Synergistic effects of exogenous melatonin and zinc oxide nanoparticles in alleviating cobalt stress in Brassica napus: insights from stress-related markers and antioxidant machinery†
Skhawat
Ali
a,
Basharat
Ali
b,
Imtiaz Ahmad
Sajid
c,
Shoaib
Ahmad
d,
Muhammad Arslan
Yousaf
a,
Zaid
Ulhassan
 a,
Kangni
Zhang
a,
Shafaqat
Ali
ef,
Weijun
Zhou
a,
Kangni
Zhang
a,
Shafaqat
Ali
ef,
Weijun
Zhou
 *a and
Bizeng
Mao
*a and
Bizeng
Mao
 *c
*c
aInstitute of Crop Science, Ministry of Agriculture and Rural Affairs Key Laboratory of Spectroscopy Sensing, Zhejiang University, Hangzhou 310058, China. E-mail: wjzhou@zju.edu.cn
bDepartment of Agricultural Engineering, Khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan 64200, Pakistan
cInstitute of Biotechnology, Ministry of Agriculture Key Lab of Molecular Biology of Crop Pathogens and Insects, Key Lab of Biology of Crop Pathogens and Insects of Zhejiang Province, Zhejiang University, Hangzhou 310058, China. E-mail: maobz@zju.edu.cn
dState Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, Jiangsu 210023, China
eDepartment of Environmental Sciences, Government College University, 38000, Faisalabad, Pakistan
fDepartment of Biological Sciences and Technology, China Medical University, Taichung 40402, Taiwan
First published on 8th November 2024
Abstract
Cobalt (Co) toxicity severely hinders plant growth, highlighting the imperative to devise strategies that promote plant growth and inhibit Co accumulation in plants. Melatonin (MT) and zinc oxide nanoparticles (ZnO NPs) have emerged as key contributors to promoting sustainable crop production and enhancing stress resistance. However, the mechanisms underlying the ameliorative effects of foliar applications of the growth regulators MT and/or ZnO NPs on Co accumulation and its associated phytotoxicity in Brassica napus remain poorly understood. To address this knowledge gap, the present study was carried out to investigate the protective roles of MT and/or ZnO NPs in promoting growth and enhancing tolerance against Co stress in B. napus. Treatment of Co (300 μM) alone significantly reduced the leaf fresh weight (33%), dry weight (49%), full plant height (51%), antioxidant enzyme activity and stomatal conductance. However, the application of MT (50 μM) with ZnO NPs (25 μM) resulted in a substantial decline in the accumulation of Co concentration and contents of MDA (33% in leaves and 26% in roots), H2O2 (41% in leaves and 35% in roots) and O2˙− (26% in leaves and 39% in roots). Additionally, combined application of MT + ZnO NPs markedly enhanced the activities of antioxidant enzymes as well as the levels of thiol compounds such as GSH, NPTs, PCs and Cys. Furthermore, the investigation of genes responsible for encoding the extrinsic proteins of photosystem II showed a marked upregulation in the expression levels of the BnPsbA, BnPsbB, BnPsbC, and BnPsbD genes. Remarkably, the synergistic application of MT and ZnO NPs proved more effective in promoting plant growth than their individual applications. Further, this study investigated the synergistic mechanisms by which MT and ZnO NPs, both individually and in combination, enhance enzymatic activities, improve photosynthetic capacity, and optimize nutrient accumulation in plants. Thus, this research provides insights into the biochemical and physiological processes underlying the ameliorative effects of these compounds on plant stress tolerance.
Environmental significanceThis study explores how MT and ZnO NPs help B. napus plants cope with Co stress. It shows that these substances can reduce Co uptake by plants, which is important for growing safer crops. Notably, the combined application of MT and ZnO NPs significantly enhanced antioxidant enzyme activities and increased levels of thiol compounds, providing better protection against Co stress. The study also found that the application upregulated the expression of genes responsible for photosystem II proteins, indicating improved plant resilience. These findings could lead to new ways of cultivating plants in regions contaminated with metal pollutants. This work provides compelling evidence for using nanoparticles to protect plant growth in water-affected areas, contributing to our understanding of sustainable agriculture in challenging environments. |
1. Introduction
Rapeseed (Brassica napus L.), commonly known as canola, is a globally significant oilseed crop cultivated for its economic and agricultural importance in achieving high yields. Over the span of twenty years, rapeseed has emerged as China's fifth most significant plantation crop, covering a vast planting area of 7.6 billion hectares.1B. napus is valued globally for its nutritional content and economic significance. Its rapid growth, extensive root network, adaptability to various soil types, and remarkable ability to accumulate toxic metal ions make it a suitable candidate for phytoremediation. These heavy metals, being non-biodegradable, accumulate in the edible parts of plants and facilitate their incorporation into the human body via the food chain, thereby causing serious health concerns and leading to many severe illnesses.2,3 According to the report of the World Health Organization (WHO), the toxic and dangerous heavy metals are mercury, nickel and cobalt.4 Natural sources of cobalt (Co) in the environment include volcanic eruptions, seawater and forest fires, while anthropogenic sources include metal smelting, combustion of fossil fuel, cobalt alloy processing and sewage.5 In plants, Co toxicity affects chlorophyll content, plant growth, and biomass by interfering with anti-oxidative enzyme activities. Exposure to Co stress induces oxidative damage in plants, which is characterized by lipid peroxidation, excessive generation of O2˙− and H2O2, as well as increased levels of MDA, a byproduct of lipid peroxidation processes. Consequently, this oxidative stress leads to cell death via DNA degradation.6,7The application of nanotechnology in agriculture, known as nano-enabled agriculture, represents a novel approach to enhancing crop productivity while simultaneously managing the discharge of agricultural chemicals into the environment.8 The use of eco-friendly and economical methods to remediate heavy metals contaminated soils is gaining popularity these days.9 One notable area garnering substantial attention is the use of nanotechnology to remediate agricultural soils contaminated by heavy metals, thereby aiming to remove these harmful contaminants.10,11 Engineered nanoparticles, particularly zinc oxide nanoparticles (ZnO NPs), have exhibited the potential to boost the cultivation of nutrient-rich plants like green vegetables and crops by mitigating heavy metal uptake, as verified by numerous studies delving into their positive impacts on crop science and agricultural production.12–14 Zinc (Zn) is an important element that is involved in the optimum growth and development of plants.15 Hence, ZnO NPs are an essential constituent of agricultural tolerance to abiotic stresses, providing plant protection from stress.16,17 ZnO NPs have been demonstrated to reduce heavy metal uptake, including cadmium, in wheat plants.18 The addition of nanoparticles could improve nutrient insufficient and boost yield production.19
Melatonin (MT), is a versatile signaling molecule found in both animals and plants. It exhibits a wide range of effects and acts as a regulator in various physiological processes. In plants, MT has been identified as an endogenous growth regulator that plays a crucial role in modulating different physiological functions and enhancing plant tolerance to various abiotic stress factors.20,21 MT treated plants maintained higher photosynthetic rate, stomatal conductance, intercellular CO2 concentration, and transpirational rate. Additionally, photosystem II (PSII)-related gene expression (psbA, psbB, psbC and psbD) was enhanced under abiotic stress.22 MT is recognized as a major scavenger of ROS in various plant species when subjected to different types of abiotic stress conditions.23,24 Previous studies have reported that the exogenous application of MT markedly improved antioxidative enzyme activities as well as the accumulation of non-enzymatic antioxidants upon biotic and abiotic stress conditions.23,25,26 With beneficial effects intact, however, the protective mechanisms of MT and/or ZnO NPs in alleviating Co toxicity in plants have been scarcely reported, so further comprehensive investigations are needed. Considering the earlier research findings, this experimental investigation aimed to evaluate the advantageous effects of MT and/or ZnO NPs in alleviating Co-induced stress in B. napus plants. Notably, this research investigated the mechanism through which MT and ZnO NPs can work together synergistically to enhance plant enzymatic activities and photosynthetic capacity.
2. Materials and methods
2.1 Characterization of zinc oxide nanoparticles (ZnO NPs)
In this study, ZnO NPs of 99% purity were procured from Cw-nano (https://www.cwnano.com). The ZnO NPs were stored under airtight conditions at 25 °C and used for the experiment. Morphology, phase purity, surface size and compositional analysis of ZnO NPs were examined by scanning electron microscopy (SEM) (TM-1000, Hitachi, Japan) and transmission electron microscopy (TEM) (JEM-1230, JEOL, Akishima, Japan) techniques, following the method described.27 Moreover, the chemical composition of ZnO NPs was determined using a field emission scanning electron microscope (FESEM) equipped with energy-dispersive X-ray spectroscopy (EDX). The instrumental technique of Fourier-transform infrared (FTIR) spectroscopy was conducted using a Bruker instrument (Germany) to identify the functional groups in the synthesized ZnO NPs.28 The crystal planes and crystal structure of ZnO NPs were measured with X-ray diffraction (XRD) (Bruker, Germany).292.2 Plant materials and growth condition
Mature and healthy seeds of B. napus (cv. ZS 758) were collected from the College of Agriculture and Biotechnology at Zhejiang University, and cobalt and melatonin were obtained from Sigma-Aldrich Co (St. Louis, MO, USA). The B. napus seeds were incubated in darkness at 25 °C for a period of three days and then transferred to 25% Hoagland nutrient solutions for further growth. After a duration of two weeks, the uniformly developing B. napus seedlings were transferred to 50% Hoagland nutrient solutions. Plants were cultivated in the greenhouse at 65% humidity, 24/16 °C as day/night temperature, a photoperiod of 16 h and a light intensity of 400 μmol m−2 s−1 for 35 days. The Hoagland solution was renewed twice a week. Each treatment was replicated three times, with four uniformly sized seedlings in each pot. The nutrient solution was consistently aerated by the aeration pump and altered every third day to prevent depletion. MT and/or ZnO NPs were set at 50 μM and 25 μM, respectively, the best concentrations were selected after the preliminary experiment. In this study, Co-treatment levels of 0 and 300 μM were chosen on the basis of a previous study.30,31B. napus at four true leaf stages (20-day-old plants) were treated with different concentrations of Co (300 μM) via Hoagland solutions, MT (50 μM) and ZnO NPs (25 μM) by foliar spraying in a half-strength Hoagland medium for the next 15 days. The experimental conditions include the following treatments: (T0) control (only nutrient solution); (T1) 50 μM MT; (T2) 25 μM ZnO NPs; (T3) 50 μM MT + 25 μM ZnO NPs; (T4) 300 μM Co (alone); (T5) 50 μM MT + 300 μM Co; (T6) 25 μM ZnO NPs + 300 μM Co; and (T7) 50 μM MT + 25 μM ZnO NPs + 300 μM Co. The details of the treatments are explained in the ESI† (Table S1†).2.3 Morphological attributes and root architecture
The healthy plants were harvested following 15 days of the treatment. Before harvesting, morphological traits were measured using scales and weighed and balanced. The detailed procedure can be found in Text S1.†2.4 Chlorophyll content and gas exchange parameters
The chlorophyll content from the sample of leaves was extracted after 15 days of treatments. The healthy plant leaves were used for this determination; fresh leaves were dipped into a solution of acetone (85% v/v) without direct light and at 4 °C until the chlorophyll was completely extracted. A spectrophotometer (UV-2600) was used for analyzing the chlorophyll contents as described.32 The gas exchange parameters were assessed using an infrared gas analyzer (IRGA) with a portable photosynthetic system (Li-Cor, 6400XT, Lincoln, NE, USA). The measurements of transpiration rate (E), stomatal conductance (gs), internal CO2 concentration (Ci), and net photosynthetic rate (Pn) were conducted following the procedure described previously.332.5 Quantifications of lipid peroxidations, reactive oxygen species (ROS) and histochemical analysis
Reactive oxygen species (ROS), hydrogen peroxide (H2O2), superoxide anion (O2˙−), and malondialdehyde (MDA) were measured in the leaves and roots and histochemical analysis in roots of B. napus using standard methodologies. For detailed procedures, refer to Text S2.†2.6 Determination of metal concentration and endogenous MT
The leaves and roots of each dry plant sample were added to a glass flask after being cut into small pieces using a grinder. The plant dry samples digestion process was conducted using the di-acid method.34 The detailed procedure of the metal content and MT can be found in the Text S3.†2.7 Estimation of the nutrient content
For the estimation of the nutrient content, plant samples from each treatment were prepared as described previously, and their concentrations were measured using inductively coupled plasma optical emission spectroscopy (ICP-OES).352.8 Estimation of antioxidant enzyme activities
Plants leaf and root samples (0.5 g) were crushed in a pre-cooled mortar and pestle in 50 mM phosphate buffer solution at pH 7.8 and ground samples were centrifuged for 20 minutes at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 revolutions per minute (rpm). After centrifugation, the resulting supernatant was transferred to a separate tube and designated as the “enzyme extract” for subsequent examination of the enzyme role. Superoxide dismutase (SOD) was analyzed using the technique outlined.36 The reaction solutions consisted of 50 mM phosphate buffer with pH 7.8, 2 μM riboflavin, 13 mM methionine, 75 μM NBT, 0.1 mM EDTA, and 100 μL of the enzyme extract, making a total volume of 3 mL. One unit of superoxide dismutase activity was defined as the amount of enzyme required to inhibit 50% of NBT reduction, determined at a wavelength of 560 nm. Catalase (CAT) activity was measured using the method described earlier.37
000 revolutions per minute (rpm). After centrifugation, the resulting supernatant was transferred to a separate tube and designated as the “enzyme extract” for subsequent examination of the enzyme role. Superoxide dismutase (SOD) was analyzed using the technique outlined.36 The reaction solutions consisted of 50 mM phosphate buffer with pH 7.8, 2 μM riboflavin, 13 mM methionine, 75 μM NBT, 0.1 mM EDTA, and 100 μL of the enzyme extract, making a total volume of 3 mL. One unit of superoxide dismutase activity was defined as the amount of enzyme required to inhibit 50% of NBT reduction, determined at a wavelength of 560 nm. Catalase (CAT) activity was measured using the method described earlier.37
2.9 Quantitative RT-PCR analysis
Fresh leaf and root samples (0.3 g) were used for total RNA extraction using the TRIzol reagent (manual) (Sangon, China). For cDNA synthesis, the Hi-script® II qRT Super Mix for qPCR with gDNA wiper kit from Vazyme Biotech Co., Ltd. was utilized. For the study of gene expression, primers were designed and are listed in Table S2.† The quantification of cDNA samples was performed using qRT-PCR on the LightCycler® 96 System from Roche, Switzerland, with the AceQ® qPCR SYBR Green Master Mix from Vazyme Biotech Co., Ltd. The quantification approach outlined by Livak and Schmittgen38 was employed.2.10 Analysis of thiol compounds
The quantification of non-protein thiol (NPT) levels, as well as the levels of the reduced glutathione (GSH) and oxidized glutathione (GSSG) were carried out.39 Phytochelatins (PCs) were calculated as the difference between the total non-protein thiol (NPT) content and the sum of GSH + GSSG levels, as follows: PCs = NPT − (GSH + GSSG). The preparation of plant samples of B. napus cultivar leaves and root samples were homogenized in 2 mL of 5% sulfosalicylic acid solution. After centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 15 min at 4 °C, 0.2 mL of supernatant was added to the reaction mixture. The reaction mixture contained 0.2 M Tris-HCl buffer at pH 2.8, and 0.15 mL of 19 mM 5,5′-dithiobis-2-nitrobenzoic acid solution. This mixture was then incubated for 20 minutes. After incubation, the light absorption of the mixture was determined using a spectrophotometer at a wavelength of 412 nm.
000 × g for 15 min at 4 °C, 0.2 mL of supernatant was added to the reaction mixture. The reaction mixture contained 0.2 M Tris-HCl buffer at pH 2.8, and 0.15 mL of 19 mM 5,5′-dithiobis-2-nitrobenzoic acid solution. This mixture was then incubated for 20 minutes. After incubation, the light absorption of the mixture was determined using a spectrophotometer at a wavelength of 412 nm.
2.11 Topographical imaging of plant using SEM and TEM
2.12 Statistical analysis
In all the experiments, three samples of the biological replicates were tested. The sample data were subjected to a one-way analysis of variance The statistical test was performed with Statistix software (version 8.0). The final data values were recorded as the mean value ± standard error. Statistical significance was assessed using Fisher's LSD test; lowercase letters differentiate significant differences at a p-value of <0.05. Graphical presentation was performed using Prism software (version 8.0).403. Results
3.1 Characterization of ZnO NPs
TEM results showed the spherical morphology of ZnO NPs with an average diameter of 33 nm (Fig. 1B). The particle size distribution of ZnO NPs was explored by scanning electron microscopy (SEM). SEM micrographs exhibited minimal agglomeration, with the ZnO NPs exhibiting a uniform spatial organization (Fig. 1A). Furthermore, the chemical composition of the ZnO NPs was investigated using scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDX) analysis (Fig. 1C). The EDX spectroscopy microanalysis displayed chemical composition of ZnO NPs samples containing strong abundance of zinc (Zn) and oxygen (O) in the targeted sample. EDX mapping further confirmed that Zn and O were the major components in the sample with strong signals (Fig. 1D). These observations verified the purity of the synthesized ZnO NPs. The XRD pattern displayed clear and distinct diffraction peaks at 2θ values corresponding to the 100, 002, 101, 102, 110, 103, 200, 112, and 201 planes (Fig. 1E). The peaks were attributed to the hexagonal phase of ZnO. Diffraction maxima were observed at 2θ values of 31.77° corresponding to the (100) plane, 34.42° for (002), 36.25° for (101), 47.54° for (102), 56.60° for (110), 62.86° for (103), 66.38° for (112), 67.96° for (201) and 77.43 for (202) (Fig. 1E). The positions of these diffraction peaks confirm the hexagonal crystal structure of the ZnO nanoparticles. FTIR spectra analysis of the sample revealed the presence of multiple absorption bands (Fig. 1F). Prominent peaks were observed at the following wavelengths: 3423, 1527, 1378, 1115, and 332 cm−1.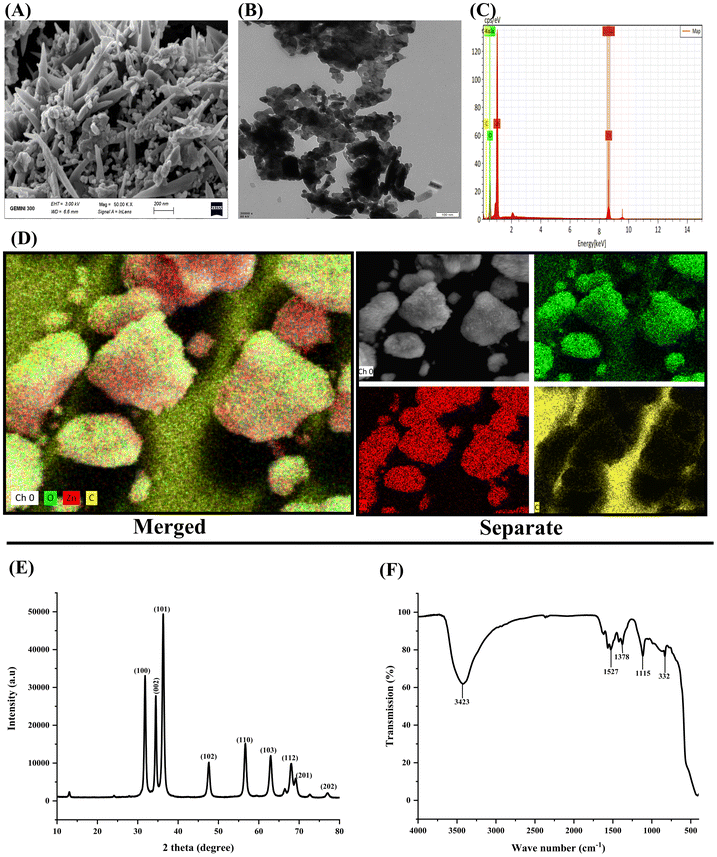 | ||
| Fig. 1 Characterizations of zinc oxide NPs: (A) SEM (scale bar = 200 nm), (B) TEM (scale bar = 200 nm), (C) EDX spectra, and (D) elemental compositions. (E) XRD and (F) FTIR of ZnO NPs. | ||
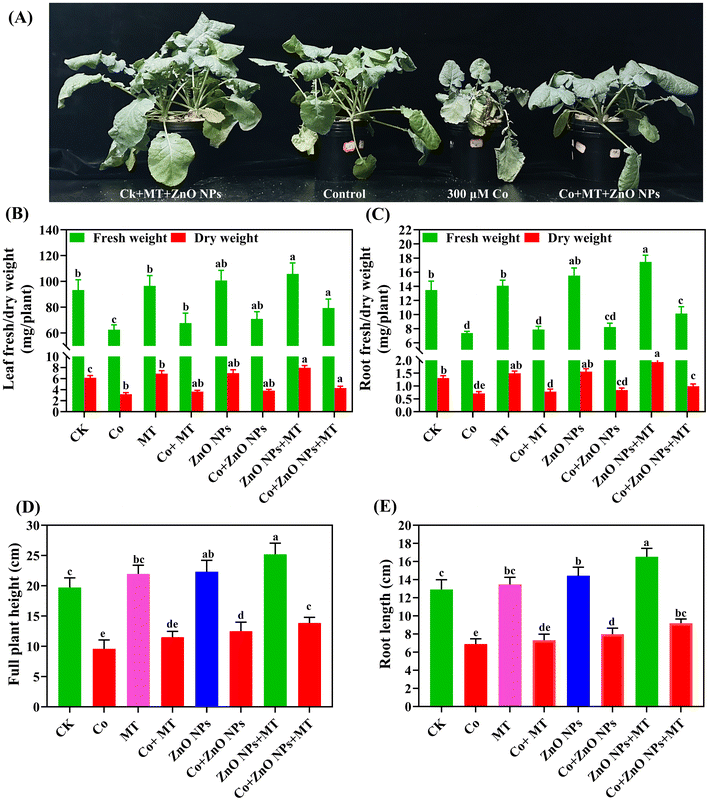
3.2 Exogenous MT and/or ZnO NPs enhanced the plant growth and photosynthetic attributes under Co stress
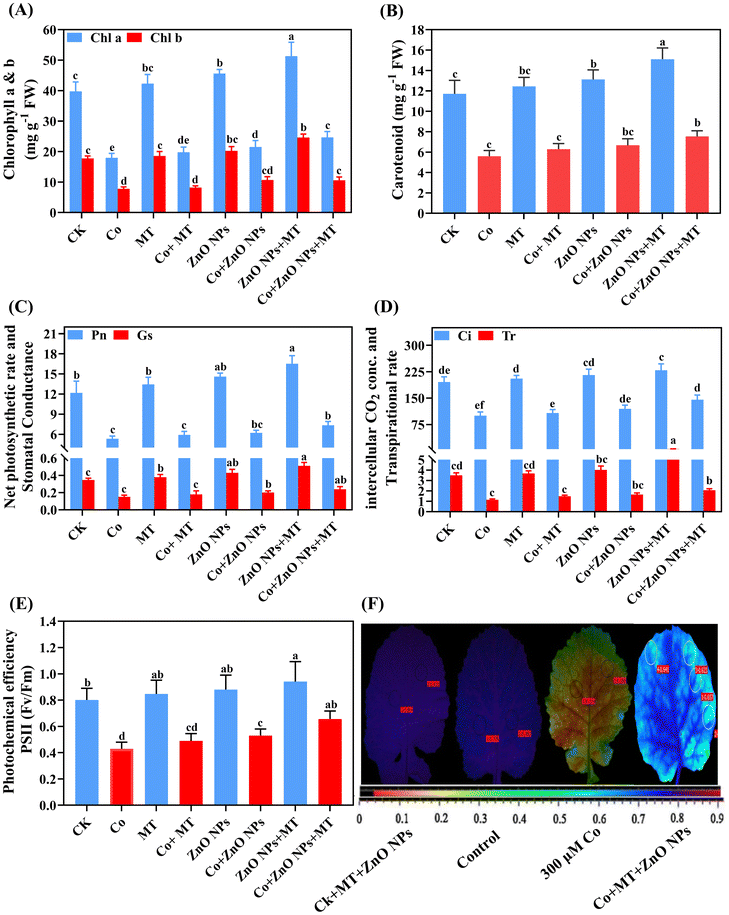
Relative to the corresponding control treatments, the concomitant application of MT and/or ZnO NPs enhanced various physiological parameters, including leaf fresh weight (LFW), leaf dry weight (LDW), root fresh weight (RFW), root dry weight (RDW), root length, overall plant height (Fig. 2), as well as the levels of chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid pigments (Fig. 3A and B). In contrast, Co supplementation alone significantly decreased the following parameters including LFW by 33%, LDW by 35%, RFW by 45%, RDW by 46%, root length (47%), overall plant height (51%), Chl a content (55%), Chl b levels (56%), and carotenoids (52%) in comparison to the respective control groups. The concurrent application of MT and/or ZnO NPs notably ameliorated the Co-induced reductions in the aforementioned parameters, as evidenced by enhancements in LFW (27%), LDW (35%), RFW (38%), RDW (39%), root length (33%), overall plant height (44%), Chl a content (38%), Chl b levels (36%), and carotenoids (35%) compared to the individual applications of MT and/or ZnO NPs. These findings demonstrated that the combined supplementation with MT and/or ZnO NPs was more efficacious than their individual applications in alleviating the deleterious impacts of Co on the growth attributes of B. napus. The individual application of MT and/or ZnO NPs slightly enhanced the gas exchange parameters, including the net photosynthetic rate (Pn), stomatal conductance (gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr), as well as the photosynthetic efficiency of photosystem II (PSII), compared to the control groups (Fig. 3C–F). In contrast, the individual application of Co significantly reduced Pn (56%), gs (57%), Ci (49%), Tr (67%), and photosynthetic efficiency of PSII (46%) when compared to their respective controls. The combined application of MT and/or ZnO NPs effectively improved the photosynthetic traits, including Pn (38%), gs (60%), Ci (45%), Tr (80%), and photosynthetic efficiency of PSII (53%), compared to the treatments with MT or ZnO NPs alone (Fig. 3C–F).3.3 Effects of MT and/or ZnO NPs on root architecture
Exposure to Co elicited significant deleterious effects on root architecture (Fig. S1†). Co-treatment resulted in reductions in root network area (29% vs. CK), number of root tips (46% vs. CK), the average diameter of roots (49% vs. CK) and surface area of roots (31% vs. CK) compared to the untreated control. However, foliar application of MT and/or ZnO NPs proved beneficial in ameliorating the adverse impacts on root morphology. The MT and/or ZnO NPs treatment exhibited an increase in root network area (12% vs. Co), the number of root tips (21% vs. Co) and the surface area of roots (25% vs. Co). Markedly, MT and/or ZnO NPs treatments promoted an increase in the average diameter of roots (48% vs. Co) compared to Co alone (Fig. S1†). Collectively, these results confirmed that MT and/or ZnO NPs application not only mitigated Co-induced stress but also conferred improvements in overall root system architecture.3.4 Exogenous MT and/or ZnO NPs enhance the endogenous melatonin and zinc levels and reduce the Co accumulation
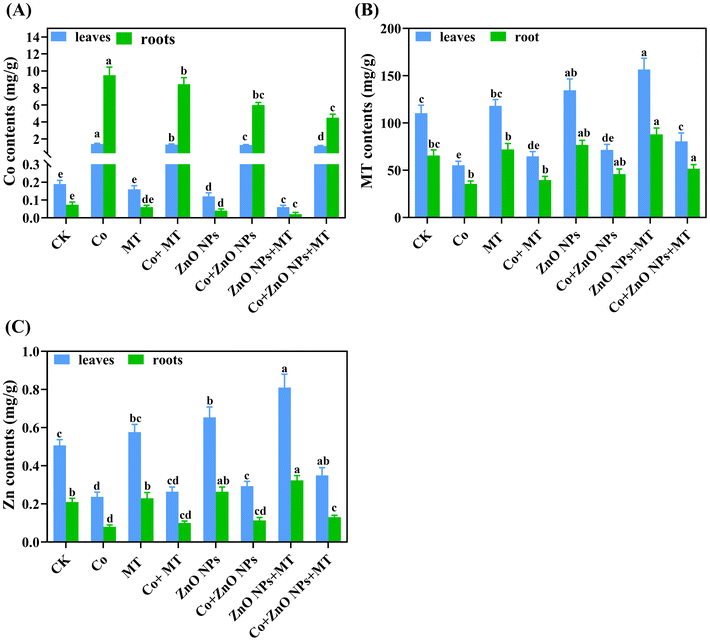
The exogenous application of MT and/or ZnO NPs slightly increased the levels of MT and Zn in both leaves and roots, suggesting their involvement in plant defense mechanisms (Fig. 4B and C). The supplementation of Co alone notably reduced the endogenous MT (50% in leaves and 46% in roots) and Zn (53% in leaves and 62% in roots) levels compared to their respective control treatments. However, the combined supply of MT and/or ZnO NPs more greatly improved the endogenous MT (46% in leaves and 45% in roots) and Zn (48% in leaves and 63% in roots) levels than their respective individual treatments under Co stress. In contrast, the Co treatments alone drastically enhanced the endogenous Co contents to 441.87 μg g−1 in leaves and 1452.29 μg g−1 in roots (Fig. 4A). These findings showed higher concentrations of Co in the roots compared to the leaves, consequently, more phytotoxic effects were observed in roots. When compared with Co-alone treatments, the co-supplementation of MT and/or ZnO NPs significantly minimized the Co accumulation in leaves by 45% and in roots by 40%, respectively (Fig. 4).3.5 Exogenous MT and/or ZnO NPs improve the macro–micro mineral nutrients in plant tissues under Co stress
Cobalt application significantly reduced the accumulation of Fe (47% in leaves and 51% in roots), Mn (56% in leaves and 45% in roots), P (55% in leaves and 56% in roots), K (51% in leaves and 43% in roots), Ca (52% in leaves and 55% in roots), and Mg (54% in leaves and 62% in roots) compared to the respective control treatments (Fig. S2†). Relative to the untreated control treatments, the exogenous application of MT and/or ZnO NPs marginally increased the contents of these mineral nutrients in both leaves and roots. Interestingly, the co-supply of MT and/or ZnO NPs effectively increased the accumulation of Fe (35% in leaves and 39% in roots), Mn (62% in leaves and 39% in roots), P (40% in leaves and 44% in roots), K (33% in leaves and 23% in roots), Ca (51% in leaves and 78% in roots), and Mg (27% in leaves and 67% in roots), when compared to Co applications alone (Fig. S2†). While the individual supply of MT and/or ZnO NPs less significantly improved the accumulation of these mineral nutrients in leaves and roots under Co stress conditions, these observations confirmed that the co-application of MT and/or ZnO NPs more efficaciously maintained the nutrient balance than MT and/or ZnO NPs alone by limiting Co accumulation in the leaves and roots of B. napus.3.6 Exogenous MT and/or ZnO NPs mitigate the oxidative damages caused by Co stress
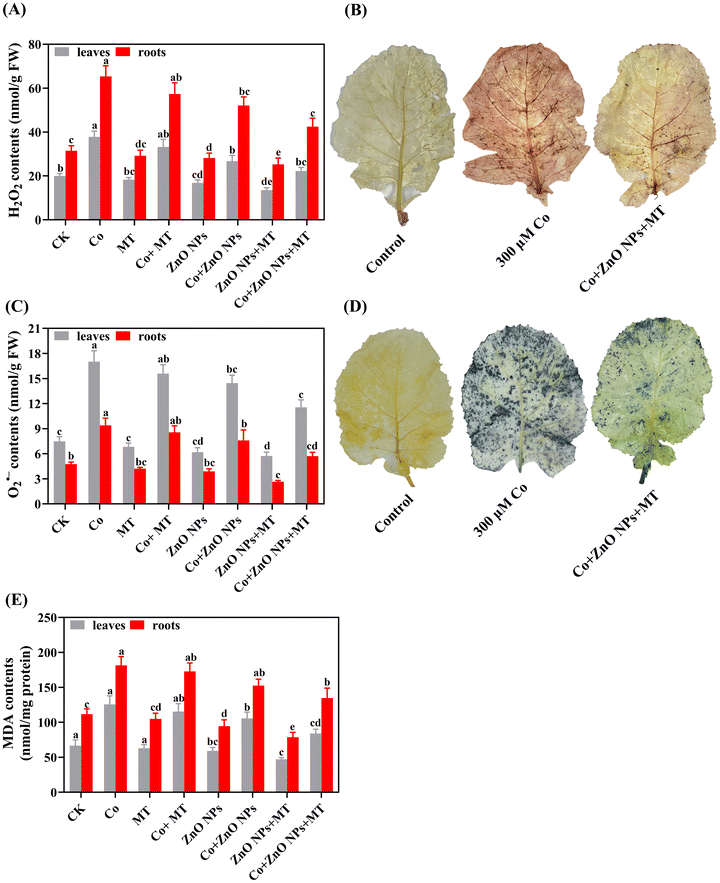
Under Co stress alone, the levels of MDA (88% in leaves and 62% in roots), H2O2 (90% in the leaves and 108% in roots), and O2˙− (127% in leaves and 97% in roots) were dramatically increased when compared to the untreated control treatments (Fig. 5A–C). Higher levels of MDA, H2O2, and O2˙− were detected in the roots compared to the leaves, indicating that Co led to elevated levels of oxidative stress in the roots. Under Co stress alone, the individual treatments of MT and/or ZnO NPs notably mitigated the upsurge in MDA, H2O2, and O2˙− levels both in leaves and roots. Markedly, the combined supplementation of MT and/or ZnO NPs substantially restricted the excessive accumulation of MDA (33% in leaves and 26% in roots), H2O2 (41% in leaves and 35% in roots), and O2˙− (26% in leaves and 39% in roots) under Co stress (Fig. 5A–C). The Co-induced oxidative damages via H2O2 and O2˙− and their alleviation effects by MT and/or ZnO NPs were further corroborated by staining the leaf with DAB and NBT, respectively. The DAB staining revealed a dark brown color in leaf tissues that were exposed to high levels of Co, indicating the presence of H2O2. This contrasted with the control treatments. Similarly, NBT staining displayed a dark blue color, indicative of O2˙−, in leaf tissues exposed to excessive Co levels compared to that with the control treatments (Fig. 5B and D). In response, the exposure to MT and/or ZnO NPs, especially their combined applications, significantly minimized the intensity of the dark brown and dark blue colors, indicating a reduction in oxidative damages induced by H2O2 and O2˙, respectively (Fig. 5B and D). These findings confirmed that the co-supply of MT and/or ZnO NPs appreciably minimized the Co-induced excessive production of oxidative stress in the leaves of B. napus (cv. ZS 758).3.7 Exogenous MT and/or ZnO NPs activated the antioxidant defense system and relate their gene expression under Co stress
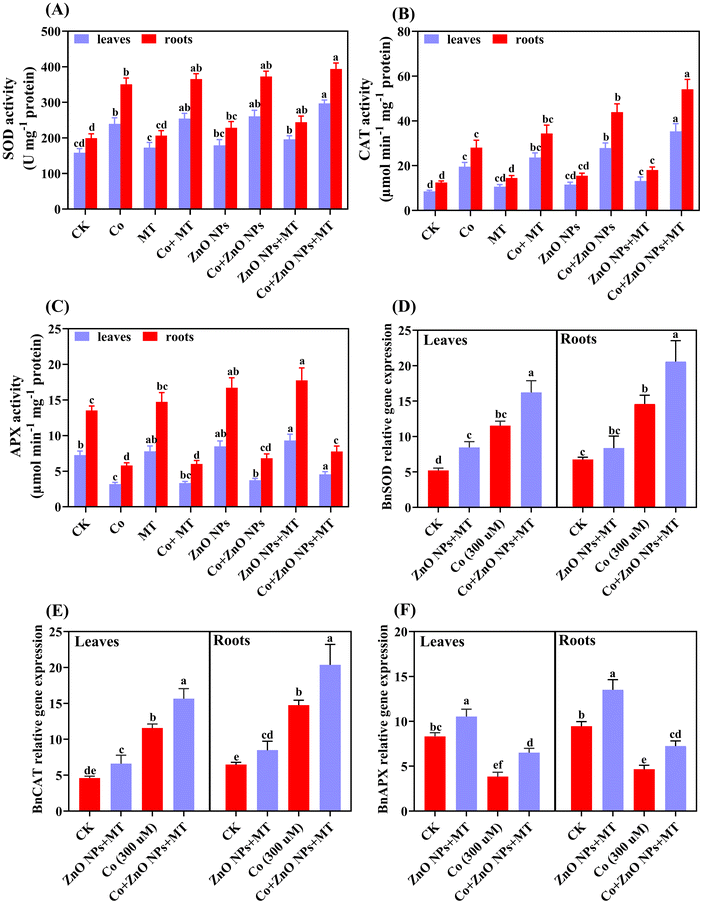
Compared to relative controls, a significant increase in the activities of SOD and CAT enzymes, and decreasing trends in the activities of APX enzymes were observed in leaves and roots under Co stress alone (Fig. 6A–C). This change resulted in a decline of SOD (51% in leaves and 76% in roots), CAT (129% in leaves and 124% in roots), and APX (56% in leaves and 57% in roots). The combined applications of MT and/or ZnO NPs escalated the activities of APX while declining the SOD and CAT activities in both leaves and roots compared to treatments with Co alone. The observations consistently showed that both MT and/or ZnO NPs were able to reverse inhibitory effects on the plant tissues caused by Co. The influence of MT and/or ZnO NPs on the expression of the antioxidant enzymes encoding genes (SOD, CAT, and APX), namely, BnSOD, BnCAT, and BnAPX, were determined in leaves and roots of B. napus under Co stress conditions (Fig. 6D–F). In comparison to untreated control treatments, the application of MT and/or ZnO NPs did not significantly affect the transcript levels of BnSOD, BnCAT, and BnAPX both in leaves and roots. The supply of Co upregulated the transcript levels of BnSOD and BnCAT, while decreasing the levels of BnAPX in both leaves and roots, as compared to those in the untreated control treatments (Fig. 6D–F). The co-applications of MT and/or ZnO NPs further upregulated or downregulated the above-mentioned transcript levels in the leaves and roots of B. napus, respectively.3.8 MT and/or ZnO NPs upregulate genes encoding core PSII proteins and defense-related genes in B. napus under Co stress
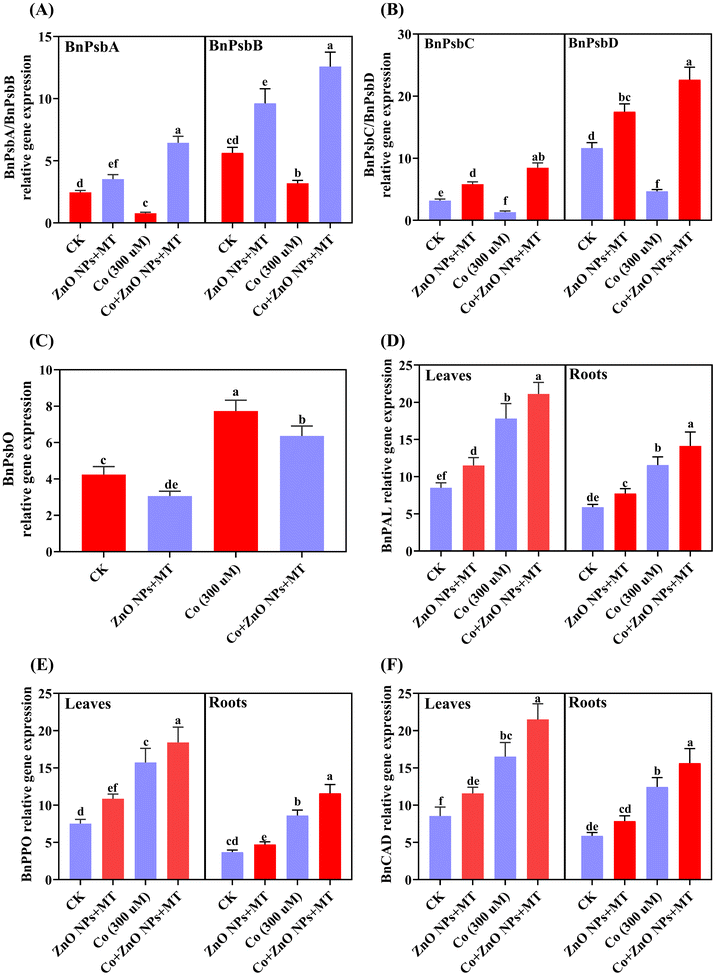
This study examined the effects of foliar application of MT and/or ZnO NPs on the expression levels of photosystem II (PSII) genes in B. napus plants under Co stress (Fig. 7A–C). Under Co stress, the expression levels of genes of the PSII, namely, BnPsbA, BnPsbB, BnPsbC, and BnPsbD, exhibited a significant downregulation compared to those in the control group. Particularly, the BnPsbA gene displayed a 3.19-fold decrease in expression, whilst BnPsbB, BnPsbC and BnPsbD genes showed 1.77-fold, 2.39-fold and 2.50-fold reductions in the expression, respectively (Fig. 7A–C). Nevertheless, the addition of MT + ZnO NPs mitigated these effects and recovered the expression of these genes relative to Co-stressed plants. The expression of the BnPsbO gene displayed an opposite behavior. When compared to the control, Co-stress significantly increased the expression of PsbO. Interestingly, foliar spray of MT and/or ZnO NPs significantly increased this effect, resulting in a 35% increase of BnPsbO expression relative to the control plants (Fig. 7C). These findings indicated that applying MT and/or ZnO NPs can modulate the transcriptional responses of key PSII genes under Co stress conditions, potentially mitigating the detrimental effects of Co on photosynthetic efficiency. Co-treatments alone elicited a marked upregulation in the transcript levels of defense-associated genes in B. napus (Fig. 7D–F). In particular, the expression of the genes BnPAL was found to be 2.09-fold higher in leaves and 1.96-fold higher expression in roots, compared to the control. BnPPO and BnCAD exhibited similar induction patterns, with 2.09/2.33-fold and 1.94/2.12-fold increases in transcript abundance in leaves and roots, respectively. Concurrently, MT and/or ZnO NPs in conjunction with Co further escalated the transcript levels of these genes. The transcript levels were augmented by 1.19/1.22-fold, 1.17/1.34-fold and 1.3/1.26-fold in leaves and roots for BnPAL, BnPPO and BnCAD, respectively, when compared to Co treatments alone (Fig. 7D–F).3.9 Applied MT and/or ZnO NPs promote the sequestration of Co by stimulating the production of chelating compounds and the enzymes responsible for their metabolism
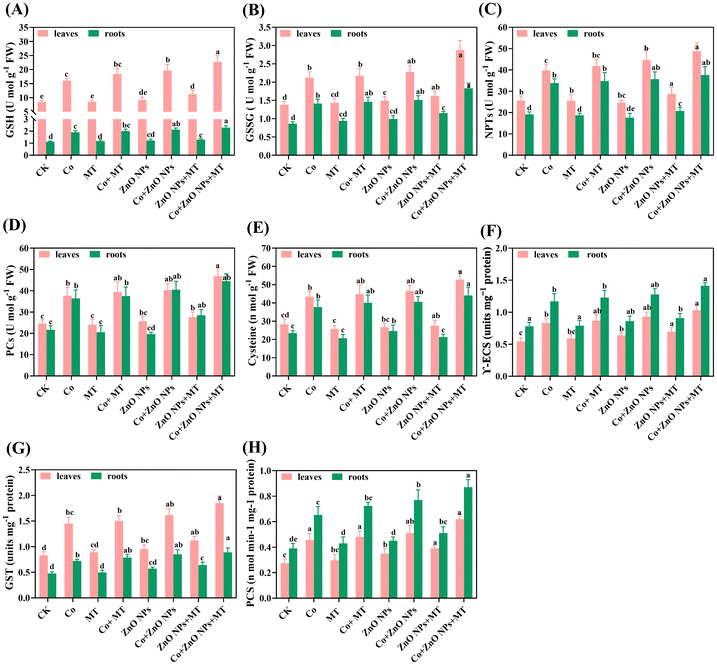
The study evaluated the biosynthesis ability of MT and/or ZnO NPs as chelating agents by examining the levels of various compounds and enzymes involved in thiol metabolism in B. napus grown under Co stress. The Co-treatment resulted in a significant increase in the levels of various compounds, including GSH, GSSG, NPTs, PCs and cysteine, as well as the γ-ECS, GST, and PCS when compared to the control plants (Fig. 8). GSH content exhibited 91% increase in leaves and 71% increase in roots, GSSG levels were elevated by 54% in leaves and 63% in roots (Fig. 8A and B). Additionally, NPTs showed an augmentation by 55% in leaves and 76% in roots and demonstrated increases in PCs (53% in leaves and 68% in roots) and cysteine (54% in leaves and 61% in roots) under Co stress alone (Fig. 8C–E). Furthermore, the enzymes involved in thiol metabolism activities were augmented by γ-ECS (53% in leaves and 50% in roots), and GST (75% in leaves and 68% in roots), while PCs increased by 67% and 57% in leaves and roots, respectively, under cobalt stress (Fig. 8F and G). Notably, the application of MT and/or ZnO NPs further enhanced the activities of these enzymes. Compared to the cobalt treatment alone; the maximum increases observed at the co-treatment concentration of MT and/or ZnO NPs were 23% and 20% for NPTs, 21% and 17% for cysteine, 24% and 21% for γ-ECS, 28% and 24% for GST, and 38% and 33%, for PCS in the leaves and roots, respectively, (Fig. 8A–H). The observed increases in thiol metabolism upon the treatments with MT and/or ZnO NPs, relative to the Co-treatment, indicated that MT and/or ZnO NPs play a pivotal role in Co detoxification in B. napus.3.10 MT and/or ZnO NPs aid in stomatal opening in response to Co stress
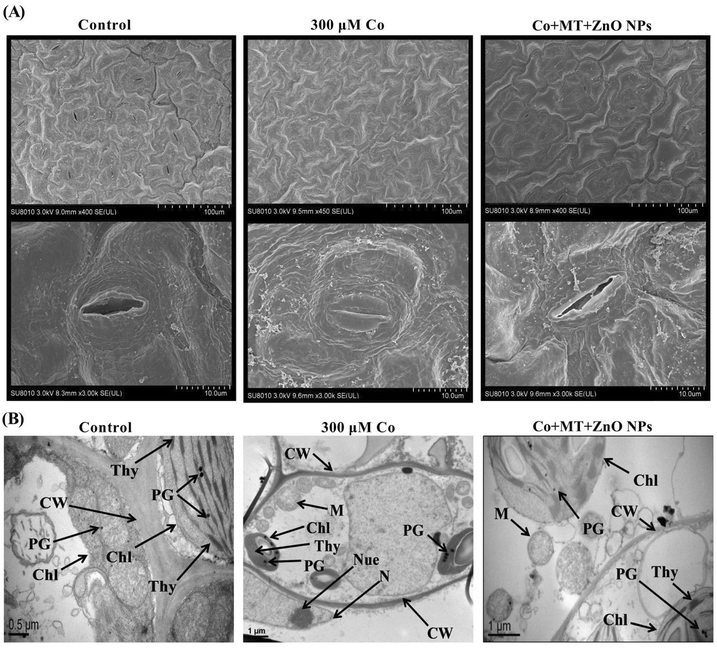
To investigate the impact of MT and/or ZnO NPs on guard cell function and stomatal aperture under Co stress, stomata on the leaf epidermis of B. napus were examined. Under Co stress conditions, substantial damage to the guard cells and stomata of B. napus was observed (Fig. 9A). In contrast, the exogenous application of MT and/or ZnO NPs significantly alleviated the Co-induced damage to the guard cells and stomata. Furthermore, an assessment of the stomatal aperture revealed that Co stress-induced stomatal closure in B. napus leaves. However, leaves treated with a combination of MT and/or ZnO NPs exhibited slightly increased stomatal opening compared to the plants exposed solely to Co stress (Fig. 9B). These findings demonstrated that MT and/or ZnO NPs effectively countered the deleterious impacts of Co on stomatal opening in B. napus.3.11 Exogenous melatonin and/or zinc oxide nanoparticles minimize the Co-induced cellular ultra-structural damages
TEM images revealed clear damage to subcellular structures and cellular protection by MT and/or ZnO NPs in B. napus leaves under Co stress (Fig. 9B). Under controlled treatments, the observed cells were well-developed and structured. The mesophyll cells of the leaf had clearly defined cell walls (CW), healthy mitochondria (M), regular arrangement of thylakoids (Thy), intact nuclear membranes (NM) in the chloroplasts, and plastoglobuli (PG) within the cytoplasm. However, upon exposure to Co stress, visible toxic symptoms were observed, such as scattered cell walls (CW), immature plastoglobuli (PG), swollen thylakoid membranes (Thy), and broken nucleolus and nucleus membranes. Remarkably, the combined treatments of MT and/or ZnO NPs significantly improved the B. napus cells from Co-mediated cellular ultrastructural damage, as evidenced by the well-recovered and developed cell walls (CW), plastoglobuli (PG), thylakoid membranes (Thy), mitochondria (M), chloroplast (Chl). These observations verified that MT and/or ZnO NPs displayed efficacy in minimizing the Co-mediated cellular ultrastructural damage in B. napus leaves.4. Discussion
Nanoparticles and growth hormones are providing novel solutions and approaches that enhance agricultural practices and plant sciences. Many investigations have elucidated that phytohormones,6,41,42 and nanoparticles,43–45 when administered separately, possess the capacity to mitigate the toxic effects induced by heavy metals in various plant species through diverse mechanisms. However, limited research has been done on mitigating Co toxicity in B. napus through the combined action mechanisms of MT and/or ZnO NPs. To address this gap, we synthesized a nano-formulation incorporating ZnO particles (Fig. 1) and MT, which can alleviate Co stress and enhance biomass production in B. napus. The results of our study indicated that supplementation of Co alone severely inhibited the plant growth attributes including growth traits (root length, leaf or root fresh and dry weight, and full height) and fresh or dry biomass production (Fig. 2). A few researchers have also documented the toxic effects of Co on plant growth and biomass production in barley and maize.46,47 The present study documents that the inhibitory effects of Coon plant roots may be the cause of the growth inhibition observed. These effects restrict the entry of the mineral nutrients, which could explain the observed inhibition. The reduction in plant growth and biomass is directly related to the enhanced uptake and translocation of Co within plant tissues. In contrast, MT and/or ZnO NPs significantly minimized the Co-induced inhibitory effects on plant growth and biomass parameters. The exogenous supply of MT and ZnO reduced the bio-accumulation of Co within plant tissues (leaves and roots) allowing them to alleviate the Co-indulged growth inhibition and thus better plant growth and development. This can be correlated with the MT-mediated modulation in anthocyanin biosynthesis and ZnO improves the plant growth attributes mainly due to the involvement of Zn ions in the functionalities of chloroplast and its development.48–50 Moreover, Co stress can also affect root architecture, as evidenced by the experiment (Fig. S1†).Similarly, foliar treatment of ZnO NPs improved the root morphology of lettuce plants.51 Another study found that nanoparticles of nSiO2, nZnO, nTiO2, nFe3O4, nCeO2, and nCuO significantly impacted the growth and root architecture of lettuce plants when exposed to Cd stress.52 The structure and characteristics of plant roots, including the root network area, number of roots, average diameter, and surface area, have an impact on the uptake of essential and non-essential elements from the growth medium (Fig. S1†). Therefore, a more enhanced root morphology may be correlated to higher MT and/or ZnO NPs uptake by plants, which could inhibit Co absorption while promoting the availability of essential micro-nutrients for the plants. Adequate supplementation of Zn ions also improves the plant growth traits on account of photosynthesis and elevated antioxidant enzyme activities.53 Photosynthetic apparatus is a clear indicator of heavy metals toxicity in plants.54 In our study, the individual supply of excessive Co levels drastically declined the levels of various photosynthetic indicators. These include photosynthetic pigments (Chl a and Chl b and carotenoids), as well as gas exchange parameters (net photosynthetic rate, stomatal conductance, intercellular CO2 concentration and transpiration rate). Additionally, the photosynthetic efficiency of PSII (Fv/Fm) was also negatively affected (Fig. 3). Potentially, the excessive deposition of Co in leaf tissues may damage the membrane structures including the photosynthetic machinery. This has been observed in electron microscopy images of leaf mesophyll cells. This may lead to chlorophyll inhibition and leaf chlorosis. The Co-mediated reduction in these photosynthetic attributes is directly linked to biomass production.47,55 In contrast, the supply of MT and/or ZnO notably enhanced the levels of photosynthetic pigments (Chl a and Chl b and carotenoids), gas exchange parameters (net photosynthetic rate, stomatal conductance, intercellular CO2 concentration and transpiration rate), and photosynthetic efficiency of PSII (Fv/Fm) under Co stress (Fig. 3). It has been reported that MT regulated the genetic expression involved in chlorophyll synthesis and thus controlled the chlorophyll contents.56 The MT applications enhanced the photosynthetic efficiency, which is related to the mineral uptake and stimulates the photosynthetic enzymes to delay the leaf senescence.25,56,57 However, ZnO NPs upregulated the Rubisco activity, which is directly linked to the photosynthetic efficiency.11 It has been documented that the exogenous supply of ZnO NPs prevented chlorophyll inhibition and enhanced the photosynthetic efficiency under heavy metals stress.47,58
Many investigations have revealed that exposure to excessive levels of abiotic stress induces alterations in the expression patterns of genes responsible for encoding vital proteins of the photosystem II (PSII) complexes such as D1, D2, CP43, and CP47, which are encoded by the PsbA, PsbB, PsbC, PsbD, and PsbO genes, respectively.59–61 This might be because PSII is the core part of the photosynthetic system that takes a hit from stress factors when plants are subjected to abiotic stresses.60,62 Our aim was to examine the effect of MT and/or ZnO NPs on the patterns of expression of the core PSII proteins in B. napus under Co stress. In particular, we examined the gene encoding D1 protein, which is the most crucial component of the PSII complex (Fig. 7). It plays an important role in the repair mechanism of oxidized PSII by facilitating the replacement of photo-damaged D1 core (PsbA) proteins with newly synthesized ones.59 However, PSII genes were significantly up-regulated in MT and/or ZnO NPs exposed plants, which is indicative of the probable involvement of Co response in stress mitigation. These findings are in agreement with earlier reports,62 that the RNAi-induced silencing of PsbO inhibited the expression of the PsbA and PsbC proteins, resulting in the loss of the variable yield of fluorescence (Fv/Fm). These findings indicate a clear relationship between the levels of expression of BnPsbO, BnPsbA, and BnPsbC genes.
In this study, individual exposure to Co significantly reduced the uptake of mineral nutrients (Fe, Mn, P, Ca, K, and Mg) especially in roots (Fig. S2†), indicating the mineral imbalance. The combined applications of MT and/or ZnO NPs significantly reduced the decrease in mineral contents in both leaves and roots when exposed to Co stress (Fig. S2†) as reported in an earlier study on soybean plants.11 This indicated that MT and/or ZnO NPs applications mediated plant growth and nutritional status of B. napus, which is correlated to the less amount of Co-bioaccumulation that results in the alleviation of Co toxicity. The individual applications of Co drastically induced the oxidative stress markers including O2˙− and H2O2 contents in the leaves and roots (Fig. 5). Earlier studies reported that Co enhanced the production of O2˙− and H2O2 in spinach.63 The excessive accumulation of ROS by Co can lead to damage to the cytoplasmic membrane and cellular macro-molecules as explained.64 In order to reduce the harmful effects of ROS-induced oxidative damage, plants activate their internal defense system to combat the toxicity of heavy metals.65 The reduced oxidative damages by the combined treatments of MT and/or ZnO NPs under Co stress, are significantly correlated to respectively higher activities in the antioxidant defense system. Our results revealed that MT or/and ZnO NPs enhance the antioxidant defense system by responding effectively in a plant treated with these exogenous components. The ROS scavenging results of MT or/and ZnO NPs were prominent and exhibited lower levels of oxidative damage and thus enhanced the plant's tolerance against various abiotic stresses.11,13,66 To understand this, we measured the antioxidative capacity of B. napus to explain how MT and/or ZnO NPs help ROS scavenging to increase the activities of both enzymatic and non-enzymatic antioxidants. Our results demonstrated that the combined treatments of MT and/or ZnO NPs positively regulated the activity of antioxidant enzymes, including SOD, CAT, APX in leaves and roots (Fig. 6A–C). The application of MT and/or ZnO NPs effectively enhanced the antioxidant enzymes' activities, as confirmed by their transcript levels (Fig. 6D–F), suggesting their functions in blocking the ROS accumulation in leaves and roots.67,68 Moreover, MDA is closely related to ROS generation,69 therefore, decreases the intactness of cell membrane, suggesting that Co remarkably enhanced B. napus to suffer from oxidative damages. The combined application of MT and/or ZnO NPs significantly reduced the accumulation of MDA, indicating that MT and/or ZnO NPs could partially maintain the integrity of the cell and B. napus could grow better under the high nutrients. The contents of three antioxidant enzyme-regulated transcript levels including BnSOD, BnCAT and BnAPX were dramatically altered under MT and/or ZnO NPs contents. Interestingly, the expression of these genes was also obviously upregulated under Co stress (Fig. 6D–F). A recent study reported that NPs may likewise regulate the gene coding for antioxidative enzymes in confronting heavy metal stress.70
The results showed that Co-stressed plants continuously accumulated thiol compounds, such as cysteine and oxidized (GSSG) and reduced glutathione (GSH), non-protein thiols (NPTs) and phytochelatins (PCs). Thiols take part in the detoxification reaction of metalloids.71,72 The MT- and/or ZnO NPs supplement enhances the Co-triggered thiols accumulation in plants (Fig. 8). Taken together, these results indicate that Co stress has stimulated the attractive mechanism of detoxification via the rise in the thiol content in plants. The augmented GSH level due to MT and/or ZnO NPs supplementation could further strengthen the activity of γ-ECS (Fig. 8A and F), which is a key enzyme in the GSH biosynthesis pathway. This enhanced GSH biosynthesis may delay leaf senescence, as recently reported in kiwifruit and apple trees.73,74 γ-ECS, GST and PCS are thiol-metabolizing enzymes involved in GSH biosynthesis and conjugation.75 The induction of enzymes of Co detoxification might be treated as compensatory mechanisms mediated by the studied organism in order to avoid the toxicity of Co. Interestingly, the presence of MT and/or ZnO NPs resulted in upregulation of the induction of Co detoxification response, which might feed into the idea that cobalt in the presence of MT and/or ZnO NPs may be involved in cobalt detoxifying mechanisms as suggested by a few previous works.25,47 The elevated levels of thiols detected in the MT treatment or ZnO NPs treatments in association with Co-treatment compared to the Co-treatment alone, suggest that the MT from the bacteria and/or the ZnO NPs provided an effective role in detoxifying Co. Furthermore, the highly accumulated chelating compounds in the plant tissue, which were PCs in roots, indicate that the root is the main site of Co detoxification (Fig. 8D).
The results of this research showed that there are distinct changes in expression levels of key genes (CAD, PAL, PPO) of B. napus (Fig. 7D–F). Also, the rise in Co levels and the introduction of MT and/or ZnO NPs further amplified this elevated gene expression. These upregulated genes represent a crucial pathway, known as the phenylpropanoid pathway, that helps plants alleviate oxidative stress.76 Interestingly, similar elevation in the secondary metabolites had been previously reported under Co-stress conditions.77 In addition, the results of current findings were also consistent with our previous findings,78 which proved that the oxidative damage resulting from the secondary metabolites was reduced. The relative expression level of the gene related to the rate-limiting biosynthesis and the transcript levels of PAL were detected in B. napus plants grown under Co and MT + ZnO NPs treatments (Fig. 7D). Moreover, in the research carried out,79 they showed that the activities of PAL, PPO, and CAD in wheat plants were also enhanced, which sounds good for removing excessive levels of ROS. These findings collectively revealed that MT and/or ZnO NPs treatment improved plant tolerance by upregulating the expression of stress-responsive genes, which in turn activated the phenylpropanoid pathway and facilitated the production of secondary metabolites. These secondary metabolites played a crucial role in mitigating oxidative stress and maintaining cellular homeostasis, thereby promoting plant survival under metal stress conditions.
The exposure to Co stress alone resulted in severe cellular damage including scattered cell walls, immature plastoglobules, lower-size starch grains, swollen thylakoid membrane, cracked cell membrane and broken nuclear membrane. These observations verified that co-supply of MT and ZnO-activated cellular protective mechanisms help B. napus plants to retain intercellular integrity (Fig. 9A). Similar cell-protecting roles by AstNPs, CuONPs and MT were found in Cd-stressed wheat plants.25,44,80 The reduction in the photosynthetic rate induced by Co-treatment could elicit the closing of stomata (Fig. 9B). This response could be attributed to the deformation of guard cells, potentially resulting from the inhibition of metabolic reactions that are essential for maintaining guard cell turgor under stress conditions induced by Co. Such inhibition of guard cell metabolism may impair the ability to sustain the necessary turgor pressure, leading to stomatal closure. On the other hand, MT and/or ZnO treatments augmented the length and width of the stomata (Fig. 9B). Our findings support previous studies,6,25,81 which showed that treatments with MT and ZnO NPs play a crucial role in maintaining cell turgor pressure. This is achieved through proline accumulation, which in turn leads to the opening of stomata. Despite Co-treatment inhibiting photosynthetic rate and causing stomatal closure as a stress response, treatments with MT and/or ZnO NPs counteract this effect by promoting guard cell turgor increase through proline accumulation. This mechanism may enable the plant to withstand drought stress by keeping the stomata open.
Conclusions
The applications of MT + ZnO NPs resulted in positive effects on the growth of B. napus plants and significantly enhanced their photosynthetic efficiency. Furthermore, the MT + ZnO NPs noticeably reduced the Co-accumulation in both the leaves and roots of B. napus plants. To reduce oxidative stress caused by Co exposure, they minimized the excessive production of ROS (H2O2 and O2˙−), and prevented peroxidation of membrane lipids induced by Co stress. Under Co stress, the activity of antioxidant enzymes (SOD, CAT and APX) is upregulated, and higher levels of transcripts for defense-related genes (PAL and PPO), genes related to photosynthesis (BnPsbA, BnPsbB, BnPsbC, BnPsbD, BnPsbO), and thiol compounds (NPTs, PCs and cysteine). This indicates that MT + ZnO NPs have the ability to enhance ROS-scavenging and improve plant resistance to Co stress. These findings could have implications for enhancing our understanding of the role of phytohormone MT and/or ZnO NPs in crop production as well as developing new techniques to produce healthy food in polluted areas, where Co pollution poses a significant challenge. Additionally, this knowledge could aid in the development of new methods for growing healthy food crops in areas affected by Co contamination, which is a major environmental issue.Data availability
All data presented in this study are available in the article or ESI.†Author contributions
Skhawat Ali: conceptualization, writing – original draft, visualization, methodology, investigation, data curation, formal analysis. Basharat Ali, Imtiaz Ahmad Sajid, Muhammad Arslan Yousaf: data curation, formal analysis, writing – review and editing. Shoaib Ahmad, Kangni Zhang, Shafaqat Ali, Zaid Ulhassan: writing – review and editing. Bizeng Mao, Weijun Zhou: supervision, methodology, writing – review and editing, funding acquisition, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Science and Technology Department of Zhejiang Province (2023C02002-3), and the Collaborative Innovation Center for Modern Crop Production co-sponsored by the Province and Ministry (CIC-MCP). We acknowledge the Zhejiang Key Laboratory of Crop Germplasm Innovation and Utilization, and Rui Sun and Weizhen Hu from the Agricultural Experiment Station of Zhejiang University for their assistance.References
- FAOSTAT, 2014, Available from: http://www.fao.org/faostat/en/#compare.
- T. Shi and Y. Wang, Heavy metals in indoor dust: spatial distribution, influencing factors, and potential health risks, Sci. Total Environ., 2021, 755, 142367, DOI:10.1016/j.scitotenv.2020.142367
.
- K. Khanna, V. L. Jamwal, S. G. Gandhi, P. Ohri and R. Bhardwaj, Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity, Sci. Rep., 2019, 9(1), 1–14, DOI:10.1038/s41598-019-41899-3
.
- M. Shafiee, R. Foroutan, K. Fouladi, M. Ahmadlouydarab, B. Ramavandi and S. Sahebi, Application of oak powder/Fe3O4 magnetic composite in toxic metals removal from aqueous solutions, Adv. Powder Technol., 2019, 30(3), 544–554, DOI:10.1016/j.apt.2018.12.006
.
- G. Genchi, G. Lauria, A. Catalano, A. Carocci and M. S. Sinicropi, Prevalence of cobalt in the environment and its role in biological processes, Biology, 2023, 12(10), 1335 CrossRef CAS
.
- S. Ali, R. A. Gill, Z. Ulhassan, N. Zhang, S. Hussain, K. Zhang and W. Zhou, Exogenously applied melatonin enhanced the tolerance of Brassica napus against cobalt toxicity by modulating antioxidant defense, osmotic adjustment, and expression of stress response genes, Ecotoxicol. Environ. Saf., 2023, 252, 114624, DOI:10.1016/j.ecoenv.2023.114624
.
- H. Samet, Alleviation of cobalt stress by exogenous sodium nitroprusside in iceberg lettuce, Chil. J. Agric. Res., 2020, 80(2), 161–170 CrossRef
.
- M. Kah, N. Tufenkji and J. C. White, Nano-enabled strategies to enhance crop nutrition and protection, Nat. Nanotechnol., 2019, 14(6), 532–540, DOI:10.1038/s41565-019-0439-5
.
- V. Shah and A. Daverey, Phytoremediation: a multidisciplinary approach to clean up heavy metal contaminated soil, Environ. Technol. Innovation, 2020, 18, 100774, DOI:10.1016/j.eti.2020.100774
.
- A. Hussain, M. Rizwan, Q. Ali and S. Ali, Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains, Environ. Sci. Pollut. Res., 2019, 26(8), 7579–7588, DOI:10.1007/s11356-019-04210-5
.
- M. Faizan, J. A. Bhat, K. Hessini, F. Yu and P. Ahmad, Zinc oxide nanoparticles alleviate the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system, Ecotoxicol. Environ. Saf., 2021, 220, 112401, DOI:10.1016/j.ecoenv.2021.112401
.
- H. Al Jabri, M. H. Saleem, M. Rizwan, I. Hussain, K. Usman and M. Alsafran, Zinc oxide nanoparticles and their biosynthesis: overview, Life, 2022, 12(4), 594 CrossRef CAS
.
- M. Faizan, J. A. Bhat, A. Noureldeen, P. Ahmad and F. Yu, Zinc oxide nanoparticles and 24-epibrassinolide alleviate Cu toxicity in tomato by regulating ROS scavenging, stomatal movement and photosynthesis, Ecotoxicol. Environ. Saf., 2021, 218, 112293, DOI:10.1016/j.ecoenv.2021.112293
.
- M. Faizan, S. Sehar, V. D. Rajput, A. Faraz, S. Afzal, T. Minkina and M. Faisal, Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress, Plants, 2021, 10(11), 2254, DOI:10.3390/plants10112254
.
- I. Cakmak and U. Á. Kutman, Agronomic biofortification of cereals with zinc: a review, Eur. J. Soil Sci., 2018, 69(1), 172–180, DOI:10.1111/ejss.12437
.
- H. M. Umair, M. Aamer, C. M. Umer, T. Haiying, B. Shahzad, L. Barbanti and H. Guoqin, The critical role of zinc in plants facing the drought stress, Agriculture, 2020, 10(9), 396, DOI:10.3390/agriculture10090396
.
- P. Venkatachalam, M. Jayaraj, R. Manikandan, N. Geetha, E. R. Rene, N. C. Sharma and S. V. Sahi, Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis, Plant Physiol. Biochem., 2017, 110, 59–69, DOI:10.1016/j.plaphy.2016.08.022
.
- A. Hussain, S. Ali, M. Rizwan, M. Z. ur Rehman, M. R. Javed, M. Imran and R. Nazir, Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants, Environ. Pollut., 2018, 242, 1518–1526, DOI:10.1016/j.envpol.2018.08.036
.
- C. O. Dimkpa, J. C. White, W. H. Elmer and J. Gardea-Torresdey, Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization, J. Agric. Food Chem., 2017, 65(39), 8552–8559, DOI:10.1021/acs.7b02961
.
- C. Sun, L. Liu, L. Wang, B. Li, C. Jin and X. Lin, Melatonin: a master regulator of plant development and stress responses, J. Integr. Plant Biol., 2021, 63(1), 126–145 CrossRef CAS
.
- R. Hardeland, Melatonin in plants–diversity of levels and multiplicity of functions, Front. Plant Sci., 2016, 7, 180784, DOI:10.3389/fpls.2016.00198
.
- Y. F. Wang, Y. Y. Guo, C. F. Zhao, H. J. Li and R. H. Zhang, Exogenous melatonin achieves drought tolerance by improving photosynthesis in maize seedlings leaves, Russ. J. Plant Physiol., 2021, 68, 718–727 CrossRef CAS
.
- R. Bhardwaj, S. Pareek, J. A. Domínguez-Avila, G. A. Gonzalez-Aguilar, D. Valero and M. Serrano, An exogenous pre-storage melatonin alleviates chilling injury in some mango fruit cultivars, by acting on the enzymatic and non-enzymatic antioxidant system, Antioxidants, 2022, 11(2), 384 CrossRef CAS
.
- M. Khan, S. Ali, H. Manghwar, S. Saqib, F. Ullah, A. Ayaz and W. Zaman, Melatonin function and crosstalk with other phytohormones under normal and stressful conditions, Genes, 2022, 13(10), 1699, DOI:10.3390/genes13101699
.
- Z. Ulhassan, Q. Huang, R. A. Gill, S. Ali, T. M. Mwamba, B. Ali and W. Zhou, Protective mechanisms of melatonin against selenium toxicity in Brassica napus: insights into physiological traits, thiol biosynthesis and antioxidant machinery, BMC Plant Biol., 2019, 19, 1–16, DOI:10.1186/s12870-019-2110-6
.
- S. Farouk and S. M. Al-Amri, Ameliorative roles of melatonin and/or zeolite on chromium-induced leaf senescence in marjoram plants by activating antioxidant defense, osmolyte accumulation, and ultrastructural modification, Ind. Crops Prod., 2019, 142, 111823, DOI:10.1016/j.indcrop.2019.111823
.
- M. Noman, T. Ahmed, S. Hussain, M. B. K. Niazi, M. Shahid and F. Song, Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri SNT22 reduced the translocation of cadmium from soil to wheat plants, J. Hazard. Mater., 2020, 398, 123175 CrossRef CAS PubMed
.
- T. Ahmed, L. Shou, J. Guo, M. Noman, Y. Qi, Y. Yao and X. Qi, Modulation of rhizosphere microbial community and metabolites by bio-functionalized nanoscale silicon oxide alleviates cadmium-induced phytotoxicity in bayberry plants, Sci. Total Environ., 2024, 933, 173068 CrossRef CAS PubMed
.
- A. M. Abdo, A. Fouda, A. M. Eid, N. M. Fahmy, A. M. Elsayed, A. M. A. Khalil and A. M. Soliman, Green synthesis of zinc oxide nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and their activity against pathogenic microbes and common house mosquito, Culex pipiens, Materials, 2021, 14(22), 6983 CrossRef CAS PubMed
.
- S. Ali, R. A. Gill, T. M. Mwamba, N. Zhang, M. T. Lv, Z. Ul Hassan and W. J. Zhou, Differential cobalt-induced effects on plant growth, ultrastructural modifications, and antioxidative response among four Brassica napus (L.) cultivars, Int. J. Environ. Sci. Technol., 2018, 15, 2685–2700, DOI:10.1007/s13762-017-1629-z
.
- S. Ali, R. A. Gill, Z. Ulhassan, U. Najeeb, M. K. Kanwar, M. Abid and W. Zhou, Insights on the responses of Brassica napus cultivars against the cobalt-stress as revealed by carbon assimilation, anatomical changes and secondary metabolites, Environ. Exp. Bot., 2018, 156, 183–196, DOI:10.1016/j.envexpbot.2018.07.004
.
-
H. K. Lichtenthaler, Chlorophylls and carotenoids-pigments of photosynthetic biomembranes, in Methods in Enzymol, ed. S. P. Colowick and N. O. Kaplan, Academic Press, San Diego, 1987, vol. 148, pp. 350–382, DOI:10.1016/0076-6879(87)48036-1
.
- M. Faizan, A. Faraz and S. Hayat, Effective use of zinc oxide nanoparticles through root dipping on the performance of growth, quality, photosynthesis and antioxidant system in tomato, J. Plant Biochem. Biotechnol., 2020, 29, 553–567, DOI:10.1007/s13562-019-00525-z
.
- M. Z. Rehman, M. Rizwan, A. Ghafoor, A. Naeem, S. Ali, M. Sabir and M. F. Qayyum, Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phytoavailability to wheat and rice under rotation, Environ. Sci. Pollut. Res., 2015, 22, 16897–16906, DOI:10.1007/s11356-015-4883-y
.
- J. Lian, J. Wu, H. Xiong, A. Zeb, T. Yang, X. Su, L. Su and W. Liu, Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.), J. Hazard. Mater., 2020, 385, 121620, DOI:10.1016/j.jhazmat.2019.121620
.
- H. Zhang, C. Guo, C. Li and K. Xiao, Cloning, characterization and expression analysis of two superoxide dismutase (SOD) genes in wheat (Triticum aestivum L.), Frontiers of Agriculture in China, 2008, 2(2), 141–149, DOI:10.1007/s11703-008-0023-5
.
-
H. Aebi, Catalase in vitro, in Methods in Enzymology, Elsevier, 1984, pp. 121–126, DOI:10.1016/S0076-6879(84)05016-3
.
- K. J. Livak and T. D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method, Methods, 2001, 25(4), 402–408, DOI:10.1006/meth.2001.1262
.
- A. Kumar, R. P. Singh, P. K. Singh, S. Awasthi, D. Chakrabarty, P. K. Trivedi and R. D. Tripathi, Selenium ameliorates arsenic-induced oxidative stress through modulation of antioxidant enzymes and thiols in rice (Oryza sativa L.), Ecotoxicology, 2014, 23, 1153–1163, DOI:10.1007/s10646-014-1257-z
.
- Z. Ulhassan, S. Yang, D. He, A. R. Khan, A. Salam, W. Azhar and W. Zhou, Seed priming with nano-silica effectively ameliorates chromium toxicity in Brassica napus, J. Hazard. Mater., 2023, 458, 131906, DOI:10.1016/j.jhazmat.2023.131906
.
- M. Qadir, A. Hussain, M. Hamayun, M. Shah, A. Iqbal and W. Murad, Phytohormones producing rhizobacterium alleviates chromium toxicity in Helianthus annuus L. by reducing chromate uptake and strengthening antioxidant system, Chemosphere, 2020, 258, 127386, DOI:10.1016/j.chemosphere.2020.127386
.
- Z. H. Dai, D. X. Guan, J. Bundschuh and L. Q. Ma, Roles of phytohormones in mitigating abiotic stress in plants induced by metal(loid)s As, Cd, Cr, Hg, and Pb, Crit. Rev. Environ. Sci. Technol., 2022, 53(13), 1310–1330, DOI:10.1080/10643389.2022.2134694
.
- M. A. Farooq, F. Islam, A. Ayyaz, W. Chen, Y. Noor, W. Hu and W. Zhou, Mitigation effects of exogenous melatonin-selenium nanoparticles on arsenic-induced stress in Brassica napus, Environ. Pollut., 2022, 292, 118473, DOI:10.1016/j.envpol.2021.118473
.
- M. Noman, T. Ahmed, S. Hussain, M. B. K. Niazi, M. Shahid and F. Song, Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri SNT22 reduced the translocation of cadmium from soil to wheat plants, J. Hazard. Mater., 2020, 398, 123175, DOI:10.1016/j.jhazmat.2020.123175
.
- Y. Li, N. Zhu, X. Liang, X. Bai, L. Zheng, J. Zhao and Y. Gao, Silica nanoparticles alleviate mercury toxicity via immobilization and inactivation
of Hg (ii) in soybean (Glycine max), Environ. Sci.: Nano, 2020, 7(6), 1807–1817, 10.1039/D0EN00091D
.
- J. L. Wa Lwalaba, G. Zvogbo, M. Mulembo, M. Mundende and G. Zhang, The effect of cobalt stress on growth and physiological traits and its association with cobalt accumulation in barley genotypes differing in cobalt tolerance, J. Plant Nutr., 2017, 40(15), 2192–2199, DOI:10.1080/01904167.2017.1346676
.
- A. Salam, A. R. Khan, L. Liu, S. Yang, W. Azhar, Z. Ulhassan, M. Zeeshan, J. Wu, X. Fan and Y. Gan, Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress, J. Hazard. Mater., 2022, 127021, DOI:10.1016/j.jhazmat.2021.127021
.
- A. R. Mir, M. Faizan, A. Bajguz, F. Sami, H. Siddiqui and S. Hayat, Occurrence and biosynthesis of melatonin and its exogenous effect on plants, Acta Soc. Bot. Pol., 2020, 89, 1–23, DOI:10.5586/asbp.8922
.
- L. Sun, Y. Wang, R. Wang, R. Wang, P. Zhang, Q. Ju and J. Xu, Physiological, transcriptomic, and metabolomic analyses reveal zinc oxide nanoparticles modulate plant growth in tomato, Environ. Sci.: Nano, 2020, 7(11), 3587–3604, 10.1039/D0EN00723D
.
- R. Hansch and R. R. Mendel, Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl), Curr. Opin. Plant Biol., 2009, 12, 259–266, DOI:10.1016/j.pbi.2009.05.006
.
- F. Gao, X. Zhang, J. Zhang, J. Li, T. Niu, C. Tang and J. Xie, Zinc oxide nanoparticles improve lettuce (Lactuca sativa L.) plant tolerance to cadmium by stimulating antioxidant defense, enhancing lignin content and reducing the metal accumulation and translocation, Front. Plant Sci., 2022, 13, 1015745, DOI:10.1007/s11356-023-29241-x
.
- N. Bano, S. Khan, Y. Hamid, M. A. Ullah, A. G. Khan, F. Bano and T. Li, Effect of foliar application of nanoparticles on growth, physiology, and antioxidant enzyme activities of lettuce (Lactuca sativa L.) plants under cadmium toxicity, Environ. Sci. Pollut. Res., 2023, 30(44), 99310–99325, DOI:10.1007/s11356-023-29241-x
.
- A. Rehman, M. Farooq, M. Asif and L. Ozturk, Supra-optimal growth temperature exacerbates adverse effects of low Zn supply in wheat, J. Plant Nutr. Soil Sci., 2019, 182(4), 656–666, DOI:10.1002/jpln.201800654
.
- P. Faseela, A. K. Sinisha, M. Brestic and J. T. Puthur, Chlorophyll a fluorescence parameter as indicators of a particular abiotic stress in rice, Photosynthetica, 2020, 58, 293–300, DOI:10.32615/ps.2019.147
.
- A. Salam, M. Rehman, J. Qi, A. R. Khan, S. Yang, M. Zeeshan and Y. Gan, Cobalt stress induces photosynthetic and ultrastructural distortion by disrupting cellular redox homeostasis in Zea mays, Environ. Exp. Bot., 2024, 217, 105562 CrossRef CAS
.
- M. A. Nawaz, Y. Jiao, C. Chen, F. Shireen, Z. Zheng, M. Imtiaz, Z. Bie and Y. Huang, Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression, J. Plant Physiol., 2018, 220, 115–127, DOI:10.1016/j.jplph.2017.11.003
.
- M. Hasan, G. J. Ahammed, L. Yin, K. Shi, X. Xia, Y. Zhou, J. Yu and J. Zhou, Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum, Front. Plant Sci., 2015, 6, 601, DOI:10.3389/fpls.2015.00601
.
- X. Pan, D. Li, Y. Fang, Z. Liang, H. Zhang, J. Z. Zhang and S. Song, Enhanced photogenerated electron transfer in a semiartificial photosynthesis system based on highly dispersed titanium oxide nanoparticles, J.
Phys. Chem. Lett., 2020, 11(5), 1822–1827 CrossRef CAS
.
- S. Sasi, J. Venkatesh, R. F. Daneshi and M. A. Gururani, Photosystem II extrinsic proteins and their putative role in abiotic stress tolerance in higher plants, Plants, 2018, 7, 100, DOI:10.3390/plants7040100
.
- F. Mehar, N. Iqbal, Z. Sehar, M. N. Alyemeni, P. Kaushik, N. A. Khan and P. Ahmad, Methyl jasmonate protects the PS II system by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants, Antioxidants, 2021, 10(8), 1216, DOI:10.3390/antiox10081216
.
- Y. V. Ivanov, P. P. Pashkovskiy, A. I. Ivanova, A. V. Kartashov and V. V. Kuznetsov, Manganese deficiency suppresses growth and photosynthetic processes but causes an increase in the expression of photosynthetic genes in Scots pine seedlings, Cell, 2022, 11(23), 3814, DOI:10.3390/cells11233814
.
- N. Varghese, O. Alyammahi, S. Nasreddine, A. Alhassani and M. A. Gururani, Melatonin positively influences the photosynthetic machinery and antioxidant system of Avena sativa during salinity stress, Plants, 2019, 8(12), 610, DOI:10.3390/plants8120610
.
- N. Pandey, G. C. Pathak, D. K. Pandey and R. Pandey, Heavy metals, Co, Ni, Cu, Zn and Cd, produced oxidative damage and evoked differential antioxidative responses in spinach, Braz. J. Plant Physiol., 2009, 21, 103–111, DOI:10.1590/S1677-04202009000200003
.
- K. B. Rejeb, M. Benzarti, A. Debez, C. Bailly, A. Savoure and C. Abdelly, NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana, J. Plant Physiol., 2015, 174, 5–15, DOI:10.1016/j.jplph.2014.08.022
.
- H. Aziz, M. Sabir, H. R. Ahmad, T. Aziz, M. Zia-ur-Rehman, K. R. Hakeem and M. Ozturk, Alleviating effect of calcium on nickel toxicity in rice, Clean: Soil, Air, Water, 2015, 43, 901–909, DOI:10.1002/clen.201400085
.
- M. Rizwan, S. Ali, B. Ali, M. Adrees, M. Arshad, A. Hussain, M. Z. Rehman and A. A. Waris, Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat, Chemosphere, 2019, 214, 269–277, DOI:10.1016/j.chemosphere.2018.09.120
.
- S. Sun, A. Liu, Z. Li, T. Guo, S. Chen and G. J. Ahammed, Anthocyanin synthesis is critical for melatonin-induced chromium stress tolerance in tomato, J. Hazard. Mater., 2023, 453, 131456 CrossRef CAS PubMed
.
- G. J. Ahammed, Z. Li, J. Chen, Y. Dong, K. Qu, T. Guo and X. Li, Reactive oxygen species signaling in melatonin-mediated plant stress response, Plant Physiol. Biochem., 2024, 108398 CrossRef CAS
.
- P. Ahmad, M. N. Alyemeni, A. A. Al-Huqail, M. A. Alqahtani, L. Wijaya, M. Ashraf, C. Kaya and A. Bajguz, Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of As and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system, Plants, 2020, 9(7), 825, DOI:10.3390/plants9070825
.
- M. Jiang, Y. Song, M. K. Kanwar, G. J. Ahammed, S. Shao and J. Zhou, Phytonanotechnology applications in modern agriculture, J. Nanobiotechnol., 2021, 19(1), 430, DOI:10.1186/s12951-021-01176-w
.
- I. V. Seregin and A. D. Kozhevnikova, Low-molecular-weight ligands in plants: role in metal homeostasis and hyper accumulation, Photosynth. Res., 2021, 150(1), 51–96, DOI:10.1007/s11120-020-00768-1
.
- S. Clemens, Metal ligands in micronutrient acquisition and homeostasis, Plant, Cell Environ., 2019, 42(10), 2902–2912, DOI:10.1111/pce.13627
.
- D. Liang, Y. Shen, Z. Ni, Q. Wang, Z. Lei, N. Xu and H. Xia, Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids, Front. Plant Sci., 2018, 9, 426, DOI:10.3389/fpls.2018.00426
.
- P. Wang, X. Sun, C. Li, Z. Wei, D. Liang and F. Ma, Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple, J. Pineal Res., 2013, 54, 292–302, DOI:10.1111/jpi.12017
.
- D. Talukdar, A metabolic diversion in the upstream thiol cascade of cysteine-deficient lentil (Lens culinaris Medik.) mutants induces arsenate tolerance by modulating downstream antioxidant defense, Turk. J. Bot., 2016, 40(4), 343–355, DOI:10.3906/bot-1506-49
.
- U. Ahmed, M. J. Rao, C. Qi, Q. Xie, H. A. Noushahi, M. Yaseen and B. Zheng, Expression profiling of flavonoid biosynthesis genes and secondary metabolites accumulation in Populus under drought stress, Molecules, 2021, 26(18), 5546, DOI:10.3390/molecules26185546
.
- A. Goossens, S. T. Häkkinen, I. Laakso, T. Seppänen-Laakso, S. Biondi, V. De Sutter, F. Lammertyn, A. M. Nuutila, H. Söderlund, M. Zabeau and D. Inzé, A functional genomics approach toward the understanding of secondary metabolism in plant cells, Proc. Natl. Acad. Sci. U. S. A., 2013, 100, 8595–8600, DOI:10.1073/pnas.1032967100
.
- M. S. Jahan, S. Guo, A. R. Baloch, J. Sun, S. Shu, Y. Wang and R. Roy, Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings, Ecotoxicol. Environ. Saf., 2020, 197, 110593, DOI:10.1016/j.ecoenv.2020.110593
.
- M. T. Dugasa, I. G. Chala and F. Wu, Genotypic difference in secondary metabolism-related enzyme activities and their relative gene expression patterns, osmolyte and plant hormones in wheat, Physiol. Plant., 2020, 168(4), 921–933, DOI:10.1111/ppl.13032
.
- A. Zeshan, M. Abdullah, M. F. Adil, D. Wei, M. Noman, T. Ahmed, S. Sehar, Y. Ouyang and I. H. Shamsi, Improvement of morpho-physiological, ultrastructural and nutritional profiles in wheat seedlings through astaxanthin nanoparticles alleviating the cadmium toxicity, J. Hazard. Mater., 2021, 12, 126511, DOI:10.1016/j.jhazmat.2021.126511
.
- S. Ahmed, M. T. Khan, A. Abbasi, I. U. Haq, A. Hina, M. Mohiuddin and Y. Li, Characterizing stomatal attributes and photosynthetic induction in relation to biochemical changes in Coriandrum sativum L. by foliar-applied zinc oxide nanoparticles under drought conditions, Front. Plant Sci., 2023, 13, 1079283, DOI:10.3389/fpls.1079283
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00821a |
| This journal is © The Royal Society of Chemistry 2025 |