Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in synthesis and application of Magnéli phase titanium oxides for energy storage and environmental remediation
S. Amanda
Ekanayake
 a,
Haoxin
Mai
a,
Haoxin
Mai
 a,
Dehong
Chen
a,
Dehong
Chen
 ab and
Rachel A.
Caruso
ab and
Rachel A.
Caruso
 *a
*a
aApplied Chemistry and Environmental Science, School of Science, STEM College, RMIT University, Melbourne, Victoria 3000, Australia. E-mail: rachel.caruso@rmit.edu.au
bCurrent Address College of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China
First published on 20th January 2025
Abstract
High-temperature reduction of TiO2 causes the gradual formation of structural defects, leading to oxygen vacancy planar defects and giving rise to Magnéli phases, which are substoichiometric titanium oxides that follow the formula TinO2n−1, with 4 ≤ n ≤ 9. A high concentration of defects provides several possible configurations for Ti4+ and Ti3+ within the crystal, with the variation in charge ordered states changing the electronic structure of the material. The changes in crystal and electronic structures of Magnéli phases introduce unique properties absent in TiO2, facilitating their diverse applications. Their exceptional electrical conductivity, stability in harsh chemical environments and capability to generate hydroxyl radicals make them highly valuable in electrochemical applications. Additionally, their high specific capacity and corrosion resistance make them ideal for energy storage facilities. These properties, combined with excellent solar light absorption, have led to their widespread use in electrochemical, photochemical, photothermal, catalytic and energy storage applications. To provide a complete overview of the formation, properties, and environmental- and energy-related applications of Magnéli phase titanium suboxides, this review initially highlights the crystal structure and the physical, thermoelectrical and optical properties of these materials. The conventional and novel strategies developed to synthesise these materials are then discussed, along with potential approaches to overcome challenges associated with current issues and future low-energy fabrication methods. Finally, we provide a comprehensive overview of their applications across various fields, including environmental remediation, energy storage, and thermoelectric and optoelectronic technologies. We also discuss promising new directions for the use of Magnéli phase titanium suboxides and solutions to challenges in energy and environment-related applications, and provide guidance on how these materials can be developed and utilised to meet diverse research application needs. By making use of control measures to mitigate the potential hazards associated with their nanoparticles, Magnéli phases can be considered as versatile materials with potential for next generation energy needs.
1. Introduction
Titanium dioxide (TiO2) is one of the most widely used semiconductors in industry owing to its high abundance, low cost, non-toxicity, chemical stability and biocompatibility.1–4 These properties have made TiO2 an excellent candidate for utilisation in a variety of diverse applications including optics, cosmetics, photovoltaic devices, photocatalysis and photoelectrochemical cells.2,4 However, the large bandgap of TiO2 (3.2 eV for anatase), which confines its light absorbance to the UV region and the high rate of electron–hole recombination, greatly limits its potential to be used in many other applications.5 Various approaches have been investigated to extend the light absorption of TiO2 into the visible region, including surface modification using organic materials, semiconductor coupling, creation of surface defects and oxygen vacancies (VO), non-metal doping and transition metal doping.6–8 Recent studies on defective titania have shown that the changes in physical and chemical properties of TiO2 such as electrical, thermal, optical and tribological properties can enhance its performance towards electrocatalytic and photocatalytic applications.9,10The introduction of defects in the lattice structure of TiO2 serves as a platform to tune its physicochemical and surface properties, making it useful in a wider range of applications. These defects can be produced by thermal annealing, electron bombardment, sputtering and via the inclusion of impurities such as Ca and H.11 Thermal annealing of TiO2 produces its reduced oxide form, due to the formation of VO and Ti interstitials. Low amounts of these vacancies (<10−4) are considered as point defects within the structure, whereas higher degrees of reduction form interstitial defects along with VO. Increasing the amount of VO during defect creation changes the O/Ti ratio in the TiO2 crystal structure, leading to the generation of titanium suboxides.12 At elevated temperatures, these VO arrange into planar defects known as crystallographic shear planes (CSPs). As the concentration of CSPs increases, the planes arrange into regular arrays known as Magnéli phases, with chemical formula TinO2n−1, where n can range from 4 to 9.12–14 Hence, the structure of Magnéli phases can be visualised as rutile chains, consisting of n number of unmodified octahedral blocks, interrupted by a CSP.15–17
Formation of VO, or its singly (VO+) or doubly (VO2+) charged components, result in delocalised electrons within the crystal structure that improve the electrical conductivity of the defective material.12 Hence, since their discovery by Arne Magnéli in 1957,18 Magnéli phase titanium suboxides have been widely used in the fields of electronics, photocatalysis, tribology, optoelectronics and thermomechanics due to their desirable features including excellent electrical and thermal conductivity, visible light absorptivity and chemical inertness.14,19–21 Magnéli phase titanium suboxides are used in various applications due to their unique structure, which imparts exceptional properties such as high electrical conductivity and strong corrosion resistance. For instance, Ti4O7, the most studied Magnéli phase, has an electrical conductivity of ∼1000 S cm−1, surpassing that of graphitic carbon (∼727 S cm−1).22 This high conductivity, combined with their oxygen evolution potential, makes Magnéli phases ideal for electrochemical applications like water splitting and cathodic protection.22 Additionally, Magnéli phases (n = 4–6) have been commercialised under the trade name Ebonex® and are utilised as electrode substrates in batteries and as catalyst supports in fuel cells and water treatment systems.23,24 Their stability in harsh chemical environments, such as fluoride-based etchants, hydrochloric acid and aqua regia, further expands their versatility in diverse electrochemical environments.25 Applications such as reactive electrochemical membranes (REMs) also take advantage of the material's ability to generate hydroxyl radicals (OH˙) through water oxidation.26 In battery applications, Magnéli phases are highly valued due to their excellent electrical conductivity and high specific capacity, allowing for significant energy storage and release. Their stability, corrosion resistance, and durability make them reliable materials for rechargeable batteries.27,28 In optics and photosensitivity-related applications, these suboxides are used for their visible light and near-infrared (NIR) photosensitivity, which results from VO created during the phase transformation from rutile to Magnéli.29 Furthermore, their high solar-to-vapour efficiency makes them suitable for photothermal applications, such as solar steam generation.30 These properties make Magnéli phases highly versatile, supporting their use in diverse fields, including energy storage, water treatment and solar energy harvesting.
Throughout the last few decades, studies have been conducted on various synthesis approaches to produce different types of titanium suboxides and examine their application in a range of areas, including for environmental remediation, energy generation or in optoelectronic devices. These aspects as well as the changes in TiO2 surface upon defect creation and their effect in catalytic applications have been previously reviewed.11,14,31–33 Although several reviews have focused on the literature related to titanium suboxides and defective (or “black”) TiO2,31,34–37 only a few have specifically addressed the synthesis and potential applications of Magnéli phase titanium suboxides.33,38,39 Some of these reviews not only discuss the ‘Magnéli phases’ but also provide a comprehensive overview of the synthesis and properties of all titanium oxides up to TiO, describing their structure, synthesis and performance. However, these reviews primarily focus on either the environmental remediation or electrochemical applications of Magnéli phase titanium suboxides, leaving their broader potential in areas such as thermoelectric and energy storage applications underexplored. To address this gap, this review provides a comprehensive overview of the literature on Magnéli phase titanium suboxides. We discuss the formation, synthesis and properties of the lower Magnéli phases (TinO2n−1, 4 ≤ n ≤ 9), along with recent advances (within the timeframe 2015–2024) in various fields, including environmental remediation, thermoelectric applications, energy storage and catalysis. In discussing the use of these materials across diverse applications, we explore the fundamental principles and mechanisms that underlie their performance, drawing connections among applications to guide readers in tailoring Magnéli phase titanium suboxides for specific uses. This review also highlights the challenges associated with their application in various fields, offering insights into potential future developments in the lesser-explored areas of Magnéli phase titanium suboxides.
2. Structure and properties of Magnéli phase titanium suboxides
2.1 Crystal structure
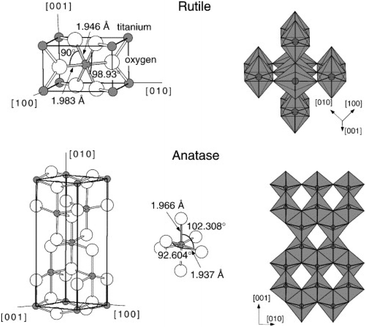 | ||
| Fig. 1 Bulk structures of rutile and anatase TiO2. Reproduced with permission from ref. 11. Copyright 2003, Elsevier. | ||
Intentional creation of defects in TiO2 with precise control of their position, concentration and distribution tailors the materials physicochemical properties and reactivity. Different types of defects introduced into TiO2 can be classified as follows.31,42,43
(I) Zero dimensional defects (point defects: oxygen and titanium vacancies and interstitials and substitutional impurities).
(II) One dimensional defects (line defects: edge or screw dislocations).
(III) Two dimensional defects (planar defects: grain and twin boundaries and stacking faults).
(IV) Three dimensional defects (volume defects: aggregates of atoms or vacancies to form precipitates or voids).
Defects in the TiO2 lattice can be introduced either by changing the O/Ti ratio or through the incorporation of high and low valence ions into the lattice to form donors and acceptors. These defects in titania can be generated during synthesis or post synthesis of the material.31,44 Some approaches used to induce defects in the TiO2 lattice include thermal annealing, electron bombardment, prolonged oxidation, partial oxidation, reducing agents (H2, C, metals), UV irradiation, high energy particle bombardment and vacuum activation.11 Calculated formation energies of point defects have shown that Ti-rich conditions favour the formation of VO and Ti interstitials, which behave as weak donors, whereas O-rich conditions lead to Ti vacancies that act as acceptors.45 Defect disorder in TiO2 as a variation of oxygen partial pressure was elucidated by Nowotny and co-workers. These findings suggested that Ti interstitials and electrons are formed at low oxygen partial pressures due to reduction of TiO2, whereas prolonged oxidation forms Ti vacancies and holes. In addition, due to the low formation enthalpy of VO and their prevalence across a broad range of stoichiometry in both reducing and oxidizing conditions, VO can be regarded as the dominant type of defect in TiO2.44
VO in metal oxides can be generated through various processes, such as thermal treatment in a vacuum or inert environment, chemical reduction at elevated temperatures, ion doping or interfacial engineering. When metal oxides undergo high-temperature treatment, lattice oxygen atoms may either desorb to release O2 (eqn (1)) or react with H2 or CO, forming H2O and CO2, respectively.46
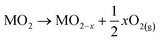 | (1) |
VO are the most common point defects in semiconducting metal oxides. This is due to their lowest formation energy among various types of defects that act as donors.47,48 Density functional theory (DFT) calculations indicate that the formation of VO introduces a defect level near the Fermi level, enhancing photoabsorption and electronic conductivity while reducing electron–hole recombination, which in turn extends the lifetimes of charge carriers. Additionally, VO can create unsaturated coordination that are more reactive, facilitating processes such as H2 evolution and CO2 reduction.46
By modifying the local atomic and electronic structures, VO act as centres for charge carrier separation, enhancing carrier separation efficiency. They can also adjust light absorption, influence the conductivity, and impact separation and surface reactions in semiconducting metal oxides.46,49 This results in changes in their chemical and physical properties including photocatalytic and photoelectrochemical effects, superconductivity, ferromagnetism, piezoelectric effect, redox activity and phase transitions.48 In addition, research has shown that these vacancies are involved in reactive oxygen species generation and acting as crucial adsorption and active sites in different applications.50
2.1.2.1 Structural changes occurring during defect creation. As shown in Fig. 2, in the rutile crystal lattice structure, each Ti atom is surrounded by six O neighbours and four Ti next-nearest neighbours while each O atom is bonded to 3 Ti atoms. Within rutile TiO2, the (110) surface is the most stable crystal surface, characterised by rows of titanium and oxygen atoms along the [001] direction.52 The titanium atoms on the surface exhibit five-fold coordination with one dangling bond and those in the bulk, six-fold coordination. Oxygen atoms can be present in-plane (bulk) or in bridging positions. Those in bulk are three-fold coordinated whereas the oxygen atoms in bridging positions are two-fold coordinated. Due to less coordination at the surface, these oxygen atoms are prone to removal during high-temperature treatments, leading to the formation of point defects.51
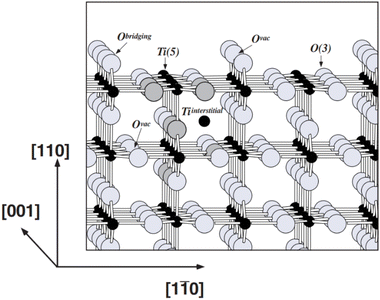 | ||
| Fig. 2 Ball and stick model of the rutile TiO2 (110) surface. Large grey balls represent oxygen atoms and small black balls represent titanium atoms. Two types of point defects that are prevalent in rutile TiO2, oxygen vacancies (Ovac) and Ti interstitials, are shown. Reprinted with permission from ref. 51. Copyright 2003, Springer Nature. | ||
During defect creation, surface oxygen atoms in the TiO2 lattice can be removed as a H2O molecule by a hydrogen reductant or O2 molecule by a non-hydrogen reductant, leading to the formation of surface VO.53,54 These VO can cause reconstruction within the TiO2 lattice or if the concentration of VO is very high it can eventually lead to lattice disorder.55 An oxygen vacancy is a double donor, where the position of the absent oxygen atom in the lattice can be occupied by two electrons leading to a neutral oxygen vacancy. Resonant photoemission spectroscopy confirms that these electrons have the ability to partially occupy the Ti 3d state, resulting in the creation of an energy state approximately 0.8 eV below the Fermi level.56 Furthermore, these electrons can also interact with neighbouring Ti4+ giving Ti3+ centres and VO+ or VO2+. Ti3+ formed on the lattice surface could react with adsorbed H2O and O2 molecules giving rise to –OH groups and O2− centres.57,58 The rearrangement of atomic positions within the lattice structure induced by VO can ultimately result in the reduction in Ti–O bond length. This in turn, can create a strain on the TiO6 octahedra leading to the formation of Ti–O systems with different octahedral packing.59
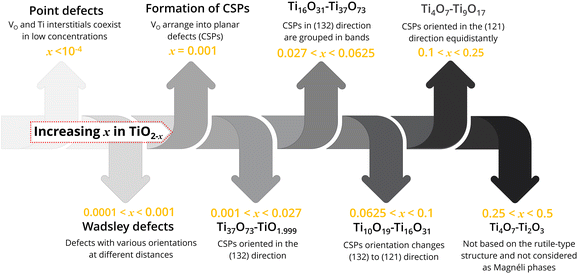
2.1.2.2 Formation of crystallographic shear planes and structural changes in TiO2−x. Defective TiO2 (TiO2−x) and titanium suboxides are formed because of VO and ionisation of these VO which at the same time correspond to the reduction of Ti4+ to Ti3+.60 Depending on the value of x in TiO2−x, the types of defects present in TiO2−x varies, along with their crystallographic structure, see Fig. 3. Slightly reduced TiO2 (TiO2−x, x < 10−4) is considered to only have point defects distributed in the matrix, where VO and Ti interstitials coexist in low concentrations.61 When the x value in TiO2−x falls between 0.001 and 0.0001, these are known as Wadsley defects. The increasing number of defects increases defect interaction and leads to defect ordering and structural transformation. Thereby, new compounds with different crystallographic structures are formed.
When the nonstoichiometry x in TiO2−x reaches a critical value of x = 10−3 it causes VO to arrange into planar defects known as CSPs.31,60 Formation of this shear structure can be considered as a way of eliminating point defects, forming regularly arranged extended planar defects.60 At CSPs, lattice planes move relative to each other, causing a collapse in the lattice structure. Such CSPs are common for d0 oxides including Ti, V, Mo and W.17 The structure of titanium suboxides with CSPs is described as a chain of rutile-like TiO6 octahedra interrupted every nth octahedron by a shear plane, where the octahedra share faces at the shear plane instead of usual edges and corners within the structure.32
When the x value in TiO2−x falls between 0.027 and 0.001, new Ti37O73–TiO1.999 structures are formed. In these structures, the CSPs are oriented in the (132) direction.31,60 When x reaches 0.0625–0.027, Magnéli phases from Ti16O31–Ti37O73 are formed. In these structures, the shear planes oriented in (132) are grouped in bands.62 When x in TiO2−x = 0.1–0.0625, higher Magnéli phases from Ti10O19 to Ti16O31 are formed. In these crystal structures, the shear plane orientation continuously changes from (132) direction to (121). When the x in TiO2−x falls between 0.25 and 0.1 (for Ti4O7–Ti9O17), CSPs are oriented in the (121) plane equidistantly, forming ordered arrays of VO.
2.1.2.3 Magnéli phases (TinO2n−1, 4 ≤ n ≤ 37). Titanium suboxides with CSPs in their structure are known as Magnéli phases and can be denoted by the formula TinO2n−1, where 4 ≤ n ≤ 37.32 Based on the concentration of CSPs (value of n), these Magnéli phases can be classified into two groups: in the series of suboxides with 10 ≤ n ≤ 37 (higher Magnéli phases), the CSP is introduced in the (132) plane in the structure of rutile TiO2, whereas the direction of the CSP changes to the (121) plane when 4 ≤ n ≤ 9 (Fig. 4). In all Magnéli phase suboxides, across the CSPs, rutile slabs are displaced relative to each other by the
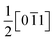 vector, also known as the displacement vector.63 Such displacement requires the removal of an oxygen plane, thereby making the CSP oxygen deficient in comparison with rutile TiO2.16 Considering the nonstoichiometry of these Magnéli phases, the y value of TiOy in higher Magnéli phases can range from 1.933 ≤ y ≤ 1.972 whereas for the lower Magnéli phases it ranges from 1.750 ≤ y ≤ 1.889.64
vector, also known as the displacement vector.63 Such displacement requires the removal of an oxygen plane, thereby making the CSP oxygen deficient in comparison with rutile TiO2.16 Considering the nonstoichiometry of these Magnéli phases, the y value of TiOy in higher Magnéli phases can range from 1.933 ≤ y ≤ 1.972 whereas for the lower Magnéli phases it ranges from 1.750 ≤ y ≤ 1.889.64
 | ||
| Fig. 4 Crystallographic shear structure of Magnéli phase titanium suboxides showing the shear plane formation along the (a) (121) plane and (b) (132) plane. Reprinted with permission from ref. 63. Copyright 2010, AIP Publishing. | ||
2.1.2.4 Lower titanium suboxides. In the octahedral unit cell of Ti3O5 (space group C2/m), Ti atoms are surrounded by O atoms and are located at three distinct sites in the crystal, two Ti3+ and one Ti4+ sites.65 The presence of two types of metallic ions within the unit cell causes a distorted octahedron due to different Ti–O distances.66 Ti2O3 is reported to have the corundum (α-Al2O3) structure, with its stoichiometry extending from TiO1.49 to TiO1.51.18,66 Its fundamental unit cell comprises of pairs of Ti3+ ions situated in octahedral sites. TiO possesses a defective NaCl crystal structure (space group Fm
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), wherein there are an equivalent number of vacancies distributed randomly on both the cation and anion sites, with its homogeneity ranging from TiO0.64 to TiO1.26.14,18
m), wherein there are an equivalent number of vacancies distributed randomly on both the cation and anion sites, with its homogeneity ranging from TiO0.64 to TiO1.26.14,18
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1] direction of the rutile structure. This direction aligns with a lattice vector of the oxygen subnet—namely, the vector [0
1] direction of the rutile structure. This direction aligns with a lattice vector of the oxygen subnet—namely, the vector [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1] that connects two oxygen atoms in the rutile crystal. This allows mapping of the dislocated atoms of that species onto atoms of the same species, while leaving the lattice positions of the oxygen atoms unchanged.67
1] that connects two oxygen atoms in the rutile crystal. This allows mapping of the dislocated atoms of that species onto atoms of the same species, while leaving the lattice positions of the oxygen atoms unchanged.67
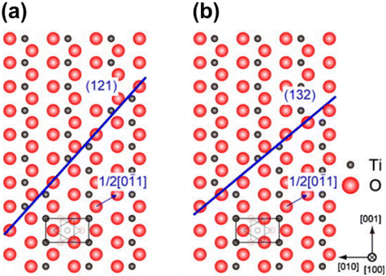
The rutile phase features an ordered arrangement of both oxygen and titanium atoms along the [001] direction. In contrast, the Magnéli phase maintains an ordered arrangement of oxygen atoms along the [001] direction but exhibits a titanium atomic mismatch along the (121) plane.69 The lattice structure of lower Magnéli phases can therefore be understood as a repeated pattern (block) of edge- and corner-sharing TiO6 rutile octahedra (as illustrated in Fig. 5(a)) that extend in two directions. However, in the third direction these blocks are disrupted by a plane of VO in the (121) direction at every nth octahedra.15,16,69 This plane is formed of face sharing TiO6 octahedra, resembling a corundum (Ti2O3) structure (Fig. 5(b)). The boundaries created by the corundum structures in Magnéli phases are known as shear planes as illustrated in Fig. 5(c).67 This layer of face sharing octahedra serves both as the last layer of one block and the first layer of the adjacent one. This causes titanium atoms of one block to relate to the unoccupied or interstitial positions of the adjacent block. As a result, the symmetry of the structure changes from tetragonal to triclinic as the size of the unit cell increases.21 Parallel rutile blocks containing n number of octahedra (referred to as pseudorutile chains) link together through the terminal face sharing octahedron,41,67,70 leading to electronic interactions between the corresponding titanium atoms.41,71 Hence, in Magnéli phase, there is an ordered arrangement of oxygen atoms along the [001] direction, while a titanium atomic mismatch occurs on the (121) plane.69
 | ||
| Fig. 5 (a) Edge, corner and face sharing orientations of TiO2 octahedra in Magnéli phase titanium suboxides. Reproduced with permission from ref. 23. Copyright 2010, Elsevier. (b) Corundum Ti2O3 viewed parallel to the c axis and (c) structure of Ti4O7, showing four-unit rutile chains along the c direction bounded by corundum structures restricted in the (001) planes. Blue spheres represent the Ti atoms while the smaller red spheres represent O atoms.67 | ||
Based on crystal structures previously reported for Magnéli phase titanium suboxides,70,72,73 Bowden et al. calculated X-ray powder diffraction patterns and compared them with experimental diffraction patterns.74 Calculated patterns for n = 4, 5 have shown good representation of the reported crystal data, though experimental patterns for n > 5 patterns were not presented due to the difficulty in obtaining their single phases.74 Crystal structure parameters for these Magnéli phase suboxides are given in Table 1.74
| Space group | a (Å) | b (Å) | c (Å) | α (Å) | β (Å) | γ (Å) | Reference | |
|---|---|---|---|---|---|---|---|---|
| Ti4O7 |
A![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
5.593 | 7.125 | 12.456 | 95.02 | 95.21 | 108.87 | 72 |
| Ti5O9 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
5.569 | 7.120 | 8.865 | 97.55 | 112.34 | 108.50 | 73 |
| Ti6O11 |
I![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
5.552 | 7.126 | 32.233 | 66.94 | 57.08 | 108.51 | 75 |
| Ti7O13 |
I![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
5.537 | 7.132 | 38.151 | 66.70 | 57.12 | 108.50 | 75 |
| Ti8O15 |
I![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
5.526 | 7.133 | 44.059 | 66.54 | 57.17 | 108.51 | 75 |
| Ti9O17 |
I![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
5.524 | 7.142 | 50.03 | 66.41 | 57.20 | 108.53 | 75 |
2.2 Physical properties of Magnéli phase titanium suboxides
| σ = enμ | (2) |
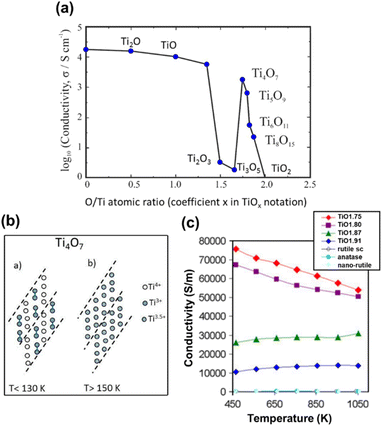 | ||
| Fig. 6 (a) Phase diagram of Magnéli phase titanium suboxides showing the variation of log of conductivity with oxygen stoichiometry. (b) Schematic representation of Ti4O7, showing the presence of Ti3+, Ti3.5+ and Ti4+ arranged in chains, interrupted by CSPs. At low temperature, Ti4O7 is non-metallic and clearly distinguishable Ti3+ and Ti4+ oxidation states are present. Reprinted with permission from ref. 32. Copyright 2022, Taylor & Francis. (c) Electrical conductivity as a function of temperature for rutile single crystals (sc), nano-rutile, anatase and different Magnéli phases. Reprinted with permission from ref. 86. Copyright 2012, Springer Nature. | ||
Magnéli phase titanium suboxides have highest conductivity for lowest n values (n = 4, 5) and exhibit phase transitions with temperature. Such transitions have been attributed to the presence of both Ti3+ and Ti4+, which provide possibilities for electron localisation at these cation sites, leading to different charge ordered states.84 Ti4O7, the most conductive Magnéli phase titanium suboxide, is reported to undergo two phase transitions with temperature. At 130 K it undergoes a semiconductor–semiconductor transition and a semiconductor–metal transition at 150 K.15,85 Similar observations had previously been made by Bartholomew et al. in their electrical property measurement of some titanium oxide Magnéli phases. They reported that Ti4O7 showed metallic behaviour at room temperature and undergoes a phase transition at 149 ± 2 K to a semiconducting phase, with a reduction in conductivity, followed by a significant decrease in magnetic susceptibility. A second transition has been observed at 125 K with three-orders of magnitude decrease in conductivity, but with no change in susceptibility.21 At low temperatures, the Ti3+ in Ti4O7 covalently bond with each other, forming bipolarons (Ti3+–Ti3+ pairs) and occupy alternate pseudorutile chains to form a low temperature (LT) semiconducting phase. A reordering of these Ti3+–Ti3+ pairs occurs during semiconductor–semiconductor transition from LT to intermediate temperature (IT). At high temperature (HT) all Ti cations are present as Ti3.5+ with no apparent pairing leading to highly conductive metallic behaviour (Fig. 6(b)).72,85,87 Watanabe et al. measured the Raman spectra of Ti4O7 as a function of temperature and observed a stable charge ordered state across the electronic phase transition temperatures.88,89 Magnetic susceptibility measurements conducted for the material showed a strong increase in paramagnetic susceptibility at 150 K where the semiconductor–metal transition takes place. However, at temperatures below 150 K, it is antiferromagnetic.90,91
Such phase transitions have also been observed in Ti5O9, at 128 K and 139 K. Although LT and IT phases are semiconducting, the conductivity of the HT phase increases with increasing temperature, making it difficult to be classified as a true metal.15,21,84 Electrical conductance measured for higher n Magnéli phases have shown that two transitions occur for Ti6O11 at 147 K and 119 K and one transition for Ti7O11 at 120 K separating regions showing non-metallic behaviour.15,21 However, conductance measurements carried out for Ti8O15 and Ti9O17 have not shown any clear steps between 4 K < T < 295 K. But a discontinuous change in the first derivative has been observed at 120 K, which was more evidently observed in Ti7O13.15 Bartholomew et al. reported that higher titanium suboxides (Ti8O15) were found to be semiconducting over the temperature range 78–295 K.21
Fan et al. compared the electrical conductivities of Ti4O7 and Ti9O17 samples prepared via hydrogen reduction. The study revealed that Ti4O7 has an electrical conductivity of 9.92 × 104 S m−1 at room temperature (RT), which decreases as temperature rises. This metallic behaviour further confirmed that Ti4O7 becomes a highly paramagnetic metal following a semiconductor-to-metal transition above 150 K, due to the delocalisation of 3d electrons from Ti3+ ions. On the other hand, Ti9O17 exhibited typical semiconductor characteristics from RT to 817 K, with electrical conductivity nearly an order of magnitude lower than that of Ti4O7 at RT, reflecting a lower level of electron doping. Furthermore, the carrier concentration of Ti4O7 was an order of magnitude higher than that of Ti9O17, with values of 17.2 × 1021 cm−3 for Ti4O7 and 1.83 × 1021 cm−3 for Ti9O17. These findings suggested that CSPs do not act as sources of electron diffraction, since the concentration of CSPs is higher in Ti4O7 compared to Ti9O17.77 Backhaus-Ricoult and co-workers reported changes in electrical conductivity as a function of temperature for ceramic powders (TiO2−x, x = oxygen deficiency) containing mixtures of Magnéli phases. TiO1.75 was composed of n = 4–5 (in TinO2n−1) phases with Ti2O3, TiO1.80 with n = 4–6 phases and Ti2O3, TiO1.87 with n = 6–8 phases and TiO1.91 composed of other (possibly higher) Magnéli phases. Their observations showed that the electrical conductivity increased with increasing oxygen deficiency (Fig. 6(c)). Ceramics with lower deficiency demonstrated semiconducting behaviour with increasing conductivity with temperature while those with Ti4O7 and Ti5O9 phases showed metallic behaviour with decreasing conductivity with increasing temperature.86 Adamaki et al. measured the AC electrical properties of Magnéli phase titanium suboxides up to 375 °C and compared them with those obtained for TiO2. Low frequency (100 Hz) electrical conductivities of Magnéli phase titanium suboxide fibres reduced at 800–1100 °C showed increased conductivity between 10−1 and 10 S m−1 in contrast to the insulating behaviour of rutile TiO2. Samples reduced at 1200–1300 °C demonstrated metallic behaviour with 103–104 S m−1 conductivity at low frequency measurements. When the measurements were conducted at a wider range of frequencies (1–105 Hz), fibres reduced at 1200 and 1300 °C behaved as conductors showing that their conductivity was frequency independent across the frequency range examined.92 The conductivity of titanium suboxides is reported to be significantly higher than that of TiO2, in a wider range of frequencies from 0.1 Hz to 1 kHz.93
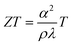 | (3) |
Thermal conductivity is a combination of two components: phonon (lattice) contribution (κl) and carrier concentration (κe). Lattice imperfections help in scattering of phonons with different wavelengths leading to a reduction in κl. Point defects, grain boundaries, phase boundaries, interfaces and dislocations are some crystal imperfections that act as phonon scattering sources.94 Thereby, a reduction in lattice thermal conductivity is reported to exhibit enhanced thermoelectric performance in the material.63,95 The large number of VO present in Magnéli phase titanium suboxides (in CSPs) serve as phonon scattering centres that can reduce the mean free path of phonons.96 This reduction reduces the lattice thermal conductivity, which in turn can affect the total thermal conductivity and thermopower.97,98The κe is estimated from electrical conductivity (σ) through the Wiedemann–Franz law, κe = σLT, where L is the Lorentz number and T is temperature. Although the Lorentz number is constant in metals, in semiconductors, it slightly depends on the carrier concentration.99 However, carrier concentration does not affect κl, suggesting that materials with good thermoelectric performance demonstrate low lattice thermal conductivity.
Harada et al. measured the variation in thermoelectric properties of a series of hot pressed Magnéli phase TiO2−x powders with change in the x value (Fig. 7(a)). From the results obtained for TiO2−x powders (x = 0.05, 0.10, 0.15 and 0.20), it was noted that the value of electrical resistivity and thermal conductivity decrease when the value of x increases, and the absolute value of the Seebeck coefficient decreases with an increase in x. However, the value of lattice thermal conductivity decreased with increasing oxygen deficiency (x) (Fig. 7(b)). Based on the dependence of electrical resistivity and lattice thermal conductivity on oxygen deficiency, it was concluded that CSPs act as sources for phonon scattering but not carrier scattering.63
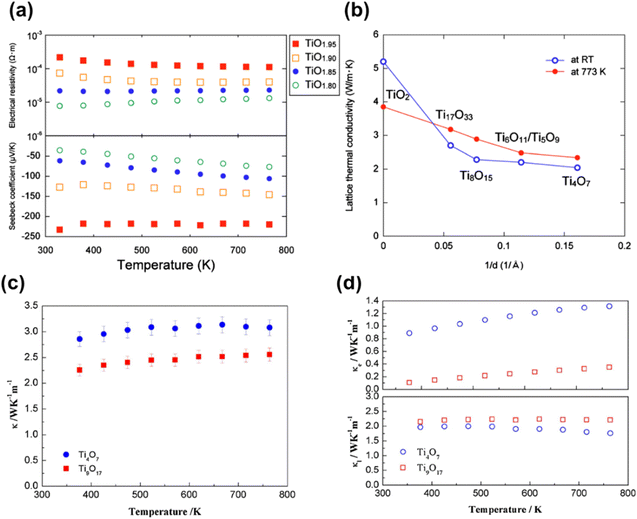 | ||
| Fig. 7 (a) Variation of electrical resistivity (top) and Seebeck coefficient (bottom) with temperature for hot pressed TiO2−x specimens. (b) Variation of lattice thermal conductivity with the density of CSPs (reciprocals of the spacing of CSPs) at RT and 500 °C. Reprinted with permission from ref. 63. Copyright 2010, AIP publishing. (c) Variation of thermal conductivity (κ), (d) electron thermal conductivity (κe) (top) and lattice thermal conductivity (κl) with temperature for Ti4O7 and Ti9O17. Reprinted with permission from ref. 77. Copyright 2018, Elsevier. | ||
Reduced rutile TiO2 and highly deficient Magnéli phase TiO2−x powders demonstrated a decrease in thermal conductivity with decreasing O/Ti ratio (i.e., increasing x). Furthermore, it was also determined that phonon scattering was mostly affected by planar defects compared to point defects and hence planar defects play a critical role in the thermal conductivity of TiO2−x materials.69 Thermoelectric studies conducted by Fan et al. showed that the thermal conductivity of Ti9O17 was lower than that of Ti4O7, demonstrating better overall thermoelectric performance compared to the latter (Fig. 7(c) and (d)).77 In measurement of thermoelectric properties of plasma spray-synthesised TiO2−x deposits, Lee et al. found the introduction of VO into substoichiometric titanium oxides with the resultant formation of Ti4O7 increases electrical conductivity but reduces the Seebeck coefficient. It was further observed that the presence of a porous, defective structure with interfaces in these deposits caused a significant reduction in through-thickness thermal conductivities.100
As shown in Fig. 8(a), stoichiometric TiO2 shows a dramatic rise in reflectance, in the range 390–420 nm. The change in the optical reflectance that occurs in this range is associated with the beginning of the tail of the absorption curve of TiO2. The optical bandgap of the material is determined from the wavelength of the inflection point of the absorption curve (edge). Due to the surface reflectivity of TiO2, the shorter wavelength region (λ < 400 nm) of its reflectance curve consists of a region of constant reflectivity. This is because if the particles are all absorbing, the absorption coefficient has minimal impact until it approaches the centre of the main absorption band. However, the particles become relatively transparent with reducing absorption coefficient at longer wavelengths (λ > 450 nm). This in turn increases the net diffuse reflectivity of the sample, due to numerous reflections and refractions occurring in the bulk of the material. Hence, when the value of the absorption coefficient is very low, diffuse reflectivity reaches 1.19
As explained in the previous paragraph, rutile based TiO2 samples show the most significant variation in reflectance between 390 and 420 nm related to their fundamental absorption edge. Reflectance on the shorter wavelength side of this is independent of the oxygen stoichiometry in TiO2, while on the long wavelength side, reflectance decreases with increasing oxygen non stoichiometry.19,101 Based on the ionisation state of VO in rutile, they can act as single or double electron donors. Hence, the decrease in reflectance with increasing VO is related to sample conductivity, which increases when the number of free electrons in the lattice increases.101 Furthermore, studies have also reported a less intense broad band at 500–600 nm, caused by reduction. This band is attributed to the presence of two-electron centred VO.102
The linear region of the slope in the reflectivity vs. wavelength plot (Fig. 8(a)) was absent for highly reduced nonstoichiometric samples. This is because the additional states created during reduction absorb light related to photon energies within the forbidden bandgap.19 Because of this, all Magnéli phases are known to be highly active under visible light, extending their absorbance from visible to the near IR region.29,103,104
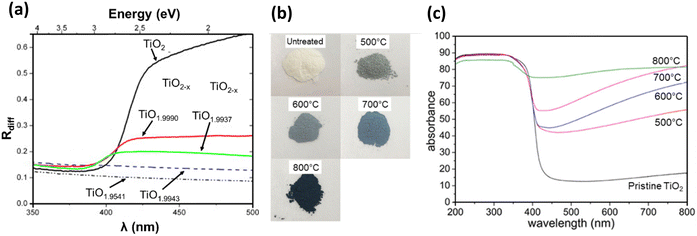 | ||
| Fig. 8 (a) Diffuse reflectance spectra of TiO2 and reduced TiO2−x showing the variation in light absorbance with changes in oxygen stoichiometry. Reprinted with permission from ref. 19. Copyright 2007, Elsevier. (b) Digital images of untreated and reduced rutile TiO2 and (c) UV-vis absorption (percentage scale) spectra of untreated and reduced TiO2 hydrogenated at 500–800 °C. Reprinted with permission from ref. 105. Copyright 2019, American Chemical Society. | ||
Liu et al. made similar observations in the optical properties of hydrogen reduced rutile TiO2 annealed at different temperatures (Fig. 8(b)). The absorbance of UV light in the λ < 400 nm region was similar for both pristine and hydrogen reduced TiO2. This similarity implies that the presence of VO on the surface of the particles has minimal impact on UV absorption. However, increasing reduction temperature resulted in higher absorption in the longer wavelength region (λ > 400 nm), attributed to the creation of oxygen defects that form mid gap states between the VB and CB. The sample reduced at the highest temperature (800 °C), comprising a mixture of Ti9O17 with rutile TiO2, showed the highest visible light absorbance, owing to the presence of the highest amount of oxygen defects (Fig. 8(c)).105
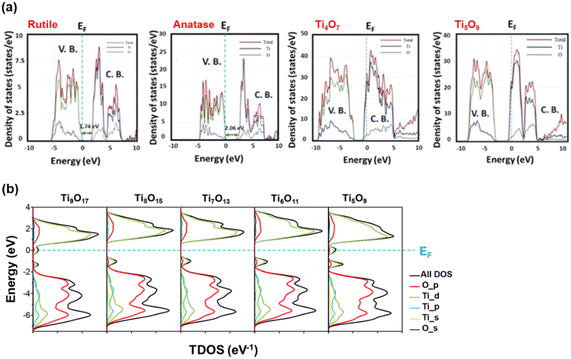 | ||
| Fig. 9 (a) Normalised DOS diagram for rutile, anatase, Ti4O7 and Ti5O9 (ref. 106) and (b) Calculated total DOS of Magnéli phase TinO2n−1 (5 ≤ n ≤ 9).30 | ||
Padilha et al. demonstrated that DFT-based calculations could identify defect levels within the bandgap region for all TinO2n−1 phases (where 2 < n < 5). These defects, similar to those in rutile TiO2, primarily involve Ti 3d orbitals and arise due to the presence of intrinsic VO. Improved descriptions of Ti 3d orbitals were obtained using either the Hubbard U parameter or hybrid functionals, which shifted these defect levels away from the CB minimum, depicting them as either shallow or deep levels.67 Slipukhina et al. conducted first-principles calculations on Ti5O9 to investigate its electronic and magnetic properties. They identified several quasi-degenerate magnetic solutions for all phases—low, intermediate and high temperatures—making it challenging to determine the precise charge distribution at various temperatures. They noted that the charge and orbital orders across all three phases were non-unique, and the formation of Ti3+–Ti3+ bipolaronic states was less likely than in Ti4O7, though not impossible. Additionally, they concluded that phase transitions in Ti5O9 result from complex interrelations between electronic correlations, electron–lattice and spin–lattice coupling, rather than structural changes alone.84
Ekanayake et al. used DFT simulations to explore the electronic and optical properties of Magnéli phase TinO2n−1 (n = 5–9). Their total DOS analysis (Fig. 9(b)) confirmed that the CB minimum is largely composed of Ti d orbitals, while the VB consists of O 2p orbitals. Their findings also indicated strong d–d correlation effects in these titanium suboxides, which led to the emergence of new bands within the Ti–O gap, thus reducing the bandgaps from the 3.2 eV observed in TiO2. Spin–orbit coupling introduced additional bands derived from Ti 3d and O 2p orbitals, especially in Ti9O17 and Ti5O9, creating high-energy mid-gap states near the Fermi level. This in turn led to further narrowing of the bandgaps of these phases contributing to metallic behaviour.30
3. Methods to synthesise Magnéli phase titanium suboxides
Synthesis approaches used to prepare Magnéli phase titanium oxides can be divided into two groups: partial reduction from TiO2 and incomplete oxidation from low valence titania species.54 Reduction approaches include hydrogen reduction, argon annealing, metal reduction, reduction with organic reductants, high energy particle reduction and electrochemical reduction. Oxidation approaches involve the use of raw Ti materials including metallic titanium, organotitanium and inorganotitanium to generate TiO2−x in an oxygen containing environment.33Fig. 10 summarises the fate of different Ti sources and the typical strategies employed in each of the synthesis methods.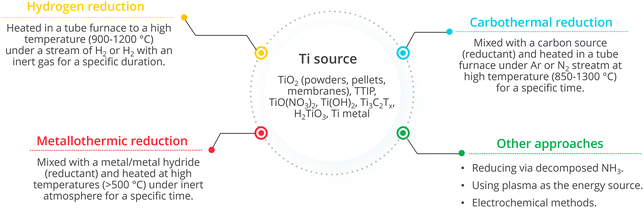 | ||
| Fig. 10 Different synthesis approaches for Magnéli phase titanium suboxides (TTIP is titanium(IV) isopropoxide and Tx indicates functional groups) and a typical process for each method. | ||
3.1 Hydrogen reduction
One of the most commonly used approaches to form Magnéli phase titanium suboxides is hydrogen reduction, which makes use of either pure H2 gas under low or high pressure, mixtures of H2/Ar or H2/N2 or the use of hydrides such as NaBH4 or CaH2.108–112 The reduction reaction occurring in the presence of hydrogen can be written as shown in eqn (4).33| nTiO2(s) + H2(g) → TinO2n−1(s) + H2O(g) | (4) |
The reaction is maintained for a specific duration (typically 2–8 h), after which the resulting product—whether in powder, pellet, or membrane form—is collected for further testing and characterisation. When different precursors, such as hydroxides, alkoxides, nitrates or MXenes, are used as the Ti source, they are first converted into TiO2 through methods like hydrolysis or hydrothermal treatment. In some cases, these precursor forms can be directly subjected to a hydrogen stream to form the desired Magnéli phases.
Table 2 summarises various studies that have made use of hydrogen reduction to prepare Magnéli phase titanium suboxides using different titanium precursors.
| Ti precursor | Synthesis/treatment of the precursor | Reduction conditions | Product composition | Ref. |
|---|---|---|---|---|
| Amorphous TiOx | Addition of H2O2 to a mixture of TiCl4 and ethylenediaminetetraacetic acid caused the decomposition of the Ti complex into colloidal TiOx. The resulting mixture after precipitating and filtering yielded amorphous TiOx | 5 g of amorphous TiOx was heated in a quartz tubular reactor at 1050 °C for 2 h with a heating rate of 5 °C min−1 under 10% H2 (Ar) stream fed at 20 cm3 min−1 | Mixture of Ti9O17 and Ti6O11 | 113 |
| Anatase TiO2 | — | Reducing anatase TiO2 powder at 950 °C for 4 h under pure H2 flow of 200 mL min−1. Ti4O7 pellet formed by reducing TiO2 pellet at 1050 °C for 2 h | Ti4O7 powder/Ti4O7 pellet | 114 |
| TiO2 pellets were initially formed by uniaxial pressing followed by sintering at 1327 °C for 5 h in air | Reduction of TiO2 pellets was conducted in a 7% H2 (Ar) atmosphere for 3.5 h from 947 to 1147 °C | Combinations of TiO2 and Ti4O7 obtained at 997 °C and 1067 °C and a mixture of Ti9O17 and Ti8O15 obtained at 1147 °C | 19 | |
| TiO2 was mixed with MilliQ water, triethanolamine and polyethylene oxide solution to form a suspension. This mixture was dip-coated on a tubular α-Al2O3 support, dried at RT and at 100 °C followed by sintering in air at 800 °C | Reduced in a tube furnace from 850 to 1050 °C (5 °C min−1 heating rate) under 30% H2 (N2) atmosphere for 2–7 h | Ti4O7/Al2O3 membrane | 115 | |
| Anatase TiO2 nanosheets | Electrostatic deposition of TiO2 nanosheets on SiO2 beads | Annealing at 950 °C for 7 h under 200 mL min−1 H2 flow | SiO2@Ti4O7 | 116 |
| Rutile TiO2 | Rutile TiO2 powders mixed with water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol (1 isopropanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and 5 wt% (w/w) polyethylene oxide binder and compressed at 20 MPa to form a plate 1, v/v) and 5 wt% (w/w) polyethylene oxide binder and compressed at 20 MPa to form a plate |
Annealing at 1050 °C for 4 h under H2 atmosphere | Monolithic porous Ti4O7 | 22 |
| Rutile TiO2 mixed with different weight percentages of PVA | Annealed at 1100 °C for 1 h at a heating rate of 3 °C min−1 under 5% H2 (Ar) atmosphere | A mixture of Ti4O7 and Ti5O9 was obtained when 75 wt% PVA and 25 wt% TiO2 was used | 117 | |
| — | Reduced at 800 °C heated at a rate of 10 °C min−1 under pure H2 gas at 1.0 L min−1 | Mixture of rutile TiO2 and Ti9O17 | 105 | |
| TiO2 (Degussa, P25) | P25 was oxidised (800 °C, 2 h) to remove any organic contaminants | Reduced in a 4% H2 (Ar) atmosphere at 1000 °C, 1100 °C and 1180 °C for 2 h at a heating rate of 1 °C min−1 | A mixture of Ti9O17/Ti6O11 was obtained at 1000 °C and Ti4O7/Ti5O9 at 1100 °C | 118 |
| Co ions deposited on TiO2 powders obtained by dispersion of CoCl6·6H2O in TiO2 powder in ethanol followed by stirring at RT, solvent evaporation at 65 °C and drying at 50 °C for 12 h | Annealed under H2 atmosphere with a flow rate of 100 mL min−1 and heated at 400–800 °C for 3 h | Ti8O15 | 119 | |
| Commercial pigment TiO2 | Mixtures of TiO2/polyethylene glycol solution compacted in a uniaxial press followed by sintering in air at 1050 °C for 1 day | Reduced at 1050 °C for 4 h | Monophasic Ti4O7 electrodes | 120 |
| TiO2 powder | — | Reduced at 1050 °C for 6 h under H2 flow in a tube furnace | Ti4O7/Ti6O11 | 121 and 122 |
| Reduced in a tube furnace at 1050 °C for 5 h in the presence of 1.0 atm H2 flow | 0.5 g of the formed Ti4O7 powder was mixed with the binder, paraffin oil and pelletised using a hydraulic press and further reduced at 1050 °C for 6 h under H2 flow to remove the binder | Ti4O7 electrode | 123 | |
| TiO2 membrane | — | Reduced in a tube furnace at 1050 °C for 30 h in a H2 atmosphere | Ti4O7/Ti6O11 REM | 124 |
| TiO2 reactive electrochemical membrane (REM) | REMs were synthesised in a tubular TiO2 ultrafiltration membrane (50 kDa), followed by cutting the membrane to 10 cm in length | REMs reduced in a tube furnace at 1050 °C under 1 atm H2 flow for 30–50 h | Ti6O11 membrane when treated for 30 h, mixture of Ti6O11 and Ti4O7 at 40 h and Ti4O7 membrane at 50 h | 26 |
| Titanium(IV) isopropoxide (TTIP) | Decomposition of TTIP vapour inside a hydrogen stream thermal aerosol hot wall reactor formed TiO2 aerosol | TiO2 was reduced by the H2 stream between 500 and 1100 °C at 100 mbar | 100% Ti4O7 at 1100 °C, 60% Ti4O7 and 21% Ti5O9 with 5.5% anatase TiO2 at 1000 °C and 24% Ti4O7, 19% Ti5O9 with 31.4% anatase TiO2 formed at 900 °C | 125 |
| TTIP solution is converted to Ti(OH)2 in water, which is further converted to titanium nitrate using HNO3. A colloidal crystal template formed of silica nanoparticles was added into the above solution followed by drying at 120 °C and further at 200 °C for 3 h | Heated under H2 flow with a flow rate of 200 mL min−1 in a tube furnace at 800 °C for 5 h | Mesoporous Ti6O11 with trace amounts of anatase TiO2 | 126 | |
| TiO(NO3)2 | Nitric acid added to a mixture of Ti(OC4H9)4)/distilled water so that Ti(OC4H9)4 would undergo hydrolysis and react with HNO3 to form TiO(NO3)2 as follows:106 | TiO(NO3)2 was then annealed in a tube furnace at 1000 °C for 6 h at a 5 °C min−1 heating rate under 200 sccm H2 flow | Ti4O7 nanoparticles | 127 |
| Ti(OC4H9)4 + 3H2O → TiO(OH)2 + 4C4H9OH | ||||
| TiO(OH)2 + 2HNO3 → TiO(NO3)2 + 2H2O | ||||
| H2Ti3O7 nanorods | Anatase TiO2 nanoparticles hydrothermally treated with NaOH at 150–180 °C for 2–5 days to form H2Ti3O7 | H2Ti3O7 nanorods are heated under a H2 atmosphere between 800 and 1050 °C for 1–4 h | Ti8O15 nanorods were obtained at 850 °C and Ti4O7 fibres at 1050 °C | 103 |
| Ti(OH)2 and anatase TiO2 | Ti(OH)2 was precipitated from H2TiCl6 and was thoroughly washed followed by drying at 100 °C. To form anatase TiO2, Ti(OH)2 was hydrothermally treated at 200–300 °C for 3 h | Reduced in a Tamman furnace under H2 atmosphere (flow rate of 5 L min−1) at 800, 900 and 1000 °C for 1, 5.5 and 27.5 h | Reduction of Ti(OH)2 and anatase TiO2 at 1000 °C for 1 h: Ti9O17 | 128 |
| Reduction of anatase TiO2 at 1000 °C for 5.5 h: mixture of Ti9O17, Ti8O15 and Ti5O9 | ||||
| MXenes (Ti3C2Tx) | Peroxo titanic acid gel, formed by a mixture of Ti3C2Tx and H2O2) was dropped on to an etched quartz glass followed by oven drying at 60 °C (where Tx indicates fucntional groups) | Annealed at 800–1000 °C under H2 atmosphere for 1 h | Magnéli phase flexible film (Ti4O7/Ti5O9 at 1000 °C and Ti6O11/Ti7O13 at 900 °C) | 129 |
The types of Magnéli phases obtained from each method indicate that the key factor in hydrogen reduction is the temperature at which these phases are generated. Generally, higher temperatures and/or longer heating times result in Ti4O7 and other lower-n TinO2n−1 phases, whereas mixed phases tend to appear at lower temperatures or with shorter heating times. Additionally, some studies have explored the mixing of different Ti sources, solvents and carbon sources before H2 reduction. For example, Krishnan et al. examined the influence of poly(vinyl alcohol) (PVA) on Magnéli phase formation. They investigated mixtures of TiO2 and PVA (25–75% PVA by weight) and subsequently annealed the samples under a hydrogen atmosphere. PVA was utilised to reduce the agglomeration of TinO2n−1 and enhance the surface area. Their findings showed that the highest reduction in agglomeration and the largest surface area (25.3 m2 g−1) were achieved with 75 wt% PVA.130 You et al. mixed TiO2 powder with water and isopropanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) before hydrogen treatment to reduce the capillary force within the powder. The resulting sample consisted of Ti4O7; however, their study did not explore the effect of varying the water-to-isopropanol ratio on the formation of Magnéli phases.131 Furthermore, while many studies employed a specific H2 stream flow rate, none have discussed its impact on particle size or the types of Magnéli phases formed. Therefore, the synthesis temperature, as well as the use of dispersants and solvents, are crucial design considerations for H2-based reduction methods to achieve the desired phase and particle size.
1, v/v) before hydrogen treatment to reduce the capillary force within the powder. The resulting sample consisted of Ti4O7; however, their study did not explore the effect of varying the water-to-isopropanol ratio on the formation of Magnéli phases.131 Furthermore, while many studies employed a specific H2 stream flow rate, none have discussed its impact on particle size or the types of Magnéli phases formed. Therefore, the synthesis temperature, as well as the use of dispersants and solvents, are crucial design considerations for H2-based reduction methods to achieve the desired phase and particle size.
As shown in Table 2, hydrogen reduction is a popular method for synthesising Magnéli phases due to its ability to operate at lower temperatures (as low as 800 °C)126 and shorter reduction times (such as 1 h)129,130, thereby saving both time and energy. This method also offers the potential to obtain Magnéli phase titanium suboxide nanoparticles, depending on the precursor used. Despite these advantages, hydrogen reduction has significant drawbacks, primarily due to the high risk of explosion associated with working with hydrogen, as well as the higher costs involved in the H2 storage and transportation.132 As a result, alternative methods have been developed for synthesising Magnéli phase titanium suboxides.
3.2 Carbothermal reduction
Reduction of TiO2 using carbon or an organic polymer under an inert atmosphere is termed carbothermal reduction and occurs according to the following reaction (eqn (5)).33| nTiO2(s) + C(s) → TinO2n−1(s) + CO(g) | (5) |
Carbothermal reduction has been widely used in recent studies owing to the safety and low cost of the method compared to other approaches. Furthermore, contact between the reactant and the reductant has shown an increased reaction rate compared to gas–solid reactions.133 It is reported that during carbothermal reduction, VO in the TiO2 lattice are initially increased followed by lattice reconstruction in the final stage.133 The ratio between TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) carbon plays a critical role in the phase of the final product, since an over stoichiometric ratio can lead to the formation of TiC or TiOxCy.134–136 Furthermore, the kinetics of the TiO2 carbothermal reduction depends heavily on the reaction temperature, initial bulk density, gas atmosphere and TiO2 grain size.134,137
carbon plays a critical role in the phase of the final product, since an over stoichiometric ratio can lead to the formation of TiC or TiOxCy.134–136 Furthermore, the kinetics of the TiO2 carbothermal reduction depends heavily on the reaction temperature, initial bulk density, gas atmosphere and TiO2 grain size.134,137
In a typical synthesis, TiO2, in the form of powder or pellets, is mixed with a carbon-based reductant such as carbon black, activated charcoal, or a polymer like PVA or polyethylene glycol (PEG). The mixing of the TiO2 and the carbon source is usually a physical process involving techniques such as ball milling, ultrasonication or manual mixing. The resulting mixtures are then heated in a tube furnace under an inert atmosphere at temperatures ranging from 850 to 1300 °C for 1 to 24 h, producing a series of products with various combinations of Magnéli phases. Table 3 summarises some research conducted on synthesising Magnéli phase titanium suboxides using the carbothermal reduction. The overall trend indicated that increasing the treatment/reduction time resulted in a higher proportion of lower Magnéli phases being formed.
| Ti precursor | Synthesis/treatment of the precursor | Reduction conditions | Product composition | Ref. |
|---|---|---|---|---|
| Anatase TiO2 | Anatase TiO2 mixed with carbon black was ball milled for 24 h at 200 rpm, dried at 80 °C to remove the solvent | Dried powders were heated in a tube furnace from 1000 to 1300 °C for 3 h under Ar flow with a flow rate of 300 mL min−1 | Anatase/Rutile/Ti9O17 at 1100 °C | 133 |
| Ti5O9/Ti4O7/λ-Ti3O5 at 1200 °C | ||||
Anatase TiO2 and polydopamine (PDA) mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio, the mixture dipped in tris–buffer solution and stirred for 48 h. TiO2@PDA composites obtained by centrifugation were washed with water and ethanol and oven dried at 80 °C for 12 h 1 mass ratio, the mixture dipped in tris–buffer solution and stirred for 48 h. TiO2@PDA composites obtained by centrifugation were washed with water and ethanol and oven dried at 80 °C for 12 h |
Heated in a tube furnace from 900 to 1200 °C under Ar flow (300 mL min−1 flow rate) for 1 h | Anatase/Ti4O7/λ-Ti3O5 at 900 °C | 133 | |
| Ti4O7/λ-Ti3O5 at 1000 °C | ||||
| TiO2 and synthetic graphite mixed with distilled water and carboxymethyl cellulose and dried overnight at 120 °C. The mixture was formed into pellets by uniaxial hydraulic press | Reduction was conducted under H2, Ar and He atmospheres (1.00 NL min−1) in a fixed bed reactor in a vertical tube furnace for 5 h | Under H2 flow: mixtures of TiO2/Ti8O15/Ti4O7 | 134 | |
| Under Ar flow: mixtures of TiO2/Ti9O17/Ti8O15/Ti5O9/Ti4O7 | ||||
| Mixtures of TiO2/carbon black were ball milled using 4.0 wt% carbon content | Heated in vacuum furnace from 800 to 1100 °C for 2 h | At 1000 °C: Ti9O17/Ti7O13/Ti6O11 | 135 | |
| At 1025 °C: Ti4O7/Ti5O9 | ||||
| A paste formed with TiO2, carbon black, water and organic binders was air oxidised at 350 °C for 2 h | Sintering at 1300 °C for 2 h under Ar atmosphere | Ti4O7/Ti5O9 | 138 | |
| — | Inside the tube of a tube furnace, a crucible containing anatase TiO2 is positioned between two crucibles containing activated charcoal. Then the samples are reduced at 1100 °C for 2–12 h | At 6 h: >90% Ti9O17 | 30 | |
| 7–8 h: mixtures of Ti9O17/Ti8O15/Ti7O13/Ti6O11 | ||||
| 9 h: mixture of Ti9O17/Ti8O15/Ti7O13/Ti6O11/Ti5O9 | ||||
| 10–12 h: phase reversal resulting in mixtures of Ti9O17/Ti8O15/Ti7O13/Ti6O11 | ||||
| Rutile TiO2 | TiO2, polyvinylpyrrolidone and water were mixed with ultrasonic radiation followed by drying and placing in a silica tube surrounded by carbon | Heated at 950 °C for 30 min by 2.45 GHz microwave irradiation under Ar atmosphere introduced into the furnace at 0.5 L min−1 | Ti4O7 nanoparticles (60 nm) | 139 |
| TiO2 and PVA were mixed 50/50 mass ratio | Heated at a rate of 5 °C min−1 at 1100 °C for 1 h under N2 atmosphere with a flow rate of 50 mL min−1 | Ti4O7 | 29 | |
| TiO2 powder | Green-body pellets were formed via uniaxial pressing of a ball-milled mixture of TiO2, polyethylene glycol and distilled water. These pellets were then sintered at 1300 °C for 4 h in air | TiO2 tablets were covered with carbon black in alumina crucibles and heated at 1300 °C (120 °C h−1) for 4–6 h under Ar flow | Ti4O7/Ti5O9/Ti6O11 and residual TiO2 | 93 |
| TiO2 powder | TiO2 fibres were formed via thermoplastic extrusion using stearic acid as the pre-coating material and polyethylene as the binder | TiO2 fibres embedded in carbon black powder were reduced at 1200 °C and 1300 °C from 1 to 24 h in an Ar atmosphere | Mixture of Ti10O19, Ti9O17, Ti8O15, Ti6O11, Ti5O9, Ti4O7, Ti3O5 and TiO2 | 92 |
| TTIP | Ti hydroxy oxide (Ti(OH)xOy was formed by the reaction between TTIP with HCl vapour. This precipitate was dispersed in glucose (C/Ti ratio = 1.07)) and dried at 50 °C | Calcined at 1000 °C for 2.5 h in a vacuum chamber (slow cooling to 500 °C followed by quenching) | Ti4O7 | 140 |
| Amorphous TiO2 is precipitated via TTIP and mixed with PVA powder | Heated from 700 to 1000 °C for 1 h under N2 atmosphere with a flow rate of 40 mL min−1 | Ti4O7 with trace amounts of anatase and rutile | 141 | |
| Titanium ethoxide | Hybrid solutions of titanium ethoxide (in absolute dry ethanol) mixed with polyethylene imine (PEI) or polyethylene glycol (PEG) were prepared followed by electrospinning the solutions | Heated from 700 to 1000 °C for 4 h under N2 atmosphere at a heating rate of 5 °C min−1 | PEG/Ti (800 °C): Ti4O7/TiN | 80 |
| PEI/Ti (900 °C): Ti8O15 | ||||
| PEI/Ti (1000 °C): Ti6O11/Ti5O9 | ||||
| Titanium ethoxide and PEG mixed with ethanol to form a gel followed by heating at 100 °C for 4–6 h | Heated at 950 °C under Ar stream with a heating rate of 4 °C min−1 | Ti4O7 | 142 |
The main trend in the materials formed via carbothermal reduction is that lower Magnéli phases are more prevalent when higher temperatures and longer durations are used for the reduction. However, for carbothermal reduction, the next most important consideration is the ratio between the Ti source and the carbon source. Toyoda et al. synthesised carbon-coated Magnéli phase titanium suboxides by heat-treating mixtures of rutile TiO2 and PVA.29 A 95/5 ratio of TiO2/PVA at 1100 °C for 1 h resulted in a mixture of rutile and Ti9O17. However, upon reducing this ratio, lower Magnéli phases were obtained, including Ti5O9 and Ti6O11 at an 80/20 ratio, and complete Ti4O7 was achieved at a 50/50 ratio. Further reducing this ratio resulted in lower titanium oxides, including Ti2O3 and Ti3O5, implying the ability to control the generated titanium suboxide phase with the reductant ratio. Similar studies were conducted by Huang et al., where glucose was used as the carbon source to reduce titanium hydroxy oxide (Ti(OH)xOy).140 The C/Ti ratio was varied from 6.49 to 1.07. At the highest C/Ti ratio, TiO was obtained in the product heat-treated at 1000 °C for 4 h; at a 2.17 ratio, TiO2 was obtained as the product after annealing at 1000 °C for 2.5 h; and at a 1.07 ratio, Ti4O7 was obtained as the product under the same conditions. These studies demonstrate that in carbothermal reduction, the temperature of heat treatment, time of annealing and the C/Ti ratio are the most important design considerations for obtaining a desired product. However, similar to hydrogen reduction, no studies have been conducted on the effect of gas flow rate, highlighting further research opportunities in this area.
As mentioned earlier, carbothermal reduction addresses many concerns associated with hydrogen reduction, particularly the high costs and safety risks related to the use and storage of hydrogen gas. However, the main drawback of carbothermal reduction is the extensive pretreatment required, involving time-consuming mixing of the TiO2 source with the carbon reductant. In addition, direct contact between TiO2 and the carbon source could also yield in unwanted byproducts such as TiC and TiOxCy, which need to be separated from Magnéli phases prior to their use. To address these issues, our group introduced a novel carbothermal reduction method that eliminates the need for pretreatment or mixing with a carbon source. Instead, this method relies on CO(g) generated under low-oxygen conditions to reduce TiO2 and form Magnéli phases.30 While the method successfully produces the desired Magnéli phases, the resulting particle sizes were in the micrometre range, limiting their potential applications. Therefore, further development of a carbothermal reduction approach that requires less/no precursor pretreatment while achieving nanoparticulate Magnéli phases remains an area of opportunity.
3.3 Metallothermic reduction
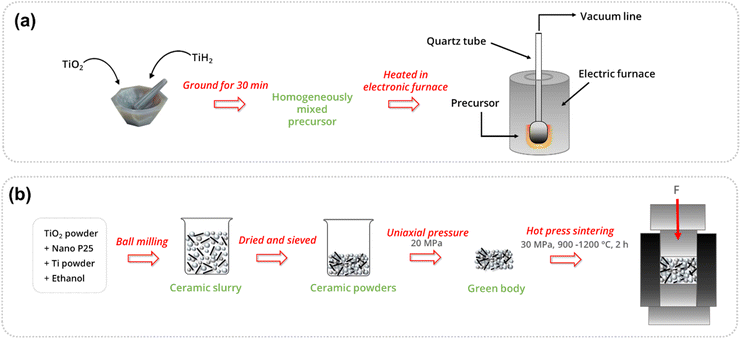
To eliminate post-synthesis purification steps for the removal of any unreacted metal oxides or carbonaceous by-products resulting from conventional reduction methodologies, metallothermic reduction has been used to synthesise titanium suboxides. This method makes use of metals or metal hydrides to reduce TiO2 at high temperatures.143 In this method, TiO2 is mixed with a reductant (either a metal or metal hydride) and the resulting mixture is heat-treated at high temperatures (typically 500 °C or above) for a certain duration, usually under an inert atmosphere, to obtain Magnéli phases. While metallothermic reduction minimises most of the post-synthesis purification compared to carbothermal reduction, the byproducts vary depending on the reductant used. When metal hydrides are used, H2 is evolved as a byproduct during the reaction. However, when metals are used as reductants, metal oxides are formed as byproducts along with the Magnéli phases. Additionally, metals like Ti and Zr, though effective as reductants, are expensive, making them unsuitable for large-scale synthesis due to the high associated costs. Therefore, there is still room for improvement in metallothermic reduction methods that do not rely on costly metals as reductants.Strobel et al. obtained Ti9O17 crystals and smaller crystals of Ti8O15 and Ti6O11 upon the reaction of TiO2 with Ti powders in a two-zone furnace using Cl2 as the transporting agent. Here it was observed that the oxygen pressure is a critical parameter in crystal growth and the temperature gradient not only influences the growth rate but also the nature of the growing phase.75 Nagao et al. reported the synthesis of Magnéli phase titania (Ti4O7 and Ti8O15), lower titanium suboxides (TiO, Ti2O3 and Ti3O5) and metallic Ti, in solid phase reaction of rutile TiO2 with TiH2 using varied TiO2/TiH2 ratios, treatment times and temperatures (Fig. 11(a)).143 The presence of TiH2 causes the generation of metallic Ti by evolving H2 at higher temperatures (∼500 °C) (eqn (6)). This metallic Ti then rapidly reacts with TiO2 in the mixture to give titanium suboxides of varied deficiencies (eqn (7)).
| TiH2 → Ti + H2 | (6) |
| Ti + (2n − 1)TiO2 → 2TinO2n−1 | (7) |
Using these conditions, Ti4O7 has been prepared with a TiO2/TiH2 ratio of 3, by treating at 600 °C for 48 h, whereas for Ti8O15, a ratio of 5 has been used at 600 °C for 72 h.143 Geng et al. synthesised Ti4O7 through in situ hot-pressed sintering of Ti and TiO2 in a single-step process. A mixture of anatase TiO2, nano P25 and Ti powders in various ratios was prepared using ethanol as the solvent. After ball milling, a ceramic slurry was obtained, which, upon drying, yielded ceramic powders. These powders were compacted into green bodies through uniaxial pressing to ensure proper loading into graphite molds. The green bodies were then hot-press sintered under a 30 MPa load at varying temperatures (900–1200 °C) for 2 h under vacuum (Fig. 11(b)). The optimal sample, with a Ti/TiO2 weight ratio of 0.09, sintered at 1000 °C, exhibited superior mechanical properties and an excellent electrical conductivity of 1129 S cm−1.144
Anatase TiO2 monoliths have been reduced using a zirconium getter to form single phase Ti6O11, Ti4O7, Ti3O5 and Ti2O3 with varied amounts of Zr between 1000 and 1180 °C at a heating rate of 100 °C h−1 for 1 day reaction time (Fig. 12(a)–(c)).81 The formation of porous TinO2n−1 monoliths is expressed in eqn (8).
| 2nTiO2 + Zr → 2TinO2n−1 + ZrO2 | (8) |
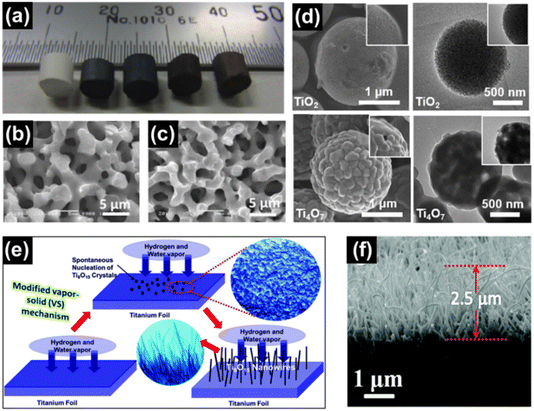 | ||
| Fig. 12 (a) Digital image of anatase TiO2, Ti6O11, Ti4O7, Ti3O5 and Ti2O3 monoliths (left to right) prepared using a sol–gel approach. SEM images of macroporous monoliths of (b) anatase TiO2 and (c) Ti4O7. Reprinted with permission from ref. 81. Copyright 2012, American Chemical Society. (d) SEM images (top left and bottom left) and TEM images (top right and bottom right) of TiO2 and Ti4O7 microspheres, respectively. Reprinted with permission from ref. 145. Copyright 2021, Elsevier. (e) Schematic illustration of the vapour–solid mechanism used for the growth of Ti8O15 nanowires and (f) SEM image of the cross section of the Ti8O15 nanowires. Reprinted with permission from ref. 146. Copyright 2015, Royal Society of Chemistry. | ||
Liu et al. made use of a mixture of mesoporous TiO2 and Ti powder (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol%) to synthesise Ti4O7 microspheres by heat treating at 850 °C for 3 h under Ar (Fig. 12(d)).145 A mixture of anatase TiO2 and Ti4O7 was obtained when CaH2 was used as the reductant to reduce P25 TiO2 (Degussa) when reacted at 500 °C for 5 h.147 Gusev et al. prepared single and multi-phase Ti4O7 and Ti6O11 oxides by reacting rutile TiO2 with Ti. Ball milling was used for mechanical activation of the starting materials followed by annealing under Ar atmosphere at 1060–1080 °C for 4 h to obtain highly conducting ceramics.148 He et al. reduced TiO2 powders with the use of chemically polished Ti foil. The Ti foil substrate was placed 6 cm away from the quartz reactor containing TiO2 in a tube furnace followed by heating at 1050 °C under H2 flow for 2 h. During the reaction, reduction of TiO2 by H2 forms H2O (g), which together with H2 (g) react with Ti to form a layer of compact Ti8O15 nanoparticles on the substrate (Fig. 12(e) and (f)).146
1 mol%) to synthesise Ti4O7 microspheres by heat treating at 850 °C for 3 h under Ar (Fig. 12(d)).145 A mixture of anatase TiO2 and Ti4O7 was obtained when CaH2 was used as the reductant to reduce P25 TiO2 (Degussa) when reacted at 500 °C for 5 h.147 Gusev et al. prepared single and multi-phase Ti4O7 and Ti6O11 oxides by reacting rutile TiO2 with Ti. Ball milling was used for mechanical activation of the starting materials followed by annealing under Ar atmosphere at 1060–1080 °C for 4 h to obtain highly conducting ceramics.148 He et al. reduced TiO2 powders with the use of chemically polished Ti foil. The Ti foil substrate was placed 6 cm away from the quartz reactor containing TiO2 in a tube furnace followed by heating at 1050 °C under H2 flow for 2 h. During the reaction, reduction of TiO2 by H2 forms H2O (g), which together with H2 (g) react with Ti to form a layer of compact Ti8O15 nanoparticles on the substrate (Fig. 12(e) and (f)).146
3.4 Other synthesis approaches
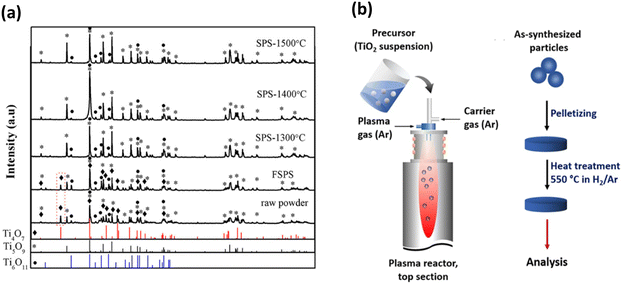 | ||
| Fig. 13 (a) XRD patterns of raw powder, FSPS sample and samples prepared by conventional SPS at different temperatures.153 (b) Preparation of TiOx nanoparticles using radiofrequency (RF) induction thermal plasma method (left) and heat treatment of the samples after synthesis to improve their electrical conductivity (right).13 | ||
Wang et al. also used SPS to fabricate Magnéli phase titanium oxides via in situ reduction with carbon powder and observed that the reduction can be easily achieved at low pressure and temperatures above 1200 °C and pointed out that a stable sintering environment without any pressure change due to gas reaction is vital for the reaction.154 SPS has been used as a densification method for Ti4O7 and Ti8O15 nano-powders by Conze and co-workers, in which the samples were heated at 1300 °C for 5 min at a heating rate of 100 °C min−1 under 60 MPa pressure using graphite dies lined with carbon foil.155 Lee et al. made use of an atmospheric plasma spray process to fabricate Magnéli phase titanium oxide deposits (n = 4–9), using TiO1.9 as the feedstock powder and by varying the hydrogen gas ratio to allow the tunability in nonstoichiometry and phase content.100 Xu et al. synthesised Magnéli phase titanium suboxides and lower suboxides (n = 2, 3) using flash synthesis by thermal plasma by varying the input power, feeding rate of the precursor H2TiO3, and the H2/Ar ratios in the gas mixture, which was used as the carrier gas.156 A radiofrequency (RF) induction plasma method was used by Arif and co-workers to form nanoparticles composed of TiO2, Ti8O15, Ti4O7 and lower titanium oxide phases. In their synthesis, a mixture of rutile TiO2 and water-isopropyl alcohol mixture was fed into the plasma reactor that made use of Ar both as the plasma and the carrier gas. To improve the conductivity of these samples, the as synthesised samples were pelletised and annealed in a vacuum furnace under 3% H2 (Ar) atmosphere (Fig. 13(b)).13
4. Potential applications of Magnéli phase titanium suboxides
Magnéli phase titanium suboxides have been studied for their potential application in a variety of fields: as electrocatalysts and photocatalysts for environmental remediation, electrodes for oxygen and hydrogen evolution and as support materials in electronic and optoelectronic devices Fig. 14, as will be discussed in the following sections. Ebonex® is the registered trademark for a range of electrically conducting and corrosion resistant suboxides of titanium, mainly Ti4O7 and Ti5O9. Ever since its discovery and commercialisation, Ebonex® has been widely used in applications that require high electrical conductivity, corrosion resistance and oxidation resistance.27 Due to the presence of highly conducting Magnéli phases, its electrical conductivity reaches up to 300 S cm−1, comparable with that of carbon. It is projected that at room temperature in a 4 mol dm−3 H2SO4 solution, the half-life of Ebonex® is 50 years.20 Due to the unique structure of Magnéli phase titanium suboxides, the TiO plane at the edges connect with the TiO2 plane via sharing faces, producing a conductive band that is enclosed by TiO2. Hence, the conductivity in Ebonex® is as a result of the TiO layers while the chemical resistance is attributed to the TiO2 planes that shield the TiO layer.27 Ebonex® is widely used as an electrode owing to its electrochemical stability in both acidic and basic media, high electrical conductivity and high overpotentials for O2 and H2 evolution.161,162 However, Ebonex® itself possesses several disadvantages that make it impractical for use without alteration. One of the drawbacks is its tendency to undergo passivation through anodic polarisation, leading to an increase in the oxygen content beyond the stoichiometric value, forming titanium(IV) oxide over time. Furthermore, the material demonstrates slow transfer kinetics, necessitating surface modification or deposition of foreign materials such as noble metals and conductive oxides, for it to be used as an efficient catalyst support.142,163 | ||
| Fig. 14 Potential applications of Magnéli phase titanium suboxides in environmental remediation and energy storage. | ||
4.1 Oxygen evolution and hydrogen evolution reactions
Photoelectrochemical water splitting is regarded as a highly desirable approach for generating sustainable hydrogen fuel, as it allows for the direct decomposition of water into hydrogen and oxygen through the absorption of sunlight.164 Electrochemical or photoelectrochemical water splitting occurs through two half reactions: oxygen evolution reaction and hydrogen evolution reaction occurring at the anode and cathode, respectively. Depending on the reaction conditions, these two half reactions are as follows.165In acidic solution:
| Cathode: 2H+ + 2e− → H22 + 2e- |
| Anode: H2O → 2H+ + ½O2 + 2e− |
| Cathode: 2H2O + 2e− → H2 + 2OH− |
| Anode: H2O → 2H+ + ½O2 + 2e− |
| Cathode: 2H2O + 2e− → H2 + 2OH− |
| Anode: 2OH−→ H2O+ + ½O2 + 2e− |
Hence, in photoelectrocatalytic water splitting, electrons facilitate the reduction of water molecules, leading to the formation of H2, while holes participate in the oxidation of water, resulting in the formation of O2 simultaneously.166
Conventional catalysts used in water splitting in acidic medium include iridium and ruthenium-based catalysts for oxygen evolution and Pt based materials for hydrogen evolution. Due to the scarcity and high cost of these materials, attention has been drawn to the development of cost effective alternatives.165 In all catalysts involved in oxygen and hydrogen evolution, the supporting material must have high electric conductivity and corrosion resistance. While carbon or graphite are commonly employed as catalyst support materials, there is emerging interest in considering Magnéli phase titanium suboxides such as Ebonex®, as catalyst supports. This is due to their notable properties including excellent oxidation resistance, high electrical conductivity (approximately 103 Ω−1 cm−1), high overpotentials for oxygen and hydrogen evolution in both acidic and basic environments, and remarkable electrochemical stability. These physicochemical properties make Magnéli phase titanium suboxides promising candidates for catalytic applications.27,161
Slavcheva et al. showed that PtCo/Ebonex® synthesised via a borohydride reduction method demonstrated better performance in the oxygen evolution reaction in alkaline medium, compared to Pt/Ebonex® and unsupported PtCo. The catalytic efficiency of these three electrodes was demonstrated by normalising the anodic current relative to the Pt content in the active layer, presented as mass activity (mA mgPt−1). The curves showed that the electrochemical behaviour of PtCo/Ebonex was significantly superior to the other two electrodes, despite containing the lowest amount of Pt (0.83 mg cm−2). This facilitated the oxygen evolution reaction to a greater extent compared to pure Pt/Ebonex (5 mg cm−2) and unsupported PtCo (3.3 mg cm−2), proving the positive influence of the support on catalytic activity. The enhanced activity of the bimetallic catalyst was attributed to surface oxide formation and electronic interactions between the metals and the catalyst support material.167 Similarly, compared to polycrystalline Pt, a Pt/Ebonex® thin film loaded Au disk showed enhanced catalytic activity for the oxygen reduction reaction in acidic solution, due to the interaction of O2 with the active sites on the surface of the catalyst.161 They conducted rotating disk electrode measurements to compare the behaviour of Ebonex/Pt and pure Pt electrodes during the oxygen reduction reaction in 0.5 mol dm−3 HClO4 solution. The obtained polarisation curves showed that the onset of O2 reduction and the half-wave potentials were significantly shifted to more positive potentials in the case of the Ebonex/Pt electrodes, indicating higher catalytic activity for O2 reduction compared to pure Pt. Stoyanova et al. measured the water splitting electrocatalytic activity of Pt–Fe/Ebonex® and Pt–Co/Ebonex® catalysts using cyclic voltammetry and steady state polarisation. Results revealed that these two catalysts show enhanced performance for the oxygen evolution reaction in polymer electrolyte membrane water electrolysis compared to pure Pt. Best catalytic properties were observed in the 2Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3Co/Ebonex® (2
3Co/Ebonex® (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 = weight ratios of precursors) with the oxygen evolution reaction reaching current densities of 230 mA cm−2 at 1.9 V. Higher activity for bimetallic-Ebonex® catalysts is attributed to the solid solution formed between the two metals causing changes in the electron density in the atoms and surface intermediate bond strength and stability of Ebonex® at high anodic potentials.168 Won et al. developed a bifunctional oxygen catalyst using Ti4O7 capable of both oxygen reduction reaction and oxygen evolution, to enhance the efficiency of a unitised regenerative fuel cell. The optimised oxygen catalyst, synthesised by depositing PtIr on a Ti4O7 support (60 wt% metal catalysts on Ti4O7) using the borohydride reduction method, showed the highest specific activity of 1.50 mA cm−2 at 1.5 V, compared to Pt/Ti4O7 and Pt/C, which exhibited specific activities of 0.26 and 0.15 mA cm−2, respectively. Moreover, PtIr/Ti4O7 demonstrated the lowest Tafel slope of 82.3 mV dec−1, compared to 104.5 and 107.3 mV dec−1 for Pt/Ti4O7 and Pt/C, respectively, highlighting its significantly superior oxygen evolution reaction activity. The enhanced performance was attributed to the synergistic effect of the PtIr phase that had a suitable composition for both oxygen evolution and oxygen reduction reactions and high stability of the Ti4O7 support in acidic medium.169
3 = weight ratios of precursors) with the oxygen evolution reaction reaching current densities of 230 mA cm−2 at 1.9 V. Higher activity for bimetallic-Ebonex® catalysts is attributed to the solid solution formed between the two metals causing changes in the electron density in the atoms and surface intermediate bond strength and stability of Ebonex® at high anodic potentials.168 Won et al. developed a bifunctional oxygen catalyst using Ti4O7 capable of both oxygen reduction reaction and oxygen evolution, to enhance the efficiency of a unitised regenerative fuel cell. The optimised oxygen catalyst, synthesised by depositing PtIr on a Ti4O7 support (60 wt% metal catalysts on Ti4O7) using the borohydride reduction method, showed the highest specific activity of 1.50 mA cm−2 at 1.5 V, compared to Pt/Ti4O7 and Pt/C, which exhibited specific activities of 0.26 and 0.15 mA cm−2, respectively. Moreover, PtIr/Ti4O7 demonstrated the lowest Tafel slope of 82.3 mV dec−1, compared to 104.5 and 107.3 mV dec−1 for Pt/Ti4O7 and Pt/C, respectively, highlighting its significantly superior oxygen evolution reaction activity. The enhanced performance was attributed to the synergistic effect of the PtIr phase that had a suitable composition for both oxygen evolution and oxygen reduction reactions and high stability of the Ti4O7 support in acidic medium.169
With the intention of developing non-platinum electrocatalysts for water splitting, Paunović and co-workers developed a Co/Ebonex® catalyst and tested it for oxygen and hydrogen evolution. The activity of the catalyst for hydrogen evolution was less than that of other electrocatalysts such as Vulcan XC-72 + TiO2 or activated multi-walled carbon nanotubes, due to the low surface area of the Magnéli phase titanium suboxides in Ebonex®. However, the Co/Ebonex® electrode showed better performance for oxygen evolution compared to CoPt/Ebonex® catalytic systems. The observed improvement is attributed to the formation of oxides on the surface of the catalyst and metal–support interaction, causing Ebonex® to act as the support material and as an active oxide electrode.170 Jović et al. studied the use of electrodeposited Ni-(Ebonex®/Ir) coatings as anode materials for the oxygen evolution reaction in alkaline solution. They observed that the pure Ni coating exhibited a drastic loss of activity after 24 h of continuous oxygen evolution at j = 50 mA cm−2 (ΔE = 395 mV), despite initially showing higher intrinsic catalytic activity for the oxygen evolution reaction compared to the composite coatings. In contrast, the oxygen evolution reaction overpotential of the Ni-(Ebonex/Ir) coatings showed only negligible changes after the stability test (ΔE = 22 mV). The OH species that were adsorbed by the active sites in Ni were transferred to the less active IrO2 particles, causing both the inherent activity and improved retention of catalytic activity of Ni-(Ebonex®/Ir) coatings.171 An amorphous TiO2−x layer containing Ti4O7 and lower titanium oxides (Ti2O3) was employed to fabricate a photoanode of black BiVO4@amorphous TiO2−x that showed a remarkable photocurrent density of 6.12 mA cm−2 at 1.23 VRHE for water oxidation and 2.5% applied bias photon-to-current efficiency for solar water splitting. The ability of the TiO2−x layer to act as an oxygen evolution catalyst, a protection layer to catalyse the water oxidation reaction and to prevent dissolution of BiVO4, promotes the efficient water splitting demonstrated by the BiVO4/TiO2−x photoanode (Fig. 15(a)-(c)).172
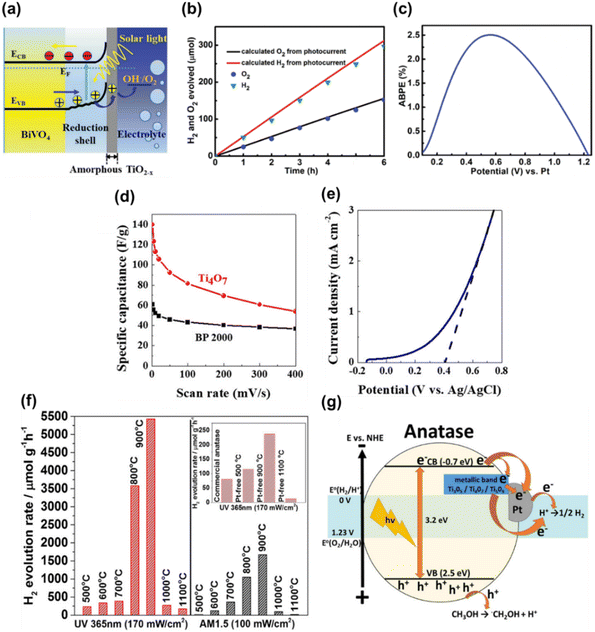 | ||
| Fig. 15 (a) Energy diagram for the transit and transfer of photogenerated charges on the b-BiVO4/TiO2−x photoanode. (b) H2 and O2 generated by the b-BiVO4/TiO2−x photoanode at 1.23 VRHE and calculated H2 and O2 amounts evolved from the photocurrent assuming 100% faradaic efficiency. (c) Applied bias-to photocurrent efficiency (APBE) of the b-BiVO4/TiO2−x photoanode obtained for solar water splitting. Reprinted with permission from ref. 172. Copyright 2019, John Wiley and Sons. (d) Specific capacitance vs. scan rate for the Ti4O7 electrode and Black Pearls 2000 carbon black electrode (BP 2000). (e) Linear sweep voltammetry curve for Ti4O7 electrodes in 0.1 M KOH solution. Reprinted with permission from ref. 140. Copyright 2018, American Chemical Society. (f) Photocatalytic hydrogen evolution from powders synthesised at temperatures 500–1000 °C under 365 nm LED and AM 1.5 solar simulator illumination. Inset: experiment conducted for anatase and powders obtained by the same synthesis method but without Pt. (g) Schematic diagram showing the band alignment between TiO2, Magnéli phase titanium suboxides and Pt. Reprinted with permission from ref. 173. Copyright 2019, American Chemical Society. | ||
Huang et al. synthesised a series of titanium suboxides (Ti2O3, Ti3O5, Ti4O7) with high surface areas (between 260 and 120 m2 g−1) via a combined sol–gel and carbothermic processes. The highest specific capacitance of 140 F g−1 was observed for the Ti4O7 electrode, with a high oxygen oxidation current above 0.5 V (Fig. 15(d) and (e)). A higher specific capacitance demonstrated the outstanding pseudocapacitance behaviour of Ti4O7, making it a promising electrochemical electrode material.140 Kolbrecka et al. studied the effect of porosity on the properties of Ti4O7 electrodes. Results revealed that the porosity of the electrodes affects the anodic current densities and the course of oxygen evolution, which is irreversible on Magnéli phase titanium suboxide electrodes. Furthermore, they also observed that the more reproducible results and high anodic current densities are obtained for the ceramic electrodes that have a high number of large pores.120
Weirzbicka et al. reported the use of anatase TiO2 and Magnéli phase titanium suboxides loaded with Pt nanoparticles for photocatalytic H2 evolution. The optimal mixed-phase particles, containing 32% anatase, 11% rutile and 57% Magnéli phases, loaded with 290 ppm of Pt, exhibited a highly efficient photocatalytic H2 evolution rate of ∼5432 μmol h−1 g−1 under UV light and 1670 μmol h−1 g−1 under AM 1.5 conditions. This was approximately 50–100 times more efficient than anatase with similar Pt loading. The enhanced photocatalytic performance of the optimised nanoparticles under both solar irradiation and UV light (λ = 365 nm) was attributed to the synergy of charge carrier formation by anatase and charge carrier separation and mobility by Magnéli phase titania (Fig. 15(f) and (g)).173 Nanoscopic inserts of Magnéli phase titanium suboxides in anatase TiO2 nanocrystals have shown to work as efficient cocatalysts in H2 generation when suspensions of these nanoparticles were used in H2O/methanol solutions. The mixed-phase particles optimised at 900 °C, consisting of 30% anatase, 25% Ti4O7 and 20% Ti5O9, achieved a direct photocatalytic H2 evolution rate of 145 μmol h−1 g−1 under AM 1.5 solar-simulated light, without the use of a cocatalyst. In comparison, pure anatase or Magnéli phases exhibited significantly lower photocatalytic H2 evolution performance. The researchers further elaborate that the activity in these mixed particles is due to the synergistic effect of anatase TiO2 acting as the light absorber and Ti4O7 as the mediator for charge separation, transport and transfer, leading to significant H2 generation without the need of an external cocatalyst.125 A Au(111)@Ti6O11 heterostructure synthesised via photoreduction showed excellent hydrogen evolution activity with a low overpotential of 49 mV at 10 mA cm−2 current density. Furthermore, it also showed 18 times higher mass activity (9.25 A mgAu−1vs. 0.51 A mgPt−1) and similar stability in acidic media compared to commercial Pt/C (20 wt%). Successful heterostructure formation enabling Au nanoparticles to better adsorb H+, increasing the number of active sites on the catalyst and the conductive Ti6O11 support material were determined as factors that lead to better performance in the Au(111)@Ti6O11 catalyst.176
4.2 Wastewater purification
| R → (R˙)+ + e− | (9) |
| M + H2O → M (OH˙) + H+ + e− | (10) |
| R + M (OH˙) → degradation by-products | (11) |
Boron doped diamond (BDD) has been a common anode material in such reactions but has high fabrication cost. This has moved attention to the use of materials such as Magnéli phase titanium suboxides, which have low fabrication costs, high oxygen evolution potential (>2.5 V vs. standard hydrogen electrode (SHE)) and chemical inertness.179,180 Magnéli titania-based electrodes have been used to successfully degrade and inactivate various organic compounds including per- and polyfluoroalkyl substances (PFASs), perfluorooctanoic acid (PFOA), phenol, N-nitrosodimethylamine, antibiotics and pathogens.181–183 However, studies have shown that the yield of OH˙ radicals generated by Magnéli phase titania-based electrodes is lower compared to that by conventional electrodes such as BDD and PbO2, due to its low interfacial charge transfer rate. To overcome this issue and to gain better performance, pure phase titanium suboxides such as Ti4O7 were introduced with the inclusion of varying amounts of foreign elements such as C, amorphous Pd clusters and Ce3+ (Fig. 16(a)–(c)).181,184
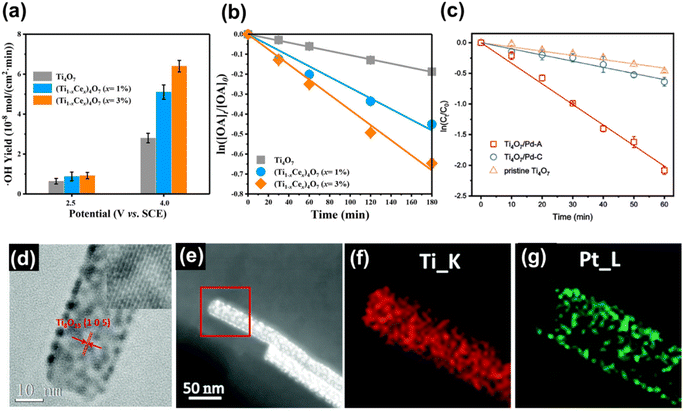 | ||
| Fig. 16 (a) Hydroxyl radical yield at different potentials and (b) oxidation of oxalate under anode polarisation at 2.0 V vs. saturated calomel electrode by Ti4O7 and (Ti(1−x)Cex)4O7 electrodes. Reprinted with permission from ref. 181. Copyright 2021, American Chemical Society. (c) Pseudo first order kinetics of PFOA degradation with pristine Ti4O7, Ti4O7/amorphous Pd and Ti4O7/crystalline Pd electrodes. Reprinted with permission from ref. 184. Copyright 2020, American Chemical Society. (d) High resolution TEM (HRTEM) image, (e) high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image, (f) and (g) HRTEM-STEM energy dispersive X-ray spectroscopy (EDS) mapping images of Pt/Ti8O15 nanowires. Reprinted with permission from ref. 146. Copyright 2015, Royal Society of Chemistry. | ||
You et al. studied the use of monolithic porous Ti4O7 electrodes for the electrochemical oxidation (degradation) of organic pollutants in industrial dyeing and finishing wastewater. Their results confirmed that the Ti4O7 electrode removed the chemical oxygen demand (COD) and dissolved organic carbon (DOC) by 66.5% and 46.7% respectively, at 8 mA cm−2 current density after 2 h of reaction. Furthermore, the bioavailability of wastewater was improved, with the ratio between the five-day biological oxygen demand: COD increasing from 0.029 to 0.28 after treatment, making it a more cost effective and environment friendly material for electrode preparation compared to PbO2 and SnO2 based electrodes.22 Wang et al. investigated the successful degradation and deactivation of antibiotics, antibiotic resistant bacteria and antibiotic resistance genes present in raw wastewater via electrooxidation using a Magnéli phase Ti4O7 anode. Multidrug-resistant Salmonella enterica serotype Typhimurium DT104 was fully inactivated, achieving a 6.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) reduction within 15 minutes at a current density of 10.0 mA cm−2. Additionally, antibiotic resistance genes such as TetG, floR and sul1, along with the class 1 integron gene (intI1) and virulence genes (invA and spvC) within the pathogen, were reduced by 99.65% to 99.94%. In the same electrochemical oxidation treatment system, the model antibiotics tetracycline and sulfadimethoxine were degraded by 97.95% and 93.42%, respectively, within 3 h.185 When used for electrochemical oxidation of perfluorooctanesulfonic acid (PFOS), the nano-Ti4O7 anode showed a better PFOS degradation rate and energy efficiency in both batch and REM operations compared to a micro-Ti4O7 anode and commercial Ebonex® (Ti9O17) anode due to its favourable pore size distribution and composition. The study found that the electroactive surface area of the anodes is linked to pores larger than 1.03 μm, suggesting a pore size threshold that limits electrochemical accessibility in Magnéli phase porous titanium suboxide materials. As a result, anodes with pore sizes just over 1 μm are likely to offer the most effective surface area for electrochemical reactions.186
reduction within 15 minutes at a current density of 10.0 mA cm−2. Additionally, antibiotic resistance genes such as TetG, floR and sul1, along with the class 1 integron gene (intI1) and virulence genes (invA and spvC) within the pathogen, were reduced by 99.65% to 99.94%. In the same electrochemical oxidation treatment system, the model antibiotics tetracycline and sulfadimethoxine were degraded by 97.95% and 93.42%, respectively, within 3 h.185 When used for electrochemical oxidation of perfluorooctanesulfonic acid (PFOS), the nano-Ti4O7 anode showed a better PFOS degradation rate and energy efficiency in both batch and REM operations compared to a micro-Ti4O7 anode and commercial Ebonex® (Ti9O17) anode due to its favourable pore size distribution and composition. The study found that the electroactive surface area of the anodes is linked to pores larger than 1.03 μm, suggesting a pore size threshold that limits electrochemical accessibility in Magnéli phase porous titanium suboxide materials. As a result, anodes with pore sizes just over 1 μm are likely to offer the most effective surface area for electrochemical reactions.186
A Ti4O7 anode was also used for the effective removal of tetracycline by electrochemical oxidation, due to the high conductivity and chemical stability of the material. For tetracycline removal, applying current densities between 0.5 and 3 mA cm−2 achieved over 90% removal across initial concentrations ranging from 1 to 50 ppm, with half-lives of 28 to 75 minutes. They further found that hydroxyl radicals generated on Ti4O7, at a rate of 2 × 10−9 mol cm−2 min−1 under 0.5 mA cm−2, contributed to at least 40% of the total tetracycline removal. Tests on Escherichia coli (E. coli) cultures confirmed that electrooxidation by the Ti4O7 anode reduced tetracycline's antimicrobial activity to undetectable levels.182 A film containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TiO2
1 TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti4O7 mixture prepared on polymethyl-meta-acrylate spectroscopy cuvettes showed effective photoanodic performance in decolourising methylene orange dye compared to TiO2 and Ti4O7 separately. The photoelectrochemical dye degradation tests indicated that TiO2 and Ti4O7 achieved decolourisation values of 35% and 46% respectively, while the TiO2/Ti4O7 mixed film achieved 53% decolourisation. The enhancement in composite film performance is attributed to the synergistic effect of photocatalytic and electrochemical activities shown by TiO2 and Ti4O7, respectively.187 Geng et al. synthesised highly ordered Ti4O7 nanotube arrays and examined their electrooxidation ability. In electrooxidizing phenol, Ti4O7 nanotube arrays showed a 1.7 times higher degradation coefficient compared to BDD, demonstrating their suitability for electrooxidation applications.180 Ti4O7 nanotube arrays achieved a 95.3% removal of phenol chemical oxygen demand, outperforming Ti4O7 particles (79.4%). This enhanced performance was attributed to the presence of Ti4O7 particulates in the nanotube arrays and their higher surface area (11.7 m2 g−1) compared to Ti4O7 particles (4.7 m2 g−1). When used for the electrochemical methanol oxidation reaction, Pt loaded Ti8O15 nanowires prepared by an electrodeposition method showed a peak current density of 28.2 mA cm−2 compared to 20.5 mA cm−2 and 17.2 mA cm−2 for Pt/C and Pt/TiO2 electrodes, respectively, demonstrating the best performance for the Magnéli titania supported electrocatalysts (Fig. 16(d)–(g)).146 A Sc2O3-Magnéli phase titanium composite electrode was prepared by Bai and co-workers using a sintering-pressing technique that demonstrated 90.16% degradation of methyl orange after 120 min of electrolysis. Optimal electrocatalytic activity was determined with a current density of 10 mA cm−2, solution pH 3 and temperature of 25 °C.188
Ti4O7 mixture prepared on polymethyl-meta-acrylate spectroscopy cuvettes showed effective photoanodic performance in decolourising methylene orange dye compared to TiO2 and Ti4O7 separately. The photoelectrochemical dye degradation tests indicated that TiO2 and Ti4O7 achieved decolourisation values of 35% and 46% respectively, while the TiO2/Ti4O7 mixed film achieved 53% decolourisation. The enhancement in composite film performance is attributed to the synergistic effect of photocatalytic and electrochemical activities shown by TiO2 and Ti4O7, respectively.187 Geng et al. synthesised highly ordered Ti4O7 nanotube arrays and examined their electrooxidation ability. In electrooxidizing phenol, Ti4O7 nanotube arrays showed a 1.7 times higher degradation coefficient compared to BDD, demonstrating their suitability for electrooxidation applications.180 Ti4O7 nanotube arrays achieved a 95.3% removal of phenol chemical oxygen demand, outperforming Ti4O7 particles (79.4%). This enhanced performance was attributed to the presence of Ti4O7 particulates in the nanotube arrays and their higher surface area (11.7 m2 g−1) compared to Ti4O7 particles (4.7 m2 g−1). When used for the electrochemical methanol oxidation reaction, Pt loaded Ti8O15 nanowires prepared by an electrodeposition method showed a peak current density of 28.2 mA cm−2 compared to 20.5 mA cm−2 and 17.2 mA cm−2 for Pt/C and Pt/TiO2 electrodes, respectively, demonstrating the best performance for the Magnéli titania supported electrocatalysts (Fig. 16(d)–(g)).146 A Sc2O3-Magnéli phase titanium composite electrode was prepared by Bai and co-workers using a sintering-pressing technique that demonstrated 90.16% degradation of methyl orange after 120 min of electrolysis. Optimal electrocatalytic activity was determined with a current density of 10 mA cm−2, solution pH 3 and temperature of 25 °C.188
The interaction of Ebonex® with the deposited metal could alter the activity or nature of the metal electrocatalyst, as studied by Dieckmann and Langer. When tested for electrogenerative oxidation of aliphatic and aromatic alcohols and formaldehyde, Pt/Ebonex® showed higher activity for methanol oxidation compared to Ni/Ebonex®, while the latter showed significant activity for formaldehyde oxidation and was slightly more polarised for benzyl alcohol.189 Chen et al. investigated the use of an Ebonex® ceramic anode for electrolytic oxidation of trichloroethylene and observed that CO2 was primarily formed with traces of CO, and no other carbon containing products.190 Under an anodic potential (Ea) of 2.5 to 4.3 V vs. silver-silver chloride electrode, trichloroethylene degradation followed first-order kinetics with respect to its concentration. The oxidation rate was pH-independent between pH 1.6 and 11. Trichloroethylene oxidation occurred exclusively on the anodic surface and became mass transport-limited at higher potentials (Ea > 4.0 V). Despite the strong anodic performance of Magnéli phase electrodes, Jing et al.191 observed a decline in the electrochemical activity of TinO2n−1 electrodes during anodic polarisation. This was attributed to be dependent on the type of electrolyte, either due to the formation of a surface TiOSO4 passivating layer or by the loss of charge carriers. Though this surface deactivation can be reversed during the discharge process, the associated ohmic drop requires pre-conditioning of the cell.191,192
Si et al. synthesised gradient titanium oxide nanowire films composed of TinO2n−1 (composed of Ti5O9, Ti4O7 and Ti3O5) and TiO2−x (anatase TiO2 with traces of rutile TiO2) (Fig. 17(a)) by an electrostatic spinning method and gradient temperature annealing, which showed excellent photothermal conversion efficiency and high photodegradation ability against simulated sewage (Fig. 17(b)). The film completely degraded 0.02 g L−1 methylene blue dye in 90 min under 2 suns and achieved a water evaporation rate of 1.833 kg m−2 h−1 under 1 sun.197 Similarly, Fujiwara et al. showed that TiO2 nanostructures containing layers of Ti4O7 and lower titanium suboxides (Ti3O5) with different Ag loadings demonstrated high photoactivity in Cr6+ reduction and methylene blue degradation under visible light (λ > 400 nm) (Fig. 17(c)). The improved performance compared to pristine materials has been attributed to the strong visible light activity of the composite due to the sub-bandgap energy tails generated from the suboxides.198 Li and co-workers synthesised TiO2–Ti5O9 nanostructures via a one-step laser ablation in liquids approach, using varying pulse energy densities during synthesis. The optimised Ti5O9–TiO2 heterojunction formed between the metal oxide phases exhibited enhanced visible light photocatalytic degradation of rhodamine B dye (λ ≥ 420 nm), nearly completely removing the dye within three hours of light irradiation. This improved performance was attributed to efficient charge separation at the phase junction and increased light absorption across a broader wavelength range.199
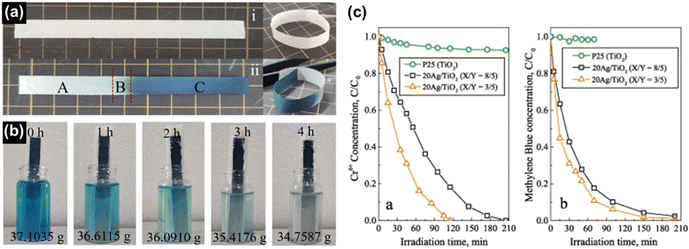 | ||
| Fig. 17 (a) Digital images of the (i) prepared pristine TiO2 and (ii) gradient titanium oxide films and (b) photographs of the change in simulated sewage with time with the treatment of gradient titanium oxide films. Reprinted with permission from ref. 197. Copyright 2022, American Chemical Society. (c) Photocatalytic reduction of Cr6+ ions (left) and methylene blue degradation (right) under visible light irradiation by TiO2 (commercial P25) and 20Ag/TiO2 prepared by flame spray pyrolysis (FSP) at X/Y = 3/5 and X/Y = 8/5. X is the rate at which the precursor solution containing TTIP, silver acetate and acetonitrile was fed through the FSP nozzle, which was then dispersed to a fine spray by Y = 5 mL min−1 oxygen. Reprinted with permission from ref. 198. Copyright 2014, Elsevier. | ||
Zhao et al. synthesised a high surface area carbon layer coated Ti4O7 and g-C3N4 composite via a wet chemical and high temperature treatment route (C@Ti4O7/g-C3N4), with its surface area 5 times higher (72.97 m2 g−1) than pristine Ti4O7 (14.83 m2 g−1). Photocatalytic degradation studies on rhodamine B, methylene blue and methyl orange under visible light showed that the activity and cyclability of C@Ti4O7/g-C3N4 outperformed that of pristine Ti4O7, C@Ti4O7 and anatase TiO2/g-C3N4 catalysts. The superior activity of the core–shell structured composite catalyst was attributed to inherent VO, efficient light absorption, a large specific surface area and enhanced charge carrier separation efficiency.196 Carbon-coated Magnéli phase titanium suboxides, prepared by heat-treating rutile TiO2 with PVA at 1100 °C under N2 flow, were studied for the photocatalytic decomposition of iminoctadine triacetate and phenol under visible light (λ > 400 nm). However, results showed that lower titanium suboxides, Ti2O3 and Ti3O5, had better photocatalytic activity compared to higher n Magnéli phases (n = 4, 5, 6, 9), implying that the presence of a higher concentration of VO is favourable for the photocatalytic activity under visible light.29
4.3 Reactive electrochemical membranes
Traditional membrane filtration faces several challenges, such as relying solely on physical separation for removing toxic pollutants, membrane fouling, decreased permeate flux, chemical usage and high energy consumption. In contrast, reactive electrochemical membranes (REMs) integrate electrochemical advanced oxidation processes (EAOPs) with physical filtration, creating a single, multifunctional water treatment system. REMs generate reactive oxygen species through EAOPs to disinfect organic contaminants without producing harmful by-products.178 Similar to the electrooxidation of organic pollutants discussed in Section 4.2.1, water in reactive electrochemical membranes is oxidised to produce OH˙ radicals on the anode surface, as shown in eqn (12) below.| H2O →OḢ + H+ + e− | (12) |
These OH˙ engage in oxidative reactions with various organic contaminants, leading to their complete degradation or conversion to different byproducts. However, for certain recalcitrant compounds that exhibit low activity towards OH˙ (such as fluorinated organics), direct oxidation can also take place during anodic oxidation. In this process, an electron is transferred from the contaminant (R) to the anode, as was shown in eqn (9).200
REMs can effectively remove waterborne pathogens and heavy metals from drinking water. Their use minimises the need for pretreatment, simplifies operation, reduces chemical consumption and lowers both process and operational costs.201 By combining EAOPs and microfiltration, REMs also mitigate membrane fouling during filtration or backwashing, enhancing flux recovery.26
Given the similarity in the working principles of anodic oxidation for organic pollutants, as discussed in Section 4.2.1, and REMs, the types of materials commonly employed are also similar. The efficiency of the process is highly dependent on the choice of anode material. Several anode materials, such as BDD, Ti/RuO2, SnO2 and PbO2, have been extensively explored for REMs. Among these, BDD has demonstrated the highest efficiency and lowest energy consumption (65 kW h kg per COD1). However, due to the high production costs involved with BDD, there has been a search for alternative anode materials for REMs.202 Magnéli phases, particularly Ti4O7, have attracted attention in this regard due to their excellent stability, high oxygen evolution potential and superior electrical conductivity.
Zaky and Chaplin demonstrated the potential use of porous and tubular Ti4O7 as REMs through the removal of a series of p-substituted phenolic pollutants. Their studies showed that these REMs were effective for both anodic oxidation and OH˙ generation, facilitating efficient removal of the phenolic compounds. Their cross-flow filtration studies, supported by DFT calculations, revealed that p-benzoquinone was mainly removed through reactions with electrochemically produced OH˙. In contrast, the removal of p-nitrophenol and p-methoxyphenol was mainly influenced by the anodic potential applied during the process.203,204 To improve the flexibility of REMs, Santos et al. used an electrospinning and electrospraying method that resulted in highly porous and flexible REMs, composed of polysulfone fibres and Ti4O7 particles. Membrane filtration experiments showed that the observed first order rate constant for phenol oxidation was 2.6 times higher in filtration mode compared to cross flow mode at a 1.0 mA cm−2 current density.205 Studies have shown that the use of REMs as cathodes prevents the formation of halogenated organic compounds that are typically produced during electrochemical oxidation and advanced oxidation processes. Magnéli phase titania samples have been investigated as suitable candidates for this purpose because of the low cost of porous monolithic structure formation and their ability to form OH˙ via water oxidation.26
As discussed above, Ti4O7 is the most conductive Magnéli phase, generating the highest amount of OH˙.206 However, to further improve OH˙ generation, many studies have focussed on modifying Ti4O7 either by increasing the electrochemical surface area or by doping or forming composites with other electrocatalysts.123 Jing et al. synthesised a ceramic, asymmetric, ultrafiltration REM composed of Ti4O7 and Ti6O11 Magnéli phases and tested their activity using humic acid and polystyrene beads as model foulants. Membrane fouling was characterised using an electrochemical impedance spectroscopy technique. A backwash mode chemical-free electrochemical regeneration process was developed based on the results of the above analyses and enabled complete recovery of a fouled membrane without the need for any chemical reagents (Fig. 18(a) and (b)).124 Degradation of sulfamethoxazole using electrochemical reduction and oxidation in single pass, flow through mode using Ti4O7 REMs and Pd–Cu doped Ti4O7 REMs were studied by Misal and co-workers. An impressive 96.1 ± 3.9% of sulfamethoxazole was removed by the Pd–Cu/Ti4O7 REM via electrochemical reduction at −1.14 V/SHE, higher than that observed for Ti4O7 and Pd/Ti4O7 REMs. However, in electrochemical oxidation, the Ti4O7 REM showed the highest removal, removing 95.7 ± 1.0% sulfamethoxazole at 2.03 V/SHE.122 Adsorption and electrochemical reduction of N-nitrosodimethylamine by carbon–Ti4O7 composite REMs was studied by Almassi and co-workers. Upon the addition of multi-walled carbon nanotubes or activated carbon to the REM, the residence times of N-nitrosodimethylamine in the REM increased by a factor of 3.8 to 5.4, leading to higher degradation.183 To study the electrochemical inactivation of E. coli at different current densities, Liang et al. studied a REM system with two highly conductive, stable and porous Ti4O7 membrane electrodes, working in dead-end filtration mode (Fig. 18(c)). Their studies showed that as the current density increased, the bacterial concentration decreased, with severe damage to the cells. Moreover, it is reported that the E. coli concentration decreased from 6.46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) to 0.18
to 0.18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 after passing through the membrane filter (Fig. 18(d)).207 To treat agriculturally contaminated water, Gayen and co-workers modified Ti4O7 REMs by depositing B-doped SnO2 to provide high overpotential in oxygen evolution. When atrazine and clothianidin were used as the model contaminants and terephthalic acid as the OH˙ probe, complete mineralisation of all compounds was achieved at 3.5 V/SHE in a single pass in the reactor with a 3.6 s residence time.121
CFU mL−1 after passing through the membrane filter (Fig. 18(d)).207 To treat agriculturally contaminated water, Gayen and co-workers modified Ti4O7 REMs by depositing B-doped SnO2 to provide high overpotential in oxygen evolution. When atrazine and clothianidin were used as the model contaminants and terephthalic acid as the OH˙ probe, complete mineralisation of all compounds was achieved at 3.5 V/SHE in a single pass in the reactor with a 3.6 s residence time.121
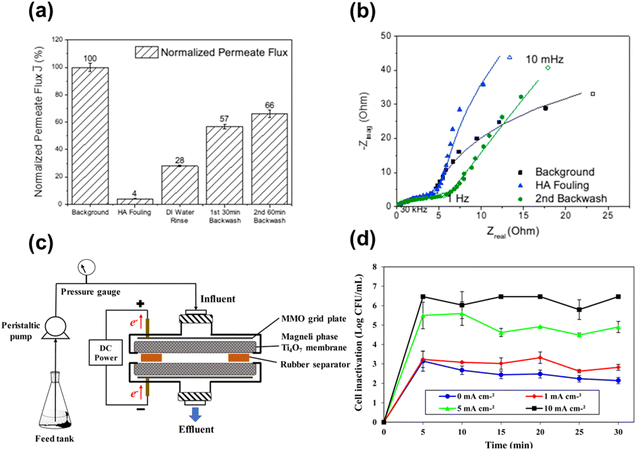 | ||
| Fig. 18 (a) Normalised permeate flux under different operational conditions for the anodic chemical-free electrochemical regeneration in backwash mode of 150 mg L−1 humic acid fouled REM and (b) electron impedance spectra obtained in the complete frequency range. Reprinted with permission from ref. 124. Copyright 2016, Elsevier. (c) REM system containing Ti4O7 ceramic membrane design and setup. (d) Effect of different current densities on the removal of E. coli by the Ti4O7 membrane filtration system. Reprinted with permission from ref. 207. Copyright 2018, Elsevier. | ||
4.4 Electronic and optoelectronic applications
The operation of resistive random-access memory (ReRAM) is based on the dielectric breakdown of an insulator, mostly metal oxides, which shows resistive switching phenomena. In the structure of a ReRAM, the insulator/semiconducting material is sandwiched between two metal electrodes. In the resistive switching process of an ReRAM, by applying pulsed voltages, the resistance of the cell can be changed significantly.208,209 Hence, devices in which the resistive switching mechanism occurs demonstrate bipolar behaviour, wherein the conducting and insulating states are alternated by applying opposite bias polarities.Kwon and co-workers probed the nanofilaments in a Pt/TiO2/Pt system during resistive switching directly by HRTEM. They observed that Ti4O7 conducting filaments formed in TiO2 implying that the formation of Magnéli phase filaments induced the observed switching (Fig. 19(a)).210 Similarly, Strachan et al. observed a Ti4O7 crystallite in the TiO2 matrix, showing that the resistance switching demonstrated by a Pt/TiO2/Pt memristor is due to TiO2 reduction and crystallisation of a metallic conducting network. They further explained that within a TiO2 matrix, the formation of Magnéli phases is thermodynamically favoured over a high concentration of randomly distributed vacancies in the material, depending on the electrochemical potential within the device (Fig. 19(b) and (c)).211 Kim et al. prepared a cross-bar type Pt/TiO2/Pt structure with improved electrical endurance characteristics and uniform low resistance state and high resistance state distribution in filamentary resistive switching. Ti4O7 and Ti5O9 conducting filaments were observed in the TiO2 thin film that lead to a stable memory window.212
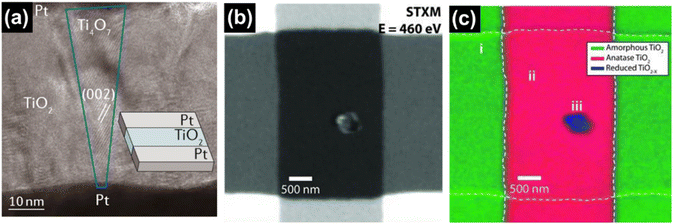 | ||
| Fig. 19 (a) HRTEM image of a nanocrystalline Ti4O7 filament (outlined in blue) in a Pt/TiO2/Pt system used in a resistive switching device. Reprinted with permission from ref. 217. Copyright 2020, Springer Nature. (b) Scanning Transmission X-ray Microscopy image of the device junction area, showing absorption contrast within the junction and (c) chemical and structural mapping of the three observed phases in the junction. Region (i) corresponds to amorphous TiO2, Region (ii) is anatase TiO2 and Region (iii) is reduced titanium suboxides TiO2−x. Reprinted with permission from ref. 211. Copyright 2010, John Wiley and Sons. | ||
Banerjee et al. demonstrated the resistive switching and complementary resistive switching behaviours in a TiOx/Al2O3-based 3D vertical crossbar ReRAM device. Stable resistive switching mechanisms in these devices were confirmed and attributed to the observation of Ti5O9 nanofilaments by HRTEM.213 As observed in these studies, formation of titanium suboxide Magnéli phases via generation of shear planes in TiO2 is related to the valence-change memory effect, which states that the reduction of transition metal ions is caused by migration of VO driven by the electrochemical potential gradient of VO.62,210–214
4.5 Batteries
Rechargeable batteries, a type of electrochemical energy storage, have garnered significant attention over the past few decades due to their higher energy efficiency compared to mechanical and chemical storage systems.215 Among various rechargeable batteries, commercial lithium-ion batteries have become particularly popular thanks to their high energy density, cyclic stability and energy efficiency. Recently, sodium and potassium have also gained similar interest as potential alternatives, due to their comparable chemical properties to lithium.216 Magnéli phase titanium suboxides are used in batteries as electrodes because of their unique electronic and electrochemical properties. These materials have high electrical conductivity and high specific capacity, suggesting that they can store and release a significant amount of energy per unit mass. In addition, Magnéli phase titanium suboxides are highly stable, corrosion resistant and durable, making them excellent candidates in rechargeable batteries.27,28 They have also demonstrated excellent cyclability, enabling them to undergo many charge–discharge cycles without a decline in electrochemical performance. Furthermore, their high thermal stability allows these materials to withstand high temperature, improving the safety and reliability of batteries and preventing overheating and short circuiting.23,24,218 As discussed in Section 4.1, Magnéli phase titanium suboxides are commonly used as catalyst supports for oxygen and hydrogen evolution reactions, primarily due to their high electrical conductivity and exceptional corrosion resistance, which ensure stability in both highly acidic and basic environments and provide significant electrochemical durability. Given that similar properties are desirable for battery applications, it is likely that Magnéli phase titanium suboxides developed for catalytic purposes could be adapted and optimised for use in battery technologies as well.Ti4O7 has been investigated as a suitable host material for sulfur loading in lithium sulfur batteries due to its high conductivity and high binding affinity towards lithium polysulfide.145 In lithium sulfur batteries, the shuttle effect and the uncontrollable deposition of lithium sulfides have been identified as factors that can result in low coulombic efficiency and capacity decay. To find a solution to this challenge, Tao et al. used a sublimation and deposition method to fabricate a high performance Ti4O7–S cathode composite by thermal diffusion of S into the Ti4O7 matrix. Results revealed higher cycling performance and reversible capacity compared to the TiO2–S cathode, due to more effective binding of Ti4O7 with S species. The Ti4O7–S cathode demonstrated high specific capacities at different C rates, achieving 1342, 1044 and 623 mA h g−1 at 0.02, 0.1 and 0.5C, respectively, along with impressive capacity retention of 99% at 100C and 0.1C (Fig. 20(a) and (b)).219 Sabbaghi and co-workers developed highly conductive Magnéli Ti4O7 nanotube arrays supported by a carbon-coated separator, to enhance the energy density and enable rapid charging and discharging in Li–S batteries. The battery demonstrated a reversible discharge capacity of 723 mA h g−1 after 500 cycles with a capacity fading rate of 0.07% per cycle at 0.5C.220 Han and Wang reported the use of graphitic carbon coated Ti9O17 as anodes for Li-ion batteries and in hybrid electrochemical cells. These materials have shown excellent cyclic stability giving a pseudocapacitive lithium-storage behaviour with a reversible capacity of 182 mA h g−1.221 Additionally, Lee et al. presented the Ti6O11/carbon nanotube composite electrode as a promising anode material for K-ion batteries. This electrode exhibited an extended cycling life of over 500 cycles at a current rate of 200 mA g−1, with a capacity retention of 76% and an impressive coulombic efficiency of 99.9%.222
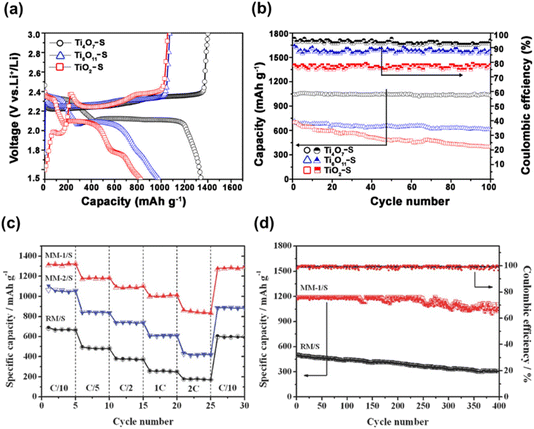 | ||
Fig. 20 (a) Initial charge–discharge curves of composite cathodes formed with TiO2, Ti4O7 and Ti6O11 at a rate of 0.02C. (b) Cyclic performance and coulombic efficiency of the cathodes for 100 cycles at 0.1C rate. Reprinted with permission from ref. 219. Copyright 2014, American Chemical Society. (c) Rate performance from C/10 to 2C of rutile TiO2 microspheres infiltrated with S (RM/S) and Ti4O7 microspheres infiltrated with S (MM-x/S) (The molar ratio of TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C during synthesis varied across the samples, with a ratio of approximately 3 C during synthesis varied across the samples, with a ratio of approximately 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for MM-1, and 3 2 for MM-1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for MM-2, depending on the volume of resol solution used) and (d) cyclability with calculated coulombic efficiency of RM/S and MM-1/S at C/5. Reprinted with permission from ref. 223. Copyright 2017, John Wiley and Sons. 4 for MM-2, depending on the volume of resol solution used) and (d) cyclability with calculated coulombic efficiency of RM/S and MM-1/S at C/5. Reprinted with permission from ref. 223. Copyright 2017, John Wiley and Sons. | ||
Wei et al. developed a sulfur cathode hosted on mesoporous Ti4O7 microspheres (70 wt% sulfur) that exhibited a high discharge capacity of 1317.6 mA h g−1 at moderate current density, with 88% capacity retention after 400 cycles, exhibiting excellent cyclability. Excellent stability attributed to the Ti–S interactions between low-coordinated Ti4O7 and lithium polysulfides, facilitated sulfur redeposition during the charging phase. Additionally, the porosity and the high electronic conductivity of the electrodes contributed to the high performance of the material.223 Furthermore, Li et al. successfully studied the feasibility of Ti4O7 for use as air–cathodes in zinc–air batteries under strong alkaline conditions due to its electrochemical durability and stability. The stability of the Ti4O7 electrode was evaluated in an O2-saturated alkaline solution through cyclic voltammetry within a potential range of −0.7 to +0.7 V vs. Hg/HgO. The Ti4O7 electrode successfully survived 5000 cycles without any significant loss in the oxygen reduction reaction peak current.114 Yao et al. used Ti4O7 as the conductive additive in a sulfur electrode and showed that sulfur/Ti4O7 had improved electrochemical properties compared to a sulfur/acetylene black electrode, in both its polysulfide absorption and its catalytic activity towards the Li/S redox reaction.127
Franko and co-workers utilised Ti4O7 as a stabilising additive for simple carbon paper cathodes to limit NaO2 degradation in sodium–oxygen batteries. Results revealed that Ti4O7 serves as a stable nucleation point for NaO2 formed in the solution, resulting in a reduced rate of NaO2 degradation. Consequently, Ti4O7-coated cathodes exhibited significantly longer cell lifetimes over many cycles compared to cathodes cast with commercial SuperP carbon black. When the Ti4O7 content in the cathode slurry (comprising Ti4O7, poly(vinylidene fluoride) and SuperP carbon in acetone) was increased to up to 90%, the cell lifetime significantly improved to 37 cycles before any notable degradation was observed.224 To evaluate the possibility of replacing carbon in electrodes, Lee et al. synthesised RuO2@Ti4O7 nanospheres that could enhance the performance of Li–O2 batteries. With Ti4O7 supporting the activation of catalytic performance of RuO2 nanoparticles during discharge–charge processes, these carbon-free RuO2@Ti4O7 nanosphere electrodes are considered as potential candidates for superior oxygen reduction and oxygenation reactions.225
4.6 Photothermal applications
Solar-driven steam generation is becoming an important approach for harnessing solar energy, as it directly converts solar energy into heat for water evaporation and facilitates energy storage.226 The photothermal materials used in solar steam evaporators can effectively capture solar energy and generate heat, which is then transferred to bulk water for water vapour generation.227 The performance of a solar steam evaporator partially depends on the photothermal material employed. Among the various types of photothermal materials, such as plasmonic nanoparticles, carbon-based materials, polymers and inorganic semiconductors, semiconductors are increasingly being modified and utilised due to their abundance and low cost.228 Magnéli phase titanium suboxides, with their excellent light absorbance across the solar spectrum and desirable thermal conductivity, have demonstrated high solar-to-vapour efficiency, making them suitable photothermal materials for solar steam generation.30The ability for Magnéli phase titanium suboxides to absorb solar light has been used in solar steam generation via a self-floating Ti4O7/yttrium stabilised zirconia (YSZ) membrane (Fig. 21(a)). While the insulating YSZ layer is conducive to water transportation, the upper Ti4O7 layer was used for photothermal conversion, so that the bilayered membrane showed a remarkable water evaporation rate of 1.86 kg m−2 h−1 under one sun (Fig. 21(b) and (c)).229 Si et al. synthesised gradient titanium oxide nanowire films composed of TiO2−x and TinO2n−1, which exhibit both photocatalytic and photothermal properties. The TinO2n−1 component of the film (containing Ti4O7 and Ti3O5) demonstrated a water evaporation rate of 1.833 kg m−2 h−1 under one sun irradiation, with an energy conversion efficiency of 88.96%.197 Xu et al. prepared Ti4O7-PVA nanocomposite hydrogels that exhibit a narrow bandgap of approximately 0.81 eV for highly efficient solar steam generation. Under one sun irradiation, this hydrogel achieved an evaporation rate of approximately 4.45 kg m−2 h−1 with an energy efficiency of around 90.69%. Additionally, the hydrogel demonstrated impressive stability, maintaining an evaporation rate of up to approximately 4.03 kg m−2 h−1 until day 20. Further experiments conducted on seawater desalination revealed negligible salt accumulation on the hydrogel's surface, enabling its use in stable purification of sewage or desalination of seawater containing multiple organic contaminants.228 Since most Magnéli phase titanium suboxides used in solar steam evaporators are based on Ti4O7, in one of our previous studies, we investigated Magnéli phases other than Ti4O7 in a solar steam evaporator and monitored its performance. We utilised a graphene oxide-based aerogel and incorporated Magnéli phase titanium suboxides (comprising 2.8% Ti5O9, 81.8% Ti6O11, 30.7% Ti7O13, 3.8% Ti8O15 and 0.9% Ti9O17) to form a solar steam evaporator. The composite aerogel (radius 2.25 cm) demonstrated a water evaporation rate of 0.832 kg m−2 h−1 under 1.0 sun with an optimised weight of 50 mg of Magnéli phase titanium suboxide particles. The calculated energy conversion efficiency for the optimised aerogel was 56.5%, which is over three times that of water without the aerogel under the same conditions.30
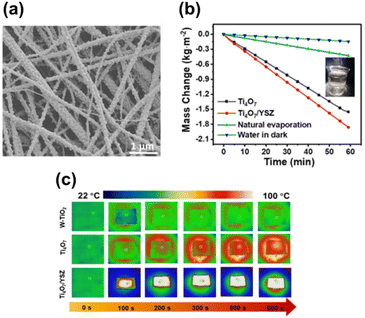 | ||
| Fig. 21 (a) SEM image of fibrous Ti4O7 membrane after calcination. (b) Rate of water evaporation from the Ti4O7 membrane with and without YSZ. (c) Temperatures on the surface of water recorded for TiO2, Ti4O7 and Ti4O7/YSZ membranes by an IR thermometer. Reprinted with permission from ref. 229. Copyright 2022, American Chemical Society. | ||
4.7 Other applications
Hydrovoltaics is a novel electricity generating technology that harnesses the ability of nanomaterials to respond to waves or the flow, dropping or evaporation of water.230 To enhance the electric power generation in a material used in hydrovoltaics, its interaction with water needs to be improved with a reduction in its internal resistance. Since VO increase the carrier concentration and attract cations, Si et al. used Magnéli phase nanocrystal film as the active material for hydrovoltaic power generation. They observed that the open circuit voltage (VOC) and short circuit current (ISC) increased with increasing oxygen vacancy concentration (which correlated to increasing annealing temperature during material synthesis). They demonstrated the use of their energy generation devices by capturing rainwater and observed that a ∼600 mV VOC was maintained for over 2.5 h (Fig. 22(a)–(c)).129 | ||
| Fig. 22 (a) Measured open circuit voltage (VOC) and (b) short circuit current (ISC) for Magnéli phase nanocrystal films prepared at different temperatures (800–1000 °C) and (c) average peak values of VOC and ISC. Reprinted with permission from ref. 129. Copyright 2021, American Chemical Society. | ||
Qing et al. synthesised a Ti4O7/polyimide composite using a heat pressing technique, which exhibited tunable dielectric properties and excellent absorbance in the high-frequency range of the GHz band. In addition, the study also demonstrated the potential of these composites as flexible absorbing coating materials. When the polyimide matrix was backfilled with 60 wt% Ti4O7 particles, the minimal reflection loss reached −49.3 dB at 13.7 GHz with a thickness of 1.25 mm. This finding further confirmed that the tunable and unique dielectric properties of Ti4O7 make it an excellent candidate for designing absorbers and for other electromagnetic applications.231 The gas sensing behaviour of a Magnéli phase containing a metal oxide layer (rutile TiO2, Ti9O17 and Ti8O15) coated on a flexible polymeric thin film was tested using NH3(g) at 12.5–100 ppm. Positive results observed in the sensing experiments were attributed to the high concentration of VO and porosity of the active layer, which can increase gas adsorption capacity and the surface for interaction.232 Fan et al. demonstrated the possibility of using Ti9O17 as a non-toxic n-type thermoelectric material,77 while Canillas et al. explored the use of Ti5O9 as a candidate electrode material in neuron growth stimulation due to its low impedance and high chemical stability.233
5. Challenges and prospects
5.1 Stability and durability
Magnéli phases are well known for their excellent chemical and electrochemical stability, making them appealing to electrochemists and electrochemical engineers. Because of these properties, Ebonex® materials have been employed both as standalone electrode materials and as substrates for various electrocatalysts.Research conducted by Smith et al. explored the stability of titanium suboxides in the Magnéli phase under harsh conditions. The study found these materials to be more durable than traditional electrodes used in large-scale electrochemical processes, including both Ti and TiO2. Magnéli phases remained stable when exposed to aggressive chemicals like fluoride-based etchants, hydrochloric acid and aqua regia. However, they experienced some degradation in more extreme environments, such as boiling phosphoric acid, concentrated HF and highly concentrated NaOH solutions (concentration > 12 M).25 Liu et al. investigated the electrochemical stability windows of Ti4O7 electrodes across acidic, neutral and alkaline aqueous solutions. Their findings demonstrate that the stability windows in all three types of solutions exceed 3.5 V, enabling the production of significant amounts of ˙OH radicals. Notably, the Ti4O7 electrode exhibited its widest stability window of 4.19 V in a 1 M NaCl solution, while the narrowest window, 3.53 V, was observed in a 1 M H2SO4 solution.234
Magnéli phase titanium suboxides show better stability in various aspects compared to carbon-based materials. Ceramics produced at high temperatures tend to be in their most stable and fully oxidised state, meaning they are less likely to oxidise further, unlike metals and carbon. In addition, in applications like electrolysis, Ebonex® electrodes demonstrate greater stability than carbon electrodes, particularly at high pH levels, where carbon materials tend to decompose.25 Li et al. investigated the stability of Magnéli phase Ti4O7 electrodes for oxygen reduction in zinc–air rechargeable batteries, as carbon materials tend to degrade due to corrosion from O2 and H2O2, and at high electrode potentials. Ti4O7, with its excellent conductivity and electrochemical stability, emerged as a strong candidate for air–cathodes in these batteries. Cyclic voltammetry and chronopotentiometric tests confirmed its stability. Raman and XPS analysis before and after testing revealed the formation of a thin TiO2 layer on the Ti4O7 surface, which likely protects the bulk material from further oxidation, enhancing its long-term stability.114
Krishnan et al. describe the use of Magnéli phase titanium suboxides as durable catalyst supports capable of withstanding high potentials in PEM fuel cells. These materials demonstrate remarkable corrosion resistance, even under very high potential conditions. The electrochemical stability of the Pt/TinO2n−1 catalyst (with Ti4O7 as the dominant phase in TinO2n−1) was investigated through cyclic voltammetry at a scan rate of 50 mV s−1. Their research highlighted that TinO2n−1 exhibits excellent stability over the potential range of −0.25 to 2.75 V vs. SHE.130 Owing to their resistance to corrosion and resilience against polarity reversal, Ebonex® electrodes are also used in electrophoresis. Their chemical inertness, especially in the presence of organic electrolytes, provides flexibility in the choice of gel types . Their large surface area allows for efficient voltage and current distribution, and they perform exceptionally well in reverse or pulsed electrophoresis, due to their high stability under polarity reversal.25
These findings suggest that, despite being synthesised through the creation of VO, Magnéli phase titanium suboxides remain stable at room temperature and in oxygen-rich environments over time. Instead of instability, they exhibit remarkable stability and durability across diverse chemical conditions, reaffirming their suitability for various applications.
5.2 Cost–benefit analysis
As detailed in the synthesis section, TiO2 is the primary feedstock for many synthesis methods of Magnéli phases. Depending on the method, different reducing agents such as hydrogen gas, carbon or metals are used. Since these reduction processes typically require high temperatures over extended periods, cost considerations largely depend on the choice of raw materials and reductants, assuming operational and energy costs remain comparable.In most reduction methods for forming Magnéli phases, TiO2 is the primary raw material, priced at approximately USD 3/kg globally in 2022.235 Considering the various reduction methods used to form Magnéli phases, the high cost of metals such as Ti (over USD 10/kg)236 and Zr (with an average import price of USD 28/kg in the United States in 2023) makes large-scale synthesis via metallothermic reduction highly impractical.
Due to the high costs associated with the storage and transportation of hydrogen, carbothermal reduction emerges as a more cost-effective method for large-scale synthesis of Magnéli phase titanium suboxides, including commercial Ebonex®. This approach is particularly advantageous as it uses activated charcoal as the reductant, which is priced at around USD 5.6/kg, based on both literature and commercial sources.237 Consequently, the relatively low cost of raw materials for producing Magnéli phase titanium suboxides offers these materials a significant cost-benefit advantage over more commonly used electrode materials like BDD in electrochemical applications.
5.3 Challenges related to the synthesis and use of Magnéli phase titanium suboxides
In the last decade, Ti3+ self-doped Magnéli phase titanium suboxides have attracted much interest in diverse fields due to their outstanding optoelectronic, photochemical and photocatalytic properties. The main current issues in the synthesis and use of Magnéli phase titanium suboxides are the high temperatures and extensive sintering required, which lead to particle coalescence and the formation of microparticles that are unsuitable for applications requiring high surface areas. Issues related to microparticle formation have been resolved by using diverse titanium precursors, such as Ti(NO3)2, and various polymeric reducing agents combined with microwave irradiation, leading to the synthesis of nanoscale Ti4O7.127,238 However, these methods still require expensive, extensive and time-consuming precursor treatments and high-temperature synthesis. To address this, several novel techniques have been developed to synthesise titanium suboxides without the need for such intense sintering. One such approach, described by Arif and co-workers, involves a thermally induced plasma process.239 While this method successfully produces Magnéli phase titanium suboxides with relatively higher surface area (52.9 m2 g−1) the use of plasma is expensive and limits its scalability. Moderate-temperature synthesis methods for Magnéli phase titanium suboxides have also been explored, but these often rely on costly reductants, such as metals and metal hydrides, making them impractical for large-scale production.143 This indicates that none of the current synthesis approaches fully meet all criteria, including nanoparticle formation, the use of low cost reductants/raw materials and the elimination of sintering. Therefore, there is a clear opportunity to develop innovative methods for synthesising nanostructured Magnéli phases at moderate temperatures without relying on costly raw materials.Recently, Ma et al. made use of 3D printing technique to synthesise a porous Magnéli phase electrode composed of Ti5O9, Ti6O11 and traces of Ti7O13, from a TiOx powder mixture and aqueous binder (Fig. 23(a) and (b)). This 3D TiOx electrode showed enhanced degradation kinetics for the probe molecules oxalic acid, terephthalic acid and paracetamol, lower accumulation of toxic by-products (hydroquinone and benzoquinone) and higher mineralisation yield compared to commercial BDD and Ti/TiOx plate anodes.240 This study demonstrates the potential of 3D printing for creating low-energy, feasible approaches for TinO2n−1 preparation, offering an alternative to conventional methods requiring high temperatures or expensive raw materials.
 | ||
| Fig. 23 (a) Porous structure and pore size of 3D printed TiOx electrode and (b) morphology of 3D TiOx as observed from SEM. Reprinted with permission from ref. 240. Copyright 2023, Elsevier. | ||
In addition to the challenges associated with the synthesis of Magnéli phase titanium suboxides, a lesser-known aspect is the potential health risks and concerns related to these materials. It is reported that Magnéli phase materials are generated as incidental nanoparticles during industrial coal combustion causing these nanomaterials to be widespread in the environment.241,242 Analyses of coal ash samples obtained from powerplants in USA and China have shown that all Magnéli phases (n = 4–9) are generated during coal burning with Ti6O11 being the most frequent.243
Toxicity tests on Magnéli phase nanomaterials report that they have potential toxicity pathways that are biologically active without photostimulation.241 These studies report that exposure and accumulation of Magnéli phases cause abnormalities in macrophages due to increased oxidative stress and mitochondrial dysfunction and also result in reduced lung function impacting airway resistance and elastance.242 Kononenko et al. studied the hazard potential of Magnéli phase titanium suboxides on A549 human lung cells. Although some potentially adverse effects of Magnéli phase nanoparticles were observed due to their cellular internalisation and biopersistance, they were considered as non-hazardous, with no increase in intracellular reactive oxygen species levels upon exposure.244 Jemec Kokalj et al. conducted a study on hazard characterisation of Magnéli TiOx using a set of ecotoxicological test organisms and human lung and liver cell lines. Since exposure to these test organisms and cell lines did not induce any biological response, Magnéli TiOx particles were considered as acutely non-hazardous.245 To mitigate the harmful effects of these nanoparticles, some countries use particle traps to capture these nanoparticles before final emission of the exhaust gas. Further assessment regarding the impact of Magnéli phase titanium suboxide particles is required to determine the feasibility of using them in large scale applications.
5.4 Potential improvement strategies and prospects
As previously discussed, the 3D printing of Magnéli phase titanium suboxide electrodes offers a promising synthesis route due to its relatively low cost and time efficiency compared to conventional approaches, which often require significant energy and extensive processing time. To date, there has been only one documented instance of Magnéli phases being synthesised through additive manufacturing. This represents a significant opportunity for further research and development, particularly in the creation of Magnéli phase electrodes for various electrochemical applications. Advancing this area could lead to more sustainable and scalable production methods for energy storage and conversion technologies.Due to the high production costs of BDD for electrochemical applications, Magnéli phase titanium suboxides and their doped counterparts are actively being investigated as alternative anode materials for the electrooxidation of organic pollutants and in REMs. While their use in these applications is currently mostly limited to the laboratory scale, we believe that electrochemical applications will soon present a promising direction for Magnéli phase titanium suboxides.
The authors of this review identified several areas related to these materials that offer significant opportunities for further development and clarification. As discussed in Section 4, photocatalytic pollutant degradation using Magnéli phase titanium suboxides has been investigated in numerous studies, with most approaches focusing on heterojunction formation between Magnéli phase TinO2n−1 and other materials. However, comprehensive studies on heterojunctions involving both organic and inorganic semiconductors are limited, revealing an evident research gap. Moreover, the charge transfer mechanisms in these heterojunctions, which are currently understood in terms of Z-scheme or semiconductor-to-metal charge transfer mechanisms, require deeper investigation. Integrating these experimental studies with theoretical insights could provide a broader understanding of charge transfer dynamics and open new avenues for utilising these materials in photocatalysis.
Further research could also explore the potential of Magnéli phases as standalone catalysts in photocatalytic water purification or with dye sensitisation. A key challenge in these applications is the narrow bandgap of Magnéli phases, which may limit their ability to generate reactive oxygen species. Additionally, the low surface area of these materials may hinder dye adsorption, thus reducing their efficacy. Our research has demonstrated the possibility of bandgap tuning to overcome this limitation, allowing Magnéli phases to reach the potentials required for the formation of superoxide and hydroxyl radicals, thereby enabling them to participate in photocatalytic reactions. Developing Magnéli phases with higher surface areas could further expand their applications, enhancing their utility in environmental and energy-related technologies. Implementing in situ characterisation could offer critical insights into the catalytic and oxidation mechanisms occurring on the surface or interface of the material across various applications. This approach may clarify real-time structural and chemical changes, thereby advancing our understanding of how Magnéli phase titanium suboxides perform under operational conditions.
Two lesser-explored applications of Magnéli phase titanium suboxides are gas sensing and hydrovoltaics. These areas hold considerable potential for industrial applications and sustainable energy generation, particularly using low-cost raw materials. Further investigation into these applications could significantly broaden the scope of Magnéli phases in environmental remediation and energy storage, contributing to the development of advanced, cost-effective solutions for global challenges.
6. Conclusion
This review has highlighted the numerous opportunities that Magnéli phase titanium suboxides present across various fields, including catalysis, batteries, REMs, and electronic and optoelectronic devices. The performance comparisons with the most common materials used in these applications indicate that Magnéli phases not only match but often exceed the performance of traditional materials for similar tasks.Despite the challenges associated with synthesising these materials for large-scale applications, innovative approaches, such as 3D printing, hold great promise. If these methods can be developed and optimised to be cost-effective, Magnéli phase materials could effectively replace conventional materials due to their excellent thermoelectric properties, optical characteristics, corrosion and oxidation resistance, and their ability to form reactive oxygen species on their surfaces.
Given their rising popularity, it is anticipated that research efforts will focus on developing higher surface area Magnéli phases through low-cost synthesis strategies, thereby broadening their use in a variety of applications. This continued exploration may address current challenges and unlock new functionalities and markets for Magnéli phases, solidifying their role as a transformative material in advanced technologies.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
S. Amanda Ekanayake: investigation, validation, formal analysis, writing – original draft. Haoxin Mai: investigation, writing – review and editing. Dehong Chen: conceptualisation, visualisation, supervision. Rachel A. Caruso: conceptualisation, funding acquisition, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding support from the Australian Research Council through the Discovery Project scheme, DP180108315.References
- Q. Guo, et al., Fundamentals of TiO2 photocatalysis: concepts, mechanisms, and challenges, Adv. Mater., 2019, 31(50), 1901997 CrossRef.
- X. Chen and A. Selloni, Introduction: Titanium Dioxide (TiO2) Nanomaterials, Chem. Rev., 2014, 114(19), 9281–9282 CrossRef PubMed.
- P. Roy, S. Berger and P. Schmuki, TiO2 Nanotubes: Synthesis and Applications, Angew. Chem., Int. Ed., 2011, 50(13), 2904–2939 CrossRef.
- R. Kaplan, et al., Simple synthesis of anatase/rutile/brookite TiO2 nanocomposite with superior mineralization potential for photocatalytic degradation of water pollutants, Appl. Catal., B, 2016, 181, 465–474 CrossRef.
- R. Miao, et al., Mesoporous TiO2 modified with carbon quantum dots as a high-performance visible light photocatalyst, Appl. Catal., B, 2016, 189, 26–38 CrossRef.
- X. Yang, et al., Synthesis of visible-light-active TiO2-based photocatalysts by carbon and nitrogen doping, J. Catal., 2008, 260(1), 128–133 CrossRef CAS.
- Y. Zhang, et al., Engineering the Unique 2D Mat of Graphene to Achieve Graphene-TiO2 Nanocomposite for Photocatalytic Selective Transformation: What Advantage does Graphene Have over Its Forebear Carbon Nanotube?, ACS Nano, 2011, 5(9), 7426–7435 CrossRef CAS PubMed.
- G.-S. Li, D.-Q. Zhang and J. C. Yu, A New Visible-Light Photocatalyst: CdS Quantum Dots Embedded Mesoporous TiO2, Environ. Sci. Technol., 2009, 43(18), 7079–7085 CrossRef CAS PubMed.
- X. Pan, et al., Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications, Nanoscale, 2013, 5(9), 3601 RSC.
- J. Nowotny, et al., Titanium dioxide for solar-hydrogen I. Functional properties, Int. J. Hydrogen Energy, 2007, 32(14), 2609–2629 CrossRef CAS.
- U. Diebold, The surface science of titanium dioxide, Surf. Sci. Rep., 2003, 48(5), 53–229 CrossRef CAS.
- H. Malik, et al., Modelling and synthesis of Magnéli Phases in ordered titanium oxide nanotubes with preserved morphology, Sci. Rep., 2020, 10(1), 8050 CrossRef CAS.
- A. F. Arif, et al., Highly conductive nano-sized Magnéli phases titanium oxide (TiOx), Sci. Rep., 2017, 7(1), 3646 CrossRef.
- B. Xu, et al., Structures, preparation and applications of titanium suboxides, RSC Adv., 2016, 6(83), 79706–79722 RSC.
- A. Inglis, et al., Electrical conductance of crystalline TinO2n-1 for n= 4–9, J. Phys. C: Solid State Phys., 1983, 16(2), 317 CrossRef CAS.
- L. A. Bursill and B. G. Hyde, Crystallographic shear in the higher titanium oxides: Structure, texture, mechanisms and thermodynamics, Prog. Solid State Chem., 1972, 7, 177–253 CrossRef CAS.
- R. A. Bennett, et al., STM and LEED observations of the surface structure of TiO2 (110) following crystallographic shear plane formation, Phys. Rev. B:Condens. Matter Mater. Phys., 1999, 59(15), 10341–10346 CrossRef CAS.
- S. Andersson, et al., Phase analysis studies on the titanium-oxygen system, Acta Chem. Scand., 1957, 11(10), 1641–1652 CrossRef CAS.
- M. Radecka, et al., Effect of oxygen nonstoichiometry on photo-electrochemical properties of TiO2−x, J. Power Sources, 2007, 173(2), 816–821 CrossRef CAS.
- W. H. Kao, P. Patel and S. L. Haberichter, Formation Enhancement of a Lead/Acid Battery Positive Plate by Barium Metaplumbate and Ebonex®, J. Electrochem. Soc., 1997, 144(6), 1907–1911 CrossRef CAS.
- R. F. Bartholomew and D. R. Frankl, Electrical Properties of Some Titanium Oxides, Phys. Rev., 1969, 187(3), 828–833 CrossRef CAS.
- S. You, et al., Monolithic Porous Magnéli-phase Ti4O7 for Electro-oxidation Treatment of Industrial Wastewater, Electrochim. Acta, 2016, 214, 326–335 CrossRef CAS.
- F. C. Walsh and R. G. A. Wills, The continuing development of Magnéli phase titanium sub-oxides and Ebonex® electrodes, Electrochim. Acta, 2010, 55(22), 6342–6351 CrossRef CAS.
- J. R. Smith, F. C. Walsh and R. L. Clarke, Electrodes based on Magnéli phase titanium oxides:the properties and applications of Ebonex“materials”, J. Appl. Electrochem., 1998, 28(10), 1021–1033 CrossRef CAS.
- J. R. Smith, F. C. Walsh and R. L. Clarke, Electrodes based on Magnéli phase titanium oxides: the properties and applications of Ebonex® materials, J. Appl. Electrochem., 1998, 28(10), 1021–1033 CrossRef CAS.
- L. Guo, Y. Jing and B. P. Chaplin, Development and Characterization of Ultrafiltration TiO2 Magnéli Phase Reactive Electrochemical Membranes, Environ. Sci. Technol., 2016, 50(3), 1428–1436 CrossRef CAS PubMed.
- K. Ellis, et al., The performance of Ebonex® electrodes in bipolar lead-acid batteries, J. Power Sources, 2004, 136(2), 366–371 CrossRef CAS.
- M. V. Simičić, et al., Influence of non-stoichiometric binary titanium oxides addition on the electrochemical properties of the barium ferrate plastic-bonded cathode for super-iron battery, Electrochim. Acta, 2017, 247, 516–523 CrossRef.
- M. Toyoda, et al., Preparation of carbon-coated Magneli phases TinO2n−1 and their photocatalytic activity under visible light, Appl. Catal., B, 2009, 88(1), 160–164 CrossRef CAS.
- S. A. Ekanayake, et al., Activated charcoal-mediated non-contact carbothermal reduction of TiO2 for controlled synthesis of Magnéli phase titanium suboxides, J. Mater. Chem. A, 2024, 12(24), 14734–14743 RSC.
- S. Jayashree and M. Ashokkumar, Switchable Intrinsic Defect Chemistry of Titania for Catalytic Applications, Catalysts, 2018, 8(12), 601 CrossRef.
- O. Berger, Understanding the fundamentals of TiO2 surfaces. Part I. The influence of defect states on the correlation between crystallographic structure, electronic structure and physical properties of single-crystal surfaces, Surf. Eng., 2022, 1–59 Search PubMed.
- A. Kumar, N. H. Barbhuiya and S. P. Singh, Magnéli phase titanium sub-oxides synthesis, fabrication and its application for environmental remediation: Current status and prospect, Chemosphere, 2022, 307, 135878 CrossRef CAS PubMed.
- B. Xu, et al., Structures, preparation and applications of titanium suboxides, RSC Adv., 2016, 6(83), 79706–79722 RSC.
- X. Wu, H. Wang and Y. Wang, A Review: Synthesis and Applications of Titanium Sub-Oxides, Materials, 2023, 16(21), 6874 CrossRef CAS.
- S. Hejazi, et al., One-dimensional suboxide TiO2 nanotubes for electrodics applications, Electrochem. Commun., 2022, 136, 107246 CrossRef CAS.
- T. S. Rajaraman, S. P. Parikh and V. G. Gandhi, Black TiO2: A review of its properties and conflicting trends, Chem. Eng. J., 2020, 389, 123918 CrossRef CAS.
- F. C. Walsh and R. G. A. Wills, The continuing development of Magnéli phase titanium sub-oxides and Ebonex® electrodes, Electrochim. Acta, 2010, 55(22), 6342–6351 Search PubMed.
- W. Yang, et al., Preparation and Electrochemical Applications of Magnéli Phase Titanium Suboxides: A Review, Chem.–Eur. J., 2024, e202402188 CrossRef PubMed.
- L. S. Dubrovinsky, et al., The hardest known oxide, Nature, 2001, 410(6829), 653–654 CrossRef.
- A. Fujishima, X. Zhang and D. A. Tryk, TiO2 photocatalysis and related surface phenomena, Surf. Sci. Rep., 2008, 63(12), 515–582 Search PubMed.
- L. Liu and X. Chen, Titanium Dioxide Nanomaterials: Self-Structural Modifications, Chem. Rev., 2014, 114(19), 9890–9918 CrossRef PubMed.
- M. K. Nowotny, et al., Defect Chemistry of Titanium Dioxide. Application of Defect Engineering in Processing of TiO2-Based Photocatalysts, J. Phys. Chem. C, 2008, 112(14), 5275–5300 CrossRef.
- M. K. Nowotny, et al., Titanium vacancies in nonstoichiometric TiO2 single crystal, Phys. Status Solidi B, 2005, 242(11), R88–R90 Search PubMed.
- Y. Lu, et al., Spatial Heterojunction in Nanostructured TiO2 and Its Cascade Effect for Efficient Photocatalysis, Nano Lett., 2020, 20(5), 3122–3129 CrossRef PubMed.
- G. Zhuang, et al., Oxygen vacancies in metal oxides: recent progress towards advanced catalyst design, Sci. China Mater., 2020, 63(11), 2089–2118 CrossRef.
- K. Zhu, et al., The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction, Nano Energy, 2020, 73, 104761 CrossRef.
- A. Sarkar and G. G. Khan, The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures, Nanoscale, 2019, 11(8), 3414–3444 RSC.
- Y. Huang, et al., Oxygen Vacancy Engineering in Photocatalysis, Sol. RRL, 2020, 4(8), 2000037 CrossRef.
- X. Pan, et al., Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications, Nanoscale, 2013, 5(9), 3601–3614 RSC.
- U. Diebold, Structure and properties of TiO2 surfaces: a brief review, Appl. Phys. A: Mater. Sci. Process., 2003, 76(5), 681–687 CrossRef.
- C. L. Pang, R. Lindsay and G. Thornton, Structure of Clean and Adsorbate-Covered Single-Crystal Rutile TiO2 Surfaces, Chem. Rev., 2013, 113(6), 3887–3948 CrossRef CAS PubMed.
- T. Sekiya, et al., Defects in Anatase TiO2 Single Crystal Controlled by Heat Treatments, J. Phys. Soc. Jpn., 2004, 73(3), 703–710 CrossRef CAS.
- X. Liu, et al., Progress in Black Titania: A New Material for Advanced Photocatalysis, Adv. Energy Mater., 2016, 6(17), 1600452 CrossRef.
- T. L. Thompson and J. T. Yates, Surface Science Studies of the Photoactivation of TiO2 New Photochemical Processes, Chem. Rev., 2006, 106(10), 4428–4453 CrossRef CAS PubMed.
- F. Parrino, et al., 2 - Properties of titanium dioxide, in Titanium Dioxide (TiO2) and Its Applications, ed. F. Parrino and L. Palmisano, Elsevier, 2021, pp. 13–66 Search PubMed.
- T. Berger, et al., Light-Induced Charge Separation in Anatase TiO2 Particles, J. Phys. Chem. B, 2005, 109(13), 6061–6068 CrossRef CAS PubMed.
- M. A. Henderson, et al., Insights into Photoexcited Electron Scavenging Processes on TiO2 Obtained from Studies of the Reaction of O2 with OH Groups Adsorbed at Electronic Defects on TiO2(110), J. Phys. Chem. B, 2003, 107(2), 534–545 CrossRef CAS.
- E. Stoyanov, F. Langenhorst and G. Steinle-Neumann, The effect of valence state and site geometry on Ti L3,2 and O K electron energy-loss spectra of TixOy phases, Am. Mineral., 2007, 92(4), 577–586 CrossRef CAS.
- Y. Dang and A. R. West, Oxygen stoichiometry, chemical expansion or contraction, and electrical properties of rutile, TiO2±δ ceramics, J. Am. Ceram. Soc., 2019, 102(1), 251–259 CrossRef CAS.
- M. A. Henderson, A surface science perspective on TiO2 photocatalysis, Surf. Sci. Rep., 2011, 66(6), 185–297 CrossRef CAS.
- M. R. K Szot, W. Speier, Z. Klusek, A. Besmehn and R. Waser, TiO2—a prototypical memristive material, Nanotechnology, 2011, 22(25), 254001 CrossRef.
- S. Harada, K. Tanaka and H. Inui, Thermoelectric properties and crystallographic shear structures in titanium oxides of the Magnèli phases, J. Appl. Phys., 2010, 108(8), 083703 CrossRef.
- L. A. Bursill and B. G. Hyde, Crystal Structures in the {132} CS Family of Higher Titanium Oxides TinO2n-1, Acta Crystallogr., Sect. B:Struct. Sci., Cryst. Eng. Mater., 1971, 27(1), 210–215 CrossRef CAS.
- E. Stoyanov, F. Langenhorst and G. Steinle-Neumann, The effect of valence state and site geometry on Ti L3,2 and O K electron energy-loss spectra of TixOy phases, Am. Mineral., 2007, 92(4), 577–586 CrossRef CAS.
- P. A. Cox, Transition metal oxides: an introduction to their electronic structure and properties, Oxford university press, 2010, vol. 27 Search PubMed.
- A. C. M. Padilha, et al., Charge storage in oxygen deficient phases of TiO2: defect Physics without defects, Sci. Rep., 2016, 6(1), 28871 CrossRef CAS.
- A. Azor-Lafarga, et al., Modified Synthesis Strategies for the Stabilization of low n TinO2n-1 Magnéli Phases, Chem. Rec., 2018, 18(7–8), 1105–1113 CrossRef CAS.
- K. M. Ok, et al., Effect of point and planar defects on thermal conductivity of TiO2−x, J. Am. Ceram. Soc., 2018, 101(1), 334–346 CrossRef CAS.
- Y. Le Page and P. Strobel, Structural chemistry of the Magnéli phases TinO2n−1, 4 ≤ n ≤ 9: II. Refinements and structural discussion, J. Solid State Chem., 1982, 44(2), 273–281 CrossRef CAS.
- J. B. Goodenough, Direct Cation- -Cation Interactions in Several Oxides, Phys. Rev., 1960, 117(6), 1442–1451 CrossRef CAS.
- M. Marezio, et al., Structural aspects of the metal-insulator transitions in Ti4O7, J. Solid State Chem., 1973, 6(2), 213–221 CrossRef CAS.
- S. Andersson, et al., The crystal structure of Ti5O9, Acta Chem. Scand., 1960, 14(5), 1161–1172 CrossRef CAS.
- M. E. Bowden, et al., Improved powder diffraction patterns for the titanium suboxides TinO2n−1 (4 ≤ n ≤ 9), Powder Diffr., 1996, 11(1), 60–68 CrossRef CAS.
- P. Strobel and Y. Le Page, Growth of Ti9O17 crystals by chemical vapor transport, J. Cryst. Growth, 1982, 56(3), 723–726 CrossRef CAS.
- W. Lei, et al., Defect engineering of nanostructures: Insights into photoelectrochemical water splitting, Mater. Today, 2022, 52, 133–160 Search PubMed.
- Y. Fan, et al., Preparation of monophasic titanium sub-oxides of Magnéli phase with enhanced thermoelectric performance, J. Eur. Ceram. Soc., 2018, 38(2), 507–513 CrossRef CAS.
- T. Bak, J. Nowotny and J. Stranger, Electrical properties of TiO2: equilibrium vs. dynamic electrical conductivity, Ionics, 2010, 16(8), 673–679 CrossRef CAS.
- M. Woydt, Tribological characteristics of polycrystalline Magnéli-typetitanium dioxides, Tribol. Lett., 2000, 8(2/3), 117–130 CrossRef CAS.
- V. Maneeratana, et al., Original Electrospun Core-Shell Nanostructured Magnéli Titanium Oxide Fibers and their Electrical Properties, Adv. Mater., 2014, 26(17), 2654–2658 CrossRef CAS.
- A. Kitada, et al., Selective Preparation of Macroporous Monoliths of Conductive Titanium Oxides TinO2n–1 (n = 2, 3, 4, 6), J. Am. Chem. Soc., 2012, 134(26), 10894–10898 CrossRef CAS.
- D. Eder and R. Kramer, Stoichiometry of “titanium suboxide”, Phys. Chem. Chem. Phys., 2003, 5(6), 1314–1319 RSC.
- S. A. Fairhurst, et al., Intrinsic paramagnetic dimers in the magneli titanium oxides, J. Magn. Reson., 1983, 54(2), 300–304 CAS.
- I. Slipukhina and M. Ležaić, Electronic and magnetic properties of the Ti5O9 Magnéli phase, Phys. Rev. B:Condens. Matter Mater. Phys., 2014, 90(15), 155133 CrossRef.
- M. Marezio, et al., Charge Localization at Metal-Insulator Transitions in Ti4O7 and V4O7, Phys. Rev. Lett., 1972, 28(21), 1390–1393 CrossRef CAS.
- M. Backhaus-Ricoult, et al., Levers for Thermoelectric Properties in Titania-Based Ceramics, J. Electron. Mater., 2012, 41(6), 1636–1647 CrossRef CAS.
- V. Eyert, U. Schwingenschlögl and U. Eckern, Charge order, orbital order, and electron localization in the Magnéli phase Ti4O7, Chem. Phys. Lett., 2004, 390(1–3), 151–156 CrossRef CAS.
- M. Watanabe, Raman spectroscopy of charge-ordered states in Magnéli titanium oxides, Phys. Status Solidi C, 2009, 6(1), 260–263 CrossRef.
- M. Watanabe and W. Ueno, Raman study of order-disorder transition of bipolarons in Ti4O7, Phys. Status Solidi C, 2006, 3(10), 3456–3459 CrossRef.
- L. K. Keys and L. N. Mulay, Magnetic Susceptibility Measurements of Rutile and the Magnéli Phases of the Ti-O System, Phys. Rev., 1967, 154(2), 453–456 CrossRef.
- L. N. Mulay and W. J. Danley, Cooperative Magnetic Transitions in the Titanium-Oxygen System: A New Approach, J. Appl. Phys., 1970, 41(3), 877–879 CrossRef.
- V. Adamaki, et al., Manufacturing and characterization of Magnéli phase conductive fibres, J. Mater. Chem. A, 2014, 2(22), 8328–8333 RSC.
- D. Regonini, et al., AC electrical properties of TiO2 and Magnéli phases, TinO2n−1, Solid State Ionics, 2012, 229, 38–44 CrossRef.
- X.-L. Shi, J. Zou and Z.-G. Chen, Advanced Thermoelectric Design: From Materials and Structures to Devices, Chem. Rev., 2020, 120(15), 7399–7515 CrossRef.
- X. Zhou, et al., Routes for high-performance thermoelectric materials, Mater. Today, 2018, 21(9), 974–988 CrossRef.
- X. Liu, et al., Controlling the Thermoelectric Properties of Nb-Doped TiO2 Ceramics through Engineering Defect Structures, ACS Appl. Mater. Interfaces, 2021, 13(48), 57326–57340 CrossRef PubMed.
- S. J. Pandey, et al., Modeling the Thermoelectric Properties of Ti5O9 Magnéli Phase Ceramics, J. Electron. Mater., 2016, 45(11), 5526–5532 CrossRef.
- D. Zhou, et al., Preparation of titanium-tantalum-oxygen composite thermoelectric ceramics through high-pressure and high-temperature method, J. Alloys Compd., 2022, 918, 165573 CrossRef.
- X. Qian, J. Zhou and G. Chen, Phonon-engineered extreme thermal conductivity materials, Nat. Mater., 2021, 20(9), 1188–1202 CrossRef PubMed.
- H. Lee, et al., Thermoelectric properties of in-situ plasma spray synthesized sub-stoichiometry TiO2−x, Sci. Rep., 2016, 6(1), 36581 CrossRef PubMed.
- F. Vratny and F. Micale, Reflectance spectra of non-stoichiometric titanium oxide, niobium oxide, and vanadium oxide, Trans. Faraday Soc., 1963, 59, 2739–2749 RSC.
- P. Coufová and H. Arend, On the nature of colour centres in oxygen-deficient BaTiO3 single crystals, Trans. Faraday Soc., 1961, 11(6), 416–423 Search PubMed.
- W.-Q. Han and Y. Zhang, Magnéli phases TinO2n−1 nanowires: Formation, optical, and transport properties, Appl. Phys. Lett., 2008, 92(20), 203117 CrossRef.
- G. Zhou, et al., Ti3+ self-doped mesoporous black TiO2/graphene assemblies for unpredicted-high solar-driven photocatalytic hydrogen evolution, J. Colloid Interface Sci., 2017, 505, 1031–1038 CrossRef CAS.
- J. Liu, et al., Pressure Dependence of Electrical Conductivity of Black Titania Hydrogenated at Different Temperatures, J. Phys. Chem. C, 2019, 123(7), 4094–4102 CrossRef CAS.
- H. Malik, et al., Modelling and synthesis of Magnéli Phases in ordered titanium oxide nanotubes with preserved morphology, Sci. Rep., 2020, 10(1), 8050 CrossRef CAS PubMed.
- M. Niu, et al., Bandgap engineering of Magnéli phase TinO2n−1: Electron-hole self-compensation, J. Chem. Phys., 2015, 143(5), 054701 CrossRef.
- X. Chen, et al., Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals, Science, 2011, 331(6018), 746–750 CrossRef CAS PubMed.
- Y. Liu, et al., Revealing the Relationship between Photocatalytic Properties and Structure Characteristics of TiO2 Reduced by Hydrogen and Carbon Monoxide Treatment, ChemSusChem, 2018, 11(16), 2766–2775 CrossRef CAS PubMed.
- W. Fang, M. Xing and J. Zhang, A new approach to prepare Ti3+ self-doped TiO2via NaBH4 reduction and hydrochloric acid treatment, Appl. Catal., B, 2014, 160–161, 240–246 CrossRef CAS.
- M. Xing, et al., Self-doped Ti3+-enhanced TiO2 nanoparticles with a high-performance photocatalysis, J. Catal., 2013, 297, 236–243 CrossRef CAS.
- X. Liu, et al., Bifunctional, Moth-Eye-Like Nanostructured Black Titania Nanocomposites for Solar-Driven Clean Water Generation, ACS Appl. Mater. Interfaces, 2018, 10(46), 39661–39669 CrossRef CAS.
- S. Siracusano, et al., Preparation and characterization of titanium suboxides as conductive supports of IrO2 electrocatalysts for application in SPE electrolysers, Electrochim. Acta, 2009, 54(26), 6292–6299 CrossRef CAS.
- X. Li, et al., Magneli phase Ti4O7 electrode for oxygen reduction reaction and its implication for zinc-air rechargeable batteries, Electrochim. Acta, 2010, 55(20), 5891–5898 CrossRef CAS.
- P. Geng and G. Chen, Magnéli Ti4O7 modified ceramic membrane for electrically-assisted filtration with antifouling property, J. Membr. Sci., 2016, 498, 302–314 CrossRef CAS.
- D. Takimoto, et al., Conductive Nanosized Magnéli-Phase Ti4O7 with a Core@Shell Structure, Inorg. Chem., 2019, 58(10), 7062–7068 CrossRef CAS.
- P. Krishnan, S. G. Advani and A. K. Prasad, Magneli phase TinO2n−1 as corrosion-resistant PEM fuel cell catalyst support, J. Solid State Electrochem., 2012, 16(7), 2515–2521 CrossRef CAS.
- I. N. Martyanov, et al., Enhancement of TiO2 visible light photoactivity through accumulation of defects during reduction–oxidation treatment, J. Photochem. Photobiol., A, 2010, 212(2), 135–141 CrossRef CAS.
- Y.-W. Lee, et al., Facile and catalytic synthesis of conductive titanium suboxides for enhanced oxygen reduction activity and stability in proton exchange membrane fuel cells, Int. J. Electrochem. Sci., 2013, 8, 9499–9507 CrossRef.
- K. Kolbrecka and J. Przyluski, Sub-stoichiometric titanium oxides as ceramic electrodes for oxygen evolution—structural aspects of the voltammetric behaviour of TinO2n−1, Electrochim. Acta, 1994, 39(11), 1591–1595 CrossRef.
- P. Gayen, et al., Electrocatalytic Reduction of Nitrate Using Magnéli Phase TiO2 Reactive Electrochemical Membranes Doped with Pd-Based Catalysts, Environ. Sci. Technol., 2018, 52(16), 9370–9379 CrossRef PubMed.
- S. N. Misal, et al., Modeling electrochemical oxidation and reduction of sulfamethoxazole using electrocatalytic reactive electrochemical membranes, J. Hazard. Mater., 2020, 384, 121420 CrossRef PubMed.
- S. Nayak and B. P. Chaplin, Fabrication and characterization of porous, conductive, monolithic Ti4O7 electrodes, Electrochim. Acta, 2018, 263, 299–310 CrossRef.
- Y. Jing, L. Guo and B. P. Chaplin, Electrochemical impedance spectroscopy study of membrane fouling and electrochemical regeneration at a sub-stoichiometric TiO2 reactive electrochemical membrane, J. Membr. Sci., 2016, 510, 510–523 CrossRef.
- M. Domaschke, et al., Magnéli-Phases in Anatase Strongly Promote Cocatalyst-Free Photocatalytic Hydrogen Evolution, ACS Catal., 2019, 9(4), 3627–3632 CrossRef.
- Y. Kuroda, et al., Templated Synthesis of Carbon-Free Mesoporous Magnéli-Phase Titanium Suboxide, Electrocatalysis, 2019, 10(5), 459–465 CrossRef.
- S. Yao, et al., Synthesis, characterization, and electrochemical performance of spherical nanostructure of Magnéli phase Ti4O7, J. Mater. Sci.: Mater. Electron., 2017, 28(10), 7264–7270 CrossRef.
- A. K. Vasilevskaia, et al., Formation of nonstoichiometric titanium oxides nanoparticles TinO2n–1 upon heat-treatments of titanium hydroxide and anatase nanoparticles in a hydrogen flow, Russ. J. Appl. Chem., 2016, 89(8), 1211–1220 CrossRef.
- P. Si, et al., Origin of Enhanced Electricity Generation on Magnéli Phase Titanium Suboxide Nanocrystal Films, ACS Appl. Energy Mater., 2021, 4(10), 10877–10885 CrossRef.
- P. Krishnan, S. G. Advani and A. K. Prasad, Magneli phase TinO2n−1 as corrosion-resistant PEM fuel cell catalyst support, J. Solid State Electrochem., 2012, 16(7), 2515–2521 CrossRef.
- S. You, et al., Monolithic Porous Magnéli-phase Ti4O7 for Electro-oxidation Treatment of Industrial Wastewater, Electrochim. Acta, 2016, 214, 326–335 CrossRef CAS.
- R. Moradi and K. M. Groth, Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis, Int. J. Hydrogen Energy, 2019, 44(23), 12254–12269 CrossRef CAS.
- F. Wang, et al., Formation mechanisms of interfaces between different TinO2n−1 phases prepared by carbothermal reduction reaction, CrystEngComm, 2019, 21(3), 524–534 RSC.
- M. A. R. Dewan, G. Zhang and O. Ostrovski, Carbothermal Reduction of Titania in Different Gas Atmospheres, Metall. Mater. Trans. B, 2009, 40(1), 62–69 CrossRef.
- R. Zhu, et al., Magnéli phase Ti4O7 powder from carbothermal reduction method: formation, conductivity and optical properties, J. Mater. Sci.: Mater. Electron., 2013, 24(12), 4853–4856 CrossRef CAS.
- O. Ostrovski, et al., Carbothermal Solid State Reduction of Stable Metal Oxides, Steel Res. Int., 2010, 81(10), 841–846 CrossRef CAS.
- R. Koc and J. S. Folmer, Carbothermal synthesis of titanium carbide usingultrafine titania powders, J. Mater. Sci., 1997, 32(12), 3101–3111 CrossRef CAS.
- C. Trellu, et al., Mineralization of organic pollutants by anodic oxidation using reactive electrochemical membrane synthesized from carbothermal reduction of TiO2, Water Res., 2018, 131, 310–319 CrossRef CAS.
- T. Takeuchi, et al., Synthesis of Ti4O7 Nanoparticles by Carbothermal Reduction Using Microwave Rapid Heating, Catalysts, 2017, 7(12), 65 CrossRef.
- S.-S. Huang, et al., Synthesis of High-Performance Titanium Sub-Oxides for Electrochemical Applications Using Combination of Sol–Gel and Vacuum-Carbothermic Processes, ACS Sustainable Chem. Eng., 2018, 6(3), 3162–3168 CrossRef CAS.
- T. Tsumura, et al., Formation of the Ti4O7 phase through interaction between coated carbon and TiO2, Desalination, 2004, 169(3), 269–275 Search PubMed.
- Q. Pang, et al., Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries, Nat. Commun., 2014, 5(1), 4759 CrossRef CAS.
- M. Nagao, et al., Magneli-Phase Titanium Suboxide Nanocrystals as Highly Active Catalysts for Selective Acetalization of Furfural, ACS Appl. Mater. Interfaces, 2020, 12(2), 2539–2547 Search PubMed.
- X. Geng, et al., Fabrication of high-performance Magnéli phase Ti4O7 ceramics by in-situ hot-pressed sintering in a single step, Mater. Today Commun., 2023, 37, 107058 CrossRef CAS.
- M. Liu, et al., Atom-economic synthesis of Magnéli phase Ti4O7 microspheres for improved sulfur cathodes for Li–S batteries, Nano Energy, 2021, 79, 105428 Search PubMed.
- C. He, et al., Direct synthesis of pure single-crystalline Magnéli phase Ti8O15 nanowires as conductive carbon-free materials for electrocatalysis, Nanoscale, 2015, 7(7), 2856–2861 Search PubMed.
- G. Zhu, et al., Black Titania for Superior Photocatalytic Hydrogen Production and Photoelectrochemical Water Splitting, ChemCatChem, 2015, 7(17), 2614–2619 CrossRef CAS.
- A. A. Gusev, et al., Conducting ceramic anodes based on titanium oxides, Chem. Sustainable Dev., 2004, 12(3), 313–318 CAS.
- C. Tang, D. Zhou and Q. Zhang, Synthesis and characterization of Magneli phases: Reduction of TiO2 in a decomposed NH3 atmosphere, Mater. Lett., 2012, 79, 42–44 Search PubMed.
- H.-P. Gou, G.-H. Zhang and K.-C. Chou, Phase evolution and reaction mechanism during reduction–nitridation process of titanium dioxide with ammonia, J. Mater. Sci., 2017, 52(3), 1255–1264 Search PubMed.
- X. Zhang, et al., Fabrication of Ti4O7 electrodes by spark plasma sintering, Mater. Lett., 2014, 114, 34–36 CrossRef CAS.
- J. Ye, et al., Temperature effect on electrochemical properties of Ti4O7 electrodes prepared by spark plasma sintering, J. Mater. Sci.: Mater. Electron., 2015, 26(7), 4683–4690 CrossRef CAS.
- M. Yu, et al., Magnéli phase titanium suboxides by Flash Spark Plasma Sintering, Scr. Mater., 2018, 146, 241–245 CrossRef CAS.
- L. Wang, et al., Study on the fabricated non-stoichiometric titanium dioxide by in situ reduction with carbon powder via spark plasma sintering, J. Mater. Sci.: Mater. Electron., 2021, 32(20), 24698–24709 CrossRef CAS.
- S. Conze, et al., Magnéli phases Ti4O7 and Ti8O15 and their carbon nanocomposites via the thermal decomposition-precursor route, J. Solid State Chem., 2015, 229, 235–242 Search PubMed.
- B. Xu, et al., Flash synthesis of Magnéli phase (TinO2n-1) nanoparticles by thermal plasma treatment of H2TiO3, Ceram. Int., 2018, 44(4), 3929–3936 CrossRef CAS.
- Z. Ertekin, U. Tamer and K. Pekmez, Cathodic electrochemical deposition of Magnéli phases TinO2n−1 thin films at different temperatures in acetonitrile solution, Electrochim. Acta, 2015, 163, 77–81 CrossRef CAS.
- C. Langlade, et al., Characterization of titanium oxide films with Magnéli structure elaborated by a sol–gel route, Appl. Surf. Sci., 2002, 186(1), 145–149 CrossRef CAS.
- M. Matsuda, et al., Magnéli Ti4O7 thin film produced by stepwise oxidation of titanium metal foil, Scr. Mater., 2021, 198, 113829 CrossRef CAS.
- S. Sun, et al., Enhanced electrochemical performance of TiO2 nanotube array electrodes by controlling the introduction of substoichiometric titanium oxides, J. Alloys Compd., 2016, 680, 538–543 CrossRef CAS.
- L. M. Vračar, et al., Electrocatalysis by nanoparticles – oxygen reduction on Ebonex/Pt electrode, J. Electroanal. Chem., 2006, 587(1), 99–107 CrossRef.
- J. Xu, et al., Insights into the electrooxidation of florfenicol by a highly active La-doped Ti4O7 anode, Sep. Purif. Technol., 2022, 291, 120904 CrossRef.
- T. Luk'yanenko, et al., Design and properties of dimensionally stable anodes on Ebonex® substrate, Vopr. Khim. Khim. Tekhnol., 2019, 2019(6), 121–127 CrossRef.
- G. Dong, L. Yan and Y. Bi, Advanced oxygen evolution reaction catalysts for solar-driven photoelectrochemical water splitting, J. Mater. Chem. A, 2023, 11(8), 3888–3903 RSC.
- B. You, et al., Enhancing Electrocatalytic Water Splitting by Strain Engineering, Adv. Mater., 2019, 31(17), 1807001 CrossRef PubMed.
- M. Ge, et al., One-dimensional TiO2 Nanotube Photocatalysts for Solar Water Splitting, Advanced Science, 2017, 4(1), 1600152 CrossRef.
- E. Slavcheva, et al., Electrocatalytic activity of Pt and PtCo deposited on Ebonex by BH reduction, Electrochim. Acta, 2005, 50(27), 5444–5448 CrossRef.
- A. Stoyanova, et al., Oxygen evolution on Ebonex-supported Pt-based binary compounds in PEM water electrolysis, Int. J. Hydrogen Energy, 2012, 37(21), 16515–16521 CrossRef.
- J.-E. Won, et al., PtIr/Ti4O7 as a bifunctional electrocatalyst for improved oxygen reduction and oxygen evolution reactions, J. Catal., 2018, 358, 287–294 CrossRef.
- P. Paunović, et al., Co-Magneli phases electrocatalysts for hydrogen/oxygen evolution, Int. J. Hydrogen Energy, 2010, 35(19), 10073–10080 CrossRef.
- B. M. Jović, et al., Ni-(Ebonex-supported Ir) composite coatings as electrocatalysts for alkaline water electrolysis. Part II: Oxygen evolution, Int. J. Hydrogen Energy, 2016, 41(45), 20502–20514 Search PubMed.
- Z. Tian, et al., Novel Black BiVO4/TiO2−x Photoanode with Enhanced Photon Absorption and Charge Separation for Efficient and Stable Solar Water Splitting, Adv. Energy Mater., 2019, 9(27), 1901287 CrossRef.
- E. Wierzbicka, et al., Magnéli Phases Doped with Pt for Photocatalytic Hydrogen Evolution, ACS Appl. Energy Mater., 2019, 2(12), 8399–8404 CrossRef CAS.
- M. Ďurovič, J. Hnát and K. Bouzek, Electrocatalysts for the hydrogen evolution reaction in alkaline and neutral media. A comparative review, J. Power Sources, 2021, 493, 229708 CrossRef.
- A. Eftekhari, Electrocatalysts for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2017, 42(16), 11053–11077 CrossRef CAS.
- G. Xiong, et al., Au(111)@Ti6O11 heterostructure composites with enhanced synergistic effects as efficient electrocatalysts for the hydrogen evolution reaction, Nanoscale, 2022, 14(10), 3878–3887 RSC.
- J. Qiao and Y. Xiong, Electrochemical oxidation technology: A review of its application in high-efficiency treatment of wastewater containing persistent organic pollutants, J. Water Proc. Eng., 2021, 44, 102308 CrossRef.
- C. Trellu, et al., Electro-oxidation of organic pollutants by reactive electrochemical membranes, Chemosphere, 2018, 208, 159–175 CrossRef CAS PubMed.
- M. Panizza and G. Cerisola, Direct And Mediated Anodic Oxidation of Organic Pollutants, Chem. Rev., 2009, 109(12), 6541–6569 CrossRef CAS.
- P. Geng, et al., Highly-Ordered Magnéli Ti4O7 Nanotube Arrays as Effective Anodic Material for Electro-oxidation, Electrochim. Acta, 2015, 153, 316–324 CrossRef CAS.
- H. Lin, et al., Defect Engineering on a Ti4O7 Electrode by Ce3+ Doping for the Efficient Electrooxidation of Perfluorooctanesulfonate, Environ. Sci. Technol., 2021, 55(4), 2597–2607 CrossRef CAS.
- S. Liang, et al., Electro-oxidation of tetracycline by a Magnéli phase Ti4O7 porous anode: Kinetics, products, and toxicity, Chem. Eng. J., 2018, 332, 628–636 CAS.
- S. Almassi, et al., Simultaneous Adsorption and Electrochemical Reduction of N-Nitrosodimethylamine Using Carbon-Ti4O7 Composite Reactive Electrochemical Membranes, Environ. Sci. Technol., 2019, 53(2), 928–937 CrossRef CAS PubMed.
- D. Huang, et al., Amorphous Pd-Loaded Ti4O7 Electrode for Direct Anodic Destruction of Perfluorooctanoic Acid, Environ. Sci. Technol., 2020, 54(17), 10954–10963 CAS.
- B. Wang, et al., Simultaneous removal of multidrug-resistant Salmonella enterica serotype typhimurium, antibiotics and antibiotic resistance genes from water by electrooxidation on a Magnéli phase Ti4O7 anode, Chem. Eng. J., 2021, 407, 127134 CrossRef CAS.
- Y. Wang, et al., Electrooxidation of perfluorooctanesulfonic acid on porous Magnéli phase titanium suboxide Anodes: Impact of porous structure and composition, Chem. Eng. J., 2022, 431, 133929 CrossRef CAS.
- V. Becerril-Estrada, et al., Study of TiO2/Ti4O7 photo-anodes inserted in an activated carbon packed bed cathode: Towards the development of 3D-type photo-electro-Fenton reactors for water treatment, Electrochim. Acta, 2020, 340, 135972 CrossRef CAS PubMed.
- H. Bai, et al., Fabrication of Sc2O3-Magneli phase titanium composite electrode and its application in efficient electrocatalytic degradation of methyl orange, Appl. Surf. Sci., 2017, 401, 218–224 CrossRef CAS.
- G. R. Dieckmann and S. H. Langer, Comparisons of Ebonex® and graphite supports for platinum and nickel electrocatalysts, Electrochim. Acta, 1998, 44(2), 437–444 CrossRef.
- G. Chen, E. A. Betterton and R. G. Arnold, Electrolytic oxidation of trichloroethylene using a ceramic anode, J. Appl. Electrochem., 1999, 29(8), 961–970 CrossRef.
- Y. Jing, et al., The roles of oxygen vacancies, electrolyte composition, lattice structure, and doping density on the electrochemical reactivity of Magnéli phase TiO2 anodes, J. Mater. Chem. A, 2018, 6(46), 23828–23839 RSC.
- C. Tran, et al., Increased discharge capacity of a Li-air activated carbon cathode produced by preventing carbon surface passivation, Carbon, 2011, 49(4), 1266–1271 CrossRef.
- Z. H. Jabbar and S. Esmail Ebrahim, Recent advances in nano-semiconductors photocatalysis for degrading organic contaminants and microbial disinfection in wastewater: A comprehensive review, Environ. Nanotechnol., Monit. Manage., 2022, 17, 100666 Search PubMed.
- A. Rafiq, et al., Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution, J. Ind. Eng. Chem., 2021, 97, 111–128 CrossRef.
- K. Nakata and A. Fujishima, TiO2 photocatalysis: Design and applications, J. Photochem. Photobiol., C, 2012, 13(3), 169–189 CrossRef.
- X. Zhao, et al., A direct oxygen vacancy essential Z-scheme C@Ti4O7/g-C3N4 heterojunctions for visible-light degradation towards environmental dye pollutants, Appl. Surf. Sci., 2020, 525, 146486 CrossRef.
- P. Si, et al., Gradient Titanium Oxide Nanowire Film: a Multifunctional Solar Energy Utilization Platform for High-Salinity Organic Sewage Treatment, ACS Appl. Mater. Interfaces, 2022, 14(17), 19652–19658 CrossRef.
- K. Fujiwara, et al., Visible-light active black TiO2-Ag/TiOx particles, Appl. Catal., B, 2014, 154–155, 9–15 CrossRef.
- L. H. Li, Z. X. Deng, J. X. Xiao and G. W. Yang, A metallic metal oxide (Ti5O9)-metal oxide (TiO2)nanocomposite as the heterojunction to enhance visible-light photocatalytic activity, Nanotechnology, 2015, 26(25), 255705 CrossRef PubMed.
- A. M. Zaky and B. P. Chaplin, Porous Substoichiometric TiO2 Anodes as Reactive Electrochemical Membranes for Water Treatment, Environ. Sci. Technol., 2013, 47(12), 6554–6563 CrossRef PubMed.
- Y. Qi, et al., Electrochemical filtration for drinking water purification: A review on membrane materials, mechanisms and roles, J. Environ. Sci., 2024, 141, 102–128 CrossRef.
- W. Wang, et al., Enhanced treatment of p-nitrophenol and coking wastewater through electrochemical and electrochemical-ozonation coupling process utilizing a novel Ti4O7 reactive electrochemical membrane anode, J. Environ. Chem. Eng., 2024, 12(3), 112549 CrossRef.
- A. M. Zaky and B. P. Chaplin, Mechanism of p-Substituted Phenol Oxidation at a Ti4O7 Reactive Electrochemical Membrane, Environ. Sci. Technol., 2014, 48(10), 5857–5867 CrossRef PubMed.
- A. M. Zaky and B. P. Chaplin, Porous Substoichiometric TiO2 Anodes as Reactive Electrochemical Membranes for Water Treatment, Environ. Sci. Technol., 2013, 47(12), 6554–6563 CrossRef.
- M. C. Santos, et al., Highly porous Ti4O7 reactive electrochemical water filtration membranes fabricated via electrospinning/electrospraying, AIChE J., 2016, 62(2), 508–524 CrossRef.
- P. C. S. Hayfield and A. Kuhn, Development of a New Material: Monolithic Ti4O7 Ebonex Ceramic, Royal Society of Chemistry, 2007 Search PubMed.
- S. Liang, et al., Electrochemical inactivation of bacteria with a titanium sub-oxide reactive membrane, Water Res., 2018, 145, 172–180 CrossRef.
- V. Gupta, et al., Resistive Random Access Memory: A Review of Device Challenges, IETE Tech. Rev., 2020, 37(4), 377–390 CrossRef.
- A. Sawa, Resistive switching in transition metal oxides, Mater. Today, 2008, 11(6), 28–36 CrossRef.
- D.-H. Kwon, et al., Atomic structure of conducting nanofilaments in TiO2 resistive switching memory, Nat. Nanotechnol., 2010, 5(2), 148–153 CrossRef PubMed.
- J. P. Strachan, et al., Direct Identification of the Conducting Channels in a Functioning Memristive Device, Adv. Mater., 2010, 22(32), 3573–3577 CrossRef PubMed.
- G. Hwan Kim, et al., Improved endurance of resistive switching TiO2 thin film by hourglass shaped Magnéli filaments, Appl. Phys. Lett., 2011, 98(26), 262901 CrossRef.
- W. Banerjee, et al., Complementary Switching in 3D Resistive Memory Array, Adv. Electron. Mater., 2017, 3(12), 1700287 CrossRef.
- R. T. Doo Seok Jeong, R. S. Katiyar, J. F. Scott, H. Kohlstedt, A. Petraru and C. S. Hwang, Emerging memories: resistive switchingmechanisms and current status, Rep. Prog. Phys., 2012, 75(7), 076502 CrossRef.
- T. Hosaka, et al., Research Development on K-Ion Batteries, Chem. Rev., 2020, 120(14), 6358–6466 CrossRef PubMed.
- L. Li, et al., Advanced Multifunctional Aqueous Rechargeable Batteries Design: From Materials and Devices to Systems, Adv. Mater., 2022, 34(5), 2104327 CrossRef.
- Z. Wang, et al., Resistive switching materials for information processing, Nat. Rev. Mater., 2020, 5(3), 173–195 CrossRef.
- M. J. Styles, et al., A furnace and environmental cell for the in situ investigation of molten salt electrolysis using high-energy X-ray diffraction, J. Synchrotron Radiat., 2012, 19(1), 39–47 CrossRef PubMed.
- X. Tao, et al., Strong Sulfur Binding with Conducting Magnéli-Phase TinO2n–1 Nanomaterials for Improving Lithium–Sulfur Batteries, Nano Lett., 2014, 14(9), 5288–5294 CrossRef.
- A. Sabbaghi, et al., Magneli Ti4O7 nanotube arrays as a free-standing efficient interlayer for fast-delivery and high-energy density lithium-sulfur batteries, J. Alloys Compd., 2023, 960, 170874 CrossRef CAS.
- W.-Q. Han and X.-L. Wang, Carbon-coated Magnéli-phase TinO2n−1 nanobelts as anodes for Li-ion batteries and hybrid electrochemical cells, Appl. Phys. Lett., 2010, 97(24), 243104 CrossRef.
- G.-W. Lee, et al., Magnéli Phase Titanium Oxide as a Novel Anode Material for Potassium-Ion Batteries, ACS Omega, 2019, 4(3), 5304–5309 CrossRef CAS PubMed.
- H. Wei, et al., Chemical Bonding and Physical Trapping of Sulfur in Mesoporous Magnéli Ti4O7 Microspheres for High-Performance Li–S Battery, Adv. Energy Mater., 2017, 7(4), 1601616 CrossRef.
- C. J. Franko, Z. Sadighi and G. R. Goward, Ti4O7-Enhanced Carbon Supports to Stabilize NaO2 in Sodium-Oxygen Batteries, J. Phys. Chem. C, 2022, 126(48), 20263–20274 CrossRef CAS.
- S. Lee, et al., Magnéli-Phase Ti4O7 Nanosphere Electrocatalyst Support for Carbon-Free Oxygen Electrodes in Lithium–Oxygen Batteries, ACS Catal., 2018, 8(3), 2601–2610 CrossRef CAS.
- V.-D. Dao, N. H. Vu and S. Yun, Recent advances and challenges for solar-driven water evaporation system toward applications, Nano Energy, 2020, 68, 104324 CrossRef.
- Y. Lin, et al., Solar steam generation based on the photothermal effect: from designs to applications, and beyond, J. Mater. Chem. A, 2019, 7(33), 19203–19227 RSC.
- X. Xu, et al., Full-spectrum-responsive Ti4O7-PVA nanocomposite hydrogel with ultrahigh evaporation rate for efficient solar steam generation, Desalination, 2024, 577, 117400 CrossRef.
- X. Qiu, et al., Interface Engineering of a Ti4O7 Nanofibrous Membrane for Efficient Solar-Driven Evaporation, ACS Appl. Mater. Interfaces, 2022, 14(49), 54855–54866 CrossRef PubMed.
- Z. Zhang, et al., Emerging hydrovoltaic technology, Nat. Nanotechnol., 2018, 13(12), 1109–1119 CrossRef PubMed.
- Y. Qing, et al., Ti3+ self-doped dark TiO2 nanoparticles with tunable and unique dielectric properties for electromagnetic applications, J. Mater. Chem. C, 2021, 9(4), 1205–1214 RSC.
- M. Gardon, et al., New procedures for building-up the active layer of gas sensors on flexible polymers, Surf. Coat. Technol., 2013, 235, 848–852 CrossRef.
- M. Canillas, et al., Physico-chemical properties of the Ti5O9 Magneli phase with potential application as a neural stimulation electrode, J. Mater. Chem. B, 2013, 1(46), 6459 RSC.
- H. J. Liu, et al., A high strength and conductivity bulk Magnéli phase Ti4O7 with superior electrochemical performance, Ceram. Int., 2022, 48(17), 25538–25546 CrossRef.
- P. Oladipupo, A. Raman and J. F. Pekny, Minimum production scale for economic feasibility of a titanium dioxide plant, J. Adv. Manuf. Process., 2023, 5(4), e10167 CrossRef.
- O. Takeda, T. Ouchi and T. H. Okabe, Recent Progress in Titanium Extraction and Recycling, Metall. Mater. Trans. B, 2020, 51(4), 1315–1328 CrossRef.
- H. A. Alhashimi and C. B. Aktas, Life cycle environmental and economic performance of biochar compared with activated carbon: A meta-analysis, Resour., Conserv. Recycl., 2017, 118, 13–26 CrossRef.
- T. Takeuchi, et al., Synthesis of Ti4O7 Nanoparticles by Carbothermal Reduction Using Microwave Rapid Heating, Catalysts, 2017, 7(2), 65 CrossRef.
- A. F. Arif, et al., Highly conductive nano-sized Magnéli phases titanium oxide (TiOx), Sci. Rep., 2017, 7(1), 3646 CrossRef.
- J. Ma, et al., Porous Magnéli phase obtained from 3D printing for efficient anodic oxidation process, Chem. Eng. J., 2023, 456, 141047 CrossRef CAS.
- Y. Yang, et al., Discovery and ramifications of incidental Magnéli phase generation and release from industrial coal-burning, Nat. Commun., 2017, 8(1), 194 CrossRef PubMed.
- D. K. McDaniel, et al., Pulmonary exposure to Magnéli phase titanium suboxides results in significant macrophage abnormalities and decreased lung function, Front. Immunol., 2019, 10, 2714 CrossRef CAS PubMed.
- Y. Yang, et al., Importance of a Nanoscience Approach in the Understanding of Major Aqueous Contamination Scenarios: Case Study from a Recent Coal Ash Spill, Environ. Sci. Technol., 2015, 49(6), 3375–3382 CrossRef CAS.
- V. Kononenko and D. Drobne, In Vitro Cytotoxicity Evaluation of the Magnéli Phase Titanium Suboxides (TixO2x−1) on A549 Human Lung Cells, Int. J. Mol. Sci., 2019, 20(1), 196 CrossRef.
- A. Jemec Kokalj, et al., The first comprehensive safety study of Magnéli phase titanium suboxides reveals no acute environmental hazard, Environ. Sci.: Nano, 2019, 6(4), 1131–1139 RSC.
| This journal is © The Royal Society of Chemistry 2025 |