Nano-silicon enhances tomato growth and antioxidant defense under salt stress†
Shuaibing
Wang
a,
Xiang
Shen
b,
Xin
Guan
a,
Li
Sun
a,
Zhongxue
Yang
b,
Dandan
Wang
a,
Yinglong
Chen
c,
Peiqiang
Li
*b and
Zhihong
Xie
 *a
*a
aNational Engineering Research Center for Efficient Utilization of Soil and Fertilizer Resources, College of Resources and Environment, Shandong Agricultural University, Tai'an City, Shandong 271018, China. E-mail: zhihongxie211@163.com
bCollege of Chemistry and Materials Science, Shandong Agricultural University, Tai'an City, Shandong 271018, China. E-mail: pqli@sdau.edu.cn
cThe UWA Institute of Agriculture, & School of Agriculture and Environment, The University of Western Australia, Perth 6009, Australia
First published on 8th October 2024
Abstract
With the rapid expansion of applications in agriculture, nanotechnology has emerged as an effective alternative for alleviating abiotic stress in plants. In this study, the effects of silicon nanoparticles (SiNPs) on Na+ accumulation and salt stress in tomatoes were investigated. The results showed that a concentration of 200 mg L−1 SiNPs significantly improved tomato growth. Furthermore, photosynthesis and chlorophyll content showed positive responses to SiNPs treatment compared to salt treatment alone. Additionally, the application of 200 mg L−1 SiNPs effectively mitigated salt-induced oxidative stress by increasing the activity of antioxidant enzymes and reducing the levels of H2O2 (by 41.59% and 34.40%) and MDA (by 45.47% and 49.99%). Simultaneously, SiNPs treatment led to significant increases in the contents of K+ and Si in tomato seedlings, while decreasing the absorption of Na+. qPCR results demonstrated that SiNPs significantly up-regulated the expression of genes related to antioxidant stress defense and salt tolerance. In summary, SiNPs hold promise as potential modifiers to enhance the response and tolerance to salt stress in tomatoes.
Environmental significanceSalt may cause secondary stress to plants, mainly osmotic imbalance, nutrient deficiency and oxidative stress, which seriously influence growth and development of plants. Silicon nanoparticles (SiNPs) can effectively improve the damage caused by salt stress on plants, and alleviate the salt-induced oxidative stress by increasing the activity of antioxidant enzymes. Besides, SiNPs are able to up-regulate the expression of salt-induced-related genes and reduce the absorption of Na+ by plants. Compared with silicon, SiNPs enhances the tolerance of plant to salt more effectively. |
1. Introduction
Tomatoes are extensively cultivated worldwide, primarily in regions where soil salinity tends to be higher.1,2 Salinity imposes secondary stresses on plants, such as osmotic imbalances, nutrient deficiency, and oxidative stress.3,4 Salt stress results in membrane degradation, decreased activity of antioxidant enzymes, cellular dysfunction, and electrolyte leakage, ultimately leading to cell death.5–7 Unfortunately, tomato seedlings are particularly susceptible to salt stress during their developmental stages,8–10 significantly impacting growth and development. Therefore, enhancing the salt tolerance of tomatoes is of paramount importance.Silicon (Si), the second most abundant element in the earth's crust and soil,11,12 while not essential for the growth and development of most plants, can effectively alleviate damage caused by various abiotic stresses.13–15 For instance, Si reduces the accumulation and transport of cadmium (Cd) in rice by down-regulating Cd transport genes and increasing the activity of antioxidant enzymes.16 Si supplementation mitigates the adverse effects of NaCl on cucumber plants by alleviating oxidative damage and regulating proline and cytokinin metabolisms.17
Over the past decade, nanotechnology has rapidly expanded its application in agriculture,18–20 providing an environmentally friendly alternative for addressing abiotic stress. Among nano materials, silicon nanoparticles (SiNPs) have garnered considerable attention due to their non-toxicity, small size, and high surface area.19,21 In practical applications, SiNPs effectively promote plant growth, photosynthesis, and improve plant stress resistance plants.22,23 Studies have shown that SiNPs enhance wheat photosynthesis, facilitate sugar synthesis and transport, and increase endogenous hormone levels.24 Furthermore, research suggests that both SiNPs and Si can enhance plant resistance to Cd stress, with SiNPs exhibiting superior efficacy in promoting plant growth, reducing oxidative damage, and decreasing Cd accumulation.25,26 This may be due to the characteristics of SiNPs (such as small size and high surface area). According to provious reports, the TEM images of Arabidopsis leaves indicated that the nanoparticles were able to enter plants through stomata and might form a physical barrier to protect the healthy growth of plants under abiotic stress conditions.19,23 Additionally, foliar sprays of SiNPs induce antioxidant production, defending lemongrass plants against salt-induced oxidative stress, SiNPs might protect wheat seedlings depend on NO-mediated triggering of antioxidant defense system, which subsequently counterbalance ROS-induced damage to photosynthesis.27 Similarly, SiNPs more effectively alleviate UV-B stress in wheat seedlings compared to Si.28 However, there are limited reports on the comparative effects of SiNPs and Si in alleviating salt stress. Therefore, this study primarily focuses on comparing the effects of SiNPs and Si on salt stress tolerance and related mechanisms in tomatoes. Additionally, a hydroponic cultivation experiment was conducted to investigate the effects of SiNPs and Si on tomato physiological parameters, including photosynthesis and the expression of salt-induced-related genes, under two salt concentrations. The findings aim to enhance our understanding of SiNPs and plant salinity stress in nano-enabled agriculture.
2. Materials and methods
2.1 Synthesis and characterization of SiNPs
According to the method outlined by Wu et al.,29 SiNPs with uniform size and good water solubility were synthesized. This process relied on the principle of easy condensation following hydrolysis, along with the addition of a reducing agent to control the reaction rate. Specifically, 1.69 g of ammonium citrate was weighed and dissolved in 50 mL of deionized water, to which 5 mL of N-[3-(trimethoxysilyl)propyl]ethylenediamine (DAMO) was added. The solution was protected under a N2 atmosphere for 20 min while being mixed and stirred. Subsequently, the solution was transferred to a 1000 mL Teflon-lined autoclave and maintained at 180 °C for 10 h. After cooling to room temperature, the solution was filtered through a 0.22 μm water filtration membrane three times and purified in a dialysis bag with a 500 Da cutoff for 24 h under light-avoiding conditions. Finally, the solution was freeze-dried to obtain SiNPs powder.The synthesized silicon nanoparticles underwent advanced materials characterization following the method described by Nallayagari et al.30 The lattice morphology and particle size of the samples were analyzed using transmission electron microscopy (TEM, JEM2100F, JEOL, Japan). Dynamic light scattering (DLS) measurements were conducted using a particle size analyzer (Zetasizer Nano S90, Malvern, UK). The functional groups present in the SiNPs were identified using Fourier transform infrared spectroscopy (FTIR, Nicolet iS50, Thermo Scientific, USA) within the range of 4000–400 cm−1.
2.2 Plant growth and treatment application
Tomato seeds (Solanum lycopersicum L.) were surface-sterilized using a 10% NaClO solution for 10 min and then rinsed thoroughly with sterile water multiple times. Subsequently, they were transferred onto a petri dish with double-layered filter paper and placed in a constant temperature incubator at 28 °C for 3 days. Care was taken to ensure that the filter paper remained damp throughout without allowing solution accumulation when tilted.The immature embryos were then transferred into planting cups containing sponge blocks and cultured for 7 days in sterile deionized water. After this period, seedlings with uniform growth were selected for transplanting into different treatment groups (all based on a 1/10 Hoagland solution). These treatments included: the control treatment (CK, 1/10 Hoagland solution), low salt + Si treatment (LS, 100 mM NaCl + Na2SiO3), low salt + SiNPs treatment (LN, 100 mM NaCl + SiNPs), high salt + Si treatment (HS, 150 mM NaCl + Na2SiO3), and high salt + SiNPs treatment (HN, 150 mM NaCl + SiNPs). The concentrations of Si and SiNPs were set at 100 mg L−1 and 200 mg L−1, respectively, based on preliminary experiment results (Fig. S1 and S2†). Detailed computational methods for calculating seed germination parameters are provided in Table S1.† Each treatment was replicated three times, with the solution being changed every 5 days. All tomato seedlings were further cultivated for 20 days in a greenhouse under conditions of 14 h of daylight and 10 h of darkness, with temperatures maintained at 26 °C during the day and 20 °C at night. The relative humidity was kept at 70%, and natural sunlight was provided for optimal growth.
2.3 Determination of plant growth parameters
Shoot length (SL) and root length (RL) were measured using a ruler after 20 d of growth under different treatments. The leaf area (LA) of the same parts was determined using an intelligent leaf area measuring instrument (YMJ-CHA3, Zhejiang Top Cloud-Agri Technology Co., Ltd., China). The fresh roots, after being rinsed, were scanned by a root analyzer to determine root basic indicators and root architecture parameters (WinRHIZO, Regent, Canada). Subsequently, the plant samples were washed with deionized water, dried on filter paper, and weighed to obtain fresh weight (FW). The samples were then dried at 105 °C in an oven for 15 min and then dried to constant weight at 75 °C to obtain dry weight (DW).2.4 Measurement of leaf photosynthetic parameters
The photosynthetic attributes of comparable sections of tomato leaves were assessed from 10:00 am to 11:00 am using a portable photosynthesis system (LI-6800, LI-COR, USA), while chlorophyll content was determined using a SPAD chlorophyll meter (SPAD-502, Minolta, Japan).2.5 Determination of antioxidant enzymes activity, MDA and H2O2
Catalase (CAT) activity, superoxide dismutase (SOD) activity, peroxidase (POD) activity, malondialdehyde (MDA), and hydrogen peroxide (H2O2) contents were determined using an assay kit from Beijing Boxbio Science & Technology Co.,Ltd., following the manufacturer's instructions.In brief, 0.1 g of fresh leaves was homogenized with 0.9 mL of ice-cold extraction buffer, followed by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The resulting supernatant was then used for determination of the respective parameters.
000 rpm for 10 min. The resulting supernatant was then used for determination of the respective parameters.
CAT activity was defined as the degradation of 1 μmol of H2O2 per g of tissue per minute. SOD activity was quantified based on the amount of cellular protein causing 50% maximum inhibition, defined as one unit of SOD activity. POD activity was determined by the amount of enzyme catalyzing the conversion of 1 μg of substrate per gram of tissue per minute at 37 °C.
2.6 Measurement of Na+, K+ and Si contents
The contents of Na+, K+, and Si were determined after the tomato seedlings were dried, ground, and digested using a microwave digestion method. The specific steps of the analysis were as follows: First, 0.1 g of plant material was weighed and placed in a digestion tank containing 7 mL of concentrated nitric acid. The mixtures in the digestion tank underwent digestion using a microwave digestion instrument (TANK-40, Sineo, China) following a plant digestion procedure. Once digestion was complete, the digestion tank was removed when the temperature decreased to room temperature. Next, the solution in the digestion tank was transferred to a 50 mL capacity bottle, and the digestion tank was rinsed with distilled water three times. The washing solution was then transferred to the capacity bottle to achieve a constant volume of 50 mL with distilled water.Standard solutions of Na+ and K+ were prepared at different concentrations to establish the standard curve. The content of Na+ and K+ in the digestion solution was determined using an inductively coupled plasma-optical emission spectrometer (ICP-OES, iCAP 7000, Thermo Scientific, USA) and expressed as mg g−1 of dry weight (mg g−1 DW).
The Si content in plants was determined using an acid–base two-step digestion method.31 First, 1 mol L−1 nitric acid solution was used for acid digestion, following the same steps as the digestion process for Na+ and K+ content determination. After acid digestion was completed and cooled to room temperature, 1.5 mol L−1 NaOH was added for base digestion. Finally, standard solutions of Si were prepared at different concentrations to establish the standard curve. The Si content in the digestion solution was determined using ICP-OES and expressed as mg g−1 DW.
2.7 Total RNA extraction and gene expression analysis
Total RNA was extracted from 0.1 g of flash-frozen fresh leaf tissues using the TransZol Up Plus RNA Kit (ER501, TransGen) following the manufacturer's instructions. First-strand cDNAs were synthesized from 1 μg of total RNA using the StarScript III All-in-one RT Mix with gDNA Remover Kit (A230, GenStar). Real-time PCR was conducted using the 2×RealStar Fast SYBR qPCR Mix (Low ROX) Kit (A304, GenStar) with a QuantStudio™ 5 Real-Time PCR System (Applied Biosystems). The thermal profile used was 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s.SlActin gene served as an internal control (Table S2†). Genes expression levels were quantified using the comparative Ct procedure.32 All assays were performed in triplicate, with three biological replicates per treatment.
2.8 Statistical analyses
R Studio (version 4.3.1) with the “agricolae” package was utilized for one-way analysis of variance (ANOVA), followed by Duncan's multiple range test (DMRT) to compare means for each environmental variable, with a significance level set at P < 0.05. Principal coordinates analysis (PCoA) was performed to assess the overall treatment effect on parameters of interest, including plant growth (FW, DW, SL, RL, and LA), photosynthesis parameters (SPAD, Pn, Tr, and Gs), antioxidant system (CAT, POD, SOD, MDA, and H2O2), as well as the content of Si, and others. For analyzing the relationship between growth parameters, photosynthesis parameters, antioxidant system, Si content, and Na+ content, redundancy analysis (RDA) was conducted. PCoA and RDA were executed using the “vegan” package in R Studio software (version 4.2.2). The “PerformanceAnalytics” package was employed for correlation analysis.3. Results
3.1 Characterization of SiNPs
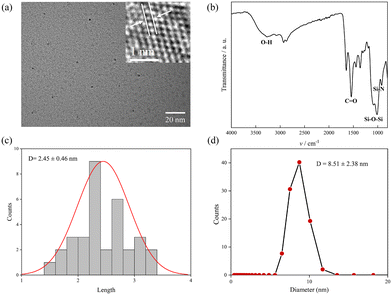
The TEM results revealed that SiNPs appeared as spherical particles with a size range of 2.45 ± 0.46 nm (Fig. 1a and c). The FTIR spectra provided evidence of the presence of diverse bioactive chemicals on the surface of SiNPs, contributing to their long-term stability (Fig. 1b). Several noticeable absorption peaks were observed in the FTIR spectra within the range of 500–3500 cm−1. The stretching vibration of Si–O–Si was observed at 1000–1200 cm−1, while the broad feature between 3000 and 3500 cm−1 was attributed to O–H stretching. The DLS diameter further confirmed the small size of the as-prepared SiNPs, with a hydrodynamic diameter of 8.51 ± 2.38 nm (Fig. 1d).3.2 Effects of Na2SiO3 and SiNPs on tomato seedlling growth under salt stress
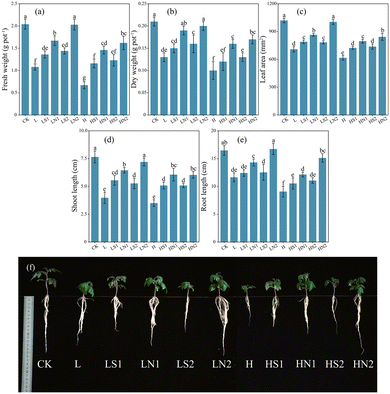
The results indicated that under both low salt and high salt stress conditions, the application of 200 mg L−1 Na2SiO3 or SiNPs could enhance the growth condition of tomato seedlings (Fig. 2), with SiNPs showing superior efficacy compared to Na2SiO3 at the same concentration. Specifically, in the LN2 treatmen, the fresh weight (FW), dry weight (DW), leaf area (LA), shoot length (SL), and root length (RL) were significantly increased by 25.00% to 40.97% (Fig. 2a–e) compared to LS2, while no significant difference was observed compared to the control (CK). Similarly, under high salt stress (150 mM NaCl), SiNPs effectively improved the growth of tomato seedlings, with growth parameters significantly higher than those under Na2SiO3 treatment at the same concentration, although they were still significantly lower than those under CK.Furthermore, root scans revealed that salt stress led to root dysplasia in tomato seedlings, while the addition of Na2SiO3 or SiNPs effectively promoted root development (Fig. S3†). Compared to the control (CK) (Table S3†)), 200 mg L−1 SiNPs under low salt stress significantly increased total root length (18.91%), root surface area (10.09%), root volume (11.52%), and mean root diameter (8.91%), with similar improvements observed under high salt stress. Additionally, SiNPs noticeably improved root architecture parameters (Table S4†)), exhibiting a similar trend to that of root basic indicators. SiNPs proved to be more effective than Na2SiO3 in enhancing root growth at the same concentration.
3.3 Effect of Na2SiO3 and SiNPs on the photosynthetic pigment of tomato seedling
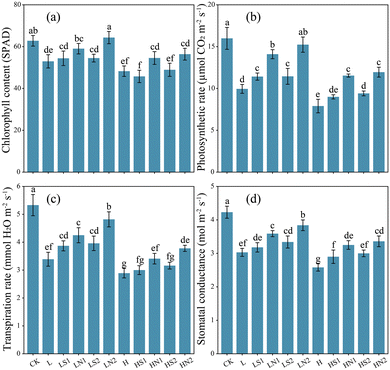
Salt stress significantly reduced the chlorophyll content in tomato leaves (Fig. 3a), and this reduction was not mitigated by the addition of Si. In contrast, SiNPs effectively increased chlorophyll content by 11.44% to 21.45% under low salt stress and 13.05% to 16.84% under high salt stress. SiNPs also mitigated the adverse effects of salt stress on plant photosynthesis (Fig. 3b–d). For instance, in the LN2 treatment, the net photosynthetic rate (Pn), transpiration rate (Tr), and stomatal conductance (Gs) were improved by 53.17%, 42.18%, and 26.73% respectively, compared to the L treatment. Similar improvements were observed under high salt stress. Na2SiO3 also increased Pn and Tr under low salt stress, albeit to a lesser extent than SiNPs.3.4 Na2SiO3 and SiNPs increased antioxidant enzymes activity and reduced salt-induced oxidative damage in tomato
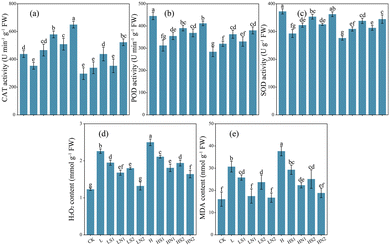
Salt stress notably decreased the activity levels of antioxidant enzymes in tomatoes (Fig. 4a–c). Treatments with Na2SiO3 and SiNPs effectively mitigated salt-induced oxidative damage by enhancing antioxidant enzyme activities and reducing the production of oxidative damaging substances compared to treatments with corresponding salt concentrations (Fig. 4).Specifically, 200 mg L−1 SiNPs significantly increased the activities of CAT (84.00%, 76.19%), POD (31.91%, 33.92%), and SOD (23.71%, 24.66%) compared to treatments with the corresponding salt concentrations. However, the differences between POD and SOD activities were not significant in treatments with 100 mg L−1 and 200 mg L−1 SiNPs. Notably, the addition of Na2SiO3 did not significantly increase CAT activity under high salt stress.
Furthermore, both Na2SiO3 and SiNPs significantly reduced the content of H2O2 and MDA (Fig. 4d and e). Specifically, 200 mg L−1 SiNPs reduced H2O2 and MDA contents to the level of the control treatment under low salt stress, with no significant difference observed between MDA contents and the control under high salt conditions.
3.5 The effect Na2SiO3 and SiNPs on potassium sodium ratio and silicon content in tomato
The results demonstrated that both Na2SiO3 and SiNPs significantly improved the potassium–sodium ratio (Fig. S4†). Specifically, under both low salt and high salt stress conditions (Fig. S4a†), the K+ content significantly increased by 16.94%, 16.24% (Na2SiO3), and 27.12%, 28.49% (SiNPs), respectively. The Na+ content in plants decreased to the level of the control (CK) when treated with 200 mg L−1 SiNPs (Fig. S4b†). Moreover, the Na+ content in plants was significantly reduced under different concentrations of salt stress when treated with Na2SiO3 and SiNPs, with SiNPs demonstrating greater effectiveness.The Si content in tomato plants increased with the increasing concentration of Na2SiO3 and SiNPs, exhibiting a dose-dependent effect (Fig. S4d†). Notably, under low salt stress, the Si content of the LS2 treatment significantly increased by 5.85% compared to LS1, while under high salt stress, the Si content of the LN2 treatment significantly increased by 33.85% compared to LN1 and by 31.16% compared to LS2. This trend was consistent across different salt stress conditions.
3.6 Relative expression of defense-related genes
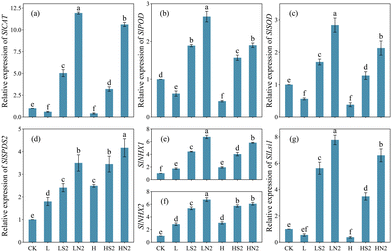
qPCR analysis was conducted on tomato genes associated with the antioxidant defense system, salt resistance induction, and silicon transport (Fig. 5). The results revealed that salt stress significantly reduced the relative expressions of SlCAT, SlPOD, and SlSOD (antioxidative defense-related genes) in above-ground parts of tomato plants (Fig. 5a–c). However, the expression of these three genes was notably upregulated in tomato plants treated with 200 mg L−1 Na2SiO3 and SiNPs. Specifically, in the LN2 treatment, their expression levels increased significantly by 19.88, 4.29, and 5.07-fold compared to the L treatment. Similarly, in the HN2 treatment, their expression levels increased significantly by 25.90, 4.52, and 5.61-fold compared to the H treatment, respectively.Under salt stress, genes related to salt resistance induction, including SlSPDS2, SlNHX1, and SlNHX2, were significantly upregulated (Fig. 5d–f), and their expression levels were further enhanced with the addition of 200 mg L−1 Na2SiO3 and SiNPs. Specifically, SlSPDS2 exhibited the highest relative expression level in the HN2 treatment, significantly increasing by 4.17-fold compared to the control. Meanwhile, the relative expression levels of SlNHX1 and SlNHX2 peaked in the LN2 treatment, significantly increasing by 6.75 and 6.74-fold, respectively.
Additionally, the expression of SlLsi1 in above-ground tomato seedlings decreased under salt stress but was highest in the LN2 treatment, significantly increasing by 7.77-fold compared to the control (Fig. 5g). The addition of Na2SiO3 also increased the relative expression of SlLsi1.
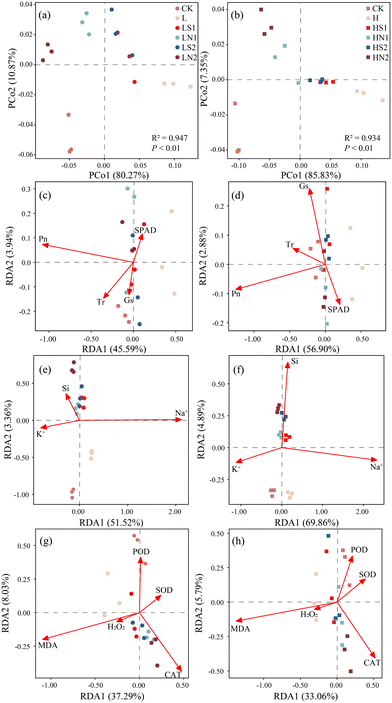
3.7 Principal coordinates analysis (PCoA) and redundancy analysis (RDA)
To comprehensively assess the effects of Na2SiO3 and SiNPs on tomato growth under salt stress, data including plant growth parameters, antioxidant system, Na+ and Si contents were analyzed using PCoA (Fig. 6a and b). The PERMANOVA test confirmed significant differences between treatments (R2 = 0.947, P < 0.01; R2 = 0.934, P < 0.01). The results revealed that the first principal axis (PCo1) accounted for 80.27% of the total variation under low salt stress. The L treatment exhibited the farthest deviation from the CK, whereas the development condition of plants in the SiNPs treatment was closer to the CK, indicating that the mitigation effect was more effective than Na2SiO3 (Fig. 6a). SiNPs treatment could alleviate salt stress more effectively under high salt stress.Furthermore, RDA was conducted to compare the effects of Na2SiO3 and SiNPs on photosynthetic parameters (Fig. 6c and d), Na+ content (Fig. 6e and f), and the antioxidant system (Fig. 6g and h) of tomato seedlings under salt stress. The first constraint axis respectively explained 45.59%, 51.52%, and 37.29% of the variation under low salt stress, and 56.90%, 69.86%, and 33.06% under high salt stress. RDA results demonstrated that Na2SiO3 and SiNPs could effectively increase the Si content of seedlings under both low and high salt stress (Fig. 6e and f) and alleviate the oxidative damage caused by salt stress (Fig. 6g and h). Correlation analysis revealed that Si content was significantly negatively correlated with MDA and H2O2 content, and significantly positively correlated with antioxidant enzyme activity (Fig. S5†). Additionally, SiNPs were more effective than Na2SiO3 in increasing Pn and SPAD, as well as reducing Na+ content in tomato (Fig. 6c–f), with Na+ showing a significant negative correlation with Pn and SPAD (Fig. S5†).
4. Discussion
Salt represents a significant hurdle to plant growth and yield formation, disrupting the physiological functions of plant cells and reducing membrane stability, ultimately leading to crop yield losses.27,33,34 The beneficial effects of Si on plant salt tolerance have been extensively studied,35 and its application in growth promotion and stress resistance has been further enhanced through nanotechnology.19,27,28 For instance, SiNPs have been shown to significantly enhance wheat biomass under Cd stress and reduce Cd content in soil, indicating potential for alleviating heavy metal stress in plants.36 SiNPs have also been observed to increase cucumber yield under water and salt stress while decreasing Na+ absorption.37 In our study, salt inhibited the growth of tomatoes, decreased chlorophyll content, and adversely affected photosynthesis. However, both Na2SiO3 and SiNPs effectively alleviated these inhibitory effects, likely due to Si's ability to retain various nutrients and water.38,39 On one hand, SiNPs promoted root growth and development under salt stress, facilitating water and mineral absorption and maintaining normal plant growth conditions for optimal photosynthesis.40,41 On the other hand, foliar deposition of SiNPs and subsequent non-toxic lignification reduced wilting and prolonged leaf retention, enhancing photosynthesis.8,42 Previous studies have shown that SiNPs could mitigate adverse salt stress effects by improving photosynthetic rate, transpiration rate, stomatal conductance, and water utilization efficiency,13,43 consistent with our findings. However, SiNPs were more effective than Na2SiO3 in mitigating salt stress effects on seedling growth, as confirmed by RDA results, possibly due to the small diameter, diverse particle morphology, large surface area, and higher reactivity and biological activity of SiNPs.Plant growth attributes were negatively affected by Na+ content, which interfered with photosynthetic activity and led to toxic ion accumulation, nutrient absorption imbalance, suspended cell division, and reactive oxygen species (ROS) production.44,45 In our study, Na2SiO3 and SiNPs significantly decreased Na+ and K+ content in tomato seedlings under salt stress, likely related to the upregulated expression of genes involved in salt resistance induction. Si can stimulate the synthesis and accumulation of polyamines in plants under salt stress, such as putrescine, spermidine, and spermidine.46S1SPDS2 serves as the primary spermidine synthase in tomatoes, and endogenous spermidine can enhance plant resistance of plants to saline–alkali stress. Plants accumulate significant levels of spermidine under salt and alkali stress to bolster their resistance to such conditions.10,47 NHX plays a crucial role in a plant's response to salt, as its abundance can enhance the salt tolerance of crops.48,49SlNHX1 and SlNHX2, being Na+/H+ antiporters, are pivotal in maintaining Na+ homeostasis by transporting Na+ from the cytoplasm to the vacuole or extracellular space.50 Additionally, Si can mitigate salt stress by influencing the transport and accumulation of Na+ and K+.22 In this study, it was observed that S1Lsi1 expression was significantly up-regulated under Na2SiO3 and SiNPs treatments, which could facilitate the accumulation and deposition of Si in tomatoes, thus reducing shoot Na+ translocation. Furthermore, correlation analysis revealed a significant positive correlation between Si content and K+ and a negative correlation with Na+ (Fig. S5†), indicating that increasing of Si content promotes the maintenance of high K+/Na+ and cellular homeostasis.
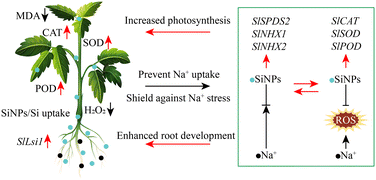
Lipid peroxidation induced by ROS represents another significant mechanism of salt toxicity in higher plants.51,52 Antioxidant enzymes play a crucial role in clearing ROS and shielding plants from oxidative stress, thereby contributing to stress tolerance.12,53 During treatments with NaCl at concentrations of 100 and 150 mM, we observed an upregulation in the contents of H2O2 and MDA, indicative of salinity-induced oxidative bursts in tomato plants, which can adversely affect plant development. SiNPs can significantly decrease the levels of H2O2 and MDA (Fig. 4d and e), hereby activating the antioxidant system and enhancing the activity of CAT, POD and SOD to mitigate ROS accumulation and associated damage (Fig. 4a–c), These findings are consistent with previous studies.25–27,54 Additionally, SiNPs treatment led to a significant upregulation of ROS defense genes (SlCAT, SlPOD and SlSOD), suggesting their involvement in enhancing ROS detoxification, reducing cell damage and death, and alleviating salt-induced inhibition (Fig. 7). Overall, these results suggest that SiNPs hold promise as a novel and effective alternative for enhancing crop growth under salt stress. However, further research is warranted to validate these findings under field conditions, as the introduction of SiNPs into the soil may lead to changes and transformations that need to be thoroughly investigated. For example, differences in the chemical properties, concentration, size or morphology of SiNPs may lead to the differences in the mechanism of nanoparticles in alleviating crop salt stress. However, most current studies focus on the effect of SiNPs of a single size and inference at mechanism level. In the future, it is necessary to evaluate and predict the optimal dose and size of SiNPs used in field, so as to make nano-silicon fertilizer both efficient and economical in sustainable agricultural applications. At the same time, multi-omics technologies, such as metabolomics, genomics and proteomics, can be used to explore the molecular mechanism of SiNPs alleviating salt toxicity in crops and regulating the absorption and transport of Na+ in crops, so that further understand how SiNPs regulates plant–microbial interaction to promote the utilization efficiency of Si, improve crop yield and enhance crop resilience.
Conclusions
The application of SiNPs significantly improved various growth parameters in tomato plants, such as the photosynthetic rate and overall plant performance. This improvement was attributed to dual mechanisms: firstly, SiNPs mitigated salt-induced oxidative stress by enhancing the activity of antioxidant enzymes and inducing the notable upregulation of ROS defense genes (SlCAT, SlPOD and SlSOD). Secondly, SiNPs influenced the accumulation of both Si and Na+ in plants, with the expression of salt-responsive genes shedding light on molecular pathways involved in SiNPs-mediated salt tolerance. Given these promising findings, it is imperative to conduct field trials to further evaluate the specific efficacy of SiNPs in rehabilitating saline–alkali land. In addition, it is necessary to monitor the whole development period of crops, and study on how SiNPs regulates plant tolerance to salt at the molecular level, including molecular recognition, signal transduction and gene expression, which will help us better understand the physiological and biochemical functions of SiNPs. Meanwhile, comparative studies between the effects of SiNPs and traditional silicon application in alleviating salt stress are warranted to elucidate their respective contributions comprehensively.Data availability
Data will be made available on request.Author contributions
Shuaibing Wang: visualization, methodology, data curation, writing – original draft, writing – review & editing; Xiang Shen: resources, methodology, formal analysis; Xin Guan: investigation, supervision; Li Sun: methodology, writing – review & editing; Zhongxue Yang: conceptualization, methodology; Dandan Wang: writing – review & editing; Yinglong Chen: investigation, writing – review & editing; Zhihong Xie: methodology, supervision, resources, writing – review & editing; Peiqiang Li: resources, conceptualization, methodology. All authors contributed to the article and approved the submitted version.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China [32270065], Shandong Provincial Natural Science Foundation [ZR2021QC175, ZR2022MD0450], the 2023 Tai'an Science and Technology Innovation “Double Ten Project” (Major Technological Research) [23JSGG01], and Shandong Province Ecological Agriculture Technology System Construction Task [SDAIT-30-04].References
- A. Cilek and S. Berberoglu, Biotope conservation in a Mediterranean agricultural land by incorporating crop modelling, Ecol. Modell., 2019, 392, 52–66 CrossRef.
- Y. Mizrahi, Effect of salinity on tomato fruit ripening, Plant Physiol., 1982, 69, 966–970 CrossRef CAS PubMed.
- K. Parvin, M. Hasanuzzaman, M. H. M. B. Bhuyan, S. M. Mohsin and M. Fujita, Quercetin Mediated Salt Tolerance in Tomato through the Enhancement of Plant Antioxidant Defense and Glyoxalase Systems, Plants, 2019, 8, 247 CrossRef CAS.
- R. Berni, M. Luyckx, X. Xu, S. Legay, K. Sergeant, J.-F. Hausman, S. Lutts, G. Cai and G. Guerriero, Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism, Environ. Exp. Bot., 2019, 161, 98–106 CrossRef CAS.
- R. Ding, Y. Li, Y. Yu, Z. Sun and J. Duan, Prospects and hazards of silica nanoparticles: Biological impacts and implicated mechanisms, Biotechnol. Adv., 2023, 69, 108277 CrossRef CAS PubMed.
- J. Bose, A. Rodrigo-Moreno and S. Shabala, ROS homeostasis in halophytes in the context of salinity stress tolerance, J. Exp. Bot., 2014, 65, 1241–1257 CrossRef CAS PubMed.
- H.-Q. Wang, X. Y. Zhao, W. Xuan, P. Wang and F.-J. Zhao, Rice roots avoid asymmetric heavy metal and salinity stress via an RBOH-ROS-auxin signaling cascade, Mol. Plant, 2023, 16, 1678–1694 CrossRef CAS PubMed.
- G. Miller, N. Suzuki, S. Ciftci-Yilmaz and R. Mittler, Reactive oxygen species homeostasis and signalling during drought and salinity stresses, Plant, Cell Environ., 2010, 33, 453–467 CrossRef CAS.
- T. Liu, X. Hu, J. Zhang, J. Zhang, Q. Du and J. Li, H2O2 mediates ALA-induced glutathione and ascorbate accumulation in the perception and resistance to oxidative stress in Solanum lycopersicum at low temperatures, BMC Plant Biol., 2018, 18, 34 CrossRef.
- P. Wang, Z. X. Abc, Y. Zhang, Y. Ma, J. Yang, F. Zhou, Y. Gao, G. Li and X. Hu, Over-expression of spermidine synthase 2 (SlSPDS2) in tomato plants improves saline-alkali stress tolerance by increasing endogenous polyamines content to regulate antioxidant enzyme system and ionic homeostasis, Plant Physiol. Biochem., 2022, 192, 172–185 CrossRef CAS PubMed.
- M. Luyckx, J.-F. Hausman, S. Lutts and G. Guerriero, Silicon and Plants: Current Knowledge and Technological Perspectives, Front. Plant Sci., 2016, 8, 411 Search PubMed.
- Y. Zhu and H. Gong, Beneficial effects of silicon on salt and drought tolerance in plants, Agron. Sustainable Dev., 2014, 34, 455–472 CrossRef CAS.
- M. Ali, S. Afzal, A. Parveen, M. Kamran, M. R. Javed, G. H. Abbasi, Z. Malik, M. Riaz, S. Ahmad, M. S. Chattha, M. Ali, Q. Ali, M. Z. Uddin, M. Rizwan and S. Ali, Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress, Plant Physiol. Biochem., 2021, 158, 208–218 CrossRef CAS PubMed.
- P. Ahmad, M. A. Ahanger, P. Alam, M. N. Alyemeni, L. Wijaya, S. Ali and M. Ashraf, Silicon (Si) Supplementation Alleviates NaCl Toxicity in Mung Bean [Vigna radiata (L.) Wilczek] Through the Modifications of Physio-biochemical Attributes and Key Antioxidant Enzymes, J. Plant Growth Regul., 2019, 38, 70–82 CrossRef CAS.
- J. Ma, H. Cai, C. He, W. Zhang and L. Wang, A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells, New Phytol., 2015, 206, 1063–1074 CrossRef CAS PubMed.
- D. Chen, D. Chen, R. Xue, J. Long, X. Lin, Y. Lin, L. Jia, R. Zeng and Y. Song, Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants, J. Hazard. Mater., 2019, 367, 447–455 CrossRef CAS PubMed.
- Y. Zhu, X. Jiang, J. Zhang, Y. He, X. Zhu, X. Zhou, H. Gong, J. Yin and Y. Liu, Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins, Plant Physiol. Biochem., 2020, 156, 209–220 CrossRef CAS PubMed.
- M. Li, L. Gao, J. C. White, C. L. Haynes, T. L. O'Keefe, Y. Rui, S. Ullah, Z. Guo, I. Lynch and P. Zhang, Nano-enabled strategies to enhance biological nitrogen fixation, Nat. Nanotechnol., 2023, 18, 688–691 CrossRef CAS.
- M. El-Shetehy, A. Moradi, M. Maceroni, D. Reinhardt, A. Petri-Fink, B. Rothen-Rutishauser, F. Mauch and F. Schwab, Silica nanoparticles enhance disease resistance in Arabidopsis plants, Nat. Nanotechnol., 2021, 16, 344–353 CrossRef CAS.
- X. Guan, X. Gao, A. Avellan, E. Spielman-Sun, J. Xu, S. Laughton, J. Yun, Y. Zhang, G. D. Bland, Y. Zhang, R. Zhang, X. Wang, E. A. Casman and G. V. Lowry, CuO Nanoparticles Alter the Rhizospheric Bacterial Community and Local Nitrogen Cycling for Wheat Grown in a Calcareous Soil, Environ. Sci. Technol., 2020, 54, 8699–8709 CrossRef CAS.
- D. Sun, H. I. Hussain, Z. Yi, J. E. Rookes, L. Kong and D. M. Cahill, Delivery of Abscisic Acid to Plants Using Glutathione Responsive Mesoporous Silica Nanoparticles, J. Nanosci. Nanotechnol., 2018, 18, 1615–1625 CrossRef CAS PubMed.
- A. Alsaeedi, H. El-Ramady, T. Alshaal, M. El-Garawani, N. Elhawat and A. Al-Otaibi, Exogenous nanosilica improves germination and growth of cucumber by maintaining K+/Na+ ratio under elevated Na+ stress, Plant Physiol. Biochem., 2018, 125, 164–171 CrossRef CAS PubMed.
- G. Guerriero, F. M. Sutera, N. Torabi-Pour, J. Renaut, J.-F. Hausman, R. Berni, H. C. Pennington, M. Welsh, A. Dehsorkhi, L. R. Zancan and S. Saffie-Siebert, Phyto-Courier, a Silicon Particle-Based Nano-biostimulant: Evidence from Cannabis sativa Exposed to Salinity, ACS Nano, 2021, 15, 3061–3069 CrossRef CAS PubMed.
- Y. Li, K. Xi, X. Liu, S. Han, X. Han, G. Li, L. Yang, D. Ma, Z. Fang, S. Gong, J. Yin and Y. Zhu, Silica nanoparticles promote wheat growth by mediating hormones and sugar metabolism, J. Nanobiotechnol., 2023, 21, 2 CrossRef CAS PubMed.
- G. Yan, H. Jin, C. Yin, Q. Huang, G. Zhou, Y. Xu, Y. He, Y. Liang and Z. Zhu, Comparative effects of silicon and silicon nanoparticles on the antioxidant system and cadmium uptake in tomato under cadmium stress, Sci. Total Environ., 2023, 904, 166819 CrossRef CAS PubMed.
- T. Ahmed, H. A. Masood, M. Noman, A. A. Al-Huqail, S. M. S. Alghanem, M. M. Khan, S. Muhammad, N. Manzoor, M. Rizwan, X. Qi, A. H. A. Abeed and B. Li, Biogenic silicon nanoparticles mitigate cadmium (Cd) toxicity in rapeseed (Brassica napus L.) by modulating the cellular oxidative stress metabolism and reducing Cd translocation, J. Hazard. Mater., 2023, 459, 132070 CrossRef CAS PubMed.
- M. Mukarram, M. M. A. Khan, D. Kurjak, A. Lux and F. J. Corpas, Silicon nanoparticles (SiNPs) restore photosynthesis and essential oil content by upgrading enzymatic antioxidant metabolism in lemongrass (Cymbopogon flexuosus) under salt stress, Front. Plant Sci., 2023, 14, 1116769 CrossRef.
- D. K. Tripathi, S. Singh, V. P. Singh, S. M. Prasad, N. K. Dubey and D. K. Chauhan, Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings, Plant Physiol. Biochem., 2017, 110, 70–81 CrossRef CAS PubMed.
- F.-G. Wu, X. Zhang, S. Kai, M. Zhang, H.-Y. Wang, J. N. Myers, Y. Weng, P. Liu, N. Gu and Z. Chen, One-Step Synthesis of Superbright Water-Soluble Silicon Nanoparticles with Photoluminescence Quantum Yield Exceeding 80%, Adv. Mater. Interfaces, 2015, 2, 1500360 CrossRef.
- A. R. Nallayagari, E. Sgreccia, R. Pizzoferrato, M. Cabibbo, S. Kaciulis, E. Bolli, L. Pasquini, P. Knauth and M. L. Di Vona, Tuneable properties of carbon quantum dots by different synthetic methods, J. Nanostruct. Chem., 2022, 12, 565–580 CrossRef CAS.
- J. A. V. A. Barros, P. F. de Souza, D. Schiavo and J. A. Nobrega, Microwave-assisted digestion using diluted acid and base solutions for plant analysis by ICP OES, J. Anal. At. Spectrom., 2016, 31, 337–343 RSC.
- K. J. Livak and T. D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method, Methods, 2001, 25, 402–408 CrossRef CAS.
- U. Ijaz, T. Ahmed, M. Rizwan, M. Noman, A. A. Shah, F. Azeem, H. F. Alharby, A. A. Bamagoos, B. M. Alharbi and S. Ali, Rice straw based silicon nanoparticles improve morphological and nutrient profile of rice plants under salinity stress by triggering physiological and genetic repair mechanisms, Plant Physiol. Biochem., 2023, 201, 107788 CrossRef CAS PubMed.
- M. Mukarram, S. Choudhary, D. Kurjak, A. Petek and M. M. A. Khan, Drought: Sensing, signalling, effects and tolerance in higher plants, Physiol. Plant., 2021, 172, 1291–1300 CrossRef CAS PubMed.
- Y.-X. Zhu, H.-J. Gong and J.-L. Yin, Role of Silicon in Mediating Salt Tolerance in Plants: A Review, Plants, 2019, 8, 147 CrossRef CAS.
- S. Ali, M. Rizwan, A. Hussain, M. Z. U. Rehman, B. Ali, B. Yousaf, L. Wijaya, M. N. Alyemeni and P. Ahmad, Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.), Plant Physiol. Biochem., 2019, 140, 1–8 CrossRef CAS PubMed.
- A. Alsaeedi, H. El-Ramady, T. Alshaal, M. El-Garawany, N. Elhawat and A. Al-Otaibi, Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake, Plant Physiol. Biochem., 2019, 139, 1–10 CrossRef CAS PubMed.
- M. R. Romero-Aranda, O. Jurado and J. Cuartero, Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status, J. Plant Physiol., 2006, 163, 847–855 CrossRef CAS.
- D. Coskun, D. T. Britto, W. Q. Huynh and H. J. Kronzucker, The Role of Silicon in Higher Plants under Salinity and Drought Stress, Front. Plant Sci., 2016, 7, 1072 Search PubMed.
- M. Haghighi and M. Pessarakli, Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage, Sci. Hortic., 2013, 161, 111–117 CrossRef CAS.
- P. Dhiman, N. Rajora, S. Bhardwaj, S. S. Sudhakaran, A. Kumar, G. Raturi, K. Chakraborty, O. P. Gupta, B. N. Devanna, D. K. Tripathi and R. Deshmukh, Fascinating role of silicon to combat salinity stress in plants: An updated overview, Plant Physiol. Biochem., 2021, 162, 110–123 CrossRef CAS PubMed.
- F. Asgari, A. Majd, P. Jonoubi and F. Najafi, Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.), Plant Physiol. Biochem., 2018, 127, 152–160 CrossRef CAS.
- M. H. Siddiqui, M. H. Al-Whaibi, M. Faisal and A. A. Al Sahli, Nano-Silicon Dioxide Mitigates The Adverse Effects Of Salt Stress On Cucurbita Pepo L, Environ. Toxicol. Chem., 2014, 33, 2429–2437 CrossRef CAS PubMed.
- G. H. Abbasi, J. Akhtar, R. Ahmad, M. Jamil, M. Anwar-ul-Haq, S. Ali and M. Ijaz, Potassium application mitigates salt stress differentially at different growth stages in tolerant and sensitive maize hybrids, Plant Growth Regul., 2015, 76, 111–125 CrossRef CAS.
- H. El Mahi, J. Perez-Hormaeche, A. De Luca, I. Villalta, J. Espartero, F. Gamez-Arjona, J. Luis Fernandez, M. Bundo, I. Mendoza, D. Mieulet, E. Lalanne, S.-Y. Lee, D.-J. Yun, E. Guiderdoni, M. Aguilar, E. O. Leidi, J. M. Pardo and F. J. Quintero, A Critical Role of Sodium Flux via the Plasma Membrane Na+/H+ Exchanger SOS1 in the Salt Tolerance of Rice, Plant Physiol., 2019, 180, 1046–1065 CrossRef CAS PubMed.
- S. S. Gill and N. Tuteja, Polyamines and abiotic stress tolerance in plants, Plant Signaling Behav., 2010, 5, 26–33 CrossRef CAS.
- C. Shang, X. Liu, G. Chen, G. Li, S. Hu, H. Zheng, L. Ge, Y. Long, Q. Wang and X. Hu, SlWRKY81 regulates Spd synthesis and Na+/K+ homeostasis through interaction with SlJAZ1 mediated JA pathway to improve tomato saline-alkali resistance, Plant J., 2024, 1774–1792 CrossRef CAS PubMed.
- N. Li, X. Wang, B. Ma, C. Du, L. Zheng and Y. Wang, Expression of a Na+/H+ antiporter RtNHX1 from a recretohalophyte Reaumuria trigyna improved salt tolerance of transgenic Arabidopsis thaliana, J. Plant Physiol., 2017, 218, 109–120 CrossRef CAS PubMed.
- A.-K. Bao, Y.-W. Wang, J.-J. Xi, C. Liu, J.-L. Zhang and S.-M. Wang, Co-expression of xerophyte Zygophyllum xanthoxylum ZxNHX and ZxVP1-1 enhances salt and drought tolerance in transgenic Lotus corniculatus by increasing cations accumulation, Funct. Plant Biol., 2014, 41, 203–214 CrossRef CAS.
- G.-Q. Wu, J.-L. Wang and S.-J. Li, Genome-Wide Identification of Na+/H+ Antiporter (NHX) Genes in Sugar Beet (Beta vulgaris L.) and Their Regulated Expression under Salt Stress, Genes, 2019, 10, 401 CrossRef CAS.
- S. S. Gill and N. Tuteja, Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants, Plant Physiol. Biochem., 2010, 48, 909–930 CrossRef CAS PubMed.
- M. Hasanuzzaman, M. H. M. B. Bhuyan, K. Parvin, T. F. Bhuiyan, T. I. Anee, K. Nahar, M. S. Hossen, F. Zulfiqar, M. M. Alam and M. Fujita, Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence, Int. J. Mol. Sci., 2020, 21, 8695 CrossRef CAS PubMed.
- J. Guo, S. Qin, Z. Rengel, W. Gao, Z. Nie, H. Liu, C. Li and P. Zhao, Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties, Ecotoxicol. Environ. Saf., 2019, 172, 380–387 CrossRef CAS PubMed.
- M. H. Siddiqui and M. H. Al-Whaibi, Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.), Saudi J. Biol. Sci., 2014, 21, 13–17 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00770k |
| This journal is © The Royal Society of Chemistry 2025 |