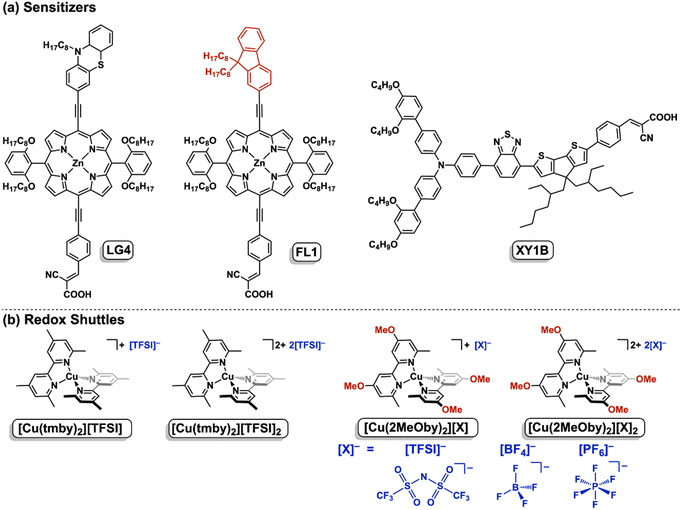
Open Access Article
Open Access ArticleMolecular engineering of porphyrin dyes and copper complexes for enhanced dye regeneration toward high-performance dye-sensitized solar cells using copper(I/II) redox shuttles†
Yuzhe Zhanga,
Tomohiro Higashino *a,
Keigo Namikawaa,
W. Ryan Osterloha and
Hiroshi Imahori
*a,
Keigo Namikawaa,
W. Ryan Osterloha and
Hiroshi Imahori *abc
*abc
aDepartment of Molecular Engineering, Graduate School of Engineering, Kyoto University, Kyoto, 615-8510, Japan. E-mail: t-higa@scl.kyoto-u.ac.jp; imahori@scl.kyoto-u.ac.jp
bInstitute for Integrated Cell-Material Sciences (WPI-iCeMS), Kyoto University, Kyoto, 606-8501, Japan
cInstitute for Liberal Arts and Sciences (ILAS), Kyoto University, Kyoto, 606-8316, Japan
First published on 21st July 2025
Abstract
Porphyrin dyes have garnered significant attention as promising photosensitizers for dye-sensitized solar cells (DSSCs) due to their exceptional light-harvesting capabilities and remarkable power conversion efficiencies (PCEs) when paired with cobalt(II/III) complex-based redox shuttles. Meanwhile, copper(I/II) complexes have emerged as new generation redox shuttles, achieving impressive open-circuit voltages (VOC) exceeding 1.0 V. However, porphyrin-based DSSCs using copper(I/II) redox shuttles have struggled with low-to-moderate PCEs, primarily due to insufficient driving forces for the dye regeneration process. In this study, we introduce FL1, a novel porphyrin dye featuring a fluorene moiety with reduced electron-donating properties, designed to ensure a sufficient driving force for dye regeneration using copper(I/II) complexes. Under optimized conditions, DSSC incorporating FL1 with a copper(I/II) complex utilizing 4,4′-dimethoxy-6,6′-dimethyl-2,2′-bipyridine [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 achieved a notable PCE of 8.30% with a VOC of 0.890 V. Furthermore, our investigation into counterion effects revealed that DSSCs employing [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2 as a redox shuttle delivered the highest PCE of 9.06% with a VOC of 0.900 V, attributed to its superior diffusion coefficient. Finally, co-sensitized DSSCs featuring FL1 and XY1B achieved an outstanding PCE of 10.9%, while retaining a high VOC of 0.945 V, setting a new benchmark efficiency for porphyrin-based DSSCs utilizing copper(I/II) redox shuttles. This breakthrough highlights the immense potential of further refining porphyrin dyes and copper(I/II) redox shuttles through energy-level engineering to optimize the driving force for dye regeneration and propel advancements in DSSC technology.
Introduction
A substantial portion of global energy consumption still relies on fossil fuels, leading to substantial CO2 emissions and pressing environmental concerns. This growing challenge has heightened the urgency for sustainable energy solutions, with solar energy emerging as a particularly compelling alternative, an abundant and virtually limitless resource derived from the sun. Among various solar technologies, organic solar cells present a promising avenue due to their cost-effective production, design flexibility, and lightweight properties.1,2 Notably, dye-sensitized solar cells (DSSCs) have captured considerable attention due to their impressive high power conversion efficiencies (PCEs), vibrant multicolor fabrication possibilities, and outstanding performance under low-light conditions.3–8Given the fundamental working principle of DSSCs, the sensitizer is a key player in facilitating light-harvesting and electron transfer (ET) processes at the dye/TiO2/electrolyte interface. To push the boundaries of DSSC efficiency, the development of sensitizers with strong light-harvesting capabilities across the visible and near-infrared spectrum is highly desirable.9–13 Moreover, the strategic incorporation of bulky substituents is crucial for suppressing charge recombination (CR) processes, thereby optimizing device performance. Porphyrin dyes have emerged as highly promising photosensitizers for DSSCs, thanks to their exceptional light-harvesting properties, adaptable electronic structures achieved through molecular engineering, and outstanding stability compared to conventional dyes.14–32 Notably, single push-pull-type porphyrin dyes designed with effective steric hindrance from bulky substituents have demonstrated impressive PCEs, reaching up to 13% when paired with tris(bipyridyl)CoII/III ([Co(bpy)3]2+/3+) as a redox shuttle. This progress underscores the immense potential of porphyrin-based sensitizers in advancing high-performance DSSCs.
Meanwhile, the redox shuttle serves as a crucial component in DSSCs, as the open-circuit voltage (VOC) is primarily determined by the potential difference between the quasi-Fermi level of TiO2 and the redox potential (Eredox) of the redox shuttle.33–35 Over the past few decades, iodide/triiodide (I−/I3−) and [Co(bpy)3]2+/3+ have been the most widely utilized redox shuttles. However, DSSCs incorporating these traditional shuttles have faced a VOC limitation, with values restricted to below 1.0 V. To address this constraint, copper(I/II) complexes have emerged as new-generation redox shuttles, offering a more positive Eredox and significantly enhancing performance. DSSCs employing suitable complementary dyes and [Cu(tmby)2]+/2+ (tmby = 4,4′-6,6′-dimethyl-2,2′-bipyridine) have achieved PCEs of up to 15%, coupled with a higher VOC exceeding 1.0 V.8,36–44 This progress emphasizes the transformative potential of copper-based redox shuttles in propelling DSSC efficiency to new heights.
In this context, DSSCs incorporating highly efficient porphyrin dyes alongside copper redox shuttles present a promising pathway for further enhancing PCE. However, despite their potential, the PCEs of porphyrin-based DSSCs using copper(I/II) complex-based redox shuttles remain below 7%, even in the case of LG4 (Fig. 1),45–47 falling significantly behind other organic dye-based DSSCs in performance. These low-to-moderate PCEs are primarily attributed to the challenge of achieving a sufficient driving force for effective dye regeneration (−ΔGreg ≥ 0.2 eV). Conventional push–pull-type porphyrin dyes typically exhibit relatively low oxidation potentials (Eox) of 0.7–0.8 V vs. NHE, limiting their compatibility with copper(I/II) complexes. In our previous studies, we demonstrated that increasing the −ΔGreg value through energy-level engineering is an effective strategy for boosting PCEs in porphyrin-based DSSCs.46 Thus, porphyrin dyes with elevated Eox values (≥0.9 V vs. NHE) would be well-suited for DSSCs utilizing copper(I/II) complexes. Additionally, ligand modification of copper(I/II) complexes emerges as another promising approach for optimizing −ΔGreg values (Fig. 2).47,48 Incorporating electron-donating substituents into the bipyridine framework shifts the Eredox value negatively, a favorable adjustment for improving porphyrin-based DSSC performance. These advancements collectively pave the way for further refining porphyrin-copper systems, driving DSSCs toward higher efficiencies.
 | ||
| Fig. 2 Energy-level engineering of both dyes and redox shuttles for achieving an efficient driving force for dye regeneration (−ΔGreg) toward enhanced cell performance. | ||
Herein, we present FL1, a novel porphyrin dye incorporating a 9,9-dioctylfluorene moiety, along with its photovoltaic performance in DSSCs employing copper(I/II) complex-based redox shuttles (Fig. 1). The reduced electron-donating nature of the 9,9-dioctylfluorene moiety, compared to the phenothiazine moiety in previously reported LG4,45 was expected to enhance the −ΔGreg value (Fig. 2), while the two bulky octyl chains at the 9-position of the fluorene skeleton were anticipated to provide a strong blocking effect against the redox shuttle. Furthermore, we aimed to further increase the −ΔGreg value by pairing FL1 with a copper(I/II) complex incorporating 4,4′-dimethoxy-6,6′-dimethyl-2,2′-bipyridine (2MeOby), [Cu(2MeOby)2]+/2+, featuring electron-donating methoxy groups (Fig. 1). Additionally, we also investigated the effect of counterions because the counterion effect of the ruthenium dyes (i.e. N719) has been reported.49 Actually, counterions play a critical role in the diffusion behavior of copper(I/II) complexes in organic solvents,50 significantly influencing the PCE of DSSCs, as high diffusion coefficients are essential for efficient charge transport within the electrolyte.50,51 To optimize charge transport, we examined three counterions, trifluoromethane-sulfonimide ([TFSI]−), tetrafluoroborate ([BF4]−), and hexafluorophosphate ([PF6]−). Through rational molecular engineering and a systematic exploration of the counterion effect, we successfully enhanced the PCE, with FL1-based DSSCs achieving an impressive PCE of 9.06%. Moreover, co-sensitization with XY1B further pushed the PCE beyond 10% while retaining a high VOC of 0.945 V, marking the first instance of porphyrin-based DSSCs with copper(I/II) complex-based redox shuttles surpassing this milestone.
Results and discussion
Synthesis and characterization
The synthesis of the porphyrin dye FL1 followed the procedure outlined in Scheme S1.† First, the Sonogashira coupling of 2-bromo-9,9′-dioctyl-9H-fluorene 1 (ref. 52) with triisopropylsilyl acetylene yielded ethynyl-substituted fluorene 2. After deprotecting the silyl group with n-tetrabutylammonium fluoride (TBAF), subsequent Sonogashira coupling with meso-bromoporphyrin 3 (ref. 19) afforded bis-ethynyl substituted porphyrin 4. Another round of triisopropylsilyl group deprotection enabled a final Sonogashira coupling with 4-iodobenzaldehyde to synthesize porphyrin 5, which then underwent Knoevenagel condensation with cyanoacrylic acid to yield FL1.Additionally, copper complexes incorporating [TFSI]− as a counterion were synthesized (Scheme S2†). The reaction of CuI with the ligand 2MeOby,53 followed by counterion exchange using LiTFSI, produced the copper(I) complex [Cu(2MeOby)2][TFSI]. In contrast, the direct reaction of Cu(TFSI)2 with 2MeOby resulted in the copper(II) complex [Cu(2MeOby)2][TFSI]2 without counterion exchange. Copper(I) complexes [Cu(2MeOby)2][X] (X = BF4 or PF6) were obtained by reacting [Cu(CH3CN)4][BF4] or [Cu(CH3CN)4][PF6] with 2MeOby (Scheme S3†). Meanwhile, the copper(II) complexes [Cu(2MeOby)2][X]2 (X = BF4 or PF6) were prepared via a two-step process: first, CuSO4·5H2O was reacted with 2MeOby, followed by counterion exchange with the respective ammonium salts (NH4BF4 or NH4PF6). Characterization of these compounds, including their copper complexes, was performed using 1H and 13C NMR, FT-IR spectroscopy, and high-resolution mass spectrometry (Fig. S1–S9†). Notably, the as-prepared copper(II) complexes contained varying proportions of their copper(I) counterparts due to unavoidable auto-reduction under ambient conditions, as Cl− ions were absent.54,55 Since Kitagawa et al. reported that the auto-reduction of copper(II) complexes occurred in ethanol,54 methanol in the reaction solvent may be a reductant for auto-reduction. On the other hand, we obtained pure copper(II) complexes by the bulk electrolysis of the corresponding copper(I) complexes, following established literature protocols.56,57 The progress of electrochemical oxidation was monitored using the current, and the electrolysis was considered complete when the relative current approached zero, indicating that the copper(I) complexes had been entirely converted to their copper(II) counterparts (Fig. S16 and S17†). Since the as-prepared copper(II) complexes contain no other impurities except for the corresponding copper(I) complexes, the actual compositions of the as-prepared copper(II) complexes were determined as [Cu(2MeOby)2][X]n (n = 1.6–1.9; X = TFSI, BF4, or PF6) by absorbance at 748 nm in acetonitrile (Fig. S19†), using the absorption coefficient of pure copper(II) complexes (vide infra). It is noteworthy that the actual compositions of the as-prepared copper(II) complexes in our laboratory were also determined as [Cu(tmby)2][TFSI]1.8 in a similar manner.
Single crystals suitable for X-ray diffraction analysis were successfully grown via vapor diffusion of Et2O into acetonitrile solutions of [Cu(2MeOby)2][TFSI] or [Cu(2MeOby)2][BF4] and by vapor diffusion of n-butanol into acetonitrile solutions of [Cu(2MeOby)2][PF6]. The resulting crystal structures (Fig. S10†) revealed Cu–N bond distances of 2.01–2.07 Å, confirming that the counterions had negligible influence on the geometry of the copper(I) complexes. The crystal data of the copper(I) complexes are also summarized in Table S1.†
Optical and electrochemical properties of porphyrin dyes
The UV/vis/NIR absorption spectra of the porphyrin dyes in tetrahydrofuran (THF) are presented in Fig. 3, with their optical properties summarized in Table 1. The absorption spectrum of FL1 exhibits light-harvesting capabilities comparable to those of LG4, with a slight 5 nm blue-shift in its lowest-energy Q-band. This shift is attributed to the fluorene moiety's weaker electron-donating ability compared to the phenothiazine moiety in LG4. The steady-state fluorescence spectrum of FL1 in THF also shows a 7 nm blue-shifted emission relative to LG4, aligning with the Q-band blue-shift observed in absorption spectra (Fig. S11†). The fluorescence lifetimes (τ), determined by time-correlated single-photon counting (TCSPC), are 1.3 ns for FL1 and 1.2 ns for LG4 (Fig. S12†). The almost identical lifetimes suggest that the replacement of the phenothiazine moiety with the fluorene moiety does not cause unfavorable aggregation tendency. The optical HOMO–LUMO gaps (E0–0) of FL1 and LG4, calculated from the intersection of their normalized absorption and emission spectra, are 1.90 eV and 1.87 eV, respectively (Table 1). Although the fluorene moiety is a weaker electron donor than the phenothiazine moiety, it has a minimal impact on FL1's overall optical properties.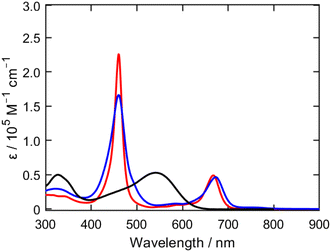 | ||
| Fig. 3 UV/vis/NIR absorption spectra of FL1 (red), LG4 (blue), and XY1B18 (black) in THF. | ||
| Dye | λabsa/nm (ε/103 M−1 cm−1) | λemb/nm | Eoxc/V | E0–0/eV | d/V | −ΔGinje/eV | −ΔGregf/eV | −ΔGregg/eV |
|---|---|---|---|---|---|---|---|---|
| a Wavelengths for Soret and Q-band maxima in THF.b Wavelengths for emission maxima in THF by excitation at Soret band maxima.c Determined by cyclic voltammetry (CV) measurements of the porphyrin-adsorbed TiO2 films (vs. the normal hydrogen electrode (NHE)).d Determined by adding the E0–0 value to the Eox (vs. NHE).e Driving forces for electron injection from the porphyrin singlet excited state (1ZnP*) to the conduction band (CB) of TiO2 (−0.5 V vs. NHE).f Driving forces for the regeneration of oxidized porphyrin dyes (ZnP˙+) by the [Cu(tmby)2]+/2+ redox couple (+0.88 V vs. NHE).g Driving forces for the regeneration of oxidized porphyrin dyes (ZnP˙+) by the [Cu(2MeOby)2]+/2+ redox couple (+0.82 V vs. NHE). | ||||||||
| FL1 | 460 (226), 668 (72) | 681 | 1.07 | 1.90 | −0.83 | 0.33 | 0.19 | 0.25 |
| LG4 | 460 (206), 673 (69) | 688 | 0.98 | 1.87 | −0.89 | 0.39 | 0.10 | 0.16 |
The electrochemical properties of the porphyrin dyes were evaluated using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) in THF with 0.1 M tetrabutylammonium hexafluorophosphate (n-Bu4NPF6) as a supporting electrolyte. The first oxidation potential (Eox) of FL1 was measured at +0.93 V vs. NHE (Fig. S13†), which is more positive than that of LG4 (+0.86 V vs. NHE), reinforcing the fluorene moiety's weaker electron-donating ability. To further assess the ET processes at the dye/TiO2/electrolyte interface, CV measurements were performed for dye-adsorbed TiO2 films (FL1/TiO2 and LG4/TiO2) as working electrodes. The Eox values of FL1/TiO2 and LG4/TiO2 were determined to be +1.07 V and +0.98 V vs. NHE, respectively (Fig. S14† and Table 1). Considering the redox potential (Eredox) of the copper(I/II) complexes [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 (+0.88 V vs. NHE, Fig. S20a†), the driving forces for dye regeneration (−ΔGreg) of the porphyrin radical cation (ZnP˙+) were calculated to be 0.19 eV for FL1 and 0.10 eV for LG4. Since previous studies suggest that −ΔGreg should be comparable to or larger than 0.20 eV for efficient dye regeneration via copper(I/II) redox shuttles,36 the larger −ΔGreg value for FL1 than for LG4 is expected to enhance dye regeneration efficiency, contributing to improved PCE. Additionally, the driving forces for electron injection (−ΔGinj) from the porphyrin excited singlet state (1ZnP*) to the conduction band (CB) of TiO2 (−0.5 V vs. NHE) were estimated using their excited-state oxidation potentials calculated using their oxidation potentials and optical HOMO–LUMO gaps. Both FL1 and LG4 possess sufficient −ΔGinj values (≥0.30 eV) to facilitate efficient electron injection into the CB of TiO2.
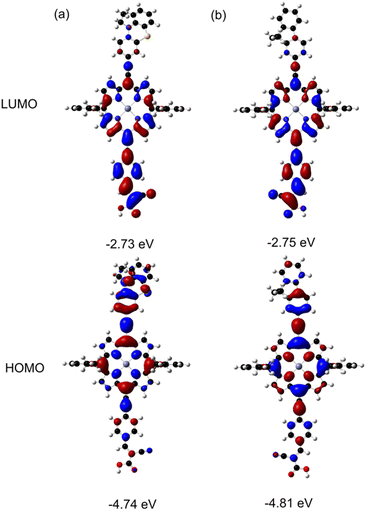
To obtain insight into the ground state geometries and electronic structures of the porphyrin dyes, we performed density functional theory (DFT) calculations on model porphyrins at the B3LYP/6-31G(d) level (Fig. 4). Both FL1 and LG4 adopt a highly planar structure, facilitating an extended π-system for effective charge delocalization. The two alkyl chains at the 9-position of the fluorene moiety in FL1 serve a dual purpose: providing a blocking effect and suppressing dye aggregation due to the enhanced steric hindrance (Fig. S15†). The highest occupied molecular orbitals (HOMOs) display significant orbital distribution on the porphyrin core and donor moieties, while the orbital distributions of the lowest unoccupied molecular orbitals (LUMOs) are mainly localized on the electron-withdrawing cyanoacrylic acid anchoring groups and porphyrin core. The HOMO level of FL1 (−4.81 eV) is slightly more negative than that of LG4 (−4.74 eV), agreeing with the weaker electron-donating ability of the fluorene moiety than the phenothiazine moiety. The calculated HOMO–LUMO energy gaps were determined to be 2.01 eV for LG4 and 2.06 eV for FL1, aligning well with the optical HOMO–LUMO gaps and electrochemical properties of these porphyrin dyes.
Optical and electrochemical properties of copper complexes
The UV/vis/NIR absorption spectra of the copper complexes [Cu(2MeOby)2][X] and [Cu(2MeOby)2][X]2 (X = TFSI, BF4, or PF6) in acetonitrile are illustrated in Fig. S18,† with their optical properties detailed in Table 2. Although we obtained the pure copper(II) complexes via electrochemical oxidation of the corresponding copper(I) complexes (vide supra), we could not isolate the pure copper(II) complexes after electrolysis. Thus, we directly used the resulting solution for absorption and electrochemical measurements of the copper(II) complexes. The pronounced absorption bands spanning 300–500 nm are characteristic of metal-to-ligand charge transfer (MLCT) transitions, a defining feature of copper complexes. In contrast, the faint absorption observed at 748 nm arises from d–d transitions, a hallmark of copper(II) complexes.54 Interestingly, [Cu(2MeOby)2][X]/[Cu(2MeOby)2][X]2 (X = TFSI, BF4, or PF6) exhibits identical absorption spectra, indicating that counter anions exert minimal influence on their optical properties.| λabsa/nm (ε/M−1 cm−1) | Db/10−5 cm2 s−1 | |
|---|---|---|
| a Wavelength of the absorption peaks in acetonitrile.b Diffusion coefficients determined via chronoamperometry. | ||
| [Cu(2MeOby)2][TFSI] | 334 (4700), 445 (4500) | 1.23 |
| [Cu(2MeOby)2][TFSI]2 | 385 (1800), 748 (110) | — |
| [Cu(2MeOby)2][BF4] | 334 (4500), 445 (4500) | 1.30 |
| [Cu(2MeOby)2][BF4]2 | 385 (1700), 748 (130) | — |
| [Cu(2MeOby)2][PF6] | 334 (4700), 445 (4600) | 2.41 |
| [Cu(2MeOby)2][PF6]2 | 385 (1700), 748 (110) | — |
To further explore the electrochemical behavior, we conducted CV measurements for both copper(I) and copper(II) complexes in acetonitrile (Fig. S20†). The copper(I) complex [Cu(2MeOby)2][TFSI] displayed a reversible oxidation peak at +0.82 V vs. NHE. Notably, the copper(II) complex [Cu(2MeOby)2][TFSI]2 exhibited a reversible reduction peak at precisely the same potential, +0.82 V, identical to the oxidation potential of its copper(I) counterpart. Consequently, the redox potential of [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 was determined to be +0.82 V vs. NHE, which is lower than that of [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 attributable to the electron-rich nature of the 2MeOby ligand with methoxy substitutions. Furthermore, the redox potentials of [Cu(2MeOby)2][BF4]/[Cu(2MeOby)2][BF4]2 and [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2 were similarly determined to be +0.82 V vs. NHE, reinforcing the notion that counterions have negligible impact on the redox behavior of the copper(I/II) complexes. Considering the Eox values of FL1/TiO2 (+1.07 V vs. NHE) and LG4/TiO2 (+0.98 V vs. NHE), the −ΔGreg values for FL1 and LG4 with [Cu(2MeOby)2]+/2+ were estimated to be 0.25 and 0.16 eV, respectively. The increase in −ΔGreg values is expected to accelerate the dye regeneration process, which, in turn, should enhance the PCE employing [Cu(2MeOby)2]+/2+ as a redox shuttle.
Diffusion behavior of copper(I) complexes
The diffusion behavior of copper(I) complexes is closely linked to mass transport in DSSCs. Since charge transport in the electrolyte involves the movement of electroinactive counterions to maintain electroneutrality, the overall mass transport of copper(I/II) complexes is predominantly governed by their counterions.51,58 The diffusion coefficients (D) of the copper(I) complexes were determined via chronoamperometry (Table 3 and Fig. S21†). For [Cu(tmby)2][TFSI] and [Cu(2MeOby)2][TFSI], the measured diffusion coefficients were 1.28 × 10−5 and 1.23 × 10−5 cm2 s−1, respectively, aligning well with previous rotating disk electrode measurement for [Cu(tmby)2][TFSI] (1.12 × 10−5 cm2 s−1).39 This consistency suggests that the ligand structure has little influence on diffusion behavior. However, a notable deviation was observed for [Cu(2MeOby)2][PF6], which exhibited a significantly higher diffusion coefficient of 2.41 × 10−5 cm2 s−1, compared to [Cu(2MeOby)2][TFSI] and [Cu(2MeOby)2][BF4] (1.30 × 10−5 cm2 s−1). This enhanced diffusion is likely attributed to weak electrostatic interaction between the positively charged copper center and the negatively charged [PF6]− counterion, facilitating superior mobility. Such favorable diffusion properties are essential for efficient charge transport within the electrolyte, even at low redox shuttle concentrations, ultimately supporting improved DSSC performance.| Dye | Electrolyte | JIPCEb/mA cm−2 | JSC/mA cm−2 | VOC/V | ff | PCE/% |
|---|---|---|---|---|---|---|
| a Photovoltaic parameters derived from the highest PCEs. The values in parentheses denote average values from three or four independent experiments. Error bars represent the standard error of the mean.b The JIPCE values were obtained by integrating the IPCE plots, and the relative discrepancies from the corresponding JSC values obtained from the J–V characteristics are provided in the parentheses. | ||||||
| LG4 | 0.2 M [Cu(tmby)2][TFSI] | 9.50 (0.7%) | 9.57 (9.35 ± 0.4) | 0.842 (0.844 ± 0.011) | 0.716 (0.716 ± 0.003) | 5.77 (5.65 ± 0.2) |
| 0.05 M [Cu(tmby)2][TFSI]2 | ||||||
| LG4 | 0.2 M [Cu(2MeOby)2][TFSI] | 10.0 (0.7%) | 10.07 (9.96 ± 0.2) | 0.821 (0.819 ± 0.002) | 0.762 (0.763 ± 0.004) | 6.30 (6.22 ± 0.1) |
| 0.05 M [Cu(2MeOby)2][TFSI]2 | ||||||
| FL1 | 0.2 M [Cu(tmby)2][TFSI] | 10.8 (3.5%) | 11.2 (10.7 ± 0.6) | 0.902 (0.895 ± 0.013) | 0.767 (0.757 ± 0.019) | 7.75 (7.28 ± 0.5) |
| 0.05 M [Cu(tmby)2][TFSI]2 | ||||||
| FL1 | 0.2 M [Cu(2MeOby)2][TFSI] | 12.3 (2.4%) | 12.6 (12.1 ± 0.4) | 0.887 (0.893 ± 0.01) | 0.730 (0.719 ± 0.009) | 8.15 (7.78 ± 0.3) |
| 0.05 M [Cu(2MeOby)2][TFSI]2 | ||||||
| FL1 | 0.1 M [Cu(tmby)2][TFSI] | 10.2 (1.9%) | 10.4 (10.1 ± 0.4) | 0.935 (0.919 ± 0.024) | 0.720 (0.735 ± 0.03) | 7.00 (6.84 ± 0.2) |
| 0.025 M [Cu(tmby)2][TFSI]2 | ||||||
| FL1 | 0.1 M [Cu(2MeOby)2][TFSI] | 11.6 (6.5%) | 12.4 (12.3 ± 0.5) | 0.890 (0.895 ± 0.006) | 0.752 (0.748 ± 0.008) | 8.30 (8.20 ± 0.2) |
| 0.025 M [Cu(2MeOby)2][TFSI]2 | ||||||
| FL1 | 0.1 M [Cu(2MeOby)2][BF4] | 12.0 (4.7%) | 12.6 (12.4 ± 0.6) | 0.913 (0.916 ± 0.003) | 0.777 (0.762 ± 0.02) | 8.94 (8.63 ± 0.3) |
| 0.025 M [Cu(2MeOby)2][BF4]2 | ||||||
| FL1 | 0.1 M [Cu(2MeOby)2][PF6] | 13.0 (1.5%) | 13.2 (12.7 ± 0.4) | 0.900 (0.910 ± 0.01) | 0.763 (0.762 ± 0.006) | 9.06 (8.80 ± 0.2) |
| 0.025 M [Cu(2MeOby)2][PF6]2 | ||||||
| XY1B | 0.1 M [Cu(2MeOby)2][PF6] | 14.1 (2.1%) | 14.4 (14.6 ± 0.2) | 0.961 (0.963 ± 0.002) | 0.752 (0.73 ± 0.02) | 10.4 (10.3 ± 0.1) |
| 0.025 M [Cu(2MeOby)2][PF6]2 | ||||||
| FL1 + XY1B | 0.1 M [Cu(2MeOby)2][PF6] | 14.4 (5.3%) | 15.2 (15.3 ± 0.1) | 0.945 (0.940 ± 0.01) | 0.756 (0.752 ± 0.005) | 10.9 (10.8 ± 0.1) |
| 0.025 M [Cu(2MeOby)2][PF6]2 | ||||||
Photovoltaic properties of DSSCs with FL1 and LG4 using [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 and [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 as redox shuttles
To optimize the fabrication conditions for DSSCs with FL1 under standard AM 1.5 conditions, we first utilized [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 as a redox shuttle. Because the as-prepared copper(II) complex contains no other impurities except for the corresponding copper(I) species (vide supra), we can prepare the electrolyte solution with an accurate concentration by adjusting the amounts of copper(I/II) complexes based on the actual composition of the copper(II) complex [Cu(tmby)2][TFSI]1.8. The electrolyte solution consisted of 0.2 M [Cu(tmby)2][TFSI], 0.05 M [Cu(tmby)2][TFSI]2, 0.1 M LiTFSI, and 0.5 M 4-tert-butylpyridine (TBP) in acetonitrile. We screened immersion solvents while maintaining a fixed immersion time of 3 hours, ultimately selecting a mixture of toluene and ethanol (EtOH) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v ratio. We then evaluated the effect of immersion time, observing that PCEs increased with extended immersion periods, reaching a peak value of 6.94% for FL1 at 3 hours (Fig. S22†). To further enhance performance, we investigated chenodeoxycholic acid (CDCA) as a co-adsorbent to suppress dye aggregation on TiO2 (Fig. S23†). The DSSC employing FL1 achieved a maximum PCE of 7.75% upon the addition of 1 equivalent of CDCA. For a comparative analysis, we fabricated DSSCs with LG4-sensitized TiO2 films under previously optimized conditions.46 The DSSC with LG4 using [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 exhibited a lower PCE of 5.77%, significantly below that with FL1. Notably, the optimized CDCA concentration was 1 equivalent for FL1 but 4 equivalents for LG4, likely due to FL1's reduced aggregation tendency arising from steric hindrance by its two alkyl chains at the 9-position of the fluorene moiety. Additionally, we fabricated DSSCs utilizing [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 as a redox shuttle, benefiting from its larger −ΔGreg values due to the less positive Eredox value (Table 1). The DSSCs employing [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 demonstrated superior PCEs (8.15% for FL1 and 6.30% for LG4) compared to those using [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2. While DSSCs with [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 exhibited slightly lower VOC values due to the less positive Eredox value, the enhanced JSC values effectively compensated for this decrease, ultimately resulting in improved PCEs.
5 v/v ratio. We then evaluated the effect of immersion time, observing that PCEs increased with extended immersion periods, reaching a peak value of 6.94% for FL1 at 3 hours (Fig. S22†). To further enhance performance, we investigated chenodeoxycholic acid (CDCA) as a co-adsorbent to suppress dye aggregation on TiO2 (Fig. S23†). The DSSC employing FL1 achieved a maximum PCE of 7.75% upon the addition of 1 equivalent of CDCA. For a comparative analysis, we fabricated DSSCs with LG4-sensitized TiO2 films under previously optimized conditions.46 The DSSC with LG4 using [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 exhibited a lower PCE of 5.77%, significantly below that with FL1. Notably, the optimized CDCA concentration was 1 equivalent for FL1 but 4 equivalents for LG4, likely due to FL1's reduced aggregation tendency arising from steric hindrance by its two alkyl chains at the 9-position of the fluorene moiety. Additionally, we fabricated DSSCs utilizing [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 as a redox shuttle, benefiting from its larger −ΔGreg values due to the less positive Eredox value (Table 1). The DSSCs employing [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 demonstrated superior PCEs (8.15% for FL1 and 6.30% for LG4) compared to those using [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2. While DSSCs with [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 exhibited slightly lower VOC values due to the less positive Eredox value, the enhanced JSC values effectively compensated for this decrease, ultimately resulting in improved PCEs.
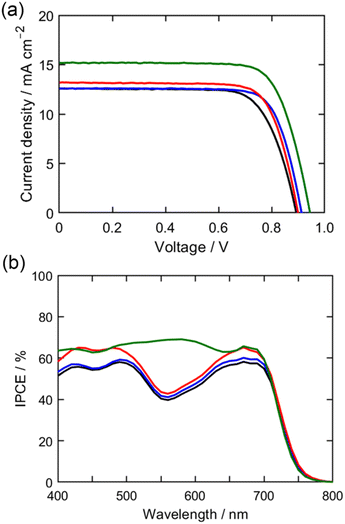
The photocurrent–voltage characteristics of DSSCs under optimized conditions are presented in Fig. 5, with the corresponding photovoltaic data listed in Table 3. The superior PCEs observed for FL1 compared to LG4 primarily stem from its higher JSC and VOC values. The enhanced JSC values are attributed to improved incident photon-to-current efficiencies (IPCEs), as illustrated in Fig. 6b. The IPCE values follow the trend: LG4 with [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 < LG4 with [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 < FL1 with [Cu(tmby)2][TFSI]2/[Cu(tmby)2][TFSI]2 < FL1 with [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2. Indeed, the JIPCE values derived from photocurrent action spectra are compatible with the measured JSC values. To further elucidate the differences in IPCE, we analyzed light-harvesting efficiency (LHE), electron injection efficiency (ϕinj), and charge collection efficiency (ηcol), based on the equation: IPCE = LHE × ϕinj × ηcol. The absorption spectra of dye-adsorbed TiO2 films, recorded without a light-scattering layer, revealed nearly identical absorption profiles for FL1 and LG4 (Fig. 6a). Taking into account the excellent absorption coefficients of the porphyrin dyes and the presence of a light-scattering layer in the actual device, the LHE values approach 100% for both dyes. Although self-quenching of the excited state due to dye aggregation on TiO2 could potentially reduce ϕinj, co-adsorption of CDCA effectively mitigates dye aggregation effects. Consequently, the higher IPCE values observed for FL1 compared to LG4 are primarily attributed to its superior ηcol values.
Given that the ηcol value correlates with the dye regeneration efficiency (φreg), we conducted microsecond time-resolved transient absorption (TA) measurements on the porphyrin-sensitized TiO2 films. The decay profiles of the porphyrin radical cation (ZnP˙+) at 800 nm were analyzed, as the electrochemical oxidation of the porphyrin dyes distinctly revealed the characteristic absorption at 700–850 nm (Fig. S24†). The φreg values were derived from the lifetimes of ZnP˙+ in the absence and presence of copper(I/II) redox shuttles (Fig. S25 and Table S2†). The estimated φreg values follow the trend: LG4 with [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 (58%) < LG4 with [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 (66%) < FL1 with [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 (71%) < FL1 with [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 (84%). This order aligns well with the trend observed for the −ΔGreg values. The highest φreg value of 84% for FL1 with [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 underscores the critical role of achieving a −ΔGreg value of 0.2 eV for an efficient dye regeneration process, even in DSSCs employing copper(I/II) redox shuttles.
To further assess CR between electrons in the CB of TiO2 and the redox shuttle, we examined current–voltage characteristics under dark conditions (Fig. S26†). The more positive voltage onsets observed for FL1 compared to LG4 indicate an effective suppression of the CR process, likely due to an enhanced blocking effect. Dye loading amounts (Γ) for FL1 and LG4, under the optimized sensitization conditions, were determined to be 9.5 × 10−11 and 1.1 × 10−10 mol cm−2, respectively. These values were obtained by measuring the absorbance of porphyrin dyes dissolved from the dye-sensitized TiO2 films into a THF/H2O (v/v = 1/1) solution with 0.1 M NaOH. The smaller Γ value for FL1 compared to LG4 suggests that the increased steric hindrance of FL1 plays a critical role in enhancing the blocking effect and suppressing dye aggregation on TiO2, primarily due to the presence of alkyl chains on the fluorene moiety. Additionally, the ET process at the dye/TiO2/electrolyte interface was investigated using electrical impedance spectroscopy (EIS) under AM 1.5 illumination under open-circuit conditions (Fig. S27†). The charge transfer resistance (Rp) at the TiO2/dye/electrolyte interface followed this trend: (32.2 Ω for LG4 with [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 < 36.8 Ω for LG4 with [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 < 42.5 Ω for FL1 with [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 < 45.0 Ω for FL1 with [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2). This order is consistent with that of the observed VOC values, reinforcing the correlation between increased Rp values for FL1 and higher VOC values in the DSSC utilizing FL1 compared to LG4.
To demonstrate the potential of our new porphyrin dye FL1 for DSSCs using copper(I/II) complex-based redox shuttles, we also measured photovoltaic performances of the DSSCs with FL1 and LG4 using the standard I−/I3− redox shuttle (Fig. S28, S29 and Table S3†). Although the DSSCs using the I−/I3− redox shuttle exhibited higher JSC values than those using [Cu(L)2][TFSI]/[Cu(L)2][TFSI]2 (L = tmby or 2MeOby) due to their larger driving forces for dye regeneration, the less positive redox potential of I−/I3− (+0.40 V vs. NHE) resulted in significantly lower VOC values. Overall, the PCEs of the DSSCs using the I−/I3− redox shuttle (5.84% for LG4 and 6.74% for FL1) are lower than those using the [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 redox shuttle. These results clearly corroborate that the combination of the porphyrin dye and the copper(I/II) complex-based redox shuttle with a sufficient −ΔGreg value is an effective means to achieve further enhancement in PCE.
Effect of electrolyte composition on photovoltaic performances
The electrolyte solution plays a crucial role in DSSCs, prompting an investigation into the effect of its composition, specifically the concentration and counterions, on the photovoltaic properties of DSSCs with FL1. We first examined the performance using a low-concentration electrolyte containing 0.1 M [CuL2][TFSI] and 0.025 M [CuL2][TFSI]2 (L = tmby or 2MeOby) for [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 and [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2. The DSSC employing [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 exhibited a slight improvement in PCE (8.30%), whereas the DSSC with [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 showed a significant decrease in PCE (7.00%) (Fig. S30† and Table 3). Reducing the concentration of copper(I/II) complexes led to a slight increase in the VOC values likely due to suppressed CR between electrons in TiO2 and the redox shuttle. Interestingly, the JSC value of the DSSC using [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 remained comparable across both low and high concentrations, suggesting its robustness. In contrast, the JSC value for [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 decreased substantially, directly contributing to its lower PCE. Considering the similar diffusion coefficients of [Cu(tmby)2][TFSI] and [Cu(2MeOby)2][TFSI], the diminished JSC value for [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 at low concentrations can likely be ascribed to an inefficient dye regeneration process. Therefore, achieving higher −ΔGreg values proves advantageous for DSSCs operating with low-concentration electrolytes.Since counterions significantly affect diffusion properties (vide supra), they are also expected to impact the photovoltaic performance of DSSCs utilizing copper(I/II) complexes. To investigate this effect, we examined different counterions at optimal concentrations of 0.1 M [Cu(2MeOby)2][X] and 0.025 M [Cu(2MeOby)2][X]2 (X = TFSI, BF4, or PF6) (Fig. 7a and Table 3). DSSCs incorporating [Cu(2MeOby)2][X]/[Cu(2MeOby)2][X]2 (X = BF4 or PF6) attained higher PCEs (8.94% for [Cu(2MeOby)2][BF4]/[Cu(2MeOby)2][BF4]2 and 9.06% for [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2) compared to [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2. Remarkably, the PCE of the DSSC employing FL1 with [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2 is the highest reported among porphyrin-based DSSCs with copper(I/II) redox shuttles. The superior PCE observed for [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2 can be attributed to its significantly improved JSC value, in line with its higher IPCE values relative to those of [Cu(2MeOby)2][BF4]/[Cu(2MeOby)2][BF4]2 and [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 (Fig. 7b). To further elucidate this enhancement, we estimated φreg values through time-resolved TA measurements conducted in the presence of copper(I/II) complexes at low concentrations (0.1 M CuI and 0.025 M CuII) (Fig. S31 and Table S4†). The φreg values followed the trend: [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 (83%) < [Cu(2MeOby)2][BF4]/[Cu(2MeOby)2][BF4]2 (84%) < [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2 (87%). Since counterions do not directly impact the Eredox value, the highest diffusion coefficient of [Cu(2MeOby)2][PF6] is likely responsible for the superior φreg, IPCE, and JSC values, ultimately leading to the highest PCE. Even though the maximum IPCE value of the DSSC using [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2 exceeded 60% due to the highest φreg value, there remains room for further improvement in the JSC value. Considering that the enhanced blocking effect can suppress the unfavorable CR process,17,18 the introduction of bulky substituents is expected to increase the ηcol value, leading to a higher JSC value.
We also measured current–voltage characteristics under dark conditions (Fig. S32†) to evaluate the impact of counterions on VOC values. The onsets of dark current for [Cu(2MeOby)2][BF4]/[Cu(2MeOby)2][BF4]2 and [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2 were more positive than those for [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2. Thus, the slightly improved VOC values observed for [Cu(2MeOby)2][BF4]/[Cu(2MeOby)2][BF4]2 and [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2 can be ascribed to more effective suppression of CR between electrons in the CB of TiO2 and the redox shuttle. Although the precise mechanism remains unclear at this stage, these findings suggest that counterions significantly influence interfacial behavior at the TiO2/dye/electrolyte interfaces, with the [TFSI]− anion potentially accelerating undesirable CR processes.
Photovoltaic properties of co-sensitized DSSCs with XY1B
The DSSC with FL1 using [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2 achieved the highest PCE among porphyrin-based DSSCs with copper(I/II) redox shuttles. However, further improvement in JSC values and overall PCEs remains possible due to relatively low IPCE values at around 550 nm. Co-sensitization with additional dyes exhibiting complementary absorption properties presents an effective strategy for achieving panchromatic absorption by addressing absorption deficits.59–61 To this end, we selected XY1B as a co-sensitizer, considering its excellent absorption profile (ε = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 at 541 nm)18 and high PCE (10.4%) in DSSCs under our optimized conditions (Fig. 2, S33† and Table 3). The co-sensitized TiO2 films were prepared via a second immersion step, wherein FL1-sensitized TiO2 films were immersed in an XY1B solution (0.1 M, 50 equiv. CDCA) in THF and ethanol solution (v/v = 1/4). Following optimization of the second immersion conditions, the co-sensitized DSSC with FL1 + XY1B exhibited an enhanced PCE, driven by improved JSC and VOC values (Fig. 7a and Table 3). The photocurrent action spectra clearly demonstrate a substantial increase in IPCE values within the 500–600 nm range, reaching 69% after co-sensitization with XY1B (Fig. 7b). This enhancement is corroborated by increased absorption observed in co-sensitized TiO2 films at approximately 550 nm (Fig. S34†). Dye loading amounts of FL1 and XY1B on the co-sensitized TiO2 were determined to be 4.7 × 10−11 and 6.2 × 10−11 mol cm−2, respectively, confirming that the JSC enhancement stems from the complementary absorption of XY1B. Furthermore, current–voltage characteristics of the co-sensitized DSSC under dark conditions displayed a positive shift in the onset voltage, agreeing with the observed increase in VOC (Fig. S32†). Ultimately, the co-sensitized DSSC with FL1 + XY1B attained an outstanding PCE of 10.9%, surpassing the highest PCE recorded for porphyrin-based DSSCs employing copper(I/II) redox shuttles, marking the first instance of exceeding 10% PCE. Since the VOC value (0.945 V) is slightly higher than the highest VOC value in the co-sensitized porphyrin-based DSSCs with [Co(bpy)3]2+/3+ (0.935 V),19 further optimization of device fabrication conditions will achieve a VOC value higher than 1.0 V. In contrast, the co-sensitized DSSC with LG4 + XY1B delivered a PCE of 8.40%, which fell below that with XY1B as a single dye (Fig. S35 and Table S5†). This reduction may be attributed to LG4's aggregation tendency, which likely quenches XY1B's excited state, thereby impairing performance. Notably, the two alkyl chains on the fluorene moiety of FL1 play a crucial role in suppressing dye aggregation, proving advantageous not only for aggregation control but also for co-sensitization.
000 M−1 cm−1 at 541 nm)18 and high PCE (10.4%) in DSSCs under our optimized conditions (Fig. 2, S33† and Table 3). The co-sensitized TiO2 films were prepared via a second immersion step, wherein FL1-sensitized TiO2 films were immersed in an XY1B solution (0.1 M, 50 equiv. CDCA) in THF and ethanol solution (v/v = 1/4). Following optimization of the second immersion conditions, the co-sensitized DSSC with FL1 + XY1B exhibited an enhanced PCE, driven by improved JSC and VOC values (Fig. 7a and Table 3). The photocurrent action spectra clearly demonstrate a substantial increase in IPCE values within the 500–600 nm range, reaching 69% after co-sensitization with XY1B (Fig. 7b). This enhancement is corroborated by increased absorption observed in co-sensitized TiO2 films at approximately 550 nm (Fig. S34†). Dye loading amounts of FL1 and XY1B on the co-sensitized TiO2 were determined to be 4.7 × 10−11 and 6.2 × 10−11 mol cm−2, respectively, confirming that the JSC enhancement stems from the complementary absorption of XY1B. Furthermore, current–voltage characteristics of the co-sensitized DSSC under dark conditions displayed a positive shift in the onset voltage, agreeing with the observed increase in VOC (Fig. S32†). Ultimately, the co-sensitized DSSC with FL1 + XY1B attained an outstanding PCE of 10.9%, surpassing the highest PCE recorded for porphyrin-based DSSCs employing copper(I/II) redox shuttles, marking the first instance of exceeding 10% PCE. Since the VOC value (0.945 V) is slightly higher than the highest VOC value in the co-sensitized porphyrin-based DSSCs with [Co(bpy)3]2+/3+ (0.935 V),19 further optimization of device fabrication conditions will achieve a VOC value higher than 1.0 V. In contrast, the co-sensitized DSSC with LG4 + XY1B delivered a PCE of 8.40%, which fell below that with XY1B as a single dye (Fig. S35 and Table S5†). This reduction may be attributed to LG4's aggregation tendency, which likely quenches XY1B's excited state, thereby impairing performance. Notably, the two alkyl chains on the fluorene moiety of FL1 play a crucial role in suppressing dye aggregation, proving advantageous not only for aggregation control but also for co-sensitization.
Stability of the DSSCs
The long-term stability of the DSSCs was assessed under white-light illumination (100 mW cm−2) at 25 °C over a 500-hour period. The DSSC with FL1 + XY1B retained 84% of its initial efficiency, outperforming the DSSC with LG4 + XY1B, which maintained 73% (Fig. S36†). The superior stability of FL1 + XY1B may have resulted from the weaker electron-donating ability of the fluorene moiety in FL1 relative to the phenothiazine moiety in LG4, a characteristic beneficial for practical use.Conclusions
We designed and synthesized a novel porphyrin dye, FL1, incorporating a fluorene skeleton with a weaker electron-donating nature relative to the phenothiazine skeleton. The Eox value of FL1/TiO2 was determined to be +1.07 V vs. NHE, ensuring a −ΔGreg value comparable to or larger than 0.2 eV. Thanks to this increased −ΔGreg value, the DSSC with FL1 achieved a higher PCE of 7.75% than that with LG4 (5.77%) when using [Cu(tmby)2][TFSI]/[Cu(tmby)2][TFSI]2 as the redox shuttle. Furthermore, the DSSC with FL1 employing [Cu(2MeOby)2][TFSI]/[Cu(2MeOby)2][TFSI]2 exhibited an even higher PCE of 8.15%, attributed to an increased −ΔGreg value (0.25 eV). This finding underscores the importance of maintaining sufficient −ΔGreg values for achieving high-performance porphyrin-based DSSCs using copper(I/II) redox shuttles. To further explore performance optimization, we investigated the influence of counterions in [Cu(2MeOby)2][X]/[Cu(2MeOby)2][X]2 (X = TFSI, BF4, or PF6) on diffusion behavior and identified [Cu(2MeOby)2][PF6] as having the highest diffusion coefficient. Consequently, the DSSC with FL1 using [Cu(2MeOby)2][PF6]/[Cu(2MeOby)2][PF6]2 attained the highest PCE of 9.06%, creating a new benchmark for porphyrin-based DSSCs employing copper(I/II) complex-based redox shuttles. Notably, co-sensitized DSSC with FL1 and the complementary dye XY1B led to an impressive PCE of 10.9%, while maintaining a high VOC of 0.945 V, setting a groundbreaking record as the first porphyrin-based DSSC employing copper(I/II) complex-based redox shuttles to exceed 10% PCE. Overall, our findings confirm that rational molecular engineering of porphyrin dyes and copper(I/II) complex-based redox shuttles with sufficient −ΔGreg values can significantly enhance photovoltaic performance. Additionally, selecting appropriate counterions with high diffusion coefficients is crucial for maximizing the charge transporting capabilities of copper(I/II) complexes. We anticipate that further refinement of porphyrin-based DSSCs, complementary dyes, and copper(I/II) redox shuttles through energy-level engineering will pave the way for next-generation solar cell technology, driving it toward top-tier performance.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
T. Higashino and H. Imahori conceived and designed this work. Y. Zhang and K. Namikawa conducted the synthesis and characterization of the products. Y. Zhang, K. Namikawa, and W. R. Osterloh performed spectroscopic and electrochemical measurements. Y. Zhang fabricated the DSSCs and evaluated their photovoltaic performances. Y. Zhang, T. Higashino, and H. Imahori co-wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the JSPS (KAKENHI Grant Numbers JP20H05841 (T. H.), JP22K05066 (T. H.), JP25K01874 (T. H.), JP20H05832 (H. I.), and JP23H00309 (H. I.)). Y. Z. thanks the China Scholarship Council (CSC) (Chinese Government Scholarship 202006250042) for funding. In this work, DFT calculations were performed using the supercomputer of ACCMS under the collaborative research program for young/women scientists, Kyoto University.Notes and references
- E. K. Solak and E. Irmak, RSC Adv., 2023, 13, 12244–12269 RSC.
- T. N. Murakami and N. Koumura, Adv. Energy Mater., 2019, 9, 1802967 CrossRef.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS.
- A. Fakharuddin, R. Jose, T. M. Brown, F. Fabregat-Santiago and J. Bisquert, Energy Environ. Sci., 2014, 7, 3952–3981 RSC.
- A. B. Muñoz-García, I. Benesperi, G. Boschloo, J. J. Concepcion, J. H. Delcamp, E. A. Gibson, G. J. Meyer, M. Pavone, H. Pettersson, A. Hagfeldt and M. Freitag, Chem. Soc. Rev., 2021, 50, 12450–12550 RSC.
- H. Zhou, M. Aftabuzzaman, Masud, S. H. Kang and H. K. Kim, ACS Energy Lett., 2025, 10, 881–895 CrossRef CAS.
- Y. Ren, D. Zhang, J. Suo, Y. Cao, F. T. Eickemeyer, N. Vlachopoulos, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Nature, 2023, 613, 60–65 CrossRef CAS PubMed.
- Y. Wu, W.-H. Zhu, S. M. Zakeeruddin and M. Grätzel, ACS Appl. Mater. Interfaces, 2015, 7, 9307–9318 CrossRef CAS.
- P. Brogdon, H. Cheema and J. H. Delcamp, ChemSusChem, 2018, 11, 86–103 CrossRef CAS PubMed.
- J.-M. Ji, H. Zhou and H. K. Kim, J. Mater. Chem. A, 2018, 6, 14518–14545 RSC.
- Y. Ren, D. Sun, Y. Cao, H. N. Tsao, Y. Yuan, S. M. Zakeeruddin, P. Wang and M. Grätzel, J. Am. Chem. Soc., 2018, 140, 2405–2408 CrossRef CAS PubMed.
- J.-M. Ji, H. J. Lee, H. Zhou, Y. K. Eom, C. H. Kim and H. K. Kim, ACS Appl. Mater. Interfaces, 2022, 14, 52745–52757 CrossRef CAS PubMed.
- H. Imahori, T. Umeyama and S. Ito, Acc. Chem. Res., 2009, 42, 1809–1818 CrossRef CAS PubMed.
- T. Higashino and H. Imahori, Dalton Trans., 2015, 44, 448–463 RSC.
- Y. Zhang, T. Higashino and H. Imahori, J. Mater. Chem. A, 2023, 11, 12659–12680 RSC.
- Y. Kurumisawa, T. Higashino, S. Nimura, Y. Tsuji, H. Iiyama and H. Imahori, J. Am. Chem. Soc., 2019, 141, 9910–9919 CrossRef CAS PubMed.
- Y. Zhang, T. Higashino, I. Nishimura and H. Imahori, ACS Appl. Mater. Interfaces, 2024, 16, 67761–67770 CrossRef CAS PubMed.
- M. Urbani, M. Grätzel, M. K. Nazeeruddin and T. Torres, Chem. Rev., 2014, 114, 12330–12396 CrossRef CAS PubMed.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629–634 CrossRef CAS.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- A. Yella, C.-L. Mai, S. M. Zakeeruddin, S.-N. Chang, C.-H. Hsieh, C.-Y. Yeh and M. Grätzel, Angew. Chem., Int. Ed., 2014, 53, 2973–2977 CrossRef CAS PubMed.
- S. H. Kang, M. J. Jeong, Y. K. Eom, I. T. Choi, S. M. Kwon, Y. Yoo, J. Kim, J. Kwon, J. H. Park and H. K. Kim, Adv. Energy Mater., 2017, 7, 1602117 CrossRef.
- J. Ji, H. Zhou, Y. K. Eom, C. H. Kim and H. K. Kim, Adv. Energy Mater., 2020, 10, 2000124 CrossRef CAS.
- H. Zhou, J.-M. Ji, H. S. Lee, Masud, M. Aftabuzzaman, D.-N. Lee, C. H. Kim and H. K. Kim, ACS Appl. Mater. Interfaces, 2023, 15, 39426–39434 CrossRef CAS PubMed.
- H. Song, Q. Liu and Y. Xie, Chem. Commun., 2018, 54, 1811–1824 RSC.
- S. Huang, Q. Li, S. Li, C. Li, H. Tan and Y. Xie, Chem. Commun., 2024, 60, 4521–4536 RSC.
- K. Zeng, Y. Lu, W. Tang, S. Zhao, Q. Liu, W. Zhu, H. Tian and Y. Xie, Chem. Sci., 2019, 10, 2186–2192 RSC.
- K. Zeng, Y. Chen, W.-H. Zhu, H. Tian and Y. Xie, J. Am. Chem. Soc., 2020, 142, 5154–5161 CrossRef CAS.
- Z. Li, Q. Lu, Y. Zhang, Q. Li, W. Wu, S. Li, H. Wang, J. Jiang, C. Li and Y. Xie, J. Mater. Chem. A, 2025, 13, 4176–4185 RSC.
- C.-C. Chen, J.-S. Chen, V. S. Nguyen, T.-C. Wei and C.-Y. Yeh, Angew. Chem., Int. Ed., 2021, 60, 4886–4893 CrossRef CAS PubMed.
- C.-C. Chen, Y.-H. Chen, V. S. Nguyen, S.-Y. Chen, M.-C. Tsai, J.-S. Chen, S.-Y. Lin, T.-C. Wei and C.-Y. Yeh, Adv. Energy Mater., 2023, 13, 2300353 CrossRef CAS.
- M. Wang, C. Grätzel, S. M. Zakeeruddin and M. Grätzel, Energy Environ. Sci., 2012, 5, 9394–9405 RSC.
- J. Cong, X. Yang, L. Kloo and L. Sun, Energy Environ. Sci., 2012, 5, 9180–9194 RSC.
- B. Pashaei, H. Shahroosvand and P. Abbasi, RSC Adv., 2015, 5, 94814–94848 RSC.
- T. Higashino and H. Imahori, ACS Energy Lett., 2022, 7, 1926–1938 CrossRef CAS.
- C. E. Housecroft and E. C. Constable, Chem. Sci., 2022, 13, 1225–1262 RSC.
- Masud and H. K. Kim, ACS Omega, 2023, 8, 6139–6163 CrossRef CAS PubMed.
- Y. Saygili, M. Söderberg, N. Pellet, F. Giordano, Y. Cao, A. B. Muñoz-García, S. M. Zakeeruddin, N. Vlachopoulos, M. Pavone, G. Boschloo, L. Kavan, J.-E. Moser, M. Grätzel, A. Hagfeldt and M. Freitag, J. Am. Chem. Soc., 2016, 138, 15087–15096 CrossRef CAS.
- Y. Cao, Y. Liu, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Joule, 2018, 2, 1108–1117 CrossRef CAS.
- Y. Ren, N. Flores-Díaz, D. Zhang, Y. Cao, J. Decoppet, G. C. Fish, J. Moser, S. M. Zakeeruddin, P. Wang, A. Hagfeldt and M. Grätzel, Adv. Funct. Mater., 2020, 30, 2004804 CrossRef CAS.
- D. Zhang, M. Stojanovic, Y. Ren, Y. Cao, F. T. Eickemeyer, E. Socie, N. Vlachopoulos, J.-E. Moser, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Nat. Commun., 2021, 12, 1777 CrossRef CAS PubMed.
- H. Rui, J. Shen, Z. Yu, L. Li, H. Han and L. Sun, Angew. Chem., Int. Ed., 2021, 60, 16156–16163 CrossRef CAS.
- S. M. Meethal, S. C. Pradhan, J. Velore, S. Varughese, R. S. Pillai, F. Sauvage, A. Hagfeldt and S. Soman, J. Mater. Chem. A, 2024, 12, 1081–1093 RSC.
- A. Colombo, G. Di Carlo, C. Dragonetti, M. Magni, A. Orbelli Biroli, M. Pizzotti, D. Roberto, F. Tessore, E. Benazzi, C. A. Bignozzi, L. Casarin and S. Caramori, Inorg. Chem., 2017, 56, 14189–14197 CrossRef CAS PubMed.
- T. Higashino, H. Iiyama, I. Nishimura and H. Imahori, Chem. Lett., 2020, 49, 936–939 CrossRef CAS.
- Y.-H. Chen, C.-C. Chen, V. S. Nguyen, X.-T. Jiang, Y.-D. Chen, M.-Y. Li, S.-Y. Chen, T.-C. Wei and C.-Y. Yeh, Cell Rep. Phys. Sci., 2024, 5, 102159 CrossRef CAS.
- T. Higashino, H. Iiyama, S. Nimura, Y. Kurumisawa and H. Imahori, Inorg. Chem., 2020, 59, 452–459 CrossRef CAS PubMed.
- G. C. Vougioukalakis, A. I. Philippopoulos, T. Stergiopoulos and P. Falaras, Coord. Chem. Rev., 2011, 255, 2602–2621 CrossRef CAS.
- L. Li, L. Zhao, X. Jiang, Z. Yu, J. liu, H. Rui, J. Shen, W. Sharmoukh, N. K. Allam and L. Sun, J. Mater. Chem. A, 2022, 10, 4131–4136 RSC.
- S. C. Pradhan, A. Hagfeldt and S. Soman, J. Mater. Chem. A, 2018, 6, 22204–22214 RSC.
- J. Ding, M. Day, G. Robertson and J. Roovers, Macromolecules, 2002, 35, 3474–3483 CrossRef CAS.
- M. Karpacheva, F. J. Malzner, C. Wobill, A. Büttner, E. C. Constable and C. E. Housecroft, Dye. Pigment., 2018, 156, 410–416 CrossRef CAS.
- S. Kitagawa, M. Munakata and A. Higashie, Inorg. Chim. Acta, 1984, 84, 79–84 CrossRef CAS.
- M. Giordano, G. Volpi, M. Bonomo, P. Mariani, C. Garino and G. Viscardi, New J. Chem., 2021, 45, 15303–15311 RSC.
- P. Ferdowsi, Y. Saygili, S. M. Zakeeruddin, J. Mokhtari, M. Grätzel, A. Hagfeldt and L. Kavan, Electrochim. Acta, 2018, 265, 194–201 CrossRef CAS.
- L. Kavan, Y. Saygili, M. Freitag, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Electrochim. Acta, 2017, 227, 194–202 CrossRef CAS.
- G. Kalaignan and Y. Kang, J. Photochem. Photobiol. C Photochem. Rev., 2006, 7, 17–22 CrossRef CAS.
- N. V. Krishna, J. V. S. Krishna, M. Mrinalini, S. Prasanthkumar and L. Giribabu, ChemSusChem, 2017, 10, 4668–4689 CrossRef CAS PubMed.
- J. M. Cole, G. Pepe, O. K. Al Bahri and C. B. Cooper, Chem. Rev., 2019, 119, 7279–7327 CrossRef CAS PubMed.
- Z. Li, Q. Li, C. Li and Y. Xie, Mater. Chem. Front., 2024, 8, 652–680 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, synthetic details, optical and electrochemical properties of porphyrin dyes and copper complexes, photovoltaic properties, HR-MS spectra, and NMR spectra. CCDC 2451025–2451027. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc03537f |
| This journal is © The Royal Society of Chemistry 2025 |