Nanoscale zero-valent iron composites for uranium-contaminated water treatment and environmental remediation: a review
Yinshan
Zhu
ab,
Shusen
Chen
c,
Ziming
Li
c,
Hao
Li
c,
Muhammad
Shaban
ab and
Changlun
Chen
 *ad
*ad
aInstitute of Plasma Physics, HFIPS, Chinese Academy of Sciences, P.O. Box 1126, Hefei 230031, PR China. E-mail: clchen@ipp.ac.cn; Fax: +86 551 65591310; Tel: +86 551 65592788
bUniversity of Science and Technology of China, Hefei 230026, PR China
cCNNC Key Lab Uranium Extract Seawater, Beijing Research Institute of Chemical Engineering Metallurgy, CNNC, Beijing 101199, PR China
dCollaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, Soochow University, Suzhou 215123, PR China
First published on 24th September 2024
Abstract
With the development of nuclear power and the nuclear industry, some uranium-containing wastewater will inevitably be released into the environment, which poses a threat to human health and the environment. Nanoscale zero-valent iron (nZVI) and its composites can be used to treat uranium-contaminated water because of its large specific surface area, many active centres and high adsorption capacity. This review proposed a matrix loaded zero-valent iron, including bimetals, inorganic materials, carbon materials, and organic and porous framework materials. The latest research progress of various nZVI-based composites in removing uranium from aqueous solutions was reviewed. A detailed introduction to the preparation methods, removal mechanisms, and environmental factors affecting the removal performance of nZVI and its composite materials was provided. The advantages and disadvantages of nZVI and its composite materials for uranium removal were analyzed, and loading methods conducive to solving the dispersion and agglomeration problems of nZVI were emphasized. Finally, the application prospects of nZVI and its composites in the remediation of uranium-contaminated water were briefly proposed, and the application prospects in environmental remediation were discussed.
Environmental significanceWith the development of nuclear power and the nuclear industry, some uranium-containing wastewater will inevitably be released into the environment, which poses a threat to human health and the environment. Nanoscale zero-valent iron (nZVI) and its composites can be used to treat uranium-contaminated water because of its large specific surface area, many active centers and high adsorption capacity. However, there is no systematic review on the removal of uranium by nZVI and its mechanisms, so it is necessary to summarize the research on nZVI and its composites in recent years. The latest research progress of various nZVI-based composites in removing uranium from aqueous solutions was reviewed. It is hoped to make more positive contributions to the promotion and application of nanoscale zero-valent iron composites for uranium-contaminated water treatment and environmental remediation. |
1. Introduction
Uranium is a crucial resource for the nuclear energy industry and comes mainly from uranium mines. The application of uranium mining in the nuclear energy industry will result in the release of uranium into the environment.1 Even though uranium is deposited on the surface of minerals as an insoluble mineral, it can move and dissolve with the host through the synergistic effects of weathering, rainfall, and biological activity, and enter the aquifer through soil–water interactions and leaching processes.2 Uranium belongs to actinide in the periodic table and is a radioactive toxic heavy metal element.3 Due to its chemical and radiation properties, even if a small amount of uranium is ingested in wastewater, it will seriously harm health. Residents may be at risk of liver cancer, bone marrow cancer and lung cancer after ingesting uranium.4 Excess uranium can lead to various health problems, such as metabolic disorders, cell death, genetic damage and so on. Under the condition of oxidation, U(VI) exists in the form of adsorption, coordination and precipitation, and has high toxicity. U(VI) is highly soluble, highly toxic, and exists in the form of UO22+, while U(IV) is relatively insoluble.5,6 The most attractive method for removing uranium from wastewater is to reduce U(VI) to U(VI). To promote the development of the nuclear industry, it is urgent to explore new materials with high efficiency, low cost, simple process and long-term recycling for the treatment of uranium-contaminated water.nZVI and its composites have been extensively studied. As a new type of nano-adsorbent, nZVI has the features of a large specific surface area and strong reducibility.7 Unlike macroscopic materials, it has unique properties such as quantum effects, surface effects, volume effects, and macroscopic quantum tunnelling effects.8 Due to its large specific surface area and surface energy, it has superior adsorption performance, high reduction activity, and abundant surface binding sites; in addition, nZVI also has unique surface and small-sized effects, which can improve its reaction activity and reaction rate. Due to the oxidation of the iron core, nZVI has the structure of iron oxide and a hydroxide shell, which makes nZVI possess the dual properties of adsorption and reduction. At the same time, the large specific surface area provides more reaction sites for nZVI particles, thus promoting the interaction between pollutants and nZVI particles.9 However, nZVI is extremely sensitive to oxidation in an aqueous solution and forms an iron ore passivation layer, which accumulates externally in nZVI and inhibits its catalytic performance for eliminating pollution. There are problems of agglomeration and passivation in practical application.10 In addition, magnetic properties of nanoparticles accelerate the aggregation of nZVI particles, leading to a decrease in the reactivity of nZVI particles, affecting the stability and adsorption efficiency of materials.11 To solve this problem, loading nZVI particles has become a focus of attention. The carriers on nanoparticles serve as “delivery vehicles”, enhancing the adsorption, pollution, and degradation capabilities of nZVI or a combination of both. These carriers have abundant contact sites, high specific surface area, and abundant interstitial structure, and usually have high adsorption capacity with uranium.12 Researchers reassembled nZVI particles using metal, polyvinylidene fluoride (PVDF), polyacrylonitrile (PAN), resin, silica, bentonite, montmorillonite, diatomaceous earth, activated carbon, and carbon nanotubes as carriers.13 The dispersion effect of the carrier was utilized to disperse nZVI on the surface and pores of the carrier, which greatly improved the agglomeration problem.14 nZVI-modified materials mainly include bimetallic, inorganic, carbon materials, and organic and porous framework materials.
To date, several reviews have described and discussed examples of nZVI-based materials removing some heavy metal ions from wastewater.15 Adsorption mechanisms of several heavy metal ions and adsorption properties of nZVI and its composites were introduced. However, there is no systematic review on the adsorption of uranium by nZVI and its mechanisms, so it is necessary to summarize the research on nZVI and its composites in recent years. Therefore, the main contents summarized in this review are as follows: (1) modification methods of nZVI and common preparation methods of nZVI-based materials, (2) the effect and principle of using nZVI and its composite materials to repair the uranium environment in water, (3) the factors affecting the removal efficiency, and (4) the prospective restoration of uranium-contaminated water.
2. Pristine nZVI
nZVI can be prepared by various methods, including physical and chemical methods.16 Physical methods include high-energy ball milling method, physical vapour deposition method, and precision milling method. Chemical methods include chemical thermal reduction, carbothermal synthesis, green synthesis, electrochemical method and liquid phase reduction.17 These physical methods are non-toxic and harmless to the environment and can control size and specific areas. However, they need high equipment and technology requirements, resulting in high energy consumption.18 Thermal reduction is one of the commonly used methods for obtaining nZVI, typically reducing goethite (α-FeOOH) or hematite (α-Fe2O3) with hydrogen at high temperatures, or decomposing Fe(CO)5 in organic solvents or argon.19 Liquid reduction is the reduction of Fe2+ or Fe3+ in solution to nZVI with a strong reducing agent such as sodium borohydride or potassium tetrahydroborate.20 This method is simple and suitable for laboratory operation, and the prepared nanoparticles have high purity, large specific surface area, uniform distribution and certain reactivity. However, in the experiment, nitrogen should be continuously introduced to avoid the oxidation of nZVI, which increases the cost, and the reducing agent has a certain toxicity. The carbothermal synthesis method utilizes reducing gases (H2, CO) generated during the thermal decomposition process of carbon-based materials (carbon black, biochar, carbon nanoparticles) to reduce iron oxide or hydrated iron ions at high temperatures to obtain Fe0.21,22 This method has advantages of low cost, easy availability of raw materials and simple operation. The green synthesis method is to use bioactive substances in plant extracts to reduce tri-ferric or ferrous to nZVI. The green nZVI is usually made of natural and biodegradable materials (plant leaves, seeds, stems, roots, fruits, and microorganisms).23 Polyphenols and flavonoids can be extracted from these plants, effectively preventing the build-up of nanoparticles.24 Compared with the traditional chemical method, the synthetic route has a lower cost and is environmentally friendly. The electrochemical synthesis method is to electrolyze the salt solution containing Fe2+/Fe3+, and the purity of nZVI is higher by cathodic deposition; however, nZVI synthesized by this method will gradually corrode in a water environment.25 The metal iron core of nZVI is wrapped in a thin shell of iron oxide/hydroxide, with Fe0 located at the centre of the core–shell, where single metal cores separate from each other through an interface oxide layer (ferrous oxide, iron oxide) and its hydroxide.26 Liu et al.27 studied the aging of nZVI prepared by liquid-phase reduction in static water. The nZVI evolved into magnetite (Fe3O4), magnetite (γ-Fe2O3) and scaly (γ-FeOOH) after 5 days. So far, nZVI has removed various pollutants, such as halogenated hydrocarbons, nitroorganic compounds, heavy metal ions, etc.28 This review discusses only the pollution treatment of uranium.Yan et al.29 used sodium borohydride and ferric chloride as raw materials to prepare nano-scale zero-valent iron by a liquid-phase reduction method. They also studied the effects of pH, hydrogen carbonate, and calcium on the removal of U(VI) by Fe0 particles synthesized under anoxic conditions. The results showed that the removal capacity and reduction rate of iron decreased significantly with the increase of pH, bicarbonate concentration, and calcium concentration. The appearance of chlorine and oxygen peaks in X-ray photoelectron spectroscopy (XPS) analysis indicated that a small amount of ferrous chloride remains, and nZVI is oxidized. XPS analysis confirmed that the redox interaction of U(VI) with nano-Fe0 resulted in the formation of UO2 and iron oxide. On this basis, Crane et al.30 reported that the removal capacity of uranium by nano-zero-valent iron reached 98% within 2 h, and the concentration of the solution remained constant after 2 days. Through XPS analysis of the extracted solids, the main mechanisms were adsorption and the chemical reduction of U(VI) to U(IV) on the surface of nZVI. The XRD image indicates that nZVI is mainly composed of metal α-Fe with a bcc structure. XPS confirms the existence of nearly stoichiometric magnetite on the surface of both materials. Similarly, Ling et al.31 enriched uranium with iron nanoparticles and achieved a removal capacity of more than 90% in 2 min at a dose of 1.0 g L−1. At the same time, a variety of structural characterization results showed that the effect of U and nZVI made the spherical structure of nZVI degraded, and uranium adhered only to the surface of nanoparticles. After the reaction, U(VI) was reduced to U(IV). Another study by Crane et al.32 showed that Ca, Na, and HCO3− ions had no significant effect on the adsorption of uranium. Hua et al.33 obtained nZVI with core–shell structure by reducing ferric chloride with sodium borohydride, and the maximum adsorption capacity of uranium was up to 4190 mg g−1 at 1.0 g L−1. Meanwhile, the adsorption performance of nZVI at low pH is weaker, which may be due to the high concentration of hydrogen ions. Hua et al.34 explored the effect of bicarbonate on the aging and reactivity of nZVI on the uranium removal. Raw nZVI and nZVIw aged in the presence of bicarbonate and nZVIw/o aged in the presence of bicarbonate were prepared for XRD analysis and microscopic characterization. The morphological changes of nZVI were observed by TEM and HAADF images. In bicarbonate (NZVIWO) deionized water, γ-FeOOH is the dominant iron phase.
Fig. 1A summarizes the removal capacity of nZVI with and without bicarbonate; with nZVI aged in 10 mM bicarbonate solution for 5 days, Fe0 is mainly converted into magnetite/magnetite (Fe3O4/Fe2O3). Due to the different reaction mechanisms between uranium, aging conditions affect the reaction activity of nZVI. Zhao et al.35 reduced and immobilized uranium with stable nZVI particles. nZVI was prepared by a liquid-phase reduction method, and carboxymethyl cellulose (CMC) and starch were used as stabilizers to stabilize nZVI. When the concentration of CMC increases, the removal efficiency decreases; for bare ZVI, starch-ZVI, and CMC-ZVI, the removal of U(VI) shows a rapid initial (<2 h) rate in the range of pH 6–9, while the removal rate of U(VI) is faster at pH 5; CMC-ZVI nanoparticles have better transport properties than starch-ZVI particles, and the removal of U(VI) is less affected by low concentration of bicarbonate, but the removal of U(VI) is inhibited significantly by a high dose of ligands. CMC-ZVI particles had a good curing effect on U, and even if exposure to air resulted in the reactivation of partially solidified uranium, they had little inhibitory effect on the activity of natural bacteria. Zheng et al.36 studied the effect of the initial concentration and addition order of U(VI)/phosphate on the removal capacity of nZVI. By observing the SEM images of nZVI in the absence and presence of U(VI) and phosphate, it is found that a single nZVI particle has a spherical core–shell structure. After reacting with U(VI) and phosphate, the size of nZVI increases, the sphericity decreases, the grain boundaries are irregular, and the surface is rough. In the absence of phosphate, the adsorption equilibrium of U(VI) by nZVI could not be reached after 180 min but reached after 40 min in the presence of phosphate, and the results show that the addition of phosphate can accelerate the removal kinetics of U(VI) by nZVI. Finally, the XPS analysis was used to understand further the co-retention mechanism of U(VI) and phosphate on the nZVI surface. Gao et al.37 studied the removal and recovery of uranium by nZVI in highly alkaline systems with carbonate. The results showed that the reduction of precipitation played a dominant role in the removal of U(VI). Powder XRD was used to detect the mineralogy of nZVI and waste nZVI samples, and the evolution process of nZVI morphology was recorded by TEM. In an anaerobic atmosphere, the removal rate of U(VI) increases with time, reaching about 100% (Fig. 1B). In contrast, in an aerobic atmosphere, the removal rate of U(VI) increases within 1 h and then decreases gradually (Fig. 1C). In the two atmospheric environments, the removal rate of U(VI) increases with the increase of the amount of nZVI. Gao et al.37 studied the removal mechanism of U(VI) in a strong alkaline system, and the reduction precipitation is responsible for the removal of U(VI) in an anaerobic atmosphere. Hua et al.38 enriched uranium from waste water with nZVI. In the study, a new uranium tailings wastewater treatment technology, such as continuous flow stirred tank reactors (CSTR), was designed. The two-stage CSTR shows high efficiency in removing U and coexisting metal/metal-like ions (except Mn). In addition, the mechanism of CSTR used for uranium enrichment, separation of radioactive waste water, and uranium removal was also studied (Fig. 1D). U(VI) is attracted by negatively charged hydroxyl groups to form a complex, which is then reduced to a low valence state.
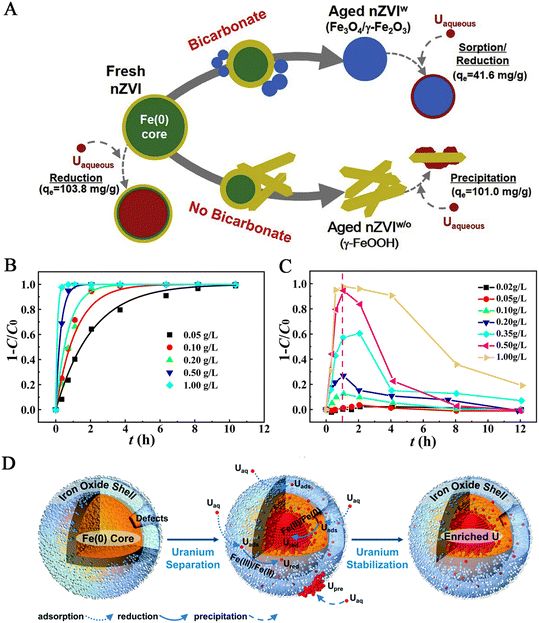 | ||
| Fig. 1 (A) Aging of ZVI with and without bicarbonate and its adsorption capacity. Reprinted with permission from ref. 34. Copyright 2018, Elsevier. Dynamics of uranium removal in an anaerobic atmosphere (B) and an aerobic atmosphere (C). Reprinted with permission from ref. 37. Copyright 2021, Elsevier. (D) Uranium enrichment mechanism. Reprinted with permission from ref. 38. Copyright 2021, Royal Society of Chemistry. | ||
Table 1 summarizes the ability of nZVI to remove uranium under different reaction conditions, uranium concentrations, and nZVI doses in the above experiments.
| Reference of iron | NZVI's mass or concentration | Uranium concentration (mg L−1) | pH | Removal capacity | Ref. |
|---|---|---|---|---|---|
| Nano Fe(0) slurry | 200 μM | 27.57 | 6.92 | 98.4% | 29 |
| Fe(0) nanoparticles | 0.1 g | 4.84 | 8.5 | 99% | 30 |
| Fe(0) nanoparticles | 1 g L−1 | 0.0023–0.88 | 3.5–8.0 | >90% | 31 |
| Fe(0) nanoparticles | 0.5 g L−1 | 1.03 | — | ≥95.7% | 32 |
| Fe(0) nanoparticles | 1.0 g L−1 | 110 | — | ≈100% | 33 |
| Fe(0) nanoparticles | 0.5 g L−1 | 52 | 6.0 | ≥97% | 34 |
| Fe(0) nanoparticles | 35 mg L−1 | 25 | 6.0 | ≈100% | 35 |
| Fe(0) original liquid suspension | 1.0 g L−1 | 45 | 6.0 | 93.36% | 36 |
| Fe(0) nanoparticles | — | 0.2 | 10.7 | 95.5 ± 3.3% | 37 |
| Fe(0) nanoparticles | 60.22 g | 0.331 | 8.5–9.0 | 99.81% | 38 |
There are many explanations for the mechanism of nZVI removing uranium, mainly Fe and Fe(II) reducing uranium to insoluble U(IV).39 NZVI has the following advantages: (1) fast degradation kinetics; (2) low dosage of reducing agent; (3) wide range of pollutant treatment; (4) better fluidity in porous materials.40
3. Supported nZVI
The high reducibility and large specific surface area of nZVI make it possess high uranium removal performance. However, the passivation and corrosion of nZVI have a negative effect on its removal performance.41 To solve these problems, the researchers modified the surface of the nZVI material and prepared composites with metals, metals–solids, inorganic materials, carbon materials, and organic and porous materials, which significantly improved the agglomeration problem and the reactivity.12,42 There are a lot of reports on the removal of uranium by modified nZVI, which has become a hot research topic and a widely concerned problem. In this paper, several kinds of modified nZVI composites are briefly introduced.3.1 Bimetallic nZVI
NZVI particles can also combine with other metal elements to form bimetallic materials and increase the redox potential of composite materials, reduce the activation energy of reactions, and accelerate the reduction rate.43,44 A bimetallic system forms a primary battery through synergistic action; Fe0 acts as an electron donor, and the doped metal acts as a catalyst, accelerating the transfer of H2 and H+ to H*.45 The doped metals can adsorb H2, which facilitates the formation of iron hydroxide as electron transfer medium, thus improving the agglomeration problem. It increases the specific surface area of the adsorbent and promotes the adsorption of heavy metal ions.46 This loading method avoids the use of a large amount of rare earth metals, reduces expensive costs, and not only increases reactivity but also prevents nZVI from being oxidized, retaining the original adsorption performance of nZVI and enhancing removal efficiency through synergistic effects. Unlike the method of loading nZVI on solid materials, the removal mechanism is different. The high potential difference between the two metals promotes electron transfer and improves electrode reaction efficiency.47Xiang et al.48 removed uranium using nZVI–Al composites. The composite material is obtained by mixing Al0 and Fe2+ and adding sodium borohydride to reduce it. Under the same experimental conditions, the removal rate of nZVI–Al can reach 97% after 30 min, which is higher than that of nZVI and Al0. This indicates that bimetallic interactions exist, and Al0 significantly avoids surface oxidation and agglomeration of nZVI. As shown in Fig. 2A, with the increase of NaCl solution concentration, the time required for the removal efficiency to reach equilibrium decreases, the total removal amount remains unchanged, the ionic strength increases, and the conductivity also increases (Fig. 2B), which further confirms the dominant process of uranium reduction. The faster the electron transfer, the faster the reaction rate. Xiang et al.48 conducted an XPS analysis, and the results showed that the composite, after reaction, contained ferric oxide and ferric oxide compounds, and the content of Fe0 was reduced. Hexavalent uranium is the main uranium species adsorbed by the composite because tetravalent uranium is oxidized, and surface corrosion products adsorb hexavalent uranium. The adsorption of U(VI) by nZVI–Al includes both intraparticle reduction and surface adsorption. As the reduction progresses, hydroxides are formed, providing –OH sites for uranium adsorption.
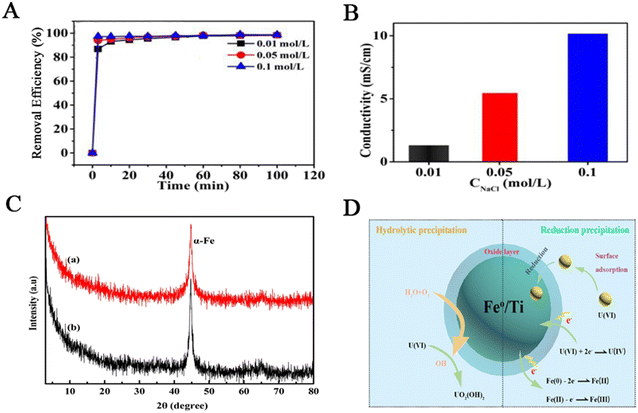 | ||
| Fig. 2 (A) The influence of pH value on the adsorption of nZVI–Al. (B) The influence of ionic strength on the adsorption and the conductivity under different NaCl concentrations. Reprinted with permission from ref. 48. Copyright 2018, Elsevier. (C) XRD spectra of nZVI (a) and FTNP (b). (D) The adsorption mechanism of FTNP. Reprinted with permission from ref. 49. Copyright 2021, Elsevier. | ||
Soon afterwards, Liao et al.49 prepared Fe/Ti bimetallic nanoparticles (FTNP) to remove uranium. Fig. 2C shows the XRD patterns of nZVI (a) and FTNP (b). The nZVI diffraction peak of FTNP is more potent than that of nZVI, indicating that nZVI is exposed in the composite material, and the degree of nZVI passivation is weakened after modification. In the XRD spectrum of FTNP after adsorption, the Fe0 diffraction peak disappears and an iron oxide peak appears. The influence of various experimental conditions on the removal ability towards U(VI) was studied, such as pH value, contact time, temperature, etc. NZVI reaches its maximum removal ability at a pH of 4.0, followed by a decrease in removal rate as the pH increases. FTNP reaches its maximum removal ability at a pH of 5.0. The more amount of adsorbent used, the better the removal effect. As the contact time increases, the removal capacity also increases and finally reaches equilibrium. When the uranium concentration is 320 mg L−1, the removal capacity of uranium reaches its maximum, 734.4 mg g−1. As the temperature increases, the removal rate increases and 298 K is the optimal temperature. Fig. 2D shows the adsorption mechanism, where Fe(0) and Fe(II) participate in the reduction process. Part of U(VI) is reduced, while the rest is hydrolyzed and precipitated.
3.2 Metal/solid material supported nZVI
However, the agglomeration problem of bimetallic particles has not been solved yet. Choosing solid materials to support bimetallic particles is an effective method to improve the dispersibility of nZVI.50 To prevent the aggregation of nZVI, it was loaded onto some carriers to improve its reactivity and adsorption capacity.51,52 Fe/Ni particles were supported on collagen fibres immobilized through bayberry tannin for uranium removal.53 The preparation process of nano Fe/Ni particles (NZFNP) is shown in Fig. 3A; the reduction of iron is attributed to its complexation with ortho phenolic hydroxyl groups on tannins, which have high reducibility. Ni2+ is adsorbed on the surface of Fe0 and reduced to Ni0 under chemical reducing agents. Bayberry tannin can enhance the stability of composite materials by chelating with metal ions through ortho phenolic hydroxyl groups. The element map shows Fe, Ni, and O elements, indicating the presence of iron oxide. The EDX image of the adsorbed NZFNP shows that it has adsorbed uranyl ions. The FT-IR spectra of the NZFNP material before and after adsorption are shown in Fig. 3B, where the phenolic hydroxyl peak of the tannin becomes wider, indicating the successful coupling of Fe3+ and phenolic hydroxyl. For the adsorbed material, the tensile vibration of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O indicates successful adsorption. When the pH is 5.0, the adsorption capacity has reached its maximum value. XPS results indicate that Fe0 and Fe(II) are reducing agents, and the removal of U(VI) is attributed to both the surface electrostatic adsorption of NZFNP and the reduction of Fe0 and Fe(II). Zhang et al.54 synthesized Fe–Ni/GO using a hydrothermal method for uranium removal from aqueous solutions. Salt solutions of iron and nickel were heated at high temperatures and freeze-dried to obtain the product. The XRD pattern of the composite material shows the formation of Ni(OH)2 and FeO(OH) as well as the formation of Fe0 and Ni0, indicating the successful synthesis of the composite material. Fig. 3C shows the isotherms at different temperatures; the fitting coefficient of the Langmuir model is high, and the adsorption is monolayer adsorption. After five cycles of adsorption, the removal rate decreased by only 7%. Fig. 3D shows the schematic diagram of the Fe–Ni/GO adsorption model for uranium. Fe0 and Ni0 form a primary cell, and electrons transfer from Fe0 to Ni0. Iron oxidation is accompanied by the conversion of U(VI) to U(IV).
O indicates successful adsorption. When the pH is 5.0, the adsorption capacity has reached its maximum value. XPS results indicate that Fe0 and Fe(II) are reducing agents, and the removal of U(VI) is attributed to both the surface electrostatic adsorption of NZFNP and the reduction of Fe0 and Fe(II). Zhang et al.54 synthesized Fe–Ni/GO using a hydrothermal method for uranium removal from aqueous solutions. Salt solutions of iron and nickel were heated at high temperatures and freeze-dried to obtain the product. The XRD pattern of the composite material shows the formation of Ni(OH)2 and FeO(OH) as well as the formation of Fe0 and Ni0, indicating the successful synthesis of the composite material. Fig. 3C shows the isotherms at different temperatures; the fitting coefficient of the Langmuir model is high, and the adsorption is monolayer adsorption. After five cycles of adsorption, the removal rate decreased by only 7%. Fig. 3D shows the schematic diagram of the Fe–Ni/GO adsorption model for uranium. Fe0 and Ni0 form a primary cell, and electrons transfer from Fe0 to Ni0. Iron oxidation is accompanied by the conversion of U(VI) to U(IV).
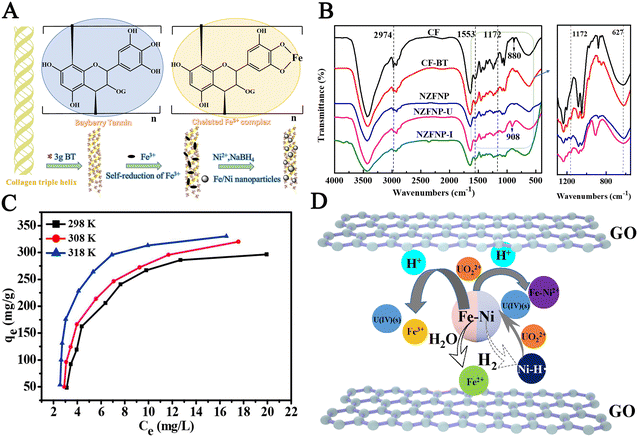 | ||
| Fig. 3 (A) The preparation process of NZFNP. (B) The FT-IR spectra of the NZFNP. Reprinted with permission from ref. 53. Copyright 2020, Elsevier. (C) The isotherms of Fe–Ni/GO at different temperatures. (D) The schematic diagram of the Fe–Ni/GO adsorption model. Reprinted with permission from ref. 54. Copyright 2019, Elsevier. | ||
3.3 nZVI supported on inorganic materials
Due to their high removal efficiency and strong adsorption capacity for uranium, inorganic materials have become an efficient material for uranium-contaminated water treatment and remediation in recent years. The hydrophobicity of these solid-phase materials can inhibit the aggregation of nano-iron, which is equivalent to an inert coating. The most common method is to load nZVI on inorganic materials, which are mainly layered double hydroxides.Pang et al.55 loaded Ca–Mg–Al layered double hydroxides on nZVI to remove uranium and prepared Ca–Mg–Al-LDH/nZVI material through in situ synthesis. SEM and TEM images indicate that Ca–Mg–Al-LDH is successfully doped in nZVI, making the composite material uniformly dispersed. The characteristic peaks of LDH and nZVI are observed in the XRD spectra of the composite material. According to the adsorption–desorption isotherm (Fig. 4A), the specific surface area of the composite is much larger than that of nZVI. Kinetic studies have shown that among the four materials LDH, Ca–Mg–Al-LDH/nZVI, nZVI and ZVI, the adsorption performance of Ca–Mg–Al-LDH/nZVI is greater than that of LDH, and the removal ability of ZVI is the weakest. Fig. 4B shows the adsorption capacity of the composite material with LDH and nZVI at different temperatures. It is evident that the composite material has the best removal effect. Pang et al. discussed the mechanism through XPS technology, proving that the mechanism is an adsorption and chemical process.55
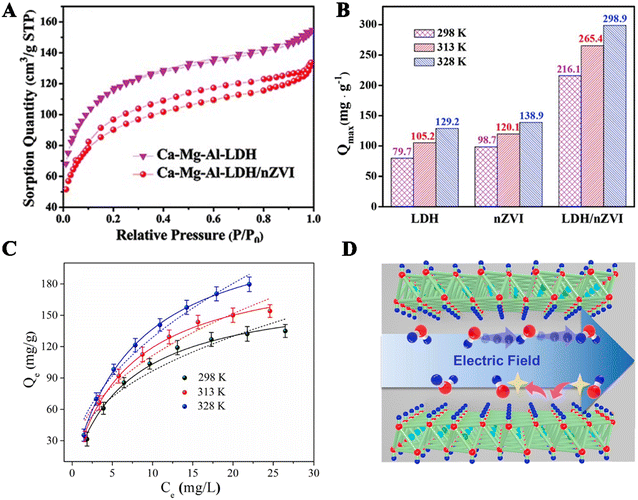 | ||
| Fig. 4 (A) Nitrogen adsorption–desorption isotherm of Ca–Mg–Al-LDH/nZVI and nZVI. (B) The ability of the three materials to remove uranium at different temperatures, pH 5.0 ± 0.1, m/V = 0.1 g L−1. Reprinted with permission from ref. 55. Copyright 2018, Royal Society of Chemistry. (C) The adsorption isotherm of the composite material (the solid line is the solid Langmuir model, and the dotted line is the Freundlich model), m/V = 0.1 g L−1, pH 5.0 ± 0.05, I = 0.01 M NaNO3. Reprinted with permission from ref. 56. Copyright 2019, Elsevier. (D) The layered structure of Zn–Al-LDH. Reprinted with permission from ref. 57. Copyright 2024, Elsevier. | ||
In addition to Ca–Mg–Al-LDH material, Ni–Mg–Al-LDH was also loaded on nZVI for uranium removal. Yu et al.56 loaded Ni–Mg–Al layered double hydroxides onto nZVI and studied their adsorption effect. SEM and TEM images indicate that the synthesis of the composite material was successful, with nZVI appearing on the surface and interlayer of the rod-shaped LDH. XPS also confirmed the successful synthesis of the composite material. In 0–60 min, the adsorption capacity increased with the increase of contact time and reached equilibrium after 60 min. Using the Langmuir model and the Freundlich model to simulate the adsorption process (Fig. 4C), the fitting coefficient of the Langmuir model is more significant than that of the Freundlich model, indicating that the adsorption is monolayer adsorption, and Qe increases with increasing temperature. In simulated wastewater, composite materials also have high adsorption capacity. Yu et al.56 conducted cyclic experiments to explore the reusability of the adsorbent, and the adsorption capacity decreased only slightly. These results indicate that Ni–Mg–Al-LDH is a good adsorption material. XPS results of Fe 2p showed that the proportion of Fe(III) increased and the proportion of Fe(II) decreased after adsorption. Fe0, Fe(II) and Fe(III) participated in the adsorption process. The change in binding energy between Mg 1s and Al 2p indicated that metal oxide groups were involved in the adsorption process. The efficient removal of uranium was the result of adsorption reduction.
The structure of layered double hydroxides such as Zn–Al-LDH is shown in Fig. 4D,57 showing a layered structure. Unlike traditional adsorbents, LDH materials have a non-toxic, harmless, and low-cost structure, and can be used as anionic soils to demonstrate adsorption performance through interlayer anion exchange. The important functional groups of these materials are conducive to the formation of LDH based composite materials, improving the adsorption capacity of materials, promoting the transfer of pollution from solution to nZVI, and increasing the reduction rate.58 This composite material has a high removal rate and less environmental pollution, which can accelerate the transfer of electrons from nZVI to pollutants.59
3.4 nZVI supported on carbon materials
Carbon-based materials used for environmental remediation include carbon nanotubes, graphene, activated carbon, biochar,60etc. The synthesis methods of iron–carbon-based composite materials include liquid-phase reduction co-precipitation (Fig. 5A), carbothermal reduction (Fig. 5B), hydrothermal carbonization (Fig. 5C), and ball milling method (Fig. 5D).61 The ball milling method involves mixing nZVI with carbon materials and propylene glycol into an open container. They are crushed into small particles in the ball milling chamber and then centrifuged and dried to obtain nanoparticles. Water and ethanol reduce rolling resistance. In the liquid-phase reduction method, NaBH4 or KBH4 solutions are used as a reducing agent. At this time, nZVI particles were generated on the carbon material.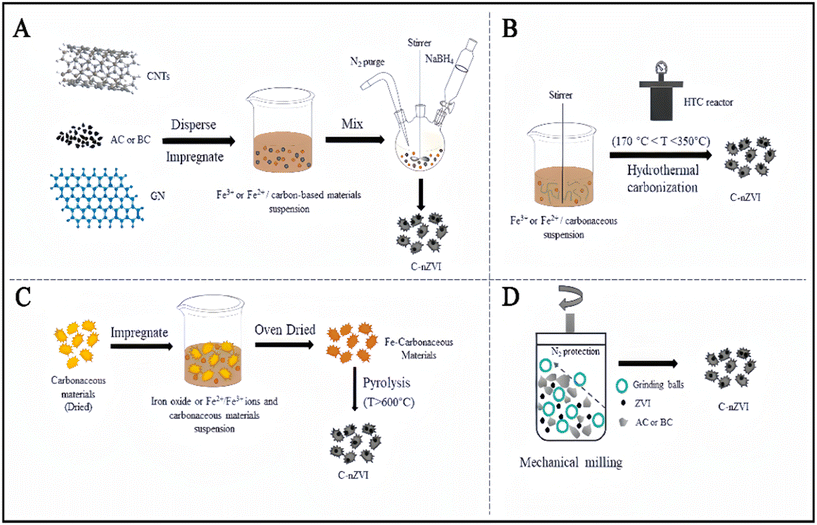 | ||
| Fig. 5 Synthesis methods of C-nZVI. (A) Liquid phase reduction co-precipitation; (B) carbothermal reduction; (C) hydrothermal carbonization; (D) ball milling. Reprinted with permission from ref. 61. Copyright 2022, Elsevier. | ||
The composite material can be separated from the mixture by filtration, and the polymer was used as a crosslinking agent during the experiment.62 The carbon thermal reduction method involves mixing a salt solution of iron with carbon materials, followed by three processes: vacuum filtration, heating in a nitrogen atmosphere, and pyrolysis. Compared with traditional liquid phase synthesis methods, it does not require expensive reagents, and carbon can support a large amount of nZVI. However, carbon carriers often have irregular pore volumes and pore structures, which affects their adsorption performance.63 Li et al.64 prepared nZVI/C using a carbon thermal reduction method. The preparation of the composite material is influenced by the molar ratio of iron to carbon and the reduction of temperature. The composition of the composite material can be observed through XRD patterns. The optimal molar ratio of iron to carbon corresponds to the absence of other impurity peaks in the composite material. The composite material reduced at 700 °C has a smaller particle size and higher particle size separation rate than materials at other temperatures. Hydrothermal carbonization is a green and environmentally friendly method for dispersing carbon materials. The reaction takes place at moderate temperatures (180–250 °C) and pressures. The reaction process is multi-step and complex, and the raw materials do not need to be dried. It can be applied to wet raw materials, but the disadvantage is that product collection is difficult and energy consumption is high. Rong et al.65 mixed banana peel juice with Fe(II) sulfate heptahydrate and then calcined it in water at 180 °C for 6 h to obtain the final product γ-Fe2O3/BC.
Sun et al.66 supported graphene oxide (GO) on nZVI to synthesize a composite material (nZVI/GO). Extended X-ray absorption fine structure (EXAFS) spectra show that the amount of uranium decreased with time. Batch experiments were conducted to study the adsorption properties of the composite materials and the effects of pH and initial uranium concentration on the adsorption capacity of the adsorbent. Fig. 6A shows that GO enhances the adsorption effect of nZVI. The removal amount of nZVI is far lower than that of nZVI/GO, and its maximum removal rate is greater than 95%. The transfer of electrons from nZVI to U(VI) is a long process, and a short time of adsorption represents that uranium adsorption is spontaneous adsorption. In the range of pH from 3.0 to 10.0, the adsorption effect of nZVI increases with the increase of pH value. When pH is greater than 7.0, the adsorption ability of nZVI/GO decreases, due to negatively charged uranium species and electrostatic repulsion. Within the range of uranium concentration studied, both adsorbents enhance their adsorption efficiency with increasing uranium concentration.
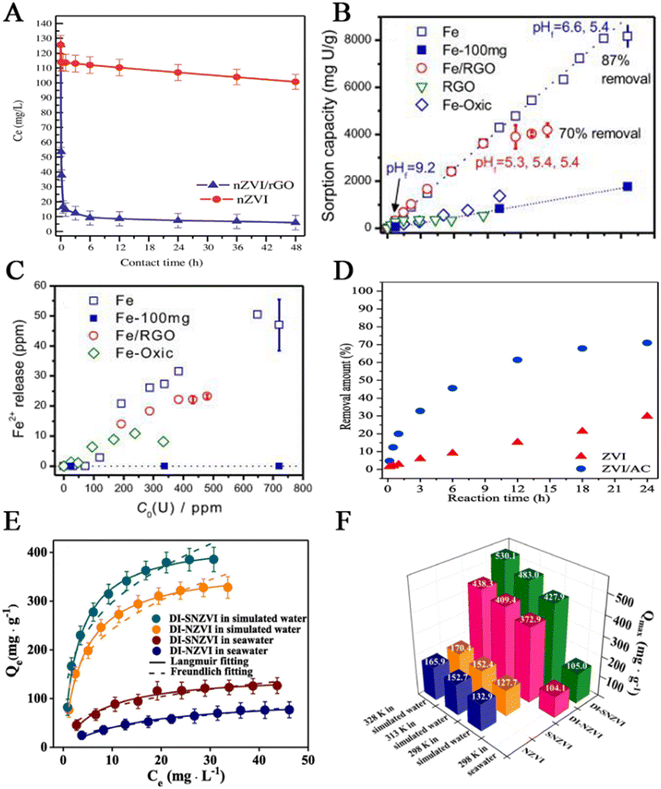 | ||
| Fig. 6 (A) The rate of uranium removal by nZVI and nZVI/GO composite materials. Reprinted with permission from ref. 66. Copyright 2014, Elsevier. Adsorption of Fe, Fe/RGO, and RGO (B) and Fe2+ concentration in solution (C) at different uranium concentrations, M = 4.0 or 100 mg, V = 50 mL, pH = 5.0, contact time = 24 h. Reprinted with permission from ref. 67. Copyright 2015, Elsevier. (D) The removal rate of uranium by nZVI and nZVI/AC at different times, C0 = 10 mg L−1, m/V = 1.2 g L−1, pH = 4.5, T = 298 K. Reproduced with permission from ref. 68. Copyright 2019, Elsevier. (E) At 298 K, the adsorption isotherm of DI-SNZVI and DI-NZVI. (F) The adsorption capacity of four materials in two water systems at different temperatures. Reprinted with permission from ref. 69. Copyright 2019, Elsevier. | ||
Very recently, a similar work was performed by Li et al.67 They fabricated Fe immobilized on rGO (Fe/rGO), measured the specific surface area using nitrogen-specific surface area analysis technology, and characterized it with SEM and XRD. BET-specific surface area analysis showed that the composite material Fe/rGO had the largest specific surface area. Batch adsorption tests showed that the time required for the Fe/rGO to completely remove 24 ppm uranium solution was less than that for Fe0 due to the increase in its surface area and the increase in oxygen-containing functional groups on its surface. Fig. 6B shows the adsorption capacity of the three materials during the reaction for 24 h under different initial uranium concentrations. When the initial uranium concentration is less than 643 ppm, the removal rate of Fe0 remained 100% and then decreased. The adsorption trend of Fe/RGO is similar to that of Fe, with an adsorption capacity of 4174 mg g−1. For Fe0, when the initial uranium concentration is less than 71 ppm, the Fe2+ concentration is almost zero. As the initial concentration of uranium increases, the concentration of Fe2+ increases, and the situation of Fe/rGO is similar to that of Fe (Fig. 6C). Subsequently, in addition to GO, activated carbon (AC) was also employed as a carbon carrier. Wang et al.68 developed an attractive adsorbent (nZVI/AC) in which nZVI was anchored on the AC surface. NZVI/AC was synthesized by a wet impregnation method. AC was added to the Fe3+ solution, and sodium borohydride was added to obtain it. Batch adsorption experiments were conducted to investigate the effects of uranium concentration, reaction time, and temperature on uranium removal. SEM and TEM images of AC, nZVI/AC, and nZVI before removal showed that the doping of AC prevented the aggregation of Fe0. Fig. 6D shows the uranium removal rate of nZVI and nZVI/AC at different times. The time varied from 0 to 24 h, and as time increased, the uranium removal rate increased. Before 6 h, the reduction amount of uranium on nZVI was greater than that of composite materials, and after 12 h, it was smaller than that of composite materials. The XRD patterns of adsorbed nZVI and nZVI/AC showed that both nZVI and nZVI/AC underwent corrosion, and the major corrosion products of the composite material were goethite and magnetite. EXAFS analysis confirmed that the adsorption mechanism was a redox reaction.
Similarly, Pang et al.69 loaded biochar onto SNZVI to prepare composite material (DI-SNZVI) for uranium removal. SEM images show that nZVI is in an aggregated state and SNZVI is uniformly dispersed. After adding derivative biochar, SNZVI is more uniformly dispersed. The nitrogen adsorption–desorption isotherms show that the specific surface areas of nZVI, SNZVI, and DI-SNZVI increase. Batch adsorption tests show that after 3 h, due to limited adsorption sites, the adsorption reached saturation, and the adsorption rates of uranium on NZVI, SNZVI, DI-NZVI, and DI-SNZVI increased in turn (Fig. 6E). During the experiment, Langmuir and Freundlich models were used to simulate the adsorption process. Compared to the Freundlich model, the Langmuir model can better simulate the adsorption process of DI-NZVI and DI-SNZVI. Regardless of whether in laboratory water or seawater, DI-SNZVI has a greater removal rate of uranium than DI-NZVI. Due to the complex composition of seawater and changes in the redox environment, seawater exhibits lower removal rates compared to laboratory water (Fig. 6F). Cyclic experiments show that after five cycles, the removal rates of both DI-SNZVI and DI-NZVI decrease, but the former decreases less, because the FeSx shell layer delays Fe0 corrosion. The antioxidant experiment shows that DI-SNZVI is more antioxidant, and its solution is not prone to yellowing. pH will affect the adsorption behaviour, but the ionic strength will not. When pH is less than 7.0, for DI-SNZVI, the adsorption rate increases with the increase of pH and then decreases. This is because when pH is less than 7.0, there are positively charged uranium species that repel each other with positively charged adsorbents, and the repulsive force decreases with the increase of pH, reaching the highest value at pH 7.0.
Wang et al.70 loaded konjac glucomannan-derived carbon aerogel (KGMC) on nZVI to remove uranium and synthesized nZVI/KGMC composite. Fig. 7A shows a schematic diagram of the synthesis of nZVI/KGMC. KGMC is assembled onto nZVI using biological assembly technology to form a 3D precursor, which is then reduced in situ to form nZVI/KGMC. SEM images of the composite material show that it has a 3D honeycomb structure, and HRTEM images show that it exhibits transparent crystal stripes. Fig. 7B shows XRD patterns of KGMC, C/nZVI and nZVI/KGMC. KGMC exhibits a graphite phase, indicating that high-temperature pyrolysis causes KGMC to carbonize and pyrolyze into graphite. The XRD pattern of nZVI/KGMC demonstrates that most of Fe is γ-Fe, and a small part is α-Fe. XPS spectra show that some of them were oxidized to iron oxide, and KGMC was on the surface of nZVI. In the uranium enrichment experiment, the maximum removal rate of the composite material reached 90.1%, and it also had a high removal rate under alkaline conditions. The existence of organic matter has little influence on its adsorption performance and can be used as a good adsorbent. When pH changes from 3.0 to 5.0, the adsorption capacity increases with increasing pH and then decreases. The adsorption process was studied by XAS, XPS, and FT-IR. The results show that electrostatic adsorption on the surface of KGMC dominated the adsorption process, and hexavalent uranium was reduced to tetravalent uranium. KGMC-coated nZVI transfers electrons, and hexavalent uranium is reduced to produce Fe2+. The reaction between Fe2+ and U(VI) continues to cause it to be reduced. During the degradation process, oxidized hydroxyl radicals are generated, and the presence of oxidized hydroxyl radicals causes organic matter to be degraded.
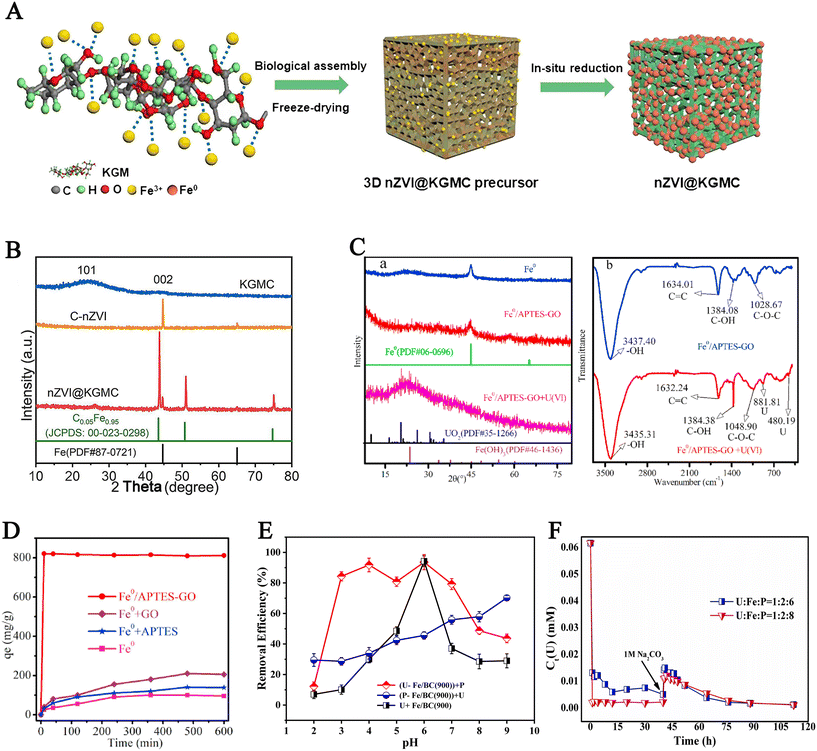 | ||
| Fig. 7 (A) The schematic diagram of the synthesis of nZVI/KGMC. (B) XRD patterns of KGMC, C/nZVI and nZVI/KGMC. Reprinted with permission from ref. 70. Copyright 2020, Elsevier. (C) XRD patterns of Fe0, Fe0/APTES–GO and Fe0/APTES–GO + U(VI) (a); FT-IR spectra of Fe/APTES–GO and Fe0/APTES–GO + U(VI) (b). (D) The change in the removal ability of Fe0, Fe0/GO, Fe0/APTES, and Fe0/APTES–GO with reaction time, C0 U(VI) = 80 mg L−1, pH 4.1, T = 30 °C. Reprinted with permission from ref. 71. Copyright 2021, Elsevier. (E) The effect of the addition orders of U and P on the removal efficiency of Fe/BC(900). (F) In the (U–Fe/BC(900)) + P system, the variation of uranium concentration with time and the desorption of uranium in the presence of sodium carbonate. Reprinted with permission from ref. 72. Copyright 2022, Elsevier. | ||
Tang et al.71 doped GO with Fe0 to remove uranium. They used 3-aminopropyltriethoxysilane (APTES) to encapsulate the composite material (Fe0/GO) synthesized from Fe0 and GO. APTES wrapped Fe0/GO and acted as a crosslinking agent. Batch adsorption experiments explored the effects of pH, temperature, and ionic strength on the adsorption efficiency of Fe0/APTES–GO. The SEM images and TEM images of the Fe0/APTES–GO composite show that GO encapsulates Fe0, and nitrogen is present in the EDS spectrum of the composite, indicating that APTES is involved in the crosslinking of GO. The XRD diffraction patterns of Fe0 and Fe0/APTES–GO both show characteristic peaks of Fe0, but due to the decrease in crystallinity of Fe0, no other peaks of iron were observed. After reacting with uranium, the Fe0 peak of Fe0/APTES–GO + U(VI) disappeared, and the UO2 peak and Fe(OH)3 characteristic peak appeared (Fig. 7C(a)). In the FT-IR spectra of Fe0/APTES–GO and nZVI/APTES–GO + U(VI), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, –OH, C–O–C, and C–OH bonds were observed. Still, in the composite material, after reacting with uranium, these bonds exhibited tensile vibrations, indicating that the reaction involved these bonds. At the same time, U–O bonds were also observed in the spectra after the reaction (Fig. 7C(b)). Fig. 7D shows the change in the removal ability of Fe0, Fe0/GO, Fe0/APTES, and Fe0/APTES–GO with reaction time, Fe0/APTES–GO reached adsorption saturation within the first ten min, and its adsorption performance is greater than that of the other three materials. The adsorption isotherm shows that when the uranium concentration is 80 mg L−1, and the temperature is 50 °C, the adsorption capacity reaches the maximum value of 1357.99 mg g−1. Tang et al. demonstrated the stability of uranium removal through leaching cumulative adsorption experiments and showed the reaction mechanism through XPS analysis.
C, –OH, C–O–C, and C–OH bonds were observed. Still, in the composite material, after reacting with uranium, these bonds exhibited tensile vibrations, indicating that the reaction involved these bonds. At the same time, U–O bonds were also observed in the spectra after the reaction (Fig. 7C(b)). Fig. 7D shows the change in the removal ability of Fe0, Fe0/GO, Fe0/APTES, and Fe0/APTES–GO with reaction time, Fe0/APTES–GO reached adsorption saturation within the first ten min, and its adsorption performance is greater than that of the other three materials. The adsorption isotherm shows that when the uranium concentration is 80 mg L−1, and the temperature is 50 °C, the adsorption capacity reaches the maximum value of 1357.99 mg g−1. Tang et al. demonstrated the stability of uranium removal through leaching cumulative adsorption experiments and showed the reaction mechanism through XPS analysis.
The last example was by Ruan et al.72 who loaded BC onto nZVI for uranium removal. Batch adsorption experiments monitored the changes in uranium concentration in the solution after the addition of adsorbents, and adsorption–desorption experiments demonstrated the stability of Fe/BC. According to the different addition order of phosphate (P), U, Fe/BC(900), the composite materials are named as (P–Fe/BC(900)) + U, (U–Fe/BC(900)) + P, and (U–P) + Fe/BC(900) systems. Fig. 7E shows the removal rate under different addition orders. It can be seen that in the (P–Fe/BC(900)) + U system when the pH is in the range of 3–7, the reaction between nZVI and P will result in a lower removal efficiency of uranium than the other two systems, but the removal rate of P is very high. The presence of P can improve the removal rate, but it will consume nZVI. Yang et al. found that with the increase in phosphate concentration, the removal efficiency of uranium increases, and increasing temperature can accelerate the adsorption process to reach equilibrium. Fig. 7F shows the variation of uranium concentration over time in the (U–Fe/BC(900)) + P system under different molar ratios of U, Fe, and P. The results show that in the presence of sodium carbonate, the desorption efficiency of uranium is very low due to the mineralization of uranyl, nZVI, and phosphate, demonstrating the long-term stability of the composite material.
The above studies all indicate that nZVI supported on carbon materials can improve the adsorption capacity of nZVI and porous carbon individually. The advantages of this modification method are as follows:73,74 (1) there are many methods for preparing porous carbon, and the raw materials are easy to obtain; (2) porous carbon has a large specific surface area, high stability, and inherent adsorption properties. After modifying nZVI, the specific surface area of the composite materials increases; (3) carbon materials can provide stable sites for nZVI, disperse nZVI, avoid the aggregation of nanoparticles, and promote the diffusion of target pollutants. The disadvantages are as follows: (1) porous carbon, such as carbon nanotubes, graphene, etc., has the characteristic of low yield; (2) the preparation of carbon materials usually requires high temperature, corrosive substances, and complex equipment,75 which consumes high energy and requires specific equipment and environmental pressure.
Table 2 summarizes the ability of nZVI loaded by metal/metal–solid/inorganic/carbon materials to remove uranium under different reaction conditions, uranium concentration, and nZVI dosage.
| Adsorbents | Adsorbent's mass or concentration | Temp. (K) | pH | Adsorption capacity | Kinetic model/isotherm model | Ref. |
|---|---|---|---|---|---|---|
| nZVI–Al | 0.36 g L−1 | 303 | 5.0 | 575 mg g−1 | PSO, PFO/Langmuir, Freundlich | 50 |
| FTNP | 0.01 g | 298 | 5.0 | 734.4 mg g−1 | PSO, PFO/Langmuir, Freundlich | 51 |
| nZFNP | 0.02 g | 328 | 5.0 | 129.5 mg g−1 | —/Langmuir | 54 |
| Fe–Ni/graphene | 0.1 g L−1 | 298 | 5.5 | 384.6 mg g−1 | PSO, PFO, IPD, Elovich/Langmuir, Freundlich | 55 |
| nZVI/Ca–Mg–Al-LDH | 0.1 g L−1 | 298 | 5.0 | 298.9 mg g−1 | PSO, PFO/Langmuir, Freundlich | 56 |
| nZVI/Ni–Mg–Al-LDH | 0.1 g L−1 | 298 | 5.0 | 176 mg g−1 | PSO, PFO/Langmuir, Freundlich | 57 |
| nZVI/GO | 2.5 g L−1 | 298 | 5.0 | 95% | PFO, PSO/— | 66 |
| Fe/RGO | 4.0 mg | — | 5.0 | 4174 mg g−1 | — | 67 |
| nZVI/AC | 1.2 g L−1 | 298 | 4.5 | 138.9 mg g−1 | PSO, PFO/Langmuir, Freundlich | 68 |
| SNZVI/DI | 0.05 g L−1 | 298 | 5.0 | 427.9 mg g−1 | PSO, PFO/Langmuir, Freundlich | 69 |
| nZVI/KGMC | 0.25 g L−1 | — | 5.0 | 720.8 mg g−1 | PSO, PFO/— | 70 |
| Fe0/APTES/GO | 0.097 g L−1 | 323 | 4.1 | 1357.9 mg g−1 | PSO, PFO/Langmuir, Freundlich | 71 |
| Fe/BC(900) | 1 g L−1 | 298 | — | 99.9% | PSO, PFO/— | 72 |
3.5 nZVI supported on organic and porous material
Metal–organic frameworks (MOFs) and organic polymers are both organic materials. MOFs are a class of crystalline porous materials, which are compounds formed by the coordination of transition metal ions/clusters and organic ligands. They have one-dimensional, two-dimensional, and three-dimensional structures, and their surface area is much larger than that of other oxides. They have advantages such as high specific surface area, high porosity, multiple chemical components, and adjustable structure.75 The structure of MOF-888 (C48H24O12Ni2) synthesized by Nguyen et al.76 is shown in Fig. 8B, which is coordinated by blue triangles (Ni(CO2)3) and hexagons (CPB unit). Due to the diene coordination function of CPB, the Ni unit has three coordination sites. In recent years, MOFs have been widely used in the adsorption field due to their highly adjustable composition and structure. Researchers have encapsulated metal nanoparticles such as nZVI in a single MOF material to form composite materials for adsorbing some environmentally polluting substances. This loading method has the following advantages: first, there are fixed metals and carbon sources in MOFs, and the composites can be obtained by carbonization.77 Second, both MOFs and nZVI have adsorption properties, and composite materials can combine their advantages.78 Organic polymers have an amorphous network structure of porous interlaced polymers, which can firmly mesh nZVI.79 Some polymers, such as polyvinyl alcohol, have specific functional hydroxyl groups, which can be complexed with metal ions. They are green, non-toxic, and harmless. In addition, coatings such as polyacrylic acid, carboxymethyl cellulose, and others attached to nZVI provide steric resistance and static electricity through surface modification, which can offset the electric and dipole attraction between particles, and improve their transmission in porous medium to stabilize nZVI.80–82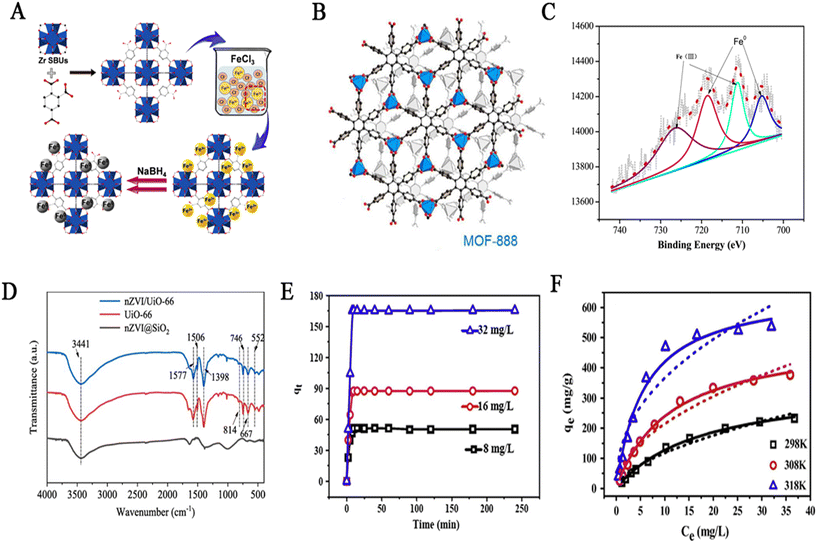 | ||
| Fig. 8 (A) The preparation process of Fe0@UiO-66-COOH. Reprinted with permission from ref. 83. Copyright 2019, American Chemical Society. (B) Structure of MOF-888. Reprinted with permission from ref. 76. Copyright 2015, American Chemical Society. (C) XPS spectra of nZVI/Zn-MOF-74. Reprinted with permission from ref. 84. Copyright 2019, Elsevier. (D) The FT-IR spectra of UiO-66, nZVI/UiO-66 and nZVI@SiO2. Reprinted with permission from ref. 85. Copyright 2020, Springer Link. (E) Effect of contact time on U(VI) sorption onto Fe–PANI–GA, pseudo-second-order kinetics. (F) Sorption isotherms of U(VI) on Fe–PANI–GA. Reprinted with permission from ref. 86. Copyright 2017, Elsevier. | ||
The adsorption mechanism mainly includes chemical adsorption (formation of iron hydroxide), physical adsorption (through organic materials), and redox reactions. Thus, the mechanism of uranium removal by Fe0@UiO-66-COOH may be that UiO-66-COOH provides a site for uranium complexation in the composite, followed by redox of nZVI and uranium to reduce U(VI) to U(IV).83 Li et al.84 used IP, XPS, and EDS techniques to analyze the adsorption mechanism of nZVI/Zn-MOF-74 composite material. U(VI) was adsorbed and converted into U(IV) by the composite materials, Fe0 was oxidized to Fe3+, and the Fe peak after absorbing U was only Fe(III). This indicates that nZVI is completely oxidized, and the oxidation and reduction processes co-occur. Infrared experiments show that there is a strong interaction between the adsorbent and uranyl. A portion of U(VI) is adsorbed on nZVI/Zn-MOF-74, while the rest is reduced. Yang et al.85 loaded UiO-66 onto nZVI and revealed the mechanism through comparative experiments and XPS spectra of nZVI/UiO-66 before and after adsorption. The mechanism of uranium adsorption on composite materials including the adsorption of U(VI) on the adsorbent and the conversion of U(VI) into U(IV) by nZVI/UiO-66. The adsorption capacity of nZVI/UiO-66 is 407.45 mg g−1, which is higher than that of various single materials, indicating a synergistic effect between the two. Chen et al.86 studied the removal mechanisms using HRTEM, EDS mapping analysis, and XRD patterns. Adsorption and reduction of precipitation are the removal mechanisms. On the one hand, U(VI) is converted into UO2 precipitate, and on the other hand, U(VI) is adsorbed through electrostatic interactions. Popescu et al.87 studied the removal mechanism of carboxymethyl cellulose (CMC)/nZVI with an ESD-R equation diagram and infrared spectrum. The reduction of U(VI) is mainly due to physical adsorption, that is, U(VI) is adsorbed on CMC, and then U(VI) is reduced to UO2 precipitate by Fe0.
Various organic and porous materials, such as UiO-66-COOH, Zn-MOF-74, UiO-66, CMC, and PANI–GA, are supported in nZVI to form a new functional composite material. Xu et al.83 encapsulated UiO-66-COOH onto nZVI for efficient treatment of U(VI). The preparation process of Fe0@UiO-66-COOH is shown in Fig. 8A. Firstly, a mixed solution of 1,2,4-benzene tricarboxylic acid and ZrCl4 is heated in a high-pressure sterilizer, cooled, washed and dried to obtain a solid. Then, the solid is added to FeCl3 solution and reduced with NaBH4. Finally, the mixed suspension is filtered, washed, and dried to obtain the product. At pH 3.0, the composite material achieved an adsorption capacity of 504 mg g−1, much higher than that of Fe0 and UiO-66-COOH, exhibiting a synergistic effect. Li et al.84 explored the new adsorbent nZVI/Zn-MOF-74 to remove U(VI) from the aqueous solution. SEM and element mapping analysis were performed on nZVI/Zn MOF-74, and it was found that Fe0 was uniformly distributed on the surface of the MOF. XPS and XRD spectra proved the existence of Fe0, while XPS spectra revealed the presence of a small amount of Fe2O3 in the composite material (Fig. 8C). Both the pseudo-first-order and pseudo-second-order kinetic models can effectively simulate the adsorption process; indicating that adsorption is physical and chemical. Different eluents were used for adsorption analysis experiments, and the elution efficiency of NaHCO3 was the best. The second and third cycles were much lower than expected due to the oxidation of Fe0. Yang et al.85 doped the metal–organic framework UiO-66 into nZVI to remove uranium from aqueous solutions. UiO-66 was synthesized from terephthalic acid and ZrCl4 as raw material, and the composite material was synthesized using a co-precipitation method. The spectrum of nZVI@SiO2 does not have obvious peaks. Fig. 8D shows FT-IR spectra of UiO-66, nZVI/UiO-66 and nZVI@SiO2. For the spectrum of UiO-66, the peaks at 667, 746 and 814 cm−1 indicate the vibration of –OH and C–H in the H2BDC ligand. The spectra of nZVI/UiO-66 and UiO-66 are similar. Because of the low loading of nZVI, the characteristic peaks of nZVI@SiO2 in nZVI/UiO-66 are not obvious. When pH increases to 6, the adsorption capacity of adsorbent increases with the increase of pH, and when pH is 7–8, the adsorption rate starts to decline. This is because the adsorption active site is protonated under acidic conditions, and the degree of protonation decreases with the decrease of acidity, so the adsorption rate starts to rise. When it increases to a certain extent, the size of uranium species is too large to enter the composite channel. Adsorption is chemical adsorption, and adsorption thermodynamic analysis shows that adsorption is a spontaneous endothermic process. Except for the interfering ions Fe3+, other interfering ions (Pb2+, Mn2+) will inhibit adsorption. Chen et al.86 supported polyaniline (PANI) and graphene aerogel (GA) on nZVI to obtain Fe–PANI–GA composite. As shown in Fig. 8E, the composite exhibits rapid adsorption kinetics, and the adsorption equilibrium can be achieved in 20 min at 298 K and pH 5.5. For adsorption thermodynamics, the adsorption of Fe–PANI–GA was in good agreement with the Langmuir isothermal adsorption model (Fig. 8F), indicating that Fe–PANI–GA adsorbed the U(VI) monolayer with an adsorption capacity of 350.47 mg g−1 at 298 K. At the same time, they studied the adsorption regeneration of Fe–PANI–GA. After five cycles of adsorption and desorption, the adsorption capacity of the composite decreased by 11.7%, and this may be due to the oxidation of iron during the cycling process, leading to a decrease in adsorption properties. Popescu et al.87 synthesized the adsorbent nZVI/CMC, which is composed of carboxymethyl cellulose and nZVI. The maximum uranium removal capacity of this adsorbent is 322.58 mg g−1. With the increase in contact time, the adsorption rate increases, reaching saturation after 30 h. In addition, they used three isothermal models of Langmuir, Freundlich and Dubin–Radushkevich to describe the adsorption process. The experimental results were most consistent with the Freundlich model. The ESD-R equation diagram shows that the adsorption was physical adsorption and a spontaneous endothermic process.
In addition to the metals, metals–solids, inorganic materials, carbon materials, and organic and porous materials mentioned above, there are also other solid materials loaded on nZVI. Table 3 summarizes the ability of nZVI loaded by organic/porous materials and other solid materials to remove uranium under different reaction conditions, uranium concentrations, and nZVI dosage.
| Adsorbents | Adsorbent's mass or concentration | Temp.(K) | pH | Adsorption capacity | Kinetic model/isotherm model | Ref. |
|---|---|---|---|---|---|---|
| Fe0/UiO-66-COOH | 0.5 g L−1 | 298 | 3.0 | 504 mg g−1 | — | 83 |
| nZVI/Zn-MOF-74 | 10 mg | 298 | 3.0 | 348 mg g−1 | PSO, PFO/Langmuir, Freundlich | 84 |
| nZVI/UiO-66 | 15 mg | 313 | 6.0 | 404.86 mg g−1 | PSO, PFO/Langmuir, Freundlich | 85 |
| Fe–PANI–GA | 0.1 g L−1 | 298 | 5.5 | 350.47 mg g−1 | PSO, PFO/Langmuir, Freundlich | 86 |
| CMC-INP | — | 298 | — | 322.58 mg g−1 | —/Langmuir, Freundlich, D–R | 87 |
| nZVI/CS | 0.1 g L−1 | 298 | 5.0 | 591.72 mg g−1 | PSO, PFO/Langmuir, Freundlich | 88 |
| nZVI/Mg(OH)2 | 150 mg L−1 | 298 | 5.0 | 98.7% | PFO/— | 89 |
| Fe@NC | 0.1 g L−1 | 303 | 4.5 | 107.95 mg g−1 | —/Langmuir, Freundlich | 90 |
| nZVI/MC800 | 0.15 g L−1 | 298 | 4.0 | 203.94 mg g−1 | PSO, PFO, IPD/Langmuir, Freundlich | 91 |
| nZVI/SC800 | 1 g L−1 | — | 3.5 | 148.99 mg g−1 | PSO, PFO/Langmuir, Freundlich | 92 |
| nZVI/PAO | 0.2 g L−1 | 298 | 5.0 | 206 mg g−1 | PSO, PFO/Langmuir, Freundlich | 93 |
| nZVI/Alk-Ti3C2Tx | 0.08 g L−1 | — | 3.5 | 1315 mg g−1 | PSO, PFO/— | 94 |
| nZVI/BS | 0.5 g L−1 | — | 6.0 | 336.3 mg g−1 | PFO/— | 95 |
| nZVI/MBenes | 0.1 g L−1 | 298 | 5.0 | 107.8 mg g−1 | PSO, PFO, IPD, Elovich/Langmuir, Freundlich | 96 |
| nZVI/SGB-NH2 | 0.2 g L−1 | 298 | 3.0 | 98.96% | — | 97 |
4. Summary and outlook
In recent decades, nZVI has been widely used in the adsorption of heavy metal ions for its high reactivity, low cost, and low toxicity. However, nZVI has strong magnetism and is prone to agglomeration in aqueous solutions, seriously affecting its adsorption performance. The application of loaded nZVI composite materials can effectively improve the aggregation problem and enhance the adsorption performance of nZVI. This article proposes five loading methods: metal loading, metal solid loading, inorganic material loading, carbon material loading, and organic and porous material loading. It summarizes the latest progress in the removal of toxic radioactive ion uranium using nZVI and nZVI-based materials and discusses in detail the preparation methods, adsorption mechanism, adsorption behaviour, and loading advantages of adsorbents. This provides extensive information for researchers to remove U(VI) through nZVI and its composite materials. Each type of material has been proved to have excellent adsorption performance. The adsorption kinetic process was simulated by a pseudo-first-order model and pseudo-second- order model, and batch adsorption experiments studied the adsorption performance. Langmuir and Freundlich models are used to fit the isotherm, and analytical techniques such as XRD, FTIR, EDS, XPS, and EXAFS are used to explore the adsorption mechanism, which mainly includes three processes: adsorption, reduction, and co-precipitation. Although nZVI and its composite materials can efficiently separate uranium from aqueous solutions, there are still some problems and challenges.(i) The mechanisms of reduction and adsorption in the treatment of uranium-contaminated water need to be further understood. From published reports, the removal mechanisms are physical adsorption and chemical adsorption, but detailed adsorption mechanisms need to be further explored to achieve more efficient adsorption reactions; (ii) there is a gap between theory and practice. nZVI-based composite materials are widely used in the experimental stage, but there is still a long way to go in practical applications such as extracting uranium from groundwater. It is necessary to establish a comprehensive and detailed uranium extraction system, and it is more important to simulate the adsorption in dynamic water; (iii) lack of information on the long-term stability and sustainability of nZVI and nZVI-based composite materials, and lack of means to extend their lifespan. Long-term experiments should be conducted to monitor long-term experimental data, observe the transformation of nZVI and nZVI-based materials over a long period, and the impact of the environment on their internal structure; (iv) more detailed understanding is needed on the specific way in which the load material adheres to nZVI and the mechanism by which it improves nZVI aggregation; (v) there are few reports on the adsorption of uranium by bimetallic and bimetallic/solid supported nZVI, and further exploration should be conducted on the role and adsorption behavior of these materials in the adsorption process; (vi) bimetals as modified materials can increase corrosion, reduce the lifespan of nZVI, and cause environmental pollution. Many modified materials are prone to oxidation, and the preparation methods for materials are complex and costly.
In the treatment of uranium-contaminated water, doping a particular material onto nZVI is an advanced and efficient method for synthesizing new iron adsorbents. The ability and potential of nZVI-based materials to remove uranium are enormous. In the future, researchers will develop more cost-effective, environmentally friendly, and efficient nZVI-based composite materials, and there are more different nZVI load materials worth exploring. With the continuous progress of the current scientific research level, we firmly believe that the remediation problem of uranium-contaminated water will be solved. We hope that this review can provide a theoretical basis and broad insights for the future use of nZVI and nZVI-based composite materials in uranium pollution remediation and further promote the development of this research field.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Yinshan Zhu: writing – original draft. Shusen Chen: project administration. Ziming Li: writing – review & editing. Hao Li: writing – review & editing. Muhammad Shaban: writing – review & editing. Changlun Chen: writing – review & editing, supervision, project administration.Conflicts of interest
The authors declare that they have no competing financial interests.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 22276194) is acknowledged. This project is funded by the Innovation Development Fund of China Seawater Uranium Extraction Technology Innovation Alliance (No. CNNC-CXLM-202220), the Jiangsu Provincial Key Laboratory of Radiation Medicine and Protection, and the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- Q. Yu, Y. Yuan, L. Feng, W. Sun, K. Lin, J. Zhang, Y. Zhang, H. Wang, N. Wang and Q. Peng, Highly efficient immobilization of environmental uranium contamination with pseudomonas stutzeri by biosorption, biomineralization, and bioreduction, J. Hazard. Mater., 2022, 424, 127758, DOI:10.1016/j.jhazmat.2021.127758.
- P. J. Nolan, S. E. Bone, K. M. Campbell, D. Pan, O. M. Healy, M. Stange, J. R. Bargar and K. A. Weber, Uranium(VI) attenuation in a carbonate-bearing oxic alluvial aquifer, J. Hazard. Mater., 2021, 412, 125089, DOI:10.1016/j.jhazmat.2021.125089.
- S. Akash, B. Sivaprakash, V. Raja, N. Rajamohan and G. Muthusamy, Remediation techniques for uranium removal from polluted environment-review on methods, mechanism and toxicology, Environ. Pollut., 2022, 302, 119068, DOI:10.1016/j.envpol.2022.119068.
- M. Ma, R. Wang, L. Xu, M. Xu and S. Liu, Emerging health risks and underlying toxicological mechanisms of uranium contamination: lessons from the past two decades, Environ. Int., 2020, 145, 106107, DOI:10.1016/j.envint.2020.106107.
- Y. Xie, C. L. Chen, X. M. Ren, X. X. Wang, H. Y. Wang and X. K. Wang, Emerging natural and tailored materials for uranium-contaminated water treatment and environmental remediation, Prog. Mater. Sci., 2019, 103, 180–234, DOI:10.1016/j.pmatsci.2019.01.005.
- J. Li, L. Zhang, J. Peng, J. Hu, L. Yang, A. Ma, H. Xia, W. Guo and X. Yu, Removal of uranium from uranium plant wastewater using zero-valent iron in an ultrasonic field, Nucl. Eng. Technol., 2016, 48, 744–750, DOI:10.1016/j.net.2016.01.021.
- J. Adusei-Gyamfi and V. Acha, Carriers for nano zerovalent iron (nZVI): synthesis, application and efficiency, RSC Adv., 2016, 6, 91025–91044, 10.1039/c6ra16657a.
- S. Li, W. Yan and W. Zhang, Solvent-free production of nanoscale zero-valent iron (nZVI) with precision milling, Green Chem., 2009, 11, 1618–1626, 10.1039/b913056j.
- Y. Sihn, S. Bae and W. Lee, Immobilization of uranium(VI) in a cementitious matrix with nanoscale zerovalent iron (nZVI), Chemosphere, 2019, 215, 626–633, DOI:10.1016/j.chemosphere.2018.10.073.
- L. Zhou, A. Li, F. Ma, H. Zhao, F. Deng, S. Pi, A. Tang and J. Yang, Combining high electron transfer efficiency and oxidation resistance in nZVI with coatings of microbial extracellular polymeric substances to enhance Sb(V) reduction and adsorption, Chem. Eng. J., 2020, 395, 125168, DOI:10.1016/j.cej.2020.125168.
- M. Song, X. Hu, T. Gu, W. Zhang and Z. Deng, Nanocelluloses affixed nanoscale zero-valent iron (nZVI) for nickel removal: synthesis, characterization and mechanisms, J. Environ. Chem. Eng., 2022, 10, 107466, DOI:10.1016/j.jece.2022.107466.
- A. Chen, C. Shang, J. Shao, J. Zhang and H. Huang, The application of iron-based technologies in uranium remediation: a review, Sci. Total Environ., 2017, 575, 1291–1306, DOI:10.1016/j.scitotenv.2016.09.211.
- H. Chen, Y. Cao, E. Wei, T. Gong and Q. Xian, Facile synthesis of graphene nano zero-valent iron composites and their efficient removal of trichloronitromethane from drinking water, Chemosphere, 2016, 146, 32–39, DOI:10.1016/j.chemosphere.2015.11.095.
- L. Dai, K. Meng, W. Zhao, T. Han, Z. Lei, G. Ma, C. Wu and H. Jia, Enhanced removal of Cd2+ by nano zero-valent iron modified attapulgite from aqueous solution: optimal design, characterization and adsorption mechanism, J. Environ. Chem. Eng., 2022, 10, 107719, DOI:10.1016/j.jece.2022.107719.
- H. Tang, J. Wang, S. Zhang, H. Pang, X. Wang, Z. Chen, M. Li, G. Song, M. Qiu and S. Yu, Recent advances in nanoscale zero-valent iron-based materials: characteristics, environmental remediation and challenges, J. Cleaner Prod., 2021, 319, 128641, DOI:10.1016/j.jclepro.2021.128641.
- H. Lu, J. Wang, S. Ferguson, T. Wang, Y. Bao and H. Hao, Mechanism, synthesis and modification of nano zerovalent iron in water treatment, Nanoscale, 2016, 8, 9962–9975, 10.1039/c6nr00740.
- Y. Li, H. Zhao and L. Zhu, Remediation of soil contaminated with organic compounds by nanoscale zero-valent iron: a review, Sci. Total Environ., 2021, 760, 143413, DOI:10.1016/j.scitotenv.2020.143413.
- Q. Li, Z. Chen, H. Wang, H. Yang, T. Wen, S. Wang, B. Hu and X. Wang, Removal of organic compounds by nanoscale zero-valent iron and its composites, Sci. Total Environ., 2021, 792, 148546, DOI:10.1016/j.scitotenv.2021.148546.
- Y. Monga, P. Kumar, R. K. Sharma, J. Filip, R. S. Varma, R. Zboril and M. B. Gawande, Sustainable synthesis of nanoscale zerovalent iron particles for environmental remediation, ChemSusChem, 2020, 13, 3288–3305, DOI:10.1002/cssc.202000290.
- M. M. Tarekegn, A. M. Hiruy and A. H. Dekebo, Nano zero valent iron (nZVI) particles for the removal of heavy metals (Cd2+, Cu2+ and Pb2+) from aqueous solutions, RSC Adv., 2021, 11, 18539–18551, 10.1039/d1ra01427g.
- Y. Du, M. Dai, I. Naz, X. Hao, X. Wei, R. Rong, C. Peng and I. Ali, Carbothermal reduction synthesis of zero-valent iron and its application as a persulfate activator for ciprofloxacin degradation, Sep. Purif. Technol., 2021, 275, 119201, DOI:10.1016/j.seppur.2021.119201.
- R. Crane and T. Scott, Nanoscale zero-valent iron: future prospects for an emerging water treatment technology, J. Hazard. Mater., 2012, 211, 112–125, DOI:10.1016/j.jhazmat.2011.11.073.
- A. R. Puthukkara P, T. Jose T and S. Lal S, Plant mediated synthesis of zero valent iron nanoparticles and its application in water treatment, J. Environ. Chem. Eng., 2021, 9, 104569, DOI:10.1016/j.jece.2020.104569.
- F. Zhu, S. Ma, T. Liu and X. Deng, Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater, J. Cleaner Prod., 2018, 174, 184–190, DOI:10.1016/j.jclepro.2017.10.302.
- C. Visentin, A. W. D. S. Trentin, A. B. Braun and A. Thomé, Nano scale zero valent iron production methods applied to contaminated sites remediation: an overview of production and environmental aspects, J. Hazard. Mater., 2021, 410, 124614, DOI:10.1016/j.jhazmat.2020.124614.
- P. Wang, F. Fu and T. Liu, A review of the new multifunctional nano zero-valent iron composites for wastewater treatment: emergence, preparation, optimization and mechanism, Chemosphere, 2021, 28, 131435, DOI:10.1016/j.chemosphere.2021.131435.
- A. Liu, J. Liu and W. Zhang, Transformation and composition evolution of nanoscale zero valent iron (nZVI) synthesized by borohydride reduction in static water, Chemosphere, 2015, 119, 1068–1074, DOI:10.1016/j.chemosphere.2014.09.026.
- L. Gong and L. Zhang, Oxyanion-modified zero valent iron with excellent pollutant removal performance, Chem. Commun., 2023, 59, 2081–2089, 10.1039/d2cc06814a.
- S. Yan, B. Hua, Z. Bao, J. Yang, C. Liu and B. Deng, Uranium(VI) removal by nanoscale zerovalent iron in anoxic batch systems, Environ. Sci. Technol., 2010, 44, 7783–7789, DOI:10.1021/es9036308.
- R. A. Crane, M. Dickinson, I. C. Popescu and T. B. Scott, Magnetite and zero-valent iron nanoparticles for the remediation of uranium contaminated environmental water, Water Res., 2011, 45, 2931–2943, DOI:10.1016/j.watres.2011.03.012.
- L. Ling and W. Zhang, Enrichment and encapsulation of uranium with iron nanoparticle, J. Am. Chem. Soc., 2015, 137, 2788–2791, DOI:10.1021/ja510488r.
- R. A. Crane, H. Pullin and T. B. Scott, The influence of calcium, sodium and bicarbonate on the uptake of uranium onto nanoscale zero-valent iron particles, Chem. Eng. J., 2015, 277, 252–259, DOI:10.1016/j.cej.2015.03.085.
- Y. Hua, D. Li, J. Zou, W. Wang, X. Wu, X. Zhang, Q. Liu, G. Zhao, M. Li, W. Zhang and J. Yang, Evolutional solid phase and solid-liquid interface uranium immobilization mechanisms by nanoscale zero-valent iron and enhanced uranium stability control strategy, Chem. Eng. J., 2023, 453, 13992, DOI:10.1016/j.cej.2022.139924.
- Y. Hua, W. Wang, X. Huang, T. Gu, D. Ding, L. Ling and W. Zhang, Effect of bicarbonate on aging and reactivity of nanoscale zerovalent iron (nZVI) toward uranium removal, Chemosphere, 2018, 201, 603–611, DOI:10.1016/j.chemosphere.2018.03.041.
- X. Zhao, W. Liu, Z. Cai, J. Fu, J. Duan, D. Zhao, M. Bozack and Y. Feng, Reductive immobilization of uranium by stabilized zero-valent iron nanoparticles: effects of stabilizers, water chemistry and long-term stability, Colloids Surf., A, 2020, 604, 125315, DOI:10.1016/j.colsurfa.2020.125315.
- H. L. Zheng, X. M. Ren, X. D. Zhang, G. Song, D. Y. Chen and C. L. Chen, Mutual effect of U(VI) and phosphate on the reactivity of nanoscale zero-valent iron (nZVI) for their co-removal, J. Mol. Liq., 2020, 297, 111853, DOI:10.1016/j.molliq.2019.111853.
- C. Gao, J. Sui, K. Chen, Z. Chen, W. Wu and Z. Guo, Efficient recovery of U(VI) from strongly alkaline solution using nanoscale zero-valent iron, J. Environ. Chem. Eng., 2021, 9, 106091, DOI:10.1016/j.jece.2021.106091.
- Y. Hua, W. Wang, N. Hu, T. Gu, L. Ling and W. Zhang, Enrichment of uranium from wastewater with nanoscale zero-valent iron (nZVI), Environ. Sci.: Nano, 2021, 8, 666–674, 10.1039/d0en01029d.
- M. Dickinson and T. B. Scott, The application of zero-valent iron nanoparticles for the remediation of a uranium-contaminated waste effluent, J. Hazard. Mater., 2010, 178, 171–179, DOI:10.1016/j.jhazmat.2010.01.060.
- X. Wang, L. Chen, L. Wang, Q. Fan, D. Pan, J. Li, F. Chi, Y. Xie, S. Yu, C. Xiao, F. Luo, J. Wang, X. Wang, C. Chen, W. Wu, W. Shi, S. Wang and X. Wang, Synthesis of novel nanomaterials and their application in efficient removal of radionuclides, Sci. China: Chem., 2019, 62, 933–967, DOI:10.1007/s11426-019-9492-4.
- L. Ren, B. Zong, R. Zhao, Y. Sun, F. Meng and R. Wang, Insights into the mechanism underlying remediation of Cr(VI) contaminated aquifer using nanoscale zero-valent iron@reduced graphene oxide, Environ. Res., 2022, 214, 11397, DOI:10.1016/j.envres.2022.113973.
- Y. Zou, X. Wang, A. Khan, P. Wang, Y. Liu, A. Alsaedi, T. Hayat and X. Wang, Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: a review, Environ. Sci. Technol., 2016, 50, 7290–7304, DOI:10.1021/acs.est.6b01897.
- X. Chen, X. Lv, Q. Yang, Y. Wang, X. Jin, J. Wang and Z. Yang, Dechlorination of carbon tetrachloride by nanoscale nickeled zero-valent iron @ multi-walled carbon nanotubes: impact of reaction conditions, kinetics and mechanism, Appl. Organomet. Chem., 2019, 33, e477, DOI:10.1002/aoc.4772.
- F. Zhu, L. Li, W. Ren, X. Deng and T. Liu, Effect of pH, temperature, humic acid and coexisting anions on reduction of Cr(VI) in the soil leachate by nZVI/Ni bimetal material, Environ. Pollut., 2017, 227, 444–450, DOI:10.1016/j.envpol.2017.04.074.
- Y. Li, X. Li, D. Han, W. Huang and C. Yang, New insights into the role of Ni loading on the surface structure and the reactivity of nZVI toward tetrabromo- and tetrachlorobisphenol A, Chem. Eng. J., 2017, 311, 173–182, DOI:10.1016/j.cej.2016.11.084.
- E. Aghaei, B. Tadesse, C. B. Tabelin and R. D. Alorro, Mercury sequestration from synthetic and real gold processing wastewaters using Fe-Al bimetallic particles, J. Cleaner Prod., 2022, 372, 133482, DOI:10.1016/j.jclepro.2022.133482.
- F. He, Z. J. Li, S. S. Shi, W. Q. Xu, H. Z. Sheng, Y. W. Gu, Y. H. Jiang and B. D. Xi, Dechlorination of excess trichloroethene by bimetallic and sulfidated nanoscale zero-valent iron, Environ. Sci. Technol., 2018, 52, 8627–8637, DOI:10.1021/acs.est.8b01735.
- S. Xiang, W. Cheng, X. Nie, C. Ding, F. Yi, A. Asiri and H. Marwani, Zero-valent iron-aluminum for the fast and effective U(VI) removal, J. Taiwan Inst. Chem. Eng., 2018, 85, 186–192, DOI:10.1016/j.jtice.2018.01.039.
- H. Liao, W. Zhu, T. Duan, Y. Zhang, G. He, Y. Wei and J. Zhou, Beaded segments like bi-metallic nano-zero-valent iron-titanium for the fast and efficient adsorption and reduction of U(VI) in aqueous solutions, Colloids Surf., A, 2021, 613, 126080, DOI:10.1016/j.colsurfa.2020.126080.
- X. W. Xing, X. M. Ren, N. S. Alharbi and C. L. Chen, Efficient adsorption and reduction of Cr(VI) from aqueous solution by santa barbara amorphous-15 (SBA-15) supported Fe/Ni bimetallic nanoparticles, J. Colloid Interface Sci., 2023, 629, 744–754, DOI:10.1016/j.jcis.2022.08.171.
- C. Nguyen, M. Tran, T. T. V. Tran and R. S. Juang, Efficient removal of antibiotic oxytetracycline from water by Fenton-like reactions using reduced graphene oxide-supported bimetallic Pd/nZVI nanocomposites, J. Taiwan Inst. Chem. Eng., 2021, 119, 80–89, DOI:10.1016/j.jtice.2021.02.001.
- X. W. Xing, X. M. Ren, N. S. Alharbi and C. L. Chen, Biochar-supported Fe/Ni bimetallic nanoparticles for the efficient removal of Cr(VI) from aqueous solution, J. Mol. Liq., 2022, 359, 119257, DOI:10.1016/j.molliq.2022.119257.
- H. Liao, J. Yu, W. Zhu, M. Kuang, T. Duan, Y. Zhang, X. Lin, X. Luo and J. Zhou, Nano-zero-valent Fe/Ni particles loaded on collagen fibers immobilized by bayberry tannin as an effective reductant for uranyl in aqueous solutions, Appl. Surf. Sci., 2020, 507, 145075, DOI:10.1016/j.apsusc.2019.145075.
- Q. Zhang, D. L. Zhao, Y. Ding, Y. Chen, F. F. Li, A. Alsaedi, T. Hayat and C. L. Chen, Synthesis of Fe-Ni/graphene oxide composite and its highly efficient removal of uranium(VI) from aqueous solution, J. Cleaner Prod., 2019, 230, 1305–1315, DOI:10.1016/j.jclepro.2019.05.193.
- H. Pang, Y. Wu, S. Huang, C. Ding, S. Li, X. Wang, S. Yu, Z. Chen, G. Song and X. Wang, Macroscopic and microscopic investigation of uranium elimination by Ca-Mg-Al-layered double hydroxide supported nanoscale zero valent iron, Inorg. Chem. Front., 2018, 5, 2657–2665, 10.1039/c8qi00779a.
- S. Yu, X. Wang, Y. Liu, Z. Chen, Y. Wu, Y. Liu, H. Pang, G. Song, J. Chen and X. Wang, Efficient removal of uranium(VI) by layered double hydroxides supported nanoscale zero-valent iron: a combined experimental and spectroscopic studies, Chem. Eng. J., 2019, 365, 51–59, DOI:10.1016/j.cej.2019.02.024.
- B. D. On, S. H. Sohn, S. M. Woo, M. K. Kwon and I. K. Park, Unveiling hygroresponsive ion conduction performances of layered double hydroxide nanosheets, Mater. Today Chem., 2024, 39, 102149, DOI:10.1016/j.mtchem.2024.102149.
- Y. Pei, W. Cheng, R. Liu, H. Di, Y. Jiang, C. Zheng and Z. Jiang, Synergistic effect and mechanism of nZVI/LDH composites adsorption coupled reduction of nitrate in micro-polluted water, J. Hazard. Mater., 2024, 464, 133023, DOI:10.1016/j.jhazmat.2023.133023.
- X. Kong, J. Chen, Y. Tang, Y. Lv, T. Chen and H. Wang, Enhanced removal of vanadium(V) from groundwater by layered double hydroxide-supported nanoscale zerovalent iron, J. Hazard. Mater., 2020, 392, 122392, DOI:10.1016/j.jhazmat.2020.122392.
- Y. Gao, Q. Wang, G. Ji and A. Li, Degradation of antibiotic pollutants by persulfate activated with various carbon materials, Chem. Eng. J., 2022, 429, 132387, DOI:10.1016/j.cej.2021.132387.
- W. Liang, G. Wang, C. Peng, J. Tan, J. Wan, P. Sun, Q. Li, X. Ji, Q. Zhang, Y. H. Wu and W. Zhang, Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: a critical review, J. Hazard. Mater., 2022, 426, 127993, DOI:10.1016/j.jhazmat.2021.127993.
- X. Wang, Y. Du and J. Ma, Novel synthesis of carbon spheres supported nanoscale zero-valent iron for removal of metronidazole, Appl. Surf. Sci., 2016, 390, 50–59, DOI:10.1016/j.apsusc.2016.08.027.
- Y. Dai, Y. Hu, B. Jiang, J. Zou, G. Tian and H. Fu, Carbothermal synthesis of ordered mesoporous carbon-supported nano zero-valent iron with enhanced stability and activity for hexavalent chromium reduction, J. Hazard. Mater., 2016, 309, 249–258, DOI:10.1016/j.jhazmat.2015.04.013.
- S. Li, J. Tang, Q. Liu, X. Liu and B. Gao, A novel stabilized carbon-coated nZVI as heterogeneous persulfate catalyst for enhanced degradation of 4-chlorophenol, Environ. Int., 2020, 138, 105639, DOI:10.1016/j.envint.2020.105639.
- X. Rong, M. Xie, L. Kong, V. Natarajan, L. Ma and J. Zhan, The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation, Chem. Eng. J., 2019, 372, 294–303, DOI:10.1016/j.cej.2019.04.135.
- Y. Sun, C. Ding, W. Cheng and X. Wang, Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron, J. Hazard. Mater., 2014, 280, 399–408, DOI:10.1016/j.jhazmat.2014.08.023.
- Z. Li, L. Wang, L. Yuan, C. Xiao, L. Mei, L. Zheng, J. Zhang, J. Yang, Y. Zhao, Z. Zhu, Z. Chai and W. Shi, Efficient removal of uranium from aqueous solution by zero-valent iron nanoparticle and its graphene composite, J. Hazard. Mater., 2015, 290, 26–33, DOI:10.1016/j.jhazmat.2015.02.028.
- M. Wang, W. Cheng, T. Wan, B. W. Hu, Y. Zhu, X. Song and Y. Sun, Mechanistic investigation of U(VI) sequestration by zero-valent iron/activated carbon composites, Chem. Eng. J., 2019, 362, 99–106, DOI:10.1016/j.cej.2018.12.138.
- H. Pang, Z. Diao, X. Wang, Y. Ma, S. Yu, H. Zhu, Z. Chen, B. Hu, J. Chen and X. Wang, Adsorptive and reductive removal of U(VI) by dictyophora indusiate-derived biochar supported sulfide NZVI from wastewater, Chem. Eng. J., 2019, 366, 368–377, DOI:10.1016/j.cej.2019.02.098.
- R. Wang, M. Li, T. Liu, X. Li, L. Zhou, L. Tang, C. Gong, X. Gong, K. Yu, N. Li, W. Zhu and T. Chen, Encapsulating carbon-coated nano zero-valent iron particles with biomass-derived carbon aerogel for efficient uranium extraction from uranium-containing wastewater, J. Cleaner Prod., 2022, 364, 132654, DOI:10.1016/j.jclepro.2022.132654.
- H. Tang, W. Cheng, Y. Yi, C. Ding and X. Nie, Nano zero valent iron encapsulated in graphene oxide for reducing uranium, Chemosphere, 2021, 278, 130229, DOI:10.1016/j.chemosphere.2021.130229.
- Y. Ruan, H. Zhang, Z. Yu, Z. Diao, G. Song, M. Su, L. Hou, D. Chen, S. Wang and L. Kong, Phosphate enhanced uranium stable immobilization on biochar supported nano zero valent iron, J. Hazard. Mater., 2022, 424, 127119, DOI:10.1016/j.jhazmat.2021.127119.
- H. Wang, S. Cai, L. Shan, M. Zhuang, N. Li, G. Quan and J. Yan, Adsorptive and reductive removal of chlorophenol from wastewater by biomass-derived mesoporous carbon-supported sulfide nanoscale zerovalent iron, Nanomaterials, 2019, 9, 1786, DOI:10.3390/nano9121786.
- M. Baikousi, Y. Georgiou, C. Daikopoulos, A. B. Bourlinos, J. Filip, R. Zboril, Y. Deligiannakis and M. A. Karakassides, Synthesis and characterization of robust zero valent iron/mesoporous carbon composites and their applications in arsenic removal, Carbon, 2015, 93, 636–647, DOI:10.1016/j.carbon.2015.05.081.
- H. Xu, K. Ye, K. Zhu, J. Yin, J. Yan, G. Wang and D. Cao, Efficient bifunctional catalysts synthesized from three-dimensional Ni/Fe bimetallic organic frameworks for overall urea electrolysis, Dalton Trans., 2020, 49, 5646–5652, 10.1039/d0dt00605j.
- P. T. K. Nguyen, H. T. D. Nguyen, H. Q. Phamp, J. Kim, K. E. Cordova and H. Furukawa, Synthesis and selective CO2 capture properties of a series of hexatopic linker-based metal-organic frameworks, Inorg. Chem., 2015, 54, 10065–10072, DOI:10.1021/acs.inorgchem.5b01900.
- L. Li, Y. He, H. Fu, X. Qu and Z. Xu, Efficient and reductive removal of bromate using a novel and stable nanoscale zero-valent iron embedded in N-doped carbon derived from metal-organic frameworks, Chemosphere, 2022, 306, 135503, DOI:10.1016/j.chemosphere.2022.135503.
- Y. Fang, J. Wen, H. Zhang, Q. Wang and X. Hu, Enhancing Cr(VI) reduction and immobilization by magnetic core-shell structured nZVI@MOF derivative hybrids, Environ. Pollut., 2020, 260, 114021, DOI:10.1016/j.envpol.2020.114021.
- P. D. Mines, J. Byun, Y. Hwang, H. A. Patel, H. R. Andersen and C. T. Yavuz, Nanoporous networks as effective stabilisation matrices for nanoscale zero-valent iron and groundwater pollutant removal, J. Mater. Chem. A, 2016, 4, 632–639, 10.1039/c5ta05025a.
- H. Li, Y. Ge and X. Zhang, High efficient removal of lead from aqueous solution by preparation of novel PPG-nZVI beads as sorbents, Colloids Surf., A, 2017, 513, 306–314, DOI:10.1016/j.colsurfa.2016.10.059.
- H. Dong, Q. He, G. Zeng, L. Tang, C. Zhang, Y. Xie, Y. Zeng, F. Zhao and Y. Wu, Chromate removal by surface-modified nanoscale zero-valent iron: effect of different surface coatings and water chemistry, J. Colloid Interface Sci., 2016, 471, 7–13, DOI:10.1016/j.jcis.2016.03.011.
- M. Mosaferi, S. Nemati, A. Khataee, S. Nasseri and A. A. Hashemi, Removal of arsenic(III, V) from aqueous solution by nanoscale zero-valent iron stabilized with starch and carboxymethyl cellulose, J. Environ. Health Sci. Eng., 2014, 12, 74, DOI:10.1186/2052-336X-12-74.
- L. Xu, D. Zhang, F. Ma, J. Zhang, A. Khayambashi, Y. Cai, L. Chen, C. Xiao and S. Wang, Nano-MOF+ technique for efficient uranyl remediation, ACS Appl. Mater. Interfaces, 2019, 11, 21619–21626, DOI:10.1021/acsami.9b06068.
- J. Li, L. Yang, J. Li, W. Yin, Y. Tao, H. Wu and F. Luo, Anchoring nZVI on metal-organic framework for removal of uranium(VI) from aqueous solution, J. Solid State Chem., 2019, 269, 16–23, DOI:10.1016/j.jssc.2018.09.013.
- F. Yang, S. Xie, G. Wang, C. Yu, H. Liu and Y. Liu, Investigation of a modified metal-organic framework UiO-66 with nanoscale zero-valent iron for removal of uranium(VI) from aqueous solution, Environ. Sci. Pollut. Res., 2020, 27, 20246–20258, DOI:10.1007/s11356-020-08381-4.
- L. Chen, S. Feng, D. Zhao, S. Chen, F. Li and C. Chen, Efficient sorption and reduction of U(VI) on zero-valent iron-polyaniline-graphene aerogel ternary composite, J. Colloid Interface Sci., 2017, 490, 197–206, DOI:10.1016/j.jcis.2016.11.050.
- I. C. Popescu, P. Filip, D. Humelnicu, I. Humelnicu, T. B. Scott and R. A. Crane, Removal of uranium(VI) from aqueous systems by nanoscale zero-valent iron particles suspended in carboxy-methyl cellulose, J. Nucl. Mater., 2013, 443, 250–255, DOI:10.1016/j.jnucmat.2013.07.018.
- Q. Zhang, D. L. Zhao, S. J. Feng, Y. Y. Wang, J. Jin, A. Alsaedi, T. Hayat and C. L. Chen, Synthesis of nanoscale zero-valent iron loaded chitosan for synergistically enhanced removal of U(VI) based on adsorption and reduction, J. Colloid Interface Sci., 2019, 552, 735–743, DOI:10.1016/j.jcis.2019.05.109.
- X. Zhang, X. Liu, Y. Peng, X. Wu, Y. Tan, Q. Zeng, Z. Song and M. Li, Controllable shell corrosion of coated nanoscale zero valent iron induces long-term potentiation of its reactivity for uranium removal, Sep. Purif. Technol., 2022, 287, 120550, DOI:10.1016/j.seppur.2022.120550.
- K. R. Zhu, G. Song, X. M. Ren and C. L. Chen, Solvent-free engineering of Fe0/Fe3C nanoparticles encased in nitrogen-doped carbon nanoshell materials for highly efficient removal of uranyl ions from acidic solution, J. Colloid Interface Sci., 2020, 575, 16–23, DOI:10.1016/j.jcis.2020.04.091.
- Z. M. Lv, S. M. Yang, L. Chen, A. Alsaedi, T. Hayat and C. L. Chen, Nanoscale zero-valent iron/magnetite carbon composites for highly efficient immobilization of U(VI), J. Environ. Sci., 2019, 76, 377–387, DOI:10.1016/j.jes.2018.06.001.
- L. Kong, Y. Zhu, M. Wang, Z. Li, Z. Tan, R. Xu, H. Tang, X. Chang, Y. Xiong and D. Chen, Simultaneous reduction and adsorption for immobilization of uranium from aqueous solution by nano-flake Fe-SC, J. Hazard. Mater., 2016, 320, 435–441, DOI:10.1016/j.jhazmat.2016.08.060.
- D. Shao, X. Wang, X. Wang, S. Hu, T. Hayat, A. Alsaedi, J. Li, S. Wang, J. Hu and X. Wang, Zero valent iron/poly(amidoxime) adsorbent for the separation and reduction of U(VI), RSC Adv., 2016, 6, 52076–52081, 10.1039/c6ra10817b.
- S. Wang, L. Wang, Z. Li, P. Zhang, K. Du, L. Yuan, S. Ning, Y. Wei and W. Shi, Highly efficient adsorption and immobilization of U(VI) from aqueous solution by alkalized MXene-supported nanoscale zero-valent iron, J. Hazard. Mater., 2021, 408, 124949, DOI:10.1016/j.jhazmat.2020.124949.
- G. Yang, L. Wei, X. Wang, X. Wu, Y. He, G. Li, T. Chen and W. Zhu, Enhancing commercially iron powder electron transport by surface biosulfuration to achieve uranium extraction from uranium ore wastewater, Inorg. Chem., 2024, 63, 1378–1387, DOI:10.1021/acs.inorgchem.3c03906.
- F. Liu, Y. Lou, F. Xia and B. Hu, Immobilizing nZVI particles on MBenes to enhance the removal of U(VI) and Cr(VI) by adsorption-reduction synergistic effect, Chem. Eng. J., 2023, 454, 140318, DOI:10.1016/j.cej.2022.140318.
- X. Zhang, H. Wen, Q. Huang, Y. Tan, Z. Sun, Y. Hua, X. Wu and M. Li, Selective removal and long-term immobilization of uranium by ultralow content Fe0 in the pores of amino functionalized silica gel, Chem. Eng. J., 2023, 461, 142015, DOI:10.1016/j.cej.2023.142015.
| This journal is © The Royal Society of Chemistry 2025 |







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times. He is also a highly cited researcher by Thomson Reuters. Researcher ID: http://www.researcherid.com/rid/H-9177-2012.
000 times. He is also a highly cited researcher by Thomson Reuters. Researcher ID: http://www.researcherid.com/rid/H-9177-2012.