Large magnetic anisotropy and a rotating cryomagnetocaloric effect in single-crystalline paramagnetic lanthanide calcium oxyborates LnCa4O(BO3)3 with Ln = Pr, Nd, Gd, Er, and Tm†
Fatiha
Azrour
a,
Romain
Viennois
 *a,
Jérôme
Long
*a,
Jérôme
Long
 ab,
Corine
Reibel
a,
Jérôme
Debray
c,
Fapeng
Yu
ab,
Corine
Reibel
a,
Jérôme
Debray
c,
Fapeng
Yu
 d,
Shujun
Zhang
d,
Shujun
Zhang
 e,
Mickaël
Beaudhuin
e,
Mickaël
Beaudhuin
 a and
Jérôme
Rouquette
a
a and
Jérôme
Rouquette
a
aICGM, Univ Montpellier, CNRS, ENSCM, Montpellier, France. E-mail: romain.viennois@umontpellier.fr; mickael.beaudhuin@umontpellier.fr; jerome.rouquette@umontpellier.fr
bInstitut Universitaire de France (IUF), 1 rue Descartes, Paris Cedex 05, 75231, France
cInstitut Néel, CNRS and Université Grenoble Alpes, BP166, Grenoble Cedex 9, F-38042, France
dState Key Laboratory of Crystal Materials, Shandong University, Jinan, China
eInstitute for Superconducting and Electronic Materials, Faculty of Engineering and Information Science, University of Wollongong, North Wollongong, Australia
First published on 12th November 2024
Abstract
Lanthanide calcium oxyborate LnCa4O(BO3)3 compounds form a family of multifunctional materials with promising nonlinear and linear optical properties and piezoelectric properties because of their polar crystal structure. In the present work, we report their anisotropic magnetic properties through the study of single-crystalline lanthanide calcium oxyborate LnCa4O(BO3)3 samples with Ln = Pr, Nd, Gd, Er and Tm. A strong magnetic anisotropy was observed, mostly originating from the strong single-ion anisotropy and varies significantly from one magnetic Ln3+ ion to another. This causes a large rotating magnetocaloric effect (RMCE) in the helium liquid temperature range for the Nd, Er and Tm compounds. For the Nd and Er compound, the RMCE is larger than the polycrystalline MCE up to the largest investigated magnetic field, 7 T, whereas the RMCE is larger than the polycrystalline value below 4 T for the Tm and Er compounds. The RMCE of the Er compound also exceeds any polycrystalline MCE in this compound family below 3 T. This shows the exceptional potential of Er-based LnCOB for RMCE applications in the liquid He temperature range.
1. Introduction
Borates, encompassing a vast array of crystalline and glassy materials with diverse B–O motifs, have emerged as a prominent material class with applications spanning various fields.1–4 Their remarkable properties include both linear and nonlinear optical behavior, making them valuable in laser and optoelectronic technologies. Additionally, they exhibit exceptional luminescence characteristics, finding use in phosphorescence and laser applications (visible and infrared).1–4 Beyond these optical properties, borates offer functionalities in flame retardancy, detergents, and even piezoelectricity.3,5Noticeably, the introduction of lanthanide ions (Ln3+) further enhances the potential of borates, particularly in laser applications.3,4 These materials also hold promise for the design of multifunctional materials, including magnetoelectric properties, as they can exhibit paramagnetism or even short-range/long-range magnetic ordering depending on the specific lanthanide element incorporated.6–18 Yet one of their limitations is their very low ordering temperatures, typically below 2 K.6–8,15
One particularly promising class of multifunctional borates is the lanthanide calcium oxyborate, LnCa4O(BO3)3 (often abbreviated as LnCOB).19–21 The intriguing aspect of LnCOB lies in the polar crystal structure belonging to the Cm space group, which paves the way for a myriad of functionalities.17–21 LnCOB materials exhibit a combination of properties, including luminescence across various ranges (infrared, visible, and ultraviolet), phosphorescence in the visible spectrum, and laser functionalities in both the visible and infrared regions.3,22–28 Furthermore, they exhibit remarkable nonlinear optical (NLO) properties3,29–33 and, crucially, retain their piezoelectric behavior even at high temperatures, a feature absent in many other materials.34–37 This unique structure is built upon two distinct types of (BO3)3− polyanions, distorted CaO6 octahedra, and distorted LnO6 octahedra, all of which assemble into 1D chains along the c-axis.17–21
Despite their diverse and promising properties, in-depth investigations into the magnetic properties of LnCOB materials, including their low-temperature heat capacity, are a relatively recent development.17,18 These studies reveal that LnCOB materials containing magnetic Ln3+ ions exhibit paramagnetic behavior, lacking long-range magnetic ordering above 2 K. A possible spin glass state has been observed in TbCa4O(BO3)3, likely arising from the quasi-1D character of the lanthanide sublattice17,18 (see also Fig. 1). Previous results were however analyzed using a mean-field approximation which could hardly consider the highly anisotropic nature of Ln3+ ions due to the presence of an unquenched orbital moment.
This work addresses these limitations by focusing on single-crystal LnCOB samples. By investigating the magnetic properties and associated anisotropy in these single crystals, we aim to obtain a more accurate and detailed understanding of the magnetic behavior in LnCOB. Understanding the anisotropic magnetic properties of LnCOB compounds is crucial not only for exploiting the potential of the rotating magnetocaloric effect (RMCE) but also for future studies on their magnetoelectric behavior. Notably, giant magnetoelectrical properties have been observed in another family of paramagnetic lanthanide borates with noncentrosymetric structures, the huntites LnAl3(BO3)4.12–15
Leveraging the strong magnetocrystalline anisotropy observed in these materials, we demonstrate a significant RMCE in LnCOB systems containing lanthanide elements with an unquenched orbital moment L ≠ 0. The RMCE is a fascinating phenomenon where a cooling effect can be achieved by simply rotating the sample in a magnetic field. The concept of RMCE was first proposed in the early 1970s,38 and it gained significant traction following the discovery of the giant RMCE in NdCo5 in 2010.39
2. Experimental details
Large single crystals of LnCa4O(BO3)3 with Ln = Pr, Nd, Gd, Er, and Tm have been grown by the Czochralski method. The details of the crystal growth and the structural characterization have been reported previously.36 For each chemical composition, a piece extracted from the single crystal ingot, was ground with an agate molar pestle.Further structural characterization has been performed by single-crystal X-ray diffraction (XRD) experiments and their analysis using the SHELX code,40 notably for determining the possible site disorder of lanthanide ions on the Ca sites and of Ca ions on the lanthanide site, as suggested by several prior studies.18,20,37,41–43 For the orientation of the single-crystalline samples, we have followed the IEEE standard on piezoelectricity which defines a specific convention for relating physical axes to crystallographic axes in piezoelectric materials.44 In this convention, the Y and Z physical axes are aligned with the crystallographic b and c axes, respectively. Thus, the X physical axis is perpendicular to the YZ plane and then completes a right-handed orthogonal system. In the present study, we will therefore show the temperature variation of the magnetic susceptibility along a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β(X), b(Y), and c(Z).
β(X), b(Y), and c(Z).
The magnetic properties have been measured with an MPMS-3S SQUID magnetometer from quantum design from 1.8 K to 300 K. Magnetic susceptibility has been measured with a magnetic field of 500 Oe under zero field cooling (ZFC) and field cooling (FC) conditions. For all the samples, we do not find any difference in the magnetic susceptibility between ZFC and FC conditions. For each single-crystalline and powdered sample, several isotherm magnetizations have been measured up to 7 T from 1.8 K up to 300 K. Magnetic characterization was conducted on rectangular parallelepiped crystals oriented along the X, Y, and Z axes as previously defined. For single-crystalline samples, we have used a horizontal rotator of the M310 model in order to determine the magnetic properties along the crystallographic directions. The data were corrected from the sample holder and the diamagnetic contributions were calculated from Pascal's constants.
For estimating the magnetocaloric effect, the magnetic entropy variation per mole ΔSm has been obtained from the application of the Maxwell thermodynamic relation to magnetization:45
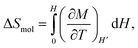 | (1) |
3. Experimental results
3.1 Crystal structure
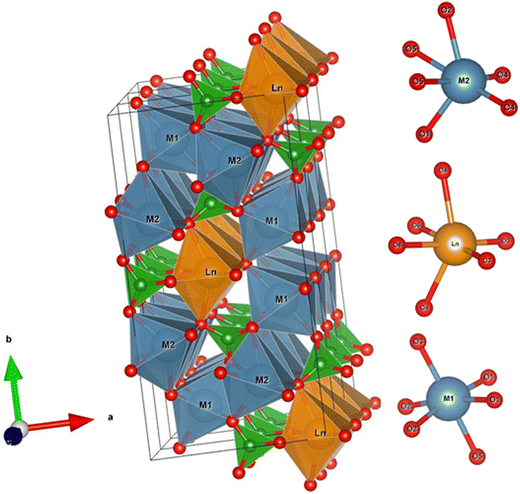
LnCOB compounds crystallize in the monoclinic space group Cm (see Fig. 1) with the general formula LnM12M22O(BO3)3. Their structure features three distinct cation sites (Ln, M1 and M2 for Ca), each coordinated by six oxygen atoms in a distorted octahedron, as can be seen in Fig. 2. These octahedra share edges, forming cationic chains that run parallel to the c-axis. Planar BO3 groups bridge these chains, oriented roughly perpendicular to the [001] direction. Typically, Ln3+ cations occupy the Ln site, while Ca2+ cations consistently fill both M1 and M2 positions. Recent studies have revealed cation disorder where Ln3+ and Ca2+ ions can interchange on the Ln and M1 sites.18,20,37,41–43 This phenomenon influences properties like electrical conductivity, thermal expansion and heat capacity.18,37,43 Interestingly, the degree of disorder depends on the size of the Ln3+ ion.18,20,37,41–43 Smaller Ln3+ cations promote a greater degree of disorder, particularly between 800 and 1100 K, likely to minimize internal stress within the crystal structure, especially along the c-axis where these cations reside.18,37,43 The crystallographic data obtained for our crystal (cif files available at the CCDC) confirm this picture as can be seen with the occupancy rate of the Ln and M1 sites given in Table 1. We determine Ln- and M1 site occupancies at 100 K and 300 K for accurate standard deviation estimation. Note that the occupancy rate of the M2 site is always 1. Notably, for the Tm compound, we found that the occupancy rate of the Ln and M1 sites is lower than that of the Er compound and higher than that of the Yb compound reported by Kelly et al.18| LnCOB | Ln | M1 | ||
|---|---|---|---|---|
| 100 K | 300 K | 100 K | 300 K | |
| Pr | 0.993 (2) | 0.985 (2) | 0.997 (12) | 0.993 (11) |
| Nd | 0.991 (2) | 0.981 (15) | 0.995 (11) | 0.999 (7) |
| Gd | 0.961 (16) | 0.959 (14) | 0.980 (8) | 0.979 (7) |
| Er | 0.803 (14) | 0.800 (10) | 0.901 (7) | 0.900 (5) |
| Tm | 0.758 (2) | 0.759 (3) | 0.879 (11) | 0.879 (14) |
As can be seen in Fig. 1 and confirmed with the interatomic distances in the different octahedra listed in Tables S1–S4 in the ESI,† the octahedra around the M1 site have the angle between the two axial O3 and O5 atoms and the M1 site only slightly smaller than 180° (about 175–177°). It is much less distorted than around the two other sites for which the same angles are 143–150° for the Ln site and 128–129° for the M2 site. This can be more clearly quantified with the distortion index for the bond lengths d (length) and the bond angle variance σ (angle)46 (see Table S6 in the ESI†). Clearly, the octahedra around the M2 site is the most distorted whereas the distortion of the octahedral around the Ln site is intermediate and decreases for smaller Ln3+ ions.
In summary, the distortion of the LnO6 octahedra decreases from the large and light lanthanide ions whereas at the same time the site disorder between the Ln3+ and the Ca2+ cations on the Ln and M1 sites increases very significantly. Both can strongly affect the anisotropic magnetic properties of LnCOB compounds.
3.2 Magnetic properties of powdered samples
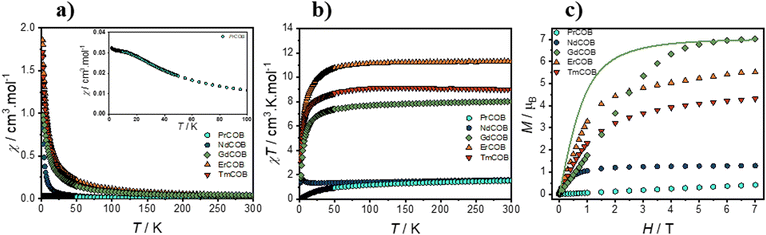
The thermal dependence of the magnetic susceptibility, χ, of powdered LnCa4O(BO3)3 (Ln = Pr, Nd, Gd, Er, and Tm) measured under 500 Oe magnetic field is shown in Fig. 2(a). The observed decrease of the magnetic susceptibility with an increase in temperature is characteristic of the paramagnetic behavior of Ln = Nd, Gd, and Er consistent with a previous report.17 Our data for Ln = Tm, which has still not been studied before (see also Fig. S1 in the ESI†), aligns with this behavior. The Pr analogue, exhibits a significant deviation at low temperature, with a broad shoulder at about 20 K as already found.17 In our sample, we notably found a minimum at about 25 K and a maximum at 5 K in dχ/dT (see Fig. S2 in the ESI†). A broad maximum at about 18 K in the magnetic contribution of Cp/T for the Pr compound was recently observed,18 which could originate from the transition between the crystal field mJ levels or the presence of some short-range ordering.In their work, Kelly and Dutton17 applied the Curie–Weiss law to a lower temperature range, which might have led to incorrect attribution of the negative Weiss constant θ values to antiferromagnetic interactions due to an isotropic approximation. Instead, to reveal the deviation from this behaviour, χT vs. T was plotted as shown in Fig. 2(b). As can be seen in Table 2, at room temperature (300 K), the χT values are in good agreement with the expected values for a single Ln3+ ion considering the free-ion approximation.47 Upon cooling, χT remains constant down to 50–100 K, before decreasing for lower temperatures for Ln = Pr, Er and Tm. This behaviour reflects the thermal depopulation of the crystal-field levels, possibly along with the presence of antiferromagnetic interactions. In this context, the absence of 1st order spin–orbit coupling for the Gd analogue makes it particularly interesting to gain insights into the nature of the magnetic interactions. Therefore, the negative deviation of χT observed below 50 K for the Gd compound suggests the presence of dominant antiferromagnetic interactions. On the other hand, the Nd compound exhibits a distinct behaviour with respect to the other materials. χT shows a maximum at around 4 K before decreasing at lower temperatures. The presence of this maximum might indicate the presence of dominant ferromagnetic interactions between the Nd3+ ions.
| LnCOB (Powder) | χT (in cm3 K mol−1) at 300 K |
M
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T (μB) T (μB) |
|---|---|---|
| PrCOB | 1.51 (1.60) | 0.42 (3.2) |
| NdCOB | 1.59 (1.64) | 1.33 (3.27) |
| GdCOB | 8.02 (7.13) | 7.02 (7) |
| ErCOB | 11.31 (11.48) | 5.51 (9) |
| TmCOB | 9.00 (7.15) | 4.39 (7) |
Fig. 2(c) shows the field dependence of magnetization for powdered LnCa4O(BO3)3 (Ln = Pr, Nd, Gd, Tm, and Er) at 1.8 K. Under a 70 kOe field, the measured magnetization values are 0.42μB, 1.28μB, 7.02μB, 4.31μB, and 5.51μB for Pr, Nd, Gd, Tm, and Er, respectively. These values agree well with previous report17 for Pr, Nd, Gd, and Er. As expected, the magnetization value for the Gd compound agrees with the value of 7.00μB expected for a single-ion value, due to the absence of orbital moment. Yet, comparison with the Brillouin function for a spin-only system (g = 2.00, S = 7/2) reveals that the experimental curve for Gd deviates from the Brillouin function at low magnetic fields. This reinforces the occurrence of antiferromagnetic interactions between the spin carriers.
In contrast, the observed magnetization values for Pr, Nd, Tm, and Er are lower than the values expected from single-ion calculations using the Landé g-factor (gJ)47 and the total angular momentum quantum number (J). This reflects the influence of single-ion anisotropy and crystal field effects on the overall magnetization behavior in these materials.
For all samples, no sign of opening of hysteresis loops could be detected indicating the absence of short- or long-range magnetic ordering.
3.3 Anisotropic magnetic properties of single-crystalline samples
With the aim of gaining insights into the magneto-crystalline anisotropy of these materials, we have studied the magnetic properties of LnCa4O(BO3)3 (Ln = Pr, Nd, Gd, Er, and Tm) on single-crystals along the different directions namely Ox, Oy, and Oz as previously defined according to the IEEE. The details of the sample orientation are described in the ESI.† Due to the absence of orbital moment for Gd, we focus next on the samples exhibiting substantial magnetic anisotropy (Pr, Nd, Er, and Tm). We observed that within a crystallographic plane, the magnetic susceptibility exhibits an oscillatory behaviour with the rotation angle and with maximum and minimum magnetic susceptibility along different crystallographic directions (Fig. S3 in the ESI†). Notably, the angular dependence is distinct for each compound, suggesting unique magnetic anisotropy for each compound, likely arising from the specific interactions of the Ln3+ ions with the crystal field. This behaviour could be explained by a crystal field model, as calculated fifty years ago in the general case,48–50 limited to the high temperature first term varying linearly with the magnetic field and with the reciprocal temperature. In this case, the anisotropy of the magnetic susceptibility at high temperature is determined only by the lowest order terms of the crystal electric field (i.e. the quadratic terms).48–50To further confirm the magnetic anisotropy, the temperature dependence of the magnetic susceptibility in Fig. 3, the χT product and the isothermal magnetization at 1.8 K are shown in Fig. 4 and 5 along the different crystallographic directions of single-crystalline LnCa4O(BO3)3 (Ln = Pr, Nd, Er, and Tm) samples. The values of χT at 300 K, μeff and of the magnetization at 7 T and 1.8 K are reported in Tables S6–S8 in the ESI.† All these measurements confirm the highly anisotropic magnetic properties of these four compounds. In contrast and as expected, the Gd-based sample exhibits a negligible dependence of the magnetic properties along different directions (see Fig. S4 of the ESI†), consistent with the isotropic character of the Gd3+ ion.
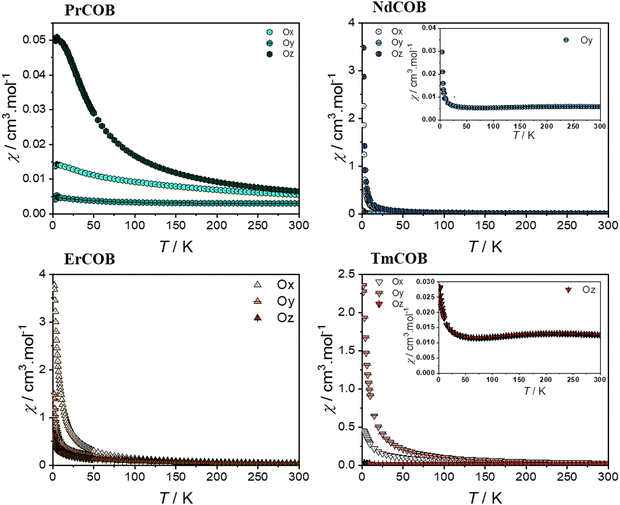 | ||
| Fig. 3 ZFC magnetic susceptibility χ of single-crystalline LnCa4O(BO3)3 (Ln = Pr, Nd, Er, and Tm) measured at 500 Oe along the different directions Ox, Oy and Oz as a function of the temperature. | ||
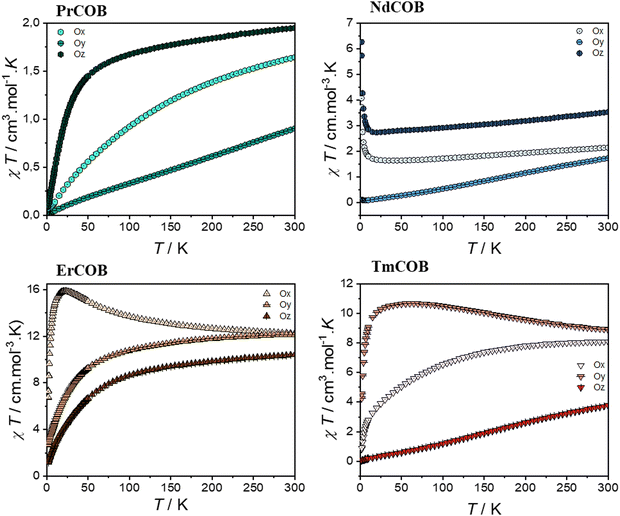 | ||
| Fig. 4 ZFC χT product of single-crystalline LnCa4O(BO3)3 (Ln = Pr, Nd, Er, and Tm) along the different directions Ox, Oy and Oz as a function of the temperature. | ||
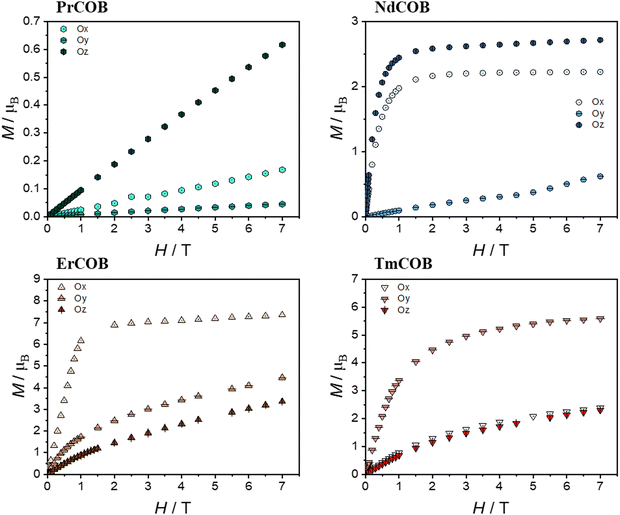 | ||
| Fig. 5 Isothermal magnetization of single-crystalline LnCa4O(BO3)3 (Ln = Pr, Nd, Er, and Tm) along the different directions Ox, Oy and Oz at 1.8 K as a function of the magnetic field. | ||
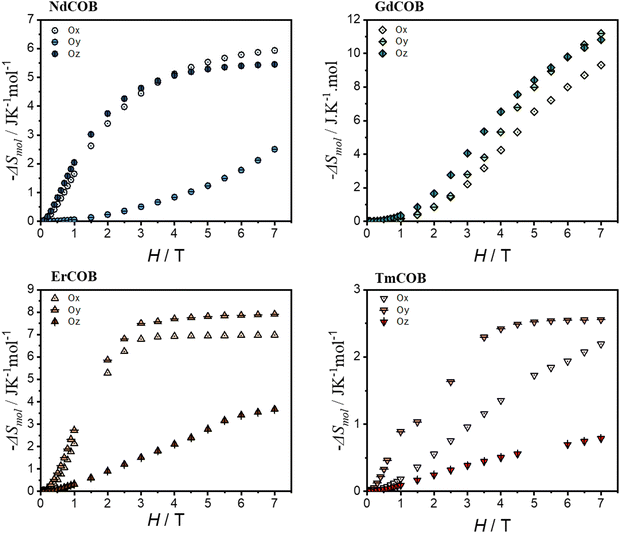
In the case of light lanthanide ions Pr3+ and Nd3+, both the magnetic susceptibility and the magnetization at 1.8 K are largest along Oz and smallest along Oy. Notably, Nd3+ is a Kramers ion (4I9/2 ground state), whereas Pr3+ is a non-Kramers ion (3H4 ground state). Both these Ln3+ ions possess an oblate 4f electron density for their largest ±mJ states.
In the case of the Nd sample, the reciprocal magnetic susceptibility varies quasi-linearly with the temperature along the Ox and Oz directions (as can be seen in Fig. S6 of the ESI†). In contrast, along the Oy direction, the magnetic susceptibility has a broad maximum at about 245 K and decreases with the temperature down to a minimum at 80 K and it increases very sharply down to 2 K. The magnetization of the Nd sample quasi-saturates at about 2.65μB and 2.15μB along Oz and Ox, whereas this is not the case for the Oy direction where it increases linearly up to about 5 T and then shows an inflexion point. In this last case, one observes a hysteresis of the magnetization of the butterfly type (see Fig. S6 of the ESI†). In the case of the Pr sample, the reciprocal magnetic susceptibility varies quasi-linearly with the temperature down to about 75 K along the Ox and Oz directions (see Fig. S6 of the ESI†) and then decreases more slowly with decreasing temperature up to saturating values whereas along the Oy direction it decreases slowly down to about 150 K and then faster. In addition, one can see in the derivative of the magnetic susceptibility that there is a minimum at about 25 K along the Ox and Oz direction as in the case of the powder (see Fig. S7 in the ESI†) but not along the Oy direction. For all three directions, the magnetization at 1.8 K varies linearly with the magnetic field and is far below the single-ion saturating value.
Heavy lanthanides Er3+ and Tm3+, which are both prolate ions, exhibit distinct magnetic anisotropy due to their differing ground state characteristics: Er3+ is a Kramers ion (4I15/2 ground state), while Tm3+ is a non-Kramers ion (3H6 ground state). This difference is reflected in their easiest magnetization directions: for Er3+, it is along Ox, whereas for Tm3+ it is along Oy. Notably, for both Er and Tm, the most difficult magnetization direction is along Oz. However, at 1.8 K, the magnetization of Tm is surprisingly similar along both Ox and Oz directions. The reciprocal magnetic susceptibility varies linearly with the temperature down to 50 K for all directions in the case of the Er compound and along Ox and Oy directions for the Tm compound (see Fig. S6 of the ESI†). For the latter compound, the magnetic susceptibility along the Oz direction looks very similar to that of the Nd along the Oy direction and with a maximum at about 215 K and a minimum at 75 K below which the magnetic susceptibility increases very fast with a decrease in temperature. This is also reflected in the χT vs. T plots which reveal the presence of a maximum for the Er compound along the Ox direction whereas it is found in the Oy direction for the Tm one. In the Er compound, the magnetization along the Ox direction almost saturates at 7.35μB, still below the single-ion saturating value, whereas magnetization is far from saturation along the two other directions. In the Tm compound, the magnetization along the Oy direction begins to approach saturation at 7 T with 5.6μB, still below the single-ion saturating value, whereas the magnetization is far from the saturation along the other two directions.
The shortest distance between the lanthanide ions is about 3.6 Å along the Oz direction (c axis). Thus, we do not expect that the magnetic exchange interaction can explain the very large magnetic anisotropy observed in LnCa4O(BO3)3 compounds, although the dipolar interactions might play a role. The Oz direction corresponds to the easy magnetization direction for the oblate Pr and Nd-based compounds. However, for the prolate lanthanide Er and Tm, the easy magnetization axis is along a direction perpendicular to the Oz direction. As we already mentioned before, the angular dependence of the magnetic susceptibility at room temperature is similar to that expected from general crystal field theory.48–50 This theory also predicts that in low-symmetry paramagnetic lanthanide compounds, the magnetic anisotropy is due to both magnetic interactions and the crystal electric field effect.48–51
In principle, it could be possible to determine the lowest order crystal field parameters (i.e. the crystal field parameters B02 and B22) in low symmetry systems from the temperature dependence of the magnetic susceptibility at high temperature in all the different crystallographic directions when it varies linearly with the reciprocal temperature.48–53 However, because of the site disorder, the Ln3+ ions are in two different crystallographic sites, making such a type of analysis of our samples impossible. At intermediate and low temperatures, the thermal variation of the magnetic susceptibility will depend also on higher order crystal field parameters. At low temperature, the magnetic anisotropy is related to the population of the mJ states which are highly anisotropic.
3.4 Magnetocaloric effect
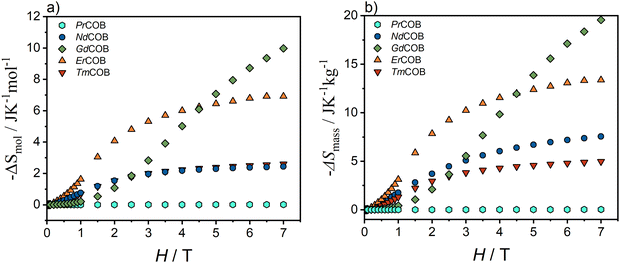
We investigated the MCE in both powdered and single-crystalline samples (Ln = Pr, Nd, Gd, Er, and Tm). The magnetization data as a function of temperature for these samples is provided in the ESI† (Fig. S8–S12). From these data, we have determined the magnetic molar entropy ΔSmol using the Maxwell thermodynamic relation to the magnetization (1). We report both the variation of the magnetic molar entropy ΔSmol and magnetic mass entropy ΔSmass between 1.8 K and 5 K for the powdered LnCa4O(BO3)3 samples for Ln = Nd, Pr, Gd, Er and Tm in Fig. 6. It turns out that the Er sample exhibits the largest magnetic entropy below 4.5 T whereas the Gd sample has the largest magnetic entropy above 4.5 T. As expected from the linear magnetization curves, the Pr sample has negligible magnetic entropy. We find smaller magnetic entropy for the Gd and Nd samples and larger magnetic entropy for the Er sample than that reported previously.17 The Tm sample has similar molar magnetic entropy to the Nd sample but smaller mass magnetic entropy than the Nd sample. One can compare our results with the magnetic entropy of Gd3Ga5O12,54–56 which is historically the most used magnetocaloric material in the development of magnetocaloric refrigerators at cryogenic temperatures.57 The magnetic molar entropy ΔSmol of the Er compound is larger than that of Gd3Ga5O12 and Dy3Ga5O12 up to about 3 T54–56 but the magnetic mass entropy ΔSmass of the Er compound is larger than that of Gd3Ga5O12 only below 1.5 T whereas it is smaller than that of Dy3Ga5O12 up to at least 5 T.Due to the highly anisotropic magnetic properties observed in single-crystalline LnCa4O(BO3)3 compounds (as discussed previously), they are promising candidates for the rotating magnetocaloric effect (RMCE). This effect allows for the manipulation of the temperature change by rotating an applied magnetic field relative to the crystallographic axes. To investigate this potential, we determined the molar magnetic entropy change (ΔSmol) along the different crystallographic directions for these single crystals. The results presented in Fig. 7 correspond to the magnetic entropy change between 1.8 K and 5 K. We did not report the magnetic entropy change of the single-crystalline Pr sample because it is very small, as in the case of the powdered Pr sample. Because at low temperature there is weak magnetic anisotropy in the case of the single-crystalline Gd sample, we also report its magnetic entropy change along the different directions in Fig. 7.
One can see that the molar magnetic entropy ΔSmol of the Nd compound is much larger along the Ox and Oz directions than along the Oy direction as well as larger than the polycrystalline value in Fig. 6. In a similar way, the molar magnetic entropy ΔSmol of the Er compound is much larger along the Ox and Oy directions than along the Oz direction as well as larger than the polycrystalline value in Fig. 6. Below 4 T, it is also larger than the polycrystalline value for the HoCa4O(BO3)3 compound.17 In the case of the Tm compound, the molar magnetic entropy ΔSmol is less anisotropic and larger in the Oy direction than in the Ox and Oz directions and only slightly larger than the polycrystalline value in Fig. 6. Finally in the case of the Gd compound, the molar magnetic entropy ΔSmol has weak anisotropy and slightly larger in the Oy and Oz direction than in the Ox direction. The polycrystalline value falls between these values.
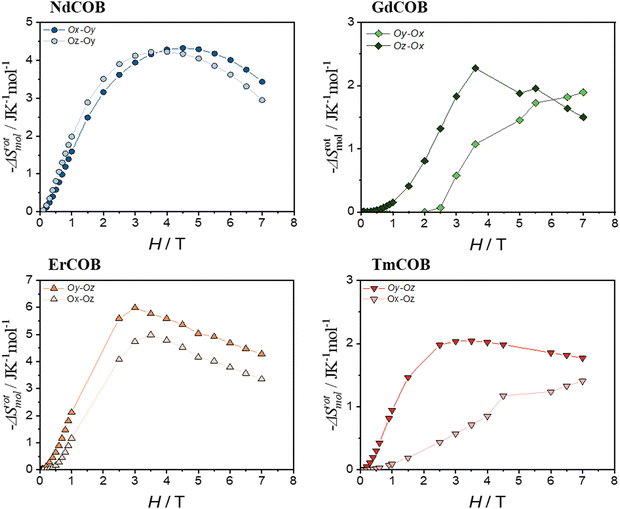
Building upon the observed anisotropy, we investigated the potential for the rotating magnetocaloric effect (RMCE) in these materials. Fig. 8 presents the rotating molar magnetic entropy change ΔSrotmol between the easy and hard magnetization directions for each LnCa4O(BO3)3 sample.
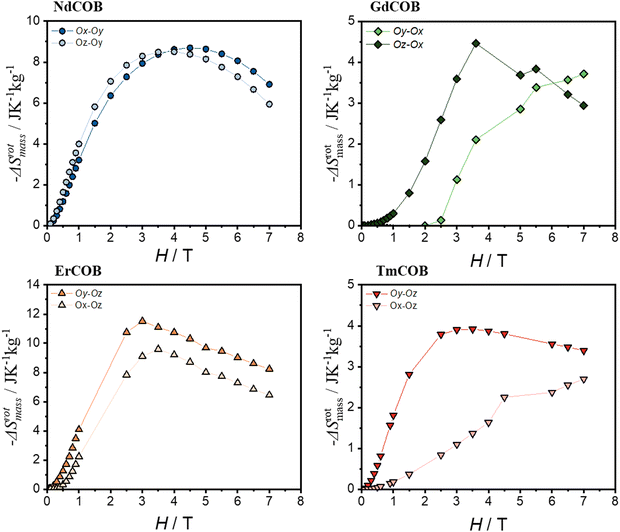
Fig. 8 demonstrates the remarkable potential of these single-crystalline LnCa4O(BO3)3 samples for the RMCE. The rotating molar magnetic entropy change (ΔSrotmol) between the easy and hard magnetization directions reaches a maximum of around 3–5 T for the three compounds Nd, Er, and Tm. The calculated change in magnetic entropy upon rotating the magnetic field ΔSrotmol significantly surpasses the values achievable in polycrystalline samples (ΔSpolymol) across the entire magnetic field range for the Nd compound. For Er and Tm, ΔSrotmol exceeds the polycrystalline magnetic molar entropy ΔSmol at lower field (below 3–4 T), particularly when rotating the field between the directions where magnetization is hardest (Oy and Oz). The Er compound shows the strongest RMCE overall, with ΔSrotmol, exceeding even the polycrystalline value reported for a similar material (HoOCa4(BO3)3) at low magnetic fields.17 These findings highlight the significant advantages of single-crystalline LnOCa4(BO3)3 for RMCE applications. In contrast, for the Gd compound, the rotating molar magnetic entropy change (ΔSrotmol) is as large as in the case of the Tm compound, but is much smaller than the polycrystalline value. In Fig. 9, we report the variation of the rotating magnetic mass entropy ΔSrotmass. This will permit us to compare it with other compounds with a RMCE.
We also focus here on the low field behaviour, which could be relevant for some future applications. Our Nd and Er samples exhibit a significant rotating magnetic entropy change (−ΔSrotmass) at 1 T, exceeding values reported for benchmark materials like NdCo5 (1.5 J K−1 kg−1) and GdCo5 (3 J K−1 kg−1) at room temperature (a giant RMCE reported in ref. 39). At 3 T, the −ΔSrotmol values for our samples are comparable or even larger than DyAl2 (4.2 J K−1 mol−1) at 24.4 K and at 2 T, but DyAl2 exhibits a higher −ΔSrotmass (19.4 J K−1 kg−1).39 While most studies report peak −ΔSrotmassabove 5 K,58–60 our work demonstrates significant −ΔSrotmass at liquid helium temperatures (around 2 T) for Nd and Er samples (6–7 J K−1 kg−1). This value is roughly half that of the best performing materials below 5 K. Notably, few Nd-based compounds are reported,58–60 and only the −ΔSrotmass of NdGa surpasses that of our Nd sample at 2 T, but at a much higher temperature (50 K).61 Similarly, only a handful of Er-based materials exhibit larger −ΔSrotmass at 5 T and liquid helium temperatures. Importantly, apart from paramagnetic KLn(MoO4)2 (Ln = Er and Tm),62,63 all reported compounds with large −ΔSrotmass exhibit magnetic ordering, with peak −ΔSrotmass occurring near their ordering temperature (ferromagnetic) or below (antiferromagnetic). Remarkably, our Er compound displays a larger ordering temperature than the paramagnetic KLn(MoO4)2 (Ln = Er and Tm) at fields exceeding 2.5 T and liquid helium temperatures.62,63 This highlights the unique nature of our materials as paramagnets exhibiting a large RMCE, with ErCOB being the most potent paramagnet with a large RMCE reported to date at liquid helium temperatures. Further work is required to assess their potential for practical applications, such as determining their refrigerant cooling power and the adiabatic temperature change with rotation.57,63
Exploring the anisotropic magnetic properties and RMCE in single-crystalline LnCa4O(BO3)3 compounds, particularly with Ln = Tb, Dy, and Ho, could be especially promising, as the Ho compound demonstrates substantially greater magnetocaloric effect (MCE) than the Er compound in polycrystalline form.17 These materials have only recently become available in single crystals.64–66 Although MCE research has largely focused on room-temperature applications,57,67 the RMCE at cryogenic temperatures remains a compelling area with applications in cryogenic research, scientific instruments, industrial gas storage, and space technology.68,69
Solid-state magnetic refrigeration could also present an environmentally friendly alternative to liquid helium for low-temperature cooling.17 A highly efficient magnetocaloric material effective below 2 K would reduce or even eliminate the need for He-3, which has particular appeal for space applications. Similarly, materials effective at fields below 2 T could allow for the use of permanent magnets, simplifying the cooling setup.59,61 In this regard, the RMCE could offer higher efficiency than the conventional MCE, enabling an efficient cooling device that operates simply by rotating the magnetocaloric material.
4. Conclusion
The present study explored the structural and magnetic properties and the magnetocaloric effect (MCE) of single-crystal lanthanide calcium oxyborates LnOCa4(BO3)3. Our findings unveil a strong influence of crystallographic anisotropy on the magnetic behavior of these materials, particularly their potential in the rotating magnetocaloric effect (RMCE), mostly originating from strong single-ion anisotropy.By performing magnetic measurements on single crystals, we have shed light on the interplay between the magnetic anisotropy of these compounds and the nature of the Ln3+ ion.
Building on these features, we have shown that single-crystalline LnCOB compounds exhibit significant magnetic anisotropy. This is evident in the dependence of both magnetization and magnetic entropy change (ΔSmol) on the direction of the applied magnetic field. This anisotropic behavior is reflected in the markedly different ΔSmol values observed along different crystallographic axes. Notably, single crystals exhibit a larger ΔSmol value compared to polycrystalline samples. This enhancement is particularly pronounced for the Nd compound, where the calculated rotating magnetic entropy change (ΔSrotmol) surpasses the polycrystalline value across the entire investigated magnetic field range. For Er and Tm, ΔSrotmol exceeds the polycrystalline value at lower fields (below 3–4 T), especially when rotating the field between the directions corresponding to hard magnetization. Among the investigated lanthanide elements, the Er compound demonstrates the most remarkable performance for the RMCE. Its ΔSrotmol value not only surpasses the polycrystalline value of LnOCa4(BO3)3 but also exceeds the ΔSpolymol value reported for a similar material, HoOCa4(BO3)3, at low fields. This signifies the exceptional potential of Er-based LnCOB compounds for RMCE applications. The ability to achieve a larger temperature change by simply rotating the magnetic field relative to the crystallographic axes presents significant advantages for targeted cooling and the development of more efficient magnetic refrigeration devices in the liquid He temperature range.
Data availability
Crystallographic data for LnOCa4(BO3)3 (Ln = Pr, Nd, Gd, Er and Tm) at T = 100 K have been deposited at the CCDC under deposition numbers 2372139, 2372141, 2372143, 2372144 and 2400871. The other data supporting this study are available in the published article and in its ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank the University of Montpellier, CNRS and PAC of ICGM. JL acknowledges the support from Institut Universitaire de France.References
- R. S. Bubnova and S. K. Filatov, High-temperature borate chemistry, Z. Kristallogr., 2013, 228, 395–428 CAS.
- H. Huppertz and D. A. Kezler, Borates: solid-state chemistry, Encyclopedia of Inorganic and Bioinorganic Chemistry in 2014 by John Wiley & Sons, Ltd.
- M. Mutailipu, K. R. Poeppelmeier and S. Pan, Borates: a rich source for optical material, Chem. Rev., 2021, 121, 1130–1202 CrossRef CAS.
- Y. Shen, S. Zhao and J. Luo, The role of cations in second-order nonlinear optical materials based on π-conjugated [BO3]3− groups, Coord. Chem. Rev., 2018, 366, 1–28 CrossRef CAS.
- S. J. Zhang and F. Yu, Piezoelectric materials for high temperature sensors, J. Am. Ceram. Soc., 2011, 94, 3153–3170 CrossRef CAS.
- P. Mukherjee, E. Suard and S. E. Dutton, Magnetic properties of monoclinic lanthanide metaborates, Ln(BO2)3, Ln = Pr, Nd, Gd, Tb, J. Phys.: Condens. Matter, 2017, 29, 405807 CrossRef CAS PubMed.
- P. Mukherjee, Y. Wu, G. I. Lampronti and S. E. Dutton, Magnetic properties of monoclinic lanthanide orthoborates, LnBO3, Ln = Gd, Tb, Dy, Ho, Er, Yb, Mater. Res. Bull., 2018, 98, 173–179 CrossRef CAS.
- A. Jesche, N. Winterhalter-Stocker, F. Hirschberger, A. Bellon, S. Bachus, Y. Tokiwa, A. Tsirlin and P. Gegenwart, Adiabatic demagnetization cooling well below the magnetic ordering temperature of the triangular antiferromagnet KBaGd(BO3)2, Phys. Rev. B, 2023, 107, 104402 CrossRef CAS.
- S. Guo, T. Kong and R. J. Cava, NaBaR(BO3)2 (R = Dy, Ho, Er and Tm); structurally perfect triangular lattice materials with two rare earth layers, Mater. Res. Express, 2019, 6, 106110 CrossRef CAS.
- S. Guo, T. Kong, W. Xie, L. Nguyen, K. Stolze, F. Cevallos and R. J. Cava, Triangular rare-earth lattice materials RbBaR(BO3)2 (R = Y, GD-Yb) and comparison to the KBaR(BO3)2 analogs, Inorg. Chem., 2019, 58, 3308–3315 CrossRef CAS PubMed.
- Y. Chen, W. Liu, J. Feng, R. Guo, F. Fan, J. Shen, G. Zhang and H. Tu, Magnetocaloric effect in LiLn6O5(BO3)3 (Ln = Gd, Tb, Dy, and Ho), Cryogenics, 2022, 124, 103476 CrossRef CAS.
- R. P. Chaudhury, B. Lorenz, Y. Y. Sun, L. N. Bezmaternykh, V. L. Temerov and C. W. Chu, Magnetoelectricity and magnetostriction due to the rare-earth moment in TmAl3(BO3)4, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 220402 CrossRef.
- K. C. Liang, R. P. Chaudhury, B. Lorenz, Y. Y. Sun, L. N. Bezmaternykh, I. A. Gudim, V. L. Temerov and C. W. Chu, Magnetoelectricity in the system RAl3(BO3)4 (R = Tb, Ho, Er, Tm), J. Phys.: Conf. Ser., 2012, 400, 032046 CrossRef.
- A. V. Malakhovskii, V. V. Sokolov and I. A. Gudim, Optical and magneto-optical spectra and electron structure of ErAl3(BO3)4 single crystal, J. Alloys Compd., 2017, 698, 364–374 CrossRef CAS.
- T. Zajarniuk, A. Szewczyk, P. Wisniewski, M. U. Gutowska, R. Puzniak, H. Szymczak, I. Gudim, V. A. Bedarev, M. I. Pashchenko, P. Tomczak and W. Szuszkiewicz, Quantum versus classical nature of the low-temperature magnetic phase transition in TbAl3(BO3)4, Phys. Rev. B, 2022, 105, 094418 CrossRef CAS.
- I. V. Bilych, M. P. Kolodyazhnaya, K. R. Zhekov, G. A. Zvyagina, V. D. Fil and I. A. Gudim, Elastic, magneoelastic, magnetopiezoelectric, and magnetodielectric characteristics of HoAl3(BO3)4, Low Temp. Phys., 2020, 46, 923–931 CrossRef CAS.
- N. D. Kelly and S. E. Dutton, Magnetic properties of quasi-one-dimensional lanthanide calcium oxyborate Ca4LnO(BO3)3, Inorg. Chem., 2020, 59, 9188–9195 CrossRef CAS.
- N. D. Kelly, S. Savvin and S. E. Dutton, Crystal structure and specific heat of calcium lanthanide oxyborate Ca4LnO(BO3)3, Z. Kristallogr., 2022, 237, 91317–91327 Search PubMed.
- T. N. Khamaganova, V. K. Trunov and B. F. Dzhurinskij, The crystal structure of calcium samarium oxide borate Ca8Sm2O2(BO3)6, Russ. J. Inorg. Chem., 1991, 36, 484–485 Search PubMed.
- A. B. Ilyukhin and B. F. Dzhurinskij, Crystal structures of double oxoborates LnCa4O(BO3)3 (Ln = Gd, Tb, Lu) and Eu2CaO(BO3), Russ. J. Inorg. Chem., 1993, 38, 847–850 Search PubMed.
- R. Norrestam, M. Nygren and J. O. Bovin, Structural investigations of new calcium-rare-earth (R) oxyborates with the composition Ca4RO(BO3)3, Chem. Mater., 1992, 4, 737–743 CrossRef CAS.
- W. van Schaik, M. M. E. van Heek, W. Middel and G. Blasse, Luminescence and energy migration in a one-dimensional Gd3+ compound: Ca4GdO(BO3)3, J. Lumin., 1995, 63, 103–115 CrossRef CAS.
- A. Lupei, A. Antic-Fidancev, G. Aka and D. Vien, Spectral and structural studies of GdCOB and YCOB, J. Alloys Compd., 2004, 380, 235–240 CrossRef CAS.
- H. C. Yang, C. Y. Li, H. He, Y. Tao, J. H. Xu and Q. Su, VUV-UV excited luminescent properties of LnCa4O(BO3)3:RE3+ (Ln = Y, La, Gd; RE = Eu, Tb, Dy, Ce), J. Lumin., 2006, 118, 61–69 CrossRef CAS.
- Z. Chen, Y. Pan, L. Xi, R. Pang, S. Huang and G. Liu, Tunable yellow-red photoluminescence and persistent afterglow in phosphors Ca4LaO(BO3)3:Eu3+ and Ca4EuO(BO3)3, Inorg. Chem., 2016, 55, 11249–11257 CrossRef CAS PubMed.
- H. Zhang, X. Meng, P. Wang, L. Zhu, X. Liu, R. Cheng, J. Dawes, P. Dekker, S. Zhang and L. Sun, Slope efficiency of up to 73% for Yb:Ca4YO(BO3)3 crystal laser pumped by laser diode, Appl. Phys. B: Lasers Opt., 1999, 68, 1147–1149 CrossRef CAS.
- H. Zhang, X. Meng, P. Wang, L. Zhu, X. Liu, X. Liu, Y. Yang, R. Wang, J. Dawes, J. Piper, S. Zhang and L. Sun, Growth of Yb-doped Ca4GdO(BO3)3 crystals and their spectra and laser properties, J. Cryst. Growth, 2001, 222, 209–214 CrossRef CAS.
- D. Vivien, F. Mougel, F. Augé, G. Aka, A. Kahn-Harai, F. Balembois, G. Lucas-Leclin, P. Georges, A. Brun, P. Aschehoug, J.-M. Benitez, N. Le Nain and M. Jacquet, Nd:GdCOB overview of its infrared, green and blue laser performances, Opt. Mater., 2001, 16, 213–220 CrossRef CAS.
- G. Aka, A. Kahn-Harari, D. Vivien, J.-M. Benitez, F. Salin and J. Godard, A new non-linear and neodymium laser self-frequency doubling crystal with congruent melting: Ca4GdO(BO3)3 (GdCOB), Eur. J. Solid State Inorg. Chem., 1996, 33, 727–736 CAS.
- G. Aka, A. Kahn-Harari, F. Mougel, D. Vivien, F. Salin, P. Coquelin, P. Colin, D. Pelenc and J. P. Damelet, Linear- and nonlinear-optical properties of a new gadolinium oxoborate crystal, Ca4GdO(BO3)3, J. Opt. Soc. Am. B, 1997, 14, 2238–2247 CrossRef CAS.
- M. Iwai, M. Kobayashi, H. Furuya, Y. Mori and T. Sasaki, Crystal growth and optical characterization of rare-earth (Re) calcium oxyborate ReCa4O(BO3)3 (Re = Y or Gd) as new nonlinear optical material, Jpn. J. Appl. Phys., 1997, 36, L276–L279 CrossRef CAS.
- S. Zhang, X. Cheng, J. Lu, G. Li, J. Lu, Z. Shao and H. Chen, Studies of the effective nonlinear coefficient of GdCa4O(BO3)3 crystal, J. Cryst. Growth, 1999, 205, 453–456 CrossRef CAS.
- Y. Liu, Z. Wang, F. Yu, H. Qi, X. Yang, X. Yu, X. Zhao and X. Xu, Angular non-critical phase-matching second-harmonic-generation characteristics of RECOB (Re = Tm, Y, Gd, Sm, Nd and La) crystals, Opt. Express, 2017, 25, 11867–11893 CrossRef CAS.
- H. Takeda, H. Nakao, S. Izukawa, H. Shimizu, T. Nishida, S. Okamura and T. Shiosaki, Growth and piezoelectric properties of R3Ga5SiO14 and RCa4O(BO3)3 (R = rare-earth elements) single-crystals, J. Alloys Compd., 2006, 408–412, 474–479 CrossRef CAS.
- S. Zhang, F. Yu, R. Xia, Y. Fei, E. Frantz, X. Zhao, D. Yuan, B. H. T. Chai, D. Snyder and T. R. Shrout, High temperature ReCOB piezocrystals: recent developments, J. Cryst. Growth, 2011, 318, 884–889 CrossRef CAS.
- F. Yu, D. Yuan, S. Zhang, Q. Lu and X. T. R. Zhao, Rare-earth calcium oxyborate piezoelectric crystals ReCa4O(BO3)3: growth and piezoelectric characterization, Crystals, 2014, 4, 241–261 CrossRef.
- M. Münchhalfen, J. Schreuer, C. Reuther, E. Mehner and H. Stöcker, Elastic, piezoelectric and dielectric properties of rare-earth calcium oxoborates RCa4O(BO3)3 (R =Er, Y, Sy, Gd, Sm, Nd, La), J. Appl. Phys., 2021, 130, 095102 CrossRef.
- R. Almeida, S. C. Freitas, R. C. Fernandes, R. Kiefe, J. P. Araujo, J. S. Amaral, J. O. Ventura, J. H. Belo and D. J. Silva, Rotating magnetocaloric effect in polycrystals-harnessing the demagnetizing effect, JPhys Energy, 2024, 6, 015020 CrossRef CAS.
- S. A. Nikitin, K. P. Skokov, Y. S. Koshkid’ko, Y. G. Pastushenkov and T. I. Ivanova, Giant rotating magnetocaloric effect in the region of spin-reorientation transition in the NdCo5 single crystal, Phys. Rev. Lett., 2010, 105, 137205 CrossRef CAS PubMed.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- G. M. Kuzmicheva, A. Y. Ageev, V. B. Rybakov, V. L. Panyutin, Y. M. Yu and V. I. Chizikov, Growth and x-ray diffraction study of YCa4O(BO3)3, Ce, Er, Yb crystals, Inorg. Mater., 2001, 37, 1051–1060 CrossRef CAS.
- E. L. Belokoneva, B. V. Mill and G. I. Ershova, Crystallization and crystal structure of LaCa4O(BO3)3, Russ. J. Inorg. Chem., 2003, 48, 5–7 CAS.
- M. Münchhalfen, J. Schreuer, C. Reuther and H. Stöcker, Influence of non-convergent cation ordering on thermal expansion of rare-earth calcium oxoborates RCa4O(BO3)3 (R =Er, Y, Sy, Gd, Sm, Nd, La), Materialia, 2022, 26, 101561 CrossRef.
- IEEE Standard on Piezoelectricity, An American National Standard, IEEE, New York, 1987, vol. 14 Search PubMed.
- J. S. Amaral and V. S. Amaral, On estimating the magnetocaloric effect from magnetization measurements, J. Magn. Magn. Mater., 2010, 322, 1552–1557 CrossRef CAS.
- K. Momma and F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- O. Kahn, Molecular magnetism, VCH Publishers, Inc., USA, 1993, 393 Search PubMed.
- P. Boutron, Anisotropie magnétique au-dessus du point d’ordre et paramètres d’environnement cristallin, J. Phys., 1969, 30, 413–419 CrossRef.
- P. Boutron, Exact calculation of the paramagnetic susceptibility of a single crystal with arbitrary crystal field and exchange interaction, Phys. Rev. B: Solid State, 1973, 7, 3226–3238 CrossRef.
- Y. L. Wang, Crystal field effect of paramagnetic Curie-Weiss temperature, Phys. Lett. A, 1971, 35, 383–384 CrossRef CAS.
- G. J. Bowden, D. S. P. Bunbury and M. A. H. McCausland, Crystal field and magnetic anisotropy in the molecular approximation. I: General considerations, J. Phys. C: Solid State Phys., 1971, 4, 1840–1854 CrossRef CAS.
- C. Rudowicz, On standardization and algebraic symmetry of the ligand field Hamiltonian for rare-earh ions at monoclinic symmetry sites, J. Chem. Phys., 1986, 84, 5045–5058 CrossRef CAS.
- N. Shohata, Magnetic properties of rare-earth gallium intermetallic compounds, J. Phys. Soc. Jpn., 1977, 42, 1873–1880 CrossRef CAS.
- T. Numazawa, K. Kamiya, T. Okano and K. Matsumoto, Magneto caloric effect in (DyxGd1−x)3Ga5O12 for adiabatic demagnetization refrigeration, Phys. B, 2003, 329–333, 1656–1657 CrossRef CAS.
- P. Mukherjee, A. C. Sackville Hamilton, H. F. Glass and S. E. Dutton, Sensitivity of magnetic properties to chemical pressure in lanthanide garnets Ln3A2X3O12, Ln = Gd, Tb, Dy, Ho, A = Ga, Sc, In, Te, X = Ga, Al, Li, J. Phys.: Condens. Matter, 2017, 29, 405808 CrossRef CAS PubMed.
- P. Mukherjee, Investigation of the magnetic and magnetocaloric properties of complex lanthanide oxides, PhD thesis, University of Cambridge, 2018 Search PubMed.
- V. Franco, J. S. Blazquez, J. J. Ipus, J. Y. Law, L. M. Moreno-Ramirez and A. Conde, Magnetocaloric effect: From materials research to refrigeration devices, Prog. Mater. Sci., 2018, 93, 112–232 CrossRef.
- H. Zhang, Y. Li, E. Liu, Y. Ke, J. Jin, Y. Long and B. Shen, Giant rotating magnetocaloric effect induced by highly texturing in polycrystalline DyNiSi compound, Sci. Rep., 2015, 5, 11929 CrossRef.
- H. Zhang, C. Xing, H. Zhou, X. Zheng, X. Miao, L. He, J. Chen, H. Lu, E. Liu, W. Han, H. Zhang, Y. Wang, Y. Long, L. van Eijk and E. Brück, Giant anisotropic magnetocaloric effect by coherent orientation of crystallographic texture and rare-earth ion moments in HoNiSi polycrystal, Acta Mater., 2020, 193, 210–220 CrossRef CAS.
- L. Wei, X. Zhang, W. Gan, C. Ding, C. Liu, L. Geng and Y. Yan, Large rotating magnetocaloric effects in polycrystalline Ni-Mn-Ga alloys, J. Alloys Compd., 2021, 874, 159755 CrossRef CAS.
- Y. Jia, T. Namiki, S. Kasai, L. Li and K. Nishimura, Magnetic anisotropy and large low field rotating magnetocaloric effect in NdGa single crystal, J. Alloys Compd., 2018, 757, 44–48 CrossRef CAS.
- V. Tkac, A. Orendacova, E. Cizmar, M. Orendac, A. Feher and A. G. Anders, Giant reversible rotating cryomagnetocaloric effect in KEr(MoO4)3 induced by a crystal-field anisotropy, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 024406 CrossRef.
- R. Tarasenko, V. Tkac, A. Orendacova, E. Cizmar, M. Orendac and A. Feher, Experimental study of the rotational magnetocaloric effect in KTm(MoO4)3, Phys. B, 2018, 538, 116–119 CrossRef CAS.
- D. Yuan, Z. Jia, J. Wang, Z. Gao, J. Zhang, X. Fu, J. Shu, Y. Yin, Q. Hu and X. Tao, Bulk growth, structure, and characterization of the new monoclinic TbCa4O(BO3)3 crystal, CrystEngComm, 2014, 16, 4008–4015 RSC.
- P. Yu, X. Pan, G. Wu, Y. Liu, F. Yu, Z. Wang and X. Xu, Crystal growth, optical, thermal and piezoelectric properties of HoCa4O(BO3)3 crystal, CrystEngComm, 2021, 23, 6574–6581 RSC.
- M. Münchhalfen, J. Schreuer, C. Reuther, E. Mehner and H. Stöcker, Elastic, piezoelectric, and dielectric properties of rare-earth calcium oxoborates RCa4O(BO3)3 (R = Er, Y, Dy, Gd, Sm, Nd, La), J. Appl. Phys., 2021, 130, 095102 CrossRef.
- M. Balli, S. Jandl, P. Fournier and A. Kedous-Lebouc, Advanced materials for magnetic cooling: Fundamentals and practical aspects, Appl. Phys. Rev., 2017, 4, 021305 Search PubMed.
- A. Elouafi, S. Ezairi, F. Lmai and A. Tizliouine, Excellent magnetocaloric effect ar cryogenic temperature in aporphous (Fe35RE65) (RE = Er, Dy and Gd) alloys, J. Mag. Mag. Mater., 2023, 588, 171381 CrossRef CAS.
- B. Wu, Y. Zhang, D. Guo, J. Wang and Z. Ren, Structure, magnetic properties and cryogenic magneto-caloric effect (MCE) in RE2FeAlO6 (RE = Gd, Dy, Ho) oxides, Ceram. Int., 2021, 47, 6290–6297 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional crystallographic data on the crystal structure of LnCOB compounds; additional data on the magnetic properties of powdered LnCOB samples; details about the orientation and the angular variation of the magnetization of the single-crystalline LnCOB samples; and additional data on the magnetic properties of the single-crystalline LnCOB samples. CCDC 2372139, 2372141, 2372143, 2372144 and 2400871. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc03249g |
| This journal is © The Royal Society of Chemistry 2025 |