Dhevalapally B. Ramachary, R. Madhavachary and M. Shiva Prasad
Org. Biomol. Chem., 2012,10, 5825-5829
DOI:
10.1039/C2OB07122C,
Communication
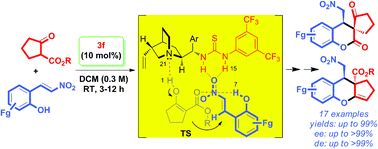
A general approach to asymmetric synthesis of highly substituted spirodihydrocoumarins with a quaternary stereocenter was achieved through neighboring ortho-hydroxyl group induced sequential Michael–lactonization reactions on 2-(2-nitrovinyl)phenols with alkyl cyclopentanone-2-carboxylates in the presence of a catalytic amount of