The intersection of nanotechnology and urban agriculture: applications of carbon dots
Hanfeng
Zhang
a,
Yue
Wang
a,
Tiantian
Zheng
a,
Ting
Li
a,
Rui
Gao
ab,
Wenzhe
Liu
ab and
Qing
Chi
 *ab
*ab
aNational Key Laboratory of Cotton Bio-breeding and Integrated Utilization, School of Agricultural Sciences, Zhengzhou University, Zhengzhou 450052, China. E-mail: qchi@zzu.edu.cn
bHenan Key Laboratory of Ion-Beam Green Agriculture Bioengineering, School of Agricultural Sciences, Zhengzhou University, Zhengzhou 450001, China
First published on 11th October 2024
Abstract
Amidst the ongoing urbanization process, the significance of urban agriculture has garnered increasing attention. With the expansion of urban population, the urban landscape broadens, and agriculture becomes more commercialized, the connection between the city and agriculture is gradually strengthened. Consequently, there is an urgent need to explore efficient, environmentally friendly, and sustainable modes of urban agricultural production. Carbon dots (CDs), emerging as a novel fluorescent nanomaterial, exhibit remarkable biocompatibility, nanoscale dimensions, low toxicity, and photoluminescence properties. These characteristics render CDs capable of safely and effectively enhancing photosynthesis efficiency, thus revealing immense application potential in the realm of urban agriculture. This paper delves into the characteristics and functionalities of CDs in various agricultural contexts, including plant photosynthesis, plant transport and storage systems, as well as biosecurity considerations. Furthermore, it explores the applications prospects of CDs in urban agriculture, aiming to provide robust support for the advancement of this vital sector.
Environmental significanceAs the urban populace expands, the urban landscape broadens, and agriculture becomes more commercialized, the nexus between the city and agriculture is gradually strengthening. Consequently, there is a pressing need to explore efficient, environmentally friendly, and sustainable modes of urban agricultural production. In this context, plant factories with artificial lighting represents an innovative and promising production system, demonstrating significant potential for stable and effective agricultural production amidst the swift pace of urbanization. However, within the plant factory energy consumption framework, lighting constitutes a significant proportion. This high reliance on artificial lighting, underscores the need for efficient energy management and optimization strategies in this domain. Carbon dots (CDs), emerging as a novel fluorescent nanomaterial, exhibit remarkable biocompatibility, nanoscale dimensions, low toxicity, and photoluminescence properties. These attributes render CDs capable of safely and effectively enhancing photosynthesis efficiency, thus revealing immense application potential in the realm of urban agriculture. |
1. Introduction
1.1 History, development, and current status of urban agriculture worldwide
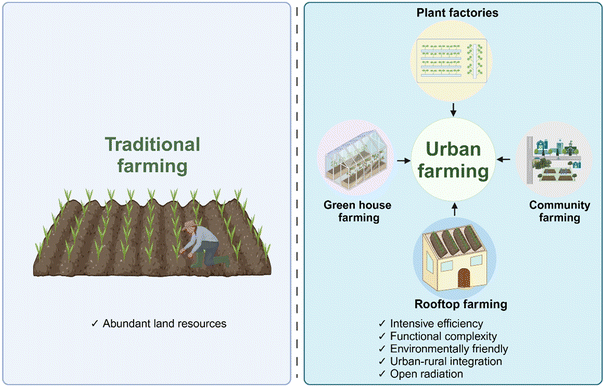
Since the mid-point of the last century, urban agriculture has garnered increasing attention from the global community. As far back as the late 19th and early 20th centuries, a British urban scholar Ebenezer Howard introduced the concept of the “Garden City”.1 In the seminal book, “Agricultural Economic Geography”, published in 1935, the Japanese geoeconomist Shiro Aoshika introduced the concept of “urban agriculture”.2 In 1957, the agricultural economist Thompson formally introduced the concept of urban agriculture in his paper titled “Urban Agriculture in Southern Japan”.3 With the acceleration of industrialization and urbanization, agriculture in urban and suburban has undergone profound transformations. To adapt this transformation, agriculture has embarked on a path of intensification, facilities-based production, and industrialization.4 In order to cater to the survival and growth of contemporary urban landscapes, urban agriculture has become a pivotal component.5–7Urban agriculture is a kind of modern agriculture which is formed in the suburbs of the city and within the economic circle of the city to meet the needs of the survival and development of the modern metropolis.8 Based on the agricultural scale, this practice can be categorized into four distinct types: greenhouse farming, plant factories, community agriculture, rooftop framing (Fig. 1). These categories not only serve diverse functions but also contribute significantly to various aspects of urban life. These functions include agricultural production, enhancement of urban landscapes, maintenance of ecological balance, promotion of cultural and educational activities, stimulation of social economy, and provision of leisure and entertainment facilities (Fig. 1).9–11
As the urbanization process has advanced in Europe, America, Japan, Singapore, and other nations, agricultural scientists and urban geographers in these regions have consecutively conducted research on urban agriculture. As its underlying principles and significance have continuously been refined and expanded, the concept of urban agriculture has gradually gained widespread acceptance worldwide.9
Since the early 1970s, the emergence and advancement of high technology and novel technologies have propelled the urban agricultural sector in Europe, Japan, and other developed nations towards increasing maturity.3 Urban agriculture in Germany originated in 1919, primarily manifesting as “citizen gardens”.12 In the 21st century, Germany has enacted an array of laws and regulations, thereby establishing the legitimate status of urban agriculture and delineating the development paradigm for civic farmers.12 Currently, “citizen farm parks” has become a crucial component of German agriculture, with their aggregate output value comprising approximately one-third of the overall agricultural output value in Germany.13 After nearly five decades of progress, Japanese urban agriculture has attained remarkable advancements. Urban farmers constitute approximately 25% of the total farming population in Japan, while the operational farmland area accounts for 27% of the total cultivable land, particularly notable for the high proportion dedicated to the cultivation of vegetables, fruits, and flowers.2,14,15 As a city-state, Singapore is renowned as the “garden city”. Despite its limited agricultural background, the urban agriculture sector has emerged as a distinct, productive, and profitable industry.16 Singapore has successfully established a comprehensive urban agriculture system, which not only ensures adequate food supply for the city but also significantly contributes to improving the degree of food self-sufficiency.
Urban agriculture has attained a certain level of maturity in several developed nations; however, globally, its development in diverse countries continues to encounter numerous challenges. These challenges not only impede the productivity and profitability of urban agriculture but also pose a significant threat to its sustainable growth.
1.2 The principal challenges in the advancement of urban agriculture
As the global population continues to expand and the expenses associated with transporting artificial fertilizers, pesticides, agricultural resources, and food itself worldwide increase, urban agriculture has emerged as a viable and beneficial complement to traditional agriculture.17 Currently, approximately 15 to 20% of global food production occurs within urban or suburban boundaries.18,19However, despite its numerous benefits, urban agriculture inevitably encounters several challenges in its development process: (1) the cost of production inputs remains significant, encompassing expenses related to land acquisition, labor force utilization, and irrigation water usage. Given the constraints on labor, irrigation facilities, and land resources in urban environments, the production costs of urban agricultural products tend to be relatively high.20,21 (2) Pertaining to environmental risks, these primarily encompass soil contamination, aquatic pollution, outbreaks of diseases and insect infestations, as well as associated public health hazards. Urban agriculture necessitates significant quantities of water resources and energy inputs, yet concomitantly, agricultural waste generated during production processes poses potential environmental implications. (3) The advancement of urban agriculture is hindered by inadequate urban planning strategies that fail to adequately account for the versatility of urban agricultural practices. This oversight is compounded by the neglect of the multifaceted benefits-beyond mere food provision-offered by urban agriculture, including the preservation of cultural heritage and agricultural biodiversity, which contribute significantly to social well-being and sustainable urban development.22,23 (4) Two primary challenges that must be addressed in the context are technological innovation and energy consumption. Science and technology serve as the vital backbone and cornerstone of urban agriculture, yet the foremost technical hurdle encountered by this sector pertains to the reduction of energy consumption.24
Amidst the myriad challenges posed by rapid urbanization to urban agriculture production, ensuring a stable supply of agricultural products to urban areas relies not only on the achievement of efficient and stable output and balanced supply from farming systems but also on shortening distribution chains to guarantee continuous product availability and accessibility.25 In this context, plant factories with artificial lighting represents an innovative and promising production system, demonstrating significant potential for stable and effective agricultural production amidst the swift pace of urbanization. Plant factories embody a multi-tiered indoor agricultural system that integrates green and sustainable crop cultivation technologies, encompassing vertical farming, optimized lighting recipes, energy-saving technologies, and intelligent control systems.26–29 These features enable agricultural production to transcend the constraints of climate and geography. Furthermore, rigorous environmental control and the enclosed space effectively prevent pests and diseases from infiltrating, which reduces pesticide and fertilizer applications.29 By producing fresh crops in an environmentally friendly manner, plant factories have exhibited robust adaptability and immense potential in tackling the most formidable challenges confronting urban agricultural science, including population growth, water scarcity, soil resource constraints, food safety, and supply chain disruptions.27,30 Thus, plant factories undeniably stand to play a significant role in spearheading the agricultural revolution, safeguarding food security, contributing to carbon neutrality, and driving the development of humanity towards a brighter future. However, within the plant factory energy consumption framework, lighting constitutes a significant proportion, accounting for approximately 80% of the total power energy consumption and approximately 25% of the overall cost. This high reliance on artificial lighting, specifically LEDs (light-emitting diodes), underscores the need for efficient energy management and optimization strategies in this domain.24,31 Hence, there remains a significant journey ahead in terms of reducing the costs and enhancing the efficiency of plant factory.
2. The significant impact of light sources and light quality on the development of plant factory
The advancement of nanotechnology presents novel opportunities to modify plant responses to light and enhance the efficacy of light absorption. Carben dots (CDs), an emerging class of carbon nanomaterials, possess distinctive characteristics. Their potential as regulators of plant photosynthesis arises from their capacity to emit red and blue light, which coincides with the primary absorption regions of plant chlorophyll.32Extensive research demonstrates that ultraviolet (UV), blue, red, far-red, and green light, all of which play crucial roles in various physiological and biochemical processes within plants.33 The composition of the spectrum determines the characteristics of light quality. Among this, red light (622–760 nm) and blue light (435–470 nm) stand out as the two primary types of light quality that play a pivotal role in driving photosynthetic biosynthesis.34 Exposure to red light positively affects the accumulation of photosynthetic pigments, enhances plant elongation, and promotes the accumulation of photosynthetic products.35–37 Blue light can stimulate chlorophyll biosynthesis, modulate stomatal aperture and chloroplast maturation, and further enhance the accumulation of secondary metabolites, including phenols and flavonoids, thereby exerting profound effects on plant growth and development.38–41 The utilization of monochromatic red or blue light alone is inadequate for supporting the requirements of typical plant growth, and any absence of these two specific light quality types results in diminished photosynthetic efficiency.42 Extensive research has demonstrated that the admixture of red and blue light in an optimal ratio can significantly enhance plant growth and photosynthesis.42–44 Therefore, the red-blue composite light is considered to be the most effective light source for promoting healthy plant growth.45 The regulation of the light source has greatly affected the development of plant factory.
Urban agriculture, whether utilizing natural or artificial lighting, has emerged as a viable solution to address the challenge posed by land scarcity.46 In contrast to traditional agricultural, urban agriculture exhibits superior production efficiency, with light sources and light quality playing a pivotal role in its successful implementation.47 Regarding the selection of lighting sources, urban agriculture, particularly exemplified by plant factory, predominantly relies on artificial illumination systems, encompassing LEDs, high-pressure sodium lamps, and fluorescent lamps (Table 1). These lighting systems facilitate the achievement of higher crop yields within shorter timeframes, effectively elongating the growing season to span an entire year, ultimately encompassing 365 days.48 Nevertheless, light sources such as high-pressure sodium lamps and fluorescent lamps consume a large amount of electrical energy and may cause light stress in plants to some extent, which ultimately makes them inefficient for optimal growth.49
| Light source | Wavelength | Advantages or disadvantages | Effect of plant | Ref. | |
|---|---|---|---|---|---|
| Natural light source | Sunlight | Ultraviolet (UV) (320–400 nm) | Affected by season and weather | Provide various wavelengths for plant growth | 50 |
| Blue (400–500 nm) | |||||
| Green (500–600 nm) | |||||
| Red (600–700 nm) | |||||
| Far-red (700–800 nm) | |||||
| Artificial light source | High-pressure sodium lamps | Yellow (565–590 nm) | No blue light | Inhibition of hypocotyl elongation | 51 |
| Orange (590–625 nm) | |||||
| Red (625–700 nm) | |||||
| Fluorescent lights | Red (635–700 nm) | Low light intensity | Reduced the risk of heat damage to plants | 50 | |
| Blue (400–499 nm) | |||||
| LEDs | 250–1000 nm (adjustable, usually red and blue) | High luminous efficiency, low power consumption, complete spectral distribution and increased the use efficiency of space | Adjustabled wavelength promotes plant photomorphogenesis | 52 | |
LED technologies have played a pivotal role in this context. In contrast to traditional light sources, LED technology offers a homogeneous and efficient production setting at low costs. This is attributed to their small size, low lamp surface temperature, high light utilization efficiency, and wide light spectrum. The ability to select and control light intensity and wavelength enables the production of highly functional and cost-effective plant products.53 Particularly, by modulating the light spectrum, it is possible to regulate light transduction mechanisms and control specific plant traits such as flowering induction, elongation, branching, secondary metabolites, nutrient status, seedling development, and cell growth54 (Table 2). Consequently, it provides a suitable lighting solution for plant factory. However, the adjustable wavelength capability of LED technology necessitates sophisticated materials, technology, and equipment support, resulting in higher costs at present.33,55
| Light quality | Wavelength | Photosynthetic photon flux density (PPFD) | Plant species | Effect | Ref. |
|---|---|---|---|---|---|
| Red | 660 nm | 300 μmol m−2 s−1 | Cucumber (Cucumis sativus L.) | Enhanced accumulation of P, K, Mn and Zn, reduced numbers and size of chloroplast and starch grain | 56 |
| Blue | 440 nm | 300 μmol m−2 s−1 | Cucumber (Cucumis sativus L.) | Blue light exhibited more effective in maintaining chloroplast ultrastructure than red light | 56 |
| Red | 660–665 nm | 250 μmol m−2 s−1 | Ginkgo biloba | Accumulated more photosynthetic pigments and increased the content of total flavonoids and flavonoids per plant | 57 |
| Blue | 460–465 nm | ||||
| Red | 600–699 nm | 250 μmol m−2 s−1 | Eggplant | Improved the morphological index, biomass accumulation and energy yield | 58 |
| Blue | 400–499 nm | ||||
| Red | 665 nm | 95 ± 5 μmol m−2 s−1 | Pepper (Capsicum annuum L.) | Blue light increased the photosynthetic performance under water deficit | 59 |
| Blue | 445 nm | ||||
| Red | 660 nm | 200 μmol m−2 s−1 | Potato | Increased the synthesis of carotenoids | 60 |
| Blue | 445 nm | ||||
| Red | 653 nm | Low 200 μmol m−2 s−1 | Lettuce | Red light had the highest QYinc under low PPFD, green light had the highest QYinc under high PPFD | 61 |
| Blue | 446 nm | High 1000 μmol m−2 s−1 | |||
| Green | 523 nm | ||||
| Red | 662 nm | 0–1800 μmol m−2 s−1 | Tomato | Improved the photosynthetic activity in plants | 62 |
| Blue | 446 nm | ||||
| Red | 660 nm | 135.29 ± 1.46–191.50 ± 0.94 μmol m−2 s−1 | Chrysanthemum (C. morifolium) | Promoted anthocyanin biosynthesis by the ratio of high red light and far red light | 63 |
| Far-red | 730 nm | ||||
| Red | 660 nm | 280 μmol m−2 s−1 | Tomato | High R/FR light-induced root exudates play a key role in chemotaxis, biofilm formation and root colonization of S. plymuthica A21-4 | 64 |
| Far-red | 730 nm | ||||
| Red | 660 nm | 200 μmol m−2 s−1 | Cucumber (Cucumis sativus L.) | Red light increased Cd sensitivity, whereas blue light enhanced Cd tolerance | 65 |
| Blue | 447 nm | ||||
| Red | 660 nm | 150 μmol m−2 s−1 | Carnation | Increased the vase life, flower numbers | 66 |
| Blue | 440 nm | ||||
| Red | 660 nm | 70–120 μmol m−2 s−1 | Saffron | Increased flower induction | 67 |
| Blue | 450 nm | ||||
| Green | 500–600 nm | ||||
| Red | 635–700 nm | 200 μmol m−2 s−1 | Strawberry | Increased plant biomass and fruit yield | 68 |
| Blue | 450–490 nm | ||||
| Red | 650–670 nm | 150 μmol m−2 s−1 | Tomato | Increased biomass of shoots and leaf area, displayed significantly higher rates of photosynthesis | 69 |
| Blue | 455–475 nm |
The current impediment that plant factory faces in its development is the significant energy consumption required to sustain LED artificial light in the system.70 Furthermore, photosynthesis remains the sole mechanism for plants to transform the radiation they intercept into biomass.71 However, according to reported findings, the conversion efficiency of the C3 plants remains approximately 0.1, whereas the conversion efficiency of C4 plants stands at around 0.13, constituting merely one-fifth of their theoretical maximum.72 In the realm of urban agriculture, the optimization of the ratio between red and blue light during plant photosynthesis, along with the enhancement of the photosynthetic energy conversion efficiency, have emerged as two pivotal challenges that are imperative for fostering plant growth and enhancing overall crop yield.
Recent research has demonstrated that CDs exhibit notable stimulatory effects on plant growth.73–75 Furthermore, CDs exhibit several noteworthy properties, including their minute size, tunable photoluminescence (PL), exceptional water solubility, biocompatibility, and insignificant toxicity.76 Notably, their PL capabilities have the potential to significantly enhance photosynthesis and thereby boost crop yield by optimizing the utilization efficiency of solar energy. Consequently, CDs emerge as effective photosynthetic enhancers with promising applications in plant factory.75
3. Structural characteristics of carbon dots
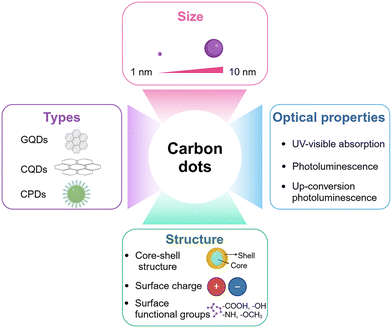
CDs, a novel class of fluorescent nanomaterials, possess sizes smaller than 10 nm.77 In 2004, the fluorescent nanomaterials were initially synthesized by researchers through electrophoresis experiments, specifically designed for the separation and purification of single-walled carbon nanotubes.78 Since that time, carbon nanomaterials exhibiting fluorescent properties have garnered significant attention. In 2006, the nanoscale carbon particles produced through laser ablation were formally designated as CDs, establishing a nomenclature that has been widely adopted in subsequent academic literature.79The CDs exhibit a nuclear-shell-like configuration arising from the hybridization of graphite carbon cores in the sp2 state and the sp3 hybridization of carbon defects, which gradually form the matrix or shell through a nucleation process.80 The carbon core typically comprises polycrystalline nanodomains, whereas the surrounding matrix encompasses an array of functional moieties, including hydroxyl (–OH), epoxy/ether (–O–), carbonyl (–CO–), and carboxylic acid (–COOH) groups.81,82 These hydrophilic functionalities endow CDs with water-solubility, thereby facilitating their integration and utilization in various aqueous environments.81,82 Later, the biocompatibility of the structure of its shell was enhanced.83 Currently, carbon-based nanomaterials, specifically CDs, are primarily categorized based on the hybridization states of carbon atoms, namely sp2 and sp3, as well as their distribution within the core or shell regions.84 These CDs are primarily classified into three distinct types: carbon quantum dots (CQDs), graphene quantum dots (GQDs), and carbonized polymer dots (CPDs).84 This classification scheme enables a more nuanced understanding of their physicochemical properties and potential applications in various fields (Fig. 2).
CDs possess distinctive physical and chemical attributes that have garnered significant attention among researchers. These attributes include fluorescence, phosphorescence, PL, water solubility, biocompatibility, resistance to photobleaching, and low toxicity.81 The origination of these properties can be traced to quantum confinement effects, edge defects, surface states, tunable band gaps, and the presence of multiple emission centers.85 By carefully selecting carbon precursors, adjusting reaction conditions, employing diverse synthesis methods, manipulating the solvent properties, and controlling the solution pH, CDs can be tailored precisely to meet the demands of specific applications.86,87 Up until now, a significant amount of literature has documented its diverse applications across various fields, including bioimaging, fluorescent sensing, cancer therapeutics, catalysis, and plant growth.88–93
4. Optical properties of carbon dots
It was demonstrated that CDs exhibit a robust absorption broadband specifically ranging from 230 nm to 270 nm within the UV absorption region, which is attributed to the π–π* transition of carbon core electrons, whereas the observed weaker absorption in the visible and near-infrared regions is ascribed to the π–π* transition of surface state electrons.94,95 Moreover, the interconnected chemical moieties potentially contribute to the absorption properties within the UV–visible spectral region.96,97CDs garnered initial interest owing to their intriguing photoluminescence properties. Typically, the precursor materials used in their preparation do not exhibit fluorescence characteristics; however, during the synthesis process, a photoluminescence center gradually emerges. Regarding the underlying mechanisms, several prevailing theories have been proposed, including the quantum size effect, surface state, molecular state, and cross-linking enhanced emission (CEE) effect.98–101 When considering the quantum size effect and surface state mechanism, the emission wavelength of CDs exhibits a redshift phenomenon as its bandgap rows become narrower.102 It was discovered that the fluorescence properties of CDs underwent a transition, shifting from blue to near-infrared regions.103 The molecular state mechanism implies that the luminescent characteristics of CDs are primarily attributed to their organic fluorophores, rather than the chemical functional groups or carbon cores residing on the surface.99 Furthermore, in order to meticulously articulate the photoluminescence properties of nonconjugated carbonized polymer dots, a mechanism termed cross-linking-enhanced emission effect was introduced. This mechanism proposes that the immobilization of the rotation and vibration of subfluorophores located on the surface of carbonized polymer dots, achieved through the presence of a crosslinked backbone and/or carbon core, significantly enhances radiative transitions, ultimately intensifying the photoluminescence properties of CDs.96 Moreover, CDs possess the remarkable property of upconversion luminescence, commonly known as up-conversion photoluminescence (UCPL), which involves the conversion of low-energy photons into high-energy photons, a unique characteristic exclusive to them.104 The upconversion phenomenon exhibited by CDs typically encompasses mechanisms such as multiphoton excitation or energy transfer. As an illustrative instance, when near-infrared light (NIR) of lower energy serves as the excitation source for CDs, these nanoparticles are capable of emitting visible light of higher energy.105 Upconversion PL is deemed effective due to its enhanced efficiency and remarkable capacity to penetrate deeply into tissues without inducing any damage to the surrounding tissues.106 This attribute renders it a promising candidate for various applications in the biological and agricultural fields. In plant factories, a large amount of energy is used to power photosynthesis, while the characteristics of CDs enhancing photosynthesis is expected to provide new solutions to solve the bottleneck of plant factories-energy consumption (Fig. 2).
5. Carbon dots and photosynthesis
5.1 Transport and accumulation of carbon dots in plants
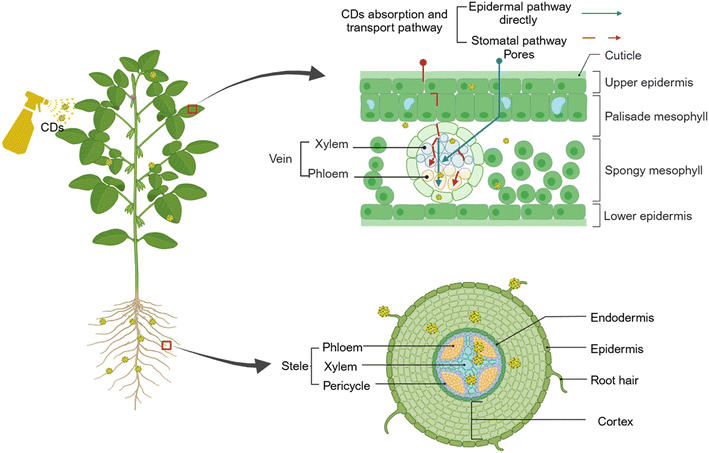
When delving into the biosafety of CDs, it is imperative to investigate the mechanisms underlying their transportation within plants. Presently, the absorption and translocation of CDs in plants, along with their potential implications on plant growth and development, have garnered considerable attention among researchers. A thorough examination of these facets will not only enhance our comprehension of the potential applications and associated risks of CDs in plants but also furnish a theoretical foundation for their potential widespread utilization in urban agriculture.It was demonstrated that the pore size of plant cell walls exhibits significant variations across different species, with a range spanning from 1.6 nm to 6.2 nm.107 The CDs fabricated through diverse methodologies typically exhibit variations in size falling below 10 nm, enabling them to traverse plant cells and be efficiently assimilated by the plant system when their size is smaller than the aperture of the cell wall.108 This assimilation process subsequently exerts a profound influence on plant growth.
In addition, leveraging the photoluminescence properties inherent in CDs, the translocation of these nanomaterials within plants can be visualized through various techniques such as fluorescence imaging, transmission electron microscopy, and Raman spectroscopy and so on.109,110 Through fluorescence microscopy, the presence of CDs was observed in the root crown cells, root cortex, vascular bundle, and mesophyll cells of maize seedlings that were treated with CDs.111 This finding demonstrates the localization of CDs within specific tissues of the maize seedlings, providing valuable insights into their distribution and potential interactions within the plant system. Laser confocal microscopy imaging unequivocally verified the penetration of CDs through the intercellular spaces of the seed coat, resulting in their accumulation within the cotyledons.112
Plants transport water and nutrients efficiently through two distinct pathways: the symplast pathway, which involves the continuous cytoplasmic network of cells; the exoplasmic pathway, which utilizes the intercellular spaces outside the cell walls.113 Nonetheless, CDs can traverse not only via the exoplasmic pathway but also through the symplastic pathway, thus exhibiting a versatile mode of transportation.114 Studies on the uptake and transport of CDs show that they can penetrate plant cells and then transport along with water and nutrients from the roots to stems and leaves, which later accumulate in different regions of the plant.115,116 Smaller-sized CDs exhibited a greater capacity for seamless transfer and penetration into cells.117 Within cells, plants absorb CDs via the exoplasmic pathway, traversing the cell wall and plasmodesmata, and subsequently penetrating the cortex to gain access to the xylem via the extracellular space.118 Utilizing laser confocal microscopy, imaging revealed that the CDs possess the capacity to adhere onto the surface of the root and subsequently infiltrate into the vascular bundle of root.112 Subsequently, these CDs were transported from the root, through the vascular system, to the stem and leaf, ultimately being detected within the leaf veins.112 The transportation of CDs from rice roots through the stems to the leaves was not only confirmed, but also subcellular localization revealed the presence of CDs within the nucleus.119
Apart from absorption through roots, CDs can also be absorbed via leaves through foliar spraying techniques. During the gas exchange process, two guard cells at the stomatal pore form an aperture with a width ranging from 3–12 μm and a length of approximately 10–30 μm.120 This intricate anatomical structure facilitates the direct entry of CDs into the plant system through the stomatal pores, thereby enhancing the efficiency of CDs utilization within plants. CDs initially penetrate leaf cells via pores present on the leaf surface, subsequently undergoing translocation to other parts of the plant via the intricate vascular network within the leaf, and even descending through the phloem (Fig. 3).121
Furthermore, the surface characteristics of CDs have the potential to significantly influence their translocation and dissemination within plant.122 Upon examination, it was observed that black solid material, identified as CDs, sporadically emerged at the apical and marginal regions of maize seedling leaves.111 Therefore, during the process of translocation within plants, CDs penetrate the root system and primarily accumulate within the nucleus of the primary root meristem.115 The transportation of nutrients and substances to the leaves occurs primarily via the vascular system, with accumulation predominantly occurring within the veins, rather than within the mesophyll system, and a minute amount of precipitation phenomenon can also be observed in the leaves during the accumulation process.112
In summary, the distribution of CDs in plants is influenced by their physical structure and superficial properties, resulting in their occurrence in multiple anatomical regions including the roots, stems, and leaves. Hence, the investigation of the safety profile of CDs in plants holds significant implications for their utilization in agricultural production.
5.2 Current knowledge on photosynthesis modulated by carbon dots in plants
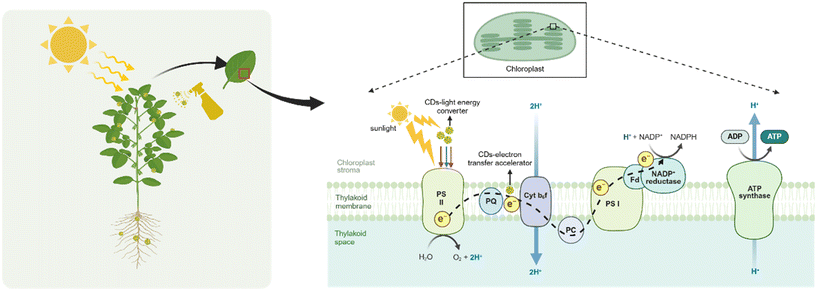
Photosynthesis, a fundamental process in plant biology, converts solar energy into chemical energy, thereby playing a pivotal role in promoting plant growth and enhancing biomass accumulation. This complex biochemical process is primarily categorized into two distinct stages: the photoreaction stage, which was occurring on the thylakoid membrane, encompassing the absorption and transformation of light energy, electron transfer mechanisms, and photosynthetic phosphorylation; the dark reaction process within the chloroplast matrix, primarily associated with the carbon assimilation process.123 Photosynthesis primarily occurs within chloroplasts, utilizing chlorophyll as the key photosynthetic pigment crucial for capturing light energy, specifically exhibiting peak absorption in the red spectrum (640–660 nm) and blue-purple spectrum (430–450 nm).124 Chlorophyll, upon excitation by light, efficiently transfers energy to the central pigment molecule, thereby initiating a photochemical reaction. This process involves the seamless transmission of absorbed light through intricate electron transport chains, ultimately reaching the photosystem-I (PS-I) and photosystem-II (PS-II) located on the thylakoid membrane.125 This meticulous orchestration ensures the efficient conversion of light energy into chemical energy within the photosynthetic apparatus. The light reaction ultimately generates adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH), which serve as the energy sources for the dark reaction of photosynthesis, specifically for the fixation of carbon dioxide (CO2).126 This process, known as CO2 assimilation, involves the utilization of energy provided by NADPH and ATP to convert CO2 into carbohydrates, ultimately leading to the synthesis of various carbohydrate molecules.126The conversion efficiency of absorbed light energy remains significantly below its theoretical potential. Consequently, optimizing the light reaction process presents a viable avenue for enhancing photosynthetic performance. This optimization involves strategies such as enhancing the absorption and transformation capabilities of light, expediting the recuperation from non-photochemical quenching, and fine-tuning the enzymes involved in the Calvin cycle.127 By implementing these improvements, it is possible to achieve a more efficient utilization of light energy, thereby bolstering photosynthetic efficiency.
CDs possess outstanding adjustable PL properties, and it has been reported that their luminous range encompasses nearly the entirety of the visible spectrum.128 CDs, regarded as artificial optical antennas, capture ultraviolet (UV) and infrared light spectra unabsorbable by plants, converting them into visible light wavelengths with plant photosynthesis, enabling chloroplasts to efficiently capture and assimilate this converted light energy, thus enhancing the rate of photosynthetic electron transfer and ultimately boosting the overall efficiency of the light reaction process.92,124,128–130 Concurrently, CDs possess the dual functionality of serving as both electron donors and acceptors, thereby expediting the photosynthetic process via the facilitation of electron transfer initially to the chloroplast and ultimately to the light-harvesting complex (Fig. 4).131
It was reported that recombined the synthesized blue fluorescent CDs with isolated chloroplasts, it is found that CDs can strongly conjugate over the surface of the chloroplast and transfer electrons towards chloroplasts by assistance of absorbed light or photons. CDs can modulate the electron transfer process to convert light energy into electrical energy and finally to the chemical energy as assimilatory power (ATP and NADPH), thereby elucidating the role of CDs in facilitating energy transfer to chloroplasts during the process of photosynthesis.132 The far-red CDs, synthesized from reduced glutathione and formamide, empower chloroplasts to harness UV-A radiation and transduce it into far-red light. This capability allows chloroplasts to directly and efficiently utilize far-red light, thereby facilitating the electron transfer from PS-II to PS-I.133 Consequently, it enhances the ATP content within the chloroplasts, contributing to the overall photosynthetic efficiency. The study demonstrated that CDs significantly impacted chlorophyll content, elevating it, facilitated light absorption, improved energy conversion efficiency within chloroplasts, and enhanced absorption and fluorescence emission spectra intensity in chloroplasts, thereby augmenting photosynthetic system activity by accelerating electron transfer.134 These findings contribute to a deeper understanding of the mechanisms involved in chloroplast function and photosynthesis. Researchers successfully synthesized magnesium and nitrogen co-doped carbon dots (Mg,N-CDs), and further demonstrated their versatility beyond photoluminescence properties by utilizing rice and ex vivo chloroplasts as model systems.128 The results revealed that CDs have the potential to enhance chlorophyll metabolism and its associated photosynthetic activity by upregulating the expression of genes involved in chlorophyll biosynthesis and turnover.128
Furthermore, CDs are capable of enhancing not only photoreactions but also the dark reactions of photosynthesis. Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) stands as a pivotal enzyme within the Calvin cycle, playing a fundamental role in carbon assimilation during the dark phase of photosynthesis.127 Consequently, the activity of RuBisCO has a direct impact on the overall rate of photosynthesis, thus highlighting its significance in this biological process. After CDs penetrate the plant mesophyll cells, they effectively enhance the enzymatic activity of RuBisCO, catalyze the first major carbon fixation reaction in Calvin cycle of photosynthesis, thereby augmenting the rate of CO2 assimilation.92,135,136 Upon treatment with 30 mg L−1 CDs, a noteworthy enhancement of 51.9% in RuBisCO enzyme activity was observed in lettuce, which is more conducive to the accumulation of carbohydrates and effectively promotes the formation of photosynthetic products.134 Therefore, the content of soluble protein is increased by 60.5%, and the content of soluble sugar is increased by 16.2% in leaf tissue.134
Researchers successfully synthesized three distinct types of blue fluorescent CDs (B-CDs) utilizing various raw materials.137 Notably, these B-CDs demonstrated a remarkable ability to traverse leaf cell membranes, resulting in efficient intracellular organelle transport, which significantly enhanced photosynthetic parameters including chlorophyll synthesis, Fd protein activity, RuBisCO enzyme functionality, and gene expression patterns.137 Consequently, the net photosynthetic rate of plants was substantially elevated, ranging from an impressive 138.5% to a staggering 350.0% increase.137 The study found that CDs in plants will be catalytically degraded by horseradish peroxidase (HRP) and hydrogen peroxide (H2O2) into phytohormone analogs and CO2.138,139 These phytohormone analogues possess the capability to regulate plant growth, while CO2 functions as a vital raw material in the Calvin cycle, contributing to the synthesis of organic matter.138,139 This enzymatic process not only plays a significant role in plant metabolism but also underscores the intricate interplay between enzymatic catalysis and plant physiology.138,139 The aforementioned findings suggest that CDs have the capacity to significantly enhance crop yield through the facilitation of photosynthetic processes in plants, thereby offering immense potential for utilization in plant factory (Table 3).
| CD precursor | Size | Optimum concentration | Plant species | Mode of application | Effect on plant photosynthesis | Ref. |
|---|---|---|---|---|---|---|
| Fluorescence | ||||||
| Glutathione and formamide | 3.8 nm | 9 μg mL−1 | Roman lettuce | Hydroponic | Increased photosynthetic efficiency by light conversion | 133 |
| Far-red | ||||||
| Citric acid and ethanolamine | 3.25 nm | 300 μg mL−1 | Rice | Foliar spray | CDs in chloroplasts could convert UV into the light coverage of photosynthesis | 92 |
| Blue and red | ||||||
| Citric acid and cysteine | 4–8 nm | 100 μg mL−1 | Mung bean | Seed pretreatment | Enhanced the photosynthesis and accumulated more carbohydrates by improving the activity of rubisco enzyme | 140 |
| Blue | ||||||
| Glucose | 5 nm | 0.56 mg ml−1 | Rice | Seed pretreatment | Enhanced the RuBisCO activity and CDs degraded to form CO2 which is then converted into carbohydrates through the Calvin cycle of photosynthesis | 139 |
| Blue | ||||||
| Natural xylose | 1.9–3.5 nm | 200 μg mL−1 | Isolated chloroplasts of fresh spinach | CDs-isolated chloroplasts mixing | The synergistic effect of fragmented graphitized structures embedded in the amorphous structure of carbon dots facilitates efficient electron transfer | 141 |
| — | ||||||
| Withered leaves | — | 0.15 mg ml−1 | Bean sprout | Seed pretreatment | Increased chlorophyll content and photosystem activity | 142 |
| Blue | ||||||
| Citric acid and ethylenediamine | 3.8 nm | 5 mg L−1 | Soybean | Foliar spray | Scavenged ROS, protected plant photosynthesis from oxidative stress and promoted carbohydrate transport | 143 |
| Blue | ||||||
| Biochar | 1–4 nm | 150 mg L−1 | Rice | Foliar spray | Improved the photosynthetic rate and stomatal conductance in relation to carbon assimilation and intrinsic water use efficiency of C3 and C4 plants | 144 |
| Blue | ||||||
| Natural waste biomass | 6–20 nm | — | Brassica chinensis L. | Foliar spray | Improved photosynthetic parameters by enhanced the ability of light energy conversion and nutrient supply can | 137 |
| Blue | ||||||
| — | 4–6 nm | 0.02 mg ml−1 | Mung bean sprout | Seed pretreatment | Enhanced the photosystem activity by accelerating the electron transfer rate | 116 |
| Yellow-green | ||||||
| Citric acid and 1,3-diaminopropane | 1.8–3.8 nm | 100 mg L−1 | Lettuce | Foliar spray | Increased photosynthetic pigment content and affected chlorophyll fluorescence parameters | 145 |
| Blue | ||||||
| Diethylenetriamine and citric acid | 2.48–3.31 nm | 300 mg L−1 | Apple | Medium treatment with CDs | As electron donors, offer a PQ-9-involved complement of PETC to improve photosynthesis | 146 |
| Blue | ||||||
| L-Cysteine and glucose | 1.5–2.7 nm | 0.066 mg ml−1 | Lettuce | Seed pretreatment | Promoted plant photosynthesis by enhanced the activity of photosystem I (PSI) | 147 |
| Blue | ||||||
| Citric acid | 2.5 ± 0.5 nm | 5 mg L−1 | Corn | Foliar spray | Improved the optical conversion and electron supply | 148 |
| Blue | ||||||
| Levofloxacin hydrochloride | 4.2 nm | 25 mg L−1 | C. pyrenoidosa | Incubation of C. pyrenoidosa with CDs | Reduced the recombination efficiency of electron–hole pairs in CDs and improved the electron transfer rate in the optical system by transferring photo-induced electrons to P680+ and QA+ | 149 |
| Blue |
6. Systematic biotoxicity and safety evaluation on carbon dots
To delve deeper into the utilization of CDs in urban agriculture, a comprehensive biosafety assessment of CDs is imperative. Presently, the biotoxicity of CDs remains a subject of debate, and its influence on plants varies significantly depending on the specific plant species and the nature of the carbon dot material combinations employed. Consequently, future investigations exploring the application of CDs in the urban agriculture must take into account their biotoxicity and safety aspects thoroughly.6.1 Safety of carbon dots in plants
To effectively translate the practical utilization of CDs in urban agriculture into reality, it is imperative for researchers to meticulously assess their potential hazards towards various organisms and the surrounding environment. Presently, the bulk of research exploring the toxicity of CDs in vivo primarily centers on animals and microorganisms, with a conspicuous scarcity of studies pertaining to their toxicity in plants.In the manufacturing of CDs, the utilization of diverse raw materials, preparation techniques, and research methodologies may exert varying impacts on plants. Specifically, CDs exhibiting differing degrees of toxicity in plants have been observed, with CDs containing amino groups demonstrating low toxicity levels while CDs possessing intricate surface functional groups exhibiting high toxicity towards plants.150 Therefore, it is crucial to carefully consider the choice of raw materials and preparation methods during the production of CDs in order to minimize potential negative impacts on plants.
In the context of safety evaluation of CDs in plants, bean sprouts were selected as a suitable model organism for investigating the toxicity of CDs due to their rapid growth rate and transparent nature, facilitating facile observation and analysis. Researchers conducted growth experiments using bean sprouts to compare the in vivo toxicity of CDs with graphene oxide (GO) and single-wall carbon nanotubes. The results demonstrated that CDs at a concentration of 100 mg L−1 were capable of enhancing the growth of mung bean sprouts without exhibiting cytotoxic effects.151 It was demonstrated that CDs have the potential to enhance growth in diverse plant species (mung bean, tomato, lettuce, Arabidopsis, peanut, wheat, corn, etc.).74,111,114,116,152–154 These findings contribute to the understanding of the potential safety of CDs in plants, providing valuable insights for future applications in agricultural.
However, some studies have documented the adverse effects of high concentrations of CDs on plant growth, indicating their potential toxicity.73 Upon investigation, it was discovered that the germination roots of mung beans underwent a significant retardation in their elongation process when the concentration of CDs exceeded 1200 mg L−1.151 Research has demonstrated that the application of CDs to plants results in their degradation into analogs of plant hormones, subsequently enhancing plant growth.32
Based on the aforementioned studies, it is implied that the potential impact of CDs on plants exhibits a concentration-dependent trend, specifically, low concentrations tend to promote plant growth, whereas higher concentrations exert inhibitory effects.32 Therefore, the optimization of CD dosages prior to their application is crucial for mitigating the potential risks associated with phytotoxicity.
6.2 The cytotoxicity of carbon dots
Currently, the primary approach employed for evaluating the biotoxicity of CDs involves the identification of diverse viable cells through techniques such as MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl-2H-tetrazolium bromide) assay or staining procedures designed to distinguish between live and dead cells.155,156 Researchers have conducted comprehensive cytotoxicity investigations on carbon spots, encompassing diverse compositions and surface structures, utilizing various mammalian cell lines as the basis for their experiments.157 The MTT assay further confirmed the non-toxic nature of CDs towards human lung cancer A549 cells and human hepatoma HepG2 cell line.158,159 Upon investigation, it was observed that the cytotoxicity exhibited by carbon spots is contingent upon the choice of surface-passivated molecules, and a more pronounced cytotoxic effect was noted at elevated concentrations.160Three surface passivation molecules (PEG1500N, PPEI-EI, PAA) were tested for cell treatment. Cytotoxicity tests on functionalized CDs found PEG1500N-functionalized CDs non-toxic, while PPEI-EI and PAA-functionalized CDs, especially at concentrations near the cytotoxicity threshold, contained higher oligo-amino polymers, leading to greater cytotoxicity.160 Functionalized CDs PEG1500N were discovered to exhibit nontoxic properties towards human renal cell lines and numerous human cancer cell lines.161,162 In conclusion, CDs exhibit relatively low toxicity towards cells, with certain materials demonstrating concentration-dependent impacts on cellular function.
6.3 Safety of carbon dots in animals and human
Upon application to plants, CDs inevitably permeate both the animal and human bodies via the intricate food chain. However, the precise implications of CDs on human health remain largely uncharted and necessitate further rigorous investigation.163 A study on mice showed that CDs were not toxic even at higher exposure levels and durations than in common optical in vivo imaging studies. No abnormalities or necrosis were found in harvested organs. The liver and spleen had higher CD concentrations but accumulation was minimal, no organ damage.159The investigation of the underlying mechanism of CDs toxicity, which necessitates the utilization of suitable in vivo toxicological models, constitutes a protracted endeavor to ensure the relevance of the outcomes to human. Given their high genetic similarity to humans, coupled with their transparency during early developmental and growth stages, zebrafish have emerged as a highly favored model organism for exploring the toxicological effects of CDs.164,165 The results demonstrated that 50% lethal dose concentration (LC50) value exceeded 100 μg ml−1, indicating that the CDs belonged to the practically non-toxic category.166 At a concentration of 150 μg mL−1, there is no obvious embryonic toxicity or teratogenic effect. However, upon exceeding a CDs concentration threshold of 200 μg ml−1, zebrafish embryos will be significantly affected, including pericardial and yolk sac edema, growth retardation, spinal curvature, and death.166 Further exploration of the neurotoxicity of zebrafish reveals that high-concentration CDs can cause neurotoxicity and multi-organ damage in zebrafish larvae. CDs can be safely utilized when the concentration is lower than 200 μg mL−1.166
After conducting a series of cytotoxicity experiments and in vivo studies, CDs were found to possess no significant biological toxicity when specific surface modifications and functional groups were introduced. Specifically, CDs functionalized with PEG1500N and those containing surface amino groups exhibited no notable toxicity. Furthermore, the biotoxicity phenomenon associated with CDs displayed a distinct concentration-dependent pattern, demonstrating nontoxicity within a specific concentration threshold range. However, given the vast array of CDs, varying preparation methods, and the intricate and diverse biological responses to CDs, further comprehensive and systematic research is necessary to thoroughly assess their biosafety and potential risks.
7. A perspective on application of carbon dots in urban agriculture
Currently, urban agriculture, an emerging agricultural modality, is experiencing a rapid surge in development across the globe, holding a pivotal position within the agricultural landscape. Nevertheless, the progression of urban agriculture is confronted with numerous challenges, including land scarcity, environmental hazards, and energy expenditure, particularly in the realm of vertical agricultural lighting systems for plant factory, where energy consumption is particularly significant.Prior investigations have demonstrated that CDs possess the capacity to enhance chloroplasts' ability to harness increased light energy, expedite the rate of electron transportation across the thylakoid membrane, upregulate RuBisCO enzyme activity, and bolster plant carbon fixation rates by facilitating chlorophyll synthesis and converting ultraviolet light into visible light, which effects collectively contribute to a notable elevation in the plant's net photosynthetic rate.92,137,167,168 Therefore, CDs have the capacity to comprehensively enhance the photosynthetic efficiency of plants, irrespective of specific crop varieties, thereby offering a novel approach to reducing costs and enhancing productivity in plant factory.
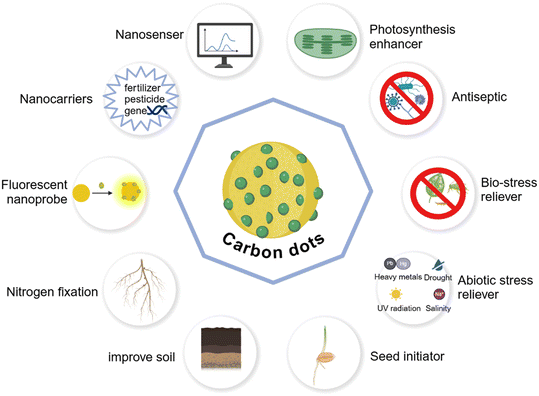
Apart from increasing photosynthetic efficiency, CDs have a wide range of agricultural science applications due to their versatility. They exhibit remarkable properties in diverse aspects, including promoting plant growth, accelerating seed germination, optimizing nitrogen nutrient uptake, effectively managing pests, and enhancing plant resilience against both biotic and abiotic stresses.75 Furthermore, their utilization as advanced fluorescent probes and nanosensors has pioneered novel avenues for plant in bioimaging, vastly enriching the research tools and application prospects of agricultural science169 (Fig. 5).
As the global population continues to expand and the demand for food intensifies, the utilization of pesticides and fertilizers has escalated accordingly. However, the extensive application of high-throughput chemical fertilizers and pesticides has reached a saturation point in terms of enhancing agricultural productivity, leading to a range of issues including environmental pollution and a decline in crop yield and quality.170 In the present context, nanotechnology has exhibited immense promise in enhancing the efficiency of pesticides and fertilizers. The controlled release and targeted delivery of nanoscale active ingredients hold significant potential for promoting sustainable agriculture and precision farming practices.171,172
In contrast to traditional fertilizers and pesticides, CDs exhibit remarkable potential in enhancing crop yield and quality, while concurrently mitigating energy consumption and mitigating environmental pollution during agricultural production. As a case in point, the application of nitrogen-doped CDs (N-CDs), serving as a water-soluble carbon nanofertilizer, has been demonstrated to significantly boost romaine lettuce biomass accumulation, enrich nutrient content, elevate photosynthetic rates, and facilitate nitrogen metabolism in plants.73 This innovative approach offers a promising alternative to conventional agricultural practices, aiming to achieve sustainable crop production with reduced environmental impact. The profound benefits associated with the application of N-CDs on romaine lettuce underscore their immense potential for fostering agricultural productivity and enhancing crop quality.
Additionally, CDs, including N-CDs, exhibit promising prospects as nano-pesticides. According to the report, N-CDs synthesized from horsetail roots demonstrated remarkable in vitro growth inhibition against plant pathogens such as Corynespora cassiicola and Phytophthora nicotianae, thus indicating their potential as environmentally friendly pesticides.173 At the same time, CDs exhibit significant advantages as sensors, demonstrating their effectiveness in detecting harmful substances, including pesticides and herbicides, within food and soil samples.174 This capability holds promise for their application in ensuring the safety of agricultural products and the preservation of soil health.
Furthermore, CDs exhibit favorable impacts in bolstering plant resilience against both abiotic and biotic stressors. Their noteworthy ROS scavenging capabilities, along with their influence on antioxidant defense mechanisms and the expression patterns of disease resistance genes, contribute significantly to enhancing the resilience of crops towards a range of abiotic or biotic challenges.143,175 Meanwhile, CDs exhibited antibacterial activity against a wide range of bacteria and fungi, thereby establishing a solid foundation for their potential utilization in crop disease management.176–178 All of these findings offer innovative approaches towards enhancing crop yields and managing diseases, thereby contributing to the advancement and sustainable development of urban agriculture.
However, to successfully apply carbon dots in urban agriculture, a series of challenges still need to be overcome. Primarily, it is essential to comprehensively assess their biotoxicity and optimize dosing to ensure agricultural safety. In addition, factors such as technical bottlenecks, economic costs, and policy supervision also need to be comprehensively considered. Future research should deeply explore the plant growth promotion mechanism of carbon dots, promote their deep integration with intelligent agricultural technologies, and at the same time strengthen toxicity risk assessment, cost control, and policy guidance to fully realize the potential of carbon dots in urban agriculture. By judiciously employing CDs, urban agriculture stands to better accommodate the demands of contemporary urban development and offer novel avenues for the realization of efficient, eco-friendly, and sustainable agricultural production techniques.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
HFZ and QC proposed the project; all the authors carried out reference searching; HFZ and YW wrote the manuscript; HFZ, YW and QC made review and final editing. All authors read and approved the final manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was funded by National Natural Science Foundation of China (32101661); Open Project of State Key Laboratory of Cotton Biology (CB2024A35). We are grateful to BioRender for providing an intuitive and powerful tool for scientific illustration. The figures in this paper have obtained permissions from BioRender.References
- F. M. Butlin and E. E. Howard, To-morrow: A Peaceful Path to Real Reform, Econ. J., 1899, 9, 71 CrossRef.
- N. Akita, Urban Services to Ecosystems: Green Infrastructure Benefits from the Landscape to the Urban Scale, Springer International Publishing, Cham, 2021, pp. 227–242 Search PubMed.
- Y. Zhou, W. Chunjui and Y. Zhou, How Does Urban Farming Benefit Participants? Two Case Studies of the Garden City Initiative in Taipei, Land, 2023, 12, 55 CrossRef.
- C. Dimitri and S. Rogus, Agriculture in urban and peri-urban areas in the United States: Highlights from the Census of Agriculture, Renew. Agr. Food Syst., 2015, 30, 64–78 CrossRef.
- K. Ackerman, P. Culligan, R. Plunz, M.-P. Sutto and L. Whittinghill, Sustainable Food Systems for Future Cities: The Potential of Urban Agriculture, The Economic and Social Review, 2014, 45, 189–206 Search PubMed.
- C. Neilson and L. Rickards, The relational character of urban agriculture: competing perspectives on land, food, people, agriculture and the city, Geogr. J., 2017, 183, 295–306 CrossRef.
- K. Colasanti, M. Hamm and C. Litjens, The City as an “Agricultural Powerhouse”? Perspectives on Expanding Urban Agriculture from Detroit, Michigan, Urban Geogr., 2012, 33, 348–369 CrossRef.
- E. Schwab, S. Caputo and J. Hernández-García, Urban Agriculture: Models-in-Circulation from a Critical Transnational Perspective, Landsc. Urban Plan., 2018, 170, 15–23 CrossRef.
- M. Dobele and A. Zvirbule, The Concept of Urban Agriculture – Historical Development and Tendencies, Rural Sustain. Res., 2020, 43, 20–26 Search PubMed.
- E. Sanyé-Mengual, I. Anguelovski, J. Oliver-Solà, J. I. Montero and J. Rieradevall, Resolving differing stakeholder perceptions of urban rooftop farming in Mediterranean cities: promoting food production as a driver for innovative forms of urban agriculture, Agric. Hum. Values, 2015, 33, 101–120 CrossRef.
- D. Despommier, The vertical farm: controlled environment agriculture carried out in tall buildings would create greater food safety and security for large urban populations, J. Verbrauch. Lebensm., 2011, 6, 233–236 CrossRef.
- Y. Ren, International Modern Urban Agriculture Experience and Its Enlightenment to China, Academic Journal of Business & Management, 2020, 2, 100–105 Search PubMed.
- T. Krikser, I. Zasada and A. Piorr, Socio-Economic Viability of Urban Agriculture—A Comparative Analysis of Success Factors in Germany, Sustainability, 2019, 11, 1999–1999 CrossRef.
- R. Iizuka, T. Kikuchi, T. Miyachi and M. Yamamoto, Food Problems and New Challenges of Urban Agriculture in Tokyo, in Tokyo as a Global City, ed. T. Kikuchi and T. Sugai, International Perspectives in Geography, 2018, pp. 137–154 Search PubMed.
- L. Kiminami, S. Furuzawa and A. Kiminami, Social Entrepreneurship, Social Business and the Multi-functionality of Urban Agriculture: Mixed Methods Research on Japan and China, SpringerBriefs in Economics, 2022, pp. 29–40 Search PubMed.
- J. A. Diehl, E. Sweeney, B. Wong, C. S. Sia, H. Yao and M. Prabhudesai, Feeding cities: Singapore's approach to land use planning for urban agriculture, Glob. Food Secur., 2020, 26, 100377 CrossRef.
- L. Xi, M. Zhang, L. Zhang, T. T. S. Lew and Y. M. Lam, Novel Materials for Urban Farming, Adv. Mater., 2022, 34, e2105009 CrossRef PubMed.
- R. P. Hall, B. Van Koppen and E. Van Houweling, The human right to water: the importance of domestic and productive water rights, Sci. Eng. Ethics, 2014, 20, 849–868 CrossRef PubMed.
- A. Abdulkadir, L. Dossa, D. Lompo, N. Abdu and H. Keulen, Characterization of urban and periurban agroecosystems in three West African cities, Int. J. Agric. Sustain., 2012, 10, 289–314 CrossRef.
- L. V. Bennedetti, P. A. de Almeida Sinisgalli, M. L. Ferreira and F. Lemes de Oliveira, Challenges to Promote Sustainability in Urban Agriculture Models: A Review, Int. J. Environ. Res. Public Health, 2023, 20, 2110 CrossRef PubMed.
- R. McDougall, P. Kristiansen and R. Rader, Small-scale urban agriculture results in high yields but requires judicious management of inputs to achieve sustainability, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 129–134 CrossRef CAS.
- J. Langemeyer, C. Madrid-Lopez, A. Mendoza Beltran and G. Villalba Mendez, Urban agriculture — A necessary pathway towards urban resilience and global sustainability?, Landsc. Urban Plan., 2021, 210, 104055 CrossRef.
- L. Pearson, L. Pearson and C. Pearson, Sustainable urban agriculture: Stocktake and opportunities, Int. J. Agric. Sustain., 2010, 8, 7–19 CrossRef.
- S. M. Petrea, M. T. Coadă, V. Cristea, L. Dediu, D. S. Cristea, A. T. Rahoveanu, A. G. Zugravu, M. M. T. Rahoveanu and D. Mocuta, A Comparative Cost – Effectiveness Analysis in Different Tested Aquaponic Systems, Agriculture and Agricultural Science Procedia, 2016, 10, 555–565 CrossRef.
- N. J. Kennard and R. H. Bamford, Urban Agriculture: Opportunities and Challenges for Sustainable Development, Zero Hunger, Springer International Publishing, Cham, 2020, pp. 929–942 Search PubMed.
- S. R. Jaeger, Vertical farming (plant factory with artificial lighting) and its produce: consumer insights, Curr. Opin. Food Sci., 2024, 56, 101145 CrossRef.
- L. Zhang, X. Yang, T. Li, R. Gan, Z. Wang, J. Peng, J. Hu, J. Guo, Y. Zhang, Q. Li and Q. Yang, Plant factory technology lights up urban horticulture in the post-coronavirus world, Hortic. Res., 2022, 9, uhac018 CrossRef.
- B. Aggarwal, N. Rajora, G. Raturi, H. Dhar, S. B. Kadam, P. S. Mundada, S. M. Shivaraj, V. Varshney, R. Deshmukh, V. T. Barvkar, P. Salvi and H. Sonah, Biotechnology and urban agriculture: A partnership for the future sustainability, Plant Sci., 2024, 338, 111903 CrossRef CAS PubMed.
- T. Kozai, G. Niu and M. Takagaki, Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, Academic Press, 2015 Search PubMed.
- X. Liu, Y. Xu, Y. Wang, Q. Yang and Q. Li, Rerouting Artificial Light for Efficient Crops Production: A Review of Lighting Strategy in PFALs, Nanjing Linye Daxue Xuebao, 2022, 12, 1021–1029 Search PubMed.
- G. L. Barbosa, F. D. Gadelha, N. Kublik, A. Proctor, L. Reichelm, E. Weissinger, G. M. Wohlleb and R. U. Halden, Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods, Int. J. Environ. Res. Public Health, 2015, 12, 6879–6891 CrossRef PubMed.
- Y. Li, X. Xu, Y. Wu, J. Zhuang, X. Zhang, H. Zhang, B. Lei, C. Hu and Y. Liu, A review on the effects of carbon dots in plant systems, Mater. Chem. Front., 2020, 4, 437–448 RSC.
- R. Paradiso and S. Proietti, Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems, J. Plant Growth Regul., 2022, 41, 742–780 CrossRef CAS.
- Z. H. Bian, Q. C. Yang and W. K. Liu, Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: a review, J. Sci. Food Agric., 2015, 95, 869–877 CrossRef CAS.
- Y. Li, G. Xin, C. Liu, Q. Shi, F. Yang and M. Wei, Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings, BMC Plant Biol., 2020, 20, 318 CrossRef CAS PubMed.
- D. Burritt and D. Leung, Adventitious shoot regeneration from Begonia×erythrophylla petiole sections is developmentally sensitive to light quality, Physiol. Plant., 2003, 118, 289–296 CrossRef CAS.
- J. Li, C. Yi, C. Zhang, F. Pan, C. Xie, W. Zhou and C. Zhou, Effects of light quality on leaf growth and photosynthetic fluorescence of Brasenia schreberi seedlings, Heliyon, 2021, 7, e06082 CrossRef CAS PubMed.
- S. Kapoor, R. Raghuvanshi, P. Bhardwaj, H. Sood, S. Saxena and O. P. Chaurasia, Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew, J. Photochem. Photobiol., B, 2018, 183, 258–265 CrossRef CAS PubMed.
- Y. Kim, H. M. Kim, H. Kim, B. R. Jeong, H.-J. Lee, H. J. Kim and S. Hwang, Ice plant growth and phytochemical concentrations are affected by light quality and intensity of monochromatic light-emitting diodes, Hortic., Environ. Biotechnol., 2018, 59, 529–536 CrossRef.
- M. Landi, M. Zivcak, O. Sytar, M. Brestic and S. I. Allakhverdiev, Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review, Biochim. Biophys. Acta, Bioenerg., 2020, 1861, 148131 CrossRef CAS PubMed.
- C. Kang, Y. Zhang, R. Cheng, E. Kaiser, Q. Yang and T. Li, Acclimating Cucumber Plants to Blue Supplemental Light Promotes Growth in Full Sunlight, Front. Plant Sci., 2021, 12, 782465 CrossRef PubMed.
- S. W. Hogewoning, G. Trouwborst, H. Maljaars, H. Poorter, W. van Ieperen and J. Harbinson, Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light, J. Exp. Bot., 2010, 61, 3107–3117 CrossRef CAS PubMed.
- Y. Li, Z. Liu, Q. Shi, F. Yang and M. Wei, Mixed red and blue light promotes tomato seedlings growth by influencing leaf anatomy, photosynthesis, CO2 assimilation and endogenous hormones, Sci. Hortic., 2021, 290, 110500 CrossRef CAS.
- Y. Li, G. Xin, M. Wei, Q. Shi, F. Yang and X. Wang, Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities, Sci. Hortic., 2017, 225, 490–497 CrossRef CAS.
- L. G. Izzo, C. Arena, V. De Micco, F. Capozzi and G. Aronne, Light quality shapes morpho-functional traits and pigment content of green and red leaf cultivars of Atriplex hortensis, Sci. Hortic., 2019, 246, 942–950 CrossRef.
- S. Song, Y. Hou, R. B. H. Lim, L. Y. F. Gaw, D. R. Richards and H. T. W. Tan, Comparison of vegetable production, resource-use efficiency and environmental performance of high-technology and conventional farming systems for urban agriculture in the tropical city of Singapore, Sci. Total Environ., 2022, 807, 150621 CrossRef CAS PubMed.
- K. Benke and B. Tomkins, Future food-production systems: Vertical farming and controlled-environment agriculture, Sustain.: Sci. Pract. Policy, 2017, 13, 13–26 Search PubMed.
- D. C. J. Neo, M. M. X. Ong, Y. Y. Lee, E. J. Teo, Q. Ong, H. Tanoto, J. Xu, K. S. Ong and V. Suresh, Shaping and Tuning Lighting Conditions in Controlled Environment Agriculture: A Review, ACS Agric. Sci. Technol., 2022, 2, 3–16 CrossRef CAS.
- W. C. Randall and R. G. Lopez, Comparison of Supplemental Lighting from High-pressure Sodium Lamps and Light-emitting Diodes during Bedding Plant Seedling Production, HortScience, 2014, 49, 589–595 Search PubMed.
- A. Trivellini, S. Toscano, D. Romano and A. Ferrante, The Role of Blue and Red Light in the Orchestration of Secondary Metabolites, Nutrient Transport and Plant Quality, Plants, 2023, 12, 2026 CrossRef CAS PubMed.
- T. W. Tibbitts, D. C. Morgan and I. J. Warrington, Growth of Lettuce, Spinach, Mustard, and Wheat Plants under Four Combinations of High-pressure Sodium, Metal Halide, and Tungsten Halogen Lamps at Equal PPFD, J. Am. Soc. Hortic. Sci., 1983, 108, 622–630 Search PubMed.
- Y. Ma, A. Xu and Z. M. Cheng, Effects of light emitting diode lights on plant growth, development and traits a meta-analysis, Hortic. Plant J., 2021, 7, 552–564 CrossRef.
- M. Anpo, H. Fukuda and T. Wada, Introduction: Artificial Light-Type Plant Factories—Outline and a Vision for the Future, Plant Factory Using Artificial Light, 2019, pp. 17–22 Search PubMed.
- D. Xu, COP1 and BBXs-HY5-mediated light signal transduction in plants, New Phytol., 2020, 228, 1748–1753 CrossRef CAS.
- G. D. Massa, H.-H. Kim, R. M. Wheeler and C. A. Mitchell, Plant Productivity in Response to LED Lighting, Hortscience, 2008, 43, 1951–1956 Search PubMed.
- Y. Miao, Q. Chen, M. Qu, L. Gao and L. Hou, Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants, Sci. Hortic., 2019, 257, 108680 CrossRef CAS.
- G. P. Wang, L. Zhang, F. L. Cao, Y. P. Ding, Q. Zhao, H. Q. Zhao and Z. Wang, Effect of red and blue light quality on growth physiological and flavonoid content of Ginkgo biloba seedlings, Nanjing Linye Daxue Xuebao, 2024, 48, 105–112 CAS.
- H. Yang, T. Wang, F. Ji, Q. Zhou and W. Jianfeng, Effects of LED light spectrum on the growth and energy use efficiency of eggplant transplants, Int. J. Agric. Biol. Eng., 2023, 16, 23–29 Search PubMed.
- A. M. Hoffmann, G. Noga and M. Hunsche, Acclimations to light quality on plant and leaf level affect the vulnerability of pepper (Capsicum annuum L.) to water deficit, J. Plant Res., 2015, 128, 295–306 CrossRef CAS.
- R. Paradiso, C. Arena, Y. Rouphael, L. d'Aquino, K. Makris, P. Vitaglione and S. De Pascale, Growth, photosynthetic activity and tuber quality of two potato cultivars in controlled environment as affected by light source, Plant Biosyst., 2019, 153, 725–735 CrossRef.
- J. Liu and M. W. van Iersel, Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms, Front. Plant Sci., 2021, 12, 619987 CrossRef PubMed.
- Y. Niu, H. Lyu, X. Liu, M. Zhang and H. Li, Photosynthesis prediction and light spectra optimization of greenhouse tomato based on response of red–blue ratio, Sci. Hortic., 2023, 318, 112065 CrossRef CAS.
- L. J. Zhou, Y. Wang, Y. Wang, A. Song, J. Jiang, S. Chen, B. Ding, Z. Guan and F. Chen, Transcription factor CmbHLH16 regulates petal anthocyanin homeostasis under different lights in Chrysanthemum, Plant Physiol., 2022, 190, 1134–1152 CrossRef CAS PubMed.
- Z. Guo, Y. Qin, J. Lv, X. Wang, T. Ye, X. Dong, N. Du, T. Zhang, F. Piao, H. Dong and S. Shen, High red/far-red ratio promotes root colonization of Serratia plymuthica A21-4 in tomato by root exudates-stimulated chemotaxis and biofilm formation, Plant Physiol. Biochem., 2024, 206, 108245 CrossRef CAS PubMed.
- Z. Guo, J. Lv, H. Zhang, C. Hu, Y. Qin, H. Dong, T. Zhang, X. Dong, N. Du and F. Piao, Red and blue light function antagonistically to regulate cadmium tolerance by modulating the photosynthesis, antioxidant defense system and Cd uptake in cucumber(Cucumis sativus L.), J. Hazard. Mater., 2022, 429, 128412 CrossRef CAS PubMed.
- M. Aalifar, S. Aliniaeifard, M. Arab, M. Zare Mehrjerdi, S. Dianati Daylami, M. Serek, E. Woltering and T. Li, Blue Light Improves Vase Life of Carnation Cut Flowers Through Its Effect on the Antioxidant Defense System, Front. Plant Sci., 2020, 11, 511 CrossRef.
- M. Orlando, A. Trivellini, M. Puccinelli, A. Ferrante, L. Incrocci and A. Mensuali-Sodi, Increasing the functional quality of Crocus sativus L. by-product (tepals) by controlling spectral composition, Hortic., Environ. Biotechnol., 2022, 63, 363–373 CrossRef CAS.
- C. Piovene, F. Orsini, S. Bosi, R. Sanoubar, V. Bregola, G. Dinelli and G. Gianquinto, Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture, Sci. Hortic., 2015, 193, 202–208 CrossRef.
- Z. Bian, N. Jiang, S. Grundy and C. Lu, Uncovering LED light effects on plant growth: New angles and perspectives – LED light for improving plant growth, nutrition and energy-use efficiency, Acta Hortic., 2018, 1227, 491–498 CrossRef.
- A. M. Beacham, L. H. Vickers and J. M. Monaghan, Vertical farming: a summary of approaches to growing skywards, J. Hortic. Sci. Biotechnol., 2019, 94, 277–283 CrossRef.
- Y. Li and F. Tao, Research Progress on the Mechanism of High Light Use Efficiency in Wheat, Chinese Journal of Agrometeorology, 2022, 43, 93–111 Search PubMed.
- X. G. Zhu, S. P. Long and D. R. Ort, Improving photosynthetic efficiency for greater yield, Annu. Rev. Plant Biol., 2010, 61, 235–261 CrossRef CAS.
- J. Tan, S. Zhao, J. Chen, X. Pan, C. Li, Y. Liu, C. Wu, W. Li and M. Zheng, Preparation of nitrogen-doped carbon dots and their enhancement on lettuce yield and quality, J. Mater. Chem. B, 2023, 11, 3113–3123 RSC.
- Z. Huang, B. Guo, Y. Zou, J. He, C. Hu, J. Zhuang and Y. Liu, Different Kinds of Citric Acid Based Carbon Dots and Their Enhancement of the Growth of Italian Lettuce, ACS Agric. Sci. Technol., 2022, 2, 684–692 CrossRef CAS.
- A. Maholiya, P. Ranjan, R. Khan, S. Murali, R. C. Nainwal, P. S. Chauhan, N. Sathish, J. P. Chaurasia and A. K. Srivastava, An insight into the role of carbon dots in the agriculture system: a review, Environ. Sci.: Nano, 2023, 10, 959–995 RSC.
- G. Ge, L. Li, D. Wang, M. Chen, Z. Zeng, W. Xiong, X. Wu and C. Guo, Carbon dots: synthesis, properties and biomedical applications, J. Mater. Chem. B, 2021, 9, 6553–6575 RSC.
- B. Wang, G. Waterhouse and S. Lu, Carbon dots: mysterious past, vibrant present, and expansive future, Trends Chem., 2022, 5, 76–87 CrossRef.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S.-Y. Xie, Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- P. Kumar, S. Dua, R. Kaur, M. Kumar and G. Bhatt, A review on advancements in carbon quantum dots and their application in photovoltaics, RSC Adv., 2022, 12, 4714–4759 RSC.
- Z. Kang and S.-T. Lee, Carbon dots: advances in nanocarbon applications, Nanoscale, 2019, 11, 19214–19224 RSC.
- M. K. Barman and A. Patra, Current status and prospects on chemical structure driven photoluminescence behaviour of carbon dots, J. Photochem. Photobiol., C, 2018, 37, 1–22 CrossRef CAS.
- Y. Liu, H. Huang, W. Cao, B. Mao, Y. Liu and Z. Kang, Advances in carbon dots: from the perspective of traditional quantum dots, Mater. Chem. Front., 2020, 4, 1586–1613 RSC.
- B. Wang, G. I. N. Waterhouse and S. Lu, Carbon dots: mysterious past, vibrant present, and expansive future, Trends Chem., 2023, 5, 76–87 CrossRef CAS.
- D. Ozyurt, M. A. Kobaisi, R. K. Hocking and B. Fox, Properties, synthesis, and applications of carbon dots: A review, Carbon Trends, 2023, 12, 100276 CrossRef CAS.
- Y. Zheng, H. Zhang, W. Li, Y. Liu, X. Zhang, H. Liu and B. Lei, Pollen derived blue fluorescent carbon dots for bioimaging and monitoring of nitrogen, phosphorus and potassium uptake in Brassica parachinensis L, RSC Adv., 2017, 7, 33459–33465 RSC.
- X. Xu, L. Mo, Y. Li, X. Pan, G. Hu, B. Lei, X. Zhang, M. Zheng, J. Zhuang, Y. Liu and C. Hu, Construction of Carbon Dots with Color-Tunable Aggregation-Induced Emission by Nitrogen-Induced Intramolecular Charge Transfer, Adv. Mater., 2021, 33, e2104872 CrossRef PubMed.
- Y. Wang, Z. Xie, X. Wang, X. Peng and J. Zheng, Fluorescent carbon-dots enhance light harvesting and photosynthesis by overexpressing PsbP and PsiK genes, J. Nanobiotechnol., 2021, 19, 260 CrossRef CAS.
- L. Peng, M. Yang, M. Zhang and M. Jia, A ratiometric fluorescent sensor based on carbon dots for rapid determination of bisulfite in sugar, Food Chem., 2022, 392, 133265 CrossRef CAS PubMed.
- D. Kim, G. Jo, Y. Chae, S. Subramani, B. Y. Lee, E. J. Kim, M. K. Ji, U. Sim and H. Hyun, Bioinspired Camellia japonica carbon dots with high near-infrared absorbance for efficient photothermal cancer therapy, Nanoscale, 2021, 13, 14426–14434 RSC.
- Q. Wu, J. Cao, X. Wang, Y. Liu, Y. Zhao, H. Wang, Y. Liu, H. Huang, F. Liao, M. Shao and Z. Kang, A metal-free photocatalyst for highly efficient hydrogen peroxide photoproduction in real seawater, Nat. Commun., 2021, 12, 483 CrossRef CAS.
- Y. Li, X. Pan, X. Xu, Y. Wu, J. Zhuang, X. Zhang, H. Zhang, B. Lei, C. Hu and Y. Liu, Carbon dots as light converter for plant photosynthesis: Augmenting light coverage and quantum yield effect, J. Hazard. Mater., 2021, 410, 124534 CrossRef CAS PubMed.
- H. Yang, C. Wang, F. Chen, L. Yue, X. Cao, J. Li, X. Zhao, F. Wu, Z. Wang and B. Xing, Foliar carbon dot amendment modulates carbohydrate metabolism, rhizospheric properties and drought tolerance in maize seedling, Sci. Total Environ., 2022, 809, 151105 CrossRef CAS PubMed.
- Y. Cao, H. Dong, Z. Yang, X. Zhong, Y. Chen, W. Dai and X. Zhang, Aptamer-Conjugated Graphene Quantum Dots/Porphyrin Derivative Theranostic Agent for Intracellular Cancer-Related MicroRNA Detection and Fluorescence-Guided Photothermal/Photodynamic Synergetic Therapy, ACS Appl. Mater. Interfaces, 2017, 9, 159–166 CrossRef CAS.
- H. Zhu, W. Zhang and S. F. Yu, Realization of lasing emission from graphene quantum dots using titanium dioxide nanoparticles as light scatterers, Nanoscale, 2013, 5, 1797–1802 RSC.
- S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang and B. Yang, The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective, Nano Res., 2015, 8, 355–381 CrossRef CAS.
- B. C. M. Martindale, G. A. M. Hutton, C. A. Caputo, S. Prantl, R. Godin, J. R. Durrant and E. Reisner, Enhancing Light Absorption and Charge Transfer Efficiency in Carbon Dots through Graphitization and Core Nitrogen Doping, Angew. Chem., Int. Ed., 2017, 56, 6459–6463 CrossRef CAS.
- X. Ding, Direct synthesis of graphene quantum dots on hexagonal boron nitride substrate, J. Mater. Chem. C, 2014, 2, 3717–3722 RSC.
- S. Li, L. Li, H. Tu, H. Zhang, D. S. Silvester, C. E. Banks, G. Zou, H. Hou and X. Ji, The development of carbon dots: From the perspective of materials chemistry, Mater. Today, 2021, 51, 188–207 CrossRef CAS.
- Y. Song, S. Zhu, S. Xiang, X. Zhao, J. Zhang, H. Zhang, Y. Fu and B. Yang, Investigation into the fluorescence quenching behaviors and applications of carbon dots, Nanoscale, 2014, 6, 4676–4682 RSC.
- S. Tao, C. Zhou, C. Kang, S. Zhu, T. Feng, S. T. Zhang, Z. Ding, C. Zheng, C. Xia and B. Yang, Confined-domain crosslink-enhanced emission effect in carbonized polymer dots, Light: Sci. Appl., 2022, 11, 56 CrossRef CAS PubMed.
- H. Ding, X.-X. Zhou, J.-S. Wei, X.-B. Li, B.-T. Qin, X.-B. Chen and H.-M. Xiong, Carbon dots with red/near-infrared emissions and their intrinsic merits for biomedical applications, Carbon, 2020, 167, 322–344 CrossRef CAS.
- H. Ding, S. B. Yu, J. S. Wei and H. M. Xiong, Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism, ACS Nano, 2016, 10, 484–491 CrossRef CAS PubMed.
- Z. Ye, X. Lin, N. Wang, J. Zhou, M. Zhu, H. Qin and X. Peng, Phonon-assisted up-conversion photoluminescence of quantum dots, Nat. Commun., 2021, 12, 4283 CrossRef CAS PubMed.
- Q. Xiang, W. Li, Y. Tan, S. Jianwei, M. Dong, J. Cheng, J. Huang, W. Zhang, Y. Gong, Q. Yang, L. Yang, H. Dong and X. Zhang, Engineering of Upconversion Carbon Dots/Metal-Organic Frameworks “Peeled Pitaya-Like” Heterostructure for Mitochondria-Targeted Photodynamic Therapy, Chem. Eng. J., 2022, 444, 136706 CrossRef CAS.
- Y. Cui, C. Zhang, L. Sun, Z. Hu and X. Liu, Simple and Efficient Synthesis of Strongly Green Fluorescent Carbon Dots with Upconversion Property for Direct Cell Imaging, Part. Part. Syst. Charact., 2015, 32, 542–546 CrossRef CAS.
- N. Carpita, D. Sabularse, D. Montezinos and D. P. Delmer, Determination of the Pore Size of Cell Walls of Living Plant Cells, Science, 1979, 205, 1144–1147 CrossRef CAS PubMed.
- Y. Zhu, Q. Zhang, Y. Li, Z. Pan, C. Liu, D. Lin, J. Gao, Z. Tang, Z. Li, R. Wang and J. Sun, Role of Soil and Foliar-Applied Carbon Dots in Plant Iron Biofortification and Cadmium Mitigation by Triggering Opposite Iron Signaling in Roots, Small, 2023, 19, e2301137 CrossRef PubMed.
- X. Yan, Q. Xu, D. Li, J. Wang and R. Han, Carbon dots inhibit root growth by disrupting auxin biosynthesis and transport in Arabidopsis, Ecotoxicol. Environ. Saf., 2021, 216, 112168 CrossRef CAS PubMed.
- Y. Gong and Z. Dong, Transfer, transportation, and accumulation of cerium-doped carbon quantum dots: Promoting growth and development in wheat, Ecotoxicol. Environ. Saf., 2021, 226, 112852 CrossRef CAS.
- J. Chen, R. Dou, Z. Yang, X. Wang, C. Mao, X. Gao and L. Wang, The effect and fate of water-soluble carbon nanodots in maize (Zea maysL.), Nanotoxicology, 2016, 10, 818–828 CrossRef CAS.
- W. Li, Y. Zheng, H. Zhang, Z. Liu, W. Su, S. Chen, Y. Liu, J. Zhuang and B. Lei, Phytotoxicity, Uptake, and Translocation of Fluorescent Carbon Dots in Mung Bean Plants, ACS Appl. Mater. Interfaces, 2016, 8, 19939–19945 CrossRef CAS.
- S. Dora, O. M. Terrett and C. Sánchez-Rodríguez, Plant–microbe interactions in the apoplast: Communication at the plant cell wall, Plant Cell, 2022, 34, 1532–1550 CrossRef.
- J. Chen, B. Liu, Z. Yang, J. Qu, H. Xun, R. Dou, X. Gao and L. Wang, Phenotypic, transcriptional, physiological and metabolic responses to carbon nanodot exposure inArabidopsis thaliana(L.), Environ. Sci.: Nano, 2018, 5, 2672–2685 RSC.
- X. Yan, J. Wang, D. Li, J. Feng, Q. Xu, H. Chen and R. Han, Response of primary root to nitrogen-doped carbon dots in Arabidopsis thaliana: alterations in auxin level and cell division activity, Environ. Sci.: Nano, 2021, 8, 1352–1363 RSC.
- H. Wang, M. Zhang, Y. Song, H. Li, H. Huang, M. Shao, Y. Liu and Z. Kang, Carbon dots promote the growth and photosynthesis of mung bean sprouts, Carbon, 2018, 136, 94–102 CrossRef CAS.
- M. Vithanage, M. Seneviratne, M. Ahmad, B. Sarkar and Y. S. Ok, Contrasting effects of engineered carbon nanotubes on plants: a review, Environ. Geochem. Health, 2017, 39, 1421–1439 CrossRef CAS.
- G. V. Lowry, A. Avellan and L. M. Gilbertson, Opportunities and challenges for nanotechnology in the agri-tech revolution, Nat. Nanotechnol., 2019, 14, 517–522 CrossRef CAS PubMed.
- H. Li, J. Huang, F. Lu, Y. Liu, Y. Song, Y. Sun, J. Zhong, H. Huang, Y. Wang, S. Li, Y. Lifshitz, S.-T. Lee and Z. Kang, Impacts of Carbon Dots on Rice Plants: Boosting the Growth and Improving the Disease Resistance, ACS Appl. Bio Mater., 2018, 1, 663–672 CrossRef CAS.
- C. Chan, D. Panzeri, E. Okuma, K. Tõldsepp, Y.-Y. Wang, G.-Y. Louh, T.-C. Chin, Y.-H. Yeh, H.-L. Yeh, S. Yekondi, Y.-H. Huang, T.-Y. Huang, T.-J. Chiou, Y. Murata, H. Kollist and L. Zimmerli, STRESS INDUCED FACTOR 2 Regulates Arabidopsis Stomatal Immunity through Phosphorylation of the Anion Channel SLAC1, Plant Cell, 2020, 32, 2216–2236 CrossRef CAS.
- J. Hong, J. R. Peralta-Videa, C. Rico, S. Sahi, M. N. Viveros, J. Bartonjo, L. Zhao and J. L. Gardea-Torresdey, Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants, Environ. Sci. Technol., 2014, 48, 4376–4385 CrossRef CAS PubMed.
- K. Qian, H. Guo, G. Chen, C. Ma and B. Xing, Distribution of different surface modified carbon dots in pumpkin seedlings, Sci. Rep., 2018, 8, 7991 CrossRef PubMed.
- A. V. Perera-Castro and J. Flexas, Recent advances in understanding and improving photosynthesis, Fac. Rev., 2020, 9, 5 Search PubMed.
- B. Guo, G. Liu, H. Wei, J. Qiu, J. Zhuang, X. Zhang, M. Zheng, W. Li, H. Zhang, C. Hu, B. Lei and Y. Liu, The role of fluorescent carbon dots in crops: Mechanism and applications, SmartMat, 2022, 3, 208–225 CrossRef CAS.
- H. Li, Y. Nakajima, E. Nango, S. Owada, D. Yamada, K. Hashimoto, F. Luo, R. Tanaka, F. Akita, K. Kato, J. Kang, Y. Saitoh, S. Kishi, H. Yu, N. Matsubara, H. Fujii, M. Sugahara, M. Suzuki, T. Masuda, T. Kimura, T. N. Thao, S. Yonekura, L.-J. Yu, T. Tosha, K. Tono, Y. Joti, T. Hatsui, M. Yabashi, M. Kubo, S. Iwata, H. Isobe, K. Yamaguchi, M. Suga and J.-R. Shen, Oxygen-evolving photosystem II structures during S1–S2–S3 transitions, Nature, 2024, 626, 670–677 CrossRef CAS PubMed.
- D. Leister, Enhancing the light reactions of photosynthesis: Strategies, controversies, and perspectives, Mol. Plant, 2023, 16, 4–22 CrossRef CAS.
- R. Li, Y. He, J. Chen, S. Zheng and C. Zhuang, Research Progress in Improving Photosynthetic Efficiency, Int. J. Mol. Sci., 2023, 24, 9286 CrossRef CAS PubMed.
- Y. Li, X. Xu, B. Lei, J. Zhuang, X. Zhang, C. Hu, J. Cui and Y. Liu, Magnesium-nitrogen co-doped carbon dots enhance plant growth through multifunctional regulation in photosynthesis, Chem. Eng. J., 2021, 422, 130114 CrossRef CAS.
- W. Li, S. Wu, H. Zhang, X. Zhang, J. Zhuang, C. Hu, Y. Liu, B. Lei, L. Ma and X. Wang, Enhanced Biological Photosynthetic Efficiency Using Light-Harvesting Engineering with Dual-Emissive Carbon Dots, Adv. Funct. Mater., 2018, 28, 1804004 CrossRef.
- X. Xu, X. Mao, J. Zhuang, B. Lei, Y. Li, W. Li, X. Zhang, C. Hu, Y. Fang and Y. Liu, PVA-Coated Fluorescent Carbon Dot Nanocapsules as an Optical Amplifier for Enhanced Photosynthesis of Lettuce, ACS Sustainable Chem. Eng., 2020, 8, 3938–3949 CrossRef CAS.
- J. H. He, Y. Y. Cheng, Q. Q. Zhang, H. Liu and C. Z. Huang, Carbon dots-based fluorescence resonance energy transfer for the prostate specific antigen (PSA) with high sensitivity, Talanta, 2020, 219, 121276 CrossRef CAS PubMed.
- S. Chandra, S. Pradhan, S. Mitra, P. Patra, A. Bhattacharya, P. Pramanik and A. Goswami, High throughput electron transfer from carbon dots to chloroplast: a rationale of enhanced photosynthesis, Nanoscale, 2014, 6, 3647–3655 RSC.
- D. Li, W. Li, H. Zhang, X. Zhang, J. Zhuang, Y. Liu, C. Hu and B. Lei, Far-Red Carbon Dots as Efficient Light-Harvesting Agents for Enhanced Photosynthesis, ACS Appl. Mater. Interfaces, 2020, 12, 21009–21019 CrossRef CAS PubMed.
- J. Hu, W. Jia, X. Wu, H. Zhang, Y. Wang, J. Liu, Y. Yang, S. Tao and X. Wang, Carbon dots can strongly promote photosynthesis in lettuce (Lactuca sativa L.), Environ. Sci.: Nano, 2022, 9, 1530–1540 RSC.
- Y. Zheng, G. Xie, X. Zhang, Z. Chen, Y. Cai, W. Yu, H. Liu, J. Shan, R. Li, Y. Liu and B. Lei, Bioimaging Application and Growth-Promoting Behavior of Carbon Dots from Pollen on Hydroponically Cultivated Rome Lettuce, ACS Omega, 2017, 2, 3958–3965 CrossRef CAS PubMed.
- A. Dutta, R. R. Dutta and S. Gogoi, in Carbon Dots in Agricultural Systems, ed. R. Khan, S. Murali and S. Gogoi, Academic Press, 2022, pp. 135–153, DOI:10.1016/B978-0-323-90260-1.00003-6.
- B. Cheng, Z. Yang, F. Chen, L. Yue, X. Cao, J. Li, H. L. Qian, X. P. Yan, C. Wang and Z. Wang, Biomass-derived carbon dots with light conversion and nutrient provisioning capabilities facilitate plant photosynthesis, Sci. Total Environ., 2023, 901, 165973 CrossRef CAS PubMed.
- H. Li, J. Huang, Y. Liu, F. Lu, J. Zhong, Y. Wang, S. Li, Y. Lifshitz, S.-T. Lee and Z. Kang, Enhanced RuBisCO activity and promoted dicotyledons growth with degradable carbon dots, Nano Res., 2019, 12, 1585–1593 CrossRef CAS.
- H. Li, J. Huang, F. Lu, Y. Liu, Y. Song, Y. Sun, J. Zhong, H. Huang, Y. Wang, S. Li, Y. Lifshitz, S. T. Lee and Z. Kang, Impacts of Carbon Dots on Rice Plants: Boosting the Growth and Improving the Disease Resistance, ACS Appl. Bio Mater., 2018, 1, 663–672 CrossRef CAS PubMed.
- M. Zhang, L. Hu, H. Wang, Y. Song, Y. Liu, H. Li, M. Shao, H. Huang and Z. Kang, One-step hydrothermal synthesis of chiral carbon dots and their effects on mung bean plant growth, Nanoscale, 2018, 10, 12734–12742 RSC.
- Z. Wang, Y. Zhang, S. Zhang, M. Ge, H. Zhang, S. Wang, Z. Chen, S. Li and C. Yang, Natural xylose-derived carbon dots towards efficient semi-artificial photosynthesis, J. Colloid Interface Sci., 2023, 629, 12–21 CrossRef CAS PubMed.
- S. Liang, M. Wang, W. Gao, S. Luo, N. Huang and Y. Qin, Fluorescent carbon dots derived from magnolia withered leaves for promoting growth and fluorescent labeling of bean sprouts, Carbon Trends, 2021, 4, 100063 CrossRef CAS.
- Y. Ji, L. Yue, X. Cao, F. Chen, J. Li, J. Zhang, C. Wang, Z. Wang and B. Xing, Carbon dots promoted soybean photosynthesis and amino acid biosynthesis under drought stress: Reactive oxygen species scavenging and nitrogen metabolism, Sci. Total Environ., 2023, 856, 159125 CrossRef CAS PubMed.
- T. L. Tan, N. A. Zulkifli, A. S. K. Zaman, M. b. Jusoh, M. N. Yaapar and S. A. Rashid, Impact of photoluminescent carbon quantum dots on photosynthesis efficiency of rice and corn crops, Plant Physiol. Biochem., 2021, 162, 737–751 CrossRef CAS PubMed.
- J. Tan, S. Zhao, J. Chen, X. Pan, C. Li, Y. Liu, C. Wu, W. Li and M. Zheng, Preparation of nitrogen-doped carbon dots and their enhancement on lettuce yield and quality, J. Mater. Chem. B, 2023, 11, 3113–3123 RSC.
- X. Jing, Y. Liu, X. Liu, Y. Zhang, G. Wang, F. Yang, Y. Zhang, D. Chang, Z.-L. Zhang, C.-X. You, S. Zhang and X.-F. Wang, Enhanced photosynthetic efficiency by nitrogen-doped carbon dots via plastoquinone-involved electron transfer in apple, Hortic. Res., 2024, 11, uhae016 CrossRef PubMed.
- E. Kou, Y. Yao, X. Yang, S. Song, W. Li, Y. Kang, S. Qu, R. Dong, X. Pan, D. Li, H. Zhang and B. Lei, Regulation Mechanisms of Carbon Dots in the Development of Lettuce and Tomato, ACS Sustainable Chem. Eng., 2021, 9, 944–953 CrossRef CAS.
- C. Wang, H. Yang, F. Chen, L. Yue, Z. Wang and B. Xing, Nitrogen-Doped Carbon Dots Increased Light Conversion and Electron Supply to Improve the Corn Photosystem and Yield, Environ. Sci. Technol., 2021, 55, 12317–12325 CrossRef CAS PubMed.
- X. Huang, J. Lin, J. Liang, E. Kou, W. Cai, Y. Zheng, H. Zhang, X. Zhang, Y. Liu, W. Li and B. Lei, Pyridinic Nitrogen Doped Carbon Dots Supply Electrons to Improve Photosynthesis and Extracellular Electron Transfer of Chlorella pyrenoidosa, Small, 2023, 19, 2206222 CrossRef CAS PubMed.
- L. Sai, S. Liu, X. Qian, Y. Yu and X. Xu, Nontoxic fluorescent carbon nanodot serving as a light conversion material in plant for UV light utilization, Colloids Surf., B, 2018, 169, 422–428 CrossRef CAS PubMed.
- X. Li, Z. Zhou, D. Lu, X. Dong, M. Xu, L. Wei and Y. Zhang, The effect of pristine carbon-based nanomaterial on the growth of green gram sprouts and pH of water, Nanoscale Res. Lett., 2014, 9, 583–589 CrossRef.
- M. Khodakovskaya, E. Dervishi, M. Mahmood, Y. Xu, Z. Li, F. Watanabe and A. S. Biris, Carbon Nanotubes Are Able To Penetrate Plant Seed Coat and Dramatically Affect Seed Germination and Plant Growth, ACS Nano, 2009, 3, 3221–3227 CrossRef CAS PubMed.
- L. Su, X. Ma, K. Zhao, C. Shen, Q. Lou, D. Yin and C. Shan, Carbon Nanodots for Enhancing the Stress Resistance of Peanut Plants, ACS Omega, 2018, 3, 17770–17777 CrossRef CAS.
- L. Xiao, H. Guo, S. Wang, J. Li, Y. Wang and B. Xing, Carbon dots alleviate the toxicity of cadmium ions (Cd2+) toward wheat seedlings, Environ. Sci.: Nano, 2019, 6, 1493–1506 RSC.
- T. Guo, X. Wang, X. Hong, W. Xu, Y. Shu and J. Wang, Modulation of the binding ability to biomacromolecule, cytotoxicity and cellular imaging property for ionic liquid mediated carbon dots, Colloids Surf., B, 2022, 216, 112552 CrossRef CAS.
- J. Wang, G. Liu, K. C. Leung, R. Loffroy, P. X. Lu and Y. X. Wang, Opportunities and Challenges of Fluorescent Carbon Dots in Translational Optical Imaging, Curr. Pharm. Des., 2015, 21, 5401–5416 CrossRef CAS PubMed.
- Y. Wang, P. Anilkumar, L. Cao, J. H. Liu, P. G. Luo, K. N. Tackett, 2nd, S. Sahu, P. Wang, X. Wang and Y. P. Sun, Carbon dots of different composition and surface functionalization: cytotoxicity issues relevant to fluorescence cell imaging, Exp. Biol. Med., 2011, 236, 1231–1238 CrossRef CAS.
- X. Zhao, J. Li, D. Liu, M. Yang, W. Wang, S. Zhu and B. Yang, Self-Enhanced Carbonized Polymer Dots for Selective Visualization of Lysosomes and Real-Time Apoptosis Monitoring, iScience, 2020, 23, 100982 CrossRef CAS.
- S. C. Ray, A. Saha, N. R. Jana and R. Sarkar, Fluorescent Carbon Nanoparticles: Synthesis, Characterization, and Bioimaging Application, J. Phys. Chem. C, 2009, 113, 18546–18551 CrossRef CAS.
- Y. Wang, P. Anilkumar, L. Cao, J.-H. Liu, P. G. Luo, K. N. Tackett, S. Sahu, P. Wang, X. Wang and Y.-P. Sun, Carbon dots of different composition and surface functionalization: cytotoxicity issues relevant to fluorescence cell imaging, Exp. Biol. Med., 2011, 236, 1231–1238 CrossRef CAS.
- Q. L. Zhao, Z. L. Zhang, B. H. Huang, J. Peng, M. Zhang and D. W. Pang, Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite, Chem. Commun., 2008,(41), 5116–5118 RSC.
- S. T. Yang, X. Wang, H. Wang, F. Lu, P. G. Luo, L. Cao, M. J. Meziani, J. H. Liu, Y. Liu, M. Chen, Y. Huang and Y. P. Sun, Carbon Dots as Nontoxic and High-Performance Fluorescence Imaging Agents, J. Phys. Chem. C Nanomater. Interfaces, 2009, 113, 18110–18114 CrossRef CAS PubMed.
- A. Khan, P. Ezati, J.-T. Kim and J. W. Rhim, Biocompatible carbon quantum dots for intelligent sensing in food safety applications: Opportunities and sustainability, Mater. Today Sustain., 2022, 100306–100314 Search PubMed.
- C. Bai and M. Tang, Progress on the toxicity of quantum dots to model organism-zebrafish, J. Appl. Toxicol., 2023, 43, 89–106 CrossRef CAS PubMed.
- A. C. Pereira, T. Gomes, M. R. Ferreira Machado and T. L. Rocha, The zebrafish embryotoxicity test (ZET) for nanotoxicity assessment: from morphological to molecular approach, Environ. Pollut., 2019, 252, 1841–1853 CrossRef CAS PubMed.
- W. Liu, G. Huang, X. Su, S. Li, Q. Wang, Y. Zhao, Y. Liu, J. Luo, Y. Li, C. Li, D. Yuan, H. Hong, X. Chen and T. Chen, Zebrafish: A Promising Model for Evaluating the Toxicity of Carbon Dot-Based Nanomaterials, ACS Appl. Mater. Interfaces, 2020, 12, 49012–49020 CrossRef CAS.
- D. Li, W. Li, H. Zhang, X. Zhang, J. Zhuang, Y. Liu, C. Hu and B. Lei, Far-Red Carbon Dots as Efficient Light-Harvesting Agents for Enhanced Photosynthesis, ACS Appl. Mater. Interfaces, 2020, 12, 21009–21019 CrossRef CAS.
- A. Guirguis, W. Yang, X. A. Conlan, L. Kong, D. M. Cahill and Y. Wang, Boosting Plant Photosynthesis with Carbon Dots: A Critical Review of Performance and Prospects, Small, 2023, 19, e2300671 CrossRef PubMed.
- C. Cao and W. Guo, Carbon dots-based fluorescent probe for the detection of imidacloprid residue in leafy vegetables, Food Chem., 2024, 435, 137578 CrossRef CAS.
- L. Liu, X. Zheng, X. Wei, Z. Kai and Y. Xu, Excessive application of chemical fertilizer and organophosphorus pesticides induced total phosphorus loss from planting causing surface water eutrophication, Sci. Rep., 2021, 11, 23015 CrossRef CAS PubMed.
- D. Wang, N. B. Saleh, A. Byro, R. Zepp, E. Sahle-Demessie, T. P. Luxton, K. T. Ho, R. M. Burgess, M. Flury, J. C. White and C. Su, Nano-enabled pesticides for sustainable agriculture and global food security, Nat. Nanotechnol., 2022, 17, 347–360 CrossRef CAS.
- C. An, C. Sun, N. Li, B. Huang, J. Jiang, Y. Shen, C. Wang, X. Zhao, B. Cui, C. Wang, X. Li, S. Zhan, F. Gao, Z. Zeng, H. Cui and Y. Wang, Nanomaterials and nanotechnology for the delivery of agrochemicals: strategies towards sustainable agriculture, J. Nanobiotechnol., 2022, 20, 11 CrossRef CAS PubMed.
- Z. Wang, Q. Liu, J. Leng, H. Liu, Y. Zhang, C. Wang, W. An, C. Bao and H. Lei, The green synthesis of carbon quantum dots and applications for sulcotrione detection and anti-pathogen activities, J. Saudi Chem. Soc., 2021, 25, 101373 CrossRef CAS.
- C. Cao and W. Guo, Carbon dots-based fluorescent probe for the detection of imidacloprid residue in leafy vegetables, Food Chem., 2024, 435, 137578 CrossRef CAS PubMed.
- L. Kuang, Y. Kang, H. Wang, R. Huang, B. Lei, M. Zhong and X. Yang, The roles of Salvia miltiorrhiza-derived carbon dots involving in maintaining quality by delaying senescence of postharvest flowering Chinese cabbage, Food Chem., 2023, 404, 134704 CrossRef CAS.
- P. Li, L. Sun, S. Xue, D. Qu, L. An, X. Wang and Z. Sun, Recent advances of carbon dots as new antimicrobial agents, SmartMat, 2022, 3, 226–248 CrossRef CAS.
- H. Wang, F. Lu, C. Ma, Y. Ma, M. Zhang, B. Wang, Y. Zhang, Y. Liu, H. Huang and Z. Kang, Carbon dots with positive surface charge from tartaric acid and m-aminophenol for selective killing of Gram-positive bacteria, J. Mater. Chem. B, 2021, 9, 125–130 RSC.
- Y. Ma, M. Zhang, H. Wang, B. Wang, H. Huang, Y. Liu and Z. Kang, N-doped carbon dots derived from leaves with low toxicity via damaging cytomembrane for broad-spectrum antibacterial activity, Mater. Today Commun., 2020, 24, 101222 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |