A catalyst-free 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines and 3-nitroindoles: an easy access to five-ring-fused tetrahydroisoquinolines†
Xihong
Liu
a,
Dongxu
Yang
a,
Kezhou
Wang
a,
Jinlong
Zhang
a and
Rui
Wang
*ab
aSchool of Life Sciences, Institute of Biochemistry and Molecular Biology, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: wangrui@lzu.edu.cn
bState Key Laboratory of Chiroscience, Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong, P. R. China. E-mail: bcrwang@polyu.edu.hk
First published on 18th October 2016
Abstract
We have reported herein a catalyst-free 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines and 3-nitroindoles by which a series of five-ring-fused tetrahydroisoquinolines featuring an indoline scaffold were obtained as single diastereomers in moderate to high yields without any additives under mild conditions. Moreover, the current method provides a novel and convenient approach for the efficient incorporation of two biologically important scaffolds (tetrahydroisoquinoline and indoline).
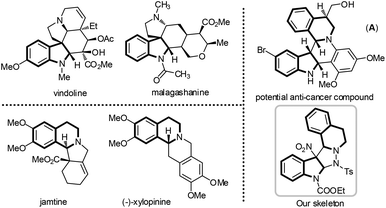
The privileged structural motifs offer synthetic and medicinal chemists a prodigious starting point to design and synthesize new classes of drug candidates. Among various naturally occurring core scaffolds, tetrahydroisoquinoline and indoline, which are widely featured in a large number of bioactive natural products and man-made compounds, have received tremendous attention from organic chemists.1,2 For instance, vindoline might contribute to the anti-diabetic effects of Catharanthus roseus,3 and jamtine has a significant anti-hyperglycemic activity (Fig. 1).4 More interesting still, compound A incorporating the two important scaffolds also exhibited efficient anti-cancer activity.5 In this respect, the development of concise and direct synthetic methods for the efficient combination of two or more privileged motifs to generate structurally complex candidate molecules is of great interest and significance.6
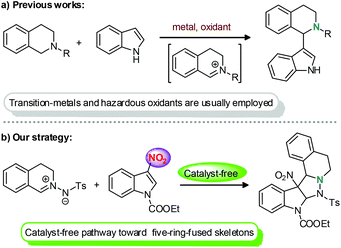
Up to now, different synthetic methods have been reported for the preparation of either polycyclic tetrahydroisoquinoline or indoline derivatives.7,8 However, the methodologies for the incorporation of two such attractive skeletons have rarely been explored.5,9 To meet such a challenge, a protocol involving indoles and either tetrahydroisoquinolines or the precursors of tetrahydroisoquinolines is undoubtedly one of the most direct strategies. In 2005, Li and co-workers first realized a direct indolation of N-aryl tetrahydroisoquinolines through a cross-dehydrogenative coupling reaction.10 And then, some related studies were also successively reported by different groups (Scheme 1a).11 However, despite these advances, such transformations still suffer from some drawbacks, such as air and moisture sensitivity, the need of stoichiometric amounts of hazardous oxidants and the use of transition metals, thus seriously limiting their further applications. For these reasons, and also in response to the principle of sustainable chemistry,12 the exploration of more cheaper, environmentally friendly, and convenient methods for the direct combination of two such privileged motifs is still in great demand. As part of our on-going efforts to synthesize C1 functionalized tetrahydroisoquinolines13 and indolines,14 we reported herein the first catalyst-free 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines and 3-nitroindoles by taking advantage of the significant superiorities of C,N-cyclic azomethine imines in the construction of polycyclic tetrahydroisoquinoline derivatives15–17 and also the great potential of 3-nitroindoles as electrophilic alkenes of a new type (Scheme 1b).18 Thus, a dearomatization of 3-nitroindoles was easily realized19 and the corresponding heterocycles containing both tetrahydroisoquinoline and indoline moieties were efficiently assembled in an atom-economical and environmentally benign manner.
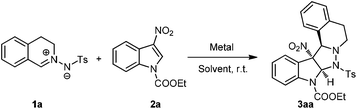
At the outset of our investigation, C,N-cyclic azomethine (1a) and 3-nitroindole (2a) were selected as model substrates to optimize the reaction conditions. The cycloaddition reaction afforded the corresponding product (3aa) in only 23% conversion in the presence of 20 mol% Cu(OTf)2 in CHCl3 after 24 h at room temperature (Table 1, entry 1). Then, some other Lewis acids including Sc(OTf)3, Zn(OTf)2 and Ni(OAc)2·4H2O were examined, and enhanced conversion was observed by using Ni(OAc)2·4H2O (Table 1, entries 2–4). We then conducted the reaction in the absence of a catalyst. And to our delight, a comparable outcome was obtained with 90% conversion (Table 1, entry 5). Encouraged by this result, the effect of solvents was checked subsequently to further improve the yield (Table 1, entries 6–13). For all of the cases, the reaction proceeded smoothly and gave the product in moderate to high conversion. The best result was obtained with ethyl acetate as the solvent. Gratifyingly, the conversion of 2a could be even increased to >95% when 1.2 equiv. of 1a was used.
| Entry | Metal (20 mol%) | Solvent | Conversionb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.1 mmol), metal (20 mol%), solvent (0.4 mL), r.t., 24 h. b Determined by 1H NMR (300 MHz) analysis of the crude reaction mixture based on 2a. c Conducted with 2h. d 1.2 equiv. of 1a was used. e The yield of the isolated product. Note: a relative configuration of the product was exhibited. | |||
| 1 | Cu(OTf)2 | CHCl3 | 23 |
| 2 | Sc(OTf)3 | CHCl3 | 8 |
| 3 | Zn(OTf)2 | CHCl3 | 44 |
| 4 | Ni(OAc)2·4H2O | CHCl3 | 89 |
| 5 | — | CHCl3 | 90 |
| 6 | — | CH2Cl2 | 87 |
| 7 | — | Toluene | 84 |
| 8 | — | THF | 84 |
| 9 | — | EtOAc | 92 |
| 10 | — | CH3CN | 88 |
| 11 | — | DMF | 84 |
| 12 | — | EtOH | 76 |
| 13 | — | MeOH | 67 |
| 14c | — | H2O | 43 |
| 15 | — | EtOAc | >95 (94) |
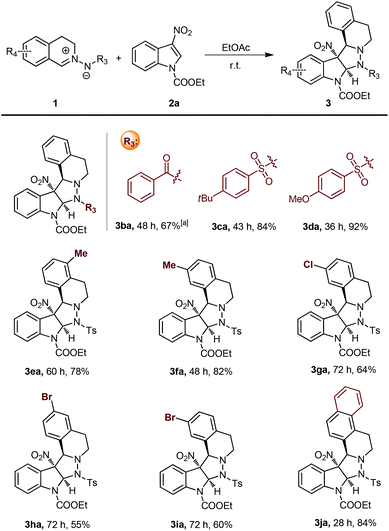
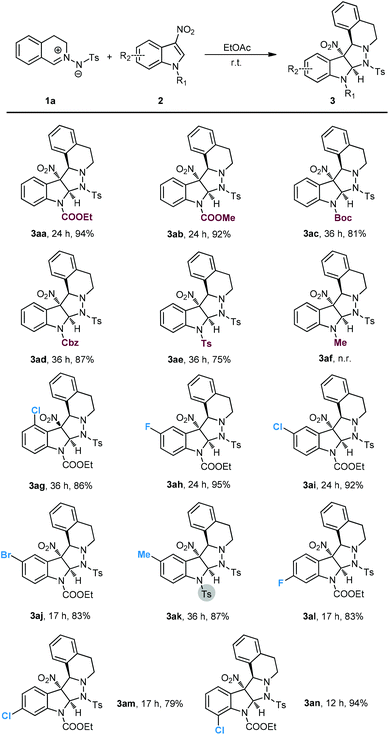
With the established optimal reaction conditions in hand, the substrate scope of the catalyst-free 1,3-dipolar cycloaddition was investigated by using 1a and a series of different substituted 3-nitroindoles. As summarized in Scheme 2, N-alkoxycarbonylated and N-Ts-protected 3-nitroindoles performed very well, and delivered the corresponding cycloadducts 3aa–3ae in high to excellent yields. N-Methyl-protected 3-nitroindole 2f failed to undergo the current transformation probably because of the reduced electrophilicity. Moreover, both electron-donating and electron-withdrawing substituents in the C5-, C6-, and C7-positions of 2 were all compatible with the standard reaction conditions (3ah–3an). It is worth noting that the 3-nitroindole 2g with a chlorine substituent at the 4-position also worked well, despite the relatively greater steric hindrance.
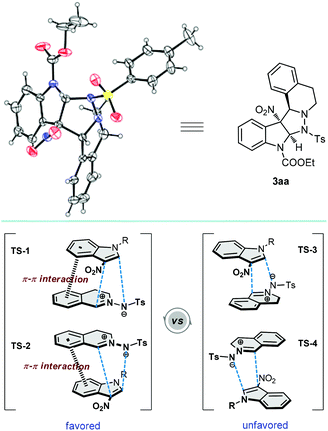
Subsequently, various structurally diverse C,N-cyclic azomethine imines were synthesized to further explore the generality of this protocol (Scheme 3). Although benzoyl hydrazine derived azomethine imine 1b showed relatively lower reactivity, the corresponding product 3ba was obtained in 67% yield when the reaction temperature was raised to 50 °C. Different substituents at the 4-position of the phenyl ring of the arylsulfonyl protecting group had little effect on the reactivities and outcomes (3ca and 3da). Besides, azomethine imines with a methyl substituent on the aromatic ring exhibited relatively higher reactivity than that with a halogen substituent. To our delight, azomethine imine 2j with a condensed-ring system also was smoothly converted into the corresponding product with a satisfactory yield. The configuration of the products was determined by X-ray analysis of 3aa (Fig. 2).20 Accordingly, related transition states (TS-1, TS-2, TS-3 and TS-4) were speculated to explain the specific stereoselectivities. The observed preference for TS-1 and TS-2 was probably caused by the π–π interaction between azomethine imines and 3-nitroindoles.21
Encouraged by the above-mentioned positive results, we then employed some other dipolarophiles including alkylidene azlactone (4) and methyleneindolinone (5) to further illustrate the compatibility of this catalyst-free 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines (Scheme 4). Gratifyingly, both of them worked well and smoothly transformed into the corresponding spirocyclic products in good to high yields with high d.r. It should be noted that an elimination of the Ts group was observed in this process.
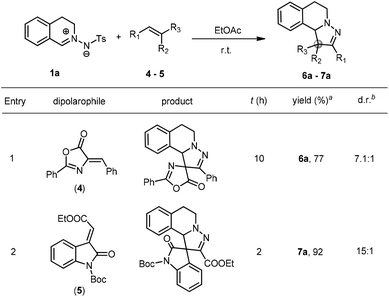 | ||
| Scheme 4 Further extension of the dipolarophiles. aIsolated yields. bDetermined by 1H NMR (300 MHz) spectroscopy of the crude mixture. See the ESI† for details. | ||
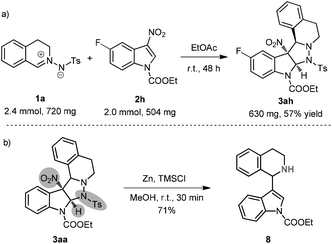
To demonstrate the practicality of this protocol, a relatively large-scale reaction of 1a and 2h was conducted. It is notable that an obvious decomposition of the corresponding cycloadduct was observed in the purification process of flash chromatography, so 3ah was isolated in a relatively lower yield with an excellent d.r. value (Scheme 5a). Besides, a representative transformation of cycloadduct 3aa was carried out (Scheme 5b). Treatment of 3aa with zinc powder and TMSCl afforded the indolationalized tetrahydroisoquinoline 8 in 71% yield.18f
Conclusions
In summary, the first catalyst-free 1,3-dipolar cycloaddition of C,N-cyclic azomethine imines and 3-nitroindoles has been realized. The method gives an easy access to a series of highly functionalized five-ring-fused tetrahydroisoquinolines featuring an indoline scaffold in excellent diastereoselectivities without any use of catalysts or additives. The current methodology is of vital significance by virtue of the mild and facile reaction conditions and also the efficient incorporation of the two intriguing skeletons of medicinal value. Besides, alkylidene azlactone and methyleneindolinone were also compatible with the current protocol and provided the corresponding spirocyclic products in high yields. Further biological investigations of the newly synthesized structurally complex ring-fused tetrahydroisoquinoline derivatives are under progress in our laboratory.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (NSFC) (grant numbers 21432003 and 21272102), the Program for Chang-jiang Scholars and Innovative Research Team in University (PCSIRT: No. IRT_15R27), and the Special Research Fund for the Doctoral Program of Higher Education (20130211130005).Notes and references
- For selected reviews, see: (a) J. D. Scott and R. M. Williams, Chem. Rev., 2002, 102, 1669 CrossRef CAS PubMed; (b) K. W. Bentley, Nat. Prod. Rep., 2004, 21, 395 RSC; (c) K. W. Bentley, Nat. Prod. Rep., 2005, 22, 249 RSC; (d) K. W. Bentley, Nat. Prod. Rep., 2006, 23, 444 RSC.
- For selected reviews, see: (a) P. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, Wiley, New York, 2nd edn, 2002 Search PubMed; (b) E. Fattorusso and O. T. Scafati, Modern Alkaloids, Wiley-VCH, Weinheim, 2008 Search PubMed; (c) M. Lachia and C. J. Moody, Nat. Prod. Rep., 2008, 25, 227 RSC. For selected examples, see: (d) W. Xie, G. Jiang, H. Liu, J. Hu, X. Pan, H. Zhang, X. Wan, Y. Lai and D. Ma, Angew. Chem., Int. Ed., 2013, 52, 12924 CrossRef CAS PubMed; (e) S. Zhao and R. B. Andrade, J. Am. Chem. Soc., 2013, 135, 13334 CrossRef CAS PubMed; (f) S. P. Lathrop and M. Movassaghi, Chem. Sci., 2014, 5, 333 RSC; (g) J. Ruchti and E. M. Carreira, J. Am. Chem. Soc., 2014, 136, 16756 CrossRef CAS PubMed; (h) Q. Li, T. Xia, L. Yao, H. Deng and X. Liao, Chem. Sci., 2015, 6, 3599 RSC; (i) A. Acharya, D. Anumandla and C. S. Jeffrey, J. Am. Chem. Soc., 2015, 137, 14858 CrossRef CAS PubMed; (j) W. Ji, L. Yao and X. Liao, Org. Lett., 2016, 18, 628 CrossRef CAS PubMed; (k) M. S. Kirillova, M. E. Muratore, R. Dorel and A. M. Echavarren, J. Am. Chem. Soc., 2016, 138, 3671 CrossRef CAS PubMed.
- (a) P. L. Feldman and H. Rapoport, J. Am. Chem. Soc., 1987, 109, 1603 CrossRef CAS; (b) D. Kato, Y. Sasaki and D. L. Boger, J. Am. Chem. Soc., 2010, 132, 3685 CrossRef CAS PubMed; (c) X.-G. Yao, F. Chen, P. Li, L. Quan, J. Chen, L. Yu, H. Ding, C. Li, L. Chen, Z. Gao, P. Wan, L. Hu, H. Jiang and X. Shen, J. Ethnopharmacol., 2013, 150, 285 CrossRef CAS PubMed.
- (a) J. Pérard-Viret, F. Souquet, M.-L. Manisse and J. Royer, Tetrahedron Lett., 2010, 51, 96 CrossRef; (b) A. Padwa and M. D. Danca, Org. Lett., 2002, 4, 715 CrossRef CAS PubMed; (c) N. S. Simpkins and C. D. Gill, Org. Lett., 2003, 5, 535 CrossRef CAS PubMed.
- Z. Chen, B. Wang, Z. Wang, G. Zhu and J. Sun, Angew. Chem., Int. Ed., 2013, 52, 2027 CrossRef CAS PubMed.
- For selected examples, see: (a) B. Tan, G. Hernández-Torres and C. F. Barbas III, J. Am. Chem. Soc., 2011, 133, 12354 CrossRef CAS PubMed; (b) F. Tan, C. Xiao, H.-G. Cheng, W. Wu, K.-R. Ding and W.-J. Xiao, Chem. – Asian J., 2012, 7, 493 CrossRef CAS PubMed; (c) V. Eschenbrenner-Lux, P. Küchler, S. Ziegler, K. Kumar and H. Waldmann, Angew. Chem., Int. Ed., 2014, 53, 2134 CrossRef CAS PubMed; (d) X. Bao, B. Wang, L. Cui, G. Zhu, Y. He, J. Qu and Y. Song, Org. Lett., 2015, 17, 5168 CrossRef CAS PubMed; (e) X. Liu, J. Zhang, L. Zhao, S. Ma, D. Yang, W. Yan and R. Wang, J. Org. Chem., 2015, 80, 12651 CrossRef CAS PubMed; (f) P. K. Mishra and A. K. Verma, Green Chem. 10.1039/c6gc02330d.
- For reviews, see: (a) M. Chrzanowska and M. D. Rozwadowska, Chem. Rev., 2004, 104, 3341 CrossRef CAS PubMed; (b) J. Stöckigt, A. P. Antonchick, F. Wu and H. Waldmann, Angew. Chem., Int. Ed., 2011, 50, 8538 CrossRef PubMed; (c) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366 CrossRef CAS PubMed; (d) C. Nájera, J. M. Sansano and M. Yus, Org. Biomol. Chem., 2015, 13, 8596 RSC; (e) W. Liu, S. Liu, R. Jin, H. Guo and J. Zhao, Org. Chem. Front., 2015, 2, 288 RSC. For selected examples, see: (f) T. Itoh, M. Miyazaki, H. Fukuoka, K. Nagata and A. Ohsawa, Org. Lett., 2006, 8, 1295 CrossRef CAS PubMed; (g) S. Murarka, I. Deb, C. Zhang and D. Seidel, J. Am. Chem. Soc., 2009, 131, 13226 CrossRef CAS PubMed; (h) L. Chen, L. Zhang, J. Lv, J.-P. Cheng and S. Luo, Chem. – Eur. J., 2012, 18, 8891 CrossRef CAS PubMed; (i) A. J. Neel, J. P. Hehn, P. F. Tripet and F. D. Toste, J. Am. Chem. Soc., 2013, 135, 14044 CrossRef CAS PubMed; (j) S. Sun, C. Li, P. E. Floreancig, H. Lou and L. Liu, Org. Lett., 2015, 17, 1684 CrossRef CAS PubMed.
- For selected reviews, see: (a) A. Steven and L. E. Overman, Angew. Chem., Int. Ed., 2007, 46, 5488 CrossRef CAS PubMed; (b) J. Kim and M. Movassaghi, Chem. Soc. Rev., 2009, 38, 3035 RSC; (c) D. Zhang, H. Song and Y. Qin, Acc. Chem. Res., 2011, 44, 447 CrossRef CAS PubMed; (d) C. C. J. Loh and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 46 CrossRef CAS PubMed; (e) L. M. Repka and S. E. Reisman, J. Org. Chem., 2013, 78, 12314 CrossRef CAS PubMed. For selected examples, see: (f) A. Martínez, M. J. Webber, S. Müller and B. List, Angew. Chem., Int. Ed., 2013, 52, 9486 CrossRef PubMed; (g) J. Zhu, Y. Liang, L. Wang, Z.-B. Zheng, K. N. Houk and Y. Tang, J. Am. Chem. Soc., 2014, 136, 6900 CrossRef CAS PubMed; (h) L. Liao, C. Shu, M. Zhang, Y. Liao, X. Hu, Y. Zhang, Z. Wu, W. Yuan and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 10471 CrossRef CAS PubMed; (i) M.-C. Tong, X. Chen, J. Li, R. Huang, H. Tao and C.-J. Wang, Angew. Chem., Int. Ed., 2014, 53, 4680 CrossRef CAS PubMed; (j) M. C. DiPoto, R. P. Hughes and J. Wu, J. Am. Chem. Soc., 2015, 137, 14861 CrossRef CAS PubMed; (k) C. Liu, J.-C. Yi, Z.-B. Zheng, Y. Tang, L.-X. Dai and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 751 CrossRef CAS PubMed.
- For examples, see: (a) W. Cao, X. Liu, J. Guo, L. Lin and X. Feng, Chem. – Eur. J., 2015, 21, 1632 CrossRef CAS PubMed; (b) S. J. Chambers, G. Coulthard, W. P. Unsworth, P. O'Brien and R. J. K. Taylor, Chem. – Eur. J., 2016, 22, 6496 CrossRef CAS PubMed.
- Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 6968 CrossRef CAS PubMed.
- (a) M. Ghobrial, M. Schnürch and M. D. Mihovilovic, J. Org. Chem., 2011, 76, 8781 CrossRef CAS PubMed; (b) W. Su, J. Yu, Z. Li and Z. Jiang, J. Org. Chem., 2011, 76, 9144 CrossRef CAS PubMed; (c) J. Dhineshkumar, M. Lamani, K. Alagiri and K. R. Prabhu, Org. Lett., 2013, 15, 1092 CrossRef CAS PubMed; (d) M. O. Ratnikov, X. Xu and M. P. Doyle, J. Am. Chem. Soc., 2013, 135, 9475 CrossRef CAS PubMed; (e) Q.-Y. Meng, J.-J. Zhong, Q. Liu, X.-W. Gao, H.-H. Zhang, T. Lei, Z.-J. Li, K. Feng, B. Chen, C.-H. Tung and L.-Z. Wu, J. Am. Chem. Soc., 2013, 135, 19052 CrossRef CAS PubMed; (f) C. Huo, C. Wang, M. Wu, X. Jia, X. Wang, Y. Yuan and H. Xie, Org. Biomol. Chem., 2014, 12, 3123 RSC; (g) J.-J. Zhong, Q.-Y. Meng, B. Liu, X.-B. Li, X.-W. Gao, T. Lei, C.-J. Wu, Z.-J. Li, C.-H. Tung and L.-Z. Wu, Org. Lett., 2014, 16, 1988 CrossRef CAS PubMed.
- For selected reviews, see: (a) I. T. Horváth and P. T. Anastas, Chem. Rev., 2007, 107, 2169 CrossRef PubMed; (b) I. T. Horváth6 and P. T. Anastas, Chem. Rev., 2007, 107, 2167 CrossRef; (c) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC; (d) C.-J. Li and P. Anastas, Chem. Soc. Rev., 2012, 41, 1413 RSC; (e) P. J. Dunn, Chem. Soc. Rev., 2012, 41, 1452 RSC.
- For our previous efforts for the synthesis of C1 functionalized tetrahydroisoquinolines, see: (a) G. Zhang, Y. Ma, S. Wang, Y. Zhang and R. Wang, J. Am. Chem. Soc., 2012, 134, 12334 CrossRef CAS PubMed; (b) G. Zhang, Y. Ma, S. Wang, W. Kong and R. Wang, Chem. Sci., 2013, 4, 2645 RSC; (c) G. Zhang, S. Wang, Y. Ma, W. Kong and R. Wang, Adv. Synth. Catal., 2013, 355, 874 CrossRef CAS; (d) G. Zhang, Y. Ma, G. Cheng, D. Liu and R. Wang, Org. Lett., 2014, 16, 656 CrossRef CAS PubMed; (e) X. Liu, J. Zhang, S. Ma, Y. Ma and R. Wang, Chem. Commun., 2014, 50, 15714 RSC; (f) Y. Ma, G. Zhang, J. Zhang, D. Yang and R. Wang, Org. Lett., 2014, 16, 5358 CrossRef CAS PubMed; (g) Q. Chen, J. Zhou, Y. Wang, C. Wang, X. Liu, Z. Xu, L. Lin and R. Wang, Org. Lett., 2015, 17, 4212 CrossRef CAS PubMed.
- For our previous works on the construction of polycyclic indolines, see: (a) H. Zhang, L. Hong, H. Kang and R. Wang, J. Am. Chem. Soc., 2013, 135, 14098 CrossRef CAS PubMed; (b) L. Wang, D. Yang, F. Han, D. Li, D. Zhao and R. Wang, Org. Lett., 2015, 17, 176 CrossRef CAS PubMed; (c) D. Yang, L. Wang, F. Han, D. Li, D. Zhao, Y. Cao, Y. Ma, W. Kong, Q. Sun and R. Wang, Chem. – Eur. J., 2014, 20, 16478 CrossRef CAS PubMed.
- (a) T. Hashimoto, Y. Maeda, M. Omote, H. Nakatsu and K. Maruoka, J. Am. Chem. Soc., 2010, 132, 4076 CrossRef CAS PubMed; (b) T. Hashimoto, M. Omote and K. Maruoka, Angew. Chem., Int. Ed., 2011, 50, 3489 CrossRef CAS PubMed; (c) T. Hashimoto, M. Omote and K. Maruoka, Angew. Chem., Int. Ed., 2011, 50, 8952 CrossRef CAS PubMed; (d) Y.-Y. Zhou, J. Li, L. Ling, S.-H. Liao, X.-L. Sun, Y.-X. Li, L.-J. Wang and Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 1452 CrossRef CAS PubMed; (e) S. Milosevic and A. Togni, J. Org. Chem., 2013, 78, 9638 CrossRef CAS PubMed; (f) D. Wang, Y. Lei, Y. Wei and M. Shi, Chem. – Eur. J., 2014, 20, 15325 CrossRef CAS PubMed; (g) W. Li, Q. Jia, Z. Du, K. Zhang and J. Wang, Chem. – Eur. J., 2014, 20, 4559 CrossRef CAS PubMed; (h) W. Li, J. Wei, Q. Jia, Z. Du, K. Zhang and J. Wang, Chem. – Eur. J., 2014, 20, 6592 CrossRef CAS PubMed; (i) C. Izquierdo, F. Esteban, A. Parra, R. Alfaro, J. Alemán, A. Fraile and J. L. G. Ruano, J. Org. Chem., 2014, 79, 10417 CrossRef CAS PubMed; (j) L. Hesping, A. Biswas, C. G. Daniliuc, C. Mück-Lichtenfeld and A. Studer, Chem. Sci., 2015, 6, 1252 RSC; (k) Z.-H. Gao, X.-Y. Chen, J.-T. Cheng, W.-L. Liao and S. Ye, Chem. Commun., 2015, 51, 9328 RSC; (l) L. Zhang, H. Liu, G. Qiao, Z. Hou, Y. Liu, Y. Xiao and H. Guo, J. Am. Chem. Soc., 2015, 137, 4316 CrossRef CAS PubMed; (m) B.-S. Li, Y. Wang, Z. Jin and Y. R. Chi, Chem. Sci., 2015, 6, 6008 RSC; (n) C. Guo, M. Fleige, D. Janssen-Müller, C. G. Daniliuc and F. Glorius, Nat. Chem., 2015, 7, 842 CrossRef CAS PubMed; (o) Y. Xu, Y. Liao, L. Lin, Y. Zhou, J. Li, X. Liu and X. Feng, ACS Catal., 2016, 6, 589 CrossRef CAS; (p) Y. Wang, Q. Wang and J. Zhu, Chem. – Eur. J., 2016, 22, 8084 CrossRef CAS PubMed; (q) X. Liu, Y. Wang, D. Yang, J. Zhang, D. Liu and W. Su, Angew. Chem., Int. Ed., 2016, 55, 8100 CrossRef CAS PubMed.
- (a) R. Na, H. Liu, Z. Li, B. Wang, J. Liu, M.-A. Wang, M. Wang, J. Zhong and H. Guo, Tetrahedron, 2012, 68, 2349 CrossRef CAS; (b) H. Kawai, Z. Yuan, E. Tokunaga and N. Shibata, Org. Lett., 2012, 14, 5330 CrossRef CAS PubMed; (c) T. Soeta, K. Tamura and Y. Ukaji, Org. Lett., 2012, 14, 1226 CrossRef CAS PubMed; (d) C. Jing, R. Na, B. Wang, H. Liu, L. Zhang, J. Liu, M. Wang, J. Zhong, O. Kwon and H. Guo, Adv. Synth. Catal., 2012, 354, 1023 CrossRef CAS PubMed; (e) T. Soeta, K. Tamura, S. Fujinami and Y. Ukaji, Org. Biomol. Chem., 2013, 11, 2168 RSC; (f) X.-Q. Hu, J.-R. Chen, S. Gao, B. Feng, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2013, 49, 7905 RSC; (g) T. Soeta, T. Ohgai, T. Sakai, S. Fujinami and Y. Ukaji, Org. Lett., 2014, 16, 4854 CrossRef CAS PubMed; (h) L. Chen, G. M. Yang, J. Wang, Q. F. Jia, J. Wei and Z. Y. Du, RSC Adv., 2015, 5, 76696 RSC; (i) Z. Li, H. Yu, Y. Feng, Z. Hou, L. Zhang, W. Yang, Y. Wu, Y. Xiao and H. Guo, RSC Adv., 2015, 5, 34481 RSC; (j) Q. Jia, L. Chen, G. Yang, J. Wang, J. Wei and Z. Du, Tetrahedron Lett., 2015, 56, 7150 CrossRef CAS; (k) L. He, L. Liu, R. Han, W. Zhang, X. Xie and X. She, Org. Biomol. Chem., 2016, 14, 6757 RSC; (l) Z. Li, H. Yu, Y. Liu, L. Zhou, Z. Sun and H. Guo, Adv. Synth. Catal., 2016, 358, 1880 CrossRef CAS; (m) Y. Lei, J.-J. Xing, Q. Xu and M. Shi, Eur. J. Org. Chem., 2016, 3486 CrossRef CAS.
- For selected examples on 1,3-DCs involving N,N′-cyclic azomethine imines, see: (a) R. Shintani and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 10778 CrossRef CAS PubMed; (b) A. Suárez, C. W. Downey and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 11244 CrossRef PubMed; (c) R. Shintani and T. Hayashi, J. Am. Chem. Soc., 2006, 128, 6330 CrossRef CAS PubMed; (d) W. Chen, W. Du, Y.-Z. Duan, Y. Wu, S.-Y. Yang and Y.-C. Chen, Angew. Chem., Int. Ed., 2007, 46, 7667 CrossRef CAS PubMed; (e) A. Chan and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 5334 CrossRef CAS PubMed; (f) N. D. Shapiro, Y. Shi and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 11654 CrossRef CAS PubMed; (g) H. Kawai, A. Kusuda, S. Nakamura, M. Shiro and N. Shibata, Angew. Chem., Int. Ed., 2009, 48, 6324 CrossRef CAS PubMed; (h) G. Zhu, W. Sun, C. Wu, G. Li, L. Hong and R. Wang, Org. Lett., 2013, 15, 4988 CrossRef CAS PubMed; (i) L. Hong, M. Kai, C. Wu, W. Sun, G. Zhu, G. Li, X. Yao and R. Wang, Chem. Commun., 2013, 49, 6713 RSC; (j) M. Hori, A. Sakakura and K. Ishihara, J. Am. Chem. Soc., 2014, 136, 13198 CrossRef CAS PubMed; (k) M. Wang, Z. Huang, J. Xu and Y. R. Chi, J. Am. Chem. Soc., 2014, 136, 1214 CrossRef CAS PubMed; (l) R.-Y. Zhu, C.-S. Wang, J. Zheng, F. Shi and S.-J. Tu, J. Org. Chem., 2014, 79, 9305 CrossRef CAS PubMed.
- (a) B. Biolatto, M. Kneeteman, E. Paredes and P. Mancini, Tetrahedron Lett., 1999, 40, 3343 CrossRef CAS; (b) T. L. S. Kishbaugh and G. W. Gribble, Tetrahedron Lett., 2001, 42, 4783 CrossRef CAS; (c) B. Biolatto, M. Kneeteman, E. Paredes and P. Mancini, J. Org. Chem., 2001, 66, 3906 CrossRef CAS PubMed; (d) I. Chataigner and S. P. Piettre, Org. Lett., 2007, 9, 4159 CrossRef CAS PubMed; (e) H. Gérard and I. Chataigner, J. Org. Chem., 2013, 78, 9233 CrossRef PubMed; (f) A. Awata and T. Arai, Angew. Chem., Int. Ed., 2014, 53, 10462 CrossRef CAS PubMed; (g) M. Andreini, M. De Paolis and I. Chataigner, Catal. Commun., 2015, 63, 15 CrossRef CAS; (h) J.-Q. Zhao, M.-Q. Zhou, Z.-J. Wu, Z.-H. Wang, D.-F. Yue, X.-Y. Xu, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2015, 17, 2238 CrossRef CAS PubMed; (i) J.-Q. Zhao, Z.-J. Wu, M.-Q. Zhou, X.-Y. Xu, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2015, 17, 5020 CrossRef CAS PubMed; (j) M. Andreini, F. Chapellas, S. Diab, K. Pasturaud, S. R. Piettre, J. Legros and I. Chataigner, Org. Biomol. Chem., 2016, 14, 2833 RSC; (k) Y. Li, F. Tur, R. P. Nielsen, H. Jiang, F. Jensen and K. A. Jørgensen, Angew. Chem., Int. Ed., 2016, 55, 1020 CrossRef CAS PubMed.
- For a review, see: (a) C.-X. Zhuo, W. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 12662 CrossRef PubMed; For examples, see: (b) Y. Lian and H. M. L. Davies, J. Am. Chem. Soc., 2010, 132, 440 CrossRef CAS PubMed; (c) Q.-F. Wu, H. He, W.-B. Liu and S.-L. You, J. Am. Chem. Soc., 2010, 132, 11418 CrossRef CAS PubMed; (d) O. Lozano, G. Blessley, T. M. del Campo, A. L. Thompson, G. T. Giuffredi, M. Bettati, M. Walker, R. Borman and V. Gouverneur, Angew. Chem., Int. Ed., 2011, 50, 8105 CrossRef CAS PubMed; (e) S. Zhu and W. C. MacMillan, J. Am. Chem. Soc., 2012, 134, 10815 CrossRef CAS PubMed; (f) H. Xiong, H. Xu, S. Liao, Z. Xie and Y. Tang, J. Am. Chem. Soc., 2013, 135, 7851 CrossRef CAS PubMed; (g) J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6802 CrossRef CAS PubMed; (h) Y.-C. Zhang, J.-J. Zhao, F. Jiang, S.-B. Sun and F. Shi, Angew. Chem., Int. Ed., 2014, 53, 13912 CrossRef CAS PubMed; (i) X. Zhang, L. Han and S.-L. You, Chem. Sci., 2014, 5, 1059 RSC; (j) X. Zhang, W.-B. Liu, H.-F. Tu and S.-L. You, Chem. Sci., 2015, 6, 4525 RSC; (k) L. Han, W. Zhang, X.-X. Shi and S.-L. You, Adv. Synth. Catal., 2015, 357, 3064 CrossRef CAS.
- CCDC 1494106 contains the supplementary crystallographic data for this paper.
- (a) L. M. Salonen, M. Ellermann and F. Diederich, Angew. Chem., Int. Ed., 2011, 50, 4808 CrossRef CAS PubMed; (b) J. Lv, L. Zhang, S. Hu, J.-P. Cheng and S. Luo, Chem. – Eur. J., 2012, 18, 799 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: CCDC 1494106. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6gc02517j |
| This journal is © The Royal Society of Chemistry 2017 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: