Crafting nature's wonders: nanoarchitectonics developments in bioinspired nanocellulose-based stimuli-responsive supramolecular matrices
Soumya Ranjan
Panda
,
Vaishakh Prasad
S.
,
Abhijit
Karmakar
* and
Apurba Lal
Koner
 *
*
Bionanotechnology Lab, Department of Chemistry, Indian Institute of Science Education and Research Bhopal, Bhopal Bypass Road, Bhauri, Bhopal-462066, Madhya Pradesh, India. E-mail: abhijitkarmakar@iiserb.ac.in; akoner@iiserb.ac.in
First published on 26th November 2024
Abstract
Stimuli-responsive supramolecular assemblies have recently gained extensive attention in the biomedical field. Research focusing mainly on bioinspired functional supramolecular materials has shown great promise for potential drug delivery applications. Such materials can be engineered into ‘smart’ materials by utilizing various external stimuli such as pH, heat, light, and magnetic fields. Combining stimuli-responsive properties with bioinspired and biodegradable nanocellulose as a supramolecular matrix can offer a synergistic strategy for targeted and on-demand delivery of therapeutic drugs. The limitations of traditional drug delivery techniques may be greatly mitigated using this combination. In this review, we aim to provide a comprehensive overview of the recent advances in the development of stimuli-responsive nanocellulose-based drug delivery systems. Finally, we have highlighted the current challenges and future perspectives in the field, emphasizing the need for further research to overcome existing barriers and fully realize the potential of stimuli-responsive nanocellulose in drug-releasing applications. Reviewing the state-of-the-art developments and identifying critical areas for future exploration will provide valuable insights for researchers and practitioners working in nanomedicine and drug delivery, fostering the advancement of innovative and effective drug-releasing technologies.
1. Introduction
Nature serves as an unparalleled source of inspiration for the design and development of bioinspired polymers, from spider silk fibroin-based materials to the sticky proteins found in mussels.1,2 The complex structures and functions seen in natural materials have been configured by millions of years of evolution, optimizing their properties to address specific environmental and biological requirements. By harnessing the structural and functional principles observed in nature, bioinspired supramolecular polymeric systems have emerged as a pivotal area of research, resulting in the creation of advanced materials with distinctive capabilities, including wound healing, tissue regeneration, stimuli-responsive matrices, and targeted drug delivery.1,3,4In a stimuli-responsive polymer, components are linked either covalently or non-covalently through an environment-sensitive bridge, prompting the polymer to alter its shape in response to specific conditions.5,6 Recent advancements have enabled both natural and synthetic bio-inspired polymers to be functionalized to perform as stimuli-responsive supramolecular hosts for therapeutics, fluorescent dyes, and biomacromolecules for their on-demand and target-specific actions.7–9 Nanocellulose (NC), a versatile and renewable nanomaterial derived from cellulose, has garnered significant attention in recent years due to its unique properties, including high surface area, ease of functionalization, biodegradability, tailor-made morphology, biocompatibility, and mechanical strength.10–12 NC is a prominent class of bioinspired polymer that can be crosslinked with itself or with other polymers to create supramolecular matrices. The term ‘nanocellulose’ refers to cellulose materials that, due to the isolation process, have at least one dimension in the nanometer range, typically ranging from 1 to 100 nanometers. NC can be prepared either by “top-down” or “bottom-up” approach. “Top-down” methods entail breaking down cellulose fiber from plant materials like wood and leftovers from agriculture and forestry using chemical, mechanical, and enzymatic means. On the other hand, “bottom-up” methods either employ biosynthetic techniques to create NC from the purest form of cellulose, which is produced by bacteria (such as Gluconacetobacter xylinus) or marine organisms like tunicates, or they accumulate nanostructured cellulose in the solution state of cellulose molecules.13,14 NC encompasses several forms, including crystalline rod-like cellulose nanocrystals (CNCs), which exhibit high mechanical strength and stiffness; longer and more entangled cellulose nanofibers (CNFs), sometimes referred to as microfibrillated cellulose (MFC), which offer enhanced flexibility and surface area; and bacterial nanocellulose (BNC), produced by bacteria and known for its high purity and water-holding capacity.15–17 Each type of NC has distinct properties and applications, contributing to the development of advanced materials in various fields. The preparations and certain features of different types of NC have been depicted schematically in Fig. 1.
 | ||
| Fig. 1 Schematic presentation of the preparations and features of different types of nanocellulose. CNF image was adapted from ref. 17 Copyright 2020, Springer Nature. | ||
Stimuli-responsive NC, which has recently been extensively explored in the biomedical field, shows promising potential for drug delivery systems. Research focuses on leveraging pH, heat, light, and magnetic fields as prominent stimuli types.18,19 By using these alterations, therapeutics can be released with precision and control, increasing the effectiveness of drug delivery systems and reducing adverse effects. Combining NC with stimuli-responsive mechanisms offers a synergistic strategy that maximizes the advantages of both materials. The stimuli-responsive qualities of NC allow for fine control over drug release patterns, and the material provides a readily functionalizable, biocompatible, and biodegradable supramolecular matrix. The limitations of traditional drug delivery techniques may be greatly mitigated using this combination.
In this review, we aim to provide a comprehensive overview of the advances in the development of stimuli-responsive NC-based drug delivery systems over the past five years. We will explore the various types of stimuli-responsive mechanisms that have been incorporated into NC matrices, including pH-responsive, thermo-responsive, magnet-responsive, and light-responsive systems, as schematically presented in Fig. 2. Furthermore, we will discuss the synthesis and functionalization strategies employed to impart stimuli-responsive properties to NC, as well as the characterization techniques used to evaluate their performance.
Moreover, this review will highlight the application of these smart NC systems in different therapeutic areas, demonstrating their potential to revolutionize drug delivery and improve patient outcomes. Finally, we will address the current challenges and future perspectives in the field, emphasizing the need for further research to overcome existing barriers and fully realize the potential of stimuli-responsive NC in drug-releasing applications. By summarizing the state-of-the-art developments and identifying key areas for future exploration, this review aims to provide valuable insights for researchers and practitioners working in the field of nanomedicine and drug delivery, fostering the advancement of innovative and effective drug-releasing technologies.
2. Thermo-responsive NC
A thermo-responsive hydrogel is made of thermo-sensitive polymeric material which exhibits sol–gel transformation in response to temperature variations. Unlike traditional hydrogels, thermosensitive hydrogels are engineered to swell or shrink in response to temperature changes. This unique property makes them particularly useful for various applications, including shape-morphing, injectable, and advanced drug carriers.20,21 Various thermoresponsive polymers, including poly(N-isopropylacrylamide) (PNIPAm), poly(dimethylaminoethyl methacrylate) (PDMAEMA), poly(di(ethylene glycol) methyl ether methacrylate) (PDEGMA), and poly(N-vinylcaprolactam) (PNVCL), exhibit distinct thermo-responsive properties and have different lower critical solution temperatures (LCSTs);22,23 however, a major challenge with existing materials is their mechanical instability, which hampers their effectiveness as drug carriers and other biomedical applications.24 To overcome such shortcomings, researchers are increasingly focusing on crosslinking NCs to develop thermo-responsive supramolecular matrices that offer enhanced mechanical stability for advanced drug delivery applications.25,26 3D hydrogel materials made up of a network of biocompatible fibers are often used as extracellular matrices (ECM) for 3D cell culture, such as spheroids or tumoroids, a model for the study of various diseases, especially cancer.27,28Curvello et al. developed a thermo-responsive supramolecular extracellular matrix by fusing collagen with nanocellulose material (COL-NC) to utilize it in epithelial intestinal organoid growth.29 COL-NC hydrogel became asymmetric and remained morphologically viable, with evidence of epithelial budding. Thermo-responsive hydrogels with LCST allow cell encapsulation in a biocompatible solution that turns into a viscoelastic microenvironment at human body temperature (37 °C) and supports cell growth.30 RGD peptide-grafted cellulose nanofibers (RGD-CNFs), even at concentrations as low as 0.1%, increased the viscosity and elastic moduli of 0.2 wt% type I collagen gels by 2.5-fold. This enhancement in moduli results in approximately 40% improvement in gelation compared to collagen alone. This thermo-responsive hydrogel was compared with Matrigel®, which is an ideal material for organoid growth along with only collagen.31 They studied the growth of organoids in each of the three substrates (Matrigel®, COL alone and COL-NC) for three days and found that measurement of cell activity of organoids in Matrigel® presents a 3.5-fold increase between day 1 and 3, whereas in COL-NC hydrogel, the increase is 2.3-fold.
In this similar context, Simchi and his colleagues utilized a bacterial nanocellulose (BNC)-based matrix to develop a thermos-responsive and injectable hydrogel, combining conjugated chitosan and poloxamers, for cardiac tissue regeneration in mammalian cardiomyocytes.32 They incorporated gold nanoparticles (AuNPs) in the BNC matrix to meet the electrophysiological performance of cardiac tissues and enhance the cell adhesion. Pluronic F127 triblock copolymer and chitosan fiber were also functionalized with the AuNP-decorated BNCs to make the hydrogel thermosensitive. To assess temperature responsiveness, the storage modulus (G′) and loss modulus (G′′) of the conjugated polymer composite were measured. The moduli values were relatively low, below 100 Pa. However, a marked change was observed at higher temperatures. At elevated temperatures, the hydrogels began to break down rapidly, with improved elasticity noted at higher concentrations of AuNPs. The crossing points of G′ and G′′ indicated the sol–gel transition (TG) occurring at 21.4 °C for 20 mM AuNPs and at 15 °C for 75 mM AuNPs. The gelatinization peak of the hydrogels was observed at approximately 37 °C for 20 mM AuNPs and 30 °C for 75 mM AuNPs, with a notable difference in the storage modulus between the two concentrations. The ionic conductivity of the hydrogels increased about two fold by increasing the gold concentration from 20 mM to 75 mM. The four-point probe assay has determined conductivity values of 2 × 10−3 S m−1 for 20 mM AuNPs and 6 × 10−2 S m−1 for 75 mM AuNPs, which is sufficient enough for cardiac tissue regeneration. In vitro degradation study and MTT assay H9C2 heart cell revealed that the hydrogel was stable and biocompatible.
Senses and co-workers have engineered a thermo-responsive nanocomposite consisting of amphiphilic Pluronic F127 triblock copolymer and cellulose nanocrystals (CNCs).33 Adding Pluronic F127 to CNCs can alter the viscoelastic behaviour, phase transition temperature, thermo-reversibility, mechanical fragility, and injectability of the composite. It also enables the gels to transition from a gel to a liquid under high shear conditions and quickly revert to their gel state at body temperature, providing new avenues for designing thermo-responsive drug delivery systems.
Postoperative peritoneal adhesions (PPAs) have serious implications for patients, surgeons, and healthcare providers as they can develop adhesive small bowel obstruction, female infertility, chronic abdominal pain, and increased surgical complexity in subsequent procedures.34
Lee and his colleagues have developed an injectable, thermo-sensitive, and anti-adhesion hydrogel containing TEMPO-oxidized cellulose nanofiber (TOCN), methyl cellulose (MC), carboxymethyl cellulose (CMC), and polyethylene glycol to mitigate PPAs.35 Schematic representation illustrating the tissue anti-adhesion mechanism of TOCN-containing injectable hydrogel has been shown in Fig. 3A. Researchers have discovered that combining carboxymethyl cellulose and polyethylene glycol (PEG) can serve as an innovative coating lubricant. This combination helps prevent undesired adhesion between adjacent tissues during surgical procedures.36,37 Their studies also depicted the higher biocompatibility of NC since TOCN 0.2 (comprising 2% methyl cellulose, 1% polyethylene glycol, 0.8% carboxymethyl cellulose, and 0.2% TOCN) exhibited cell viability of 89.24 ± 3.76% for RBMSCs (rat bone marrow-derived mesenchymal stem cells) and 91.25 ± 2.47% for L929 fibroblast cells at day 14. Moreover, the gelation efficiency of their material improved with increasing amounts of TOCN, leading to shorter gelation times and slower degradation rates observed at 37 °C. During the 2-week experimental period, rats treated with thermosensitive hydrogel TOCN 0.2 and the positive control (commercial gel) did not experience any adhesion between the wall and cecum. However, the negative control group (rats treated with normal saline) showed intensified adhesion by day 14 compared to day 7 [Fig. 3B]. Traces of the commercial gel remained on day 7.
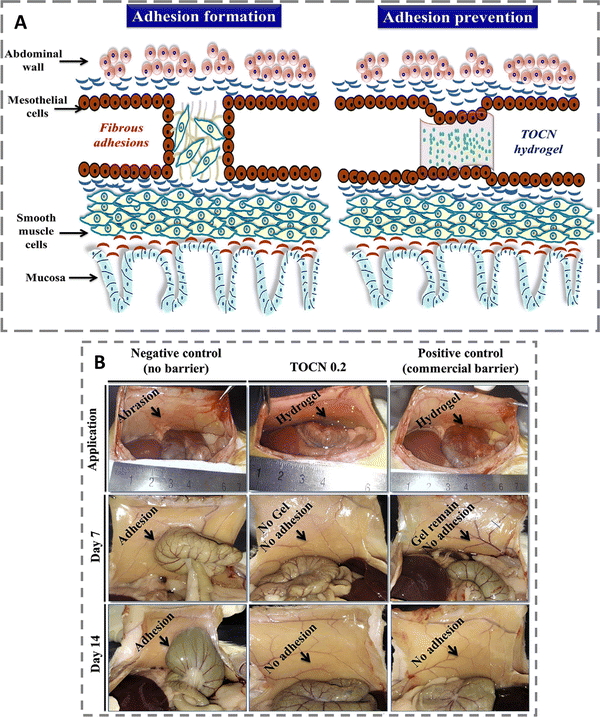 | ||
| Fig. 3 (A) Schematic representation illustrating the tissue anti-adhesion mechanism of TOCN-containing injectable hydrogel. (B) Evaluation of the antiadhesion efficacy of TOCN 0.2 hydrogel through a rat side wall-cecal abrasion model. Defects were created on the peritoneal wall and cecum and healing parts were indicated by arrows. Reproduced with permission from ref. 35 Copyright 2019, Elsevier. | ||
Thermo-responsive NC hydrogels offer many advantages, such as controlled drug release and adaptability to environmental changes, but they also face several disadvantages and limitations such as higher temperature sensitivity, delayed response time and limited range of showing thermo-responsiveness. Moreover, crosslinking with other temperature sensitive polymers with NC leads to lower drug loading capacity and limits the biocompatibility. Although several applications are reported for thermosensitive injectable hydrogel, in vivo application of these nanomaterials is still in its infancy. Addressing these limitations involves ongoing research and development to enhance the performance, stability, and versatility of thermoresponsive NC hydrogels in various applications.
3. pH-responsive NC
Environmental pH greatly influences NC-based hydrogels since their supramolecular self-assemblies rely on the electrostatic interactions between the chains or particles within the hydrogel. Current methods for preparing NC include mechanical, acid hydrolysis, oxidation, and enzymatic techniques, which introduce various ionizable surface groups such as hydroxyl, sulfonic acid, aldehyde, or carboxyl groups. Meanwhile, these ionizable groups can either release or absorb protons depending on the surrounding pH level38e.g., the TEMPO oxidation method introduces carboxylic acid groups onto the side chains, with a pKa around 4.8,39 below which the carboxyl groups remain predominantly in their COOH form. At pH levels above the pKa, these groups deprotonate to the carboxylate form, which increases ionic repulsion between the nanofibrils leading to hydrogel swelling. Various regions of the human body have different pH values; for instance, body fluids generally have a pH of around 7.4, while gastric juice is much more acidic, with a pH ∼2.0. Additionally, lesion tissues often exhibit pH changes, such as tumors with extracellular pH around 6.5 and intracellular pH approximately 5.0.40 As a result, researchers have frequently utilized NC as a pH-responsive hydrogel for controlled drug release applications.41,42Nia et al. developed pH-responsive trifunctional biopolymer-based BHNC-β-CD-EDA (BBE) nanosponges by combining ethylene diamine conjugated beta-cyclodextrin (β-CD-EDA) and bifunctional hairy nanocellulose (BHNC), as shown in Fig. 4. Here hairy nanocellulose (HNC) having high surface area has been developed by periodate oxidation43,44 of cellulose nanocrystals (CNCs) where cellulose chains were cleaved in the amorphous regions, leaving the soluble amorphous regions attached to the nanorods on both ends. Their approach involved modifying the crystalline regions of cellulose chains with a dialdehyde moiety, followed by partial oxidation to create HNC composites with both aldehyde and acid functionalities. They have also investigated the pH responsiveness of this nanocomposite and the results revealed that their composite exhibited sustained release of DOX molecules up to 8 days at pH 5.5. Moreover, the BBE–DOX conjugate exerted higher cytotoxicity towards the MDA-MB-231 breast cancer cells than free DOX molecules.
 | ||
| Fig. 4 (A) Diagram of the one-pot synthesis method for polymerized hyper-cross-linked BBE. (B) Left figure depicts the FE-SEM image of left-handed helical BBE1/4 fibrils; right figure shows its possible explanation of helical fibril formation by HNC rods (green with hairs), cross-linked at their ends by β-CD-EDA (yellow with red carbamate-EDA arms). Reproduced with permission from ref. 45 Copyright 2023, American Chemical Society. | ||
Cubosomes, a self-assembled lipid-based liquid crystalline structure, are often considered as drug delivery vehicles owing to their unique properties, including solid mucoadhesiveness and high specific surface area. Moreover, these carriers are capable of encapsulating hydrophilic, hydrophobic, and amphiphilic molecules.46,47 Despite such features, the cubosome's liquid crystalline structures cannot withstand the dry environment. To overcome this difficulty, Ferreira et al. merged cubosomes with BNC in an in situ process, which are mechanically robust, and further treated these BNC capsules with a pH-responsive polymer like alginate through electrostatic interactions to create a system designed for pH-sensitive and colon-specific drug delivery applications.48 In order to further validate the pH-triggered delivery system, tetracycline hydrochloride (TCH), a representative hydrophilic drug, was tested. At pH 1.2, drug release is constrained, likely because the drug is trapped within the alginate chains during the coating process. At pH levels between 6 and 8, the sustained release of cubosomes occurs mainly through diffusion via the pores of the BC capsule, thanks to the pH-responsive properties of alginate. This alginate-cellulose-cubosome delivery system presents distinct benefits compared to traditional drug carriers such as liposomes and polymer nanoparticles, due to its specialized composition and functionality.
Yuyeon et al. and team discovered a cleaver technique for pH-responsive targeted cancer therapy by combining two nature inspired biomacromolecules.49 They first functionalized two different types of single-stranded DNA encoding the DOX incorporated i-motif or aptamer sequence and the T7 promoter sequence to synthesize the circular DNAs (containing pH-responsive and cell targeting sequence). After this, the DNA polymer network was further decorated with CNCs [Fig. 5] to form the polymeric DNA nanostructure-CNCs complex (pDCs). Since endosomes and tumor environments typically have pH values of 5 and 6.4, respectively, drug release was assessed at these pH levels. The pDCs displayed significant pH- and DNA density-dependent DOX release profiles (approximately 85% at pH 5.0) and efficient cancer-targeted intracellular delivery and anticancer therapy within HeLa cells.
 | ||
| Fig. 5 Schematic illustration of the step-by-step formation process of the functional polymeric DNA nanostructure-CNCs complex. Reproduced with permission from ref. 49 Copyright 2024, Elsevier. | ||
Nishiguchi and Taguchi engineered a novel NC composite for bone regeneration based on the pure supramolecular interaction between bisphosphonated-nanocellulose (pNC) and β-tricalcium phosphate (β-TCP) through Ca2+ ions.50 The pNC-β-TCP composite regulates osteoclast and osteoblast activity for bone regeneration, with bisphosphonate groups enabling reversible crosslinking with β-TCP, enhancing mechanical properties and thixotropic behaviour. Bisphosphonate groups in pNC form ion-cross-linkages with calcium ions at pH 7 (pK1 = 2.5, pK2 = 6.5). However, when the pH drops to around 4.5 due to osteoclast activity, these linkages are disrupted. This results in the release of nanofibers containing pharmacologically active bisphosphonates in response to osteoclasts, a phenomenon known as osteoclast-responsive behaviour, which targets osteoclast precursors locally. The pharmacological activity of pNC-β-TCP was examined by differentiating RAW264.7 cells into osteoclasts in its presence. pNC-β-TCP at 2.5 mg mL−1 significantly reduced osteoclast formation in a dose-dependent manner without cytotoxicity up to 5 mg mL−1. It also inhibited pit formation on apatite plates, suggesting reduced bone resorption through bisphosphonate pathways. Additionally, pNC-β-TCP showed enhanced adhesion strength to dentin (ivory plates) compared to controls.
Anirudhan et al. have followed a strategy to fabricate the surface of chemically modified graphene oxide (MGO) with amine functionalized nanodextran (AND) and carboxylated NC via a layer by layer supramolecular self-assembly process.51 In this self-assembly, two alternate charged nanoparticles i.e., anionic CNC and cationic AND exhibited electrostatic interactions with graphene layers. This whole system was utilized for the encapsulation and pH-responsive delivery of anticancer drug curcumin (CUR). The pH sensitive ionic bond between negatively charged carboxylic acid groups of CNC and positively charged amino groups of AND plays a crucial role here. This nanocomposite exhibited almost 86.4% loading capacity for CUR, which is similar to that of other graphene-based materials,52,53 and 89.0% of the drug was released in a sustained manner (within 48 h) at acidic pH (∼5.5). Moreover, CUR loaded MGO-AND/CNC showed high cytotoxicity as compared to bare CUR in HCT116 cells.
Zhu and his co-workers grafted polyethylenimine (PEI) onto carboxylated cellulose nanofibers (CNF-COOH) to develop a nanosized biomass-based pH-responsive cellulose nanofiber (CNF-PEI) with excellent biocompatibility.54 The CNF-COOH had antibacterial rates of 6.0% for E. coli and 1.1% for L. monocytogenes, as depicted in Fig. 6A and B. However, the antibacterial efficacy of CNF-PEI was significantly higher at pH 7, achieving rates of 93.9% against E. coli and 94.0% against L. monocytogenes. This suggests that CNF-PEI exhibited excellent antibacterial properties. According to their pH-response studies, it was observed that specifically, at a pH of 3.0, mimicking the acidic environment of the stomach, the aerogel released 133.76 mg g−1 of DOX over 10 h. The release amount decreased at higher pH levels: 41.35 mg g−1 at pH 5.0, reaching equilibrium in 5 h, and 39.02 mg g−1 at pH 7.4. The findings indicated that the CNF-PEI aerogel exhibits pH-responsive drug release capabilities, making it a promising candidate for clinical use in treating gastric cancer.
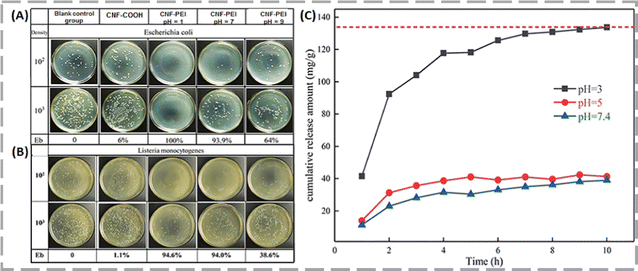 | ||
| Fig. 6 Evaluation of antibacterial activity of CNF-COOH and CNF-PEI against E. coli (A) and L. monocytogenes (B), along with the cumulative DOX release profile from CNF-PEI aerogel under different pH conditions at 37 °C (C). Reproduced with permission from ref. 54 Copyright 2020, American Chemical Society. | ||
4. Photo-responsive NC
Light has a remarkable demand as external stimuli for biomedical applications due to its non-invasive nature, precise control over wavelength, intensity, and the lack of need for additional reagents for remote activation. This makes it an ideal choice for various applications in the field, including imaging, diagnostics, and therapeutic interventions.55,56 Researchers often doped photothermal materials into hydrogels due to their exceptional ability to absorb light at designated wavelengths and convert it to heat. This process allows for targeted and controlled application of thermal energy, which can induce specific changes in the hydrogel, such as alterations in its structure or the release of drugs.57,58 Near-infrared (NIR)-responsive hydrogels are particularly preferred among different light wavelengths because they can penetrate deeply into tissues, exhibit minimal scattering, and have a lower risk of causing harm in in vivo applications.59,60 Bioinspired NC materials are often functionalized with light-absorbing substances to enhance their performance. These substances include carbon-based nanomaterials such as graphene oxide and carbon dots, metal-based nanoparticles, polydopamine polymers, macrocyclic compounds like protoporphyrin IX, and small molecules such as indocyanine green and azobenzene.38,61,62 These photo-responsive hybrid NCs function in two distinct ways: (1) photothermal therapeutic release, where the temperature is elevated to disrupt thermolabile crosslinks and alter the material's shape, and (2) photothermal ablation of cancer cells, where the temperature is raised beyond 42 °C to induce cell death. In the following paragraphs, we have depicted how photo-responsive NC matrices have been applied in various fields, ranging from wound healing to photothermal chemotherapy.The primary emphasis in the design of wound dressing materials has been on improving antibacterial efficacy, often at the expense of hemostatic function. Therefore, there is a pressing need for new approaches that not only achieve swift hemostasis but also combat bacterial infections and eliminate biofilm barriers to support effective wound healing.
Shi et al. developed a white and NIR dual light-responsive wound dressing material with endogenous antibacterial cellulose nanofibril (CNF)-based hydrogel, shortly named CNF-DLRIHWD.63 In a tailor-made design of CNF-DLRIHWD, white light-responsive CNF protoporphyrin IX (CNF-PpIX) and the antibacterial CNF polyaminopropyl biguanide (CNF-PAPB) were assembled as the core framework, complemented by Prussian blue nanoparticles (PBNPs) for NIR responsiveness, Pluronic® F127 (F127) for temperature sensitivity, and hydroxypropyl methyl cellulose (HPMC) was introduced as a binder [Fig. 7]. This material exhibited an excellent photothermal conversion efficiency, i.e., 46.3%. Interestingly, CNF-DLRIHWD effectively demonstrated a temperature-sensitive sol–gel–sol transition, enabled by the thermos-responsive properties of F127, transitioning from a fluid sol at 25 °C to a semi-solid gel at 36 °C to 45 °C, and back to a fluid sol at 47 °C. Moreover, CNF-DLRIHWD demonstrated significantly enhanced antibacterial activity under NIR irradiation compared to its dark state, achieving over 99.9% inhibition against E. coli, S. aureus, and MRSA, due to the ROS generation facilitated by the in-built porphyrin ring upon NIR exposure. They also investigated the in vivo hemostatic efficacy of CNF-DLRIHWD using a hemorrhaging liver rat model, demonstrating that bleeding cessation occurred within 63 seconds. This effect was facilitated by CNF-DLRIHWD forming an in situ gel on the liver tissue surface, resulting in a significant reduction of blood loss to 286.4 mg. The matrix skeleton of CNF-DLRIHWD has the ability to bind with Fe3+ in the blood, thereby promoting coagulation factor XII activation and enhancing platelet aggregation, which contributes to achieving rapid hemostasis.
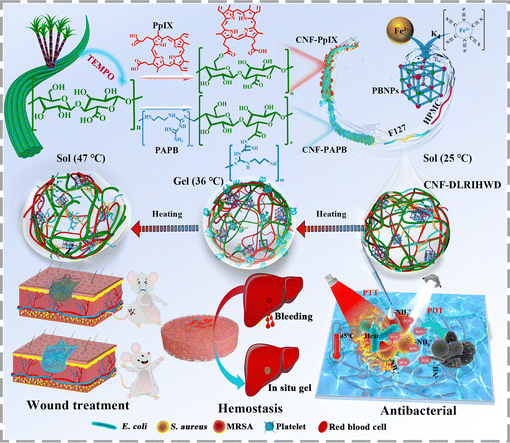 | ||
| Fig. 7 Schematic representation of dual light-responsive CNF-based hydrogel wound dressing (CNF-DLRIHWD) and its application in first-aid hemostasis and wound healing. Reproduced with permission from ref. 63 Copyright 2019, Elsevier. | ||
Do et al. hybridized CNCs with folic acid, carbon dots (Cdots) and DOX for both targeted tumor cell imaging and photodynamic/photothermal chemotherapy.62 Folic acid was conjugated with 3-aminopropyltriethoxysilane (APTES) functionalized CNCs and then Cdots and DOX were loaded onto this nanocomposite through electrostatic interactions. Incorporating carbon dots improved both the emission characteristics (excited at 370 nm, emitting at 450 nm) and the photothermal effectiveness of this nanocomposite. Consequently, temperature increases varied with the wavelength of the LED: approximately 6 °C at 450 nm, 11–12 °C at 650 nm, and up to 14 °C at 808 nm. This nanocomposite also demonstrated pH-responsive release of DOX. At pH 5.6, the release of DOX from DOX-CNC/APTES/FA and DOX-CNC/APTES/FA/Cdots increased over time, reaching 88.1 ± 2.7% and 67.3 ± 0.6%, respectively, after 96 h of incubation. Liu et al. have developed polydopamine (PDA) coated cellulose nanocrystal (CNC)-based systems for NIR-responsive photothermal chemotherapy.64 Herein, CNCs made up of bamboo pulp were conjugated with PDA and Ag nanoparticles And incorporated into a poly(N-isopropylacrylamide) (PNIPAM) matrix to fabricate PDA@CNC/PNIAPM nanocomposite hydrogels with controllable performance in response to NIR stimuli. Tian et al. utilized cellulose nanocrystals (CNCs) as a nanocarrier for the co-drug delivery of β-cyclodextrin (β-CD) encapsulated CUR and gold nanoparticles (AuNPs).65 This nanosystem exhibited remarkable loading capacity of 31.4 μg mg−1 and a heat-promoted release rate of 77.63% with the help of AuNPs. They also studied the NIR-responsive photothermal efficacy of the AuNP-CNC-β-CD/CUR nanosystem and it was found that under NIR light (804 nm) with an intensity of 0.8 W cm−2 the temperature increased from 24.5 to 50.8 °C with increasing irradiation time. Furthermore, a cytotoxicity assay on the MCF-7 cell line revealed that the antitumor potency of CUR was enhanced in the AuNP-CNC-β-CD/CUR nanosystem.
You et al. developed a pH- and UV-responsive cellulose-based hydrogel (ASB@AZO-CNC) with controlled and sustained release properties, as shown in Scheme 1.66 Using azoxystrobin (ASB) as a model fungicide, the hydrogel achieved up to 89.44% drug release at pH 5.5, with a 6.4-fold increase in release rate under UV irradiation compared to non-irradiated conditions. This research highlights the potential of such cellulose-based materials for creating advanced fungicide carriers with intelligent, controlled-release capabilities.
 | ||
| Scheme 1 Synthetic scheme illustrating the route of preparation and light-controlled adsorption process of the ASB@AZO-CNC hydrogel. Reproduced with permission from ref. 66 Copyright 2024, Royal Society of Chemistry. | ||
Liu et al. engineered a composite hydrogel, ZIF-8@PCNFs-Cur, by incorporating ZIF-8 nanoparticles and curcumin (Cur) onto PDA-modified cellulose nanofibers (CNFs).67 Their composite hydrogel exhibited a high drug encapsulation efficiency of 82 wt% and a drug-loading capacity of 4.5 wt%. The material displayed strong mechanical properties and sustained release of the drug over a period of 107 hours upon exposure to near-infrared (NIR) light. Moreover, the release rate was accelerated under acidic conditions. The release mechanism was found to follow abnormal transport, highlighting the intricate interactions within the hydrogel that control drug diffusion.
While photoresponsive NC hydrogels offer considerable benefits for controlled drug delivery and various biomedical applications, they still face limitations. Most research has focused on designing these hydrogels for the NIR-I (650 to 950 nm) range, which has limited tissue penetration, confining their use primarily for superficial tissues like the skin rather than internal organs.68–70 On the other hand, the NIR-II (1000 to 1700 nm) range can penetrate deeper into tissues, and research on NIR-II-responsive NC is still in its early stages, presenting challenges in advancing this technology for broader applications. Moreover, the intricate design of photothermal-responsive NC-based hydrogels often leads to stability issues and uncontrolled release of therapeutics, potentially impacting their long-term effectiveness and reliability. In addition to these, effective use of photo-responsive NC hydrogels often requires specialized light sources for activation, which can be cumbersome and impractical for some applications. Research is on-going to address these challenges and improve the performance of NC-based light-sensitive drug carriers.
5. Magnetic field-responsive NC
Skin is the largest organ in the human body, comprising approximately 16% of the body weight and fulfilling many crucial bodily functions.71 Skin cancer/melanoma is by far the most common type of cancer in humans.Sailaja et al. engineered magnet-responsive nanocellulose films (MFs) by integrating superparamagnetic iron oxide nanoparticles (SPIONPs) with chitosan-TEMPO-oxidized NC which was further loaded with doxorubicin (DOX) drug for smart treatment of melanoma skin cancer [Fig. 8].72 They designed different MFs by varying the ratio of cellulose and SPION. Among all these magnetic strips, the MF4 where the SPION content was higher had the highest magnetic saturation (MS) value of 23.3 emu g−1.
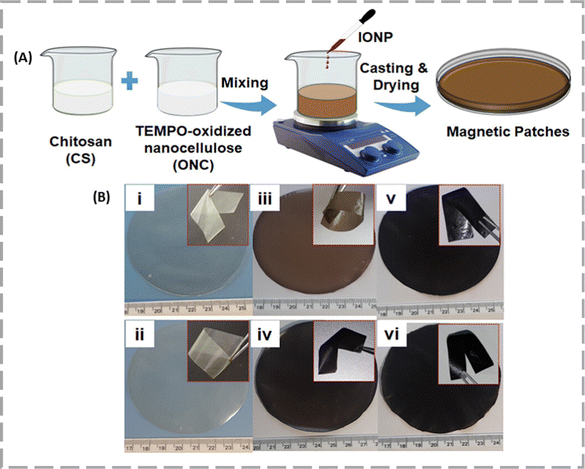 | ||
| Fig. 8 (A) Schematic representation of the preparation of magnetic patches. (B) Images of (i) CS0, (ii) CS4, (iii) MF1, (iv) MF2, (v) MF3, and (vi) MF4. The inset figures depict the flexibility of the patches. Reproduced with permission from ref. 72 Copyright 2023, American Chemical Society. | ||
So, it was selected for further magnetic hyperthermia induced DOX release experiments. The DOX-loaded sample (D-MF4) exhibited a magnetic saturation of 21.2 emu g−1 and increased the temperature to 42.2 °C within 7 minutes, with a specific loss power (SLP) of 4.70 W g−1. Drug release studies revealed that, without a magnetic field, 65% of the drug was released over 28 days, while a 50 mT magnetic field triggered 12.3% release within 1 h. Cytotoxicity assessments using an MTT assay showed that D-MF4 reduced L929 cell viability by 12.56% after 24 h and 71.17% after 5 days, and B16F10 melanoma cell viability by 13.02% and 79.55% at the same intervals, indicating greater toxicity towards B16F10 melanoma cells.
Yusefi et al. designed solid Fe3O4 nanofiller functionalized cellulose nanowhiskers (CNWs) to synthesize magnetic CNWs. This nanoconjugate was used as a carrier for anticancer agent 5-fluorouracil. The nanocomposites exhibited high thermal stability and drug encapsulation efficiency (92 ± 3.58%). Moreover, it showed potency to kill colorectal cancer cells.73
6. Multi-responsive NC
6.1 Thermo- and pH-responsive
He et al. and team discovered a novel dual-stimuli responsive (pH and temperature) NC material by combining carboxylated NC fibers prepared from Bagasse pulp fiber (PF) with a mixture of hyperbranched polyethylenimine (HPEI) and isobutyramide (IBAm).74 The new NC formed material was coined as TOCNFs-HPEI-IBAm, which was further applied as an anti-bacterial agent. TOCNF-HPEI-IBAm demonstrated effective dual-response performance across varying temperatures and pH levels. AFM measurements of TOCNF-HPEI-IBAm at temperatures of 20 °C and 40 °C, and pH values of 3, 7, and 10, revealed that its arithmetic average roughness (Ra) increased from 11.9 nm at pH 3 to 22.8 nm at pH 7, and further to 90.5 nm at pH 10, compared to PF. Furthermore, the Ra of TOCNF-HPEI-IBAm at 40 °C increased first and then decreased (60.7, 83.4, and 77.4 nm) when the pH value increased. This is due to the formation of intramolecular and intermolecular hydrogen bonds between the amide groups of TOCNF-HPEI-IBAm at different temperatures and pH values. This group also checked the anti-bacterial properties of TOCNF-HPEI-IBAm by subjecting it to the two most common bacteria available in the environment, i.e., Escherichia coli (E. coli) and Listeria monocytogenes (L. monocytogenes). The antibacterial activity of TOCNF-HPEI-IBAm against E. coli was 97.89% at pH 3, 97.05% at pH 7, and 96.84% at pH 10. For L. monocytogenes, the activity was 99.47% at pH 3, 99.82% at pH 7, and 99.64% at pH 10, indicating high efficacy across all pH levels. On the basis of these results, it was found that TOCNF-HPEI-IBAm with a high amino density (8.58 mmol g−1) possessed excellent antibacterial properties (≥97%). The DOX loading content of TOCNF-HPEI-IBAm was 642.52 mg g−1. At pH = 3.0, the maximum release amount of DOX drugs (39.30%) was reached at the 9th hour, and the release amount reached equilibrium at the 11th hour.Yuan Li et al. discovered a novel dual-stimuli responsive (pH and temperature) NC material by combining NC with pH responsive monomer acrylic acid, (AA) or acrylamide (AM) with a thermal responsive monomer N-isopropylacrylamide (NIPAM).75 Due to the presence of these two monomers in the cellulose material, it showed both pH and thermal stimuli-responsive behaviour. This material was further applied in delivering 5-fluorouracil (5-Fu) drug. The 5-FU drug loaded [NFC-g-(AA/AM)]-g-NIPAM hydrogel and non-loaded hydrogels showed different morphological features. The 5-FU drug loaded [NFC-g-(AA/AM)]-g-NIPAM hydrogel exhibited sustained release of the drug, reaching the maximum releasing amount within 5 h, but at pH 7 and 40 °C, 5-FU was rapidly released in the first hour and the maximum release rate is achieved within 30 min, and then it slowly fluctuates again in the following 6 h. Again, the pH was changed to 4.3, the release process was completed in 5 h at 40 °C and pH 4.3, and the equilibrium reached after 5 h of shaking, showing a rapid release behaviour under acidic conditions.
6.2 Thermo- and photo-responsive NC
Dong et al. discovered dual-responsive cellulose nanofibers (CNs) (extracted from cotton linter pulps) with hyaluronic acid methacrylate (CNF + HAMA). CN + HAMA hydrogels consisting of CNs and HAMA were designed to achieve temperature and UV dual-responsiveness (Fig. 9).76 The dual responsiveness of the CN + HAMA hydrogels could induce gelation at body temperature. The CN + HAMA mixture can be in a liquid state at a low temperature of 4 °C and solidified due to the self-association interactions between cellulose aggregates, as the temperature increased to 37 °C in a reversible manner. Similar behaviour was observed under UV exposure, i.e., when the solution of CN + HAMA was irradiated with UV light for 10 s to 10 min at different time durations it changed its physical state from liquid to solid. The thermal responsiveness characteristics of CN + HAMA hydrogel precursors helped it to adjust and physically cross-link during 3D printing by thermal gelation, which further helped to maintain the shapes of the printed structures. The CN + 1% HAMA hydrogel, identified as the optimal concentration of HAMA for 3D printing, was standardized using different time periods of UV irradiation. This composite demonstrated excellent biocompatibility with the L929 cells.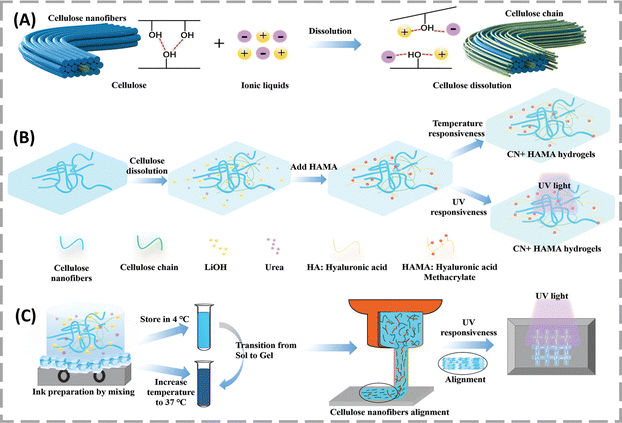 | ||
| Fig. 9 Schematic representation of the synthesis mechanism of the CN + HAMA hydrogels. Illustration of the dissolution mechanism of cellulose (A) and synthesis and curing process of the CN + HAMA hydrogels (B) and (C). Reproduced from ref. 76. | ||
The CN + 1% HAMA hydrogel features a ridge and groove nano-surface that directs cell growth through contact guidance, showcasing its potential in tissue engineering. In vivo safety was assessed by implanting the hydrogel in Kunming mice and observing the inflammatory response over 10 weeks. H&E staining showed no toxicity or inflammation at the implantation site, and immunofluorescence for T-cells and macrophages confirmed a significant reduction in immune cell signals after 3 weeks. These results indicate that the hydrogel is biocompatible and does not cause tissue damage.
6.3 pH- and magnet-responsive NC
In this context, Sumitha et al. have demonstrated a biodegradable, multi-stimuli responsive NC-based drug delivery system for DOX delivery in cancer cells.77 They integrated phosphorylated cellulose nanofibers (PNC) with superparamagnetic iron oxide nanoparticles (SPIONs) and subsequently loaded DOX onto the nanocellulose. A hydrogen bond formed between the –NH2 groups of DOX and the –OH groups of nanocellulose facilitates the better loading of drugs. This nanocomposite was capable of exhibiting both pH-dependent Dox delivery and magnetic field-induced photothermal therapy at the tumor site [Fig. 10]. The higher PNC content in PNC-IO-2 (IO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PNC = 74
PNC = 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26) compared to PNC-IO-1 (IO
26) compared to PNC-IO-1 (IO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PNC = 85
PNC = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) led to a higher DOX loading capacity. The release of DOX was notably found to be lower at pH 7.4 than at pH 5.6, the pH of tumor cells. After 5 days, the cumulative DOX release at pH 5.6 was 93.6%, 83.8%, and 87.5% for IO-DOX (without PNC), PNC-IO-1-DOX, and PNC-IO-2-DOX, respectively, while at pH 7.4, the release percentages were 51%, 31%, and 33%. Furthermore, applying a magnetic field to the samples IO, PNC-IO-2, and PNC-IO-2-DOX induced hyperthermia by increasing the temperature at the tumor site to over 40 °C within 4 minutes.
15) led to a higher DOX loading capacity. The release of DOX was notably found to be lower at pH 7.4 than at pH 5.6, the pH of tumor cells. After 5 days, the cumulative DOX release at pH 5.6 was 93.6%, 83.8%, and 87.5% for IO-DOX (without PNC), PNC-IO-1-DOX, and PNC-IO-2-DOX, respectively, while at pH 7.4, the release percentages were 51%, 31%, and 33%. Furthermore, applying a magnetic field to the samples IO, PNC-IO-2, and PNC-IO-2-DOX induced hyperthermia by increasing the temperature at the tumor site to over 40 °C within 4 minutes.
 | ||
| Fig. 10 (A) Schematic illustration of the preparation of DOX loaded magnetic cellulose nanofibers. (B) Functionalization of DOX and SPIONs with cellulose nanofibers. (C) Illustration of the release of DOX loaded magnetic cellulose nanofibers within tumour cells. Reproduced with permission from ref. 77 Copyright 2021, Elsevier. | ||
6.4 pH-, thermo-, and photo-responsive NC
Surgical removal of the tumor, followed by effective post-operative wound healing, is the most common way to treat skin cancer.78,79 However, irregular wound surfaces can cause a variety of post-operative complications.80 To overcome this challenge, Zhao et al. developed a shape-adaptable, multi stimuli-responsive, bioinspired in situ hydrogel to kill residual tumour cells and resist bacterial infection.81 In this research, a multifunctional liquid wound dressing (CNF-ILWD) responsive to pH, temperature, and NIR stimuli was developed. It contains a thermosensitive polymer poly(N-isopropylacrylamide) (PNIPAm) and pH-responsive CNF substrate as on/off switches. The CNF nanocavities enable the loading of multiple drugs, thus reducing drug dosage and frequency. This makes CNF-ILWD combined with a photothermal agent indocyanine green (ICG) and DOX, an ideal treatment for irregular tumour postoperative wounds.The CNF-ILWD@ICG&DOX showed excellent controllable temperature rise from 42–50 °C which is effective for killing cancer cells. It was shown that the temperature increase could also stimulate the drug release from the dressing and accelerate the treatment of tumour cells. ICG also produced ROS upon NIR irradiation, thereby increasing the rate of bacterial death. This enables a wound treatment strategy that combines both PTT and PDT. The release rate of DOX from CNF-ILWD@DOX increased as the pH decreased from 7.4 to 3.0. In the pH range of 5.5 to 6.5, similar to the tumour microenvironment, the release rate increased dramatically between 37–45 °C with NIR laser stimulation at pH 5.5, reaching a maximum release rate of 96.7%. Compared with the control, CNF-ILWD@ICG + NIR presented antibacterial activity rates of 98.9, 99.4, and 99.5% against E. coli, S. aureus, and MRSA, respectively. Drug-loaded hydrogels also destroyed bacterial biofilms as compared to the control, thus facilitating the release of ICG and DOX into the wound.
Liu et al. prepared a NIR-responsive porous composite hydrogel, MPDA@graphene oxide (GO)/CNFs, through a physical crosslinking technique for controlled drug delivery.82 They observed that drug release was enhanced by near-infrared (NIR) light and pH changes. The pH-responsive behavior of the hydrogel could enable targeted drug release in acidic environments, such as those found at bacteria-infected sites.
We have presented a summary of the stimuli-responsive nanocellulose materials according to their types, sources, other added components, drug payload, stimuli type, and, applications in Table 1.
| Type of NC | Type of plants | Added material | Drug payload | Stimuli type | Applications | Ref. |
|---|---|---|---|---|---|---|
| CNF | Bleached eucalyptus kraft pulp | Collagen embedded NC | — | Temperature | Matrix for the growth of intestinal organoids | 29 |
| CNF | Cotton linter pulps | Hyaluronic acid methacrylate | — | Temperature and light | Used for cell growth and soft tissue repair | 76 |
| BNC | Purchased | Chitosan, Pluronic F-127 and gold nanoparticles | — | Temperature | Treatment of myocardial infarction by cardiac tissue generation | 32 |
| CNC | Purchased | Cellulose nanocrystals were decorated with DNA. | DOX | pH | Used for targeted cancer treatment | 49 |
| CNF | Ciona intestinalis (a marine vertebrate) | Double crosslinked with collagen fibrils | Dexamethasone | — | Used for corneal treatment | 83 |
| Pineapple peel carboxymethyl NC hydrogels | Pineapple peel | Poly-vinyl alcohol | Naringin | pH and magnetic | Used for drug delivery system | 84 |
| Wood pulp | N-Isopropylacrylamide acrylic acid or acrylamide | 5-Fluorouracil | pH and temperature | Used for drug delivery system | 75 | |
| CNF | Bagasse pulp fiber | Hyperbranched polyethylenimine and isobutyramide groups | Dexamethasone | pH and temperature | Used as an anti-bacterial agent. | 74 |
| CNF | Cotton linter pulps | Bisphosphonated nanocellulose and β-tricalcium phosphate | — | pH | Used as a bone substitute for bone regeneration | 50 |
| CNF | Agave sisalana plant leaves | Superparamagnetic iron oxide nanoparticles and chitosan | DOX | Magnetic | Melanoma treatment | 72 |
| CNF | Purchased | Acrylamide, acrylic acid and polydopamine | Rhodamine 6G | pH | Used for transdermal drug delivery system | 85 |
| CNF | Bagasse pulp | Polyethylenimine | DOX | pH | Used for drug delivery system and as an anti-bacterial agent. | 54 |
| CNF | Wood pulp | Poly(N-isopropylacrylamide), amino hyperbranched polyamine and indocyanine green | DOX | pH, temperature and NIR | Used for wound dressing for multiple synergistic therapy of the postoperative infected wound | 81 |
| CNF & CNC | Purchased | Poly(N-isopropylacrylamide) | DOX | Temperature | Used for in vivo therapeutic administration and elastic wound dressing applications | 86 |
| CNF | Cotton linter pulps | κ-Carrageenan oligosaccharides nanoparticles | Surfactin and herbmedotcin | Temperature | Used as an antibacterial and anti-inflammatory agent for the treatment of periodontitis. | 87 |
| BNC | Komagataeibacter xylinus bacteria | Sodium alginate and six cations (manganese, cobalt, copper, zinc, silver, and cerium) | Six cations (manganese, cobalt, copper, zinc, silver, and cerium) | pH | Used for antibacterial wound dressing | 88 |
| CNF | Coconut husk | Polypropylene glycol (PPG), polycaprolactone (PCL) diol and (PEG) polyethylene glycol. | BSA and lysozyme protein | Temperature | Used for drug delivery system | 89 |
| CNC | Purchased | Graphene oxide with AND | Curcumin | pH | Used for drug delivery system | 51 |
| CNF | Purchased | Cellulose, methyl cellulose and PEG | pH and temperature | Anti-adhesive in post-operative tissue regeneration | 35 | |
| CNF | Agave sisalana leaves | SPIONs | DOX | pH and magnet | Used for drug delivery system | 77 |
| BNC | Komagataeibacter medellinensis bacteria | Pluronic F127 and sodium alginate | Tetracycline hydrochloride (TCH) | pH | Used for colon-targeted drug delivery system | 48 |
| CNF | Purchased | PNIPAM and sodium alginate | Tetracycline hydrochloride (TCH) | Temperature | Used for antibacterial drug delivery system | 26 |
| CNC | Softwood kraft pulp sheets | Beta cyclodextrin ethylenediamine conjugate (β-CD-EDA) | DOX | pH | Used for anticancer drug delivery system | 45 |
| CNC | Purchased | Pluronic F127 | — | Temperature | Injectable thermosensitive gel | 33 |
| CNF | Bagasse pulp | Protoporphyrin IX, PAPB, Prussian blue nanoparticles, Pluronic® F127 (F127), hydroxypropyl methyl cellulose | — | White and NIR light | Drug resistant bacteria infected wound healing | 63 |
| CNC | Bamboo pulp | Polydopamine and poly(N-isopropylacrylamide) | 5-Fluorouracil | NIR light | Photothermal therapy and anticancer drug delivery | 64 |
| CNC | Cotton fiber | AuNP and β-CD | Curcumin | NIR light | Photothermal therapy and anticancer drug delivery | 65 |
| CNF & CNC | Bleached softwood kraft pulp | Mesoporous poly-dopamine (MPDA) nanoparticles and polyethyleneimine-modified cellulose nanocrystals | Tetracycline hydrochloride | Light and pH | Used for drug delivery system | 90 |
| CNC | Purchased | Polydopamine coated mesoporous organosilica nano-particles | — | pH | Used as a drug delivery system in lung cancer therapy | 91 |
7. Conclusions and future perspectives
NC, the most abundant natural biopolymer on earth, is also recognized for its low density, biocompatibility, and biodegradability. NC hydrogels share structural similarities, such as porosity and an interconnected framework, with collagen and fibrinogen present in the extracellular matrix. Researchers have also combined other polymer and environment-responsive connectors to make these composite materials sensitive towards external stimuli. Among different types of NC, cellulose nanocrystals (CNCs) possess excellent properties, including a large surface area of approximately 250 m2 g−1, high tensile strength of 7500 MPa, and a high Young's modulus of up to 140 GPa.92 Despite their nano dimensions, starch and NCs exhibit better biodegradability in aqueous environments than their larger counterparts. Due to the mechanical stiffness, these CNCs are widely used as tissue regeneration therapy. Influenced by surface charge, CNC suspension can spontaneously form anisotropic chiral nematic liquid crystal phases above a critical concentration, which is responsible for unique optical properties of the material. On the other hand, BNCs have demonstrated its potential in various biomedical applications due to its high in vivo biocompatibility, water solubility, purity, porosity, and flexibility, with the absence of hemicellulose and lignin. Additionally, bacterial NC has a significant capacity to retain water, making it suitable for hydrophilic ingredients. Due to their high surface area and porosity, CNFs are utilized to create stimuli-responsive hydrogels by combining them with various other polymers. Due to the presence of oxygen functionalities, NC itself can show pH sensitivity to some extent as well provide ample opportunity for functionalization with other components. The drug binding and release time of NC-based drugs can vary based on the supramolecular interactions with the NC matrices.Recently, the positive results regarding drug delivery systems have demonstrated the importance and interest of academia and companies in investing in the development of NC-based products. Nevertheless, proficient chemical modifications of NC, while preserving their fundamental properties such as crystallinity, dimensions, and mechanical properties, continue to pose challenges as NC fibers exhibit a marked affinity for water, resulting in hygroscopic behavior that can compromise the dimensional stability of CNF-based materials. The elevated viscosity of CNF suspensions presents processing and handling complications, particularly in large-scale applications. Moreover, unchecked degrees of functionalization may compromise structural integrity during composite formation with other polymers. Furthermore, the size reduction achieved through the conversion of cellulose into NC significantly amplifies hydrogen bonding, consequently leading to the aggregation of NC particles. This effect can be further exacerbated by the coordination of drugs on the exterior of NC, thereby impeding dispersibility and solubility. As such, it becomes imperative to devise sophisticated surface modification and fiber pretreatment techniques to surmount these limitations and augment specific attributes. The present state of NC hydrogel materials is defined by their relatively soft characteristics and restricted long-term effectiveness. Further exploration and advancement of a targeted drug delivery system utilizing NC hydrogels at the cellular level could significantly propel the treatment of specific diseases, notably cancer. Thorough in vivo and long-term biocompatibility studies encompassing diverse biological models and the properties of NC are yet to be conducted. Additionally, the pH and temperature-dependent sol–gel behavior of NC-based composite materials have been widely employed for drug delivery applications, yet certain stimuli such as light in the NIR-II region and ultrasound therapy remain unexplored. In a broader context, the challenge lies in scaling up the production process and manufacturing of NC to achieve the optimal price-performance ratio for its diverse applications. We have provided a schematic overview of the current landscape and future prospects of NCs as drug delivery vehicles in Fig. 11.
Abbreviations
| 5-Fu | 5-Fluorouracil |
| AA | Acrylic acid |
| AM | Acrylamide |
| AND | Aminated nanodextran |
| APTES | Aminopropyltriethoxysilane |
| ASB | Azoxystrobin |
| AuNPs | Gold nanoparticles |
| AZO | Azobenzene |
| BBE | BHNC-β-CD-EDA |
| BC | Bacterial cellulose nanofibers |
| BHNC | Bifunctional hairy nanocellulose |
| BNC | Bacterial nanocellulose |
| Cdots | Carbon dots |
| CMC | Carboxymethyl cellulose |
| CN | Cellulose nanofiber |
| CNCs | Cellulose nanocrystals |
| CNF | Cellulose nanofibril |
| CNF-COOH | Carboxylated cellulose nanofiber |
| DLRIHWD | Dual light-responsive in situ hydrogel wound dressing |
| CNF-PAPB | Polyaminopropyl biguanide |
| CNF-PpIX | CNF protoporphyrin IX |
| COL-NC | Collagen with nanocellulose material |
| CUR | Curcumin |
| DOX | Doxorubicin |
| ECM | Extracellular matrices |
| G′ | Storage modulus |
| G′′ | Loss modulus |
| HAMA | Hyaluronic acid methacrylate |
| HNCs | Hairy nanocellulose composites |
| HPEI | Hyperbranched polyethylenimine |
| HPMC | Hydroxypropyl methyl cellulose |
| IBAm | Isobutyramide |
| LCST | Lower critical solution temperature |
| MC | Methyl cellulose |
| MFs | Magnet-responsive nanocellulose films |
| MGO | Modified pGraphene oxide |
| M s | Magnetic saturation |
| NIPAM | N-Isopropylacrylamide |
| PBNPs | Prussian blue nanoparticles |
| pDCs | Polymeric DNA nanostructure-CNCs complex |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| pNC | Bisphosphonated-nanocellulose |
| PNC | Phosphorylated cellulose nanofiber |
| PNIPAM | Poly(N-isopropylacrylamide) |
| PPAs | Postoperative peritoneal adhesions |
| R a | Average roughness |
| RBMSCs | Rat bone marrow-derived mesenchymal stem cells |
| RGD-CNFs | RGD peptide-grafted cellulose nanofibers |
| SLP | Specific loss power |
| SPIONPs | Superparamagnetic iron oxide nanoparticles |
| TCH | Tetracycline hydrochloride |
| TG | Sol–gel transition |
| TOCN | TEMPO-oxidized cellulose nanofiber |
| β-CD | β-Cyclodextrin |
| β-TCP | β-Tricalcium phosphate |
Author contributions
All authors enthusiastically participated in writing this.Data availability
Data can be obtained upon request from the author.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We wish to acknowledge the passion and hard work and impressive contribution of all the researchers who are working on nanocellulose-based stimuli responsive matrices. AK would like to thank BRNS for funding and CCRAS (Ministry of Ayush) for the postdoctoral fellowship of AK. SRP acknowledges IISER Bhopal for his doctoral fellowship. We acknowledge the infrastructural support from IISER Bhopal.References
- L. Montero de Espinosa, W. Meesorn, D. Moatsou and C. Weder, Chem. Rev., 2017, 117, 12851–12892 CrossRef CAS PubMed.
- R. R. Naik and S. Singamaneni, Chem. Rev., 2017, 117, 12581–12583 CrossRef CAS PubMed.
- S. Gao, G. Tang, D. Hua, R. Xiong, J. Han, S. Jiang, Q. Zhang and C. Huang, J. Mater. Chem. B, 2019, 7, 709–729 RSC.
- M. S. Ganewatta, Z. Wang and C. Tang, Nat. Rev. Chem., 2021, 5, 753–772 CrossRef CAS PubMed.
- X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042–6065 RSC.
- M. A. C. Stuart, W. T. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk and M. Urban, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- H. Ning, C. Monroe, S. Gibbons, B. Gaskey and P. Flater, Int. Mater. Rev., 2024, 09506608241252498 Search PubMed.
- X. Wang, X. Shi, S. Yin, J. Zheng, Y. Song and H. Sun, ACS Mater. Lett., 2024, 6, 3770–3793 CrossRef CAS.
- M. A. Abdelhamid, M.-R. Ki and S. P. Pack, Int. J. Mol. Sci., 2024, 25, 4678 CrossRef CAS.
- B. Thomas, M. C. Raj, J. Joy, A. Moores, G. L. Drisko and C. Sanchez, Chem. Rev., 2018, 118, 11575–11625 CrossRef CAS PubMed.
- W. Chen, H. Yu, S.-Y. Lee, T. Wei, J. Li and Z. Fan, Chem. Soc. Rev., 2018, 47, 2837–2872 RSC.
- B. Alonso-Lerma, L. Barandiaran, L. Ugarte, I. Larraza, A. Reifs, R. Olmos-Juste, N. Barruetabeña, I. Amenabar, R. Hillenbrand and A. Eceiza, Commun. Mater., 2020, 1, 57 CrossRef.
- M. Abba, B. B. Nyakuma, Z. Ibrahim, J. B. Ali, S. I. A. Razak and R. Salihu, J. Nat. Fibers, 2022, 19, 4368–4379 CrossRef CAS.
- C. Sharma, N. K. Bhardwaj and P. Pathak, Cellulose, 2022, 29, 7177–7191 CrossRef CAS.
- M. Y. Khalid, A. Al Rashid, Z. U. Arif, W. Ahmed and H. Arshad, J. Mater. Res. Technol., 2021, 14, 2601–2623 CrossRef CAS.
- T. M. S. U. Gunathilake, Y. C. Ching, C. H. Chuah, N. Abd Rahman and N.-S. Liou, Int. J. Biol. Macromol., 2020, 158, 670–688 CrossRef.
- C. Chingakham, O. Manaf, A. Sujith and V. Sajith, Appl. Nanosci., 2020, 10, 795–806 CrossRef CAS.
- A. Gelmi and C. E. Schutt, Adv. Healthcare Mater., 2021, 10, 2001125 CrossRef CAS.
- Y. Ai, Z. Lin, W. Zhao, M. Cui, W. Qi, R. Huang and R. Su, J. Mater. Chem. B, 2023, 11, 7004–7023 RSC.
- Y. Deng, J. Xi, L. Meng, Y. Lou, F. Seidi, W. Wu and H. Xiao, Eur. Polym. J., 2022, 180, 111591 CrossRef CAS.
- J. Hu, Y. Chen, Y. Li, Z. Zhou and Y. Cheng, Biomaterials, 2017, 112, 133–140 CrossRef CAS.
- M. A. Ward and T. K. Georgiou, Polymers, 2011, 3, 1215–1242 CrossRef CAS.
- R. Nigmatullin, V. Gabrielli, J. C. Muñoz-García, A. E. Lewandowska, R. Harniman, Y. Z. Khimyak, J. Angulo and S. J. Eichhorn, Cellulose, 2019, 26, 529–542 CrossRef CAS.
- X. Xu, Y. Liu, W. Fu, M. Yao, Z. Ding, J. Xuan, D. Li, S. Wang, Y. Xia and M. Cao, Polymers, 2020, 12, 580 CrossRef CAS PubMed.
- E. Gicquel, C. Martin, Q. Gauthier, J. Engström, C. Abbattista, A. Carlmark, E. D. Cranston, B. Jean and J. Bras, Biomacromolecules, 2019, 20, 2545–2556 CrossRef CAS.
- R. Goyal, S. Sahu, S. Mitra, R. Niranjan, R. Priyadarshini, R. Yadav and B. Lochab, ACS Appl. Polym. Mater., 2024, 6, 1348–1361 CrossRef CAS.
- M. J. Kratochvil, A. J. Seymour, T. L. Li, S. P. Paşca, C. J. Kuo and S. C. Heilshorn, Nat. Rev. Mater., 2019, 4, 606–622 CrossRef CAS.
- J. A. Hubbell, Biotechnology, 1995, 13, 565–576 CAS.
- R. Curvello, D. Alves, H. E. Abud and G. Garnier, Mater. Sci. Eng., C, 2021, 124, 112051 CrossRef CAS.
- A. Mellati, S. Dai, J. Bi, B. Jin and H. Zhang, RSC Adv., 2014, 4, 63951–63961 RSC.
- Z. Gan, X. Qin, H. Liu, J. Liu and J. Qin, Bioact. Mater., 2023, 28, 386–401 CAS.
- H. Tohidi, N. Maleki-Jirsaraei, A. Simchi, F. Mohandes, Z. Emami, L. Fassina, F. Naro, B. Conti and F. Barbagallo, Materials, 2022, 15, 5122 CrossRef CAS PubMed.
- E. Kushan and E. Senses, ACS Appl. Bio Mater., 2021, 4, 3507–3517 CrossRef CAS PubMed.
- S. Soltany, Naunyn-Schmiedeberg's Arch. Pharmacol., 2021, 394, 317–336 CrossRef CAS PubMed.
- T. Sultana, H. Van Hai, C. Abueva, H. J. Kang, S.-Y. Lee and B.-T. Lee, Mater. Sci. Eng., C, 2019, 102, 12–21 CrossRef CAS.
- N. S. Capanema, A. A. Mansur, A. C. de Jesus, S. M. Carvalho, L. C. de Oliveira and H. S. Mansur, Int. J. Biol. Macromol., 2018, 106, 1218–1234 CrossRef CAS.
- M. S. Rahman, M. S. Hasan, A. S. Nitai, S. Nam, A. K. Karmakar, M. S. Ahsan, M. J. Shiddiky and M. B. Ahmed, Polymers, 2021, 13, 1345 CrossRef CAS PubMed.
- S. Hu, Y. Zhi, S. Shan and Y. Ni, Carbohydr. Polym., 2022, 275, 118741 CrossRef CAS PubMed.
- G. Chinga-Carrasco and K. Syverud, J. Biomater. Appl., 2014, 29, 423–432 CrossRef PubMed.
- S. Bazban-Shotorbani, M. M. Hasani-Sadrabadi, A. Karkhaneh, V. Serpooshan, K. I. Jacob, A. Moshaverinia and M. Mahmoudi, J. Controlled Release, 2017, 253, 46–63 CrossRef CAS PubMed.
- L. Ning, C. You, Y. Zhang, X. Li and F. Wang, Compos. Commun., 2021, 26, 100739 CrossRef.
- Y. Li, C. Wang, Y. Luan, W. Liu, T. Chen, P. Liu and Z. Liu, J. Appl. Polym. Sci., 2022, 139, 51647 CrossRef CAS.
- O. M. Vanderfleet and E. D. Cranston, Nat. Rev. Mater., 2021, 6, 124–144 CrossRef CAS.
- H. Yang and T. G. van de Ven, Cellulose, 2016, 23, 1791–1801 CrossRef CAS.
- M. Heidari Nia, S. Ashkar, J. G. Munguia-Lopez, J. Kinsella and T. G. van de Ven, Biomacromolecules, 2023, 24, 2100–2117 CrossRef CAS.
- H. M. Barriga, M. N. Holme and M. M. Stevens, Angew. Chem., Int. Ed., 2019, 58, 2958–2978 CrossRef CAS PubMed.
- Z. Karami and M. Hamidi, Drug Discovery Today, 2016, 21, 789–801 CrossRef CAS PubMed.
- F. V. Ferreira, N. Z. Ezazi, C. G. Otoni, A. C. Aguiar, J. R. Bianchi, J. H. Lopes, D. M. dos Santos, L. G. Greca, H. S. Barud and H. A. Santos, ACS Appl. Polym. Mater., 2024, 6, 3708–3720 CrossRef CAS.
- Y. Lee, K. Nam, Y. M. Kim, K. Yang, Y. Kim, J.-W. Oh and Y. H. Roh, Carbohydr. Polym., 2024, 340, 122270 CrossRef CAS PubMed.
- A. Nishiguchi and T. Taguchi, Biomacromolecules, 2019, 20, 1385–1393 CrossRef CAS PubMed.
- T. Anirudhan, F. Shainy and J. P. Thomas, Int. J. Biol. Macromol., 2019, 135, 776–789 CrossRef PubMed.
- D. De, C. K. Das, D. Mandal, M. Mandal, N. Pawar, A. Chandra and A. N. Gupta, ACS Appl. Bio Mater., 2020, 3, 6284–6296 CrossRef CAS PubMed.
- L. A. Al-Ani, F. A. Kadir, N. M. Hashim, N. M. Julkapli, A. Seyfoddin, J. Lu, M. A. AlSaadi and W. A. Yehye, Heliyon, 2020, 6, e05360 CrossRef CAS.
- H. He, M. Cheng, Y. Liang, H. Zhu, Y. Sun, D. Dong and S. Wang, J. Agric. Food Chem., 2020, 68, 3518–3527 CrossRef CAS.
- Q. Ye, R. Wang, S. Yan, B. Chen and X. Zhu, J. Mater. Chem. B, 2022, 10, 2617–2627 RSC.
- F. Ercole, T. P. Davis and R. A. Evans, Polym. Chem., 2010, 1, 37–54 RSC.
- A. Karmakar, A. Silswal and A. L. Koner, J. Mater. Chem. B, 2024, 12, 4785–4808 RSC.
- Y. Liu, H. Wang, S. Li, C. Chen, L. Xu, P. Huang, F. Liu, Y. Su, M. Qi and C. Yu, Nat. Commun., 2020, 11, 1724 CrossRef CAS.
- F. Hao, L. Wang, B. Chen, L. Qiu, J. Nie and G. Ma, ACS Appl. Mater. Interfaces, 2021, 13, 46938–46950 CrossRef CAS PubMed.
- S. Zhao, L. Zhang, L. Deng, J. Ouyang, Q. Xu, X. Gao, Z. Zeng and Y. N. Liu, Small, 2021, 17, 2103003 CrossRef CAS PubMed.
- R. Nasseri, C. Deutschman, L. Han, M. Pope and K. Tam, Mater. Today Adv., 2020, 5, 100055 CrossRef.
- T. T. A. Do, S. Grijalvo, T. Imae, M. J. Garcia-Celma and C. Rodríguez-Abreu, Carbohydr. Polym., 2021, 270, 118366 CrossRef CAS PubMed.
- X. Shi, Z. Chen, Y. He, Q. Lu, R. Chen, C. Zhao, D. Dong, Y. Sun and H. He, Carbohydr. Polym., 2022, 297, 120042 CrossRef CAS PubMed.
- X. Liu, Y. Wu, Q. Lin, J. Cheng, F. Lin, L. Tang, B. Huang and B. Lu, Cellulose, 2021, 28, 2255–2271 CrossRef CAS.
- Y. Tian, D. Jia, M. Dirican, M. Cui, D. Fang, C. Yan, J. Xie, Y. Liu, C. Li and J. Fu, Biomacromolecules, 2022, 23, 960–971 CrossRef CAS.
- C. You, X. Ji, H. Lin, N. Ma, W. Wei, L. Long, L. Ning and F. Wang, Environ. Sci.: Nano, 2024, 11, 1442–1451 RSC.
- Y. Liu, Y. Huo, M. Li, C. Qin and H. Liu, Cellulose, 2022, 29, 379–393 CrossRef.
- Y. Yang, K. Long, Y. Chu, H. Lu, W. Wang and C. Zhan, Adv. Funct. Mater., 2024, 2402975 CrossRef CAS.
- R. Roy, A. Khan, T. Dutta and A. L. Koner, J. Mater. Chem. B, 2022, 10, 5352–5363 RSC.
- K. Pal, V. Sharma, D. Sahoo, N. Kapuria and A. L. Koner, Chem. Commun., 2018, 54, 523–526 RSC.
- V. Jayarama Reddy, S. Radhakrishnan, R. Ravichandran, S. Mukherjee, R. Balamurugan, S. Sundarrajan and S. Ramakrishna, Wound Repair Regen., 2013, 21, 1–16 CrossRef.
- N. Sumitha, N. G. Krishna and G. Sailaja, ACS Appl. Polym. Mater., 2023, 5, 9170–9179 CrossRef CAS.
- M. Yusefi, K. Shameli, M. S. Lee-Kiun, S.-Y. Teow, H. Moeini, R. R. Ali, P. Kia, C. J. Jie and N. H. Abdullah, Int. J. Biol. Macromol., 2023, 233, 123388 CrossRef CAS PubMed.
- H. He, X. Shi, W. Chen, R. Chen, C. Zhao and S. Wang, J. Agric. Food Chem., 2020, 68, 7425–7433 CrossRef CAS PubMed.
- Y. Li, L. Zhang, Z. Song, F. Li and D. Xie, Int. J. Biol. Macromol., 2022, 223, 11–16 CrossRef CAS PubMed.
- L. Dong, M. Liang, Z. Guo, A. Wang, G. Cai, T. Yuan, S. Mi and W. Sun, Int. J. Bioprint., 2022, 8, 126–139 Search PubMed.
- N. Sumitha, S. Sreeja, P. G. Varghese and G. Sailaja, Mater. Chem. Phys., 2021, 273, 125108 CrossRef CAS.
- P. Kavyashree, R. Aswini, A. Karmakar and A. L. Koner, Chem. – Asian J., 2024, 19, e202400427 CrossRef PubMed.
- A. Testori, I. Stanganelli, L. Della Grazia and L. Mahadavan, Surg. Oncol., 2004, 13, 211–221 CrossRef CAS.
- D. C. Ziogas, A. Martinos, D.-P. Petsiou, A. Anastasopoulou and H. Gogas, Cancers, 2022, 14, 2873 CrossRef CAS PubMed.
- C. Zhao, R. Chen, Z. Chen, Q. Lu, H. Zhu, Q. Bu, J. Yin and H. He, ACS Appl. Mater. Interfaces, 2021, 13, 51578–51591 CrossRef CAS PubMed.
- Y. Liu, Q. Fan, Y. Huo, C. Liu, B. Li and Y. Li, ACS Appl. Mater. Interfaces, 2020, 12, 57410–57420 CrossRef CAS PubMed.
- M. Xeroudaki, M. Rafat, P. Moustardas, A. Mukwaya, S. Tabe, M. Bellisario, B. Peebo and N. Lagali, Acta Biomater., 2023, 172, 234–248 CrossRef CAS PubMed.
- H. Dai, H. Zhang, L. Ma, H. Zhou, Y. Yu, T. Guo, Y. Zhang and H. Huang, Carbohydr. Polym., 2019, 209, 51–61 CrossRef CAS PubMed.
- Y. Li, M. Yao, Y. Luo, J. Li, Z. Wang, C. Liang, C. Qin, C. Huang and S. Yao, ACS Appl. Mater. Interfaces, 2023, 15, 5883–5896 CrossRef CAS PubMed.
- Q. Sun, S. Tao, G. Bovone, G. Han, D. Deshmukh, M. W. Tibbitt, Q. Ren, P. Bertsch, G. Siqueira and P. Fischer, Adv. Healthcare Mater., 2024, 2304287 CrossRef CAS PubMed.
- A. Johnson, F. Kong, S. Miao, H.-T. V. Lin, S. Thomas, Y.-C. Huang and Z.-L. Kong, Sci. Rep., 2020, 10, 18037 CrossRef CAS PubMed.
- M. Shahriari-Khalaji, S. Hong, G. Hu, Y. Ji and F. F. Hong, Polymers, 2020, 12, 2683 CrossRef CAS PubMed.
- Y. Leow, Y. J. Boo, M. Lin, Y. C. Tan, R. Z. R. Goh, Q. Zhu, X. J. Loh, K. Xue and D. Kai, Carbohydr. Polym., 2024, 323, 121453 CrossRef CAS.
- Y. Liu, Q. Fan, Y. Huo, M. Li, H. Liu and B. Li, Cellulose, 2021, 28, 6953–6966 CrossRef CAS.
- L. Li, S. Li, S. Xu, G. Zeng, G. Dai, L. He, Q. Yan, Y. Wang, Y. Meng and W. Xiong, Process Biochem., 2024, 142, 223–232 CrossRef CAS.
- N. T. U. Culsum, C. Melinda, I. Leman, A. Wibowo and Y. W. Budhi, Mater. Today Commun., 2021, 26, 101817 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |






