Cr2TiC2Tx MXene as an adsorbent material in ultrasonic-assisted d-μ-solid phase extraction for trace detection of heavy metals†
Saman
Bagheri
 *,
Rashmeet Kaur
Khurana
,
Md. Ibrahim
Kholil
*,
Rashmeet Kaur
Khurana
,
Md. Ibrahim
Kholil
 ,
Michael J.
Loes
,
Michael J.
Loes
 ,
Shengyuan
Luo
,
Shengyuan
Luo
 and
Alexander
Sinitskii
and
Alexander
Sinitskii
 *
*
Department of Chemistry and Nebraska Center for Materials and Nanoscience, University of Nebraska-Lincoln, Lincoln, Nebraska 68588, USA. E-mail: sbagheri2@unl.edu; sinitskii@unl.edu
First published on 6th November 2024
Abstract
MXenes are a large family of two-dimensional transition metal carbides, nitrides, and carbonitrides. While MXenes have great potential for applications in analytical chemistry, most of the studies in this field are focused on Ti3C2Tx, the most popular MXene material. For example, several studies employed Ti3C2Tx as an adsorbent for the trace detection of toxic analytes, but there is limited knowledge on the utility of other MXene materials for this application. In this work, we investigated the potential of Cr2TiC2Tx, one of the least studied MXenes, for application as an adsorbent material in ultrasonic-assisted dispersive micro solid-phase extraction (d-μ-SPE) method for the detection of heavy metals at trace levels in food and soil samples. We synthesized large monolayer flakes of Cr2TiC2Tx and characterized it by a variety of microscopic and spectroscopic techniques. Cr2TiC2Tx MXene showed remarkable performance in the d-μ-SPE method with the detection limits of 0.09 and 1.9 ng mL−1, and dynamic ranges of 0.3–90 μg L−1 and 6–120 μg L−1 for cadmium (Cd2+) and lead (Pb2+) ions, respectively. The great performance of Cr2TiC2Tx MXene as an adsorbent for the trace detection of heavy metals highlights the importance of investigating other MXenes beyond Ti3C2Tx for analytical applications.
1. Introduction
MXenes are an emerging family of two-dimensional materials with a general formula of Mn+1XnTx, where M is a transition metal, such as Ti, Mo, V, etc., X is carbon and/or nitrogen, n = 1, 2, 3, 4, or 5, and Tx represents the surface functional groups.1–3 Since their discovery in 2011,4 MXenes have grown into a large field of research.1–3 Significant effort in this field has been dedicated to developing new MXene materials by experimenting with various combinations of M and X elements. This effort has experimentally advanced over 40 different MXenes.1–3 However, research on MXene applications has been predominantly focused on Ti3C2Tx, the most studied MXene to date.1–3 The general preference for Ti3C2Tx stems from its straightforward synthesis, which has been thoroughly optimized over the last decade to yield large monolayer flakes of high quality.5–7One of the MXene materials that received less attention than Ti3C2Tx is Cr2TiC2Tx. It is synthesized by etching Cr2TiAlC2, a layered quaternary MAX phase, where a Ti layer is sandwiched between two Cr–C layers (⋯Cr–C–Ti–C–Cr–Al⋯). While the transition metals mostly occupy different layers, a recent study demonstrated that a certain number of Cr atoms may also be present in the Ti layer.8 The first detailed study on the synthesis and physical properties of Cr2TiC2Tx was reported by Hantanasirisakul and co-workers.9 In that study, the authors etched Cr2TiAlC2 and exfoliated it into Cr2TiC2Tx using a mixture of HF/HCl as the etchant and TMAOH as the intercalating agent. For delamination, the sample was sonicated and a solution of monolayer MXene flakes with lateral sizes in sub-μm range was collected. Building upon this foundational work, we optimized the synthesis process and produced Cr2TiC2Tx flakes with larger sizes in μm range, with minimal degradation and defects.
Trace detection of contaminating agents and toxins using MXenes is still a relatively uncharted territory. The studies reported so far overwhelmingly utilized Ti3C2Tx10–17 and explored applications such as electrochemical sensors, purification, and extraction.18–22 Considering the pool of published literature, there remains a significant gap in the exploration and understanding of other MXene materials, such as Cr2TiC2Tx, for which there is no case study on their performance in trace detection of heavy metals. In this work, we investigated the potential of Cr2TiC2Tx MXene as an adsorbent in ultrasonic-assisted dispersive micro solid-phase extraction (d-μ-SPE) for the trace detection of heavy metal ions, Cd2+ and Pb2+.
The structure of this work is demonstrated in Scheme 1, which shows that we first synthesized Cr2TiC2Tx (I), and then utilized it for the adsorption of Cd2+ and Pb2+ ions (II) that were present in trace amounts in analytical samples. After the extraction of the MXene material, the heavy metal ions were desorbed from Cr2TiC2Tx flakes using an acid (III), which resulted in preconcentrated samples that were suitable for analysis by flame atomic absorption spectrometry (FAAS, IV). After the desorption of Cd2+ and Pb2+ ions, the MXene material can be reused for preconcentration of heavy metal ions from other analytical samples. We studied the adsorption properties, structural stability, and efficiency of Cr2TiC2Tx in detecting trace concentrations of Cd2+ and Pb2+ ions. We demonstrate that Cr2TiC2Tx exhibits attractive properties as an adsorbent in ultrasonic-assisted d-μ-SPE, offering high sensitivity, stability, and performance.
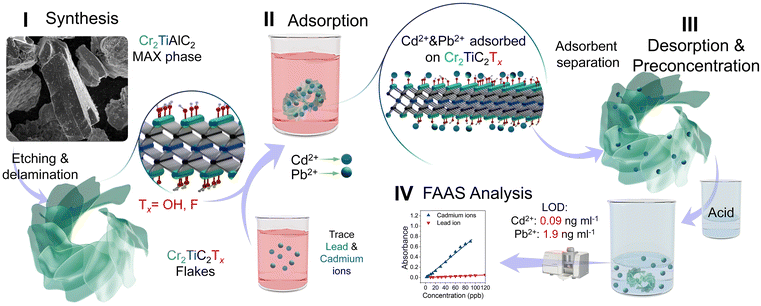 | ||
| Scheme 1 Schematic illustration of the application of Cr2TiC2Tx MXene as a solid phase in ultrasonic-assisted d-μ-SPE for heavy metal detection. | ||
2. Experimental
2.1. Reagents and materials
Ti (99%, 325 mesh), Al (99%, 325 mesh), and Cr (99.9%, 325 mesh) were purchased from Alfa Aesar. Concentrated hydrochloric acid (HCl), nitric acid (HNO3), and acetic acid (CH3COOH) were purchased from Thermo Fisher Scientific. Tetramethylammonium hydroxide (TMAOH, 25 wt% in methanol), hydrofluoric acid (48–52% HF), lead nitrate (Pb(NO3)2), and cadmium nitrate tetrahydrate (Cd(NO3)2·4H2O) were purchased from Sigma-Aldrich. Heavy metal stock solutions were prepared by dissolving appropriate amounts of the metal salts in double-distilled water.2.2. Synthesis
For the Cr2TiAlC2 MAX phase synthesis, the elemental precursors, Cr, Ti, Al, and graphite, taken at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9, were thoroughly mixed using a pestle and mortar, transferred into an alumina crucible, and annealed at 1450 °C under the flow of argon (450 sccm) for 8 h. The prepared MAX phase was crushed and sieved to obtain particles with sizes of 30–40 μm.
1.9, were thoroughly mixed using a pestle and mortar, transferred into an alumina crucible, and annealed at 1450 °C under the flow of argon (450 sccm) for 8 h. The prepared MAX phase was crushed and sieved to obtain particles with sizes of 30–40 μm.
For the synthesis of Cr2TiC2Tx MXene, 6 g of the MAX phase was slowly added to a solution containing 18 mL of HF, 32 mL of HCl, and 45 mL of H2O and stirred at 600 rpm with a 2-inch stir bar for 48 h at room temperature (25 °C). The etching product was washed with deionized (DI) water to reach pH 7 before intercalation. Then, 6 mL of TMAOH solution (25 wt% in methanol) was mixed with 24 mL of H2O and used to intercalate multilayer MXene particles for 24 h at room temperature. After the intercalation, the solution was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and washed with DI water to pH 7 using a 500 mL round-bottom centrifuge tube. Finally, the Cr2TiC2Tx MXene was delaminated in 50 mL of DI water through mild shaking for 20–30 min, centrifuged at 1500 rpm for 5 min, and vacuum dried for further experiments.
000 rpm and washed with DI water to pH 7 using a 500 mL round-bottom centrifuge tube. Finally, the Cr2TiC2Tx MXene was delaminated in 50 mL of DI water through mild shaking for 20–30 min, centrifuged at 1500 rpm for 5 min, and vacuum dried for further experiments.
2.3. Materials characterization
Concentrations of cadmium and lead ions were analyzed by a 3100 PerkinElmer FAAS spectrometer using an air-acetylene flame. Cadmium (228.8 nm) and lead (283.3 nm) hollow cathode lamps were used as radiation sources. The pH measurements were performed at 25 ± 1 °C using a digital Accumet AB150 ion analyzer supplied with a combined glass-calomel electrode. The morphology of MXene samples was studied by scanning electron microscopy (SEM) using an FEI Nova NanoSEM 450 instrument at the accelerating voltage of 5 kV. Transmission electron microscopy (TEM), scanning transmission electron microscopy (STEM), selected area electron diffraction (SAED), and energy-dispersive X-ray (EDX) spectroscopy were performed using an FEI Tecnai Osiris instrument (200 kV) on MXene flakes deposited on lacey carbon TEM grids.Brunauer–Emmett–Teller (BET) surface area analysis was performed using a Micrometrics ASAP 2460 surface area and porosimetry analyzer by recording nitrogen adsorption isotherms at −196 °C. Before the adsorption experiments, all samples were dried at 120 °C for 24 h under N2. The standard BET procedure involving the nitrogen adsorption–desorption data collected at a relative equilibrium pressure interval was used to calculate the specific surface areas of the samples. The amount of nitrogen adsorbed at the relative pressure of 0.985 was used to estimate the total pore volume. X-ray diffraction (XRD) patterns were recorded by a PANalytical Empyrean powder diffractometer with Ni-filtered Cu Kα radiation operated at 40 kV and 30 mA using a step size of 0.03° and 1 s dwelling time. The chemical analysis of the prepared samples was carried out by X-ray photoelectron spectroscopy (XPS) using a Thermo Scientific K-alpha X-ray photoelectron spectrometer with monochromatic Al Kα (1486.6 eV) radiation and a flood gun for charge compensation.
3. Results and discussion
3.1. Cr2TiC2Tx synthesis and characterization
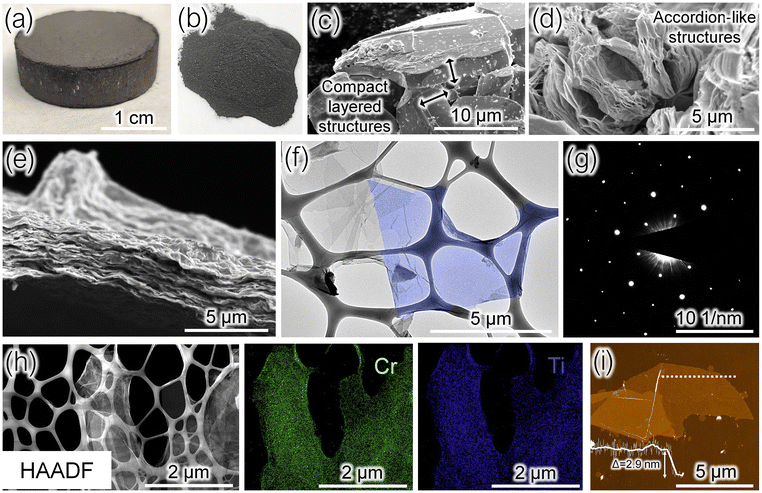
After the synthesis, Cr2TiAlC2 was crushed into a powder, and a fraction of particles with sizes of 30–40 μm was collected by sieving. The progression of these steps is demonstrated in Fig. 1, where we show an optical image of a sintered Cr2TiAlC2 pellet in Fig. 1a, a crushed MAX phase powder in Fig. 1b, and an SEM image of the sieved particles in Fig. 1c.After collecting powdered Cr2TiAlC2 material, the MAX phase was etched in a mixture of HF, HCl, and H2O for 48 h. Upon completion, the compact MAX phase particles transformed into MXene accordion-like structures7,23 as aluminum atoms were removed from Cr2TiAlC2. An SEM image of representative accordion-like structures with split MXene layers is shown in Fig. 1d. The delamination of accordion-like structures into monolayer Cr2TiC2Tx flakes was performed as described in the Experimental section, resulting in an aqueous suspension of MXene sheets. A vacuum filtration of this suspension produced a uniform Cr2TiC2Tx film that is shown in the SEM image in Fig. 1e. The film consists of stacked MXene flakes with no signs of MAX phase particles. The TEM analysis in Fig. 1f also confirms the formation of uniform Cr2TiC2Tx flakes with lateral sizes of a few μm. The SAED pattern in Fig. 1g demonstrates the hexagonal arrangement of the diffraction spots, corresponding to the structure of Cr2TiC2Tx. The expected uniform distribution of Ti and Cr elements in Cr2TiC2Tx flakes was confirmed by the STEM-EDX analysis in Fig. 1h. Finally, the monolayer character of MXene flakes was confirmed by AFM. An AFM image of a monolayer Cr2TiC2Tx flake with a lateral size of 10 μm and a thickness of about 2.9 nm is shown in Fig. 1i. Similar thicknesses of about 2.5 nm were previously reported for the monolayer flakes of Ti3C2Tx MXene deposited on Si/SiO2 substrates, while bilayer Ti3C2Tx flakes have significantly larger AFM thicknesses of >4 nm.5,24,25
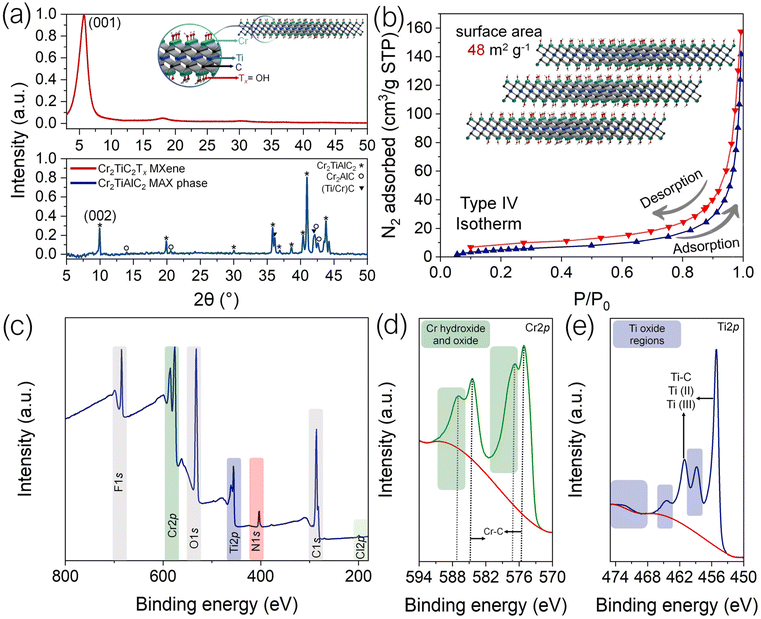
Cr2TiAlC2 and Cr2TiC2Tx were characterized by powder XRD, and the obtained patterns are presented in Fig. 2a. The quality of the parent MAX phase has a significant effect on the MXene synthesis.6,7,26 The produced Cr2TiAlC2 MAX phase was highly crystalline, and no significant impurity phases were recorded. The minor impurities in Cr2TiAlC2 were Cr2AlC and (Ti/Cr)C, which are labeled accordingly in Fig. 2a. The sample for the XRD analysis of Cr2TiC2Tx was prepared by vacuum filtering of suspension of MXene flakes and had a layered structure similar to the one shown in Fig. 1e. XRD pattern for the Cr2TiC2Tx film shows a major (001) diffraction peak at 2θ = 5.9°, which corresponds to the interlayer spacing between the MXene flakes of about 1.5 nm. There are also two smaller peaks at 2θ = 18.1° and 31.2° that represent higher-order (003) and (005) diffractions, respectively, and also originate from the layered structure of the stacked MXene flakes. No diffraction peaks related to the parent MAX phase or other possible crystalline impurities were recorded.
Fig. 2b shows BET data for a dried powder of Cr2TiC2Tx flakes. The sample shows a type IV adsorption isotherm with no plateau at high relative pressure (0.8–1), which is observed in mesoporous and macroporous systems. The BET surface area calculated for this sample is 48 m2 g−1, which is in accordance with reports on other MXene structures.17
Finally, we studied Cr2TiC2Tx by XPS to investigate the functional groups in the MXene material and its stability during preparation and post-synthesis processing. The results for the freshly prepared Cr2TiC2Tx MXene are presented in Fig. 2c–e. The XPS data are consistent with the previous report on Cr2TiC2Tx.9 The major peaks in the XPS survey spectrum in Fig. 2c confirm the presence of Cr, Ti, C, F, and O in Cr2TiC2Tx, suggesting the –F, –OH, and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups that are typical for MXenes produced by HF etching.27 The presence of these functional groups is important for the adsorption of heavy metal cations on the surface of MXene sheets. Fig. S1† shows the deconvoluted spectra of F 1s and O 1s. The XPS F 1s spectrum exhibits a dominant peak at 684.4 eV corresponding to Cr–F and a minor shoulder at 686.6 eV, which we interpret as residual fluoride impurities, such as AlF3. For O 1s, we assigned the components at 529.7, 531, 532.1, and 533.7 eV to Cr(Ti)–Ox, Cr–O, Cr–OH, and Cr–H2O, respectively.9,28 The N 1s peak that is observed in the survey spectrum can be explained by the use of TMAOH as intercalating agent, while the minor Cl 2p peak is present because of the use of HCl for the etching. The high-resolution spectra of Cr 2p and Ti 2p demonstrate the major metal carbide peaks but also suggest Cr–O and Ti–O bonding. Since we did not observe any visible signs of the MXene degradation in microscopic images, which is typically manifested as oxide particles and pinholes,25,29 these peaks can be attributed to the presence of oxygen in the C layers of Cr2TiC2Tx, which was established previously.8
O functional groups that are typical for MXenes produced by HF etching.27 The presence of these functional groups is important for the adsorption of heavy metal cations on the surface of MXene sheets. Fig. S1† shows the deconvoluted spectra of F 1s and O 1s. The XPS F 1s spectrum exhibits a dominant peak at 684.4 eV corresponding to Cr–F and a minor shoulder at 686.6 eV, which we interpret as residual fluoride impurities, such as AlF3. For O 1s, we assigned the components at 529.7, 531, 532.1, and 533.7 eV to Cr(Ti)–Ox, Cr–O, Cr–OH, and Cr–H2O, respectively.9,28 The N 1s peak that is observed in the survey spectrum can be explained by the use of TMAOH as intercalating agent, while the minor Cl 2p peak is present because of the use of HCl for the etching. The high-resolution spectra of Cr 2p and Ti 2p demonstrate the major metal carbide peaks but also suggest Cr–O and Ti–O bonding. Since we did not observe any visible signs of the MXene degradation in microscopic images, which is typically manifested as oxide particles and pinholes,25,29 these peaks can be attributed to the presence of oxygen in the C layers of Cr2TiC2Tx, which was established previously.8
3.2. Cr2TiC2Tx as a solid phase in d-μ-SPE for trace heavy metal detection
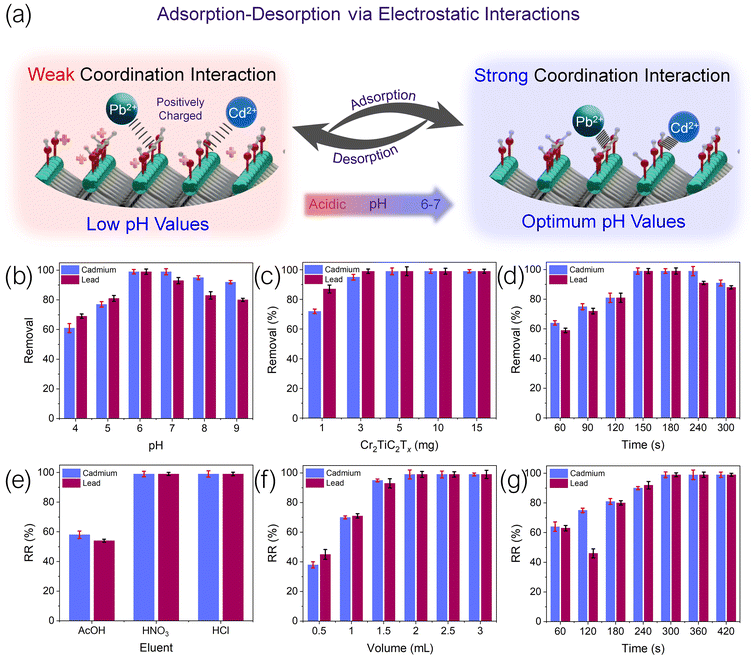
The scheme of coordination of heavy metal cations to the surface functional groups of Cr2TiC2Tx is shown in Fig. 3a. According to the literature on related adsorbent materials,30–32 this is likely the main adsorption mechanism, and it is expected to be pH-dependent. For Pb2+ and Cd2+ ions, the optimal pH was found to be 6 (Fig. 3b). The abundance of protons at low pH values results in positively charged MXene sheets, interfering with the adsorption of cations (Fig. 3a, left panel) and leading to less effective removal of heavy metals. At high pH values, lead and cadmium precipitate as insoluble oxides/hydroxides. The optimal removal of Pb2+ and Cd2+ was observed at pH 6 (Fig. 3b), when the adsorption of heavy metal cations was most efficient (Fig. 3a, right panel). A recent study suggests a possible presence of chromium vacancies in Cr2TiC2Tx,33 which may also provide adsorption sites for Pb2+ and Cd2+ ions. However, given the likely scarceness of these vacancies compared to the surface functional groups, we still expect the adsorption mechanism shown in Fig. 3a to be dominant in the d-μ-SPE process.
Fig. 3c demonstrates that Cr2TiC2Tx exhibits a great adsorption behavior, as evident by its high removal percentage at only 1 mg of adsorbent, especially for Pb2+ ions, with removal efficiency reaching 90%. Although complete removal was reached with 3 mg of Cr2TiC2Tx adsorbent, we chose 10 mg as the optimized adsorbent mass due to the difficulty in Cr2TiC2Tx recovery and the possible loss of material during the desorption steps. The d-μ-SPE method heavily relies on the efficiency of the dispersion of the adsorbent, which in this study was performed by sonication. We reached the highest removal in 150 s, and after 240 s the adsorption–desorption equilibrium shifts toward desorption and reduces the removal efficiency (Fig. 3d). Thus, 150 s was selected as the optimized sonication time for the adsorption step.
The desorption step is affected by the type of eluent, the volume and concentration of the eluent, and the sonication time for the dispersion of the adsorbent. As was the case for the adsorption step, we optimized the abovementioned factors, and the results are summarized in Fig. 3e–g. The most important factor for the desorption is the eluent type. As shown schematically in Fig. 3a, the coordination of heavy metals to the surface groups of Cr2TiC2Tx weakens in acidic environment, resulting in desorption of cations from MXene sheets. Fig. 3e demonstrates that for acetic acid as a representative weak acid, the recovery of heavy metal ions was less than 60%. However, strong acids, such as HCl and HNO3, successfully desorbed Pb2+ and Cd2+ ions. HCl was selected as the eluent for the next steps as a safer strong acid. The concentration of the acids for the desorption experiments was fixed at 2 M, however, the volume was optimized. Using HCl as the eluent, volume range of 0.5–3 mL was tested, and 2 mL was sufficient to yield the highest recovery (Fig. 3f). Finally, desorption of heavy metals from the Cr2TiC2Tx surface was achieved by sonicating the recovered adsorbent from the adsorption step in 2 mL of HCl (2 M) for 300 s (Fig. 3g). Lower sonication times were not sufficiently effective, leading to lower recovery percentages, while higher sonication times lead to deterioration of the adsorbent during the adsorption–desorption cycles.30–32
In summary, based on the results of the system optimization experiments, the optimized conditions for the adsorption of heavy metal ions on MXene sheets include pH 6, an adsorbent mass of 10 mg, and sonication time of 150 s. For the desorption step, the optimized procedure involves the use of 2 mL of 2 M HCl with sonication time of 300 s.
| Sample | Element | Added (μg g−1) | Found (μg L−1) ((mean ± ts)/N1/2) N = 3, PR = 0.05 | RR (%) |
|---|---|---|---|---|
| a Not added. b Not detected. | ||||
| Farm soil | Cd | NAa | 7.96 ± 0.32 | — |
| 25 | 32.98 ± 0.21 | 100.10 | ||
| Pb | NA | 30.53 ± 0.27 | — | |
| 25 | 55.14 ± 0.35 | 98.44 | ||
| Shrimp | Cd | NA | 8.13 ± 0.33 | — |
| 25 | 33.11 ± 0.51 | 99.92 | ||
| Pb | NA | NDb | — | |
| 25 | 25.13 ± 0.25 | 100.52 | ||
| Cod fish | Cd | NA | 6.13 ± 0.21 | — |
| 25 | 31.27 ± 0.12 | 100.56 | ||
| Pb | NA | ND | — | |
| 25 | 25.12 ± 0.26 | 100.48 | ||
| Coffee beans | Cd | NA | ND | — |
| 25 | 25.12 ± 0.12 | 100.48 | ||
| Pb | NA | ND | — | |
| 25 | 25.09 ± 0.11 | 100.36 | ||
| Rice | Cd | NA | 5.68 | — |
| 25 | 30.58 ± 0.12 | 99.60 | ||
| Pb | NA | ND | — | |
| 25 | 25.18 ± 0.25 | 100.72 | ||
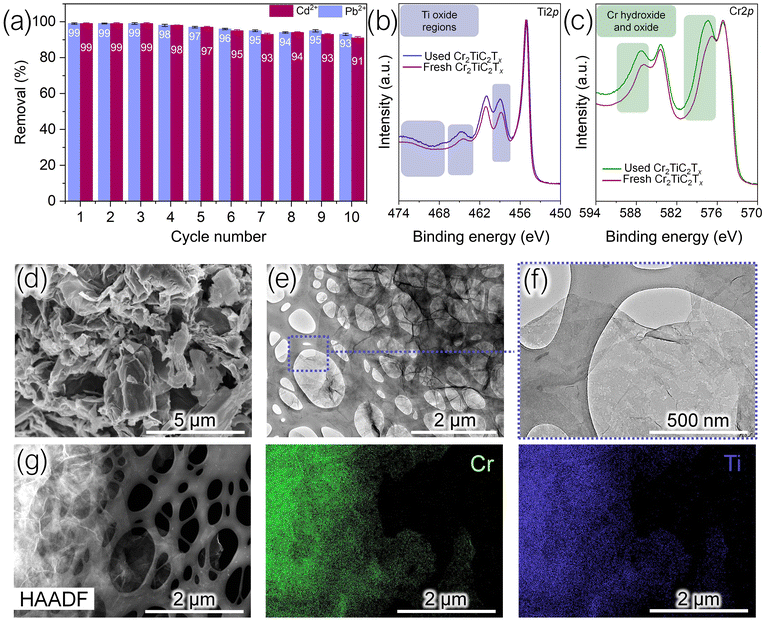
The stability of the MXene adsorbent over several cycles of adsorption and desorption was tested and an acceptable performance of about 96% of the initial performance was recorded for up to six cycles. By the tenth cycle, the performance decreased to 91% (Fig. 4a), likely due to sonication and loss of adsorbent. After the sixth cycle, XPS and electron microscopy were performed on the recovered Cr2TiC2Tx samples (Fig. 4b–g). The XPS Ti 2p and Cr 2p spectra of the MXene recovered after six cycles look very similar to the spectra of the freshly prepared Cr2TiC2Tx (Fig. 4b and c). While there is a noticeable increase of the XPS signal in the oxide regions of the spectra after six d-μ-SPE cycles, the change is rather small compared to oxidized MXene samples reported in literature,25,29 where the metal oxide peaks become much more intense than the metal carbide peaks. Therefore, the XPS results suggest modest degradation of Cr2TiC2Tx after six d-μ-SPE cycles.
SEM image of Cr2TiC2Tx that was recovered after six d-μ-SPE cycles (Fig. 4d) shows that the material retains its sheet-like morphology. Although the flakes appear aggregated and crumpled, this appearance is typical for dried MXene samples. A closer inspection of these flakes by TEM (Fig. 4e and f) demonstrates mesoscopic holes and tears, suggesting some degradation of Cr2TiC2Tx during the cycles, although we did not observe oxide particles decorating the flake edges, which typically appear when the oxidation of MXene sheets becomes more extensive.25,29,34 Some tearing of Cr2TiC2Tx flakes could be caused by sonication5,7 that was extensively used in the d-μ-SPE cycles. The STEM-EDX analysis (Fig. 4g) of the recovered MXene sample shows uniform elemental distribution of Cr and Ti, similar to the freshly prepared Cr2TiC2Tx. Overall, these results suggest modest degradation of Cr2TiC2Tx from cycle to cycle, so that the adsorbent material can be reused multiple times for efficient d-μ-SPE.
Finally, the developed Cr2TiC2Tx-based d-μ-SPE was compared with other methods and materials from recent literature, as summarized in Table 2. The Cr2TiC2Tx-based d-μ-SPE method shows a high performance and often exceeds the LOD values reported for other functionalized nanomaterials.
| Material | Method | LOD (Cd2+) (ng mL−1) | LOD (Pb2+) (ng mL−1) | Ref. |
|---|---|---|---|---|
| MOF-derived BCN | Electrochemical | 0.41 | 0.93 | 35 |
| MWCNT-2Fe3O4@SiO2-SH | VA-DMSPE-GFAAS | 0.09 | — | 36 |
| Ag modified ZnO | d-μ-SPE-FAAS | — | 32.7 | 37 |
| B. subtilis-MWCNT | SPE-ICP-OES | — | 0.024 | 38 |
| KCC-1 | d-μ-SPE-GFAAS | 0.02 | 0.18 | 39 |
| Magnetic orange peel powder | DMSS-FAAS | — | 2.64 | 40 |
| NH2/SH-functionalized Ti3C2Tx | d-μ-SPE-FAAS | 0.12 | 2.30 | 17 |
| Cr2TiC2Tx | d-μ-SPE-FAAS | 0.09 | 1.9 | This work |
4. Conclusions
This work demonstrates that Cr2TiC2Tx MXene is an efficient adsorbent for the d-μ-SPE method for trace detection of heavy metals in real-life samples. We synthesized a high-quality Cr2TiC2Tx and demonstrated uniform monolayer flakes with sizes in the μm range. The bulk MXene material had a BET surface area of 48 m2 g−1. We tested Cr2TiC2Tx for the adsorption and desorption of Pb2+ and Cd2+ ions and determined the following optimized conditions: for the adsorption step, pH 6, 10 mg of Cr2TiC2Tx as adsorbent, and sonication time of 150 s, and for the desorption step, 2 mL of 2 M HCl and sonication time of 300 s. Cr2TiC2Tx was shown as an efficient tool for preconcentrating heavy metal ions from agricultural samples followed by FAAS analysis. The developed method, Cr2TiC2Tx-d-μ-SPE, holds a great promise with attractive figures of merits. The LOD values for Cd2+ and Pb2+ ions were 0.09 and 1.9 ng mL−1, with large linear dynamic ranges of 0.3–90 and 6–110 ng mL−1, respectively. After the use, the MXene can be recovered for another d-μ-SPE, and in six consecutive cycles the material showed a performance of at least 96% of the first analytical experiment. By the tenth cycle, the performance decreased to 91%, likely due to sonication and loss of adsorbent. The results of experiments with interfering ions showed a promising Cr2TiC2Tx-d-μ-SPE performance with relative recoveries in a range of 99.67–102.34% and 99.78–101.18% for lead and cadmium ions, respectively.This study shows that Cr2TiC2Tx is highly efficient, selective, and reliable adsorbent that can open new avenues for addressing environmental and food safety challenges. Future studies of Cr2TiC2Tx could explore the possibility of its surface functionalization to improve the performance of the material as an adsorbent and tailor it for specific analytical applications. Furthermore, while most studies in the field of MXenes focus on Ti3C2Tx, this work demonstrates that there is a great promise in exploring other MXene materials as well. For example, we demonstrate that as an adsorbent for the d-μ-SPE method, Cr2TiC2Tx had better LOD values than NH2/SH-functionalized Ti3C2Tx that we tested in our previous study (Table 2).17 This result warrants an investigation of other MXene materials beyond Ti3C2Tx for analytical applications.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The work was supported by the National Science Foundation through awards OIA-2044049 (MXene synthesis) and OSI-2329159 (MXene characterization). Some experiments were performed using the instrumentation at the UNL instrumentation facilities supported by the National Science Foundation (award ECCS-2025298), the Nebraska Research Initiative, and the Nebraska Center for Energy Sciences Research.References
- Y. Gogotsi and B. Anasori, ACS Nano, 2019, 13, 8491–8494 CrossRef PubMed.
- B. Anasori and Y. Gogotsi, 2D Metal Carbides and Nitrides (MXenes): Structure, Properties and Applications, Springer International Publishing, 2019 Search PubMed.
- A. Vahidmohammadi, J. Rosen and Y. Gogotsi, Science, 2021, 372, eabf1581 CrossRef PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef PubMed.
- A. Lipatov, M. Alhabeb, M. R. Lukatskaya, A. Boson, Y. Gogotsi and A. Sinitskii, Adv. Electron. Mater., 2016, 2, 1600255 CrossRef.
- T. S. Mathis, K. Maleski, A. Goad, A. Sarycheva, M. Anayee, A. C. Foucher, K. Hantanasirisakul, C. E. Shuck, E. A. Stach and Y. Gogotsi, ACS Nano, 2021, 15, 6420–6429 CrossRef PubMed.
- M. Shekhirev, J. Busa, C. E. Shuck, A. Torres, S. Bagheri, A. Sinitskii and Y. Gogotsi, ACS Nano, 2022, 16, 13695–13703 CrossRef PubMed.
- P. P. Michałowski, M. Anayee, T. S. Mathis, S. Kozdra, A. Wójcik, K. Hantanasirisakul, I. Jóźwik, A. Piątkowska, M. Możdżonek, A. Malinowska, R. Diduszko, E. Wierzbicka and Y. Gogotsi, Nat. Nanotechnol., 2022, 17, 1192–1197 CrossRef PubMed.
- K. Hantanasirisakul, B. Anasori, S. Nemsak, J. L. Hart, J. Wu, Y. Yang, R. V. Chopdekar, P. Shafer, A. F. May, E. J. Moon, J. Zhou, Q. Zhang, M. L. Taheri, S. J. May and Y. Gogotsi, Nanoscale Horiz., 2020, 5, 1557–1565 RSC.
- D. Mohanadas, R. Rohani, Y. Sulaiman, S. A. Bakar, E. Mahmoudi and L.-C. Zhang, Mater. Today Sustainability, 2023, 22, 100411 CrossRef.
- Y. Sheth, S. Dharaskar, V. Chaudhary, M. Khalid and R. Walvekar, Chemosphere, 2022, 293, 133563 CrossRef PubMed.
- Z. Othman, H. R. Mackey and K. A. Mahmoud, Chemosphere, 2022, 295, 133849 CrossRef PubMed.
- M. Bilal and I. Ihsanullah, J. Water Process Eng., 2022, 49, 103010 CrossRef.
- M. Kandeel, A. Turki Jalil, M. Hadi Lafta, S. Ziyadullaev and Y. Fakri Mustafa, Microchem. J., 2022, 183, 108121 CrossRef.
- A. Dhillon, N. Singh, M. Nair and D. Kumar, Chemosphere, 2022, 303, 135166 CrossRef PubMed.
- A. S. Jatoi, N. M. Mubarak, Z. Hashmi, N. H. Solangi, R. R. Karri, Y. H. Tan, S. A. Mazari, J. R. Koduru and A. Alfantazi, Chemosphere, 2023, 313, 137497 CrossRef PubMed.
- S. Bagheri, R. Chilcott, S. Luo and A. Sinitskii, Langmuir, 2022, 38, 12924–12934 CrossRef PubMed.
- A. Parihar, N. K. Choudhary, P. Sharma and R. Khan, Environ. Pollut., 2023, 316, 120695 CrossRef PubMed.
- X. Zhang, D. An, Z. Bi, W. Shan, B. Zhu, L. Zhou, L. Yu, H. Zhang, S. Xia and M. Qiu, J. Electroanal. Chem., 2022, 911, 116239 CrossRef.
- K. Rizwan, A. Rahdar, M. Bilal and H. M. N. Iqbal, Chemosphere, 2022, 291, 132820 CrossRef PubMed.
- S. Hao, C. Liu, X. Chen, B. Zong, X. Wei, Q. Li, H. Qin and S. Mao, J. Hazard. Mater., 2021, 418, 126301 CrossRef PubMed.
- X. Zhu, B. Liu, H. Hou, Z. Huang, K. M. Zeinu, L. Huang, X. Yuan, D. Guo, J. Hu and J. Yang, Electrochim. Acta, 2017, 248, 46–57 CrossRef.
- S. Bagheri, A. Lipatov, N. S. Vorobeva and A. Sinitskii, ACS Nano, 2023, 17, 18747–18757 CrossRef PubMed.
- A. Lipatov, H. Lu, M. Alhabeb, B. Anasori, A. Gruverman, Y. Gogotsi and A. Sinitskii, Sci. Adv., 2018, 4, eaat0491 CrossRef PubMed.
- H. Pazniak, I. A. Plugin, M. J. Loes, T. M. Inerbaev, I. N. Burmistrov, M. Gorshenkov, J. Polcak, A. S. Varezhnikov, M. Sommer, D. V. Kuznetsov, M. Bruns, F. S. Fedorov, N. S. Vorobeva, A. Sinitskii and V. V. Sysoev, ACS Appl. Nano Mater., 2020, 3, 3195–3204 CrossRef.
- C. E. Shuck, M. Han, K. Maleski, K. Hantanasirisakul, S. J. Kim, J. Choi, W. E. B. Reil and Y. Gogotsi, ACS Appl. Nano Mater., 2019, 2, 3368–3376 CrossRef.
- J. Halim, K. M. Cook, M. Naguib, P. Eklund, Y. Gogotsi, J. Rosen and M. W. Barsoum, Appl. Surf. Sci., 2016, 362, 406–417 CrossRef.
- P. A. Maughan, L. Bouscarrat, V. R. Seymour, S. Shao, S. J. Haigh, R. Dawson, N. Tapia-Ruiz and N. Bimbo, Nanoscale Adv., 2021, 3, 3145–3158 RSC.
- M. J. Loes, S. Bagheri, N. S. Vorobeva, J. Abourahma and A. Sinitskii, ACS Appl. Nano Mater., 2023, 6, 9226–9235 CrossRef.
- Y. Zhang, L. Wang, N. Zhang and Z. Zhou, RSC Adv., 2018, 8, 19895–19905 RSC.
- B. M. Jun, S. Kim, J. Heo, C. M. Park, N. Her, M. Jang, Y. Huang, J. Han and Y. Yoon, Nano Res., 2018, 12, 471–487 CrossRef.
- K. Rasool, R. P. Pandey, P. A. Rasheed, S. Buczek, Y. Gogotsi and K. A. Mahmoud, Mater. Today, 2019, 30, 80–102 CrossRef.
- S. Bagheri, M. J. Loes, A. Lipatov, K. Acharya, T. R. Paudel, H. Lu, R. Khurana, M. I. Kholil, A. Gruverman and A. Sinitskii, Matter, 2024, 7, 4281–4296 CrossRef.
- A. Sarycheva, M. Shanmugasundaram, A. Krayev and Y. Gogotsi, ACS Nano, 2022, 16, 6858–6865 CrossRef PubMed.
- R. Huang, J. Lv, J. Chen, Y. Zhu, J. Zhu, T. Wågberg and G. Hu, J. Hazard. Mater., 2023, 442, 130020 CrossRef PubMed.
- P. d. S. Morales, P. M. dos Santos, A. E. De Carvalho and M. Z. Corazza, Food Chem., 2022, 368, 130823 CrossRef PubMed.
- O. Ozalp and M. Soylak, Talanta, 2023, 253, 124082 CrossRef CAS.
- S. Ozdemir, A. Dündar, N. Dizge, E. Kılınç, D. Balakrishnan, K. S. Prasad and N. Senthilkuma, Chemosphere, 2023, 317, 137840 CrossRef CAS PubMed.
- M. Behbahani, G. Rabiee, S. Bagheri and M. M. Amini, Microchem. J., 2022, 183, 107951 CrossRef CAS.
- F. A. Borges, L. M. Costa, C. e. R. T. Tarley, G. d. F. a. L. Martins and E. C. Figueiredo, Food Chem., 2023, 413, 135676 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr02556c |
| This journal is © The Royal Society of Chemistry 2025 |