A recyclable metal–organic framework for ammonia vapour adsorption†
Tu N.
Nguyen
 *ab,
Ian M.
Harreschou
*ab,
Ian M.
Harreschou
 c,
Jung-Hoon
Lee
c,
Jung-Hoon
Lee
 d,
Kyriakos C.
Stylianou
d,
Kyriakos C.
Stylianou
 *c and
Douglas W.
Stephan
*c and
Douglas W.
Stephan
 *e
*e
aHelen Scientific Research and Technological Development Co., Ltd, Ho Chi Minh City, Vietnam
bInstitut des Sciences et Ingénierie Chimiques (ISIC), Ecole Polytechnique Fédérale de Lausanne (EPFL Valais), Sion, Switzerland. E-mail: ngoctu.nguyen@epfl.ch
cDepartment of Chemistry, Oregon State University, Corvallis, OR 97331, USA. E-mail: kyriakos.stylianou@oregonstate.edu
dComputational Science Research Center, Korea Institute of Science and Technology (KIST), Seoul 02792, Republic of Korea
eDepartment of Chemistry, University of Toronto, Toronto, ON M5S 3H6, Canada. E-mail: douglas.stephan@utoronto.ca
First published on 17th July 2020
Abstract
Herein, we present a new strategy to design metal–organic frameworks (MOFs) as adsorbents for ammonia (NH3) vapour. The linking ligand is functionalized with a sterically hindered Lewis acidic boron (B) centre, allowing efficient capture of NH3 and easy recycling of the MOF by simply heating at low temperature. The recycled MOF material can be used for NH3 capture for at least 5 cycles without losing its crystallinity or its luminescence properties.
Toxic gases, such as NH3, CO, or H2S, cause an immediate danger to life even at ppm concentrations.1 For example, the exposure limit of NH3 in industrial settings recommended by the US Occupational Safety and Health Administration is 25 ppm.2 Therefore, developing adsorption materials capable of capturing these gases could offer a strategy of great importance for the protection of the environment, workplace safety and public health.
Porous materials with relatively high surface areas such as zeolites and activated carbons have been the traditional adsorbents for toxic gases.3 During the last decade, metal–organic frameworks (MOFs) and covalent-organic frameworks (COFs), which often possess high porosity and pore-surface tunability, have emerged as adsorbents that are superior to zeolites and activated carbons.4 It is well known that the sole reliance on the materials’ porosity is not sufficient for effective capture of gas/vapour.5,6 The adsorption rate and capacity for gas molecules can be increased when the pore surface incorporates sites for coordination, acid–base interactions, electrostatic interactions, π–π stacking, or H-bonding. The incorporation of these sites either on the pore surface or at the nodes within the scaffold of MOFs and COFs has been widely examined. A large number of MOFs and COFs have integrated Brønsted and Lewis acidic groups, e.g. –COOH, –SO3H, –PO3H2, –OH, open metal sites, or boroxine rings7 have been reported as promising NH3 sorbent due to the strong acid–base interaction.8a–g
Strong interactions of the gas molecules with the metal nodes or organic linkers of the MOF and COF adsorbents could cause collapse of the materials’ frameworks9 or difficulties in recycling the adsorbents.10 For example, in the case of the boronic acid-based COF-10, adsorbed NH3 can only completely be removed by heating at 200 °C for 12 hours at 0.1 Torr,8a presumably a result of strong B-NH3 binding.
An ideal porous material for recyclable NH3 adsorption should provide interactions that are neither too strong nor too weak, allowing both capture and release. In molecular chemistry, such reversible interactions have been achieved for Lewis acid–base adducts via the introduction of steric demands, affording so-called “frustrated Lewis pairs”.11 Applying this strategy to MOFs for NH3 capture, bulky Lewis acidic B centers were introduced into a MOF. While this enhances the electrophilicity of the pores, the steric demanding environment about B deters strong dative binding thus facilitating subsequent release and thus a recyclable adsorbent.
Targeting recyclable NH3 binding, the highly stable MOF, SION105-Eu with the chemical formula of ([Eu(tctb)3(H2O)]) (Fig. 1, left) was selected. This MOF contains the linking ligand tctb3− with a central sterically hindered Lewis acidic B centre (Fig. 1, right) and has been shown to adsorb CO2, with an uptake capacity of ∼1.9 mmol g−1 at 195 K and 1 bar, and a BET surface area of 216 m2 g−1 (Fig. S4, ESI†), and has been utilized for fluoride ion detection in drinking water, and as a catalyst for CO2 transformation.12 Herein, we demonstrate this MOF also captures NH3 vapour but at the same time, releases it upon heating, affording a stable, recyclable MOF for NH3 adsorption.
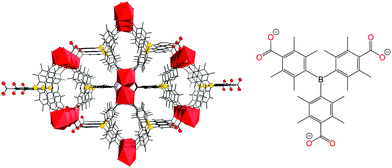 | ||
| Fig. 1 (left) Structure of SION105-Eu, with Eu ions shown as red polyhedra and H atoms omitted for clarity; (right) ligand tctb3−. | ||
SION105-Eu was synthesized based on a solvothermally synthetic procedure, by heating Eu(NO3)3·6H2O (1 equivalent) and tris(p-carboxylic acid)tridurylborane (H3tctb) (1 equivalent) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of DMF
1 mixture of DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O at 120 °C for 72 hours.12a The crystalline powder was filtered, soaked in MeOH for three days, filtered again, and then dried in oven at 120 °C. The purity of the sample was confirmed by PXRD, in which the experimental pattern matched well with the simulation based on the single-crystal structure (Fig. S1, ESI†). The MOF powder is expected to be hydrophobic due to the presence of a large number of –CH3 groups on the ligand, which was confirmed by the measurement of the contact angle (119.86°) with a water drop (Fig. S2, ESI†). The MOF floats in water (Fig. S3, ESI†), and the exposure to water left the crystallinity of the sample unaltered as confirmed by PXRD (Fig. S1, ESI†).
H2O at 120 °C for 72 hours.12a The crystalline powder was filtered, soaked in MeOH for three days, filtered again, and then dried in oven at 120 °C. The purity of the sample was confirmed by PXRD, in which the experimental pattern matched well with the simulation based on the single-crystal structure (Fig. S1, ESI†). The MOF powder is expected to be hydrophobic due to the presence of a large number of –CH3 groups on the ligand, which was confirmed by the measurement of the contact angle (119.86°) with a water drop (Fig. S2, ESI†). The MOF floats in water (Fig. S3, ESI†), and the exposure to water left the crystallinity of the sample unaltered as confirmed by PXRD (Fig. S1, ESI†).
The activated sample of SION105-Eu was first subjected to NH3 gas adsorption under dry conditions, with the isotherms measured at 303 and 313 K (Fig. S5, ESI†). At ∼1 bar and 303 K, the MOF adsorbs 5.7 mmol g−1 NH3. The isosteric heat of NH3 adsorption calculated based on the Clausius–Clapeyron relation is −28.7 kJ mol−1 for the coverage of 1.5 mmol g−1 and slightly decreases at higher adsorption amounts (Fig. S6, ESI†), suggesting a good interaction between NH3 and the MOF.
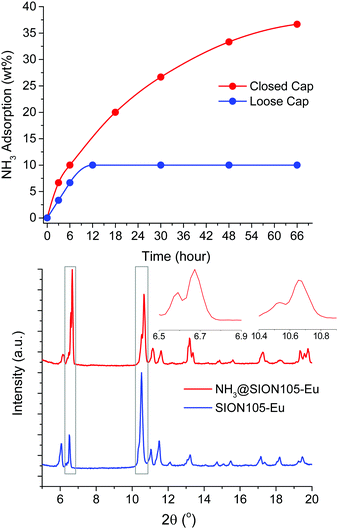
The ability of the material to capture of NH3 vapour from an aqueous NH3 solution was tested at room temperature (Fig. S7, ESI†). 100 mg of the powder in a 2 mL vial was placed inside a 50 mL vessel. A second vial containing 3.0 mL of aqueous NH3 solution (28 wt%) was also placed into the larger vessel. With the cap loose, the capture of NH3 by the MOF was assessed at ambient pressure while higher pressures were achieved with the cap tightly closed. The amount of NH3 vapour adsorbed by the sample was gravimetrically recorded as a function of time (Fig. 2). The MOF adsorbs up to ∼10 wt% after 6 hours, and ∼36 wt% after 66 hours when the vessel is tightly closed. Adsorption is slower and reaches saturation of ∼10 wt% after 12 hours when the cap is loose. These findings are consistent with the adsorption of NH3 being pressure dependent. The thermal gravimetric analysis (TGA) of the sample collected after 6 hours of NH3 adsorption in a closed vessel (NH3@SION105-Eu) shown in Fig. S8 (ESI†) also illustrates the weight loss of around 10 wt% at below 100 °C, corresponding to the release of NH3. As the MOF unit molecular weight is 709.43 g mol−1, 10 wt% (5.9 mmol g−1) is similar to the value obtained with dry NH3 gas, and indicates an average molar ratio of NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B of ca. 4
B of ca. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This uptake is similar to that seen for Zn(INA)2 (∼6 mmol g−1),8b and Al-PMOF (∼7.6 mmol g−1) at room temperature and 1 bar.8g In the present case, this suggests the presence of the Lewis acidic B centres prompts non-stoichiometric capture of NH3 molecules, presumably facilitated by hydrogen bonding interactions among NH3 molecules. In contrast, exposure of SION105-Eu to pure water showed negligible adsorption, suggesting that capture of more basic NH3 is enhanced by the electrophilic nature of the MOF.
1. This uptake is similar to that seen for Zn(INA)2 (∼6 mmol g−1),8b and Al-PMOF (∼7.6 mmol g−1) at room temperature and 1 bar.8g In the present case, this suggests the presence of the Lewis acidic B centres prompts non-stoichiometric capture of NH3 molecules, presumably facilitated by hydrogen bonding interactions among NH3 molecules. In contrast, exposure of SION105-Eu to pure water showed negligible adsorption, suggesting that capture of more basic NH3 is enhanced by the electrophilic nature of the MOF.
A suspension containing ∼2 mg of the ground MOF powder in 200 μL of THF was deposited onto a filter-paper plate and allowed to dry (Fig. S9, ESI†). The luminescence emission measurements of this sample were made in the presence and absence of NH3 vapour. After 20 minutes of exposure to NH3 vapour, the luminescence peak at ∼615 nm, characteristic for the transition 5D0 → 7F2 of Eu3+, decreased in intensity by ∼30% (Fig. S10, ESI†). Since lanthanide luminescence emission is induced by the antenna effect,13 this partial quenching is consistent with a rather weak electrostatic interaction between the B centre of the antenna ligand tctb3− and NH3.
The PXRD pattern of the MOF sample collected after 6 hours of NH3 adsorption (NH3@SION105-Eu) showed the preservation of crystallinity, although the two original peaks in the regions of 2θ = 6.5–6.9° and 10.4–10.9° were split, and their intensities were altered (Fig. 2, bottom). This also suggests only weak electrostatic or van der Waals interactions between the MOF and NH3 as the formation of the B-NH3 adduct would be expected to lead to quaternization of B and a drastic perturbation of the PXRD pattern. It is noteworthy that van der Waals interactions have detected for frustrated Lewis acidic boron–olefin interactions.14 The B environment in trimesitylborane (Mes3B) is similar to that in SION105-Eu and indeed, exposure of Mes3B to NH3 showed no evidence of adduct formation by 11B-NMR spectra (Fig. S15, ESI†).15
The FTIR spectrum of the NH3@SION105-Eu (Fig. S16, ESI†) shows the appearance of N–H stretching bands at 3300–3500 cm−1, confirming the retention of NH3 within the MOF. In contrast, the MOF MIL103-Eu which is highly porous, but does not contain B shows no evidence of NH3 adsorption in the FTIR spectrum (Fig. S18, ESI†). This further supports the notion that the electrophilic pores in SION105-Eu facilitates NH3 adsorption.
To assess the stability of SION105-Eu over the course of adsorption, the powdered MOF was exposed to NH3 vapour (generated by an aqueous NH3 solution (28 wt%) in a tightly closed vessel) for 6, 12, and 66 hours. The samples were subsequently heated at 75 °C in an oven for 30 min. In each case, the original mass of the sample was retrieved. PXRD measurements showed that the crystallinity was unaltered after 6 hours, slightly decreased after 12 hours and fully degraded after 66 hours of exposure to NH3 (Fig. S19, ESI†). Similar experiments with the well-known MOFs, HKUST-1 (Cu) and MOF-5 (Zn) showed that these MOFs completely lost crystallinity in less than 1 hour of exposure to aqueous NH3 (Fig. S20 and S21, ESI†). Indeed, while a number of transition metal-based MOFs8b are reported to be stable in the presence of dry NH3 gas, they decomposed in “wet” NH3 vapour, thus precluding recycling.9 The present data demonstrate that SION105-Eu offers superior stability in this regard.
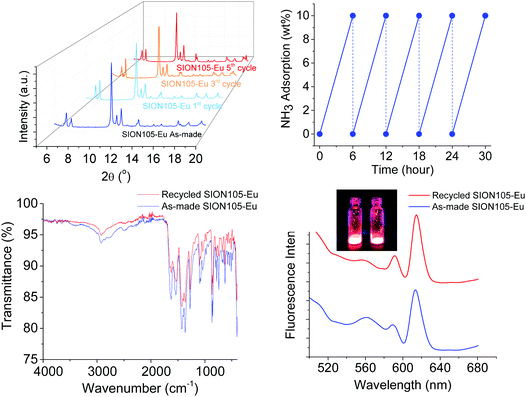
SION105-Eu was subjected to adsorption of NH3 for 6 hours in a closed cap vessel and the NH3 subsequently liberated by heating at 75 °C for 30 min; this process was repeated 5 times. The NH3 adsorption capacity remains unchanged. The PXRD pattern, FTIR spectrum and luminescence emission of the MOF were unaltered after the 5th cycle of NH3 capture, being essentially the same as those derived from the original material (Fig. 3). These observations affirm that SION105-Eu is recyclable for NH3 capture.
Density functional theory (DFT) calculations were performed to confirm the electrostatic mechanism of SION105-Eu (see details in ESI†). It was revealed that no covalent bonds were formed between NH3 molecules and the MOF scaffold as expected, and the computed heat of NH3 adsorption was determined to be −40.9 kJ mol−1 at zero coverage, which is close to the value of −28.7 kJ mol−1 derived from the Clausius–Clapeyron equation. Notably, most works reported MOFs for NH3 adsorption do not include the isosteric heat. Both the experimental and computed values for SION105-Eu are much lower than the value reported for the [SrOOC]17-COF (−91.2 kJ mol−1) due to the presence of the open Sr-sites in the latter that strongly interact with NH3.8f
The above results demonstrate that the MOF SION105-Eu has several key features that may be further exploited in the design of stable and recyclable materials for toxic gas capture. Firstly, the use of lanthanide ions in the +3 oxidation state provides hard Lewis acids that associate strongly with hard donor atoms such as O from carboxylate ligands, providing stability in the presence of substrate molecules, in the present case, NH3. Similarly, the incorporation of the bulky duryl groups on the Lewis acidic B centre within tctb3− ligand precludes strong acid–base B–N interactions, again making the MOF structure robust in the presence of NH3. However, the presence of the B on the linkers also makes the pores electrophilic, prompting electrostatic attraction of NH3 and thus its capture.
In conclusion, this work demonstrates a strategy for the design of materials for toxic gas adsorption based on the incorporation of sterically encumbered, electrophilic B sites in SION105-Eu. The resulting highly stable MOF is shown to capture NH3 vapour. Moreover, this binding is reversible with simple heating to 75 °C, affording a recyclable material for NH3 capture. Efforts to tune the Lewis acidicity of the boron centers for improved uptake capacity and recyclability of related MOFs will be undertaken. In addition, practical applications of such materials are also being targeted via the shaping of the MOF powder16 into pellets or beads.
TNN thanks Helen Co., Ltd for support. JHL's work was supported by the KIST Institutional Program (Project No. 2E30460). KCS thanks the Department of Chemistry at Oregon State University for support through start-up funding. Computational resources provided by KISTI Supercomputing Centre (Project No. KSC-2019-CRE-0149) are gratefully acknowledged. The authors thank Dr Nhat Truong Nguyen and Dr Andrzej Gładysiak for collecting the PXRD patterns, Mr Walter Liang for the FTIR spectra, and Ms Karlee Bamford for discussion.
Conflicts of interest
There are no conflicts to declare.Notes and references
- WHO, Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease, 2016 Search PubMed.
- Programmer, Occupational Safety and Health Guideline for Ammonia, https://www.cdc.gov/niosh/docs/81-123/pdfs/0028-rev.pdf.
- (a) A. Srinivasan and M. W. Grutzeck, Environ. Sci. Technol., 1999, 33, 1464–1469 CrossRef CAS; (b) M. Gonçalves, L. Sánchez-García, E. D. Oliveira Jardim, J. Silvestre-Albero and F. Rodríguez-Reinoso, Environ. Sci. Technol., 2011, 45, 10605–10610 CrossRef PubMed.
- (a) N. S. Bobbitt, M. L. Mendonca, A. J. Howarth, T. Islamoglu, J. T. Hupp, O. K. Farha and R. Q. Snurr, Chem. Soc. Rev., 2017, 46, 3357–3385 RSC; (b) M. R. Tchalala, P. M. Bhatt, K. N. Chappanda, S. R. Tavares, K. Adil, Y. Belmabkhout, A. Shkurenko, A. Cadiau, N. Heymans, G. De Weireld, G. Maurin, K. N. Salama and M. Eddaoudi, Nat. Commun., 2019, 10, 1328 CrossRef CAS PubMed; (c) P. Wang, Q. Xu, Z. Li, W. Jiang, Q. Jiang and D. Jiang, Adv. Mater., 2018, 30, 1801991 CrossRef PubMed; (d) A. Gładysiak, T. N. Nguyen, M. Spodaryk, J.-H. Lee, J. B. Neaton, A. Züttel and K. C. Stylianou, Chem. – Eur. J., 2019, 25, 501–506 Search PubMed; (e) B. Valizadeh, T. N. Nguyen, B. Smit and K. C. Stylianou, Adv. Funct. Mater., 2018, 28, 1801596 CrossRef.
- E. Barea, C. Montoro and J. A. R. Navarro, Chem. Soc. Rev., 2014, 43, 5419–5430 RSC.
- P. G. Boyd, A. Chidambaram, E. García-Díez, C. P. Ireland, T. D. Daff, R. Bounds, A. Gładysiak, P. Schouwink, S. M. Moosavi, M. M. Maroto-Valer, J. A. Reimer, J. A. R. Navarro, T. K. Woo, S. Garcia, K. C. Stylianou and B. Smit, Nature, 2019, 576, 253–256 CrossRef CAS PubMed.
- Y. Wang, X. Zhao, H. Yang, X. Bu, Y. Wang, X. Jia, J. Li and P. Feng, Angew. Chem., Int. Ed., 2019, 58, 6316–6320 CrossRef CAS PubMed.
- (a) C. J. Doonan, D. J. Tranchemontagne, T. G. Glover, J. R. Hunt and O. M. Yaghi, Nat. Chem., 2010, 2, 235–238 CrossRef CAS PubMed; (b) Y. Chen, C. Yang, X. Wang, J. Yang, K. Ouyang and J. Li, J. Mater. Chem. A, 2016, 4, 10345–10351 RSC; (c) A. Gładysiak, T. N. Nguyen, J. A. R. Navarro, M. J. Rosseinsky and K. C. Stylianou, Chem. – Eur. J., 2017, 23, 13602–13606 CrossRef; (d) G. Barin, G. W. Peterson, V. Crocellà, J. Xu, K. A. Colwell, A. Nandy, J. A. Reimer, S. Bordiga and J. R. Long, Chem. Sci., 2017, 8, 4399–4409 RSC; (e) A. J. Rieth and M. Dincă, J. Am. Chem. Soc., 2018, 140, 3461–3466 CrossRef CAS PubMed; (f) Y. Yang, M. Faheem, L. Wang, Q. Meng, H. Sha, N. Yang, Y. Yuan and G. Zhu, ACS Cent. Sci., 2018, 4, 748–754 CrossRef CAS PubMed; (g) S. Moribe, Z. Chen, S. Alayoglu, Z. H. Syed, T. Islamoglu and O. K. Farha, ACS Mater. Lett., 2019, 1, 476–480 CrossRef CAS.
- G. W. Peterson, G. W. Wagner, A. Balboa, J. Mahle, T. Sewell and C. J. Karwacki, J. Phys. Chem. C, 2009, 113, 13906–13917 CrossRef CAS PubMed.
- L. Hamon, C. Serre, T. Devic, T. Loiseau, F. Millange, G. Férey and G. D. Weireld, J. Am. Chem. Soc., 2009, 131, 8775–8777 CrossRef CAS PubMed.
- D. W. Stephan, Science, 2016, 354, aaf7229 CrossRef PubMed.
- (a) F. M. Ebrahim, T. N. Nguyen, S. Shyshkanov, A. Gładysiak, P. Favre, A. Zacharia, G. Itskos, P. J. Dyson and K. C. Stylianou, J. Am. Chem. Soc., 2019, 141, 3052–3058 CrossRef CAS PubMed; (b) S. Shyshkanov, T. N. Nguyen, F. M. Ebrahim, K. C. Stylianou and P. J. Dyson, Angew. Chem., Int. Ed., 2019, 58, 5371–5375 CrossRef CAS PubMed.
- T. N. Nguyen, F. M. Ebrahim and K. C. Stylianou, Coord. Chem. Rev., 2018, 377, 259–306 CrossRef CAS.
- X. Zhao and D. W. Stephan, J. Am. Chem. Soc., 2011, 133, 12448–12450 CrossRef CAS PubMed.
- H. C. Brown and V. H. Dodson, J. Am. Chem. Soc., 1957, 79, 2302–2306 CrossRef CAS.
- B. Valizadeh, T. N. Nguyen and K. C. Stylianou, Polyhedron, 2018, 145, 1–15 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details and experimental data. See DOI: 10.1039/d0cc00741b |
| This journal is © The Royal Society of Chemistry 2020 |