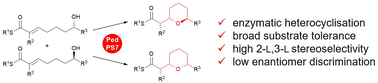
Intramolecular oxa-Michael addition-catalysing cyclases are widespread in polyketide biosynthetic pathways. Although they have significant potential in biotechnology and chemoenzymatic synthesis of chiral heterocycles, they have only scarcely been studied. Here, we present detailed investigations on the selectivity profile of the pyran synthase PedPS7 showing that it combines broad substrate tolerance with high selectivity for the formation of up to two new stereocentres and relaxed precursor stereoisomer discrimination. Two of the four possible tetrahydropyran stereoisomers are reliably accessible by this enzyme. The results indicate fundamental differences between the individual subtypes of intramolecular oxa-Michael addition-catalysing cyclases.