 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile N–C bond cleavage and arene reduction by a transient uranium(II) complex†
R. A. Keerthi Shivaraam a,
Leonor Maria
a,
Leonor Maria b,
Thayalan Rajeshkumarc,
Rosario Scopelliti
b,
Thayalan Rajeshkumarc,
Rosario Scopelliti d,
Ivica Živković
d,
Ivica Živković e,
Andrzej Sienkiewicz
e,
Andrzej Sienkiewicz ef,
Laurent Maron
ef,
Laurent Maron c and
Marinella Mazzanti
c and
Marinella Mazzanti *a
*a
aInsititut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland. E-mail: marinella.mazzanti@epfl.ch
bCentro de Química Estrutural, Institute of Molecular Sciences, Instituto Superior Técnico, Universidade de Lisboa, 2695-066 Bobadela, Portugal
cLaboratoire de Physique et Chimie des Nano-objets, Institut National des Sciences Appliquées, 31077 Toulouse, Cedex 4, France
dX-Ray Diffraction and Surface Analytics Platform, Institute of Chemical Sciences and Engineering (ISIC), École Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
eLaboratory for Quantum Magnetism, Institute of Physics, Ecole Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland
fADSresonances SàrL, 1920 Martigny, Switzerland
First published on 2nd July 2025
Abstract
Complexes of uranium(II) remain extremely rare and their reactivity is practically unexplored. Here we report that the reduction of the heteroleptic bis-aryloxide U(III) complex [U(κ6-{(tBu2ArO)2Me2-cyclam})I], A, yields a rare and highly reactive U(II) intermediate that enables a rare example of intramolecular uranium mediated N–C cleavage and effects arene reduction resulting in the isolation of the U(IV) complex [U{κ5-((tBu2ArO)Me2-cyclam)}{κ2-(tBu2ArOCH2)}] (2) and of the inverse–sandwich complex [{U(κ5-{(tBu2ArO)2Me2-cyclam})}2(μ-η6:η6-benzene)] (3) respectively. Moreover, the U(II) solvent-dependent reactivity results in the formation of a putative U–N2 complex in diethyl ether. Computational, EPR and magnetic studies indicate the electronic structure of 3 to be an equilibrium between two possible electronic structures very close in energy: (U(IV)–arene4−–U(IV) and U(III)–arene2−–U(III)). These results indicate that polydentate amine-phenolate ligands can be used to access highly reactive U(II) intermediates and that provides evidence that U(II) species are involved in the formation of inverse sandwich complexes.
Introduction
The recent discoveries of molecular compounds of uranium in the +2 oxidation state1–11 have opened a route to explore new reactivity in uranium chemistry. Notably, it has resulted in a few unambiguous examples of single-metal oxidative addition examples involving both two and four electron transfer to N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (e.g. in azobenzene, Fig. 1a and b) and C
N (e.g. in azobenzene, Fig. 1a and b) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (e.g. in phenylacetylene) π-bonds.8,12 Examples of both intramolecular13–16 C–H activation and intermolecular17 C–H oxidative addition (Fig. 1c) by putative U(II) intermediates have also been reported. More recently examples of two-electron oxidative atom and group transfer reactions at a well-defined uranium(II) centre have also been reported.9,18 These examples suggest that oxidative addition reactivity may be easily accessible for uranium compounds in the +2 oxidation state, but the study of these complexes is limited by their scarce number and their high reactivity.
C (e.g. in phenylacetylene) π-bonds.8,12 Examples of both intramolecular13–16 C–H activation and intermolecular17 C–H oxidative addition (Fig. 1c) by putative U(II) intermediates have also been reported. More recently examples of two-electron oxidative atom and group transfer reactions at a well-defined uranium(II) centre have also been reported.9,18 These examples suggest that oxidative addition reactivity may be easily accessible for uranium compounds in the +2 oxidation state, but the study of these complexes is limited by their scarce number and their high reactivity.
Single-metal oxidative–additions reactions play a key role in d-block metal chemistry and in transition metal mediated catalysis.19 In contrast, these reactions are hard to reach in f element chemistry because of their limited access to two electron redox couples and multielectron transfer.20,21 Notably, uranium mediated oxidative addition reactions have been for a long time limited to single electron transfer. Fewer cases of N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, N
N, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N–N oxidative additions involving multiple electron transfer are also known, although the reaction intermediates remain in many case unidentified.22–27 Redox active ligands8,9,28–31 and sterically induced reductions (SIR)32,33 have provided a successful alternative metal–ligand cooperative approach to implement oxidative addition at a single metal centre.
N and N–N oxidative additions involving multiple electron transfer are also known, although the reaction intermediates remain in many case unidentified.22–27 Redox active ligands8,9,28–31 and sterically induced reductions (SIR)32,33 have provided a successful alternative metal–ligand cooperative approach to implement oxidative addition at a single metal centre.
In contrast, examples of metal-based oxidative addition reactions at a single uranium centre, not involving oxidative atom or group transfer, remain scarce12,20,21 but recent reports8,12,17 indicate that U(II) compounds are well poised to develop this chemistry.
Moreover, U(II) species are also likely intermediates in the formation of uranium inverse sandwich complexes;34–36 however evidence of their involvement is still lacking due to the high reactivity of putative intermediate species. Notably, prior to the isolation of the first example of a U(II) complex by Evans in 2013,2 attempts to isolate molecular complexes of uranium in the +2 oxidation state by reduction of the U(III) analogues in aromatic solvents had resulted in the isolation of inverse sandwich complexes with uranium in a formal +2 oxidation state. However, further spectroscopic and computational studies indicated that the electronic structure of these complexes is better described as U(III)–arene2−–U(III) or U(IV)–arene4−–U(IV).36,37 The possible involvement of intermediate “U(II)” species in the formation of inverse sandwich complexes still remains to be proven.
So far, stabilisation of U(II) species has been attained with bulky cyclopentadienyl derivatives preventing access to the metal centre2–5,14,15,38 or with arene anchored polydentate ligands that can establish strong delta bonding interactions with the uranium centre.1–10 The only example of a U(II) complex with a strong N-donor ligand set –N((SiMe3)2)3 that did not present high steric crowding4 was isolated at low temperature and was found to be extremely reactive towards C–H activation of the –N(SiMe3)2 ligand resulting in the cyclometalated U(III) complex [U{N(SiMe3)2}2(κ2-C,N–CH2SiMe2NSiMe3)].39 Amine-phenolate ligands of different charge, bulk and denticity were shown to be well suited for isolating well defined and highly reactive U(III) complexes40,41 but have so far failed to produce complexes of uranium in lower oxidation state. We became interested in investigating U(III) to U(II) reduction using the polydentate aminophenolate ligand (tBu2ArO)2Me2-cyclam. This was prompted by the reported ability of the heteroleptic bis-aryloxide U(III) precursor [U(κ6-{(tBu2ArO)2Me2-cyclam})I], A41 to cleave azobenzene yielding the U(VI) bis–imido complex [U{(tBu2OAr)2Me2-cyclam}(NPh)2] and the U(IV) byproduct [U{(tBu2OAr)2Me2-cyclam}I]I. While a different mechanism was proposed based on the seminal work of Burns,26 the possibility of a U(II) intermediate in this reaction cannot be ruled out. Here we provide evidence that reduction of the complex A produces a highly reactive U(II) intermediate enabling arene reduction and the first example of uranium(II) mediated N–C cleavage. We also report the synthesis and reduction of the analogous complex [U(κ6-{(tBu2ArO)2Me2-cyclam})(OSi(OtBu)3)] (1) to elucidate the importance of the presence of a reactive halide position for generating neutral U(II) species supported by non-carbon based ligands. It should be noted that, to date, most reported U(II) complexes are “ate” complexes probably due to the rarity of stable heteroleptic U(III) precursors.3,7
Results and discussion
Synthesis of a heteroleptic U(III) complex
With the bis-aryloxide U(III) precursor [U(κ6-{(tBu2ArO)2Me2-cyclam})I] (A) in hand, we first prepared the heteroleptic dark red brown U(III) complex [U(κ6-{(tBu2ArO)2Me2-cyclam})(OSi(OtBu)3)] (1) in 56% yield from the salt metathesis reaction between A and KOSi(OtBu)3 (see Scheme 1 below). | ||
| Scheme 1 Synthesis of complexes 1, 2 and 3 (bonds involved in oxidative addition are drawn in orange). | ||
Single crystals suitable for X-ray diffraction (Fig. 2) were grown from slow evaporation of a solution of 1 in pentane at −40 °C, while bulk isolation was performed from a concentrated reaction mixture in hexane at −40 °C. The 1H NMR spectrum of 1 in d8-THF at −40 °C (Fig. S4†) displays 35 peaks between 43 ppm and −50 ppm, which corresponds well with the 1H NMR spectrum of the crude reaction mixture (Fig. S3†) in d8-THF at −40 °C, indicating a straightforward ligand displacement reaction. The solid-state molecular structure of 1 (Fig. 2) features a seven-coordinate U(III) ion bound by two oxygens from the two aryloxide arms, four nitrogen atoms of the cyclam ring and a tris(tert-butoxy)siloxide ligand in a distorted pentagonal bipyramidal geometry. The U–Oaryloxide bond distances in 1 (2.251(4) Å and 2.309(4) Å), although slightly longer presumably to the presence of the siloxide ligand, are still comparable to the U(III) precursor A41 (2.223(4) Å and 2.263(3) Å). The U–Ncylam bond distances in 1 (2.797(6) Å, 2.824(5) Å, 2.909 (6) Å, 2.953(6) Å; average U–Ncylam = 2.87(6) Å) are slightly longer than those in A (2.721(5) Å − 2.809(5) Å; average U–Ncylam = 2.76(5) Å).
 | ||
| Fig. 2 Molecular structure of complex 1 with thermal ellipsoids drawn at the 50% probability level. Methyl groups of tBu group and hydrogen atoms have been omitted for clarity. | ||
Reduction of U(III) complexes
The reduction of the U(III) complexes A41 and 1 was pursued in order to explore the possibility of accessing a uranium(II) complex. The 1H NMR spectrum in d8-THF of the reaction mixture obtained after reacting 1 with 1–2.2 KC8 at −40 °C (Fig. S7a and S8b†) after 14 hours showed the presence of the resonances of 1 and of minor decomposition products suggesting that its reduction was not possible in the investigated conditions.In contrast, the reaction of a violet solution of complex A with excess KC8 (2.2 equiv.) in d8-THF at −40 °C led to the complete disappearance of the signals assigned to A in the 1H NMR spectrum (Fig. S12†) and the appearance of a new set of resonances. Single crystals of the U(IV) complex [U{κ5-((tBu2ArO)Me2-cyclam)}{κ2-(tBu2ArOCH2)}] (2) suitable for X-ray diffraction were isolated from a concentrated reaction mixture in THF at −40 °C (Fig. 3). The easier reduction of A compared to 1 is most likely due to the presence of the reactive iodide ligand that is easily eliminated upon reduction with KC8. In contrast, reduction of the complex 1 would require the formation of a “ate” complex which is not accessible in the reducing conditions used.
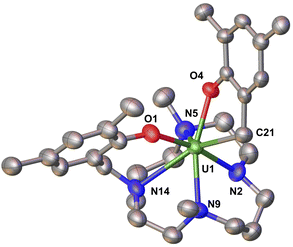 | ||
| Fig. 3 Molecular structure of complex 2 with thermal ellipsoids drawn at the 50% probability level. Methyl groups of tBu group and hydrogen atoms have been omitted for clarity. | ||
Analytically pure complex 2 was isolated in 45% yield from a concentrated hexane solution of the reaction mixture. The 1H NMR spectrum of 2 in d8-THF at −40 °C features 39 peaks from 230 ppm to −273 ppm (Fig. S13†). Complex 2 was observed as the major species formed even when the reaction was conducted in the presence of one equiv. of 2.2.2-cryptand. The solid-state structure of 2 shows that the reduction of A with KC8 did not lead the formation of a stable divalent uranium species as anticipated, but resulted in a U(IV) complex derived from the reductive C–N cleavage of one ligand arm.
Notably, the benzylic C–N bond connecting the cyclam ring to the aryloxide arm is cleaved forming a new C–U bond. The formation of complex 2 is likely to involve a highly reactive putative U(II) species that undergoes intramolecular oxidative addition of the C–N bond to yield the respective U(IV) complex. The removal of the bound iodide in A as KI upon reduction opens a coordination site at the uranium centre that likely facilitates the intramolecular oxidative addition reaction, resulting in the seven-coordinate complex in 2. Monitoring the reduction of K2(tBu2ArO)2Me2-cyclam with 2.2 equiv. KC8 by 1H NMR spectroscopy (see ESI and Fig. S29†) did not show any evolution, and only unreacted starting material was observed; thereby ruling out the possibility of a ligand reduction occurring without metal assistance. The proposed oxidative addition mechanism invoking a putative U(II) species is further corroborated by the stability of the U(III) complex 1 under similar reducing conditions as those used for the formation of 2.
Stoichiometric oxidative addition of the C–N bond to transition metals is well established and has been used to develop catalytic methodologies that have emerged recently as a powerful strategy for the preparation or utilization of nitrogen-containing compounds.42–47 However, complex 2 represents only the second example of an oxidative addition of a C–N bond mediated by a uranium complex48 and the first promoted by a monometallic putative uranium(II) species. In the only reported example of oxidative C–N addition to an f-element, Liddle and coworkers showed that the reduction of the mononuclear U(IV) complex [U(TsXy)(Cl)(THF)] [TsXy = HC-(SiMe2NAr)3; Ar = 3,5-Me2C6H3] in hexane yielded the bimetallic U(IV)/U(IV) [U{HC(SiMe2Ar)2(SiMe2-μ-N)}(μ-η1:η1-Ar)U-(TsXy)] as a result of a bimolecular reductive C–N bond cleavage by a putative U(III) intermediate.48 In the latter example only a one-electron transfer from each metal is involved resulting in an overall unchanged oxidation state. In contrast, the formation of 2 requires a two-electron transfer process at a single metal centre, a putative U(II), which results in the overall oxidation state of the metal increases by +1 on going from U(III) to U(IV) reminiscent of transition metal promoted C–N oxidative addition.
The solid-state molecular structure of 2 (Fig. 3) reveals a uranium ion in the +4 oxidation state with an overall coordination number of seven, wherein the metal center is bound by three neutral amino nitrogen atoms from the cyclam ring, two anionic oxygens from the aryloxide arms, one anionic amido nitrogen and one monoanionic carbon atom resulting from the benzylic C–N bond cleavage. The U–Oaryloxide bond distances in 2 (U–Oaryloxide = 2.177(6), 2.219(6) Å) are shorter than those in A (2.223(4) and 2.263(3) Å) in agreement with the ionic radii contraction expected for a U(IV) ion. The U–Ncyclam-amine bond distances in 2 (2.599(9) − 2.779(9) Å; av. U–Ncyclam-amine = 2.68(2) Å) are also shorter than those in A (2.721(5) Å − 2.809(5) Å; av. U–Ncyclam = 2.76(5) Å), but considerably longer than the U–Ncyclam-amido bond distance (2.265(6) Å) in 2.41 The U–Ncyclam-amido bond distance (2.265(6) Å) in 2 compares well with previously reported U(IV) amido bond distances.49–51 The newly formed U(IV)–Ccylam bond distance (2.505(11) Å) is in the range of previously reported U(IV)-alkyl bond distances (2.4–2.5 Å)52–57 and is similar to that found in a eight coordinate U-alkyl compound (2.5134(7) Å).58
Given the highly reactive nature of the putative U(II) species generated upon reduction we sought to investigate if dinitrogen reduction reactivity could compete with N–C cleavage by conducting the reduction of A with KC8 (2.2 equiv.) in a less coordinating solvent (diethyl ether) under N2. The reaction was carried out at −40 °C for 3 days and resulted in the formation of an insoluble purple species containing N2-bound uranium species that was stable under vacuum. Dissolution of the purple species in THF or in benzene resulted in N2 release and formation of complex 2 (see ESI, Fig. S27†) and the inverse sandwich complex [{U(κ5-{(tBu2ArO)2Me2-cyclam})}2(μ-η6:η6-benzene)], 3 respectively (see infra and ESI, Fig. S26†).
We were not able to isolate any N2-bound uranium complex, but we found that quenching the reaction mixture, resulting from the reduction reaction of A with 2.2 equiv. of KC8 in diethyl ether −40 °C under N2, with a strong acid (HCl in diethyl ether) revealed (upon quantitative integration performed with dimethyl sulfone as the internal standard) the formation of ammonium chloride in 20% yield (0.2 equiv. per complex A used) (see ESI†). When larger excess of KC8 was used (6–10 equiv.) ammonium chloride was obtained after quenching in similar yield (20–30%). Performing the reaction under 15N2 also yielded ammonium chloride (15NH4Cl) in 30% yield. In contrast, no ammonium chloride formation was observed when the same reaction was performed under argon or when the reduction was carried out in hexane under dinitrogen. Moreover, the 1H NMR spectrum measured in d8-toluene at −40 °C of the reaction mixture obtained after reaction of A with 2.2 equiv. of KC8 in diethyl ether −40 °C under argon showed the resonances assigned to 2 (Fig. S24b†).
Finally, the reduction of the complex A in benzene prevented the C–N oxidative addition from occurring leading instead to the isolation of the inverse sandwich complex [{U(κ5-{(tBu2ArO)2Me2-cyclam})}2(μ-η6:η6-benzene)] (3) in 32% yield, formed from the reduction of the benzene molecule by the putative U(II) intermediate. Analogous uranium inverse sandwich complexes have been reported by several groups34,36,37,59–63 and are considered to be U(II) synthons because they can function as four electron reductants with diverse substrates; spectroscopic and structural studies led to the assignment of their electronic structure as consistent of U(III) centres and a dianionic bridging arene. Single crystals of 3 suitable for X-ray diffraction were isolated from a concentrated reaction mixture in toluene at −40 °C (see Fig. 4). The reduction of A was also performed in hexane (both at −40 °C and r.t.) to isolate the putative U(II). Reduction of A in hexane with 2.2 equiv. KC8 at r.t. over three days resulted in a dark red brown suspension. The 1H NMR spectrum (Fig. S23†) of the crude reaction mixture in d6-benzene at r.t showed the presence of complex 3 as the only identifiable species suggesting that the low solubility of the putative U(II) complex in hexane prevents the reductive cleavage of the ligand. Dissolution of the residue in a more polar solvent such as d8-THF resulted in the observation of the signals assigned to complex 2 by 1H NMR spectroscopy (see ESI, Fig. S16c†), signifying that the intramolecular C–N cleavage step is instantaneous in d8-THF.
 | ||
| Fig. 4 Molecular structure of complex 3 with thermal ellipsoids drawn at the 50% probability level. Methyl groups of tBu group and hydrogen atoms have been omitted for clarity. | ||
The solid-state molecular structure of 3 (see Fig. 4) reveals that each uranium metal centre is coordinated by two aryloxide oxygen atoms and two nitrogen atoms of the cyclam ring, with a benzene molecule bridging the two uranium centres. In each half of the complex, only two out of the four nitrogen atoms in the cyclam ring interact with the uranium ion in order to accommodate the benzene moiety: thereby exhibiting a conformationally flexible cyclam backbone. The U–Oaryloxide bond lengths (2.207(3) Å and 2.229(4) Å) are comparable to those found in complex A (2.223(4) Å and 2.263(3) Å). The average U–Carene (2.61(2) Å) and Carene–Carene (1.430(14) Å) bond lengths are consistent with the previously reported diuranium(III) inverse sandwich complexes.34,36,37,63
EPR and magnetism studies
To further elucidate the electronic structure of complex A, 2 and 3, SQUID magnetometry (Fig. 5) and EPR (Fig. 6) studies were undertaken. Complex A has a magnetic moment of 3.97μB (χM = 6.58 × 10−3 emu mol−1; χT = 1.97 emu K mol−1) at 300 K and 2.61μB (χM = 4.25 × 10−1 emu mol−1; χT = 8.50 × 10−1 emu K mol−1) at 2 K. The observed behaviour is similar to that reported for other U(III) compounds with an f3 electronic configuration corresponding to 4I9/2 ground state40,64 In such U(III) complexes the magnetic moment decreases steadily with decrease in temperature to reach a non-zero value corresponding to a doublet state for the Kramers ion. On the other hand, complex 2 has a magnetic moment of 2.98μB (χM = 3.70 × 10−3 emu mol−1; χT = 1.11 emu K mol−1) at 300 K and 1.00μB (χM = 6.27 × 10−2 emu mol−1; χT = 1.25 × 10−1 emu K mol−1) at 2 K. The decrease in magnetic moment is more abrupt for complex 2 below 50 K, when compared to complex A. Such a decrease in magnetic moment below 50 K is well documented for various U(IV) complexes reported in the literature.64 The magnetic moment for 2 does not approach zero at 2 K, which could indicate the presence of a non-singlet ground state.65 Nonetheless, the magnetic data for 2 compare well with those of several U(IV) complexes reported previously.66–69 In comparison, the measured magnetic moment of 3 is of 3.32μB (χM = 4.58 × 10−3 emu mol−1; χT = 1.37 emu K mol−1) at 300 K and 1.04μB (χM = 6.70 × 10−2 emu mol−1; χT = 1.34 × 10−1 emu K mol−1) at 2 K per uranium ion, while per complex it is 4.69μB (χM = 9.17 × 10−3 emu mol−1; χT = 2.75 emu K mol−1) at 300 K and 1.46μB (χM = 1.34 × 10−1 emu mol−1; χT = 2.68 × 10−1 emu K mol−1) at 2 K. Although the decrease in magnetic moment with the decrease in temperature in 3 is quite different from both complexes A and 2, the low value of its magnetic moment at 2 K is close to that of the U(IV) complex 2. This correlates well with DFT studies (vide infra) with complex 3 calculated to have a U(IV)–arene4−–U(IV) ground state but with a very close in energy U(III)–arene2−–U(III) state. Considering that an EPR signal is also observed (see next section) that can be assigned to the U(III) species is likely that both configurations contribute to the magnetic response. The observed behaviour is rather different from that reported for the analogous arene-bridged complex [(μ-toluene)U2(N[tBu]Ar)4] (Ar = 3,5-C6H3Me2) that presented a strong antiferromagnetic behaviour below 125 K and lower magnetic moment in all 5–300 K temperature range (1.57μB at 300 K and 0.25μB at 5 K for one uranium center).36,61The EPR spectrum of complex A at 6 K features g-values of 4.75, 0.82 and 0.65 at 6 K (Fig. 6). EPR spectra with rhombic signals and similar low field g-factors have been reported for heteroleptic uranium(III) complexes supported by tris(3,5- dimethyl-1-pyrazolyl)borate ligands or amide ligands.70,71
While assignment of oxidation state is not trivial in inverse-sandwich complexes, EPR data measured in the solid state (Fig. 6) and in frozen solution (see ESI†) at 6 K show for complex 3 the presence of a signal in agreement with the presence of a f3 U(III) rather that EPR silent f2 U(IV) or f4 U(II) ions. The EPR spectrum of 3 at 6 K features g-values of 2.80, 2.60 and 0.8 (Fig. 6), which following spin quantification accounts for 40% of the total expected spins. Similar EPR spectra have been reported for monometallic uranium(III) complexes featuring strong donor ligands.8,72,73 The observation of this EPR profile is suggestive of a U(III)–arene2−U(III) structure for complex 3 similar to the structure proposed for the inverse sandwich complex [(μ-toluene)U2(N[tBu]Ar)4] complex.61 The presence of such species is corroborated by the computational studies indicating the possible existence of U(III)–arene2−U(III) and U(IV)–arene4−U(IV) structures in equilibrium. Moreover, the EPR spectrum of the putative U(II) species obtained as highly insoluble solid upon reacting A with 2.2 KC8 in hexane was also recorded to further characterize the nature of this intermediate. The EPR spectrum shows a radical-like signal due to the presence of unreacted KC8 at g = 2.0021 and two additional signals of very low intensity at g = 2.82 and 2.52. The signals are significantly shifted with respect to the spectrum of complex A (a small residual signal corresponding to A is also observed (Fig. S40†)). The observed low intensity signals (4% integrated spins) could be tentatively assigned to the U(II) species generated upon reduction. The possibility that the signal could be arising from metallic impurity contained in KC8 (ref. 74) was ruled out by measuring the solid state EPR of the same batch of KC8 which revealed only an asymmetric signal at g = 2.0020, thus pointing to the itinerant electrons in the highly conducting particles of KC8. Several U(II) complexes were reported to possess a 5f4 configuration1,7,8,11 that did not feature any metal based EPR signal. However, recently Evans and co-workers reported EPR studies on 10 U(II) complexes having a 5f36d1 electronic configuration that showed display two-line axial signals with g‖ = 2.04 and g⊥ = 2.00.75 The reported studies suggested that the presence of EPR signals can be used to differentiate 5f36d1 and 5f4 configurations in U(II) complexes.
The almost silent EPR spectrum of the product of the reduction of A in hexane indicate that a U(II) species is formed which is mostly in a 5f4 configuration. The observed low intensity signals could suggest some contribution of the 5f36d1 configuration, because of the f–d mixing also found in the computed structure (see below). To further corroborate our hypothesis the precipitate obtained from the reduction reaction mixture was suspended in benzene and the EPR spectrum of the resulting dark red brown solution (following filtration to remove excess KC8 and graphite) was measured at 6 K. The signal corresponding to the U(II) intermediate disappeared, and the signal corresponding to complex 3 was observed in the EPR spectrum recorded (Fig. S27†). These results provide further evidence of the involvement of a U(II) species in both reduction of benzene observed in 3 and the N–C cleavage observed in the case of 2.
Computational studies
DFT studies were carried out (B3PW91 functional) to give some insights on the formation of complex 2 and the electronic structure of complex 3. First of all the formation of the U(II) intermediate B was investigated computationally. Although disproportionation of the starting complex A is prohibitively high (68.0 kcal mol−1 in enthalpy and 68.9 kcal mol−1 in Gibbs Free Energy), the reduction of complex A to form the transient complex B is endothermic by 23.4 kcal mol−1 in enthalpy (19.5 kcal mol−1 in Gibbs Free Energy). In THF solution, this reaction is becoming endothermic by 15.2 kcal mol−1 in enthalpy (11.1 kcal mol−1 in Gibbs Free energy). This makes this intermediate plausible and it is in line with the fact that this transient species was not experimentally stabilised. The electronic structure of B clearly indicates a U(II) system in a S = 2 ground state. The associated electronic configuration is a mixture of the 5f4 and 5f3d1 configurations since three pure 5f orbitals are occupied and the fourth one is a mixture (60–40) between the 5f0 and 6d0 atomic orbitals. This electronic structure fits the EPR data and the hypothesis given experimentally. The formation of complex 2 from complex B was thus investigated (Fig. 7). In complex B, the coordination of both nitrogen and oxygen induces a slight elongation of the C–N bond (1.50 Å) with respect to simple amines (1.47 Å). Complex B can easily reach a C–N bond breaking transition state (TS2) with an accessible barrier of 16.4 kcal mol−1 in enthalpy (16.9 kcal mol−1 in Gibbs Free Energy). At the transition state, the C–N bond is already broken (1.88 Å) while the U–N distance is decreased to 2.55 Å. Following the intrinsic reaction coordinate, it yields the very stable complex 2 (−49.0 kcal mol−1 in enthalpy, −49.4 kcal mol−1 in Gibbs Free Energy).The electronic structure of complex 3 was finally investigated computationally using the same methodology. Two spin states were considered, namely a S = 3 (two U(III) centres) and S = 2 (two U(IV) centres) spin states. The S = 2 (quintet) is slightly more stable than the S = 3 (septet) by 4.8 kcal mol−1 in enthalpy (2.5 kcal mol−1 in Gibbs Free Energy). This TS is located on the triplet Potential Energy Surface (PES) and corresponds to a U(IV). This small energy difference indicates that these two structures could be in equilibrium and that clearly both can be populated at room temperature, in agreement with the observed experimental magnetism. The comparison between the optimized geometry in both spin states and the experimental one clearly shows that the quintet geometry is the one that is in best agreement with the X-ray (see Table S4†). In both cases, the unpaired spin density plots (Fig. S47k and S48k†) are located at the two uranium centres only, indicating either two U(IV) for the quintet or two U(III) for the septet. The main difference between the two spin states is the presence of two doubly occupied δ-bonds (HOMO and HOMO − 1) for the quintet while only one is occupied (HOMO − 1) for the septet (Fig. S47 and S48†). The situation is different for [(μ-toluene)U2(N[Ad]Ar)4] (Ar = 3,5-C6H3Me2), since the quintet is found to be the ground state while the septet is 15.3 kcal mol−1 higher in enthalpy (16.9 kcal mol−1 in Gibbs Free Energy). Therefore, there is no equilibrium between the two spin states, explaining the difference in magnetism observed with complex 3.
Conclusion
In conclusion reduction of the heteroleptic aminephenolate complex A in apolar solvents produces a highly reactive transient neutral “U(II)” species (B). In contrast, upon replacement of the iodide ligand in A with a siloxide to yield complex 1, the reduction of the uranium centre is not observed even in presence of excess reducing agent with only minor decomposition products formed.In polar solvents B undergoes a unique example of C–N oxidative addition to a U(II) center to yield complex 2. The transient complex B was also found to reduce benzene to yield the inverse sandwich complex 3. Computational, EPR and magnetic studies indicate the presence for 3 of an equilibrium between two possible electronic structures very close in energy (U(IV)–arene4−–U(IV) and U(III)–arene2−–U(III)). These results indicate that polydentate amine-phenolate ligands can be used to access highly reactive U(II) intermediates and that U(II) species are involved in the formation of inverse sandwich complexes. Moreover the “U(II)” intermediate was found to react with N2 yielding ammonia (even if in low yield) upon acidification. Future studies will be directed to tune the stability and the reactivity of the U(II) intermediate by ligand design.
Data availability
Synthetic details, analytical data including depictions of all spectra and coordinate data of all computationally optimised species, are documented in the ESI.† Crystallographic data is made available via the CCDC. The data that support the findings of this study are openly available in the zenodo repository at https://doi.org/10.5281/zenodo.15801184.†Author contributions
R. A. K. S designed and carried out all the experiments and analysed the data; L. M. carried out the ligand synthesis and some of the experiments and analysed the data. M. M. designed and supervised the project and analysed the data; T. R. and L. M. carried out the computational study; R. S. measured and analyzed the X-ray data, I. Z. measured and analysed the magnetic data; A. S. measured and analysed the EPR data; R. A. K. S and M. M. wrote the manuscript with contributions of all authors, and all authors have given approval for the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge support from the Swiss National Science Foundation grant number 212723 and 217133 and the Ecole Polytechnique Fédérale de Lausanne (EPFL). LM is a senior member of the Institut Universitaire de France. CalMip is acknowledged for a generous grant of computing time. Leonor Maria acknowledges support from Fundação para a Ciencia e a Tecnologia (FCT) through projects UIDB/00100/2020 (CQE) and LA/P/0056/2020 (IMS). We thank Dr Maxime Tricoire for assistance with EPR data collection and analysis.Notes and references
- H. S. La Pierre, A. Scheurer, F. W. Heinemann, W. Hieringer and K. Meyer, Synthesis and Characterization of a Uranium(II) Monoarene Complex Supported by delta Backbonding, Angew. Chem., Int. Ed., 2014, 53, 7158–7162 CrossRef CAS.
- M. R. MacDonald, M. E. Fieser, J. E. Bates, J. W. Ziller, F. Furche and W. J. Evans, Identification of the +2 Oxidation State for Uranium in a Crystalline Molecular Complex, K(2.2.2-Cryptand) (C5H4SiMe3)(3)U, J. Am. Chem. Soc., 2013, 135, 13310–13313 CrossRef CAS.
- F. S. Guo, N. Tsoureas, G. Z. Huang, M. L. Tong, A. Mansikkamaki and R. A. Layfield, Isolation of a Perfectly Linear Uranium(II) Metallocene, Angew. Chem., Int. Ed., 2020, 59, 2299–2303 CrossRef CAS PubMed.
- A. J. Ryan, M. A. Angadol, J. W. Ziller and W. J. Evans, Isolation of U(II) compounds using strong donor ligands, C5Me4H and N(SiMe3)(2), including a three-coordinate U(II) complex, Chem. Commun., 2019, 55, 2325–2327 RSC.
- D. N. Huh, J. W. Ziller and W. J. Evans, Chelate-Free Synthesis of the U(II) Complex, (C5H3(SiMe3)(2))(3)U (1−), Using Li and Cs Reductants and Comparative Studies of La(II) and Ce(II) Analogs, Inorg. Chem., 2018, 57, 11809–11814 CrossRef CAS PubMed.
- D. N. Huh, C. J. Windorff, J. W. Ziller and W. J. Evans, Synthesis of uranium-in-cryptand complexes, Chem. Commun., 2018, 54, 10272–10275 RSC.
- B. S. Billow, B. N. Livesay, C. C. Mokhtarzadeh, J. McCracken, M. P. Shores, J. M. Boncella and A. L. Odom, Synthesis and Characterization of a Neutral U(II) Arene Sandwich Complex, J. Am. Chem. Soc., 2018, 140, 17369–17373 CrossRef CAS PubMed.
- M. Keener, R. A. K. Shivaraam, T. Rajeshkumar, M. Tricoire, R. Scopelliti, I. Zivkovic, A. S. Chauvin, L. Maron and M. Mazzanti, Multielectron Redox Chemistry of Uranium by Accessing the plus II Oxidation State and Enabling Reduction to a U(I) Synthon, J. Am. Chem. Soc., 2023, 145, 16271–16283 Search PubMed.
- C. Deng, J. F. Liang, R. Sun, Y. Wang, P. X. Fu, B. W. Wang, S. Gao and W. L. Huang, Accessing five oxidation states of uranium in a retained ligand framework, Nat. Commun., 2023, 14, 4657 CrossRef CAS.
- N. Tsoureas and I. Vagiakos, Recent Advances in Low Valent Thorium and Uranium Chemistry, Inorganics, 2024, 12, 275 CrossRef CAS.
- M. D. Straub, E. T. Ouellette, M. A. Boreen, R. D. Britt, K. Chakarawet, I. Douair, C. A. Gould, L. Maron, I. Del Rosal, D. Villarreal, S. G. Minasian and J. Arnold, A Uranium(II) Arene Complex That Acts as a Uranium(I) Synthon, J. Am. Chem. Soc., 2021, 143, 19748–19760 CrossRef CAS.
- D. K. Modder, C. T. Palumbo, I. Douair, R. Scopelliti, L. Maron and M. Mazzanti, Single metal four-electron reduction by U(II) and masked “U(II)” compounds, Chem. Sci., 2021, 12, 6153–6158 RSC.
- H. S. La Pierre, H. Kameo, D. P. Halter, F. W. Heinemann and K. Meyer, Coordination and Redox Isomerization in the Reduction of a Uranium(III) Monoarene Complex, Angew. Chem., Int. Ed., 2014, 53, 7154–7157 CrossRef CAS PubMed.
- J. C. Wedal, J. W. Ziller, F. Furche and W. J. Evans, Synthesis and Reduction of Heteroleptic Bis(cyclopentadienyl) Uranium(III) Complexes, Inorg. Chem., 2022, 61, 7365–7376 CrossRef CAS PubMed.
- J. C. Wedal, S. Bekoe, J. W. Ziller, F. Furche and W. J. Evans, C-H Bond Activation via U(II) in the Reduction of Heteroleptic Bis(trimethylsilyl)amide U(III) Complexes, Organometallics, 2020, 39, 3425–3432 CrossRef CAS.
- M. A. Boreen, C. S. Z. Ye, A. Kerridge, K. N. McCabe, B. A. Skeel, L. Maron and J. Arnold, Does Reduction-Induced Isomerization of a Uranium(III) Aryl Complex Proceed via C–H Oxidative Addition and Reductive Elimination across the Uranium(II/IV) Redox Couple?, Inorg. Chem., 2022, 61, 8955–8965 CrossRef CAS.
- W. Fang, Y. F. Li, T. Z. Zhang, T. Rajeshkumar, I. del Rosal, Y. Zhao, T. W. Wang, S. O. Wang, L. Maron and C. Q. Zhu, Oxidative Addition of E–H (E = C, N) Bonds to Transient Uranium(II) Centers, Angew. Chem., Int. Ed., 2024, 63, e202419987 Search PubMed.
- C. Deng, Y. L. Li, Y. Wang and W. L. Huang, Two-Electron Oxidative Atom and Group Transfer Reactions at a Well-Defined Uranium(II) Center, Angew Chem. Int. Ed. Engl., 2025, 64, e202419987 CrossRef CAS PubMed.
- J. F. Hartwig, Organotransition Metal Chemistry, University Science Books, New York, 2010 Search PubMed.
- B. M. Gardner, C. E. Kefalidis, E. Lu, D. Patel, E. J. L. McInnes, F. Tuna, A. J. Wooles, L. Maron and S. T. Liddle, Evidence for single metal two electron oxidative addition and reductive elimination at uranium, Nat. Commun., 2017, 8, 1898 CrossRef PubMed.
- E. Lu and S. T. Liddle, Uranium-mediated oxidative addition and reductive elimination, Dalton Trans., 2015, 44, 12924–12941 RSC.
- C. L. Webster, J. W. Ziller and W. J. Evans, Reactivity of U3+ Metallocene Allyl Complexes Leads to a Nanometer-Sized Uranium Carbonate, (C5Me5)(2)U (6)(mu-kappa(1):kappa(2)-CO3)(6), Organometallics, 2013, 32, 4820–4827 CrossRef CAS.
- D. R. Hartline, S. T. Löftier, D. Fehn, J. M. Kasper, F. W. Heinemann, P. Yang, E. R. Batista and K. Meyer, Uranium-Mediated Peroxide Activation and a Precursor toward an Elusive Uranium cis-Dioxo Fleeting Intermediate, J. Am. Chem. Soc., 2023, 145, 8927–8938 CrossRef CAS PubMed.
- I. Korobkov, S. Gambarotta and G. P. A. Yap, A highly reactive uranium complex supported by the calix 4 tetrapyrrole tetraanion affording dinitrogen cleavage, solvent deoxygenation, and polysilanol depolymerization, Angew. Chem., Int. Ed., 2002, 41, 3433–3436 CrossRef CAS.
- I. Korobkov, S. Gambarotta and G. P. A. Yap, Highly reactive uranium(III) polypyrrolide complexes: intramolecular C–H bond activation, ligand isomerization, and solvent deoxygenation and fragmentation, Organometallics, 2001, 20, 2552–2559 CrossRef CAS.
- B. P. Warner, B. L. Scott and C. J. Burns, A simple preparative route to bis(imido)-uranium(VI) complexes by the direct reductions of diazenes and azides, Angew. Chem., Int. Ed., 1998, 37, 959–960 CrossRef CAS.
- M. Falcone, L. Chatelain, R. Scopelliti, I. Zivkovic and M. Mazzanti, Nitrogen reduction and functionalization by a multimetallic uranium nitride complex, Nature, 2017, 547, 332–335 CrossRef CAS PubMed.
- J. J. Kiernicki, P. E. Fanwick and S. C. Bart, Utility of a redox-active pyridine(diimine) chelate in facilitating two electron oxidative addition chemistry at uranium, Chem. Commun., 2014, 50, 8189–8192 RSC.
- E. M. Matson, S. R. Opperwall, P. E. Fanwick and S. C. Bart, “Oxidative Addition” of Halogens to Uranium(IV) Bis(amidophenolate) Complexes, Inorg. Chem., 2013, 52, 7295–7304 CrossRef CAS.
- D. P. Halter, F. W. Heinemann, L. Maron and K. Meyer, The role of uranium-arene bonding in H2O reduction catalysis, Nat. Chem., 2018, 10, 259–267 CrossRef CAS PubMed.
- N. H. Anderson, S. O. Odoh, Y. Y. Yao, U. J. Williams, B. A. Schaefer, J. J. Kiernicki, A. J. Lewis, M. D. Goshert, P. E. Fanwick, E. J. Schelter, J. R. Walensky, L. Gagliardi and S. C. Bart, Harnessing redox activity for the formation of uranium tris(imido) compounds, Nat. Chem., 2014, 6, 919–926 CrossRef CAS PubMed.
- W. J. Evans and B. L. Davis, Chemistry of tris(pentamethylcyclopentadienyl) f-element complexes, (C5Me5)(3)M, Chem. Rev., 2002, 102, 2119–2136 CrossRef CAS PubMed.
- W. J. Evans, S. A. Kozimor and J. W. Ziller, (C5Me5)(2)U (mu-Ph)(2)BPh2 as a four electron reductant, Chem. Commun., 2005, 4681–4683 RSC.
- S. T. Liddle, Inverted sandwich arene complexes of uranium, Coord. Chem. Rev., 2015, 293, 211–227 CrossRef.
- P. L. Diaconescu and C. C. Cummins, Diuranium inverted sandwiches involving naphtalene and cyclooctatetraene, J. Am. Chem. Soc., 2002, 124, 7660–7661 CrossRef CAS.
- P. L. Diaconescu, P. L. Arnold, T. A. Baker, D. J. Mindiola and C. C. Cummins, Arene-bridged diuranium complexes: Inverted sandwiches supported by delta backbonding, J. Am. Chem. Soc., 2000, 122, 6108–6109 Search PubMed.
- W. J. Evans, S. A. Kozimor, J. W. Ziller and N. Kaltsoyannis, Structure, reactivity, and density functional theory analysis of the six-electron reductant, (C5Me5)(2)U (2)(mu-eta(6):eta(6)-C6H6), synthesized via a new mode of (C5Me5)(3)M reactivity, J. Am. Chem. Soc., 2004, 126, 14533–14547 Search PubMed.
- C. J. Windorff, M. R. MacDonald, K. R. Meihaus, J. W. Ziller, J. R. Long and W. J. Evans, Expanding the Chemistry of Molecular U2+ Complexes: Synthesis, Characterization, and Reactivity of the {C5H3(SiMe3)(2) (3)U}(−) Anion, Chem.–Eur. J., 2016, 22, 772–782 CrossRef CAS PubMed.
- D. K. Modder, R. Scopelliti and M. Mazzanti, Accessing a Highly Reducing Uranium(III) Complex through Cyclometalation, Inorg. Chem., 2024, 63, 9527–9538 CrossRef CAS.
- I. Castro-Rodriguez and K. Meyer, Small molecule activation at uranium coordination complexes: control of reactivity via molecular architecture, Chem. Commun., 2006, 1353–1368 Search PubMed.
- L. Maria, I. C. Santos, V. R. Sousa and J. Marcalo, Uranium(III) Redox Chemistry Assisted by a Hemilabile Bis(phenolate) Cyclam Ligand: Uranium-Nitrogen Multiple Bond Formation Comprising a trans-{RN
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U(VI)
U(VI)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR}(2+) Complex, Inorg. Chem., 2015, 54, 9115–9126 Search PubMed.
NR}(2+) Complex, Inorg. Chem., 2015, 54, 9115–9126 Search PubMed. - A. N. Desnoyer and J. A. Love, Recent advances in well-defined, late transition metal complexes that make and/or break C–N, C–O and C–S bonds, Chem. Soc. Rev., 2017, 46, 197–238 RSC.
- J. García-Cárceles, K. A. Bahou and J. F. Bower, Recent Methodologies That Exploit Oxidative Addition of C–N Bonds to Transition Metals, ACS Catal., 2020, 10, 12738–12759 CrossRef.
- K. B. Ouyang, W. Hao, W. X. Zhang and Z. F. Xi, Transition-Metal-Catalyzed Cleavage of C–N Single Bonds, Chem. Rev., 2015, 115, 12045–12090 CrossRef CAS.
- O. V. Ozerov, C. Y. Guo, V. A. Papkov and B. M. Foxman, Facile oxidative addition of N–C and N–H bonds to monovalent rhodium and iridium, J. Am. Chem. Soc., 2004, 126, 4792–4793 CrossRef CAS PubMed.
- T. M. Cameron, K. A. Abboud and J. M. Boncella, Unusual molybdenum mediated C–N bond activation, Chem. Commun., 2001, 1224–1225 RSC.
- J. B. Bonanno, T. P. Henry, D. R. Neithamer, P. T. Wolczanski and E. B. Lobkovsky, Arylamine C–N bond oxidative addition to (silox)(3)Ta (silox = (t)Bu(3)SiO), J. Am. Chem. Soc., 1996, 118, 5132–5133 Search PubMed.
- D. Patel, F. Moro, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, A Formal High Oxidation State Inverse-Sandwich Diuranium Complex: A New Route to f-Block-Metal Bonds, Angew. Chem., Int. Ed., 2011, 50, 10388–10392 CrossRef CAS PubMed.
- S. Fortier, J. L. Brown, N. Kaltsoyannis, G. Wu and T. W. Hayton, Synthesis, Molecular and Electronic Structure of U–V(O) N(SiMe3)(2) (3), Inorg. Chem., 2012, 51, 1625–1633 CrossRef CAS PubMed.
- C. J. Windorff, J. M. Sperling, T. E. Albrecht-Schönzart, Z. L. Bai, W. J. Evans, A. N. Gaiser, A. J. Gaunt, C. A. P. Goodwin, D. E. Hobart, Z. K. Huffman, D. N. Huh, B. E. Klamm, T. N. Poe and E. Warzecha, A Single Small-Scale Plutonium Redox Reaction System Yields Three Crystallographically-Characterizable Organoplutonium Complexes, Inorg. Chem., 2020, 59, 13301–13314 CrossRef CAS PubMed.
- E. J. Schelter, R. L. Wu, B. L. Scott, J. D. Thompson, T. Cantat, K. D. John, E. R. Batista, D. E. Morris and J. L. Kiplinger, Actinide Redox-Active Ligand Complexes: Reversible Intramolecular Electron-Transfer in U(dpp-BIAN)(2)/U(dpp-BIAN)(2)(THF), Inorg. Chem., 2010, 49, 924–933 CrossRef CAS PubMed.
- S. Fortier, B. C. Melot, G. Wu and T. W. Hayton, Homoleptic Uranium(IV) Alkyl Complexes: Synthesis and Characterization, J. Am. Chem. Soc., 2009, 131, 15512–15521 CrossRef CAS.
- L. A. Seaman, J. R. Walensky, G. Wu and T. W. Hayton, In Pursuit of Homoleptic Actinide Alkyl Complexes, Inorg. Chem., 2013, 52, 3556–3564 CrossRef CAS.
- P. L. Diaconescu, A. L. Odom, T. Agapie and C. C. Cummins, Uranium-group 14 element single bonds: isolation and characterization of a uranium(IV) silyl species, Organometallics, 2001, 20, 4993–4995 CrossRef CAS.
- S. Duhovic, J. V. Oria, S. O. Odoh, G. Schreckenbach, E. R. Batista and P. L. Diaconescu, Investigation of the Electronic Structure of Mono(1,1′-Diamidoferrocene) Uranium(IV) Complexes, Organometallics, 2013, 32, 6012–6021 CrossRef CAS.
- E. J. Schelter, J. M. Veauthier, C. R. Graves, K. D. John, B. L. Scott, J. D. Thompson, J. A. Pool-Davis-Tournear, D. E. Morris and J. L. Kiplinger, 1,4-Dicyanobenzene as a scaffold for the preparation of bimetallic actinide complexes exhibiting metal-metal communication, Chem.–Eur. J., 2008, 14, 7782–7790 CrossRef CAS PubMed.
- K. C. Jantunen, C. J. Burns, I. Castro-Rodriguez, R. E. Da Re, J. T. Golden, D. E. Morris, B. L. Scott, F. L. Taw and J. L. Kiplinger, Thorium(IV) and uranium(IV) ketimide complexes prepared by nitrile insertion into actinide-alkyl and -aryl bonds, Organometallics, 2004, 23, 4682–4692 CrossRef CAS.
- B. S. Newell, T. C. Schwaab and M. P. Shores, Synthesis and Characterization of a Novel Tetranuclear 5f Compound: A New Synthon for Exploring U(IV) Chemistry, Inorg. Chem., 2011, 50, 12108–12115 CrossRef CAS PubMed.
- A. J. Wooles, D. P. Mills, F. Tuna, E. J. L. McInnes, G. T. W. Law, A. J. Fuller, F. Kremer, M. Ridgway, W. Lewis, L. Gagliardi, B. Vlaisavljevich and S. T. Liddle, Uranium(III)–carbon multiple bonding supported by arene delta-bonding in mixed-valence hexauranium nanometre-scale rings, Nat. Commun., 2018, 9, 2097 CrossRef PubMed.
- D. P. Mills, F. Moro, J. McMaster, J. van Slageren, W. Lewis, A. J. Blake and S. T. Liddle, A delocalized arene-bridged diuranium single-molecule magnet, Nat. Chem., 2011, 3, 454–460 CrossRef CAS PubMed.
- B. Vlaisayljevich, P. L. Diaconescu, W. L. Lukens Jr, L. Gagliardi and C. C. Cummins, Investigations of the Electronic Structure of Arene-Bridged Diuranium Complexes, Organometallics, 2013, 32, 1341–1352 CrossRef.
- M. J. Monreal, S. I. Khan, J. L. Kiplinger and P. L. Diaconescu, Molecular quadrangle formation from a diuranium mu-eta(6),eta(6)-toluene complex, Chem. Commun., 2011, 47, 9119–9121 RSC.
- W. J. Evans, C. A. Traina and J. W. Ziller, Synthesis of Heteroleptic Uranium (mu-eta(6):eta(6)-C6H6)(2-) Sandwich Complexes via Facile Displacement of (eta(5)-C5Me5)(1-) by Ligands of Lower Hapticity and Their Conversion to Heteroleptic Bis(imido) Compounds, J. Am. Chem. Soc., 2009, 131, 17473–17481 CrossRef CAS PubMed.
- D. R. Kindra and W. J. Evans, Magnetic Susceptibility of Uranium Complexes, Chem. Rev., 2014, 114, 8865–8882 CrossRef CAS PubMed.
- J. A. Seed, L. Birnoschi, E. L. Lu, F. Tuna, A. J. Wooles, N. F. Chilton and S. T. Liddle, Anomalous magnetism of uranium(IV)-oxo and -imido complexes reveals unusual doubly degenerate electronic ground states, Chem, 2021, 7, 1666–1680 CAS.
- I. Castro-Rodriguez, H. Nakai and K. Meyer, Multiple-bond metathesis mediated by sterically pressured uranium complexes, Angew Chem. Int. Ed. Engl., 2006, 45, 2389–2392 CrossRef CAS PubMed.
- S. C. Bart, F. W. Heinemann, C. Anthon, C. Hauser and K. Meyer, A New Tripodal Ligand System with Steric and Electronic Modularity for Uranium Coordination Chemistry, Inorg. Chem., 2009, 48, 9419–9426 CrossRef CAS PubMed.
- O. P. Lam, P. L. Feng, F. W. Heinemann, J. M. O'Connor and K. Meyer, Charge-separation in uranium diazomethane complexes leading to C–H activation and chemical transformation, J. Am. Chem. Soc., 2008, 130, 2806–2816 CrossRef CAS PubMed.
- E. M. Matson, M. D. Goshert, J. J. Kiernicki, B. S. Newell, P. E. Fanwick, M. P. Shores, J. R. Walensky and S. C. Bart, Synthesis of Terminal Uranium(IV) Disulfido and Diselenido Compounds by Activation of Elemental Sulfur and Selenium, Chem.–Eur. J., 2013, 19, 16176–16180 CrossRef CAS PubMed.
- J. A. Seed, M. Gregson, F. Tuna, N. F. Chilton, A. J. Wooles, E. J. L. McInnes and S. T. Liddle, Rare-Earth- and Uranium-Mesoionic Carbenes: A New Class of f-Block Carbene Complex Derived from an N-Heterocyclic Olefin, Angew. Chem., Int. Ed., 2017, 56, 11534–11538 CrossRef CAS PubMed.
- N. J. Wolford, X. J. Yu, S. C. Bart, J. Autschbach and M. L. Neidig, Ligand effects on electronic structure and bonding in U(III) coordination complexes: a combined MCD, EPR and computational study, Dalton Trans., 2020, 49, 14401–14410 RSC.
- W. W. Lukens, M. Speldrich, P. Yang, T. J. Duignan, J. Autschbach and P. Kogerler, The roles of 4f-and 5f-orbitals in bonding: a magnetochemical, crystal field, density functional theory, and multi-reference wavefunction study, Dalton Trans., 2016, 45, 11508–11521 RSC.
- D. Pividori, M. E. Miehlich, B. Kestel, F. W. Heinemann, A. Scheurer, M. Patzschke and K. Meyer, Uranium Going the Soft Way: Low-Valent Uranium(III) Coordinated to an Arene-Anchored Tris-Thiophenolate Ligand, Inorg. Chem., 2021, 60, 16455–16465 Search PubMed.
- A. Ambrosi, C. K. Chua, B. Khezri, Z. Sofer, R. D. Webster and M. Pumera, Chemically reduced graphene contains inherent metallic impurities present in parent natural and synthetic graphite, Proc. Natl. Acad. Sci. USA, 2012, 109, 12899–12904 CrossRef CAS PubMed.
- J. C. Wedal, W. N. G. Moore, W. W. Lukens and W. J. Evans, Perplexing EPR Signals from 5f36d1 U(II) Complexes, Inorg. Chem., 2024, 63, 2945–2953 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: For detailed syntheses, spectroscopic, magnetic and crystallographic data. CCDC 2411215, 2411216, 2411217. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc03694a |
| This journal is © The Royal Society of Chemistry 2025 |




