Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Safe-by-design assessment of an SiO2@ZnO multi-component nanomaterial used in construction†
A.
Brunelli
 *a,
A.
Serrano-Lotina
b,
M. A.
Bañares
*a,
A.
Serrano-Lotina
b,
M. A.
Bañares
 b,
V.
Alcolea-Rodriguez
b,
V.
Alcolea-Rodriguez
 b,
M.
Blosi
c,
A.
Costa
c,
S.
Ortelli
c,
W.
Peijnenburg
de,
C.
Fito
f,
E. G.
Fernandez
f,
J. S.
Hermosilla
f,
L. G.
Soeteman-Hernández
b,
M.
Blosi
c,
A.
Costa
c,
S.
Ortelli
c,
W.
Peijnenburg
de,
C.
Fito
f,
E. G.
Fernandez
f,
J. S.
Hermosilla
f,
L. G.
Soeteman-Hernández
 e,
I.
Garmendia Aguirre
g,
H.
Rauscher
e,
I.
Garmendia Aguirre
g,
H.
Rauscher
 g,
F.
Murphy
h,
V.
Stone
h,
J.
Balbuena
i,
J. M. L.
Cormano
i,
L.
Pizzol
g,
F.
Murphy
h,
V.
Stone
h,
J.
Balbuena
i,
J. M. L.
Cormano
i,
L.
Pizzol
 j,
D.
Hristozov
j,
A.
Marcomini
a and
E.
Badetti
j,
D.
Hristozov
j,
A.
Marcomini
a and
E.
Badetti
 *a
*a
aDepartment of Environmental Sciences, Informatics and Statistics, Ca' Foscari University of Venice, Via Torino 155, Venice, 30172, Italy. E-mail: andrea.brunelli@unive.it; elena.badetti@unive.it
bSpectroscopy and Industrial Catalysis, Instituto de Catálisis y Petroleoquímica, CSIC-ICP, Marie Curie 2, E-28049-Madrid, Spain
cNational Research Council, Institute of Science, Technology and Sustainability for Ceramic Materials ISSMC-CNR (Former ISTEC-CNR), Faenza, Italy
dInstitute of Environmental Science, Leiden University, Leiden, The Netherlands
eNational Institute for Public Health and the Environment (RIVM), Center for Safety of Substances and Products, Bilthoven, The Netherlands
fITENE, C/Albert Einstein, 1, Paterna, 46980, Valencia, Spain
gEuropean Commission, Joint Research Centre (JRC), Ispra, Italy
hInstitute of Biological Chemistry, Biophysics and Bioengineering, Heriot-Watt University, Edinburgh, UK
iFCIAC Centro de Innovación Andaluz para la Construcción Sostenible, Cordoba, Spain
jGreenDecision Srl, Venice, Italy
First published on 23rd October 2024
Abstract
Safety aspects of chemicals/materials are transversal in all sustainability dimensions, representing a pillar at the early innovation stages of the European Commission's “safe and sustainable by design” (SSbD) framework for chemicals and materials. The first three of the five SSbD framework steps cover different safety aspects, namely, hazard assessment based on intrinsic properties (step 1), occupational health and safety (including exposure) assessment during the production/processing phase (step 2) and exposure in the final application phase (step 3). The goal of this work was to identify a set of characterization tools/procedures to support the operationalization of the first three safety steps in multi-component nanomaterials (MCNMs), applying the findings to an SiO2 core–ZnO shell MCNM. The safety of this MCNM, which is used as an additive to silicate/calcium hydroxide mortar to improve air quality through photocatalytic NOx removal, was investigated from different perspectives along its value chain. Existing and newly generated data on its hazard profile were collected, the exposure of workers during its synthesis was assessed, and potential exposure to hazardous substances during its final application phase was investigated. In step 1, physico-chemical properties, hazard classification and cytotoxicity assays were considered. In step 2, a three-tiered established methodology for evaluating occupational exposure assessment was performed. Lastly, in step 3, the release of inorganic substances from MCNM-based mortars in the final application phase was investigated. Safety assessment according to the SSbD framework was performed by selecting tools and procedures suitable for application in the early innovation stage, resulting in a preliminary hazard assessment of MCNMs and a suggestion for redesigning a step in the process.
Environmental significanceMulticomponent nanomaterials (MCNMs) integrate several functions into one material, leading to innovative applications. SiO2@ZnO MCNMs incorporated in mortars increase the mechanical strength of the material while providing photocatalytic activity for NOx removal. However, the combination of these multiple features may also lead to unexplored risks, making it necessary to carefully evaluate their human health and environmental aspects before entering the market. In this context, a safety assessment of SiO2@ZnO MCNMs using setting tools/procedures to operationalize the safety aspects of the safe and sustainable by design (SSbD) framework is needed for the creation of safer and functional materials. The main goal of this approach, in the early phase of the design process, is to decrease the likelihood of adverse impacts on human health and the environment. |
1. Introduction
A wide array of studies has identified that the hazards of engineered nanomaterials (NMs) varies considerably according to their physicochemical characteristics.1–3 This information has been useful in their risk assessment, regulatory considerations and the development of safe by design (SbD) strategies. However, science and technology are progressing at an increasing speed, developing new unconventional materials with the aim to address key global challenges such as energy provision, climate change, reducing pollution and improving human health. With regard to EU goals defined under the European Green Deal policy, prioritized key enabling technologies include advanced materials, such as multi-component nanomaterials (MCNMs), which consist of at least two components with a size in the nanometer range. MCNMs are widely used in several fields, including targeted drug delivery,4 multicomponent medical sensing,5 heterogeneous catalysis6 and multifunctional coatings in construction. The latter enables enhanced exterior durability and mechanical strength7 as well as photocatalytic activity using TiO2–ZnO MCNMs for NOx removal.8 However, the benefits from their use can be marred by uncertainties about their potential risks to human health and the environment, given that it is difficult to identify and assess these materials owing to their multicomponent nature.9,10 Indeed, upon contact with biological or environmental systems, the components of MCNMs could exhibit differing rates of degradation or interaction to induce combined effects (e.g. antagonistic, synergetic and potentiation effects).Thus, to guide the innovation process for chemicals and materials, including MCNMs, the Joint Research Centre (JRC)11 proposed a safe and sustainable by design (SSbD) framework, which forms the basis of the recommendation by the European Commission (EC).12 This framework was developed by considering SbD methods and frameworks to diagnose potential risks from the use of NMs in different commercial products.13,14 Recently, Sudheshwar et al.15 reviewed the existing studies prior to the SSbD framework, showing that the SbD concept is originally linked to the nanotechnology sector. They also noted that the 89 SbD studies examined predominantly addressed human safety aspects over environmental issues, with only 14 of them being relevant to the SSbD framework. These studies were compared based on aspects such as the tools used/developed, the applicability domain, suggested guidance, life cycle stages addressed, case study presence/absence, and link to the SSbD framework. With respect to previous SbD frameworks, the main novelty of the SSbD by the EC is the integration of safety and sustainability aspects of chemicals and materials with a desirable function (or service) as early as possible in the innovation process. This premarket approach considers safety, environmental, social and economic aspects along the entire life cycle of a chemical/material and consists of 5 steps, as follows: i) hazard assessment; ii) human health and safety aspects in the production and processing phase; iii) human health and environmental aspects in the final application phase; iv) environmental sustainability assessment; and v) social and economic sustainability assessment. The framework proposes the integral assessment of all steps in an iterative manner as the innovation proceeds. This means that all steps should be addressed together in the different innovation stages, which leads to an iterative approach of the framework as the innovation proceeds and more data become available. In this work, we focused on safety as a transversal aspect of sustainability, and specifically steps 1–3 of the SSbD framework. In the case of MCNM, this is already challenging and not straightforward and requires specific attention to the physical, chemical and toxicological characterization procedures to be used and the exposure assessments to be performed for MCNMs.
Given the current lack of knowledge on the safety aspects of nano-enabled products and the need for effective characterization procedures and suitable exposure assessments for MCNMs according to the SSbD approach, it is crucial to identify physical, chemical and toxicological testing strategies for the safety assessment of new materials throughout the design phase before they enter the market. These strategies must be able to carefully evaluate their hazard profile in line with the policy initiatives launched by the European Green Deal and regulatory requirements.
The case study used is an SiO2 core–ZnO-shell MCNM embedded in a cement mortar for the photocatalytic decontamination of NOx in air. In this study, according to the safety pillar of the SSbD framework, a safety assessment of the MCNM was performed in the very early stages of the design process. This work was performed in the frame of the European H2020 SUNSHINE project (https://h2020sunshine.eu), which strives to develop an overarching approach for the SSbD of MCNMs, including demonstration via industrially relevant case studies.16
2. Materials and methods
2.1 SUNSHINE implementation of the safety dimension within the SSbD framework
The first three steps of the SSbD framework cover different aspects of chemicals/materials safety, for which indicators, criteria and an evaluation system need to be defined. In detail, step 1 aims at investigating the intrinsic properties of a chemical/material to determine its hazard profile before further assessing its safety during use. This is based, when available, on hazard classes and categories established within the Classification, Labelling and Packaging (CLP) Regulation 1227/2008 as well as Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation 1907/2006. Step 2 deals with the human health and safety aspects in the chemical/material production and processing phases, investigating whether the chemical/material of interest can pose any risk to workers before application. This step involves the use of available models to assign scores/levels to the related variables including hazard of chemicals, frequency and duration of exposure, amount of chemical used/present, physico-chemical properties, operational conditions, and type of existing risk management measures. Based on the results, the risk estimation is performed. Lastly, step 3 covers the human health and environmental aspects in the final application phase, identifying the likelihood of the exposure and the potential exposure routes and related toxicity impacts. Within SUNSHINE, the safety aspects for the advanced SiO2@ZnO MCNM were covered, as summarized below. With regards to step 1 of the SSbD framework, physico-chemical characterization of both individual NMs and MCNM, hazard assessment of the MCNM precursors and preliminary in vitro assays with NMs and MCNM were performed (cf. 3.2). In step 2, an occupational exposure assessment was carried out through an air monitoring campaign (cf. 3.3). Concerning step 3, the potential leaching of inorganic substances from both the pristine powders and the MCNM-based mortars was assessed by dissolution experiments (cf. 3.4). Fig. 1 shows the relation between the safety dimension assessment of the SSbD framework and the corresponding implementation within the SUNSHINE project.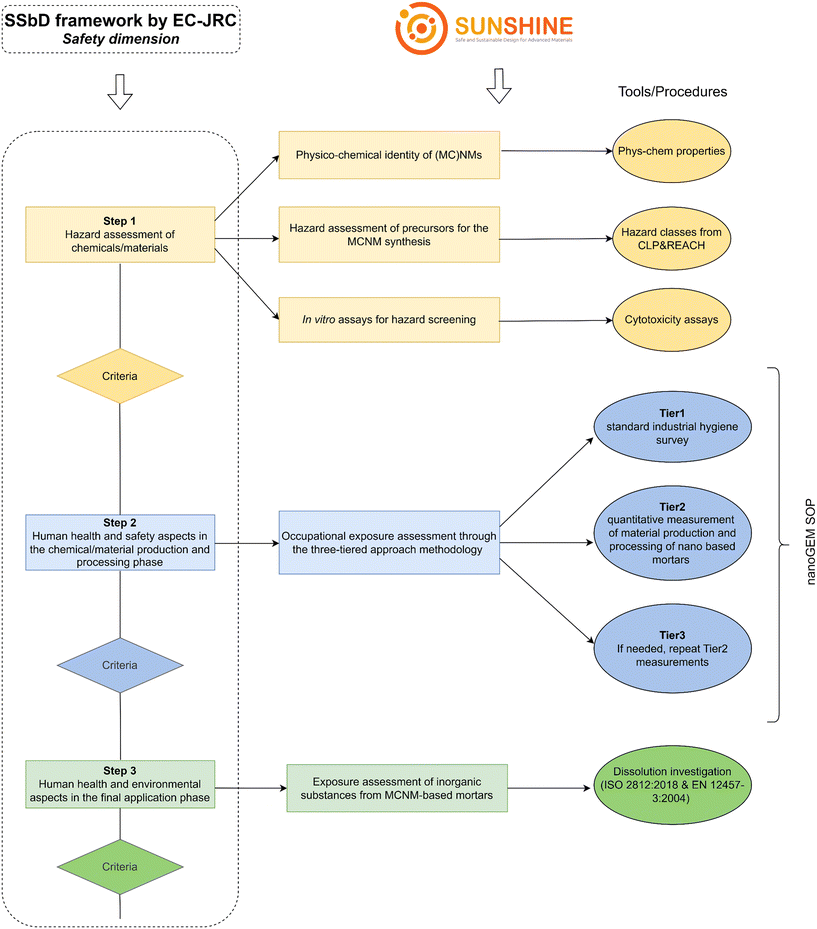 | ||
| Fig. 1 Safety dimension assessment of the SSbD framework (in dashed rectangle) and corresponding implementation within SUNSHINE. Tools/procedures for each step are displayed in ellipses. | ||
2.2 Case study materials
The pristine SiO2@ZnO MCNMs were synthetized by the CIAC Company (Cordoba, Spain), starting from commercial mesoporous SiO2 NM powder with an average particle diameter of 20 nm (99.5% purity, NanoAmor, TX, USA) and ZnAc2·2H2O (purity 99.5%, ITW Reagents S.r.l.). In brief, zinc acetate dihydrate was deposited on the SiO2 NM core in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio through a calcination process at 600 °C (heating rate of 100 °C h−1) for 2 h, and then hand ground with agate mortar for 15 min, to form the desired core–shell structure with ZnO NM as the outer shell. In addition, a ZnO NM was also synthesized as a comparable individual component (benchmark) material by following the same calcination procedure at 600 °C from zinc acetate dihydrate in the absence of SiO2.
2 ratio through a calcination process at 600 °C (heating rate of 100 °C h−1) for 2 h, and then hand ground with agate mortar for 15 min, to form the desired core–shell structure with ZnO NM as the outer shell. In addition, a ZnO NM was also synthesized as a comparable individual component (benchmark) material by following the same calcination procedure at 600 °C from zinc acetate dihydrate in the absence of SiO2.
Concerning the MCNM-enabled product, the mortar used for testing was Morcemsec® Capa Fina CR CSIV W2 (Grupo PUMA S.L., Cordoba, Spain) based on Portland cement (between 9 and <40 wt%) and calcium dihydroxide (<5 wt%). The formulation of the MCNM-based mortars was performed by adding the SiO2@ZnO MCNM powder to commercial mortar, obtaining homogeneous dust, and then mixing it with water, following the procedure described in EN 196-1:2016 (ref. 17) before placing in a mold for 48 h. Afterwards, according to the curing conditions of EN 1015-11:2019,18 the samples were exposed to 95 ± 5% relative humidity and 20 ± 2 °C for 5 days and 65 ± 5% relative humidity and 20 ± 2 °C for an additional 21 days.
2.3 Hazard assessment of the chemicals/materials (step 1)
The XRD patterns were recorded using an X'Pert PRO diffractometer by PANalytical and data were collected in the 2θ range of 5° to 55° at a time step of 50 s. The quantification of the elemental composition was performed using an ICP-OES 5100 – Vertical Dual View apparatus coupled with a OneNeb nebulizer (Agilent Technologies, Santa Clara, CA, USA), using a mixture of 10% v/v HNO3, 10% v/v H2SO4 and 1% v/v HF (ultrapure analytical grade from Merck, Darmstadt, Germany) to ensure complete digestion. Calibration curves were obtained with 0.1, 1, 10 and 100 mg L−1 of Si and Zn standards prepared in ultrapure water using the same procedure applied to the samples.
The reactivity of both individual and MCNMs and the degree of coverage of SiO2 by ZnO was assessed via temperature programmed reaction (TPRx) using methanol as the probe molecule. The potential reaction products include carbon dioxide (CO2) for materials with basic sites, methyl formate (HCOOCH3) for materials possessing bifunctional basic-redox sites, formaldehyde (HCHO) for materials with redox sites, dimethoxymethane ((CH3O)2CH2) for materials featuring bifunctional acid-redox sites, and dimethyl ether (CH3OCH3) for materials with acidic sites. The reaction products were detected using a Pfeiffer OmniStar mass spectrometer. The experimental procedure involved four phases including pretreatment, initial purge, methanol chemisorption, and TPRx. Pretreatment involved heating the sample from 100 °C to 450 °C at a rate of 10 °C min−1 with the introduction of 150 mL min−1 of synthetic air to eliminate surface moisture and impurities. Following pretreatment, an initial purge with inert gas (150 mL min−1 of Ar) at 100 °C was conducted until impurities were no longer detected in the mass spectra. Subsequently, methanol chemisorption was carried out with a methanol/argon mixture at a constant temperature of 100 °C, concluding when a stable methanol signal was observed in the mass spectrum (m/z = 31). The final phase, TPRx, involved maintaining the methanol/argon flow, while increasing the temperature from 100 °C to 450 °C at a rate of 10 °C min−1. The experiment utilized a sample of 0.1 g (mesh size: 25–100 μm) diluted with 0.5 g of SiC (black 180, Navarro SiC S.A.) to ensure a uniform temperature distribution.
In this study, a frequently used cell line was employed (THP-1 monocyte cells; ATCC (TIB 202), passage number 15–20), which was treated to differentiate the cells to behave like mature macrophages. A standard operating procedure (SOP) was utilised to culture the cells and treat them with controls, NMs or MCNMs to enhance the reproducibility of the results and comparison with previous studies using the same SOP (https://www.patrols-h2020.eu/publications/sops/index.php).26
SiO2@ZnO MCNM, SiO2 NM or ZnO NM were suspended at a concentration of 1 mg mL−1 in phosphate buffered saline with 0.05% w/v bovine serum albumin (BSA) and dispersed by sonication using an ultrasonicating water bath for 16 min. Dispersion was followed immediately by dilution in RPMI medium (without serum) to obtain the required final concentrations (final concentration 0–100 μg mL−1). The treatments were added to each well of a 96-well plate containing cells prior to incubation at 37 °C in the presence of 5% CO2 for 24 h.
The deposited dose was not assessed as this was not deemed appropriate for hazard screening at such an early innovation stage given that it is important to keep the experimental work manageable and affordable.
Cytotoxicity was assessed as a decrease in cell viability to indicate short term hazard, but more importantly it allowed the selection of appropriate concentrations to investigate the production of IL-1β at sublethal concentrations.23 Cell viability was assessed using the Alamar blue (ThermoFisher, catalog number: DAL1025) assay, which measures the ability of cells to reduce the non-fluorescent dye 7-hydroxy-3H-phenoxazin-3-one 10-oxide (resazurin) to the fluorescent product resorufin. A decrease in the ability to generate the fluorescent product (excitation/emission wavelengths of 560/590 nm) was used as the measure of cytotoxicity. Data were expressed as % cell viability. The control cells were treated with RPMI medium (without FCS) without particles added. The production of IL-1β was measured in the cell culture supernatant, according to the R&D Human IL-1β/IL-1F2 DuoSet protocol (catalog numbers: DY201-05).
2.4 Human health and safety aspects in the chemical/material production and processing phase (step 2)
The NMs/chemicals used and the steps of the process to synthesize the SiO2@ZnO MCNM are displayed in Scheme 1. Regarding the evaluation of the occupational safety and health (OSH) aspects in the production and processing of (MC)NMs, a three-tiered methodology, already recommended by different authors or institutions,27–31 was applied, as depicted in Fig. 2.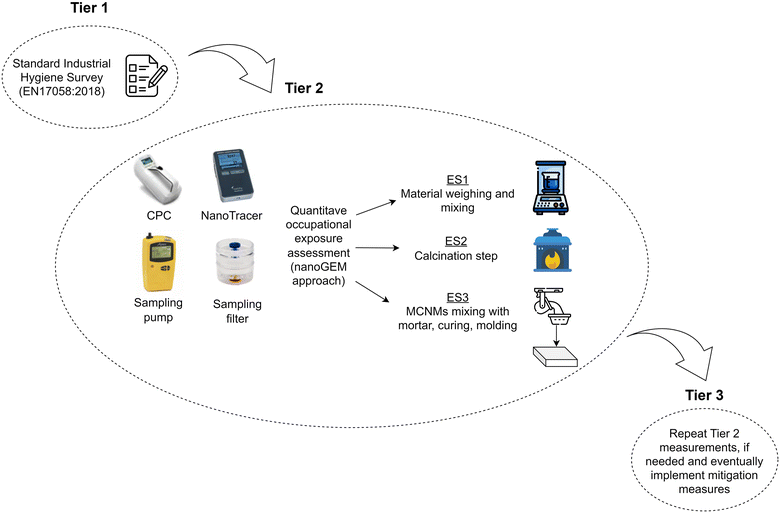 | ||
| Fig. 2 Schematic of the three-tiered methodology approach applied to assess the occupational safety and health (OSH) aspects in the production and processing of (MC)NMs. ES = exposure scenario. | ||
In the first tier, a standard industrial hygiene survey through questionnaires following the EN 17058:2018 (ref. 32) “workplace exposure – assessment of exposure by inhalation of nano-objects and their aggregates and agglomerates” was filled in by CIAC Foundation and included in the ESI† (Tables S1 and S2), for gathering information on potential sources/points of (MC)NMs emission. The target areas, processes or tasks from which any (MC)NMs can be released were identified.
Afterwards, tier two covered the occupational exposure assessment at different stages of the material production and processing of mortars with embedded SiO2@ZnO MCNM, performing a quantitative exposure assessment. An overview of the exposure scenarios (ES) and the contributing exposure scenarios (CES), which refer to specific activities where the release of (MC)NMs may take place, were identified and listed in Table 1, according to ECHA guidance documents.33,34 The maps of each ES are displayed in Fig. S1–S3.†
| Exposure scenario (ES) | Contributing exposure scenario (CES) | (MC)NMs |
|---|---|---|
| ES1 (material synthesis) | 1. Background measurement | SiO2 |
| 2. ZnAc2·2H2O weighing | ||
| 3. SiO2 weighing | ||
| 4. SiO2 addition | ||
| 5. Local exhaust ventilation | ||
| 6. 1st ZnAc2·2H2O addition | ||
| 6. 2nd ZnAc2·2H2O addition | ||
| ES2_I (calcination) | 7. Background measurement | SiO2, ZnO |
| 8. Moving the dispersion | ||
| 9. Muffle opening | ||
| ES2_II (calcination) | 10. High temperature ramp | SiO2, ZnO |
| ES3 (mortar formulation) | 11. Background measurement | SiO2, ZnO |
| 12. Low temperature ramp | ||
| 13. Mortar addition to the stirring tank | ||
| 14. Additive addition to the stirring tank | ||
| 15. Additive addition to the stirring tank + stirring | ||
| 16. Test specimen unmolding |
In particular, the potential exposure to (MC)NMs was evaluated over around 50 min during both their synthesis (i.e., material weighing and calcination) and the (MC)NM-based mortar formulation. The particle number and mass concentrations both in air and in the personal breathing zone (i.e., PBZ, around 30 cm from the respiratory tract) of the workers was determined by using a portable condensation particle counter (CPC, model TSI 3007) and NanoTracer PNT 1000. To gain insights into the possible transport of particles through the air, measurements were performed at around 0.5–1 m (near field, NF) and 5–7 m from the source (far field, FF). Regarding the chemical composition and morphology of the particles potentially released during the monitoring campaign, a Tygon tube (length 1 m) at 30 cm from the mouth of the worker was set to monitor particle release near the breathing zone. Moreover, two high-flow peristaltic pumps (Casella, model APEX) containing a polycarbonate HEPA filter with a diameter of 37 mm and pore size of 0.4 μm, were fixed on the lab coat of the worker at 30 cm from their mouth to collect the particles in air during the monitoring campaign. Subsequently, the filters were observed by scanning electronic microscopy analysis using a field emission scanning electron microscope (FESEM, Carl Zeiss Sigma NTS, Germany). Elemental analysis was performed by image analysis using FESEM coupled to an energy dispersive X-ray micro-analyser (EDS, mod. INCA).
Finally, according to the standard operating procedure published as part of the three-tiered methodology,27 the criterion used to evaluate the results from tier 2 was the comparison of the particle concentration values obtained during the different activities monitored and the particle background concentrations using eqn (1), as follows:
| Cnet − Cbg > 3·S2(DBI) | (1) |
2.5 Human health and environmental aspects in the final application phase (step 3)
Firstly, a preliminary release study of Zn and Si from both individual and MCNM pristine powders was conducted. In detail, stock dispersions of (MC)NMs (i.e., SiO2, ZnO and SiO2@ZnO) at 0.5 g L−1 (20 mg in 40 mL ultrapure water, UPW) were sonicated with an ultrasound probe (UP-200S Hielscher Ultrasonics GmbH, Germany) in an ice bath for 4 min (power = 80%, frequency = 0.5 and sonication energy = 43 J mL−1 (ref. 35)). Afterwards, ultrafiltration of each sample was performed using Amicon Ultra 15 mL centrifugal 3k filters (Millipore) at 0.5, 1, 3, 24 and 48 h after sample preparation and acidified with 2% HNO3 prior to the ICP-OES analysis. The calibration curves and limits of detection (LOD) are reported in the ESI.†
Subsequently, the potential release of hazardous substances from the MCNM-based mortars was studied. In brief, the dissolution of Zn, Si and Ti was estimated from cement mortars with different percentages of MCNMs (i.e., 1% or 5% of SiO2@ZnO, and as references, 1% or 5% of TiO2 and 1% or 5% of silica-fume), according to the ISO 2812:2007 on Paints and varnishes – determination of resistance to liquids-Part 2: Water immersion method36 and to EN 12457-3:2004 for leaching evaluation.37 Before the release experiment, each mortar tested (weight: 29 ± 0.7 g, size: 4 × 4 cm) was subjected to a thorough cleaning process with compressed air to get rid of potential impurities on its surface and weighed. After this pre-treatment, each mortar was totally immersed in 200 mL of UPW, placed on an upside-down glass petri dish to avoid contact with the bottom of the beaker and magnetically stirred during the test. Leachates were collected at different intervals (i.e., 0.5, 3, 24, 48 and 72 h, which correspond to the typical time points used in (eco)toxicity assays) and filtered through a 0.2 μm mesh filter. Afterwards, each sample was acidified with 2% HNO3, and then analysed by ICP-OES to determine the concentration of Zn, Si and Ti.
3. Results and discussion
3.1 Physicochemical characterization
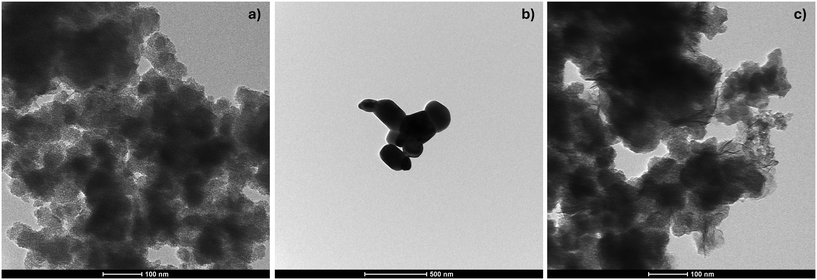
Among the intrinsic properties that can influence the toxicity and modulate the intensity of adverse effects exerted by the (MC)NMs,38 chemical composition, constituent particle size, morphology, and crystallinity were considered as the most relevant and were investigated in detail. Regarding the chemical composition, the ICP-OES analysis estimated that SiO2 accounted for 80% of the total MCNM, while ZnO for the remaining 20%. The particle size and shape of the (MC)NMs were determined by TEM (Fig. 3), showing highly agglomerated constituent SiO2 particles, with an average particle size of around 20 nm (Fig. 3a). ZnO NM (Fig. 3b), synthesized as benchmark material, showed elliptic/roundish shape particles with an average particle size of <200 nm. Fig. 3c displays the TEM image of MCNM, which is constituted of agglomerated particles similar to that observed for SiO2 NM but with the presence of a non-homogeneous coating.The crystalline structure of MCNM was determined via XRD analysis, showing that the SiO2 core was amorphous, whereas the ZnO shell revealed a crystalline phase, as observed for the individual components (Fig. S4†).
To gather further information on the coating, energy dispersive X-ray (EDX) mapping, reactivity analysis via MeOH-TPRx and scanning transmission electron microscopy (STEM) analysis were also performed. The EDX mapping of the single elements, i.e., Si (Fig. S5c†), Zn (Fig. S5d†), O (Fig. S5f†), and the overlayed mapping of Si–Zn (Fig. S5e†) confirmed that MCNM was an Si core–Zn shell structure with a non-homogeneous coverage. Finally, the reactivity analysis via MeOH-TPRx provided insight into the primary reactivity of the surface reactive sites of (MC)NMs. SiO2 (Fig. S6a†) exhibited acidic reactivity, as indicated by the formation of dimethyl ether (DME, green trace). ZnO (Fig. S6b†) displayed redox character, as evidenced by the formation of formaldehyde. Lastly, the presence of ZnO on the surface of SiO2 resulted in a system that has both acidic and redox properties (DME and HCHO formation, respectively) (Fig. S6c†). This reactive profile indicated that silica (acidic) is not totally covered by ZnO. Therefore, SiO2@ZnO exhibited reactive properties similar to that of its individual components ZnO and SiO2, without any distinct effects arising from the interaction between SiO2 and ZnO being clearly observed. Finally, the non-homogeneous coverage of SiO2 by ZnO was also corroborated by STEM (Fig. S7†).
3.2 Hazard properties evaluation
According to the SSbD framework, the hazard assessment of chemicals/materials to synthesize the SiO2@ZnO MCNM was performed considering the information gathered from the physico-chemical characterization and the criteria defined in Table 3 of the SSbD framework11 (i.e., criterion H1 = most harmful substances, criterion H2 = substances of concern, and criterion H3 = other hazard properties) based on the hazard classes and categories determined through CLP and REACH.The SiO2 NM powder (CAS no. 7631-86-9), which was used to improve the stability, compatibility, and dispersibility of the cementitious material with respect to its bulk phase,39 was classified as non-hazardous according to the current Globally Harmonized System of Classification and Labelling of Chemicals (GHS). However, given that there is still no final consensus on the risk assessment of NMs,40,41 this classification may be updated by the European Chemicals Agency (ECHA) if new insights on their toxicity are provided.
Alternatively, the hazard identification of ZnAc2·2H2O (CAS no. 557-34-6) showed both health and environmental hazards, i.e., acute toxicity (oral, category 4, H302), serious eye damage (category 1, H318) and long-term (chronic) aquatic hazard (category 2, H411). The workflow to assess the hazard of the chemicals used for the synthesis of SiO2@ZnO MCNM is displayed in Fig. 4.
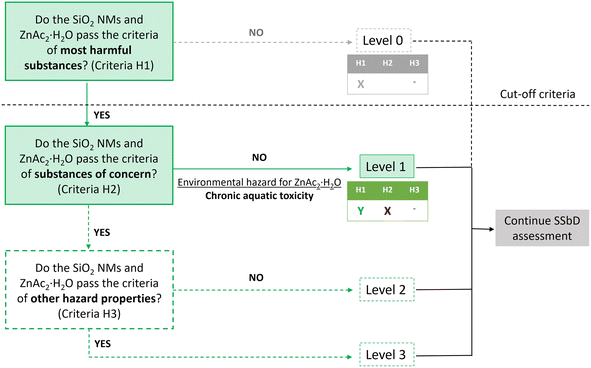 | ||
| Fig. 4 Workflow to assess hazard of SiO2@ZnO MCNM based on step 1 of the SSbD framework. The pathway followed is represented by solid lines and colored rectangles. | ||
Therefore, based on the three criteria defined in Table 3 of the SSbD framework,11 SiO2 NMs and ZnAc2·2H2O pass the H1 but not H2 criteria, which refers to substances of concern described in the Chemical Strategy for Sustainability (CSS) and not already included in the H1 criteria. In addition to these criteria, the framework suggests the use of an evaluation system, which includes 4 levels for step 1, moving from level 0 (chemicals or materials that do not pass hazard criterion H1 (e.g., considered most harmful substances) to level 3 (chemicals or materials that pass all the safety criteria in step 1). Accordingly, the NMs and chemicals used for the synthesis of SiO2@ZnO MCNM belong to level 1, corresponding to chemicals or materials that pass hazard criterion H1 but not criterion H2 (i.e., ZnAc2·2H2O has the potential to induce chronic adverse effects to aquatic life).
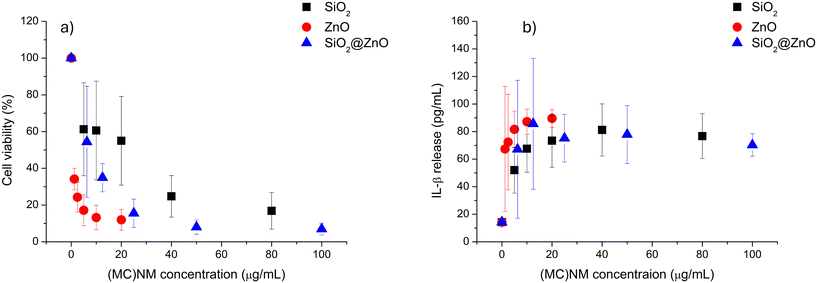
In addition to the preliminary evaluation of the hazard properties, the cytotoxicity of the (MC)NMs was assessed and the results are displayed in Fig. 5. Fig. 5a shows the viability of THP-1 cells exposed to SiO2@ZnO, SiO2 or ZnO NMs for 24 h. Viability is expressed as a percentage of control over a range of exposure concentrations from 0 (control) to 100 μg mL−1. All three materials induced a concentration-dependent decrease in viability, with the ZnO material being the most potent and SiO2 being the least potent. Considering that MCNM is a combination of both ZnO and SiO2, and that the curve for MCNM was between the curves for the individual materials, the interaction is additive rather than synergistic or antagonistic. The production of the pro-inflammatory cytokine IL-1β by the NM- and MCNM-treated THP-1 cells (Fig. 5b) also exhibited a concentration-dependent effect. For this particular indicator, there was no significant difference between any of the materials assessed, suggesting that none of the components dominated the pro-inflammatory activity of MCNM and there was no mixture effect.
The in vitro assessment of the MCNM hazard indicated that MCNM was more reflective of the cytotoxicity and pro-inflammatory responses induced by ZnO NM rather than SiO2 compared at the same mass exposure concentration. This suggested the responses are driven by the ZnO NM component. There is no evidence of an enhanced hazard response due to the SiO2 NM; however, a slight increase in response compared to ZnO alone means some interaction between the NM components may impact the bioactivity of ZnO when in MCNM form. Considering the worst-case scenario, it can be assumed in the early innovation stages that the hazard of MCNM is comparable to that of ZnO. Further work in a wider array of human and environmental models for both acute and longer-term exposure will be required in later innovation stages for regulatory risk assessment.
3.3 MCNM production and processing phase: human health and safety aspects
The survey to identify the potential sources/points of (MC)NM emission is included in the ESI.† According to the results obtained, the OSH assessment during the material synthesis (weighing and calcination) and the (MC)NM-based mortar formulation was carried out using the monitoring procedure described in the Materials and methods section. The background levels of particle number measured over at least 15 min for the three ES considered are summarized in Table S3† and the corresponding SEM-EDX images are displayed in Fig. S8–S13.† The results in Table S3† show that the background levels were in the rage of 3000 to 6000 # cm−3 for ES1, 4500 to 9500 # cm−3 for ES2 and 2450 to 4500 # cm−3 for ES3. The SEM-EDX images of the background measured for the three exposure scenarios considered revealed the presence of agglomerated particles in the nm–μm range and their main composition was C, O and Si, but Ca, Al, K and Fe were also detected. Zn was only identified in the mortar formulation laboratory (ES3).The results of the monitoring campaign through portable air monitoring devices for the three ES, showing particle concentration (number of particles per cm3) vs. time, are displayed in Fig. 6.
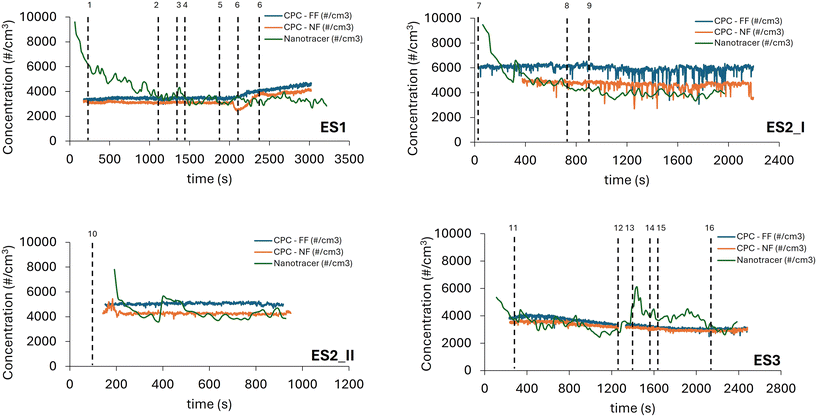 | ||
| Fig. 6 Particle number concentration for different exposure scenarios considered (ES1, ES2_1, ES2_2, and ES3). Dot lines refer to a specific contributing exposure scenario (CES) listed in Table 1. CPC-FF = condensation particle counter-far field (5–7 m from the source) and CPC-NF (0.5–1 m from the source) = condensation particle counter-near field. | ||
Regarding the measurements for ES1, corresponding to weighing ZnAc2·2H2O and SiO2 powders, Fig. 6 shows that the particle concentration was relatively stable during the whole activity monitored and did not exceed 5000 # cm−3. The only significant increment in the values recorded was observed when ZnAc2·2H2O was added. As observed for ES1, the monitoring campaign for ES2 related to the calcination step again revealed that the particle concentration was relatively stable over time, even if all the values started at least 1000 # cm−3 higher than that in ES1. Concerning the mortar formulation step (ES3), the results in Fig. 6 highlight that while the CPC values were approximately the same during all the activities monitored, the data collected by the NanoTracer presented a peak reaching 6000 # cm−3. This is related to the addition of mortar to the stirring tank. According to the NanoGEM Standard Operation Procedures (SOP) for assessing exposure to nanomaterials,27 a peak is statistically significant as follows:
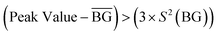 | (2) |
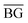 is the mean value of the background and S2(BG) is the standard deviation of the background.
is the mean value of the background and S2(BG) is the standard deviation of the background.
Therefore, given that 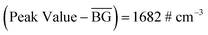 and (3 × S2(BG)) = 1354 # cm−3, the peak recorded by NanoTracer was considered statistically significant.
and (3 × S2(BG)) = 1354 # cm−3, the peak recorded by NanoTracer was considered statistically significant.
The corresponding SEM-EDX images obtained during the monitoring campaign are displayed in Fig. S14–S19,† highlighting the presence of some agglomerated particles in the micrometer size range, mainly composed of C, O, Si, while a very low signal corresponding to Zn emerged, which was not present in the background samples.
Subsequently, the results from the monitoring campaign were assessed by adopting the approach reported by Asbach et al., 2014 (ref. 27) as the criteria to follow, where the values of the background (Table S3†) and data from tier 2 were compared (Tables S4–S6†). Based on this comparison, no significant exposure to particles (i.e., almost all agglomerates in the μm size range) in any of the three exposure scenarios was determined. A further comparison was also performed between the results included in this work and some recommended limit values (recommended benchmark level – RBL or nano reference values – NRV) proposed by various international bodies, as reported in the literature.42 For example, according to the Institute for Occupational Safety and Health of the German Social Accident Insurance (IFA), considering an average airborne concentration during an 8 h workday, the RBL values are 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 # cm−3 (from 1 to 100 nm) for bio persistent granular NMs with a density of >6000 kg m−3 and 40
000 # cm−3 (from 1 to 100 nm) for bio persistent granular NMs with a density of >6000 kg m−3 and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 # cm−3 for particles with a density of <6000 kg m−3. Following the RBL values proposed by IFA, the Social and Economic Council of the Netherlands (SER) proposed the same values, referred to as NRV. According to the results obtained, it can be concluded that none of the exposure scenarios investigated generate a particle concentration higher than the RBL/NRV values. However, considering the continuous improvements in the risk assessment of advanced materials, including NMs, a further SSbD measure to mitigate the potential exposure to SiO2 NMs in powder form through inhalation would be its replacement with colloidal SiO2, but ensuring that the same functionality of the SiO2@ZnO MCNM is achieved.
000 # cm−3 for particles with a density of <6000 kg m−3. Following the RBL values proposed by IFA, the Social and Economic Council of the Netherlands (SER) proposed the same values, referred to as NRV. According to the results obtained, it can be concluded that none of the exposure scenarios investigated generate a particle concentration higher than the RBL/NRV values. However, considering the continuous improvements in the risk assessment of advanced materials, including NMs, a further SSbD measure to mitigate the potential exposure to SiO2 NMs in powder form through inhalation would be its replacement with colloidal SiO2, but ensuring that the same functionality of the SiO2@ZnO MCNM is achieved.
3.4 MCNM final application phase: human health and environmental aspects
To assess the potential exposure to the (MC)NMs under investigation in the final application phase, the release of potentially hazardous metals (i.e., Zn, Si, and Ti) from both the pristine materials, used as the reference from which the release could be maximum, and the embedded mortars was evaluated. Considering the application of mortars on the surface of buildings that are subject to different weathering agents such as raining events, which can wash out undesirable and harmful substances, their potential release was evaluated considering the worst-case scenario. This test involved the total immersion of a mortar brick in UPW over time, as described by the standard leaching test methods ISO 2812:2018 for mortar immersion conditions and EN 12457-3:2004 for leaching evaluation.43 The results obtained from these experiments are reported below.The dissolution of the (MC)NM powder dispersed in UPW was investigated at room temperature by ICP-OES at 0.5, 1, 3, 24 and 48 h after preparation of the dispersion and the values are displayed in Fig. 7. The data are expressed as % of undissolved Zn and Si normalized by the molecular weight for each (MC)NM, considering the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of SiO2
1 ratio of SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO for the MCNM, as initially determined by ICP-OES. The results showed that Si dissolution in UPW, from both individual and MCNM, was negligible over time (around 2%), while Zn dissolution revealed some differences between ZnO and SiO2@ZnO MCNM only at 48 h, reaching around 9% and 6%, respectively. This slight difference could be ascribed to their particle size and surface chemistry, which are generally recognized to be the main physico-chemical parameters affecting the colloidal behaviour of NMs.44,45 Specifically, as the particle size decreases, the surface area and number of reactive surface sites increase, which often results in different behavior in NMs with respect to their bulk state. In the case of the ZnO NMs investigated in this study, their particle size was <200 nm with an elliptic/roundish shape, while in the case of the core–shell structure, ZnO was present as a thin coating on the surface of SiO2 NMs.
ZnO for the MCNM, as initially determined by ICP-OES. The results showed that Si dissolution in UPW, from both individual and MCNM, was negligible over time (around 2%), while Zn dissolution revealed some differences between ZnO and SiO2@ZnO MCNM only at 48 h, reaching around 9% and 6%, respectively. This slight difference could be ascribed to their particle size and surface chemistry, which are generally recognized to be the main physico-chemical parameters affecting the colloidal behaviour of NMs.44,45 Specifically, as the particle size decreases, the surface area and number of reactive surface sites increase, which often results in different behavior in NMs with respect to their bulk state. In the case of the ZnO NMs investigated in this study, their particle size was <200 nm with an elliptic/roundish shape, while in the case of the core–shell structure, ZnO was present as a thin coating on the surface of SiO2 NMs.
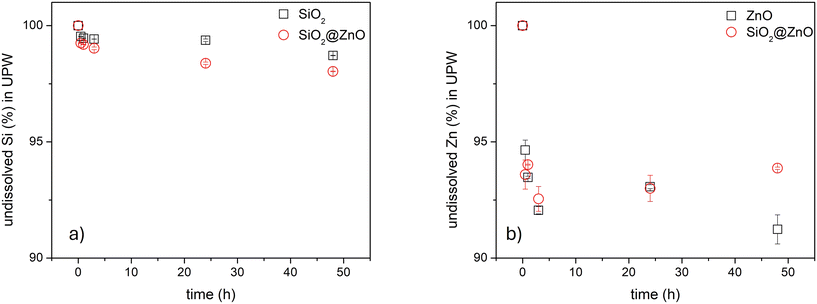 | ||
| Fig. 7 Dissolution of Si and Zn from both individual and MCNM. a) Si% from SiO2 NM and SiO2@ZnO MCNM. b) Zn% from ZnO NM and SiO2@ZnO MCNM. | ||
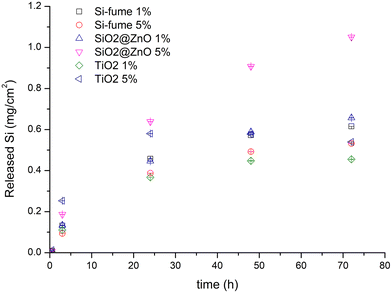
Moving from the synthesis to the use phase, the potential release of hazardous substances from (MC)NMs embedded in mortars was investigated. According to the experimental design followed in this work, no release of Zn and Ti from the mortars immersed in UPW after 72 h was detected, while the Si release data (expressed as mg of Si per cm2 of mortar) are displayed in Fig. 8. The results of this experiment allowed us to discriminate whether the source of Si was the mortar itself or the MCNMs embedded in the mortar, showing that the mortar sample with a higher percentage of MCNM (5% SiO2@ZnO) was the only material that released a different Si amount than the others, especially at 48 and 72 h. Therefore, the difference between the maximum amount of Si released from the 5% SiO2@ZnO mortar and that released by the mortars without Si-based NMs allowed the determination of the amount of Si ascribable to the MCNM. For example, at 72 h, this amount ranged from around 0.4 to 0.6 mg cm−2, suggesting the maximum Si release of 4% with respect to the maximum amount potentially released by the MCNM.
3.5 SbD assessment summary
The combination of experimental data and literature information reported in this work allowed us to assess the safety of the SiO2@ZnO MCNM. The results from the hazard assessment (step 1) are comprised of information from its basic physicochemical characteristics, preliminary evaluation of hazard properties and rapid cytotoxicity assays. The results of this first step showed the following: a) the MCNM was composed of an SiO2 NP core, with non-homogeneous coverage of ZnO, with a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SiO2
1 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO ratio; b) the hazard evaluation of the components used for the MCNM synthesis reached level 1 (according to the SSbD framework evaluation system), given that ZnAc2·H2O can induce chronic environmental toxicity; and c) the in vitro assessment of the MCNM suggested that its hazard can be comparable to that of the ZnO NPs tested. The results from the air monitoring campaign (step 2) did not highlight any exposure of workers to particles for the three exposure scenarios considered, disclosing that the particle concentration recorded was always below the recommended RBL/NRV thresholds proposed by the IFA German Institute and by the SER Dutch advisory body. Lastly, the release experiments from the mortars with embedded MCNM (step 3) showed only the slight release of Si from the mortar loaded with the highest MCNM concentration. Therefore, considering the worst-case scenario, these SbD data suggest that this SiO2@ZnO MCNM can be considered similar to <200 nm round-shape ZnO NPs for its cytotoxicity response but it does not pose significant concerns resulting from the occupational exposure assessment as well as from the final application phase. These outcomes, not only including the new experimental data but also the approach to assess the safety dimension of MCNM, can be very useful to different stakeholders, including value chain actors in the building sector such as governments, private investors, construction companies and real estate agents. Moreover, researchers, policy makers and the European Commission can take advantage of this case study to guide future efforts toward the sustainability assessment of advanced materials.
ZnO ratio; b) the hazard evaluation of the components used for the MCNM synthesis reached level 1 (according to the SSbD framework evaluation system), given that ZnAc2·H2O can induce chronic environmental toxicity; and c) the in vitro assessment of the MCNM suggested that its hazard can be comparable to that of the ZnO NPs tested. The results from the air monitoring campaign (step 2) did not highlight any exposure of workers to particles for the three exposure scenarios considered, disclosing that the particle concentration recorded was always below the recommended RBL/NRV thresholds proposed by the IFA German Institute and by the SER Dutch advisory body. Lastly, the release experiments from the mortars with embedded MCNM (step 3) showed only the slight release of Si from the mortar loaded with the highest MCNM concentration. Therefore, considering the worst-case scenario, these SbD data suggest that this SiO2@ZnO MCNM can be considered similar to <200 nm round-shape ZnO NPs for its cytotoxicity response but it does not pose significant concerns resulting from the occupational exposure assessment as well as from the final application phase. These outcomes, not only including the new experimental data but also the approach to assess the safety dimension of MCNM, can be very useful to different stakeholders, including value chain actors in the building sector such as governments, private investors, construction companies and real estate agents. Moreover, researchers, policy makers and the European Commission can take advantage of this case study to guide future efforts toward the sustainability assessment of advanced materials.
4. General remarks and conclusions
This work falls within the context of implementing the European Commission Chemicals Strategy for Sustainability and focused on the safety dimension aspects of the SSbD framework proposed by the JRC. The stepwise approach followed can be considered one of the activities envisaged by the European Commission to support the testing and potential refinement of the framework, which was developed for chemicals and materials in general, and not specifically for advanced materials. The MCNM studied here is novel and innovative, and hence the existing data required for applying the framework are scarce. For example, the evaluation of hazard properties in step 1 of the framework requires data, which are not yet available from CLP and REACH registrations. Therefore, the framework encourages the use of New Approach Methodologies (NAMs), such as in vitro and in silico assays, as relevant means to support the innovation process in the early stages because even if they are not fully validated for regulatory purposes, NAMs can provide valuable information that is relatively economical to obtain.46 Hence, faster and more affordable in vitro assays or in silico tools (in this case specifically for MCNMs) can be valuable to gather this information in the early innovation stages. Regarding these tools, the selection of the most suitable benchmark with which to compare the in vitro results of MCNM is crucial but a challenge at the same time because it depends greatly on the physicochemical characteristics of the MCNM and how it may interact with the surroundings.The following tools/procedures related to experimental activities were identified to operationalize the safety aspects of the SSbD framework to evaluate the safety of the SiO2@ZnO MCNM in the early innovation stage including basic physicochemical characterization, hazard classification, rapid in vitro assays, the three-tiered methodology developed by the nanoGEM research project and the release investigation of potential hazardous substances from MCNM-based mortars. It is worth noting that except for the procedure addressing step 3 of the SSbD framework, chosen specifically for this case study, the tools identified in this study are generally applicable. As a result, they can be effectively utilized for implementing the safety aspect of the SSbD framework for MCNMs.
Concerning the re-design phase of this MCNM, a valid alternative to decrease the exposure of workers during the synthesis of MCNM, acting in the early-stage design will be the replacement of the SiO2 powder with colloidal SiO2, ensuring the same material functionality. Therefore, this approach can have a positive impact in the early stage of the design phase of chemicals/materials, steering innovation towards the green industrial transition, beyond current regulatory compliance.
In line with the framework, further assessments are ongoing to include the estimation of the environmental, social and economic impacts to address the other steps not covered in this work. For example, even if recycling of building materials is constantly increasing, it is worth noting that the end-of-life phase of MCNM-based mortars was not considered in this work, although it may be a critical step contributing to a more comprehensive safety and sustainability assessment. The ongoing research activity within SUNSHINE is now focusing on integrating the information generated herein with the screening level approach already developed by project partners,16 with the aim to include all these information in the SUNSHINE SSbD e-infrastructure. This methodology will provide an interactive tool to meet the expectations of stakeholders along the value chain of this MCNM, which can also be extended to other advanced materials. The digital integration of information through the e-infrastructure will help producers, both large companies and small and medium enterprises, to clearly identify criticalities along the entire MCNM life cycle in which to take action by eliminating hazardous materials, replacing them with less hazardous ones and disclosing the environmental, economic and social impacts compared to a benchmark. Overall, we believe that this approach is consistent with the Green Deal ambition towards a zero-pollution toxic-free environment to adequately protect citizens and the environment.
Disclaimer
The content expressed in this paper is solely the opinion of the authors and does not necessarily reflect the opinion of their institutions.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was performed in the frame of the European Union's Horizon 2020 research and innovation programme under SUNSHINE (Safe and Sustainable Design for Advanced Materials) project (G.A. No. 952924).References
- Y.-W. Huang, M. Cambre and H.-J. Lee, The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms, Int. J. Mol. Sci., 2017, 18, 2702 CrossRef.
- K. Luyts, D. Napierska, B. Nemery and P. H. M. Hoet, How physico-chemical characteristics of nanoparticles cause their toxicity: complex and unresolved interrelations, Environ. Sci.: Processes Impacts, 2013, 15, 23–38 RSC.
- A. Sukhanova, S. Bozrova, P. Sokolov, M. Berestovoy, A. Karaulov and I. Nabiev, Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties, Nanoscale Res. Lett., 2018, 13, 44 CrossRef PubMed.
- N. M. Tran, A. N. Nguyen, J. Bae, J. Kim, D. Kim and H. Yoo, Recent strategies for constructing hierarchical multicomponent nanoparticles/metal–organic framework hybrids and their applications, Nanoscale Adv., 2023, 5, 3589–3605 RSC.
- D. Li, F. Guo and L. Qi, Gold Nanoarrow-Based Core–Shell and Yolk–Shell Nanoparticles for Surface-Enhanced Raman Scattering, ACS Appl. Nano Mater., 2022, 5, 126–132 CrossRef CAS.
- Y. Xiang, Y. Huang, B. Xiao, X. Wu and G. Zhang, Magnetic yolk-shell structure of ZnFe2O4 nanoparticles for enhanced visible light photo-Fenton degradation towards antibiotics and mechanism study, Appl. Surf. Sci., 2020, 513, 145820 CrossRef CAS.
- G. F. Huseien, Potential Applications of Core-Shell Nanoparticles in Construction Industry Revisited, Appl. Nano, 2023, 4, 75–114 CrossRef.
- A. Speziale, J. F. González-Sánchez, B. Taşcı, A. Pastor, L. Sánchez, C. Fernández-Acevedo, T. Oroz-Mateo, C. Salazar, I. Navarro-Blasco, J. M. Fernández and J. I. Alvarez, Development of Multifunctional Coatings for Protecting Stones and Lime Mortars of the Architectural Heritage, Int. J. Archit. Herit., 2020, 14, 1008–1029 CrossRef.
- F. Zhang, Z. Wang, W. J. G. M. Peijnenburg and M. G. Vijver, Review and Prospects on the Ecotoxicity of Mixtures of Nanoparticles and Hybrid Nanomaterials, Environ. Sci. Technol., 2022, 56, 15238–15250 CrossRef CAS.
- D. Pozzi, G. Caracciolo, L. Digiacomo, V. Colapicchioni, S. Palchetti, A. L. Capriotti, C. Cavaliere, R. Zenezini Chiozzi, A. Puglisi and A. Laganà, The biomolecular corona of nanoparticles in circulating biological media, Nanoscale, 2015, 7, 13958–13966 RSC.
- C. Caldeira, R. Farcal, A. I. Garmendia, L. Mancini, D. Tosches, A. Amelio, K. Rasmussen, H. Rauscher, S. J. Riego and S. Sala, Safe and sustainable by design chemicals and materials - Framework for the definition of criteria and evaluation procedure for chemicals and materials, EUR 31100 EN, Publications Office of the European Union, Luxembourg, 2022, DOI:10.2760/487955, JRC128591, ISBN 978-92-76-53264-4, https://publications.jrc.ec.europa.eu/repository/handle/JRC128591.
- European Commission, Commission recommendation of 8.12.2022 establishing a European assessment framework for ‘safe and sustainable by design’ chemicals and materials, Brussels, 2022, 8.12.2022 C (2022) 8854 final Search PubMed.
- A. Kraegeloh, B. Suarez-Merino, T. Sluijters and C. Micheletti, Implementation of Safe-by-Design for Nanomaterial Development and Safe Innovation: Why We Need a Comprehensive Approach, Nanomaterials, 2018, 8, 239 CrossRef PubMed.
- L. Yan, F. Zhao, J. Wang, Y. Zu, Z. Gu and Y. Zhao, A Safe-by-Design Strategy towards Safer Nanomaterials in Nanomedicines, Adv. Mater., 2019, 31, 1805391 CrossRef CAS PubMed.
- A. Sudheshwar, C. Apel, K. Kümmerer, Z. Wang, L. G. Soeteman-Hernández, E. Valsami-Jones, C. Som and B. Nowack, Learning from Safe-by-Design for Safe-and-Sustainable-by-Design: Mapping the current landscape of Safe-by-Design reviews, case studies, and frameworks, Environ. Int., 2024, 183, 108305 CrossRef CAS.
- L. Pizzol, A. Livieri, B. Salieri, L. Farcal, L. G. Soeteman-Hernández, H. Rauscher, A. Zabeo, M. Blosi, A. L. Costa, W. Peijnenburg, S. Stoycheva, N. Hunt, M. J. López-Tendero, C. Salgado, J. J. Reinosa, J. F. Fernández and D. Hristozov, Screening level approach to support companies in making safe and sustainable by design decisions at the early stages of innovation, Clean. Environ. Syst., 2023, 10, 100132 CrossRef.
- European Committee for Standardization, EN 196-1:2016 - Methods of Testing Cement - Part1: Determination of Strength, 2016.
- European Committee for Standardization, EN 1015-11:2019 - Methods of test for mortar for masonry - Part 11: Determination of flexural and compressive strength of hardened mortar, 2019.
- K. Rasmussen, H. Rauscher, A. Mech, J. Riego Sintes, D. Gilliland, M. González, P. Kearns, K. Moss, M. Visser, M. Groenewold and E. A. J. Bleeker, Physico-chemical properties of manufactured nanomaterials - Characterisation and relevant methods. An outlook based on the OECD Testing Programme, Regul. Toxicol. Pharmacol., 2018, 92, 8–28 CrossRef CAS.
- H. Bresch, V.-D. Hodoroaba, A. Schmidt, K. Rasmussen and H. Rauscher, Counting Small Particles in Electron Microscopy Images - Proposal for Rules and Their Application in Practice, Nanomaterials, 2022, 12, 2238 CrossRef CAS PubMed.
- European Commission, Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006, Official Journal of the European Union L 353, 31.12.2008, 2008, pp. 1–1355 Search PubMed.
- European Commission, Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC, Official Journal of the European Union, L 396, 30.12.2006, 2006, pp. 1–849 Search PubMed.
- N. Ruijter, L. G. Soeteman-Hernández, M. Carrière, M. Boyles, P. McLean, J. Catalán, A. Katsumiti, J. Cabellos, C. Delpivo, A. Sánchez Jiménez, A. Candalija, I. Rodríguez-Llopis, S. Vázquez-Campos, F. R. Cassee and H. Braakhuis, The State of the Art and Challenges of In Vitro Methods for Human Hazard Assessment of Nanomaterials in the Context of Safe-by-Design, Nanomaterials, 2023, 13, 472 CrossRef CAS PubMed.
- H. M. Braakhuis, A. G. Oomen and F. R. Cassee, The State of the Art and Challenges of In Vitro Methods for Human Hazard Assessment of Nanomaterials in the Context of Safe-by-Design, Toxicol. Appl. Pharmacol., 2016, 299, 3–7 CrossRef CAS PubMed.
- B. Sun, X. Wang, Z. Ji, R. Li and T. Xia, NLRP3 Inflammasome Activation Induced by Engineered Nanomaterials, Small, 2013, 9, 1595–1607 CrossRef CAS.
- https://www.patrols-h2020.eu/publications/sops/index.php .
- C. Asbach, T. A. J. Kuhlbusch, B. Stahlmecke, H. Kaminski, H. J. Kiesling, M. Voetz, D. Dahmann, U. Götz, N. Dziurowitz and S. Plitzko, Measurement and monitoring strategy for assessing workplace exposure to airborne nanomaterials, in Safety of Nanomaterials along Their Lifecycle: Release, Exposure, and Human Hazards, 2014, pp. 233–246 Search PubMed.
- M. Methner, L. Hodson and C. Geraci, Nanoparticle emission assessment technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials--part A, J. Occup. Environ. Hyg., 2010, 7, 127–132 CrossRef CAS PubMed.
- OECD, Strategies, techniques and sampling protocols for determining the concentrations of manufactured nanomaterials in air at the workplace, Series on the Safety of Manufactured nanomaterials N° 82, ENV/JM/MONO, 2017, vol. 30.
- OECD, Series on the safety of manufactured nanomaterials number 11 - Emission assessment for identification of sources and release of airborne manufactured nanomaterials in the workplace: compilation of existing guidance, ENV/JM/MONO, 2009, vol. 16.
- BAuA, BG RCI, IFA, IUTA, TUD and VCI, Tiered Approach to an Exposure Measurement and Assessment of Nanoscale Aerosols Released from Engineered Nanomaterials in Workplace Operations, 2011 Search PubMed.
- European Committee for Standardization, EN17058:2018 - Workplace exposure - Assessment of exposure by inhalation of nano-objects and their aggregates and agglomerates, 2018.
- European Chemicals Agency, An illustrative example of the exposure scenarios to be annexed to the safety data sheet. Part 1, Introductory note, European Chemicals Agency, 2017 Search PubMed.
- European Chemicals Agency, Guidance on information requirements and chemical safety assessment – Chapter 14: occupational exposure assessment, version 3.0 - August 2016, European Chemicals Agency, 2016 Search PubMed.
- G. M. DeLoid, J. M. Cohen, G. Pyrgiotakis and P. Demokritou, Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials, Nat. Protoc., 2017, 12, 355–371 CrossRef CAS.
- International Organization for Standardization, ISO 2812-1:2017(en), Paints and varnishes - Determination of resistance to liquids — Part 1: Immersion in liquids other than water, 2017.
- UNI EN 12457-3, 2004, Characterization of waste - Leaching - Compliance test for leaching of granular waste materials and sludges - Part 3: Two stage batch test at a liquid to solid ratio of 2 l/kg and 8 l/kg for materials with high solid content and with particle size below.
- OECD, Physical-chemical decision framework to inform decisions for risk assessment of manufactured nanomaterials, ENV/JM/MONO, 2019, vol. 12.
- S. Gupta, Smart Nanoconcretes and Cement-Based Materials, in Smart Nanoconcretes and Cement-Based Materials, ed. M. S. Liew, P. Nguyen-Tri, T. A. Nguyen and S. Kakooei, Elsevier, 2020, pp. 601–617 Search PubMed.
- W. Brand, P. C. E. van Kesteren, R. J. B. Peters and A. G. Oomen, Issues currently complicating the risk assessment of synthetic amorphous silica (SAS) nanoparticles after oral exposure, Nanotoxicology, 2021, 15, 905–933 CAS.
- P. C. E. van Kesteren, F. Cubadda, H. Bouwmeester, J. C. H. van Eijkeren, S. Dekkers, W. H. de Jong and A. G. Oomen, Novel insights into the risk assessment of the nanomaterial synthetic amorphous silica, additive E551, in food, Nanotoxicology, 2015, 9, 442–452 CrossRef CAS PubMed.
- M. Visser, I. Gosens, D. Bard, P. van Broekhuizen, G. Janer, E. Kuempel, M. Riediker, U. Vogel and S. Dekkers, Towards health-based nano reference values (HNRVs) for occupational exposure: Recommendations from an expert panel, NanoImpact, 2022, 26, 100396 CrossRef CAS PubMed.
- A. Brunelli, L. Calgaro, E. Semenzin, V. Cazzagon, E. Giubilato, A. Marcomini and E. Badetti, Leaching of nanoparticles from nano-enabled products for the protection of cultural heritage surfaces: a review, Environ. Sci. Eur., 2021, 33, 48 CrossRef.
- E. A. Meulenkamp, Size Dependence of the Dissolution of ZnO Nanoparticles, J. Phys. Chem. B, 1998, 102, 7764–7769 CrossRef CAS.
- A. Albanese, P. S. Tang and W. C. W. Chan, The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems, Annu. Rev. Biomed. Eng., 2012, 14, 1–16 CrossRef CAS PubMed.
- E. Abbate, I. Garmendia Aguirre, G. Bracalente, L. Mancini, D. Tosches, K. Rasmussen, M. Bennett, H. Rauscher and S. Sala, Sustainable by Design chemicals and materials - Methodological Guidance, Publications Office of the European Union, Luxembourg, 2024, DOI:10.2760/28450, JRC138035.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00352g |
| This journal is © The Royal Society of Chemistry 2025 |