An ester-substituted polyfluorene derivative for light-emitting electrochemical cells: bright blue emission and its application in a host–guest system†
Go
Nagatsu
a,
Tomo
Sakanoue
*a,
Shizuka
Tane
b,
Fumihiro
Yonekawa
b and
Taishi
Takenobu
 *a
*a
aDepartment of Applied Physics, Nagoya University, Furo, Chikusa, Nagoya 464-8603, Japan. E-mail: sakanoue@nagoya-u.jp; takenobu@nagoya-u.jp
bNippon Chemical Industrial Co., Ltd, 9-11-1, Kameido, Koto, Tokyo 136-8515, Japan
First published on 8th March 2018
Abstract
The unique interaction of electrons and ions in film blends of light-emitting polymers and electrolytes can realize the operation of light-emitting electrochemical cells (LECs) in which voltage-induced dynamic p–n homojunctions achieve efficient light emission. A crucial issue is the compatibility between the light-emitting polymers and electrolytes; the phase incompatibility of polymers and electrolytes makes it difficult to prepare uniform film blends suitable for the light-emitting layers of LECs. Here, we report that the introduction of ester groups into a light-emitting polymer improves phase compatibility with an ionic liquid-based electrolyte with high electrochemical stability. The resulting uniform film blends of the light-emitting polymer and electrochemically stable ionic liquid contributed significantly to making LECs more efficient and brighter; 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 of luminance with a high efficiency of 9 cd A−1 was demonstrated. Furthermore, we found that the ester-substituted polymer could be applied as a host material such that efficient LECs based on a host–guest system were also demonstrated.
000 cd m−2 of luminance with a high efficiency of 9 cd A−1 was demonstrated. Furthermore, we found that the ester-substituted polymer could be applied as a host material such that efficient LECs based on a host–guest system were also demonstrated.
Introduction
There is growing interest in the fabrication of solution-processed optoelectronic devices, particularly to reduce the manufacturing costs and energy consumption.1,2 Polymeric semiconductors are soluble in common organic solvents and have low temperature processibility; therefore, they are considered promising materials for achieving such green manufacturing of optoelectronic devices in the near future. Tremendous effort has been applied to develop high performance polymeric semiconductors and high consistency roll-to-roll manufacturing techniques; as a result, organic light-emitting diode (OLED)-based displays and lighting produced through a solution-process are now almost ready for the market. To further exploit the advantages associated with the solution-processibility of semiconducting polymers, researchers have furthered their exploration of novel devices which are more suitable for printing techniques, as current printed OLEDs require severe control of the fabrication process: carefully controlled nanometer-scale multi-layered structures are required, not to mention air-unstable metallic electrodes for energy level alignment to achieve efficient charge injection.Light-emitting electrochemical cells (LECs) are a promising alternative to OLEDs with structures consisting of simple, single emitting layers that are composed of blends of light-emitting polymers and electrolytes.3–7 The presence of the electrolyte enables LECs to operate in a unique fashion that is distinct from OLEDs; LECs are less sensitive to the thickness of the emitting layers and energy level alignment at the metal/emitting layer interfaces. Therefore, they possess a strong advantage for printed manufacturing with great flexibility in structural design and a high tolerance in the film thickness.8 These unusual characteristics, distinct from conventional electronic devices, are bestowed by the electrochemical process of ion rearrangement occurring in the emitting layers: the application of a voltage induces electrochemically-doped dynamic p–n homojunctions. The high charge density increases the conductivity of the emitting layers and guarantees the balance of the number of electrons and holes for efficient recombination. Such ion-mediated high conductivity and balanced electron/hole ratio in LECs may be a key for future high-performance light sources, with notable advantages in manufacturing. In fact, such features of LECs make it possible to use unique techniques and materials in their fabrication: a fully solution-based roll process and a lamination process have been applied to demonstrate the feasibility of flexible LECs;8 carbon nanotube-based sheets have been applied for the electrodes to realize wire-based LECs.9 We can go beyond unique lighting devices; for example, we have demonstrated high current injection greater than 2 kA cm−2, which suggests that LECs might be used as a platform device for electrically driven polymer lasers.10 Such demonstrations strongly indicate the potential of LECs for future application to unique, low-cost and high-performance organic light sources.
However, there remains a difficulty associated with LECs; it is difficult to use two different types of charge carriers of electrons and ions. Since materials exhibiting efficient transport for both electronic and ion charges have not yet been developed, materials in LECs are usually based on a blend of conventional light-emitting materials and electrolytes; however, the polarity of these two materials is very different, making it very difficult to make the uniform film blend necessary for efficient electron–ion interaction and uniform light emission. Therefore, a careful selection of the combination of light-emitting materials and electrolytes is paramount in preparing a uniform film blend.
This has resulted in the testing of many electrolyte materials in LECs. The most widely used electrolytes are polyether-based materials, including conventional PEO, oligoethers, and crown ethers.11–13 The other candidates are ionic liquids (ILs); thus, there are several options regarding electrolytes for LECs.14 In contrast, the options for light-emitting polymers are very limited due to generally low electrolyte compatibilities. Most light-emitting polymers are composed of phenyl groups and alkyl chains with a low polarity, resulting in phase separation with polyethers. Although some polyphenylenevinylene-based polymers, “Super Yellow” (SY-PPV, Merck PDY-132) and MEH-PPV, show good compatibility, the number of useful polymers for LECs has been limited by phase incompatibility, placing considerable difficulty in finding a good combination of electrolytes and polymers.15–17
To address this issue, we previously developed an IL-based electrolyte to solve the phase incompatibility issue and realized the use of a wide range of light-emitting polymers developed for OLEDs to LECs.14 We designed an IL of tetrabutylphosphonium-dibutylphosphonate (P4444-DBP) to exhibit a small polarity, which was found to greatly improve the polymer compatibility and enable the fabrication of high performance LECs with higher luminance and efficiency and lower driving voltage than a PLED using the same light-emitting polymer. However, a drawback remained in P4444-DBP: although it showed good compatibility with various light-emitting polymers, it is not the best material from the viewpoint of electrochemical stability as there are several ILs having a much wider electrochemical stability window (ESW) than P4444-DBP.
It has been reported in previous works that ILs of quaternary alkyl ammonium or quaternary alkyl phosphonium cations with fluorinated anions such as trifluoromethanesulfonate (Tf), bis(trifluoromethane)sulfonamide (TFSA), and tris(perfluoroalkyl)trifluorophosphate (FAP) have a wide ESW.18–20 In our previous study, we found that P4444-DBP had an electrochemical window of ∼5.3 V, which was narrower than that of trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)amide (P66614-TFSA) of ∼5.9 V, clearly indicating the advantage of using a TFSA anion for electrochemical stability.12 Therefore, in the present study, we focused on the possibility of applying a light-emitting polymer with high compatibility in fluorinated ILs to LECs. We found that using a polar ester group substituent improved the IL compatibility, such that the ester-substituted polymer dissolved in P66614-TFSA, a fluorinated IL. Such a high compatibility allowed us to prepare high performance LECs exhibiting a small turn-on voltage and a notably high luminance of ∼60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m2 with a high efficiency, 9 cd A−1. Furthermore, we investigated the potential use of the same blue-emitting polymer for fabricating LECs based on host–guest systems and the effect of the emitting dopants on charge transport characteristics.
000 cd m2 with a high efficiency, 9 cd A−1. Furthermore, we investigated the potential use of the same blue-emitting polymer for fabricating LECs based on host–guest systems and the effect of the emitting dopants on charge transport characteristics.
Results and discussion
Effect of ester substituents
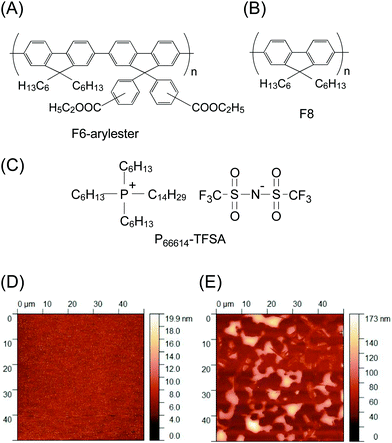
The polymer material tested in this study is poly(fluorene-co-arylfluorene), in which an ethyl ester group on the arylfluorene unit (F6-arylester) (Fig. 1A) is substituted. The π-conjugated backbone of F6-arylester is not very different from the most conventional fluorene polymer, poly (9,9-dioctylfluorene) (F8) (Fig. 1B); the absorption and photoluminescence spectra of F6-arylester are similar in spectral shape to those of F8, showing a deep blue emission (ESI,† Fig. S1A). The idea of attaching an ester group to the fluorene copolymers is derived from work by Susan et al. who reported the fabrication of “ion gels” by using poly(methyl methacrylate) (PMMA) as a network polymer in an IL consisting of 1-ethyl-3-methylimidazolium bis(trifluoromethane sulfonyl)amide (EMIM-TFSA).21 Interestingly, PMMA and EMIM-TFSA IL blended well in any mixing ratio, creating a completely compatible binary system. The mechanism behind this extremely high compatibility has not yet been elucidated, but there was evidence of interaction of the TFSA anion with PMMA: the ester groups on PMMA are thought to be the key for compatibility. We thus introduced ester groups into the polyfluorene derivatives.To check the IL compatibility of F6-arylester, we first carried out a simple dissolution test with P66614-TFSA (Fig. 1C); a small amount of the F6-arylester powder was simply submerged into the IL and heated at 90 °C for 12 hours, and then filtered using a 0.2 μm membrane filter to exclude the residual non-dissolved solid-state polymer powder. This results in IL emission in the deep blue region under ultraviolet irradiation (ESI,† Fig. S1B). Furthermore, the PL in the IL was distinct from that of the powder state. This clearly indicates the dissolution of F6-arylester and high compatibility in ILs based on the TFSA anion, though they did not show the complete compatibility reported in the case of PMMA and EMIM-TFSA.21
Importantly, the high compatibility of the ILs and F6-arylester was also clearly observed in the film blends prepared by a simple spin-coating method. Fig. 1D shows an atomic force microscope (AFM) image of a film blend of F6-arylester and P66614-TFSA blended with a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio by weight. The AFM image in Fig. 1D, the phase image (ESI,† Fig. S1C) and the optical microscopy image (ESI,† Fig. S1E) showed no apparent structures. The root mean square (RMS) roughness was less than 5 nm, indicating little phase separation in the film blend. To clarify the effect of the ester group, we also prepared a film blend of F8 and P66614-TFSA, in which we found a clear difference from the case of F6-arylester. The film blend of F8 and P66614-TFSA showed severe phase separation, as the P66614-TFSA was repelled on the surface of the F8 film (Fig. 1E and ESI,† Fig. S1D, F), clearly indicating the effectiveness of using ester substituents to obtain uniform film blends.
1 ratio by weight. The AFM image in Fig. 1D, the phase image (ESI,† Fig. S1C) and the optical microscopy image (ESI,† Fig. S1E) showed no apparent structures. The root mean square (RMS) roughness was less than 5 nm, indicating little phase separation in the film blend. To clarify the effect of the ester group, we also prepared a film blend of F8 and P66614-TFSA, in which we found a clear difference from the case of F6-arylester. The film blend of F8 and P66614-TFSA showed severe phase separation, as the P66614-TFSA was repelled on the surface of the F8 film (Fig. 1E and ESI,† Fig. S1D, F), clearly indicating the effectiveness of using ester substituents to obtain uniform film blends.
Device characteristics of F6-arylester-based LECs
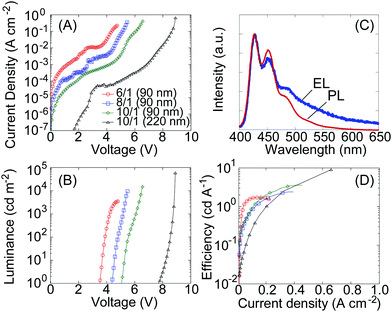
The uniform film blend of F6-arylester and P66614-TFSA enabled the construction of high performance blue-emitting LECs with high luminance and efficiency. 90 nm film blends were prepared on transparent indium–tin–oxide (ITO)-coated glass substrates by spin-coating, with silver contacts added as counter electrodes. The blend ratio of F6-arylester to P66614-TFSA was varied; polymer/IL ratios of 6/1, 8/1 and 10/1 were investigated to understand the effect of IL concentration on LEC performance. Fig. 2A and B show the current density–voltage (J–V) and luminance–voltage (L–V) characteristics of F6-arylester-based LECs with various polymer/IL ratios. Note that the electrical characteristics of LECs are often measured with a fixed voltage or current as the ion rearrangement takes some time to achieve full doping of the polymers; however, our IL-based LECs showed a rather fast response (ESI,† Fig. S2), which allowed us to measure the characteristics in voltage scan mode at a slow scan rate of about 50 mV s−1. The LEC based on the 6/1 ratio showed the lowest turn-on voltage, around 3.6 V, almost the same as the energy gap between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of F6-arylester as determined from the ultraviolet-visible (UV-Vis) absorption edge of the film. This matching of the HOMO–LUMO energy gap EHOMO–LUMO (3.5 eV) to the turn-on voltage Von (3.6 V) indicates that efficient charge injection and transport were achieved in the LEC. The electroluminescent (EL) spectrum showed pure blue emission with a peak wavelength of 426 nm, which is similar to the PL spectrum of F6-arylester (Fig. 2C). The EL spectrum showed a longer spectral tale than in the PL spectrum. Such a difference in EL and PL is widely observed in polyfluorene derivatives and has been studied for a long time. It is considered to be due to keto-defects, excimer emission and/or aggregation of molecules.22An increase in polymer concentration, i.e., polymer/IL ratios of 8/1 and 10/1, resulted in a higher Von, 4.3 V and 5 V, respectively. This indicates that there must be unfavorable voltage drops for charge injection and/or transport. Although this resulted in an increase in driving voltage, the maximum luminance achieved was increased with less IL. The maximum luminance was about 3600 cd m−2 with a polymer/IL ratio of 6/1, but this was increased to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cd m−2 and 19
500 cd m−2 and 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 with ratios of 8/1 and 10/1, respectively. The achieved current density is improved slightly with decreasing IL concentration; this results in an improvement in efficiency from 1.8 cd A−1 to 3.6 cd A−1 when reducing the IL concentration from 6/1 to 10/1 (Fig. 2D).
000 cd m−2 with ratios of 8/1 and 10/1, respectively. The achieved current density is improved slightly with decreasing IL concentration; this results in an improvement in efficiency from 1.8 cd A−1 to 3.6 cd A−1 when reducing the IL concentration from 6/1 to 10/1 (Fig. 2D).
We ascribed this increase in the driving voltage and the improvement in efficiency on lowering the IL concentration to an increase in the thickness of the non-doped depletion region at the p–n homojunction. Since the depleted emission zone has the highest resistivity in the LECs, the increase in driving voltage suggests a thicker depletion region, resulting in a higher resistance. The increase of efficiency with decreasing IL concentration also suggests a thicker emission zone: when the depleted junction region is thin, excitons generated in the junction can migrate to the doped regions where they are quenched non-radiatively. In contrast, when the thickness of the depletion region is thicker than the exciton migration length, the quenching can be suppressed and the efficiency improves. In fact, increase of the efficiency of LECs was reported previously on widening the emission zone of LECs by decreasing ion concentrations.23–25 To confirm that the increase in the driving voltage and efficiency is due to the widening of the emission zone, we prepared an LEC with an emitting layer thickness of 220 nm. The device resulted in a further increase in the driving voltage and efficiency and gave a higher luminance; the turn on voltage becomes high, at around 7 V, while a notably high luminance of around 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 was obtained at 8.8 V with a high efficiency of 9 cd A−1 (Fig. 2A, B and D). The results suggest that the thickness of the depletion region in LECs depends on the concentration of IL and the thickness of the emitting layers. This is somewhat different from our previous blue-emitting LECs based on a polyfluorene-spiro copolymer that showed little dependence on IL concentration. We assume that the IL dependent characteristics of the F6-arylether/P66614-TFSA blend are due to the strong interaction between the TFSA anion and ester groups. This traps the TFSA anions and suppresses the growth of doping layers in F6-arylether/P66614-TFSA, resulting in thickness and IL concentration-dependent characteristics.
000 cd m−2 was obtained at 8.8 V with a high efficiency of 9 cd A−1 (Fig. 2A, B and D). The results suggest that the thickness of the depletion region in LECs depends on the concentration of IL and the thickness of the emitting layers. This is somewhat different from our previous blue-emitting LECs based on a polyfluorene-spiro copolymer that showed little dependence on IL concentration. We assume that the IL dependent characteristics of the F6-arylether/P66614-TFSA blend are due to the strong interaction between the TFSA anion and ester groups. This traps the TFSA anions and suppresses the growth of doping layers in F6-arylether/P66614-TFSA, resulting in thickness and IL concentration-dependent characteristics.
Application in host–guest based LECs
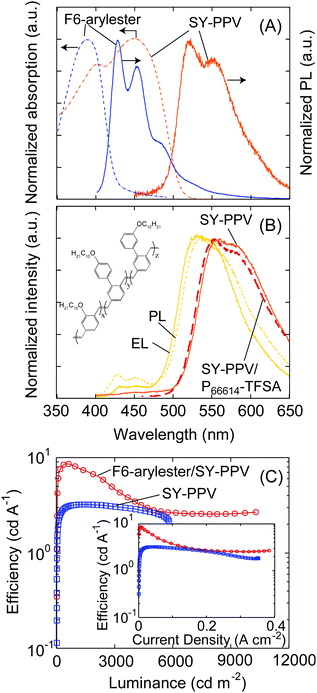
One of the important aspects of the development of blue-emitting devices is their use as a host material due to the wide HOMO–LUMO gap. As widely studied in OLED research, the host–guest system is a key technique that is important for using efficient emitters of phosphorescent or thermally activated delayed fluorescent (TADF) materials and for tuning the emission colors.26–28 The use of host–guest systems is thus also widely investigated in LECs; however, the effect of adding emitting dopants on the efficiency and transport characteristics is not thoroughly discussed.29–33 We therefore prepared LECs with F6-arylester as a host material and SY-PPV as a guest emitter for the model system given the excellent overlap of the emission spectra of F6-arylester and absorption of SY-PPV (Fig. 3A), so that efficient Förster resonance energy transfer can be expected. The film blend of 5 wt% SY-PPV doped F6-arylester and P66614-TFSA (mass ratio of F6- arylester/SY-PPV/P66614-TFSA = 1/0.05/0.17) showed a yellow emission mostly derived from the PL spectra of SY-PPV in solution (Fig. 3A and B), indicating efficient energy transfer from F6-arylester to SY-PPV and well-isolated, uniform dispersion of the SY-PPV molecules into the F6-arylester. We note that the P66614-TFSA did not influence the optical properties of SY-PPV as the PL of the SY-PPV/P66614-TFSA blend showed the same spectrum as that of neat SY-PPV (Fig. 3B). Furthermore, the PLQY of SY-PPV was improved in the F6-arylester film compared to the film blend of SY-PPV and P66614-TFSA, i.e., the PLQY of a SY-PPV/P66614-TFSA film blend was 0.34, while that of F6-arylester:SY-PPV/P66614-TFSA was improved to 0.75 due to the suppression of concentration quenching. These results suggest that higher efficiency can be expected with electrical excitation.In fact, the F6-arylester/SY-PPV-based LECs showed a yellow EL emission whose spectrum was approximately 92% dominated by the SY-PPV, indicating efficient energy transfer from F6-arylester to SY-PPV. Importantly, the EL efficiency was improved compared to a SY-PPV/P66614-TFSA-based LEC, whose J–V and L–V characteristics are shown in the ESI,† Figs. S2A and B: the standard SY-PPV/P66614-TFSA LEC showed a maximum efficiency of 3.2 cd A−1, while the LEC based on F6-arylester/SY-PPV/P66614-TFSA showed 8.2 cd A−1 (Fig. 3C), resulting in a higher efficiency using a host–guest system. We note that the efficiency of the host–guest system was decreased with increasing current density and became almost constant over 5000 cd m−2. Such a behavior was not observed in the standard SY-PPV-based or F6-arylester-based LECs. The origin of this is not clearly understood. A recent study on host–guest LECs by Tang et al. suggests that emitter doping creates traps that may lead to imbalanced electron/hole mobilities.33 This could change the recombination zone close to the p/n-doped regions where the excitons are severely quenched, and thus can be a possible origin of the lowering of emission efficiency under high luminance of our host–guest LECs.
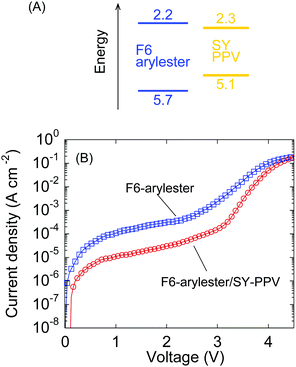
To understand the effect of the SY-PPV dopant on the charge transport characteristics, we compared the J–V characteristics to the LEC without SY-PPV, i.e., the standard F6-arylester-based LEC with the same polymer/IL ratio. The cyclic voltammetry measurements revealed that the HOMO of SY-PPV is located at a 0.6 eV shallower energy level than that of F6-arylester, while the LUMO of SY-PPV is not very different (Fig. 4A). This suggested that the guest molecule, SY-PPV, can be considered to be a deep trap for hole transport. In fact, similar host–guest OLEDs based on the system of SY-PPV doped F8, which have almost the same HOMO–LUMO levels as F6-arylester, showed a significant decrease in conductivity with SY-PPV doping: the current density of the F8-based OLED was decreased by nearly three orders of magnitude by the traps created by SY-PPV.27 In contrast, in our LECs, the effect of the hole traps of SY-PPV was surprisingly small. The current density was smaller using the SY-PPV dopant in low voltage regions; however, the difference in current density was reduced with increasing voltage, becoming almost zero at around 4.5 V (Fig. 4B). This suggests that the effect of the deep traps of SY-PPV is negligible in LECs with high enough voltage.
We believe that the smallness of the trapping effect of SY-PPV is due to the characteristics of electrochemical doping in LECs. The electrochemically doped charge density in LECs is on the order of 1019–1020 cm−3 while the charge density in OLEDs is 1015–1016 cm−3, such that the doping density in LECs is thought to be high enough to fill the traps made by SY-PPV. Therefore, the p-doped region still has a high conductivity compared to the depletion region of the p–n homojunction, where the resistance of the LECs is determined. Furthermore, the voltage is concentrated in the thin depletion region, such that the high electric field can assist efficient charge transport by the Poole–Frenkel effect.34,35 Although further study of charge transport in the host–guest systems is required, we stress that the introduction of a host–guest system into LECs can increase emission efficiency with little drawbacks for electronic charge transport due to the dynamic electrochemical doping and electric field concentration at the p–n homojunction. This indicates a high degree of utility for host–guest systems in LECs such that we anticipate LECs with phosphorescent or TADF dopants in the future.
Conclusions
We found that an ester-substituted blue-emitting polyfluorene derivative of F6-arylester achieved high compatibility with P66614-TFSA, an IL with high stability. This enabled us to obtain uniform film blends of these two materials in which an electrochemically induced p–n junction was formed for efficient light emission, achieving a high performance with a maximum luminance efficiency of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 and 9 cd A−1, respectively. The wide HOMO–LUMO property of F6-arylester was further applied by using it as a host material for SY-PPV. The PLQY as well as the EL efficiency was improved as the host–guest system avoided concentration quenching. Furthermore, we found that the emitter guest molecules did not act as traps. This is distinct from conventional OLEDs and would be a unique intrinsic characteristic of LECs due to high charge density, which is high enough to fill the trap states of the guest molecules. We believe that such wide HOMO–LUMO polymers compatible with electrochemically stable ILs can accelerate LEC development, by enabling the application of a wide range of efficient emitting guest molecules such as phosphorescent materials and thermally activated delayed fluorescent (TADF) materials, both promising materials as part of an emerging class of efficient emitters.
000 cd m−2 and 9 cd A−1, respectively. The wide HOMO–LUMO property of F6-arylester was further applied by using it as a host material for SY-PPV. The PLQY as well as the EL efficiency was improved as the host–guest system avoided concentration quenching. Furthermore, we found that the emitter guest molecules did not act as traps. This is distinct from conventional OLEDs and would be a unique intrinsic characteristic of LECs due to high charge density, which is high enough to fill the trap states of the guest molecules. We believe that such wide HOMO–LUMO polymers compatible with electrochemically stable ILs can accelerate LEC development, by enabling the application of a wide range of efficient emitting guest molecules such as phosphorescent materials and thermally activated delayed fluorescent (TADF) materials, both promising materials as part of an emerging class of efficient emitters.
Experimental section
Materials
Light-emitting polymers, F6-arylester (Mw = 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), F8 (Mw = 150
000), F8 (Mw = 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and SY-PPV (Mw > 1
000) and SY-PPV (Mw > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were supplied by Sumitomo Chemical, Lumtec and Merck, respectively, and were used as received. The IL of P66614-TFSA was supplied by Sigma-Aldrich.
000) were supplied by Sumitomo Chemical, Lumtec and Merck, respectively, and were used as received. The IL of P66614-TFSA was supplied by Sigma-Aldrich.
Characterization of materials and film blends
UV-Vis absorption spectra were recorded on a VARIAN Carry 5000 spectrophotometer. PL and EL spectra were recorded using a multichannel spectrograph (Lambda Vision SA-100). An ultraviolet-emitting LED with an emission spectrum centred at 365 nm was used as an excitation light source for PL measurements. The PLQY of the polymer films was measured using a calibrated integrating sphere with a multichannel spectrograph (Hamamatsu PMA-11). The excitation wavelengths for PLQY measurements on SY-PPV/P66614-TFSA and F6-arylester:SY-PPV/P66614-TFSA films were 444 nm and 388 nm, respectively. AFM measurements of the film blends were carried out using a Nanosurf FlexAFM. The dissolution tests of polymers in P66614-TFSA were carried out by placing the polymer powders directly into P66614-TFSA in vials. The concentration of the polymers was set to 0.5 mg ml−1. The vials were then heated to 90 °C for 12 hours under a nitrogen atmosphere. It should be noted that some undissolved powder remained at the bottom of the vials after the heating process. The remaining powders were filtered using a 0.2 μm membrane filter.Fabrication and characterization of LECs
The devices were fabricated on ITO-coated glass substrates. The substrates were sonicated in acetone and isopropanol, followed by ultraviolet-ozone cleaning. The active layer blends of polymers and ILs were prepared by spin-coating at 1000 rpm for 60 s in a glovebox. Chlorobenzene solutions of each polymer and IL were prepared separately and mixed together in the required ratios just before spin-coating. The solution concentrations of F6-arylester-LECs and F6-arylester/SY-PPV were 6 mg ml−1, except for the 220 nm-thick F6-arylester LEC that was prepared from a high-concentration solution of 15 mg ml−1. SY-PPV-based LECs were prepared from a solution of 6.5 mg ml−1 concentration. The samples were subsequently annealed at 90 °C for 30 minutes, followed by the preparation of silver contacts by thermal evaporation. The completed device was measured in air with a source measure unit (Agilent B2912A). Luminance was monitored using a calibrated Si photodiode equipped with an eye response filter (Hamamatsu S9219-01).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Sumitomo Chemical Co., Ltd for the supply of F6-arylester. The authors also thank Hiroshi Ito and Hisaaki Tanaka for the fruitful discussions. This work was supported by JSPS KAKENHI Grant Numbers JP17H02765, JP26102012, and JP25000003.References
- R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. D. Santos, J. L. Bredas, M. Logdlund and W. R. Salaneck, Nature, 1999, 397, 121 CrossRef CAS.
- H. Zheng, Y. Zheng, N. Liu, N. Ai, Q. Wang, S. Wu, J. Zhou, D. Hu, S. Yu, S. Han, W. Xu, C. Luo, Y. Meng, Z. Jiang, Y. Chen, D. Li, F. Huang, J. Wang, J. Peng and Y. Cao, Nat. Commun., 2013, 4, 1971 Search PubMed.
- Q. Pei, G. Yu, C. Zhang, Y. Yang and A. J. Heeger, Science, 1995, 269, 1086 CrossRef CAS PubMed.
- S. Meier, D. Tordera, A. Pertegas, C. Roldan-Carmona, E. Orti and H. Bolink, Mater. Today, 2014, 17, 217 CrossRef CAS.
- S. Chen, G. Wantz, L. Bouffier and J. Gao, ChemElectroChem, 2016, 3, 392 CrossRef CAS.
- J. Liang, L. Li, X. Niu, Z. Yu and Q. Pei, Nat. Photonics, 2013, 7, 817 CrossRef CAS.
- A. Sandstrom, H. F. Dam, F. C. Krebs and L. Edman, Nat. Commun., 2012, 3, 1002 CrossRef PubMed.
- A. Sandstrom, A. Asadpoordarvish, J. Enevold and L. Edman, Adv. Mater., 2014, 26, 4975 CrossRef PubMed.
- Z. Zhang, K. Guo, Y. Li, X. Li, G. Guan, H. Li, Y. Luo, F. Zhao, Q. Zhang, B. Wei, Q. Pei and H. Peng, Nat. Photonics, 2015, 9, 233 CrossRef CAS.
- T. Sakanoue, J. Li, H. Tanaka, R. Ito, S. Ono, S. Kuroda and T. Takenobu, Adv. Mater., 2017, 29, 1606392 CrossRef PubMed.
- M. J. Jafari, J. Liu, I. Engquist and T. Ederth, ACS Appl. Mater. Interfaces, 2017, 9, 2747 CAS.
- F. P. Wenzl, C. Suess, P. Pachler, A. Hasse, E. J. W. List, P. Poelt, D. Somitsch, P. Knoll, U. Scherf and G. Leising, Solid State Ionics, 2004, 169, 161 CrossRef CAS.
- Y. Cao, Q. Pei, M. R. Andersson, G. Yu and A. Heeger, J. Electrochem. Soc., 1997, 12, 317 CrossRef.
- T. Sakanoue, F. Yonekawa, K. Albrecht, K. Yamamoto and T. Takenobu, Chem. Mater., 2017, 29, 6122 CrossRef CAS.
- S. Tang, J. Mindemark, C. M. G. Araujo, D. Brandell and L. Edman, Chem. Mater., 2014, 26, 5083 CrossRef CAS.
- L. Edman, Electrochim. Acta, 2005, 50, 3878 CrossRef CAS.
- F. P. Wenzl, P. Pachler, C. Suess, A. Haase, E. J. W. List, P. Poelt, D. Somitsch, P. Knoll, U. Scherf and G. Leising, Adv. Funct. Mater., 2004, 14, 441 CrossRef CAS.
- M. Shamsipur, A. A. M. Beigi, M. Teymouri, S. M. Pourmortazavi and M. Irandoust, J. Mol. Liq., 2010, 157, 43 CrossRef CAS.
- K. Tsunashima and M. Sugiya, Electrochemistry, 2007, 75, 734 CrossRef CAS.
- N. V. Ignat’ev, U. W. Biermann, A. Kucheryna, G. Bissky and H. Willner, J. Fluorine Chem., 2005, 126, 1150 CrossRef.
- M. A. B. H. Susan, T. Kaneko, A. Noda and M. Watanabe, J. Am. Chem. Soc., 2005, 127, 4976 CrossRef CAS PubMed.
- Y. Honmou, S. Hirata, H. Komiyama, J. Hiyoshi, S. Kawauchi, T. Iyoda and M. Vacha, Nat. Commun., 2014, 5, 4666 CAS.
- J. Fang, P. Matyba and L. Edman, Adv. Funct. Mater., 2009, 19, 2671 CrossRef CAS.
- S. van Reenen, P. Matyba, A. Dzwilewski, R. A. J. Janssen, L. Edman and M. Kemerink, Adv. Funct. Mater., 2011, 21, 1795 CrossRef CAS.
- F. AlTal and J. Gao, Org. Electron., 2015, 18, 1 CrossRef CAS.
- F. J. Quites, G. C. Faria, J. C. Germino and T. D. Z. Atvars, J. Phys. Chem. A, 2014, 118, 10380 CrossRef CAS PubMed.
- M. U. Hassan, Y. C. Liu, K. U. Hasan, H. Butt, J. F. Chang and R. H. Friend, Appl. Mater. Today, 2015, 1, 45 CrossRef.
- M. U. Hassan, Y. C. Liu, K. U. Hasan, H. Butt, J. F. Chang and R. H. Friend, Nano Energy, 2016, 21, 62 CrossRef CAS.
- S. Tang, J. Pan, H. Buchholz and L. Edman, ACS Appl. Mater. Interfaces, 2011, 3, 3384 CAS.
- Y. Xiong, L. Li, J. Liang, H. Gao, S. Chou and Q. Pei, Mater. Horiz., 2015, 2, 338 RSC.
- H. C. Su, C. C. Wu, F. C. Fang and K. T. Wong, Appl. Phys. Lett., 2006, 89, 261118 CrossRef.
- Y. Nishikitani, D. Takizawa, H. Nishide, S. Uchida and S. Nishimura, J. Phys. Chem. C, 2015, 119, 28701 CAS.
- S. Tang, A. Sandström, P. Lundberg, T. Lanz, C. Larsen, S. van Reenen, M. Kemerink and L. Edman, Nat. Commun., 2017, 8, 1190 CrossRef PubMed.
- L. Pautmeier, R. Richert and H. Bassler, Synth. Met., 1990, 37, 271 CrossRef CAS.
- B. R. Huang, C. C. Liao, W. C. Ke, Y. C. Chang, H. P. Huang and N. C. Chen, J. Appl. Phys., 2014, 115, 113705 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: UV-Vis and PL spectra, photograph of the ionic liquid, optical micrographs and phase images of the polymer/ionic liquid blends, and J–V–L characteristics of the LECs. See DOI: 10.1039/c7qm00623c |
| This journal is © the Partner Organisations 2018 |