Formation of hydrocarbons and carbon oxides in MXene reactions with water under varying oxidative conditions†
Shuohan
Huang
 *a,
Guanglei
Xiang
a and
Vadym N.
Mochalin
*a,
Guanglei
Xiang
a and
Vadym N.
Mochalin
 *bc
*bc
aState Key Laboratory of Advanced Fiber Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China. E-mail: huangs@dhu.edu.cn
bDepartment of Chemistry, Missouri University of Science & Technology, Rolla, MO 65409, USA. E-mail: mochalinv@mst.edu
cDepartment of Materials Science & Engineering, Missouri University of Science & Technology, Rolla, MO 65409, USA
First published on 21st February 2025
Abstract
Titanium carbide/carbonitride MXenes have garnered significant attention due to their remarkable properties, versatile solution processability, and broad range of potential applications. However, when exposed to the environment, MXenes are susceptible to degradation, which ultimately leads to the formation of metal oxides, a process that may be regarded as either disadvantageous or beneficial, depending on the application of a MXene and our knowledge about the underlying mechanisms. Therefore, it is very important to understand the reactivity of MXenes in different environments and conditions. Although researchers have made efforts to understand MXene degradation in air and water, our knowledge of the involved processes and even products of degradation remains incomplete. Here, we study the degradation of MXenes (Ti2CTx, Ti3C2Tx, and Ti3CNTx) under various oxidative conditions, in the presence of hydrogen peroxide, oxygen, ambient air, and argon. Gaseous products of MXene degradation in an aqueous environment were examined using gas chromatography (GC) equipped with a thermal conductivity detector (TCD) and a flame ionization detector (FID) working in series. In addition to methane and carbon dioxide, gaseous products including higher hydrocarbons were identified and analyzed. This research further deepens our understanding of the fundamental chemistry of MXenes.
Introduction
Two-dimensional (2D) transition metal carbides and nitrides, known as MXenes, exhibit a very broad range of compositions compared to other 2D materials.1 MXenes have the general formula Mn+1XnTx (n = 1–4), where M represents an early transition metal, X can be carbon, nitrogen, or oxygen, and T denotes surface terminations such as –OH, –F, and –O–. Over the past decade following their discovery, MXenes have demonstrated an exceptional combination of physical and chemical properties, leading to a broad spectrum of potential applications including electromagnetic interference (EMI) shielding,2,3 terahertz spectroscopy and communications,4 wear reduction and lubrication,5,6 energy storage,7–9 molecular/ionic sieving,10,11 sensors,12–14 composites,15,16 and many others.17–19 However, since their discovery, MXenes have been known to be unstable, particularly in aqueous colloidal solutions, spontaneously transforming into the corresponding metal oxides over time, a process that usually results in loss of their functional properties,20 but also provides opportunities to make new materials.13,21–23Initial reports have been focused on studying and preventing oxidation as the main mechanism of MXene degradation. After the initial discovery of MXene hydrolysis24 increasing attention has been directed toward understanding the chemical interactions between MXenes and water.20,25,26 In our own subsequent work, analysis of the gaseous products was established as a sensitive and unambiguous technique to detect and monitor MXene degradation,27 revealing that carbon and nitrogen of MXenes predominantly form CH4 and NH3, respectively. These experimental discoveries sparked the interest of theorists in using quantum chemical modeling to understand the underlying processes. Wu et al.28 investigated the interaction between water molecules and the basal plane of the Ti3C2O2 MXene using ab initio molecular dynamics (AIMD) simulations at room temperature (RT). Their findings revealed that the H2O attack begins with the attachment (chemisorption) of a water molecule onto a Ti atom. This is followed by breaking Ti–C bonds and deprotonation of the water molecule, resulting in the formation of Ti–OH on the Ti3C2O2 surface and the elimination of an H3O+ into the aqueous phase.
Both chemical composition and surface functionalization significantly influence MXene stability. More recently, Song et al.29 used AIMD to investigate the interaction between water and the defective Ti3C2O2 MXene at RT. Their findings reveal that water molecules occupy the O vacancies and dissociate into OH groups and hydronium ions. The hydroxyl groups increase the negative charge on the MXene surface, which repels water O atoms, hindering their attachment to the Ti sites, thereby reducing the likelihood of further reactions. Additionally, they observed that a high concentration of –F groups on the MXene surface could prevent its degradation. Nesterova et al.30 utilized enhanced sampling AIMD to study the role of terminating groups in the chemical stability of the Ti3C2Tx MXene during its contact with water. Their findings showed that the reactivity of Ti towards water depends on the local coordination of Ti sites and the chemical composition of the MXene surface.
Despite these theoretical advancements, AIMD simulations are limited to extremely short time scales, typically picoseconds, meaning they can only capture the very first steps of MXene–water reactions. However, given that MXene degradation is likely a multistep process involving concurrent hydrolysis and oxidation reactions as well as ionic equilibria and polycondensations ultimately leading to transition metal oxides observed as the final products, the theoretical approaches alone cannot fully resolve the reaction mechanisms. This highlights the need for experimental studies to investigate the long-term evolution of MXenes under various environmental conditions. Experimental data not only provide direct evidence of reaction products but also serve as a critical input for refining computational models, ultimately improving our understanding of MXene reactivity.
Studies of the reactions of metal carbides with water have a long history, with metal carbides traditionally being divided into two main classes: those that readily react with water or dilute acids, and those that do not.31,32 The carbides decomposable by water or aqueous mineral acids can be further subdivided into three groups: (a) forming methane during hydrolysis, like Fe3C, Be3C, and Al4C3; (b) forming acetylene upon hydrolysis, like the carbides of alkali, alkaline earth, and rare earth metals, where C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C linkages exist in the crystal lattice (e.g., CaC2); and (c) forming propyne on hydrolysis, like Mg2C3, because of C–C
C linkages exist in the crystal lattice (e.g., CaC2); and (c) forming propyne on hydrolysis, like Mg2C3, because of C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C linkages present in its lattice.32 Bulk TiC does not belong to any of these groups; it is inert towards water and is therefore traditionally classified, along with almost all other transition metal carbides, as non-reacting. This classification, although valid for bulk carbides, is being challenged when applied at the nanoscale to 2D forms of transition metal carbides, as we now know that titanium carbide MXenes spontaneously and completely react with water (hydrolysis) under ambient conditions (temperature, pH, and oxidizing strength), forming hydrocarbons and carbon oxides, thus showing dramatically different chemical properties compared to bulk TiC in the key reaction used for classification of carbides, i.e., their hydrolysis.
C linkages present in its lattice.32 Bulk TiC does not belong to any of these groups; it is inert towards water and is therefore traditionally classified, along with almost all other transition metal carbides, as non-reacting. This classification, although valid for bulk carbides, is being challenged when applied at the nanoscale to 2D forms of transition metal carbides, as we now know that titanium carbide MXenes spontaneously and completely react with water (hydrolysis) under ambient conditions (temperature, pH, and oxidizing strength), forming hydrocarbons and carbon oxides, thus showing dramatically different chemical properties compared to bulk TiC in the key reaction used for classification of carbides, i.e., their hydrolysis.
In view of the above, careful identification of gaseous products of MXene hydrolysis and oxidation is important not only for a better understanding of MXene chemistry and ways to improve performance in applications, but it also may help us to clarify chemical properties and classification of metal carbides broadly defined. Our initial studies hypothesized about the evolution of CO, CO2, CH4, and H2 during MXene hydrolysis, and experimentally confirmed the formation of CO2 and CH4.24,27,33 Later, Doo et al.34 detected the signals of CH4, CO, CO2, and HF during the degradation of the Ti3C2Tx MXene in water using gas chromatography coupled with time-of-flight mass spectrometry. These results establish a firm basis for the idea that tracing the carbon containing products of MXene hydrolysis can provide valuable information about the fundamental mechanisms of MXene reactivity. To emphasize this idea, we mention parallels with a large body of research on products of bulk carbide hydrolysis. Initial studies of hydrolysis of Mn3C carbide by Moissan revealed only CH4 and H2 as gaseous products, but later studies demonstrated that treatment of manganese carbide samples with hydrochloric acid solutions liberates only part of the carbide's carbon as volatile hydrocarbons, with free carbon and liquid hydrocarbons also being formed in the reaction.35 It is not unlikely that MXene hydrolysis, in addition to already reported CH4 and CO2, may yield other products, albeit in smaller quantities. Moreover, the specific ratio of concentrations of different carbon containing products may depend on the nature of 2D carbide, properties of the environment, and conditions of the hydrolytic degradation, for example, oxidation strength and acidity of the environment, temperature, etc.
In this study, we investigated the volatile carbonaceous degradation products of 3 different titanium MXenes (Ti2CTx, Ti3CNTx, and Ti3C2Tx) exposed to various oxidation environments (aqueous H2O2, O2, air, and Ar) using gas chromatography with a thermal conductivity detector (TCD) and a flame ionization detector (FID) working in series. The GC analysis of these products detected higher hydrocarbons in addition to methane, providing further important insights into the fundamental chemistry of 2D transition metal carbides and carbonitrides (MXenes).
Methods
Synthesis of MAX phases and MXenes
Ti2AlC was synthesized by mixing TiC (2 μm size powder, 99.5%, Alfa Aesar), Ti (−325 mesh, 99.5%, Alfa Aesar), and Al (−325 mesh, 99.5%, Alfa Aesar) powders in a molar ratio of 0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.15
1.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05. To prevent the formation of transition metal binary carbides, the initial concentration of Al was adjusted to be slightly higher than the stoichiometric amount.36 The mixture of dry powders was mechanically blended for 12 hours at RT, then heated at 10 °C min−1 to 1400 °C and held at this temperature for 4 hours under Ar flow. Ti3AlC2 was synthesized by mixing Ti, Al, and graphite powders (−325 mesh, 99%, Alfa Aesar) at a molar ratio of 3
1.05. To prevent the formation of transition metal binary carbides, the initial concentration of Al was adjusted to be slightly higher than the stoichiometric amount.36 The mixture of dry powders was mechanically blended for 12 hours at RT, then heated at 10 °C min−1 to 1400 °C and held at this temperature for 4 hours under Ar flow. Ti3AlC2 was synthesized by mixing Ti, Al, and graphite powders (−325 mesh, 99%, Alfa Aesar) at a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.88. This mixture was also blended for 12 hours and then heated at 10 °C min−1 to 1550 °C and held for 2 hours under Ar flow. Ti3AlCN was synthesized by mixing Ti, AlN (10 μm size powder, 98%, Sigma-Aldrich), and graphite powders at a molar ratio of 3
1.88. This mixture was also blended for 12 hours and then heated at 10 °C min−1 to 1550 °C and held for 2 hours under Ar flow. Ti3AlCN was synthesized by mixing Ti, AlN (10 μm size powder, 98%, Sigma-Aldrich), and graphite powders at a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was blended for 12 hours at RT, heated at 10 °C min−1 to 1550 °C, and held at this temperature for 2 hours under Ar flow. The resulting MAX phase ceramic samples were manually crushed using a mortar and pestle.
1. The mixture was blended for 12 hours at RT, heated at 10 °C min−1 to 1550 °C, and held at this temperature for 2 hours under Ar flow. The resulting MAX phase ceramic samples were manually crushed using a mortar and pestle.
The synthesis of Ti2CTx and Ti3C2Tx MXenes followed previously described methods.24,33 The as-synthesized MAX phase powder (Ti2AlC or Ti3AlC2, 0.3 g, 325 mesh, particle size <38 μm) was gradually added to an etchant solution prepared by dissolving 0.6 g of LiF (97%, Alfa Aesar) in 6 ml of 9 M HCl (36 wt%, Alfa Aesar) in a loosely capped plastic 50 mL centrifuge tube. The mixture was stirred for 36 hours at 35 °C. For the synthesis of the Ti3CNTx MXene from the Ti3AlCN MAX phase, 0.3 g of Ti3AlCN powder (325 mesh, particle size <38 μm) was slowly added to an etchant solution prepared by dissolving 0.8 g of LiF in 10 ml of 9 M HCl in a 50 mL plastic centrifuge tube. The mixture was stirred for 18 hours at 40 °C. After etching, the mixtures were washed several times by repeated centrifugation and addition of fresh portions of deionized water until the pH of the supernatant reached approximately 6. Aqueous colloidal solutions of MXenes were obtained by mild ultrasonication at RT for 30 minutes in an ultrasonic bath (Bransonic M2800H) followed by centrifugation for 1 hour at 3500 rpm (Thermo Fisher Scientific Sorvall ST8 Centrifuge). The concentration of the freshly prepared MXene colloidal solutions was approximately 5 mg mL−1.
Characterization of MAX phases and MXenes
X-ray diffraction (XRD) patterns of Ti3AlC2, Ti3AlCN, and Ti2AlC MAX phases, as well as Ti3C2Tx, Ti3CNTx, and Ti2CTx MXenes were obtained using a Bruker D8 Discover X-ray diffractometer (Diffrac Commander) with Cu Kα radiation (U = 40 kV and I = 40 mA). The measurements were recorded over a 2θ range from 5° to 85°.UV–vis spectra of MXene colloidal solutions were measured in a quartz cuvette (1 cm path length) using a PerkinElmer Lambda 35 UV–vis spectrometer, covering a wavelength range of 400–850 nm.
Raman spectra of the MXenes were collected using a Renishaw InVia Raman microscope equipped with a 532 nm laser. Spectra were obtained with a 50× objective and a 1200 lines per mm grating, with an exposure time of 30 s, 1% laser power, and 10 accumulations.
Degradation of MXene samples under different environmental conditions
Four 6 mL aliquots of each MXene solution were transferred into separate 10 ml headspace vials. To the first vial, 100 μL of 30% H2O2 (Certified ACS, Thermo Fisher Scientific) was added. The remaining three vials were purged either with O2 gas (UN1072 UHP, Airgas, 99.994%), ambient compressed air (laboratory-grade, moisture and CO2-free, filtered) or Ar gas (UN1006 UHP300, Airgas, 99.999%), correspondingly, by bubbling the gas through the solution for 10 minutes (Fig. S1†). Immediately after purging, the vials were sealed, placed upside down to prevent gas escape through the cap, and stored at room temperature for approximately 1 day for the Ti2CTx MXene and approximately 5 days for Ti3CNTx and Ti3C2Tx MXenes, before the aliquots of the gas phase were collected for analysis. For each environmental condition, the experiment was performed at least three times to ensure reproducibility of the results. The variability of gas composition measurements was within an acceptable range, with standard deviations provided in the ESI (Table S1†).Analysis of gases formed during MXene degradation
Gas aliquots collected from the MXene samples in water under different atmospheres were analyzed using a Thermo Scientific Trace-1300 gas chromatograph. The instrument was equipped with a fused silica capillary column (Carboxen 1006 PLOT, 30 m length, 0.53 mm diameter, 30 μm thickness) coupled in series with TCDs and FIDs. Helium was used as both carrier and reference gas with flow rates of 4 and 4.3 mL min−1, respectively. The parameters for the GC analysis were set as follows. For the first detector in series (TCD), the reference gas flow rate was 4.3 mL min−1, the purge flow rate was maintained at 4 mL min−1, and the TCD filament temperature was set to 280 °C. For the FID, the hydrogen gas, makeup gas (N2), and airflow rates were 35, 40, and 350 mL min−1, respectively, and the detector temperature was 250 °C. The column temperature was held at 50 °C for 4 minutes, then ramped up to 100 °C at a rate of 20 °C min−1 and held for 20 minutes. For each measurement, 0.1 mL of gas was manually injected into a GC injector using a 1 mL gastight syringe (Hamilton).Computational modeling of MXenes and bulk carbides
Atomistic models of monolayer Ti2C(OH)2, Ti3C2(OH)2, and Ti3CN(OH)2, as well as bulk CaC2 were built and fully optimized using density functional theory (DFT) implemented in CASTEP.37 Full geometry optimization with cell optimization was performed with the GGA PBE functional using ultrasoft pseudopotentials with Koelling–Hammon relativistic treatment with a plane-wave energy cut-off of 380 eV, 3 × 3 × 1 k points on a Monkhorst–Pack grid, and SCF tolerance of 5 × 10−7 eV per atom. Geometry was converged within 5 × 10−6 eV per atom energy, 0.01 eV A−1 force, 0.02 GPa stress, and 5 × 10−4 Å displacement. The parameters of the DFT optimized models are provided in Fig. S2.† These models were used to calculate the carbon–carbon pair distribution functions.Results and discussion
Fabrication of MXene dispersions involves etching of aluminum from MAX phases, followed by delamination of multilayer MXenes in aqueous solutions. XRD analysis of Ti3AlC2, Ti3AlCN, and Ti2AlC MAX phases, along with their corresponding MXenes, confirms good quality of our MAX phases2,24 and successful formation of MXenes, as evidenced by the shift of the (002) peak (Fig. 1a–c). UV–vis spectra show characteristic plasmon resonance peaks at ∼758, 655, and 501 nm for Ti3C2Tx, Ti3CNTx, and Ti2CTx dispersions, respectively (Fig. 1d–f).38 Raman spectra of the MXenes (Fig. 1g–i), obtained after drying from freshly prepared colloidal solutions, show the expected vibration modes of MXenes, with patterns consistent with the reported literature for Ti3C2Tx, Ti3CNTx, and Ti2CTx MXenes.24,39 The full-range Raman spectra in Fig. S3† show that the fresh MXenes do not exhibit any significant signals corresponding to sp2 or amorphous carbon. Overall, the results of XRD, UV-vis, and Raman characterization confirm the successful synthesis of MXenes. Furthermore, the morphology of the tested samples was examined using AFM to show that all MXene samples were in a similar size range and had similar surface morphology (Fig. S4†).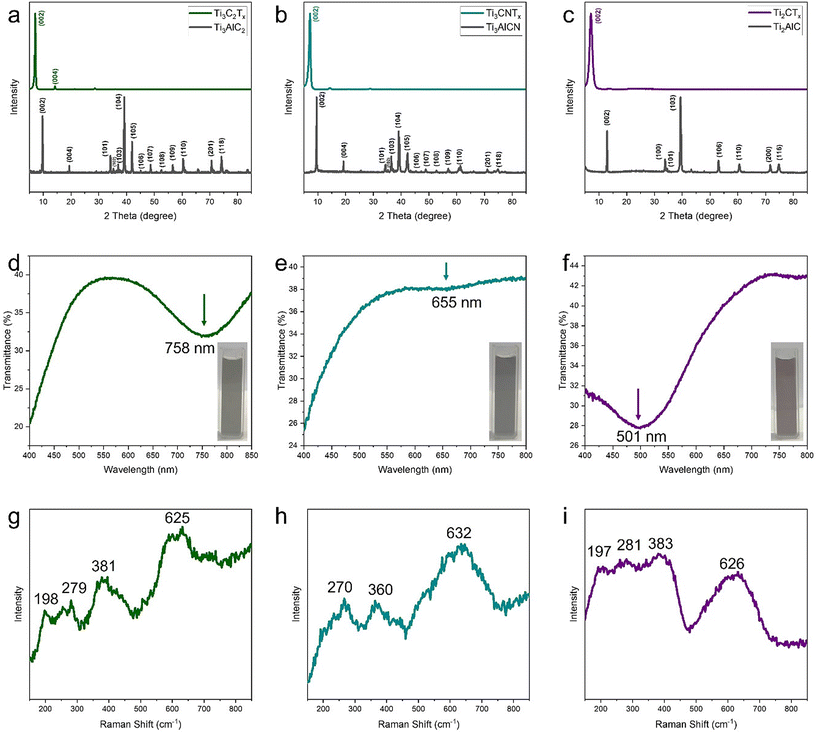 | ||
| Fig. 1 (a–c) XRD patterns of Ti3AlC2, Ti3AlCN, and Ti2AlC MAX phases and their corresponding MXenes. (d–f) UV-vis spectra and (g–i) Raman spectra of Ti3C2Tx, Ti3CNTx, and Ti2CTx MXenes, respectively. | ||
An example of gas chromatography results (Fig. 2) shows the composition of the gaseous products from hydrolysis of the Ti3C2Tx MXene in the presence of H2O2. On comparing the retention time of the detected compounds with the corresponding reference gases, we see signals of CO, CH4, and CO2 with the TCD, while the FID detected CO, CH4, C2H2 (acetylene), C2H4 (ethylene), and C2H6 (ethane), identified by the coincidence of the retention time of the corresponding peaks with the retention time of the corresponding reference gases under the same conditions. The FID is much more sensitive to hydrocarbons than the TCD; therefore, it is not unusual that smaller concentrations of hydrocarbons can be seen with the FID while going undetected with the TCD. The green-colored area in the bottom panel, which is a zoomed-in view of the corresponding narrow area of the FID signal labeled in the middle panel, highlights the peaks of the three mentioned C2 hydrocarbons and a CO peak. We note that this is the first time when in addition to the previously detected gaseous products (CO, CO2, and CH4), C2 hydrocarbons were detected among the degradation products of MXenes.
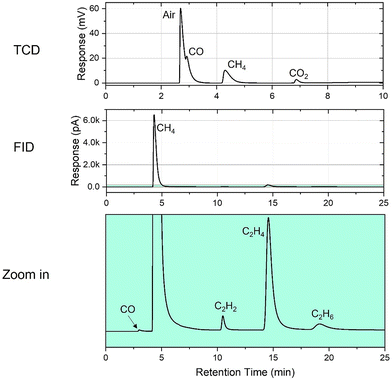 | ||
| Fig. 2 Gas chromatographic analysis of gaseous products collected from hydrolysis of Ti3C2Tx MXene in the presence of H2O2. | ||
Among metal carbides that react with water, LiC2, CaC2, SrC2, and BaC2 produce pure C2H2; Al4C3 and Be2C produce pure CH4; Mn3C forms a mixture of H2 and CH4; and rare-earth carbides such as CeC, LaC, YC2, and ThC2 react with cold water forming a complex gas mixture containing H2, C2H2, C2H4, and CH4.31 Based on the experimental results, titanium carbide MXenes exhibit properties similar to the above listed reactive metal carbides, rather than to their non-reacting bulk “cousin” TiC.
In the literature on bulk carbide hydrolysis, the formation of CH4 is typically explained by the presence of methylene (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) radicals, which are primary products that undergo hydrogenation and/or polymerization to methane or hydrocarbon molecules with more carbon atoms.32 For example, C2H6 is usually discussed as formed through the dimerization of CH3˙ radicals, which are present among hydrolysis products in small amounts. C2H2 is usually associated with preexisting C
CH2) radicals, which are primary products that undergo hydrogenation and/or polymerization to methane or hydrocarbon molecules with more carbon atoms.32 For example, C2H6 is usually discussed as formed through the dimerization of CH3˙ radicals, which are present among hydrolysis products in small amounts. C2H2 is usually associated with preexisting C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C linkages, and C2H4 with C
C linkages, and C2H4 with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linkages in the crystalline structure of carbides. However, as shown by radial distribution function analysis (Fig. 3), distances between any pair of carbon atoms in the MXene structure greatly exceed the C–C, C
C linkages in the crystalline structure of carbides. However, as shown by radial distribution function analysis (Fig. 3), distances between any pair of carbon atoms in the MXene structure greatly exceed the C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or C
C or C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond length (fully OH terminated models were considered, but changing surface terminations from OH to O, F, or a mixture thereof does not change this conclusion). The shortest C–C distances measured with the optimized models are 3.089 Å for Ti3C2(OH)2, 3.081 Å for Ti2C(OH)2, and 3.073–3.093 Å for Ti3CN(OH)2 in contrast to 1.253 Å for CaC2. As expected, Ti3CN(OH)2 shows more disorder manifested by slightly broader peaks in its C–C radial distribution function (Fig. 3b); therefore, we list a range of shortest distances for this MXene.
C bond length (fully OH terminated models were considered, but changing surface terminations from OH to O, F, or a mixture thereof does not change this conclusion). The shortest C–C distances measured with the optimized models are 3.089 Å for Ti3C2(OH)2, 3.081 Å for Ti2C(OH)2, and 3.073–3.093 Å for Ti3CN(OH)2 in contrast to 1.253 Å for CaC2. As expected, Ti3CN(OH)2 shows more disorder manifested by slightly broader peaks in its C–C radial distribution function (Fig. 3b); therefore, we list a range of shortest distances for this MXene.
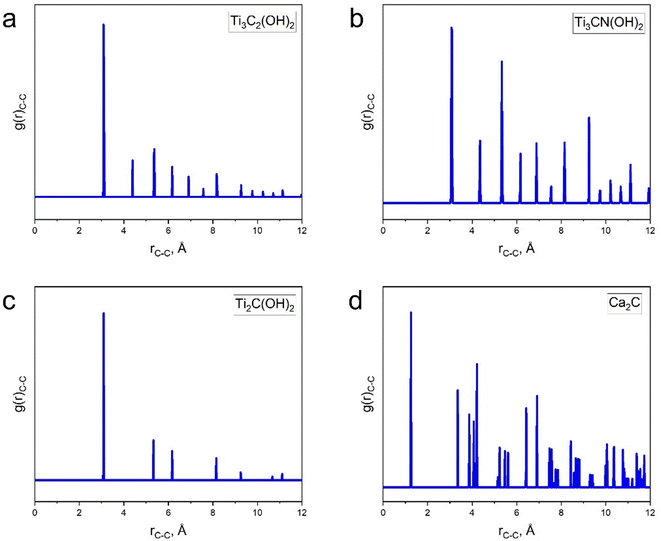 | ||
| Fig. 3 C–C pair distribution functions calculated from atomistic models of (a) Ti3C2(OH)2, (b) Ti3CN(OH)2, (c) Ti2C(OH)2 MXenes and (d) bulk CaC2, which were optimized with density functional theory. | ||
Because there is no existing carbon–carbon linkages in the crystalline structure of these MXenes, the most plausible way to form C2 or higher hydrocarbons during MXene hydrolysis is through secondary post-hydrolytic reactions, e.g., radical recombination, which is consistent with the low concentration of these larger molecules that we detected. Their low concentration explains why they were not detected before. Similar radical processes may be involved in the formation of free carbon during oxidative MXene degradation, as reported in some studies.40–42 However, no solid carbon signals were detected in our experiments after the complete degradation of MXenes in water, leading to the conclusion that all MXene carbon atoms probably end up in molecular species.27,43
The formation of CO in hydrolysis of metal carbides is rarely reported. For instance, only 0.22% of CO was detected when Mn3C was hydrolyzed by water at 20 °C.35 CO and CO2 are likely generated from the direct oxidation of MXene carbon by either H2O or dissolved O2. By considering MXene as an ionic crystal, the ionic nature of TixCy, i.e., 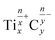 , suggests that electrons can be transferred from Ti atoms to C atoms within the MXene lattice. In the presence of the oxidizer, e.g. O2, once the C atoms gain electrons from the Ti atoms, they become more prone to reducing water, leading to the formation of carbon oxides.44
, suggests that electrons can be transferred from Ti atoms to C atoms within the MXene lattice. In the presence of the oxidizer, e.g. O2, once the C atoms gain electrons from the Ti atoms, they become more prone to reducing water, leading to the formation of carbon oxides.44
Having identified the main and minor gaseous products, we further investigate their quantitative composition. A commercially available standard mixture of different gases was used to determine the gas composition. According to the manufacturer, this mixture included 1.00 mol% of C2H2, 1.00 mol% of CO2, 1.00 mol% of CO, 1.02 mol% of C2H6, 1.00 mol% of C2H4, and 0.999 mol% of CH4 (Scotty48, 48 Liters@300 psig, 21 °C, analyzed gases). When no strong oxidizers are present in the aqueous MXene colloidal solution, the majority of carbon atoms in Ti3C2Tx convert into CH4. As the oxidation potential of the environment increases, the formation of carbon oxides also increases. As shown in Fig. 4a, the addition of H2O2 to Ti3C2Tx aqueous solution resulted in the production of 54.33 mol% CH4, 2.21 mol% C2 hydrocarbons, and 43.46 mol% carbon oxides. For samples purged with and kept under an O2 atmosphere, the measurements showed 88.51 mol% CH4, 3.94 mol% C2 hydrocarbons, and 7.54 mol% carbon oxides (Fig. 4b). In the ambient air environment, the results were 91.09 mol% CH4, 4.45 mol% C2 hydrocarbons, and 4.45 mol% carbon oxides (Fig. 4c). Ti3C2Tx aqueous colloids purged with Ar gas produced 93.44 mol% CH4, 4.42 mol% C2 hydrocarbons, and 2.14 mol% carbon oxides (Fig. 4d). Overall, as the environment became more reducing, the amount of CH4 among the gas products increased, the amount of C2 hydrocarbons remained relatively constant and small, while the formation of carbon oxides decreased. This is consistent with hydrolysis becoming a more pronounced pathway of MXene degradation compared with oxidation under these conditions. Among the detected C2 hydrocarbons, C2H4 was consistently formed in greater quantities than C2H6 and C2H2, with their amounts remaining relatively similar. Therefore, the nature and content of hydrolysis products of MXenes in water depend on the oxidation potential of the environment.
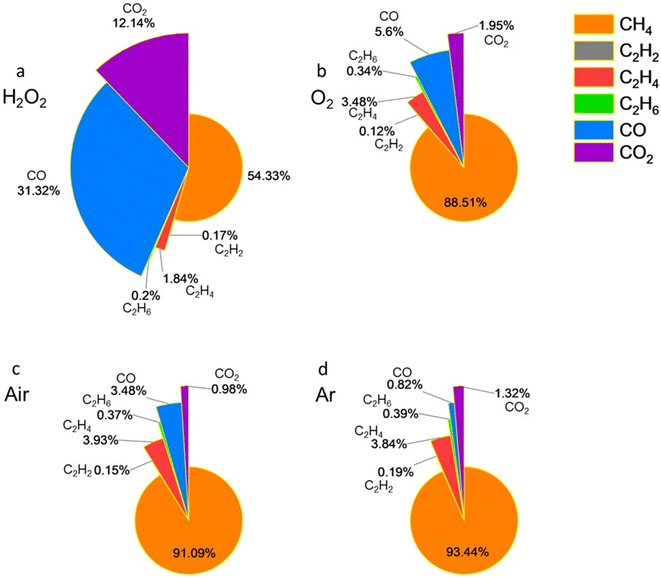 | ||
| Fig. 4 Gaseous product composition of Ti3C2Tx aqueous solution under the conditions of (a) H2O2, (b) O2, (c) air, and (d) Ar. | ||
As shown in Fig. 5a, the addition of H2O2 to Ti3CNTx aqueous colloidal solution resulted in the production of 33.38 mol% CH4, 2.14 mol% C2 hydrocarbons, and 64.48 mol% carbon oxides. Under an O2 atmosphere, the measurements gave 90.84 mol% CH4, 3.37 mol% C2 hydrocarbons, and 5.79 mol% carbon oxides (Fig. 5b). In an air environment, the results were 95.32 mol% CH4, 2.53 mol% C2 hydrocarbons, and 2.15 mol% carbon oxides (Fig. 5c). For Ti3CNTx samples purged with Ar gas, the analysis showed 96.75 mol% CH4, 2.17 mol% C2 hydrocarbons, and 1.08 mol% CO2 (well above ∼0.04% naturally occurring in ambient air), with no CO detected (Fig. 5d).
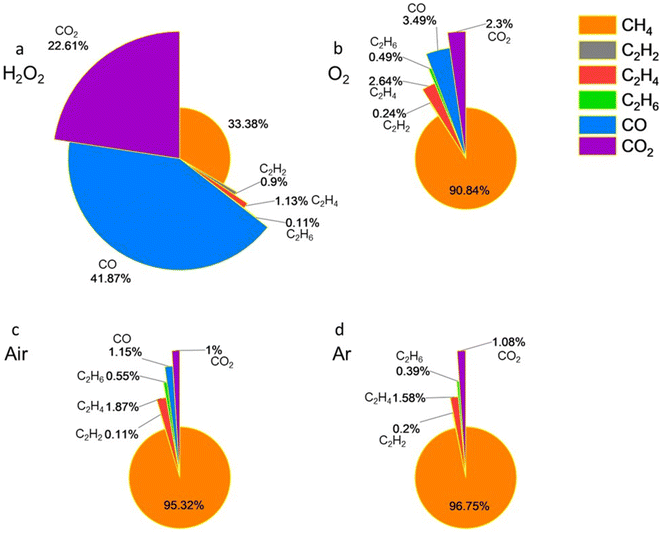 | ||
| Fig. 5 Gaseous product composition of Ti3CNTx aqueous solution under the conditions of (a) H2O2, (b) O2, (c) air, and (d) Ar. | ||
For the Ti3CNTx MXene with the addition of H2O2, only approximately 33 mol% of CH4 was formed, while more than 64 mol% of gaseous products were carbon oxides. As noted in our prior studies,27 compared to Ti3C2Tx, random substitution of C atoms with N in Ti3CNTx increases the reactivity of the material, making it more susceptible to oxidation. Correspondingly, the composition of gases evolved from Ti3CNTx under O2, air, and Ar atmospheres shows a typical ratio of products observed for other studied MXenes above, except that the percentage of CH4 increased compared to Ti3C2Tx in all three environments (O2, air, and Ar), indicating an even higher reactivity of Ti3CNTx in hydrolysis compared to its oxidation, as CH4 is a product of hydrolysis.
The addition of H2O2 to the Ti2CTx aqueous solution (Fig. 6a) resulted in the production of 75.72 mol% CH4, 5.41 mol% C2 hydrocarbons, and 18.87 mol% carbon oxides. Under an O2 atmosphere, 97.86 mol% CH4, 0.58 mol% C2 hydrocarbons, and 1.56 mol% CO2 were formed (with no CO detected, Fig. 6b). In an air environment, the results were 98.94 mol% CH4, 0.32 mol% C2 hydrocarbons, and 0.74 mol% CO2 (with no CO detected, Fig. 6c). For Ti2CTx samples purged with Ar gas, GC analysis showed 99.48 mol% CH4, 0.18 mol% C2 hydrocarbons, and 0.34 mol% CO2 (with no CO detected, Fig. 6d). It is worth noting that trace amounts of C2H2, specifically 0.0012 mol%, 0.0004 mol%, and 0.0004 mol%, were still detectable among the degradation products of the Ti2CTx MXene in contact with O2, air, and Ar atmospheres, respectively. But these amounts are insignificant and therefore we do not show them in Fig. 6b–d.
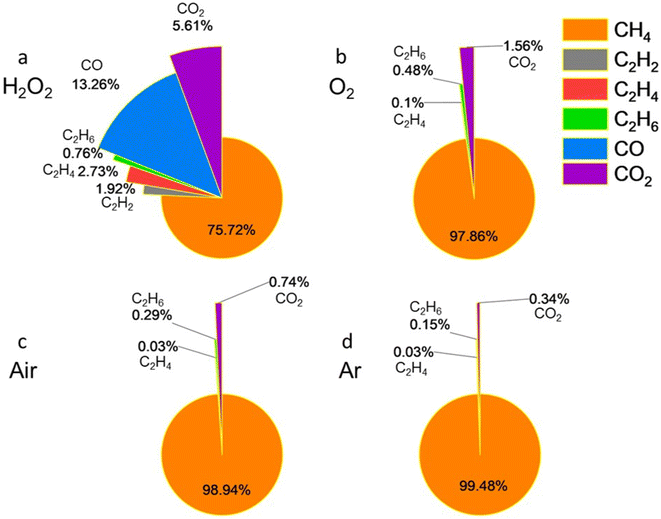 | ||
| Fig. 6 Gaseous product composition of Ti2CTx aqueous solution under the conditions of (a) H2O2, (b) O2, (c) air, and (d) Ar. | ||
For Ti2CTx, more CH4 was produced compared to the other MXenes. This might be explained assuming that higher hydrocarbons are formed in MXene hydrolysis via radical recombination processes, and since the Ti2CTx monolayer contains only one atomic layer of carbon whereas Ti3C2Tx and Ti3CNTx both contain two, the likelihood of recombination of two radicals formed in the process of stepwise C–Ti bonds breaking during Ti2CTx degradation is significantly lower compared to other studied MXenes with a thicker monolayer that provides a higher local concentration of C atoms and therefore higher likelihood of radical recombination. When the probability for recombination is low, it is more likely that all carbon atoms will eventually saturate their unsaturated valences by a step-wise process of binding hydrogen from water and forming CH4, as observed for Ti2CTx.
Interestingly, small but measurable quantities of H2 were detected among degradation products of Ti2CTx in aqueous colloids under both air and Ar atmospheres (Fig. 7). We never observed H2 formation with other MXenes and it is not quite clear how it can be formed. One hypothetical source of H2 might be H+, which was evidenced in MXene degradation by a drop in pH of solutions over time from freshly made until their complete degradation.27 These protons in solution could in turn originate either from not completely removed HF species or from the reactions of functional groups (at least some of the Tx contain hydrogen atoms, e.g., OH), or they could be protons formed by the attack of H2O on MXenes, when OH groups of water are attached to Ti atoms forming Ti(OH)n (n = 1–4) and hydrogen atoms can either attach to C atoms in MXenes forming hydrocarbons or, if there are not enough C atoms, will form protons that can be solvated by water and reduced to H2 later. In the latter case, when there are not enough C atoms for the reaction with all H atoms from the attacking water molecules, one cannot exclude recombination of H atoms from water with the direct formation of H2. It is not clear at present which of these processes (if any) results in the detected H2, but it is noted that we only detect H2 signal from Ti2CTx samples under air and, to a larger extent, under Ar atmospheres, i.e., when the oxidation strength of the environment is relatively low, which agrees with the abovementioned hypotheses.
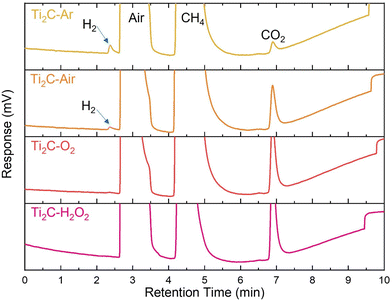 | ||
| Fig. 7 GC-TCD analysis of gaseous products from the hydrolysis of the Ti2CTx MXene in different environments. | ||
While the detection of C2 hydrocarbons among the products of MXene hydrolysis is interesting, we note that even more complex products have been observed in hydrolysis of some of the bulk carbides in the past. For example, bulk uranium carbide, U2C3, when reacted with water yields significant amount of liquid and solid hydrocarbons in addition to a gaseous mixture containing methane, ethylene, and hydrogen.31,45 Similarly, more complex products, including higher hydrocarbons, may also be anticipated in hydrolysis of MXenes, especially of those MXenes which have not been studied yet and thus, warrant further investigation in future research.
Conclusions
In conclusion, this study explores the degradation behavior of titanium containing MXenes (Ti2CTx, Ti3C2Tx, and Ti3CNTx) in water across various oxidation environments, including hydrogen peroxide, oxygen, air, and argon. Gas chromatography analysis revealed the formation of gaseous products such as methane, carbon oxides, and, for the first time, C2 hydrocarbons (ethylene, acetylene, and ethane), broadening our understanding of MXene chemistry. According to our results, methane is the dominant product under reducing conditions, while the production of carbon oxides increases in the environments with higher oxidation potential. This research offers insights into the hydrolysis and oxidative degradation mechanisms of MXenes, in comparison with bulk metal carbides, thereby challenging our traditional knowledge of carbide chemistry. We believe that this information will also contribute to a more rational development of various applications of MXenes.Author contributions
Shuohan Huang: writing – original draft, methodology, data curation, conceptualization, and funding acquisition; Guanglei Xiang: formal analysis, writing – review and editing, and validation; Vadym N. Mochalin: writing – review and editing, conceptualization, supervision, and funding acquisition.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (2232022D-03) and the National Natural Science Foundation of China (No. 52402102). We acknowledge funding support for this research from the National Science Foundation (DMREF-2324156).References
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- A. Iqbal, F. Shahzad, K. Hantanasirisakul, M.-K. Kim, J. Kwon, J. Hong, H. Kim, D. Kim, Y. Gogotsi and C. M. Koo, Science, 2020, 369, 446–450 CrossRef CAS PubMed.
- C. Liu, Y. Ma, Y. Xie, J. Zou, H. Wu, S. Peng, W. Qian, D. He, X. Zhang, B.-W. Li and C.-W. Nan, ACS Appl. Mater. Interfaces, 2023, 15, 4516–4526 CrossRef CAS PubMed.
- G. Li, N. Amer, H. A. Hafez, S. Huang, D. Turchinovich, V. N. Mochalin, F. A. Hegmann and L. V. Titova, Nano Lett., 2020, 20, 636–643 CrossRef CAS PubMed.
- S. Huang, K. C. Mutyala, A. V. Sumant and V. N. Mochalin, Mater. Today Adv., 2021, 9, 100133 CrossRef CAS.
- A. Rosenkranz, M. C. Righi, A. V. Sumant, B. Anasori and V. N. Mochalin, Adv. Mater., 2023, 35, 2207757 CrossRef CAS PubMed.
- J. Yang, M. Li, S. Fang, Y. Wang, H. He, C. Wang, Z. Zhang, B. Yuan, L. Jiang, R. H. Baughman and Q. Cheng, Science, 2024, 383, 771–777 CrossRef CAS PubMed.
- M. Lai, C. Zhao, D. Wang, R. Gao, P. Cai, L. Sun, Q. He, H. Peng, H. Zhang, F. Xu, C. Hu, K. Liang and C. J. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 55555–55568 CAS.
- W. Zhang, C. Zeng, M. Zhang, C. Zhao, D. Chao, G. Zhou and C. Zhang, Angew. Chem., Int. Ed., 2025, 64, e202413728 CrossRef CAS PubMed.
- X. Zhu, M. Lou, J. Chen, X. Fang, S. Huang and F. Li, Appl. Surf. Sci., 2023, 625, 157194 CrossRef CAS.
- Y. Li, X. Zhu, Q. Gao, Y. Bai, M. Lou, S. Huang, F. Li and B. Van der Bruggen, J. Membr. Sci., 2024, 712, 123236 CrossRef CAS.
- H. Zhang, Y. He, Y. Xia, Y. Chen, Y. Wang, R. Weng, T. Zhang and S. Huang, Mater. Today Chem., 2024, 38, 102088 CrossRef CAS.
- S. Chertopalov and V. N. Mochalin, ACS Nano, 2018, 12, 6109–6116 CrossRef CAS PubMed.
- Q. He, C. Zhao, H. Chen, T. Wu, C. Zeng, Y. Chen and C. Zhang, J. Mater. Chem. A, 2024, 12, 25622–25642 RSC.
- L. Bi, W. Perry, R. Wang, R. Lord, T. Hryhorchuk, A. Inman, O. Gogotsi, V. Balitskiy, V. Zahorodna, I. Baginskiy, S. Vorotilo and Y. Gogotsi, Adv. Funct. Mater., 2023, 34, 2312434 CrossRef.
- A. Baimenov, C. Daulbayev, S. G. Poulopoulos and V. N. Mochalin, Mater. Today, 2024, 78, 75–91 CrossRef CAS.
- S. Abdolhosseinzadeh, R. Schneider, M. Jafarpour, C. Merlet, F. Nüesch, C. Zhang and J. Heier, Adv. Electron. Mater., 2025, 11, 2400170 CrossRef CAS.
- T. Guo, D. Zhou, S. Deng, M. Jafarpour, J. Avaro, A. Neels, J. Heier and C. Zhang, ACS Nano, 2023, 17, 3737–3749 CrossRef CAS PubMed.
- T. Guo, D. Zhou, M. Gao, S. Deng, M. Jafarpour, J. Avaro, A. Neels, E. Hack, J. Wang, J. Heier and C. Zhang, Adv. Funct. Mater., 2023, 33, 2213183 CrossRef CAS.
- K. P. Marquez, K. M. D. Sisican, R. P. Ibabao, R. A. J. Malenab, M. A. N. Judicpa, L. Henderson, J. Zhang, K. A. S. Usman and J. M. Razal, Small Sci., 2024, 4, 2400150 CrossRef CAS.
- P. Ridley, C. Gallano, R. Andris, C. E. Shuck, Y. Gogotsi and E. Pomerantseva, ACS Appl. Energy Mater., 2020, 3, 10892–10901 CrossRef CAS.
- M. A. K. Purbayanto, M. Chandel, D. Bury, A. Wójcik, D. Moszczyńska, A. Tabassum, V. N. Mochalin, M. Naguib and A. M. Jastrzębska, Langmuir, 2024, 40, 21547–21558 CrossRef CAS PubMed.
- M. A. K. Purbayanto, D. Bury, M. Chandel, Z. D. Shahrak, V. N. Mochalin, A. Wójcik, D. Moszczyńska, A. Wojciechowska, A. Tabassum, M. Naguib and A. M. Jastrzębska, ACS Appl. Mater. Interfaces, 2023, 15, 44075–44086 CrossRef CAS PubMed.
- S. Huang and V. N. Mochalin, Inorg. Chem., 2019, 58, 1958–1966 CrossRef CAS PubMed.
- F. Cao, Y. Zhang, H. Wang, K. Khan, A. K. Tareen, W. Qian, H. Zhang and H. Ågren, Adv. Mater., 2022, 34, 2107554 CrossRef CAS PubMed.
- R. A. Soomro, P. Zhang, B. Fan, Y. Wei and B. Xu, Nanomicro Lett., 2023, 15, 108 CAS.
- S. Huang and V. N. Mochalin, ACS Nano, 2020, 14, 10251–10257 CrossRef CAS PubMed.
- T. Wu, P. R. C. Kent, Y. Gogotsi and D.-e. Jiang, Chem. Mater., 2022, 34, 4975–4982 CrossRef CAS.
- H. Song and D.-e. Jiang, Nanoscale, 2023, 15, 16010–16015 RSC.
- V. Nesterova, V. Korostelev and K. Klyukin, J. Phys. Chem. Lett., 2024, 15, 3698–3704 CrossRef CAS PubMed.
- G. N. H, Nature, 1896, 54, 357–357 CrossRef.
- G. L. Putnam and K. A. Kobe, Chem. Rev., 1937, 20, 131–143 CrossRef CAS.
- S. Huang, V. Natu, J. Tao, Y. Xia, V. N. Mochalin and M. W. Barsoum, J. Mater. Chem. A, 2022, 10, 22016–22024 RSC.
- S. Doo, A. Chae, D. Kim, T. Oh, T. Y. Ko, S. J. Kim, D.-Y. Koh and C. M. Koo, ACS Appl. Mater. Interfaces, 2021, 13, 22855–22865 CrossRef CAS PubMed.
- W. R. Myers and W. Fishel, J. Am. Chem. Soc., 1945, 67, 1962–1964 CrossRef CAS.
- M. N. Abdelmalak, MXenes: A new family of two-dimensional materials and its application as electrodes for Li-ion batteries, Drexel University, 2014 Search PubMed.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr. – Cryst. Mater., 2005, 220, 567–570 CrossRef CAS.
- K. Shevchuk, A. Sarycheva and Y. Gogotsi, MRS Bull., 2022, 47, 545–554 CrossRef CAS.
- S. Huang and V. N. Mochalin, Inorg. Chem., 2022, 61, 9877–9887 CrossRef CAS PubMed.
- H. Ghassemi, W. Harlow, O. Mashtalir, M. Beidaghi, M. Lukatskaya, Y. Gogotsi and M. L. Taheri, J. Mater. Chem. A, 2014, 2, 14339–14343 RSC.
- M. Naguib, O. Mashtalir, M. R. Lukatskaya, B. Dyatkin, C. Zhang, V. Presser, Y. Gogotsi and M. W. Barsoum, Chem. Commun., 2014, 50, 7420–7423 RSC.
- Y. Liu, Z. Shi, T. Liang, D. Zheng, Z. Yang, Z. Wang, J. Zhou and S. Wang, InfoMat, 2024, 6, e12536 CrossRef CAS.
- B. Ahmed, D. H. Anjum, M. N. Hedhili, Y. Gogotsi and H. N. Alshareef, Nanoscale, 2016, 8, 7580–7587 RSC.
- Y. Hori and T. Mukaibo, Bull. Chem. Soc. Jpn., 1968, 41, 1983–1989 CrossRef CAS.
- S. El Jamal, M. Johnsson and M. Jonsson, ACS Omega, 2021, 6, 24289–24295 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr04937c |
| This journal is © The Royal Society of Chemistry 2025 |
