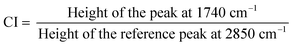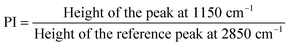Open Access Article
Open Access ArticleInsights in photocatalytic/Fenton-based degradation of microplastics using iron-modified titanium dioxide aerogel powders†
Guru Karthikeyan
Thirunavukkarasu
 *a,
Monika
Motlochová
*a,
Monika
Motlochová
 a,
Dmytro
Bavol
a,
Anna
Vykydalová
a,
Jaroslav
Kupčík
a,
Michal
Navrátil
a,
Dmytro
Bavol
a,
Anna
Vykydalová
a,
Jaroslav
Kupčík
a,
Michal
Navrátil
 a,
Kaplan
Kirakci
a,
Kaplan
Kirakci
 a,
Eva
Pližingrová
a,
Dana
Dvoranová
b and
Jan
Šubrt
a
a,
Eva
Pližingrová
a,
Dana
Dvoranová
b and
Jan
Šubrt
a
aInstitute of Inorganic Chemistry of the Czech Academy of Sciences, Husinec-Řež 250 68, Czech Republic. E-mail: thirunavukkarasu@iic.cas.cz
bInstitute of Physical Chemistry and Chemical Physics, Faculty of Chemical and Food Technology, Slovak University of Technology in Bratislava, Bratislava, SK-812 37, Slovak Republic
First published on 18th December 2024
Abstract
Microplastic (MP) pollution has become a serious environmental problem in the current decade. Unfortunately, wastewater treatment plants are not favorable for treating MPs. Therefore, it is necessary to develop methodologies to treat MPs in water efficiently. Photocatalytic (PC) and photo-Fenton (PF) processes are among the promising treatment methodologies that utilize reactive oxygen species (ROS) to degrade MPs. In this study, TiO2 aerogel powders (TiAP) were prepared by lyophilization and subsequent annealing of peroxo-titanic acid gels, followed by modification with Fe at the surface for the PC/PF-based degradation of MPs. Fe-modification on TiAP boosts the PC activity and activates the PF-based process in the presence of H2O2. The degradation of polystyrene (PS) MPs was evaluated using attenuated total reflection infrared (ATR-IR) spectroscopy, total organic carbon (TOC) analysis, thermogravimetric analysis coupled with differential scanning calorimetry and mass spectrometry (TGA-DSC/MS), nuclear magnetic resonance (NMR) spectroscopy, and high-performance liquid chromatography with high-resolution mass spectrometry (HPLC-HRMS). Photo-induced degradation of the PS MPs was evaluated by monitoring the changes in the carbonyl/peroxyl index (CI/PI) recorded by ATR-IR spectroscopy and the mass loss measured by TGA-DSC/MS techniques. Interestingly, the samples with higher CI value changes affected the total mass residue, while samples with lower changes in the CI value did not alter the total mass residue after the photo-induced treatment. Further, NMR spectra confirmed the formation of new peaks due to the oxidative degradation of PS MPs, especially between 0.8 and 1.3 ppm. Additionally, by-products formed after the photo-induced treatment process analyzed by the HPLC-HRMS technique indicate the degradation of PS MPs. The indirect techniques of electron paramagnetic resonance (EPR) spectroscopy revealed the ROS contributing to the oxidation of PS MPs during the PC and PF treatment process using Fe-modified TiAP. This study's findings have the potential to significantly influence future research and environmental policies by providing better insights into preparing efficient nanostructures for photo-induced degradation of MPs.
Environmental significanceWater bodies throughout the world are being affected by microplastics that cause adverse effects on the environment. Conventional water treatment methodologies do not efficiently remove microplastics from water. Therefore, eventually, these microplastics end up in the food cycle and potentially cause harm to all living organisms. This study deals with understanding the use of iron-modified titanium dioxide aerogel powders for the photocatalytic and photo-Fenton-based processes for the efficient degradation of microplastics in water. |
1. Introduction
Over the past decades, excessive use and disposal of plastic products have seriously threatened the environment.1–3 By 2025, around 250 million tons of plastics could be accumulated in rivers and oceans.3–5 Eventually, these plastics are fragmented into micro/nano sizes through various chemical, physical, and biological processes.6–9 Plastics with a size range between 5 mm and 1 μm have been classified as microplastics (MPs), whereas less than 1 μm are nanoplastics (NPs).6,9–11 The toxic additives (plasticizers, pigments, stabilizers, etc.) added during plastic manufacturing are ultimately released into the environment during the MP/NP transport cycle.12 Further, MPs/NPs could also adsorb other toxic organic and inorganic pollutants in the environment, thereby increasing their potency.7,13,14 Regardless of their size, they are classified as pollutants of emerging concern owing to their toxicity and negative impact on water bodies.15–17Unfortunately, wastewater treatment methodologies in municipal treatment plants are not well equipped to treat water containing MPs/NPs.18 In fact, these municipal treatment plants are one of the sources of microplastic pollutants.19,20 Currently, techniques such as physical separation (membrane separation, adsorption, and coagulation), biological techniques (membrane bioreactors and biological degradation), and advanced oxidation processes (photocatalytic (PC) process, Fenton-based process, and ozonation) are being developed for the treatment of MPs/NPs in water.21–24 Physical separation techniques are non-destructive in nature, i.e., MPs/NPs are transferred from the water to another medium (filters, adsorbents/coagulants), whereas biological treatment methodologies require optimal environmental conditions.10 However, eliminating MPs/NPs by degrading them to non-toxic products through an advanced oxidative degradation process is a more efficient process than the physical separation of MPs.10,11,21,22 Moreover, reaction conditions such as treatment time, catalyst concentration, and light intensity could be manipulated based on the pollutant type and treatment methodologies.25
Generally, oxidative degradation of MPs by photoaging via the generation of reactive oxygen species (ROS) is very slow, and hence, the UV/H2O2 system was introduced to enhance their degradation.26,27 But still, even with the addition of H2O2, the degradation of microplastics could take days.27 Hence, photocatalysts were used to enhance the degradation of microplastics.28 The TiO2 semiconductor is a popular photocatalyst worldwide due to its non-toxicity, availability, recyclability, and affordability.29 TiO2 exists in three polymorphs (anatase, rutile, and brookite), where anatase is the most suitable for photocatalytic degradation of organic pollutants.29–31 Upon irradiation of light of suitable wavelength (λ = 275 to 405 nm) on TiO2, e−/h+ are produced, which subsequently react with surface water and oxygen to form ROS.32–35 The hydroxyl radical (HO˙) is the main ROS species responsible for the decomposition of pollutants.32–36 This process of pollutant degradation has been used for the degradation of various complex organic pollutants.35 L. P. Domínguez-Jaimes et al. have investigated the degradation of polystyrene (PS) NPs using electrochemically anodized TiO2 nanotubular layers.11 However, the TiO2 anatase phase has a bandgap of 3.2 eV, thereby limiting its ability to absorb only UV light, and has a faster e−/h+ recombination rate, which hinders the production of (HO˙) radicals.30 Hence, mixed anatase and rutile phases are prepared to improve e−/h+ separation in TiO2 nanostructures.37–39 Further, metal/non-metal modifications are performed on TiO2 to improve light absorption in the visible region and promote e−/h+ separation, thereby improving the photocatalytic properties.40–43 Among the dopants, Fe2+/Fe3+ ions are quite attractive as they improve photocatalytic properties and are well known for their Fenton/photo-Fenton (PF) active properties.40,44,45 Particularly, Fe2+/Fe3+ undergoes a redox process with H2O2 to form HO˙ radicals that decompose pollutants.46 Ortiz et al. have studied the Fenton oxidation of different types of MPs, such as polyethylene terephthalate (PET), polyethylene (PE), polyvinyl chloride (PVC), polypropylene (PP), and expanded PS (EPS), as target pollutants.47 The non-toxicity and availability of Fe make it a suitable material for the oxidation process.44,45 Liu B. et al. have analyzed the degradation products of PS and nylon 6 obtained with Fe2+ Fenton reagents and activation with peroxymonosulfate.48 Therefore, modification of mixed-phase TiO2 nanostructures with Fe could be attractive for the degradation of microplastics.
In this work, peroxo-titanic acid gels synthesized by the precipitation of titanyl oxysulfate with tert-butylamine are freeze-dried and then subsequently annealed at 800 °C to form mixed-phase TiO2 aerogel powders (TiAP). Further, TiAP is modified at the surface with different concentrations of Fe through the wet-impregnation technique. Therefore, the Fe-modified TiAP could exhibit both photocatalytic and photo-Fenton processes for the degradation of MPs. Indeed, Fe modification could produce additional ROS through the combination of photocatalytic and photo-Fenton processes, which could enhance the degradation of MPs. PS MPs are chosen as a model pollutant due to their ready accumulation in living organisms through respiratory, dermal, and digestive tracts.49,50 PS is used in food packaging, toys, cup covers, storage, etc.,49 therefore, it is necessary to understand effective ways to degrade PS MPs using different photo-induced processes. There are only a handful of reports on photocatalytic/Fenton-based degradation of MPs.10,11,47 These reports deal with the evaluation of PS MP degradation through separate photocatalytic/Fenton-based processes by investigating the changes in the carbonyl and peroxyl indexes (CI/PI), mass loss after degradation, and degradation by-products.10,11,47 However, our work investigates photocatalytic/Fenton-based degradation of PS MPs in one system using Fe-modified TiAP. Briefly, the relationship of the changes in the CI value of PS MPs with the polymer mass loss and the degradation products formed after photo-induced treatment processes are discussed. Hence, our work will bridge the knowledge gap in understanding MP degradation using photocatalytic/Fenton-based processes.
2. Materials and methods
2.1. Materials
Titanium(IV) oxysulfate (TiOSO4·xH2O, min. 29% Ti as TiO2, Sigma-Aldrich), hydrogen peroxide (H2O2, aqueous solution, p.a., 30%, penta), and tert-butylamine (TBA, 98%, Sigma-Aldrich) were used for the preparation of TiAP. Iron(II) sulfate heptahydrate (99% penta) was used as a precursor for Fe modification. Polystyrene beads (Mw ∼192![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma Aldrich), acetone (T.G., Svero Chema), tetrahydrofuran (A.G., penta), dimethylacetamide (DMA, 99%, Sigma Aldrich), and dialysis bags (Membra-Cel™, 44 mm, MWCO-14
000, Sigma Aldrich), acetone (T.G., Svero Chema), tetrahydrofuran (A.G., penta), dimethylacetamide (DMA, 99%, Sigma Aldrich), and dialysis bags (Membra-Cel™, 44 mm, MWCO-14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Roth) were used for the synthesis of PS MPs. Perchloric acid (68%, Lachner) and sodium hydroxide (penta) were used to adjust the pH during the photo-Fenton processes. Isopropyl alcohol (IPA, penta) and p-benzoquinone (BQ, ≥98%, Sigma-Aldrich) were used as scavengers during the photo-induced degradation of PS MPs.
000, Roth) were used for the synthesis of PS MPs. Perchloric acid (68%, Lachner) and sodium hydroxide (penta) were used to adjust the pH during the photo-Fenton processes. Isopropyl alcohol (IPA, penta) and p-benzoquinone (BQ, ≥98%, Sigma-Aldrich) were used as scavengers during the photo-induced degradation of PS MPs.
2.2. Instrumental techniques
Crystalline phases of the prepared TiAP and Fe-modified TiAP were analyzed by X-ray diffraction (XRD, Co radiation Malvern PANalytical Empyrean III). The surface area of the photocatalysts was measured using the Brunauer–Emmett–Teller method in a Quantachrome Nova 4200e instrument. Typically, the photocatalysts were degassed at 25 °C for 72 h, and then subsequently, N2 adsorption–desorption isotherms were measured at −196 °C. The shape and morphology of the samples were imaged using high-resolution scanning electron microscopy (HR-SEM, FEI Nova NanoSEM 450 microscope equipped with an Everhart–Thornley secondary electron detector, a through-lens detector, and an energy-dispersive X-ray analysis detector) at an accelerating voltage of 15 kV. The samples with higher and lower changes in the CI values were imaged using a high-resolution transmission electron microscope (HR-TEM, Talos F200X, Thermo Scientific, USA) with an FEG (field emission gun) operated at 200 kV, which combines scanning TEM (STEM) and TEM imaging and equipped with 4 in-column SDD Super-X windowless detectors for EDX chemical analysis and elemental mapping. Samples for HR-TEM were prepared by gently dropping a small drop of dilute (water) sample suspension on a standard TEM grid covered with carbon foil, followed by drying at ambient temperature before use. Attenuated total reflection infrared (ATR-IR) spectra were recorded by a Nicolet Nexus 670 FTIR spectrometer on a diamond crystal in the region from 400–4000 cm−1. Thermogravimetric analysis (TGA) coupled with differential scanning calorimetry (DSC) and mass spectrometry (MS) were carried out to monitor the mass loss and gas evolution during the whole degradation of PS MPs. TGA-DSC-MS were performed on a NETZSCH (STA449 F1 Jupiter) instrument coupled with a gas chromatograph (GC Agilent Technologies, 7890B) and mass detector (MS Agilent Technologies, 5977B MSD). Thermoanalytical measurements were carried out in an inert atmosphere (argon, 50 cm3 min−1), in an open crucible made of α-Al2O3, and the initial sample mass was approx. 5 mg (crushed to fine powders). Samples were heated from 35 °C to 600 °C with a heating rate of 5 °C min−1, enabling a detailed study of the decomposition process. The gaseous products were led by a heated transfer line (T = 150 °C) directly to the MS, and subsequently, gas analysis was done in SIM mode at the intensity (A) of individually selected fragments for PS, namely: m/z = 51, 78, 103, and 104. 1H Nuclear magnetic resonance (NMR) spectra were recorded using a JEOL 600 MHz NMR spectrometer. All the samples were dissolved (∼20 mg mL−1) in d-chloroform (99.8%, Acros Organics) by sonication (several minutes) followed by centrifugation to remove the photocatalysts. Subsequently, NMR spectra of supernatant solutions were recorded. The zeta potentials were determined by laser Doppler electrophoresis using a Zetasizer Nano ZS analyzer (Malvern, UK). High-performance liquid chromatography with high-resolution mass spectrometry (HPLC-HRMS) was used to identify the by-products formed before and after the photo-induced treatment process. A description of the methodology used in the HPLC-HRMS technique is given in the ESI.†2.3. Synthesis of microplastics
Polystyrene (PS) MPs prepared by the dialysis technique were used as a model pollutant.51 Typically, 85 mg of PS beads were dissolved in 30 mL of organic solvent (10 mL of dimethylacetamide, 10 mL of tetrahydrofuran, and 10 mL of acetone). Later, the dissolved PS beads were poured into a dialysis bag and immersed in 1.5 L of water for 24 h, forming PS MPs. 1.5 L of water was replaced 5 times during 24 h to ensure complete removal of organic solvent. Subsequently, only the suspensions (∼30 mL) of PS MPs were taken, whereas larger aggregates of the PS particles were discarded. For all photo-induced degradation processes, freshly prepared PS MP suspensions were used. The amount of PS MPs present in the suspensions after the dialysis process is approx. 30 mg (±5% error), i.e., the weight is measured after centrifugation of PS MP solution followed by separating the pellets and drying at room temperature.2.4. Synthesis of photocatalysts
2.5. Photo-induced degradation experiments
The photocatalytic experiments were done using freshly prepared PS MP solution. The PS MP solution was diluted with 300 mL of water and suspended with 60 mg of photocatalyst followed by irradiation under UVA light (λmax = 365 nm, 8 W, Sylvania, flux density of 6.24 mW cm−2 measured by UVA probe #28949; ILT 1400-A photometer) for 480 min with constant stirring and air bubbling. During the photo-induced degradation experiments, the final concentrations of PS MPs and photocatalysts were 0.1 mg mL−1 and 0.2 mg mL−1, respectively. Photo-Fenton experiments were carried out with similar conditions to the photocatalytic process, except the pH was changed (2, 3, 5, 7, and 9), and 100 mM H2O2 was added every 1 h throughout the process.54 At predetermined time intervals (0, 60, 120, 180, 240, and 480 min), 10 mL of the samples were collected and subsequently filtered to separate the solid and the liquid samples. Finally, the solid samples collected at 0 min (before treatment) and 480 min (after photocatalytic/Fenton-based processes) were analyzed by HR-SEM, HR-TEM, ATR-IR, TGA-DSC/MS, and NMR techniques. Total organic carbon content analysis (TOC, Shimadzu TNM-L ROHS) was used to quantify the organic carbon in the filtered solutions (liquid part). The sampling methodology for evaluating the PS MP degradation is given in Fig. S1.†2.6. Evaluation of MP degradation by ATR-IR spectroscopy
The solid samples collected during the photocatalytic and photo-Fenton processes were dried at room temperature (25 °C) before analysis by ATR-IR spectroscopy. ATR-IR spectroscopy was used to calculate the CI and PI, respectively.10 The CI and PI values indicate the oxidative degradation of MPs, i.e., the cleavage of the carbonyl (1740 cm−1) and peroxyl (1150 cm−1) chains during the oxidation process. The CI and PI values were calculated using the following equation.Additionally, the reference peak at 2850 cm−1 belongs to the C–H stretching of CH2 groups, which is used as an internal standard to evaluate the degradation of PS MPs. Furthermore, the peak heights are normalized at 2850 cm−1 for optimal comparison of all the measured spectra. Importantly, values of the peak height are calculated using Gaussian fitting profiling.
3. Results and discussion
XRD patterns of TiAP and Fe-modified TiAP are shown in Fig. 1A. A mixture of TiO2 anatase (reference code: 01-071-1166) and rutile phases (reference code: 01-072-1148) was identified for all the prepared samples. Indeed, 2θ diffraction patterns at approx. 25.33, 37.81, 48.05, 53.87, and 55.08° 2θ correspond to anatase crystal structure ((101), (004), (200), (105), and (211)), whereas 2θ diffraction patterns at approx. 27.46, 36.08, and 41.25° 2θ correspond to rutile crystal structure diffraction planes ((110), (101), and (111)). In all the samples, different compositions of anatase and rutile phases were identified and tabulated in Table 1. TiAP has 9% rutile phase, whereas Fe-modified samples TiAP0.01Fe, TiAP0.1 Fe, and TiAP1Fe have 24%, 23%, and 13% rutile content, respectively. Mainly, Fe-modification was performed through a two-step annealing process, resulting in the development of the rutile phase during the second annealing step (Fig. S2a†). However, the increase in the Fe-content (TiAP1Fe) stabilizes the anatase phase and inhibits rutile content formation. Nevertheless, the combined effects of anatase and rutile phases promote e−/h+ separation and are known to increase the photocatalytic activity.37,38,55 Notably, mixed phases of approx. 70–90% anatase and 10–30% rutile are reported to have better photocatalytic properties.38,39,55,56 Further, the surface area of the prepared photocatalysts was analyzed by the Brunauer–Emmett–Teller method (Fig. S2b†) and also tabulated in Table 1. TiAP had a surface area of 17.98 m2 g−1, whereas Fe modification along with the two-step annealing process contributed to the slight reduction in the surface area of the samples. This minor reduction in the surface area also correlates with our other publication on similar aerogel powders with Ce modification.53| Sample | Anatase (%) | Anatase crystallite size (nm) | Surface area (m2 g−1) |
|---|---|---|---|
| TiAP | 91 | 51.4 | 17.98 |
| TiAP0.01Fe | 76 | 51.9 | 10.49 |
| TiAP0.1Fe | 77 | 76.7 | 11.51 |
| TiAP1Fe | 87 | 56.5 | 17.58 |
| TiAP_2 step annealed | 74 | 56.3 | 12.23 |
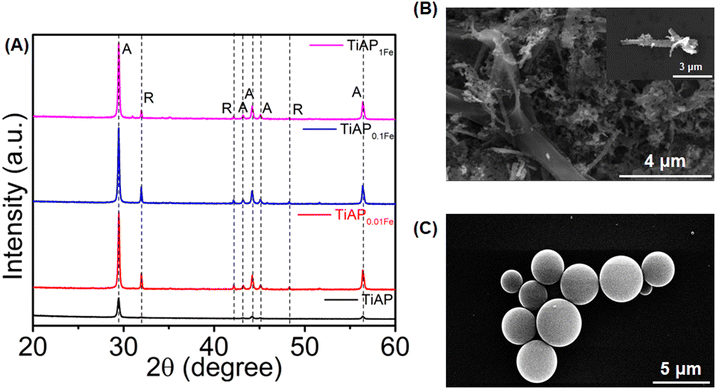
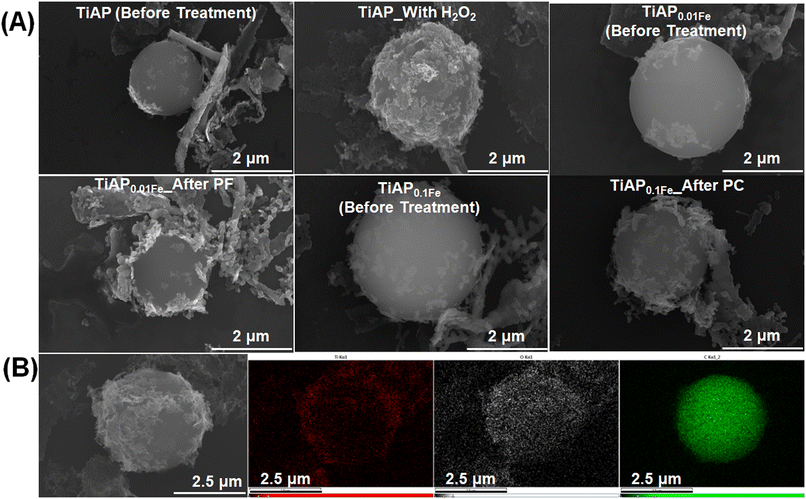
Fig. 1B and C show the HR-SEM images of representative TiAP and polystyrene samples. TiAP has a leaf-like morphology composed of multiple nanoparticles grouped in a structure. The average diameter of an individual nanoparticle ranges from 200 to 400 nm. Further, the PS MPs have a spherical morphology with a diameter ranging from 2 to 4.5 μm.
Before investigating the photo-induced degradation behaviour of PS MPs using non-modified TiAP and Fe-modified TiAP, they were tested with caffeine, which was used as a model organic pollutant. Since quantification of the degradation of PS MPs is not straightforward, combinations of analysis techniques such as ATR-IR spectroscopy, TOC, and TGA techniques10,11,57 are required. For example, the degradation efficiency for pollutants, such as dyes, caffeine, pesticides, etc., could be evaluated through UV-vis spectrometry, high-performance liquid chromatography, and TOC techniques.10,53,58,59 Therefore, caffeine was used as a model organic pollutant to understand the degradation trend through photocatalytic and photo-Fenton-based processes using TiAP and Fe-modified TiAP (Fig. S3†). Accordingly, TiAP0.1Fe showed the highest caffeine degradation extent in photocatalytic (∼45%) and photo-Fenton-based processes (∼99%) after 120 min of UVA light irradiation. Hence, photo-induced degradation of PS MPs was discussed in detail for TiAP and TiAP0.1Fe.
Firstly, the photo-induced degradation of PS MPs (solid samples) was monitored by ATR-IR spectroscopy by monitoring the changes in the carbonyl and peroxyl groups (Fig. S4 and S5†). Noticeably, the oxidation reaction leads to chain scission, cross-linking, and discoloration of PS MPs.60 The oxidation reaction in PS compounds occurs due to the formation of HO˙, ROO˙ (peroxyl radical), and POO˙ (polystyryl radicals).60,61 The POO˙ radicals that exist in an excited state are converted to ROO˙ radicals under reaction with oxygen.27,61 HO˙ radicals and ROO˙ radicals are responsible for chain scission of the polymers that increase the CI.10,27,61 The degradation of PS MPs occurs in multidirectional pathways, leading to the formation of multiple polymer moieties and various intermediates.61 Eventually, the end products of the PS MP degradation in all the pathways are CO2 and H2O.10,11 Possible pathways for photo-induced oxidation (photocatalytic and photo-Fenton processes) using TiO2 structures (TiAP and Fe-modified TiAP) of PS MPs are given below.10,61
| TiO2 + O2 + e− (hv) → O2˙− |
| TiO2 + H2O + h+ (hv) → HO˙ + H+ |
| HO˙ + ∼CH2CHPh − CH2∼ → ∼CH2C˙ PhCH2∼ (macro-radical) + H2O |
| HO˙ + ∼CH2CHPh − CH2∼ → ∼C˙ HCHPhCH2∼ + H2O |
| ∼CH2C˙ PhCH2∼ + O2 → ∼CH2C(O2˙)PhCH2∼ (peroxyl radical) |
| ∼CH2C˙ PhCH2∼ + O2 → ∼CH2C(OOH)PhCH2∼ (hydroperoxide) |
| ∼CH2C(O2˙)PhCH2∼ + hv → ∼CH2C(O˙)PhCH2∼ (alkoxyl radical) |
| ∼CH2C(O˙)PhCH2∼ + ∼CHPh∼ (neighbouring moeity) → ∼CH2C(OH)PhCH2~ |
| ∼CH2C(O˙)PhCH2∼ → ∼CH2C(O)CH2∼ + Ph˙ |
| ∼CH2C(O˙)PhCH2∼ → ∼CH2C(O)Ph∼ + ∼C˙ H2 − CHPh∼ |
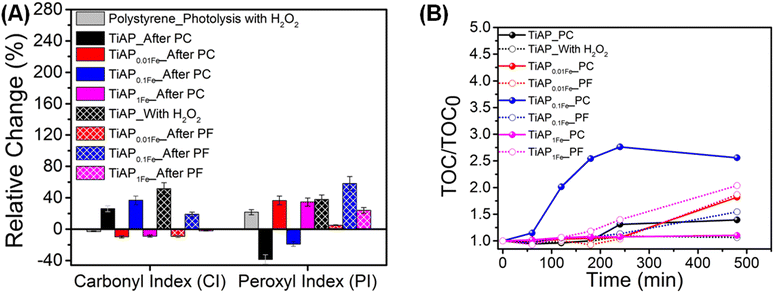
The chain scission of PS MPs during the photo-induced oxidation process will form multiple polymer moieties that could influence the changes in the carbonyl and peroxyl groups in the ATR-IR spectra. The CI and PI values were calculated using the ratio changes in carbonyl (at 1740 cm−1) and peroxyl groups (at 1150 cm−1) in comparison to internal standards (reference group of C–H stretching of the –CH2– group) at 2850 cm−1.10 Therefore, the CI and PI value changes indicate the oxidative degradation of PS MPs. Relative changes in CI and PI were calculated with respect to the non-treated samples (Fig. 2A and Table S1†). The photocatalytic process by TiAP showed approx. 26% increase in CI and a 38% decrease in PI values, whereas TiAP with H2O2 showed approx. 52% and 39% increase in CI and PI values, respectively. The decrease in PI could indicate that the degradation did not occur in the direction of forming peroxyl-containing moieties; instead, peroxyl radicals are eventually converted to alkoxyl radicals that form carbonyl groups. Notably, Fe-modified TiAP samples show different changes in CI and PI values for photocatalytic and photo-Fenton processes. For instance, photocatalytic and photo-Fenton processes with TiAP0.01Fe and TiAP1Fe showed a decreased CI and increased PI value. This implies that one of the PS degradation pathways is due to the formation of the peroxyl group. Additionally, the decrease in CI could indicate another pathway of PS degradation, i.e., based on the Norrish-I mechanism that forms carbon monoxide during the photo-induced degradation process.63–65 Ghanadi et al.64 reported a decrease in the CI value for the HDPE polymer observed after the first few days of exposure to UV radiation. TiAP0.1Fe showed the highest CI and PI values, increasing to approximately 36% and 58% for the photocatalytic and photo-Fenton processes, respectively.
To further solidify the versatility of Fe modification on TiAP, especially for the photo-Fenton-based degradation of PS MPs, the homogenous photo-Fenton process containing Fe2+ ions was carried out at different pH values (2, 3, 5, 7, and 9). Equivalent concentrations of Fe2+ present (∼12.6 μM Fe2+ salt) in TiAP0.1Fe were used for the homogenous Fenton process. Accordingly, higher changes in CI and PI values were observed for the TiAP0.1Fe system in all tested pH ranges (Fig. S6†). Therefore, it's evident that the presence of TiAP along with Fe2+ assists in the efficient degradation of PS MPs. Moreover, at pH 2, both CI and PI values had higher changes. Notably, the calculations of CI and PI through carbonyl and peroxyl groups are only semi-quantitative methods for analyzing the polymer oxidation process.10 Hence, additional methodologies are required to quantify PS degradation efficiency. Fig. 2B shows the carbon content monitored throughout the PS degradation (liquid part) process for all the samples. The liquids collected for TOC analysis showed an increasing carbon content throughout the reaction. The photocatalytic process with TiAP0.1Fe showed the highest increase in the carbon content. Typically, the detection of higher carbon content in the liquid part is due to the release of carbon chains during the polymer photo-degradation process. A representative liquid part (TiAP_PC) collected after 480 min and 24 h of irradiation was analyzed by NMR, and proton signals between 0.8 and 1.9 ppm belonging to aliphatic groups were observed (Fig. S7a†). Therefore, ROS generated during the photocatalytic degradation were used to break the PS MPs into aliphatic chains. However, with prolonged irradiation (at least 24 h), the broken polymer chains eventually mineralized to CO2 and H2O.10,11 Indeed, the longer irradiation time (24 h) using TiAP resulted in approx. 53% mineralization of the PS MPs (Fig. S7b†). Since this work deals with understanding the photocatalytic/Fenton processes in non-modified and Fe-modified TiAP, the TOC experiments were not carried out for a longer treatment time for other samples. The summaries of reported research work on CI, PI, and mineralization extent according to their photocatalytic conditions are tabulated in Table S2.† Our reported CI, PI, and mineralization extent are similar to other reported studies on the photo-induced degradation of PS MPs.
HR-SEM and HR-TEM images before the treatment and after the photocatalytic and photo-Fenton-based process are shown in Fig. 3 and 4 and S8,† respectively. The representative EDX mapping in Fig. 3B shows that the spherical-shaped particles are carbon (green) belonging to the PS MPs, and Ti (red) covers the surface of PS MPs. O (white) is present throughout the samples that belong to PS MPs and TiO2 photocatalysts. Interestingly, after 480 min of both photocatalytic/Fenton-based processes, the photocatalyst (TiAP and Fe-modified TiAP) covers the surface of the PS MPs. This coverage of the photocatalysts around the PS MPs could facilitate an attack of the HO˙ radicals on the PS MPs, leading to their degradation.61
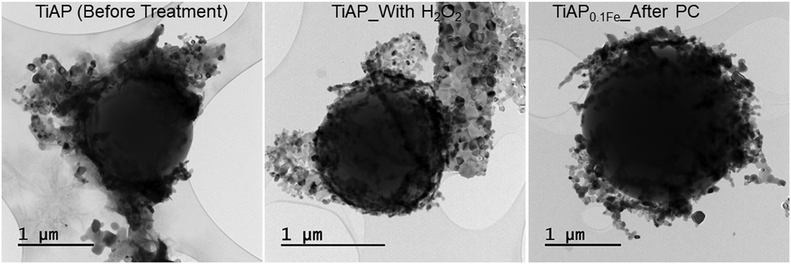 | ||
| Fig. 4 Representative HR-TEM images of TiAP before treatment and samples with higher changes in the CI value (TiAP_With H2O2 and TiAP0.1Fe_After PC). | ||
The thermal decomposition of the PS MPs before and after the photo-induced processes using non-modified TiAP, TiAP0.01Fe, and TiAP0.1Fe was investigated using TGA/DSC-MS (Table 2, Fig. S9–S11†). Particularly, the mass loss was recorded for the samples with the highest and lowest changes in CI value (Fig. 2A). Further, the thermal decomposition of unmodified peroxo-titanate powders without PS MPs was investigated in detail in our previous study.52,66,67 Evidently, all the samples before and after the photo-induced processes showed two-step thermal decomposition. In the first step, between 30 and 200 °C, the evolution of adsorbed water from the surface of the samples was observed. Then, in the second step, the decomposition of PS MPs with a DTG curve maximum at approx. 440 °C was observed to be connected with the evolution of PS fragments with m/z of 51, 78, 103, and 104.68,69 Further, hardly any changes in the DSC curve were observed during the measurements (Fig. S9–S11†). The glass transition temperature of PS was hardly observed around 100 °C70 because of the simultaneous thermal analysis measurements. Also, there are the same changes around the temperature of the main degradation step, which is approx. at 440 °C. The mass residue is 53–68% for the measured samples, i.e., around 32–47% of the PS MPs were released during the thermal decomposition process. However, it could be observed that there is a clear difference in the total mass residue for the samples with the highest change in the CI value (TiAP_With H2O2 and TiAP0.1Fe_After PC). For instance, before the treatment, non-modified TiAP and TiAP0.1Fe had retained approx. 61% and 56% of their initial mass, respectively. However, after the photo-induced treatment process, the mass residue increased to approx. 68% for TiAP_With H2O2 and 65% for TiAP0.1Fe_After PC. Since PS MPs were already degraded during the photo-induced treatment process, there is a higher mass residue for the samples with higher changes in the CI value. In other words, PS MPs were partially removed during the photo-degradation process, and a higher percentage of the materials' mass belongs to the photocatalysts (TiAP or Fe-modified TiAP). I. Nabi et al.57 also showed that in the case of PS MP-modified TiO2 films, 100% of the material mass belongs to TiO2 after the photocatalytic treatment. Additionally, the samples with lower changes in CI value (TiAP0.01Fe_After PF and TiAP0.1Fe_After PF) showed no significant differences in the total mass residue before and after the photo-induced treatment process (Table 2 and Fig. S11†). Furthermore, the samples with higher changes in the PI values (TiAP0.1Fe_After PF) did not affect the mass residue in the TGA/DSC-MS spectra. Hence, it could be understood that the changes in the CI values are necessary to affect the mass residue after the photo-induced treatment process. Importantly, to confirm the reproducibility of the experiments, TGA/DSC-MS measurements were repeated 5 times for a representative sample (TiAP_With H2O2), and the differences between particular measurements were ±2.10%. Therefore, the chosen evaluation methodology can be considered reliable due to the negligible error in TGA-DSC/MS measurements.
| Sample | T onset (°C) | R 600°C (%) |
|---|---|---|
| TiAP (before treatment) | 400 | 60.81 |
| TiAP_With H2O2 | 407 | 67.90 |
| TiAP0.1Fe (before treatment) | 414 | 56.36 |
| TiAP0.1Fe_After PC | 419 | 64.70 |
| TiAP0.1Fe_After PF | 416 | 53.05 |
Interestingly, preliminary studies showed that the photo-Fenton-based process using TiAP0.1Fe showed the highest caffeine degradation efficiency among all the treated samples. However, a similar degradation efficiency in the photo-Fenton process could not be obtained for PS MPs. This could be because the mechanism of caffeine and PS MP adsorption onto the photocatalysts differs between these two pollutants. For instance, zeta potential measurements (Fig. S12†) showed that the surface charge of caffeine at acidic pH (∼3) and near neutral pH (∼6) is close to zero.71–73 Further, the surface charge of Fe-modified TiO2 (TiAP and Fe-modified TiAP) remains positive for both neutral and acidic pH, respectively.74 During the photo-induced degradation process in water, the caffeine molecule adsorbs onto TiO2 (TiAP and Fe-modified TiAP) through hydrogen bond formation.75,76 Therefore, caffeine degradation is better for photocatalytic and photo-Fenton processes using TiAP and Fe-modified TiAP. However, the surface charge of PS MPs changes at near neutral and acidic pH. Zeta potential measurements (Fig. S12a†) showed that the surface charge of PS MPs at near neutral and acidic pH is negative and positive, respectively.71,72 Hence, owing to the better electrostatic interaction between PS MPs and photocatalysts (TiAP and TiAP-Fe) at near neutral pH a higher degradation efficiency is exhibited. Meanwhile, at acidic pH, both photocatalysts and PS MPs showed positive surface charges, and hence, the interaction between them became weaker, thereby affecting the degradation efficiency. To confirm the interaction of a positively charged photocatalyst with another positively charged pollutant, methylene blue (MB) was chosen as a model organic cationic pollutant.77 Interestingly, almost similar MB degradation efficiency (∼61%) was obtained between photocatalytic and photo-Fenton processes at acidic pH using TiAP0.1Fe (Fig. S12b†). This indicates that electrostatic repulsion inhibits the photo-Fenton degradation process at acidic pH. Indeed, the degradation efficiency is related to the surface charge of the pollutant and the photocatalyst.58,77 Nevertheless, TiAP0.1Fe showed good photocatalytic degradation of PS MPs among all the tested samples. Even though the photo-Fenton process was weaker, it still exhibited oxidative degradation of PS MPs.
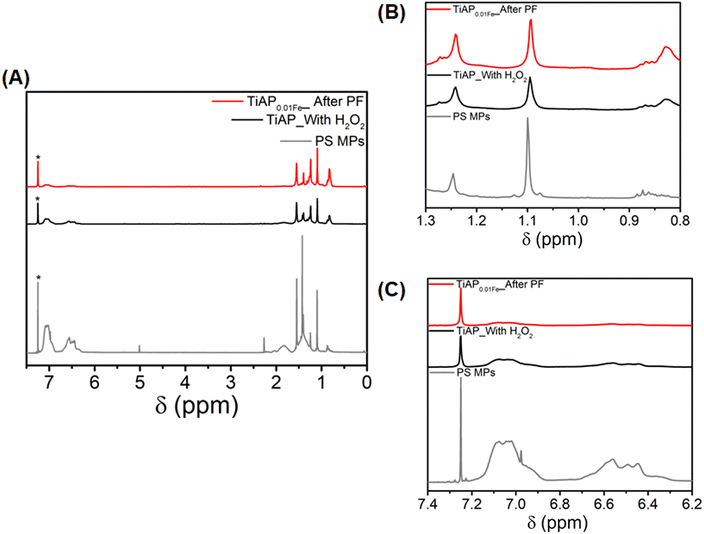
Proton NMR spectra were recorded for the as-prepared PS MPs, TiAP_With H2O2, and TiAP0.01Fe_After PF (Fig. 5). Typically, as-prepared PS MPs prepared for this study have one set of peaks between 6.3 and 7.2 ppm belonging to aromatic protons of the styrene moiety and between 0.8 and 1.9 ppm belonging to aliphatic protons.78 Already, oxidized moieties of aromatic alcohols are present in the as-prepared PS MPs at 2.3 and 5 ppm.78 During the photo-induced degradation, polymer chains undergo scissions, which could be observed in the NMR spectrum. Indeed, new NMR peaks formed for TiAP_With H2O2 and TiAP0.01Fe_After PF, especially at 0.82 and 1.27 ppm (Fig. 5B and C). Furthermore, during the photo-induced oxidation process, the cleavage of aliphatic and aromatic alcohol groups, i.e., the peaks at 1.83, 2.26, and 5.01 ppm, was absent in the treated samples. Additionally, proton NMR spectra recorded for the homogenous Fenton process using Fe2+ at pH 2 and pH 9 (Fig. S13†) revealed only the formation of new peaks of aliphatic protons (at 0.83 and 1.28 ppm). In contrast, negligible changes occurred in aliphatic and aromatic alcohol groups. This difference in NMR spectra also could indicate that these polymer chains were only partially affected during the homogenous photo-Fenton degradation process. Importantly, NMR spectra could be used to identify the oxidative degradation of the PS MPs but not to determine the extent of the degradation efficiency. Therefore, the samples, irrespective of their degradation efficiency, showed the oxidation of the polymer chains in the PS MPs.
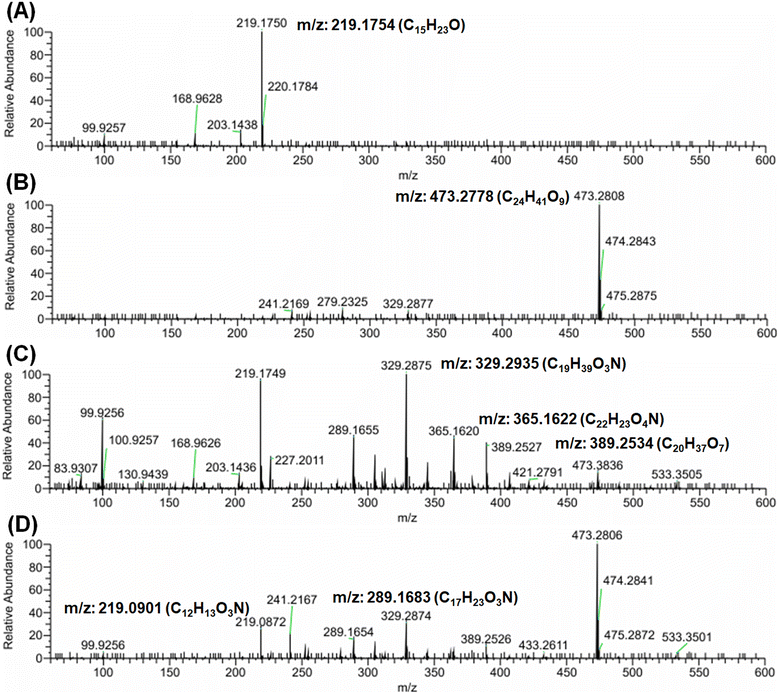
Complementary to NMR spectra, HPLC-HRMS confirmed the oxidation of PS MPs through photo-induced processes (Fig. 6 and S14 and S15†). HPLC-HRMS measurements were carried out in both negative and positive modes for non-treated PS MPs and samples with the highest change in CI value (TiAP_With H2O2). Interestingly, the spectra for PS MPs and TiAP_With H2O2 could be obtained only from the negative mode, indicating that by-products are polar compounds that ionize by deprotonation. Further, clear differences in chromatogram profiles were observed for PS MPs and TiAP_With H2O2, at the wavelength of 220 nm and 280 nm, thereby confirming the photo-induced oxidation of PS MPs (Fig. S14 and S15†). Additionally, the m/z spectrum showed the formation of additional peaks, which could be possibly due to the degradation of PS MPs (Fig. 6). The m/z at 219, 289, 329, 365, and 389 are closely related to the phenyl groups, esters, and organic acids, which are common oxidative degradation by-products.10,11 Based on m/z, plausible intermediates formed are shown in Fig. S16.† Therefore, the formation of degradation by-products confirms the photo-induced degradation of PS MPs.
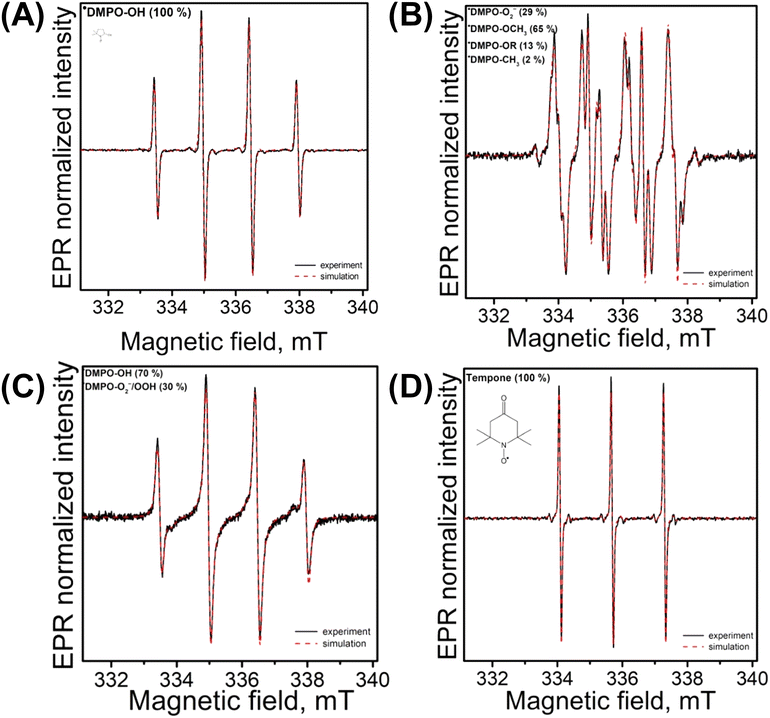
The main ROS responsible for the degradation of PS MPs during the photoinduced degradation process using TiAP0.1Fe were determined by electron paramagnetic resonance (EPR) spectroscopy. The EPR spin trapping technique utilizing 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was used to identify the transient paramagnetic species formed upon exposure of the aerated dispersions of TiAP0.1Fe. The immediate formation of the characteristic 4-line signal upon LED@365 nm exposure, assigned to the ˙DMPO–OH spin-adduct with spin-Hamiltonian parameters (aN = 1.501 mT, aβH = 1.477 mT, a13C(6x13C) = 0.737 mT, g = 2.0057; Fig. 7A) in the reaction system TiAP0.1Fe/DMPO/water, was observed. The spin-adduct represents the HO˙ formed upon (i) oxidation of HO−/H2O adsorbed on the photocatalyst's surface and (ii) cascade complex reactions via O2˙−/H2O2 trapped by the DMPO spin trap.79,80 The dominant pathway – genuine spin trapping – was supported by adding an aprotic dimethylsulfoxide (DMSO) solvent into an aqueous suspension of TiAP0.1Fe (20% vol. DMSO). The LED@365 nm exposure led to the generation of two spin-adducts, and the 6-line signal was attributed to carbon-centered ˙DMPO–CH3 (aN = 1.325 mT, aβH = 2.320 mT, g = 2.0057, crel = 65%) along with ˙DMPO–OH (aN = 1.495 mT, aβH = 1.442 mT, g = 2.0057, crel = 35%; data not shown). The rapid reaction of HO˙ with DMSO produces methyl radicals, detectable in the reaction with DMPO as the corresponding spin-adduct ˙DMPO–CH3. The rapid dismutation of the superoxide radical anion and low stability of ˙DMPO–O2−/OOH in water limit the detection of O2˙− in an aqueous environment. To support its formation upon exposure, the experiments were performed in DMSO, a well-known solvent stabilizing the superoxide radical anions.79 The LED@365 nm exposure of the aerated DMSO suspension of TiAP0.1Fe in the presence of DMPO resulted in the generation of four spin-adducts (Fig. 7B): ˙DMPO–O2− (aN = 1.287 mT, aβH = 1.037 mT, aγH = 0.140 mT, g = 2.0057, crel = 29%) produced by the reaction of O2˙− with DMPO and ˙DMPO–OCH3 (aN = 1.329 mT, aβH = 0.799 mT, aγH = 0.162 mT, g = 2.0057, crel = 56%), ˙DMPO–OR (aN = 1.390 mT, aβH = 1.162 mT, g = 2.0057, crel = 13%), and ˙DMPO–CH3 (aN = 1.438 mT, aβH = 2.127 mT, g = 2.0057, crel = 2%).79,80 The production of the ˙DMPO–OCH3 adduct is initiated by generated ROS (mainly HO˙), which immediately attack the DMSO molecules, forming methyl radicals, and the rapid reaction of these methyl radicals with molecular oxygen results in the generation of peroxomethyl radicals serving as a source of ˙DMPO–OCH3 spin-adducts. The main source of ROS is adsorbed H2O/HO− naturally occurring on the photocatalyst surface.79,80
The addition of H2O2 to the aerated aqueous suspension of TiAP0.1Fe in the presence of DMPO upon LED@365 nm exposure resulted in the generation of two EPR signals assigned to ˙DMPO–OH (crel = 70%) and ˙DMPO–O2−/OOH (aN = 1.456 mT, aβH = 1.129 mT, aγH = 0.158 mT, g = 2.0057, crel = 30%) (Fig. 7C). The presence of the second-mentioned spin-adduct in aqueous media is relatively scarce; under given experimental conditions, HOO˙ is formed, and the oxidation/reduction of H2O2 by photogenerated holes and electrons leads to the effective formation of molecular oxygen.81 The higher amount of molecular oxygen was evidenced by the presence of small bubbles in the EPR quartz cell and broadened lines of EPR signals were observed. The higher concentration of O2 (and consecutive formation of O2˙−) and the occurrence of HOO˙ enable the more effective generation of the ˙DMPO–O2−/OOH spin-adduct.82 It should be noted here that an analogous experiment without a photocatalyst in the reaction system resulted only in the formation of ˙DMPO–OH, and no presence of other spin-adducts was found.
The photoinduced oxidation of sterically hindered amines, e.g., 4-oxo-2,2,6,6-tetramethylpiperide N-oxyl (TMPO), leads to the formation of stable free nitroxide 4-oxo-2,2,6,6-tetramethylpiperidone (Tempone). Fig. 7D represents experimental and simulated EPR spectra of stable free nitroxide Tempone formed upon LED@365 nm exposure in the reaction system TiAP0.1Fe/TMPO/water/air (aN = 1.611 mT, a13C(6x13C) = 0.641 mT, g = 2.0055). The main species responsible for the oxidation is singlet oxygen (1O2), however, ROS or other reactive species should be considered.79 The addition of sodium azide, a widely used water-soluble 1O2 quencher,79 led to a significant drop in EPR signal intensity (80% decrease), supporting the 1O2 formation under experimental conditions. Additionally, the formation of the ROS during the photo-induced degradation process was complemented by scavenging experiments. IPA (HO˙)53,83 and BQ (O2˙− and 1O2)53,84,85 were used as scavengers for photocatalytic and photo-Fenton degradation processes, respectively (Fig. S17a†). The presence of both IPA and BQ reduced the changes in the CI and PI values during the photo-catalytic/Fenton process using TiAP0.1Fe, thereby confirming the generation of these ROS (HO˙, O2˙−, and 1O2) in both the photocatalytic and photo-Fenton systems. Additionally, probably due to the quenching effect of the scavenger, proton NMR spectra (Fig. S17b†) also showed only the formation of new peaks at 0.83 and 1.28 ppm, whereas no changes in the aromatic and aliphatic alcohol groups were observed. Therefore, modification of TiAP with Fe produces ROS by both the photocatalytic and photo-Fenton processes, thereby enhancing the degradation efficiency of PS MPs (Fig. S18†). The ROS cleave polystyrene into intermediates (detected by HPLC-HRMS) which finally mineralize into CO2 and H2O. This work will eventually lead to an understanding of using efficient nanostructures to study the degradation of PS MPs in the environment.
Conclusions
TiO2 nanostructures were successfully prepared as aerogel powders and further modified at the surface with Fe for photocatalytic and photo-Fenton-based PS MP degradation. The changes in CI and PI values corresponding to the photo-induced degradation of PS MPs were observed with TiAP, and Fe-modified TiAP (0.01–1 at%) were monitored by ATR-IR spectroscopy technique. Accordingly, TiAP_With H2O2 and TiAP0.1Fe_After PC showed the highest change in the CI value, i.e., these two samples exhibited the highest degradation of PS MPs. Owing to the better electrostatic interaction between the PS MPs and catalyst (TiAP and TiAP0.1 Fe) a higher degradation efficiency was achieved. In contrast, in the photo-Fenton-based system, the electrostatic interaction between PS MPs and Fe-modified TiAP (TiAP0.1Fe) became weaker, lowering the degradation efficiency. Even though Fe modification improves e−/h+ separation, the electrostatic interaction between the pollutant and catalyst plays a vital role in the degradation of organic pollutants. Further, TGA/DSC-MS showed that the samples with higher CI value changes showed significant residual mass changes after the photo-induced treatment process. In contrast, samples with lower changes in the CI value did not trigger a residual mass change. Subsequently, NMR spectral peaks related to the oxidative degradation of PS MPs were observed for the samples with higher and lower changes in the CI values, confirming the breakage of the polymer chains. Furthermore, HPLC-HRMS spectra showed the formation of degradation by-products after the photo-induced degradation process for the sample with a higher change in the CI value. Finally, TOC results for more extended treatment time revealed the eventual mineralization of the PS MPs. EPR spectroscopy and scavenging experiments show the generation of ROS in photocatalytic (HO˙, O2˙−, and 1O2) and photo-Fenton (HO˙, HOO˙, and O2˙−) systems using TiAP0.1Fe. This study will assist in preparing efficient nanostructures and choosing better photo-induced processes for treating MPs in water.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Guru Karthikeyan Thirunavukkarasu: conceptualization, investigation, methodology, visualization, writing-original draft, writing-review & editing. Monika Motlochová: methodology, visualization, writing-review & editing. Dmytro Bavol: investigation, methodology, writing-review & editing. Anna Vykydalová: investigation, methodology, writing-review & editing. Jaroslav Kupčík: investigation, methodology, writing-review & editing. Michal Navrátil: investigation, methodology, writing-review & editing. Kaplan Kirakci: methodology, writing-review & editing. Eva Pližingrová: methodology, writing-review & editing. Dana Dvoranová: investigation, methodology, writing-review & editing. Jan Šubrt: investigation, methodology, writing-review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by i) Research Infrastructure NanoEnviCz, supported by the Ministry of Education, Youth and Sports of the Czech Republic under Project No. LM2018124 and ii) Programme to Support Prospective Human Resources – post Ph.D. candidates from the Czech Academy of Sciences under project no: L200322301. This work was partially financially supported by Scientific Grant Agency of the Slovak Republic (Project VEGA 1/0422/24) (Dana Dvoranová).Notes and references
- L. M. Heidbreder, I. Bablok, S. Drews and C. Menzel, Tackling the plastic problem: A review on perceptions, behaviors, and interventions, Sci. Total Environ., 2019, 668, 1077–1093 CrossRef CAS PubMed.
- P. Dauvergne, Why is the global governance of plastic failing the oceans?, Global Environ. Change, 2018, 51, 22–31 CrossRef.
- D. K. A. Barnes, F. Galgani, R. C. Thompson and M. Barlaz, Accumulation and fragmentation of plastic debris in global environments, Philos. Trans. R. Soc., B, 2009, 364, 1985–1998 CrossRef CAS.
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan and K. L. Law, Plastic waste inputs from land into the ocean, Science, 2015, 347, 768–771 CrossRef CAS.
- J. O. Babayemi, I. C. Nnorom, O. Osibanjo and R. Weber, Ensuring sustainability in plastics use in Africa: consumption, waste generation, and projections, Environ. Sci. Eur., 2019, 31, 60 CrossRef.
- B. R. Kiran, H. Kopperi and S. Venkata Mohan, Micro/nano-plastics occurrence, identification, risk analysis and mitigation: challenges and perspectives, Rev. Environ. Sci. Biotechnol., 2022, 21, 169–203 CrossRef PubMed.
- S.-A. Strungaru, R. Jijie, M. Nicoara, G. Plavan and C. Faggio, Micro- (nano) plastics in freshwater ecosystems: Abundance, toxicological impact and quantification methodology, TrAC, Trends Anal. Chem., 2019, 110, 116–128 CrossRef CAS.
- H. Shi, J. Frias, A. El-Din, H. Sayed, G. E. De-la-Torre, M.-C. Jong, S. A. Uddin, R. Rajaram, S. Chavanich, A. Najii, M. D. Fernández-Severini, Y. S. Ibrahim and L. Su, Small plastic fragments: A bridge between large plastic debris and micro- & nano-plastics, TrAC, Trends Anal. Chem., 2023, 168, 117308 CrossRef CAS.
- S. Monira, R. Roychand, M. A. Bhuiyan and B. K. Pramanik, Role of water shear force for microplastics fragmentation into nanoplastics, Environ. Res., 2023, 237, 116916 CrossRef CAS PubMed.
- P. García-Muñoz, P. H. Allé, C. Bertoloni, A. Torres, M. U. De La Orden, J. M. Urreaga, M.-A. Dziurla, F. Fresno, D. Robert and N. Keller, Photocatalytic degradation of polystyrene nanoplastics in water. A methodological study, J. Environ. Chem. Eng., 2022, 10, 108195 CrossRef.
- L. P. Domínguez-Jaimes, E. I. Cedillo-González, E. Luévano-Hipólito, J. D. Acuña-Bedoya and J. M. Hernández-López, Degradation of primary nanoplastics by photocatalysis using different anodized TiO2 structures, J. Hazard. Mater., 2021, 413, 125452 CrossRef.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling, J. Hazard. Mater., 2018, 344, 179–199 CrossRef CAS.
- F. Yu, C. Yang, Z. Zhu, X. Bai and J. Ma, Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment, Sci. Total Environ., 2019, 694, 133643 CrossRef CAS.
- Y. Yu, W. Y. Mo and T. Luukkonen, Adsorption behaviour and interaction of organic micropollutants with nano and microplastics – A review, Sci. Total Environ., 2021, 797, 149140 CrossRef CAS.
- V.-G. Le, M.-K. Nguyen, H.-L. Nguyen, C. Lin, M. Hadi, N. T. Q. Hung, H.-G. Hoang, K. N. Nguyen, H.-T. Tran, D. Hou, T. Zhang and N. S. Bolan, A comprehensive review of micro- and nano-plastics in the atmosphere: Occurrence, fate, toxicity, and strategies for risk reduction, Sci. Total Environ., 2023, 904, 166649 CrossRef CAS PubMed.
- V. Kumar, E. Singh, S. Singh, A. Pandey and P. C. Bhargava, Micro- and nano-plastics (MNPs) as emerging pollutant in ground water: Environmental impact, potential risks, limitations and way forward towards sustainable management, Chem. Eng. J., 2023, 459, 141568 CrossRef CAS.
- S. Lambert and M. Wagner, in Freshwater Microplastics, ed. M. Wagner and S. Lambert, Springer International Publishing, Cham, 2018, vol. 58, pp. 1–23 Search PubMed.
- A. S. Reddy and A. T. Nair, The fate of microplastics in wastewater treatment plants: An overview of source and remediation technologies, Environ. Technol. Innovation, 2022, 28, 102815 CrossRef CAS.
- M. Sadia, A. Mahmood, M. Ibrahim, M. K. Irshad, A. H. A. Quddusi, A. Bokhari, M. Mubashir, L. F. Chuah and P. L. Show, Microplastics pollution from wastewater treatment plants: A critical review on challenges, detection, sustainable removal techniques and circular economy, Environ. Technol. Innovation, 2022, 28, 102946 CrossRef CAS.
- K. Conley, A. Clum, J. Deepe, H. Lane and B. Beckingham, Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year, Water Res.:X, 2019, 3, 100030 CAS.
- S. H. Paiman, S. F. Md Noor, N. Ngadi, A. H. Nordin and N. Abdullah, Insight into photocatalysis technology as a promising approach to tackle microplastics pollution through degradation and upcycling, Chem. Eng. J., 2023, 467, 143534 CrossRef CAS.
- N. D. O. Dos Santos, R. Busquets and L. C. Campos, Insights into the removal of microplastics and microfibres by Advanced Oxidation Processes, Sci. Total Environ., 2023, 861, 160665 CrossRef CAS PubMed.
- R. Y. Krishnan, S. Manikandan, R. Subbaiya, N. Karmegam, W. Kim and M. Govarthanan, Recent approaches and advanced wastewater treatment technologies for mitigating emerging microplastics contamination – A critical review, Sci. Total Environ., 2023, 858, 159681 CrossRef CAS.
- N. A. Khan, A. H. Khan, E. A. López-Maldonado, S. S. Alam, J. R. López López, P. F. Méndez Herrera, B. A. Mohamed, A. E. D. Mahmoud, A. Abutaleb and L. Singh, Microplastics: Occurrences, treatment methods, regulations and foreseen environmental impacts, Environ. Res., 2022, 215, 114224 CrossRef CAS.
- Z. Yang, Y. Li and G. Zhang, Degradation of microplastic in water by advanced oxidation processes, Chemosphere, 2024, 357, 141939 CrossRef CAS.
- X. Shi, Z. Chen, X. Liu, W. Wei and B.-J. Ni, The photochemical behaviors of microplastics through the lens of reactive oxygen species: Photolysis mechanisms and enhancing photo-transformation of pollutants, Sci. Total Environ., 2022, 846, 157498 CrossRef CAS PubMed.
- S. Dong, X. Yan, Y. Yue, W. Li, W. Luo, Y. Wang, J. Sun, Y. Li, M. Liu and M. Fan, H2O2 concentration influenced the photoaging mechanism and kinetics of polystyrene microplastic under UV irradiation: Direct and indirect photolysis, J. Cleaner Prod., 2022, 380, 135046 CrossRef CAS.
- J. Ge, Z. Zhang, Z. Ouyang, M. Shang, P. Liu, H. Li and X. Guo, Photocatalytic degradation of (micro)plastics using TiO2-based and other catalysts: Properties, influencing factor, and mechanism, Environ. Res., 2022, 209, 112729 CrossRef CAS PubMed.
- Y. Kakuma, A. Y. Nosaka and Y. Nosaka, Difference in TiO2 photocatalytic mechanism between rutile and anatase studied by the detection of active oxygen and surface species in water, Phys. Chem. Chem. Phys., 2015, 17, 18691–18698 RSC.
- T. Luttrell, S. Halpegamage, J. Tao, A. Kramer, E. Sutter and M. Batzill, Why is anatase a better photocatalyst than rutile? - Model studies on epitaxial TiO2 films, Sci. Rep., 2014, 4, 4043 CrossRef.
- V. Etacheri, C. Di Valentin, J. Schneider, D. Bahnemann and S. C. Pillai, Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments, J. Photochem. Photobiol., C, 2015, 25, 1–29 CrossRef CAS.
- R. Ghamarpoor, A. Fallah and M. Jamshidi, Investigating the use of titanium dioxide (TiO2) nanoparticles on the amount of protection against UV irradiation, Sci. Rep., 2023, 13, 9793 CrossRef CAS PubMed.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Understanding TiO 2 Photocatalysis: Mechanisms and Materials, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- Q. Guo, C. Zhou, Z. Ma and X. Yang, Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges, Adv. Mater., 2019, 31, 1901997 CrossRef CAS PubMed.
- D. Chen, Y. Cheng, N. Zhou, P. Chen, Y. Wang, K. Li, S. Huo, P. Cheng, P. Peng, R. Zhang, L. Wang, H. Liu, Y. Liu and R. Ruan, Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review, J. Cleaner Prod., 2020, 268, 121725 CrossRef CAS.
- P. Jiang, T. Zhou, J. Bai, Y. Zhang, J. Li, C. Zhou and B. Zhou, Nitrogen-containing wastewater fuel cells for total nitrogen removal and energy recovery based on Cl·/ClO· oxidation of ammonia nitrogen, Water Res., 2023, 235, 119914 CrossRef CAS PubMed.
- W. R. Siah, H. O. Lintang, M. Shamsuddin and L. Yuliati, High photocatalytic activity of mixed anatase-rutile phases on commercial TiO 2 nanoparticles, IOP Conf. Ser.:Mater. Sci. Eng., 2016, 107, 012005 Search PubMed.
- K. Almashhori, T. T. Ali, A. Saeed, R. Alwafi, M. Aly and F. E. Al-Hazmi, Antibacterial and photocatalytic activities of controllable (anatase/rutile) mixed phase TiO2 nanophotocatalysts synthesized via a microwave-assisted sol–gel method, New J. Chem., 2020, 44, 562–570 RSC.
- Y. Zhang, J. Chen and X. Li, Preparation and Photocatalytic Performance of Anatase/Rutile Mixed-Phase TiO2 Nanotubes, Catal. Lett., 2010, 139, 129–133 CrossRef CAS.
- N. R. Reddy, P. M. Reddy, N. Jyothi, A. S. Kumar, J. H. Jung and S. W. Joo, Versatile TiO2 bandgap modification with metal, non-metal, noble metal, carbon material, and semiconductor for the photoelectrochemical water splitting and photocatalytic dye degradation performance, J. Alloys Compd., 2023, 935, 167713 CrossRef CAS.
- T. Zhou, J. Wang, S. Chen, J. Bai, J. Li, Y. Zhang, L. Li, L. Xia, M. Rahim, Q. Xu and B. Zhou, Bird-nest structured ZnO/TiO2 as a direct Z-scheme photoanode with enhanced light harvesting and carriers kinetics for highly efficient and stable photoelectrochemical water splitting, Appl. Catal., B, 2020, 267, 118599 CrossRef CAS.
- T. Zhou, S. Chen, L. Li, J. Wang, Y. Zhang, J. Li, J. Bai, L. Xia, Q. Xu, M. Rahim and B. Zhou, Carbon quantum dots modified anatase/rutile TiO2 photoanode with dramatically enhanced photoelectrochemical performance, Appl. Catal., B, 2020, 269, 118776 CrossRef CAS.
- T. Zhou, L. Li, J. Li, J. Wang, J. Bai, L. Xia, Q. Xu and B. Zhou, Electrochemically reduced TiO2 photoanode coupled with oxygen vacancy-rich carbon quantum dots for synergistically improving photoelectrochemical performance, Chem. Eng. J., 2021, 425, 131770 CrossRef CAS.
- S. Gowrisankaran, G. K. Thirunavukkarasu, H. Makarov, T. Roch, G. Plesch, M. Motola, G. Mailhot, M. Brigante and O. Monfort, New insights into the mechanism of coupled photocatalysis and Fenton-based processes using Fe surface-modified TiO2 nanotube layers: The case study of caffeine degradation, Catal. Today, 2023, 413–415, 114027 CrossRef CAS.
- J. P. Ribeiro and M. I. Nunes, Recent trends and developments in Fenton processes for industrial wastewater treatment – A critical review, Environ. Res., 2021, 197, 110957 CrossRef CAS PubMed.
- M. I. Maldonado, P. C. Passarinho, I. Oller, W. Gernjak, P. Fernández, J. Blanco and S. Malato, Photocatalytic degradation of EU priority substances: A comparison between TiO2 and Fenton plus photo-Fenton in a solar pilot plant, J. Photochem. Photobiol., A, 2007, 185, 354–363 CrossRef CAS.
- D. Ortiz, M. Munoz, J. Nieto-Sandoval, C. Romera-Castillo, Z. M. De Pedro and J. A. Casas, Insights into the degradation of microplastics by Fenton oxidation: From surface modification to mineralization, Chemosphere, 2022, 309, 136809 CrossRef CAS.
- B. Liu, Q. Jiang, Z. Qiu, L. Liu, R. Wei, X. Zhang and H. Xu, Process analysis of microplastic degradation using activated PMS and Fenton reagents, Chemosphere, 2022, 298, 134220 CrossRef CAS PubMed.
- K. Kik, B. Bukowska and P. Sicińska, Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms, Environ. Pollut., 2020, 262, 114297 CrossRef CAS.
- D. Barceló, Y. Picó and A. H. Alfarhan, Microplastics: Detection in human samples, cell line studies, and health impacts, Environ. Toxicol. Pharmacol., 2023, 101, 104204 CrossRef.
- C. Zhang, J. W. Chung and R. D. Priestley, Dialysis Nanoprecipitation of Polystyrene Nanoparticles, Macromol. Rapid Commun., 2012, 33, 1798–1803 CrossRef CAS.
- B. Komárková, M. Motlochová, V. Slovák, P. Ecorchard, P. Bezdička, D. Bavol and J. Šubrt, Effect of amines on (peroxo)titanates: characterization and thermal decomposition, J. Therm. Anal. Calorim., 2022, 147, 5009–5022 CrossRef.
- G. K. Thirunavukkarasu, O. Monfort, M. Motola, M. Motlochová, M. Gregor, T. Roch, M. Čaplovicová, A. Y. Lavrikova, K. Hensel, V. Brezová, M. Jerigová, J. Šubrt and G. Plesch, Ce ion surface-modified TiO 2 aerogel powders: a comprehensive study of their excellent photocatalytic efficiency in organic pollutant removal, New J. Chem., 2021, 45, 4174–4184 RSC.
- H.-M. Feng, J.-C. Zheng, N.-Y. Lei, L. Yu, K. H.-K. Kong, H.-Q. Yu, T.-C. Lau and M. H. W. Lam, Photoassisted Fenton Degradation of Polystyrene, Environ. Sci. Technol., 2011, 45, 744–750 CrossRef CAS PubMed.
- W.-K. Wang, J.-J. Chen, X. Zhang, Y.-X. Huang, W.-W. Li and H.-Q. Yu, Self-induced synthesis of phase-junction TiO2 with a tailored rutile to anatase ratio below phase transition temperature, Sci. Rep., 2016, 6, 20491 CrossRef CAS PubMed.
- M. Buchalska, M. Kobielusz, A. Matuszek, M. Pacia, S. Wojtyła and W. Macyk, On Oxygen Activation at Rutile- and Anatase-TiO2, ACS Catal., 2015, 5, 7424–7431 CrossRef CAS.
- I. Nabi, A.-U.-R. Bacha, K. Li, H. Cheng, T. Wang, Y. Liu, S. Ajmal, Y. Yang, Y. Feng and L. Zhang, Complete Photocatalytic Mineralization of Microplastic on TiO2 Nanoparticle Film, iScience, 2020, 23, 101326 CrossRef CAS PubMed.
- G. K. Thirunavukkarasu, S. Gowrisankaran, M. Caplovicova, L. Satrapinskyy, M. Gregor, A. Lavrikova, J. Gregus, R. Halko, G. Plesch, M. Motola and O. Monfort, Contribution of photocatalytic and Fenton-based processes in nanotwin structured anodic TiO2 nanotube layers modified by Ce and V, Dalton Trans., 2022, 51, 10763–10772 RSC.
- I. Konstantinou, Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: intermediates and degradation pathways, Appl. Catal., B, 2003, 42, 319–335 CrossRef CAS.
- E. Yousif and R. Haddad, Photodegradation and photostabilization of polymers, especially polystyrene: review, Springerplus, 2013, 2, 398 CrossRef PubMed.
- T. J. Kemp and R. A. McIntyre, Influence of transition metal-doped titanium(IV) dioxide on the photodegradation of polystyrene, Polym. Degrad. Stab., 2006, 91, 3010–3019 CrossRef CAS.
- S. Goldstein, D. Aschengrau, Y. Diamant and J. Rabani, Photolysis of Aqueous H2O2 : Quantum Yield and Applications for Polychromatic UV Actinometry in Photoreactors, Environ. Sci. Technol., 2007, 41, 7486–7490 CrossRef CAS PubMed.
- A. L. Andrady, J. E. Pegram and Y. Tropsha, Changes in carbonyl index and average molecular weight on embrittlement of enhanced-photodegradable polyethylenes, J. Environ. Polym. Degrad., 1993, 1, 171–179 CrossRef CAS.
- M. Ghanadi and L. P. Padhye, Revealing the long-term impact of photodegradation and fragmentation on HDPE in the marine environment: Origins of microplastics and dissolved organics, J. Hazard. Mater., 2024, 465, 133509 CrossRef CAS.
- C. Srivastava, B. Alcock, A. Strandlie and S. A. Grammatikos, Comparison of the effect of thermal and hygrothermal sub-Tg aging on the durability and appearance of multilayered filament wound composite structure, Polym. Test., 2024, 131, 108326 CrossRef CAS.
- M. Palkovská, V. Slovák, J. Šubrt, J. Boháček and J. Havlín, Thermal decomposition of a peroxopolytitanic acid cryogel: TA/MS study, Thermochim. Acta, 2017, 647, 1–7 CrossRef.
- B. Komárková, P. Ecorchard, M. Motlochová, V. Slovák, X. Vislocká, D. Smržová, M. Kormunda, P. Bezdička, K. Lušpai, M. Šimunková, D. Dvoranová, M. Šrámová Slušná, L. Belháčová and J. Šubrt, Effect of amines on the peroxo-titanates and photoactivity of annealed TiO2, Arabian J. Chem., 2022, 15, 103808 CrossRef.
- E. Dümichen, P. Eisentraut, C. G. Bannick, A.-K. Barthel, R. Senz and U. Braun, Fast identification of microplastics in complex environmental samples by a thermal degradation method, Chemosphere, 2017, 174, 572–584 CrossRef.
- J. Wang, J. Lee, E. E. Kwon and S. Jeong, Quantitative analysis of polystyrene microplastic and styrene monomer released from plastic food containers, Heliyon, 2023, 9, e15787 CrossRef CAS.
- J. Rieger, The glass transition temperature of polystyrene: Results of a round robin test, J. Therm. Anal., 1996, 46, 965–972 CrossRef CAS.
- H. Xu and L. B. Casabianca, Probing driving forces for binding between nanoparticles and amino acids by saturation-transfer difference NMR, Sci. Rep., 2020, 10, 12351 CrossRef CAS PubMed.
- A. Homola and R. O. James, Preparation and characterization of amphoteric polystyrene latices, J. Colloid Interface Sci., 1977, 59, 123–134 CrossRef CAS.
- F. M. Onaga Medina, M. B. Aguiar, M. E. Parolo and M. J. Avena, Insights of competitive adsorption on activated carbon of binary caffeine and diclofenac solutions, J. Environ. Manage., 2021, 278, 111523 CrossRef CAS.
- B. Babić, J. Gulicovski, Z. Dohčević-Mitrović, D. Bučevac, M. Prekajski, J. Zagorac and B. Matović, Synthesis and characterization of Fe3+ doped titanium dioxide nanopowders, Ceram. Int., 2012, 38, 635–640 CrossRef.
- M. Francoeur, A. Ferino-Pérez, C. Yacou, C. Jean-Marius, E. Emmanuel, Y. Chérémond, U. Jauregui-Haza and S. Gaspard, Activated carbon synthetized from Sargassum (sp) for adsorption of caffeine: Understanding the adsorption mechanism using molecular modeling, J. Environ. Chem. Eng., 2021, 9, 104795 CrossRef CAS.
- M. Ptaszkowska-Koniarz, J. Goscianska and R. Pietrzak, Synthesis of carbon xerogels modified with amine groups and copper for efficient adsorption of caffeine, Chem. Eng. J., 2018, 345, 13–21 CrossRef CAS.
- F. Azeez, E. Al-Hetlani, M. Arafa, Y. Abdelmonem, A. A. Nazeer, M. O. Amin and M. Madkour, The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles, Sci. Rep., 2018, 8, 7104 CrossRef.
- A. Ceccarini, A. Corti, F. Erba, F. Modugno, J. La Nasa, S. Bianchi and V. Castelvetro, The Hidden Microplastics: New Insights and Figures from the Thorough Separation and Characterization of Microplastics and of Their Degradation Byproducts in Coastal Sediments, Environ. Sci. Technol., 2018, 52, 5634–5643 CrossRef CAS.
- D. Dvoranová, Z. Barbieriková and V. Brezová, Radical Intermediates in Photoinduced Reactions on TiO2 (An EPR Spin Trapping Study), Molecules, 2014, 19, 17279–17304 CrossRef.
- Z. Barbieriková, D. Dvoranová and V. Brezová, in Materials Science in Photocatalysis, Elsevier, 2021, pp. 125–138 Search PubMed.
- J. R. Harbour, J. Tromp and M. L. Hair, Photogeneration of hydrogen peroxide in aqueous TiO2 dispersions, Can. J. Chem., 1985, 63, 204–208 CrossRef CAS.
- I. Papailias, N. Todorova, T. Giannakopoulou, D. Dvoranová, V. Brezová, D. Dimotikali and C. Trapalis, Selective removal of organic and inorganic air pollutants by adjusting the g-C3N4/TiO2 ratio, Catal. Today, 2021, 361, 37–42 CrossRef CAS.
- C. Wang, R. Sun, R. Huang and H. Wang, Superior fenton-like degradation of tetracycline by iron loaded graphitic carbon derived from microplastics: Synthesis, catalytic performance, and mechanism, Sep. Purif. Technol., 2021, 270, 118773 CrossRef CAS.
- I. Gutiérrez, S. G. Bertolotti, M. A. Biasutti, A. T. Soltermann and N. A. García, Quinones and hydroxyquinones as generators and quenchers of singlet molecular oxygen, Can. J. Chem., 1997, 75, 423–428 CrossRef.
- X. Zheng, J. Yuan, J. Shen, J. Liang, J. Che, B. Tang, G. He and H. Chen, A carnation-like rGO/Bi2O2CO3/BiOCl composite: efficient photocatalyst for the degradation of ciprofloxacin, J. Mater. Sci.: Mater. Electron., 2019, 30, 5986–5994 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00818a |
| This journal is © The Royal Society of Chemistry 2025 |