Open Access Article
Open Access ArticleSuperior magnetocapacitance in ferro/ferrimagnetic Fe3O4/Fe/Fe3C integrated N-doped carbon hybrid nanostructures under mild magnetic fields†
Gwan Hyeon
Park‡
a,
Junbeom
Maeng‡
a,
Tanwir
Ansari
b,
Jungseub
Ha
a,
Suresh
Pittala
c,
Jaehyun
Heo
a,
Subin
Kim
a,
Jae Hyeong
Yu
d,
Sandya Rani
Mangishetti
 *a and
Won Bae
Kim
*a and
Won Bae
Kim
 *ad
*ad
aDepartment of Chemical Engineering, Pohang University of Science and Technology (POSTECH), 77 Cheongam-ro, Nam-gu, Pohang-si, Gyeongsangbuk-do 37673, Republic of Korea. E-mail: smangisetti@gmail.com; kimwb@postech.ac.kr
bDepartment of Metallurgy and Materials Engineering, Indian Institute of Technology Madras, Chennai, 600036, India
cDepartment of Physics, Dayananda Sagar University, Bengaluru, 562112, India
dDepartment of Battery Engineering, Graduate Institute of Ferrous & Eco Materials Technology (GIFT), Pohang University of Science and Technology (POSTECH), 77 Cheongam-ro, Nam-gu, Pohang, Gyeongbuk 37673, Republic of Korea
First published on 3rd April 2025
Abstract
To address the low energy density challenges of supercapacitors, two key strategies were implemented. The first involved designing Fe3O4/Fe/Fe3C wrapped by N-doped carbon onto N-doped carbon nanosheets (Fe@NCNS) using a template free, scalable, simple pyrolysis process. The second strategy focused on enhancing energy storage capacitance using external magnetic fields (MFs). The optimized Fe@NCNS hybrid nanostructure demonstrated a specific capacitance of 1327.3 F g−1 at 1.5 A g−1 with an excellent rate capability of 88.3% at 45 A g−1 in a three-electrode system. Under a mild MF strength of 6 mT, the specific capacitance significantly increased to 2057.3 F g−1, attributed to the reduced electrode and electrolyte resistance based on magnetoresistance and magnetohydrodynamics. The constructed asymmetric supercapacitor (ASC) demonstrated a significant energy density of 181.9 W h kg−1. Furthermore, a remarkable enhancement in energy density was recorded, reaching 263.7 W h kg−1, representing a 1.45-fold increase when subjected to a 6 mT MF. This ASC device exhibits exceptional cycle stability after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. It is worth highlighting that the specific capacitance and energy and power densities achieved under varying MFs surpass those of most metal oxide-based hybrid structures reported to date. These findings indicate significant potential for the advancement of efficient energy storage devices, including memory devices.
000 cycles. It is worth highlighting that the specific capacitance and energy and power densities achieved under varying MFs surpass those of most metal oxide-based hybrid structures reported to date. These findings indicate significant potential for the advancement of efficient energy storage devices, including memory devices.
Introduction
The increasing demand for eco-friendly portable electronics has intensified the need for sustainable energy storage devices.1–3 Supercapacitors have garnered significant research interest due to their fast charging–discharging capabilities, long cycle stability, and high-power densities.2,4–6 However, supercapacitors face challenges related to their low energy density.7,8 In this regard, recent research has identified two significant strategies to efficiently address this issue. The first strategy involves exploring cost-effective, scalable hybrid anode electrode materials with suitable physicochemical properties.9–12 The second strategy focuses on enhancing energy storage performance in the presence of external magnetic fields.13–15 Therefore, designing electrode materials with excellent physical, electrochemical, and magnetic properties, while enhancing capacitive performance under a magnetic field, is a crucial approach to overcoming the low energy density of asymmetric supercapacitors.16–19In this context, considering the efficiency of electrode materials, various transition metal oxides have been investigated as anode electrodes for supercapacitor assembly.20–24 Among metal oxides, iron oxide (Fe3O4) has garnered significant attention due to its affordability, high theoretical capacitance, reasonable conductivity, and abundant availability.25–28 Despite these compelling characteristics, Fe3O4-based electrode materials for supercapacitors face significant challenges, including poor rate capability, low cycle stability, substantial irreversibility and capacity loss.29,30 These issues primarily stem from factors such as low conductivity, volume changes, and rapid dissolution or aggregation during the continuous electrolyte ion adsorption/desorption process.31
In light of these challenges, recent studies have focused on developing Fe3O4-based electrodes with innovative architectures to enhance electrochemical performance. For example, Jianan Zhang et al. developed Fe3O4@Fe3C–C yolk–shell nanospindle anode electrodes for lithium-ion batteries, demonstrating improved energy storage performance.32 Similarly, Wang Qichen showed that Fe/Fe3C@C nanoparticles, encased in a nitrogen-doped graphene and CNT network, can increase the power density of zinc–air batteries.33 Yang Hou and his team synthesized N-doped porous Fe/Fe3C@C nanoboxes supported on graphene sheets, derived from metal organic frameworks for an efficient ORR.34 However, most of these studies employ costly, multi-step strategies involving soft or hard templates and harsh post-treatment processes to create hybrid core–shell structures, which limit their practical applications. Moreover, while there have been significant advancements in the application of iron-based core–shell nanostructures for batteries and energy conversion, reports on their utilization as anode electrode materials to address energy density limitations in asymmetric supercapacitors remain scarce.
To address the aforementioned challenges, we developed Fe3O4/Fe/Fe3C wrapped by N-doped carbon on N-doped carbon nanosheets (Fe@NCNS) through a simultaneous self-assembly process combined with a scalable, efficient, and straightforward pyrolysis process. During the pyrolysis process, variations in reaction temperature and duration influenced the production of hybrid nanostructures with distinct compositions and morphologies. Our findings reveal that the encapsulation of well-developed heteroatom-doped carbon effectively prevents the degradation of Fe3O4/Fe/Fe3C nanoparticles during continuous charging–discharging cycles, while enhancing the interfacial area, electronic conductivity, mechanical stability, and electrochemical kinetics. The fabricated asymmetric supercapacitor, using Fe@NCNS as the anode, demonstrates exceptional energy and power density, along with improved cycling stability.
Furthermore, the introduction of a magnetic field has the potential to significantly enhance the performance of supercapacitors. For instance, Wang et al.35 and Pal et al.36 demonstrated enhanced performance upon the application of an external magnetic field, revealing a reduction in interfacial resistance that facilitated the transport of electrons and ions. The importance of compositing pseudocapacitive materials with carbon, along with considerations of surface area and magnetic properties, was emphasized for enhancing electrochemical performance under magnetic field applications. Therefore, the assembled supercapacitor system using the developed magnetic hybrid nanostructure has demonstrated enhanced performance, which can be attributed to phenomena such as: (i) increased energy density resulting from magnetocapacitance, (ii) improved ionic conductivity driven by magnetohydrodynamics (MHD), and (iii) reduced charge transfer resistance caused by magnetoresistance. In this study, we further examined the significant effects of various external magnetic fields (2 mT, 4 mT, and 6 mT) on the electrochemical properties of the developed magnetic core–shell hybrid electrode material, elucidating the underlying mechanisms through the analysis of experimental electrochemical studies.
Electrochemical studies revealed that Fe@NCNS exhibited magnetocapacitance under a 6 mT magnetic field in a three-electrode system, achieving an enhancement in specific capacitance of up to 55%. The constructed asymmetric supercapacitor demonstrates an exceptional energy density of 263.7 W h kg−1 and a power density of 1.58 kW kg−1, along with a remarkable cycle stability of 96.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles under a 6 mT magnetic field. These results indicate that the application of external magnetic fields can significantly enhance the performance of supercapacitors without requiring materials replacement. The reason for the enhanced kinetics has been elucidated through the synergistic effects of reduced solution resistance (Rs), ion diffusion resistance, and charge transfer resistance (Rct) at the electrode–electrolyte interface. The application of the external magnetic field led to a decrease in solution resistance and Warburg impedance (Wi), attributed to the MHD effect, as well as a decrease in electrode resistance (directly associated with Rct) due to the observed magnetoresistance properties.
000 cycles under a 6 mT magnetic field. These results indicate that the application of external magnetic fields can significantly enhance the performance of supercapacitors without requiring materials replacement. The reason for the enhanced kinetics has been elucidated through the synergistic effects of reduced solution resistance (Rs), ion diffusion resistance, and charge transfer resistance (Rct) at the electrode–electrolyte interface. The application of the external magnetic field led to a decrease in solution resistance and Warburg impedance (Wi), attributed to the MHD effect, as well as a decrease in electrode resistance (directly associated with Rct) due to the observed magnetoresistance properties.
Experimental
Materials
Iron nitrate nanohydrate (Fe(NO3)3·9H2O) (99%), melamine, N-methyl-2-pyrrolidone (NMP), and potassium hydroxide (KOH) were purchased from Merck. Graphite powder (≥99%) was bought from Sigma Aldrich. Camachile fruit peel was collected from an Asian market.Synthesis of Fe3O4/Fe/Fe3C wrapped by N-doped carbon onto N-doped carbon nanosheets (Fe@NCNS)
A mixture of pulverized biowaste from camachile fruit peel, melamine, and iron nitrate nano hydrate (Fe (NO3)3·9H2O) precursors in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 mass ratio was added to deionized water and left to stir magnetically for 6 h. Later, we dried this solution mixture under vacuum conditions for 12 h at 60 °C. We first heat treated the dried precursor mixture at 600 °C for 30 minutes, then raised the temperature to 850 °C and maintained it there for 2.5 h under a 160 sccm argon gas atmosphere. Following the completion of the synthesis process, we used the collected sample as it is, without resorting to any harsh post treatment procedures for cleaning. We conducted different controlled experiments, such as variations in operating temperatures and durations, to explore the synergistic role of temperature operating conditions in the formation of a favorable hybrid core shell nanostructure. The pyrolysis conditions are shown in Table S1.†
0.25 mass ratio was added to deionized water and left to stir magnetically for 6 h. Later, we dried this solution mixture under vacuum conditions for 12 h at 60 °C. We first heat treated the dried precursor mixture at 600 °C for 30 minutes, then raised the temperature to 850 °C and maintained it there for 2.5 h under a 160 sccm argon gas atmosphere. Following the completion of the synthesis process, we used the collected sample as it is, without resorting to any harsh post treatment procedures for cleaning. We conducted different controlled experiments, such as variations in operating temperatures and durations, to explore the synergistic role of temperature operating conditions in the formation of a favorable hybrid core shell nanostructure. The pyrolysis conditions are shown in Table S1.†
Results & discussion
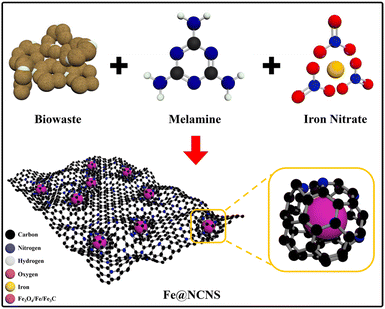
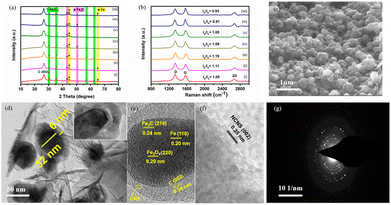
Scheme 1 illustrates the synthesis process of Fe@NCNS from pulverized camachile fruit peel, a type of bio-waste. Fe@NCNS can be easily synthesized in a one-step process by mixing the bio-waste with melamine and iron nitrate nonahydrate, followed by pyrolysis. Through this method, we can easily synthesize a hybrid nanostructure of Fe3O4/Fe/Fe3C with ferro/ferrimagnetic properties, encapsulated by N-doped carbon on an N-doped carbon nanosheet conductive support.X-ray diffraction (XRD) patterns of the produced materials under different pyrolysis conditions are presented in Fig. 1a. All the XRD patterns exhibit a slightly broadened characteristic diffraction peak at around 26.6°, corresponding to the (002) plane of graphitic carbon. This high-intensity diffraction peak results from the overlapping characteristic humps of the graphitic carbon shell and the layered graphene-like 2D carbon nanosheets present in the hybrid nanostructure. The diffraction peaks observed at 30.2°, 35.4°, 57.0°, and 62.6° correspond to the (220), (311), (511), and (400) crystal planes of Fe3O4 (JCPDS No. 19-0629). Peaks detected at 42.8° and 43.9° correspond to the (121) and (210) crystal planes of Fe3C (JCPDS No. 03-0989), while peaks at 44.5° and 65.0° correspond to the (110) and (200) planes of Fe (JCPDS No. 87-0721), respectively. The XRD patterns indicate that the α-Fe and Fe3C phases become increasingly dominant at higher reaction temperatures and longer durations (reaction conditions are given in Table S1†). This suggests that as the reaction temperature and duration increase, Fe3O4 is progressively converted into Fe, then Fe3C, and subsequently into α-Fe.
The formation of layered graphene-like 2D carbon nanosheets is further validated by Raman spectroscopy (Fig. 1b). The metallic Fe and Fe3C present can act as catalysts for the formation of graphitic carbon on their surfaces and facilitate the graphitization of biowaste, ultimately improving the electrical conductivity of the resulting hybrid nanostructure. The Fe@NCNS synthesized under different reaction conditions exhibited a D band, a G band, and a 2D band peak, which are characteristic peaks of graphitic carbon structures. A peak position shift was observed under varying reaction conditions, indicating structural changes. Under a reaction condition of 850 °C for 2.5 hours, the hybrid nanostructure showed a significantly lower ID/IG ratio compared to other operating conditions. This can be attributed to the successful integration of core nanoparticles embedded in thin crystalline carbon shells into the graphitic carbon nanosheets. Based on these findings, we identified 850 °C for 2.5 hours as the optimal operating condition for the formation of a well-integrated hybrid nanostructure. Consequently, we anticipate that this hybrid structure could exhibit excellent electrochemical stability during electrochemical studies. Table S2† lists the D, G, and 2D band peak positions and the ID/IG ratios for the synthesized materials.
The FE-SEM images of Fe@NCNS reveal that variations in reaction temperature or duration significantly influenced the morphological properties of the developed hybrid nanostructures, as shown in Fig. S1.† Varying these parameters resulted in aggregated, non-uniform particle distributions and uneven wrapping nanostructures on the carbon surface. Nonetheless, pyrolysis conducted at 850 °C for 2.5 hours demonstrates uniform morphological characteristics. The FE-SEM image (Fig. 1c) shows that the Fe3O4/Fe/Fe3C nanoparticles covered with N-doped carbon are well-encased in N-doped carbon nanosheet layers, forming an integrated hybrid nanostructure. This indicates that the core shell nanostructures are uniformly and seamlessly integrated into the carbon nanosheet surface. Elemental mapping analysis and energy dispersive spectroscopy (EDS) studies further demonstrate the uniform distribution of carbon (C), oxygen (O), nitrogen (N), and iron (Fe) elements within the hybrid nanostructure (Fig. S2†).
The transmission electron microscopy (TEM) images (Fig. 1d–f) reveal that each individual core–shell structure is composed of Fe3O4/Fe/Fe3C nanoparticles with an average size of approximately 32 nm. These core nanoparticles are completely enclosed by thin carbon shells with an average thickness of around 6 nm. The formed 2D carbon nanosheets exhibit characteristic features akin to layered graphene, including sharp edges and surface wrinkles, as confirmed by the HR-TEM image (Fig. 1e–f). The formation of Fe3C appears to strengthen the connection between the Fe3O4/Fe/Fe3C nanoparticles and the carbon shells. The HR-TEM images show that the core exhibits lattice fringes with d-spacing values of 0.20 nm and 0.29 nm, corresponding to the (110) and (220) crystal planes of the Fe and Fe3O4 phases, respectively. Additionally, at the peripheries of the core, a d-spacing of 0.24 nm is observed, attributed to the (210) crystal planes of Fe3C. Graphitic carbon shells surround and interconnect the core nanoparticles, exhibiting a characteristic d-spacing of 0.34 nm, which corresponds to the C (002) planes. Lattice fringes with a d-spacing of 0.35 nm are associated with the (002) planes of the carbon nanosheets. The corresponding selected area diffraction (SAED) pattern is presented in Fig. 1g. As a result, this hybrid material is expected to exhibit improved electrochemical stability in the electrolyte and rapid charge transport through the dual-conducting system of N-doped carbon shell and carbon nanosheets, leading to enhanced electrochemical performance.
The textural properties of the synthesized hybrid nanostructure under optimized reaction conditions were ascertained through the analysis of N2 adsorption–desorption isotherms. Fig. S3a† presents characteristics of both type I and type IV isotherms with an H3 hysteresis loop, indicating micro- and mesoporous characteristics. The Fe@NCNS exhibits a high surface area of 632 m2 g−1 and a pore volume of 0.48 cm3 g−1. These results indicate that the advanced hybrid nanostructure mitigated the agglomeration of the individual components, resulting in an enhanced surface area. Pore size distribution analysis revealed a narrow size pore distribution ranging from 1 to 150 nm, as determined from the desorption isotherms using the BJH method (Fig. S3b†), highlighting the hierarchical porous nature of the material. The material comprises mesopores with an average pore diameter of 3.55 nm. Consequently, the increased surface area associated with a hierarchical porous structure is expected to improve the redox reaction processes while reducing the diffusion path length for electrolyte ions and electrons. Table S3† provides the values for the pore size and surface area. The physical properties of porous N-doped carbon nanosheets (PNCNS) are analyzed using XRD, Raman, SEM, BET and BJH analyses. The results are presented in the ESI (Fig. S4†).
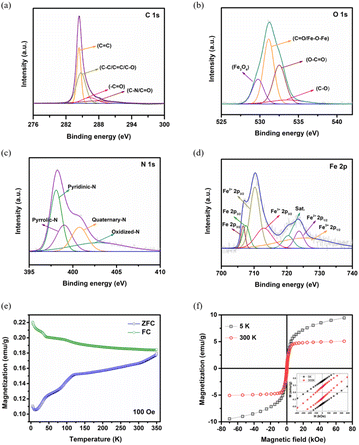
XPS analysis was conducted to elucidate the elemental bonding configuration and chemical composition of Fe@NCNS. The existence of C, N, O, and Fe was confirmed from the survey spectrum (Fig. S5†). The deconvoluted C 1s spectra (Fig. 2a) display three prominent peaks at 284.3 eV, 284.7 eV, and 286.3 eV, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C/C
C, C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–O, and C–N/C–O bonds. The characteristic peaks at 529.7 eV, 531.1 eV, 532.5 eV, and 533.6 eV in the high resolution O 1s spectra (Fig. 2b) are attributed to Fe3O4, C
C/C–O, and C–N/C–O bonds. The characteristic peaks at 529.7 eV, 531.1 eV, 532.5 eV, and 533.6 eV in the high resolution O 1s spectra (Fig. 2b) are attributed to Fe3O4, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/Fe–O–Fe, O–C
O/Fe–O–Fe, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O bonds, respectively. In the deconvoluted high resolution N 1s spectra (Fig. 2c), four distinct peaks are observed at binding energies of 398.3 eV for pyridinic-N (N-6), 399.5 eV for pyrrolic-N (N-5), 400.8 eV for quaternary N (N–Q), and 403.1 eV for oxidized N (N–Ox). The peak at 398.3 eV may also correspond to Fe–Nx bonding. The inclusion of N-6 and N–Q is crucial for enhancing the conductivity of the resultant hybrid nanostructure. Each of these four nitrogen species significantly contributes to the improvement of the electrochemical performance of the hybrid nanostructured electrode. Specifically, the high concentration of pyrrolic and pyridinic nitrogen plays a vital role in pseudocapacitive redox reactions, resulting in superior electrochemical performance. The high resolution Fe 2p spectrum (Fig. 2d) is deconvoluted into seven characteristic peaks. The peaks observed at 712.6 eV and 725.4 eV correspond to the Fe 2p3/2 and Fe 2p1/2 states of Fe3+ in Fe3O4, while those at 710.3 eV and 723.7 eV correspond to the Fe 2p3/2 and Fe 2p1/2 states of Fe2+ in Fe3O4, respectively. The peaks at 706.8 eV and 708.5 eV are associated with Fe 2p3/2 in metallic Fe and Fe3C, respectively. The presence of these distinctive characteristic peaks substantiates that this hybrid nanostructure comprises Fe, Fe3C, and Fe3O4 within a nitrogen incorporated carbon matrix.
O, and C–O bonds, respectively. In the deconvoluted high resolution N 1s spectra (Fig. 2c), four distinct peaks are observed at binding energies of 398.3 eV for pyridinic-N (N-6), 399.5 eV for pyrrolic-N (N-5), 400.8 eV for quaternary N (N–Q), and 403.1 eV for oxidized N (N–Ox). The peak at 398.3 eV may also correspond to Fe–Nx bonding. The inclusion of N-6 and N–Q is crucial for enhancing the conductivity of the resultant hybrid nanostructure. Each of these four nitrogen species significantly contributes to the improvement of the electrochemical performance of the hybrid nanostructured electrode. Specifically, the high concentration of pyrrolic and pyridinic nitrogen plays a vital role in pseudocapacitive redox reactions, resulting in superior electrochemical performance. The high resolution Fe 2p spectrum (Fig. 2d) is deconvoluted into seven characteristic peaks. The peaks observed at 712.6 eV and 725.4 eV correspond to the Fe 2p3/2 and Fe 2p1/2 states of Fe3+ in Fe3O4, while those at 710.3 eV and 723.7 eV correspond to the Fe 2p3/2 and Fe 2p1/2 states of Fe2+ in Fe3O4, respectively. The peaks at 706.8 eV and 708.5 eV are associated with Fe 2p3/2 in metallic Fe and Fe3C, respectively. The presence of these distinctive characteristic peaks substantiates that this hybrid nanostructure comprises Fe, Fe3C, and Fe3O4 within a nitrogen incorporated carbon matrix.
The magnetic properties of Fe@NCNS depend on the magnetic nature of the core Fe3O4/Fe/Fe3C species and the heteroatom-doped carbon network. At an applied field of 100 Oe, the temperature-dependent magnetization (M–T) curves were obtained in both the “zero field cooled” (ZFC) and “field cooled” (FC) modes, as shown in Fig. 2e. The curves reveal a clear bifurcation in the ZFC–FC data from 350 K to 5 K, indicating high-temperature magnetic ordering and spin irreversibility in the sample. Such loops are the characteristic of ferro- and ferrimagnetic materials.37 The ZFC curve shows an increase in magnetization as the temperature increases to 350 K, with anomalies observed around 33 K and 90 K. The FC curve exhibits higher magnetization values than the ZFC curve at all temperatures and shows a decrease in magnetization as the temperature increases, with an anomaly around 33 K. These anomalies may result from the temperature dependence of Fe sublattices in Fe@NCNS as each sublattice can exhibit its own temperature-dependence of magnetization. Typically, Fe3O4 nanoparticles of a finite size display superparamagnetic behavior.38
However, this behavior is not observed in the M–T curves, suggesting that Fe@NCNS exhibits ferro- and ferrimagnetic properties. The field-dependent magnetization curves of the samples, measured at 5 K and 300 K by sweeping the field to ±70 kOe, are depicted in Fig. 2f, with an enlarged portion near the origin shown as the inset. The magnetization values align with the observations from the M–T curves. The figure clearly demonstrates finite magnetization values with an opening in the loop tending towards saturation at both 5 K and 300 K, indicating ferro- and ferri magnetism (FM) above room temperature. The loop at 5 K exhibits a larger opening, indicating high anisotropy, while the loop at 300 K shows a narrower opening, suggesting decreased magnetic anisotropy. At low temperatures, the alignment of spins in the field direction results in a high saturation magnetization of approximately 9.5 emu g−1 at 5 K. Thermal fluctuations cause a decline in the value to approximately 5.1 emu g−1 at 300 K. Similarly, the magnetic coercivity (HC) decreases from 260 kOe at 5 K to 102 kOe at 300 K, while the magnetic remanence (Mr) reduces from 0.48 emu g−1 at 5 K to 0.23 emu g−1 at 300 K. Given that Fe@NCNS contains Fe3O4/Fe/Fe3C species within heteroatom-doped carbon network, it exhibits mixed interactions. Consequently, this hybrid nanostructure demonstrates a combination of ferromagnetic and ferrimagnetic properties.
Electrochemical studies
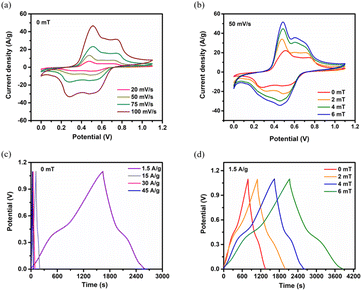
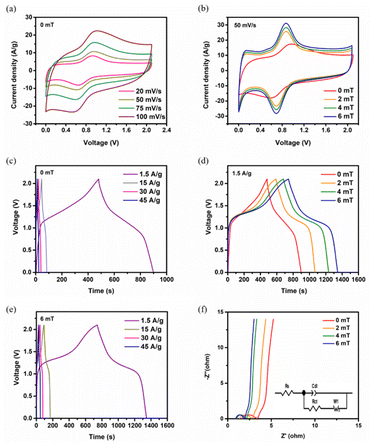
Electrochemical studies were conducted in a three electrode and asymmetric supercapacitor assembly using 3 M KOH electrolyte to assess the electrochemical performance of the Fe@NCNS electrode for supercapacitor applications. Additionally, to explore the impact of MFs on the electrochemical performance of the supercapacitors, we evaluated the same systems under external MFs of 2 mT, 4 mT, and 6 mT, respectively. Fig. 3a illustrates the cyclic voltammetry (CV) curves of the Fe@NCNS electrode within a three electrode system at varying scan rates, revealing a symmetric, slightly deviating rectangular shape with redox reaction peaks. The observed redox peaks correspond to the faradaic transitions involving Fe0, Fe2+, and Fe3+ species during the reduction and oxidation processes occurring in the charge–discharge cycle. The concurrent contribution of electric double layer capacitance (EDLC) and faradaic processes to the energy storage mechanism accounts for the substantial integral area under the CV curves. Fig. 3b presents the comparative CV curves in the same system subjected to different small magnetic fields (0 mT, 2 mT, 4 mT and 6 mT) at a scan rate of 50 mV s−1. A noticeable change in the integral area and a peak shift in the CV curves are evident under these external MFs. The CV curves exhibit an expanded integral area as the MF increases from 0 mT to 6 mT. This enlarged integral area in the presence of an external magnetic field results from enhanced ion and charge transportation across the electrode–electrolyte interface, leading to changes in the electric double layer at the electrode surface. The observed variations can primarily be attributed to the magnetohydrodynamic (MHD) effect,39 which accelerates electron transfer at the electrode–electrolyte interface and increases the ion transportation rate within the electrolyte solution. Furthermore, we observed only subtle changes in the CV shape between 0 mT and 6 mT. Specifically, the anodic peak shifted slightly toward the positive potential, while the cathodic peak shifted slightly toward the negative potential. These findings clearly indicate that both the formation of the core–shell hybrid nanostructure and the application of external magnetic fields during the electrochemical studies significantly enhanced the electrochemical performance.Fig. 3c presents the galvanostatic charge–discharge (GCD) curves at different current densities in the absence of a magnetic field. The symmetric nonlinear behavior further validates the existence of both EDLC and pseudocapacitance characteristics. The lower IR drop at higher current densities signifies reduced ion transport resistance and an expedited current–voltage response, indicating good capacitive performance. An ultrahigh specific capacitance of 1327.3 F g−1 is achieved at a current density of 1.5 A g−1, with a retention of 88.3% at a current density of 45 A g−1, demonstrating excellent rate capability. This is among the highest specific capacitance and rate capabilities reported for Fe3O4-based hybrid electrodes to date (the reported results are compared and listed in Table S4†). This high specific capacitance likely results from the interconnected hierarchical core–shell hybrid nanostructure, which facilitates rapid electron migration throughout the carbon framework and ensures swift ion access to the active sites. Fig. 3d illustrates the comparative GCD profiles at a current density of 1.5 A g−1 under magnetic fields of 0 mT, 2 mT, 4 mT and 6 mT, respectively. The discharge curves exhibit no noticeable voltage drop throughout the discharge process as the magnetic fields strength increases from 0 mT to 6 mT, suggesting a low internal resistance of the electrode. An increase in discharge time is observed with the external magnetic fields, suggesting an improvement in specific capacitance derived from magnetocapacitance. A significant increase in specific capacitance is observed, increasing from 1327.3 F g−1 to 1607.0, 1924.5 and 2057.3 F g−1 under MF strengths of 0 mT, 2 mT, and 4 mT, and 6 mT, respectively, at a current density of 1.5 A g−1. Notably, a substantial 55% increase in specific capacitance is achieved as the MF strength increased from 0 mT to 6 mT. The coulombic efficiencies remain comparable to those achieved without the presence of MFs. These findings indicate that the MF influences both the pseudocapacitive electrode–electrolyte interface and the double layer region, leading to an enhancement in magnetocapacitance.
To comprehend the electrochemical behavior of the Fe@NCNS electrode and investigate the impact of magnetic mechanism under varying MF strengths, we conducted electrochemical impedance spectroscopy (EIS) analysis for the resistance and reaction kinetics. Fig. S6† presents the Nyquist plots both with and without the application of external MFs. The measured Rs, Rct and Warburg impedance (Wi) values are 0.52 Ω, 4.65 Ω, and 2.5 Ω respectively, in the absence of MFs, indicating the high conductivity of the hybrid electrode material. The Nyquist plots shifted towards lower resistance values in the presence of MFs (the fitted resistance values are listed in Table S5†). Based on the observations, it can be concluded that in the absence of a magnetic field, electrolyte ions move towards the electrode surface due to electrostatic forces. However, when subjected to varying magnetic fields, an additional Lorentz force influences the movement of ions, perpendicular to the magnetic field lines. As a result, more electrolyte ions migrate towards the electrode surface, leading to electrolyte convection within the bulk electrolyte and subsequently decreasing Rs. A reduction in Rs from 0.52 Ω to 0.30 Ω was observed at 6 mT. Similarly, Rct decreased from 4.65 Ω to 2.93 Ω, indicating that the magnetized electrodes exhibit low charge transfer resistance across the electrode–electrolyte interface, which significantly enhances the rate capability. The low Warburg impedance 1.56 Ω signifies a rapid ion diffusion process. Notably, this hybrid electrode displayed more pronounced vertical lines in the low frequency region as the external MFs increased from 2 mT to 6 mT, compared to their absence. The observed results suggest that 6 mT represents the optimal value for achieving high specific capacitance.
To investigate the influence of the external MF on the electrochemical characteristics of supercapacitor devices, an asymmetric supercapacitor (ASC) was constructed utilizing Fe@NCNS as the positive electrode and PNCNS as the negative electrode with a 3 M KOH electrolyte, operating within the voltage range of 0 V to 2.1 V. The CV and GCD profiles at different scan rates and current densities for the ASC are presented in Fig. 4a and b, respectively. The typical comparative CV curves at 50 mV s−1 and GCD curves at 1.5 A g−1, both in the absence and presence of MFs, are shown in Fig. 4b and c, respectively. Additionally, GCD profiles at different current densities under 0 mT, 2 mT, 4 mT and 6 mT are shown in Fig. 4d, e, S7a, and S7b,† respectively. Under external MFs, the CV and GCD curves exhibited changes similar to those observed in the three-electrode analysis, revealing an increased integral area under the CV curves and a longer discharge time under the GCD curves as MF strengths increased. The specific capacitance of the ASC was measured to be 297.0 F g−1 in the absence of a MF and subsequently increased to 341.5 F g−1, 395.1 F g−1, and 430.6 F g−1 under the influence of 2 mT, 4 mT, and 6 mT MFs, respectively, at a current density of 1.5 A g−1. A capacitance increase of nearly 45% was noted at a current density of 1.5 A g−1 under 6 mT. The measured rate capabilities were 81.7%, 80.8%, 82.3%, and 82.8% under 0 mT, 2 mT, 4 mT, and 6 mT MFs, respectively, at a current density of 45 A g−1. These findings indicate that a MF strength of 6 mT yields the best electrochemical performance for the constructed ASC device.
Fig. 4f shows the Nyquist plots with and without the presence of MFs. The impedance spectra for the constructed supercapacitor systems demonstrate a shift towards lower resistance values as the MF strength increases. This phenomenon is primarily attributed to the magnetoresistance of the electrode, which shows a decrease in resistivity with increasing magnetic field strengths.40 The negative magnetoresistance of the electrode plays a key role in facilitating rapid interfacial charge transfer throughout the charging–discharging process. The external MF, combined with the local magnetic field generated by the ferro/ferrimagnetic hybrid electrode material, influences the motion and diffusion of electrolyte ions through the Lorentz force. This interaction diminishes the concentration gradient within the Nernst diffusion layer, leading to the MHD effect, which enhances electrolyte ion transport. As a result, electrolyte ions gain more effective access to redox active centers, increasing the capacitance. The Rs values decreased from 0.62 Ω to 0.30 Ω and the Rct values decreased from 5.99 Ω to 3.20 Ω as the MF increased from 2 mT to 6 mT. These result suggest excellent capacitive behavior with reduced charge transfer resistance.
To further understand the kinetic properties of the ASC device under MFs, we computed the capacity contribution derived from the CV profiles of the ASC at 0 mT and 6 mT, respectively. At a scan rate of 20 mV s−1, the capacity contribution observed under 0 mT is 59.8%, (Fig. S9a†), which decreases to 24.9% under 6 mT (Fig. S9b†). These findings suggest that the kinetics of the ASC under MFs is primarily governed by a diffusion controlled process. These observations indicate that the created core–shell hybrid nanostructure features enhanced ion diffusion and transport capabilities, providing ample and efficient charge transportation channels via adsorption–desorption processes. When external MFs are applied, the mobility of ions in the electrolyte is enhanced through the interaction of the Lorentz force and the electric field force, resulting in a reduction in the ion diffusion length, which consequently improves the electrochemical performance of the ASC device. Furthermore, we anticipate that MFs may have contributed to variation in electron spin energy states, and we intend to investigate these effects in our future research.
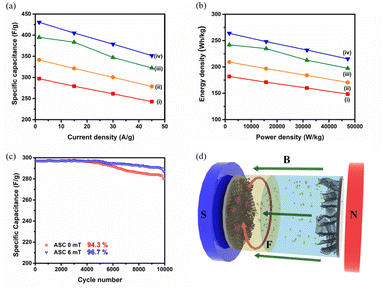
Fig. 5a shows the rate capability performance for the constructed supercapacitor systems under different MFs, highlighting the influence of magnetocapacitance. The plot illustrating energy density versus power density (Ragone plot) for the fabricated supercapacitor devices is presented in Fig. 5b. The ASC device fabricated with the Fe@NCNS positive electrode delivers a remarkable energy density of 181.9 W h kg−1 at a power density of 1.57 kW kg−1 at a current density of 1.5 A g−1, while maintaining an energy density of 148.5 W h kg−1 at a power density of 47.2 kW kg−1 at 45 A g−1. An outstanding energy density of 263.7 W h kg−1 is achieved under a 6 mT external MF at a current density of 1.5 A g−1. Table S6† compares the electrochemical performance of the hybrid electrodes under the influence of external MFs. The specific capacitance and energy and power densities obtained under zero and varied MFs are listed in Table S7.† Notably, these values exceed those of most metal oxide-based hybrid structures reported to date (Table S8† lists the comparative results).
To evaluate the durability of the electrode material, the cycle stability of the constructed ASC devices was measured using GCD measurement over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a current density of 1.5 A g−1 under 0 mT and 6 mT, respectively. An ASC fabricated using Fe@NCNS shows a specific capacitance of 280.1 F g−1, retaining 94.3% of its initial capacitance after 10
000 cycles at a current density of 1.5 A g−1 under 0 mT and 6 mT, respectively. An ASC fabricated using Fe@NCNS shows a specific capacitance of 280.1 F g−1, retaining 94.3% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, demonstrating durability and good reversibility during continuous charging–discharging processes (Fig. 5c). In general, metal oxide-based hybrid nanostructures exhibit unsatisfactory cycling performance due to poor conductivity and degradation during continuous cycling. However, in our study, the developed core–shell hybrid nanostructures displayed remarkable cycle stability and rate capability. Furthermore, the cycling stability of the hybrid electrode was studied under a 6 mT MF to assess its influence on long-term energy storage performance. An improvement in cycle stability was observed, increasing to 96.7% from 94.3% after 10
000 cycles, demonstrating durability and good reversibility during continuous charging–discharging processes (Fig. 5c). In general, metal oxide-based hybrid nanostructures exhibit unsatisfactory cycling performance due to poor conductivity and degradation during continuous cycling. However, in our study, the developed core–shell hybrid nanostructures displayed remarkable cycle stability and rate capability. Furthermore, the cycling stability of the hybrid electrode was studied under a 6 mT MF to assess its influence on long-term energy storage performance. An improvement in cycle stability was observed, increasing to 96.7% from 94.3% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Fe@NCNS, exhibiting ferro/ferrimagnetic properties, demonstrates novel effects of magnetocapacitance and magnetohydrodynamics under an external mild magnetic field (Fig. 5d). This can be considered an effective solution for addressing the low energy density of supercapacitors.
000 cycles. Fe@NCNS, exhibiting ferro/ferrimagnetic properties, demonstrates novel effects of magnetocapacitance and magnetohydrodynamics under an external mild magnetic field (Fig. 5d). This can be considered an effective solution for addressing the low energy density of supercapacitors.
Conclusions
In this research, Fe3O4/Fe/Fe3C wrapped by N-doped carbon onto N-doped carbon nanosheets (Fe@NCNS) was successfully developed through a simple one-step pyrolysis process with biowaste. We systematically investigated the impact of pyrolysis conditions on the microstructural and morphological properties, resulting in the development of a well-integrated ferro/ferrimagnetic hybrid nanostructure with beneficial physicochemical properties. This study examines the electrochemical properties of this hybrid nanostructure for supercapacitor half-cell and full-cell assembly. In this ferro/ferrimagnetic hybrid nanostructure, the synergistic effect from the interaction between the core active species and heteroatom-doped conductive carbon network leads to high capacitance, exceptional rate capability, and remarkable cycle stability. Fe@NCNS exhibited a superior specific capacitance of 1327.3 F g−1 at a current density of 1.5 A g−1, demonstrating a rate capability of 88.3% at a current density of 45 A g−1. Additionally, the electrochemical performance of this hybrid electrode, which combines ferrimagnetic and ferromagnetic properties, was further evaluated under mild external magnetic fields ranging from 2 to 6 mT. In comparison to the absence of a MF, a 6 mT MF exhibited a 55% enhancement in capacitance at a current density of 1.5 A g−1.Furthermore, Fe@NCNS served as an efficient positive electrode for ASC assembly, exhibiting a significant energy density of 181.9 W h kg−1. A substantial improvement in energy density was observed, achieving 263.7 W h kg−1, representing a 1.45-fold enhancement in the presence of a 6 mT MF. This ASC device exhibited a cycle stability of 94.3% at 0 mT and 96.7% at 6 mT after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The enhanced performance is attributed to the magnetohydrodynamic (MHD) effect, changes in electrode resistance, and the localized magnetic environment of the materials under magnetic fields. These findings underscore the potential of this approach for energy storage and conversion devices, providing cost-effective and stable solutions for practical applications. This study presents an innovative strategy for developing magnetic anode materials designed for high-energy storage devices. The results observed under both zero and external magnetic fields indicate that these advanced electrode materials could pave the way for a new generation of energy storage technologies.
000 cycles. The enhanced performance is attributed to the magnetohydrodynamic (MHD) effect, changes in electrode resistance, and the localized magnetic environment of the materials under magnetic fields. These findings underscore the potential of this approach for energy storage and conversion devices, providing cost-effective and stable solutions for practical applications. This study presents an innovative strategy for developing magnetic anode materials designed for high-energy storage devices. The results observed under both zero and external magnetic fields indicate that these advanced electrode materials could pave the way for a new generation of energy storage technologies.
Data availability
Because of the privacy concerns, the raw data underpinning this research is not publicly available. The data and figures supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A5A1084921), the NRF grant funded by the Korean government (MSIT) (RS-2024-00355916), and Korea Institute for Advancement of Technology(KIAT) grant funded by the Korea Government (MOTIE) (RS-2024-00419413, HRD Program for Industrial Innovation).References
- T. Bertaglia, C. M. Costa, S. Lanceros-Méndez and F. N. Crespilho, Mater. Adv., 2024, 5, 7534–7547 Search PubMed.
- J. Sun, B. Luo and H. Li, Adv. Energy Sustainable Res., 2022, 3(6), 2100191 CrossRef.
- A. González, E. Goikolea, J. A. Barrena and R. Mysyk, Renew. Sustainable Energy Rev., 2016, 58, 1189–1206 CrossRef.
- A. Shuja, H. R. Khan, I. Murtaza, S. Ashraf, Y. Abid, F. Farid and F. Sajid, J. Alloys Compd., 2024, 1009, 176924 CrossRef CAS.
- J. Zhang, M. Gu and X. Chen, Micro Nano Eng., 2023, 21, 100229 CrossRef.
- D. P. Chatterjee and A. K. Nandi, J. Mater. Chem. A, 2021, 9, 15880–15918 RSC.
- H. Rashid Khan and A. Latif Ahmad, J. Ind. Eng. Chem., 2024, 141, 46–66 CrossRef.
- K. Dissanayake and D. Kularatna-Abeywardana, J. Energy Storage, 2024, 96, 112563 CrossRef.
- A. Yousaf, S. A. Khan and M. Koç, Cem. Concr. Compos., 2025, 155, 105809 CrossRef CAS.
- M. M. Baig, M. A. Khan, I. H. Gul, S. Javaid and S. M. Baig, J. Electron. Mater., 2023, 52, 5775–5794 CAS.
- N. Kumar, S. Bin Kim, S. Y. Lee and S. J. Park, Nanomaterials, 2022, 12(20), 3708 CAS.
- S. Najib and E. Erdem, Nanoscale Adv, 2019, 1, 2817–2827 RSC.
- S. Pal, S. Majumder, S. Dutta, S. Banerjee, B. Satpati and S. De, J. Phys. D: Appl. Phys., 2018, 51, 375501 CrossRef.
- S. Raj K A and C. S. Rout, J. Mater. Chem. A, 2023, 11, 5495–5519 RSC.
- K. Roy, P. Devi and P. Kumar, Nano Energy, 2021, 87, 106119 CrossRef CAS.
- S. Reenu, L. Phor, A. Kumar and S. Chahal, J. Energy Storage, 2024, 84(part B), 110698 CrossRef.
- E. Lichtfouse, J. Schwarzbauer and D. Robert, Environmental Chemistry for a Sustainable World, Springer, 2011 Search PubMed.
- L. Qu, P. Wang, B. Motevalli, Q. Liang, K. Wang, W. Jiang, J. Z. Liu and D. Li, Adv. Mater., 2024, 36(35), 2404232 CrossRef CAS PubMed.
- E. S. Greenhalgh, S. Nguyen, M. Valkova, N. Shirshova, M. S. P. Shaffer and A. R. J. Kucernak, Compos. Sci. Technol., 2023, 235, 109968 CrossRef CAS.
- X. Zhang, J. Luo, P. Tang and J. Fransaer, et al. , Nano Energy, 2017, 31, 311–321 CrossRef CAS.
- C. Xiong and Y. Su, Adv. Sustainable Syst., 2025, 9(1), 2400578 CAS.
- D. P. Dubal, W. B. Kim and C. D, J. Phys. Chem. Solids, 2012, 73(1), 18–24 CAS.
- S. K. Kang, M. Kim, G. H. Park, J. Ji, S. Hong and W. B. Kim, Adv. Funct. Mater., 2024, 35(2), 2408986 Search PubMed.
- A. Kumar, H. K. Rathore, D. Sarkar and A. Shukla, Electrochem. Sci. Adv., 2022, 2(6), e2100187 CAS.
- S. Tanaka, Y. V. Kaneti, N. L. W. Septiani, S. X. Dou, Y. Bando, Md. S. A. Hossain, J. Kim and Y. Yamauchi, Small Methods, 2019, 3(5), 1800512 Search PubMed.
- J. A. Goudar, S. N. Thrinethra, S. Chapi, M. V. Murugendrappa, M. R. Saeb and M. Salami-Kalajahi, Adv. Energy Sustainable Res., 2024, 2400271 Search PubMed.
- G. M. Tomboc, B. Tesfaye Gadisa, M. Jun, N. K. Chaudhari, H. Kim and K. Lee, Chem.-Asian J., 2020, 15(11), 1628–1647 CAS.
- I. Inamuddin, T. Altalhi and S. M. Adnan, Electroceramics for High Performance Supercapicitors, Wiley, 2023 Search PubMed.
- S. Sun, J. Li, C. Xu, T. Zhai and H. Xia, J. Mater. Chem. A, 2022, 10, 19231–19253 RSC.
- P. Pazhamalai, V. Krishnan, M. S. Mohamed Saleem, S. J. Kim and H. W. Seo, Nano Convergence, 2024, 11, 30 CrossRef CAS PubMed.
- R. Liu, A. Zhou, X. Zhang, J. Mu, H. Che, Y. Wang, T. Wang, Z. Zhang and Z. Kou, Chem. Eng. J., 2021, 412, 128611 CrossRef CAS.
- J. Zhang, K. Wang, Q. Xu, Y. Zhou, F. Cheng and S. Guo, ACS Nano, 2015, 9(3), 3369–3376 CrossRef CAS PubMed.
- Q. Wang, Y. Lei, Z. Chen, N. Wu, Y. Wang, B. Wang and Y. Wang, J. Mater. Chem. A, 2018, 6, 516–526 RSC.
- Y. Hou, T. Huang, Z. Wen, S. Mao, S. Cui and J. Chen, Adv. Energy Mater., 2014, 4(11), 1400337 CrossRef.
- G. Wang, H. Xu, L. Lu and H. Zhao, J. Energy Chem., 2014, 23(6), 809–815 CrossRef.
- S. Pal, S. Majumder, S. Dutta, S. Banerjee, B. Satpati and S. De, J. Phys. D: Appl. Phys., 2018, 51, 375501 CrossRef.
- X. L. Liu, Y. Yang, J. P. Wu, Y. F. Zhang, H. M. Fan and J. Ding, Chin. Phys. B, 2015, 24, 127505 Search PubMed.
- G. Gao, X. Liu, R. Shi, K. Zhou, Y. Shi, R. Ma, E. Takayama-Muromachi and G. Qiu, Cryst. Growth Des., 2010, 10(7), 2888–2894 CAS.
- S. Gao, J. Fan, K. Cui, Z. Wang, T. Huang, Z. Tan, C. Niu, W. Luo and Z. Chao, Small, 2024, 20(20), 2308212 CAS.
- J. Zhu, M. Chen, H. Wei, N. Yerra, N. Haldolaarachchige, Z. Luo, D. P. Young, T. C. Ho, S. Wei and Z. Guo, Nano Energy, 2014, 6, 180–192 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ta01387a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |