Haijin Guo, Yunyun Liu, Chengping Wen and Jie-Ping Wan
Green Chem., 2022,24, 5058-5063
DOI:
10.1039/D2GC01644C,
Communication
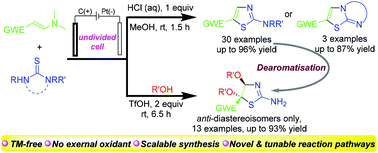
An electrochemical method for the synthesis of 2-aminothiazoles via aryl ring construction using enaminones and thioureas is reported. The cascade enamine C–H thiolation and C–N amination constitutes a major transformation for the thiazole ring formation. Moreover, a simple modification of electrochemical conditions enables the tunable dearomatization of the thiazole ring via vicinal dialkoxylation, leading to the synthesis of 4,5-dialkoxyl thiazolines bearing a quaternary carbon center with excellent diastereoselectivity.