. Mater., 2021, 31(51), 2105845, DOI:10.1002/adfm.202105845
.
- S. Kaza, L. C. Yao, P. Bhada-Tata and F. Van Woerden, What a waste 2.0: A global snapshot of solid waste management to 2050, World Bank, Washington, DC, 2018 Search PubMed.
- Y. M. Lozano and M. C. Rillig, Effects of microplastic fibers and drought on plant communities, Environ. Sci. Technol., 2020, 54(10), 6166–6173, DOI:10.1021/acs.est.0c01051
.
- J. Gigault, B. Pedrono, B. Maxit and A. Ter Halle, Marine plastic litter: The unanalyzed nano-fraction, Environ. Sci.: Nano, 2016, 3, 346–350, 10.1039/c6en00008h
.
- M. C. Rillig, Microplastic in terrestrial ecosystems and the soil, Environ. Sci. Technol., 2012, 46, 6453–6454, DOI:10.1021/es302011r
.
- H. Zhao, X. Huang, L. Wang, X. Zhao, F. Yan, Y. Yang, G. Li, P. Gao and P. Ji, Removal of polystyrene nanoplastics from aqueous solutions using a novel magnetic material: Adsorbability, mechanism, and reusability, Chem. Eng. J., 2022, 430(4), 133122, DOI:10.1016/j.cej.2021.133122
.
- K. Duis and A. Coors, Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects, Environ. Sci. Eur., 2016, 28, 2, DOI:10.1186/s12302-015-0069-y
.
- L. M. Hernandez, N. Yousefi and N. Tufenkji, Are There Nanoplastics in Your Personal Care Products?, Environ. Sci. Technol. Lett., 2017, 4(7), 280–285, DOI:10.1021/acs.estlett.7b00187
.
- Z. Akdogan and B. Guven, Microplastics in the environment: A critical review of current understanding and identification of future research needs, Environ. Pollut., 2019, 254, 113011, DOI:10.1016/j.envpol.2019.113011
.
- M. A. Browne, P. Crump, S. J. Niven, E. Teuten, A. Tonkin, T. Galloway and R. Thompson, Accumulation of microplastic on shorelines woldwide: Sources and sinks, Environ. Sci. Technol., 2011, 45(21), 9175–9179, DOI:10.1021/es201811s
.
- K. Kannan and K. Vimalkumar, A review of human exposure to microplastics and insights into microplastics as obesogens, Front. Endocrinol., 2021, 12, 724989, DOI:10.3389/fendo.2021.724989
.
- T. Wang, C. Yu, Q. Chu, F. Wang, T. Lan and J. Wang, Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films, Chemosphere, 2020, 244, 125491, DOI:10.1016/j.chemosphere.2019.125491
.
- I. E. Napper and R. C. Thompson, Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions, Mar. Pollut. Bull., 2016, 112(1–2), 39–45, DOI:10.1016/j.marpolbul.2016.09.025
.
- J. Sun, X. Dai, Q. Wang, M. C. van Loosdrecht and B. J. Ni, Microplastics in wastewater treatment plants: Detection, occurrence and removal, Water Res., 2019, 152, 21–37, DOI:10.1016/j.watres.2018.12.050
.
- P. L. Ngo, B. K. Pramanik, K. Shah and R. Roychand, Pathway, classification and removal efficiency of microplastics in wastewater treatment plants, Environ. Pollut., 2019, 255(2), 113326, DOI:10.1016/j.envpol.2019.113326
.
- R. C. Thompson, Y. Olsen, R. P. Mitchell, A. Davis, S. J. Rowland, A. W. John, D. McGonigle and A. E. Russell, Lost at sea: Where is all the plastic?, Science, 2004, 304(5672), 838, DOI:10.1126/science.1094559
.
-
M. T. Khan, Y. L. Cheng, S. Hafeez, Y. F. Tsang, J. Yang and A. Nawab, Microplastics in Wastewater, in Handbook of Microplastics in the Environment, ed. T. Rocha-Santos, M. Costa and C. Mouneyrac, Springer, 2020, pp. 1–33, DOI:10.1007/978-3-030-10618-8_39-1
.
- M. Pivokonsky, L. Cermakova, K. Novotna, P. Peer, T. Cajthaml and V. Janda, Occurrence of microplastics in raw and treated drinking water, Sci. Total Environ., 2018, 643, 1644–1651, DOI:10.1016/j.scitotenv.2018.08.102
.
- F. Murphy, C. Ewins, F. Carbonnier and B. Quinn, Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment, Environ. Sci. Technol., 2016, 50(11), 5800–5808, DOI:10.1021/acs.est.5b05416
.
- Z. Wang, T. Lin and W. Chen, Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP), Sci. Total Environ., 2020, 700, 134520, DOI:10.1016/j.scitotenv.2019.134520
.
- J. Talvitie, A. Mikola, A. Koistinen and O. Setälä, Solutions to microplastic pollution-removal of microplastics from wastewater effluent with advanced wastewater treatment technologies, Water Res., 2017, 123, 401–407, DOI:10.1016/j.watres.2017.07.005
.
- C. M. Rochman, E. Hoh, B. T. Hentschel and S. Kaye, Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris, Environ. Sci. Technol., 2013, 47(3), 1646–1654, DOI:10.1021/es303700s
.
- J. P. da Costa, A. Paço, P. S. Santos, A. C. Duarte and T. Rocha-Santos, Microplastics in soils: Assessment, analytics and risks, Environ. Chem., 2019, 16(1), 18–30, DOI:10.1071/EN18150
.
- A. McCormick, T. J. Hoellein, S. A. Mason, J. Schluep and J. J. Kelly, Microplastic is an abundant and distinct microbial habitat in an urban river, Environ. Sci. Technol., 2014, 48(20), 11863–11871, DOI:10.1021/es503610r
.
- M. Camacho, A. Herrera, M. Gómez M, A. Acosta-Dacal, I. Martínez, L. A. Henríquez-Hernández and O. P. Luzardo, Organic pollutants in marine plastic debris from Canary Islands beaches, Sci. Total Environ., 2019, 662, 22–31, DOI:10.1016/j.scitotenv.2018.12.422
.
- G. E. De-la-Torre, Microplastics: an emerging threat to food security and human health, J. Food Sci. Technol., 2020, 57, 1601–1608, DOI:10.1007/s13197-019-04138-1
.
- C. Duan, T. Ma, J. Wang and Y. Zhou, Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review, J. Water Process Eng., 2020, 37, 101339, DOI:10.1016/j.jwpe.2020.101339
.
- B. Gupta, R. S. Ambekar, R. M. Tromer, P. S. Ghosal, R. Sinha, A. Majumder, P. Kumbhakar, P. M. Ajayan, D. S. Galvao, A. K. Gupta and C. S. Tiwary, Development of a schwarzite-based moving bed 3D printed water treatment system for nanoplastic remediation, RSC Adv., 2021, 11, 19788–19796, 10.1039/D1RA03097C
.
- N. P. Ivleva, A. C. Wiesheu and R. Niessner, Microplastic in aquatic ecosystems, Angew. Chem., Int. Ed., 2017, 56, 1720–1739, DOI:10.1002/anie.201606957
.
- A. Kundu, N. P. Shetti, S. Basu, K. R. Reddy, M. N. Nadagouda and T. M. Aminabhavi, Identification and removal of micro- and nano-plastics: Efficient and cost-effective methods, Chem. Eng. J., 2021, 421, 129816, DOI:10.1016/j.cej.2021.129816
.
- P. Romphophak, O. Faikhaw, S. Sairam, P. Thuptimdang and C. Coufort-Saudejaud, Removal of microplastics and nanoplastics in water treatment processes: A systematic literature review, J. Water Process Eng., 2024, 64, 105669, DOI:10.1016/j.jwpe.2024.105669
.
- G. Chellasamy, R. M. Kiriyanthan, T. Maharajan, A. Radha and K. Yun, Remediation of microplastics using bionanomaterials: A review, Environ. Res., 2022, 208, 112724, DOI:10.1016/J.ENVRES.2022.112724
.
- M. Sajid, I. Ihsanullah, M. T. Khan and N. Baig, Nanomaterials-based adsorbents for remediation of microplastics and nanoplastics in aqueous media: A review, Sep. Purif. Technol., 2023, 305, 122453, DOI:10.1016/j.seppur.2022.122453
.
- P. S. Goh, H. S. Kang, A. F. Ismail, W. H. Khor, L. K. Quen and D. Higgins, Nanomaterials for microplastic remediation from aquatic environment: Why nano matters?, Chemosphere, 2022, 299, 134418, DOI:10.1016/j.chemosphere.2022.134418
.
- M. T. Ekvall, M. Lundqvist, E. Kelpsiene, E. Šileikis, S. B. Gunnarsson and T. Cedervall, Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products, Nanoscale Adv., 2019, 1, 1055–1061, 10.1039/c8na00210j
.
- M. Fischer and B. M. Scholz-Böttcher, Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography-mass spectrometry, Environ. Sci. Technol., 2017, 51, 5052–5060, DOI:10.1021/acs.est.6b06362
.
- A. Pellis, C. Gamerith, G. Ghazaryan, A. Ortner, E. H. Acero and G. M. Guebitz, Ultrasound-enhanced enzymatic hydrolysis of poly(ethylene terephthalate), Bioresour. Technol., 2016, 218, 1298–1302, DOI:10.1016/j.biortech.2016.07.106
.
- J. Gago, F. Galgani, T. Maes and R. C. Thompson, Microplastics in seawater: Recommendations from the marine strategy framework directive implementation process, Front. Mar. Sci., 2016, 3, 219, DOI:10.3389/fmars.2016.00219
.
- Y. Zhang, H. Jiang, K. Bian, H. Wang and C. Wang, A critical review of control and removal strategies for microplastics from aquatic environments, J. Environ. Chem. Eng., 2021, 9, 105463, DOI:10.1016/j.jece.2021.105463
.
- Y. J. Chen, Y. Chen, C. Miao, Y. R. Wang, G. K. Gao, R. X. Yang, H. J. Zhu, J. H. Wang, S. L. Li and Y. Q. Lan, Metal-organic framework-based foams for efficient microplastics removal, J. Mater. Chem. A, 2020, 8, 14644–14652, 10.1039/D0TA04891G
.
- H. Wan, J. Wang, X. Sheng, J. Yan, W. Zhang and Y. Xu, Removal of polystyrene microplastics from aqueous solution using the metal-organic framework material of ZIF-67, Toxics, 2022, 10, 70, DOI:10.3390/toxics10020070
.
- S. Modak, M. Kasula and M. R. Esfahani, Nanoplastics Removal from Water using metal−organic framework: Investigation of adsorption mechanisms, kinetics, and effective environmental parameters, ACS Appl. Eng. Mater., 2023, 1, 744–755, DOI:10.1021/acsaenm.2c00174
.
- X. Peng, M. Urso, M. Kolackova, D. Huska and M. Pumera, Biohybrid magnetically driven microrobots for Sustainable Removal of micro/nanoplastics from the aquatic environment, Adv. Funct. Mater., 2024, 34, 2307477, DOI:10.1002/adfm.202307477
.
- M. K. Devi, N. Karmegam, S. Manikandan, R. Subbaiya, H. Song, E. E. Kwon, B. Sarkar, N. Bolan, W. Kim, J. Rinklebe and M. Govarthanan, Removal of nanoplastics in water treatment processes: A review, Sci. Total Environ., 2022, 845, 157168 CrossRef
.
- A. A. Mohana, S. M. Farhad, N. Haque and B. K. Pramanik, Understanding the fate of nano-plastics in wastewater treatment plants and their removal using membrane processes, Chemosphere, 2021, 284, 131430, DOI:10.1016/j.chemosphere.2021.131430
.
- B. K. Pramanik, S. K. Pramanik and S. Monira, Understanding the fragmentation of microplastics into nano-plastics and removal of nano/microplastics from wastewater using membrane, air flotation and nano-ferrofluid processes, Chemosphere, 2021, 282, 131053, DOI:10.1016/j.chemosphere.2021.131053
.
- M. Enfrin, J. Lee, P. Le-Clech and L. F. Dumee, Kinetic and mechanistic aspects of ultrafiltration membrane
fouling by nano- and microplastics, J. Membr. Sci., 2020, 601, 117890, DOI:10.1016/j.memsci.2020.117890
.
- P. Ribao, J. Corredor, M. J. Rivero and I. Ortiz, Role of reactive oxygen species on the activity of noble metal-doped TiO2 photocatalysts, J. Hazard. Mater., 2019, 372, 45–51, DOI:10.1016/j.jhazmat.2018.05.026
.
- L. P. Domínguez-Jaimes, E. I. Cedillo-González, E. Luévano-Hipólito, J. D. Acuña-Bedoya and J. Hernández-López, Degradation of primary nanoplastics by photocatalysis using different anodized TiO2 structures, J. Hazard. Mater., 2021, 413, 125452, DOI:10.1016/j.jhazmat.2021.125452
.
- R. Gusain, K. Gupta, P. Joshi and O. P. Khatri, Adsorptive removal of photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review, Adv. Colloid Interface Sci., 2019, 272, 102009, DOI:10.1016/j.cis.2019.102009
.
- C. Zhang, C. Lai, G. Zeng, D. Huang, C. Yang, Y. Wang, Y. Zhou and M. Cheng, Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution, Water Res., 2016, 95, 103–112, DOI:10.1016/j.watres.2016.03.014
.
- D. Qin, Y. Zhou, W. Wang, C. Zhang, G. Zeng, D. Huang, L. Wang, H. Wang, Y. Yang, L. Lei, S. Chen and D. He, Recent advances in two-dimensional nanomaterials for photocatalytic reduction of CO2: insights into performance, theories and perspective, J. Mater. Chem. A, 2020, 8, 19156–19195, 10.1039/D0TA07460H
.
- C. Zhang, Y. Zhou, W. Wang, Y. Yang, C. Zhou, L. Wang, L. Lei, D. He, H. Luo and D. Huang, Formation of Mo2C/hollow tubular g-C3N4 hybrids with favorable charge transfer channels for excellent visible light-photocatalytic performance, Appl. Surf. Sci., 2020, 527, 146757, DOI:10.1016/j.apsusc.2020.146757
.
- Y. Yang, X. Li, C. Zhou, W. Xiong, G. Zeng, D. Huang, C. Zhang, W. Wang, B. Song, X. Tang, X. Li and H. Guo, Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review, Water Res., 2020, 184, 116200, DOI:10.1016/j.watres.2020.116200
.
- T. S. Tofa, K. L. Kunjali, S. Paul and J. Dutt, Visible light photocatalytic degradation of microplastic residues with xinc oxide nanorods, Chem. Lett., 2019, 17, 1341–1346, DOI:10.1007/s10311-019-00859-z
.
- Z. Ouyang, Y. Yang, C. Zhang, S. Zhu, L. Qin, W. Wang, D. He, Y. Zhou, H. Luo and F. Qin, Recent advances in photocatalytic degradation of plastics and plastic-derived chemicals, J. Mater. Chem. A, 2021, 9, 13402, 10.1039/D0TA12465F
.
- B. Cao, S. Wan, Y. Wang, H. Guo, M. Ou and Q. Zhong, Highly-efficient visible-light-driven photocatalytic H2 evolution integrated with microplastic degradation over MXene/ZnxCd1-xS photocatalyst, J. Colloid Interface Sci., 2022, 605, 311–319, DOI:10.1016/j.jcis.2021.07.113
.
- Y. H. Lai, P. W. Yeh, M. J. Jhong and P. C. Chuang, Solar-driven hydrogen evolution in alkaline seawater over earth-abundant g-C3N4/CuFeO2 heterojunction photocatalyst using microplastic as a feedstock, Chem. Eng. J., 2023, 475, 146413, DOI:10.1016/j.cej.2023.146413
.
- I. Nabi, A. U. Bacha, K. Li, H. Cheng, T. Wang, Y. Liu, S. Ajmal, Y. Yang, Y. Feng and L. Zhang, Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film, iScience, 2020, 23, 101326, DOI:10.1016/j.isci.2020.101326
.
- J. Kang, L. Zhou, X. Duan, H. Sun, Z. Ao and S. Wang, Degradation of cosmetic microplastics via functionalized carbon, Matter, 2019, 1, 745–758, DOI:10.1016/j.matt.2019.06.004
.
- C. Shi, S. Zhang, J. Zhao, J. Ma, H. Wu, H. Sun and S. Cheng, Experimental study on removal of microplastics from aqueous solution by magnetic force effect on the magnetic sepiolite, Sep. Purif. Technol., 2022, 288, 120564, DOI:10.1016/j.seppur.2022.120564
.
- J. Grbic, B. Nguyen, E. Guo, J. B. You, D. Sinton and C. M. Rochman, Magnetic extraction of microplastics from environmental samples, Environ. Sci. Technol. Lett., 2019, 6, 68–72, DOI:10.1021/acs.estlett.8b00671
.
- X. Li, B. Liu, Y. Lao, P. Wan, X. Mao and F. Chen, Efficient magnetic harvesting of microalgae enabled by surface-initiated formation of iron nanoparticles, Chem. Eng. J., 2021, 408, 127252, DOI:10.1016/j.cej.2020.127252
.
- Y. Tang, S. Zhang, Y. Su, D. Wu, Y. Zhao and B. Xie, Removal of microplastics from aqueous solutions by magnetic carbon nanotubes, Chem. Eng. J., 2021, 406, 126804, DOI:10.1016/j.cej.2020.126804
.
- X. Guo and J. Wang, The chemical behaviors of microplastics inmarine environment: A review, Mar. Pollut. Bull., 2019, 142, 1–14, DOI:10.1016/j.marpolbul.2019.03.019
.
- Z. Zhang and Y. Chen, Current research and perspective ofmicroplastics (MPs) in soils (dusts), rivers (lakes), and marine environments in China, Ecotoxicol. Environ. Saf., 2020, 202, 110976, DOI:10.1016/j.ecoenv.2020.110976
.
- X. Huang, D. Y. Zemlyanov, S. Diaz-Amaya, M. Salehi, L. Stanciu and A. J. Whelton, Competitive heavy metal adsorption onto new and aged polyethylene under various drinking water conditions, J. Hazard. Mater., 2020, 385, 121585, DOI:10.1016/j.jhazmat.2019.121585
.
- D. Elkhatib, V. Oyanedel-Craver and E. Carissimi, Electrocoagulation applied for the removal of microplastics from wastewater treatment facilities, Sep. Purif. Technol., 2021, 276, 118877, DOI:10.1016/j.seppur.2021.118877
.
- M. Kiendrebeogo, M. K. Estahbanati, A. K. Mostafazadeh, P. Drogui and R. D. Tyagi, Treatment of microplastics in water by anodic oxidation: A case study for polystyrene, Environ. Pollut., 2021, 269, 116168, DOI:10.1016/j.envpol.2020.116168
.
- W. Perren, A. Wojtasik and Q. Cai, Removal of microbeads from wastewater using electrocoagulation, ACS Omega, 2018, 3, 3357–3364, DOI:10.1021/acsomega.7b02037
.
- M. Shen, Y. Zhang, E. Almatrafi, T. Hu, C. Zhou, B. Song, Z. Zeng and G. Zeng, Efficient removal of microplastics from wastewater by an electrocoagulation process, Chem. Eng. J., 2022, 428, 131161, DOI:10.1016/j.cej.2021.131161
.
- F. Miao, Y. Liu, M. Gao, X. Yu, P. Xiao, M. Wang, S. Wang and X. Wang, Degradation of polyvinyl chloride microplastics via an electro-fenton-like system with a TiO2/graphite cathode, J. Hazard. Mater., 2020, 399, 123023, DOI:10.1016/j.jhazmat.2020.123023
.
- A. Lissaneddine, M. N. Pons, F. Aziz, N. Ouazzani, L. Mandi and E. Mousset, A critical review on the electrosorption of organic compounds in aqueous effluent–influencing factors and engineering considerations, Environ. Res., 2022, 204, 112128, DOI:10.1016/j.envres.2021.112128
.
- J. G. da Costa, J. M. Costa and A. F. de Almeida Neto, Recent advances and future applications in electro-adsorption technology: an updated review, J. Environ. Chem. Eng., 2021, 9, 106355, DOI:10.1016/j.jece.2021.106355
.
- C. S. Grant, Electrokinetic separation of ultrafine materials: A review, Sep. Purif. Methods, 2008, 22, 55–91, DOI:10.1080/15422119308544977
.
- Q. Chen and Y. J. Yuan, A review of polystyrene bead manipulation by dielectrophoresis, RSC Adv., 2019, 9, 4963–4981 RSC
.
- Y. He, D. Zhao, H. Lin, H. Huang, H. Li and Z. Guo, Design of diamond anodes in electrochemical degradation of organic pollutants, Curr. Opin. Electrochem., 2022, 32, 100878, DOI:10.1016/j.coelec.2021.100878
.
- C. T. Yavuz, A. Prakash, J. T. Mayo and V. L. Colvin, Magnetic separation: From steel plants to biotechnology, Chem. Eng. Sci., 2009, 64, 2510–2521, DOI:10.1016/j.ces.2008.11.018
.
- J. Talvitie, A. Mikola, A. Koistinen and O. Setälä, Solutions to MP pollution removal of MPs from wastewater effluent with advanced wastewater treatment technologies, Water Resour., 2017, 123, 401–440, DOI:10.1016/j.watres.2017.07.005
.
- B. Bhushan and Y. C. Jung, Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction, Prog. Mater. Sci., 2011, 56, 1–108, DOI:10.1016/j.pmatsci.2010.04.003
.
- O. Rius-Ayra, O. Bouhnouf-Riahi and N. Llorca-Isern, Superhydrophobic and sustainable nanostructured powdered iron for the efficient separation of oil-in-water emulsions and the capture of microplastics, ACS Appl. Mater. Interfaces, 2020, 12, 45629–45640, DOI:10.1021/acsami.0c13876
.
- W. Li, S. Liu, K. Huang, S. Qin, B. Liang and J. Wang, Wang, Preparation of magnetic Janus microparticles for the rapid removal of microplastics from water, Sci. Total Environ., 2023, 903, 166627, DOI:10.1016/j.scitotenv.2023.166627
.
- X. Li, L. Chen, D. Cui, W. Jiang, L. Han and N. Niu, Preparation and application of Janus nanoparticles: recent development and prospects, Coord. Chem. Rev., 2022, 454, 214318, DOI:10.1016/j.ccr.2021.214318
.
- J. Yang, Y. Liu, J. Li, M. Zuo, W. Li, N. Xing, C. Wang and T. Li, γ-Fe2O3@AgmSiO2-NH2 magnetic Janus micromotor for active water remediation, Appl. Mater. Today, 2021, 25, 101190, DOI:10.1016/j.apmt.2021.101190
.
- Z. Feng, K. Yan, J. Li, X. Xu, T. Yuan, T. Wang and J. Zheng, Magnetic janus particles as a multifunctional drug delivery system for paclitaxel in efficient cancer treatment, Mater. Sci. Eng., C, 2019, 104, 110001, DOI:10.1016/j.msec.2019.110001
.
- L. Yue, W. Pu, T. Zhao, J. Zhuang and S. Zhao, A high performance magnetically responsive janus nano-emulsifier: preparation, emulsification characteristics, interfacial rheology, and application in emulsion flooding, J. Pet. Sci. Eng., 2022, 208, 109478, DOI:10.1016/j.petrol.2021.109478
.
- X. Shi, X. Zhang, W. Gao, Y. Zhang and D. He, Removal of microplastics from water by magnetic nano-Fe3O4, Sci. Total Environ., 2022, 802, 149838, DOI:10.1016/j.scitotenv.2021.149838
.
- Y. Chen, L. Ouyang, N. Liu, F. Li, P. Li, M. Sun, H. Qin, Y. Li, X. Xiang and L. Wu, pH-responsive magnetic artificial melanin with tunable aggregation-induced stronger magnetism for rapid remediation of plastic fragments, J. Hazard. Mater., 2022, 435, 128962, DOI:10.1016/j.jhazmat.2022.128962
.
- H. Ye, Y. Wang, X. Liu, D. Xu, H. Yuan, H. Sun, S. Wang and X. Ma, Magnetically steerable iron oxides-manganese dioxide core–shell micromotors for organic and microplastic removal, J. Colloid Interface Sci., 2021, 588, 510–521, DOI:10.1016/j.jcis.2020.12.097
.
- Y. Zhang, Z. Dong, Z. Deng, S. Shi, C. Tang and X. Hu, An iron “nano-fishnet” for the rapid removal and surface clean-up of micro/nanoplastics from seawater, Environ. Sci.: Nano, 2023, 10, 2566, 10.1039/d3en00344b
.
- L. M. Martin, J. Sheng, P. V. Zimba, L. Zhu, O. Fadare, C. Haley, M. Wang, T. D. Phillips, J. Conkle and W. Xu, Testing an iron oxide nanoparticles-based method for magnetic separation of nanoplastics and microplastics from water, Nanomaterials, 2022, 12, 2348, DOI:10.3390/nano12142348
.
- M. Sun, W. Chen, X. Fan, C. Tian, L. Sun and H. Xie, Cooperative recyclable magnetic microsubmarines for oil and microplastic removal from water, Appl. Mater. Today., 2020, 20, 100682, DOI:10.1016/j.apmt.2020.100682
.
- R. Yan, S. Lin, W. Jiang, L. Zhang, W. Zhao and Q. Sui, Effect of aggregation behaviour on microplastic removal by manetic Fe3O4 nanoparticles, Sci. Total Environ., 2023, 898, 165431, DOI:10.1016/j.scitotenv.2023.165431
.
- H. P. Wang, X. H. Huang, J. N. Chen, M. Dong, C. Z. Nie and L. Qin, Modified superhydrophobic magnetic Fe3O4 nanoparticles for removal of microplastics in liquid foods, Chem. Eng. J., 2023, 476, 146562, DOI:10.1016/j.cej.2023.146562
.
- S. Kouchakipour, M. Hosseinzadeh, M. Z. Qaretapeh and K. Dashtian, Sustainable large-scale Fe3O4/carbon for enhanced polystyrene nanoplastics removal through magnetic adsorption coagulation, J. Water Process Eng., 2024, 58, 104919, DOI:10.1016/j.jwpe.2024.104919
.
- Y. Zhang, J. Duan, R. Liu, E. Petropoulos, Y. Feng, L. Xue, L. Yang and S. He, Efficient magnetic capture of PE microplastic from water by PEG modified Fe3O4 nanoparticles: Performance, kinetics, isotherms and influence factors, J. Environ. Sci., 2025, 147, 677–687, DOI:10.1016/j.jes.2023.07.025
.
- M. Mon, R. Bruno, J. F. Soria, D. Armentano and E. Pardo, Metal-organic framework technologies for water remediation: towards a sustainable ecosystem, J. Mater. Chem. A, 2018, 6, 4912–4947, 10.1039/C8TA00264A
.
- Y. Zhang, S. Yuan, X. Feng, H. Li, J. Zhou and B. Wang, Preparation of nanofibrous metal-organic framework filters for efficient air pollution control, J. Am. Chem. Soc., 2016, 138, 5785–5788, DOI:10.1021/jacs.6b02553
.
- X. Ma, Y. Chai, P. Li and B. Wang, Metal-organic framework films and their potential applications in environmental pollution control, Acc. Chem. Res., 2019, 52, 1461–1470, DOI:10.1021/acs.accounts.9b00113
.
- Y. Wang, M. Zhao, J. Ping, B. Chen, X. Cao, Y. Huang, C. Tan, Q. Ma, S. Wu and Y. Yu, Bioinspired designed of ultrathin 2D bimetallic metal-organic framework nanosheets used as biomimetic enzymes, Adv. Mater., 2016, 28, 4149–4155, DOI:10.1002/adma.201600108
.
- M. Haris, M. W. Khan, A. Zavabeti and N. Mahmood, Self-assembly of C@FeO nanopillars on 2D-MOF for simultaneous removal of microplastics and dissolved contaminants from water, Chem. Eng. J., 2023, 455, 140390, DOI:10.1016/j.cej.2022.140390
.
- H. Wan, J. Yan, C. Guo, Q. Cui and W. Zhang, Synthesis of core-heteroshell structure for ZIF-67/VTM and its efficient activation of peroxymonosulfate in treatment of levofloxacin from an aqueous solution, Environ. Res., 2022, 204, 111986, DOI:10.1016/j.envres.2021.111986
.
- D. Pedrero, G. Edo, F. F. Pinas, R. Rosal and S. Aguado, Efficient removal of nanoplastics from water using mesoporous metal organic frameworks, Sep. Purif. Technol., 2024, 333, 125816, DOI:10.1016/j.seppur.2023.125816
.
- Q. Liu, Y. Chen, Z. Chen, Y. Xle, H. Yu, S. Yuan, Y. Guo, Y. Cheng, H. Qlan and W. Yao, Rapid magnetization and removal of microplastics from environment and food based on magnetic metal-organic framework Fe3O4@SiO2@MIL-53(Al), Environ. Sci. Pollut. Res., 2023, 30, 117373–117389, DOI:10.1007/s11356-023-30314-0
.
- L. Han, J. Ma, H. Lin, C. Chen, J. Teng, B. Li, D. Zhao, Y. Xu, W. Yu and L. Shen, A novel flower-like nickel-metal-organic framework (Ni-MOF) membrane for efficient multi-component pollutant removal by gravitation, Chem. Eng. J., 2023, 470, 144311, DOI:10.1016/j.cej.2023.144311
.
- F. Pasanen, R. O. Fuller and F. Maya, Fast and simultaneous removal of microplastics and plastic derived endocrine disruptors using a magnetic ZIF-8 nanocomposite, Chem. Eng. J., 2023, 455, 140405, DOI:10.1016/j.cej.2022.140405
.
- Y. Gao, Q. Liu, Y. Zeng, H. Li, C. Hu and C. Wang, Synthesis of a novel microplastic trap with abundant oxime groups based on MOF-545 post-engineering for the environmental pollution control and water remediation, J. Cleaner Prod., 2023, 430, 139678, DOI:10.1016/j.jclepro.2023.139678
.
- X. R. Huang, H. H. Zhao, X. F. Hu, F. H. Liu, L. Wang, X. Zhao, P. C. Gao and P. H. Ji, Optimization of preparation technology for modified coal fly ash and its adsorption properties for Cd2+, J. Hazard. Mater., 2020, 392, 122461, DOI:10.1016/j.jhazmat.2020.122461
.
- S. B. Wang, Y. Boyjoo, A. Choueib and Z. H. Zhu, Removal of dyes from aqueous solution using fly ash and red mud, Water Res., 2005, 39, 129–138, DOI:10.1016/j.watres.2004.09.011
.
- H. Zhao, X. Huang, L. Wang, X. Zhao, F. Yan, Y. Yang, G. Li, P. Gao and P. Ji, Removal of polystyrene nanoplastic from aqueous solutions using a novel magnetic material: Adsorbability, mechanism, and reusability, Chem. Eng. J., 2022, 430, 133122, DOI:10.1016/j.cej.2021.133122
.
- O. G. Apul and T. Karanfil, Adsorption of synthetic organic contaminants by carbon nanotubes: a critical review, Water Res., 2015, 68, 34–55, DOI:10.1016/j.watres.2014.09.032
.
- O. G. Apul, Y. Zhou and T. Karanfil, Mechanisms and modeling of halogenated aliphatic contaminant adsorption by carbon nanotubes, J. Hazard. Mater., 2015, 295, 138–144, DOI:10.1016/j.jhazmat.2015.04.030
.
- J. Zhang, R. Li, G. Ding, Y. Wang and C. Wang, Sorptive removal of phenanthrene from water by magnetic carbon nanomaterials, J. Mol. Liq., 2019, 293, 111540, DOI:10.1016/j.molliq.2019.111540
.
- Y. Tang, S. Zhang, Y. Su, D. Wu, Y. Zhao and B. Xie, Removal of microplastics from aqueous solutions by magnetic carbon nanotubes, Chem. Eng. J., 2021, 406, 126804, DOI:10.1016/j.cej.2020.126804
.
- Q. Li, Z. Chen, H. Wang, H. Yang, T. Wen, S. Wang, B. Hu and X. Wang, Removal of organic compounds by nanoscale zero-valent iron and its composites, Sci. Total Environ., 2021, 792, 148546, DOI:10.1016/j.scitotenv.2021.148546
.
- B. A. Geim, Graphene: status and prospects, Science, 2009, 324, 1530–1534, DOI:10.1126/science.1158877
.
- G. Peng, M. Xiang, W. Wang, Z. Su, H. Liu, Y. Mao, Y. Chen and P. Zhang, Engineering 3d graphene-like carbon-assembled layered double oxide for efficient microplastic removal in a wide pH range, J. Hazard. Mater., 2022, 433, 128672, DOI:10.1016/j.jhazmat.2022.128672
.
- A. Batool and S. Valiyaveettil, Surface functionalized cellulose fibres- A reniewable adsorbent for removal of plastic nanoparticles from water, J. Hazard. Mater., 2021, 413, 125301, DOI:10.1016/j.jhazmat.2021.125301
.
- C. Sun, Z. Wang, L. Chen and F. Li, Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with
different functional groups, Chem. Eng. J., 2020, 393, 124796, DOI:10.1016/j.cej.2020.124796
.
- C. Sun, Z. Wang, H. Zheng, L. Chen and F. Li, Biodegradable and re-usable sponge materials made from chitin for efficient removal of microplastics, J. Hazard. Mater., 2021, 420, 126599, DOI:10.1016/j.jhazmat.2021.126599
.
- Z. Wang, C. Sun, F. Li and L. Chen, Fatigue resistance, re-usable and biodegradable sponge materials from plant protein with rapid water adsorption capacity for microplastic removal, Chem. Eng. J., 2021, 415, 129006, DOI:10.1016/j.cej.2021.129006
.
- R. Wang, L. Zhang, B. Chen and X. Zhu, Low-pressure driven electrospun membrane with tuned surface charge for efficient removal of polystyrene nanoplastics from water, J. Membr. Sci., 2020, 614, 118470, DOI:10.1016/j.memsci.2020.118470
.
- M. Zhang, J. Yang, Z. Kang, X. Wu, L. Tang, Z. Qiang, D. Zhang and X. Pan, Removal of micron-scale microplastics particles from different waters with efficient tool of surface-functionalized microbubbles, J. Hazard. Mater., 2021, 404, 124095, DOI:10.1016/j.jhazmat.2020.124095
.
- B. Zheng, B. Li, H. Wan, X. Lin and Y. Cai, Coral-inspired environmental durability aerogels for micron-sized plastic particles removal in aquatic environment, J. Hazard. Mater., 2022, 413, 128611, DOI:10.1016/j.jhazmat.2022.128611
.
- G. Zhou, X. Huang, H. Xu, Q. Wang, M. Wang, Y. Wang, Q. Li, Y. Zhang, Q. Ye and J. Zhang, Removal of polystyrene nanoplastics from water by CuNi carbon material: the role of adsorption, Sci. Total Environ., 2022, 820, 153190, DOI:10.1016/j.scitotenv.2022.153190
.
- J. A. González, M. E. Villanueva, L. L. Piehl and G. J. Copello, Development of a chitin/ graphene oxide hybrid composite for the removal of pollutant dyes: adsorption and desorption study, Chem. Eng. J., 2015, 280, 41–48, DOI:10.1016/j.cej.2015.05.112
.
- H. Bai, H. Wang, J. Zhang, C. Wu, J. Zhang, Y. Xiang and S. Lu, Simultaneously enhancing ionic conduction and mechanical strength of poly(ether sulfones)-poly (vinyl pyrrolidone) membrane by introducing graphitic carbon nitride nanosheets for high temperature proton exchange membrane fuel cell application, J. Membr. Sci., 2018, 558, 26–33, DOI:10.1016/j.memsci.2018.04.039
.
- V. Siipola, S. Pflugmacher, H. Romar, L. Wendling and P. Koukkari, Low-cost biochar adsorbents for water purification including microplastic removal, Appl. Sci., 2020, 10, 788, DOI:10.3390/app10030788
.
- Z. A. Ganie, N. Khandelwal, E. Tiwari, N. Singh and G. K. Darbha, Biochar-facilitated remediation of nanoplastic contaminated water: Effects of pyrolysis temperature induced surface modifications, J. Hazard. Mater., 2021, 417, 126096, DOI:10.1016/j.jhazmat.2021.126096
.
- N. Singh, N. Khandelwal, Z. A. Ganie, E. Tiwari and G. K. Darbha, Eco-friendly magnetic biochar: an effective trap for nanoplastics of varying surface functionality and size in the aqueous environment, Chem. Eng. J., 2021, 418, 129405, DOI:10.1016/j.cej.2021.129405
.
- J. Wang, C. Sun, Q. X. Huang, Y. Chi and J. H. Yan, Adsorption and thermal degradation of microplastics from aqueous solution by Mg/Zn modified biochars, J. Hazard. Mater., 2021, 419, 126486, DOI:10.1016/j.jhazmat.2021.126486
.
- Y. Shi, J. Du, T. Zhao, B. Feng, H. Bian, S. Shan, J. Meng, P. Christie, M. H. Wong and J. Zhang, Removal of nanoplastics from aqueous solutions by aggregation using reusable magnetic biochar modified with cetyltrimethylammonium bromide, Environ. Pollut., 2023, 318, 120897, DOI:10.1016/j.envpol.2022.120897
.
- J. Li, X. Chen, S. Yu and M. Cui, Removal of pristine and aged microplastics from water by magnetic biochar: adsorption and magnetization, Sci. Total Environ., 2023, 875, 162647, DOI:10.1016/j.scitotenv.2023.162647
.
- A. S. Magid, M. S. Islam, Y. Chen, L. Weng, J. Li, J. Ma and Y. Li, Enhanced adsorption of polystyrene nanoplastics (PSNPs) onto oxidized corncob biochar with high pyrolysis temperature, Sci. Total Environ., 2021, 784, 147115, DOI:10.1016/j.scitotenv.2021.147115
.
- X. Wang, Y. Dan, Y. Diao, F. Liu, H. Wang and W. Sang, Transport and retention of microplastics in saturated porous media with peanut shell biochar (PSB) and MgO-PSB amendment: Co-effects of cations and humic acid, Environ. Pollut., 2022, 305, 119307, DOI:10.1016/j.envpol.2022.119307
.
- J. Wu, C. Yang, H. Zhao, J. Shi, Z. Liu, C. Li and F. Song, Efficient removal of microplastics from aqueous solutions by a novel magnetic biochar: performance, mechanism, and reusability, Environ. Sci. Pollut. Res., 2023, 30, 26914–26928, DOI:10.1007/s11356-022-24130-1
.
- A. Subair, P. K. Lakshmi, S. Chellappan and C. Chinghakham, Removal of polystyrene microplastics using biochar-based continuous flow fixed-bed column, Environ. Sci. Pollut. Res., 2024, 31, 13753–13765, DOI:10.1007/s11356-024-32088-5
.
- Z. Wang, M. Sedighi and A. L. Langton, Filtration of microplastic spheres by biochar: removal efficiency and immobilization mechanisms, Water Res., 2020, 184, 116165, DOI:10.1016/j.watres.2020.116165
.
- M. Tong, T. Li, M. Li, L. He and Z. Ma, Cotransport and deposition of biochar with different sized-plastic particles in saturated porous media, Sci. Total Environ., 2020, 713, 136387, DOI:10.1016/j.scitotenv.2019.136387
.
- M. Ahmad, N. M. Lubis, M. Usama, J. Ahmad, M. I. Al-Wabel, H. A. Al-Swadi and M. I. Rafique, Scavenging microplastics and heavy metals from water using jujube waste-derived biochar in fixed-bed column trials, Environ. Pollut., 2023, 325, 122319, DOI:10.1016/j.envpol.2023.122319
.
- A. Uheida, H. G. Mejia, M. A. Rehim, W. Hamd and J. Dutta, Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system, J. Hazard. Mater., 2021, 406, 124299, DOI:10.1016/j.jhazmat.2020.124299
.
- R. Jiang, G. Lu, Z. Yan, J. Liu, D. Wu and Y. Wang, Microplastic degradation of hydroxy-rich bismuth oxychloride, J. Hazard. Mater., 2021, 405, 124247, DOI:10.1016/j.jhazmat.2020.124247
.
- H. Ye, Y. Wang, D. Xu, X. Liu, S. Liu and X. Ma, Design and fabrication of micro/nanomotors for environmental and sensing applications, Appl. Mater. Today, 2021, 23, 101007, DOI:10.1016/j.apmt.2021.101007
.
- Z. Chen, L. Li, L. Hao, Y. Hong and W. Wang, Hormesis-like growth and photosynthetic physiology of marine diatom Phaeodactylum tricornutum Bohlin exposed to polystyrene microplastics, Front. Environ. Sci. Eng., 2022, 16, 1–13, DOI:10.1007/s11783-021-1436-0
.
- T. Kurinomaru, A. Inagaki, M. Hoshi, C. Nakamura and H. Yamazoe, Protein microswimmers capable of delivering cells for tissue engineering applications, Mater. Horiz., 2020, 7, 877–884, 10.1039/c9mh01799b
.
- S. M. Mousavi, S. Hermanova, Y. Ying, J. Plutnar and M. Pumera, A maze in plastic wastes: Autonomous motile photocatalytic microrobots against microplastics, ACS Appl. Mater. Interfaces, 2021, 13, 25102–25110, DOI:10.1021/acsami.1c04559
.
- K. Khairudin, N. F. Bakar and M. S. Osman, Magnetically recyclable flake-like BiOI-Fe3O4 microswimmers for fast and efficient degradation of microplastics, J. Environ. Chem. Eng., 2022, 10, 108275, DOI:10.1016/j.jece.2022.108275
.
- X. Liu, Y. Yang, S. Wan, S. Li, M. Ou, F. Song, X. Fan and Q. Zhong, Tuning the surface hydrophilicity of a C3N4 nanosheet for efficient photocatalytic H2 evolution coupled with microplastic degradation, Int. J. Hydrogen Energy, 2023, 48, 27599–27610, DOI:10.1016/j.ijhydene.2023.04.016
.
- P. Amato, M. Fantauzzi, F. Sannino, I. Ritacco, G. Santoriello, M. F. Camellone, C. Imparato, A. Bifulco, G. Vitiello, L. Caporaso, A. Rossi and A. Aronne, Indirect daylight oxidative degradation of polyethylene microplastics by a bio-waste modified TiO2-based material, J. Hazard. Mater., 2024, 463, 132907, DOI:10.1016/j.jhazmat.2023.132907
.
- M. C. Tarazona, C. Siligardi, H. A. Carreon-Lopez, J. E. Valdez-Cerda, P. Pozzi, G. Kaushik, J. F. Villarreal-Chiu and E. I. Cedillo-Gonzalez, Low environmental impact remediation of microplastics: Visible-light photocatalytic degradation of PET microplastics using bio-inspired C, N-TiO2/SiO2 photocatalysts, Mar. Pollut. Bull., 2023, 93, 115206, DOI:10.1016/j.marpolbul.2023.115206
.
- J. Jeyaraj, V. Baskaralingam, T. Stalin and I. Muthuvel, Mechanistic vision on polypropylene microplastics degradation by solar radiation using TiO2 nanoparticle as photocatalyst, Environ. Res., 2023, 233, 116366, DOI:10.1016/j.envres.2023.116366
.
- C. A. Rojas-Guerrero, M. Villanueva-Rodriguez, J. L. Guzman-Mar, A. Hernandez-Ramirez, E. I. Cedillo-Gonzalez, F. E. Longoria-Rodriguez and L. Hinojosa-Reyes, Solar photocatalytic degradation of polyethylene terephthalate nanoplastics: Evaluation of the applicability of the TiO2/MIL-100(Fe) composite material, J. Environ. Chem. Eng., 2023, 11, 110415, DOI:10.1016/j.jece.2023.110415
.
- L. Wang, A. Kaeppler, D. Fischer and J. Simmchen, Photocatalytic TiO2 micromotors for removal of microplastics and suspended matter, ACS Appl. Mater. Interfaces, 2019, 11, 32937–32944, DOI:10.1021/acsami.9b06128
.
- X. Wang, Z. Zhu, J. Jiang, R. Li and J. Xiong, Preparation of heterojunction C3N4/WO3 photocatalyst for degradation of microplastics in water, Chemosphere, 2023, 337, 139206, DOI:10.1016/j.chemosphere.2023.139206
.
- J. D. Acuna-Bedoya, E. Luevano-Hipolito, E. I. Cedillo-Gonzalez, L. P. Dominguez-Jaimes, A. M. Hurtado and J. M. Hernandez-Lopez, Boosting visible-light photocatalytic degradation of polystyrene nanoplastics with immobilized CuxO obtained by anodization, J. Environ. Eng., 2021, 9, 106208, DOI:10.1016/j.jece.2021.106208
.
- R. Miandad, R. Kumar, M. A. Barakat, C. Basheer, A. S. Aburiazaiza, A. S. Nizami and M. Rehan, Untapped conversion of plastic waste char into carbon-metal LDOs for the adsorption of Congo red, J. Colloid Interface Sci., 2018, 511, 402–410, DOI:10.1016/j.jcis.2017.10.029
.
- H. Song, D. C. Tsang, G. Kwon, E. E. Kwon and D. W. Cho, Coupling carbon dioxide and magnetite for the enhanced thermolysis of polyvinyl chloride, Sci. Total Environ., 2019, 696, 133951, DOI:10.1016/j.scitotenv.2019.133951
.
- S. Ren, X. Xu, Z. S. Zhu, Y. Yang, W. Tian, K. Hu, S. Zhong, J. Yi, X. Duan and S. Wang, Catalytic transformation of microplastics to functional carbon for catalytic peroxymonosulfate activation: Conversion mechanism and defect of scavenging, Appl. Catal., B, 2024, 342, 123410, DOI:10.1016/j.apcatb.2023.123410
.
- R. Kumar, Tertiary and quaternary recycling of thermoplastics by additive manufacturing approach for thermal sustainability, Mater. Today: Proc., 2021, 37, 2382–2386, DOI:10.1016/j.matpr.2020.08.183
.
- A. Lee and M. S. Liew, Tertiary recycling of plastics waste: an analysis of feedstock, chemical and biological degradation methods, J. Mater. Cycles Waste Manage., 2021, 23, 32–43 CrossRef
.
- A. Fivga and I. Dimitriou, Pyrolysis of plastic waste for production of heavy fuel substitute: a techno-economic assessment, Energy, 2018, 149, 865–874, DOI:10.1016/j.energy.2018.02.094
.
- F. Pinto, R. André, M. Miranda, D. Neves, F. Varela and J. Santos, Effect of gasification agent on co-gasification of rice production wastes mixtures, Fuel, 2016, 180, 407–416, DOI:10.1016/j.fuel.2016.04.048
.
- D. K. Omol, O. Acaye, D. F. Okot and O. Bongomin, Production of fuel oil from municipal plastic wastes using thermal and catalytic pyrolysis, J. Energy Res. Rev., 2020, 4, 1–8, DOI:10.9734/jenrr/2020/v4i230120
.
- G. Naidu, N. Nagar and K. M. Poluri, Mechanistic insights into cellular and molecular basis of protein-nanoplastic interactions, Small, 2024, 20, 2305094, DOI:10.1002/smll.202305094
.
- Y. Mao, S. Fan, X. Li, J. Shi, M. Wang, Z. Niu and G. Chen, Trash to treasure: electrocatalytic upcycling of polyethylene terephthalate (PET) microplastic to value-added products by Mn0.1Ni0.9Co2O4-δ RSFs spinel, J. Hazard. Mater., 2023, 457, 131743, DOI:10.1016/j.jhazmat.2023.131743
.
- F. Ozyonar and B. Karagozoglu, Operating cost analysis and treatment of domestic wastewater by electrocoagulation using aluminum electrodes, Pol. J. Environ. Stud., 2011, 20, 173 Search PubMed
.
- K. S. Hashim, A. Shaw, R. Al Khaddar, M. O. Pedrola and D. Phipps, Iron removal, energy consumption and operating cost of electrocoagulation of drinking water using a new flow column reactor, J. Environ. Manage., 2017, 189, 98–108, DOI:10.1016/j.jenvman.2016.12.035
.
- R. Sridhar, V. Sivakumar, V. P. Immanuel and J. P. Maran, Treatment of pulp and paper industry bleaching effluent by electrocoagulant process, J. Hazard. Mater., 2011, 186, 1495–1502, DOI:10.1016/j.jhazmat.2010.12.028
.
- R. Xu, Z. Yang, Y. Niu, D. Xu, J. Wang, J. Han and H. Wang, Removal of microplastics and attached heavy metals from secondary effluent of wastewater treatment plant using interpenetrating bipolar plate electrocoagulation, Sep. Purif. Technol., 2022, 290, 120905, DOI:10.1016/j.seppur.2022.120905
.
- L. Vuori and M. Ollikainen, How to remove microplastics in wastewater? A cost-effectiveness analysis, Ecol. Econ., 2022, 192, 107246, DOI:10.1016/j.ecolecon.2021.107246
.
- Z. Chen, J. Fang, W. Wei, H. H. Ngo, W. Guo and B. J. Ni, Emerging adsorbents for micro/nanoplastics removal from contaminated water: Advances and perspectives, J. Cleaner Prod., 2022, 371, 133676, DOI:10.1016/j.jclepro.2022.133676
.
- I. Ali, X. Tan, Y. Xie, C. Peng, J. Li, I. Naz, Z. Duan, P. Wan, J. Huang, J. Liang, Z. Rui and Y. Ruan, Recent innovations in microplastics and nanoplastics removal by coagulation technique: Implementations, knowledge gaps and prospects, Water Res., 2023, 245, 120617, DOI:10.1016/j.watres.2023.120617
.
Footnote |
| † Both the authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |