Open Access Article
Open Access ArticleLiMg(IO3)3: an excellent SHG material designed by single-site aliovalent substitution†
Jin
Chen
 ab,
Chun-Li
Hu
*a,
Fei-Fei
Mao
ab,
Chun-Li
Hu
*a,
Fei-Fei
Mao
 c,
Xiao-Han
Zhang
a,
Bing-Ping
Yang
a and
Jiang-Gao
Mao
c,
Xiao-Han
Zhang
a,
Bing-Ping
Yang
a and
Jiang-Gao
Mao
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China. E-mail: mjg@fjirsm.ac.cn; clhu@fjirsm.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100039, P. R. China
cNanjing Agricultural University, Nanjing 210095, P. R. China
First published on 15th October 2019
Abstract
An excellent second harmonic generation (SHG) material, LiMg(IO3)3 (LMIO), has been elaborately designed from Li2MIV(IO3)6 (MIV = Ti, Sn, and Ge) by aliovalent substitution of the central MIV cation followed by Wyckoff position exchange. The new structure sustains the ideal-alignment of (IO3)− groups. Importantly, LMIO exhibits an extremely strong SHG effect of roughly 24 × KH2PO4 (KDP) under 1064 nm laser radiation or 1.5 × AgGaS2 (AGS) under 2.05 μm laser radiation, which is larger than that of α-LiIO3 (18 × KDP). The replacement of MIV with Mg2+ without d–d electronic transitions induces an obviously larger band gap (4.34 eV) with a short absorption edge (285 nm). This study shows that single-site aliovalent substitution provides a new synthetic route for designing SHG materials.
Introduction
Noncentrosymmetric (NCS) or polar crystals have attracted scientific endeavors since they are potential candidates as SHG materials.1,2 Metal iodates with polar units, (IO3)− or (IO4)3−, are a class of important compounds which can exhibit excellent SHG performance.3 Numerous synthetic efforts have been made and afforded a number of iodates with outstanding SHG properties,3–8 such as BiO(IO3) (12.5 × KDP)7a and GdI5O14 (15 × KDP).8d However, generally, the larger SHG effects of iodates are usually accompanied by smaller band gaps. Recently, aliovalent substitution has become an effective approach for exploring novel SHG crystals.9,10 Aliovalent substitution of BiO(IO3) gave BiFSeO3, which has the highest SHG effect among selenites reported.9a The novel route of aliovalent substitution involving three atom sites has expended d0-TM cations to post main group cations, such as from V5+ to Ga3+.10 In this way, isostructural compounds sustained both large polarizability and a wide band gap.10 For example, α- and β-Ba2[GaF4(IO3)2(IO3)] (6 × KDP; 4.61 and 4.31 eV)10a have clearly larger band gaps and slightly weaker SHG signals than α- and β-Ba2[VO2F4(IO3)2(IO3)] (9 × KDP; 2.59 and 2.55 eV).9dRecently, a serial of isostructural SHG iodates, namely Li2MIV(IO3)6 (MIV = Ti, Sn, and Ge), were reported, whose structures feature well-arranged (IO3)− units. The regulation of Ti4+, Sn4+ and Ge4+ cations induces SHG effects shifting from 17×, 15×, to 32 × KDP and band gaps changing from 3.0, 3.9 to 3.89 eV for Ti, Sn and Ge compounds, respectively.11 Interestingly, in these structures, both Li+ and M4+ cations are octahedrally coordinated but they occupy different Wyckoff positions. The Li+ cation site on 2b and the LiO6 octahedra are bridged by iodate groups into a 3D [Li(IO3)6]5− framework. M4+ cations located at 2a are only 50% occupied and 0D [MIV(IO3)6]2− polyanions were formed. The full occupation of MIV would be not consistent with the colorless and insulating crystals, which had been discussed in detail by P. Shiv Halasyamani.11b We consider that aliovalent substitution of the M4+ cation with two divalent metal ions may result in LiMII(IO3)3 with a completely ordered structure, and this expands the family of this class of metal iodates with excellent SHG effects. Additionally, the use of alkaline-earth cations can avoid the unfavorable d–d electronic transitions for the transition metal ions and favors the blue-shift of the absorption edge. By using such a single-site aliovalent substitution method, LiMg(IO3)3 (LMIO) was designed from Li2MIV(IO3)6via replacing the defect-containing M4+ cation with an ordered Mg2+ cation followed by Wyckoff position exchange. In LMIO, a 3D [Mg(IO3)6]4− anionic framework is formed instead of 0D [MIV(IO3)6]2− anions. LMIO displays an extremely large SHG response (24 × KDP under 1064 nm laser radiation or 1.5 × AGS under 2.05 μm laser radiation), large band gap (4.34 eV), high LDT (46 × AGS) and good thermal stability (>500 °C).
Results and discussion
Polar LMIO was prepared by hydrothermal reactions of LiCl, MgCl2, I2O5 and 3% HCl solution at 230 °C for 3 days. Its purity was checked by PXRD study and EDS elemental analyses (Fig. S1 and S2†).LMIO crystallizes in the NCS and polar space group P63 (no. 173). The asymmetric unit of LMIO contains one Li, one Mg, one I, and three O atoms. Both Li and Mg atoms occupy sites with 3-fold axis symmetry whereas the remaining atoms are located at the normal sites. Both Li+ and Mg2+ cations connect with six O atoms to form LiO6 and MgO6 octahedra, and each octahedron links with six (IO3)− groups which are ideally aligned along the c direction, leading to very large polarizability. The lengths of the Li–O bond (2.075(6) and 2.088(5) Å) and Mg–O bond (2.076(2) and 2.111(2) Å) are pretty close indicating that the distortions of LiO6 and MgO6 are very small. The I5+ atom is three coordinated in a triangular-pyramidal geometry with I–O bond lengths of 1.7966(19), 1.809(2) and 1.815(2) Å. BVS calculations exhibit the values of 1.14 (Li), 2.03 (Mg) and 5.06 (I), verifying that these cations are in oxidation states of +1, +2 and +5, respectively.
Neighboring LiO6 octahedra are inter-linked into a 1D chain along the c-axis via face-sharing (Fig. 1a) whereas neighboring MgO6 octahedra are bridged by iodate groups into a 3D [Mg(IO3)6]4− anionic framework with 1D tunnels of Mg6I6 12-membered rings (MRs) (Fig. 1b). The overall structure of LMIO can be viewed as the 1D chains of face-sharing LiO6 octahedra inserted into the 1D tunnels of the 3D [Mg(IO3)6]4− anionic framework (Fig. 1c). Because that a process of Wyckoff position exchange occurred as the structural evolution from Li2MIV(IO3)6 to LMIO and different occupancy of the cation at the 2a position (fully occupied Li+ in LMIO but 50% occupied M4+ in Li2MIV(IO3)6), it is interesting to note that there is in the structures of Li2MIV(IO3)6 (M = Ti, Sn, Ge), neighboring LiO6 octahedra or [MIV(IO3)6]2− anions are not interconnected, that is they are isolated.11
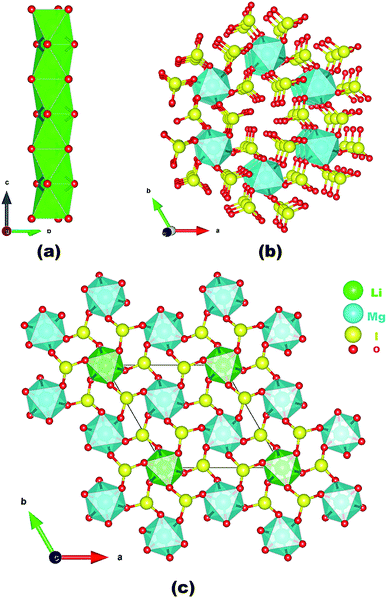 | ||
| Fig. 1 A 1D chain of face-sharing LiO6 octahedra along the c-axis (a); the 3D [Mg(IO3)6]4− anionic framework along the c-axis (b); and the view of the structure of LMIO along the c-axis (c). | ||
The ionic radii of the six-coordinated Li+, Mg2+, Ti4+, Sn4+, and Ge4+ are 0.076, 0.072, 0.0605, 0.069, and 0.053 nm, respectively.12 Obviously, the ionic radius of Mg2+ is much closer to that of Li+ than that of M4+. Thus, Wyckoff position exchange occurred during the aliovalent substitution of M4+ with Mg2+ cations. In addition, our calculations of total energy show that LMIO (−12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816.63 eV) is more energy-favorable than the one adopting the Li2MIV(IO3)6 (M = Ti, Sn, Ge) structural type (−12814.03 eV). Attempts to replace Li+ in LMIO with Na+ to get an isostructural sodium phase were not successful, probably owing to the much larger size of Na+ (0.102 nm). We consider that a disordered M3+ cation, such as Al3+ and Ga3+ with 67% occupancy, may replace the M4+ cation in Li2MIV(IO3)6 (M = Ti, Sn, Ge).
816.63 eV) is more energy-favorable than the one adopting the Li2MIV(IO3)6 (M = Ti, Sn, Ge) structural type (−12814.03 eV). Attempts to replace Li+ in LMIO with Na+ to get an isostructural sodium phase were not successful, probably owing to the much larger size of Na+ (0.102 nm). We consider that a disordered M3+ cation, such as Al3+ and Ga3+ with 67% occupancy, may replace the M4+ cation in Li2MIV(IO3)6 (M = Ti, Sn, Ge).
The calculations of bond strain index (BSI) and global instability index (GII) values gave small values of 0.030 (BSI) and 0.079 (GII) for LMIO, which indicated that the structure of LMIO is unstrained and stable.11b,13 The corresponding BSI and GII values of Li2MIV(IO3)6 (M = Ti, Sn, Ge) are much larger than those of LMIO (Table S3†), which are consistent with the disorder in Li2MIV(IO3)6 (M = Ti, Sn, Ge).
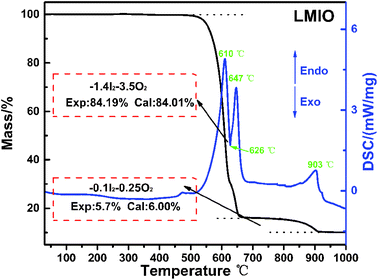
Thermogravimetric analysis (TGA) reveals the high thermal stability (>500 °C) of LMIO (Fig. 2). It shows two steps of weight loss in 500–650 °C (sharp) and 750–910 °C (smooth), and both are related to the release of I2 and O2. The DSC curves exhibit three endothermic peaks at 610, 647 and 903 °C and one exothermic peak at 626 °C. Compared to Li2MIV(IO3)6 (M = Ti, Sn, Ge) (∼400 °C), LMIO has higher thermal stability.11
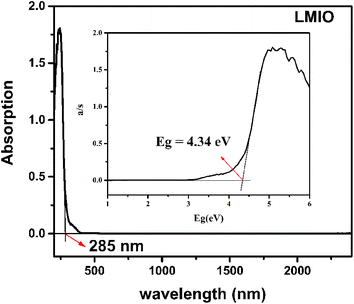
The IR spectrum shows that LMIO is transparent in the wavelength region of 2.5–11.2 μm (4000–890 cm−1) (Fig. S3†). The UV-Vis-IR study revealed the absorption edge of 285 nm for LMIO (Fig. 3). Hence, the transparent range (0.28–11.2 μm) of LMIO covers the UV, Visible and mid-IR regions. The band gap of LMIO (4.34 eV) is significantly wider than those of Li2MIV(IO3)6 (M = Ti, Sn, Ge) (3.0, 3.9 and 3.86 eV for Ti, Sn and Ge compounds, respectively).11
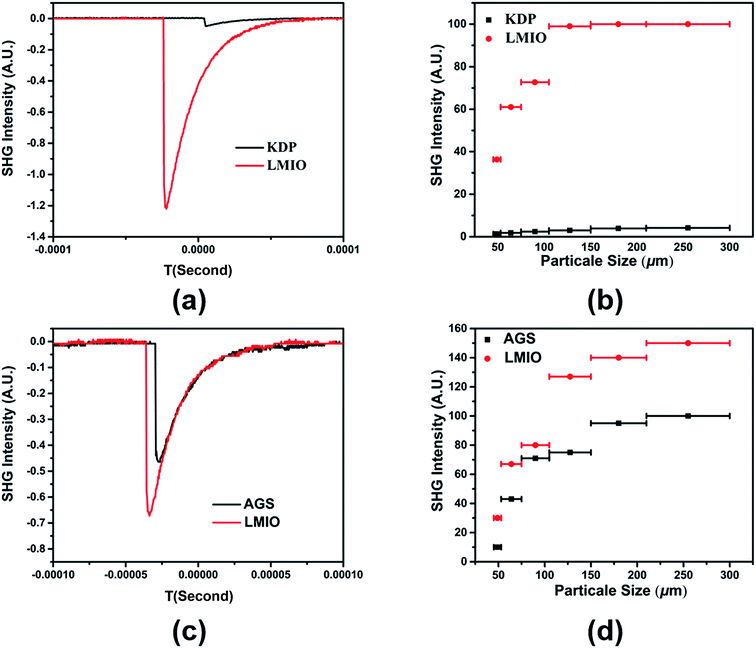
Powder SHG experiments indicate that LMIO is a phase-matchable SHG material with remarkable SHG signals of 24 × KDP under 1064 nm laser radiation or 1.5 × AGS under 2.05 μm laser radiation (Fig. 4). Compared with commercial α-LiIO3 (18 × KDP), LMIO possesses an obviously enhanced SHG signal. The SHG response of LMIO is also much larger than those of Li2Ti(IO3)6 (17 × KDP) and Li2Sn(IO3)6 (15 × KDP) but is slightly smaller than that of Li2Ge(IO3)6 (32 × KDP). Eliminating the influence induced by the disorder effect, we adopt the Gaussian09 program to calculate the first-order hyperpolarizabilities of [Mg(IO3)6]4− and [MIV(IO3)6]2− anions (M = Ti, Sn and Ge), which are obtained to be 3047, 2982, 2602 and 1707 a.u. for Ge, Mg, Sn, and Ti, respectively, and such a trend is roughly consistent with results from the SHG measurements (Li2Ge(IO3)6 > LMIO > Li2Sn(IO3)6 ≈ Li2Ti(IO3)6). Notably, LMIO is the only NLO material featuring excellent optical nonlinearity (>20 × KDP) and a near-UV absorption edge (<300 nm).
LDT experiments revealed a large value of 101.86 MW cm−2 for LMIO, which is about 46 times that of AGS (2.22 MW cm−2). This value is comparable to those of Li2MIV(IO3)6 (M = Ti, Sn, Ge) (49×, 41×, and 42 × AGS for Ti, Sn and Ge compounds, respectively) and α-LiIO3 (54 × AGS).11d
Theoretical calculations based on DFT methods were applied for understanding the source of the outstanding SHG effect. Band structure calculations revealed that LMIO is an indirect band gap compound with a gap of 3.35 eV (Fig. S4†), which is much smaller than the measured value (4.34 eV). Hence scissor of 0.99 eV was applied during the subsequent optical property analyses.
The partial density of states (PDOS) was studied for the energy band assignment and bond interaction (Fig. 5). In the whole energy region, full overlapping of the electronic states between I atoms and O atoms is observed, indicating strong interactions in I–O bonds. The band gap of LMIO is determined by IO3 units because the nonbonding orbitals from I and O atoms dominate the highest VB; meanwhile the empty orbitals from I and O atoms define the lowest CB.
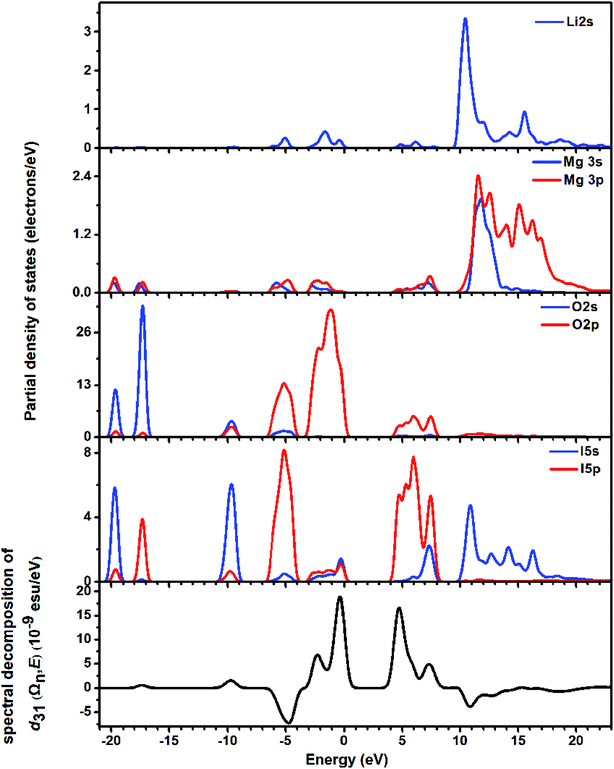 | ||
| Fig. 5 The partial density of states (the upper four panels) and the spectral decomposition of d31 (the bottommost panel) for LMIO. | ||
Calculations of the SHG coefficient of LMIO revealed the largest SHG tensor d31 of 2.02 × 10−8 esu at 1064 nm. This result is consistent with our experimental value (24 × KDP). In addition, the refractive index curves of LMIO were recorded. It shows large anisotropy with large birefringence at 1064 nm (0.22), ensuring phase-matching abilities (Fig. S5†).
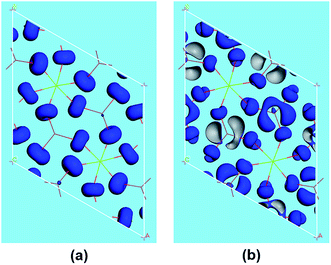
The SHG origin of LMIO has been further explored from spectral decomposition and SHG density. The spectral decomposition plot of d31 (the bottom panel in Fig. 5) indicates that the main SHG-contributed regions are located at the upper part of the VB and the lower part of the CB, corresponding to O-2p nonbonding states and unoccupied I-5p and O-2p states, respectively. There are small negative contributions from the VB regions of −6.3 to −3.6 eV and the CB region of 10–15 eV. Intuitively, for the SHG density (Fig. 6), the major SHG-contributors are the O-2p nonbonding orbitals in the VB and the unoccupied O-2p and I-5p orbitals in the CB. Accordingly, the IO3 building units are the leading source of the large SHG coefficient. Numerically, the SHG-contributed percentages of Li+, MgO6 and IO3 groups are 0.43%, 8.94% and 90.6%, respectively. Hence, it is concluded that the very strong SHG response of LMIO originates predominantly from the ideal-alignment of (IO3)− groups.
Conclusions
In conclusion, via the aliovalent substitution of disordered MIV (50% occupancy) with fully ordered Mg2+ and the exchange of Wyckoff positions, a promising SHG material, LiMg(IO3)3 (LMIO), has been obtained. It displays a strong SHG effect (24 × KDP) with a phase matching ability and large band gap (4.34 eV). LMIO also has superior thermal stability (>500 °C) and LDT properties (46 × AGS). This novel approach of replacement of MIV by MII atoms further expands the family of iodate-based SHG materials. Our future research efforts will be devoted to aliovalent substitution of MIV with defect containing MIII (66.7% occupancy) cations.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Our work has been supported by the National Natural Science Foundation of China (No. 21231006, 91622112, 21875248 and 21773244), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000), and the 100 Talents Project of Fujian Province. We thank Bingxuan Li at FJIRSM for their help with the laser-induced damage threshold (LDT) tests.Notes and references
-
(a) S. P. Guo, X. Cheng, Z. D. Sun, Y. Chi, B. W. Liu, X. M. Jiang, S. F. Li, H. G. Xue, S. Deng, V. Duppel, J. Khler and G. C. Guo, Angew. Chem., Int. Ed., 2019, 58, 8087–8091 (
Angew. Chem.
, 2019
, 131
, 8171–8175
) CrossRef CAS PubMed
; (b) H. M. Zhou, L. Xiong, L. Chen and L. M. Wu, Angew. Chem., Int. Ed., 2019, 58, 9979–9983 ( Angew. Chem. , 2019 , 131 , 10084–10088 ) CrossRef CAS PubMed
; (c) B. Zhang, E. Tikhonov, C. Xie, Z. Yang and S. Pan, Angew. Chem., Int. Ed., 2019, 58, 11726–11730 ( Angew. Chem. , 2019 , 131 , 11852–11856 ) CrossRef CAS PubMed
; (d) L. Kang, X. Zhang, F. Liang, Z. Lin and B. Huang, Angew. Chem., Int. Ed., 2019, 58, 10250–10254 ( Angew. Chem. , 2019 , 131 , 10356–10360 ) CrossRef CAS PubMed
; (e) B. L. Wu, C. L. Hu, F. F. Mao, R. L. Tang and J. G. Mao, J. Am. Chem. Soc., 2019, 141, 10188–10192 CrossRef CAS PubMed
; (f) J. Mark, J. Wang, K. Wu, J. G. Lo, S. Lee and K. Kovnir, J. Am. Chem. Soc., 2019, 141, 11976–11983 CrossRef CAS PubMed
.
-
(a) K. M. Ok, Acc. Chem. Res., 2016, 49, 2774–2785 CrossRef CAS PubMed
; (b) Z. Xia and K. R. Poeppelmeier, Acc. Chem. Res., 2017, 50, 1222–1230 CrossRef CAS PubMed
; (c) M. Mutailipu, M. Zhang, Z. Yang and S. Pan, Acc. Chem. Res., 2019, 52, 791–801 CrossRef CAS PubMed
; (d) S. P. Guo, Y. Chi and G. C. Guo, Coord. Chem. Rev., 2017, 355, 44–57 CrossRef
.
-
(a) M. S. Wickleder, Chem. Rev., 2002, 102, 2011–2088 CrossRef CAS PubMed
; (b) C. L. Hu and J. G. Mao, Coord. Chem. Rev., 2015, 288, 1–17 CrossRef CAS
; (c) C. F. Sun, B. P. Yang and J. G. Mao, Sci. China: Chem., 2011, 54, 911–922 CrossRef CAS
.
-
(a) R. E. Sykora, K. M. Ok, P. S. Halasyamani and T. E. Albrecht-Schmitt, J. Am. Chem. Soc., 2002, 124, 1951–1957 CrossRef CAS PubMed
; (b) Y. H. Li, G. P. Han, H. W. Yu, H. Li, Z. H. Yang and S. L. Pan, Chem. Mater., 2019, 31, 2992–3000 CrossRef CAS
.
-
(a) R. E. Sykora, K. M. Ok, P. S. Halasyamani, D. M. Wells and T. E. Albrecht-Schmitt, Chem. Mater., 2002, 14, 2741–2749 CrossRef CAS
; (b) B. P. Yang, C. L. Hu, X. Xu, C. F. Sun, J. H. Zhang and J. G. Mao, Chem. Mater., 2010, 22, 1545–1550 CrossRef CAS
; (c) C. F. Sun, C. L. Hu, X. Xu, B. P. Yang and J. G. Mao, J. Am. Chem. Soc., 2011, 133, 5561–5572 CrossRef CAS PubMed
; (d) H. W. Yu, M. L. Nisbet and K. R. Poeppelmeier, J. Am. Chem. Soc., 2018, 140, 8868–8876 CrossRef CAS PubMed
.
-
(a) C. F. Sun, C. L. Hu, X. Xu, J. B. Ling, T. Hu, F. Kong, X. F. Long and J. G. Mao, J. Am. Chem. Soc., 2009, 131, 9486–9487 CrossRef CAS PubMed
; (b) F. F. Mao, C. L. Hu, J. Chen, R. L. Tang, B. L. Wu and J. G. Mao, Chem. Commun., 2019, 55, 6906–6909 RSC
; (c) H. X. Tang, Y. X. Zhang, C. Zhou, R. B. Fu, H. Lin, Z. J. Ma and X. T. Wu, Angew. Chem., Int. Ed., 2019, 58, 3824–3828 ( Angew. Chem. , 2019 , 131 , 3864–3868 ) CrossRef CAS PubMed
.
-
(a) S. D. Nguyen, J. Yeon, S. H. Kim and P. S. Halasyamani, J. Am. Chem. Soc., 2011, 133, 12422–12425 CrossRef CAS PubMed
; (b) F. F. Mao, C. L. Hu, X. Xu, D. Yan, B. P. Yang and J. G. Mao, Angew. Chem., Int. Ed., 2017, 129, 2183–2187 ( Angew. Chem. , 2017 , 129 , 2183–2187 ) CrossRef
.
-
(a) K. M. Ok and P. S. Halasyamani, Angew. Chem., Int. Ed., 2004, 43, 5489–5491 (
Angew. Chem.
, 2004
, 116
, 5605–5607
) CrossRef CAS PubMed
; (b) X. Xu, C. L. Hu, B. X. Li, B. P. Yang and J. G. Mao, Chem. Mater., 2014, 26, 3219–3230 CrossRef CAS
; (c) F. F. Mao, C. L. Hu, J. Chen, B. L. Wu and J. G. Mao, Inorg. Chem., 2019, 58, 3982–3989 CrossRef CAS PubMed
; (d) J. Chen, C. L. Hu, F. F. Mao, B. P. Yang, X. H. Zhang and J. G. Mao, Angew. Chem., Int. Ed., 2019, 58, 11666–11669 ( Angew. Chem. , 2019 , 131 , 11792–11795 ) CrossRef CAS PubMed
.
-
(a) M. L. Liang, C. L. Hu, F. Kong and J. G. Mao, J. Am. Chem. Soc., 2016, 128, 9433–9436 CrossRef PubMed
; (b) X. L. Cao, C. L. Hu, F. Kong and J. G. Mao, Inorg. Chem., 2015, 54, 3875–3882 CrossRef CAS PubMed
; (c) X. L. Cao, C. L. Hu, X. Xu, F. Kong and J. G. Mao, Chem. Commun., 2013, 49, 9965–9967 RSC
; (d) X. H. Dong, L. Huang, C. F. Hu, H. M. Zeng, Z. Lin, X. Wang, G. H. Zou and K. M. OK, Angew. Chem., Int. Ed., 2019, 58, 6528–6534 ( Angew. Chem. , 2019 , 131 , 6598–6604 ) CrossRef CAS PubMed
; (e) F. Yang, L. Huang, X. Zhao, L. Huang, D. Gao, J. Bi, X. Wang and G. Zou, J. Mater. Chem. C, 2019, 7, 8131–8138 RSC
.
-
(a) J. Chen, C. L. Hu, F. F. Mao, J. H. Feng and J. G. Mao, Angew. Chem., Int. Ed., 2019, 58, 2098–2102 (
Angew. Chem.
, 2019
, 131
, 2120–2124
) CrossRef CAS PubMed
; (b) F. You, F. Liang, Q. Huang, Z. Hu, Y. Wu and Z. Lin, J. Am. Chem. Soc., 2019, 141, 748–752 CrossRef CAS PubMed
.
-
(a) H. Y. Chang, S. H. Kim, P. S. Halasyamani and K. M. Ok, J. Am. Chem. Soc., 2009, 131, 2426–2427 CrossRef CAS PubMed
; (b) H. Y. Chang, S. H. Kim, K. M. Ok and P. S. Halasyamani, J. Am. Chem. Soc., 2009, 131, 6865–6873 CrossRef CAS PubMed
; (c) H. Y. Kim, T. T. Tran, P. S. Halasyamani and K. M. Ok, Inorg. Chem. Front., 2015, 2, 361–368 RSC
; (d) B. P. Yang, C. L. Hu, X. Xu and J. G. Mao, Inorg. Chem., 2016, 55, 2481–2487 CrossRef CAS PubMed
; (e) F. F. Mao, C. L. Hu, J. Chen and J. G. Mao, Chem. Mater., 2018, 30, 2443–2452 CrossRef CAS
; (f) H. M. Liu, X. X. Jiang, X. X. Wang, L. Yang, Z. S. Lin, Z. G. Hu, X. G. Meng, X. G. Chen and J. G. Qin, J. Mater. Chem. C, 2018, 6, 4698–4705 RSC
.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef
.
-
(a) C. Preiser, J. Losel, I. D. Brown, M. Kunz and A. Skowron, Acta Crystallogr., Sect. B: Struct. Sci., 1999, B55, 698–711 CrossRef CAS PubMed
; (b) A. Salinas-Sanchez, J. L. Garcia-Munoz, J. Rodriguez-Carvajal, R. SaezPuche and J. L. Martinez, J. Solid State Chem., 1992, 100, 201–211 CrossRef CAS
; (c) I. D. Brown, The Chemical Bond in Inorganic Chemistry: The Bond Valence Model, Oxford University Press, Oxford, U.K., 1st edn, 2002 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1951165. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc04832d |
| This journal is © The Royal Society of Chemistry 2019 |