The interaction of CO with a copper(ii) chloride oxy-chlorination catalyst
Abstract
The interaction of CO with an attapulgite-supported, KCl modified CuCl2 catalyst has previously been examined using a combination of XANES, EXAFS and DFT calculations. Exposing the catalyst to CO at elevated temperatures leads to the formation of CO2 as the only identifiable product. However, phosgene production can be induced by a catalyst pre-treatment stage, where the supported CuCl2 sample is exposed to a diluted stream of dichlorine; subsequent CO exposure at ∼643 K then leads to phosgene production. This communication describes a series of FTIR based micro-reactor measurements, coupled with characterisation measurements utilising TEM, XRD and XPS to define the nature of the catalyst at different stages of the reaction coordinate. The CuCl2 catalyst is able to support Deacon activity 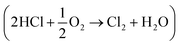 , establishing this work with the possibility of utilising the oxy-chlorination of CO to produce phosgene. Continuous dosing of CO at elevated temperatures over the chlorine pre-dosed CuCl2 catalyst shows diminishing phosgene production as a function of time-on-stream, indicating surface chlorine supply to be rate-limiting under the reaction conditions studied. A pictorial reaction scheme is proposed to account for the surface chemistry observed.
, establishing this work with the possibility of utilising the oxy-chlorination of CO to produce phosgene. Continuous dosing of CO at elevated temperatures over the chlorine pre-dosed CuCl2 catalyst shows diminishing phosgene production as a function of time-on-stream, indicating surface chlorine supply to be rate-limiting under the reaction conditions studied. A pictorial reaction scheme is proposed to account for the surface chemistry observed.

- This article is part of the themed collection: Reaction mechanisms in catalysis


 Please wait while we load your content...
Please wait while we load your content...