Emerging investigator series: CeO2/CuO nanostructured composite with enhanced antimicrobial properties and low cytotoxicity to human keratinocytes in vitro†
Svetlana
Vihodceva
 *a,
Andris
Šutka
*a,
Andris
Šutka
 a,
Mairis
Iesalnieks
a,
Mairis
Iesalnieks
 a,
Liga
Orlova
a,
Arturs
Pludonis
a,
Liga
Orlova
a,
Arturs
Pludonis
 a,
Maarja
Otsus
b,
Mariliis
Sihtmäe
b,
Heiki
Vija
b,
Alexandra
Nefedova
cd,
Angela
Ivask
a,
Maarja
Otsus
b,
Mariliis
Sihtmäe
b,
Heiki
Vija
b,
Alexandra
Nefedova
cd,
Angela
Ivask
 c,
Anne
Kahru
c,
Anne
Kahru
 b and
Kaja
Kasemets
b
b and
Kaja
Kasemets
b
aInstitute of Materials and Surface Engineering, Faculty of Natural Sciences and Technology, Riga Technical University, Paula Valdena 7, LV-1048 Riga, Latvia. E-mail: Svetlana.Vihodceva@rtu.lv
bNational Institute of Chemical Physics and Biophysics, Laboratory of Environmental Toxicology, Akadeemia tee 23, 12618 Tallinn, Estonia
cInstitute of Molecular and Cell Biology, University of Tartu, Riia 23, 51010 Tartu, Estonia
dInstitute of Physics, University of Tartu, W. Ostwaldi 1, 50411, Tartu, Estonia
First published on 22nd November 2024
Abstract
This research presents a synthesis method for the CeO2/CuO nanostructured composite, which has potential applications as an antimicrobial material in the production of antimicrobial surface coatings, for example, for high-touch surfaces. The antimicrobial efficacy, mode of action, and potential cytotoxicity of CeO2/CuO towards the human immortalized keratinocyte cell line in vitro were studied compared to those of CuO, CeO2, and ionic Cu (a solubility control). The used synthesis method resulted in a CeO2/CuO nanostructured composite with a mean particle size of 27 nm and a specific surface area of 80.3 m2 g−1. The composite had a significant proportion (54%) of non-lattice oxygen species, highlighting the presence of substantial surface defects crucial for generating reactive oxygen species (ROS). The antimicrobial properties of CeO2/CuO, CuO, and CeO2 were assessed at six concentrations from 1 to 1000 mg L−1 in deionized water. The CeO2/CuO composite exhibited antibacterial efficacy at a minimum bactericidal concentration (MBC) of 100 mg L−1 towards Escherichia coli already after 2 h of contact and towards Pseudomonas aeruginosa and Staphylococcus aureus after 4 h of contact, whereas after 24 h of exposure, the antibacterial efficacy to all three bacterial strains was evident already at a MBC = 10 mg L−1. Fungi Candida albicans proved less susceptible than bacteria (24 h MBC = 100 mg L−1). Thus, the CeO2/CuO composite showed significant antibacterial efficacy against Gram-negative and Gram-positive bacteria, being at the same time safe to human keratinocytes in vitro in the case of which even 1000 mg L−1 caused no harmful effects after 2 h exposure and 500 mg L−1 caused no cytotoxicity after 24 h exposure. CeO2/CuO caused abiotic and biotic ROS production in all the tested environments. ROS production in deionized water was the most remarkable. Shedding of Cu-ions from CeO2/CuO was moderate and depended on the test environment, varying from 0.3 to 1 mg L−1, and considering the MBC of ionic Cu for microorganisms was not the main contributor to the antimicrobial activity of CeO2/CuO. The CeO2/CuO composite exhibited no acute toxicity to the environmentally relevant bacterium Vibrio fischeri. These findings indicate that CeO2/CuO's high ROS production is its primary antimicrobial mechanism and that due to its low cytotoxicity to human keratinocytes, it can be considered a promising antimicrobial agent.
Environmental significanceAntimicrobial resistance and toxicity concerns necessitate the development of new, safer antimicrobial materials. The CeO2/CuO nanostructured composite exhibits significant antimicrobial efficacy against Gram-negative and Gram-positive pathogenic bacteria models and fungus, requiring significantly less material than CuO alone to achieve similar antimicrobial effects. The high proportion of non-lattice oxygen species in CeO2/CuO enhances reactive oxygen species production, driving its antimicrobial activity. Importantly, this composite demonstrates low cytotoxicity to human keratinocytes in vitro and no acute toxicity to the environmentally relevant bacterium Vibrio fischeri. These properties indicate that CeO2/CuO is a promising antimicrobial agent. |
1. Introduction
Antimicrobial resistance (AMR) has become a global health concern, with severe social and economic effects, and the development of new antimicrobial materials is urgently needed.1In the EU, in 2021, Escherichia coli was the most frequently reported bacterial species exhibiting AMR, accounting for 39.4% of all AMR pathogens.2 Also, Staphylococcus aureus is a common cause of infections in healthcare facilities, and alarmingly, people with methicillin-resistant S. aureus (MRSA) infections are 64% more likely to die than people with drug-sensitive infections.3 In addition, more than a billion people worldwide are affected by fungal infections, especially those induced by Candida sp., contributing to more than 1.6 million deaths annually.4
Metal and metal oxide micro/nanoparticles (M/NPs) are among the promising materials investigated for their antimicrobial properties,5–7 mainly due to their multimodal mechanisms of action8,9via (i) direct binding to microorganisms' cell surfaces, (ii) induction of oxidative stress, and (iii) cellular adverse effects due to the release of metal ions.10,11 The multimodal range of these antimicrobial activities of M/NPs may lower the probability of developing resistance compared to conventional antibiotics.12
According to the earlier published (eco)toxicological database NanoE-Tox,13 the most popular antimicrobial M/NPs also used in commercial products, i.e. Ag, ZnO, and CuO, showed the highest antimicrobial properties and cytotoxicity. As the awareness regarding the potential health risks posed by antimicrobial materials is on the rise, there is a growing demand for the development of safer M/NP materials.
Shape, size, surface modification, morphology, concentration – all these properties influence the antimicrobial and toxic properties of M/NPs.14 Recent achievements in nanotechnology have led to the development of nanostructured materials consisting of two or more metal/metal oxide components. These materials possess unique physicochemical properties that make them applicable in many areas;15 for instance, the antimicrobial efficiency of one metal oxide can be complemented by the low cytotoxicity of another, making them promising candidates for medical applications.14 One example of two metal oxide materials is the CeO2/CuO composite material that up to now has mostly been used in catalytic applications.16–18
Indeed, this material has the interface between Cu and Ce, which provides multifunctional sites with distinctive chemical characteristics.16–18 This material enhances catalytic activity by combining effects like redox facilitation, defect formation, and interfacial reactivity.16–18 These effects are attributed to doping low-valent copper ions into CeO2, which generates oxygen vacancies due to the difference in charge, therefore augmenting the number of defects compared to the pure phase of CeO2,16–18 The latter may also contribute to enhanced antimicrobial activity, thereby justifying further investigation into the CeO2/CuO composite's antimicrobial potential. Importantly, CuO NPs are widely recognized for their antimicrobial properties, which have been extensively studied and validated in the scientific literature.13 However, CeO2 NPs have not shown antimicrobial properties and cytotoxicity,5 but rather possess anti-inflammatory and immunomodulatory properties.19
So far, limited research exists on the antimicrobial activity and mechanisms of CeO2/CuO composites. There are few studies mainly relying on agar diffusion tests, and with scarce mode of action information.20–23 Furthermore, the toxicity/safety assessment conducted in parallel with the antimicrobial studies is needed to evaluate the potential applications of CeO2/CuO.
This paper evaluates the antimicrobial properties of the CeO2/CuO nanostructured composite compared to pristine CeO2 and CuO M/NPs against pathogenic bacterial models Gram-negative bacteria E. coli and P. aeruginosa, Gram-positive bacteria S. aureus, and fungi C. albicans. It also evaluates ecotoxicity on the naturally luminescent environmental bacterium Vibrio fischeri and cytotoxicity on human keratinocytes (HaCaT cells) in vitro.
2. Materials and methods
2.1. Materials
Cerium(III) nitrate hexahydrate (Ce(NO3)3·6H2O, 99.5%, USA) was purchased from Acros Organics. Copper(II) nitrate hemi(pentahydrate) (CuN2O6·2.5H2O, ≥98%, Germany), high molecular weight chitosan (>75% deacetylated chitin, poly/D glucosamine, Netherlands), 2′,7′-dichlorofluorescin diacetate (C24H16Cl2O7, ≥98%, Israel), acetic acid (≥99.8%, Germany), glucose (Germany) and hydrogen peroxide solution (H2O2, 30%, Germany) were purchased from Sigma Aldrich. High-purity deionized (DI) water was obtained from a Milli-Q® system (Merck Millipore, Darmstadt, Germany) and used for all the tests. Tryptone, yeast extract, and agar were obtained from LabM (Lancashire, UK).Cytotoxicity testing: Dulbecco's modified Eagle medium (DMEM) with 4.5 g L−1 glucose and L-glutamine without sodium pyruvate (Corning, USA) supplemented with 10% inactivated fetal bovine serum (FBS, PAN Biotech, Germany), penicillin–streptomycin (100×, Thermo Fisher, USA), neutral red (3-amino-7-dimethylamino-2-methyl-phenazine hydrochloride, Carl Roth, Germany), phosphate-buffered saline (PBS, prepared in house according to the general recipe), glacial acetic acid (99.8%, Sigma-Aldrich, Germany), dimethylsulfoxide (DMSO, HPLC grade, 99.7%, Honeywell, USA), and trypsin/EDTA 0.05% solution in PBS (PAN Biotech, Germany).
2.2. Synthesis of CeO2, CuO, and CeO2/CuO
CeO2, CuO, and CeO2/CuO powders were synthesized by adding 1.16 g of copper(II) nitrate and/or 2.17 g of cerium(III) nitrate (for the synthesis of CuO, CeO2/CuO, and CeO2, respectively) to DI water and vigorously stirring with a magnetic stirrer at room temperature for 30 min. Then 1.5 g of chitosan was added and the mixtures were stirred for another 30 min. Then, 3 ml of acetic acid was added. The solution was stirred till the complete dissolution of chitosan. The solution was added dropwise into 0.5 M NaOH to form gel beads. Afterward, the beads were washed several times with DI water until neutral pH was reached and frozen at −20 °C. After de-frosting, the beads were dried in a tube vacuum furnace at 60 °C. The resultant products were annealed at 500 °C for 1 h and cooled to room temperature. According to the literature, CeO2/CuO catalysts calcined at 500 °C have higher activity.242.3. Physicochemical characterization
The phase composition of the synthesized powders was analyzed by powder XRD analysis (Rigaku MiniFlex X-ray diffractometer, Tokyo, Japan) with Cu Kα (λ 1.540593 Å) radiation in the scan range 5–90°. The morphology and size of particles and elemental composition were characterized using a field emission scanning electron microscope (FESEM, Lyra, FEI NanoSEM 650, Eindhoven, Netherland) coupled with an energy dispersive spectrometer (EDS, TEAM™ Integrated EDS with Apollo X SDD) for X-ray microanalysis. X-ray photoelectron spectroscopy (XPS, Escalab Xi+, Thermo Scientific) analysis was performed using monochromatic Al K1 as the X-ray source with a spot size of 500 μm; peak fitting was performed using Advantage 5.9925 software, and the advantageous carbon peak at 284.8 eV was chosen as the calibration point. The Brunauer–Emmett–Teller (BET) analysis was conducted using a QuantaChrome NOVA 1200 (USA). The average hydrodynamic diameter (dh) and zeta (ζ) potential of powders in DI water (50 mg L−1) were measured in a 1 mL DTS1061 folded capillary cell using a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) immediately after preparation (0 h) and after 24 h incubation at room temperature using the Zetasizer Nano ZS (Malvern Instruments Ltd, Worcestershire, UK).The shedding of Ce and Cu-ions from the synthesised materials was conducted in the respective testing media. For that, CeO2, CuO, and CeO2/CuO suspensions (100 mg L−1) were incubated for 2 and 24 h in: (i) DI water as described in section 2.4., (ii) 2% NaCl as described in section 2.5., and (iii) DMEM as described in section 2.7. Then, 3 mL of each suspension was centrifuged (Sigma3–16, Sigma, Germany) for 20 min at 9500 rpm using ultrafiltration tubes Sartorius VivaSpin 6, 5000 MWCO PES (Sartorius Stedim Biotech GmbH, Goettingen, Germany), and 2375 μL of the filtrate was removed and mixed with 125 μL concentrated HNO3. The concentration of Ce and Cu in the supernatant was analyzed by total reflection X-ray fluorescence spectroscopy (TRXF) using a Picofox S2 (Bruker AXS Microanalysis GmbH, Karlsruhe, Germany, a detection limit of Cu 30 μg L−1). The Spectra software (AXS Microanalysis GmbH, Karlsruhe, Germany) was used to quantify the copper content.
2.4. Spot test for estimating minimum bactericidal concentrations (MBCs)
The stock suspensions of CeO2, CuO, and CeO2/CuO (4 g L−1) were prepared in DI water and homogenized using an ultrasonic probe (Branson Digital Sonifier®, Danbury, CT, USA) at 40 W for 1 min immediately after preparation. Before the testing, the stocks were diluted in DI water to obtain the test concentrations. The nominal concentrations used for testing were 1, 10, 100, 250, 500, and 1000 mg L−1.The minimum bactericidal concentration (MBC), defined as the lowest tested concentration (mg L−1) that completely inhibited visible colony formation of the Gram-negative bacteria E. coli ATCC 25922 and P. aeruginosa ATCC 27583, Gram-positive bacteria S. aureus ATCC 6538, and fungi C. albicans ATCC 10231 (the American Type Culture Collection, ATCC), was determined using a cell viability test – “spot” assay in DI water, following the methodology described previously.25–27 In brief, the exponentially growing microorganisms were exposed to CeO2, CuO, and CeO2/CuO in DI water in 96-well microplates (Falcon) at room temperature. After 2, 4, and 24 h of exposure, 3 μL of the microbial culture was spotted onto toxicant-free Luria–Bertani (LB) nutrient agar medium (for bacteria) or yeast-extract-peptone-dextrose (YPD) medium (for C. albicans). The seeded agar plates were incubated for 24 h (for bacteria) and 48 h (for C. albicans) at 30 °C in the dark, and then the colony's formation was evaluated visually.
2.5. Bioluminescence inhibition test with environmental bacteria Vibrio fischeri
A kinetic V. fischeri bioluminescence inhibition test (“A Flash assay”) following the ISO 21338:2010 protocol28 was performed at 20 °C.27 In brief, CeO2, CuO, and CeO2/CuO suspensions were prepared in 2% NaCl. A freeze-dried V. fischeri reagent 1243-157 BioToxTM (Environmental Bio-Detection Products Inc., Canada) was reconstituted with 2% NaCl, and 100 μL of bacterial suspension was added to 100 μL of the tested compound on a 96-well white polypropylene microplate (Greiner Bio-One, Austria).Bacterial luminescence was continuously recorded during the first 15 s after dispensing (no additional sample mixing) and once again after 30 min incubation. The microplate Luminometer Orion II (Berthold Detection Systems, Pforzheim, Germany), controlled by the Simplicity version 4.2 Software, was used to dispense bacteria and record luminescence. After the bioluminescence inhibition assay, bacteria were further incubated at 20 °C and 3 μL of bacterial suspension was pipetted as a spot on Beneckea Harvey (BH) nutrient agar medium (per L: yeast extract 3 g, tryptone 5 g, glycerol (99%) 2 mL, NaCl 30 g Na2HPO4·12H2O 9.45 g, KH2PO4 1 g, (NH4)2HPO4 0.5 g, MgSO4·7H2O 0.3 g, agar 15 g) after 1 and 24 h of exposure. The inoculated agar plates were then incubated in the dark for 48 h at room temperature, and the MBC of the tested compounds was visually determined.
2.6. Confocal laser scanning microscopy (CLSM) analysis
To visualize microbial interactions with CeO2, CuO, or CeO2/CuO, microorganisms from an exponentially growing culture were suspended in DI water (OD600nm = 0.5) and exposed to 100 mg L−1 for 2 h at room temperature. Then, cell suspensions were stained with Syto9 nucleic acid stain (final concentration 5 μM) for 15 min in the dark. 20 μL of stained suspension was spread on a microscopy slide (Deltalab, Spain) and dried at 37 °C for 15 min. The dried samples were immersed in Mowiol mounting medium (Sigma-Aldrich, Germany), covered with a cover glass, and examined using a Zeiss LSM800 CLSM (Zeiss, Jena, Germany) with excitation/emission track settings of 488 nm/505–550 nm to visualize Syto9-stained cells and a reflection mode at 640 nm to visualize CeO2, CuO, and CeO2/CuO. Images were processed with ZEN Software (Carl Zeiss Microscopy, Jena, Germany).2.7. Cytotoxicity evaluation
The cytotoxicity of CeO2, CuO, CeO2/CuO, and Cu ions was tested on the human immortalized keratinocyte cell line HaCaT (ATCC, USA) following the ISO 10993-5 protocol,29 with a recommended cellular toxicity cut-off value of 30%. The cells were cultivated in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin at 37 °C and 5% CO2. Cell suspensions were seeded onto 96-well plates in 100 μL at approximately 105 cells per mL and incubated overnight to form a half-confluent monolayer. The next day, the medium was removed from the plate, and 100 μL of either fresh medium (control) or medium containing different concentrations of CeO2, CuO, and CeO2/CuO was added. After 2 or 24 h of exposure, the suspension containing particles was aspirated and replaced with 100 μL of fresh cell culture medium. The cells were again incubated overnight at 37 °C with 5% CO2. Neutral red (NR) assay30 was used to assess cell viability after 24 h of exposure. 40 μg mL−1 solution of the solid NR dye in PBS was added to each well of the microplate, and the plate was incubated for 2 h at 37 °C and 5% CO2. Then, the medium was aspirated, and the cells were washed with 150 μL PBS. To dissolve NR dye, 100 μL of solubilization solution containing 50% ethanol, 49% DI water, and 1% glacial acetic acid was added, and the plate was shaken for 10 min at 200 rpm. Subsequently, absorbance was measured at 540 nm using a Tecan Infinity plate reader. Blank samples, which contained no cells, served as a reference. For positive control to demonstrate the functionality of the assay, cells were treated with 10 and 20% DMSO in the cell growing medium. All the assays were conducted at least in triplicate.To visually evidence the changes in HaCaT cells after exposure to the test compounds, cells exposed to 1000 mg L−1 of CeO2, CuO, and CeO2/CuO for 24 h were washed with PBS, fixed with 4% formaldehyde in PBS and kept under PBS. Then, the cells were stained for 30 min using 0.1% Triton X-100, 8 μM Hoechst, and 0.16 μM Phalloidin 568 (ThermoFisher). Images were taken with an Olympus BX61 microscope and XM10 camera with 40× magnification and 365/530 and 515/590 excitation/emission filter sets and a bright field. Images were processed using the Imaris program (Oxford Instruments).
2.8. Abiotic and biotic reactive oxygen species (ROS) measurement
An analysis of CeO2, CuO, and CeO2/CuO-induced ROS under abiotic (i.e. without the cells) and biotic (i.e. in the presence of the cells) conditions was performed as described previously.27 In the case of abiotic ROS, fluorescent dye 2′,7′-dichlorofluorescin diacetate DCF-DA (450 μL of 1.3 mM in ethanol) was deacetylated to DCF by reacting with 1800 mL of 0.01 M NaOH for 30 min in the dark. Subsequently, 100 μL of CeO2, CuO, and CeO2/CuO suspension and 100 μL of DCF solution were added to each well of a 96-well black microplate, and fluorescence was registered at excitation and emission wavelengths of 485 nm and 527 nm, respectively, using the Fluoroskan Ascent FL microplate reader (Thermo Labsystems, Finland).To determine biotic ROS production, microorganisms were prepared as described in section 2.4 and exposed to CeO2, CuO, and CeO2/CuO suspensions (1–1000 mg L−1) and positive control (H2O2) in DI water for 2 and 24 h. After incubation, DCF-DA working solution (50 μL, 250 μg mL−1 in 25 mM Na-phosphate buffer, pH 7.2) was added and incubated for 1 h at room temperature in the dark. Fluorescence was recorded at excitation and emission wavelengths of 485 nm and 527 nm, respectively, using the Fluoroskan Ascent FL microplate reader (Thermo Labsystems, Finland). ROS production in HaCaT was determined in cells exposed to different concentrations (10–1000 mg L−1) of CeO2, CuO, and CeO2/CuO for 2 and 24 h on black-walled 96-well microplates as described in the case of the cytotoxicity test (section 2.7.). Then, DCF-DA working solution was added to cells incubated for 30 min at 37 °C with 5% CO2, the dye was removed, fresh cell culture medium (DMEM) was added, and fluorescence was recorded at excitation and emission wavelengths of 485 nm and 527 nm, respectively, using an Infinite M200 Pro plate reader (Tecan).
Abiotic and biotic ROS levels were calculated as a relative change in fluorescence signal compared to the test medium (abiotic ROS) or non-exposed microorganism/HaCaT cells (biotic ROS).
2.9. Statistical analysis
Statistically significant differences between the test results were analysed using one-way ANOVA followed by Tukey's post hoc test for multiple comparisons, with normality assessed using the Shapiro–Wilk test and QQ plot, all performed in GraphPad Prism 10.3.1 at α = 0.05.3. Results and discussion
3.1. X-ray diffraction (XRD) analysis
Powder XRD patterns were recorded for all the samples. Fig. 1 shows the XRD data of CeO2, CuO, and CeO2/CuO. The XRD pattern confirms (Fig. 1) that the CeO2/CuO composite was prepared successfully; the presence of a CuO phase is evident alongside the CeO2 standard card ICDD 01-071-4807. The reflexes of this secondary phase correspond to the CuO standard card ICDD 99-000-3666, confirming that the second phase is exclusively CuO without other impurities.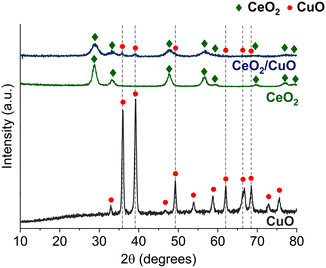 | ||
| Fig. 1 XRD patterns of CuO (ICDD 01-073-6234), CeO2 (ICDD 00-034-0394) and CeO2/CuO (CeO2 ICDD 01-071-4807; CuO ICDD 99-000-3666). ESI† Fig. S1. | ||
3.2. Field emission scanning electron microscopy (FESEM), energy dispersive spectroscopy (EDS), and Brunauer–Emmett–teller (BET) analysis
The morphology, particle size, and elemental analysis of CeO2, CuO, and CeO2/CuO obtained from FESEM studies are shown in Fig. 2. All the powders consisted of micro/nanoparticle aggregates (Fig. 2A–H) with CeO2 having a mean particle size of approximately 10 nm (Fig. 2B and C), CuO with a mean particle size of about 111 nm (Fig. 2E and F) and CeO2/CuO with a mean particle size of around 27 nm (Fig. 2H and I). FESEM images show that CuO particles exhibit higher particle size heterogeneity than the other materials (Fig. 2D–F). According to BET, the specific surface area (SSA) of CeO2 and CeO2/CuO was 90.1 m2 g−1 ± 0.85 and 80.3 ± 0.14 m2 g−1, respectively, while CuO had a SSA of 3.4 ± 0.14 m2 g−1. The decrease in CuO SAA can be explained by the increased porosity and particle size (Fig. 2D–F).31Fig. 2(L) shows the EDS elemental composition of CeO2/CuO (EDS spectrum image Fig. S1†); analysis confirmed a homogeneous distribution of Cu and Ce within the CeO2/CuO composite, as confirmed by EDS mapping images (Fig. 2J and K); this suggests that the used synthesis method ensures a homogeneous distribution of CeO2 and CuO within the composite.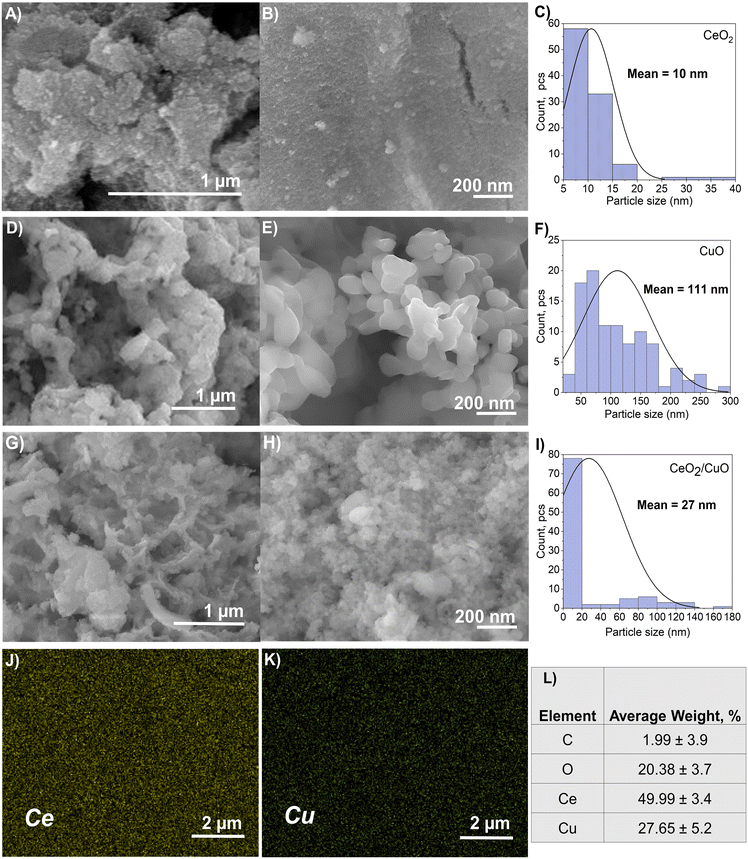 | ||
| Fig. 2 Field emission scanning electron microscopy (FESEM) images and particle size distribution histograms determined from the FESEM images measuring 100 particles by ImageJ software (A–C) CeO2; (D–F) CuO; (G–I) CeO2/CuO. The energy dispersive spectroscopy EDS elemental mapping of CeO2/CuO, the elements Ce (J) and Cu (K), and table with the EDS weight percentage of elements in CeO2/CuO (L). Example of particle size measurement and a representative EDS spectrum image can be found in the ESI† (Fig. S2). | ||
3.3. X-ray photoelectron spectroscopy (XPS)
To analyse the surface compositions and chemical states of the elements in the CeO2/CuO composite, we first conducted an XPS analysis of CeO2 and CuO (Fig. 3). C 1s spectra (Fig. 3A) of CeO2 revealed large amounts of surface contaminants in the form of carbonates. The presence of carbonates can be attributed to the thermal decomposition of chitosan during the synthesis, resulting from its organic composition. This correlates well with O 1s spectra (Fig. 3B), where we can observe the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O maximum at 528.1 eV. The total C
O maximum at 528.1 eV. The total C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O concentration on the CeO2 surface is estimated to be around 7%. Peaks at 532.6 and 534.3 (Fig. 3B) correspond to oxygen bonds that are not fully coordinated. They can be attributed to defective oxygen places in the crystal lattice, crystalline water, or –OH groups, which form in a few first monolayers on the oxide surface.32 The total amount of non-lattice oxygen species is 19%. The Ce 3d (Fig. 3C) signal shows a large amount of satellite signals, which is typical of transition metals.33 CuO formed a much cleaner phase with a low amount of surface contaminants in the form of carbonate. The amount of non-lattice oxygen species is close to CeO2, around 23%. Cu 3p spectra (Fig. 3F) did not reveal the presence of lower oxidation states with 2p1/2–2p3/2 peak splitting of 20 eV, corresponding to the oxidation state of the CuO phase.34
O concentration on the CeO2 surface is estimated to be around 7%. Peaks at 532.6 and 534.3 (Fig. 3B) correspond to oxygen bonds that are not fully coordinated. They can be attributed to defective oxygen places in the crystal lattice, crystalline water, or –OH groups, which form in a few first monolayers on the oxide surface.32 The total amount of non-lattice oxygen species is 19%. The Ce 3d (Fig. 3C) signal shows a large amount of satellite signals, which is typical of transition metals.33 CuO formed a much cleaner phase with a low amount of surface contaminants in the form of carbonate. The amount of non-lattice oxygen species is close to CeO2, around 23%. Cu 3p spectra (Fig. 3F) did not reveal the presence of lower oxidation states with 2p1/2–2p3/2 peak splitting of 20 eV, corresponding to the oxidation state of the CuO phase.34
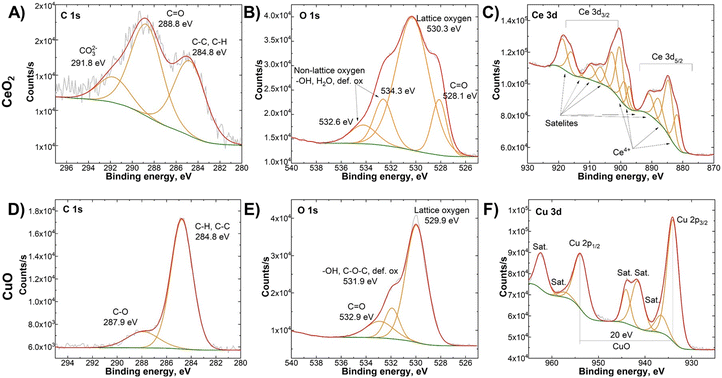 | ||
| Fig. 3 X-ray photoelectron spectroscopy (XPS) spectra of CeO2 (A–C – upper row) and CuO (D–F – lower row). XPS survey graphs can be found in the ESI† (Fig. S3). | ||
Fig. 4 shows the C 1s, O 1s, Ce 3d, and Cu 2p XPS spectra of CeO2/CuO. The Ce 3d (Fig. 4D) and Cu 2p (Fig. 4C) XPS spectra revealed no significant differences from pure oxide phases. However, the O 1s (Fig. 4B) spectra reveal a large presence of non-lattice oxygen species of 54% in comparison to 19–23% in pure oxide phases, revealing a significant presence of surface defects in the CeO2/CuO composite. Based on the findings reported in the literature, the addition of copper to CeO2/CuO leads to an increase in defects compared to pure CeO2.17,18 However, relatively high non-lattice oxygen species CuO also show that synthesis parameters allow obtaining M/NPs with significant surface defects.
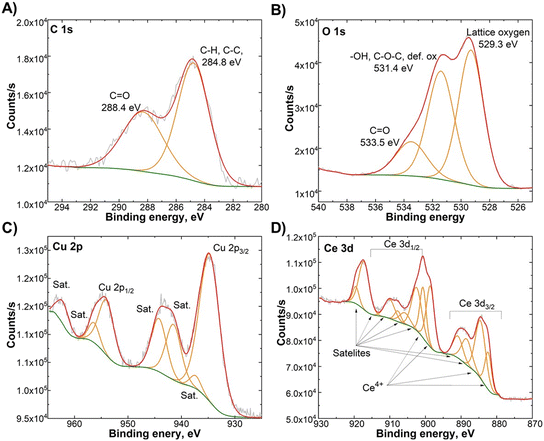 | ||
| Fig. 4 X-ray photoelectron spectroscopy (XPS) spectra of the CeO2/CuO composite: A) C 1s, B) O 1s, C) Cu 2p, and D) Ce 3d. XPS survey graph can be found in the ESI† (Fig. S3). | ||
3.4. ζ-Potential, hydrodynamic size and solubility of the synthesized materials
The hydrodynamic size of CeO2, CuO, and CeO2/CuO depended on the dispersion medium being smallest in DI water (344–782 nm) and DMEM (188–992 nm) and largest in 2% NaCl (up to 2200 nm). The hydrodynamic size remarkably increased in time: 24 h incubation in 2% NaCl and DMEM increased the hydrodynamic size even to 10–30 μm (Table 1).| pH | ζ-Potential, mV | Polydispersity index (Pdl) | Hydrodynamic size, nm | Solubilized Cu from 100 mg L−1 M/NPs, mg L−1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1000 mg L−1 | As prepared | 24 h | As prepared | 24 h | As prepared | 24 h | 2h | 24 h | |
| n.a. not measured.a Measured without CO2 at room temperature. | |||||||||
| In DI water | |||||||||
| CeO2 | 7.20 | −22.9 | 15.4 | 0.34 | 0.57 | 344 | 539 | 0 | 0 |
| CuO | 6.90 | −10.7 | −5.2 | 0.41 | 0.55 | 604 | 879 | 0.20 | 0.24 |
| CeO2/CuO | 6.80 | 4.0 | 9.1 | 0.63 | 0.56 | 762 | 1006 | 0.35 | 0.32 |
| In 2% NaCl | |||||||||
| CeO2 | 6.99 | −1.4 | n.a. | 0.47 | 0.47 | 1980 | 28736 | n.a. | n.a. |
| CuO | 7.00 | −5.8 | n.a. | 0.87 | 0.19 | 2200 | 10308 | 0.50 | 0.98 |
| CeO2/CuO | 7.02 | −3.4 | n.a. | 0.81 | 0.19 | 1840 | 10407 | 0.53 | 1.00 |
| In DMEM | |||||||||
| CeO2 | 8.54a | −9.2 | 13.3 | 0.13 | 0.08 | 189 | 1484 | n.a. | n.a. |
| CuO | 8.48a | −19.3 | −5.9 | 0.08 | 0.20 | 993 | 1288 | 0.15 | 0.23 |
| CeO2/CuO | 8.42a | 9.6 | 7.7 | 0.05 | 0.26 | 227 | 1810 | 0.88 | 0.74 |
The ζ-potential of as-prepared suspensions of CuO and CeO2 in DI water, 2% NaCl and DMEM was negative. However, the CeO2/CuO exhibited a slightly positive surface charge in both DI water and DMEM, but showed a negative ζ-potential in 2% NaCl (Table 1). Notably, surface charge plays a significant role in driving antimicrobial activity: as the bacterial surface has a negative charge, positively charged particles are more tightly associated with microbial cells than negatively charged particles.27,35,36 In addition, in our previous studies, we showed that positively charged hematite NPs (but not negatively charged ones) in DI water showed higher antimicrobial activity towards Gram-negative bacteria E. coli than to Gram-positive bacteria S. aureus.27
It is widely accepted that the toxic effect of well-known antimicrobial M/NPs such as CuO, ZnO, and nAg is also driven by metal ions dissolved and released from those particles.37 However, not all metal oxides are soluble. For example, CeO2 has extremely low solubility and undergoes partial dissolution only under specific conditions such as low pH.38 Also in this study we did not detect any released Ce from CeO2/CuO after 2 and 24 h incubation in DI water (data not shown).
Concerning the shedding of Cu-ions from CuO and CeO2/CuO there were differences depending on the incubation/toxicity testing medium. More specifically, if 100 mg L−1 or CuO or CeO2/CuO was incubated for 2 and 24 h in DI water, the concentration of Cu-ions was about 0.2–0.3 mg Cu per L and did not change in time. In 2% NaCl, however, the shedding of Cu-ions increased from 0.5 mg Cu per L at 2 h to 1 mg Cu per L in 24 h (Table 1). Interestingly, the shedding of Cu ions from CeO2/CuO in DMEM medium was up to 6 times higher than from CuO, though the final concentration of Cu-ions from CeO2/CuO in DMEM was lower (0.7–0.8 mg Cu per L) than in 2% NaCl (1 mg Cu per L) (Table 1). The latter can be explained by Cu2+ binding to organic ligands present in the cell culture medium, leading to reduced bioavailability and cytotoxicity.39,40 Overall, the observed differences in solubility, surface charge, and agglomeration between CuO and CeO2/CuO likely contributed to their different toxic effects on microbes and human keratinocytes.
3.5. Antibacterial activity
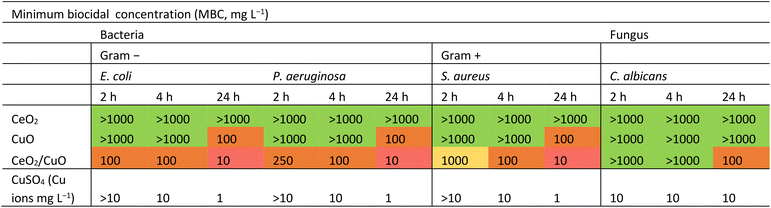
According to MBC values (Table 2), pristine CeO2 did not exhibit antibacterial activity against any tested bacteria, even at the highest concentration (1000 mg L−1). Our findings align with some of the previously published results, showing no significant antibacterial activity of CeO2 against S. aureus and E. coli.5 Different from pure CeO2, CeO2/CuO exhibited antibacterial activity against all tested bacteria after 4 and 24 h with MBC of 100 mg L−1 and 10 mg L−1, respectively (Table 2). Moreover, compared with CuO, CeO2/CuO also had higher antibacterial activity at all the tested timepoints (Table 2). In the current study, CeO2/CuO showed higher antimicrobial activity against Gram-negative bacteria E. coli and P. aeruginosa than against Gram-positive bacteria S. aureus.Thus, combining non-bactericidal CeO2 with CuO yielded a CeO2/CuO composite with enhanced antibacterial properties.
Similar to findings in other studies, which have demonstrated that composites often achieve superior performance compared to their components,14 our study also reveals that the CeO2/CuO composite requires less material than CuO alone to achieve comparable antibacterial activity against both Gram-negative and Gram-positive bacteria. The fact that CeO2/CuO required less material than CuO to achieve the same antibacterial activity against Gram-negative bacteria and Gram-positive bacteria offers practical application benefits and may potentially lower the environmental impact. However, further experiments and investigations are needed to fully assess and confirm these environmental advantages.
3.6. Antifungal activity
Neither CeO2 nor CuO showed antifungal activity against C. albicans up to 1000 mg L−1. Only CeO2/CuO showed some antifungal properties with 24 h MBC of 100 mg L−1 (Table 2). Thus, the enhanced antifungal efficacy of CeO2/CuO, though at higher concentrations than the antibacterial ones (Table 2), was observed. The lower sensitivity of C. albicans to CuO can be explained by the fact that C. albicans has resistance to Cu ions due to its predominant use as a P1-type ATPase (Cu transporter) to counteract the toxicity of copper ions.41We suggest that the higher antibacterial and antifungal efficacy of CeO2/CuO compared to CuO and CeO2 most likely originated from a combination of slightly positive surface charge, higher SSA than CuO (section 3.2.), and Cu dissolution (Table 1). However, considering the MBC values for Cu ions (4 and 24 h MBC was 10 mg Cu per L and 1 mg Cu per L for bacteria, respectively, and for C. albicans 4 and 24 h MBC = 10 mg Cu per L; Table 2) and Cu ions shedding from CuO (2 h 0.20 mg Cu per L and 24 h 0.24 mg Cu per L, section 3.2., Table 1) and CeO2/CuO (2 h 0.35 mg Cu per L and 24 h 0.32 mg Cu per L, section 3.2., Table 1), the released Cu-ions were not the main contributor to the antimicrobial action of CeO2/CuO.
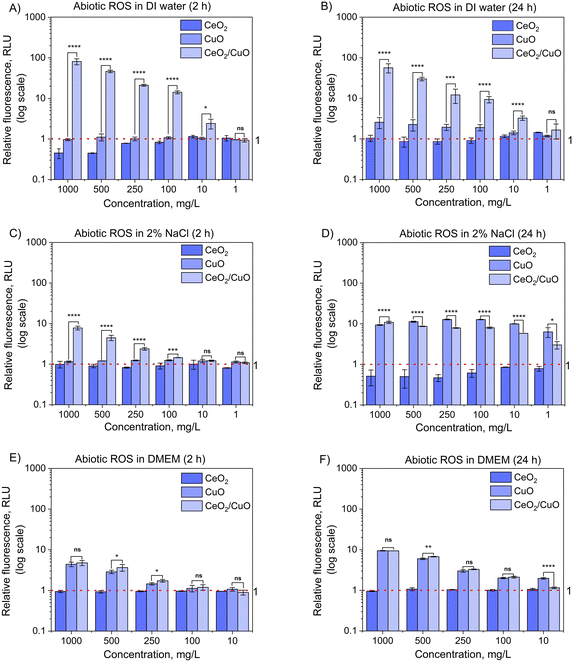
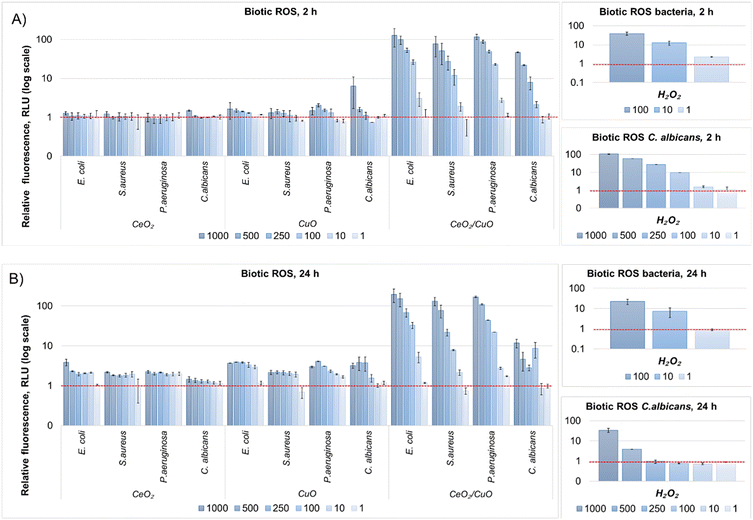
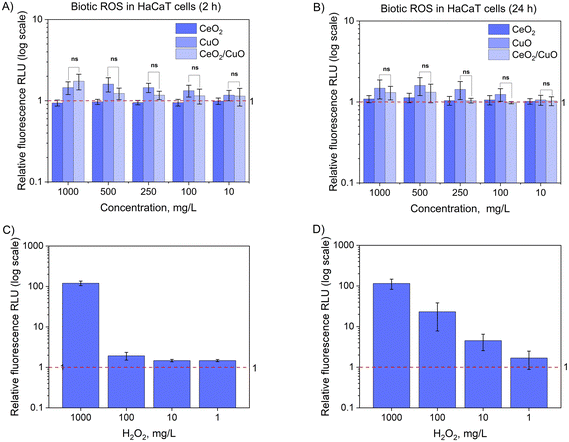
3.7. Abiotic and biotic reactive oxygen species (ROS)
Due to the importance of ROS in the antibacterial activity of metal oxide M/NPs,42 the ability of CeO2, CuO, and CeO2/CuO to produce ROS under abiotic conditions (i.e. no living cells present) and biotic (i.e. with living cells) conditions was studied.The fact that CuO exhibited variable ROS production depending on the test medium and treatment time indicates that environmental conditions play a significant role in CuO ROS production and may also affect the antimicrobial activity of this material.
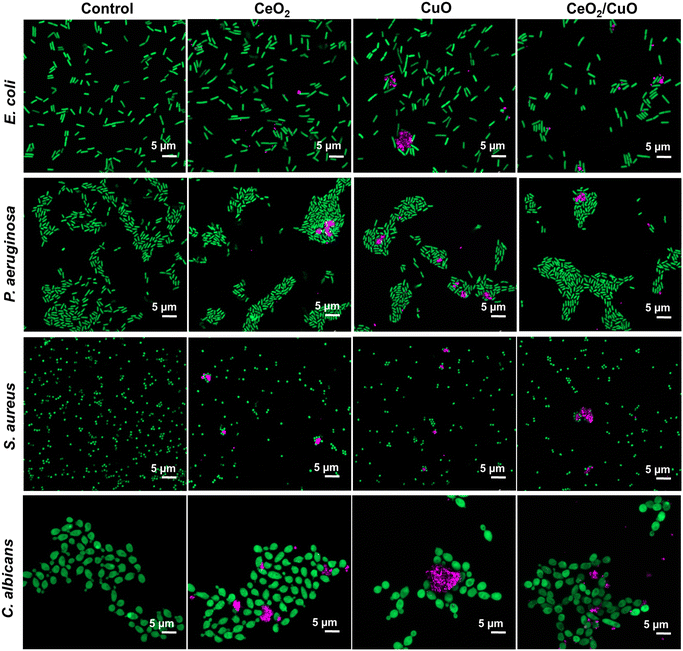
3.8. Confocal laser scanning microscopy (CLSM)
CLSM was used to visualize the interactions between the studied materials and microorganisms. When used in reflection mode, this method enables the simultaneous detection of fluorescently stained bacteria and fungi, along with reflective signals from M/NPs.35CLSM imaging showed CeO2, CuO, and CeO2/CuO interactions with microorganisms after 2 h exposure in DI water (Fig. 7). The concentration of M/NPs used for microscopy was 100 mg L−1, which for CeO2/CuO corresponded 2 h MBC value (Table 2).
Although enhanced cellular interactions of CeO2/CuO were expected because of their slightly positive surface charge (Table 1), there were no differences in their cellular association pattern compared to CeO2 and CuO. CLSM images also confirmed the results of DLS (Table 1) on the heterogeneity in size of all tested M/NPs.
3.9. Toxicity to environmental bacteria Vibrio fischeri
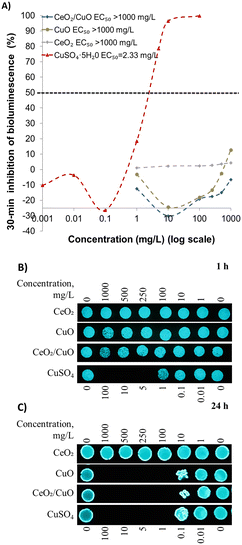
The toxicity of CeO2, CuO, and CeO2/CuO towards environmentally relevant bacterium V. fischeri was evaluated by a 15 s and 30 min bioluminescence inhibition assay (a flash Assay). After the flash test the bacteria were further incubated in the test suspensions with M/NPs for 1 and 24 h and bacterial viability was assessed by the colony formation ability on an agarised growth medium. The V. fischeri bioluminescence rapidly decreases in response to toxicants in a dose-effect manner, providing a quick, cost-effective, and sensitive screening method for toxicity. Its response to toxicants correlates reasonably well with other ecotoxicity tests (such as algal and crustacean tests).46,47 Our results showed that during the first 15 s and 30 min of exposure neither CeO2, CuO, nor CeO2/CuO inhibited V. fischeri bioluminescence at concentrations up to 1000 mg L−1, showing no acute adverse effects (Fig. 8A). Importantly, the positive control, Cu-ions, showed high toxicity; 30 min EC50 (half-effective concentration) was 2.33 mg Cu per L (Fig. 8A).We also evaluated the viability of V. fischeri after prolonged exposure to CeO2, CuO, and CeO2/CuO for 1 and 24 h using a spot test. The results showed that exposure for 1 h did not induce toxicity (i.e. bacteria yielded colonies on the agarised medium). However, after 24 h of exposure, CeO2 still did not induce any toxic effects, but there was no growth of bacteria exposed to CuO and CeO2/CuO at 100 mg L−1 and higher (Fig. 8B and C) being different from the spot-test results for other microorganisms shown in Table 2. where the toxic effects of CeO2/CuO were not comparable to that of CuO. We suggest that possibly in the case of V. fischeri the toxicity of both CuO and CeO2/CuO is driven by released Cu ions. The latter could be due to the relatively high shedding of Cu-ions in 2% NaCl (section 3.4., Table 1). Test results of the experiment with CuSO4 as a Cu ion source show that Cu ions' 24 h MBC = 1 mg L−1 (Fig. 8C). This value is comparable to the solubility of Cu ions from CuO (24 h 0.98 mg Cu per L) and CeO2/CuO (24 h 1 mg Cu per L) (section 3.4, Table 1), demonstrating the crucial role of Cu ion release in toxicity to V. fischeri. Also, in an earlier study, it was shown that V. fischeri is sensitive to Cu ions.48 In follow-up studies to establish the ecotoxicity of the composite, more detailed tests can be conducted to assess the broader environmental impact using other ecologically relevant species (e.g., algae, crustaceans).
3.10. Cytotoxicity to human keratinocytes in vitro
Cytotoxicity towards human cells is an important parameter, especially for materials intended to be used as antimicrobials in medical settings. In essence, an antimicrobial material should exhibit a balance between effectively targeting and eliminating harmful microorganisms while being safe for non-target organisms. The cytotoxicity of CeO2, CuO, and CeO2/CuO was assessed using an in vitro assay with the human keratinocyte cell line (HaCaT). Keratinocytes were chosen due to their importance in the case of a skin exposure scenario, and following ISO 10993-5 recommendation,29 a decrease of cell viability by less than 30% was considered non-cytotoxic. The results indicated that pristine CeO2 did not demonstrate any cytotoxicity even at the highest tested concentration of 1000 mg L−1 (Fig. 9).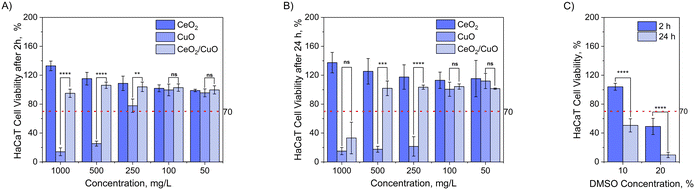 | ||
| Fig. 9 Viability of the HaCaT keratinocyte cell line after incubation with CeO2, CuO, and CeO2/CuO for A) 2 h and B) 24 h, performed in triplicate/quadruplicate. DMSO (C) was used as a positive control. The asterisk (*) represents the p-value of the statistical test. No significant difference (NS) means that the p-value >0.05. One asterisk indicates that the p-value ≤0.05. Two asterisks indicate that the p-value ≤0.01. Three asterisks mean the p-value is ≤0.001, and four asterisks mean the p-value is ≤0.0001. Visualisation of the neutral red uptake (NRU) assay with HaCaT cells (Fig. S6†) and viability of the HaCaT keratinocytes cell line after incubation with Cu ions (Fig. S7†) can be found in the ESI.† | ||
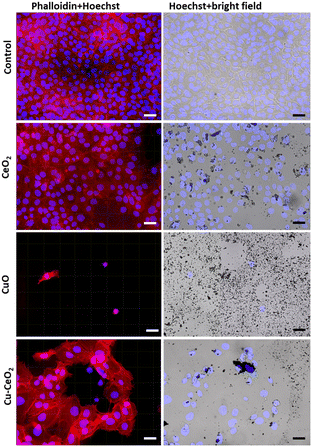
These results agree with most of the previously published cytotoxicity data demonstrating that CeO2 NPs act as low cytotoxicity anti-inflammatory agents49,50 and cellular antioxidants across multiple compartments.51 Interestingly though, also other clearly contradictory cellular effects involving oxidative stress induction and some cytotoxicity52 have been suggested for CeO2. However, the level of cell death due to CeO2 M/NPs has been indeed relatively low reaching only up to 20%53 and therefore, according to our classification,29 no cytotoxicity would have been reported in this case. In order to visually confirm that the cell morphology was not affected even at the highest CeO2 concentration, microscopy images were taken from CeO2 exposed cells after 24 h of exposure (Fig. 10). Evidently, no effects on cell morphology were detected due to exposure to CeO2, although deposition of M/NPs on cells was observed under bright field (Fig. 10).
Similar to CeO2, CeO2/CuO showed no cytotoxicity after 2 h, but after 24 h cytotoxicity was detected only at the highest tested concentration, 1000 mg L−1 (Fig. 9). Microscopical visualization confirmed that indeed, some of the HaCaT cells were damaged after 24 h exposure to 1000 mg L−1 of CeO2/CuO (Fig. 10) and that there was a significant particle deposition on and near the cells. Comparison of the antimicrobial effects showed that CeO2/CuO exhibited 24 h MBC towards bacteria E. coli, S. aureus, and P. aeruginosa already at 10 mg L−1 and towards fungus C. albicans at 100 mg L−1 (section 3.5., Table 2). Therefore, due to the difference between MBC and cytotoxicity values, CeO2/CuO could be considered a promising antimicrobial material. Compared to CeO2/CuO, CuO showed significantly higher cytotoxicity already after 2 h of exposure, where only 25% of cells survived the exposure to 500 mg L−1 (Fig. 9A). After 24 h of exposure, notable cytotoxicity of CuO was detected already at 250 mg L−1 (Fig. 9B). Apparent cellular damage can also be seen under microscopy, where after 24 h of exposure to 1000 g L−1 CuO only a few attached but likely dead cells were observed (Fig. 10).
Indeed, the cytotoxic effects of CuO NPs are well known, and this effect has been attributed both to abiotic copper ion release and the internalization of CuO NPs and their intracellular dissolution.37 In our study, internalization of CuO was not possible to be determined, but evident deposition of those particles on cells was seen under a microscope. The fact that the antimicrobial effects and cytotoxicity of CuO were detected at comparable concentrations54 suggests that these M/NPs would not be preferred for antimicrobial use. Interestingly, no clear ROS signal was detected in the case of any M/NP exposure (Fig. 11), and thus, oxidative stress was likely not the main cause of cell death in the presence of CuO and higher concentrations of CeO2/CuO. HaCaT cells were used as a model cell line for dermal exposure. However, the use of only one cell line does not allow to reveal all potential modes of action of the tested M/NPs and a thorough toxicological characterization would require the use of different cell types as well as observation of subtoxic endpoints.
4. Conclusions
Our study demonstrated that the synthesised CeO2/CuO nanostructured composite (but not CuO and CeO2 individually) demonstrated remarkable antimicrobial efficiency. Indeed, the CeO2/CuO composite exhibited antibacterial efficacy at a MBC of 100 mg L−1 upwards and showed antibacterial efficacy towards E. coli already after 2 h of contact and towards P. aeruginosa and S. aureus after 4 h of contact. Fungus C. albicans was inhibited after a longer contact time (24 h MBC = 100 mg L−1). Importantly, CeO2/CuO displayed no cytotoxic effects on HaCaT cells after 2 h even at 1000 mg L−1 and after 24 h at 500 mg L−1. The antimicrobial action of CeO2/CuO was mainly due to remarkable ROS production induced by a high proportion (54%) of non-lattice oxygen species due to increased surface defects within the composite. Thus, CeO2/CuO may be a promising composite material for antimicrobial surface coatings.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
SV – investigation, validation, visualization, writing – original draft, conceptualization, formal analysis, methodology. AŠ – project administration, writing – review and editing. MI – formal analysis, visualization, writing – original draft. LO, AP, MO, MS, HV – formal analysis. AN – formal analysis, writing – review and editing. AI – methodology, validation, writing – review and editing. AK – methodology, writing – review and editing. KK – methodology, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the M-ERA.NET funding mechanism (Project CaFeOx) No. ES RTD/2021/11. This work was also supported by the European Cooperation in Science and Technology (COST) Action CA18130 ENFORCE-TXRF and COST Action CA20130 EURO-MIC, the Estonian Research Council grant PRG749, TEM-TA55 and the Estonian Ministry of Education and Research grant TK210.References
- A European One Health Action Plan Against Antimicrobial Resistance (AMR) 2017 plan https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf Search PubMed.
- Antimicrobial resistance in the EU/EEA (EARS-Net) - Annual epidemiological report for 2021 https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2021 Search PubMed.
- World health organization https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance Search PubMed.
- A. Rokas, Evolution of the human pathogenic lifestyle in fungi, Nat. Microbiol., 2022, 7, 607–619 CrossRef CAS PubMed.
- A. Nefedova, K. Rausalu, E. Zusinaite, A. Vanetsev, M. Rosenberg, K. Koppel, S. Lilla, M. Visnapuu, K. Smits, V. Kisand, T. Tätte and A. Ivask, Antiviral efficacy of cerium oxide nanoparticles, Sci. Rep., 2022, 12, 18746 CrossRef CAS.
- T. Yadavalli and D. Shukla, Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections, Nanomed.: Nanotechnol., Biol. Med., 2017, 13, 219–230 CrossRef CAS PubMed.
- T. Xia, M. Kovochich, M. Liong, L. Mädler, B. Gilbert, H. Shi, J. I. Yeh, J. I. Zink and A. E. Nel, Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties, ACS Nano, 2008, 2, 2121–2134 CrossRef CAS.
- K. Maduray and R. Parboosing, Metal Nanoparticles: a Promising Treatment for Viral and Arboviral Infections, Biol. Trace Elem. Res., 2021, 199, 3159–3176 CrossRef CAS PubMed.
- H. Kotrange, A. Najda, A. Bains, R. Gruszecki, P. Chawla and M. M. Tosif, Metal and Metal Oxide Nanoparticle as a Novel Antibiotic Carrier for the Direct Delivery of Antibiotics, Int. J. Mol. Sci., 2021, 22, 9596 CrossRef CAS PubMed.
- M. Milovanovic, A. Arsenijevic, J. Milovanovic, T. Kanjevac and N. Arsenijevich, Nanoparticles in Antiviral Therapy, Antimicrob. Nanoarchitecton., 2017, 383–410 CAS.
- X. Yang, Q. Yu, W. Gao, X. Tang, H. Yi and X. Tang, The mechanism of metal-based antibacterial materials and the progress of food packaging applications: A review, Ceram. Int., 2022, 48, 34148–34168 CrossRef CAS.
- L. Strasfeld and S. Chou, Antiviral Drug Resistance: Mechanisms and Clinical Implications, Infect. Dis. Clin. North Am., 2010, 24, 413–437 CrossRef.
- K. Juganson, A. Ivask, I. Blinova, M. Mortimer and A. Kahru, NanoE-Tox: New and in-depth database concerning ecotoxicity of nanomaterials, Beilstein J. Nanotechnol., 2015, 6, 1788–1804 CrossRef CAS.
- S. Stankic, S. Suman and F. Haque, et al., Pure and multi metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties, J. Nanobiotechnol., 2016, 14, 73 CrossRef PubMed.
- Y. Mao, T. J. Park and S. S. Wong, Synthesis of classes of ternary metal oxidenanostructures, Chem. Commun., 2005, 5721–5735 RSC.
- M. Konsolakis, The role of Copper–Ceria interactions in catalysis science: Recent theoretical and experimental advances, Appl. Catal., B, 2016, 198, 49–66 CrossRef CAS.
- X. Zhang, L. Su and Y. Kong, et al., CeO2 nanoparticles modified by CuO nanoparticles for low-temperature CO oxidation with high catalytic activity, J. Phys. Chem. Solids, 2020, 147, 109651 CrossRef CAS.
- Y. Du, F. Gao, Y. Zhou, H. Yi, X. Tang and Z. Qi, Recent advance of CuO-CeO2 catalysts for catalytic elimination of CO and NO, J. Environ. Chem. Eng., 2021, 9, 106372 CrossRef CAS.
- A. Ahmad, M. S. Javed, S. Khan, T. M. Almutairi, A. A. A. Mohammed and R. Luque, Green synthesized Ag decorated CeO2 nanoparticles: Efficient photocatalysts and potential antibacterial agents, Chemosphere, 2023, 310, 136841 CrossRef CAS PubMed.
- S. M. F. Khyrun, A. Jegatha Christy, R. Usha, L. C. Nehru and S. Suresh, Novel solution combustion synthesis of CeO2/CuO nanocomposite for photocatalytic and biological applications, Opt. Mater., 2023, 139, 113756 CrossRef CAS.
- A. Norbert, S. M. Alappatt, S. S. John, S. Shaji, S. K. Remillard, U. P. Deshpande and R. R. Philip, Phytosynthesized Cu-Doped Cerium Oxide Nanoparticles for Antibacterial Application, Phys. Status Solidi A, 2023, 220, 2200731 CrossRef CAS.
- E. P. Gusliani, R. Yetria, S. Syukri, A. Labanni and S. Arief, Highly antimicrobial activity of cerium oxide nanoparticles synthesized using Moringa oleifera leaf extract by a rapid green precipitation method, J. Mater. Res. Technol., 2021, 15, 2355–2364 CrossRef.
- S. K. Kannan and M. Sundrarajan, A Green Approach for the Synthesis of a Cerium Oxide Nanoparticle: Characterization and Antibacterial Activity, Int. J. Nanosci., 2014, 13, 1450018 CrossRef CAS.
- X. Zheng, X. Zhang, X. Wang, S. Wang and S. Wu, Preparation and characterization of CuO/CeO2 catalysts and their applications in low-temperature CO oxidation, Appl. Catal., A, 2005, 295, 142–149 CrossRef CAS.
- K. Kasemets, S. Suppi, K. Künnis-Beres and A. Kahru, Toxicity of CuO Nanoparticles to Yeast Saccharomyces cerevisiae BY4741 Wild-Type and Its Nine Isogenic Single-Gene Deletion Mutants, Chem. Res. Toxicol., 2013, 26, 356–367 Search PubMed.
- S. Suppi, K. Kasemets, A. Ivask, K. Künnis-Beres, M. Sihtmäe, I. Kurvet, V. Aruoja and A. Kahru, A novel method for comparison of biocidal properties of nanomaterials to bacteria, yeasts and algae, J. Hazard. Mater., 2015, 286, 75–84 CrossRef CAS.
- S. Vihodceva, A. Šutka, M. Sihtmae, M. Rosenberg, M. Otsus, I. Kurvet, K. Smits, L. Bikse, A. Kahru and K. Kasemets, Antibacterial Activity of Positively and Negatively Charged Hematite (α-Fe2O3) Nanoparticles to Escherichia coli, Staphylococcus aureus and Vibrio fischeri, Nanomaterials, 2021, 11, 652 CrossRef CAS PubMed.
- ISO 21338:2010—Water Quality—Kinetic Determination of the Inhibitory Effects of Sediment, Other Solids and Coloured Samples on the Light Emission of Vibrio fischeri (Kinetic Luminescent Bacteria Test), International Organization for Standardization, Geneva, Switzerland, 2010 Search PubMed.
- ISO 10993-5:2009 Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity https://www.iso.org/standard/36406.html Search PubMed.
- G. Ates, T. Vanhaecke, V. Rogiers and R. M. Rodrigues, Assaying Cellular Viability Using the Neutral Red Uptake Assay, Methods Mol. Biol., 2017, 1601, 19–26 CrossRef CAS.
- S. Hussaini and J. Dvorkin, Specific surface area versus porosity from digital images: High-porosity granular samples, J. Pet. Sci. Eng., 2021, 108961 CrossRef CAS.
- M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. St. C. Smart, Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn, Appl. Surf. Sci., 2010, 257, 887–898 CrossRef CAS.
- R. Eloirdi, P. Cakir, F. Huber, A. Seibert, R. Konings and T. Gouder, X-ray photoelectron spectroscopy study of the reduction and oxidation of uranium and cerium single oxide compared to (U-Ce) mixed oxide films, Appl. Surf. Sci., 2018, 457, 566–571 CrossRef CAS.
- J. Yuan, J. J. Zhang, M. P. Yang, W. J. Meng, H. Wang and J. X. Lu, CuO Nanoparticles Supported on TiO2 with High Efficiency for CO2 Electrochemical Reduction to Ethanol, Catalysts, 2018, 8, 171 CrossRef CAS.
- K. Kasemets, S. Käosaar, H. Vija, U. Fascio and P. Mantecca, Toxicity of differently sized and charged silver nanoparticles to yeast Saccharomyces cerevisiae BY4741: a nano-biointeraction perspective, Nanotoxicology, 2019, 13, 1041–1059 CrossRef CAS.
- W. Zhang, J. Hughes and Y. Chen, Impacts of Hematite Nanoparticle Exposure on Biomechanical, Adhesive, and Surface Electrical Properties of Escherichia coli Cells, Appl. Environ. Microbiol., 2012, 78, 3905–3915 CrossRef CAS PubMed.
- O. Bondarenko, K. Juganson, A. Ivask, K. Kasemets, M. Mortimer and A. Kahru, Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review, Arch. Toxicol., 2013, 87, 1181–1200 CrossRef CAS PubMed.
- T. V. Plakhova, A. Y. Romanchuk, S. N. Yakunin, T. Dumas, S. Demir and S. Wang, et al., Solubility of Nanocrystalline Cerium Dioxide: Experimental Data and Thermodynamic Modeling, J. Phys. Chem. C, 2016, 120, 22615–22626 CrossRef CAS.
- A. Thit, H. Selck and H. F. Bjerregaard, Toxic mechanisms of copper oxide nanoparticles in epithelial kidney cells, Toxicol. In Vitro, 2015, 29, 1053–1059 CrossRef CAS.
- C. Gunawan, W. Y. Teoh, C. P. Marquis and R. Amal, Cytotoxic Origin of Copper(II) Oxide Nanoparticles: Comparative Studies with Micron-Sized Particles, Leachate, and Metal Salts, ACS Nano, 2011, 5, 7214–7225 CrossRef CAS.
- P. J. Riggle and C. A. Kumamoto, Role of a Candida albicans P1-Type ATPase in Resistance to Copper and Silver Ion Toxicity, J. Bacteriol., 2000, 182, 4899–4905 CrossRef CAS.
- D. M. Aruguete, B. Kim and M. F. Hochella, et al., Antimicrobial nanotechnology: its potential for the effective management of microbial drug resistance and implications for research needs in microbial nanotoxicology, Environ. Sci.: Processes Impacts, 2013, 15, 93–102 RSC.
- M. Das, S. Patil, N. Bhargava, J. F. Kang, L. M. Riedel, S. Seal and J. J. Hickman, Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons, Biomaterials, 2007, 28, 1918–1925 CrossRef CAS.
- X. Liu, J. Tang, L. Wang and J. Giesy, Mechanisms of oxidative stress caused by CuO nanoparticles to membranes of the bacterium Streptomyces coelicolor M145, Ecotoxicol. Environ. Saf., 2018, 158, 123–130 CrossRef CAS.
- V. Prasanna and R. Vijayaraghavan, Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark, Langmuir, 2015, 31, 9155–9162 CrossRef PubMed.
- K. A. D. Kusumahastuti, M. Sihtmäe, V. Aruoja, N. Gathergood and A. Kahru, Ecotoxicity profiling of a library of 24 l-phenylalanine derived surface-active ionic liquids (SAILs), Sustainable Chem. Pharm., 2021, 19, 100369 CrossRef.
- V. Aruoja, M. Sihtmäe, H.-C. Dubourguier and A. Kahru, Toxicity of 58 substituted anilines and phenols to algae Pseudokirchneriella subcapitata and bacteria Vibrio fischeri: Comparison with published data and QSARs, Chemosphere, 2011, 84, 1310–1320 CrossRef CAS.
- I. Kurvet, A. Ivask, O. Bondarenko, M. Sihtmäe and A. Kahru, LuxCDABE—Transformed Constitutively Bioluminescent Escherichia coli for Toxicity Screening: Comparison with Naturally Luminous Vibrio fischeri, Sensors, 2011, 11, 7865–7878 CrossRef CAS.
- M. J. Akhtar, M. Ahamed and H. Alhadlaq, Anti-Inflammatory CeO2 Nanoparticles Prevented Cytotoxicity Due to Exogenous Nitric Oxide Donors via Induction Rather Than Inhibition of Superoxide/Nitric Oxide in HUVE Cells, Molecules, 2021, 6, 5416 CrossRef PubMed.
- C. Eilenberger, F. Selinger, M. Rothbauer, Y. Lin, A. Limbeck, B. Schädl, J. Grillari, N. S. Kavok, V. K. Klochkov, Y. V. Malyukin, V. Margitich and P. Ertl, Cytotoxicity, Retention, and Anti-inflammatory Effects of a CeO2 Nanoparticle-Based Supramolecular Complex in a 3D Liver Cell Culture Model, ACS Pharmacol. Transl. Sci., 2021, 4, 101–106 CrossRef CAS PubMed.
- S. Singh, A. Kumar, A. Karakoti, S. Seal and W. T. Self, Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxidenanoparticles, Mol. BioSyst., 2010, 6, 1813–1820 RSC.
- G. Cheng, W. Guo, L. Han, E. Chen, L. Kong, L. Wang, W. Ai, N. Song, H. Li and H. Chen, Cerium oxide nanoparticles induce cytotoxicity in human hepatoma SMMC-7721 cells via oxidative stress and the activation of MAPK signaling pathways, Toxicol. In Vitro, 2013, 27, 1082–1088 CrossRef CAS PubMed.
- M. Sandeep and A. K. Pandey, Cerium Oxide Nanoparticles Induced Toxicity in Human Lung Cells: Role of ROS Mediated DNA Damage and Apoptosis, BioMed Res. Int., 2014, 891934 Search PubMed.
- A. L. Kubo, G. Vasiliev, H. Vija, J. Krishtal, V. Tõugu, M. Visnapuu, V. Kisand, A. Kahru and O. M. Bondarenko, Surface carboxylation or PEGylation decreases CuO nanoparticles' cytotoxicity to human cells in vitro without compromising their antibacterial properties, Arch. Toxicol., 2020, 94, 1561–1573 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00501e |
| This journal is © The Royal Society of Chemistry 2025 |