Stretchable/flexible silver nanowire electrodes for energy device applications
Jinwook
Jung†
a,
Hyunmin
Cho†
ab,
Recep
Yuksel
c,
Dongkwan
Kim
a,
Habeom
Lee
d,
Jinhyeong
Kwon
e,
Phillip
Lee
f,
Junyeob
Yeo
g,
Sukjoon
Hong
h,
Husnu Emrah
Unalan
 i,
Seungyong
Han
*j and
Seung Hwan
Ko
i,
Seungyong
Han
*j and
Seung Hwan
Ko
 *abk
*abk
aApplied Nano and Thermal Science Lab, Department of Mechanical Engineering, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul, 08826, Republic of Korea
bInstitute of Advanced Machinery and Design (SNU-IAMD), Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea
cCenter for Multidimensional Carbon Materials (CMCM), Institute for Basic Science (IBS) Ulsan, 44919, Republic of Korea
dSchool of Mechanical Engineering, Pusan National University, 2 Busandaehag-ro, 63Beon-gil, Geumjeong-gu, Busan, 46241, Republic of Korea
eManufacturing System R&BD Group, Korea Institute of Industrial Technology (KITECH), 89 Yangdaegiro-gil, Ipjang-myon, Seobuk-gu, Cheonan, Chungcheongnam-do 31056, Republic of Korea
fPhotoelectronic Hybrid Research Center, Korea Institute of Science and Technology, Seoul, 02792, Republic of Korea
gNovel Applied Nano Optics Lab, Department of Physics, Kyungpook National University, 80 Daehak-ro, Pookgu, Daegu 41566, Republic of Korea
hDepartment of Mechanical Engineering, Hanyang University, 55 Hanyangdaehak-ro, Sangnok-gu, Ansan, Gyeonggi-do 15588, Republic of Korea
iDepartment of Metallurgical and Materials Engineering, Middle East Technical University, Ankara 06800, Turkey
jMultiscale Bio-inspired Technology Lab, Department of Mechanical Engineering, Ajou University, 206 World cup-ro, Yeongtong-gu, Suwon, Gyeonggi-do 16499, Republic of Korea. E-mail: sy84han@ajou.ac.kr; maxko@snu.ac.kr
kInstitute of Engineering Research, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea
First published on 12th August 2019
Abstract
Research on sustainable and high-efficiency energy devices has recently emerged as an important global issue. These devices are now moving beyond the form of a bulk, rigid platform to a portable, flexible/stretchable format that is easily available in our daily lives. Similar to the development of an active layer for the production of next-generation energy devices, the fabrication of flexible/stretchable electrodes for the easy flow of electrons is also very important. Silver nanowire electrodes have high electronic conductivity even in a flexible/stretchable state due to their high aspect ratio and percolation network structures compared to conventional electrodes. Herein, we summarize the research in the field of flexible/stretchable electronics on energy devices fabricated using silver nanowires as the electrodes. Additionally, for a systematic presentation of the current research trends, this review classifies the surveyed research efforts into the categories of energy production, storage, and consumption.
1. Introduction
Going beyond the rigid substrates represented by the silicon wafer has long been an important goal in the research and development of electronic devices. Albeit in primitive forms, new types of electronic devices on non-rigid substrates are now not only being studied in research labs but also being introduced to the market (e.g., curved displays, recent foldable phones from CES 2019). While these flexible devices are attracting intense public attention, the benefits provided by the use of a non-rigid substrate are currently limited because these devices are still built based on rigid platforms with only a tiny fraction of non-rigid components. Meanwhile, the development of stretchable electronics has become one of the most recent research trends in the field of non-rigid electronics, and it is expected to broaden the application of electronic devices even further to human-attachable patches and soft robotics; nevertheless, these applications are even more technically challenging than the usual flexible electronics.1–4While there is still much room for improvement, as mentioned above, we believe that the successful development of electronic devices on non-rigid substrates (i.e., flexible/stretchable devices) will indeed give rise to innovative changes in human–electronic device interactions. Since they are fabricated on lightweight substrates,5–7 such electronic devices will be much more portable, and the high degree of freedom with regard to the device shape and structure will enable the easy integration of different electronic devices with each other or with other systems, including living organisms. Although they are not yet available, the substrates, constituent electronic materials, and relevant processing/fabrication technologies are expected to be inexpensive compared to their conventional counterparts, leading to the reduction of the overall production cost of the electronic devices through the economies of scale arising from their huge production volume.8,9 In this regard, advances in the development of flexible/stretchable devices will allow the ubiquitous use of electronics for a wide variety of purposes, and thus, the prospects for the future development of flexible/stretchable devices are very much in line with such well-known concepts as ubiquitous networking,10 the Internet of Things (IoT), and wearable devices.11
We note that these well-known ideas share a common requirement of self-sustainability that becomes more critical as the application scope of electronic devices expands. The energy-related components of a self-sustainable device can be divided into three subcategories of harvesting, consumption, and storage systems. To stand alone without a connection to an external power supply, each device should first be able to harvest electrical energy from its surroundings by utilizing, for example, light, wind, water, heat, mechanical movement, or static electricity. Then, the device consumes energy to perform the desired task, while the remaining energy must be stored electrochemically or electrophysically for future use to complete the energy cycle. While a huge number of individual flexible/stretchable energy devices have been reported for harvesting, consumption, and storage, integrated frameworks, such as stretchable electrodes, must be developed to efficiently incorporate these individual components in a single flexible/stretchable electronic device. Two kinds of strategies are used to fabricate stretchable electrodes, and combining the two dramatically increases the stretchability.12 One is forming stretchable structures, such as serpentine or pre-strain structures, which minimizes stress and suppresses crack propagation.13 The other strategy is replacing conventional inorganic materials with alternative materials, such as graphene sheets,14–17 carbon nanotubes (CNTs),14,18–20 conductive polymers,21–24 and metal nanostructures.25–29 These materials are stretchable due to their nano/molecular structures and stress delocalization effect. Although each material has advantages, such as chemical stability, adhesion properties, and electrical conductivity, good optoelectrical properties are essential for optoelectronic applications. In the unstrained condition, optoelectrical properties such as transparency and related sheet resistance are dependent on the amount of materials and affect the stretchability of electrodes. Thus, for applications that need extremely high stretchability, large amounts of electrode materials are deposited onto substrates to maintain many electrical conduction paths in the strained condition. Therefore, to compare the properties of the various stretchable electrodes, a comparison of conductivity, transparency, sheet resistance, and stretchability is summarized in Table 1.
| Energy generation | Energy storage | Energy consumption | ||||||
|---|---|---|---|---|---|---|---|---|
| Materials | Solar cell (OPV) | Nanogenerator (TENG) | Supercapacitor | Battery (Li ion) | Fuel cell | OLED | Sensor (mechanical) | Joule heater (transparent) |
| Power conversion efficiency | Power density | Areal capacitance | Areal capacity | Power density | Current efficiency | Maximum gauge factor (GF) | Figure of merit | |
| Mechanical strain (bend radius) | Mechanical strain (stretch) | Mechanical strain (stretch or bend radius) | Mechanical strain (stretch or bend radius) | Mechanical strain (bend radius or bend degree) | Mechanical strain (bend radius) | Mechanical strain (stretch or compression) | Mechanical strain (stretch or bend radius) | |
| a Calculated from graph. | ||||||||
| Ag NWs | 7.21%139 | 500 mW m−2 (ref. 69) | ∼0.21 mF cm−2 (with Au)86 | 3.2 mA h cm−2 (ref. 97) | 117 mW cm−2 (ref. 111) | 4.91 cd A−1 (ref. 119) | 14 (ref. 140) | 74.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a136 a136 |
| Bend 0.48 mm (ref. 139) | Stretch 300%69 | Stretch 60%86 | 1 mm (ref. 97) | 150 mm (ref. 111) | Bend 1 mm (ref. 119) | Stretch 70%140 | Stretch 60%136 | |
| Cu NWs | 3.11%141 | N/A | N/A | 6.6 mA h cm−2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 142 142 |
N/A | N/A | ∼0.7 kPa−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143 143 |
40.67 (ref. 144) |
| Bend 3 mm (ref. 137) | N/A | N/A | Bend 10 mm (ref. 142) | N/A | N/A | Compression 60%143 | Bend 3 mma![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144 144 |
|
| Graphene | 2.91%145 | 2500 mW m−2 (ref. 146) | 3.67 mF cm−2 (ref. 147) | 0.96 mA h cm−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 148 148 |
0.004 mW cm−2 (ref. 149) | 30.2 cd A−1 (ref. 150) | 14 (ref. 151) | 73.06 (ref. 152) |
| Bend 3 mm (ref. 145) | Stretch 150%146 | Bend 2.5 mm (ref. 147) | 2 mm (ref. 148) | 180° (ref. 149)149 | Bend 7.5 mm (ref. 150) | Stretch 7.1%151 | Stretch 4%a152 | |
| CNTs | 3.91%153 | <1000 mW m−2 (ref. 154) | 45 mF cm−2 (ref. 155) | 2.2 mA h cm−2 (ref. 156) | 145.2 mW cm−2 (ref. 157) | 0.85 cd A−1 (ref. 158) | 0.82 (ref. 159) | 36.68 (ref. 160) |
| Bend 5 mm (ref. 153) | Stretch 30%154 | Bend 0.8 mm (ref. 155) | 2 mm (ref. 156) | 50° (ref. 157) | Bend 2 mm (ref. 158) | Stretch 200%159 | N/A | |
| PEDOT:PSS | 2%161 | 4.06 mW m−2 (ref. 162) | 667.5 mF cm−2 (ref. 163) | N/A | N/A | N/A | 396 (ref. 164) | N/A |
| Bend 10 mm (ref. 161) | Stretch 100%162 | Bend 3 mm (ref. 163) | N/A | N/A | N/A | Stretch 1.2%164 | N/A | |
Among the many schemes explored for obtaining flexible/stretchable platforms toward self-sustainable devices, we suggest that silver nanowires (Ag NWs), especially in their percolative network form, are a promising candidate to achieve this objective. In earlier research on Ag NW-based electronics, Ag NWs have attracted much attention as a transparent conducting material because of their high conductivity combined with high transparency, as described in previous sections. In addition to their superior optoelectrical properties, the percolating structure of Ag NWs has high mechanical stability and thus can maintain a stable conducting path during bending30 and stretching31 under high mechanical stress. It was also found that the percolation structure of very long silver nanowires (v-Ag NWs) can endure larger stress than the structure formed by short Ag NWs.29 Lee et al. reported that by transferring v-Ag NWs onto a pre-strained Ecoflex substrate, the electrode can accommodate a strain of more than 460% without a notable change in the resistance.29 Because of these advantages, Ag NWs have been extensively studied as a key material in flexible/stretchable applications, and in this review, we will focus on three of their applications for self-sustainable devices: energy generation, energy storage, and energy consumption devices.
2. Fabrication processes and properties of Ag NW electrodes
Before we discuss the Ag NW electrode-based energy generation devices, we briefly introduce the fabrication and deposition process of Ag NWs. Ag NWs are usually synthesized by hydrothermal growth and a polyol process. The polyol process, which uses ethylene glycol (EG) as a solvent and reducing agent, is most extensively used. In the synthesis process, polyvinylpyrrolidone (PVP) is one of the most important chemicals, and it behaves as a surfactant of nanowires. Thus, silver ions are selectively reduced in length, and a high aspect ratio can be obtained. The high aspect ratio of Ag NWs is an important parameter for obtaining a high transparent conductor electrode (TCE) figure-of-merit (FoM). Furthermore, the great length of NWs allows them to withstand more stress, and the small diameter induces low haze.32 To achieve these properties, the precursors, surfactant, and growth temperature must be appropriately selected, and the growth time needs to be controlled.32–34 Additionally, strategies such as successive multistep growth (SMG) can be used to synthesize v-Ag NWs.35,36 In addition to the Ag NW synthesis methods, the uniform formation of Ag NWs on target substrates is also an important process. Since Ag NWs are stored in solutions, such as ethanol, isopropyl alcohol, or deionized water (DI water), they can be directly deposited onto stretchable/flexible substrates by drop casting,37 spin coating,38 dip coating,39 or brush painting40 (Fig. 2(a)). For uniform coating, the vacuum filtration method29 can also be used for Ag NWs if the electrode is not large. For scalable coating, doctor blading,41 Meyer rod coating,26 and spray coating42 technologies have also been introduced by many researchers. Each technology requires different optimization processes, but usually, the hydrophilic properties of substrates are important to ensure the good adhesion of Ag NWs onto substrates; thus, plasma treatment is usually applied to substrates. During these processes, uniform heating is also important to avoid agglomeration or the coffee ring effect, which decreases the uniformity of electrodes.26 Moreover, by introducing a lamination or embedding process, the electrodes can also be deposited at the target device in the dry condition, which minimizes potential damage from the solvent of the Ag NW solution. Besides synthesis and deposition processes, the optoelectrical properties of electrodes can be further enhanced by various junction enhancement methods, such as thermal annealing,43 plasmonic welding,44 pressure-induced welding,45 chemical soldering,46 and capillary force-assisted soldering.47 In addition to optoelectrical applications, which just need adequate flexibility with good transparency, some devices require highly stretchable electrodes regardless of their transparency. Usually, a large amount of Ag NWs makes more percolation paths, which maintains good electrical conductivity in a highly stretchable state. Besides using a large amount of Ag NWs, some researchers have used pre-strained elastomers as substrates to fabricate electrodes that maintain their high conductivity in the strained state. In addition to the factors mentioned earlier, others such as reduced surface roughness, bandgap engineering, current spreading, and enhanced mechanical/chemical stability are further discussed in the following sections.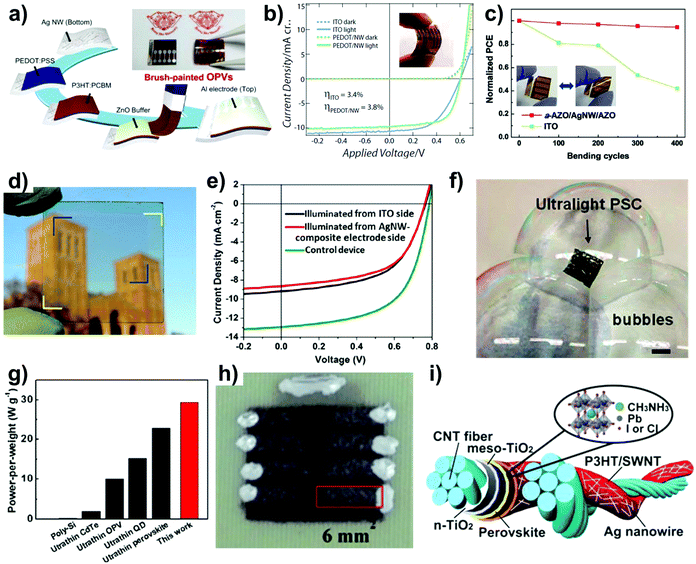 | ||
| Fig. 2 Solar cells using Ag NWs as transparent electrodes. (a) Schematic fabrication process of brush-painted organic solar cells that represents the easy processing of Ag NWs.40 (b) I–V curves of solar cells fabricated on flexible substrates. PEDOT/NWs electrode has higher efficiencies compared to ITO-based control devices.48 (c) Normalized PCE change with cyclic bending test. The Ag NWs-based cell shows lower degradation than the ITO-based cell.57 (d) Photograph of a visibly transparent solar cell that has high transparency in the visible region.62 (e) I–V curve of top illumination and bottom illumination of a transparent solar cell.62 (f) Ultralight PSC fabricated on a 1.3 μm-thick PEN film floats onto bubbles. Scale bar is 1 cm.52 (g) Ultralight PSC with Ag NWs has the highest power-per weight value (29.4 W g−1) among other lightweight cells.52 (h) Spray-coated organic solar cells on fabrics.64 (i) Schematic structure of double-twisted fiber-based solar cells.65 | ||
3. Energy generation
To date, electrical power generation has been largely carried out by extremely large-scale energy generation systems, such as thermal, hydroelectric, and nuclear power stations, but it is clear that different approaches must be used for wearable/portable devices (Fig. 1). Solar cells and nanogenerators have attracted intense attention for use as wearable/portable energy generation devices because they can directly convert light energy or mechanical energy into electrical energy. In general, high transparency combined with high conductivity is mainly required for solar cells, and the high mechanical stability of electrodes is mainly required for nanogenerators. However, since Ag NWs have both characteristics at the same time, flexible solar cells and transparent nanogenerators can be made with Ag NW electrodes.3.1. Solar cells
Ag NW networks have been focused for alternative transparent electrode for solar cells due to their high conductivity combined with high transparency, high robustness under bending, and fabrication by a simple solution process. Thus, Ag NW-based electrodes can be used to replace rigid, heavy glass substrates with flexible and lightweight polymers or metal foil substrates because the Ag NW deposition process is fully compatible with polymer substrates due to the use of low-temperature solution-based processes.41 However, Ag NW electrodes itself are cannot be used for solar cells due to low efficiency thus proper optimization processes are needed.48 Briefly, appropriate current spreading, low surface roughness, and high chemical stabilities are needed. Most of the problems are improved by coating additional materials,48–53 pressing,54 or embedding.50 For the application of film-based substrates to enhance the fabrication speed using a roll-to-roll (R2R) process, mechanical robustness in the bent state is another important factor to be considered. In general, it is known that the power conversion efficiency (PCE) of solar cells using Ag NW electrodes are lower or similar to that of ITO based control devices when fabricated onto the glass substrate.41,48,55,56 On the other hand, Gaynor et al., reported that solar cells using Ag NW electrodes has higher PCE compared to ITO based control devices due to mechanical robustness of Ag NW electrodes (Fig. 2(b)).48 Here, the PCE is calculated according to the following:| η = (FF × |Jsc| × Voc)/Pin, |
Such superior performance is due to the high robustness of Ag NWs compared to ITO when deposited onto flexible substrates. The sheet resistance of the Ag NW-based transparent electrodes is stable down to a curvature of 2 mm, while that of ITO is unstable for curvatures below 9 mm. In turn, this sheet resistance change affects the PCE of the fabricated solar cells. Lee et al. reported that Ag NW-based solar cells were highly stable and maintained 94% of their initial PCE after 400 bending cycles, while ITO-based solar cells maintained only 42% of their initial PCE (Fig. 2(c)).57 In addition, another important advantage of using Ag NW electrodes is that they can be utilized as both the top electrode and bottom electrode in contrast to the conventional solar cell devices that utilize an ITO bottom electrode and an opaque top electrode. Exploiting this advantage, Gaynor et al. used Ag NW network electrodes as the top electrode while using opaque metal as the bottom electrode. Their study demonstrated the potential of Ag NW network electrodes for metal foil substrate-based solar cells suitable for mass production.54 In addition to the opaque solar cells obtained using Ag NWs, semi-transparent solar cells can be fabricated by combining a bottom transparent conducting electrode or bottom Ag NW electrode with a top Ag NW electrode.58–61
When using these top/bottom transparent electrodes, the appropriate selection of the active layer that is sensitive to light in either the UV or NIR region enables the fabrication of visibly transparent solar cells that are suitable for portable/wearable applications from an aesthetic point of view62 (Fig. 2(d)). The fabricated device has an average light transmittance of 61% over the 400–650 nm wavelength range. It can be used with either top-side illumination (3.82%) or bottom-side illumination (4.02%); however, in both cases, the PCE is lower than that of an opaque control device (6.03%) due to the absence of reflection at the opaque electrode62 (Fig. 2(e)).
In addition to aesthetics, the weight of the solar cell is another important factor enabling its use in various applications, such as drones, power aviation models, and weather balloons,63 because a low solar cell weight can decrease the power consumption in these applications. To obtain lightweight solar cells, Kang et al. used orthogonal Ag NW networks that had low surface roughness compared to the random network structure, thus eliminating the need for thick buffer oxide layers.52 They deposited PH1000 and PEDOT:PSS layers onto the orthogonal Ag NW network, and these layers fully covered the NW network. This structure shows similar PCE values on a 120 μm-thick PEN film (13.05%) and a 1.3 μm-thick PEN foil (12.85%), but the PCE is lower than that of a glass-based solar cell (16.25%). The device on the 1.3 μm-thick PEN foil is light enough to float (4.37 g m−2) on bubbles (Fig. 2(f)) and shows the highest power-per-weight ratio of 29.4 W g−1 among the various lightweight solar cells52 (Fig. 2(g)). With the development of lightweight solar cells based on flexible films, stretchable and wearable solar cells started to attract much attention. For this application, some researchers used non-planar substrates, such as fabric or fibers, to fabricate wearable solar cells. Arumugam et al. fabricated organic solar cells on woven polyester cotton fabric by sequentially coating the fabric with Ag NWs, ZnO NP, P3HT:ICBA, and PEDOT:PSS by the spray-coating method, thus proving the feasibility of the low-cost fabrication of wearable solar cells (Fig. 2(h)).64 Other types of substrates such as fibers were also used for solar cell fabrication. Li et al. fabricated fibrous solar cells by twisting two fibers. In this structure, by applying Ag NWs to the contact region, the average PCE was significantly increased from 1.2% (for a cell that did not use Ag NW electrodes) to 2.4%.65 Thus, it has been shown that Ag NW electrodes display easy processability, a combination of high transparency and conductivity, and high robustness under mechanical deformation, making future Ag NW-based flexible/wearable solar cell applications highly promising.
Through this section, it has been shown that Ag NWs electrode display easy processability, a combination of high transparency and conductivity, and high robustness under mechanical deformation. Especially for the recent research trend of solar cells, which is based on solution processed perovskite solar cells, prospects of solution processed Ag NWs electrode is highly promising. In addition to these trends, Ag NWs are expected to contribute to the widespread use of wearable solar cells because research has shown that they are suitable electrodes for wearable energy production devices.
3.2. Nanogenerators
Compared to solar cells that, as of now, have been developed for several decades, nanogenerators are an emerging technology for the generation of electrical energy from various types of waste mechanical energy. Since many of the devices under development are based on lightweight and flexible substrates, such as polymer films, the use of nanogenerators is another possible approach for realizing wearable energy generation systems. In order to realize wearable nanogenerators, fabricated devices need to be highly flexible or stretchable to conformably attached to human skin or clothes. Additional requirement is high mechanical stabilities which can endure repeated stress. Thus the Ag NW electrode is highly appropriate for this application, since it can be applied to various substrates, such as films, elastomers, and fabrics with high mechanical stabilities by various strategies such as photonic welding66 and embedding.67–69The most common device types are piezoelectric nanogenerators (PENGs), which harvest the energy generated from strained piezoelectric materials, and triboelectric nanogenerators (TENGs), which harvest the energy from contact triboelectrification and electrostatic induction. Although in and of themselves, Ag NWs have shown negligible energy harvesting performance, they can affect the efficiency of nanogenerators by changing either the phase70 or the surface potential of the partially embedded Ag NWs/polymer film structures.68,71 However, it was also demonstrated that the amount of Ag NWs in fully embedded structures has nearly no effect on the triboelectric efficiency when a sufficient amount of Ag NWs is used.68 Therefore, Ag NWs are now mainly used to create stable contact on stretchable elastomer or non-planar substrates, such as fabric or paper, rather than to increase the device efficiency. For instance, Jeong et al. fabricated a nanocomposite PENG composed of silicone rubber, ferroelectric Pb(Mg1/3Nb2/3)O3-PbTiO3 (PMN-PT) particles, and carbon nanotubes (MWCNTs). Then, the contact of the fabricated PENG was formed by v-Ag NWs, and it was shown that this contact was highly stable in the stretched state compared to that obtained using short Ag NWs. The generated PENG operates by multiple modes, such as stretching, twisting, folding, and crumpling, and it thus can be applied for harvesting various mechanical motions (Fig. 3(a)). For example, the PENG was attached onto clothes such as stockings that need to be stretched, and it generated electrical energy from the human knee bending motion that induced the stretching of the PENG72 (Fig. 3(b)). In a similar study, Chen et al. fabricated stretchable transparent nanocomposites by spray coating Ag NW electrodes onto BaTiO3/polydimethylsiloxane (PDMS) active layers and embedding with PDMS. This embedding structure protects the Ag NWs from oxidation and endows it with stability under deformation.67 The above-mentioned embedding structure is important for maintaining high conductivity in a strained state. To analyze the effect of Ag NW embedding in a polymeric material, Kang et al. fabricated an embedded structure of Ag NWs in SU-8 and PDMS in order to fabricate a mechanically robust TENG.68 In this study, they demonstrated that the embedded structures were highly stable during bending and tape tests. Likewise, Lai et al. obtained a super stretchable TENG by fully embedding Ag NWs into an Ecoflex elastomer (Fig. 3(c)). Then, the devices could be operated as a single-electrode TENG that generated energy from contact with human skin. Due to its embedded structure and highly stretchable electrode, this device was stable when stretched to 300% strain and maintained its original performance when stretched by a factor of 1000.69
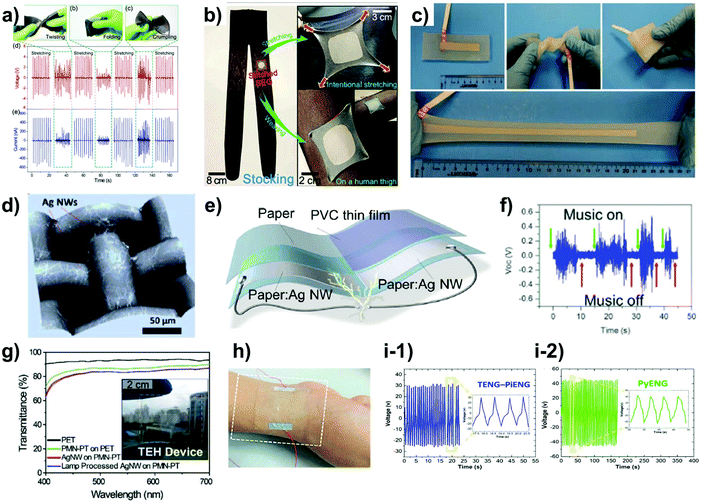 | ||
| Fig. 3 Nanogenerators for wearable application. (a) Photograph and energy generation properties of nanocomposite piezoelectric nanogenerators (NC-PENG) by stretching, twisting, folding and crumpling.72 (b) Photograph of NC-PENG attached onto a stocking for harvesting of biomechanical energy.72 (c) Photograph of a triboelectric nanogenerator (TENG) composed of embedded Ag NWs in Ecoflex and stable under twisting, folding and stretching.69 (d) 3D optical microscope image of Ag NWs directly coated onto the polyester textile.73 (e) Schematic image of paper-based TENG in which a conductive paper:Ag NWs layer is sandwiched between two normal papers.76 (f) Sound energy harvesting properties of paper-based TENG due to its low weight.76 (g) Highly transparent piezoelectric nanogenerator (PENG) fabricated using a flash-lamp-induced welding process.66 (h) Photograph of transparent and flexible tribo-piezo-pyroelectric hybrid energy generator.78 (i-1) and (i-2) Energy generation properties of TENG-PENG and PyENG modes.78 | ||
To increase the utilization of nanogenerators in various wearable applications, non-planar substrates, such as textiles and fabrics, have also been used as substrates. Wu et al. fabricated an e-textile by blade coating Ag NWs onto a polyester textile (Fig. 3(d)) followed by the application of an additional graphene oxide (GO) coating and the chemical reduction to form reduced graphene oxide (rGO). The GO layer enhanced the adhesion and stability of the Ag NW electrodes, enabling the fabrication of highly conductive and mechanically stable e-textiles. Due to its coating consisting of two kinds of polymers with different electron affinities, the fabricated electrodes could be used as a TENG operating through rubbing.73 Likewise, Guo et al. created an e-cloth by dip coating Ag NWs onto a nylon cloth. The fabricated electrodes were encapsulated by PDMS to protect the Ag NWs from chemical or mechanical deformation. Then, Voc and Isc were optimized by further adjusting the surface chemistry by coating fluoroalkylsilane onto the PDMS surface and using a polyethylene terephthalate (PET)/Al foil as the counter electrode.74 Zhang et al. fabricated a conductive fabric by dip coating Ag NWs onto a glass fiber followed by PDMS encapsulation to fabricate a hybridized nanogenerator by combining a TENG and an electromagnetic generator (EMG). Using an appropriate commercial transformer, the two energy generation systems were successfully hybridized and integrated with a bus grip to harvest wasted biomechanical energy.75 In addition to the textile/fabric substrates, paper-based TENGs have also been reported. Wu et al. fabricated conductive paper by dip coating Ag NWs onto paper and encapsulating the top and bottom sides by attaching regular paper to enhance the oxidation stability of the device (Fig. 3(e)). Since the fabricated conductive paper had no TENG functionality, a PVC film was attached to the sandwich structures in order to modify the surface potentials. The fabricated TENG could generate energy through the contact-separation mode and the rubbing mode. In addition to these modes of operation, it could harvest environmental vibration energy, such as sound (Fig. 3(f)) and wind energy, due to the softness and low weight of the paper.76
In addition to its applicability to the various substrates mentioned above, the transparency of Ag NWs enables their use in transparent energy generators, which are favorable from the aesthetic point of view. Park et al. fabricated a stable Ag NW network on a PET film by flash lamp welding and used a PMN-PT single crystal layer as a piezoelectric active layer to obtain a transparent PENG (Fig. 3(g)).66 Similarly, transparent TENGs have attracted much attention. Lee et al. fabricated a transparent electrode by spin-coating Ag NWs on PDMS followed by coating PEDOT:PSS and poly-urethane-acrylate (PUA) layers. Then, the fabricated TENG was attached to human skin and fabric to demonstrate wearable applications.77 In addition to transparent PENG and TENG, a hybrid transparent nanogenerator was also reported. Sun et al. reported a transparent hybridized nanogenerator that generates energy from piezoelectric, triboelectric, and pyroelectric effects. They fabricated a high-FoM transparent electrode by patterning Ag NWs using leaf-venation (LV) and encapsulated the electrode with PDMS. Since ferroelectric polyvinylidene fluoride (PVDF), which has pyroelectric properties, was used as the active material, the fabricated device can harvest mechanical energy using the TENG-PENG mechanism (Fig. 3(i–1)) and the pyroelectric energy generated from the change in the spontaneous polarization of the material arising from the changes in the temperature (Fig. 3(i-2)).78 The above literature survey shows that Ag NWs are highly suitable electrode materials for the fabrication of flexible/stretchable nanogenerators that require highly stable properties in the mechanically strained state. Since this application area has only recently been explored, there is a lot of room for development and, therefore, research efforts on Ag NW-based nanogenerators will certainly multiply.
In summary, many nanogenerators had been fabricated on the basis of conventional electrodes such as opaque Al foil because transparency is not needed. However, if the transparency of electrode is given with high mechanical robustness, it can be expected to have various functionalities by combining with various energy consuming devices. In addition to transparency, various researches have been reported that nanogenerators based on elastomer, textile, and papers are possible by using Ag NWs thus it is highly promising for wearable energy generation application. Since the related research area has just begun, the performance/functionality of the nanowire-based nanogenerators will be gradually increased.
4. Energy storage
Various types of energy storage devices such as supercapacitors, batteries, and fuel cells are currently available, and these have different power density, energy density, and life cycle characteristics so that each device type has its own advantages. Therefore, in this section, we will survey supercapacitors, batteries, and fuel cells in which Ag NWs were used as electrodes in order to obtain flexible devices. Generally, functionality of storing electrical energies and mechanical, chemical stability are important for electrode of each device. Though Ag NW electrode has low functionality in storing electrical energies, coating of appropriate material onto the electrode can enhance its functionality and chemical stability while maintaining the high mechanical robustness needed for flexible energy storage devices. Therefore, we will discuss various strategies of fabricating Ag NW electrodes for flexible energy storage systems.4.1 Supercapacitors
In general, supercapacitors requires high capacitance and high electrochemical stabilities. However, the intrinsic advantages of Ag NW electrodes provide multiple functionalities such as flexibility, stretchability and transparency. In addition, combined with appropriate active materials, Ag NWs appear to be one of the most promising current collectors or charge transfer layers for supercapacitors79,80 and also can greatly boost performance of supercapacitor. Usually, the energy storage efficiency of the supercapacitors increases sharply when the electrode active materials are in close contact with the current collectors.81 In light of this, 1D core–shell and 2D thin-film nanostructures of Ag NWs (so-called networks) are used to fabricate efficient supercapacitors because, in this case, the electrode active materials can be directly deposited by various means onto the surface of the Ag NWs without using polymeric binders and conductive additives. It is known that the use of such binders and additives leads to inefficient charge collection and waste of active materials due to the decrease in the active material volume fraction and increase in the total electrode thickness. For instance, Yuksel et al. presented a core–shell architecture in which molybdenum oxide (MoO2) was used as the pseudocapacitive shell layer,81 obtaining specific capacitance of 500.7 F g−1 at a current density of 0.25 A g−1. Yuksel et al. also fabricated another core shell structure in order to obtain a flexible supercapacitor using electrodeposition processes for the deposition of conformal and coaxial nickel hydroxide (Ni(OH)2) flakes onto Ag NWs.82 The fabricated Ag NWs/Ni(OH)2 core–shell networks demonstrated high mechanical stability on PET substrates for 1000 bending cycles (Fig. 4(a)). The strong adhesion of Ag NWs to PET substrates and the interface compatibility of the electrodeposited Ni(OH)2 shell and Ag NW core led to excellent mechanical stability. The Ag NWs/Ni(OH)2 electrodes showed a specific capacitance of 1165.2 F g−1 at a current density of 3 A g−1.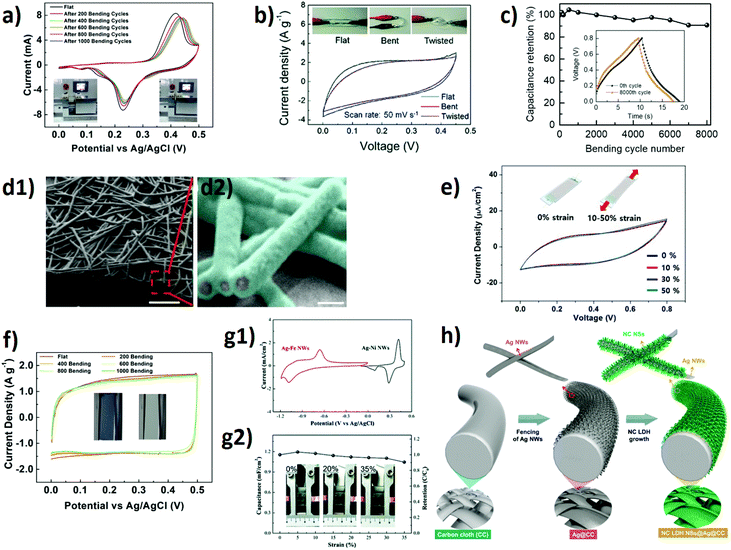 | ||
| Fig. 4 Supercapacitors using Ag NW electrodes. (a) CV results for flexible Ag NWs/Ni(OH)2 nanocomposite electrodes following various bending cycles at a scan rate of 20 mV s−1. Insets are the photos of the homemade bending setup,82 (b) CV curves of Ag NWs/Ni(OH)2 nanosheets under different mechanical conditions (flat, bent, and twisted). The insets show the photographs of the corresponding conditions,83 (c) mechanical stability of the Ag NWs/Ni(OH)2-PEIE/PEDOT:PSS device up to 8000 bending cycles (bending radius of 1 mm). The inset shows the first and last charge–discharge cycles.85 (d1–2) SEM images of Ag/Au/PPy core–shell NW. False colored SEM image shows PPy coating on the Ag/Au NWs surface. Scale bars in (d1–2) show 1 μm and 100 nm, respectively.87 (e) CV curves of the Ag/Au/PPy core–shell NWs supercapacitors at a scan rate of 50 mV s−1 when subjected to the indicated strain rates. The inset shows a schematic illustration of strained and unstrained supercapacitors,87 (f) CV results for flexible electrodes after every 200 bending cycles. Photographs of the electrode at −0.6 V (left) and at 0.5 V (right).89 (g1) CV curves of Ag–Ni NWs and Ag–Fe NWs at a scan rate of 100 mV s−1 in 1 M KOH aqueous electrolyte. (g2) Change in the specific capacitance of the device with increasing strain. The insets show photographs of the device under various strain levels.92 (h) Schematic illustration showing the fabrication steps of the NC-LDH-NSs@Ag NWs@CC electrodes.93 | ||
Du et al. reported a composite electrode based on drop-casted Ag NWs and Ni(OH)2 nanosheets on PDMS.83 A specific capacitance of 78 F g−1 at a current density of 0.5 A g−1 and a capacitance retention of 45.5% after 1500 cycles were reported. This capacitance loss was attributed to the phase transformation of Ni(OH)2 during the charge–discharge cycles. A remarkable relative capacitance drop of 46% after 300 bending cycles (bending angle: 180°) was observed for these electrodes (Fig. 4(b)). Such poor electrochemical stability of the Ag NWs may be due to the adverse effect of exposure to the electrolyte ions during the electrochemical processes; therefore, a conformal coating layer on NWs may protect them from the degradation due to electrolyte ions. Sheng et al. presented a 5 nm-thick zinc oxide (ZnO) protective layer on Ag NW networks, where vertically aligned, two-dimensional cobalt hydroxide (Co(OH)2) nanosheets were used as the electrode active material. This rationally-designed electrode architecture provided a remarkable enhancement in the performance of the hybrid electrode, and the device showed a high areal capacitance, of 5.18 mF cm−2, and a long cycle life, of up to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.84 In another study, Ginting et al. reported a hybrid electrode composed of Ag NWs, pseudocapacitive Ni(OH)2-polyethylenimine ethoxylated (PEIE) composites, and PEDOT:PSS. The fabricated device showed a high areal capacitance, of 3.3 mF cm−2, and a capacitance retention of 90% was achieved after 8000 cycles for bending down to a radius of 1 mm (Fig. 4(c)).85
000 cycles.84 In another study, Ginting et al. reported a hybrid electrode composed of Ag NWs, pseudocapacitive Ni(OH)2-polyethylenimine ethoxylated (PEIE) composites, and PEDOT:PSS. The fabricated device showed a high areal capacitance, of 3.3 mF cm−2, and a capacitance retention of 90% was achieved after 8000 cycles for bending down to a radius of 1 mm (Fig. 4(c)).85
One of the problems in the use of Ag NW networks as supercapacitor electrodes is their limited potential window arising from the electrochemical instability of Ag. This issue also limits the energy and power densities of the devices. To enlarge the voltage window of Ag NWs-based devices, Lee et al. utilized a conformal and few-nanometers-thick gold (Au) coating on Ag NWs. Highly stretchable supercapacitors based on both chemically and electrochemically stable Ag–Au core–shell NW (AACS NW) network electrodes were demonstrated.86 Direct electropolymerization of pyrrole, which has an oxidation potential higher than that of Ag, occurred on these current collectors with improved electrochemical stability, as reported by the same group (SEM images presented in Fig. 4(d1–2)).87 The Ag/Au/PPy core–shell NWs network on flexible PET and PDMS substrates showed superior mechanical performance under various bending and stretching cycles. An areal specific capacitance of 0.58 mF cm−2 was reported for the supercapacitors. Due to its stable coating layers and superior Ag NW stretchability, no changes in the cyclic voltammetry (CV) characteristics were observed for these devices on PDMS substrates when subjected to tensile strains up to 50% (Fig. 4(e)).
The Ag NW networks on transparent and flexible substrates such as PET and PDMS are also superior platforms for the realization of flexible electrochromic supercapacitors. These devices allow visual determination of the remaining energy and charged state through the changes in their color. Through spray-coating of electrochromic conducting polymers onto Ag NWs networks, Yuksel et al. obtained two supercapacitor electrodes with different color options, one with an optical modulation from green to transparent and the other from blue to transparent.88,89 Electrochemical measurements were complemented by spectroelectrochemical measurements thanks to the transparent nature of Ag NWs networks. Electrodes also showed good bendability, such that the electrochemical performance of the bent electrodes was found to be almost identical to that of their flat counterparts upon repetitive bending up to 1000 cycles (Fig. 4(f)). On the inorganic side, Shen et al. demonstrated flexible electrochromic Ag NW/WO3 supercapacitor electrodes on PDMS-coated PET substrates.90 The electrodes showed a high coloration efficiency (80.2 cm2 C−1), with a gravimetric capacitance of 138.2 F g−1 at a scan rate of 10 mV s−1.
To explore advantageous synergistic effects in energy storage devices, various electrochemically active materials with different mechanical properties have been developed and assembled in the form of nanocomposites. For instance, Chen et al. reported hierarchical nanocomposite films of rGO and aligned polyaniline (PANI) nanoarrays on Ag NWs-coated PET substrates.91 The resulting nanocomposite films showed an areal capacitance of 4.50 mF cm−2, indicating the synergistic effect of rGO and PANI nanoarray for improving the charge storage capacity relative to the control devices (0.32 mF cm−2 for the bare rGO film, and 1.94 mF cm−2 for the PANI film at a current density of 0.1 mA cm−2). An areal capacitance of 2.76 mF cm−2 was reported at a current density of 0.3 mA cm−2, with a capacitance retention of 98.2% after 600 bending cycles.
The energy density of a supercapacitor is directly proportional to its operational potential window; thus, it is highly important to design devices with enlarged operational potential windows. To extend the operational potential window, an alternative strategy of fabricating a device with an asymmetrical configuration, where two different electrodes are used in a single supercapacitor, is required. Park et al. reported highly stretchable asymmetric supercapacitors (ASCs) with Ag–Ni and Ag–Fe core–shell NW electrodes.92 The fabricated ASCs operated at 1.6 V, stretched to 35% strain with no performance loss, and showed a capacitance retention of 92% after 5000 cycles (Fig. 4(g1–2)). Due to their high operating voltage of 1.6 V, these devices achieved outstanding energy and power densities, of 0.35 mW h cm−3 and 267 mW cm−3, respectively.
Meanwhile, Yu's group demonstrated a hybrid supercapacitor electrode composed of nickel-cobalt-layered double hydroxide nanosheets on Ag NW-fenced carbon cloth (NC-LDH-NSs@Ag NWs@CC).93 In this structure, the hydrophilic Ag NWs acted as the scaffold material, which increased the conductivity and mass loading of the electrodeposited NC-LDH-NSs (Fig. 4(h)). The fabricated electrodes were assembled with activated-carbon-coated CCs to form flexible ASCs. A slight increment in the capacitance of the bent device was attributed to the decrease in the distance between the electrodes. The fabricated hybrid electrodes showed high energy (78.8 μW h cm−2) and power densities (12.1 mW cm−2) and operated within a large potential window, of 0–1.6 V.
It is evident that Ag NW networks are ideal electrode materials for flexible, transparent, and stretchable supercapacitors, where the NWs improve the charge transport and, in some cases, eliminate the need for additional current collectors. Provided that irreversible oxidation of Ag and the operating voltage limit are avoided for the Ag NW-based supercapacitors, research on the novel electrode morphologies and complementary electrode materials will continue to attract increasing attention.
4.2 Batteries
Currently, lithium-ion batteries are the most widely used energy storage devices, due to their large energy density and long-life cycle. The energy density of a battery is dictated by the thermodynamics and chemistry of the materials and their systems. However, another important parameter for lithium ion batteries is the volume change of the constituent materials and systems due to the uptake and release of the lithium ions that result in mechanical stresses. This not only degrades the long-term energy storage performance of the battery but also has a significant effect on safety. In particular, for a flexible battery used in variable-shape conditions, such a change in the volume of the electrode may introduce additional safety problems. In case of Ag NW electrode, which has three-dimensional nanostructure, can be free from the problems associated with the volume contraction and expansion of lithium ion batteries during alloying process. In addition, silver is known to form alloys with a high percentage of Li; thus, it can be highly promising material for flexible/stretchable battery. Since flexibility/stretchability of Ag NW is highly required for this application, transparency is rather important.Lee et al. fabricated nanostructures based on silver, gold, and silver–gold alloy nanowires (Fig. 5(a)) using a biological system, and its electrochemical performance was measured to confirm its applicability as a lithium ion battery electrode.94 It was found that the capacity retention was improved compared to thin-film electrodes, and the extent of this capacity improvement depends on the nanomaterial size, alloy ratio, and surface treatment. Although the use of silver as a main constituent material of a commercialized battery is limited due to the high cost of silver, its use will increase due to the increasing requirements for the stability of the battery related to the development of wearable devices. Phattharasupakun et al. showed that nanowire-based nanostructures can improve the stability of lithium-ion batteries.95 A 3D interconnected freestanding silver nanowire aerogel (Ag NWA) was fabricated by freeze-drying the nanowires dispersed in DI water (Fig. 5(b) and (c)). The Ag NWA can store a significant amount of Li by forming a Li–Ag NWA alloy (Li–Ag NWA), and the volume expansion caused by this process does not cause any problems due to its structural characteristics. In practice, Li–Ag NWA/LFP cells showed superior performance compared to the Li/LFP cells using Li plates, particularly for the specific capacities at high rates and cycling stability. Thus, it was shown that the 3D freestanding Ag NWA can be an ideal host for regulating, accommodation, and compensating metallic Li in order to achieve safe and flexible Li-ion batteries.
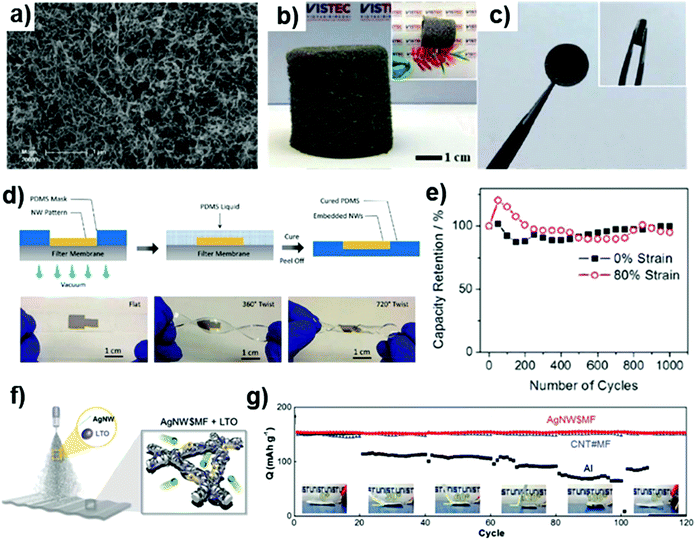 | ||
Fig. 5 Fabrication of battery using Ag NW electrodes. (a) SEM image of CTAB-stabilized Au0.67Ag0.33 alloy nanowire network.94 Digital images of (b) as-synthesized Ag NW aerogel(Ag NWA) and (c) freestanding Ag NWA electrode.95 (d) Illustrations of the lithographic filtration method for the fabrication of stretchable Ag NW electrodes with embedded structures. The digital images show examples of the soft, stretchable electrodes in relaxed and twisted states. (e) Cyclic stability of the suggested stretchable electrodes at 0% and 80% strains.96 (f) The ultrasonic spray method used to fabricate the NW@MF structures. Well-dispersed mixtures of active materials and Ag NWs (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) in water were ultrasonicated within the nozzle at 120 kHz and then sprayed onto PET nonwoven mats.97 (g) Capacity retention of folded cells at different angles at 1 C.97 2, w/w) in water were ultrasonicated within the nozzle at 120 kHz and then sprayed onto PET nonwoven mats.97 (g) Capacity retention of folded cells at different angles at 1 C.97 | ||
Ag NW electrodes can provide not only a solution to the lithium ion batteries’ volume change problem but also provide additional functionality for the batteries. Silver–zinc batteries have comparable specific energy to Li-ion batteries and ensure high safety due to their non-toxic constituent materials such as water-based electrolytes. This makes silver–zinc batteries very valuable as energy storage devices for wearable applications. In many wearable devices, functionality such as flexibility or stretchability is required, and the energy storage devices must also have this functionality. Yan et al. implemented a silver–zinc battery based on a stretchable Ag NW electrode.96 The stretchable electrode was fabricated using a lithographic filtration method and have a structure in which Ag NWs are embedded in PDMS (Fig. 5(d)). This electrode provides both the electroactivity and electron conductivity necessary for the batteries. The Ag NWs on the surface of the PDMS reacts with the electrolyte, and the Ag NWs in the PDMS act as the current collector. In the electrochemical measurements, the areal capacity increases slightly with strain due to the additional exposure of new Ag NWs from beneath the surface. The full silver–zinc battery was fabricated, and it maintained its electrochemical performance even up to 80% stretching (Fig. 5(e)).
Other types of electrodes for flexible batteries were fabricated by Hwang et al.97 They presented a process for fabricating electrodes in which Ag NWs are wound around the microfiber mats (Fig. 5(f)). The ultrasonic spray method was used to attach the Ag NWs to the microfibers of the mats. Prior to the spray process, the lithium titanate (LTO) active material was mixed with the Ag NW ink. By adjusting the wettability of spray ink, the Ag NWs and LTO were firmly attached to the microfibers in the mat. During the spray process, the ductile Ag NWs were wound around the microfibers. The electrode showed an extreme flexibility, and it could withstand not only bending but also folding. In fact, the LED connected to the electrode was stably driven with no flickering during the repeated bending of the electrode. Moreover, by measuring the mass of the electrode, it was confirmed that no Ag NWs and LTO were detached from the microfiber mats during 1000 folding cycles. Additionally, the lithium ion batteries made using these electrodes maintained their capacity during 100 folding cycles (Fig. 5(g)). This demonstrated the suitability of Ag NWs for use as a battery electrode in wearable devices.
Nanowires can also be used to improve the electrical and electrochemical performance characteristics as well as the stability and mechanical durability of batteries.98 Graphene and CNTs are now widely used in the production of flexible electrodes and flexible energy devices. In particular, highly porous graphene aerosol (GA) is highly advantageous as an electrode for energy storage devices. Hu et al. introduced an electrode in which the Ag NWs are integrated into the three-dimensional GA structure through a low-temperature hydrothermal self-assembly method.98 The Ag NWs at the electrode prevent the restacking between the graphene sheets constituting the GA, thereby preventing the reduction of the surface area where the electrochemical reaction occurs. Moreover, since the Ag NWs provide a continuous electron conduction path for the GA, the high-temperature heat treatment process, which has been mainly used to increase the electrical conductivity of GA, can be replaced with the low-temperature hydrothermal self-assembly method. Therefore, the suggested process and the structure can be effectively applied in the fabrication of flexible batteries to a flexible substrate that is sensitive to high temperatures. In summary, flexibility/stretchability and three dimension structures of Ag NW electrode is highly advantageous for wearable batteries, though the energy density needs to be improved.
4.3 Fuel cells
Although fuel cells are sometimes regarded as an energy generation system because they generate electrical energy from fuels such as hydrogen or methanol, it is more appropriate to categorize them as an energy storage system in this review. This is because they can provide stable electrical energy to the energy consumption devices regardless of any condition except for the supply of fuels. Compared to batteries and supercapacitors, fuel cells have high energy density and high energy-conversion efficiency. Moreover, due to their open systems, they have low environmental impact and generate power continuously as long as the fuel is supplied.99 To exploit these strengths, flexible fuel cells have been developed for wearable/portable electronics and intelligent vehicles.100 While various strategies have been used to obtain the required flexibility of the fuel cell, most of them only focused on the flexibility of the substrate in the beginning of the fuel cell fabrication process. However, for the future application such as wearable/portable electronics, the stable power generation capabilities at the mechanically bended state is highly required. In order to successively provide flexibility of fuel cell itself, the current collector must be mechanically stable. For instance, Ito et al. fabricated a microscale direct methanol fuel cell (μ-DMFC) using polysulfone (PSF) with 10 holes for embedding 10 cells.101 Although this was considered the first report of a flexible fuel cell, in fact each cell was not flexible. Since then, several studies utilizing other flexible polymers such as polyester sheet102 and cyclic olefin polymer (COP)103 have been reported, but these studies focused only on the development of novel fabrication processes and cost reduction rather than on the evaluation of the power density under mechanical deformation. The change in the power density was analyzed for the first time by Weinmueller et al. They proposed a DMFC based on SU-8 and showed that the power density decrease under bending is due to the increase in the inner resistance caused by the microfracture in the metallization layer.104 To improve the flexibility of the current collector, Hsu et al. proposed a flexible proton exchange membrane fuel cell (PEMFC) by applying a carbon fiber as the current collector, but the fabricated cell exhibited very limited flexibility.105Then, by introducing high flexibility of Ag NW electrode as the current collector, a substantially flexible fuel cell was fabricated. Chang et al. reported the flexible polymer electrolyte fuel cell (PEFC) using PDMS and Ag NWs.106 Since the membrane electrode assembly (MEA) is already soft, the PEFC will be bendable if the remaining rigid parts including the flow channel, the current collector, and the endplate are fabricated using a flexible material. Fig. 6(a) shows a cross-sectional schematic of the flexible PEFC.107 A percolation network of long Ag NWs was applied to the flow-channel-patterned PDMS, which can be fabricated in an all-in-one “PDMS pad” component, suggesting the possibility of a significantly simplified fabrication process. Additionally, the PEFC showed a low operation temperature, which facilitates the use of flexible polymers such as PDMS. The device obtained using this process can operate in bent condition, and it showed an open circuit voltage of 1.035 V (Fig. 6(b)).
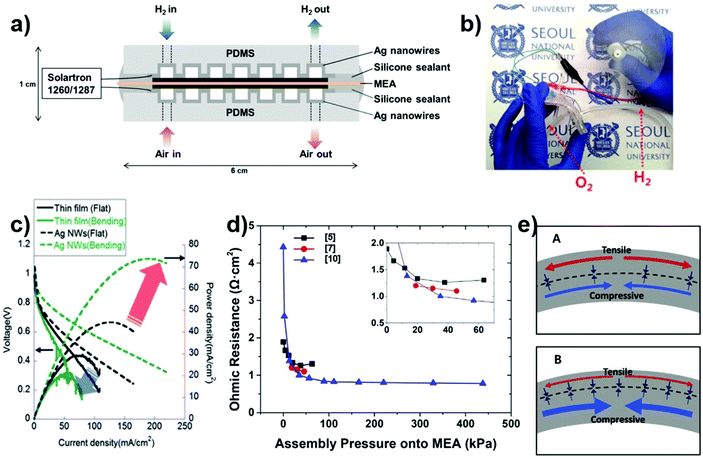 | ||
| Fig. 6 Fabrication of fuel cell based on flexible Ag NW electrodes. (a) Cross-sectional schematic of a flexible fuel cell with Ag NW current collector based on flow channel-structured PDMS.107 (b) Photograph of a motor operated with power provided by a fuel cell under bending. 25 °C hydrated fuel gases (H2, O2) supplied actively to the bendable fuel cell stack.106 (c) Fuel cell performance, using thin film and Ag NWs percolation network current collectors, comparison in bent conditions (solid line: thin film, dotted line: Ag NWs percolation).106 (d) Quantitative comparison of ohmic resistance at various assembly pressures. The inset shows magnified graphs of the low assembly pressure range.109 (e) Internal stress schematics of bendable fuel cells; (A) symmetric and (B) asymmetric stack assemblies.110 | ||
Interestingly, while the metal film current collector-based PEFC showed a decrease in the power density, the Ag NW current collector-based PEFC showed better performance with a higher degree of bending (Fig. 6(c)). The degraded performance of the metal-film-based fuel cell arises from the delamination of the metal film under bending, implying that thin metal films are not appropriate for use as the current collectors in flexible fuel cells.108 The improved performance of the Ag NW current collector-based PEFC is due to the mechanical stability of the Ag NW percolation network and the pressure on the MEA, resulting in a reduced ohmic loss. The variation of the ohmic resistance is a function of assembly pressure, enabling direct (in situ) measurement of the pressure on the MEA109 (Fig. 6(d)). The efficiency changes corresponding to the inner stress favor other performance-enhancing strategies such as asymmetric stack assemblies (Fig. 6(e))110 and stiffness control of the endplate.111 Meanwhile, the robust Ag NW current collector facilitated the performance analyses of the flexible PEFC under torsion107 and the mixed loads.112 Moreover, Ag NWs have been used to achieve a flexible catalyst for fuel cells;113–116 however, this is outside the scope of the present review. Ag NW-based fuel cells have been successfully developed with excellent mechanical properties of the electrodes, but points for improvement remain. In terms of the stable voltage supply, the problem of performance change according to mechanical deformation can be compensated by controlling the fuel flow by means of a feedback system using a strain sensor. In addition, when the fuel leakage problem of the flow-channel and the problem of bonding between the current collector and MEA are solved, it seems possible to develop a stretchable fuel cell suitable for stretchable Ag NW-based current collector.
5. Energy consumption
Since the percolation networks formed by Ag NWs are highly transparent and highly robust under strain, they can be used for nearly all wearable devices. However, compared to the other two types of energy devices, flexibility and stretchability are highly important for the energy consumption devices because these characteristics enable entirely new functions by allowing the design of devices that are highly conformal to a human body. Among the various types of applications, in this review, we will address the Ag NW-based organic light emitting diodes (OLED), sensors, and heaters. In addition to the flexibility and stretchability, there are various requirements, such as current spreading, low surface roughness, and good oxidation stability, for these applications and optimization processes will be addressed in each subsection below.5.1 OLEDs
OLEDs which convert electrical energies to light energy generally needs high performance transparent electrodes, appropriate selection of active materials and bandgap engineering. Moreover, its competitor inorganic-based light emitting diodes are relatively fragile at mechanical strain, flexible OLEDs have been explosively studied by many research groups. For that reason, the Ag NW network is highly advantageous for flexible OLEDs due to its intrinsic advantages. However, it also requires the use of various optimization processes to obtain low surface roughness, current path uniformity, strong adhesion to the substrate, and high chemical stability. In this chapter, we will cover various optimization processes for successfully applying Ag NWs electrode to OLEDs.Band structure is highly important for efficient light emission. Li et al. reported on the Ag NW-based flexible OLED devices fabricated by plasma treatment of Ag NWs on the photo-curable resin (NOA63).117 In this process, the thin dielectric polymer polyvinylpyrrolidone (PVP) layer coated onto Ag NWs was removed, which induced the change in the Ag NWs work function from 5.32 to 5.84 eV as shown in Fig. 7(a–i). This removal of the PVP is mainly due to the heat generated at the Ag NWs, which is accompanied by minimal substrate damage. In addition to the band structure changes, the area of the contact between Ag NWs increases during the plasma treatment, decreasing the sheet resistance of the electrode and leading to an increase in the maximum luminance and current efficiency of the OLEDs (Fig. 7(a-ii)). Similarly, various methods, such as mechanical pressing118 and intense pulsed light (IPL) treatment,119,120 have been reported for improving the junction between the nanowires. Lian et al. applied pressure to Ag NWs to weld junctions in order to achieve low surface roughness and high adhesion between the Ag NWs and the substrates. However, a few cracks were observed at the connection point, which has an undesirable effect on the electrical conductivity. Due to the large surface energy of the fresh cracks, organics such as PVP are attached on the Ag NWs surfaces as capping agents. Fig. 7(b) shows that the cracks on the connection point are completely restored with the CaCl2 solution treatment, which can further decrease surface roughness. The surface roughness also significantly affects the efficiency of the device because similar to solar cells, an uneven substrate could increase the short-circuit current and leakage currents.121 In the case of the IPL treatment, which selectively welds the junction by a plasmonic effect, the welding not only affects the junctions, but also decreases the surface roughness values and increases surface adhesion. Lee et al. reported an Ag NWs network conductor for OLEDs fabricated by IPL without either high pressure or temperature treatment.119 In addition to the excellent electrical conductivity and optical transparency, the IPL-treated Ag NWs networks show superior mechanical stability, which was investigated by the measurements of the ultimate bending radius, and repeat bending and tape peel-off tests, to demonstrate their suitability for emerging flexible and stretchable optoelectronic devices. The rate of change of the sheet resistance (ΔRs) for the IPL-treated Ag NWs network after 2000 bending cycles was only 2.6%. When the IPL-treated Ag NWs network was used in the OLED electrode, no color distortion was observed, and the observed electroluminescent characteristics were comparable to or higher than those of other OLED devices with flexible PET/ITO and PET/indium zinc oxide (IZO) electrodes (Fig. 7(c)). During this IPL treatment, a partial embedding of Ag NWs into polymer substrates was observed, increasing their mechanical stability. Song et al. reported observing this embedding of Ag NWs into their colorless-polyimide (cPI) substrates,120 and the improved surface roughness reduces the leakage current. The leakage current of the IPL-irradiated Ag NWs network (7 μA cm−2) was significantly lower than that of the non-irradiated network (130 μA cm−2) at 2.0 V reverse bias voltage (Fig. 7(d-i and ii)). In addition to this IPL treatment-induced embedded structure, forming embedded structures using a UV-curable polymer can also be used to obtain a smooth surface. Sim et al. demonstrated an OLED fabricated using embedded Ag NWs in a UV-curable resin and R2R processing for large-area fabrication (Fig. 7(e)). The embedded Ag NWs flexible electrode shows good mechanical and chemical stability as well as low surface roughness. After the bending test, the surface roughness and sheet resistance changed only slightly, showing suitability for use as a flexible OLED electrode.121
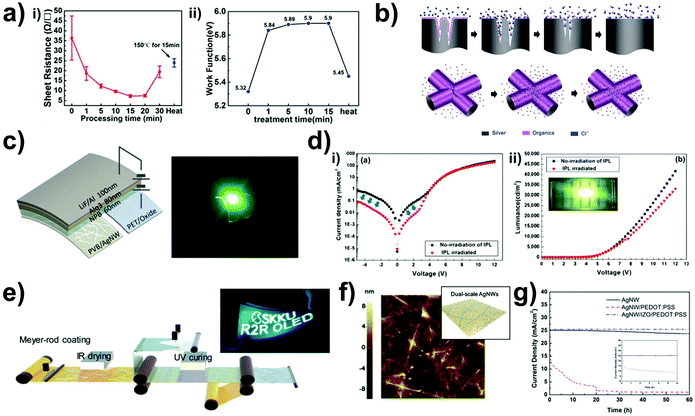 | ||
| Fig. 7 Ag NWs-based OLED with transparent electrode. (a-i) Sheet variation of the Ag NWs, (a-ii) the work function of Ag NWs with plasma treatment time as the anode.117 (b) Schematic of the crack renovation with the CaCl2 treatment.118 (c) Schematic of the OLED device and digital camera image of PVB/Ag NWs-based OLEDs operation under compressive mechanical stress.119 (d) Measured characteristics for the OLEDs with and without IPL exposure (8 times of 500 μs exposure): (i) current density-bias voltage, (ii) luminescence-bias voltage.120 (e) Schematic for the R2R process used to fabricate large-area flexible OLEDs, and a light-emission image obtained under bending stress.121 (f) AFM topology information and schematic for the dual-scale Ag NWs.122 (g) Current density changes under current stressing for different conductive films.123 | ||
For this embedded structure, a uniform distribution of Ag NWs can increase the performance of the OLEDs. Lee et al.122 fabricated a dual-scale metal nanowire network that is also embedded into a UV-curable resin (Fig. 7(f)). The long and thick Ag NWs served as the skeleton for carrier transport, and the short and thin Ag NWs interconnected the long and thick Ag NWs across large network voids. After the deposition of the dual-scale NWs network, the network was patterned by direct laser ablation for an arbitrary designed OLED shape without the use of a photo-mask and wet etching process.
Coating of oxide materials such as IZO onto the Ag NWs is another approach for increasing chemical stability of Ag NWs that prevents the oxidation of Ag NWs (Fig. 7(g)). Yun et al. fabricated PEDOT:PSS/IZO/Ag NWs structures to prevent oxidation of Ag NWs and to improve the interconnection of Ag NWs and their binding to the substrate.123 The IZO layer does not degrade the mechanical stability during and after the buckling test. The hybrid conductor-based OLED shows enhanced and stable performance under mechanical stress at the current density of 25 mA cm−2 for up to 60 h, whereas that of the control devices decreases to half of its initial current density within 1 h at the same experimental conditions. Thus, the performance of Ag NW-based flexible OLEDs have been drastically improved recently through the adoption of these various strategies, demonstrating the great potential of Ag NWs for use in wearable OLED applications.
As an electrode component of stretchable and flexible OLED, the surface roughness and work function engineering of Ag NWs as anode were key issues. The electrical instability caused by electrical short through an active material penetration and nanowire network breakdown is related with OLED operation under the mechanical stress/strain. To improve the lifetime and external quantum efficiency (EQE), the anode/hole transport layer (HTL) interface design is also important; research on the morphologies and active materials of the interface will realize the electrically stable and high-efficiency OLED.
5.2 Sensors
The sensitivity and gauge factor are the main parameters for the evaluation of the sensor performance and Ag NW electrode is highly advantageous for various type of sensor application since its relative resistance–strain curve can be tuned by various methods such as Ag NWs density and structures. In detail, sensor electrodes are divided into two categories of the stable conductor in the active material and in the sensor itself. For instance, Ag NW-based transparent conductive electrodes on flexible substrates were actively employed as simple electrodes or in functionalized form. Here, we survey the latest sensor applications such as strain, chemical and health monitoring sensors obtained using Ag NW-based transparent electrodes and a flexible/stretchable substrate. In fact, typical Ag NWs showed weak adhesion strength when placed directly on the flexible/stretchable substrates, and therefore, usually embedded structures or composites are formed to improve the mechanical stability of the electrodes in various bending/stretching conditions to enable practical applications. Therefore, in this section, the Ag NW-based electrodes for sensor applications formed through embedding or in composite form are reviewed.Yao et al. have reported a wearable hydration sensor fabricated by facile drop-casting of Ag NWs onto PDMS (Fig. 8(a)).124 The simple interdigitated pattern of Ag NWs on the PDMS substrate was placed directly on human skin to show dry/wet states by measuring the impedance on the human skin. Since the Ag NW network on PDMS in this study was consistent with only a single-side, plain electrode structure, it was connected to a rigid electric circuit board to show the developed functionalities such as electrocardiogram (ECG) and skin impedance measurements and motion detection in the wireless condition. Similarly, a single-side structure of Ag NW electrode on the target substrate was demonstrated by Youn et al.125 A vacuum filtration process along with a 3D-printed mold was used to achieve an Ag NWs-embedded cPI electrode with a lower surface roughness (Fig. 8(b)). The electrode fabricated in this study exhibited good resistance behavior from −20 to 20 °C. The resultant electrode was applied as a real-time temperature monitoring sensor for blood packages by combining with an external wireless controller system. In addition to this embedded structure, a thin-film device has a remarkably broadened range of possible applications because it can make conformal contact with various targets such as skin. For example, skin-attachable loudspeakers and microphones were developed by Kang et al. by using orthogonally aligned Ag NWs on a nanometer-thick polymer substrate (Fig. 8(c)).126 The low thickness of the nanomembrane allowed conformal contacts with surfaces in various conditions such as human skin, line-patterned 3D PDMS microstructures, and micropyramid-patterned 3D microstructures. Since the Ag NW nanomembrane structure can definitely affect and/or detect an imperceptible vibration, it was utilized as a speaker and voice analyzer.
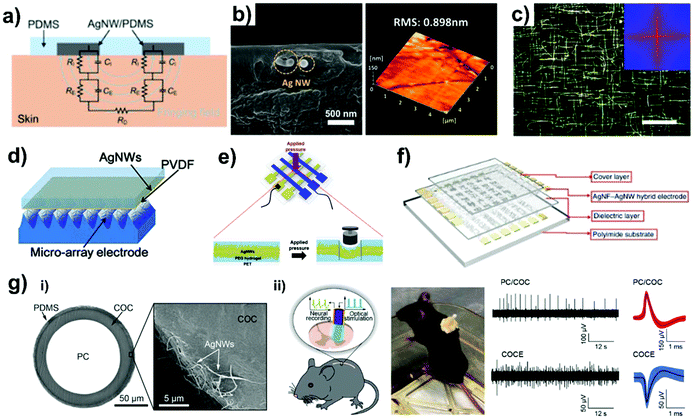 | ||
| Fig. 8 Sensor using Ag NWs. (a) Schematic image of the Ag NWs-based sensor placed on the skin with the fringing field infiltrating the upper skin layer.124 (b) Cross-sectional SEM image and AFM topology information for the p-Ag NWs/cPI electrode.125 (c) Dark-field optical and FFT microscope image for the orthogonal Ag NWs corresponding to its Ag NWs network structure.126 (d) Schematic of Ag NWs-embedded PVDF top electrode and microarray PDMS bottom electrode for the capacitive pressure sensor.127 (e) Schematic illustration of the Ag NW-PEG-PET sandwich structured resistive pressure sensor.128 (f) Capacitive pressure sensor array with silver nanofiber–silver nanowires (Ag NF–Ag NWs) hybrid electrode.129 (g-i) Cross-sectional SEM image of flexible neural probes. (g-ii) Probing spinal cord electrophysiology with Ag NWs-based neural probes.131 | ||
Typical single-layered Ag NW electrode sensors have a simple structure and operate mainly by measuring the electrical resistance changes caused by an external stimulus. Despite these single-layer Ag NW electrodes having shown good mechanical and electrical performance in several studies, their long-term chemical stability and durability in harsh conditions are unknown. In contrast to a single layer structure, a double-layered structure provided highly improved stability by protecting the Ag NW electrodes with polymers and by minimizing the exposed areas of the electrodes. Therefore, most of the developed sensors have a bilayer structure and can read signals through electrical capacitance variations. Shuai et al. demonstrated a capacitive pressure sensor by combining two layers of Ag NW-embedded PDMS substrates (Fig. 8(d)).127 The relative capacitance signals were improved using two different types of electrodes such as the planar-shape top electrode and the buckled-shape bottom electrode. Interestingly, the electrodes were fabricated by two different solution-based fabrication processes of vacuum filtration for planar top electrode and drop casting for the microarray bottom electrode. The proposed capacitive pressure sensor was obtained in the form of 3 × 3 or 5 × 5 pixels within the same area (30 × 30 mm2) to detect multi-pressure conditions. A double-layered resistive-type pressure sensor was also fabricated by Ko et al., where each of the single-layered Ag NW electrodes were directly overlapped.128Fig. 8(e) shows that a photolithography-patterned polyethylene glycol (PEG) hydrogel intermediate layer efficiently held the Ag NW network on the PET substrate. When a pressure stimulus was applied on the sensor, the displacement gap between the electrodes became narrower and the sensor showed low electrical resistance and a response through a higher electrical current value. In this case, each of the Ag NW electrodes was directly overlapped while the PEG intermediate layer and PET substrate encapsulated the electrodes, and high electrical conductivity was obtained for up to 48 h in chronoamperometry measurements. Thus, the Ag NWs-PEG pressure sensor was obtained using a simple fabrication process and showed good mechanical stability, high sensitivity, low pressure sensing threshold, and fast response time, making it suitable for the development of transparent and flexible electronic applications.
To obtain a smart sensor systems, An et al. functionalized a transparent Ag NW electrode by combining a tactile pressure sensor and skin temperature sensor in a single device (Fig. 8(f)).129 Their fabrication of this smart sensor system began with a precise deposition of Ag NWs and Ag nanofibers (Ag NF) hybrid network on a cPI substrate using photolithography-assisted electrospinning and electro spraying. Then, various materials including SiO2 as the dielectric layer, metal oxide semiconductor and PEDOT:PSS were deposited and patterned. The assembled sensor was passivated with several different types of thin cover layers such as glass slide, PET, or cellulose nanofiber film (CNF), respectively. The CNF-covered device recorded the highest output voltage value compared to ITO electrodes with difference thicknesses of glass covering layers. Finally, the device was integrated with a readout circuit and expanded to obtain a multiplexed finger sensor array for high-performance, high-reliability fingerprint sensor.
Unlike embedded Ag NW structures on the flexible/stretchable substrates, Ag NW-based composite electrodes mixed with different functional materials show different physical and chemical properties. Li et al. reported a flexible ammonia (NH3) sensor made from a PEDOT:PSS and Ag NW composite film.130 Although intact PEDOT:PSS was widely used as an active material for NH3 gas sensor applications, it showed slow response sensitivity and low relative resistance changes. To achieve the desired combination between PEDOT:PSS and Ag NWs, several different concentrations of Ag NWs were added to PEDOT:PSS film. Since the insertion of Ag NWs into the PEDOT:PSS film increases the surface roughness, d the absorption of the NH3 molecules to the resultant film is enhanced. As a result, the relative resistance changes before and after the NH3 exposure was increased as well. The fabricated Ag NW-PEDOT:PSS film was utilized in a food freshness monitoring sensor.
In a similar approach, in a pioneering work, the Ag NW containing composite was implanted by Lu et al. in a bioelectric application for the measurement of the spinal cord signal in mice (Fig. 8(g)).131 The fiber probe was first produced from a thermal drawing of a flexible optical fiber as the structural core of the probe. Prior to dip-coating of Ag NWs and PDMS for the fiber, respectively, the diameter of the optical fiber was reduced after thermal drawing to several micrometers. The processed optical fiber had a multilayer structure consisting of an inner polycarbonate (PC) core, intermediated cyclic olefin copolymer (COC) cladding layer, and an Ag NWs and PDMS outer coating layer. Due to the processed fiber, the flexible neural probe maintained its good optical property and gained the desired electrical property. Moreover, a COC elastomer (COCE) also treated by using the same process and the COCE core fiber showed appropriate mechanical and electrical properties when used as a stretchable neural probe. The fabricated Ag NW coated fibers optically stimulated neural activity in the mouse spinal cord and simultaneously recorded an electrophysiology response. Thus, in that study, flexible and stretchable concentric probes were developed by using an Ag NWs–polymer composite for the optical and electrophysiological examination of spinal cord circuits in the mouse model. These studies have demonstrated that the superior properties of Ag NWs can enable entirely new applications that cannot be implemented using rigid and opaque electrode materials.
The conductive nanowire as Ag NWs based sensor takes a role such as electrical properties change (resistance, capacitance, inductor) of the nanowire or NWs network itself and current path between sensing active materials for signal detecting. The variation of NWs network structure, multiscale morphology, and interface between Ag NWs and sensing material,132,133 is a major factor for high sensitive sensor with self-powered system,134 which will be enhanced by researches on nanomaterial and structure engineering.
5.3 Joule heaters
According to Joule's law, the conductive material radiates heat under an applied bias voltage, and it can be easily shown that the output power varies with applied voltage and resistance of electrodes. Since the mechanism is simple, Ag NWs electrode itself can work as heater and provides various functionalities such as flexibility/stretchability and transparency. Though the mechanism is simple, there are several requirements for commercialization. For example, the oxidation of electrode affects resistance degradation thus the controllability of the Joule heater can decrease unexpectedly. In a stretchable and flexible Joule heater, the issue of uncertainty and instability under mechanical stress/strain and exposure to chemicals remains challenging, and it must be addressed to enable the practical use of these devices. This has motivated many researchers to focus on the fabrication method of a uniform and stable electrode.Kim et al. reported a uniformly interconnected Ag NW network for a flexible and transparent Joule heater.135 To improve the solution dispersion and uniform coating of Ag NWs, a solution-based synthetic method was used with small-diameter Ag NWs and exfoliated clay platelets (Fig. 9(a)). By obtaining a nanowire composite with nanoscale clay platelets and Ag NWs, the Ag NWs network was uniformly interconnected over a large-area through scalable solution method.
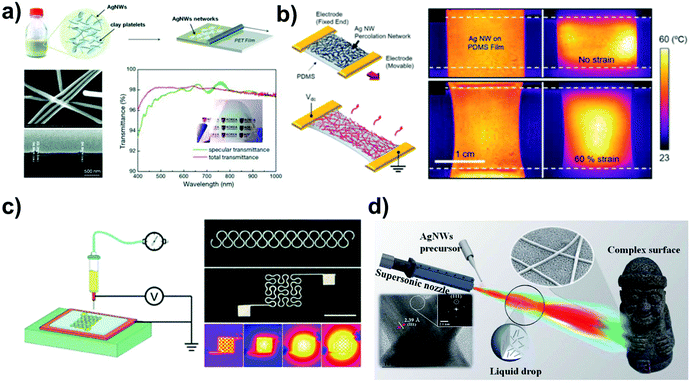 | ||
| Fig. 9 Stretchable and flexible Joule-heater with Ag NWs. (a) Fabrication procedures for transparent and flexible electrodes with Ag NWs and clay platelets.135 (b) Schematic and infrared camera thermal image for the highly stretchable and transparent heater with Ag NWs/PDMS.136 (c) EHD printing setup and large-scale Ag NWs pattern with high resolution. Scale bar, 5 mm.137 (d) Schematic images of supersonic cold-spray deposition onto a 3D structure; SEM and TEM image of self-fused Ag NWs.138 | ||
Moreover, the clay coating enhanced electrical performance by creating a contiguously interlinked and overlapping network, thereby minimizing the change in the conductivity of the Ag NWs network. The uniformly dispersed Ag NWs ink is advantageous for mechanical flexibility and scalability to a R2R system. In another study of a mechanically stable Ag NWs-based Joule heater, Hong et al. demonstrated a highly stretchable and transparent heater based on an Ag NW network partially embedded into a stretchable PDMS substrate (Fig. 9(b)).136 The Ag NW-based stretchable heater showed stable operation under 60% strain, bending, and twisting. This partial embedding morphology was obtained mainly due to the mechanical pressure and evaporation of the residual solvent during the vacuum-transfer process. The swelling between the solvent and oxidized PDMS led to the partial embedding structure with applied pressure. Moreover, overheating at the Ag NW interconnections is prevented due to the deep penetration morphology of PDMS. The local heating was investigated by selective laser ablation patterning, which can easily manipulate the heating region without using the conventional photolithography process. In order to enhance throughput of Ag NW electrodes, several other direct printing processes were developed for the fabrication of flexible and stretchable heaters using Ag NWs inks. Cui et al. demonstrated high-resolution and high-throughput Ag NWs network patterns using an electrohydrodynamic (EHD) printing process on a wide range of substrates such as paper, PET, glass, and PDMS (Fig. 9(c)).137 The rheological behavior of the Ag NW inks including viscosity, applied pressure, voltage, standoff distance, printing speed, nozzle size, and Ag NW concentration are important for the EHD printing resolution. A flexible Ag NW network heater was fabricated with a uniform temperature distribution and was printed onto lab-use gloves, showing stable heating performance under mechanical strain. In order to coat Ag NWs on the three dimensional structures not only for 2D structures, Lee et al. applied a supersonic spray method with self-fused Ag NW junctions (Fig. 9(d)).138 Ag NWs were mixed into a gas stream and homogeneously deposited on the both 2D and 3D substrates. The flexible Ag NW network that was self-fused by supersonic spraying showed low sheet resistance (Rs < 10 Ω sq−1), high transparency (Tr > 95%), and low haze (h < 1%). When voltage was not supplied, the Ag NW network behaved as a thermal insulator, preventing the emission of body heat from glove by trapping air, enabling its use in medical applications.
Overall, though there has been extensive research on heater application, the heater properties can be dramatically changed with the surrounding condition and uniformity of the heating performance, causing drastic temperature increase, which is directly connected with safety. For practical application, energy consumption efficiency, thermal stability, and uniform heating will be improved with research on fabrication processes, heating materials, and structure engineering. In addition, the unified measurement standard for flexible/stretchable heaters is needed under mechanical stress and strain to evaluate and develop the characteristics of the heater device.
6. Conclusion and prospective
In this article, we reviewed the energy generation, storage, and consumption devices fabricated using Ag NW electrodes with superior properties under strain. Using these Ag NW electrodes, bulky and rigid devices were transformed into lightweight and soft devices that are highly appropriate for portable and wearable applications. Even though the solution-based deposition process is suitable for use with flexible substrates, it also requires a range of entirely different fabrication processes and materials; thus, advances for a wide range of methods, device types, and applications have been achieved in an explosively growing research effort by many researchers. For example, PCEs of solar cells have been increased by the development of solar cell technologies that are highly advantageous for use in flexible/stretchable devices and that achieve performance comparable to that of rigid control devices. For the energy storage devices for wearable applications, different types of devices have their own advantages, so that they have been competitively developed accordingly. For example, supercapacitors have short charging times and long cycle life, while batteries are advantageous due to their high energy densities. Flexible/stretchable fuel cells are alternative energy supply devices for wearable applications, because they can supply energy continuously as long as fuel is supplied. Compared to the previous two kinds of energy devices, energy consumption devices (OLEDs, sensors, and heaters) using Ag NWs have been most extensively studied, since flexibility or stretchability of these devices gives them other functionalities and applications that cannot be realized on rigid substrates. For instance, highly flexible OLEDs are expected for implantable applications such as devices for photo stimulation of cells, neurons, and tissues. In case of highly flexible sensors, which have extremely low thicknesses, they make conformal contact with various contacts so they have high potential for epidermal or implantable devices that measure physiological signals. Similarly, Joule heaters based on flexible/stretchable substrates can be used for wearable heaters; these devices transfer heat to human body more effectively for heatable clothes or thermal therapy. Although many advances have been made for each one of these device types, there is lack of research on integrated systems. For the development of self-sustainable wearable devices, the three types of devices must be combined into a single integrated platform. An integrated system necessitates unified fabrication process and materials to simplify the whole fabrication process and lower fabrication prices.Additionally, we forecast that the three major fields that require energy devices on flexible or stretchable will be the sensors for IoT concept, soft robotics, and human-attachable healthcare devices. Being flexible and wireless is the common feature that is desirable for these applications, while the main concerns regarding the energy devices might be different for each area. Various sensors are the key component for the concept of IoT, and the total number of sensors is anticipated to rapidly increase to create an efficient network of smart devices. Connecting a powerline to every sensor is impractical; therefore, the power consumption for IoT sensors should be as low as possible through the miniaturization and optimization of the hardware and software. For a self-sustainable IoT sensor that performs its task for an extended period, an efficient energy harvesting device appears to be one of the important issues. Since the location and the surrounding environment of the sensor may vary, energy-harvesting devices should be prepared to harvest specific types of energy that is available at installation locations. The energy storage device is becoming more important for soft robotics due to the emergence of the concept of ‘untethered robots’ that operate under various actuation methods. Some of the actuation methods are triggered by external sources, but a large number of studies still require the connection to the power supply together with the requirement of large work/power density. Besides the actuation, energy devices are required within the soft robots for sensing and processing purposes as well. For human-attachable healthcare purposes, the importance of the energy consumption components summarized in this review is also high for thermotherapy and i/o purposes. We, therefore, suggest that the future research on flexible/stretchable energy devices should consider its subject of application as well as required performance and facile integration.
In that point of view, we believe that Ag NWs are appropriate as a platform material that combines and connects the three different kinds of wearable energy devices into a single integrated system, because they are highly advantageous due to their soft substrate-suitable low-temperature solution processing and superior optical, mechanical, and electrical properties described in this review. In conclusion, the significance of integrated systems is increasing with the development of IoT technology, and we hope this review will give insights into new approaches for the use of Ag NWs in self-sustainable wearable devices.
Author contributions
S. Han and S. H. Ko designed the concept of the review subject. J. Jung led the study of the energy generation section with P. Lee, J. Yeo, and S. Hong. S. Han led the sub-subject of the energy storage with R. Yuksel, H. Lee, and H. E. Unalan. H. Cho led the energy consumption part with J. Kwon and D. Kim. S. H. Ko organized this works and all authors wrote the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is supported by the National Research Foundation of Korea (NRF) Grant funded through the Basic Science Research Program (2017R1A2B3005706, NRF-2016R1A5A1938472, NRF-2017K2A9A1A06025786) and Creative Materials Discovery Program (NRF-2016M3D1A1900035). S. Han was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1C1C1007629) and Ajou University research fund. S. Hong was supported by an NRF grant funded through the Basic Science Research Program (NRF-2017R1C1B1008847). J. Yeo was supported by the NRF grant funded by the Korea government (MSIT) (NRF-2018R1A6A1A06024970) and IITP grant funded by the Korea government (MSIP) (No. 2017000910001100). P. Lee was supported by the Technology Development Program to Solve Climate Changes (2015M1A2A2056824).References
- J. A. Rogers, T. Someya and Y. Huang, Science, 2010, 327, 1603–1607 CrossRef CAS PubMed.
- D. H. Kim and J. A. Rogers, Adv. Mater., 2008, 20, 4887–4892 CrossRef CAS.
- Y. Sun, W. M. Choi, H. Jiang, Y. Y. Huang and J. A. Rogers, Nat. Nanotechnol., 2006, 1, 201 CrossRef CAS PubMed.
- T. Cheng, Y. Zhang, W. Y. Lai and W. Huang, Adv. Mater., 2015, 27, 3349–3376 CrossRef CAS PubMed.
- M. Kaltenbrunner, M. S. White, E. D. Głowacki, T. Sekitani, T. Someya, N. S. Sariciftci and S. Bauer, Nat. Commun., 2012, 3, 770 CrossRef PubMed.
- T. Chen, S. Wang, Z. Yang, Q. Feng, X. Sun, L. Li, Z. S. Wang and H. Peng, Angew. Chem., Int. Ed., 2011, 50, 1815–1819 CrossRef CAS PubMed.
- L. Wang, D. Chen, K. Jiang and G. Shen, Chem. Soc. Rev., 2017, 46, 6764–6815 RSC.
- F. C. Krebs, J. Fyenbo and M. Jørgensen, J. Mater. Chem., 2010, 20, 8994–9001 RSC.
- S. Bae, H. Kim, Y. Lee, X. Xu, J.-S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. R. Kim and Y. I. Song, Nat. Nanotechnol., 2010, 5, 574 CrossRef CAS PubMed.
- Y.-D. Lee and W.-Y. Chung, Sens. Actuators, B, 2009, 140, 390–395 CrossRef CAS.
- D.-H. Kim, N. Lu, R. Ma, Y.-S. Kim, R.-H. Kim, S. Wang, J. Wu, S. M. Won, H. Tao and A. Islam, Science, 2011, 333, 838–843 CrossRef CAS PubMed.
- S. Han, M. K. Kim, B. Wang, D. S. Wie, S. Wang and C. H. Lee, Adv. Mater., 2016, 28, 10257–10265 CrossRef CAS PubMed.
- S. Xu, Y. Zhang, J. Cho, J. Lee, X. Huang, L. Jia, J. A. Fan, Y. Su, J. Su, H. Zhang, H. Cheng, B. Lu, C. Yu, C. Chuang, T.-i. Kim, T. Song, K. Shigeta, S. Kang, C. Dagdeviren, I. Petrov, P. V. Braun, Y. Huang, U. Paik and J. A. Rogers, Nat. Commun., 2013, 4, 1543 CrossRef PubMed.
- M. Kaempgen, G. Duesberg and S. Roth, Appl. Surf. Sci., 2005, 252, 425–429 CrossRef CAS.
- X. Wang, L. Zhi and K. Müllen, Nano Lett., 2008, 8, 323–327 CrossRef CAS PubMed.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706 CrossRef CAS PubMed.
- C. Yan, J. Wang, W. Kang, M. Cui, X. Wang, C. Y. Foo, K. J. Chee and P. S. Lee, Adv. Mater., 2014, 26, 2022–2027 CrossRef CAS PubMed.
- D. Zhang, K. Ryu, X. Liu, E. Polikarpov, J. Ly, M. E. Tompson and C. Zhou, Nano Lett., 2006, 6, 1880–1886 CrossRef CAS PubMed.
- C. Yu, C. Masarapu, J. Rong, B. Wei and H. Jiang, Adv. Mater., 2009, 21, 4793–4797 CrossRef CAS PubMed.
- Z. Yu, X. Niu, Z. Liu and Q. Pei, Adv. Mater., 2011, 23, 3989–3994 CrossRef CAS PubMed.
- Y. H. Kim, C. Sachse, M. L. Machala, C. May, L. Müller-Meskamp and K. Leo, Adv. Funct. Mater., 2011, 21, 1076–1081 CrossRef CAS.
- S. I. Na, S. S. Kim, J. Jo and D. Y. Kim, Adv. Mater., 2008, 20, 4061–4067 CrossRef CAS.
- D. J. Lipomi, J. A. Lee, M. Vosgueritchian, B. C. K. Tee, J. A. Bolander and Z. Bao, Chem. Mater., 2012, 24, 373–382 CrossRef CAS.
- J. G. Tait, B. J. Worfolk, S. A. Maloney, T. C. Hauger, A. L. Elias, J. M. Buriak and K. D. Harris, Sol. Energy Mater. Sol. Cells, 2013, 110, 98–106 CrossRef CAS.
- S. De, T. M. Higgins, P. E. Lyons, E. M. Doherty, P. N. Nirmalraj, W. J. Blau, J. J. Boland and J. N. Coleman, ACS Nano, 2009, 3, 1767–1774 CrossRef CAS PubMed.
- L. Hu, H. S. Kim, J.-Y. Lee, P. Peumans and Y. Cui, ACS Nano, 2010, 4, 2955–2963 CrossRef CAS PubMed.
- T. Akter and W. S. Kim, ACS Appl. Mater. Interfaces, 2012, 4, 1855–1859 CrossRef CAS PubMed.
- J. Jung, H. Lee, I. Ha, H. Cho, K. K. Kim, J. Kwon, P. Won, S. Hong and S. H. Ko, ACS Appl. Mater. Interfaces, 2017, 9, 44609–44616 CrossRef CAS PubMed.
- P. Lee, J. Lee, H. Lee, J. Yeo, S. Hong, K. H. Nam, D. Lee, S. S. Lee and S. H. Ko, Adv. Mater., 2012, 24, 3326–3332 CrossRef CAS PubMed.
- Z. Yu, Q. Zhang, L. Li, Q. Chen, X. Niu, J. Liu and Q. Pei, Adv. Mater., 2011, 23, 664–668 CrossRef CAS PubMed.
- F. Xu and Y. Zhu, Adv. Mater., 2012, 24, 5117–5122 CrossRef CAS PubMed.
- S. M. Bergin, Y.-H. Chen, A. R. Rathmell, P. Charbonneau, Z.-Y. Li and B. J. Wiley, Nanoscale, 2012, 4, 1996–2004 RSC.
- Y. Sun, Y. Yin, B. T. Mayers, T. Herricks and Y. Xia, Chem. Mater., 2002, 14, 4736–4745 CrossRef CAS.
- T. Cheng, Y.-Z. Zhang, W.-Y. Lai, Y. Chen, W.-J. Zeng and W. Huang, J. Mater. Chem. C, 2014, 2, 10369–10376 RSC.
- J. Lee, P. Lee, H. Lee, D. Lee, S. S. Lee and S. H. Ko, Nanoscale, 2012, 4, 6408–6414 RSC.
- J. H. Lee, P. Lee, D. Lee, S. S. Lee and S. H. Ko, Cryst. Growth Des., 2012, 12, 5598–5605 CrossRef.
- J.-Y. Lee, S. T. Connor, Y. Cui and P. Peumans, Nano Lett., 2008, 8, 689–692 CrossRef CAS PubMed.
- D.-S. Leem, A. Edwards, M. Faist, J. Nelson, D. D. C. Bradley and J. C. de Mello, Adv. Mater., 2011, 23, 4371–4375 CrossRef CAS PubMed.
- C. Sachse, L. Müller-Meskamp, L. Bormann, Y. H. Kim, F. Lehnert, A. Philipp, B. Beyer and K. Leo, Org. Electron., 2013, 14, 143–148 CrossRef CAS.
- S.-B. Kang, Y.-J. Noh, S.-I. Na and H.-K. Kim, Sol. Energy Mater. Sol. Cells, 2014, 122, 152–157 CrossRef CAS.
- J. Krantz, M. Richter, S. Spallek, E. Spiecker and C. J. Brabec, Adv. Funct. Mater., 2011, 21, 4784–4787 CrossRef CAS.
- S.-E. Park, S. Kim, D.-Y. Lee, E. Kim and J. Hwang, J. Mater. Chem. A, 2013, 1, 14286 RSC.
- M. Lagrange, D. P. Langley, G. Giusti, C. Jiménez, Y. Bréchet and D. Bellet, Nanoscale, 2015, 7, 17410–17423 RSC.
- E. C. Garnett, W. Cai, J. J. Cha, F. Mahmood, S. T. Connor, M. G. Christoforo, Y. Cui, M. D. McGehee and M. L. Brongersma, Nat. Mater., 2012, 11, 241 CrossRef CAS PubMed.
- S. J. Lee, Y.-H. Kim, J. K. Kim, H. Baik, J. H. Park, J. Lee, J. Nam, J. H. Park, T.-W. Lee, G.-R. Yi and J. H. Cho, Nanoscale, 2014, 6, 11828–11834 RSC.
- S.-P. Chen and Y.-C. Liao, Phys. Chem. Chem. Phys., 2014, 16, 19856–19860 RSC.
- J. Lee, P. Lee, H. B. Lee, S. Hong, I. Lee, J. Yeo, S. S. Lee, T.-S. Kim, D. Lee and S. H. Ko, Adv. Funct. Mater., 2013, 23, 4171–4176 CrossRef CAS.
- W. Gaynor, G. F. Burkhard, M. D. McGehee and P. Peumans, Adv. Mater., 2011, 23, 2905–2910 CrossRef CAS PubMed.
- A. Kim, Y. Won, K. Woo, C.-H. Kim and J. Moon, ACS Nano, 2013, 7, 1081–1091 CrossRef CAS PubMed.
- A. Kim, Y. Won, K. Woo, S. Jeong and J. Moon, Adv. Funct. Mater., 2014, 24, 2462–2471 CrossRef CAS.
- A. Kim, H. Lee, H.-C. Kwon, H. S. Jung, N.-G. Park, S. Jeong and J. Moon, Nanoscale, 2016, 8, 6308–6316 RSC.
- S. Kang, J. Jeong, S. Cho, Y. J. Yoon, S. Park, S. Lim, J. Y. Kim and H. Ko, J. Mater. Chem. A, 2019, 7, 1107–1114 RSC.
- X. Dai, Y. Zhang, H. Shen, Q. Luo, X. Zhao, J. Li and H. Lin, ACS Appl. Mater. Interfaces, 2016, 8, 4523–4531 CrossRef CAS PubMed.
- W. Gaynor, J.-Y. Lee and P. Peumans, ACS Nano, 2010, 4, 30–34 CrossRef CAS PubMed.
- J.-Y. Lee, S. T. Connor, Y. Cui and P. Peumans, Nano Lett., 2010, 10, 1276–1279 CrossRef CAS PubMed.
- M. Song, D. S. You, K. Lim, S. Park, S. Jung, C. S. Kim, D.-H. Kim, D.-G. Kim, J.-K. Kim, J. Park, Y.-C. Kang, J. Heo, S.-H. Jin, J. H. Park and J.-W. Kang, Adv. Funct. Mater., 2013, 23, 4177–4184 CrossRef CAS.
- E. Lee, J. Ahn, H.-C. Kwon, S. Ma, K. Kim, S. Yun and J. Moon, Adv. Energy Mater., 2018, 8, 1702182 CrossRef.
- Z. M. Beiley, M. G. Christoforo, P. Gratia, A. R. Bowring, P. Eberspacher, G. Y. Margulis, C. Cabanetos, P. M. Beaujuge, A. Salleo and M. D. McGehee, Adv. Mater., 2013, 25, 7020–7026 CrossRef CAS PubMed.
- G. Y. Margulis, M. G. Christoforo, D. Lam, Z. M. Beiley, A. R. Bowring, C. D. Bailie, A. Salleo and M. D. McGehee, Adv. Energy Mater., 2013, 3, 1657–1663 CrossRef CAS.
- Y. Fang, Z. Wu, J. Li, F. Jiang, K. Zhang, Y. Zhang, Y. Zhou, J. Zhou and B. Hu, Adv. Funct. Mater., 2018, 28, 1705409 CrossRef.
- F. Guo, P. Kubis, T. Stubhan, N. Li, D. Baran, T. Przybilla, E. Spiecker, K. Forberich and C. J. Brabec, ACS Appl. Mater. Interfaces, 2014, 6, 18251–18257 CrossRef CAS PubMed.
- C.-C. Chen, L. Dou, R. Zhu, C.-H. Chung, T.-B. Song, Y. B. Zheng, S. Hawks, G. Li, P. S. Weiss and Y. Yang, ACS Nano, 2012, 6, 7185–7190 CrossRef CAS PubMed.
- M. Kaltenbrunner, G. Adam, E. D. Głowacki, M. Drack, R. Schwödiauer, L. Leonat, D. H. Apaydin, H. Groiss, M. C. Scharber, M. S. White, N. S. Sariciftci and S. Bauer, Nat. Mater., 2015, 14, 1032 CrossRef CAS PubMed.
- S. Arumugam, Y. Li, S. Senthilarasu, R. Torah, A. L. Kanibolotsky, A. R. Inigo, P. J. Skabara and S. P. Beeby, J. Mater. Chem. A, 2016, 4, 5561–5568 RSC.
- R. Li, X. Xiang, X. Tong, J. Zou and Q. Li, Adv. Mater., 2015, 27, 3831–3835 CrossRef CAS PubMed.
- J. H. Park, G.-T. Hwang, S. Kim, J. Seo, H.-J. Park, K. Yu, T.-S. Kim and K. J. Lee, Adv. Mater., 2017, 29, 1603473 CrossRef PubMed.
- X. Chen, K. Parida, J. Wang, J. Xiong, M.-F. Lin, J. Shao and P. S. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 42200–42209 CrossRef CAS PubMed.
- H. Kang, H. Kim, S. Kim, H. J. Shin, S. Cheon, J.-H. Huh, D. Y. Lee, S. Lee, S.-W. Kim and J. H. Cho, Adv. Funct. Mater., 2016, 26, 7717–7724 CrossRef CAS.
- Y.-C. Lai, J. Deng, S. Niu, W. Peng, C. Wu, R. Liu, Z. Wen and Z. L. Wang, Adv. Mater., 2016, 28, 10024–10032 CrossRef CAS PubMed.
- S. Cheon, H. Kang, H. Kim, Y. Son, J. Y. Lee, H.-J. Shin, S.-W. Kim and J. H. Cho, Adv. Funct. Mater., 2018, 28, 1703778 CrossRef.
- H. Kang, H. T. Kim, H. J. Woo, H. Kim, D. H. Kim, S. Lee, S. Kim, Y. J. Song, S.-W. Kim and J. H. Cho, Nano Energy, 2019, 58, 227–233 CrossRef CAS.
- C. K. Jeong, J. Lee, S. Han, J. Ryu, G.-T. Hwang, D. Y. Park, J. H. Park, S. S. Lee, M. Byun, S. H. Ko and K. J. Lee, Adv. Mater., 2015, 27, 2866–2875 CrossRef CAS PubMed.
- C. Wu, T. W. Kim, F. Li and T. Guo, ACS Nano, 2016, 10, 6449–6457 CrossRef CAS PubMed.
- Y. Guo, K. Li, C. Hou, Y. Li, Q. Zhang and H. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 4676–4683 CrossRef CAS PubMed.
- K. Zhang, Z. L. Wang and Y. Yang, ACS Nano, 2016, 10, 4728–4734 CrossRef CAS PubMed.
- C. Wu, T. W. Kima, S. Sung, J. H. Park and F. Li, Nano Energy, 2018, 44, 279–287 CrossRef CAS.
- B.-Y. Lee, S.-U. Kim, S. Kang and S.-D. Lee, Nano Energy, 2018, 53, 152–159 CrossRef CAS.
- J.-G. Sun, T.-N. Yang, C.-Y. Wang and L.-J. Chen, Nano Energy, 2018, 48, 383–390 CrossRef CAS.
- J. Kwon, Y. D. Suh, J. Lee, P. Lee, S. Han, S. Hong, J. Yeo, H. Lee and S. H. Ko, J. Mater. Chem. C, 2018, 6, 7445–7461 RSC.
- X. Liu, D. Li, X. Chen, W.-Y. Lai and W. Huang, ACS Appl. Mater. Interfaces, 2018, 10, 32536–32542 CrossRef CAS PubMed.
- R. Yuksel, S. Coskun and H. E. Unalan, Electrochim. Acta, 2016, 193, 39–44 CrossRef CAS.
- R. Yuksel, S. Coskun, Y. E. Kalay and H. E. Unalan, J. Power Sources, 2016, 328, 167–173 CrossRef CAS.
- H. Du, Y. Pan, X. Zhang, F. Cao, T. Wan, H. Du, R. Joshi and D. Chu, Nanoscale Adv., 2019, 1, 140–146 RSC.
- H. Sheng, X. Zhang, Y. Ma, P. Wang, J. Zhou, Q. Su, W. Lan, E. Xie and C. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 8992–9001 CrossRef CAS PubMed.
- R. T. Ginting, M. M. Ovhal and J.-W. Kang, Nano Energy, 2018, 53, 650–657 CrossRef CAS.
- H. Lee, S. Hong, J. Lee, Y. D. Suh, J. Kwon, H. Moon, H. Kim, J. Yeo and S. H. Ko, ACS Appl. Mater. Interfaces, 2016, 8, 15449–15458 CrossRef CAS PubMed.
- H. Moon, H. Lee, J. Kwon, Y. D. Suh, D. K. Kim, I. Ha, J. Yeo, S. Hong and S. H. Ko, Sci. Rep., 2017, 7, 41981 CrossRef CAS PubMed.
- R. Yuksel, S. Coskun, G. Gunbas, A. Cirpan, L. Toppare and H. E. Unalan, J. Electrochem. Soc., 2017, 164, A721–A727 CrossRef CAS.
- R. Yuksel, A. Ekber, J. Turan, E. Alpugan, S. O. Hacioglu, L. Toppare, A. Cirpan, G. Gunbas and H. E. Unalan, Electroanalysis, 2018, 30, 266–273 CrossRef CAS.
- L. Shen, L. Du, S. Tan, Z. Zang, C. Zhao and W. Mai, Chem. Commun., 2016, 52, 6296–6299 RSC.
- F. Chen, P. Wan, H. Xu and X. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 17865–17871 CrossRef CAS PubMed.
- S. Park, A. W. M. Tan, J. Wang and P. S. Lee, Nanoscale Horiz., 2017, 2, 199–204 RSC.
- S. C. Sekhar, G. Nagaraju and J. S. Yu, Nano Energy, 2017, 36, 58–67 CrossRef CAS.
- Y. J. Lee, Y. Lee, D. Oh, T. Chen, G. Ceder and A. M. Belcher, Nano Lett., 2010, 10, 2433–2440 CrossRef CAS PubMed.
- N. Phattharasupakun, J. Wutthiprom, S. Duangdangchote and M. Sawangphruk, Chem. Commun., 2019, 55, 5689–5692 RSC.
- C. Yan, X. Wang, M. Cui, J. Wang, W. Kang, C. Y. Foo and P. S. Lee, Adv. Energy Mater., 2014, 4, 1301396 CrossRef.
- C. Hwang, W.-J. Song, J.-G. Han, S. Bae, G. Song, N.-S. Choi, S. Park and H.-K. Song, Adv. Mater., 2018, 30, 1705445 CrossRef PubMed.
- S. Hu, T. Han, C. Lin, W. Xiang, Y. Zhao, P. Gao, F. Du, X. Li and Y. Sun, Adv. Funct. Mater., 2017, 27, 1700041 CrossRef.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS PubMed.
- X. Wang and G. Shi, Energy Environ. Sci., 2015, 8, 790–823 RSC.
- T. Ito, K. Kimura and M. Kunimatsu, Electrochem. Commun., 2006, 8, 973–976 CrossRef CAS.
- J. Wheldon, W.-J. Lee, D.-H. Lim, A. B. Broste, M. Bollinger and W. H. Smyrl, Electrochem. Solid-State Lett., 2009, 12, B86–B89 CrossRef CAS.
- S. Tominaka, H. Nishizeko, J. Mizuno and T. Osaka, Energy Environ. Sci., 2009, 2, 1074–1077 RSC.
- C. Weinmueller, G. Tautschnig, N. Hotz and D. Poulikakos, J. Power Sources, 2010, 195, 3849–3857 CrossRef CAS.
- F.-K. Hsu, M.-S. Lee, C.-C. Lin, Y.-K. Lin and W.-T. Hsu, J. Power Sources, 2012, 219, 180–187 CrossRef CAS.
- I. Chang, T. Park, J. Lee, M. H. Lee, S. H. Ko and S. W. Cha, J. Mater. Chem. A, 2013, 1, 8541–8546 RSC.
- T. Park, I. Chang, J. Lee, S. H. Ko and S. W. Cha, ECS Trans., 2014, 64, 927–934 CrossRef CAS.
- I. Chang, M. H. Lee, J.-H. Lee, Y.-S. Kim and S. W. Cha, Int. J. Precis. Eng. Manuf., 2013, 14, 501–504 CrossRef.
- T. Park, I. Chang, J. H. Jung, H. B. Lee, S. H. Ko, R. O'Hayre, S. J. Yoo and S. W. Cha, Energy, 2017, 134, 412–419 CrossRef CAS.
- I. Chang, T. Park, J. Lee, H. B. Lee, S. Ji, M. H. Lee, S. H. Ko and S. W. Cha, Int. J. Hydrogen Energy, 2014, 39, 7422–7427 CrossRef CAS.
- I. Chang, T. Park, J. Lee, H. B. Lee, S. H. Ko and S. W. Cha, Int. J. Hydrogen Energy, 2016, 41, 6013–6019 CrossRef CAS.
- T. Park, I. Chang, H. B. Lee, S. H. Ko and S. W. Cha, Int. J. Hydrogen Energy, 2017, 42, 1884–1890 CrossRef CAS.
- S. M. Alia, K. Duong, T. Liu, K. Jensen and Y. Yan, ChemSusChem, 2012, 5, 1619–1624 CrossRef CAS PubMed.
- A. J. Lemke, A. W. O'Toole, R. S. Phillips and E. T. Eisenbraun, J. Power Sources, 2014, 256, 319–323 CrossRef CAS.
- M. Kostowskyj, R. Gilliam, D. Kirk and S. Thorpe, Int. J. Hydrogen Energy, 2008, 33, 5773–5778 CrossRef CAS.
- L. Zeng, T. Zhao and L. An, J. Mater. Chem. A, 2015, 3, 1410–1416 RSC.
- J. Li, Y. Tao, S. Chen, H. Li, P. Chen, M.-z. Wei, H. Wang, K. Li, M. Mazzeo and Y. Duan, Sci. Rep., 2017, 7, 16468 CrossRef PubMed.
- L. Lian, D. Dong, S. Yang, B. Wei and G. He, ACS Appl. Mater. Interfaces, 2017, 9, 11811–11818 CrossRef CAS PubMed.
- D. J. Lee, Y. Oh, J.-M. Hong, Y. W. Park and B.-K. Ju, Sci. Rep., 2018, 8, 14170 CrossRef PubMed.
- C.-H. Song, K.-H. Ok, C.-J. Lee, Y. Kim, M.-G. Kwak, C. J. Han, N. Kim, B.-K. Ju and J.-W. Kim, Org. Electron., 2015, 17, 208–215 CrossRef CAS.
- H. Sim, C. Kim, S. Bok, M. K. Kim, H. Oh, G.-H. Lim, S. M. Cho and B. Lim, Nanoscale, 2018, 10, 12087–12092 RSC.
- J. Lee, K. An, P. Won, Y. Ka, H. Hwang, H. Moon, Y. Kwon, S. Hong, C. Kim, C. Lee and S. H. Ko, Nanoscale, 2017, 9, 1978–1985 RSC.
- H. J. Yun, S. J. Kim, J. H. Hwang, Y. S. Shim, S.-G. Jung, Y. W. Park and B.-K. Ju, Sci. Rep., 2016, 6, 34150 CrossRef CAS PubMed.
- S. Yao, A. Myers, A. Malhotra, F. Lin, A. Bozkurt, J. F. Muth and Y. Zhu, Adv. Healthcare Mater., 2017, 6, 1601159 CrossRef PubMed.
- D.-Y. Youn, U. Jung, M. Naqi, S.-J. Choi, M.-G. Lee, S. Lee, H.-J. Park, I.-D. Kim and S. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 44678–44685 CrossRef CAS PubMed.
- S. Kang, S. Cho, R. Shanker, H. Lee, J. Park, D.-S. Um, Y. Lee and H. Ko, Sci. Adv., 2018, 4, eaas8772 CrossRef PubMed.
- X. Shuai, P. Zhu, W. Zeng, Y. Hu, X. Liang, Y. Zhang, R. Sun and C.-p. Wong, ACS Appl. Mater. Interfaces, 2017, 9, 26314–26324 CrossRef CAS PubMed.
- Y. Ko, D. Kim, G. Kwon and J. You, Micromachines, 2018, 9, 438 CrossRef PubMed.
- B. W. An, S. Heo, S. Ji, F. Bien and J.-U. Park, Nat. Commun., 2018, 9, 2458 CrossRef PubMed.
- S. Li, S. Chen, B. Zhuo, Q. Li, W. Liu and X. Guo, IEEE Electron Device Lett., 2017, 38, 975–978 CAS.
- C. Lu, S. Park, T. J. Richner, A. Derry, I. Brown, C. Hou, S. Rao, J. Kang, C. T. Moritz and Y. Fink, Sci. Adv., 2017, 3, e1600955 CrossRef PubMed.
- L. Wang, S. Chen, W. Li, K. Wang, Z. Lou and G. Shen, Adv. Mater., 2019, 31, 1804583 CrossRef PubMed.
- Z. Lou, L. Wang, K. Jiang and G. Shen, Nano Today, 2019, 26, 176–198 CrossRef CAS.
- Z. Lou, L. Li, L. Wang and G. Shen, Small, 2017, 13, 1701791 CrossRef PubMed.
- T. Kim, Y. W. Kim, H. S. Lee, H. Kim, W. S. Yang and K. S. Suh, Adv. Funct. Mater., 2013, 23, 1250–1255 CrossRef CAS.
- S. Hong, H. Lee, J. Lee, J. Kwon, S. Han, Y. D. Suh, H. Cho, J. Shin, J. Yeo and S. H. Ko, Adv. Mater., 2015, 27, 4744–4751 CrossRef CAS PubMed.
- Z. Cui, Y. Han, Q. Huang, J. Dong and Y. Zhu, Nanoscale, 2018, 10, 6806–6811 RSC.
- J.-G. Lee, J.-H. Lee, S. An, D.-Y. Kim, T.-G. Kim, S. S. Al-Deyab, A. L. Yarin and S. S. Yoon, J. Mater. Chem. A, 2017, 5, 6677–6685 RSC.
- M. Song, J. H. Park, C. S. Kim, D.-H. Kim, Y.-C. Kang, S.-H. Jin, W.-Y. Jin and J.-W. Kang, Nano Res., 2014, 7, 1370–1379 CrossRef CAS.
- M. Amjadi, A. Pichitpajongkit, S. Lee, S. Ryu and I. Park, ACS Nano, 2014, 8, 5154–5163 CrossRef CAS PubMed.
- H. Zhai, Y. Li, L. Chen, X. Wang, L. Shi, R. Wang and J. Sun, Nano Res., 2018, 11, 1895–1904 CrossRef CAS.
- Z. Yin, S. Cho, D.-J. You, Y.-k. Ahn, J. Yoo and Y. S. Kim, Nano Res., 2018, 11, 769–779 CrossRef CAS.
- X. Xu, R. Wang, P. Nie, Y. Cheng, X. Lu, L. Shi and J. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 14273–14280 CrossRef CAS PubMed.
- H. Zhai, R. Wang, X. Wang, Y. Cheng, L. Shi and J. Sun, Nano Res., 2016, 9, 3924–3936 CrossRef CAS.
- Z. Liu, J. Li and F. Yan, Adv. Mater., 2013, 25, 4296–4301 CrossRef CAS PubMed.
- H. Chen, Y. Xu, J. Zhang, W. Wu and G. Song, Nano Energy, 2019, 58, 304–311 CrossRef CAS.
- M. F. El-Kady, V. Strong, S. Dubin and R. B. Kaner, Science, 2012, 335, 1326–1330 CrossRef CAS PubMed.
- J.-G. Wang, D. Jin, R. Zhou, X. Li, X.-r. Liu, C. Shen, K. Xie, B. Li, F. Kang and B. Wei, ACS Nano, 2016, 10, 6227–6234 CrossRef CAS PubMed.
- F. Shen, D. Pankratov, A. Halder, X. Xiao, M. D. Toscano, J. Zhang, J. Ulstrup, L. Gorton and Q. Chi, Nanoscale Adv., 2019, 1, 2562–2570 RSC.
- T.-H. Han, Y. Lee, M.-R. Choi, S.-H. Woo, S.-H. Bae, B. H. Hong, J.-H. Ahn and T.-W. Lee, Nat. Photonics, 2012, 6, 105 CrossRef CAS.
- S.-H. Bae, Y. Lee, B. K. Sharma, H.-J. Lee, J.-H. Kim and J.-H. Ahn, Carbon, 2013, 51, 236–242 CrossRef CAS.
- J. Kang, H. Kim, K. S. Kim, S.-K. Lee, S. Bae, J.-H. Ahn, Y.-J. Kim, J.-B. Choi and B. H. Hong, Nano Lett., 2011, 11, 5154–5158 CrossRef CAS PubMed.
- I. Jeon, K. Cui, T. Chiba, A. Anisimov, A. G. Nasibulin, E. I. Kauppinen, S. Maruyama and Y. Matsuo, J. Am. Chem. Soc., 2015, 137, 7982–7985 CrossRef CAS PubMed.
- S. Park, H. Kim, M. Vosgueritchian, S. Cheon, H. Kim, J. H. Koo, T. R. Kim, S. Lee, G. Schwartz, H. Chang and Z. Bao, Adv. Mater., 2014, 26, 7324–7332 CrossRef CAS PubMed.
- A. W. Lang, J. F. Ponder, A. M. Österholm, N. J. Kennard, R. H. Bulloch and J. R. Reynolds, J. Mater. Chem. A, 2017, 5, 23887–23897 RSC.
- Z. Chen, J. W. To, C. Wang, Z. Lu, N. Liu, A. Chortos, L. Pan, F. Wei, Y. Cui and Z. Bao, Adv. Energy Mater., 2014, 4, 1400207 CrossRef.
- F. Ning, X. He, Y. Shen, H. Jin, Q. Li, D. Li, S. Li, Y. Zhan, Y. Du and J. Jiang, ACS Nano, 2017, 11, 5982–5991 CrossRef CAS PubMed.
- L. Hu, J. Li, J. Liu, G. Grüner and T. Marks, Nanotechnology, 2010, 21, 155202 CrossRef PubMed.
- T. Yamada, Y. Hayamizu, Y. Yamamoto, Y. Yomogida, A. Izadi-Najafabadi, D. N. Futaba and K. Hata, Nat. Nanotechnol., 2011, 6, 296 CrossRef CAS PubMed.
- Y. Kim, H. R. Lee, T. Saito and Y. Nishi, Appl. Phys. Lett., 2017, 110, 153301 CrossRef.
- C.-K. Cho, W.-J. Hwang, K. Eun, S.-H. Choa, S.-I. Na and H.-K. Kim, Sol. Energy Mater. Sol. Cells, 2011, 95, 3269–3275 CrossRef CAS.
- Z. Wen, Y. Yang, N. Sun, G. Li, Y. Liu, C. Chen, J. Shi, L. Xie, H. Jiang, D. Bao, Q. Zhuo and X. Sun, Adv. Funct. Mater., 2018, 28, 1803684 CrossRef.
- D. Ni, Y. Chen, H. Song, C. Liu, X. Yang and K. Cai, J. Mater. Chem. A, 2019, 7, 1323–1333 RSC.
- N. Liu, G. Fang, J. Wan, H. Zhou, H. Long and X. Zhao, J. Mater. Chem., 2011, 21, 18962–18966 RSC.
Footnote |
| † These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2019 |