Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Magnesium plasmonics for UV applications and chiral sensing†‡
Hyeon-Ho
Jeong
ab,
Andrew G.
Mark
a and
Peer
Fischer
*ac
aMax Planck Institute for Intelligent Systems, Heisenbergstr. 3, 70569 Stuttgart, Germany
bInstitute of Materials, École Polytechnique Fédérale de Lausanne (EPFL), CH-1015 Lausanne, Switzerland
cInstitute for Physical Chemistry, University of Stuttgart, Pfaffenwaldring 55, 70569 Stuttgart, Germany. E-mail: fischer@is.mpg.de
First published on 15th September 2016
Abstract
We demonstrate that chiral magnesium nanoparticles show remarkable plasmonic extinction- and chiroptical-effects in the ultraviolet region. The Mg nanohelices possess an enhanced local surface plasmon resonance (LSPR) sensitivity due to the strong dispersion of most substances in the UV region.
Nanoparticles whose size is smaller than the wavelength of light can give rise to light-induced collective electron oscillations, known as local surface plasmon resonance (LSPR).1,2 The resonance wavelength in LSPR is sensitive to the environment, which is the basis for a number of sensing applications.2 Improvements in sensitivity have been achieved by engineering the particles' size, shape, and material composition,3,4 and the mode of detection.5,6 Studies to date have primarily focussed on increasing the shift of the resonance for a given change in the refractive index. However, in many applications the quantity of ultimate interest is not the medium's refractive index, but the concentration of some chemical species.7,8 One particularly promising route to improve the concentration sensitivity is to exploit the natural refractive index dispersion of the molecule (analyte) of interest. Most molecules show notably higher and more dispersive refractive indices at short wavelengths, because of electronic resonances (transitions) in the UV region. Changes in the concentration of the analyte therefore yield larger changes in the refractive index at shorter wavelengths, and therefore larger shifts in the plasmon resonance. However, this also requires a plasmonic probe that shows a UV resonance.
Here, we introduce what we believe are the first magnesium (Mg) nanoparticles for UV plasmonics and LSPR sensing. The Mg nanoparticles are grown with a helical shape. Their exceptionally strong chiroptical response in the near UV region (e.g. circular dichroism, CD = ∼13° at λ = ∼437 nm in solution, see the ESI,† Fig. S2) gives rise to a concomitant enhancement of the LSPR sensitivity.
In an earlier study,5 we developed a chiral plasmonic sensing approach that is based on a chiral nanoprobe (i.e. it measures the differential extinction between left-handed (LH) and right-handed (RH) circularly polarised light using CD spectroscopy, i.e. CD ∝ EL − ER). The change in response to the change in the refractive index of the medium surrounding the nanoparticles is tracked, as schematically shown in Fig. 1. CD-based sensing with chiral nanoparticles offers two distinct advantages over traditional polarisation independent measurements: firstly, the CD signal of a chiral absorber naturally comprises multiple spectral features compared to an extinction peak, and each feature can be tracked. This provides high sensing flexibility and fidelity. In particular, the zero crossings (CD = 0°) where the LH and RH extinctions are equal are ideal for tracking, because they are intrinsically narrow and possess high figures of merit.5 Secondly, the chiral particles provide a strong optical contrast, even in the presence of optically dense fluids, as any achiral background signals are cancelled. Hence, chiral nanoparticles can even be “seen” in strongly absorbing physiological fluids (e.g. blood).9
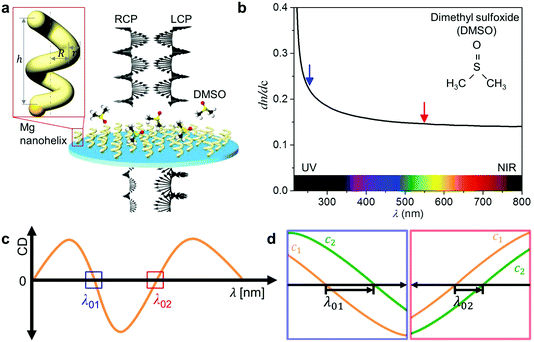 | ||
| Fig. 1 Sensing using UV chiral plasmonics: (a) schematic that illustrates the interaction of left-handed (LH) nanohelices with left- and right-handed circularly polarised light (LCP, RCP) in the presence of dimethyl sulfoxide (DMSO). The inset shows the schematic view of a 2-turn left-handed Mg nanohelix showing dimensions of the seed particle and the nanostructure that grows upon it (h: height, R: major radius, r: minor radius). (b) Change in the refractive index of the DMSO solution in response to a change in its concentration (dn·dc−1) as a function of λ,12,13 which is larger in the UV (blue arrow) than in the visible (red arrow) region. (c) A CD spectrum of LH nanohelices as a function of λ. Two boxes indicate zero crossing points, λ01 (blue: in UV) and λ02 (red: in visible). (d) Each panel indicates the resonance shifts at λ01 (left panel) and λ02 (right panel) where the concentrations of the molecules are varied between c1 and c2 (c1 < c2). | ||
The chiral LSPR sensor operates by tracking the shift in the zero crossing of the CD signal in response to changes in n, the refractive index of the local medium, which surrounds the particles. The resonance condition is5
εr(λ*) = −![[small chi, Greek, macron]](https://www.rsc.org/images/entities/char_e0d8.gif) n2, n2, | (1) |
![[small chi, Greek, macron]](https://www.rsc.org/images/entities/char_e0d8.gif) is a shape-dependent factor. For sensing tasks undertaken in the visible to NIR regime typical of conventional plasmonics, eqn (1) is reasonable (ESI,† Fig. S1).8,10 However, for measurements in the UV region, the dispersion of the medium (solvent and analyte) n(λ) needs to be considered, such that
is a shape-dependent factor. For sensing tasks undertaken in the visible to NIR regime typical of conventional plasmonics, eqn (1) is reasonable (ESI,† Fig. S1).8,10 However, for measurements in the UV region, the dispersion of the medium (solvent and analyte) n(λ) needs to be considered, such thatεr(λ*) = −![[small chi, Greek, macron]](https://www.rsc.org/images/entities/char_e0d8.gif) [n(λ*)]2. [n(λ*)]2. | (2) |
In LSPR sensing it is customary to define a refractive index sensitivity, which describes the change in the resonance position (wavelength) as a function of refractive index5
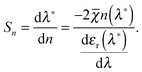 | (3) |
However, another important factor in assessing sensitivity is to determine by how much the tracking wavelength λ* changes in response to the volume concentration c of the species of interest
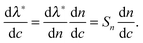 | (4) |
| n = n2c + n1·(1 − c), | (5) |
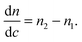 | (6) |
Consider dimethyl sulfoxide (DMSO) in water (Fig. 1b).12,13 Within the visible region the refractive index of DMSO is everywhere higher than that of water, so the sign of eqn (6) is always positive. But dn·dc−1 at 600 nm is only 0.144 RIU, whereas at 250 nm it is 0.229 RIU, almost 60% higher. Thus, enhanced sensitivity to changes in solute concentration (eqn (4)) can be achieved at shorter wavelengths, and in particular through measurements in the UV region.
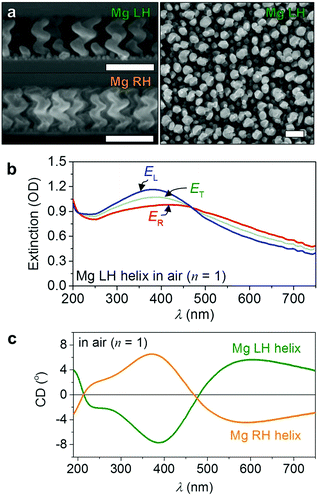
Aluminium (Al) nanoparticles have thus far been favoured for UV plasmonic applications,14,15 even though theory predicts that Mg provides substantially higher far-field absorption efficiency and higher near-field electric field enhancements.16 Furthermore, Mg is abundant, biocompatible, and lightweight (two-thirds lighter than Al).17 However, to the best of our knowledge, there is no report of Mg nanoparticles for UV plasmonics.18 Here we show how we can grow large numbers of chiral Mg nanoparticles using a previously reported fabrication method that we term nano glancing angle deposition (nanoGLAD), which allows precise control of size, shape, and material composition of the nanoparticles at the wafer-level.19–21 Briefly, an array of gold nanoseeds is first patterned on a 1 cm2 quartz glass using block-copolymer micelle nanolithography (BCML).22 Then, in a second step, Mg is evaporated onto the Au nanoseeds at an angle α = 87° and T = ∼100 K in a vacuum chamber with a base pressure P = 1 × 10−6 mbar.23–28 The substrate is continuously rotated during deposition, which, because of the high angle, leads to the growth of a nanohelix on each Au nanoseed by a self-shadowing effect.19,29,30 Due to the fast diffusion of the Mg adatoms, we were not able to shape nanohelices with pure Mg (see the ESI,† Fig. S2), so we co-deposited 8% of titanium (Ti).31 Doping Ti into the Mg matrix does not significantly change the optical properties, but it improves the structural quality of the nanostructures.32 Moreover, as the nanoparticle becomes more Ti rich, the LSPR sensitivity is enhanced since Ti flattens the real part of the dielectric function of the composite.5 The increase in damping also somewhat broadens the lines, but this effect does not diminish the overall improvement.33 Finally, the nanohelices are coated with a thin layer of HfO2 by atomic layer deposition (ALD, Savannah 100, Cambridge NanoTech).34 The 4 nm thin oxide layer improves the chemical resistance of the particles.14Fig. 2a shows scanning electron microscopy (SEM) images of arrays of two different chiralities of 8% Ti-doped Mg nanohelices (here samples are prepared on Si wafers for imaging). Helices with opposite chiralities are grown on different patterned glass substrates (as well as wafers) and during different depositions, but display very similar structural dimensions. The height, major radius, and minor radius of the helices are 142 nm, 26 nm, and 18 nm respectively. Fig. 2b shows the extinction (upper panel) and CD (lower panel) spectra of Mg LH and RH nanohelices obtained using a Jasco J-810 CD spectrometer. The helices exhibit chiroptical responses that saturate the spectrometer even in its largest amplitude detection range, so the responses were measured with an external lock-in-amplifier (UHFL, Zurich Instruments) referenced to the photoelastic modulator (PEM) and synchronized with the monochromator. In accordance with theoretical predictions,16 we have observed an extraordinarily large short-wavelength extinction (∼1 OD at λ = ∼385 nm) and optical activity (CD = ∼8° at λ = ∼380 nm) (see also the ESI,† Fig. S3). The spectral features of both chiralities are well matched across the entire spectrum (max, min, and two zero crossings).
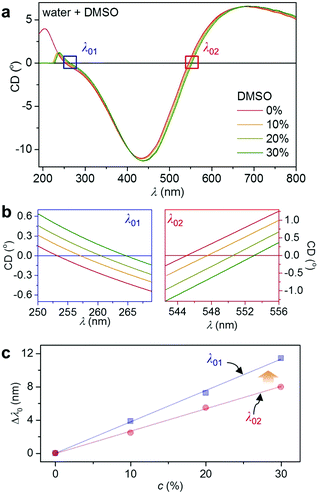
UV-enhanced LSPR refractive index sensing is demonstrated with a DMSO–water system. In neat DMSO, CD spectra of the LH Mg nanohelices (Fig. 3) are shifted relative to the Mg spectra in air (Fig. 2b), because of the relative increase of the refractive index (see Fig. 1b and the ESI,† Fig. S1). Fig. 3 shows CD spectra of the Mg LH nanohelices in DMSO–water mixtures varying from 0 to 30%. DMSO shows strong electronic absorption below 230 nm. We track the CD resonances at the two zero crossings λ01 and λ02 (see the ESI,† Fig. S4 for the extinction spectra). The crossings' wavelengths shift in response to increasing DMSO concentration, as shown in Fig. 3c. Both depend linearly on the concentration of DMSO, but crucially, the shift of Δλ01 in the UV region is larger than the shift at the longer wavelength Δλ02. This is in agreement with the 1.6 times larger dn·dc−1 in the UV compared to the visible region, which in turn induces a larger plasmonic shift (see Fig. 1b). These results clearly show that UV plasmonics is promising for high sensitivity LSPR due to the dispersion of the medium.
In conclusion, this communication reports what we believe are the first Mg nanoparticles that have been used for plasmonics in water. Mg nanohelices were fabricated using nanoGLAD and their chiroptical response is exceptionally strong in the UV region. This feature is crucial for the detection of molecules as in the UV region the dispersion of the medium surrounding the plasmonic particle gives rise to larger refractive index changes. UV sensing may thus enable single molecule detection with a plasmonic nanosensor.35 It should be possible to increase the LSPR sensitivity further through engineering the dielectric function of Mg nanoparticles.5
Apart from UV sensing, Mg nanoparticles are promising for resonance coupling effects for chiral sensing36–39 and plasmon resonance energy transfer (PRET) applications,40–42 since the plasmonic resonance of Mg in the UV approaches molecular resonances.
We are grateful to the Nanostructuring Laboratory, Max Planck Institute for Solid State Research, for ALD access. We thank M. Alarcon-Correa, T.-C. Lee, and J. G. Gibbs for helpful discussions and C. Miksch for assistance with the sample preparation. This work was supported by the Max Planck–EPFL Centre for Molecular Nanoscience and Technology and the European Research Council under ERC Grant agreement 278213.
Notes and references
- R. H. Ritchie, Phys. Rev., 1957, 106, 874–881 CrossRef CAS.
- K. A. Willets and R. P. Van Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267–297 CrossRef CAS PubMed.
- H. Chen, X. Kou, Z. Yang, W. Ni and J. Wang, Langmuir, 2008, 24, 5233–5237 CrossRef CAS PubMed.
- D. E. Charles, D. Aherne, M. Gara, D. M. Ledwith, Y. K. Gun'ko, J. M. Kelly, W. J. Blau and M. E. Brennan-Fournet, ACS Nano, 2010, 4, 55–64 CrossRef CAS PubMed.
- H.-H. Jeong, A. G. Mark, M. Alarcon-Correa, I. Kim, P. Oswald, T.-C. Lee and P. Fischer, Nat. Commun., 2016, 7, 11331 CrossRef CAS PubMed.
- N. Maccaferri, K. E. Gregorczyk, T. V. A. G. de Oliveira, M. Kataja, S. van Dijken, Z. Pirzadeh, A. Dmitriev, J. Åkerman, M. Knez and P. Vavassori, Nat. Commun., 2015, 6, 6150 CrossRef CAS PubMed.
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. Van Duyne, Nat. Mater., 2008, 7, 442–453 CrossRef CAS PubMed.
- K. M. Mayer and J. H. Hafner, Chem. Rev., 2011, 111, 3828–3857 CrossRef CAS PubMed.
- H.-H. Jeong, A. G. Mark, T.-C. Lee, M. Alarcón-Correa, S. Eslami, T. Qiu, J. G. Gibbs and P. Fischer, Nano Lett., 2016, 16, 4887–4894 CrossRef CAS PubMed.
- M. M. Miller and A. A. Lazarides, J. Phys. Chem. B, 2005, 109, 21556–21565 CrossRef CAS PubMed.
- W. Heller, J. Phys. Chem., 1965, 69, 1123–1129 CrossRef CAS.
- I. Z. Kozma, P. Krok and E. Riedle, J. Opt. Soc. Am. B, 2005, 22, 1479–1485 CrossRef CAS.
- G. M. Hale and M. R. Querry, Appl. Opt., 1973, 12, 555–563 CrossRef CAS PubMed.
- M. W. Knight, N. S. King, L. Liu, H. O. Everitt, P. Nordlander and N. J. Halas, ACS Nano, 2014, 8, 834–840 CrossRef CAS PubMed.
- G. Maidecchi, G. Gonella, R. Proietti Zaccaria, R. Moroni, L. Anghinolfi, A. Giglia, S. Nannarone, L. Mattera, H.-L. Dai, M. Canepa and F. Bisio, ACS Nano, 2013, 7, 5834–5841 CrossRef CAS PubMed.
- J. M. Sanz, D. Ortiz, R. Alcaraz de la Osa, J. M. Saiz, F. González, A. S. Brown, M. Losurdo, H. O. Everitt and F. Moreno, J. Phys. Chem. C, 2013, 117, 19606–19615 CAS.
- L.-Y. Chen, J.-Q. Xu, H. Choi, M. Pozuelo, X. Ma, S. Bhowmick, J.-M. Yang, S. Mathaudhu and X.-C. Li, Nature, 2015, 528, 539–543 CrossRef CAS PubMed.
- F. Sterl, N. Strohfeldt, R. Walter, R. Griessen, A. Tittl and H. Giessen, Nano Lett., 2015, 15, 7949–7955 CrossRef CAS PubMed.
- A. G. Mark, J. G. Gibbs, T.-C. Lee and P. Fischer, Nat. Mater., 2013, 12, 802–807 CrossRef CAS PubMed.
- J. G. Gibbs, A. G. Mark, T.-C. Lee, S. Eslami, D. Schamel and P. Fischer, Nanoscale, 2014, 6, 9457–9466 RSC.
- J. G. Gibbs, A. G. Mark, S. Eslami and P. Fischer, Appl. Phys. Lett., 2013, 103, 213101 CrossRef.
- R. Glass, M. Möller and J. P. Spatz, Nanotechnology, 2003, 14, 1153 CrossRef CAS.
- K. Robbie, M. J. Brett and A. Lakhtakia, Nature, 1996, 384, 616 CrossRef CAS.
- G. Nair, H. J. Singh and A. Ghosh, J. Mater. Chem. C, 2015, 3, 6831–6835 RSC.
- H.-H. Jeong, A. G. Mark, T.-C. Lee, K. Son, W. Chen, M. Alarcón-Correa, I. Kim, G. Schütz and P. Fischer, Adv. Sci., 2015, 2, 1500016 CrossRef.
- S. H. Lee, D. P. Singh, J. H. Sung, M.-H. Jo, K. C. Kwon, S. Y. Kim, H. W. Jang and J. K. Kim, Sci. Rep., 2016, 6, 19580 CrossRef CAS PubMed.
- J. Deng, J. Fu, J. Ng and Z. Huang, Nanoscale, 2016, 8, 4504–4510 RSC.
- S. Xie, J. Yang, X. Xiao, Y. Hou, J. Du, L. Pang, X. Li and F. Gao, Nanoscale Res. Lett., 2015, 10, 1–9 CrossRef CAS PubMed.
- H.-H. Jeong, A. G. Mark, J. G. Gibbs, T. Reindl, U. Waizmann, J. Weis and P. Fischer, Nanotechnology, 2014, 25, 235302 CrossRef PubMed.
- J. H. Singh, G. Nair, A. Ghosh and A. Ghosh, Nanoscale, 2013, 5, 7224–7228 RSC.
- Y. He, Y. Zhao and J. Wu, Appl. Phys. Lett., 2008, 92, 063107 CrossRef.
- G. K. Larsen, Y. He, J. Wang and Y. Zhao, Adv. Opt. Mater., 2014, 2, 245–249 CrossRef.
- T. G. Habteyes, S. Dhuey, E. Wood, D. Gargas, S. Cabrini, P. J. Schuck, A. P. Alivisatos and S. R. Leone, ACS Nano, 2012, 6, 5702–5709 CrossRef CAS PubMed.
- D. Walker, B. T. Käsdorf, H.-H. Jeong, O. Lieleg and P. Fischer, Sci. Adv., 2015, 1, 1500501 Search PubMed.
- P. Zijlstra, P. M. R. Paulo and M. Orrit, Nat. Nanotechnol., 2012, 7, 379–382 CrossRef CAS PubMed.
- W. Ma, H. Kuang, L. Xu, L. Ding, C. Xu, L. Wang and N. A. Kotov, Nat. Commun., 2013, 4, 3689 Search PubMed.
- Y. He, K. Lawrence, W. Ingram and Y. Zhao, Chem. Commun., 2016, 52, 2047–2050 RSC.
- E. Hendry, T. Carpy, J. Johnston, M. Popland, R. V. Mikhaylovskiy, A. J. Lapthorn, S. M. Kelly, L. D. Barron, N. Gadegaard and M. Kadodwala, Nat. Nanotechnol., 2010, 5, 783–787 CrossRef CAS PubMed.
- K. M. McPeak, C. D. van Engers, S. Bianchi, A. Rossinelli, L. V. Poulikakos, L. Bernard, S. Herrmann, D. K. Kim, S. Burger, M. Blome, S. V. Jayanti and D. J. Norris, Adv. Mater., 2015, 27, 6244–6250 CrossRef CAS PubMed.
- Y. Choi, T. Kang and L. P. Lee, Nano Lett., 2009, 9, 85–90 CrossRef CAS PubMed.
- J. Li, S. K. Cushing, F. Meng, T. R. Senty, A. D. Bristow and N. Wu, Nat. Photonics, 2015, 9, 601–607 CrossRef CAS.
- M. L. Brongersma, N. J. Halas and P. Nordlander, Nat. Nanotechnol., 2015, 10, 25–34 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc06800f |
| ‡ Part of the issue “chirality at the Nanoscale”. |
| This journal is © The Royal Society of Chemistry 2016 |