Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of weathering and simulated gastric fluid exposure on cellular responses to polystyrene particles†
Liyuan
Gong
 *a,
Animesh
Pan
*a,
Animesh
Pan
 b,
Takeshi
Matsuo
d,
Hemalatha
Kanniyappan
c,
Irene
Andreu
b,
Takeshi
Matsuo
d,
Hemalatha
Kanniyappan
c,
Irene
Andreu
 b,
Alan
Rothman
e,
Geoffrey D.
Bothun
b,
Alan
Rothman
e,
Geoffrey D.
Bothun
 b,
Mathew
Mathew
c and
Yang
Lin
b,
Mathew
Mathew
c and
Yang
Lin
 *a
*a
aDepartment of Mechanical, Industrial and Systems Engineering, University of Rhode Island, Kingston, RI 02881, USA. E-mail: liyuan_gong@uri.edu; yanglin@uri.edu
bDepartment of Chemical Engineering, University of Rhode Island, Kingston, RI 02881, USA
cDepartment of Department of Biomedical Sciences, University of Illinois Chicago, Chicago, IL 60607, USA
dDepartment of Mechanical and Systems Engineering, Okayama University, Okayama, 700-8530, Japan
eDepartment of Cell and Molecular Biology, University of Rhode Island, Kingston, RI 02881, USA
First published on 11th October 2024
Abstract
Micro and nanoplastics pose a growing environmental threat with complex implications for human health. Despite the extensive research on the cytotoxicities of microplastics, gaps remain in understanding cellular responses to the interplay between environmental weathering and physiological processes. This study aims to fill this knowledge gap by evaluating and comparing the in vitro cellular responses to pristine polystyrene particles, particles weathered under UV light in DI water and seawater, and particles with subsequent incubation in simulated gastric fluid (SGF). In this study, Fourier transform infrared spectroscopy in attenuated total reflection mode (ATR-FTIR), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS) were implemented to conduct surface chemistry and morphology characterizations of the particles. The combination of these techniques allowed a comprehensive understanding of surface morphology and chemistry alterations due to the weathering degradation and SGF incubation. Results showed nitrogen and carbonyl groups formed on weathered particles, and seawater-weathered particles showed a more pronounced weathering degree. After SGF incubation, stronger nitrogen and amide groups were detected on the surface of weathered particles, and more organic matter was attached. Two cell lines that are widely used for the evaluation of microplastic cytotoxicity were used, RAW264.7 macrophage and Caco-2 intestine epithelial cells. Results showed weathered and SGF-treated particles enhanced macrophage metabolic activity, viability, and pro-inflammatory effects compared to pristine particles. Elevated reactive oxygen species (ROS) generation was detected for all particle groups. Weathered particles caused higher cytotoxicity effects on Caco-2 cells and damaged tight junction integrity. The organic matter formation from the SGF incubation protected tight junction integrity and reduced cytotoxicity. These findings highlight the importance of taking both environmental and physiological factors into account for a more comprehensive assessment of microplastic toxicity.
Environmental significanceStudying weathered microplastics and physiologically representative microplastics holds crucial environmental importance, particularly amidst the growing concern over the pervasive threats that microplastics pose to both environmental ecosystems and human health. Our research addresses significant knowledge gaps by integrating weathering and simulated physiological digestion environments to assess the impact of these particles on various cell lines. The findings underscore the imperative of incorporating weathering and biological processes for a comprehensive understanding of microplastics' cytotoxicity and implications for public health risk assessment. |
1. Introduction
The pervasive issue of micro and nanoplastic pollution has emerged as a rising global concern, with complex implications spanning environmental, ecological, and public health domains. These microplastic particles (<5 mm in diameter1), frequently undergo extensive environmental weathering and biological exposure, leading to substantial alterations in particle physical and chemical properties,2 resulting in significant variations in their adsorption and uptake,3,4 distribution and fate,5,6 and influences on animal behaviors.7,8 However, previous research on microplastic cytotoxicity assessment has predominantly focused on using pristine particles, with only a handful of studies utilizing artificially weathered microplastics,9–12 and fewer incorporating biological processes.13–15 This underscores the critical need for further research to bridge the knowledge gap and enhance our understanding of the combined impacts on microplastics resulting from both environmental and biological factors, which are essential for conducting accurate public health risk assessments related to microplastics.The process of aging or weathering plastics in natural environments involves complex interactions of physical, chemical, and biological degradation.1,16 The interplay of these natural forces breaks down larger plastics into microplastics, altering their physicochemical properties. Weathering mechanisms are typically classified into hydrolysis, photooxidation, chemical oxidation, microbial degradation, organic matter adsorption, and others.17 Each degradation mechanism has distinct effects on particle properties, resulting in particle heterogeneity and complex variations in their impact on toxicological assessments.18 Consequently, research utilizing laboratory-controlled artificially weathered microplastics for more representative toxicology evaluations is on the rise. To name a few, Englert et al. compared the hepatotoxicity in human liver cells between pristine and UV-weathered micro – and nano polystyrene (PS) particles. It was found that UV-weathered PS particles induced cytotoxicity after 24 hours of incubation at a low concentration of 0.1 μg ml−1, but other cellular stress responses were only detected for cells exposed to particles at higher concentrations. It was also found that micro-sized (3 μm) PS particles caused more pronounced cytotoxicity than nano-sized PS particles (25 and 100 nm).11 Recently Koner et al. evaluated the cytotoxicity of naturally aged polypropylene (PP) particles and non-aged PP particles on RAW264.7 murine macrophage cells. Decreased cell viability was found for both types of PP particles, although aged-PP particles induced less cellular oxidative stress, membrane damage, and apoptosis compared with non-aged PP particles.19 While numerous studies have noted increased cytotoxicity and cellular stress resulting from weathered microplastics,10,20,21 a few have reported similar findings indicating that weathered microplastics may not necessarily exhibit more adverse effects than pristine microplastics.22,23 The contrasting cellular impacts are likely due to the variations in experimental setups, whereas cellular responses to microplastics are dependent on many key variables, such as microplastic particle size,24,25 concentration,26–28 and type.29,30 Additionally, the absence of standardized laboratory weathering methods reduces the comparability of research findings.17 Therefore, further experiments are required to address existing research gaps, incorporating comprehensive assessments of key variables to enhance our understanding and evaluation of the toxicity of weathered microplastics.
In addition, the physiological environment can significantly alter particle properties, thereby affecting their interactions with cells. Ingestion is one of the primary routes for microplastics to enter human bodies,31 and several studies have evaluated cellular responses to microplastics treated with simulated digestion fluids. Paul et al. studied Caco-2 cellular responses to four types of nanoplastics that were processed by simulated saliva, gastric, and intestinal fluids. Decreased cellular interactions and increased transportation across cellular barriers were found for digested particles. It concluded that the organic matter formed on the particles during the digestion process enhanced the translocation through the Caco-2 epithelium.32 Similarly, Liu et al.33 observed corona formation on polystyrene microplastic after incubation in simulated digestion fluids, and reduced cytotoxicity and transport function disorder, but increased pro-inflammatory effects of Caco-2 cells. Wang et al. found enhanced hepatic cytotoxicity of polystyrene nanoparticles after chemically transformed by simulated gastric fluid.34 Studies also investigated other physiological responses to micro- and nanoplastic treated with simulated digestion fluids, for example, lipid digestion,35,36 and interaction with microbiota.37,38 However, all these studies utilized pristine particles, neglecting the potential effects of particle aging and chemical transformations in digestion fluids. Given that prior research has highlighted the impact of protein corona on cellular behaviors, it is crucial to integrate both weathering and simulated digestion processes into studies, as environmental weathering can substantially modify protein corona composition and subsequently alter cellular responses.39,40 Despite this importance, studies combining environmental weathering and simulated in vivo digestion processes remain relatively scarce.
Herein, our study aims to elucidate the cumulative impact of weathering and physiological digestion on microplastic toxicity. Specifically, 10 μm polystyrene (PS) microplastic particles underwent a simulated weathering process under UV exposure in deionized (DI) water and seawater. Subsequently, pristine and weathered particles were subjected to simulated gastric fluid (SGF) to partially replicate the digestive environment. The interplay between weathering and physiological digestion is a complex process in real-world scenarios, where ingested particles are rarely pristine.41 In this study, we aim to simulate real-life conditions by first weathering the particles and then exposing them to SGF. While weathering and digestion can occur concurrently in nature, isolating these factors in our study allows us to examine the physicochemical changes that occur at each stage and assess their distinct impacts on cellular responses. To understand the physicochemical alterations at each stage, particle surface chemistry and physical properties were analyzed using multiple techniques including SEM-EDS imaging analysis, FTIR and Raman spectroscopy, and XPS analysis. Additionally, polystyrene particles are widely used as a model for studying microplastic cytotoxicity across a broad size range, from various sources, with different aging protocols,42–45 and it is one of the most abundant microplastics in nature and found in human tissue.46 We decided to use 10-micron polystyrene particles as a starting point for the weathering, which allowed us to investigate the particle properties changes more in detail. Microplastics within this size range have been found abundant in tissue but cytotoxicity is less explored.47 This selection helps provide continuity with previous studies while addressing unexplored aspects of particle transformations to better predict micro- and nanoplastic cytotoxicities. To evaluate the biological impacts, the cellular responses of RAW264.7 and Caco-2 cells were assessed after incubation with these particles for 24 hours. These cell lines were chosen for their established utility in assessing diverse properties of PS microplastic particles.48–54 Cell viability, reactive oxygen species (ROS) generation, pro-inflammatory cytokine TNFα secretion, tight junction integrity, and particle uptake were evaluated at two common concentrations found in the literature.55–58 Overall, the findings from this study contribute to filling the existing knowledge gap by providing a detailed characterization of PS particles after weathering and SGF treatment, and by evaluating their impacts on cellular responses. These insights underscore the importance of conducting comprehensive studies using representative environmental and physiological models for accurate microplastic toxicity assessments.
2. Experimental
2.1. Microplastic particle preparation
PS particles with a diameter of 9.5–11.5 μm (Cospheric, Santa Barbara, CA) were incubated in DI water (ACS reagent grade, Fisher Scientific, USA) and surface seawater (Narragansett Bay, RI, USA). Surface seawater was first passed through a 63 μm stainless steel filter into a glass jar. It was then treated with 30% stabilized H2O2 in the dark for 48 hours to digest organic matter, such as bacteria, to eliminate potential biological contaminants and microbial and particle interactions.59 Both waters were filtered using a 0.22 μm membrane at the final step. Subsequently, 150 mg of PS particles were added to glass beakers containing 300 mL of water samples. The beakers were then exposed to UV-A of 340 nm wavelength (maximum irradiance of 0.89 W m−2 nm−1) for 500 hours in a UV chamber (Q-Lab, Westlake, OH, USA) (Fig. S1, ESI†), followed similar weathering protocols with a few previous studies.60,612.2. SEM-EDS analysis
Particle surface morphology and elemental analysis were investigated using a field emission scanning electron microscope (Zeiss Sigma VP FE-SEM, Oberkochen, Germany). Initially, PS particles were centrifuged at 6000 rpm for 15 minutes. The supernatant was removed and 2 μL of the particle-containing solution was pipetted onto a silicon wafer. SEM-EDS elemental mapping was performed with the PS particles as-is under 10 kV acceleration voltage. For better morphology imaging, the samples were subsequently coated with a 2 nm-thick gold layer using a Cressington 108 sputter coater (Cressington, Watford, UK). Uncoated particles were used for the EDS analysis.2.3. ATR-FTIR analysis
The characterization of surface chemical functional groups was investigated using an FTIR spectrophotometer equipped with ATR (Shimadzu IRTracer-100, Kyoto, Japan). Particles were centrifuged at 6000 rpm for 15 minutes and the supernatant solution was removed. Subsequently, 2 μL of the solution was placed onto the ATR prism and left to dry completely. The final spectrum was collected at an integration time of 120 seconds, covering a wavenumber range from 400 cm−1 to 4000 cm−1, and plotted as % transmission.2.4. Micro Raman spectroscopic analysis
Raman spectroscopy was also performed to investigate the surface chemical functional groups. We used a micro-Raman spectrometer (WITec Alpha 300R+, Ulm, Germany) equipped with a 100X objective with a numerical aperture of 0.9, a 532 nm laser source set at 10 mW power, and a CCD detector with 600 lines per cm grating. 1 μL of the particle solution was pipetted onto a silicon wafer and left to dry completely. An integration time of 5 seconds and 100 accumulations were employed for the acquisition of a single spectrum. The spectra were processed using WITec Control Five software (version 5). The final spectrum wavenumber range was 300 cm−1 to 3600 cm−1.2.5. XPS analysis
Particle surface elemental composition was analyzed by XPS. The K-Alpha system from Thermo Fisher Scientific with an Al Kα source was used. Samples were cast onto silicon wafers and left to dry in a desiccator completely. The investigation of elemental composition was conducted using the survey scan function.2.6. SGF particle incubation
SGF solution is commonly utilized in biological studies to replicate in vivo gastric digestive conditions.62–64 To prepare the SGF, 0.2% w/v NaCl and 0.7% v/v HCl were added to ultrapure water and adjusted to achieve a final pH near 2.5. 10 mg of porcine gastric mucosal pepsin (P7012, Sigma Aldrich, St. Louis, Missouri, USA) was then added to 10 mL of the buffer solution.62,65 The solution was thoroughly mixed using a vortex mixer and filtered through a 0.22 μm membrane. For the particle incubation, 1 mL of the prepared SGF was pipetted into centrifuge tubes containing the pristine PS particles, DI water-weathered PS particles and seawater-weathered PS particles. The particles were incubated at 37 °C for four hours to simulate the average transit time of food in the stomach.66 Following incubation, the particles were centrifuged, rinsed with PBS, and subsequently resuspended in cell culture medium to prepare for cellular analysis.2.7. Cellular viability analysis
RAW 264.7 cells (ATCC number TIB-71) were cultured in T75 flasks using DMEM high glucose medium containing 10% heat-inactivated fetal bovine serum (FBS) and maintained at 37 °C in a humidified atmosphere (5% CO2, 95% air). The cells were passaged to passages 5 or 6 (P5 or P6) for all experiments. Caco-2 cells (ATCC HTB-37) were cultured in the same medium supplemented with penicillin–streptomycin and reached 85% confluency for the experiments. The Cell Counting Kit-8 (CCK-8) assay kit (96990, Sigma Aldrich, St. Louis, Missouri, USA) was utilized to assess cell viability in both cell lines. Cells were seeded in 96-well plates at a density of 1 × 104 cells per mL. Following the initial 24 hour incubation, 100 μL of particle-containing medium was added to each well. After 24 hour incubation, the cells were thoroughly washed with phosphate-buffered saline (PBS 1×) and then replenished with 100 μL of fresh medium. All subsequent test procedures were conducted following the manufacturer's protocol. The final absorbance was measured using a microplate reader (SpectraMax M2, Molecular Devices, San Jose, CA, USA).2.8. Intracellular ROS analysis
The Invitrogen total ROS assay kit (88-5930-74, Thermo Fisher, Waltham, Massachusetts, USA) was used to measure reactive oxygen species (ROS) generation of RAW 264.7 cells after 24 hour incubation with particles. Cells were initially seeded in 96-well plates at a density of 1 × 104 cells per ml. Subsequently, the cells were stained with the ROS test reagent according to the manufacturer's protocol, and then cells were thoroughly washed with PBS, and particles were added and incubated for 24 hours. Fluorescent images of 500 μm × 500 μm area of each well were captured using Opera Phenix high content screening system (Perkin Elmer, Waltham, Massachusetts, USA) in the FITC channel. Fluorescent intensity was calculated using ImageJ.2.9. Pro-inflammatory cytokine TNFα analysis
Standard mouse TNFα (tumor necrosis factor α) and human TNFα ELISA kits (BioLegend, San Diego, CA, USA) were used to quantify the secretion of TNFα for RAW264.7 and Caco-2 cells. Cells were seeded in 96-well plates at a density of 1 × 104 cells per ml. Subsequently, PS particles were added to the wells. Following a 24 hour incubation, 50 μL of cell culture supernatant was harvested for cytokine level determination. All procedures followed the manufacturer's protocol.2.10. Caco-2 tight junction integrity and particle uptake analysis
Immunofluorescent analysis of the Caco-2 tight junction was done using ZO-1 antibody (green) (61-7300, Thermo Fisher Scientific). Cells were fixed with 4% paraformaldehyde for 15 minutes and permeabilized in 0.1% Triton X-100 in PBS for 10 minutes, followed by blocking with 1% BSA. Then, cells were incubated with Goat anti-Rabbit IgG (heavy chain), Alexa Fluor 488 conjugate (#A27034, Thermo Fisher Scientific). Cell nuclei were counter-stained with DAPI (D1306, Thermo Fisher Scientific) afterward. The Opera Phenix high-content screening system was utilized for imaging with a 63× water objective.2.11. Statistical analysis
OriginLab (version 2024, Northampton, MA, USA) was used to plot spectrum graphs. GraphPad Prism software (version 6.0; La Jolla, CA, USA) was utilized for creating graphs and conducting statistical analyses. Each experimental group consisted of three replicates. Data are presented as means ± standard error of the mean (S.E.M). Two-way ANOVA tests are performed for data analysis. Significance levels were denoted as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.3. Results
3.1. Physicochemical characterization of PS particles
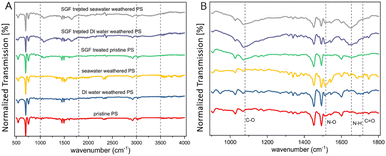
Particle surface chemical functional groups were investigated using ATR-FTIR and Raman spectroscopy techniques. Pristine particles and weathered particles exhibited similar FTIR spectra looking at the full spectrum range (Fig. 1A). However in the wavenumber range between 1000 cm−1 and 1800 cm−1 (Fig. 1B), a broadband peak around 1094 cm−1, associated with the C–O carboxyl group can be seen for SGF-treated particles,67 and a minor peak at 1716 cm−1, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl group can be seen for DI water-weathered and seawater-weathered particles.68 The formation of these chemical functional groups has been frequently reported as indicative of the chemical transformations that polymers undergo during photooxidation weathering.69–71 The minimal change observed in the IR spectrum for C–O carboxyl groups, coupled with a significant increase in C
O carbonyl group can be seen for DI water-weathered and seawater-weathered particles.68 The formation of these chemical functional groups has been frequently reported as indicative of the chemical transformations that polymers undergo during photooxidation weathering.69–71 The minimal change observed in the IR spectrum for C–O carboxyl groups, coupled with a significant increase in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups for the weathered particles, suggests a rapid transition from C–O to C
O groups for the weathered particles, suggests a rapid transition from C–O to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. This may indicate that in aqueous weathering conditions, unstable C–O groups quickly oxidize to C
O. This may indicate that in aqueous weathering conditions, unstable C–O groups quickly oxidize to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, progressing to an advanced oxidation stage of polystyrene. This result is consistent with previous studies on polystyrene weathered in aqueous conditions,72–75 which differs from observations of polystyrene weathered in dry conditions where C–O formation is often more pronounced.76,77 Additionally, a distinct peak near 1530 cm−1 can be detected for particles of seawater-weathered, SGF-treated DI water-weathered, and SGF-treated seawater-weathered. This peak is indicative of the formation of N–O stretching.78 A strong broad IR band within the range of 1600–1700 cm−1 can be seen for SGF-particles, this IR peak range is identified as primary amine scissors group N–H2.79 The formation of these nitrogen-containing groups suggests the attachment of organic matter to the particles. Moreover, the SGF-treated seawater-weathered particles exhibited strong broadband in the FTIR and Raman spectra within the wavenumber range of 2500–3300 cm−1, and the seawater-weathered particles also showed a very weak indication of this band. This signifies the presence of O–H stretching (Fig. S2, ESI†).80
O, progressing to an advanced oxidation stage of polystyrene. This result is consistent with previous studies on polystyrene weathered in aqueous conditions,72–75 which differs from observations of polystyrene weathered in dry conditions where C–O formation is often more pronounced.76,77 Additionally, a distinct peak near 1530 cm−1 can be detected for particles of seawater-weathered, SGF-treated DI water-weathered, and SGF-treated seawater-weathered. This peak is indicative of the formation of N–O stretching.78 A strong broad IR band within the range of 1600–1700 cm−1 can be seen for SGF-particles, this IR peak range is identified as primary amine scissors group N–H2.79 The formation of these nitrogen-containing groups suggests the attachment of organic matter to the particles. Moreover, the SGF-treated seawater-weathered particles exhibited strong broadband in the FTIR and Raman spectra within the wavenumber range of 2500–3300 cm−1, and the seawater-weathered particles also showed a very weak indication of this band. This signifies the presence of O–H stretching (Fig. S2, ESI†).80
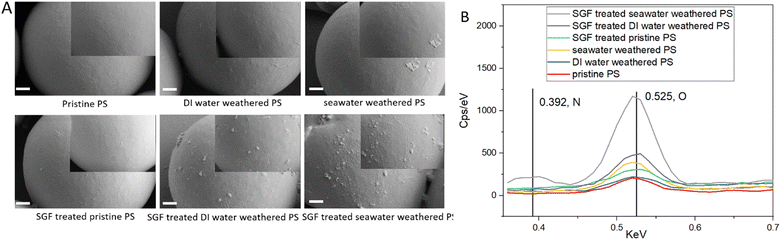
The formation of oxygen-bonding groups and nitrogen-bonding groups indicates the oxidation and attachment of organic matter on the particle surface. Therefore, the surface morphology and elemental compositions of PS particles were further investigated using SEM and EDS mapping analysis. Fig. 2 shows the surface morphology of different types of particles and the atomic % of oxygen and nitrogen composition on the particle surface. The pristine PS particle has the lowest amount of oxygen and nitrogen detected on the surface. Also, a smooth surface can be seen. Both weathered particles showed increased oxygen and nitrogen on the surface, and seawater-weathered particles showed much higher oxygen than DI-water-weathered particles. The seawater-weathered particles showed some organic matter attachment to the particle surface which can be seen better in the SEM images at high magnification on gold-coated particles (Fig. S3†). The SGF treatment strongly increased the oxygen and nitrogen composition of DI water-weathered particles and seawater-weathered particles, but not so much for the pristine particles. Both DI water-weathered and seawater-weathered particles showed clear attachment of organic matters and slight deformation (Fig. 2 and S3†). In a recent study done by Jiménez-Arroyo et al., organic matter was also seen on the polylactic acid (PLA) particles after the simulated gastric digestion phase.81 In addition, the more abundant organic matter formed on the weathered particles is in agreement with previous studies.82,83
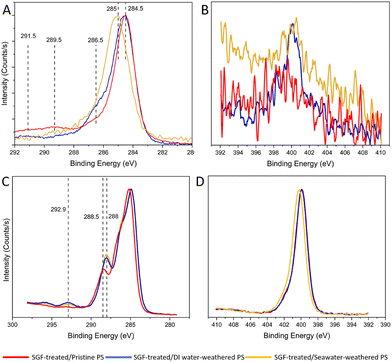
XPS analysis was done to further validate the degree of surface oxidation and elemental composition of the particles. Characteristic signals of polystyrene, specifically the C–C and C–H bonds are shown at a binding energy of 285 eV for pristine PS and 284.5 eV for weathered PS particles. The C–O bond, indicative of either ether or alcohol groups, was detected in weathered particles at 286.5 eV but was absent in pristine particles.4 Seawater-weathered particles exhibited a more pronounced peak at 289.5 eV, representative of carbon from the –O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O– group (Fig. 3A).84 In the N1s region (Fig. 3B), the pristine PS showed little surface nitrogen, while both DI water- and seawater-weathered particles exhibited prominent nitrogen peaks at 400 eV.85 In addition, the C1s spectra for SGF-treated particles exhibited new peaks at 288 eV or 288.5 eV (Fig. 3C), which are indicative of the C
C–O– group (Fig. 3A).84 In the N1s region (Fig. 3B), the pristine PS showed little surface nitrogen, while both DI water- and seawater-weathered particles exhibited prominent nitrogen peaks at 400 eV.85 In addition, the C1s spectra for SGF-treated particles exhibited new peaks at 288 eV or 288.5 eV (Fig. 3C), which are indicative of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups associated with acyl groups in proteins.86 Furthermore, all SGF-treated particles exhibited strong nitrogen peaks in the N1s region (Fig. 3D). Moreover, the XPS survey revealed that PS particles weathered in seawater also contained trace amounts of magnesium and sodium, along with other elements typical of seawater exposure (Fig. S4†). In summary, the oxygen-containing groups on the weathered particles verified the oxidation from the weathering effects, and the increased oxygen and nitrogen-containing groups further confirmed the existence of organic matter on the surface of the particles.
O groups associated with acyl groups in proteins.86 Furthermore, all SGF-treated particles exhibited strong nitrogen peaks in the N1s region (Fig. 3D). Moreover, the XPS survey revealed that PS particles weathered in seawater also contained trace amounts of magnesium and sodium, along with other elements typical of seawater exposure (Fig. S4†). In summary, the oxygen-containing groups on the weathered particles verified the oxidation from the weathering effects, and the increased oxygen and nitrogen-containing groups further confirmed the existence of organic matter on the surface of the particles.
3.2. Cell viability analysis of RAW 264.7
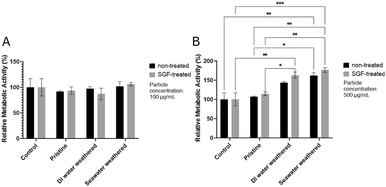
Macrophages are a critical component of the innate immune system, playing a central role in regulating cellular responses to microplastic exposure.87,88 To study microplastic cytotoxicity to macrophages, cells were incubated with different samples for 24 hours at two concentrations (100 and 500 μg ml−1), to provide comparisons across studies as well as represent real-world accumulation effects.89Cell viability was evaluated using the CCK-8 kit. The CCK-8 assay relies on the reduction of a water-soluble tetrazolium salt (WST-8) by cellular dehydrogenases in viable cells to produce an orange-colored formazan dye. The amount of formazan dye generated is directly proportional to the number of living cells. This method provides a measure of cellular metabolic activity, and an indirect representation of cell viability as only metabolically active cells are capable of reducing WST-8. Therefore, increased formazan production can indicate enhanced cellular viability or metabolic activity, making CCK-8 an effective tool for assessing the cytotoxicity of microplastics. It has been widely used to evaluate the cytotoxicity of microplastics.90–92
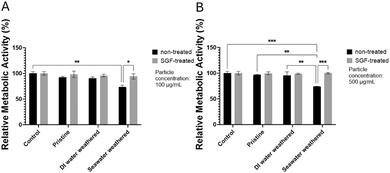
Fig. 4A illustrates that at the lower concentration, cell metabolic activity (viability) remains consistent across all samples. Neither the weathering nor SGF treatments had discernibly different impacts on the viability compared to pristine particles. None of the particle types caused any significant adverse impacts on the viability. This result is in agreement with the findings in the literature.93 Interestingly, at the higher concentration (Fig. 4B), a noticeable increase in macrophage cell metabolic activity (viability) was observed after a 24 hour incubation with PS particles. Specifically, weathered PS particles resulted in significantly higher viability compared to pristine particles, with the highest viability seen in seawater-weathered and SGF-treated seawater-weathered particles. Although, the SGF treatment further slightly enhanced cell viability in each group, the differences are not prominent. The increased cell metabolic activity at this concentration level (500 μg ml−1) after 24 hours of exposure is similar to some of the previous findings, possibly due to the short exposure time.19,94 A recent study also showed increased cell viability for THP-1 macrophages after given PS particles (1 μm) that were processed with in vitro simulated digestion juice.95 Although many of the studies reported increased cytotoxicity or reduced viability of cells to PS particles even after short exposure time, typically 24 hours, the cytotoxicity of PS particles is highly dependent on particle size, particle shape, surface functional groups, and cell types. Additionally, exposure to microplastics has been found to increase cell proliferation to promote pro-inflammatory responses.96–98
3.3. ROS generation of RAW264.7
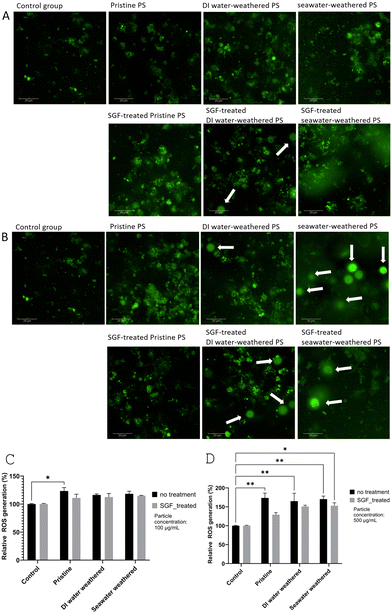
Reactive oxygen species (ROS) are chemically reactive molecules containing oxygen, including a variety of substances such as peroxides, superoxide, and hydroxyl radicals. Typically, ROS is a natural byproduct of the normal metabolism of oxygen, yet under certain conditions, ROS levels can increase dramatically, which can result in significant damage to cell structures.99 Previous studies suggest that the presence of microplastics can trigger the overproduction of ROS, leading to cellular and tissue damage.100–103ROS generation at the 24 hour endpoint was evaluated for both concentrations using the high-content screening imaging technique. It was observed weathered particles and SGF-treated particles are stained due to the surface oxidation effect and cause inaccuracy in the fluorescent intensity analysis104 (Fig. S5†). Therefore, the intensity of particles was subtracted using ImageJ, and absolute fluorescent intensity for cellular ROS was measured. The final cellular ROS from each test group was the average of nine randomly selected areas for the three replicas of each test group, three areas in each well. Fig. 5A and B are example images of one area showing FITC fluorescent intensity for each type of particle group. Fig. 5B shows the weathered particles, and SGF-treated weathered particles having fluorescent on the surface (pointed by arrows).
Fig. 5C shows the quantified relative cellular ROS production after being treated with particles at 100 μg ml−1. Elevated ROS generation is observed for all groups compared to the control group. Both DI water-weathered and seawater-weathered groups showed a significant increase in ROS generation compared to the control group. Cells treated with pristine particles did not show significantly higher ROS than the control group. At the same time, no significant difference was found comparing the weathered particles with pristine particles, although a slight increase can be seen. The effect of SGF treatment was also not obvious. At the 500 μg ml−1 concentration level, all particle groups showed higher ROS generation than the control group, which is consistent with the observations from the lower concentration level. Similarly, only a small increase in ROS generation can be seen for the weathered particles compared to the pristine particles. Though the difference is not significant, the increase in ROS generation is consistent with the previous findings that compared between weathered PS particles and pristine PS particles.104,105 The increase in ROS is also consistent with the increased metabolic activity seen in the weathered groups but not in the SGF-treated groups. Interestingly, a slight decrease in ROS generation can be seen for the SGF-treated particles, compared to the non-SGF-treated particles, this is likely due to the existence of the organic matter that acted as a protective shield for the cells against the particles.106
3.4. Pro-inflammatory cytokine TNFα analysis of RAW264.7
Microplastic particles have been found to induce pro-inflammatory responses in macrophages. Specifically, it has been found that PS microplastic particles can activate macrophages and affect the secretion of inflammation biomarkers.107,108 TNFα is considered a representative pro-inflammatory biomarker for macrophages due to its pivotal role in initiating and amplifying inflammatory responses. TNF-α production reflects the activation and inflammatory state of these cells. Therefore, it is a valuable biomarker to assess the pro-inflammatory effects of PS microplastic particles on RAW264.7 cells.To study the pro-inflammatory responses of RAW264.7 to the different types of PS particles used in this study, TNFα secretion after 24 hours of exposure to PS particles was evaluated. The absolute concentration level was derived from the standard curve that is developed based on the manufacturer's protocol (Fig. S6†). A significant elevation (P < 0.0001) was observed for all particle types compared with the control group, despite the particle concentrations or types (Fig. 6). The TNFα secretion of the weathered PS particles did not appear to significantly differ from that of the pristine groups. This phenomenon was also seen in a previous study where pristine PS particles (2 μm) were found to induce a higher level of TNFα secretion than the artificially weathered particles.107 To evaluate the effects of SGF treatment, there was a significant decrease in TNFα secretion of the SGF-treated seawater-weathered particles compared with the seawater-weathered particles, a similar trend can be seen for the SGF-treated pristine particles compared with pristine particles. The reduced secretion of TNFα for the SGF-treated groups is likely due to the attachment of organic matter on the surface of the particles, as the organic matter can act as a protein corona layer, which has been used to mitigate nanoparticle cytotoxicity.109 Additionally, several studies have reported protein corona or bio-corona formed on the surface of micro- and nanoparticles and have mitigated inflammatory responses of macrophages.110–112
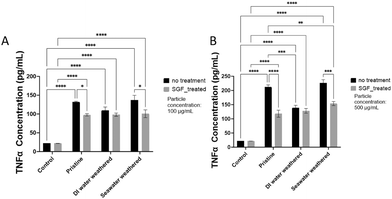 | ||
| Fig. 6 RAW264.7 TNFα secretion at 24 hour endpoint. A. TNFα secretion for low-concentration particle groups (100 μg ml−1); B. TNFα secretion for high-concentration particle groups (500 μg ml−1). | ||
3.5. Cell viability analysis of Caco-2
Caco-2 cells, derived from human colon carcinoma, have become a valuable tool for investigating various aspects of intestinal physiology and pathophysiology.113 Unsurprisingly, these cells have also been extensively utilized to study the intestinal cellular effects (e.g., inflammation, oxidative stress, and alterations in barrier function) of microplastics over the past years.114–116 In addition, the simulation of a more realistic digestive environment is also crucial to the study of microplastic impact on Caco-2 cells, as factors such as pH levels and protein enzymes can potentially alter the properties of microplastics and affect intestinal cellular responses.14The evaluation of Caco2 cellular viability was performed to understand the impact of exposure to the different types of PS particles. It was observed that at both concentrations, seawater-weathered particles exhibited reduced cellular metabolic activity and cell viability compared to other types of particles (Fig. 7). This decrease in viability for the seawater group, relative to both pristine and DI water-weathered groups, may be attributed to the deformation of seawater particles, coupled with the stronger surface oxidation effects.117,118 Interestingly, the SGF treatments reversed this impact, as a significantly increased metabolic activity and viability was seen in the SGF-treated seawater-weathered group than the seawater-weathered group. This is also in agreement with other studies that little cytotoxicity effects from microplastic particles to Caco-2 cells in most cases,119–121 and protein corona can further act as the protective mechanism for the particles and reduce cytotoxicity to Caco-2 cells.122
 | ||
| Fig. 7 Caco2 cell metabolic activity (viability) after incubation with particles for 24 hours. Relative metabolic activity for A. low and B. high concentrations. | ||
3.6. Pro-inflammatory cytokine TNFα analysis of Caco-2
TNFα is also often used as a biomarker to evaluate the inflammatory states of Caco-2 cells after exposure to the different types of PS particles.123,124 In this study, TNFα secretion of Caco-2 cells after 24 hours of incubation with particles were measured. A slightly increased TNFα secretion was detected for all particle groups compared to the control group at both concentrations, however, the difference is not significant (Fig. 8). The variation of TNFα secretion at the two concentrations is small. In addition, the reduction of TNFα secretion for the SGF-treated groups is also negligible. The differences in the pro-inflammatory weathered particles and SGF-treated particles on Caco-2 cells are not detectable at the concentrations tested in this study. Previous studies have reported that the effects of pristine particles do not have significant effects on Caco-2 cells.122,125 Similarly, TNFα secretion of Caco-2 cells treated pristine and UV-oxidization have also been found negligible.126 However, it has been found at a much higher concentration (1 mg ml−1), the cytotoxicity of microplastic particles to Caco-2 cells is significantly increased,127 and longer exposure to microplastic particles has also been observed to increase cytotoxicity and alter inflammatory responses.128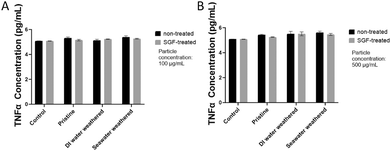 | ||
| Fig. 8 Caco2 TNFα secretion after 24 hours of incubation with particles. A and B. TNFα secretion for groups of low (100 μg ml−1) and high concentration (500 μg ml−1) respectively. | ||
3.7. Tight junction integrity and particle uptake analysis of Caco-2
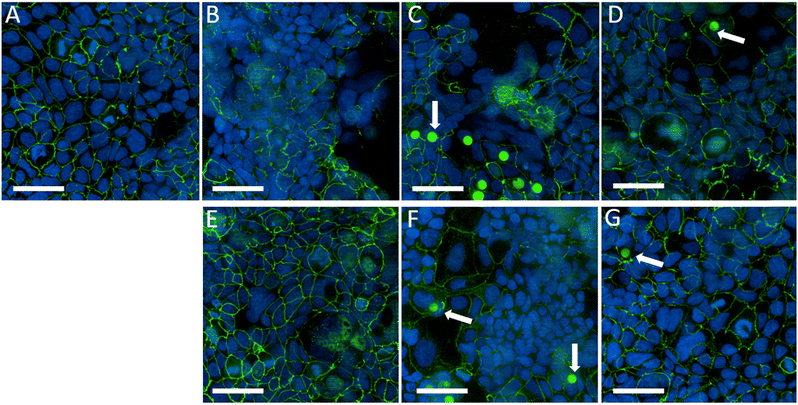
Caco-2 cells often serve as an established in vitro model of the intestinal epithelium, allowing researchers to simulate and understand how microplastic particles might impact gut barrier function. The integrity of tight junctions in Caco-2 cells is critical for maintaining the selective permeability of the intestinal barrier, regulating the transport of nutrients, ions, and other molecules while preventing the entry of pathogens and toxins into the bloodstream.In immunofluorescent imaging analysis, the damages of tight junction integrity could manifest as gaps, discontinuities, or irregularities in the fluorescent staining pattern along the cell borders (green). Such changes suggest a loss of tight junction integrity, which can have implications for barrier function and cell–cell interactions. Increased permeability associated with damaged tight junctions may allow microplastic particles or other substances to cross the intestinal barrier more readily, potentially triggering inflammatory responses or other diseases.129 In addition, counter-staining with DAPI can help visualize nuclei morphology and fragmented nuclei often indicate cell death, which can be used as validation for the cytotoxicity effects.
Therefore in this study, we evaluated the tight junction integrity of Caco-2 cells after 24 hours of incubation of the different types of PS particles to evaluate the effects on barrier integrity from the weathering and SGF-treatment. Fig. 9 shows random fields of view acquired for each treatment condition at the height of 2 μm from the bottom of the well to avoid operator bias. Fig. 9B–D all showed disruptions of tight junctions compared to the control group in Fig. 9A, indicating damage to barrier integrity after the cells are incubated with pristine, DI water-weathered, and seawater-weathered particles for 24 hours. So far there have been few studies directly investigating barrier function to weathered microplastics, although it has been found that carboxylated PS particles induced barrier function disruption, which can explain the damages we see with the weathered particles, as the carboxylic functional groups formed on the surface of the particles.130
The SGF-treated groups all showed intact tight junction integrity, especially for SGF-treated pristine particles and SGF-treated seawater-weathered particles. Recently it has been reported that microplastic particles undergo a simulated digestion process that mitigates barrier disruption, which aligns with our findings.122
For particle uptake analysis, due to the stronger surface oxidation effects, weathered particles and SGF-treated weathered particles were stained with Alexa Fluor 488 and showed green fluorescence (pointed by arrows). However, the presence of the particles does not sufficiently indicate particles are uptaken by the cells, even though the cells were washed thoroughly during the staining process, and particles can be seen in the 3D images from Z-stack imaging (Fig. S7†). Microplastic particle uptake by Caco-2 cells has been studied previously, and it has been found that the uptake of particles is size-dependent, and 10 μm PS particles have much less uptake efficiency than the smaller-sized particles.120 Therefore, the remaining particles shown in the images are likely due to the increased permeability of the barrier function of Caco-2 cells, which has been seen in previous studies. Further studies using permeability models, such as quantitative TEER (trans-epithelial electrical resistance) measurement of gut-on-chip and transwell models are suggested to validate the findings.
4. Discussions
In recent years, the study of micro- and nano-plastic cytotoxicity has gained significant attention, though findings vary due to factors like particle type, size, shape, and different cell lines used. Dose-dependent cellular responses to microplastics have also been reported.131,132 Despite numerous studies, a critical gap remains in exploring environmentally and physiologically relevant particles. The effects of weathering and physiological interactions on particle properties and their influence on cellular responses have been largely overlooked, compromising the accuracy of cytotoxicity assessments. To address this gap, we designed a study investigating the effects of environmental weathering and simulated gastric fluid treatment on microplastics, focusing on their physiochemical changes and cellular impacts.In this study, we employed a laboratory-controlled weathering protocol to artificially age PS particles. To mimic real-world conditions and explore different weathering scenarios, PS particles were incubated in various water sources (DI water and seawater) in a UV chamber for 500 hours. Following this, both pristine and weathered particles underwent SGF treatment to simulate the early stages of digestion upon ingestion. The particles were then diluted into two concentration groups: 100 μg mL−1, aligning with concentrations commonly used in microplastic cytotoxicity studies,133–135 and 500 μg mL−1, chosen to represent an extreme condition from the experimental aspect to provide more insights at a high concentration range, and reflect the potential accumulation effects suggested by estimates of adult microplastic consumption.89,136 Our primary goals were to investigate the physiochemical changes in particles weathered under different conditions and evaluate cellular responses of RAW264.7 and Caco-2 cells to both pristine and weathered PS particles, with and without SGF treatment.
Initially, particles subjected to weathering in various water sources exhibited disparate degrees of degradation. Specifically, particles incubated in seawater displayed more pronounced weathering effects compared to those in DI water, as evidenced by heightened surface oxidation observed through FTIR, SEM-EDS, and XPS analyses. Following SGF treatment, a considerable accumulation of organic matter was observed on the particle surfaces, accompanied by noticeable deformations in the weathered particles. Weathered microplastics undergo a series of chemical transformations, often characterized by the formation of carboxyl (COOH) and carbonyl groups on their surface.137 These alterations are indicative of the initial stages of photooxidative degradation in the environment. When exposed to environmental stressors such as sunlight, microplastics, particularly PS particles, undergo photooxidation, leading to the incorporation of oxygen-containing functional groups on their surfaces as shown on the FTIR spectrum.138 This process can be accelerated in aqueous environments due to the presence of reactive oxygen species generated by the combination of UV radiation and water molecules.139 Seawater, with its higher salinity and diverse array of organic and inorganic compounds, provides an environment that can contribute to accelerated weathering of microplastics compared to DI water.140,141 Additionally, exposure to UV radiation in seawater enhances the photooxidation process, resulting in more pronounced weathering effects on PS particles.75,142 The observation of minimal changes in C–O bonds coupled with significant increases in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and overall oxidation in the IR spectrum underscores the necessity for comprehensive, time-resolved weathering studies. Such studies should compare results under various conditions, an approach that has been rarely implemented in existing literature.75,142
O groups and overall oxidation in the IR spectrum underscores the necessity for comprehensive, time-resolved weathering studies. Such studies should compare results under various conditions, an approach that has been rarely implemented in existing literature.75,142
Following incubation in SGF, PS particles exhibited a notable accumulation of organic matter on their surfaces. This phenomenon can be attributed to the adsorption of organic compounds present in the SGF onto the particle surfaces. Specifically, the pepsin used in this experiment interacts with the PS particles, leading to the deposition of organic material. Interestingly, pristine PS particles demonstrated comparatively lower levels of organic matter accumulation after SGF exposure, likely due to their smoother surface. In contrast, weathered PS particles, with their increased surface roughness and higher abundance of functional groups, provided more sites for organic matter attachment, and the potentially changed surface charge that can all contribute to the enhanced deposition.143 However, more comprehensive studies are needed to understand the binding mechanism between organic compounds for different types of microplastics, as it has been found that weathered particles do not always enhance the adsorption of organic compounds.144 Furthermore, the weakened physiochemical structural integrity from the weathering effects facilitates the enzymatic degradation process in the SGF acidic environment for the weathered particles, therefore, changes in particles are easily deformed in the weathered particles compared to the pristine particles.145,146 As a result, all these alterations in particle physiochemical properties can affect particle–cell interactions and lead to distinct cellular responses compared to pristine particles.
Our findings revealed notable variations in the cellular responses of RAW264.7 and Caco-2 cells to the different particles. Moreover, distinct effects were observed regarding the influence of weathering and SGF treatment, along with particle concentration on these cellular responses. For the RAW264.7 macrophages, we evaluated cellular viability (metabolic activity), ROS generation, and TNFα secretion at 24 hours endpoint incubation with particles. When the macrophages were given particles at lower concentration levels, there was no significant variation in cellular viability between the test groups and the control group. However, the effects of the particles became prominent at the higher concentration level. Overall, weathered particles significantly increased cellular metabolic activity, with seawater-weathered particles exhibiting the most pronounced effect. SGF treatment further amplified this increase in metabolic activity slightly. This phenomenon could be attributed to several factors. Firstly, macrophages possess innate mechanisms for phagocytosis and degradation of foreign particles, the presence of PS particles may have triggered the upregulation of cellular processes involved in particle clearance, leading to enhanced cell metabolic activity (viability).147,148 Moreover, the surface characteristics of the PS particles play a crucial role in determining their interaction with macrophages. Weathered particles, with their increased surface roughness and abundance of functional groups, may have facilitated more efficient recognition and engulfment by macrophages compared to pristine particles, consequently promoting macrophage metabolic activity.149,150 Additionally, the SGF treatment slightly increased macrophage metabolic activity, possibly by altering the surface properties of the particles and the formation of organic matter that could also mitigate cellular responses.151 It is noteworthy to discuss that previous studies reported increased cytotoxicity effects associated with microplastic exposure, it is essential to consider the discrepancies in particle size used. Previous studies predominantly focused on nanoplastics, where smaller particle sizes were found to induce higher cytotoxicity than larger particles.152 Furthermore, it has also been reported that PS particles have a higher chance to aggregate in salty aquatic environments, which further increases particle size and reduces contact with the cells.153 This discrepancy highlights the critical role of particle size in influencing cellular responses and emphasizes the need to include nanoplastics in future studies using the developed protocol for comparison. Additionally, the duration of incubation may also influence macrophage metabolic activity and viability dynamics. Increased metabolic activity might occur within the initial 24 hours of exposure, possibly due to the activation of cellular defense mechanisms. However, with longer incubation periods, cellular stresses may accumulate, leading to a subsequent decrease in metabolic activity (viability).154 The formation of organic matter on the particle surface has been shown to mitigate cytotoxicity, whether it takes the form of an eco-corona or a protein corona.155 Typically, these layers shield the particles with organic compounds, resulting in particle aggregation, alteration of particle surface charge, and ultimately reduced particle–cell interaction and uptake.156 This mechanism often leads to decreased cytotoxicity. The organic matter observed on the particle surfaces in this study is presumed to consist primarily of degraded polystyrene residues and, predominantly, pepsin from the SGF, which has been shown to mitigate cytotoxicity to macrophages.157 And the results showed that this protective behavior from pepsin attachment is also seen for Caco-2 cells.
Reactive oxygen species (ROS) are key indicators of cellular oxidative stress when cells encounter foreign particles and have been widely investigated as biomarkers for micro and nanoplastic-induced cellular responses.158,159 Our results align with previous findings showing that PS particles stimulate macrophages to produce elevated levels of ROS. Since ROS is a natural byproduct of cellular metabolism, particularly in active cells, additional oxidative stress markers are needed to determine whether the observed ROS increase is due to oxidative stress, heightened metabolic activity, or both. In our study, increased ROS generation was seen with both pristine and weathered particles, it may be due to a shift to anaerobic glycolysis in response to oxidative stress.160 The effects of SGF treatment varied across particle types, and the slight increase in cell metabolic activity was not correlated with higher ROS levels. This suggests that SGF may have reduced cellular oxidative stress, allowing cells to resume normal metabolic activity, or that other intriguing dynamics between macrophage ROS and metabolic pathways are at play that need further investigation.161,162 Additionally, ROS elevation was detected across the two concentrations utilized in this study, which is widely acknowledged to be highly dose-dependent.163 Although, we observed no significant variation in ROS generation between weathered and pristine particles, indicating the weathering effect alone under our experimental conditions did not necessarily increase cellular oxidative stress compared with pristine particles, which further signaling the importance to conduct comprehensive study and standardized protocol for micro and nanoplastic cytotoxicity evaluation.
Our findings showed all types of PS particles induced pro-inflammatory responses in macrophages. This heightened inflammatory state may be attributed to several factors, including the activation of innate immune pathways in response to the presence of foreign particles, and the physical interaction between PS particles and macrophages triggering inflammatory signaling cascades.164 Previous studies also showed enhanced inflammatory responses of macrophages to microplastic particles.165 Interestingly, the weathering effect on PS particles did not significantly alter their inflammatory potential compared to pristine particles. However, our results revealed a substantial reduction in inflammatory responses, particularly TNFα secretion, following SGF treatment. This observation suggests a potential mitigating effect of SGF treatment on the inflammatory properties of PS particles. The increase in TNFα secretion may also explain the increase in glycolysis metabolic activity and viability of the macrophages.166 Firstly, the process of phagocytosis may stimulate cellular signaling pathways associated with cell survival and cytokine production, thereby promoting macrophage viability and TNF-α secretion.167 Moreover, the physicochemical properties of PS particles, such as their size, surface charge, and surface chemistry, can influence their interaction with macrophages and subsequent cellular responses.154 It is also possible that certain characteristics of the PS particles may elicit a favorable response from macrophages, leading to enhanced viability and cytokine production, and specifically, the SGF-treated particles further change these responses, either by enhancing the same or altering the signaling pathways. The pro-inflammatory effects on Caco-2 cells were not detected in this study, possibly due to the increase in cell death. Therefore, it is also recommended to study pro-inflammatory effects on Caco-2 cells at lower concentrations, typically a concentration that does not induce significant cell death.
A significant route for microplastic particles to enter the human body is through the transepithelial pathway, which differs from the endocytosis mechanism observed in Caco-2 cells. Rather than being internalized by cells, micro and nanoplastics can breach the epithelial barrier, primarily through disruption of tight junction integrity.168 In this study, 10-micron-sized particles were utilized, and notable impairment of tight junction integrity was observed, particularly in the case of weathered particles. This observation suggests that weathering processes may exacerbate the ability of micro and nanoplastics to compromise the epithelial barrier, facilitating their translocation across epithelial cell layers.167 Moreover, the presence of organic matter, particularly pepsin, appeared to play a protective role in preserving tight junction integrity, particularly evident for pristine particles. Organic matter, such as pepsin, present in the SGF, may exert a stabilizing effect on tight junction proteins, thereby mitigating the disruptive effects of micro and nanoplastic exposure on epithelial barrier function.168 It is possible that pepsin, along with other organic compounds, forms a barrier or coating on the particle surface, shielding it from direct interaction with tight junction proteins.156 Additionally, organic matter may modulate cellular signaling pathways involved in tight junction regulation, thereby preserving barrier integrity. Maintaining barrier integrity is crucial for the protective function of intestinal epithelial cells. The significant alterations observed due to weathering and SGF treatment underscore the necessity of assessing the impact of microplastics on Caco-2 or other intestinal epithelial cells using environmentally and physiologically representative microplastics. Employing such models can offer valuable insights into the translocation mechanisms of microplastics within the body, thereby enhancing our understanding of their potential health implications.
5. Conclusion
This study dived into the combination effects of weathered and SGF-incubated microplastic on RAW264.7 and Caco-2 cells. The weathering effect and SGF treatment altered the physical properties and surface chemistry of the particles, consequently inducing distinct cellular responses compared to pristine particles. Seawater-weathered groups underwent more complex surface chemical alterations compared to the DI water-weathered group. SGF incubation enhanced organic matter attachment on particles, with weathered particles having more pronounced organic matter attachment. Overall, weathering effects led to increased macrophage metabolic activity, ROS production, and TNFα secretion, enhancing cell viability. Weathered particles exhibited higher cytotoxicity toward Caco-2 cells compared to pristine particles. The incubation with SGF, which facilitated the formation of organic matter on the particles, further amplified macrophage metabolic activity and viability, while mitigating the cytotoxicity in Caco-2 cells for both pristine and weathered particles. Additionally, SGF treatment helped preserve the tight junction barrier integrity of Caco-2 cells, reducing the disruptive effects of the particles. Based on our findings, future studies should include time-dependent analysis to better clarify the effects of weathering on particle transformations and their biological impacts, as well as exploring weathering and SGF effects on cellular metabolic pathways linked to cellular responses.This study has underscored the importance of combining environmental and physiological factors for a more comprehensive understanding of microplastic cytotoxicity and offers several potential avenues for future research. We employed weathered microplastics and physiologically relevant SGF to enhance the representation of real-world conditions. This in vitro study provides valuable insights into micro- and nanoplastics toxicity and predicts their potential in vivo behavior. Further validation with in vivo studies is essential. Ongoing work should apply the protocol to a broader size range, including submicron and nanoplastics, to deepen our understanding of their toxicity across scales.
Data availability
The authors confirm that data supporting the findings of the study are available within the article and upon request.Author contributions
Conceptualization of the study: Y. L., L. G. Sample preparation: L. G., T. M. Data collection: L. G. Data analysis: L. G., A. P., Y. L. Experiments: L. G. Results interpretation: L. G., A. P., H. K., Y. L. Manuscript writing: L. G., A. P., Y. L. Manuscript review: L. G., Y. L., I. A., A. P., H. K., M. M., G. B., A. R. Project supervision: Y. L., I. A., G. B., A. R.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was supported through the Water Resources Research Act Program funded by the U.S. Geological Survey (G21AP10622-02), the National Science Foundation (NSF, Grant 2347408), and Lin's Start-up Funds. The FTIR, Raman, and FE-SEM data were acquired at the RI Consortium for Nanoscience and Nanotechnology, a URI College of Engineering core facility partially funded by the National Science Foundation EPSCoR, Cooperative Agreement #OIA-1655221. The authors thank Professor Arun Shukla for generously allowing us to use their UV weathering chamber for an extended time. The authors thank the helpful comments from the peers and reviewers on this paper. Especially, the authors thank Dr. Matthew Cabral, Dr Weizhou Yue, Dr. Jie Shen, and Dr. HuiFang Liu for their mentoring and support.Notes and references
- W. He, S. Liu, W. Zhang, K. Yi, C. Zhang, H. Pang, D. Huang, J. Huang and X. Li, Recent Advances on Microplastic Aging: Identification, Mechanism, Influence Factors, and Additives Release, Sci. Total Environ., 2023, 889, 164035, DOI:10.1016/j.scitotenv.2023.164035.
- V. Fernández-González, J. M. Andrade-Garda, P. López-Mahía and S. Muniategui-Lorenzo, Impact of Weathering on the Chemical Identification of Microplastics from Usual Packaging Polymers in the Marine Environment, Anal. Chim. Acta, 2021, 1142, 179–188, DOI:10.1016/j.aca.2020.11.002.
- X. Zhang, M. Xia, J. Zhao, Z. Cao, W. Zou and Q. Zhou, Photoaging Enhanced the Adverse Effects of Polyamide Microplastics on the Growth, Intestinal Health, and Lipid Absorption in Developing Zebrafish, Environ. Int., 2022, 158, 106922, DOI:10.1016/j.envint.2021.106922.
- A. F. R. M. Ramsperger, V. K. B. Narayana, W. Gross, J. Mohanraj, M. Thelakkat, A. Greiner, H. Schmalz, H. Kress and C. Laforsch, Environmental Exposure Enhances the Internalization of Microplastic Particles into Cells, Sci. Adv., 2020, 6(50), eabd1211, DOI:10.1126/sciadv.abd1211.
- Y. Qiu, S. Zhou, C. Zhang, L. Chen, W. Qin and Q. Zhang, Vertical Distribution and Weathering Characteristic of Microplastics in Soil Profile of Different Land Use Types, Sci. Total Environ., 2023, 905, 166902, DOI:10.1016/j.scitotenv.2023.166902.
- J. N. Hanun, F. Hassan and J.-J. Jiang, Occurrence, Fate, and Sorption Behavior of Contaminants of Emerging Concern to Microplastics: Influence of the Weathering/Aging Process, J. Environ. Chem. Eng., 2021, 9(5), 106290, DOI:10.1016/j.jece.2021.106290.
- B. Rios-Fuster, P. Arechavala-Lopez, K. García-Marcos, C. Alomar, M. Compa, E. Álvarez, M. M. Julià, A. Solomando Martí, A. Sureda and S. Deudero, Experimental Evidence of Physiological and Behavioral Effects of Microplastic Ingestion in Sparus Aurata, Aquat. Toxicol., 2021, 231, 105737, DOI:10.1016/j.aquatox.2020.105737.
- L. Félix, P. Carreira and F. Peixoto, Effects of Chronic Exposure of Naturally Weathered Microplastics on Oxidative Stress Level, Behaviour, and Mitochondrial Function of Adult Zebrafish (Danio Rerio), Chemosphere, 2023, 310, 136895, DOI:10.1016/j.chemosphere.2022.136895.
- P. Liu, Y. Shi, X. Wu, H. Wang, H. Huang, X. Guo and S. Gao, Review of the Artificially-Accelerated Aging Technology and Ecological Risk of Microplastics, Sci. Total Environ., 2021, 768, 144969, DOI:10.1016/j.scitotenv.2021.144969.
- S. Jeon, D.-K. Lee, J. Jeong, S. I. Yang, J.-S. Kim, J. Kim and W.-S. Cho, The Reactive Oxygen Species as Pathogenic Factors of Fragmented Microplastics to Macrophages, Environ. Pollut., 2021, 281, 117006, DOI:10.1016/j.envpol.2021.117006.
- F. H. Englert, F. A. Mueller, B. Dugershaw-Kurzer, V. M. Kissling, S. Boentges, G. S. Gupta, G. A. Fontana, S. Diedrich, L. Suter-Dick, S. J. Sturla and T. Buerki-Thurnherr, Environmentally Relevant UV-Light Weathering of Polystyrene Micro- and Nanoplastics Promotes Hepatotoxicity in a Human Cell Line, Environ. Sci.: Nano, 2023, 10(6), 1644–1659, 10.1039/D3EN00044C.
- S. Jeon, J. H. Jeon, J. Jeong, G. Kim, S. Lee, S. Kim, M. Maruthupandy, K. Lee, S. I. Yang and W.-S. Cho, Size- and Oxidative Potential-Dependent Toxicity of Environmentally Relevant Expanded Polystyrene Styrofoam Microplastics to Macrophages, J. Hazard. Mater., 2023, 459, 132295, DOI:10.1016/j.jhazmat.2023.132295.
- Y. Ma, J. Y. C. Koh, H. K. Lim, P. Shi and C. Y. Tay, Elucidating the Size-Dependency of In Vitro Digested Polystyrene Microplastics on Human Intestinal Cells Health and Function, Macromol. Chem. Phys., 2022, 223(13), 2100454, DOI:10.1002/macp.202100454.
- S. Liu, X. Wu, W. Gu, J. Yu and B. Wu, Influence of the Digestive Process on Intestinal Toxicity of Polystyrene Microplastics as Determined by in Vitro Caco-2 Models, Chemosphere, 2020, 256, 127204, DOI:10.1016/j.chemosphere.2020.127204.
- L. Wang, Y. Wang, M. Xu, J. Ma, S. Zhang, S. Liu, K. Wang, H. Tian and J. Cui, Enhanced Hepatic Cytotoxicity of Chemically Transformed Polystyrene Microplastics by Simulated Gastric Fluid, J. Hazard. Mater., 2021, 410, 124536, DOI:10.1016/j.jhazmat.2020.124536.
- O. S. Alimi, D. Claveau-Mallet, R. S. Kurusu, M. Lapointe, S. Bayen and N. Tufenkji, Weathering Pathways and Protocols for Environmentally Relevant Microplastics and Nanoplastics: What Are We Missing?, J. Hazard. Mater., 2022, 423, 126955, DOI:10.1016/j.jhazmat.2021.126955.
- O. S. Alimi, D. Claveau-Mallet, R. S. Kurusu, M. Lapointe, S. Bayen and N. Tufenkji, Weathering Pathways and Protocols for Environmentally Relevant Microplastics and Nanoplastics: What Are We Missing?, J. Hazard. Mater., 2022, 423, 126955, DOI:10.1016/j.jhazmat.2021.126955.
- C. Wang, J. Zhao and B. Xing, Environmental Source, Fate, and Toxicity of Microplastics, J. Hazard. Mater., 2021, 407, 124357, DOI:10.1016/j.jhazmat.2020.124357.
- S. Koner, A. Mukherjee and N. Chandrasekaran, Elucidating the Effects of Naturally Weathered Aged-Polypropylene Microplastics and Newly Procured Polypropylene Microplastics on Raw 264.7 Macrophages, Environ. Sci.: Nano, 2024, 11(3), 983–999, 10.1039/D3EN00742A.
- M. Völkl, V. Jérôme, A. Weig, J. Jasinski, N. Meides, P. Strohriegl, T. Scheibel and R. Freitag, Pristine and Artificially-Aged Polystyrene Microplastic Particles Differ in Regard to Cellular Response, J. Hazard. Mater., 2022, 435, 128955, DOI:10.1016/j.jhazmat.2022.128955.
- S. Jeon, J. H. Jeon, J. Jeong, G. Kim, S. Lee, S. Kim, M. Maruthupandy, K. Lee, S. I. Yang and W.-S. Cho, Size- and Oxidative Potential-Dependent Toxicity of Environmentally Relevant Expanded Polystyrene Styrofoam Microplastics to Macrophages, J. Hazard. Mater., 2023, 459, 132295, DOI:10.1016/j.jhazmat.2023.132295.
- J. C. Prata, C. Venâncio, A. V. Girão, J. P. da Costa, I. Lopes, A. C. Duarte and T. Rocha-Santos, Effects of Virgin and Weathered Polystyrene and Polypropylene Microplastics on Raphidocelis Subcapitata and Embryos of Danio Rerio under Environmental Concentrations, Sci. Total Environ., 2022, 816, 151642, DOI:10.1016/j.scitotenv.2021.151642.
- M. Peng, M. Vercauteren, C. Grootaert, A. I. Catarino, G. Everaert, A. Rajkovic, C. Janssen and J. Asselman, Bioenergetic Effects of Pristine and Ultraviolet-Weathered Polydisperse Polyethylene Terephthalate and Polystyrene Nanoplastics on Human Intestinal Caco-2 Cells, Sci. Total Environ., 2024, 908, 168267, DOI:10.1016/j.scitotenv.2023.168267.
- B. Wu, X. Wu, S. Liu, Z. Wang and L. Chen, Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 cells, Chemosphere, 2019, 221, 333–341, DOI:10.1016/j.chemosphere.2019.01.056.
- Y. Chen, Y. Ling, X. Li, J. Hu, C. Cao and D. He, Size-Dependent Cellular Internalization and Effects of Polystyrene Microplastics in Microalgae P. Helgolandica Var. Tsingtaoensis and S. Quadricauda, J. Hazard. Mater., 2020, 399, 123092, DOI:10.1016/j.jhazmat.2020.123092.
- M. U. Ijaz, S. Shahzadi, A. Samad, N. Ehsan, H. Ahmed, A. Tahir, H. Rehman and H. Anwar, Dose-Dependent Effect of Polystyrene Microplastics on the Testicular Tissues of the Male Sprague Dawley Rats, Dose-Response, 2021, 19(2), 15593258211019882 CrossRef CAS.
- D. C. Bobori, K. Feidantsis, A. Dimitriadi, N. Datsi, P. Ripis, S. Kalogiannis, I. Sampsonidis, G. Kastrinaki, N. M. Ainali and D. A. Lambropoulou, Dose-Dependent Cytotoxicity of Polypropylene Microplastics (PP-MPs) in Two Freshwater Fishes, Int. J. Mol. Sci., 2022, 23(22), 13878 CrossRef CAS PubMed.
- Q. Wan, J. Li and Y. Chen, Comparative Growth and Cellular Responses of Toxigenic Microcystis Exposed to Different Types of Microplastics at Various Doses, Environ. Pollut., 2021, 290, 117950 CrossRef CAS.
- W. A. da Silva Brito, D. Singer, L. Miebach, F. Saadati, K. Wende, A. Schmidt and S. Bekeschus, Comprehensive in Vitro Polymer Type, Concentration, and Size Correlation Analysis to Microplastic Toxicity and Inflammation, Sci. Total Environ., 2023, 854, 158731, DOI:10.1016/j.scitotenv.2022.158731.
- Q. Wan, J. Li and Y. Chen, Comparative Growth and Cellular Responses of Toxigenic Microcystis Exposed to Different Types of Microplastics at Various Doses, Environ. Pollut., 2021, 290, 117950, DOI:10.1016/j.envpol.2021.117950.
- P. Li and J. Liu, Micro (Nano) Plastics in the Human Body: Sources, Occurrences, Fates, and Health Risks, Environ. Sci. Technol., 2024, 58(7), 3065–3078, DOI:10.1021/acs.est.3c08902.
- M. B. Paul, L. Böhmert, A. F. Thünemann, K. Loeschner, L. Givelet, C. Fahrenson, A. Braeuning and H. Sieg, Influence of Artificial Digestion on Characteristics and Intestinal Cellular Effects of Micro-, Submicro- and Nanoplastics, Food Chem. Toxicol., 2024, 184, 114423, DOI:10.1016/j.fct.2023.114423.
- S. Liu, X. Wu, W. Gu, J. Yu and B. Wu, Influence of the Digestive Process on Intestinal Toxicity of Polystyrene Microplastics as Determined by in Vitro Caco-2 Models, Chemosphere, 2020, 256, 127204, DOI:10.1016/j.chemosphere.2020.127204.
- L. Wang, Y. Wang, M. Xu, J. Ma, S. Zhang, S. Liu, K. Wang, H. Tian and J. Cui, Enhanced Hepatic Cytotoxicity of Chemically Transformed Polystyrene Microplastics by Simulated Gastric Fluid, J. Hazard. Mater., 2021, 410, 124536, DOI:10.1016/j.jhazmat.2020.124536.
- H. Tan, T. Yue, Y. Xu, J. Zhao and B. Xing, Microplastics Reduce Lipid Digestion in Simulated Human Gastrointestinal System, Environ. Sci. Technol., 2020, 54(19), 12285–12294, DOI:10.1021/acs.est.0c02608.
- G. M. DeLoid, X. Cao, R. Coreas, D. Bitounis, D. Singh, W. Zhong and P. Demokritou, Incineration-Generated Polyethylene Micro-Nanoplastics Increase Triglyceride Lipolysis and Absorption in an in Vitro Small Intestinal Epithelium Model, Environ. Sci. Technol., 2022, 56(17), 12288–12297 CrossRef CAS PubMed.
- A. Tamargo, N. Molinero, J. J. Reinosa, V. Alcolea-Rodriguez, R. Portela, M. A. Bañares, J. F. Fernández and M. V. Moreno-Arribas, PET Microplastics Affect Human Gut Microbiota Communities during Simulated Gastrointestinal Digestion, First Evidence of Plausible Polymer Biodegradation during Human Digestion, Sci. Rep., 2022, 12(1), 528 CrossRef CAS PubMed.
- Y. Peng, J. Lu, L. Fan, W. Dong and M. Jiang, Simulated Gastrointestinal Digestion of Two Different Sources of Biodegradable Microplastics and the Influence on Gut Microbiota, Food Chem. Toxicol., 2024, 185, 114474 CrossRef CAS.
- T. Du, X. Yu, S. Shao, T. Li, S. Xu and L. Wu, Aging of Nanoplastics Significantly Affects Protein Corona Composition Thus Enhancing Macrophage Uptake, Environ. Sci. Technol., 2023, 57(8), 3206–3217 CrossRef CAS.
- J. Wen, H. Sun, Z. Liu, X. Zhu, Z. Qin, E. Song and Y. Song, Aging Processes Dramatically Alter the Protein Corona Constitution, Cellular Internalization, and Cytotoxicity of Polystyrene Nanoplastics, Environ. Sci. Technol. Lett., 2022, 9(11), 962–968 CrossRef CAS.
- Z. Yuan, R. Nag and E. Cummins, Human Health Concerns Regarding Microplastics in the Aquatic Environment - From Marine to Food Systems, Sci. Total Environ., 2022, 823, 153730, DOI:10.1016/j.scitotenv.2022.153730.
- B. Wu, X. Wu, S. Liu, Z. Wang and L. Chen, Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 Cells, Chemosphere, 2019, 221, 333–341 CrossRef CAS PubMed.
- V. Mattioda, V. Benedetti, C. Tessarolo, F. Oberto, A. Favole, M. Gallo, W. Martelli, M. I. Crescio, E. Berio and L. Masoero, Pro-Inflammatory and Cytotoxic Effects of Polystyrene Microplastics on Human and Murine Intestinal Cell Lines, Biomolecules, 2023, 13(1), 140 CrossRef CAS PubMed.
- N. Aloi, A. Calarco, G. Curcuruto, M. Di Natale, G. Augello, S. C. Carroccio, P. Cerruti, M. Cervello, A. Cuttitta, P. Colombo and V. Longo, Photoaging of Polystyrene-Based Microplastics Amplifies Inflammatory Response in Macrophages, Chemosphere, 2024, 364, 143131, DOI:10.1016/j.chemosphere.2024.143131.
- X. Wang, X.-M. Ren, H. He, F. Li, K. Liu, F. Zhao, H. Hu, P. Zhang, B. Huang and X. Pan, Cytotoxicity and Pro-Inflammatory Effect of Polystyrene Nano-Plastic and Micro-Plastic on RAW264. 7 Cells, Toxicology, 2023, 484, 153391 CrossRef CAS PubMed.
- S. Massardo, D. Verzola, S. Alberti, C. Caboni, M. Santostefano, E. Eugenio Verrina, A. Angeletti, F. Lugani, G. M. Ghiggeri, M. Bruschi, G. Candiano, N. Rumeo, M. Gentile, P. Cravedi, S. La Maestra, G. Zaza, G. Stallone, P. Esposito, F. Viazzi, N. Mancianti, E. La Porta and C. Artini, MicroRaman Spectroscopy Detects the Presence of Microplastics in Human Urine and Kidney Tissue, Environ. Int., 2024, 184, 108444, DOI:10.1016/j.envint.2024.108444.
- G. Zhou, Q. Wu, P. Tang, C. Chen, X. Cheng, X.-F. Wei, J. Ma and B. Liu, How Many Microplastics Do We Ingest When Using Disposable Drink Cups?, J. Hazard. Mater., 2023, 441, 129982, DOI:10.1016/j.jhazmat.2022.129982.
- S. Liu, X. Wu, W. Gu, J. Yu and B. Wu, Influence of the Digestive Process on Intestinal Toxicity of Polystyrene Microplastics as Determined by in Vitro Caco-2 Models, Chemosphere, 2020, 256, 127204 CrossRef CAS.
- X. Yu, M. Lang, D. Huang, C. Yang, Z. Ouyang and X. Guo, Photo-Transformation of Microplastics and Its Toxicity to Caco-2 Cells, Sci. Total Environ., 2022, 806, 150954 CrossRef CAS.
- N. D. Saenen, M. S. Witters, I. Hantoro, I. Tejeda, A. Ethirajan, F. Van Belleghem and K. Smeets, Polystyrene Microplastics of Varying Sizes and Shapes Induce Distinct Redox and Mitochondrial Stress Responses in a Caco-2 Monolayer, Antioxidants, 2023, 12(3), 739 CrossRef CAS PubMed.
- B. Wu, X. Wu, S. Liu, Z. Wang and L. Chen, Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 Cells, Chemosphere, 2019, 221, 333–341 CrossRef CAS.
- J. Chen, X. Chen, Y. Xuan, H. Shen, Y. Tang, T. Zhang and J. Xu, Surface Functionalization-Dependent Inflammatory Potential of Polystyrene Nanoplastics through the Activation of MAPK/NF-KB Signaling Pathways in Macrophage Raw 264.7, Ecotoxicol. Environ. Saf., 2023, 251, 114520, DOI:10.1016/j.ecoenv.2023.114520.
- J. Hwang, D. Choi, S. Han, S. Y. Jung, J. Choi and J. Hong, Potential Toxicity of Polystyrene Microplastic Particles, Sci. Rep., 2020, 10(1), 7391, DOI:10.1038/s41598-020-64464-9.
- X. Wang, X.-M. Ren, H. He, F. Li, K. Liu, F. Zhao, H. Hu, P. Zhang, B. Huang and X. Pan, Cytotoxicity and Pro-Inflammatory Effect of Polystyrene Nano-Plastic and Micro-Plastic on RAW264.7 Cells, Toxicology, 2023, 484, 153391, DOI:10.1016/j.tox.2022.153391.
- Y. S. Eltemsah and T. Bøhn, Acute and Chronic Effects of Polystyrene Microplastics on Juvenile and Adult Daphnia Magna, Environ. Pollut., 2019, 254, 112919, DOI:10.1016/j.envpol.2019.07.087.
- J. Hwang, D. Choi, S. Han, J. Choi and J. Hong, An Assessment of the Toxicity of Polypropylene Microplastics in Human Derived Cells, Sci. Total Environ., 2019, 684, 657–669, DOI:10.1016/j.scitotenv.2019.05.071.
- Y. He, J. Li, J. Chen, X. Miao, G. Li, Q. He, H. Xu, H. Li and Y. Wei, Cytotoxic Effects of Polystyrene Nanoplastics with Different Surface Functionalization on Human HepG2 Cells, Sci. Total Environ., 2020, 723, 138180, DOI:10.1016/j.scitotenv.2020.138180.
- R. Gautam, J. Jo, M. Acharya, A. Maharjan, D. Lee, P. B. Kc, C. Kim, K. Kim, H. Kim and Y. Heo, Evaluation of Potential Toxicity of Polyethylene Microplastics on Human Derived Cell Lines, Sci. Total Environ., 2022, 838, 156089, DOI:10.1016/j.scitotenv.2022.156089.
- F. Pfeiffer and E. K. Fischer, Various Digestion Protocols within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction, Front. Environ. Sci., 2020, 8, 572424 CrossRef.
- S. Burrows, J. Colwell, S. Costanzo, S. Kaserzon, E. Okoffo, F. Ribeiro, S. O'Brien, T. Toapanta, C. Rauert, K. V. Thomas and T. Galloway, UV Sources and Plastic Composition Influence Microplastic Surface Degradation: Implications for Plastic Weathering Studies, J. Hazard. Mater. Adv., 2024, 14, 100428, DOI:10.1016/j.hazadv.2024.100428.
- X. Han, R. D. Vogt, J. Zhou, B. Zheng, X. Yu, J. Feng and X. Lu, Increased Cu (II) Adsorption onto UV-Aged Polyethylene, Polypropylene, and Polyethylene Terephthalate Microplastic Particles in Seawater, Front. Mar. Sci., 2021, 8, 770606 CrossRef.
- C. Siri, Y. Liu, T. Masset, W. Dudefoi, D. Oldham, M. Minghetti, D. Grandjean and F. Breider, Adsorption of Progesterone onto Microplastics and Its Desorption in Simulated Gastric and Intestinal Fluids, Environ. Sci.: Processes Impacts, 2021, 23(10), 1566–1577 RSC.
- T. H. Jeong, K. B. Kim, S. Y. Kim, Y. R. Kim, J. H. Kim, T. M. T. Pham, T. H. Ha, S. J. Ahn, M.-D. Kim and Y. H. Kim, Pepsin-Induced Modification of Silver Nanoparticles in Simulated Gastric Fluid, Colloid Interface Sci. Commun., 2021, 44, 100491, DOI:10.1016/j.colcom.2021.100491.
- Y. Sun, T. Zhen, Y. Li, Y. Wang, M. Wang, X. Li and Q. Sun, Interaction of Food-Grade Titanium Dioxide Nanoparticles with Pepsin in Simulated Gastric Fluid, LWT--Food Sci. Technol., 2020, 134, 110208, DOI:10.1016/j.lwt.2020.110208.
- X. Peng, T. Yang, S. Guo, J. Zhou, G. Chen, Z. Zhu and J. Tan, Revealing Chemical Release from Plastic Debris in Animals' Digestive Systems Using Nontarget and Suspect Screening and Simulating Digestive Fluids, Environ. Pollut., 2024, 348, 123793, DOI:10.1016/j.envpol.2024.123793.
- S. Hellmig, F. Von Schöning, C. Gadow, S. Katsoulis, J. Hedderich, U. R. Fölsch and E. Stüber, Gastric Emptying Time of Fluids and Solids in Healthy Subjects Determined by 13C Breath Tests: Influence of Age, Sex and Body Mass Index, J. Gastroenterol. Hepatol., 2006, 21(12), 1832–1838, DOI:10.1111/j.1440-1746.2006.04449.x.
- V. Otero, D. Sanches, C. Montagner, M. Vilarigues, L. Carlyle, J. A. Lopes and M. J. Melo, Characterisation of Metal Carboxylates by Raman and Infrared Spectroscopy in Works of Art, J. Raman Spectrosc., 2014, 45(11–12), 1197–1206 CrossRef CAS.
- V. Ţucureanu, A. Matei and A. M. Avram, FTIR Spectroscopy for Carbon Family Study, Crit. Rev. Anal. Chem., 2016, 46(6), 502–520 CrossRef.
- P. Liu, L. Qian, H. Wang, X. Zhan, K. Lu, C. Gu and S. Gao, New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes, Environ. Sci. Technol., 2019, 53(7), 3579–3588 CrossRef CAS.
- M. Zvekic, L. C. Richards, C. C. Tong and E. T. Krogh, Characterizing Photochemical Ageing Processes of Microplastic Materials Using Multivariate Analysis of Infrared Spectra, Environ. Sci.: Processes Impacts, 2022, 24(1), 52–61 RSC.
- C. Campanale, I. Savino, C. Massarelli and V. F. Uricchio, Fourier Transform Infrared Spectroscopy to Assess the Degree of Alteration of Artificially Aged and Environmentally Weathered Microplastics, Polymers, 2023, 15(4), 911 CrossRef CAS.
- Z. Ren, X. Gui, Y. Wei, X. Chen, X. Xu, L. Zhao, H. Qiu and X. Cao, Chemical and Photo-Initiated Aging Enhances Transport Risk of Microplastics in Saturated Soils: Key Factors, Mechanisms, and Modeling, Water Res., 2021, 202, 117407, DOI:10.1016/j.watres.2021.117407.
- X. Wang, H. Zheng, J. Zhao, X. Luo, Z. Wang and B. Xing, Photodegradation Elevated the Toxicity of Polystyrene Microplastics to Grouper (Epinephelus Moara) through Disrupting Hepatic Lipid Homeostasis, Environ. Sci. Technol., 2020, 54(10), 6202–6212, DOI:10.1021/acs.est.9b07016.
- L. Cai, J. Wang, J. Peng, Z. Wu and X. Tan, Observation of the Degradation of Three Types of Plastic Pellets Exposed to UV Irradiation in Three Different Environments, Sci. Total Environ., 2018, 628–629, 740–747, DOI:10.1016/j.scitotenv.2018.02.079.
- V. Fernández-González, J. M. Andrade-Garda, P. López-Mahía and S. Muniategui-Lorenzo, Impact of Weathering on the Chemical Identification of Microplastics from Usual Packaging Polymers in the Marine Environment, Anal. Chim. Acta, 2021, 1142, 179–188, DOI:10.1016/j.aca.2020.11.002.
- H.-Y. Kim, J. Ashim, S. Park, W. Kim, S. Ji, S.-W. Lee, Y.-R. Jung, S. W. Jeong, S.-G. Lee, H.-C. Kim, Y.-J. Lee, M. K. Kwon, J.-S. Hwang, J. M. Shin, S.-J. Lee, W. Yu, J.-K. Park and S.-K. Choi, A Preliminary Study about the Potential Risks of the UV-Weathered Microplastic: The Proteome-Level Changes in the Brain in Response to Polystyrene Derived Weathered Microplastics, Environ. Res., 2023, 233, 116411, DOI:10.1016/j.envres.2023.116411.
- N. F. A. Biber, A. Foggo and R. C. Thompson, Characterising the Deterioration of Different Plastics in Air and Seawater, Mar. Pollut. Bull., 2019, 141, 595–602, DOI:10.1016/j.marpolbul.2019.02.068.
- K. Hadjiivanov and H. Knözinger, FTIR Study of CO and NO Adsorption and Coadsorption on a Cu/SiO 2 Catalyst: Probing the Oxidation State of Copper, Phys. Chem. Chem. Phys., 2001, 3(6), 1132–1137 RSC.
- A. Sadat and I. J. Joye, Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins, Appl. Sci., 2020, 10(17), 5918 CrossRef CAS.
- P. Ragavendran, D. Sophia, C. Arul Raj and V. K. Gopalakrishnan, Functional Group Analysis of Various Extracts of Aerva Lanata (L.,) by FTIR Spectrum, Pharmacologyonline, 2011, 1, 358–364 Search PubMed.
- C. Jiménez-Arroyo, A. Tamargo, N. Molinero, J. J. Reinosa, V. Alcolea-Rodriguez, R. Portela, M. A. Bañares, J. F. Fernández and M. V. Moreno-Arribas, Simulated Gastrointestinal Digestion of Polylactic Acid (PLA) Biodegradable Microplastics and Their Interaction with the Gut Microbiota, Sci. Total Environ., 2023, 902, 166003, DOI:10.1016/j.scitotenv.2023.166003.
- J. Wen, H. Sun, Z. Liu, X. Zhu, Z. Qin, E. Song and Y. Song, Aging Processes Dramatically Alter the Protein Corona Constitution, Cellular Internalization, and Cytotoxicity of Polystyrene Nanoplastics, Environ. Sci. Technol. Lett., 2022, 9(11), 962–968, DOI:10.1021/acs.estlett.2c00650.
- T. Du, X. Yu, S. Shao, T. Li, S. Xu and L. Wu, Aging of Nanoplastics Significantly Affects Protein Corona Composition Thus Enhancing Macrophage Uptake, Environ. Sci. Technol., 2023, 57(8), 3206–3217, DOI:10.1021/acs.est.2c05772.
- K. Ranganathan, A. Morais, I. Nongwe, C. Longo, A. F. Nogueira and N. J. Coville, Study of Photoelectrochemical Water Splitting Using Composite Films Based on TiO2 Nanoparticles and Nitrogen or Boron Doped Hollow Carbon Spheres as Photoanodes, J. Mol. Catal. A: Chem., 2016, 422, 165–174, DOI:10.1016/j.molcata.2015.10.024.
- G. Zorn, L.-H. Liu, L. Árnadóttir, H. Wang, L. J. Gamble, D. G. Castner and M. Yan, X-Ray Photoelectron Spectroscopy Investigation of the Nitrogen Species in Photoactive Perfluorophenylazide-Modified Surfaces, J. Phys. Chem. C, 2014, 118(1), 376–383 CrossRef CAS.
- J. Y. Fang and X. C. Wan, XPS Analysis of Tea Plant Leaf and Root Surface, Guangpuxue Yu Guangpu Fenxi, 2008, 28(9), 2196–2200 CAS.
- X. Wang, X.-M. Ren, H. He, F. Li, K. Liu, F. Zhao, H. Hu, P. Zhang, B. Huang and X. Pan, Cytotoxicity and Pro-Inflammatory Effect of Polystyrene Nano-Plastic and Micro-Plastic on RAW264.7 Cells, Toxicology, 2023, 484, 153391, DOI:10.1016/j.tox.2022.153391.
- J. Chen, Z. Xu, Y. Liu, A. Mei, X. Wang and Q. Shi, Cellular Absorption of Polystyrene Nanoplastics with Different Surface Functionalization and the Toxicity to RAW264.7 Macrophage Cells, Ecotoxicol. Environ. Saf., 2023, 252, 114574, DOI:10.1016/j.ecoenv.2023.114574.
- N. H. Mohamed Nor, M. Kooi, N. J. Diepens and A. A. Koelmans, Lifetime Accumulation of Microplastic in Children and Adults, Environ. Sci. Technol., 2021, 55(8), 5084–5096, DOI:10.1021/acs.est.0c07384.
- G. Jiao, X. He, X. Li, J. Qiu, H. Xu, N. Zhang and S. Liu, Limitations of MTT and CCK-8 Assay for Evaluation of Graphene Cytotoxicity, RSC Adv., 2015, 5(66), 53240–53244 RSC.
- Y. Zhang, S. Wang, V. Olga, Y. Xue, S. Lv, X. Diao, Y. Zhang, Q. Han and H. Zhou, The Potential Effects of Microplastic Pollution on Human Digestive Tract Cells, Chemosphere, 2022, 291, 132714 CrossRef CAS.
- J. Chen, Z. Xu, Y. Liu, A. Mei, X. Wang and Q. Shi, Cellular Absorption of Polystyrene Nanoplastics with Different Surface Functionalization and the Toxicity to RAW264.7 Macrophage Cells, Ecotoxicol. Environ. Saf., 2023, 252, 114574, DOI:10.1016/j.ecoenv.2023.114574.
- J. Rudolph, M. Völkl, V. Jérôme, T. Scheibel and R. Freitag, Noxic Effects of Polystyrene Microparticles on Murine Macrophages and Epithelial Cells, Sci. Rep., 2021, 11(1), 15702, DOI:10.1038/s41598-021-95073-9.
- H. Brouwer, M. Porbahaie, S. Boeren, M. Busch and H. Bouwmeester, The in Vitro Gastrointestinal Digestion-Associated Protein Corona of Polystyrene Nano- and Microplastics Increases Their Uptake by Human THP-1-Derived Macrophages, Part. Fibre Toxicol., 2024, 21(1), 4, DOI:10.1186/s12989-024-00563-z.
- W. Kwon, D. Kim, H.-Y. Kim, S. W. Jeong, S.-G. Lee, H.-C. Kim, Y.-J. Lee, M. K. Kwon, J.-S. Hwang, J. E. Han, J.-K. Park, S.-J. Lee and S.-K. Choi, Microglial Phagocytosis of Polystyrene Microplastics Results in Immune Alteration and Apoptosis in Vitro and in Vivo, Sci. Total Environ., 2022, 807, 150817, DOI:10.1016/j.scitotenv.2021.150817.
- J. C. Aguilar-Guzmán, K. Bejtka, M. Fontana, E. Valsami-Jones, A. M. Villezcas, R. Vazquez-Duhalt and A. G. Rodríguez-Hernández, Polyethylene Terephthalate Nanoparticles Effect on RAW 264.7 Macrophage Cells, Microplast. Nanoplast., 2022, 2(1), 9, DOI:10.1186/s43591-022-00027-1.
- V. Collin-Faure, M. Vitipon, A. Torres, O. Tanyeres, B. Dalzon and T. Rabilloud, The Internal Dose Makes the Poison: Higher Internalization of Polystyrene Particles Induce Increased Perturbation of Macrophages, Front. Immunol., 2023, 14 DOI:10.3389/fimmu.2023.1092743.
- Z. Yu, Q. Li, J. Wang, Y. Yu, Y. Wang, Q. Zhou and P. Li, Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field, Nanoscale Res. Lett., 2020, 15(1), 115 CrossRef CAS PubMed.
- A. Das, The Emerging Role of Microplastics in Systemic Toxicity: Involvement of Reactive Oxygen Species (ROS), Sci. Total Environ., 2023, 895, 165076, DOI:10.1016/j.scitotenv.2023.165076.
- S. Li, Y. Ma, S. Ye, S. Tang, N. Liang, Y. Liang and F. Xiao, Polystyrene Microplastics Trigger Hepatocyte Apoptosis and Abnormal Glycolytic Flux via ROS-Driven Calcium Overload, J. Hazard. Mater., 2021, 417, 126025, DOI:10.1016/j.jhazmat.2021.126025.
- S. Giri and A. Mukherjee, Eco-Corona Reduces the Phytotoxic Effects of Polystyrene Nanoplastics in Allium Cepa: Emphasizing the Role of ROS, Environ. Exp. Bot., 2022, 198, 104850, DOI:10.1016/j.envexpbot.2022.104850.
- A. Banerjee and W. L. Shelver, Micro-and Nanoplastic Induced Cellular Toxicity in Mammals: A Review, Sci. Total Environ., 2021, 755, 142518 CrossRef CAS PubMed.
- N. D. Saenen, M. S. Witters, I. Hantoro, I. Tejeda, A. Ethirajan, F. Van Belleghem and K. Smeets, Polystyrene Microplastics of Varying Sizes and Shapes Induce Distinct Redox and Mitochondrial Stress Responses in a Caco-2 Monolayer, Antioxidants, 2023, 12(3) DOI:10.3390/antiox12030739.
- M. Völkl, V. Jérôme, A. Weig, J. Jasinski, N. Meides, P. Strohriegl, T. Scheibel and R. Freitag, Pristine and Artificially-Aged Polystyrene Microplastic Particles Differ in Regard to Cellular Response, J. Hazard. Mater., 2022, 435, 128955 CrossRef PubMed.
- V. Escamilla-Rivera, M. Uribe-Ramirez, S. Gonzalez-Pozos, O. Lozano, S. Lucas and A. De Vizcaya-Ruiz, Protein Corona Acts as a Protective Shield against Fe3O4-PEG Inflammation and ROS-Induced Toxicity in Human Macrophages, Toxicol. Lett., 2016, 240(1), 172–184 CrossRef CAS.
- M. Völkl, V. Jérôme, A. Weig, J. Jasinski, N. Meides, P. Strohriegl, T. Scheibel and R. Freitag, Pristine and Artificially-Aged Polystyrene Microplastic Particles Differ in Regard to Cellular Response, J. Hazard. Mater., 2022, 435, 128955, DOI:10.1016/j.jhazmat.2022.128955.
- S. Jeon, D.-K. Lee, J. Jeong, S. I. Yang, J.-S. Kim, J. Kim and W.-S. Cho, The Reactive Oxygen Species as Pathogenic Factors of Fragmented Microplastics to Macrophages, Environ. Pollut., 2021, 281, 117006, DOI:10.1016/j.envpol.2021.117006.
- C. Corbo, R. Molinaro, A. Parodi, N. E. Toledano Furman, F. Salvatore and E. Tasciotti, The Impact of Nanoparticle Protein Corona on Cytotoxicity, Immunotoxicity and Target Drug Delivery, Nanomedicine, 2015, 11(1), 81–100, DOI:10.2217/nnm.15.188.
- Y. K. Lee, E.-J. Choi, T. J. Webster, S.-H. Kim and D. Khang, Effect of the Protein Corona on Nanoparticles for Modulating Cytotoxicity and Immunotoxicity, Int. J. Nanomed., 2015, 97–113 CAS.
- R. Leibe, I.-L. Hsiao, S. Fritsch-Decker, U. Kielmeier, A. M. Wagbo, B. Voss, A. Schmidt, S. D. Hessman, A. Duschl and G. J. Oostingh, The Protein Corona Suppresses the Cytotoxic and Pro-Inflammatory Response in Lung Epithelial Cells and Macrophages upon Exposure to Nanosilica, Arch. Toxicol., 2019, 93, 871–885 CrossRef CAS.
- I. Ali, X. Tan, C. Peng, I. Naz, Y. Zhang, A. Hernández, R. Marcos, R. Pervez, Z. Duan and Y. Ruan, Eco- and Bio-Corona-Based Microplastics and Nanoplastics Complexes in the Environment: Modulations in the Toxicological Behavior of Plastic Particles and Factors Affecting, Process Saf. Environ. Prot., 2024 DOI:10.1016/j.psep.2024.04.035.
- I. Ali, X. Tan, C. Peng, I. Naz, Y. Zhang, A. Hernández, R. Marcos, R. Pervez, Z. Duan and Y. Ruan, Eco- and Bio-Corona-Based Microplastics and Nanoplastics Complexes in the Environment: Modulations in the Toxicological Behavior of Plastic Particles and Factors Affecting, Process Saf. Environ. Prot., 2024 DOI:10.1016/j.psep.2024.04.035.
- B. Wu, X. Wu, S. Liu, Z. Wang and L. Chen, Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 cells, Chemosphere, 2019, 221, 333–341, DOI:10.1016/j.chemosphere.2019.01.056.
- J. Domenech, M. de Britto, A. Velázquez, S. Pastor, A. Hernández, R. Marcos and C. Cortés, Long-Term Effects of Polystyrene Nanoplastics in Human Intestinal Caco-2 Cells, Biomolecules, 2021, 11(10) DOI:10.3390/biom11101442.
- M. Herrala, M. Huovinen, E. Järvelä, J. Hellman, P. Tolonen, M. Lahtela-Kakkonen and J. Rysä, Micro-Sized Polyethylene Particles Affect Cell Viability and Oxidative Stress Responses in Human Colorectal Adenocarcinoma Caco-2 and HT-29 Cells, Sci. Total Environ., 2023, 867, 161512, DOI:10.1016/j.scitotenv.2023.161512.
- Q. Shi, J. Tang, X. Liu and R. Liu, Ultraviolet-Induced Photodegradation Elevated the Toxicity of Polystyrene Nanoplastics on Human Lung Epithelial A549 Cells, Environ. Sci.: Nano, 2021, 8(9), 2660–2675, 10.1039/D1EN00465D.
- J. Wang, H. Tian, Y. Shi, Y. Yang, F. Yu, H. Cao, L. Gao and M. Liu, The Enhancement in Toxic Potency of Oxidized Functionalized Polyethylene-Microplastics in Mice Gut and Caco-2 Cells, Sci. Total Environ., 2023, 903, 166057, DOI:10.1016/j.scitotenv.2023.166057.
- S. Wu, M. Wu, D. Tian, L. Qiu and T. Li, Effects of Polystyrene Microbeads on Cytotoxicity and Transcriptomic Profiles in Human Caco-2 Cells, Environ. Toxicol., 2020, 35(4), 495–506, DOI:10.1002/tox.22885.
- V. Stock, L. Böhmert, E. Lisicki, R. Block, J. Cara-Carmona, L. K. Pack, R. Selb, D. Lichtenstein, L. Voss, C. J. Henderson, E. Zabinsky, H. Sieg, A. Braeuning and A. Lampen, Uptake and Effects of Orally Ingested Polystyrene Microplastic Particles in Vitro and in Vivo, Arch. Toxicol., 2019, 93(7), 1817–1833, DOI:10.1007/s00204-019-02478-7.
- M. Peng, M. Vercauteren, C. Grootaert, A. I. Catarino, G. Everaert, A. Rajkovic, C. Janssen and J. Asselman, Bioenergetic Effects of Pristine and Ultraviolet-Weathered Polydisperse Polyethylene Terephthalate and Polystyrene Nanoplastics on Human Intestinal Caco-2 Cells, Sci. Total Environ., 2024, 908, 168267, DOI:10.1016/j.scitotenv.2023.168267.
- S. Liu, X. Wu, W. Gu, J. Yu and B. Wu, Influence of the Digestive Process on Intestinal Toxicity of Polystyrene Microplastics as Determined by in Vitro Caco-2 Models, Chemosphere, 2020, 256, 127204, DOI:10.1016/j.chemosphere.2020.127204.
- Y. Chen, A. M. Williams, E. B. Gordon, S. E. Rudolph, B. N. Longo, G. Li and D. L. Kaplan, Biological Effects of Polystyrene Micro-and Nano-Plastics on Human Intestinal Organoid-Derived Epithelial Tissue Models without and with M Cells, Nanomedicine, 2023, 50, 102680 CrossRef CAS PubMed.
- D. Xu, Y. Ma, C. Peng, Y. Gan, Y. Wang, Z. Chen, X. Han and Y. Chen, Differently Surface-Labeled Polystyrene Nanoplastics at an Environmentally Relevant Concentration Induced Crohn's Ileitis-like Features via Triggering Intestinal Epithelial Cell Necroptosis, Environ. Int., 2023, 176, 107968 CrossRef CAS PubMed.
- R. Lehner, W. Wohlleben, D. Septiadi, R. Landsiedel, A. Petri-Fink and B. Rothen-Rutishauser, A Novel 3D Intestine Barrier Model to Study the Immune Response upon Exposure to Microplastics, Arch. Toxicol., 2020, 94(7), 2463–2479, DOI:10.1007/s00204-020-02750-1.
- S. Jeon, J. H. Jeon, J. Jeong, G. Kim, S. Lee, S. Kim, M. Maruthupandy, K. Lee, S. I. Yang and W.-S. Cho, Size- and Oxidative Potential-Dependent Toxicity of Environmentally Relevant Expanded Polystyrene Styrofoam Microplastics to Macrophages, J. Hazard. Mater., 2023, 459, 132295, DOI:10.1016/j.jhazmat.2023.132295.
- M. Herrala, M. Huovinen, E. Järvelä, J. Hellman, P. Tolonen, M. Lahtela-Kakkonen and J. Rysä, Micro-Sized Polyethylene Particles Affect Cell Viability and Oxidative Stress Responses in Human Colorectal Adenocarcinoma Caco-2 and HT-29 Cells, Sci. Total Environ., 2023, 867, 161512, DOI:10.1016/j.scitotenv.2023.161512.
- S. Wu, M. Wu, D. Tian, L. Qiu and T. Li, Effects of Polystyrene Microbeads on Cytotoxicity and Transcriptomic Profiles in Human Caco-2 Cells, Environ. Toxicol., 2020, 35(4), 495–506, DOI:10.1002/tox.22885.
- T. Luo, D. Wang, Y. Zhao, X. Li, G. Yang and Y. Jin, Polystyrene Microplastics Exacerbate Experimental Colitis in Mice Tightly Associated with the Occurrence of Hepatic Inflammation, Sci. Total Environ., 2022, 844, 156884, DOI:10.1016/j.scitotenv.2022.156884.
- G. M. DeLoid, X. Cao, D. Bitounis, D. Singh, P. M. Llopis, B. Buckley and P. Demokritou, Toxicity, Uptake, and Nuclear Translocation of Ingested Micro-Nanoplastics in an in Vitro Model of the Small Intestinal Epithelium, Food Chem. Toxicol., 2021, 158, 112609, DOI:10.1016/j.fct.2021.112609.
- Y. Deng, H. Chen, Y. Huang, Y. Zhang, H. Ren, M. Fang, Q. Wang, W. Chen, R. C. Hale and T. S. Galloway, Long-Term Exposure to Environmentally Relevant Doses of Large Polystyrene Microplastics Disturbs Lipid Homeostasis via Bowel Function Interference, Environ. Sci. Technol., 2022, 56(22), 15805–15817 CrossRef CAS.
- C. Zhang, J. Wang, Z. Pan, S. Wang, L. Zhang, Q. Wang, Q. Ye, A. Zhou, S. Xie and F. Zeng, A Dosage-Effect Assessment of Acute Toxicology Tests of Microplastic Exposure in Filter-Feeding Fish, Fish Shellfish Immunol., 2021, 113, 154–161 CrossRef CAS.
- B. Wu, X. Wu, S. Liu, Z. Wang and L. Chen, Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 cells, Chemosphere, 2019, 221, 333–341, DOI:10.1016/j.chemosphere.2019.01.056.
- H. Brouwer, M. Porbahaie, S. Boeren, M. Busch and H. Bouwmeester, The in Vitro Gastrointestinal Digestion-Associated Protein Corona of Polystyrene Nano- and Microplastics Increases Their Uptake by Human THP-1-Derived Macrophages, Part. Fibre Toxicol., 2024, 21(1), 4, DOI:10.1186/s12989-024-00563-z.
- J. Chen, X. Chen, Y. Xuan, H. Shen, Y. Tang, T. Zhang and J. Xu, Surface Functionalization-Dependent Inflammatory Potential of Polystyrene Nanoplastics through the Activation of MAPK/ NF-KB Signaling Pathways in Macrophage Raw 264.7, Ecotoxicol. Environ. Saf., 2023, 251, 114520, DOI:10.1016/j.ecoenv.2023.114520.
- K. Senathirajah, S. Attwood, G. Bhagwat, M. Carbery, S. Wilson and T. Palanisami, Estimation of the Mass of Microplastics Ingested – A Pivotal First Step towards Human Health Risk Assessment, J. Hazard. Mater., 2021, 404, 124004, DOI:10.1016/j.jhazmat.2020.124004.
- P. Liu, X. Zhan, X. Wu, J. Li, H. Wang and S. Gao, Effect of Weathering on Environmental Behavior of Microplastics: Properties, Sorption and Potential Risks, Chemosphere, 2020, 242, 125193 CrossRef CAS PubMed.
- V. Fernández-González, J. M. Andrade-Garda, P. López-Mahía and S. Muniategui-Lorenzo, Impact of Weathering on the Chemical Identification of Microplastics from Usual Packaging Polymers in the Marine Environment, Anal. Chim. Acta, 2021, 1142, 179–188 CrossRef PubMed.
- K. Zhu, H. Jia, Y. Sun, Y. Dai, C. Zhang, X. Guo, T. Wang and L. Zhu, Long-Term Phototransformation of Microplastics under Simulated Sunlight Irradiation in Aquatic Environments: Roles of Reactive Oxygen Species, Water Res., 2020, 173, 115564 CrossRef CAS PubMed.
- J. P. Da Costa, A. R. Nunes, P. S. M. Santos, A. V. Girao, A. C. Duarte and T. Rocha-Santos, Degradation of Polyethylene Microplastics in Seawater: Insights into the Environmental Degradation of Polymers, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2018, 53(9), 866–875 CrossRef CAS.
- J. Duan, N. Bolan, Y. Li, S. Ding, T. Atugoda, M. Vithanage, B. Sarkar, D. C. W. Tsang and M. B. Kirkham, Weathering of Microplastics and Interaction with Other Coexisting Constituents in Terrestrial and Aquatic Environments, Water Res., 2021, 196, 117011 CrossRef CAS PubMed.
- C. Campanale, I. Savino, C. Massarelli and V. F. Uricchio, Fourier Transform Infrared Spectroscopy to Assess the Degree of Alteration of Artificially Aged and Environmentally Weathered Microplastics, Polymers, 2023, 15(4) DOI:10.3390/polym15040911.
- J. Zhang, Q. Zhang, J. P.-Y. Maa, X. Shen, J. Liang, L. Yu, L. Ge and G. Wang, Effects of Organic Matter on Interaction Forces between Polystyrene Microplastics: An Experimental Study, Sci. Total Environ., 2022, 844, 157186, DOI:10.1016/j.scitotenv.2022.157186.
- R. Liu, Y. Wang, Y. Yang, L. Shen, B. Zhang, Z. Dong, C. Gao and B. Xing, New Insights into Adsorption Mechanism of Pristine and Weathered Polyamide Microplastics towards Hydrophilic Organic Compounds, Environ. Pollut., 2023, 317, 120818, DOI:10.1016/j.envpol.2022.120818.
- H. Luo, C. Liu, D. He, J. Xu, J. Sun, J. Li and X. Pan, Environmental Behaviors of Microplastics in Aquatic Systems: A Systematic Review on Degradation, Adsorption, Toxicity and Biofilm under Aging Conditions, J. Hazard. Mater., 2022, 423, 126915 CrossRef CAS.
- M. E. González-López, A. S. M. del Campo, J. R. Robledo-Ortíz, M. Arellano and A. A. Pérez-Fonseca, Accelerated Weathering of Poly (Lactic Acid) and Its Biocomposites: A Review, Polym. Degrad. Stab., 2020, 179, 109290 CrossRef.
- W. Kwon, D. Kim, H.-Y. Kim, S. W. Jeong, S.-G. Lee, H.-C. Kim, Y.-J. Lee, M. K. Kwon, J.-S. Hwang and J. E. Han, Microglial Phagocytosis of Polystyrene Microplastics Results in Immune Alteration and Apoptosis in Vitro and in Vivo, Sci. Total Environ., 2022, 807, 150817 CrossRef CAS.
- S. D. Merkley, H. C. Moss, S. M. Goodfellow, C. L. Ling, J. L. Meyer-Hagen, J. Weaver, M. J. Campen and E. F. Castillo, Polystyrene Microplastics Induce an Immunometabolic Active State in Macrophages, Cell Biol. Toxicol., 2022, 38(1), 31–41 CrossRef CAS.
- S. Koner, A. Mukherjee and N. Chandrasekaran, Elucidating the Effects of Naturally Weathered Aged-Polypropylene Microplastics and Newly Procured Polypropylene Microplastics on Raw 264.7 Macrophages, Environ. Sci.: Nano, 2024 Search PubMed.
- S. D. Merkley, H. C. Moss, S. M. Goodfellow, C. L. Ling, J. L. Meyer-Hagen, J. Weaver, M. J. Campen and E. F. Castillo, Polystyrene Microplastics Induce an Immunometabolic Active State in Macrophages, Cell Biol. Toxicol., 2022, 38(1), 31–41, DOI:10.1007/s10565-021-09616-x.
- H. He, K. Liu, Z. Guo, F. Li, Z. Liao, X. Yang, X. Ren, H. Huang, B. Huang and X. Pan, Photoaging Mechanisms of Microplastics Mediated by Dissolved Organic Matter in an Iron-Rich Aquatic Environment, Sci. Total Environ., 2023, 860, 160488 CrossRef CAS PubMed.
- A. Banerjee and W. L. Shelver, Micro-and Nanoplastic Induced Cellular Toxicity in Mammals: A Review, Sci. Total Environ., 2021, 755, 142518 CrossRef CAS PubMed.
- S. Li, H. Liu, R. Gao, A. Abdurahman, J. Dai and F. Zeng, Aggregation Kinetics of Microplastics in Aquatic Environment: Complex Roles of Electrolytes, PH, and Natural Organic Matter, Environ. Pollut., 2018, 237, 126–132, DOI:10.1016/j.envpol.2018.02.042.
- D. Choi, J. Bang, T. Kim, Y. Oh, Y. Hwang and J. Hong, In Vitro Chemical and Physical Toxicities of Polystyrene Microfragments in Human-Derived Cells, J. Hazard. Mater., 2020, 400, 123308 CrossRef CAS PubMed.
- V. Stock, L. Böhmert, E. Lisicki, R. Block, J. Cara-Carmona, L. K. Pack, R. Selb, D. Lichtenstein, L. Voss, C. J. Henderson, E. Zabinsky, H. Sieg, A. Braeuning and A. Lampen, Uptake and Effects of Orally Ingested Polystyrene Microplastic Particles in Vitro and in Vivo, Arch. Toxicol., 2019, 93(7), 1817–1833, DOI:10.1007/s00204-019-02478-7.
- S. Matic, D. H. D'Souza, T. Wu, P. Pangloli and V. P. Dia, Bovine Milk Exosomes Affect Proliferation and Protect Macrophages against Cisplatin-Induced Cytotoxicity, Immunol. Invest., 2020, 49(7), 711–725 CrossRef CAS.
- S. Jeon, D.-K. Lee, J. Jeong, S. I. Yang, J.-S. Kim, J. Kim and W.-S. Cho, The Reactive Oxygen Species as Pathogenic Factors of Fragmented Microplastics to Macrophages, Environ. Pollut., 2021, 281, 117006, DOI:10.1016/j.envpol.2021.117006.
- B. Wu, X. Wu, S. Liu, Z. Wang and L. Chen, Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 cells, Chemosphere, 2019, 221, 333–341, DOI:10.1016/j.chemosphere.2019.01.056.
- S. Li, Y. Ma, S. Ye, S. Tang, N. Liang, Y. Liang and F. Xiao, Polystyrene Microplastics Trigger Hepatocyte Apoptosis and Abnormal Glycolytic Flux via ROS-Driven Calcium Overload, J. Hazard. Mater., 2021, 417, 126025, DOI:10.1016/j.jhazmat.2021.126025.
- A. Viola, F. Munari, R. Sánchez-Rodríguez, T. Scolaro and A. Castegna, The Metabolic Signature of Macrophage Responses, Front. Immunol., 2019, 10 DOI:10.3389/fimmu.2019.01462.
- A. Y. Andreyev, Y. E. Kushnareva, N. N. Starkova and A. A. Starkov, Metabolic ROS Signaling: To Immunity and Beyond, Biochemistry, 2020, 85, 1650–1667 CAS.
- S. Jeon, D.-K. Lee, J. Jeong, S. I. Yang, J.-S. Kim, J. Kim and W.-S. Cho, The Reactive Oxygen Species as Pathogenic Factors of Fragmented Microplastics to Macrophages, Environ. Pollut., 2021, 281, 117006, DOI:10.1016/j.envpol.2021.117006.
- V. Mattioda, V. Benedetti, C. Tessarolo, F. Oberto, A. Favole, M. Gallo, W. Martelli, M. I. Crescio, E. Berio and L. Masoero, Pro-Inflammatory and Cytotoxic Effects of Polystyrene Microplastics on Human and Murine Intestinal Cell Lines, Biomolecules, 2023, 13(1), 140 CrossRef CAS.
- X. Wang, X.-M. Ren, H. He, F. Li, K. Liu, F. Zhao, H. Hu, P. Zhang, B. Huang and X. Pan, Cytotoxicity and Pro-Inflammatory Effect of Polystyrene Nano-Plastic and Micro-Plastic on RAW264. 7 Cells, Toxicology, 2023, 484, 153391 CrossRef CAS.
- A. Marrocco and L. A. Ortiz, Role of Metabolic Reprogramming in Pro-Inflammatory Cytokine Secretion from LPS or Silica-Activated Macrophages, Front. Immunol., 2022, 13 DOI:10.3389/fimmu.2022.936167.
- N. R. M. Beijer, A. Dehaut, M. P. Carlier, H. Wolter, R. M. Versteegen, J. L. A. Pennings, L. de La Fonteyne, H. Niemann, H. M. Janssen and B. G. Timmermans, Relationship between Particle Properties and Immunotoxicological Effects of Environmentally-Sourced Microplastics, Front. Water, 2022, 4, 866732 CrossRef.
- X. Shi, T. Xu, M. Gao, Y. Bi, J. Wang, Y. Yin and S. Xu, Combined Exposure of Emamectin Benzoate and Microplastics Induces Tight Junction Disorder, Immune Disorder and Inflammation in Carp Midgut via Lysosome/ROS/Ferroptosis Pathway, Water Res., 2024, 121660 CrossRef CAS.
- Q. Li, L. Jiang, J. Feng, X. Wang, X. Wang, X. Xu and W. Chu, Aged Polystyrene Microplastics Exacerbate Alopecia Associated with Tight Junction Injuries and Apoptosis via Oxidative Stress Pathway in Skin, Environ. Int., 2024, 108638 CrossRef CAS PubMed.
- A. Toschi, B. Rossi, B. Tugnoli, A. Piva and E. Grilli, Nature-Identical Compounds and Organic Acids Ameliorate and Prevent the Damages Induced by an Inflammatory Challenge in Caco-2 Cell Culture, Molecules, 2020, 25(18), 4296 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00444b |
| This journal is © The Royal Society of Chemistry 2025 |