Yao Li, Bimalendu Roy and Xinyu Liu
Chem. Commun., 2011,47, 8952-8954
DOI:
10.1039/C1CC13264D,
Communication
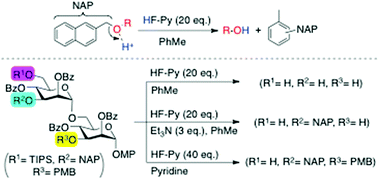
A new method for selective cleavage of 2-naphthylmethyl (NAP) ether utilizing 10–20 molar excess of HF/