Silicon oxide microchips functionalized with fluorescent probes for quantitative real-time glutathione sensing in living cells†
Saman
Bagherpour
 ab,
Patricia
Vázquez
cd,
Marta
Duch
e,
Juan
Pablo Agusil
ab,
Patricia
Vázquez
cd,
Marta
Duch
e,
Juan
Pablo Agusil
 e,
José Antonio
Plaza
e,
Mariano
Redondo-Horcajo
c,
Teresa
Suárez
c and
Lluïsa
Pérez-García
e,
José Antonio
Plaza
e,
Mariano
Redondo-Horcajo
c,
Teresa
Suárez
c and
Lluïsa
Pérez-García
 *ab
*ab
aDepartament de Farmacologia, Toxicologia i Química Terapèutica, Universitat de Barcelona, Av. Joan XXIII 27-31, Barcelona, 08028, Spain. E-mail: mlperez@ub.edu
bInstitut de Nanociència i Nanotecnologia IN2UB, Universitat de Barcelona, Barcelona, 08028, Spain
cCentro de Investigaciones Biológicas Margarita Salas, CIB (CSIC), Madrid, 28040, Spain
dDepartamento de Bioquímica y Biología Molecular, Facultad de Medicina, Universidad Complutense de Madrid, Madrid, 28040, Spain
eInstituto de Microelectrónica de Barcelona, IMB-CNM (CSIC), Campus UAB, Cerdanyola del Vallès, Barcelona, 08193, Spain
First published on 20th December 2024
Abstract
Glutathione (GSH) plays a vital role in the regulation of intracellular functions which alterations in physiological glutathione levels are associated to various diseases. Molecular bioimaging is a sensitive method for GSH detection, but challenges persist in the development of fluorescent probes, mainly concerning long-term tracking of intracellular GSH concentration because of aggregation of molecular probes and their washout in cells. Engineered nanomaterials have shown great promise for increasing the disease diagnosis accuracy. Microchips generated by advanced microfabrication techniques can be applied in designing biomedical devices due to control over size, shape, and bioactive coatings utilization. In the current work, the synthesis and characterization of two GSH probes, Bdpy1 and Bdpy2, is reported, each offering irreversible and reversible GSH reactions, respectively. These GSH probes are immobilized on silicon oxide microchips (SOμC), micro-fabricated using photolithographic techniques, to give SOμC-Bdpy1 and SOμC-Bdpy2. Both functionalized microchips exhibited sensitivity to GSH, and, notably, the reversible SOμC-Bdpy2 showed less time dependency, making it more suitable for long-term intracellular GSH sensing. In vitro experiments in HeLa cells reveal both SOμC-Bdpy1 and SOμC-Bdpy2 were internalized in living cells, showing SOμC-Bdpy2 more reliable results (due to its less time dependency) for quantifying intracellular GSH. Remarkably, the intracellular GSH measurement was monitored by SOμC-Bdpy2 for 48 h, indicating the functionalized microchips capability to detect GSH amount in different time intervals. This study introduces a promising approach for long term quantification of intracellular GSH, overcoming the limitation of fluorescent probes and offering valuable insights into microchip-based sensing methodologies.
1. Introduction
Biological thiols, such as glutathione (GSH), cysteine (Cys), and homocysteine (Hcy), are essential for preserving the proper redox state of biological processes. GSH, a tripeptide of glutamate, cysteine, and glycine, is the main nonprotein thiol in living cells. For healthy individuals, cellular concentrations of GSH range from 0.5 to 10 mM, and its concentrations in physiological fluids are approximately 2 to 12 μM.1 GSH has several important cellular functions related to metabolism, antioxidant defense, regulation of intracellular redox status, protein glutathionylation, as well as mitochondrial function and integrity.2 Abnormal concentration levels of GSH are associated to various diseases including oxidative stress resulting in cancer,3 neurotoxicity,4 heart problems,5 Alzheimer's,6 aging related deseases.7Molecular imaging based on fluorescent probes is assumed to be a sensitive technique providing online fluorescence sensing of GSH.8–10 Although these probes have made great progress in the field, they still face a number of challenges for the long-term tracking of intracellular GSH levels due to the aggregation of fluorescent probes in cellular environments, leading to fluorescence aggregation-caused quenching11,12 in long-term intervals, or their rapid washout from cells. On the other hand, nanostructured materials have attracted considerable attention owing to their exceptional properties, becoming integral components in the development of highly sensitive chemo- and biosensors.13–18 Recent years have witnessed the development of nanomaterials-based GSH sensors.19–22 However, despite these advancements, concerns persist regarding toxicity, realtime, and long-term studies of intracellular GSH detection. Moreover, the use of non-reversible nanomaterials complicates efforts to conduct thorough studies on the interactions of nanomaterials within biological systems systems.23
Recently, microparticles have shown great potential for studying their interactions with cellular environments and for possible applications as intracellular micrometric devices.24–26 Microchips generated by advanced microfabrication techniques can be applied in the design of new biomedical devices due to providing exceptional advantages. These advantages include precise control over size and shape, which allows for the generation of devices tailored to specific biomedical applications.27 Additionally, improved surface design and chemical functionalization capability enhance the performance and integration of these devices within biological systems. More importantly, the use of bioactive coatings can significantly improve the efficacy and targeting of drug delivery systems.28
Silicon oxide microchips (SOμC) can be produced by employing photolithographic processes providing great platforms for digging into the complicated nature of cellular processes. In contrast to chemically fabricated microparticles, it has been demonstrated that by the utilization of microelectronic-based approaches, micrometric-sized particles of various materials, shapes, and sizes achieve lower polydispersity in the aqueous media.29 They can provide new insights into both individual cell activities and their population dynamics. Moreover, the biocompatibility of these types of microchips allows them to integrate effortlessly into biological environments without causing adverse interactions. Their uniformity, easy identification, and long-term existence within cells make them invaluable tools for studying intricate biological systems and achieving long-term results. Furthermore, their versatility appears through, as they may be customized with specialized functionalities to meet a variety of research objectives. It has been also shown that they are able to interact with living cells,30 applying for the cells tracking the micrometric tags,31 and employing the functionalized microchips as the intracellular pH sensor.28 Cuboids silicon oxide microchips (3 × 3 × 0.5 μm3), among other microchips types, offer a straightforward platform for immobilizing biomedical compounds because the observation of these microchips is easy even under bright-field microscopy.32 Additionally, as previously described, the cell internalization of these microchips is relatively harmless, and they are able to stay inside of cells for extended periods33 which facilitates the long-term tracking potentiality. In terms of the proportion of cells with internalized microchips, cuboids demonstrated an uptake rate of almost 50% with a particle![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cell 10
cell 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, and the average number of particles taken up per cell ranged from roughly 1 to 1.5 particles per cell. We have also shown that the cellular uptake of cuboid microchips did not show major alterations in cellular metabolism indicating the low cytotoxicity of these type of microchips.34,35 Based on our research experience, the physicochemical properties of compounds can be maintained after conjugation with these types of microchips.28,36,37 Consequently, surface functionalization of silicon oxide microchips is able to increase the selective delivery of fluorescent probes to specific cells to realize effective intracellular imaging for the long-term tracking purposes.
1 ratio, and the average number of particles taken up per cell ranged from roughly 1 to 1.5 particles per cell. We have also shown that the cellular uptake of cuboid microchips did not show major alterations in cellular metabolism indicating the low cytotoxicity of these type of microchips.34,35 Based on our research experience, the physicochemical properties of compounds can be maintained after conjugation with these types of microchips.28,36,37 Consequently, surface functionalization of silicon oxide microchips is able to increase the selective delivery of fluorescent probes to specific cells to realize effective intracellular imaging for the long-term tracking purposes.
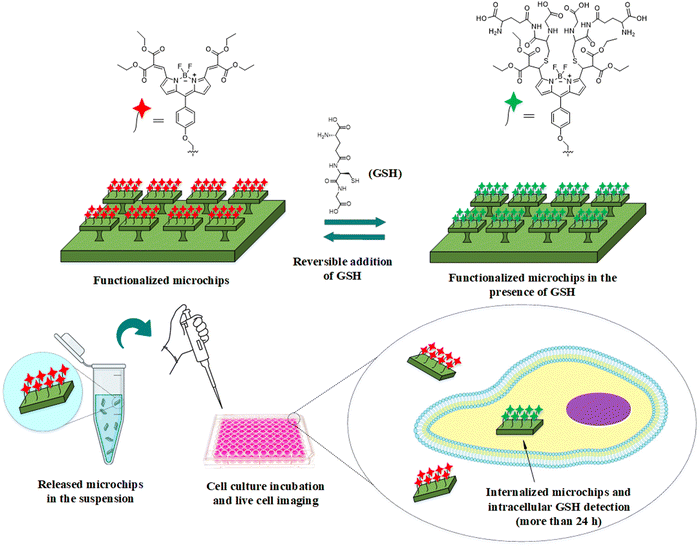
We report, for the first time, a micrometric sensing platform based on microfabricated silicon oxide microchips functionalized with either reversible or irreversible fluorescent sensing probes that has been developed and applied for intracellular recognition of GSH in living cells. This innovative approach addresses the limitations commonly associated with traditional fluorescent probes when used in solution within biological environments for accurate quantification and long-term tracking of GSH. By integrating these advanced sensing platforms, we aim to achieve precise intracellular and real-time quantification of GSH levels, overcoming existing challenges of molecular probes in solution and enabling enhanced monitoring of this biomolecule in complex biological systems. In this study, we used BODIPY functionalized silicon oxide microchips as a device for intracellular GSH sensing (Fig. 1). First, two types of BODIPY-based probes as the GSH sensors, called Bdpy1 and the reversible probe Bdpy2, were synthesized and immobilized onto the surface of cuboid SOμC previously microfabricated using the photolithographic techniques. The capability of the prepared SOμC-Bdpy1 and SOμC-Bdpy2 for long-term tracking of GSH was evaluated first in GSH aqueous solution during different time intervals and at various intracellular range of GSH levels. Then, the sensitivity of functionalized microchips was investigated through in vitro experiments in HeLa cells for the quantification and long-term tracking of intracellular GSH.
2. Results and discussion
2.1 Synthesis, characterization and optical properties in solution of GSH probes Bdpy1 and Bdpy2
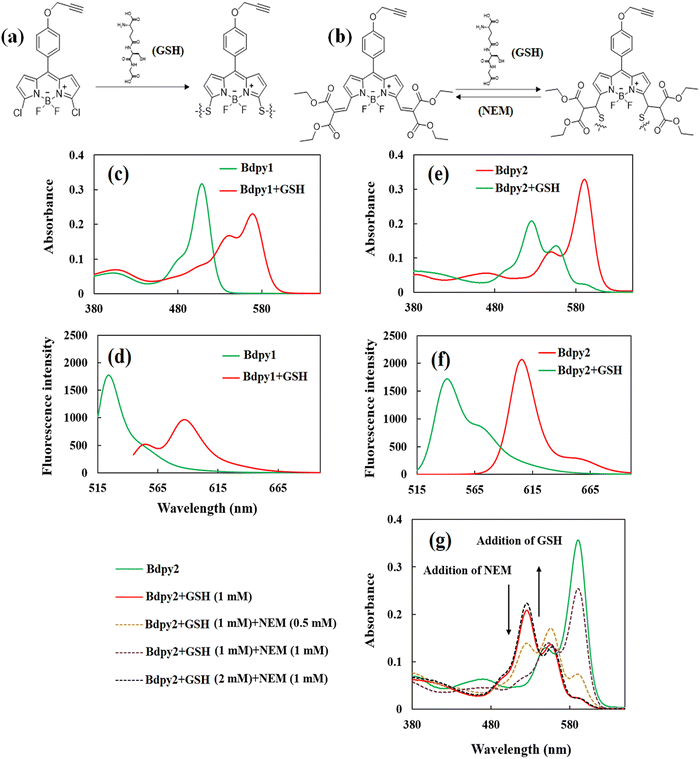
Two molecular probes, Bdpy1 (Fig. 2a) and Bdpy2 (Fig. 2b) were selected and synthesized as conjugable GSH probes. Bdpy1 incorporates (a) chlorine substituents for irreversible reaction with GSH and (b) a propargyloxy moiety at the para position of the meso phenyl substituent for conjugation to the surface of SOμCs. The new Bdpy2, instead, incorporates diethyl malonate electron-withdrawing groups, connected via exocyclic double bonds, which provides a specific Michael addition reversible reaction between the vinyl group and GSH. The synthesis and all chemical characterizations of the conjugable Bdpy1 has been reported previously by us.38 As for conjugable Bdpy2, it was synthesized as a four-step sequence (Scheme S1, ESI†) starting with the condensation of diethyl 2-((1H-pyrrol-2-yl)methylene)malonate (1) and 4-(prop-2-yn-1-yloxy)benzaldehyde (2) to give dipyrromethane (3) followed by oxidation to yield dipyrromethene (4) as shown in the ESI† (Scheme S1). Then, reaction of 4 with boron trifluoride afforded Bdpy2. The synthesized compounds were characterized by 1H NMR and 13C NMR spectroscopies, electrospray ionization mass spectrometry (ESI MS), and Fourier transform infrared (FT-IR) (Fig. S1-1–S1-12 in ESI†).Before immobilization of GSH probes on the surface of microchips, their optical properties in solution were investigated using UV-vis absorption and fluorescence spectroscopies experiments both in the absence and presence of GSH. As it can be seen in Fig. 2, the maximum absorbance (Fig. 2c) and emission (Fig. 2d) Bdpy1 are at λex = 508 nm and λem = 525 nm, respectively. In the presence of GSH (1 mM), the new absorbance peak at λex = 572 nm (λem = 585 nm) appears correspond to the creation of a new chromophore as the consequence of the di-substitution reaction of the thiol group in GSH and chlorines Bdpy1 (Fig. 2(a)). Fig. S2 in the ESI,† shows the absorption spectrum of GSH in solution (λmax ∼ 190 nm) in the absence of Bdpy1, and the GSH solution does not exhibit any emission (data not shown). In the case of the reversible probe of Bdpy2, its absorption peak exhibits at λex = 590 nm and the emission is at λem = 608 nm. This difference in the absorption and emission of Bdpy2 compared with Bdpy1 is attributed to the increase conjugation caused by the diethyl malonate groups, connected to the BODIPY core via exocyclic double bonds at the 3,5-positions, which extend the delocalization of the pi-system, causing a shift towards longer wavelengths in the absorption band.39 However, in the presence GSH, a new absorption peak emerged at λex = 525 nm (Fig. 2(e)) with the emission at 545 nm (Fig. 2(f)). The Michael type addition between the vinyl group and GSH in Bdpy2 (Fig. 2(b)) disrupts the conjugated system extension, leading to an approximate blue shift in both the absorption and fluorescence bands. In both Bdpy1 and Bdpy2, the mono-substituted probes with GSH exhibited λex = 542 nm (λem = 555 nm) and λex = 556 nm (λem = 570 nm) after 30 min, respectively (data not shown). The reverse process for Bdpy2 was tracked by observing the subsequent reduction in the λex = 525 nm absorption upon addition of 0.5 mM and 1 mM of N-ethylmaleimide (NEM), which reacts with GSH irreversibly, to deplete GSH and then GSH levels were restored by adding more GSH (Fig. 2(g)). Throughout the consumption of GSH and its subsequent restoration, the fluctuations in the absorbance closely mirrored the changes in GSH concentrations. Fig. S3 in the ESI,† also shows maximum absorption of Bdpy2 upon the addition of different concentrations of GSH and NEM over 12 h time intervals. The emission spectra also confirmed the consumption and restoration of Bdpy2 in the same conditions applied for absorption experiments (data not shown). This alignment underscores the remarkably reversible character of the interaction between compound Bdpy2 and GSH. The reversibility of Bdpy2 is attributed to the combined influence of two –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in diethyl malonate groups, which enhance the acidity of the αC–H bond, facilitating the rapid elimination of thiol adducts at physiological pH while simultaneously accelerating the rate of thiol addition.40
O in diethyl malonate groups, which enhance the acidity of the αC–H bond, facilitating the rapid elimination of thiol adducts at physiological pH while simultaneously accelerating the rate of thiol addition.40
2.2 Fabrication and functionalization of silicon oxide microchips with GSH probes
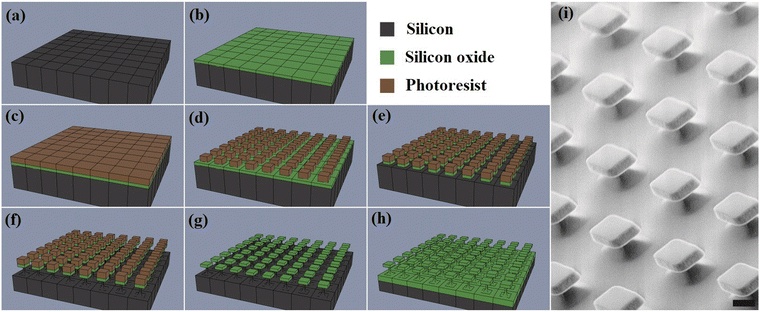
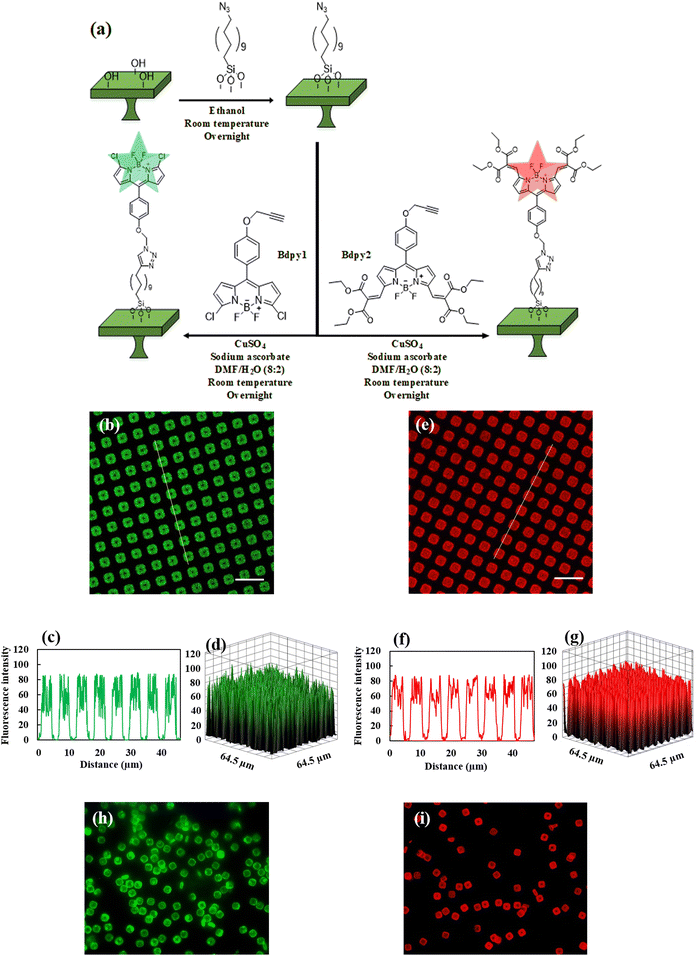
The silicon oxide microchips (SOμC) were fabricated using microelectronics techniques summarized in, Fig. 3, over a 100-mm-Φ silicon wafer, Fig. 3a. First, a 1-μm-thick silicon oxide layer was thermally grown (Fig. 3b). This layer will be the structural material of the microchips. The next processes were performed to define the lateral dimensions of the chips. For that purpose, a photoresist layer was spun on the wafer (Fig. 3c), and a photolithographic process was performed to define the lateral dimensions of the chips to 3 × 3 μm2 (Fig. 3d). Then, the silicon oxide layer was patterned by a dry etching process (Fig. 3e). At this stage, the devices were defined and the next processes were performed to prepare the release of the chips from the wafer. A quasi-isotropic silicon etching was performed to define pillars underneath the silicon oxide chips (Fig. 3f). Next the photoresist was removed (Fig. 3g), and the whole wafer was oxidized (Fig. 3h) to convert the silicon pillars in silicon oxide pillars. At this technological stage, the microchips remain physically anchored to the substrate for their posterior chemical functionalization. Fig. 3i exhibits the SEM image of fabricated silicon oxide microchips anchored to the silicon substrate. When required, the chips can be removed from the wafer by mechanical peel-off28 using a water-soluble mounting media layer and subsequently recollected in Eppendorf tubes.In order to immobilize Bdpy1 or Bdpy2, both silicon oxide surfaces (SOS) and substrate anchored micro-fabricated SOμC were first functionalized using a siloxane linker incorporating a terminal azide group capable of reacting with the alkyne group in the propargyloxy moiety of Bdpy1 or Bdpy2 (Fig. 4a) to obtain SOS-Bdpy1, SOS-Bdpy2, SOμC-Bdpy1, and SOμC-Bdpy2.
Contact angle measurements were used to evaluate the success of the functionalization of the silicon oxide surfaces. Table S1 in the ESI,† exhibits the values obtained for different surfaces, and Fig. S4 (ESI†) also shows the corresponding contact angle images. The non-functionalized silicon oxide surface (SOS) exhibited hydrophilicity with a contact angle of 39° ± 3°. After functionalization with the azide linker (SOS-N3), the contact angle increased to 90° ± 2°, indicating enhanced hydrophobicity. After conjugation, a reduced contact angle of 72° ± 3° was found for Bdpy1 conjugated surface (SOS-Bdpy1) and 68° ± 2° for Bdpy2 conjugated surface (SOS-Bdpy2), confirming successful Bdpy1 and Bdpy2 conjugation. Moreover, more contact angle reduction was observed after incubation of SOS-Bdpy1 and SOS-Bdpy2 in GSH aqueous solution (SOS-Bdpy1-GSH and SOS-Bdpy2-GSH), which were measured 54° ± 3° and 51° ± 2°, respectively, indicating more hydrophilicity of both surfaces after reacting GSH with Bdpy1 and Bdpy2 conjugated to the surface. In contrast, SOS surface, which does not have azide linker on its surface, showed minimal contact angle changes in the presence of Bdpy1 and Bdpy2 solutions, indicating adsorption is negligible.
The extent of functionalization on silicon oxide surfaces was characterised by fluorescence microscopy and images are shown in Fig. S5 (ESI†), showing limited fluorescence in the green emission for SOS and SOS-N3, while SOS-Bdpy1 displayed strong green fluorescence confirming successful Bdpy1 conjugation. Also, the images for non-functionalized silicon oxide surfaces in the presence of Bdpy1 (Fig. S5, ESI†) reveal the presence of some aggregates lacking uniform adsorption. Fluorescence images were captured from the SOS-Bdpy1 in the absence and presence of GSH (SOS-Bdpy1-GSH), as seen in Fig. S6 (ESI†), with the absence of GSH resulting in strong green emission (λem = 498–531 nm) and no red emission (λem = 565–632 nm), while in the presence of GSH, green emission decreased, and red emission appeared, indicating a reaction between GSH and Bdpy1 conjugated on the silicon oxide surface.
Laser scanning confocal microscopy (LSCM) confirmed the successful functionalization of silicon oxide microchips attached to the surface (Fig. 4) with either Bdpy1 (λex = 488 nm, λem = 498–531 nm selected according to emission Bdpy1 spectrum) or Bdpy2 (λex = 590 nm, λem = 595–630 nm selected according to emission Bdpy2 spectrum). Whereas non-functionalized silicon oxide microchips (SOμC) attached to the surface showed no fluorescence emission (Fig. S7, ESI†), both functionalized microchips SOμC-Bdpy1 and SOμC-Bdpy2 still attached to the surface exhibited strong fluorescence emission (Fig. 4b and c), which is clearly reflected by their fluorescence intensity profiles as well as 3D diagrams (Fig. 4c–g).
A mounting medium, which solidifies at the room temperature, was employed to mechanically remove SOμC from the wafer28 and release them into aqueous suspension, as depicted in Scheme S2 in the ESI.†Fig. 4h and i display fluorescence microscopy images of SOμC-Bdpy1 and SOμC-Bdpy2 after being released into suspension (PBS, pH = 7.4), revealing a clear fluorescence emission for both SOμC-Bdpy1 and SOμC-Bdpy2 microchips in contrast to the non-functionalized SOμC (Fig. S8 in the ESI†). These images confirm that neither conjugation to microchips nor release of microchips from wafers to suspension affects the optical properties of Bdpy1 and Bdpy2, providing reliable platforms to perform sensing experiments.
Flow cytometry was also employed to quantify SOμC-Bdpy1 in the suspension of released functionalized microchips (Fig. S9 in the ESI†). Fig. S9a and b (ESI†) display the results of flow cytometry studies related to SOμC and SOμC-Bdpy1. While Fig. S9a (ESI†) illustrates that the non-functionalized SOμC lack fluorescence, Fig. S9b (ESI†) reveals that most functionalized SOμC are highly fluorescent. From particle count in the suspension, a concentration of ca. 5000 microchips per μL (Fig. S9c, ESI†), a value aligned with the size of the wafer utilized to prepare functionalized SOμC and suggesting an almost quantitative yield for the release process of the microchips into suspension.
Using florescence microscopy, we also examined the photobleaching of fluorescently labelled microchips in suspension. The data in Fig. S9d (ESI†) illustrate that fluorescence intensity remains almost unvaried for a period of at least 14 days, and only after 21 days a moderate loose of fluorescence intensity is observed. Fig. S10 and S11 (ESI†) show the fluorescence microscopy images and 3D surface plots of SOμC-Bdpy1 and SOμC-Bdpy2, respectively, during similar time intervals. These results indicate the high photostability of the fluorophore on the surface of the chips, and their feasibility for real-time monitoring studies.
Finally, to gain quantitative surface coverage of SOμC-Bdpy1, a 1 cm2 silicon oxide piece of wafer (SOμC anchored to the surface) was functionalized as previously described, and the fluorescence intensity of the initial and final Bdpy1 solutions used for conjugation were measured (at emission wavelength of λem = 525 nm). Fig. S12 and S13 (ESI†) display the fluorescence spectra before and after surface functionalization and calibration curve, respectively, and the data acquired from spectrofluorometric measurements allowed to determine the monolayer density to be ca. 9.7 × 1015 molecules cm−2, a value consistent with previous studies.31
2.3 Sensing capability of functionalized microchips toward GSH in suspension
Fig. S14 (ESI†) shows the fluorescence microscopy images and the fluorescence intensity profiles for SOμC-Bdpy1 after 1 h to 12 h incubation in the GSH solution (λex = 488 nm, λem = 498–531 nm). As for the Bdpy1-GSH conjugates at the surface of SOμC, λex = 560 nm and λem = 565–632 nm were chosen. By increasing the time from 1 h to 12 h, the enhancement of the fluorescence intensity in λem = 565–632 nm is clearly observed with a reduction, which is also evident from the fluorescence intensity profiles. This observation results from the kinetics inherent to the GSH conjugation process away from the equilibrium, which is consistent with the nature of Bdpy1 as an irreversible GSH probe. Consequently, the number of Bdpy1-GSH conjugates at the surface of microchips increases with time resulting in higher fluorescence intensity. The time-dependent ratiometric fluorescence is also shown in Fig. S14(f) (ESI†), which results from the medium fluorescence intensity of about 200 microchips in different time intervals incubations. For SOμC-Bdpy2, the excitation and emission wavelengths were set at λex = 590 nm, λem = 595–630 nm and λex = 528 nm, λem = 530–580 nm for Bdpy2 and Bdpy2-GSH, respectively. As it can be seen in Fig. S15 (ESI†), it is clear that there is an increase in fluorescent intensity at λem = 530–580 nm, which is associated with Bdpy2-GSH, from 1 h to 6 h. However, between 6 h to 12 h, there is no considerable change in fluorescent intensity in either for Bdpy2 or the Bdpy2-GSH. The ratiometric calculations (Fig. S15(f), ESI†) also confirms the fluorescent images, leading to the conclusion that after 6 hours, the Bdpy2 molecules on the surface of microchips have reached an equilibrium state with GSH solution. By comparing the result of time dependent experiments for SOμC-Bdpy1 and SOμC-Bdpy2, as well as the ratiometric signal calculations, a comparable difference between the ratiometric values in the same time intervals is obvious which can be related to the irreversibility and reversibility nature of two different functionalized microchips which make reversible microchips more suitable for long-term detection of GSH.Concentration dependence was also studied for SOμC-Bdpy1 and SOμC-Bdpy2 microchips incubating them overnight and 24 h at 37 °C with different concentrations of GSH (1, 3, 5, 7, and 9 mM in PBS solution), and, then, were analyzed by the fluorescence microscopy (Fig. S16 and S17 in the ESI†). Fig. S16 (ESI†) shows that incrementing of GSH concentration from 1 mM to 9 mM, the fluorescence intensity in the green emission for SOμC-Bdpy1 is gradually reduced followed by the increase in red emission which is also seen by the fluorescence intensity profile plots. Fig. S16f (ESI†) also illustrates the ratiometric signal calculations of fluorescence intensity in each concentration of GSH after overnight incubation time. Fig. S16f (ESI†) shows a linear trend for the average of ratiometric calculations resulting from the microchips in each concentration of GSH. On the other hand, the experiments with 24 h of incubation (Fig. S17, ESI†) revealed increasing in ratiometric signal compared to overnight incubation time at the same concentrations of GSH for SOμC-Bdpy1.
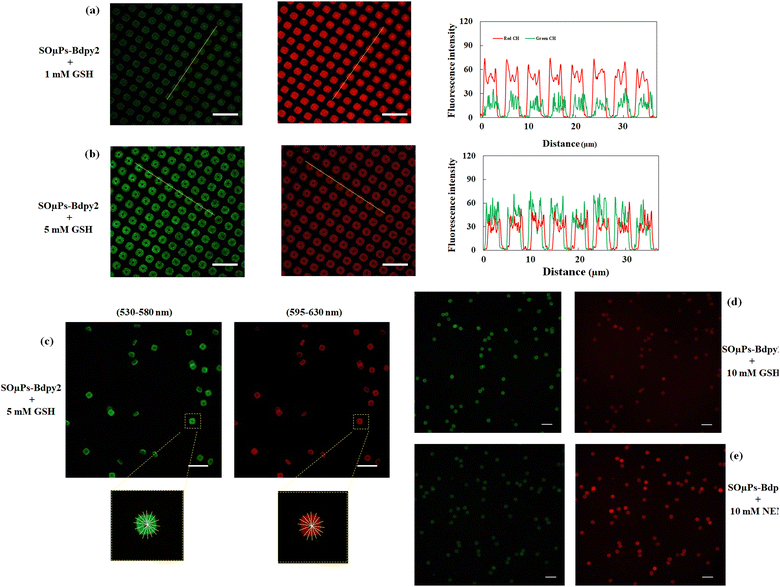
Similar experiments were performed for SOμC-Bdpy2, and Fig. 5(a) and (b) exhibit SOμC-Bdpy2 attached to surface and incubated in 1 mM and 5 mM GSH PBS solution, respectively, which the change of ratiometric fluorescent intensity in different concentrations of GSH is evident. Fig. S18 and S19 (ESI†) also show that the ratiometric fluorescence increases with concentration of GSH during overnight, and the difference between overnight and 24 h values is negligible. The results of these experiments are compatible with the irreversible and reversible nature of both fluorescent probes. For SOμC-Bdpy1, which incorporates an irreversible probe, there is no equilibrium within different time intervals at the same concentration of GSH, resulting in a change in the ratiometric fluorescent intensity over time. On the other hand, SOμC-Bdpy2, containing a reversible probe, reaches equilibrium within 12 hours, but the effect of increasing incubation time, is negligible, supporting the use of SOμC-Bdpy2 for in vitro experiments and long-term tracking of intracellular GSH.
The selectivity of SOμC-Bdpy1 and SOμC-Bdpy2 for GSH detection respect thiol containing cysteine (Cys) and Homocysteine (Hcy), possessing distinct roles in cellular function and metabolism, was also examined. For this purpose, the microchips were incubated in 1 mM solutions of either Cys or Hcy overnight. Fig. S20 (ESI†) exhibits a negligible change of fluorescence intensity indicating the high selectivity exhibited by SOμC-Bdpy1 for GSH detection. Fig. S21 (ESI†) also shows comparable results for a similar experiments using SOμC-Bdpy2. These results manifest an high selectivity of SOμC-Bdpy1 and SOμC-Bdpy2 for the GSH detection, especially taking into consideration the much higher intracellular concentration of GSH compared to the Cys and Hcy.41–43 SOμC-Bdpy1 and SOμC-Bdpy2 exhibited comparable fluorescent intensity when they are incubated in PBS alone or cell culture medium during overnight incubation time indicating that there is no negative effect on the application SOμC-Bdpy1 and SOμC-Bdpy2 in the presence of cell culture medium (Fig. S22, ESI†).
It was also necessary to validate the ratiometric sensitivity of released microchips toward GSH with the microchips detached from the surface. For this purpose, SOμC-Bdpy1 (Fig. S23, ESI†) and SOμC-Bdpy2 (Fig. 5(c)) released microchips in suspension were incubated overnight in a 5 mM GSH in culture medium, and the ratiometric signal of the released microchips was measured using the average of eight fluorescence intensity profiles across each microchip. Since the orientation of microchips in the suspension is different from the x axis projection of microscope, it is of high importance to select and focus exclusively on the surface of microchips which are in flat orientation.
The average ratiometric signal for SOμC-Bdpy1 (Fig. S23, ESI†) was ca. 1.7 ± 0.2 (ca. 50 SOμC-Bdpy1) which showed a close ratiometric consistency with the particles attached to the surface incubated in 5 mM GSH in culture medium during overnight according to Fig. S16f (ESI†). Regarding the SOμC-Bdpy2 (Fig. 5(c)), the ratiometric calculations also was ca. 1.3 ± 0.1 (ca. 50 SOμC-Bdpy2) which is comparable with the ratiometric signal calculations experiments of microchips on the surface in Fig. S18f (ESI†).
The reversibility of SOμC-Bdpy2 was also evaluated by incubating released microchips with 10 mM GSH solution (Fig. 5(d)) and then incubating them in a 10 mM NEM solution (Fig. 5(e)) while using the cell culture medium. Fig. 5(c) shows that after incubation in the NEM solution, the fluorescence of SOμC-Bdpy2 is recovered, indicating that, even though Bdpy2 has been immobilized on the surface of microchips, this molecule maintains its reversibility nature to detect GSH changes on the microchip surface even at different concentration of GSH (Fig. S23, ESI†).
2.4 Functionalized microchips performance in living cells
HeLa cells were incubated for overnight with either SOμC-Bdpy1 or SOμC-Bdpy2 to analyse their in vitro sensitivity and quantify intracellular GSH concentration in real-time. HeLa cells readily internalize the microchips and using a 0.5–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of microchip
1 ratio of microchip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
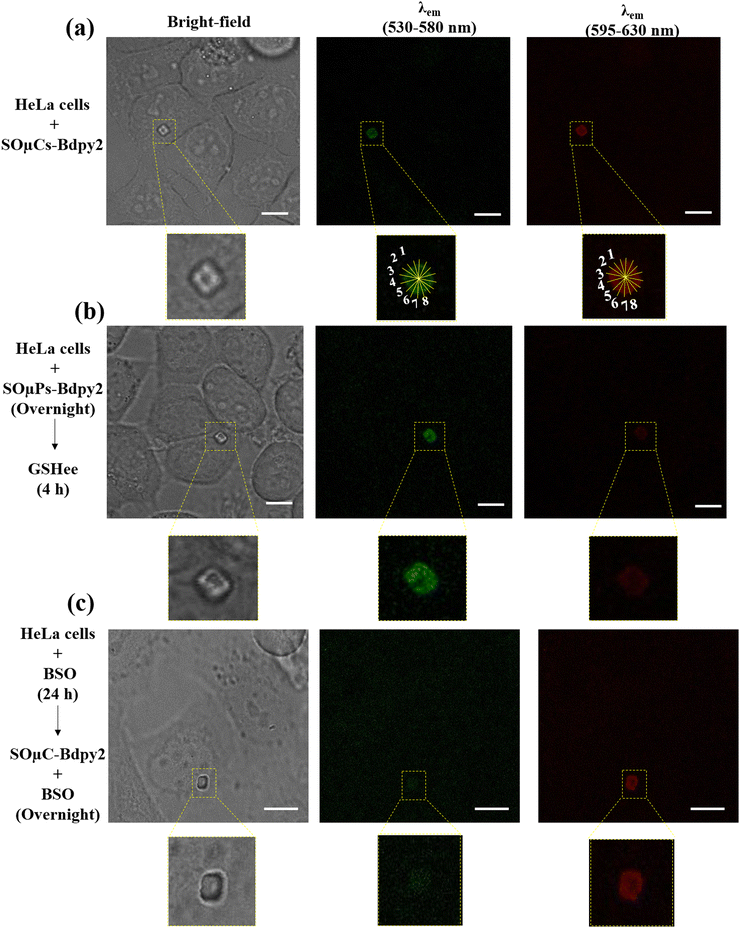
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cell, around 15% of the cells had one internalized microchip. Microchip internalization was confirmed by CLSM in living cells (Fig. 6a) as well as cell viability, always over 95%, as estimated using Cell tracker. The fluorescence intensity of internalized SOμC-Bdpy1 and SOμC-Bdpy2 was quantified to assess intracellular GSH concentration. To estimate the GSH concentration in HeLa cells, the ratiometric signal of the internalized SOμC-Bdpy1 and SOμC-Bdpy2 was measured applying the average of eight fluorescence intensity profiles across each microchip. The average fluorescence signal ratio for each internalized SOμC-Bdpy1 was calculated as shown in Fig. S25 (ESI†), and then, the average fluorescence signal ratio for all internalized SOμC-Bdpy1 was converted to GSH concentration by using the ratiometric curve resulting from the SOμC-Bdpy1 in the GSH solution for overnight (Fig. S16f, ESI†). These calculations manifested that the internalized SOμC-Bdpy1 exhibited the GSH intracellular level of ca. 3.1 ± 0.6 mM. The ratiometric calculation also indicated that ca. 73% of internalized SOμC-Bdpy1 show intracellular GSH concentration of ca. 2–3 mM while ca. 18% and 9% of internalized SOμC-Bdpy1 quantifies the intracellular GSH concentration ca. 3–4 mM and ca. 4–5.8 mM respectively (Table S3 and Fig. S27, ESI†). A similar calculation was also done for SOμC-Bdpy2 internalized in HeLa cells (Fig. 6(a) and Fig. S26 and Table S4 (ESI†)) and average ratiometric fluorescent intensity was converted to GSH concentration by applying the ratiometric curve (Fig. S18f, ESI†) of these functionalized microchips in the GSH solution. The ratiometric calculations from SOμC-Bdpy2 also indicated that the internalized microchips were able to detect intracellular GSH ca. 3.7 ± 1 mM which is more consistent with the GSH level reported in the literature44,45 and it showed that ca. 23%, ca. 45%, and ca. 32% of internalized SOμC-Bdpy2 exhibited the intracellular GSH concentration of ca. 2–3 mM, ca. 3–4 mM, and 4–5.8 mM, respectively (Table S5 and Fig. S27, ESI†). On account of the fact that the ratiometric curves resulting from SOμC-Bdpy1 and SOμC-Bdpy2 in the GSH solution are related to the same exposure time of microchips to the GSH solution for all particles, in the case of internalized microchips, the time of internalization and the period of the presence of functionalized microchips inside of the cells may be different during overnight incubation or even intracellular GSH might change during different cell states. For this reason, it could be concluded that the ratiometric curve resulting from the SOμC-Bdpy2 can be more reliable for the intracellular GSH quantification compared to the SOμC-Bdpy1. Moreover, it is concluded that SOμC-Bdpy2 is a good candidate not only for quantifying GSH levels even in individual cells, which is another advantage of these functionalized microchips, but also for distinguishing GSH levels between different cells. HeLa cells were also incubated with SOμC-Bdpy2 for 48 h, and as it can be seen in Fig. S28 (ESI†), the result of the ratiometric calculation signal is ca. 0.96 ± 0.07 (3.8 ± 0.3 mM) which is comparable with the result of internalized SOμC-Bdpy2 during overnight incubation time, demonstrating the potentiality of SOμC-Bdpy2 to yield reliable GSH measurements during different time interval and long-term tracking of intracellular GSH. The sensitivity and reversibility of SOμC-Bdpy2 was also studied toward the variations in GSH concentrations in living cells. For this purpose, living cells were incubated with SOμC-Bdpy2 overnight followed by incubation with 10 mM glutathione ethyl ester (GSHee), which is a cell-permeable derivative of GSH,46 for 4 h (Fig. 6(b) and Fig. S29a, ESI†) which exhibited the increment of the ratiometric fluorescent intensity (ca. 2.4 ± 0.3) which was more than this value for cells in the control condition (ca. 0.92 ± 0.2). The performance of SOμC-Bdpy2 in lower intracellular GSH level was also studied with the addition of NEM, which is a GSH scavenger. The difficulty for working with NEM in the biological environment is that the cells cannot tolerate in high concentration of NEM for 4 h incubation even though the reduction in the ratiometric signal is evident as it can be seen in Fig. S29(b) (ESI†). For this reason, living cells were incubated 24 h with 1 mM buthionine sulphoximine (BSO), which reduces levels of glutathione in living cells,47 and then were incubated with SOμC-Bdpy2 during overnight in presence of 1 mM of BSO. As it can be seen in Fig. 6(c), the fluorescence signal in the λem = 595–630 nm is considerably higher than λem = 530–580 nm resulting in the lower ratiometric signal compared to the internalized SOμC-Bdpy2 in the control condition. In this experiment, the fluorescent signal recovery in the range of λem = 530–580 nm was also confirmed by the subsequent addition of GSHee (10 mM, 4 hours of incubation time), as shown in Fig. S30 (ESI†). To investigate the performance of SOμC-Bdpy2 for long-term tracking, HeLa cells were also incubated with SOμC-Bdpy2 for overnight followed by incubation with BSO in the presence of SOμC-Bdpy2 for overnight (Fig. S31(a), ESI†) and subsequent incubation with GSHee for 4 h (Fig. S31(b), ESI†). Fig. S31 (ESI†) exhibits the change in ratiometric signals under these conditions, indicating the flexibility of SOμC-Bdpy2 (changing the ratiometric signal with the increment and reduction of GSH concentration) for long-term tracking of GSH levels in living cells.
cell, around 15% of the cells had one internalized microchip. Microchip internalization was confirmed by CLSM in living cells (Fig. 6a) as well as cell viability, always over 95%, as estimated using Cell tracker. The fluorescence intensity of internalized SOμC-Bdpy1 and SOμC-Bdpy2 was quantified to assess intracellular GSH concentration. To estimate the GSH concentration in HeLa cells, the ratiometric signal of the internalized SOμC-Bdpy1 and SOμC-Bdpy2 was measured applying the average of eight fluorescence intensity profiles across each microchip. The average fluorescence signal ratio for each internalized SOμC-Bdpy1 was calculated as shown in Fig. S25 (ESI†), and then, the average fluorescence signal ratio for all internalized SOμC-Bdpy1 was converted to GSH concentration by using the ratiometric curve resulting from the SOμC-Bdpy1 in the GSH solution for overnight (Fig. S16f, ESI†). These calculations manifested that the internalized SOμC-Bdpy1 exhibited the GSH intracellular level of ca. 3.1 ± 0.6 mM. The ratiometric calculation also indicated that ca. 73% of internalized SOμC-Bdpy1 show intracellular GSH concentration of ca. 2–3 mM while ca. 18% and 9% of internalized SOμC-Bdpy1 quantifies the intracellular GSH concentration ca. 3–4 mM and ca. 4–5.8 mM respectively (Table S3 and Fig. S27, ESI†). A similar calculation was also done for SOμC-Bdpy2 internalized in HeLa cells (Fig. 6(a) and Fig. S26 and Table S4 (ESI†)) and average ratiometric fluorescent intensity was converted to GSH concentration by applying the ratiometric curve (Fig. S18f, ESI†) of these functionalized microchips in the GSH solution. The ratiometric calculations from SOμC-Bdpy2 also indicated that the internalized microchips were able to detect intracellular GSH ca. 3.7 ± 1 mM which is more consistent with the GSH level reported in the literature44,45 and it showed that ca. 23%, ca. 45%, and ca. 32% of internalized SOμC-Bdpy2 exhibited the intracellular GSH concentration of ca. 2–3 mM, ca. 3–4 mM, and 4–5.8 mM, respectively (Table S5 and Fig. S27, ESI†). On account of the fact that the ratiometric curves resulting from SOμC-Bdpy1 and SOμC-Bdpy2 in the GSH solution are related to the same exposure time of microchips to the GSH solution for all particles, in the case of internalized microchips, the time of internalization and the period of the presence of functionalized microchips inside of the cells may be different during overnight incubation or even intracellular GSH might change during different cell states. For this reason, it could be concluded that the ratiometric curve resulting from the SOμC-Bdpy2 can be more reliable for the intracellular GSH quantification compared to the SOμC-Bdpy1. Moreover, it is concluded that SOμC-Bdpy2 is a good candidate not only for quantifying GSH levels even in individual cells, which is another advantage of these functionalized microchips, but also for distinguishing GSH levels between different cells. HeLa cells were also incubated with SOμC-Bdpy2 for 48 h, and as it can be seen in Fig. S28 (ESI†), the result of the ratiometric calculation signal is ca. 0.96 ± 0.07 (3.8 ± 0.3 mM) which is comparable with the result of internalized SOμC-Bdpy2 during overnight incubation time, demonstrating the potentiality of SOμC-Bdpy2 to yield reliable GSH measurements during different time interval and long-term tracking of intracellular GSH. The sensitivity and reversibility of SOμC-Bdpy2 was also studied toward the variations in GSH concentrations in living cells. For this purpose, living cells were incubated with SOμC-Bdpy2 overnight followed by incubation with 10 mM glutathione ethyl ester (GSHee), which is a cell-permeable derivative of GSH,46 for 4 h (Fig. 6(b) and Fig. S29a, ESI†) which exhibited the increment of the ratiometric fluorescent intensity (ca. 2.4 ± 0.3) which was more than this value for cells in the control condition (ca. 0.92 ± 0.2). The performance of SOμC-Bdpy2 in lower intracellular GSH level was also studied with the addition of NEM, which is a GSH scavenger. The difficulty for working with NEM in the biological environment is that the cells cannot tolerate in high concentration of NEM for 4 h incubation even though the reduction in the ratiometric signal is evident as it can be seen in Fig. S29(b) (ESI†). For this reason, living cells were incubated 24 h with 1 mM buthionine sulphoximine (BSO), which reduces levels of glutathione in living cells,47 and then were incubated with SOμC-Bdpy2 during overnight in presence of 1 mM of BSO. As it can be seen in Fig. 6(c), the fluorescence signal in the λem = 595–630 nm is considerably higher than λem = 530–580 nm resulting in the lower ratiometric signal compared to the internalized SOμC-Bdpy2 in the control condition. In this experiment, the fluorescent signal recovery in the range of λem = 530–580 nm was also confirmed by the subsequent addition of GSHee (10 mM, 4 hours of incubation time), as shown in Fig. S30 (ESI†). To investigate the performance of SOμC-Bdpy2 for long-term tracking, HeLa cells were also incubated with SOμC-Bdpy2 for overnight followed by incubation with BSO in the presence of SOμC-Bdpy2 for overnight (Fig. S31(a), ESI†) and subsequent incubation with GSHee for 4 h (Fig. S31(b), ESI†). Fig. S31 (ESI†) exhibits the change in ratiometric signals under these conditions, indicating the flexibility of SOμC-Bdpy2 (changing the ratiometric signal with the increment and reduction of GSH concentration) for long-term tracking of GSH levels in living cells.
These results point out that SOμC-Bdpy2 can be used as hybrid micromaterial for real time intracellular GSH sensing, providing more reliable results compared to SOμC-Bdpy1. Moreover, SOμC-Bdpy2 can be considered as advantageous alternative to organic fluorescent probes for the quantitative calculation of intracellular GSH for various reasons, since (a) they are compatible with aqueous suspension, thus avoiding the use of organic solvents often required to solubilize molecular probes, (b) the precise shape and size of these micro-fabricated microchips, which can be identified by fluorescence microscopy, can eliminate the difficulties related to disturbance by background auto-fluorescence from biomolecules in living systems, (c) they are biocompatible, and (d) allow intracellular measurements not only of cell populations, but of individual cells. Overall, these microchips can provide new opportunities for long-term tracking of biomolecules in living systems, which organic fluorescence probes are unable to perform.
Compared to all previous works that have utilized silicon-based materials for GSH sensing,23 the current study introduces a significant advancement by incorporating a reversible sensing system. This innovative feature not only enhances the practicality of the sensor but also enables dynamic, real-time monitoring of intracellular GSH levels. Such a capability is particularly valuable in biological and medical research, as it allows for continuous tracking of GSH fluctuations within live cells, providing deeper insights into cellular processes and oxidative stress mechanisms.
3. Conclusions
Silicon oxide microchips, functionalized with synthesized Bdpy1 and Bdpy2, were developed as devices for intracellular GSH detection. Initially, Bdpy1 and Bdpy2, were designed, synthesized, and characterized using relevant techniques. The ability of Bdpy1 and Bdpy2 toward sensing GSH was confirmed through UV-vis absorption and fluorescence spectroscopies, and Bdpy2 exhibited a reversible nature toward GSH conjugation. Cuboid silicon oxide microchips were fabricated using photolithographic processes, and Bdpy1 and Bdpy2 were immobilized on their surfaces, resulting in SOμC-Bdpy1 and SOμC-Bdpy2. Fluorescence microscopy verified surface functionalization, and the capability of the functionalized microchips for GSH detection was assessed, showing sensitivity and linear ratiometric curves in the range of intracellular GSH levels (1 to 9 mM). Ratiometric signal change for SOμC-Bdpy2 became less significant with prolonged incubation, making it more suitable for biological experiments. Notably, SOμC-Bdpy2 exhibited reversibility with varying GSH concentrations during addition and reduction using NEM. In vitro experiments revealed internalization of both SOμC-Bdpy1 and SOμC-Bdpy2 in living cells. Ratiometric signal calculations indicated intracellular GSH concentrations quantified by SOμC-Bdpy1 was ca. 3.1 ± 0.6 mM and for SOμC-Bdpy2 was quantified ca. 3.7 ± 1 mM. Within SOμC-Bdpy2, approximately 23% of cells were in the concentrations of 2–3 mM, and 77% were in the range of 3–5.8 mM.Consequently, SOμC-Bdpy2 offers several advantages over organic fluorescent probes for accurately measuring intracellular GSH. They are compatible with aqueous suspensions, eliminating the need for organic solvents typically required to dissolve molecular probes. In addition, the precisely defined shape and size of these micro-fabricated microchips, which can be detected using fluorescence microscopy. They are also biocompatible and enable intracellular measurements not only in cell populations but also in individual cells. These microchips present new opportunities for long-term monitoring of biomolecules in living systems, a capability that organic fluorescence probes lack.
4. Experimental
4.1 Materials and general methods
Materials and general methods are explained in the ESI.†4.2 Synthesis of Bdpy2 probe
Bdpy2 synthesis procedure is explained in the ESI,† in Section S1.4.3 SOμC microchips fabrication
100-mm-Φ silicon wafers are use as initial substrates. First, a numerical code is marked in the wafers for their identification using a diamond tip scriber. Then the wafers are cleaned. The fabrication process starts by a thermal silicon oxidation of 1 μm at 1100 °C (TS-Series V, Tempress). Then a standard photolithographic process is performed by using a stepper (Nikon i12D) and using 1.2-μm-thick positive photoresist HIPR 6512 (Fujifilm). The exposed silicon oxide layer is dry etched (AMI ETCH P-5000). Then, with the photoresist and silicon oxide masks, the underneath silicon is etched (∼2.12 μm DRIE Alcatel 601E) by using a quasi-isotropic etching recipe to define the pillars. Once defined the structures, the photoresist is removed. Finally, the wafer is 1.5-μm-thick thermally oxidized to convert the silicon anchoring structures onto silicon oxide structures.4.4 Functionalization of silicon oxide surfaces and SOμC microchips with 11-azidoundecyltriethoxysilane
A solution of 11-azidoundecyltriethoxysilane (3.5 × 10−3 mmol, 1.1 mg) in ethanol (1 mL) was prepared. The silicon oxide surface and wafer containing SOμC with an area of 2 cm2 was immersed in the prepared solution. The reaction continued overnight with a slight agitation (150 rpm) at room temperature. Then, the samples were rinsed with absolute ethanol three times and dried using nitrogen.4.5 Conjugation of Bdpy1 or Bdpy2 to linker functionalized surface or microchips anchored to the surface and release of microchips from surfaces
In order to immobilize Bdpy1 or Bdpy2 surface or SOμC using the click chemistry reaction, the proportions of compound Bdpy1 or Bdpy2, CuSO4, and sodium ascorbate were 1, 0.1, and 1, respectively. For this purpose, 1 mL solution (DMF(8)/H2O(2)), containing 0.2 mM of Bdpy1, 0.02 mM CuSO4, and 0.2 mM sodium ascorbate, was used. For preparing this solution, first stock solutions of Bdpy1 or Bdpy2 (0.25 mM) in DMF, CuSO4 (0.2 mM) in Milli-Q water, and sodium ascorbate (2 mM) in Milli-Q water were prepared. Then, the functionalized surface with liker and functionalized microchips with linker anchored to the surface was immersed in 0.8 mL of the prepared compound Bdpy1 or Bdpy2 solution. Afterward, 0.1 mL of CuSO4 stock solution was added to the solution followed by slight agitation under dark. Then, 0.1 mL of the sodium ascorbate stock solution was added to the reaction solution. The reaction was continued overnight at room temperature. The surface was rinsed with a solvent with the same proportion of the reaction solvent (DMF(8)/H2O(2)) to remove unreacted Bdpy1 or Bdpy2, CuSO4, and sodium ascorbate from the surface. Then, the surface washed with DCM to remove the unreacted Bdpy1 and Bdpy2 completely from the surface and dried using nitrogen.In order to release microchips from the wafer, a sufficient volume of Fluoromount™ aqueous mounting media (Fluoromount, Thermo-Fischer) was placed on top of the substrate. Then, the Fluoromount™ was solidified overnight at room temperature. Afterward, the solidified Fluoromount™ containing microchips was separated from the silicon substrate and, next, dissolved in Mili-Q water. The suspension was centrifuged (5 min and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) in order to remove the supernatant.
000 rpm) in order to remove the supernatant.
4.6 Microchips characterization with CLSM
To acquire the confocal laser scanning microscopy (CLSM) images of microchips A LEICA TCS SP8 STED 3X (Leica Microsystems GmbH) as used. The acquisition conditions for SOμC-Bdpy1 were in λex = 488 nm, λem = 498–531 nm, and for SOμC-Bdpy1-GSH were in λex = 560 nm, λem = 565–632 nm. The acquisition conditions for SOμC-Bdpy2 were also in λex = 528 nm, λem = 530–580 nm and for SOμC-Bdpy2-GSH were in (λex = 590 nm, λem = 595–630 nm).To study the capability of SOμC-Bdpy1 and SOμC-Bdpy2, as well as calculating the ratiometric signals, SOμC-Bdpy1 and SOμC-Bdpy2 were incubated in GSH solution (PBS, pH = 7.4, 37 °C) during different time intervals and GSH concentration. The selectivity experiments toward Cys and Hcy were also performed using Cys and Hcy solutions (1 mM, PBS, pH = 7.4, 37 °C). Moreover, SOμC-Bdpy2 were incubated with 5 or 10 mM of GSH during overnight and after remove the medium and SOμC-Bdpy2 were incubated for 4 h with 5 or 10 mM of NEM, respectively.
4.7 Cell culture conditions, incubation with microchips, and image processing
HeLa cell line (CCL-2) was provided from ATCC and was grown in standard DMEM medium supplemented with 10% FBS, 1% penicillin–streptomycin and 1% of glutamine and kept at 37 °C and 5% CO2 conditions. HeLa cells were incubated with microchips (SOμC-Bdpy1 and SOμC-Bdpy2) in glass-bottom p35 plates (Grenier) to be easily observed under the confocal microscope. Typically, 10.000 cells per cm2 were seeded and after 24 h to 48 h of growing, SOμC-Bdpy1 or SOμC-Bdpy2 were added. The ratio of microchip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cell used was 0.5
cell used was 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and incubation in a DMEM small volume was performed for overnight to improve the contact of the chip with the cells and facilitate their entry. After, more culture medium was added when it was necessary, the cells were incubated with 1 mM of BSO (L-buthionine sulphoximine, B2515; MERCK) during 48 h. Cell viability was analyzed by incubating cells with Cell Tracker Green (CFDA; Molecular Probes, Invitrogen) for 15 min at 37 °C. When stated, 10 mM of GSH (GSHee, G1404; Sigma-Aldrich) or 100 μM of NEM (N-ethylmaleimide) (NEM, 203030; ThermoScientific, Pierce) were added during 4 h before CLSM.
1 and incubation in a DMEM small volume was performed for overnight to improve the contact of the chip with the cells and facilitate their entry. After, more culture medium was added when it was necessary, the cells were incubated with 1 mM of BSO (L-buthionine sulphoximine, B2515; MERCK) during 48 h. Cell viability was analyzed by incubating cells with Cell Tracker Green (CFDA; Molecular Probes, Invitrogen) for 15 min at 37 °C. When stated, 10 mM of GSH (GSHee, G1404; Sigma-Aldrich) or 100 μM of NEM (N-ethylmaleimide) (NEM, 203030; ThermoScientific, Pierce) were added during 4 h before CLSM.
Cells were observed and CLSM images were taken under a LEICA TCS SP8 STED 3X (Leica Microsystems GmbH). The conditions of acquisition for SOμC-Bdpy1 were set in λex = 488 nm, λem = 498–531 nm and for SOμC-Bdpy1-GSH were set in λex = 560 nm, λem = 565–632 nm. For SOμC-Bdpy2, the conditions were also set in (λex = 528 nm, λem = 530–580 nm) and for SOμC-Bdpy2-GSH were set in λex = 590 nm, λem = 595–630 nm. All the images were acquired with a z-step set at 1 μm. Laser intensity was adjusted to obtain all images in the fluorescence linear range and photomultiplier tube (PMT) gain and offset were optimized so the images never showed saturated pixels. CLSM images of maximal projections were processed using the ImageJ48 software.
Author contributions
Saman Bagherpour: data curation, methodology, conceptualization, investigation, visualisation, and writing – original draft, review & editing. Patricia Vázquez: data curation, methodology, investigation, and writing – review & editing. Marta Duch and Juan Pablo Agusil: microfabrication methodology. José Antonio Plaza: methodology, investigation, funding acquisition, and writing – review & editing. Mariano Redondo-Horcajo: data curation. Teresa Suárez: methodology, investigation, and supervision. Lluïsa Pérez-García: methodology, conceptualization, investigation, visualisation, supervision, funding acquisition, and writing – original draft, review & editing.Data availability
Data and additional information is available upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Projects PID2020-115663GB-C3 and PID2023-146658NB-C32 were funded by MCIN/AEI/10.13039/501100011033 and by FEDER, EU. We also thank AGAUR (Generalitat de Catalunya) for a grant to consolidated research groups 2021 SGR 01085. S. B. thanks Generalitat de Catalunya for a predoctoral FISDUR scholarship.References
- M. Hanko, Ľ. Švorc, A. Planková and P. Mikuš, Anal. Chim. Acta, 2019, 1062, 1–27 CrossRef CAS PubMed.
- G. Wu, Y. Z. Fang, S. Yang, J. R. Lupton and N. D. Turner, J. Nutr., 2004, 134, 489–492 CrossRef CAS PubMed.
- A. Bansal and M. Celeste Simon, J. Cell Biol., 2018, 217, 2291–2298 CrossRef CAS.
- S. J. James, W. Slikker, S. Melnyk, E. New, M. Pogribna and S. Jernigan, Neurotoxicology, 2005, 26, 1–8 CrossRef CAS.
- S. C. Lu, Mol. Aspects Med., 2009, 30, 42–59 CrossRef CAS.
- C. B. Pocernich and D. A. Butterfield, Biochim. Biophys. Acta, Mol. Basis Dis., 2012, 1822, 625–630 CrossRef CAS.
- T. Homma and J. Fujii, Curr. Drug Metab., 2015, 16, 560–571 CrossRef CAS PubMed.
- X. Xia, Y. Qian and B. Shen, J. Mater. Chem. B, 2018, 6, 3023–3029 RSC.
- X.-X. Chen, L.-Y. Niu, N. Shao and Q.-Z. Yang, Anal. Chem., 2019, 91, 4301–4306 CrossRef CAS.
- H.-J. Xiang, H. P. Tham, M. D. Nguyen, S. Z. Fiona Phua, W. Q. Lim, J.-G. Liu and Y. Zhao, Chem. Commun., 2017, 53, 5220–5223 RSC.
- W. Z. Yuan, P. Lu, S. Chen, J. W. Y. Lam, Z. Wang, Y. Liu, H. S. Kwok, Y. Ma and B. Z. Tang, Adv. Mater., 2010, 22, 2159–2163 CrossRef CAS PubMed.
- J. Zhang, N. Wang, X. Ji, Y. Tao, J. Wang and W. Zhao, Chem. – Eur. J., 2020, 26, 4172–4192 CrossRef CAS.
- C. Caltagirone, A. Bettoschi, A. Garau and R. Montis, Chem. Soc. Rev., 2015, 44, 4645–4671 RSC.
- Z. G. Khan, M. R. Patil, S. N. Nangare, A. G. Patil, S. H. S. Boddu, R. S. Tade and P. O. Patil, J. Nanostruct. Chem., 2022, 12, 1053–1074 CrossRef.
- S. A. Zaidi and J. H. Shin, Anal. Methods, 2016, 8, 1745–1754 RSC.
- J. Zeng, X.-W. Hua, Y.-W. Bao and F.-G. Wu, ACS Appl. Nano Mater., 2021, 4, 6083–6092 CrossRef CAS.
- J. Zhu, J. Shen, B. Hu, L. Yang and C. Jiang, Anal. Chem., 2022, 94, 1126–1134 CrossRef CAS PubMed.
- B. Hu, J. Zhu, J. Shen, L. Yang and C. Jiang, Anal. Chem., 2022, 94, 7559–7566 CrossRef CAS.
- W. Li, J. Wang, J. Zhu and Y.-Q. Zheng, J. Mater. Chem. B, 2018, 6, 6858–6864 RSC.
- V. K. Singh, P. K. Yadav, S. Chandra, D. Bano, M. Talat and S. H. Hasan, J. Mater. Chem. B, 2018, 6, 5256–5268 RSC.
- Z. Jin, G. Xu, Y. Niu, X. Ding, Y. Han, W. Kong, Y. Fang, H. Niu and Y. Xu, J. Mater. Chem. B, 2020, 8, 3513–3518 RSC.
- T. Han, J. Yang, Y. Wang, Y. Cao, Y. Wang, H.-Y. Chen and J.-J. Zhu, Electrochim. Acta, 2021, 381, 138281 CrossRef CAS.
- S. Bagherpour and L. Pérez-García, J. Mater. Chem. B, 2024, 12, 8285–8309 RSC.
- P. Decuzzi, B. Godin, T. Tanaka, S.-Y. Lee, C. Chiappini, X. Liu and M. Ferrari, J. Controlled Release, 2010, 141, 320–327 CrossRef CAS PubMed.
- J. Key, A. L. Palange, F. Gentile, S. Aryal, C. Stigliano, D. Di Mascolo, E. De Rosa, M. Cho, Y. Lee, J. Singh and P. Decuzzi, ACS Nano, 2015, 9, 11628–11641 CrossRef CAS PubMed.
- R. Mathaes, G. Winter, A. Besheer and J. Engert, Int. J. Pharm., 2014, 465, 159–164 CrossRef CAS PubMed.
- M. I. Arjona, M. Duch, A. Hernández-Pinto, P. Vázquez, J. P. Agusil, R. Gómez-Martínez, M. Redondo-Horcajo, E. Amirthalingam, L. Pérez-García, T. Suárez and J. A. Plaza, Adv. Mater., 2022, 34, 2109581 CrossRef CAS.
- N. Torras, J. P. Agusil, P. Vázquez, M. Duch, A. M. Hernández-Pinto, J. Samitier, E. J. De La Rosa, J. Esteve, T. Suárez, L. Pérez-García and J. A. Plaza, Adv. Mater., 2016, 28, 1449–1454 CrossRef CAS PubMed.
- J. P. Agusil, N. Torras, M. Duch, J. Esteve, L. Pérez-García, J. Samitier and J. A. Plaza, Adv. Funct. Mater., 2017, 27, 1605912 CrossRef.
- E. Fernández-Rosas, R. Gómez, E. Ibañez, L. Barrios, M. Duch, J. Esteve, J. A. Plaza and C. Nogués, Biomed. Microdevices, 2010, 12, 371–379 CrossRef.
- O. Penon, S. Novo, S. Durán, E. Ibañez, C. Nogués, J. Samitier, M. Duch, J. A. Plaza and L. Pérez-García, Bioconjugate Chem., 2012, 23, 2392–2402 CrossRef CAS.
- M. E. Alea-Reyes, M. Rodrigues, A. Serrà, M. Mora, M. L. Sagristá, A. González, S. Durán, M. Duch, J. A. Plaza, E. Vallés, D. A. Russell and L. Pérez-García, RSC Adv., 2017, 7, 16963–16976 RSC.
- S. Durán, M. Duch, T. Patiño, A. Torres, O. Penon, R. Gómez-Martínez, L. Barrios, J. Esteve, C. Nogués, L. Pérez-García and J. A. Plaza, Sens. Actuators, B, 2015, 209, 212–224 CrossRef.
- G. Bruce, S. Bagherpour, M. Duch, J. A. Plaza, S. Stolnik and L. Pérez-García, ACS Biomater. Sci. Eng., 2024, 10, 5689–5700 CrossRef CAS.
- G. Bruce, S. Bagherpour, M. Duch, J. A. Plaza, S. Stolnik and L. Pérez-García, Microchim. Acta, 2024, 191, 554 CrossRef CAS.
- D. Limón, J. E. Hornick, K. Cai, Y. Beldjoudi, M. Duch, J. A. Plaza, L. Pérez-García and J. F. Stoddart, ACS Nano, 2022, 16, 5358–5375 CrossRef.
- S. Giraldo, M. E. Alea-Reyes, D. Limón, A. González, M. Duch, J. A. Plaza, D. Ramos-López, J. de Lapuente, A. González-Campo and L. Pérez-García, J. Pharm., 2020, 12, 1–19 Search PubMed.
- S. Bagherpour, P. Vázquez, M. Redondo-Horcajo, T. Suárez, J. A. Plaza and L. Pérez-García, Part. Part. Syst. Charact., 2024, 41, 2400134 CrossRef CAS.
- H. Liu, W. Song, S. Zhang, K. S. Chan, Z. Guo and Z. Shen, Chem. Sci., 2020, 11, 8495–8501 RSC.
- E. H. Krenske, R. C. Petter and K. N. Houk, J. Org. Chem., 2016, 81, 11726–11733 CrossRef CAS PubMed.
- T. Hasan, R. Arora, A. K. Bansal, R. Bhattacharya, G. S. Sharma and L. R. Singh, Exp. Mol. Med., 2019, 51, 1–13 CrossRef CAS.
- A. H. Hainsworth, N. E. Yeo, E. M. Weekman and D. M. Wilcock, Biochim. Biophys. Acta, Mol. Basis Dis., 2016, 1862, 1008–1017 CrossRef CAS PubMed.
- H. Song, J. Zhang, X. Wang, Y. Zhou, C. Xu, X. Pang and X. Peng, Sens. Actuators, B, 2018, 259, 233–240 CrossRef CAS.
- K. Umezawa, M. Yoshida, M. Kamiya, T. Yamasoba and Y. Urano, Nat. Chem., 2017, 9, 279–286 CrossRef CAS.
- X. Jiang, Y. Yu, J. Chen, M. Zhao, H. Chen, X. Song, A. J. Matzuk, S. L. Carroll, X. Tan, A. Sizovs, N. Cheng, M. C. Wang and J. Wang, ACS Chem. Biol., 2015, 10, 864–874 CrossRef CAS PubMed.
- F. Magata, A. Ideta, F. Matsuda, M. Urakawa and Y. Oono, Theriogenology, 2021, 167, 37–43 CrossRef CAS.
- B. Zechmann, M. Müller and G. Zellnig, Protoplasma, 2006, 227, 197–209 CrossRef CAS.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, Nat. Methods, 2012, 9, 671–675 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01859a |
| This journal is © The Royal Society of Chemistry 2025 |