Aminophenol–formaldehyde particles containing hydrophilic benzenoid-amine for a highly efficient solar-thermal water harvester†
Rongtai
Yu
*ab,
Jianchao
Xie
a,
Fangfen
Jin
a,
Weiwei
Lu
a,
Mingzhu
Jin
a,
Xinyang
He
a,
Ashok K.
Nanjundan
 bc,
Chengzhong
Yu
bc,
Chengzhong
Yu
 *b and
Xiaodan
Huang
*b and
Xiaodan
Huang
 *b
*b
aSchool of Materials Science and Engineering, Jingdezhen Ceramic University, Jingdezhen, Jiangxi 333403, P. R. China. E-mail: yurongtai@jci.edu.cn
bAustralian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, Queensland 4072, Australia. E-mail: x.huang@uq.edu.au; c.yu@uq.edu.au
cSchool of Engineering, University of Southern Queensland, Springfield, QLD 4300, Australia
First published on 17th December 2024
Abstract
Solar-driven interfacial evaporation systems hold great potential for addressing clean water scarcity and wastewater purification challenges. However, low water yield and the presence of contaminants in wastewater remain significant obstacles. This study introduces wide light-absorbing hydrophilic aminophenol–formaldehyde (APF) resin particles with π-conjugated and π-stacked benzenoid–quinoid donor–acceptor couples as light absorbers to enhance solar-to-vapor conversion efficiency. The incorporation of hydrophilic amine groups led to a 30% increase in the evaporation rate and a 32% reduction in the evaporation enthalpy. The carbonized APF-based evaporator achieved a high evaporation rate of 2.89 kg m−2 h−1 and 3.07 kg m−2 h−1 from sewage and simulated seawater, respectively, under natural solar irradiance (0.7 suns). Furthermore, solar vapor generation rates reached 16.22 kg m−2 h−1 and 13.98 kg m−2 h−1 from sewage and simulated seawater under 3.9 suns. The APF-based evaporator also demonstrated exceptional stability and durability in solar-to-vapor conversion.
Introduction
The generation of clean water from sources such as seawater or wastewater is critical for human survival and societal development, particularly in the face of increasing water scarcity. Over 1.1 billion people worldwide are currently facing a drinking water crisis.1–4 To meet the growing demand for clean water, technologies like membrane-based reverse osmosis and thermal distillation have been developed to purify water from various sources. However, these methods are hindered by high energy consumption, secondary pollution, and operational complexities such as fuel combustion and membrane fouling, which limit their widespread adoption.5–7Solar-driven interfacial evaporation (SDIE) technology offers a green and sustainable solution by using solar energy absorbed by specialized materials to evaporate and purify water.8–11 This approach has gained attention as a promising strategy to combat water scarcity. Various materials have been explored as solar absorbers, including plasmonic particles,12–14 semiconductors,15,16 carbon-based materials,17–20 and π-conjugated polymers.11,21,22 The mechanisms for solar-to-heat conversion differ among these materials, encompassing phonon–phonon relaxation for plasmonic particles, tunable energy bands for semiconductors, electron–electron scattering in carbon-based materials, and the unique π-conjugated structures of polymers.
Despite significant research progress in recent years, the SDIE technology still faces challenges such as low vapor yield and inadequate light absorption, which are limited by the efficiency of solar absorbers.10,11 Additionally, complex pollutants present in wastewater can be harmful and toxic to solar absorbers, further impeding the efficient conversion of solar energy to heat.23,24 Consequently, achieving high solar-to-vapor yield and environmental tolerance simultaneously under real wastewater conditions and natural solar irradiance remains challenging. Conventional solar absorbers exhibit a limited range of rate (vapor yield, ≤1.6 kg m−1 h−1) under natural sunlight (solar flux, ≤1 kW m−2).17,18,22 Zhou et al. demonstrated that introducing a hydratable polymer led to a drop in water evaporation enthalpy, transforming the water phase from bulk water through molecular interactions between water and the polymer.22,23 Additionally, hydrophilic composites have been shown to assist in salt rejection, making them useful in practical applications.25,26 However, these materials relied on complex composites consisting of hydrophilic polymers (e.g., polyvinyl alcohol and chitosan,22 alginate,23 polyimide,25 and polyvinyl alcohol26) integrated with solar absorbers (e.g., polypyrrole,22 graphene,23 porous graphitic carbon,25 and carbon nanotubes26). These combinations require intricate synthesis protocols and introduce heat transfer resistance, thereby reducing evaporation efficiency. The development of novel materials that inherently combine hydrophilic functional groups and efficient solar-absorbing properties remains a significant challenge. In 2019, the Shiraishi group introduced a resorcinol–formaldehyde resin polymer featuring π-conjugated and π-stacked benzenoid–quinoid couples, which offered broad light absorption and strong light absorption capacity.27 This breakthrough sheds light on the potential of advanced resin materials in enhancing the performance of solar-powered water harvesters, paving the way for more efficient and resilient water purification technologies.
Here, an evaporator utilizing an aminophenol–formaldehyde (APF) resin polymer particles with naturally hydrophilic amine groups has been developed, demonstrating a significant reduction in water evaporation enthalpy. The hydrophilic amine groups modify the interaction between APF particles and surrounding water molecules, leading to lower energy consumption during the vaporization process (Fig. 1). Moreover, the electrostatic repulsion between the amine groups and cations in wastewater facilitates effective salt rejection. Incorporating π-conjugated and π-stacked benzenoid–quinoid couples within APF particles further enhances the material's light absorption capabilities. The evaporator achieved notable results, with vapor yields of 2.89 kg m2 h−1 from sewage and 3.07 kg m2 h−1 from simulated seawater under natural solar irradiance (0.7 suns). These yields increased to 16.22 kg m2 h−1 and 13.98 kg m2 h−1, respectively, under intensified solar irradiance (3.9 suns). This innovative polymer-based evaporator not only exhibits high efficiency but also maintains environmental friendliness and stability in purifying water from real wastewater, positioning it as a promising candidate for SDIE technology.
Materials and methods
Materials
3-Aminophenol (98%), resorcinol (99%), formaldehyde solution (37 wt%), ammonia aqueous solution (25 wt%), sodium chloride (A. R.), calcium chloride (A. R.), and magnesium chloride (A. R.) were ordered in Shanghai Aladdin Biochemical Technology Co., Ltd (China). All the reagents were used as received without further purification.Preparation of phenolic resins
APF resin particles were synthesized based on a previously reported method, with slight modifications.28 Specifically, 3-aminophenol (3-AP, 0.1 g) and ammonium hydroxide (NH4OH, 0.05 ml, 28%) were added to a flask containing deionized water (30 ml) at room temperature. After the 3-AP dissolved, a formaldehyde solution (0.1 ml) was added to the mixture, followed by vigorous agitation for 30 minutes. The resulting solids were collected and washed three times with deionized water. These synthesized APF resin particles are denoted as “P” in the figures.The APF resin particles were carbonized in a tubular furnace at 800 °C for 2 hours under a mixed nitrogen and water vapor environment. Water vapor was generated by passing nitrogen gas through a water-filled gas-washing bottle. The resulting carbonized aminophenol–formaldehyde resins are referred to as “CP” in the figures. The CP sample maintains its spherical morphology with a diameter of approximately 200 nm, and elemental mapping analysis reveals that it primarily contains carbon, nitrogen, and oxygen, which are inherited from the APF resin (Fig. S1†).
For comparison, resorcinol–formaldehyde resins were synthesized using a similar procedure to the aminophenol–formaldehyde resins, with the substitution of 3-AP (0.1 g) with resorcinol (0.1 g). The polymer resulting from this synthesis is denoted as “RF” in the figures.
Water evaporation performance under natural solar irradiance
 | (1) |
Mass change and evaporation rate are also used to evaluate the performance of the evaporator.
The mass change of water was calculated by the following formula.29
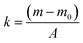 | (2) |
Evaporation rate of water:30
 | (3) |
| ΔHvapΔm0 = ΔHequΔmg | (4) |
Results and discussion
Water extraction under natural solar irradiance
The diffuse reflectance of APF particles showed strong light absorption up to 800 nm, indicating a low bandgap (Fig. 2a). These phenolic polymers, known for their cost-effectiveness as semiconductor photocatalysts, have demonstrated excellent performance in photocatalytic hydrogen peroxide production through benzenoid–quinoid donor–acceptor pairs, achieving over 1.0% solar energy-to-chemical energy conversion efficiency.27,32,33 The donor–acceptor pairs enhance both light absorption and mass transfer.33 Designing a highly hydrophilic light absorber with benzenoid–quinoid donor–acceptor pairs for water evaporation is both an intriguing and significant development.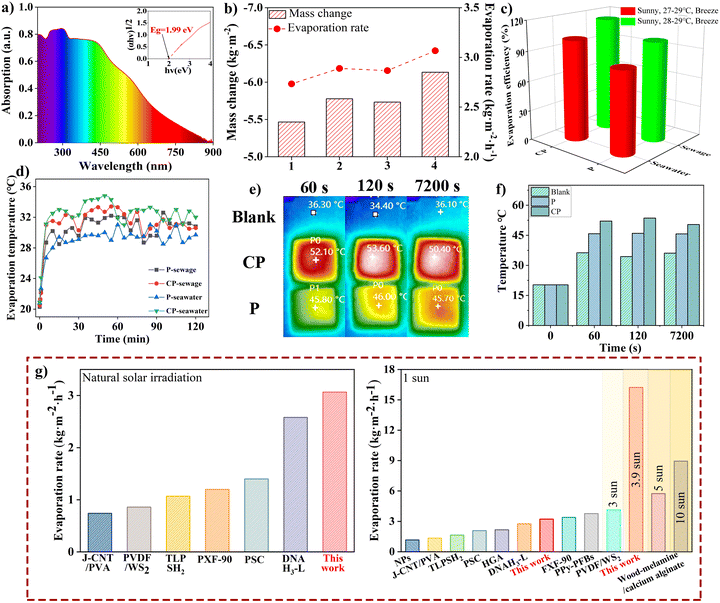 | ||
| Fig. 2 Water evaporation performance of APF resin particles under natural solar irradiance. (a) Diffuse reflectance UV-vis spectra of APF resin particles, with the Tauc plot shown in the inset. (b) Water evaporation tests using pristine and carbonized APF particles in both sewage and simulated seawater (No. 1 and 2 represent sewage evaporation using pristine and carbonized APF, respectively, while No. 3 and 4 correspond to simulated seawater evaporation using pristine and carbonized APF, respectively). (c and d) Evaporation efficiency and surface temperature of phenolic resin under solar irradiance. (e and f) IR thermal images of phenolic resin coatings without water delivery and their respective temperature curves. (g) Comparison of photothermal conversion performance of the APF-based evaporator under natural solar irradiance and a solar simulator, benchmarked against previously reported materials.9,26,38–45 | ||
The precursor 3-AP, used in the synthesis of APF resin particles, contains an inherent amino group in the meta-position of the benzene ring. As a result, APF particles naturally possess electron–donating –NH2 groups.34 Notably, replacing resorcinol with 3-AP as a precursor retains a similar polymerization process, yielding a π-conjugated and π-stacked donor–acceptor structure (Fig. S3†).34–36 N-doping not only enhances photothermal conversion efficiency but also increases hydrophilicity due to the presence of hydrophilic amine groups.36,37
The APF resin particles-based water harvester was constructed and tested under natural solar irradiance using sewage and simulated seawater as water sources. Under solar irradiance of 0.7 kW m−2 for two hours, the evaporator demonstrated higher evaporation rates for simulated seawater compared to sewage. The evaporation rates were 2.87 kg m−2 h−1 for the polymer and 3.07 kg m−2 h−1 for the carbonized APF for seawater, whereas for sewage, they were 2.73 kg m−2 h−1 and 2.89 kg m−2 h−1, respectively (Fig. 2b and ESI Fig. S4†). These rates were calculated after subtracting the blank control data, where no phenolic resins were coated on the filter paper under the same conditions. Interestingly, the carbonized APF exhibited a higher evaporation rate, likely due to the increase in π-conjugated benzenoid–quinoid formation, which occurs via nucleophilic addition and the removal of the methylol group during carbonization.27 This process led to a 5% increase in the evaporation rate. Although the evaporation rate for sewage was 6% lower than that of simulated seawater, the solar-to-vapor conversion efficiency for sewage was higher (Fig. 2c). Surface temperature changes were also monitored during water harvesting using an infrared thermal imaging camera (Fig. 2d). The surface temperature of the phenolic resin evaporator increased rapidly from 20 °C to approximately 30 °C within the first 10 minutes, eventually reaching nearly 35 °C under natural solar irradiance. Notably, the rate of temperature rise in the carbonized resins was higher compared to the non-carbonized ones.
To examine the photothermal conversion efficiency of APF particles in the water evaporator, the surface temperature of the coating was measured before device fabrication, isolating the effects of water desorption. Under natural solar irradiation (0.7 kW m−2), the dry state surface temperature of the coating rapidly increased to 45.8 °C for the polymer and 52.1 °C for the carbonized polymer within 60 seconds (Fig. 2e and f). The temperature then stabilized with a difference of no more than 2 °C, indicating the high efficiency of solar energy conversion to heat in the evaporator. The surface temperature of the phenolic resin coating was significantly higher than that of the filter paper substrate, which reached only 36.3 °C, further demonstrating the strong photothermal conversion and solar responsiveness of the aminophenol–formaldehyde resins. Moreover, Fig. 2g shows that the APF-based resin evaporator achieved exceptionally high evaporation rates under both natural solar and simulated 1.0 sun irradiances (3.17 kg m−2 h−1), and compared to previous studies, highlighting its effectiveness as a solar-powered water harvester.
Contribution of the hydrophilic benzenoid amine
Intrigued by the superior evaporation performance of APF resin particles, we conducted further experiments to explore the contribution of amine groups in the evaporation process. Both resorcinol and 3-AP, the precursors for synthesizing phenolic resins, undergo a hydrothermal reaction where formaldehyde reacts with the 3-AP/resorcinol in the presence of an ammonia catalyst. The polymerization processes of these two precursors are similar, resulting in the formation of APF and RF resins.34–36 In addition to the reported π-conjugated and π-stacked benzenoid–quinoid donor–acceptor structures in these resins,9,26,38–45 the key distinction between APF and RF is the presence of an amine group in APF, absent in RF.27,32,46 Therefore, we synthesized APF and RF particles following a straightforward protocol. FTIR spectra analysis confirmed the presence of benzenoid and quinoid dimers in both types of particles, with the amine group in the benzenoid amine unit specifically identified in APF particles (Fig. S3†).RF, APF and carbonized APF were employed as photothermal conversion materials in constructing the evaporator for a water harvester under a solar simulator with a light intensity of 3.9 kW m−2 (current of 15 A). The photothermal conversion efficiency of all APF-based materials surpassed that of the RF evaporator, resulting in higher evaporation rates of 16.09 and 16.24 kg m−2 h−1 for APF polymer and carbonized APF, respectively, compared to 12.2 kg m−2 h−1 for the RF polymer (Fig. 3a and S5†). The APF-based evaporator achieved a significantly higher evaporating temperature, with a temperature difference of over 14 °C compared to the RF evaporator (Fig. S5a and b†). The improved evaporation rate, attributed to the presence of benzenoid amine groups in the APF particles, indicates that despite the similar polymerization and composition between APF and RF polymers, the former exhibited a 30% increase in evaporation efficiency.47
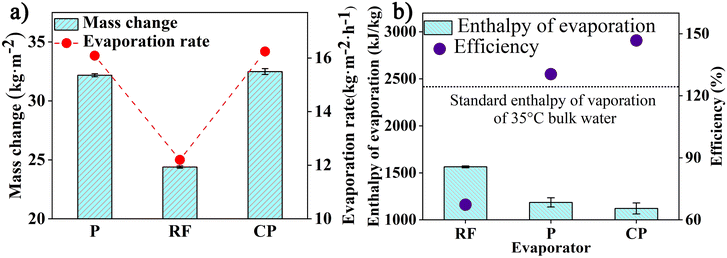 | ||
| Fig. 3 The effect of hydrophilic benzenoid-amine on evaporation performance under a solar simulator. (a) Evaporation rate and mass change. (b) The evaporation enthalpy and efficiency. | ||
Zhou et al. reported that water evaporation by a polymer evaporator could classify water into bound, intermediate, and free water, based on intermolecular hydrogen bonding, such as water/polymer and weakened water/water interactions.22 Studies have shown that intermediate water vaporizes more easily and with less energy than bulk water.11,48 The amount of intermediate water is directly affected by the hydrophilic groups in the evaporator, with greater hydratability seen with highly hydratable –NH2 groups.49 To evaluate the impact of –NH2 groups' hydratability on water evaporation, the vaporization enthalpy was calculated by comparing the RF evaporator to the spontaneous evaporation of bulk water. The results revealed that the water evaporation enthalpy for the phenolic resins evaporator was considerably lower than the standard enthalpy of bulk water, indicating a change in the water state caused by the phenolic resins, reducing the energy needed for evaporation (Fig. 3b). The enhanced hydratability from –NH2 groups led to a 32% reduction in the enthalpy of evaporation between the APF and RF evaporator, with an additional decrease observed in the carbonized APF evaporator. Furthermore, increasing the amount of APF polymer in the coating continued to lower the equivalent water evaporation enthalpy, suggesting that the quantity of intermediate water depends on the amount of APF polymer. However, excessive coating could interfere with the water–polymer interaction (Fig. S6†).
Laboratory test of light harvesting and photothermal conversion
To further investigate the performance of APF particles-based evaporator, further studies were conducted focusing on various factors influencing photothermal conversion efficiency using a solar simulator. In Movie S1 (ESI†), the steam flow velocity from the APF-based evaporator surface is visibly depicted, showing its efficient vapor generation. The results in Fig. S7 and S8† reveal the effect of the amount of APF applied to the coating on both the evaporation rate and surface temperature. The APF-based evaporator exhibited a remarkable vapor generation rate of 16.09 kg m−2 h−1 for APF polymer with a coating of 4.7 mg/2.25 cm2 and 17.02 kg m−2 h−1 for carbonized APF with a coating of 1 mg/2.25 cm2, under an irradiance of 3.9 kW m−2. All evaporation rates were normalized against blank bulk water, demonstrating the high efficiency of the APF-based evaporator, surpassing previously reported values (Fig. 2g). Interestingly, while thicker coatings do not increase surface temperature, they affect the interaction between water and the polymer (Fig. S7d†).The photothermal conversion performance was also evaluated under different illumination intensities (Fig. S9†). As the light intensity increased from 2.4 to 6.7 kW m−2 (10 to 20 A), the equilibrium evaporation rate rose from 11.29 to 18.67 kg m−2 h−1 for the APF polymer and from 12.42 to 23.80 kg m−2 h−1 for carbonized APF. These results outperformed most evaporation materials reported in the literature (Fig. 2g). The enhanced performance can be attributed to the π-conjugated and π-stacked benzenoid–quinoid donor–acceptor pairs and hydrophilic amine groups, which improve light absorption and photothermal conversion efficiency while reducing the energy required for water evaporation. Moreover, the evaporator's heat localization, aided by the insulating foam, minimized heat loss to the bulk water, further boosting efficiency.
The design of interfacial solar evaporators plays a critical role in optimizing water evaporation efficiency, often overlooked in favor of material properties. A well-balanced water delivery system is essential to prevent issues like excessive wetting or overheating, which can arise from mismatches between photothermal conversion efficiency and water supply at the surface.50 In this study, adjusting the width of cotton towels to regulate water delivery helped manage these imbalances. As shown in Fig. S10,† an optimal evaporation rate of 16.07 kg m−2 h−1 was achieved with a towel width of 1.2 cm, whereas wider (2.4 cm) or narrower (0.6 cm) widths resulted in lower evaporation rates of 13.67 kg m−2 h−1 and 13.82 kg m−2 h−1, respectively. Excessive water delivery lowered surface temperatures, while insufficient water supply caused excessive heating (Fig. S10c†), demonstrating the importance of precisely controlling water transport to match photothermal demands.
The low-band gap APF polymer semiconductors, with their strong absorption up to 800 nm (Fig. 2a), show promise for photothermal conversion across a broad wavelength range.27 Investigating the photothermal response under monochromatic light (435 nm, 500 nm, and 700 nm), the APF polymer maintained a notable evaporation rate, achieving 1.67 kg m−2 h−1 under 435 nm light and 1.18 kg m−2 h−1 under 700 nm light (Fig. S11a and b†). Despite longer wavelengths producing lower interfacial temperatures (Fig. S11c†), the evaporation rate under 700 nm light was similar to that under full-spectrum solar irradiance (Fig. 2g). Airflow also significantly impacted performance,51,52 with wind (generated from the xenon lamp exhaust), the interfacial temperature remained around 40 °C, while without wind (blocking the vent), it reached approximately 60 °C (Fig. S12†). This resulted in double the water evaporation mass under wind conditions compared to still air (Fig. S12a†).
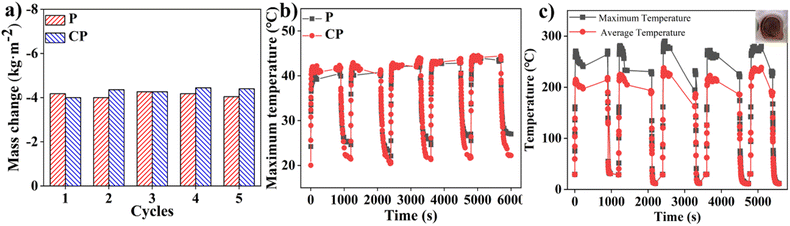
The APF-based evaporator demonstrated strong durability and stability in solar-to-vapor conversion. After five cycles of evaporation testing, the vapor generation rate and interfacial temperatures remained steady, indicating minimal degradation in performance (Fig. 4a and b). In a dry-state test (without water), the carbonized APF coating reached temperatures exceeding 200 °C, with peak temperatures around 280 °C, causing scorching on the filter paper substrate (Fig. 4c). These results highlight the robustness of the APF-based evaporator and its potential for applications in water purification and sewage treatment, maintaining consistent solar-to-vapor conversion and stability under various conditions.
Water harvesting in practical wastewater
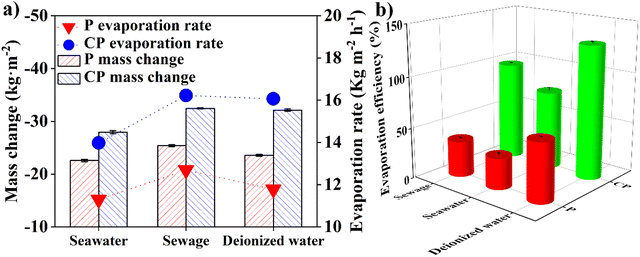
Solar harvester technology holds great promise for water purification by producing clean water. In addition, solar-energy evaporators have demonstrated high performance in water-salt separation,10,26,51–53 antifouling, selective water extraction, and photocatalytic degradation of organic pollutants.23,54 This study evaluated the evaporation performance of an APF-based interfacial water evaporator for seawater desalination and wastewater purification, using deionized water as a control. Under 3.9 suns irradiance, the evaporation rate was 12.69 kg m−2 h−1 for sewage and 11.78 kg m−2 h−1 for deionized water with the APF polymer-based evaporator, and 16.22 kg m−2 h−1 for sewage and 16.07 kg m−2 h−1 for deionized water with the carbonized APF evaporator (Fig. 5a, S12 and S13†). However, the evaporation efficiency of deionized water was higher than that of sewage, at 58.04% versus 35.42% for the APF polymer-based evaporator, and 131.11% versus 97.33% for the carbonized APF evaporator (Fig. 5b). The higher efficiency for deionized water might benefit from its simple chemical composition, while the presence of pollutants in sewage would have an adverse effect on the evaporation efficiency.Under 3.9 suns irradiance, the evaporation rate for seawater was lower than for sewage and deionized water, at 11.29 kg m−2 h−1 for the APF polymer-based evaporator and 13.98 kg m−2 h−1 for the carbonized APF evaporator (Fig. 5a). Interestingly, under natural solar irradiance (0.7 suns), an opposite trend in evaporation rate between seawater and sewage was observed (Fig. 2b). Furthermore, seawater demonstrated the lowest evaporation efficiency (Fig. 5b). The interfacial temperature for the seawater evaporator, whether using the APF polymer-based or carbonized APF evaporator, was the highest (Fig. S13†). These results suggest that metal ions may impede rapid water transport, raising the interfacial temperature due to insufficient water delivery. However, the water transport rate was not significantly impacted under 0.7 suns irradiation, resulting in no significant reduction in seawater evaporation rate under natural sunlight. The appearance of white salt crystallization on the carbonized APF evaporator post-experiment provides further evidence of metal ion transportation to the evaporator surface (Fig. S14†).
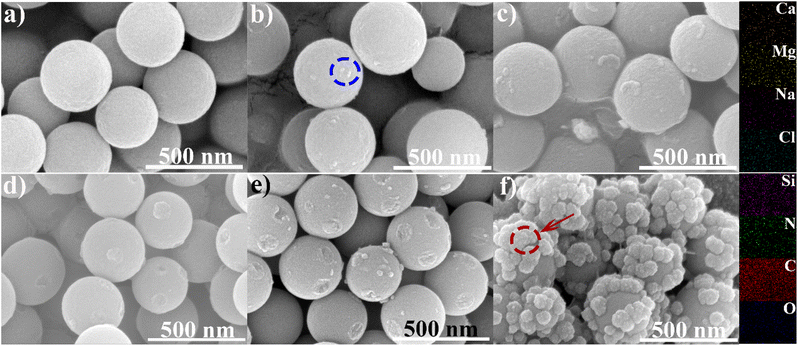
Additionally, the characteristics of the sewage influenced the interfacial temperature, with the amount of evaporating material also playing a role (Fig. S15†). FTIR analysis showed an increase in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O infrared peak, attributed to CO2 interference following evaporation, while the amine peak showed noticeable weakening or disappearance (Fig. S16†).28,55–57 Scanning electron microscopy (SEM) images revealed uniformly spherical structures before evaporation (Fig. 6a and d), with surface spots on the carbonized APF, attributed to H2O/N2 etching (Fig. 6d). EDS and mapping confirmed that the spots in Fig. 6b were due to salt crystallization (Table S1†). Notably, large, grainy, round precipitates were observed during sewage evaporation with the carbonized APF evaporator, which was also present on the surface of particles from the APF-based evaporator (Fig. 6c, f and S17†). EDS and mapping identified this precipitate as potential silica compounds (Table S2†), indicating that dissolved silicon species in the sewage were precipitated during water evaporation. The surface precipitations on either APF or carbonized APF particles would bring down the solar energy absorption of evaporator devices and reduce their contact with water environments, thereby lowering the evaporation efficiencies in simulated seawater and sewage.
O infrared peak, attributed to CO2 interference following evaporation, while the amine peak showed noticeable weakening or disappearance (Fig. S16†).28,55–57 Scanning electron microscopy (SEM) images revealed uniformly spherical structures before evaporation (Fig. 6a and d), with surface spots on the carbonized APF, attributed to H2O/N2 etching (Fig. 6d). EDS and mapping confirmed that the spots in Fig. 6b were due to salt crystallization (Table S1†). Notably, large, grainy, round precipitates were observed during sewage evaporation with the carbonized APF evaporator, which was also present on the surface of particles from the APF-based evaporator (Fig. 6c, f and S17†). EDS and mapping identified this precipitate as potential silica compounds (Table S2†), indicating that dissolved silicon species in the sewage were precipitated during water evaporation. The surface precipitations on either APF or carbonized APF particles would bring down the solar energy absorption of evaporator devices and reduce their contact with water environments, thereby lowering the evaporation efficiencies in simulated seawater and sewage.
Conclusion
A novel SDIE system, based on a wide light-absorbing APF-based phenolic resin with hydrophilic amine groups, has been developed, achieving an impressive evaporation rate ranging from 12.42 kg m−2 h−1 to 23.80 kg m−2 h−1 at power densities of 2.4 to 6.7 kW m−2 for carbonized APF. This system also exhibited a notable evaporation rate of 1.18 kg m−2 h−1 even under 700 nm monochromatic light. Additionally, efficient solar-to-vapor conversion was demonstrated using sewage and simulated seawater under natural solar irradiance. The dry carbonized APF-based coating reached an average interfacial temperature exceeding 200 °C without the need for a water supply. The hydrophilic amine groups contributed to a significantly higher evaporating temperature, resulting in a temperature difference of over 14 °C compared to the RF evaporator. This temperature increase led to improved evaporation efficiency and a reduction in evaporation enthalpy. The APF-based phenolic resin evaporators show strong potential in addressing challenges related to sewage purification and water scarcity.Data availability
The data supporting this article have been included as part of the main manuscript and the ESI.†Author contributions
The original concept for the study was conceived by Rongtai Yu, who also prepared the published work, including manuscript writing. Rongtai Yu and Xiaodan Huang provided critical review, commentary, and revisions throughout both the pre- and post-publication stages. Jianchao Xie conducted the experiments and data collection, while Fangfen Jin, Weiwei Lu, Mingzhu Jin, and Xinyang He contributed to the materials preparation, reagent sourcing, and investigation process. Chengzhong Yu and Ashok K. Nanjundan offered guidance and participated in discussions.Conflicts of interest
The authors declare no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge the financial support provided by the National Natural Science Foundation of China (52060009). This work used the Queensland node of the NCRIS-enabled Australian National Fabrication Facility (ANFF). The authors acknowledge the support from the Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, the University of Queensland for technical assistance.References
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS.
- X. Han, W. Wang, K. Zuo, L. Chen, L. Yuan, J. Liang, Q. Li, P. M. Ajayan, Y. Zhao and J. Lou, Nano Energy, 2019, 60, 567–575 CrossRef CAS.
- Y. Hu, H. Ma, M. Wu, T. Lin, H. Yao, F. Liu, H. Cheng and L. Qu, Nat. Commun., 2022, 13, 4335 CrossRef CAS.
- Y. Wang, Y. Zhou, P. Han, G. Qi, D. Gao, L. Zhang, C. Wang, J. Che, Y. Wang and S. Tao, Environ. Sci. Technol., 2024, 58, 3812–3822 CrossRef CAS PubMed.
- P. V. Chitrao, P. K. Bhoyar, K. Chopra and R. Divekar, Commercial viability of Solar Thermal Hyper distillation of Waste Water—SAINNOVA, A Case Study, Information and Communication Technology for Competitive Strategies (ICTCS 2021), 2023, vol. 401, pp. 333–340 Search PubMed.
- M. Gao, L. Zhu, C. K. Peh and G. W. Ho, Energy Environ. Sci., 2019, 12, 841–864 RSC.
- E. Jones, M. Qadir, M. T. H. Van Vliet, V. Smakhtin and S.-M. Kang, Sci. Total Environ., 2019, 657, 1343–1356 CrossRef CAS.
- C. Chen, Y. Kuang and L. Hu, Joule, 2019, 3, 683–718 CrossRef CAS.
- X. Li, P. Wang, Q. Lu, H. Yao, C. Yang, Y. Zhao, J. Hu, H. Zhou, M. Song, H. Cheng, H. Dai, X. Wang and H. Geng, Water Res., 2023, 244, 120447 CrossRef CAS.
- X. Chen, N. Yang, Y. Wang, H. He, J. Wang, J. Wan, H. Jiang, B. Xu, L. Wang, R. Yu, L. Tong, L. Gu, Q. Xiong, C. Chen, S. Zhang and D. Wang, Adv. Mater., 2022, 34, 2107400 CrossRef CAS.
- F. Zhao, X. Zhou, Y. Shi, X. Qian, M. Alexander, X. Zhao, S. Mendez, R. Yang, L. Qu and G. Yu, Nat. Nanotechnol., 2018, 13, 489–495 CrossRef CAS.
- H. Li, H. Zhu, S. Zhang, N. Zhang, M. Du and Y. Chai, Small Struct., 2020, 1, 2000033 CrossRef.
- L. Zhou, Y. Tan, D. Ji, B. Zhu, P. Zhang, J. Xu, Q. Gan, Z. Yu and J. Zhu, Sci. Adv., 2016, 2, 1501227 CrossRef PubMed.
- L. Zhou, Y. Tan, J. Wang, W. Xu, Y. Yuan, W. Cai, S. Zhu and J. Zhu, Nat. Photonics, 2016, 10, 393–398 CrossRef CAS.
- Y. Shi, R. Li, Y. Jin, S. Zhuo, L. Shi, J. Chang, S. Hong, K.-C. Ng and P. Wang, Joule, 2018, 2, 1171–1186 CrossRef CAS.
- J. Wang, Y. Li, L. Deng, N. Wei, Y. Weng, S. Dong, D. Qi, J. Qiu, X. Chen and T. Wu, Adv. Mater., 2016, 29, 1603730 CrossRef PubMed.
- H. Ren, M. Tang, B. Guan, K. Wang, J. Yang, F. Wang, M. Wang, J. Shan, Z. Chen, D. Wei, H. Peng and Z. Liu, Adv. Mater., 2017, 29, 1702590 CrossRef PubMed.
- X. Yang, Y. Yang, L. Fu, M. Zou, Z. Li, A. Cao and Q. Yuan, Adv. Funct. Mater., 2017, 28, 174505 Search PubMed.
- M. Zhu, Y. Li, G. Chen, F. Jiang, Z. Yang, X. Luo, Y. Wang, S. D. Lacey, J. Dai, C. Wang, C. Jia, J. Wan, Y. Yao, A. Gong, B. Yang, Z. Yu, S. Das and L. Hu, Adv. Mater., 2017, 29, 1704107 CrossRef.
- Z. Gan, X. Wu, M. Meng, X. Zhu, L. Yang and P. K. Chu, ACS Nano, 2014, 8, 9304–9310 CrossRef CAS PubMed.
- Y. Guo, H. Lu, F. Zhao, X. Zhou, W. Shi and G. Yu, Adv. Mater., 2020, 32, 1907061 CrossRef CAS.
- X. Zhou, F. Zhao, Y. Guo, B. Rosenberger and G. Yu, Sci. Adv., 2019, 5, eaaw5484 CrossRef CAS PubMed.
- X. Hao, H. Yao, P. Zhang, Q. Liao, K. Zhu, J. Chang, H. Cheng, J. Yuan and L. Qu, Nat. Water, 2023, 1, 982–991 CrossRef CAS.
- Z. Yang, D. Li, K. Yang, L. Chen, J. Wang, X. Zhu and B. Chen, Environ. Sci. Technol., 2023, 57, 13047–13055 CrossRef CAS PubMed.
- M. Kim, K. Yang, Y. S. Kim, J. C. Won, P. Kang, Y. H. Kim and B. G. Kim, Carbon, 2020, 164, 349–356 CrossRef CAS.
- H. Jian, Q. Qi, W. Wang and D. Yu, Sep. Purif. Technol., 2021, 264, 118459 CrossRef CAS.
- Y. Shiraishi, T. Takii, T. Hagi, S. Mori, Y. Kofuji, Y. Kitagawa, S. Tanaka, S. Ichikawa and T. Hirai, Nat. Mater., 2019, 18, 985–993 CrossRef CAS.
- R. Yu, X. Huang, Y. Liu, Y. Kong, Z. Gu, Y. Yang, Y. Wang, W. Ban, H. Song and C. Yu, Adv. Sci., 2020, 7, 202000393 Search PubMed.
- Y. Li, Y. Shi, H. Wang, T. Liu, X. Zheng, S. Gao and J. Lu, Carbon Energy, 2023, 5, e331 CrossRef CAS.
- H. Wang, Y. Bo, M. Klingenhof, J. Peng, D. Wang, B. Wu, J. Pezoldt, P. Cheng, A. Knauer, W. Hua, H. Wang, P. A. Van Aken, Z. Sofer, P. Strasser, D. M. Guldi and P. Schaaf, Adv. Funct. Mater., 2023, 202310942 Search PubMed.
- X. Wu, Y. Lu, X. Ren, P. Wu, D. Chu, X. Yang and H. Xu, Adv. Mater., 2024, 2313090 CrossRef CAS PubMed.
- Y. Shiraishi, M. Matsumoto, S. Ichikawa, S. Tanaka and T. Hirai, J. Am. Chem. Soc., 2021, 143, 12590–12599 CrossRef CAS.
- L. Yuan, C. Zhang, J. Wang, C. Liu and C. Yu, Nano Res., 2021, 14, 3267–3273 CrossRef CAS.
- D.-S. Bin, Z.-X. Chi, Y. Li, K. Zhang, X. Yang, Y.-G. Sun, J.-Y. Piao, A.-M. Cao and L.-J. Wan, J. Am. Chem. Soc., 2017, 139, 13492–13498 CrossRef CAS PubMed.
- X. Wang, X. Yang, C. Zhao, Y. Pi, X. Li, Z. Jia, S. Zhou, J. Zhao, L. Wu and J. Liu, Angew. Chem., 2023, 62, e202302829 CrossRef CAS.
- Y. Pi, L. Cui, W. Luo, H. Li, Y. Ma, N. Ta, X. Wang, R. Gao, D. Wang, Q. Yang and J. Liu, Angew. Chem., Int. Ed., 2023, 62, e202307096 CrossRef CAS PubMed.
- J. Ma, J. Yu, G. Chen, Y. Bai, S. Liu, Y. Hu, M. Al-Mamun, Y. Wang, W. Gong, D. Liu, Y. Li, R. Long, H. Zhao and Y. Xiong, Adv. Mater., 2023, 35, 2302537 CrossRef CAS.
- N. Wei, Z. Li, Q. Li, E. Yang, R. Xu, X. Song, J. Sun, C. Dou, J. Tian and H. Cui, J. Colloid Interface Sci., 2021, 588, 369–377 CrossRef CAS PubMed.
- Y. Chen, H. Qiu, X. Li, Q. Tong, M. Jensen, Q. Li and N. Wang, Appl. Surf. Sci., 2022, 582, 152483 CrossRef CAS.
- L. Chen, J. Ren, J. Gong, J. Qu and R. Niu, Chem. Eng. J., 2023, 454, 140383 CrossRef CAS.
- W. Cai, W. Wang, J. Ji, Y. Wang, Z. Wang, J. Mao, J. Wang, M. Zhang, Y. Liu and Q. Chen, Sci. Total Environ., 2024, 928, 172597 CrossRef CAS.
- J. Xiong, J. Yi, S. Peng, Z. Yang, Y. Wu, W. Wang, S. Lv, J. Peng, C. Xue, X. Min, M. Li and T. Nakamura, J. Cleaner Prod., 2022, 379, 134683 CrossRef CAS.
- P. Wang, Q. Cui, Q. Zeng, Q. Jiang and Q. Ren, Sol. Energy, 2023, 250, 59–69 CrossRef CAS.
- Y. Lin, Z. Chen, L. Fang, M. Meng, Z. Liu, Y. Di, W. Cai, S. Huang and Z. Gan, Nanotechnology, 2018, 30, 015402 CrossRef PubMed.
- Y. Xu, S. Dong, Y. Sheng, C. Liu, F. Xing, Y. Di and Z. Gan, J. Mater. Chem. A, 2022, 11, 1866–1876 RSC.
- Y. Shiraishi, T. Hagi, M. Matsumoto, S. Tanaka, S. Ichikawa and T. Hirai, Commun. Chem., 2020, 3, 169 CrossRef CAS.
- X. Bai, L. Guo, T. Jia and Z. Hu, J. Mater. Chem. A, 2024, 12, 13116–13126 RSC.
- X. Zhou, F. Zhao, Y. Guo, Y. Zhang and G. Yu, Energy Environ. Sci., 2018, 11, 1985–1992 RSC.
- A. Bernkop-Schnürch, C. Paikl and C. Valenta, Pharm. Res., 1997, 14, 917–922 CrossRef.
- Y. Bu, Y. Zhou, W. Lei, L. Ren, J. Xiao, H. Yang, W. Xu and J. Li, J. Mater. Chem. A, 2022, 10, 2856–2866 RSC.
- K. Zhu, Q. Liao, X. Hao, H. Yao, J. Bai, T. Guang, T. Lin, H. Cheng and L. Qu, Adv. Mater., 2023, 35, 2211932 CrossRef CAS.
- Y. Liu, X. Tan, Z. Liu, E. Zeng, J. Mei, Y. Jiang, P. Li, W. Sun, W. Zhao, C. Tian, Y. Dong, Z. Xie and C. Wang, Small, 2024, 2400796 CrossRef CAS PubMed.
- W. Li, J. Tang, Z. Song, X. Yang, X. Gong, H. Wang, X. Liu and W. Liu, Desalination, 2024, 576, 117366 CrossRef CAS.
- L. Su, X. Liu, W. Xia, B. Wu, C. Li, B. Xu, B. Yang, R. Xia, J. Zhou, J. Qian and L. Miao, J. Colloid Interface Sci., 2023, 650, 613–621 CrossRef CAS PubMed.
- R. Yu, Y. Wang, X. Xu, Q. Zheng, W. Jiang, J. Yu, H. Wang, Y. Kong, C. Yu and X. Huang, J. Colloid Interface Sci., 2024, 660, 859–868 CrossRef CAS PubMed.
- P. J. Larkin, IR and Raman Spectra–Structure Correlations, 2018, pp. 85–134 Search PubMed.
- P. J. Larkin, General Outline for IR and Raman Spectral Interpretation, 2018, pp. 135–151 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06763k |
| This journal is © The Royal Society of Chemistry 2025 |