 Open Access Article
Open Access ArticleComposite revolution: unleashing the potential of polymers in sustainable energy and environmental applications
Arun Varghese ab,
Kalathiparambil Rajendra Pai Sunajadevi
ab,
Kalathiparambil Rajendra Pai Sunajadevi *ac and
Dephan Pinheiro
*ac and
Dephan Pinheiro ac
ac
aDepartment of Chemistry, Christ University, Bangalore-560029, Karnataka, India. E-mail: sunajadevi.kr@christuniversity.in
bDepartment of Chemistry, Faculty of Engineering and Technology, Jain University, Bangalore 562112, India
cCentre for Renewable Energy and Environmental Sustainability, Christ University, Bangalore 560 029, India
First published on 8th May 2025
Abstract
The rising demand for sustainable solutions to global energy and environmental challenges has accelerated research into advanced functional materials. Conductive polymer composites based on polyaniline (PANI), polypyrrole (PPy), poly(3,4-ethylenedioxythiophene) (PEDOT), and chitosan have emerged as promising candidates due to their tunable properties, environmental compatibility, and multifunctionality. This review highlights the energy and environmental applications of polymer-based mixed metal oxide catalysts. These composites show excellent performances in supercapacitance and water splitting applications, offering both efficient energy storage and hydrogen generation solutions and eco-friendly fuel alternatives. Using adsorption and corrosion inhibition techniques, water pollution and corrosion have also been addressed. Polymers such as PANI, PPy, PEDOT, and chitosan, when integrated with metal oxides, heteroatoms, and carbonaceous materials, enhance the functional properties of the composites. These materials demonstrate significant potential in supercapacitors, water splitting, adsorption, and corrosion resistance. The review provides a comparative analysis of different composites, helping readers understand how the incorporation of various components can improve performances. The review emphasizes sustainable approaches to tackle the current energy and environmental issues through advanced polymer-based catalytic systems.
Introduction
The energy needs of the day are diverse and multifaceted, encompassing electricity generation, transportation, heating, cooling, and industrial processes.1 Population growth, urbanization, and economic development continue to drive up energy demand globally, particularly in emerging economies. The march towards cleaner sources of energy, such as renewable energy and nuclear power, is essential to mitigate the environmental impact of fossil fuel consumption and combat climate change. Additionally, improving energy efficiency across sectors can reduce waste and enhance sustainability.2–5Addressing society's energy and environmental needs requires navigating complex challenges and seizing opportunities for innovation and collaboration. Key challenges include decoupling the entrenched dependency on fossil fuels, improving the inadequate infrastructure for renewable energy deployment, overcoming the economic barriers towards sustainability initiatives, and bypassing geopolitical tensions over energy resources.6,7
The Sustainable Development Goals (SDGs), established by the United Nations in 2015, are a set of 17 interconnected targets aimed at addressing pressing global issues such as climate change, inequality, environmental degradation, and sustainable living (Fig. 1). SDG 6 focuses on clean water and sanitation, emphasizing universal access to safe water and improved sanitation to prevent disease, improve quality of life, and support other SDGs.8 With increasing water pollution and limited freshwater availability, technologies like adsorption and photocatalytic degradation are prioritized for effective water purification. Achieving SDG 6 not only improves health and ecosystems but also drives socio-economic growth, requiring collaborative action, innovative water technologies, and effective resource allocation.9
 | ||
| Fig. 1 Sustainable development goals declared by the United Nations. Reproduced from ref. 10 with permission from Wiley, copyright 2024. | ||
Similarly, SDG 7 promotes affordable and clean energy to ensure reliable and sustainable energy access.11,12 This goal is crucial for powering essential services and transitioning from fossil fuels to renewable sources, despite challenges like poor infrastructure and funding. Technologies such as electrocatalytic water splitting for clean hydrogen production and supercapacitors for energy storage are pivotal to this shift.13 Complementing these, SDG 9 emphasizes Industry, Innovation, and Infrastructure as pillars for economic and technological progress.14 Advancements in supercapacitors, fuel cells, and water remediation methods support these goals by fostering innovation and sustainability. Ultimately, addressing energy and environmental challenges demands a holistic approach, combining technology, policy, and global cooperation to ensure a sustainable and resilient future.
As environmental concerns and global energy demands continue to rise, there is a greater emphasis on finding new materials that can meet these two issues. Polymer-based composites have become the most promising of the many materials being investigated because of their exceptional blend of adaptability, adjustable characteristics, and functional performance. Particularly, bio-derived polymers like chitosan and conductive polymers like polyaniline (PANI), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene) (PEDOT) have attracted a lot of interest. When combined or utilized separately, these materials present encouraging opportunities for the creation of multipurpose platforms designed for environmentally friendly energy conversion and clean-up.15,16
S. Peter et al. reported a review on the usage of chitin and chitosan in energy and environmental applications.17 A review of the energy storage applications of biopolymers like cellulose, chitosan, and lignin is also reported.18 A comparative study of the usage of polymers supported nanocomposites in environmental applications was reported by X. Zhao and coworkers.19 A review of the nanocellulose based polymers is reported for energy conversion applications.20 A study on carbon based polymer nanocomposites was reported for energy storage applications.21 A review of functionalized nanoparticle loaded polymers for water splitting, solar cells, and carbon dioxide capture studies was also reported.22 A comparison table of various reported reviews is given in Table 1. In this review, we have focused on the various polymer composites like PANI, PPy, PEDOT, and chitosan composites for applications like supercapacitance, electrocatalytic water splitting, adsorption, and corrosion inhibition studies. A comprehensive study of these different polymers for four different applications in a single review will be of great help to researchers who are in their early stages. The discussions on sustainable development goals are also included in the review. Detailed descriptions of the applications and the importance of polymer based composites are carried out in the study. Finally, comparative studies on the usage of these composites for various energy and environmental applications are given in the review. As we strive for sustainable development, we must explore innovative technologies that meet our energy needs and minimize the environmental impact. This is where the pivotal role of supercapacitors, water splitting, corrosion, and adsorption studies in addressing these critical issues acquires significance.
| Sl. no. | Composite | Applications | Ref. |
|---|---|---|---|
| 1 | Chitin and chitosan | Energy and environmental | 17 |
| 2 | Biopolymers | Energy storage | 18 |
| 3 | Polymers supported nanocomposites | Catalytic degradation and sensing | 19 |
| 4 | Nanocellulose based composites | Energy conversion devices | 20 |
| 5 | Carbon based polymers | Energy storage studies | 21 |
| 6 | Functionalized nanoparticle loaded polymers | Water splitting, solar cells, CO2 capture | 22 |
| 7 | Polyaniline, polyoyrrole, PEDOT, and chitosan | Supercapacitance, water splitting, adsorption, and Corrosion inhibition | This study |
Energy and environmental applications
Supercapacitors
Supercapacitors, also known as “ultracapacitors” or “electrochemical capacitors,” have emerged as effective storage devices of energy due to their high power and energy density, rapid charging capabilities, and excellent stability. Unlike conventional capacitors that store energy through electrostatic attraction between opposite charges on conductive plates, supercapacitors utilize electrochemical double-layer capacitance (EDLC) or pseudocapacitance mechanisms. These mechanisms empower supercapacitors to achieve significantly higher energy densities compared to conventional capacitors while offering rapid charge and discharge rates akin to batteries.23,24 At the core of supercapacitor technology lies the development of an electrochemical double layer at the interface between an electrode and an electrolyte solution. When a voltage is applied across the electrodes immersed in the electrolyte, ions from the electrolyte crowd around the electrode–electrolyte interface and create a double layer of charges. This process occurs swiftly and is reversible, enabling supercapacitors to exhibit high power density characteristics crucial for applications requiring rapid energy storage and release cycles.25Supercapacitors store electrical energy through two primary mechanisms: electrostatic double-layer capacitance and pseudocapacitance. Electrostatic double-layer capacitance involves the physical separation of charges at the electrode–electrolyte interface. Similar to static electricity storage in conventional capacitors, this mechanism is significantly enhanced by utilizing high-surface-area electrode materials and efficient electrolytes.26 Meanwhile, pseudocapacitance involves redox reactions at the electrode surface, where ions in the electrolyte undergo faradaic processes, leading to additional charge storage. This mechanism, which is akin to batteries but distinguished by faster charge transfer kinetics, complements electrostatic double-layer capacitance to achieve higher energy densities. Common pseudocapacitive materials in supercapacitors include transition metal oxides and conducting polymers, known for their higher capacitance values than purely electrostatic storage.27 The combination of these mechanisms gives rise to another type of supercapacitor called hybrid supercapacitor.28 The types of supercapacitors are illustrated in Fig. 2. The choice of electrode materials heavily influences the performance characteristics of supercapacitors. Carbon-based materials such as graphene compounds, activated carbon, and carbon nanotubes dominate due to their high specific surface area, excellent electrical conductivity, and chemical stability in various electrolytes. These materials facilitate the efficient formation of the electrostatic double-layer and contribute significantly to the high capacitance values and rapid charge–discharge capabilities of supercapacitors.29
 | ||
| Fig. 2 (A) EDLC (B) pseudo and (C) hybrid supercapacitors. Reproduced from ref. 28 with permission from Wiley, copyright 2022. | ||
Supercapacitors find diverse applications across industries and technological domains owing to their unique attributes. In renewable energy systems, supercapacitors serve as energy storage devices for mitigating power output fluctuations from solar panels and wind turbines. Supercapacitors also enhance portable electronic devices, acting as auxiliary power sources during peak demand periods, thereby extending battery life and improving device performance.30,31 In transportation, supercapacitors enhance the efficiency of regenerative braking systems in electric vehicles by swiftly storing and releasing energy during braking cycles. This not only enhances vehicle efficiency but also prolongs battery life by reducing stress on the energy storage system.32 Additionally, supercapacitors are integral to hybrid electric vehicles, providing bursts of high power for acceleration and complementing the energy density of batteries. The integration of supercapacitors into electronics and consumer goods optimizes energy management systems and enable innovative functionalities like rapid charging for mobile devices, instantaneous energy storage for wearable electronics, and reliable backup power for critical equipment. Their ability to operate across a wide temperature range and endure numerous charge–discharge cycles makes supercapacitors suitable for demanding environments where durability and reliability are paramount.33
Supercapacitor applications span transportation, renewable energy, electronics, and industrial sectors, contributing to enhanced energy efficiency, reduced environmental impact, and the emergence of new technological innovations.34,35 Advancements in supercapacitor technology continue to be driven by research aimed at improving energy density, enhancing power density, and optimizing cost-effectiveness. Innovations in electrode materials, electrolyte formulations, and device architecture pave the way for supercapacitors with higher energy storage capacities and extended operational lifetimes. Emerging trends include the development of flexible supercapacitors for wearable electronics, the integration of supercapacitors into structural components for smart materials applications, and the exploration of hybrid systems that combine the strengths of supercapacitors and batteries. As research continues to push the boundaries of supercapacitor performance and scalability, these devices are poised to play a pivotal role in transforming our society towards a sustainable energy future.
Electrocatalytic water splitting
Electrocatalytic water splitting, or electrolysis, is a process that uses electrical energy to decompose water into hydrogen and oxygen gases. Hydrogen, known as the cleanest form of energy, holds tremendous potential as a versatile fuel source for transportation, power generation, and industrial applications.36,37 However, the widespread adoption of hydrogen fuel faces cost, efficiency, and infrastructure challenges. Through rigorous research and development in water splitting studies, scientists aim to overcome these barriers by improving catalysts, electrolytes, and system design. By leveraging advances in water splitting technology, we can harness the abundant energy of sunlight or renewable electricity to produce clean hydrogen fuel, thereby reducing our dependence on fossil fuels. Electrocatalytic water splitting stands at the forefront of renewable energy conversion, aiming to harness electricity from sustainable sources to produce clean hydrogen fuel. This process involves intricate mechanisms encompassing both the hydrogen (HER) and oxygen evolution reactions (OER).38,39 The electrocatalytic overall water splitting studies produce hydrogen and oxygen gases by lowering the activation energy barriers for HER and OER using the electrocatalysts.40The HER starts with the reduction of protons (H+) from water to form hydrogen gas (H2). There are three steps in the mechanism of HER leading to the production of hydrogen in a basic medium. Volmer step (eqn (1)), Heyrovsky step (eqn (2)), and Tafel step (eqn (3)) are the three basic steps in hydrogen production. The Tafel slope values for the Volmer, Heyrovsky step, and Tafel reaction steps are 120, 40, and 30 mV dec−1, respectively.41
| H3O+ + e → Hads + H2O | (1) |
| H3O+ + Hads + e → H2 + H2O | (2) |
| Hads + Hads → H2 | (3) |
This reaction predominantly takes place on the catalyst surface, where protons from the aqueous electrolyte solution adsorb onto active sites. The process involves the adsorption of protons, followed by the transfer of electrons present in the external circuit to the catalyst surface, facilitating the reduction of protons to hydrogen atoms. These hydrogen atoms subsequently combine to form H2 molecules, which desorb from the catalyst surface into the gas phase. The effectiveness of the HER is contingent upon several factors, including the catalyst's active sites, surface area, electronic structure, and interactions with the electrolyte. Catalysts with optimized properties enhance the kinetics of hydrogen production.
In water electrolysis, OER occurs at the anode with a thermodynamic voltage of 1.23 V, while the HER happens at the cathode with a thermodynamic voltage of 0 V. The total voltage required for water decomposition in electrolysis is 1.23 V.42,43 The schematic representation of overall water splitting is given in Fig. 3.
 | ||
| Fig. 3 Schematic representation of overall water splitting. Reproduced from ref. 43 with permission from Wiley, copyright 2021. | ||
In an alkaline medium, the HER taking place at the cathodic site is given by eqn (4).
| 2H+ + 2e → H2 | (4) |
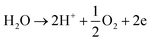 | (5) |
| 2H2O → 2H2 + O2 | (6) |
The optimization of electrocatalytic overall water splitting involves balancing the performance of HER and OER catalysts to maximize hydrogen production efficiency. Strategies for improving efficiency include innovative design and engineering of the catalyst to enhance active sites and surface areas for both reactions. Additionally, electrolyte optimization plays a critical role in facilitating proton and ion transport while mitigating parasitic reactions and catalyst degradation. Fine-tuning operational conditions such as pH, temperature, and applied potential further enhances catalyst activity and stability over extended electrochemical cycles.
Research in electrocatalytic overall water splitting continues to advance through the development of novel materials, deeper insights into reaction mechanisms, and the scaling up of electrochemical systems for practical applications. By leveraging the synergistic effects of materials science, electrochemistry, and renewable energy technologies, electrocatalytic overall water splitting represents a cornerstone in enabling a sustainable hydrogen economy and addressing global energy challenges.44,45 The electrocatalytic HER holds significant importance within electrochemistry, particularly in advancing technologies aimed at generating clean hydrogen fuel from water using renewable energy sources. This reaction is pivotal across various applications such as water electrolysis, hydrogen fuel cells, and renewable energy storage, where efficient and sustainable hydrogen production is crucial.
Adsorption studies
Adsorption is a process where molecules or ions from a fluid phase adhere to the surface of a solid or liquid, known as the adsorbent. This phenomenon arises from the attractive forces between the adsorbate and the surface of the adsorbent.46 Various factors, including the nature of the adsorbate and adsorbent, surface properties, temperature, pressure, and the presence of other substances in the fluid phase, influence the adsorption mechanisms. Adsorbent materials, such as conducting polymers, activated carbon, zeolites, and metal–organic frameworks, exhibit high surface areas and optimized pore structures, enabling efficient removal of pollutants, contaminants, and toxins from air and water.47,48 Adsorption studies focus on optimizing adsorbent materials, understanding adsorption mechanisms, and designing cost-effective treatment processes. By leveraging the power of adsorption, we can address pressing environmental challenges, such as air pollution, water scarcity, and contamination, while promoting public health and ecological sustainability.49–52 A graphical representation of the adsorption process is shown in Fig. 4. | ||
| Fig. 4 Schematic representation of adsorption of dye molecules using graphene. Reproduced from ref. 53 with permission from Elsevier, copyright 2018. | ||
Understanding adsorption mechanisms is pivotal to numerous scientific disciplines, encompassing chemistry, materials science, environmental science, and engineering. It serves as the foundation for applications spanning purification and separation processes, catalysis, drug delivery systems, and ecological remediation strategies.53,54 Adsorption can be broadly categorized into physical and chemical adsorption, each characterized by distinct mechanisms and energetics.55 External factors such as temperature and pressure also impact adsorption mechanisms. In physical adsorption, lower temperatures typically favour adsorption because they decrease the thermal energy available to adsorbate molecules, enhancing their likelihood to adhere to the adsorbent surface. Conversely, in chemisorption, higher temperatures may facilitate the activation of chemical bonds, thereby promoting adsorption. Pressure influences adsorption by altering the concentration of adsorbate molecules in the fluid phase and affecting adsorbate–adsorbent interactions.56 Physical adsorption, or physisorption, entails relatively weak intermolecular forces like van der Waals forces, hydrogen bonding, and dipole–dipole interactions between the adsorbate molecules and the surface of the adsorbent. These forces arise due to temporary or induced dipoles in both the adsorbate and adsorbent materials. Physisorption is typically non-specific and reversible, occurring on any surface irrespective of its chemical composition. The strength of physisorption increases with rising surface area and diminishes with temperature, as higher temperatures provide adequate thermal energy to overcome weak van der Waals forces, thereby causing desorption of the adsorbate molecules.57 Chemical adsorption, or chemisorption, on the other hand, involves stronger interactions between the adsorbate and the surface of the adsorbent, often through chemical bonds such as ionic, covalent, or metallic bonds. Chemisorption necessitates a specific chemical affinity between the adsorbate and the surface, frequently involving electron transfer or sharing between molecules and surface atoms or ions. This type of adsorption is typically more specific and irreversible compared to physisorption. Chemisorption processes often demand activation energy to initiate bond formation or breaking, which can be influenced by factors such as temperature, pressure, and the presence of catalysts.46,58
The adsorbent surface's physical and chemical properties, surface area, porosity, surface charge, and surface functional groups are critical in determining adsorption capacity and selectivity. Higher porosity and surface area provide more efficient active sites for adsorption, whereas surface charge and functional groups can influence interaction strength and specificity between the adsorbent and adsorbate molecules.59 The study of adsorption mechanisms often employs advanced experimental techniques and theoretical models to elucidate the underlying processes. Experimental methods encompass isotherm studies, measuring equilibrium adsorption capacity as a function of adsorbate concentration at constant temperature, and kinetic studies, examining the rate at which adsorption equilibrium is achieved over time. Adsorption mechanisms are multifaceted and dependent on a complex interplay of factors, including intermolecular forces, surface properties, temperature, pressure, and chemical affinity.60 Understanding these mechanisms is essential for designing and optimizing adsorption processes across various industries, such as environmental remediation, water purification, gas separation, catalysis, and biomedical applications. Advancements in experimental techniques and theoretical modelling continuously expand the knowledge of adsorption mechanisms, paving the way for innovative solutions to global challenges in sustainability and public health.61
Corrosion inhibition studies
Corrosion, the gradual degradation of materials due to chemical reactions with the environment, poses significant challenges to infrastructure integrity, safety, and environmental sustainability. From pipelines and bridges to industrial equipment and marine structures, corrosion costs billions of dollars annually and poses risks to public health and the environment. Corrosion studies play a vital role in understanding the mechanism of corrosion and developing effective mitigation strategies, such as protective coatings, corrosion inhibitors, and advanced materials. By combating corrosion, we can prolong the lifespan of infrastructure, conserve resources, and minimize environmental pollution, contributing to savings in cost, sustainable development, and economic prosperity.62Electrochemical corrosion inhibition studies encompass a wide range of research focused on mitigating the damaging effects of corrosion on metallic materials. Corrosion, an electrochemical process, results in the deterioration of metals through oxidation reactions in the presence of an electrolyte. This issue presents significant challenges across various sectors, such as transportation, infrastructure, marine, and aerospace industries, where metal structures are constantly exposed to harsh environmental conditions. The primary goal of corrosion inhibition studies is to devise effective strategies and materials that can reduce corrosion rates and prolong the lifespan of metal components.63 The foundation of electrochemical corrosion inhibition studies lies in comprehending the underlying mechanisms and dynamics of corrosion process. Corrosion typically proceeds through simultaneous anodic dissolution and cathodic reactions on metal surfaces immersed in electrolytes. Anodic dissolution involves the release of metal ions into the electrolyte solution, while cathodic reactions, such as oxygen reduction or hydrogen evolution, consume electrons to maintain charge equilibrium. This electrochemical nature of corrosion forms the basis for evaluating the efficacy of corrosion inhibitors.64,65 A schematic representation of corrosion inhibitor on copper substrate is shown in Fig. 5.
 | ||
| Fig. 5 Schematic representation of corrosion inhibition on copper surface. Reproduced from ref. 65 with permission from RSC, copyright 2014. | ||
One of the fundamental techniques employed in electrochemical corrosion inhibition studies is electrochemical impedance spectroscopy (EIS). EIS enables the characterization of the electrical properties of metal–electrolyte interfaces by measuring impedance responses across a range of frequencies. Analyzing impedance spectra helps researchers understand how inhibitors modify the electrochemical behavior of metal surfaces, thereby impeding corrosion processes. Another widely used method in corrosion inhibition studies is potentiodynamic polarization. By comparing polarization curves obtained with and without inhibitors, researchers can quantify the extent to which inhibitors suppress corrosion rates and shift the corrosion potential towards more noble values. This information is critical for elucidating the mechanism of action of inhibitors and optimizing their formulation for practical applications.66,67
The selection and design of corrosion inhibitors play a pivotal role in electrochemical corrosion inhibition studies. Corrosion inhibitors are chemical compounds or coatings that adsorb onto metal surfaces, forming protective barriers that impede the electrochemical reactions responsible for corrosion. The effectiveness of inhibitors hinges on several factors, including their chemical composition, molecular structure, adsorption affinity to metal surfaces, and ability to modify corrosion potential and current density (CD).68 Organic compounds such as organic acids, amines, polymers, and heterocyclic compounds are commonly employed as corrosion inhibitors due to their capacity to form stable complexes with metal ions or adsorb onto metal surfaces through non-covalent interactions. Inorganic inhibitors, such as metal oxides, chromates, phosphates, and molybdates, are also utilized for their ability to develop passive oxide layers on metal surfaces, thereby preventing further corrosion.69,70 Hybrid inhibitors, which integrate organic and inorganic components, have gained attention for their synergistic effects in enhancing corrosion protection. Designing effective inhibitors involves a systematic approach encompassing screening candidate compounds through laboratory tests, evaluating their performance under simulated environmental conditions, and optimizing their formulation for specific applications.71 The evaluation of corrosion inhibitors extends beyond laboratory experiments to encompass field studies and practical applications. Accelerated corrosion testing methodologies, including salt spray testing, humidity chamber testing, and immersion testing in corrosive environments, are utilized to simulate severe operating conditions and assess the durability of inhibitors over extended periods.72
The significance of electrochemical corrosion inhibition studies transcends corrosion control to encompass broader implications for sustainability, safety, and economic viability. By reducing corrosion rates and extending the service life of metal structures, corrosion inhibitors contribute to substantial cost savings associated with the maintenance, repair, and replacement of corroded components. Moreover, corrosion inhibition is crucial in bolstering the reliability and safety of critical infrastructure such as bridges, pipelines, offshore platforms, and industrial equipment, thereby minimizing the risk of catastrophic failures and environmental contamination.73 Electrochemical corrosion inhibition studies epitomize a multidisciplinary field that integrates chemistry, materials science, electrochemistry, and engineering principles to devise effective strategies for mitigating corrosion in diverse industrial applications. Through advanced electrochemical techniques and systematic evaluation methodologies, researchers continue to innovate and optimize corrosion inhibitors to meet the evolving demands of modern industries for sustainable and resilient infrastructure. As the global emphasis on environmental sustainability and resource conservation grows, the development of effective corrosion inhibitors and their applications is set to play a pivotal role in ensuring the longevity and performance of metallic materials across a wide spectrum of challenging environments.
Polymer based composites for energy and environmental applications
Conducting polymers are polymers with highly π-conjugated polymeric chains and are also called ‘synthetic metals.’ Some of the important conducting polymers used are polyacetylene, polyaniline (PANI), polypyrrole (PPy), polythiophene, poly(para-phenylene), chitosan, poly(3,4-ethylenedioxythiophene) (PEDOT), and polyfuran. These conducting polymers have readily tunable bandgaps, good redox chemistry or electroactivity, high flexibility, and good processability over inorganic nanomaterials.74 The studies on conducting polymers started in the 1970s.75 The polymer-based nanocomposites are prepared by reinforcing polymers with nanoparticles. Nanoparticles have strong activation and inter-particle interactions, which lead to strong aggregation tendencies that hinder the possibilities of exploring the properties and applications. This can be avoided by coating or grafting polymers onto the nanoparticle surface; thus, the polymers can hold the functional groups of nanoparticles, which prevents their aggregation.76PANI, a conducting polymer, when combined with TiO2 nanocomposite, shows enhanced energy applications like photocatalysis, energy storage, anti-corrosion, and supercapacitance.77,78 Polyvinyl alcohol and chitosan have gained the attention of scientists due to their less expensive, non-toxic, biodegradable, non-carcinogenic, bio-compatible, and water-soluble properties.79,80 PVA was combined with TiO2 to produce good photocatalytic and antibacterial results.81 Polymeric carbon nitride is a semiconductor that has a bandgap of 5 eV.82 It is the oldest synthetic polymer reported, showing high photocatalytic activity for hydrogen production and good absorption properties in the range of visible light. These carbon nitride-sensitized TiO2 nanotube arrays showed significantly improved photoelectrochemical and photocatalytic properties than TiO2 nanotube arrays.83 Z–Z-scheme heterojunction photocatalyst was made by the combination of polypyrrole, Ag, and inorganic particles. Conductive polymer, PPy, has high absorption coefficients in visible light, increased mobility of charges, and good environmental stability. This was doped with silver (Ag) since it can act as an electron mediator.84 Polyaniline was combined with zinc oxide and copper oxide to degrade organic pollutants. In another study, polyaniline was combined with TiO2 and CuO for degradation of 4-chlorophenol.85,86
Antibacterial studies of polymer-based nanocomposites have been conducted.87 Polyvinyl alcohol was combined with magnesium oxide and silicon carbide, which showed better antibacterial properties.88 Polypyrrole chitosan composite showed high inhibition rates against E. coli bacteria.89 PANI is another important polymer used in antibacterial studies of polymer-based nanocomposites.90
Conducting polymers-based supercapacitors are made from PANI, polypyrrole, and polythiophene.91 Supercapacitors created by combining PANI with graphene showed higher capacitance than individual PANI and graphene.92 PANI is lightweight, low cost, high conductivity, has good flexibility, and is environmentally friendly. Combining PANI with metal oxides will enhance their cyclic stability. Polypyrrole is another conducting polymer with safety excellent conductivity and higher density than other CPs.93 Heavy metals, like cobalt, cadmium, chromium, and lead, have been absorbed from aqueous solutions using polymers and polymer composites. Polymers like PANI and PPy combine with other metal oxides, like SiO2 and TiO2, to perform adsorption activities.94,95 Polyvinyl alcohol-based composites have been used to study adsorption properties to remove copper ions from an aqueous solution.96 Polymer-based nanocomposites were also used for corrosion studies.62 Polyvinyl acetate and PANI were effectively used in corrosion resistance studies.97
Recently, various studies on polymer-based mixed metal oxides have been reported. Polypyrrole–CuO–ZnO composites were used for oxygen-sensing studies.98 PANI–CuO–TiO2–SiO2 composites were used for ammonia sensing studies.99 PANI–CuO–ZnO composites were used in degradation studies.86 PANI–CuO–TiO2 composites were used for the degradation of 4-chlorophenol. In another study, PANI–ZnO–CuO composites were used for the degradation of organic pollutants.85,86
The combination of conducting polymers with suitable elements will enhance functionality due to the synergy between the elements, and efficient composites can be fabricated for various energy and environmental applications. Polymer based composites are used in various energy and environmental applications. Polymers like PANI,100 PPy,101 PEDOT,102 and chitosan103 have been explored widely by scientists for different applications. The excellent surface area, conductive nature, and redox properties make them better candidates for energy and environmental applications. Incorporating these polymers with various substances like metal, metal oxides, and heteroatoms gives rise to efficient composites and is used in supercapacitance, electrocatalytic water splitting, adsorption, and corrosion inhibition studies. The energy and environmental needs of society require multifaceted solutions that integrate scientific innovation, technological advancement, and ecological safety. Supercapacitors, water splitting, corrosion, and adsorption studies are critical for addressing these complex challenges and for shaping a more sustainable future. Investing in research, education, and collaboration can harness these technologies' transformative potential to meet society's evolving needs while safeguarding the planet for future generations.
Polyaniline composites for energy and environmental applications
In 1834, Ferdinand Runge reported the polymerization of aniline to get aniline black. However, PANI did not attract significant scientific attention until the 1980s, spurred by pioneering research on conductive polymers by Alan J. Heeger, Alan G. MacDiarmid, and Hideki Shirakawa, which eventually earned them the Nobel Prize in Chemistry in the year 2000.104 PANI's conductive properties are highly dependent on its oxidation state, which can be adjusted through doping processes. This versatility makes PANI particularly appealing for various applications. Among the earliest and most impactful uses of PANI is in energy storage, notably in rechargeable batteries and supercapacitors. PANI's high conductivity and environmental stability enhance the performance and longevity of these devices. Additionally, its ability to undergo reversible redox reactions makes it an excellent candidate for electrode materials in batteries and supercapacitors.105In environmental applications, PANI has shown considerable promise in removing pollutants from water and air. It can adsorb and degrade organic pollutants, heavy metals, and dyes, making it an effective material for environmental remediation. Furthermore, PANI's conductive nature allows it to be used in sensors for detecting environmental toxins and pollutants, enabling real-time monitoring of environmental quality.106 The combination of PANI with other compounds, such as graphene and metal oxides, has led to the creation of advanced composites with enhanced properties for both energy and environmental applications. These composites often demonstrate improved electrical conductivity, mechanical strength, and chemical stability.107 The evolution of PANI from a chemical curiosity to a pivotal material underscores the importance of interdisciplinary research and innovation. As researchers continue to discover new ways to improve the properties and performance of PANI, its role in addressing some of the most critical energy and environmental challenges of our time is likely to expand further.
Polyaniline composites for supercapacitance studies
Various metal oxides were combined with PANI to be used as electrodes for supercapacitor applications. Iron oxide derived from the used ink cartridges was combined with PANI, which showed a specific capacitance (CSP) of 286 F g−1 when 4 M KOH was used as the electrolyte.100 The electrochemical polymerization method was used to fabricate the PANI/molybdenum oxide working electrode on carbon cloth. This material showed a CSP of 841.6 F g−1 at a CD of 0.5 A g−1.108 A CSP of 376 F g−1 and a capacitance retention of 95.3% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles were realised for a working electrode made of PANI and copper oxide.109 D. J. Ahirrao et al. have studied the efficiency of PANI/manganese oxide composite, which showed a CSP of 605 F g−1 at a CD of 1 A g−1. In this study, the PANI showed a CSP of 515 F g−1, and manganese oxide showed a CSP of 141 F g−1.110 D. A. L. Almeida et al. studied the effect of PANI's energy storage efficiency by incorporating various metal oxides like iron, nickel, and manganese. Out of these, they found that PANI/manganese oxide composites showed a comparatively higher efficiency with a CSP of 115.7 F g−1.111 PANI/CuO composite utilized for energy storage studies showed a CSP of 424 F g−1 (Fig. 6).112
000 cycles were realised for a working electrode made of PANI and copper oxide.109 D. J. Ahirrao et al. have studied the efficiency of PANI/manganese oxide composite, which showed a CSP of 605 F g−1 at a CD of 1 A g−1. In this study, the PANI showed a CSP of 515 F g−1, and manganese oxide showed a CSP of 141 F g−1.110 D. A. L. Almeida et al. studied the effect of PANI's energy storage efficiency by incorporating various metal oxides like iron, nickel, and manganese. Out of these, they found that PANI/manganese oxide composites showed a comparatively higher efficiency with a CSP of 115.7 F g−1.111 PANI/CuO composite utilized for energy storage studies showed a CSP of 424 F g−1 (Fig. 6).112
 | ||
| Fig. 6 The supercapacitance analysis using PANI/CuO composite. Reproduced from ref. 112 with permission from Elsevier, copyright 2020. | ||
In another study, zinc cobalt oxide was combined with PANI to give a CSP of 1055 F g−1 at 1 A g−1 and capacitance retention of 85.7% at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.113 PANI was combined with cobalt oxide to give a CSP of 985 F g−1, whereas pristine cobalt oxide showed a CSP of 278 F g−1.114 When PANI was combined with manganese oxide, the CSP value demonstrated was 323.7 F g−1 at a scan rate of 5 mV s−1.115 In another study, PANI combined with iron oxide showed a CSP of 572 F g−1 at a CD of 0.5 A g−1.116 M. M. Mezgebe et al. evaluated the efficiency of PANI with manganese oxide and found it to have a CSP of 665 F g−1 (Fig. 7a–d).117 PANI combined with cobalt oxide has shown a CSP of 1151 F g−1 at a CD of 3 A g−1, with a capacitance retention of 92% at 5000 cycles.118
000 cycles.113 PANI was combined with cobalt oxide to give a CSP of 985 F g−1, whereas pristine cobalt oxide showed a CSP of 278 F g−1.114 When PANI was combined with manganese oxide, the CSP value demonstrated was 323.7 F g−1 at a scan rate of 5 mV s−1.115 In another study, PANI combined with iron oxide showed a CSP of 572 F g−1 at a CD of 0.5 A g−1.116 M. M. Mezgebe et al. evaluated the efficiency of PANI with manganese oxide and found it to have a CSP of 665 F g−1 (Fig. 7a–d).117 PANI combined with cobalt oxide has shown a CSP of 1151 F g−1 at a CD of 3 A g−1, with a capacitance retention of 92% at 5000 cycles.118
 | ||
| Fig. 7 The galvanostatic charge–discharge graphs of PANI/manganese oxide composite evaluated for charge storage capacity. Reproduced from ref. 117 with permission from Elsevier, copyright 2019. | ||
Different compounds like metal–organic framework (MOF), graphene oxide (GO), and reduced graphene oxide (rGO) were combined with PANI and metal oxides to form ternary composites. These composites were used as electrodes for energy storage studies. D. Qin et al. have studied the energy storage capacity of PANI combined with ZnO and Zn–Co MOF and observed a CSP of 458.9 F g−1 at a CD of 1 A g−1. They have also constructed an asymmetric supercapacitor with activated carbon as the anionic electrode and the studied material as the cationic electrode.119 When PANI was combined with copper oxide and rGO, a CSP of 534.5 F g−1 was shown at a CD of 1 A g−1.120 PANI, when combined with titanium oxide and tin oxide, showed a CSP of 540 F g−1. This composite showed a capacitance retention of 85% after 6000 cycles.121 K. Y. Yasoda et al. combined PANI and manganese oxide with GO, which showed a CSP of 829 F g−1. The presence of GO has increased the efficiency of PANI towards energy storage.122 PANI incorporated with nickel ferrite spinel in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio has shown a maximum CSP of 758 F g−1. It showed a maximum capacitance retention of 97% at 10
1 weight ratio has shown a maximum CSP of 758 F g−1. It showed a maximum capacitance retention of 97% at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.123 A composite having a core–shell intercalation structure was synthesized by Y. Ma et al. The composite was PANI/manganese oxide/nickel oxide with GO. A CSP of 396 F g−1 at a CD of 0.5 A g−1. It also showed a cyclic stability of 82.6% at 3000 cycles.124 PANI, when combined with nickel cobalt oxide, showed a CSP of 561.2 F g−1 at 10 A g−1. This was combined with a negative MXene electrode for an asymmetric supercapacitor, which showed a cyclic stability of 86.2% at 3000 cycles. The efficient redox kinetics and better dynamic equilibrium between the positive and negative electrodes in the two-electrode system are responsible for the energy storage capacity.125 PANI combined with manganese oxide and N-doped carbon showed a specific capacity (CS) of 410 C g−1. When manganese oxide was replaced with tin oxide, the CS value increased to 534 C g−1. Both the composites showed more than 97% capacitance retention after 5000 cycles.126 PANI/GO combined manganese and molybdenum oxide was used as a working electrode for electrochemical analysis of energy storage. The CSP of this electrode was found to be 596 F g−1.127
000 cycles.123 A composite having a core–shell intercalation structure was synthesized by Y. Ma et al. The composite was PANI/manganese oxide/nickel oxide with GO. A CSP of 396 F g−1 at a CD of 0.5 A g−1. It also showed a cyclic stability of 82.6% at 3000 cycles.124 PANI, when combined with nickel cobalt oxide, showed a CSP of 561.2 F g−1 at 10 A g−1. This was combined with a negative MXene electrode for an asymmetric supercapacitor, which showed a cyclic stability of 86.2% at 3000 cycles. The efficient redox kinetics and better dynamic equilibrium between the positive and negative electrodes in the two-electrode system are responsible for the energy storage capacity.125 PANI combined with manganese oxide and N-doped carbon showed a specific capacity (CS) of 410 C g−1. When manganese oxide was replaced with tin oxide, the CS value increased to 534 C g−1. Both the composites showed more than 97% capacitance retention after 5000 cycles.126 PANI/GO combined manganese and molybdenum oxide was used as a working electrode for electrochemical analysis of energy storage. The CSP of this electrode was found to be 596 F g−1.127
A hierarchical porous ruthenium cobalt oxide combined with PANI composite was utilized as the working electrode to study the efficiency of energy storage. This composite demonstrated a CS of 1028.7 C g−1 at a CD of 1 A g−1. This composite also showed a high capacity retention of 93.2% even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.128 The prepared PANI/copper manganite composite working electrode by A. S. A. Almalki showed a CSP of 1181 F g−1 at a CD of 1 A g−1 and demonstrated 95% capacitance retention at 5000 cycles.129 PANI/nanowire-shaped cobalt oxide grown on nickel foam was used as an electrode for an asymmetric supercapacitor, which showed a CS of 1113 C g−1 at a CD of 3 A g−1. The cyclic stability showed by this composite was 86.3% at 10
000 cycles.128 The prepared PANI/copper manganite composite working electrode by A. S. A. Almalki showed a CSP of 1181 F g−1 at a CD of 1 A g−1 and demonstrated 95% capacitance retention at 5000 cycles.129 PANI/nanowire-shaped cobalt oxide grown on nickel foam was used as an electrode for an asymmetric supercapacitor, which showed a CS of 1113 C g−1 at a CD of 3 A g−1. The cyclic stability showed by this composite was 86.3% at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.130 In another study, PANI/cobalt oxide combined with rGO showed an enhanced capacitance of 1982 F g−1 when a scan rate of 10 mV s−1 was employed. The device fabricated using a two-electrode system shows a CSP of 662 F g−1 at a CD of 1 A g−1.131 PANI was combined with oxides of nickel, cobalt, and praseodymium to study the efficiency of energy storage. It was seen that the synthesized composite gave a CSP of 905 F g−1 at a CD of 1 A g−1.132 PANI combined with molybdenum oxide showed a CSP of 606 F g−1 at a CD of 1 A g−1.133 Ni–Co–Mn MOF combined with rGO and PANI has shown a CSP of 1007 F g−1 at a CD of 1 A g−1.134
000 cycles.130 In another study, PANI/cobalt oxide combined with rGO showed an enhanced capacitance of 1982 F g−1 when a scan rate of 10 mV s−1 was employed. The device fabricated using a two-electrode system shows a CSP of 662 F g−1 at a CD of 1 A g−1.131 PANI was combined with oxides of nickel, cobalt, and praseodymium to study the efficiency of energy storage. It was seen that the synthesized composite gave a CSP of 905 F g−1 at a CD of 1 A g−1.132 PANI combined with molybdenum oxide showed a CSP of 606 F g−1 at a CD of 1 A g−1.133 Ni–Co–Mn MOF combined with rGO and PANI has shown a CSP of 1007 F g−1 at a CD of 1 A g−1.134
PANI combined with various metal oxides has shown substantial promise as an electrode material for supercapacitor applications, owing to its high pseudocapacitance and environmental stability. Notably, composites such as PANI/zinc–cobalt oxide (1055.0 F g−1), PANI/ruthenium–cobalt oxide (1028.7 F g−1), and PANI/Ni–Co–Mn–MOF/rGO (1007.0 F g−1) demonstrated superior CSP values due to the synergistic effects between the redox-active metal oxides and the conductive polymer matrix. The integration of binary and ternary metal oxides, as well as the inclusion of conductive additives like rGO, enhances the charge storage capability, cycling stability, and surface area of the composites. Overall, PANI-based hybrid materials emerge as efficient candidates for high-performance supercapacitors, especially when tailored with multi-component oxide systems.
Polyaniline composites for water splitting studies
Composites containing PANI have been utilized as electrocatalysts for water splitting studies to tackle the energy related issues in our society. PANI and tungstic acid hybrid was annealed in a vacuum to produce a tungsten carbide/carbon composite by C. Liu et al.135 The synthesized catalyst was utilized for electrocatalytic hydrogen evolution reaction (HER) studies. The composite showed an overpotential (OP) of 220 mV at 10 mA cm−2 and a Tafel slope value of 93 mV dec−1. Iron-doped PANI was synthesized to evaluate the overall water-splitting efficiency and showed an OP of 235 mV for oxygen evolution reaction (OER) studies.136 C. Ray et al. utilized PANI to synthesize nitrogen-doped cobalt–nickel nitride on carbon cloth for an overall water-splitting reaction. The OPs shown by this electrocatalyst at 10 mA cm−2 for HER and OER were 68 and 247 mV, respectively.137Molybdenum carbide combined with nitrogen-doped carbon matrix was utilized for electrocatalytic HER studies. The study was carried out in both acidic and alkaline electrolytes, and the catalyst showed OPs of 81 and 89 mV in the alkaline and acidic mediums.138 Nitrogen-doped carbon–iron phosphide composite was used as the catalyst for overall water splitting studies. It showed an OP of 193 mV for HER and 302 mV for OER studies. The potential required to reach a CD of 10 mA cm−2 when a two electrode assembly, formed for overall water splitting, was found to be 1.63 V.139 R. Djara et al. studied the effect of variation in the concentration of oxidants and doping agents utilized for the chemical oxidative synthesis of PANI towards HER analysis. It was found that when HCl was used as a doping agent and ammonium persulfate as the oxidant, the lowest OP of 690 mV was observed.140 Thermal activation of PANI/GO and ammonium hexafluorophosphate was carried out to obtain N, P, and F-doped graphene, which was used for electrochemical water splitting studies. This composite showed an OP of 520 mV at a CD of 10 mA cm−2.141
A carbon nanostructured PANI–nickel composite was synthesized through a chemical oxidative polymerization technique and evaluated for HER and OER activity. OPs of 190 mV and 360 mV were shown by this catalyst for HER and OER, respectively.142 Nickel–cobalt metal phosphide-coated PANI composite was utilized for HER study and showed an OP of 80.6 mV at a CD of 10 mA cm−2.143 Carbon dots combined with PANI were explored for overall water splitting. The OER studies showed that an OP of 150 mV was required to reach a CD of 30 mA cm−2. In the case of HER, the OP required to reach a CD of 20 mA cm−2 was found to be 65 mV (Fig. 8).144
 | ||
| Fig. 8 Overall water splitting studies using PANI/carbon dot composite. Reproduced from ref. 144 with permission from ACS, copyright 2021. | ||
PANI was combined with iridium for HER studies, which required an OP of 36 mV to reach a CD of 10 mA cm−2. PANI combined with ruthenium was utilized for OER study, which required an OP of 240 mV. An overall potential of 1.45 V was required by the catalyst when overall water splitting was carried out.145 PANI combined with nickel–iron layered double hydroxide was utilized for OER studies, which utilized an OP of 220 mV, with a Tafel slope of 44 mV dec−1.146 Cobalt molybdenum sulfide/PANI composite required OPs of 98 and 250 mV at 10 mA cm−2 for HER and OER, respectively. The efficiency of this composite was due to the presence of a defect-rich structure and the conductive nature of PANI that directs the electron transfer.147 Cobalt-incorporated PANI was analyzed for the OER study using glassy carbon and nickel foam substrates. When glassy carbon was used, an OP of 341 mV was required, whereas when nickel foam was used, the OP was reduced to 251 mV.148 Cobalt-doped tungsten selenide/PANI composite required OPs of 308 and 360 mV for HER and OER analyses, respectively.149 When cobalt-doped molybdenum selenide was combined with PANI, OPs of 196 and 385 mV were required for HER and OER, respectively.150 The onset potential for OER was found to be 134 mV when zinc–cobalt/cellulose acetate/PANI composite was used as an electrocatalyst.151 When sulfonated PANI was combined with cobalt, an OP of 312 mV was required for OER analysis.152 Copper molybdenum sulfide particles combined with rGO/PANI were utilized for OER, which required an OP of 395 mV.153
The metal oxides were combined with PANI in various studies to be used as the electrocatalysts for water splitting studies. PANI/manganese molybdenum oxide composite was utilized for overall water splitting studies. The OPs required for HER and OER studies were 155 mV and 410 mV, respectively, for the composite.154 PANI/tungsten trioxide composite required an OP of 289 mV at 50 mA cm−2 for OER study.155 PANI was combined with cobalt oxide to analyze its efficiency towards OER, and it required an OP of 258 mV to attain a CD of 10 mA cm−2.156 Molybdenum/carbon/nickel electrocatalyst synthesized from PANI/molybdenum oxide was utilized for HER studies. The catalyst showed an onset potential of 44 mV at 1 mA cm−2. The Tafel slope value was found to be 49 mV dec−1, and the OP value was found to be 60 mV. The authors claim this is a potential earth-abundant catalyst for hydrogen generation.157
PANI and its composites have shown notable electrocatalytic activity in both the HER and OER. Pristine PANI exhibits a high HER OP (690 mV), indicating limited catalytic efficiency. However, upon hybridization with suitable materials, significant reductions in OP are observed. For instance, PANI/carbon dots (65 mV for HER, 150 mV for OER), PANI/nickel–cobalt phosphide (80.6 mV for HER), and PANI/tungstic acid (220 mV for HER) display marked improvements, illustrating the role of synergistic effects and enhanced active surface area. For OER, PANI/nickel (360 mV) and PANI/manganese molybdenum oxide (410 mV) show promising performance. The inclusion of multi-metallic oxides, phosphides, and carbon-based nanostructures improves conductivity, boosts active site availability, and facilitates electron transfer, making PANI-based composites efficient bifunctional catalysts for overall water splitting.
Polyaniline composites for adsorption studies
The high surface area and excellent porous nature of PANI have made it an excellent adsorbent substance for removing toxic organic substances that cause pollution from water sources. The incorporation of substances like metal oxides into the PANI matrix has improved the adsorptive nature of PANI. Wood dust was used to synthesize ZnO through the green synthetic method, and it was incorporated into PANI for adsorptive removal studies of red-XGRL dye. 200 mg L−1 of the dye solution was used, and the optimum pH for the composite was 11. The composite showed a maximum adsorption capacity of 70.4 mg g−1 toward the dye solution. The study confirmed the fitness towards pseudo second order kinetics, Langmuir adsorption isotherm, and exothermic behavior of the adsorption process.158 PANI/ZnO composite was synthesized using wheat straw and was used for the adsorptive removal of blue-XGRRL dye. The composite showed a maximum adsorption capacity of 55.8 mg g−1 towards a dye solution with a concentration of 100 mg L−1. The adsorption process followed pseudo second order kinetics and Langmuir adsorption isotherm.159In another study, PANI/ZnO adsorbent was chemically synthesized using formic acid. This composite was utilized to remove methylene orange dye from wastewater. Maximum removal efficiency of 97% was shown by the adsorbent within a period of 40 min.160 Methyl orange dye removal was carried out by using PANI/ZnO composite, synthesized by A. Deb et al.161 The maximum adsorption capacity was found to be 240.8 mg g−1. When ultrasonication was carried out, the adsorption equilibrium was obtained within 15 min, which proves the efficiency of the ultrasonication method in the adsorption process. Using response surface methodology, the maximum removal efficiency of methyl orange dye was found to be 99%.
The synthesis of PANI/manganese oxide/niobium pentoxide composite was carried out using the chemical oxidative polymerization technique in an acidic medium. This was used as the adsorbent for the removal of methyl orange from an aqueous solution, which showed a maximum removal efficiency of 97.3%.162 PANI was combined with iron oxide to remove basic blue dye from water samples. A maximum adsorption capacity of 78.1 mg g−1 was shown by the composite. The adsorption process followed pseudo second order kinetics. It was also found that the adsorption process is exothermic in nature.163 Acid blue 40 dye was removed from water sources using PANI/iron oxide adsorbent. A higher maximum adsorption capacity of 216.9 mg g−1 was observed for this composite.164 PANI/iron oxide was also used for the removal of chromium and nickel ions from the wastewater.165
Various organic pollutants like p-nitrophenol and resorcinol were removed from the wastewater by using PANI/nickel oxide composite. Freundlich and Temkin adsorption isotherms were best fitted for the process, and it followed pseudo second order kinetics. A maximum adsorption capacity of 19.53 mg g−1 for resorcinol and 29.78 mg g−1 for p-nitrophenol was observed.166 PANI was combined with titanium dioxide to remove methyl orange dye. In this study, physical adsorption occurred between the dye molecules and the adsorbent.167 In another study, polyacrylonitrile/PANI/TiO2 fiber composite was synthesized through the electrospinning method. The Langmuir adsorption isotherm was the best fit for the adsorption process between congo red dye and the adsorbent. This membrane was also used to remove chromium ions from water sources.168
Nickel oxide and manganese oxide were incorporated into PANI to remove methyl orange dye through chemical oxidative polymerization of aniline using ammonium persulfate as the oxidant. Pure PANI has a removal efficiency of 53%, whereas the metal oxides-doped PANI showed an enhanced removal efficiency of 97%. The maximum adsorption capacity of metal oxides doped PANI was found to be 248.4 mg g−1.169 Iron, manganese, and zirconium oxides were combined with PANI for the removal studies of methyl red dye. A maximum adsorption capacity of 434.7 mg g−1 was seen for the adsorbent.170 PANI/CuO/ZnO composite was synthesized to carry out the adsorptive removal of congo red dye. The negative values for the Gibbs free energy data suggest the spontaneity of the adsorption process.171 Iron oxide, zirconium oxide, and manganese ferrite were incorporated into the PANI matrix to study the adsorptive efficiency of a dye solution containing methyl orange and eosin yellow dyes.172 Hollow PANI combined with manganese dioxide and iron oxide has been utilized for the adsorptive removal of malachite green and congo red dyes (Fig. 9).173 From the studies, it is understood that PANI is an excellent candidate for various energy and environmental applications. Table 2 gives details of the adsorption studies reported using polymer composites.
 | ||
| Fig. 9 Adsorptive studies using PANI/manganese dioxide/iron oxide on toxic dyes. Reproduced from ref. 173 with permission from ACS, copyright 2021. | ||
| Sl. no. | Composite | Dye | Maximum adsorptive capacitance (mg g−1) | Ref. |
|---|---|---|---|---|
| 1 | PANI/zinc oxide | RedXGRL | 78.4 | 151 |
| 2 | PANI/zincoxide | BlueXGRRL | 55.8 | 152 |
| 3 | PANI/zinc oxide | Methyl orange | 240.8 | 154 |
| 4 | PANI/iron oxide | Basic blue | 78.1 | 156 |
| 5 | PANI/iron oxide | Acid blue 40 | 216.9 | 157 |
| 6 | PANI/nickel oxide | Resorcinol | 19.5 | 159 |
| 7 | PANI/nickel oxide/manganese oxide | Methyl orange | 248.4 | 162 |
Polypyrrole composites for energy and environmental applications
PPy was successfully synthesized by Donald Weiss and his team in 1963. In 1915, Angelo Angeli had already reported the synthesis of Pyrrole black, which was the early form of PPy.174 The good conducting nature, electrochemical behavior, and excellent surface area of PPy have allowed scientists to use composites with PPy for energy applications.Polypyrrole composites for supercapacitance studies
Introducing metal oxides into PPy has improved the efficiency of composites in applications for energy storage. The incorporation of molybdenum oxide into the PPy matrix was studied by M. Sun et al. The high CSP and layered structure of this transition metal oxide, along with the fast charging–discharging capacity of PPy makes it an efficient candidate in charge storage applications.175 Bismuth oxide was also utilized to combine with PPy composite to be used as an electrode for supercapacitance study. The electrolyte used in this study was lithium sulfate, and the CS shown by this compound was found to be 360 F g−1.176 Gadolinium oxide was combined with PPy and polyindole to check the supercapacitance activity. It was found that the activity of the PPy composite was comparatively higher than that of the polyindole composite. The PPy composite showed a CSP of 341.6 F g−1, whereas the polyindole composite showed a lesser CSP of 305.5 F g−1. The GCD curves of this study confirm the pseudocapacitance nature of this composite.177 Manganese oxide on carbon cloth was utilized as the cathode, and the PPy/Mxene composite was utilized as the anode in a study to form asymmetric supercapacitors. This fabricated energy storage device gave a CSP of 75.3 F g−1 at a scan rate of 10 mV s−1.32 Iron oxide combined with PPy on carbon cloth showed a CSP of 640 F g−1 at 1 mA cm−2.101 Iron oxide derived from the used ink cartridges is combined with PPy, which showed a CSP of 319 F g−1 when a 4 M KOH was used as the electrolyte.100Cobalt oxide, which is flower shaped, was combined with PPy and showed a CSP of 6.81 F cm−2 at a CD of 5 mA cm−2. It also demonstrated a capacitance retention of 95% at 1000 cycles.178 PPy, when combined with nickel oxide, has a CSP of 595 F g−1 with a capacitance retention of 80.7% after 1000 cycles was observed.179 PPy incorporated with vanadium pentoxide showed a CSP of 172 F g−1.180 PPy was combined with honeycomb-like hematite to function as an electrode for studies related to storing energy. This PPy/iron oxide composite showed a CSP of 1167.8 F g−1 at a CD of 1 A g−1. The retention of capacitance of this composite was studied for 3000 cycles, and it showed a capacitance retention of 97.1%.181 Cobalt oxide, when combined with PPy, showed a CSP of 155.5 mA h g−1.182 A highly porous cobalt oxide was combined with PPy for a supercapacitance study. The electrode made by this composite exhibited a CSP of 1292.2 F g−1 at a CD of 1 A g−1.183 In another study, porous iron oxide was incorporated into the PPy matrix, which produced an extensive conductive network for energy storage applications. This composite showed a CSP of 215.12 mF cm−2 at 1.1 mA cm−2.184
The carbon-based materials were also combined with PPy to act as electrodes for supercapacitance studies. Carbon nanotubes combined with PPy/manganese oxide showed a CSP of 214 F g−1. The carbon nanotube acted as flexible and conductive backing in this composite, whereas the role of metal oxide was to increase the surface area. The interface of PPy also plays a vital role in the energy storage activity of the composite.185 Multiwalled carbon nanotube and manganese oxide were incorporated into the PPy matrix, which demonstrated a CSP of 272.7 F g−1.186 PPy/manganese oxide/graphene/copper hydroxide composite was synthesized by H. N. Miankushki et al. In this study, the prepared composite showed a CSP of 370 F g−1 in 6 M KOH solution.187 PPy was combined with iron oxide and GO, which showed a CSP of 163 F g−1 at 10 A g−1. The electrolyte used in this study was lithium nitrate.188 The study on the energy storage capacity of PPy/rGO doped manganese ferrite was carried out by S. Ishaq. It was found that the composite demonstrated a CSP of 232 F g−1 at a scan rate of 5 mV s−1.189 One pot hydrothermal method was utilized to synthesize PPy/rGO/cobalt oxide composite, which was used for supercapacitor applications. This study analyzed the influence of an anodic surfactant (sodium lauryl sulfate) on the synthesized composite. It was seen that with the addition of surfactant, the CSP values increased from 1206 to 1663 F g−1.190 PPy/manganese oxide combined with carbon fiber paper was developed as a flexible supercapacitor with a CSP of 69.3 F cm−3 (Fig. 10a–d).191
 | ||
| Fig. 10 Supercapacitance studies using a flexible PPy/manganese oxide combined with carbon fiber supercapacitor. Reproduced from ref. 191 with permission from Nature, copyright 2013. | ||
The ternary composites using PPy also showed good results in supercapacitance studies. Zinc oxide and tin oxide were combined with GO/PPy by V. Molahalli et al. The composite showed a CSP of 392 F g−1 at a 75 mV s−1 scan rate.192 The silver oxide was combined with PPy/Zn–Co MOF, which showed a CSP of 420 F g−1 at a CD of 1 A g−1. This composite showed a cyclic stability of 84.5% capacitive retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.193 In another study, PPy was combined with rGO and iron oxide, which gave CSP of 442 F g−1 at a CD of 1 A g−1.194 Nitrogen–sulfur co-doped iron oxide was combined with PPy for supercapacitor applications. The CSP demonstrated by this composite was 866 F g−1 at 1 A g−1.195 A co-electrodeposition method was carried out to synthesize PPy/nickel oxide/cobalt oxide in the form of nanosheets. The electrode using this composite demonstrated a CSP of 1123 F g−1 at a CD of 1 A g−1.196 PPy based composites showed efficiency in energy storage studies. Table 3 gives details of the supercapacitance studies reported using polymer composites.
000 cycles.193 In another study, PPy was combined with rGO and iron oxide, which gave CSP of 442 F g−1 at a CD of 1 A g−1.194 Nitrogen–sulfur co-doped iron oxide was combined with PPy for supercapacitor applications. The CSP demonstrated by this composite was 866 F g−1 at 1 A g−1.195 A co-electrodeposition method was carried out to synthesize PPy/nickel oxide/cobalt oxide in the form of nanosheets. The electrode using this composite demonstrated a CSP of 1123 F g−1 at a CD of 1 A g−1.196 PPy based composites showed efficiency in energy storage studies. Table 3 gives details of the supercapacitance studies reported using polymer composites.
| Sl. no. | Composite | Specific capacitance (F g−1) | Ref. |
|---|---|---|---|
| 1 | PANI/iron oxide | 286.0 | 93 |
| 2 | PANI/copper oxide | 376.0 | 102 |
| 3 | PANI/manganese oxide | 115.7 | 104 |
| 4 | PANI/cobalt oxide | 985.0 | 107 |
| 5 | PANI/manganese oxide | 665.0 | 110 |
| 6 | PANI/zinc–cobalt oxide | 1055.0 | 106 |
| 7 | PANI/copper oxide-rGO | 534.5 | 113 |
| 8 | PANI/titanium oxide/tin oxide | 540.0 | 114 |
| 9 | PANI/manganese oxide/GO | 829.0 | 115 |
| 10 | PANI/ruthenium–cobalt oxide | 1028.7 | 121 |
| 11 | PANI/Ni–Co–Mn–MOF/rGO | 1007.0 | 127 |
| 12 | PPy/bismuth oxide | 360.0 | 169 |
| 13 | PPy/gadolinium oxide | 341.6 | 170 |
| 14 | PPy/cobalt oxide | 1292.2 | 176 |
| 15 | PPy/manganese oxide–carbon nanotubes | 214.0 | 178 |
| 16 | PPy/rGO/manganese ferrite | 232.0 | 182 |
| 17 | PPy/rGO/cobalt oxide | 1663.0 | 183 |
| 18 | PPy/nickel oxide/cobalt oxide | 1123.0 | 189 |
PPy-based metal oxide composites have also exhibited excellent electrochemical behaviour, with certain combinations outperforming even their PANI counterparts. In particular, the PPy/rGO/cobalt oxide composite achieved an outstanding CSP of 1663.0 F g−1, followed by PPy/cobalt oxide (1292.2 F g−1) and PPy/nickel oxide/cobalt oxide (1123.0 F g−1), highlighting the efficiency of cobalt-rich systems in enhancing charge storage. The addition of nanostructured oxides, carbon nanotubes, and rGO to the PPy matrix facilitates improved electron transport, mechanical strength, and electroactive surface area. These findings underscore the versatility of PPy as a conductive polymer and its potential to be engineered with advanced nanostructures for sustainable and efficient supercapacitor applications.
Polypyrrole composites for water splitting studies
The water splitting studies were also carried out using various PPy based composites. Composites of PPy combined with metal oxides are reported for energy production studies. Samarium oxide was combined with PPy to be used as a bifunctional catalyst through the hydrothermal method for both HER and OER applications. The OPs required by the catalyst for HER and OER were observed as 206 and 272 mV at a CD of 10 mA cm−2.197 Nickel/nickel ferrite combined with PPy was utilized for overall water splitting. The presence of PPy improves the rate of flow of ions and their interaction between the electrode and the electrolyte. The interaction between the conducting polymer and the nickel/nickel ferrite increases the reaction efficiency of the surface active sites. The OPs required for HER and OER by the catalyst were found to be 127 and 265 mV at a CD of 10 mA cm−2.198 Cobalt oxide was combined with PPy for OER studies, and it showed an OP of 610 mV. The metal oxide enhances the electrocatalytic active surface area and electrochemical activity of the whole system.199 When iridium oxide was combined with PPy, an OP of 291.3 mV was required to reach 10 mA cm−2.200 Nitrogen-doped carbon and cobalt oxide were combined and incorporated into PPy for electrochemical OER studies. This composite required an OP of 120 mV for OER analysis.201 Nickel oxide was combined with PPy to be used as an electrocatalyst for OER analysis through the electrodeposition technique. It required an OP of 610 mV at a pH of 9.2.202PPy was incorporated with walnut-shaped nickel hydroxide for OER studies. It showed an OP of 461 mV and a Tafel slope value of 89 mV dec−1.203 Flower shaped cobalt–nickel sulfide/PPy composite was synthesized for utilization in water splitting reactions. It was observed that the OPs required by the catalysts were 63 and 207 mV for HER and OER, respectively.204 Iron–phosphorus nanorods were incorporated into PPy for HER studies, which required an OP of 103.1 mV.205 An electrodeposition technique was carried out for the synthesis of PPy, which was introduced with phosphotungstic anion on nickel foam. The OPs required by the catalyst were found to be 48 and 236 mV at a CD of 10 mA cm−2 for HER and OER, respectively.206 PPy was combined with iron, tungsten, and cobalt doped cobalt oxide for OER analysis, which showed an OP of 400 mV to reach 10 mA cm−2 CD.207 Cobalt phosphide containing PPy was utilized to synthesize N–O–P-doped hollow carbon composite through pyrolysis. This was utilized for HER analysis with an OP of 290 mV.208 PPy/zinc tungstate was synthesized as a bifunctional electrocatalyst for water splitting with OPs of 76 and 84 mV towards Her and OER, respectively.209
In another study, PPy was combined with cobalt–nickel ferrite and rGO to obtain a catalyst for OER study. It required an OP of 371 mV for OER analysis.210 Cobalt oxide incorporated PPy/carbon nanocomposite was utilized as an electrode material for water splitting, which required OPs of 340 and 490 mV for OER and HER, respectively.211 PPy/ZIF-67 MOF was utilized for the synthesis of cobalt oxide/nitrogen-doped carbon nanotubes. The synthesized electrocatalyst required an OP of 200 mV for OER analysis.212 PPy/cobalt hydroxide/rGO required an OP of 350 mV to attain a CD of 10 mA cm−2 during OER analysis.213 The combination of PPy with various metal oxides, heteroatoms, carbonaceous compounds, and MOFs enhances the properties of the polymer for water splitting studies. Table 4 gives details of the electrocatalytic water splitting studies reported using polymer composites.
| Sl. No. | Composite | Overpotenital (mV) | Ref. | |
|---|---|---|---|---|
| HER | OER | |||
| 1 | PANI | 690 | 133 | |
| 2 | PANI/tungstic acid | 220 | 128 | |
| 3 | PANI/iron | 235 | 129 | |
| 4 | Nitrogen doped-cobalt nickel nitride | 68 | 247 | 130 |
| 5 | PANI/nickel | 190 | 360 | 135 |
| 6 | PANI/nickel–cobalt phosphide | 80.6 | 136 | |
| 7 | PANI/carbon dots | 65 | 150 | 137 |
| 8 | PANI/ruthenium | 240 | 138 | |
| 9 | PANI/rGO/copper–molybdenum sulfide | 395 | 146 | |
| 10 | PANI/manganese molybdenum oxide | 155 | 410 | 147 |
| 11 | PPy/samarium oxide | 206 | 272 | 190 |
| 12 | PPy/nickel–nickel ferrite | 127 | 265 | 191 |
| 13 | PPy/cobalt oxide | 610 | 192 | |
| 14 | PPy/zinc tungstate | 76 | 84 | 202 |
| 15 | PPy/cobalt nickel ferrite/rGO | 371 | 203 | |
| 16 | PPy/cobalt hydroxide/rGO | 350 | 206 | |
| 17 | PPy/carbon–cobalt oxide | 490 | 340 | 204 |
PPy-based composites also exhibit promising HER and OER catalytic behavior. Notably, PPy/zinc tungstate demonstrates excellent bifunctional activity with very low OPs (76 mV for HER and 84 mV for OER), highlighting its efficiency. Other HER-active materials include PPy/nickel–nickel ferrite (127 mV) and PPy/samarium oxide (206 mV), while for OER, PPy/cobalt hydroxide/rGO (350 mV) and PPy/cobalt nickel ferrite/rGO (371 mV) display respectable values. However, some composites like PPy/cobalt oxide show a higher OER OP (610 mV), indicating room for improvement. The integration of rare-earth oxides, ferrites, and rGO into the PPy matrix enhances conductivity, electrochemical activity, and stability, confirming the viability of PPy-based composites for sustainable water electrolysis applications.
Other polymer composites for energy and environmental applications
Many other polymers like PEDOT, chitosan, and polythiophene were also reported for energy and environmental applications. The composites of these polymers were synthesized by incorporating various substances like metal oxides, carbonaceous compounds like graphene, and heteroatoms.PEDOT composites
The supercapacitance studies using PEDOT based composites have caught the attention of researchers. A fiber-based supercapacitor was developed by combining PEDOT with vanadium pentoxide, showing a CSP of 60 mF cm−2.214 Copper oxide nanowires were incorporated into the PEDOT matrix, which showed a remarkable CSP of 907.5 mF cm−2 at a CD of 3 mA cm−2.215 Cobalt ferrite was introduced into PEDOT to analyze the efficiency of charge storage capacity. It showed a CS of 299.2 mA h g−1 at a CD of 1 A g−1. A cyclic stability of 91.7% capacitance retention was seen after 2000 cycles for this composite.216 PEDOT, when combined with molybdenum oxide, shows a CSP of 365 F g−1 at 1 A g−1.217 When iron oxide is incorporated into PEDOT, a CSP of 338 mF cm−2 at a CD of 1 mA cm−2 is obtained.218 PEDOT was combined with tin oxide and evaluated for charge storage applications in 2 M sulphuric acid. The composite showed a CSP of 126 F g−1, which was evident from the GCD studies.219 In 2014, cobalt oxide nanowires and manganese oxide were introduced into PEDOT to analyze its efficiency towards supercapacitor application.220 In 2008, F. J. Liu introduced manganese oxide into PEDOT for energy storage studies. This composite showed a maximum CSP of 372 F g−1.221 Manganese oxide is a common metal oxide used for various energy storage applications and is combined with PEDOT for synthesizing efficient composites for supercapacitance applications.222–224When carbon nanotubes were introduced into PEDOT/MnO2, an even higher CSP of 427 F g−1 was obtained.225 Iron oxide, when combined with graphene and PEDOT showed a CSP of 153 F g−1 at 0.1 A g−1. The cyclic stability was only seen up to 3500 cycles for this composite.226 PEDOT/graphene/molybdenum oxide flexible supercapacitor was fabricated, and it showed a CSP of 94 F g−1. The role of PEDOT in this study was to behave as both a current collector and a binder.102 When combined with titanium oxide and rGO, PEDOT showed a CSP of 102.6 F g−1. The binary rGO/titanium oxide in this study showed a CSP of 98.8 F g−1. The improvement in the CSP of the final composite is due to the addition of a conducting polymer.227 Nickel cobalt ferrite, when combined with rGO and PEDOT, gave a CSP of 1286 F g−1. The synergistic effect between the carbonaceous compound, conducting polymer, and nickel cobalt ferrite is responsible for the better efficiency towards energy storage capacity.228
The HER studies using PEDOT composites were also reported. Pt/sulfonated graphene sheets/PEDOT were synthesized for HER analysis, and it was found that a lower OP of 40 mV was required to reach a CD of 10 mA cm−2. Even though the composite shows efficiency towards HER studies, the high cost of Pt makes it less feasible.229 Pt/polyacrylic acid/PEDOT composite was utilized for HER analysis, which required an OP of 84 mV.230 Cobalt–molybdenum selenide combined with PEDOT was utilized for overall water splitting. The OPs required for HER and OER were 20 and 153 mV, respectively.231 Cobalt–nickel sulfide/PEDOT composite was also utilized as a bifunctional electrocatalyst, which required OPs of 90 and 270 mV for HER and OER, respectively.232 The synergy between the various compounds incorporated into the polymer matrix and the PEDOT polymer has improved the electrochemical efficiency of the polymers.
Chitosan composites
The chitosan composites have been extensively used in anticorrosion studies. There are also energy-related studies that have been reported using chitosan composites. The composites of chitosan with metal oxides are used for supercapacitance and water splitting studies. Chitosan/iron oxide combined with activated carbon was used as an electrode for supercapacitor application. This electrode showed a CSP of 54.4 F g−1.233 Nitrogen-doped nickel oxide incorporated into porous carbon structure derived from nitrogen-doped chitosan was utilized for supercapacitance studies, which showed a CSP of 614.6 F g−1.234 When combined with copper oxide and zinc oxide, chitosan showed a CSP of 638.3 F g−1 and a capacitance retention of 86.98% at 5000 cycles.235 Cobalt/nickel oxide combined with graphitic porous carbon composite was derived from chitosan and was utilized for energy storage studies. It showed a CSP of 1266.7 F g−1 at a CD of 1 A g−1.236 Copper oxide and copper metal were combined with chitosan to study the energy storage capacity, and it showed a CSP of 2479 F g−1 at 0.5 A g−1.237 Nickel cobalt oxide sulfide used as an electrode for the supercapacitance study showed an energy density of 55.3 W h kg−1 at a power density of 400 W kg−1.238 Manganese cobalt oxide nanoneedle combined with carbon aerogel derived from chitosan showed an energy density of 84.3 W h kg−1 at a power density of 600 W kg−1.239The chitosan composites combined with metal oxides and heteroatoms were utilized for electrocatalytic water splitting studies. Nickel cobaltite combined with chitosan was utilized as the electrode for electrocatalytic water splitting. The OPs required for HER and OER were observed as 240 and 310 mV, respectively.240 In another study, manganese cobalt oxide was combined with chitosan for the water splitting reaction. The overall water splitting reaction required a potential of 1.57 V to reach a CD of 10 mA cm−2. The OPs required for HER and OER were found to be 180 and 258 mV, respectively.241 Chitosan/Prussian blue nanocomposite was coated on nickel foam to be used as an electrocatalyst for water-splitting studies. It was observed that OPs of 146 and 272 mV were required for HER and OER analysis, respectively, to reach a CD of 10 mA cm−2.242 Chitosan combined with nickel oxide required an OP of 240 mV for OER analysis.243 Nitrogen-doped chitosan matrix with tungsten oxide was utilized for overall water splitting, with OPs of 60 and 306 mV towards HER and OER, respectively.244
Chitosan is a polymer that is extensively used for corrosion inhibition studies. Many studies have reported using various chitosan composites for mitigating the corrosion effect. The inhibitors prevent the metal surface from undergoing corrosion (Fig. 11).245 The chitosan was evaluated as an inhibitor on mild steel under acidic conditions, which showed an inhibition efficiency of 77%.103 The combination of chitosan and metal oxides has been proven as an effective candidate in corrosion inhibition studies. Chitosan/TiO2 composite was utilized as an inhibitor on mild steel under acidic conditions, which showed an inhibition efficiency of 97.1%.246 Chitosan was combined with iron oxide to evaluate its efficiency in an acidic medium. The medium used was 15% HCl, and the composite showed an inhibition efficiency of 95.4%.246 When copper oxide was combined with chitosan, the inhibition efficiency was 90.3% when studied under a 5% HCl medium on carbon steel.63 Chitosan/GO/zinc oxide composite was evaluated for corrosion inhibition, and an inhibition efficiency of 85.6% was found in 1 M HCl solution. Thus, it is understood that the combination of polymers with suitable materials will enhance the efficiency towards energy and environmental applications.
 | ||
| Fig. 11 Illustration of the inhibition action on metal surface. Reproduced from ref. 245 with permission from ACS, copyright 2024. | ||
Conclusions
This review discussed the importance of polymer based composites for energy and environmental applications. Polymer-based composites have shown significant promise in advancing energy and environmental technologies. Their customizable properties, including high surface area and conductivity, have led to notable improvements in energy storage within supercapacitors. In the realm of water splitting, these composites have enhanced catalytic efficiency, thereby supporting more effective hydrogen production. When applied to adsorption, polymer composites exhibit high selectivity and capacity for removing pollutants, making them highly effective for water treatment and environmental cleanup. Likewise, in corrosion inhibition, the incorporation of polymers into composite materials offers a sustainable and efficient solution for protecting metal surfaces. These diverse applications highlight the flexibility and potential of polymer-based composites in tackling critical energy and environmental challenges.The future of polymer-based composites in energy and environmental applications depends on advancing their performance through innovative material design and fabrication methods. Breakthroughs in nanotechnology, molecular engineering, and hybrid systems are expected to yield composites with greater efficiency and multifunctionality. Additionally, developing biodegradable and renewable polymers will be crucial for aligning these materials with sustainability objectives. Ensuring that these composites are scalable, cost-effective, and ready for real-world applications will be key to moving them from the lab to industry. As the demand for clean energy and environmental solutions continues to grow, polymer-based composites are set to play a pivotal role in shaping next-generation technologies.
Author contributions
Arun Varghese (Scopus ID-57205701115, ORCiD-0000-0002-6298-6739) conceptualization, data curation, methodology, writing – original draft. Kalathiparambil Rajendra Pai Sunajadevi (Scopus ID-55450509500, ORCiD-0000-0001-7826-1620) investigation, supervision, writing – reviewing and editing. Dephan Pinheiro (Scopus ID-57203248645, ORCiD-0000-0002-1921-248X) writing – reviewing and editing.Data availability
The data will be made available on request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to Christ University for providing the resources and facilities.References
- P. Wang, H. Wang, W.-Q. Chen and S. Pauliuk, Fundam. Res., 2022, 2, 392–395 Search PubMed.
- U. Riaz, S. M. Ashraf and J. Kashyap, Polym. – Plast. Technol. Eng., 2015, 54, 1850–1870 Search PubMed.
- N. K. Daniel, A. Varghese, K. R. Sunaja Devi, S. J. Chundattu and A. Sreekanth, Colloids Surf., A, 2024, 703, 135177 Search PubMed.
- Z. Wang and B. Mi, Environ. Sci. Technol., 2017, 51, 8229–8244 Search PubMed.
- K. P. Chaitra, T. P. Vinod, A. Varghese and K. R. Sunajadevi, Chem. Eng. J., 2024, 499, 156049 Search PubMed.
- M. I. A. Abdel Maksoud, R. A. Fahim, A. E. Shalan, M. Abd Elkodous, S. O. Olojede, A. I. Osman, C. Farrell, A. H. Al-Muhtaseb, A. S. Awed, A. H. Ashour and D. W. Rooney, Environ. Chem. Lett., 2021, 19, 375–439 Search PubMed.
- A. Boretti, Int. J. Hydrogen Energy, 2022, 47, 14371–14374 Search PubMed.
- T. H. Le, Y. Kim and H. Yoon, Polymers, 2017, 9(4), 150 Search PubMed.
- G. Joseph, S. D. Kalathiparambil Rajendra Pai, A. Varghese, D. Pinheiro, M. K. Mohan and S. J. Chundattu, J. Mol. Struct., 2024, 1308, 138095 Search PubMed.
- A. Varghese and S. Devi K R, Adv. Sustainable Syst., 2024, 2300575, 1–11 Search PubMed.
- Sustainable Development Goals: Their Impacts on Forests and People, ed. P. Katila, C. J. Pierce Colfer, W. de Jong, G. Galloway, P. Pacheco and G. Winkel, Cambridge University Press, 2019 Search PubMed.
- J. D. Sachs, G. Schmidt-Traub, M. Mazzucato, D. Messner, N. Nakicenovic and J. Rockström, Nat. Sustainable, 2019, 2, 805–814 Search PubMed.
- W. Schramade, J. Appl. Corp. Financ., 2017, 29, 87–99 Search PubMed.
- L. Wang, Y. Zhang, L. Chen, H. Xu and Y. Xiong, Adv. Mater., 2018, 30, 1–12 Search PubMed.
- A. Fleming, R. M. Wise, H. Hansen and L. Sams, Mar. Policy, 2017, 86, 94–103 Search PubMed.
- R. B. Swain, A Critical Analysis of the Sustainable Development Goals, in Handbook of Sustainability Science and Research, ed. W. Leal Filho, World Sustainability Series, Springer, Cham, 2018, pp. 341–355 Search PubMed.
- S. Peter, N. Lyczko, D. Gopakumar, H. J. Maria, A. Nzihou and S. Thomas, Waste Biomass Valorization, 2021, 12, 4777–4804 Search PubMed.
- L. S. Anthony, M. Vasudevan, V. Perumal, M. Ovinis, P. B. Raja and T. N. J. I. Edison, J. Environ. Chem. Eng., 2021, 9, 105832 Search PubMed.
- X. Zhao, L. Lv, B. Pan, W. Zhang, S. Zhang and Q. Zhang, Chem. Eng. J., 2011, 170, 381–394 Search PubMed.
- D. Lasrado, S. Ahankari and K. Kar, J. Appl. Polym. Sci., 2020, 48959 Search PubMed.
- S. S. Siwal, Q. Zhang, N. Devi and V. K. Thakur, Polymers, 2020, 12, 505 Search PubMed.
- T. A. Saleh, N. P. Shetti, M. M. Shanbhag, K. Raghava Reddy and T. M. Aminabhavi, Mater. Sci. Energy Technol., 2020, 3, 515–525 Search PubMed.
- S. Kulandaivalu, Energies, 2019, 12, 2107 Search PubMed.
- A. G. Olabi, M. A. Abdelkareem, T. Wilberforce and E. T. Sayed, Renewable Sustainable Energy Rev., 2021, 135, 110026 Search PubMed.
- J. Theerthagiri, R. A. Senthil, P. Nithyadharseni, S. J. Lee, G. Durai, P. Kuppusami, J. Madhavan and M. Y. Choi, Ceram. Int., 2020, 46, 14317–14345 Search PubMed.
- Z. S. Iro, C. Subramani and S. S. Dash, Int. J. Electrochem. Sci., 2016, 11, 10628–10643 Search PubMed.
- J. Banerjee, K. Dutta, M. A. Kader and S. K. Nayak, Polym. Adv. Technol., 2019, 30, 1902–1921 Search PubMed.
- A. Dutta, S. Mitra, M. Basak and T. Banerjee, Energy Storage, 2022, 5(1), e339 Search PubMed.
- R. Vinodh, R. S. Babu, S. Sambasivam, C. V. V. M. Gopi, S. Alzahmi, H.-J. Kim, A. L. F. de Barros and I. M. Obaidat, Nanomaterials, 2022, 12, 1511 Search PubMed.
- A. Varghese, K. R. Sunaja Devi, S. Mathew, B. Saravanakumar and D. Pinheiro, Mater. Today Proc., 2023 DOI:10.1016/j.matpr.2023.12.003.
- A. Varghese, S. Devi K R, F. Kausar and D. Pinheiro, Mater. Today Chem., 2023, 29, 101424 Search PubMed.
- X. Li, H. Xie, Y. Feng, Y. Qu, L. Zhai, H. Sun, X. Liu and C. Hou, J. Mater. Sci.: Mater. Electron., 2023, 34, 1878 Search PubMed.
- T. Jayaraman, A. P. Murthy, V. Elakkiya, S. Chandrasekaran, P. Nithyadharseni, Z. Khan, R. A. Senthil, R. Shanker, M. Raghavender, P. Kuppusami, M. Jagannathan and M. Ashokkumar, J. Ind. Eng. Chem., 2018, 64, 16–59 Search PubMed.
- Z. Yin and Q. Zheng, Adv. Energy Mater., 2012, 2, 179–218 Search PubMed.
- A. Varghese, K. Rajendra, P. Sunajadevi, D. Pinheiro, B. Saravanakumar and M. Selvaraj, Int. J. Hydrogen Energy, 2025, 123, 238–246 Search PubMed.
- F. Kausar, A. Varghese, D. Pinheiro and S. Devi K R, Int. J. Hydrogen Energy, 2022, 47, 22371–22402 Search PubMed.
- B. You and Y. Sun, Acc. Chem. Res., 2018, 51, 1571–1580 Search PubMed.
- X. Peng, C. Pi, X. Zhang, S. Li, K. Huo and P. K. Chu, Sustainable Energy Fuels, 2019, 3, 366–381 Search PubMed.
- A. Varghese, S. Devi K R and D. Pinheiro, Int. J. Hydrogen Energy, 2023, 48(76), 29552–29564 Search PubMed.
- X. Luo, X. Tan, P. Ji, L. Chen, J. Yu and S. Mu, EnergyChem, 2023, 5, 100091 Search PubMed.
- S. Ji, Z. Yang, C. Zhang, Z. Liu, W. W. Tjiu, I. Y. Phang, Z. Zhang, J. Pan and T. Liu, Electrochim. Acta, 2013, 109, 269–275 Search PubMed.
- S. Anantharaj, S. R. Ede, K. Karthick, S. Sam Sankar, K. Sangeetha, P. E. Karthik and S. Kundu, Energy Environ. Sci., 2018, 11, 744–771 Search PubMed.
- X. Chen, J. Yang, Y. Cao, L. Kong and J. Huang, ChemElectroChem, 2021, 8, 4427–4440 Search PubMed.
- M. Nemiwal, T. C. Zhang and D. Kumar, Int. J. Hydrogen Energy, 2021, 46, 21401–21418 Search PubMed.
- A. Varghese, S. Devi K R, D. Pinheiro and M. K. Mohan, Surf. Interfaces, 2023, 41, 103221 Search PubMed.
- P. P. Nayak, A. Varghese, S. Devi K R and D. Pinheiro, J. Appl. Polym. Sci., 2024, 141, 1–12 Search PubMed.
- G. Ö. Kayan and A. Kayan, J. Polym. Environ., 2021, 29, 3477–3496 Search PubMed.
- A. Kayan, Adv. Compos. Hybrid Mater., 2019, 2, 34–45 Search PubMed.
- S. Jadoun, J. P. Fuentes, B. F. Urbano and J. Yáñez, J. Environ. Chem. Eng., 2023, 11, 109226 Search PubMed.
- M. Ahmadian and M. Jaymand, Coord. Chem. Rev., 2023, 486, 215152 Search PubMed.
- S. Samsami, M. Mohamadizaniani, M.-H. Sarrafzadeh, E. R. Rene and M. Firoozbahr, Process Saf. Environ. Prot., 2020, 143, 138–163 Search PubMed.
- S. Mathew, A. Varghese, S. Devi K. R. and D. Pinheiro, Next Sustainable, 2024, 4, 100049 Search PubMed.
- A. Y. W. Sham and S. M. Notley, J. Environ. Chem. Eng., 2018, 6, 495–504 Search PubMed.
- A. Varghese, K. R. Sunaja Devi and D. Pinheiro, Mater. Today Commun., 2023, 35, 105739 Search PubMed.
- P. E. Ohale, K. Chukwudi, J. N. Ndive, M. E. Michael, M. N. Abonyi, M. M. Chukwu, C. C. Obi, C. E. Onu, C. A. Igwegbe and C. O. Azie, Results Surf. Interfaces, 2023, 13, 100157 Search PubMed.
- J. P. Gaspard, Interfacial Aspects of Phase Transformations, Springer Netherlands, Dordrecht, 1982, pp. 103–118 Search PubMed.
- S. Singh, S. Perween and A. Ranjan, J. Environ. Chem. Eng., 2021, 9, 105149 Search PubMed.
- M. T. Rahman, T. Kameda, S. Kumagai and T. Yoshioka, React. Kinet., Mech. Catal., 2017, 120, 703–714 Search PubMed.
- Z. Wang, X. Wang and Y. Yang, Adv. Mater., 2024, 36(4), 2301721 Search PubMed.
- M. T. Baig and A. Kayan, Int. J. Biol. Macromol., 2024, 280, 135676 Search PubMed.
- A. Intisar, A. Ramzan, S. Hafeez, N. Hussain, M. Irfan, N. Shakeel, K. A. Gill, A. Iqbal, M. Janczarek and T. Jesionowski, Chemosphere, 2023, 336, 139203 Search PubMed.
- H. Wang, D. Gao, Y. Meng, H. Wang, E. Wang and Y. Zhu, Prog. Org. Coat., 2015, 82, 74–80 Search PubMed.
- P. S. Umoren, D. Kavaz and S. A. Umoren, Sustainability, 2022, 14, 7981 Search PubMed.
- A. K. Keerthana and P. M. Ashraf, Appl. Nanosci., 2020, 10, 1061–1071 Search PubMed.
- L. Guo, W. Dong and S. Zhang, RSC Adv., 2014, 4, 41956–41967 Search PubMed.
- S. John, A. Joseph, M. Kuruvilla and T. Sajini, J. Bio-Tribo-Corrosion, 2017, 3, 3 Search PubMed.
- A. Varghese, K. R. Sunaja Devi, D. Pinheiro and J. Jomy, J. Environ. Chem. Eng., 2024, 113824 Search PubMed.
- Q. Wang, R. Wang, Q. Zhang, C. Zhao, X. Zhou, H. Zheng, R. Zhang, Y. Sun and Z. Yan, Molecules, 2023, 28, 2832 Search PubMed.
- P. Kesari, G. Udayabhanu, A. Roy and S. Pal, Int. J. Biol. Macromol., 2023, 225, 1323–1349 Search PubMed.
- W. v Baeckmann, Handbook of Cathodic Corrosion Protection, Elsevier, 1997, pp. 1–26 Search PubMed.
- S. Sivakumar, P. K. Selvaraj and S. Selvaraj, Asian J. Chem., 2018, 30, 2043–2048 Search PubMed.
- H. R. Bakhsheshi-Rad, E. Hamzah, W. S. Ying, M. Razzaghi, S. Sharif, A. F. Ismail and F. Berto, Materials, 2021, 14, 1930 Search PubMed.
- P. D. Desai, C. B. Pawar, M. S. Avhad and A. P. More, Vietnam J. Chem., 2023, 61, 15–42 Search PubMed.
- X. Lu, W. Zhang, C. Wang, T. Wen and Y. Wei, Prog. Polym. Sci., 2011, 36, 671–712 Search PubMed.
- C. K. Chiang, S. C. Gau, C. R. Fincher, Y. W. Park and A. G. Macdiarmid, Phys. Rev. Lett., 1977, 39, 1098–1101 Search PubMed.
- S. Mallakpour, M. Dinari and E. Azadi, Int. J. Polym. Anal. Charact., 2015, 20, 82–97 Search PubMed.
- Y. Li, Y. Yu, L. Wu and J. Zhi, Appl. Surf. Sci., 2013, 273, 135–143 Search PubMed.
- K. M. Ismayil, A. Varghese and R. Antony, J. Elastomers Plast., 2020, 52, 103–116 Search PubMed.
- C. Ramesh, M. Hariprasad, V. Ragunathan and N. Jayakumar, Eur. J. Appl. Eng. Sci. Res., 2012, 1, 201–206 Search PubMed.
- X. Zang, R. Jiang, H.-Y. Zhu, Q. Wang, Y.-Q. Fu, D.-X. Zhao, J.-B. Li and H. Liu, Sep. Purif. Technol., 2024, 330, 125521 Search PubMed.
- D. Tekin, D. Birhan and H. Kiziltas, Mater. Chem. Phys., 2020, 123067 Search PubMed.
- M. Deifallah, P. F. McMillan and F. Corà, J. Phys. Chem. C, 2008, 112, 5447–5453 Search PubMed.
- X. Zhou, F. Peng, H. Wang, H. Yu and Y. Fang, Chem. Commun., 2011, 47, 10323–10325 Search PubMed.
- X. Liu and L. Cai, Appl. Surf. Sci., 2018, 445, 242–254 Search PubMed.
- R. Nekooie, T. Shamspur and A. Mostafavi, J. Photochem. Photobiol., A, 2021, 407, 113038 Search PubMed.
- S. Rajendran, R. Pachaiappan, T. K. A. Hoang, S. Karthikeyan, L. Gnanasekaran, S. Vadivel, M. Soto-Moscoso and M. A. Gracia-Pinilla, J. Hazard. Mater., 2021, 416, 125989 Search PubMed.
- S. Vignesh, S. Suganthi, J. Kalyana Sundar, V. Raj and P. R. Indra Devi, Appl. Surf. Sci., 2019, 479, 914–929 Search PubMed.
- H. Ahmed, A. Hashim and H. M. Abduljalil, Egypt. J. Chem., 2019, 62, 1167–1176 Search PubMed.
- M. Soleimani, M. Ghorbani and S. Salahi, Int. J. Nanosci. Nanotechnol., 2016, 12, 191–197 Search PubMed.
- G. Sharma, D. Pathania, M. Naushad and N. C. Kothiyal, Chem. Eng. J., 2014, 251, 413–421 Search PubMed.
- I. Shown, A. Ganguly, L. C. Chen and K. H. Chen, Energy Sci. Eng., 2015, 3, 2–26 Search PubMed.
- W. Raza, F. Ali, N. Raza, Y. Luo, K. Kim and J. Yang, Nano Energy, 2018, 52, 441–473 Search PubMed.
- V. Kumaravel, S. C. Pillai and P. Forouzandeh, Catalysts, 2020, 10, 969 Search PubMed.
- G. Zhao, X. Huang, Z. Tang, Q. Huang, F. Niu and X. Wang, Polym. Chem., 2018, 9, 3562–3582 Search PubMed.
- D. Duranoĝlu, I. G. Buyruklardan Kaya, U. Beker and B. F. Şenkal, Chem. Eng. J., 2012, 181–182, 103–112 Search PubMed.
- L. Li, Z. Wang, P. Ma, H. Bai, W. Dong and M. Chen, J. Polym. Res., 2015, 22, 1–10 Search PubMed.
- R. C. Patil and S. Radhakrishnan, Prog. Org. Coat., 2006, 57, 332–336 Search PubMed.
- H. Albaris and G. Karuppasamy, J. Mater. Sci.: Mater. Electron., 2019, 30, 9989–9998 Search PubMed.
- Q. W. Zengyuan Pang, Q. Nie, P. Lv, J. Yu and F. Huang, IOPscience, 2017, 2, 0–31 Search PubMed.
- P. M. Jambhale, V. N. Narwade, M. Shariq, K. A. Bogle and M. D. Shirsat, J. Electron. Mater., 2024, 53, 3117–3127 Search PubMed.
- C. Wu, Z. Pei, M. Lv, D. Huang, Y. Wang and S. Yuan, Molecules, 2023, 28, 434 Search PubMed.
- S. L. Marasso, P. Rivolo, R. Giardi, D. Mombello, A. Gigot, M. Serrapede, S. Benetto, A. Enrico, M. Cocuzza, E. Tresso and C. F. Pirri, Mater. Res. Express, 2016, 3, 065001 Search PubMed.
- M. Srivastava, S. K. Srivastava, Nikhil, G. Ji and R. Prakash, Int. J. Biol. Macromol., 2019, 140, 177–187 Search PubMed.
- S. C. Rasmussen, An Int. J. Hist. Chem. Subst., 2017, 1, 99–109 Search PubMed.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 Search PubMed.
- M. Ayad and S. Zaghlol, Chem. Eng. J., 2012, 204–205, 79–86 Search PubMed.
- U. Gazal, I. Khan, M. A. Usmani and A. H. Bhat, Polymer-based Nanocomposites for Energy and Environmental Applications, Elsevier, 2018, pp. 315–332 Search PubMed.
- R. Sun, Y. Pei, H. Ren, Y. Jin, J. Hu, Q. Wang, X. Wang and M. Yan, J. Appl. Polym. Sci., 2023, 141(12), e55111 Search PubMed.
- P. Supchocksoonthorn, S. Witchakul, W. Pholauyphon, T. Jorn-am, P. Janpauk, N. Sirisit, X. Liang, S. Song, T. Sangtawesin and P. Paoprasert, J. Power Sources, 2023, 587, 233724 Search PubMed.
- D. J. Ahirrao and N. Jha, AIP Conf. Proc., 2017, 1832, 050168 Search PubMed.
- D. A. L. Almeida, C. O. P. Silva, D. A. Graves, R. M. Sanches, R. C. Firmino, A. F. Sardinha, N. G. Ferreira, M. Gonçalves, A. C. C. Migliano and E. S. Gonçalves, Electrochim. Acta, 2023, 472, 143369 Search PubMed.
- S. P. Ashokkumar, H. Vijeth, L. Yesappa, M. Niranjana, M. Vandana and H. Devendrappa, Inorg. Chem. Commun., 2020, 115, 107865 Search PubMed.
- B. Zhou, Z. Li, D. Qin, Q. Zhang, M. Yu and C. Yang, J. Alloys Compd., 2023, 956, 170327 Search PubMed.
- I. I. Misnon, K. Manickavasakam, N. Nordin and R. Jose, Int. J. Appl. Ceram. Technol., 2023, 20, 2030–2042 Search PubMed.
- Y. Wang, Y. Wang, C. Wang and Y. Wang, J. Compos. Sci., 2021, 5, 129 Search PubMed.
- T. Prasankumar, B. R. Wiston, C. R. Gautam, R. Ilangovan and S. P. Jose, J. Alloys Compd., 2018, 757, 466–475 Search PubMed.
- M. M. Mezgebe, K. Xu, G. Wei, S. Guang and H. Xu, J. Alloys Compd., 2019, 794, 634–644 Search PubMed.
- C. P. Sandhya, R. Baig E, S. Pillai, C. Molji, A. Aravind and S. J. Devaki, Electrochim. Acta, 2019, 324, 134876 Search PubMed.
- D. Qin, B. Zhou, Z. Li and C. Yang, J. Mol. Struct., 2024, 1309, 138140 Search PubMed.
- L. He, J. Liu, L. Yang, Y. Song, M. Wang, D. Peng, Z. Zhang and S. Fang, Electrochim. Acta, 2018, 275, 133–144 Search PubMed.
- R. Bolagam, R. Boddula and P. Srinivasan, ChemistrySelect, 2017, 2, 65–73 Search PubMed.
- K. Y. Yasoda, M. S. Kumar and S. K. Batabyal, Ionics, 2020, 26, 2493–2500 Search PubMed.
- G. Singh, Y. Kumar and S. Husain, Energy Storage, 2024, 6, e525 Search PubMed.
- Y. Ma, Y. Fu, N. Wei, J. Zhu, H. Niu, C. Qin and X. Jiang, J. Appl. Polym. Sci., 2023, 140, e54672 Search PubMed.
- X. Li, H. Xie, Y. Feng, Y. Qu, L. Zhai, H. Sun, X. Liu and C. Hou, J. Appl. Polym. Sci., 2023, 140, e54580 Search PubMed.
- S. Vargheese, G. Dhakal, R. S. Kumar, R. T. R. Kumar, J.-J. Shim and Y. Haldorai, J. Energy Storage, 2023, 71, 108145 Search PubMed.
- R. Kalpana and P. Subbramaniyan, Int. Res. J. Multidiscip. Technovation, 2024, 40–50 Search PubMed.
- T. Kavinkumar, T. R. Naveenkumar and B. Neppolian, J. Alloys Compd., 2024, 982, 173741 Search PubMed.
- A. S. Almalki, J. Mater. Sci.: Mater. Electron., 2024, 35, 581 Search PubMed.
- S. Sahoo, G. Dhakal, W. K. Kim, Y. R. Lee and J.-J. Shim, J. Energy Storage, 2023, 73, 109061 Search PubMed.
- A. R. Athira, T. Merin, K. Anupama, K. A. Ann Mary and T. S. Xavier, Diamond Relat. Mater., 2022, 130, 109475 Search PubMed.
- M. Naveed, T. Munawar, M. Shahid, F. Mukhtar, A. Maqbool, M. Riaz, S. Manzoor, M. Naeem and F. Iqbal, Ceram. Int., 2021, 47, 18497–18509 Search PubMed.
- Y. Sui, Y. Ma, Y. Gao, J. Song, Y. Ye, H. Niu, W. Ma, P. Zhang and C. Qin, New J. Chem., 2021, 45, 10654–10663 Search PubMed.
- M. A. Marwat, S. Ishfaq, K. M. Adam, B. Tahir, M. H. Shaikh, M. F. Khan, M. R. Abdul Karim, Z. U. Din, S. Abdullah and E. Ghazanfar, RSC Adv., 2024, 14, 2102–2115 Search PubMed.
- C. Liu, J. Zhou, Y. Xiao, L. Yang, D. Yang and D. Zhou, Int. J. Hydrogen Energy, 2017, 42, 29781–29790 Search PubMed.
- Y. Dong, J. Feng and G. Li, Macromol. Chem. Phys., 2017, 18(22), 1700359 Search PubMed.
- C. Ray, S. C. Lee, B. Jin, A. Kundu, J. H. Park and S. Chan Jun, J. Mater. Chem. A, 2018, 6, 4466–4476 Search PubMed.
- J.-T. Ren, L. Chen, C.-C. Weng, G.-G. Yuan and Z.-Y. Yuan, ACS Appl. Mater. Interfaces, 2018, 10, 33276–33286 Search PubMed.
- J. Tong, T. Li, L. Bo, W. Li, Y. Li and Y. Zhang, ChemElectroChem, 2019, 6, 3437–3444 Search PubMed.
- R. Djara, Y. Holade, A. Merzouki, N. Masquelez, D. Cot, B. Rebiere, E. Petit, P. Huguet, C. Canaff, S. Morisset, T. W. Napporn, D. Cornu and S. Tingry, J. Electrochem. Soc., 2020, 167, 066503 Search PubMed.
- J. Zhang and L. Dai, Angew. Chem., Int. Ed., 2016, 55, 13296–13300 Search PubMed.
- R. Djara, Y. Holade, A. Merzouki, M.-A. Lacour, N. Masquelez, V. Flaud, D. Cot, B. Rebiere, A. van der Lee, J. Cambedouzou, P. Huguet, S. Tingry and D. Cornu, Front. Chem., 2020, 8, 385 Search PubMed.
- J. Zhang, Y. Li, Z. Wang, Y. Wang, F. Wang and M. Chen, Nanotechnology, 2020, 31, 445401 Search PubMed.
- X. Gu, Z. Chen, Y. Li, J. Wu, X. Wang, H. Huang, Y. Liu, B. Dong, M. Shao and Z. Kang, ACS Appl. Mater. Interfaces, 2021, 13, 24814–24823 Search PubMed.
- R. Djara, M.-A. Lacour, A. Merzouki, J. Cambedouzou, D. Cornu, S. Tingry and Y. Holade, Polymers, 2021, 13, 190 Search PubMed.
- J. Zhang, H. Zhang and Y. Huang, Appl. Catal., B, 2021, 297, 120453 Search PubMed.
- S. Mathew, J.-H. Sim, R. Rajmohan, O. L. Li and Y.-R. Cho, Electrochim. Acta, 2022, 403, 139586 Search PubMed.
- S. Sriram, S. Mathi, B. Vishnu, B. Karthikeyan and J. Jayabharathi, ChemistrySelect, 2022, 7(8), e202104516 Search PubMed.
- S. Cogal, G. Celik Cogal, M. Mičušík, M. Kotlár and M. Omastová, Int. J. Hydrogen Energy, 2024, 49, 689–700 Search PubMed.
- S. Cogal, G. Celik Cogal, M. Mičušík, A. Michalcova, M. Šlouf and M. Omastová, J. Electroanal. Chem., 2023, 946, 117728 Search PubMed.
- M. W. Fazal, F. Zafar, M. Asad, F. Mohammad, H. Al Sulami, H. Khalid, A. A. Abdelwahab, M. U. Ur Rehman, N. Akhtar, W. A. El-Said, S. Hussain and M. A. Shenashen, ACS Appl. Energy Mater., 2023, 6, 2739–2746 Search PubMed.
- M. Vijayarangan, S. Mathi, A. Gayathri, J. Jayabarathi and V. Thanikachalam, ChemistrySelect, 2022, 7(43), e202203206 Search PubMed.
- A. Singh, A. Singh, G. Kociok-Köhn, R. Bhimireddi, A. Singh, A. K. Singh, A. Kumar and M. Muddassir, Appl. Organomet. Chem., 2022, 36(6), e6683 Search PubMed.
- M. S. Tamboli, S. A. Patil, A. M. Tamboli, S. S. Patil, N. T. N. Truong, K. Lee, C. S. Praveen, N. K. Shrestha, C. Park and B. B. Kale, Dalton Trans., 2022, 51, 6027–6035 Search PubMed.
- A. M. Alenad, T. A. M. Taha, M. Abdullah, A. G. Abid, S. Manzoor, R. Y. Khosa, H. M. T. Farid, S. Trukhanov, M. I. Sayyed, D. Tishkevich and A. Trukhanov, J. Electroanal. Chem., 2023, 941, 117550 Search PubMed.
- S. S. Dakave, G. A. Bhinge, N. N. Kengar, A. D. Teli and C. M. Kanamadi, Mater. Today Proc., 2023 DOI:10.1016/j.matpr.2022.12.246.
- J. Zhang, X. Meng, J. Zhao and Z. Zhu, ChemCatChem, 2014, 6, 2059–2064 Search PubMed.
- T. Aliyam, R. Munir, G. Albasher, M. Zahid, T. Samreen, M. Ghamkhar, M. Yaseen, F. Younas and S. Noreen, Polym. Bull., 2024, 81, 8251–8284 Search PubMed.
- T. Aliyam, R. Munir, G. Albasher, M. Yaseen, M. Zahid, M. Shakeel and S. Noreen, Colloid Polym. Sci., 2024, 302, 409–431 Search PubMed.
- S. M. Alardhi, A. H. Abdalsalam, A. A. Ati, M. H. Abdulkareem, A. A. Ramadhan, M. M. Taki and Z. Y. Abbas, Polym. Bull., 2024, 81, 1131–1157 Search PubMed.
- A. Deb, M. Kanmani, A. Debnath, K. L. Bhowmik and B. Saha, Ultrason. Sonochem., 2019, 54, 290–301 Search PubMed.
- K. M. Obaid, A. S. Abbas and Y. F. Al-Khafaji, Indones. J. Chem., 2023, 23, 1270 Search PubMed.
- A. Muhammad, A.-H. A. Shah, S. Bilal and G. Rahman, Materials, 2019, 12, 1764 Search PubMed.
- A. Muhammad, A. ul H. A. Shah and S. Bilal, Materials, 2019, 12, 2854 Search PubMed.
- A. Muhammad, A. ul H. A. Shah and S. Bilal, Appl. Sci., 2020, 10, 2882 Search PubMed.
- B. Goswami and D. Mahanta, Colloids Surf., A, 2019, 582, 123843 Search PubMed.
- J. N. Ramteke, N. V. Nerkar and S. B. Kondawar, Springer Proceedings in Physics, 2020, 242, pp. 87–94 Search PubMed.
- B. Xu, X. Wang, Y. Huang, J. Liu, D. Wang, S. Feng, X. Huang and H. Wang, Chem. Eng. J., 2020, 399, 125749 Search PubMed.
- L. I. A. Ali, H. K. Ismail, H. F. Alesary and H. Y. Aboul-Enein, Int. J. Environ. Sci. Technol., 2021, 18, 2031–2050 Search PubMed.
- B. Saha, A. Debnath and B. Saha, J. Indian Chem. Soc., 2022, 99, 100635 Search PubMed.
- T. B. Gelaw, B. K. Sarojini and A. K. Kodoth, J. Polym. Environ., 2022, 30, 4086–4101 Search PubMed.
- A. Deb, A. Debnath, K. Bhowmik, S. Rudra Paul and B. Saha, Int. J. Environ. Anal. Chem., 2023, 103, 5938–5956 Search PubMed.
- S. Dutta, S. K. Srivastava, B. Gupta and A. K. Gupta, ACS Appl. Mater. Interfaces, 2021, 13, 54324–54338 Search PubMed.
- C. Rasmussen Seth, Bull. Hist. Chem., 2015, 40, 45–55 Search PubMed.
- M. Sun, Y. Zhang, Z. Liang, R. Zhang, K. Zhang, Y. Lin, D. Wang and T. Xie, New J. Chem., 2024, 48, 6723–6732 Search PubMed.
- E. Karaca, J. Energy Storage, 2023, 71, 108210 Search PubMed.
- Y. Senjaliya, N. Oad, S. Chakroborty, B. Tripathi, P. Chandra, P. K. Tripathi, A. Solanki, I. A. Darwish, S. Rawat, A. Barik, R. Kapadia and N. Asthana, J. Mol. Struct., 2024, 1307, 137919 Search PubMed.
- X. Wang, L. Jia, Q. Liu, J. Liu, X. Guo, X. Jing and J. Wang, Colloids Surf., A, 2016, 506, 646–653 Search PubMed.
- W. Ji, J. Ji, X. Cui, J. Chen, D. Liu, H. Deng and Q. Fu, Chem. Commun., 2015, 51, 7669–7672 Search PubMed.
- X. Sun, Q. Li and Y. Mao, Electrochim. Acta, 2015, 174, 563–573 Search PubMed.
- P.-Y. Tang, L.-J. Han, A. Genç, Y.-M. He, X. Zhang, L. Zhang, J. R. Galán-Mascarós, J. R. Morante and J. Arbiol, Nano Energy, 2016, 22, 189–201 Search PubMed.
- C. Yang, H. Chen and C. Guan, Nanomaterials, 2019, 9, 586 Search PubMed.
- Z. Zhang, X. Su, Y. Zhu, Z. Fang, X. Luo and Z. Chen, Nanotechnology, 2020, 31, 255403 Search PubMed.
- Y. Xue, N. Gao, H. Ma, Y. Wang and C. Liu, Mater. Today Commun., 2024, 40, 109445 Search PubMed.
- P. Li, Y. Yang, E. Shi, Q. Shen, Y. Shang, S. Wu, J. Wei, K. Wang, H. Zhu, Q. Yuan, A. Cao and D. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 5228–5234 Search PubMed.
- A. H. P. de Oliveira, M. L. F. Nascimento and H. P. de Oliveira, Mater. Res., 2016, 19, 1080–1087 Search PubMed.
- H. N. Miankushki, A. Sedghi and S. Baghshahi, J. Solid State Electrochem., 2018, 22, 3317–3329 Search PubMed.
- C. R. Mariappan, V. Gajraj, S. Gade, A. Kumar, S. Dsoke, S. Indris, H. Ehrenberg, G. V. Prakash and R. Jose, J. Electroanal. Chem., 2019, 845, 72–83 Search PubMed.
- S. Ishaq, M. Moussa, F. Kanwal, M. Ehsan, M. Saleem, T. N. Van and D. Losic, Sci. Rep., 2019, 9, 5974 Search PubMed.
- A. R. Athira and T. S. Xavier, J. Mater. Sci.: Mater. Electron., 2024, 35, 509 Search PubMed.
- J. Tao, N. Liu, W. Ma, L. Ding, L. Li, J. Su and Y. Gao, Sci. Rep., 2013, 3, 2286 Search PubMed.
- V. Molahalli, V. S. Bhat, A. Shetty, D. Hundekal, A. Toghan and G. Hegde, J. Energy Storage, 2023, 69, 107953 Search PubMed.
- Q. Wang, C. Han, G. Tang, L. Liu, T. Li and Y. Han, J. Alloys Compd., 2023, 931, 167510 Search PubMed.
- J. Vigneshwaran, S. Abraham, B. Muniyandi, T. Prasankumar, J. T. Li and S. Jose, Surf. Interfaces, 2021, 27, 101572 Search PubMed.
- M. Zhu, Q. Chen, J. Tang, W. Wei and S. Li, Appl. Surf. Sci., 2019, 480, 582–592 Search PubMed.
- Z.-M. Shen, X.-J. Luo, Y.-Y. Zhu and Y.-S. Liu, J. Energy Storage, 2022, 51, 104475 Search PubMed.
- A. M. Tawfeek, K. Jabbour, A. G. Abid, M. U. Nisa, S. Manzoor, B. Shabbir, M. Y. ur Rehman, T. Munawar, M. Sillanpää and M. N. Ashiq, J. Sol-Gel Sci. Technol., 2024 DOI:10.1007/s10971-023-06288-3.
- L. Jia, G. Du, D. Han, Y. Wang, W. Zhao, Q. Su, S. Ding and B. Xu, Chem. Eng. J., 2023, 454, 140278 Search PubMed.
- D. V. Morales, C. N. Astudillo, V. Anastasoaie, B. Dautreppe, B. F. Urbano, B. L. Rivas, C. Gondran, D. Aldakov, B. Chovelon, D. André, J.-L. Putaux, C. Lancelon-Pin, S. Sirach, E.-M. Ungureanu, C. Costentin, M.-N. Collomb and J. Fortage, Sustainable Energy Fuels, 2021, 5, 4710–4723 Search PubMed.
- J. Zhang, S. Liu, H. Wang, Q. Xia and X. Huang, Int. J. Hydrogen Energy, 2020, 45, 33491–33499 Search PubMed.
- S. Farid, S. Ren, D. Tian, W. Qiu, J. Zhao, L. Zhao, Q. Mao and C. Hao, Int. J. Hydrogen Energy, 2020, 45, 31926–31941 Search PubMed.
- D. V. Morales, C. N. Astudillo, Y. Lattach, B. F. Urbano, E. Pereira, B. L. Rivas, J. Arnaud, J.-L. Putaux, S. Sirach, S. Cobo, J.-C. Moutet, M.-N. Collomb and J. Fortage, Catal. Sci. Technol., 2018, 8, 4030–4043 Search PubMed.
- Q. Li, H. He, Z. Zheng, L. Zhang, J. Chen, S. Li, B. Zhang, J. Zhang, S. Luo and A. Xie, Ionics, 2022, 28, 4341–4351 Search PubMed.
- Y. Song, P. Hong, T. Li, G. Ma, Q. Deng, Y. Zhou and Y. Zhang, J. Colloid Interface Sci., 2022, 618, 1–10 Search PubMed.
- H. Pei, L. Zhang, G. Zhi, D. Kong, Y. Wang, S. Huang, J. Zang, T. Xu, H. Wang and X. Li, Chem. Eng. J., 2022, 433, 133643 Search PubMed.
- L. Wu, J. Cen, A. Ali, J. Li and P. Kang Shen, J. Electroanal. Chem., 2022, 904, 115877 Search PubMed.
- Q. Hu, Y. Liu, L. Ma, X. Zhang and H. Huang, J. Appl. Electrochem., 2018, 48, 1189–1195 Search PubMed.
- S. Huang, Y. Meng, Y. Cao, S. He, X. Li, S. Tong and M. Wu, Appl. Catal., B, 2019, 248, 239–248 Search PubMed.
- K. Brijesh, K. Bindu, D. Shanbhag and H. S. Nagaraja, Int. J. Hydrogen Energy, 2019, 44, 757–767 Search PubMed.
- X. Jia, Y. Zhang, D. Guo, L. Zhang, L. Wang and L. Zhou, Ionics, 2020, 26, 1885–1894 Search PubMed.
- S. S. Jayaseelan, N. Bhuvanendran, Q. Xu and H. Su, Int. J. Hydrogen Energy, 2020, 45, 4587–4595 Search PubMed.
- S. Farid, W. Qiu, J. Zhao, X. Song, Q. Mao, S. Ren and C. Hao, J. Electroanal. Chem., 2020, 858, 113768 Search PubMed.
- H. Mao, X. Guo, Y. Fu, Z. Cao, D. Sun, B. Wang, Y. Zhang, Q. Fan and X.-M. Song, Appl. Surf. Sci., 2019, 485, 554–563 Search PubMed.
- Q. Xu, C. Lu, S. Sun and K. Zhang, J. Phys. Chem. Solids, 2019, 129, 234–241 Search PubMed.
- A. Abas, H. Sheng, Y. Ma, X. Zhang, Y. Wei, Q. Su, W. Lan and E. Xie, J. Mater. Sci.: Mater. Electron., 2019, 30, 10953–10960 Search PubMed.
- K. Song, R. Yang, X. Chen, X. Wang, G. Chen and N. Zhao, Appl. Surf. Sci., 2021, 542, 148670 Search PubMed.
- S. Muduli, T. K. Pani, K. K. Garlapati and S. K. Martha, J. Energy Storage, 2024, 95, 112396 Search PubMed.
- Y. Wang, T. Zhang, X. Zheng, X. Tian and S. Yuan, ACS Appl. Mater. Interfaces, 2023, 15, 59413–59421 Search PubMed.
- M. Lee and J. Bae, Bull. Korean Chem. Soc., 2015, 36, 2101–2106 Search PubMed.
- X. Xia, D. Chao, Z. Fan, C. Guan, X. Cao, H. Zhang and H. J. Fan, Nano Lett., 2014, 14, 1651–1658 Search PubMed.
- F.-J. Liu, J. Power Sources, 2008, 182, 383–388 Search PubMed.
- X. Shen, T. Wang, X. Wei and S. Li, ACS Appl. Energy Mater., 2022, 5, 2596–2605 Search PubMed.
- P. S. Shewale and K.-S. Yun, J. Alloys Compd., 2022, 911, 164939 Search PubMed.
- X. Shen, X. Wei, T. Wang, S. Li and H. Li, Mater. Chem. Front., 2022, 6, 2894–2904 Search PubMed.
- Y. Hou, Y. Cheng, T. Hobson and J. Liu, Nano Lett., 2010, 10, 2727–2733 Search PubMed.
- E. Pardieu, S. Pronkin, M. Dolci, T. Dintzer, B. P. Pichon, D. Begin, C. Pham-Huu, P. Schaaf, S. Begin-Colin and F. Boulmedais, J. Mater. Chem. A, 2015, 3, 22877–22885 Search PubMed.
- R. Maharsi, A. F. Arif, T. Ogi, H. Widiyandari and F. Iskandar, RSC Adv., 2019, 9, 27896–27903 Search PubMed.
- K. Hareesh, S. R. Rondiya, N. Y. Dzade, S. D. Dhole, J. Williams and S. Sergey, J. Sci.: Adv. Mater. Devices, 2021, 6, 291–301 Search PubMed.
- A. Abdolmaleki, Z. Mohamadi, A. A. Ensafi, N. Z. Atashbar and B. Rezaei, Int. J. Hydrogen Energy, 2018, 43, 8323–8332 Search PubMed.
- A. J. Hackett, J. Malmström and J. Travas-Sejdic, ACS Appl. Energy Mater., 2019, 2, 1436–1444 Search PubMed.
- S. Cogal, S. Ramani, V. R. Bhethanabotla and J. N. Kuhn, ChemCatChem, 2021, 13, 2017–2024 Search PubMed.
- H. Liu, D. Zhao, M. Dai, X. Zhu, F. Qu, A. Umar and X. Wu, Chem. Eng. J., 2022, 428, 131183 Search PubMed.
- A. Witecka, P. Pietrzyk-Thel, M. Krajewski, K. Sobczak, A. Wolska and A. Jain, J. Alloys Compd., 2024, 985, 174040 Search PubMed.
- K. Chen, J. Liu, H. Bian, J. Wei, W. Wang and Z. Shao, Nanotechnology, 2020, 31, 335713 Search PubMed.
- M. Gouda, M. M. Khalaf, M. F. Abou Taleb and H. M. Abd El-Lateef, J. Appl. Polym. Sci., 2024, 55293 Search PubMed.
- Y. Liu, C. Xiang, H. Chu, S. Qiu, J. McLeod, Z. She, F. Xu, L. Sun and Y. Zou, J. Mater. Sci. Technol., 2020, 37, 135–142 Search PubMed.
- Y. Xi, Z. Xiao, H. Lv, H. Sun, S. Zhai and Q. An, J. Colloid Interface Sci., 2023, 630, 525–534 Search PubMed.
- P. Hao, J. Tian, Y. Sang, C.-C. Tuan, G. Cui, X. Shi, C. P. Wong, B. Tang and H. Liu, Nanoscale, 2016, 8, 16292–16301 Search PubMed.
- P. Hao, Z. Zhao, L. Li, C.-C. Tuan, H. Li, Y. Sang, H. Jiang, C. P. Wong and H. Liu, Nanoscale, 2015, 7, 14401–14412 Search PubMed.
- S. S. Medany, A. Nafady, R. A. Soomro and M. A. Hefnawy, Sci. Rep., 2024, 14, 2453 Search PubMed.
- L. Xia, J. Wang, L. Bo, W. Shi, Y. Zhang, Y. Shen, X. Ji, X. Guan, Y. Wang and J. Tong, Chem. Eng. J., 2023, 467, 143464 Search PubMed.
- T. Dhanasekaran, A. Bovas and T. P. Radhakrishnan, ACS Appl. Mater. Interfaces, 2023, 15, 6687–6696 Search PubMed.
- U. Divya Madhuri and T. P. Radhakrishnan, ChemElectroChem, 2019, 6, 1984–1989 Search PubMed.
- J. Chen, H. Yang, X. Sang, Z. Su, D. Li and Q. Wang, Solid State Sci., 2018, 83, 23–30 Search PubMed.
- V. Díaz-Jiménez, G. Gómez-Sánchez, N. V. Likhanova, P. Arellanes-Lozada, O. Olivares-Xometl, I. V. Lijanova and J. Arriola-Morales, ACS Omega, 2024, 9, 27798–27831 Search PubMed.
- P. Kesari, G. Udayabhanu, A. Roy and S. Pal, Int. J. Biol. Macromol., 2023, 225, 1323–1349 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |
