Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Arsenic removal from Peruvian drinking water using milk protein nanofibril–carbon filters: a field study†
Sreenath
Bolisetty
 *ab,
Akram
Rahimi
ab and
Raffaele
Mezzenga
*ab,
Akram
Rahimi
ab and
Raffaele
Mezzenga
 *ac
*ac
aETH Zurich, Department of Health Sciences and Technology, Schmelzbergstrasse 9, 8092 Zurich, Switzerland
bBluAct Technologies GmbH, Dufauxstrasse 57, 8152 Opfikon, Switzerland. E-mail: sreenath@bluact.com; Tel: +41 763456017
cETH Zurich Department of Materials, Wolfgang-Pauli-Strasse 10, 8093 Zurich, Switzerland. E-mail: raffaele.mezzenga@hest.ethz.ch; Tel: +41 44 632 9140
First published on 30th September 2021
Abstract
The tap water quality in Peru fails to meet the World Health Organization (WHO) drinking water standards; consequently, the local population in Peru has been exposed over the last few years to harmful arsenic levels through water consumption. A field study was conducted in Peru between 2019 and 2020 using granular adsorbers and hybrid membranes based on the technology using milk protein nanofibril–carbon hybrid materials previously introduced in the literature by us (S. Bolisetty and R. Mezzenga, Nat. Nanotechnol., 2016, 11, 365). The performance was analyzed across 28 households as well as 3 community-based water treatment plants in some of the Peruvian arsenic burdened regions, covering a range of groundwater arsenic concentrations between 11 μg L−1 and 1.1 mg L−1. Three different kinds of filtration units were installed including the combined granular media and hybrid membrane (type I), hybrid membrane alone (type II), and granular media (type III), to determine the effectiveness of different filtration setups for arsenic removal in different regions. The arsenic removal by household filtration units and community filtration units shows a removal efficiency exceeding 99% at various initial arsenic concentrations for a duration up to nine months. In addition, it is shown that an aqueous NaOH solution can be used to regenerate the adsorbent, extending its operational lifetime and the overall capacity for As removal. High purification efficiency, very low cost, safety, little to no energy requirement, possibility of regeneration and simplicity of operation make this technology suitable for arsenic removal from drinking water in a broad range of urban and rural areas.
Water impactThe metalloid arsenic in groundwater has been considered as the most serious inorganic contamination in drinking water. Over several hundred million people in more than 70 countries are affected by arsenic contamination in drinking water. The milk protein nanofibril-based technology has shown extraordinary results for the removal of both arsenite As(III) and arsenate As(V). This work reports a field study in several regions of Peru on drinking water purification using protein nanofibril–carbon-based products. Household faucet filters, community-based pilot treatment and large-scale plant studies have shown very efficient long-term removal of arsenic. |
1 Introduction
Highly toxic arsenic contamination in drinking water is responsible for serious public health effects, called arsenicosis, upon prolonged consumption.1 It has been proved that chronic exposure to arsenic is associated with an increased risk of skin, bladder and lung cancer, as well as with skin lesions, respiratory disease, and neurological and cardiovascular diseases.2 Today millions of people in many parts of the world are still exposed to an elevated level of arsenic through contaminated water consumption. The World Health Organization (WHO) proposes a 10 μg L−1 arsenic threshold as a drinking water guideline; however, many low–middle income countries are not able to cope with the WHO standards due to high purification costs. Consequently, some governments adjusted their national drinking water standards up to 50 μg L−1 in arsenic affected areas.3–5Peru has many mineral-rich regions with high arsenic contamination in their natural groundwater and surface drinking water, which can be due to both volcanic (natural pollution) and man-made mining activities (anthropomorphic pollution). Particularly, the expansion of the mineral extraction industry in different parts of Peru is adversely affecting the quality of the Peruvian water supply.6,7 It has been assessed that there are approximately 1.6 million Peruvians residing in regions with poor quality drinking water within 5 km of active or historical mining operations.8 As can be observed in Fig. 1a, several other heavy metals such as lead, zinc, cadmium, mercury, copper and chromium are also released into the water sources in Peru due to the mining industry.2,9 In 2000, it was estimated that more than a million Peruvian people living in both rural and urban areas have been consuming arsenic-rich water (exceeding 50 μg L−1 arsenic) for a long period of time (20–30 years).10,11 The Ministry of Health (MoH) in Peru reported that the regions of Tacna, Arequipa and Moquegua, especially the districts of Candarave, Inclan and Jorge Basadre, have a high level of arsenic contamination, up to 1.1 ppm, 110 times higher than the WHO drinking water limit.
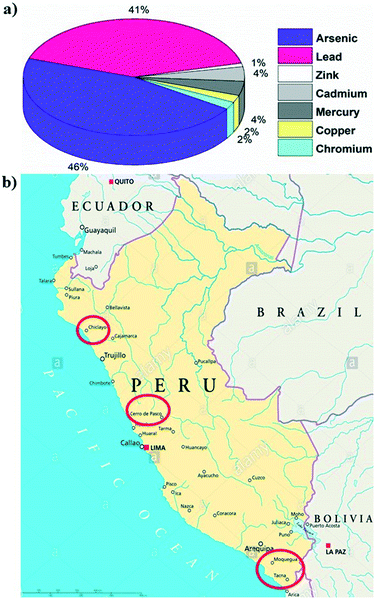 | ||
| Fig. 1 a) Different metal percentages in a water sample in Peru9 (MINSA 2016). b) Site location map for the Peru field study. The study locations are marked by the red circles (Tacna, Moquegua, Cerro de Pasco, and Chiclayo). | ||
So far, several water treatment approaches including reverse osmosis, coagulation/filtration, adsorption and ion exchange have been developed for arsenic removal.12–16 Despite these advances, none of these technologies is currently applied on a broad scale in Peru because each of them requires sophisticated technical systems, and none of them is found to suit the reality in this moderate-income region of the world. Accordingly, identifying a suitable, low energy consumption, highly efficient and safe technology that does not require large investments and can be used at the household level, specifically in close proximity of the mining areas, has become urgent.
Protein nanofibrils generated from inexpensive milk proteins have shown unique binding properties to adsorb, via metal–ligand binding interactions, any type of heavy metal ion and metalloids, including arsenic, from polluted water in a non-selective, very general and unprecedented way.17–24 This new technology is radically distinct from the current technologies and shows promise to bring a ground-breaking solution to this long-standing problem. The major difference is in the functioning mode, which is more reminiscent of an adsorption process operated with membrane filters and granular media of great capacity, rather than filtration carried out with specific selectivity (e.g. exchange resins) or size-dependent traditional membranes with high operating pressure (e.g. reverse osmosis, nanofiltration, and ultrafiltration). Furthermore, the technology is sustainable and energy-neutral (it can work with simple gravitational pressure), has no secondary pollution side effects and is of very low cost, which make it globally accessible worldwide.
In this work we benchmark and validate this technology under real operating conditions in one of the most demanding settings for arsenic removal, in both urban and rural areas of Peru. This field study is particularly aimed at assessing the long-time performance of granular media and the hybrid membrane based on protein fibrils in the treatment of groundwater and surface water with a range of typical dissolved species concentrations for both household applications and community-based large-scale water treatments. In total, we installed three different types of filtration units for the Peru field study starting from April 2019 to July 2020: type I – household installation of combined membrane and granular media, type II – household installation of the membrane alone, and type III – community installation of the granular media.
2 Experimental section
The technology used to produce whey protein nanofibril–carbon hybrid membranes and granular media has been deeply discussed in numerous earlier publications and will not be further discussed here. In short, hybrid membranes and granular media were produced and kindly provided by BluAct Technologies GmbH, following earlier publication and patent protocols.17,18,24–26Fig. 2a and b show a representative image of the hybrid membrane (see Fig. 2a) and granular media (see Fig. 2b), respectively.The field study was executed in three different regions highlighted in the map shown in Fig. 1b with highly variable concentrations of arsenic in the area of Tacna (15–50 ppb), Moquegua (10–35 ppb) and Inclan (60–450 ppb) in Southern Peru and Cerro de Pasco (3–5 ppb), Ticapampa (250–1100 ppb) and Chiclayo (5–100 ppb) in Northern Peru. Water samples were collected before and after the filtration at regular intervals in household and large-scale filtration units having various water supply systems from April 2019 until July 2020. The samples were collected directly from the inlet and outlet of the filtration process and stored without any reagents. All the samples have been acidified using 0.1 M HNO3 before analysis by AAS. The samples were analyzed for the residual arsenic concentration before and after the filtration at ETH Zurich using atomic absorption spectroscopy on a AAS240Z Zeeman graphite furnace (GTA 120) equipped with a PSD 120 programmable sample dispenser. In this study we measured the total arsenic content without distinguishing As (III) and As (V) in the water, before and after the treatment. The measurements were performed in triplicate, and the results were averaged. A separate calibration was done by measuring the standard solutions with various concentration regimes. The physicochemical parameters of the water before and after filtration is shown in ESI Table S2.†
In total, three different types of water filtration units were installed and studied in detail (see Fig. 2c–f). For the household water treatment, type I household installation relies on both the membrane (geometry of the filters: 20 × 10 cm; number of filters used: 3) and granular media while type II household installation relies on the membrane alone. The instructions were given to the people in the houses about the usage of the filtration setup. Water was pumped from spring water reservoirs into the filter membrane or granular media, with a consecutive water throughput of 0.5–2 L min−1. It must be emphasized that no chemicals were added in this study. The details of the performance over the field study period at different arsenic contamination levels are discussed in the Results and discussion section below.
For the large-scale community units, only the granular media were filled directly inside the column (type III) as a fixed bed. The community units were installed by replacing the existing charcoal with our granular media (see Fig. 2e and f). The community filtration units in Inclan and EPS Tacna were designed specifically by BluAct Technologies GmbH. The Inclan community filtration unit also has the prefiltration units of the stone and sand filters, with the granular media. The spring water reservoir directly passed through the media via simple gravitation pressure without any external energy. The filtered water passes directly to the automatic closing reservoir tank. 20 kg granular media are used for the community-based water filtration setups (see Fig. 2e and f). The plants are operated in a continuous mode with automatic stops when not in use. Additionally, the other water parameters such as pH, turbidity, conductivity, and temperature are also recorded. The change in turbidity of the water before and after filtration is visible in Fig. 2g. The exact address of all installations is recorded and shown in Table S1 in the ESI.† A total of 28 household (types I and II) and 3 community-based (type III) water filtration units were installed (see Fig. 2h).
Hair and nail study: toenail and hair samples were collected from volunteers in Tacna, Ticapampa and Inclan to establish a correlation between the exposure to arsenic via groundwater and the accumulated arsenic in the body. The nail and hair samples were cleaned for analysis by following the procedure outlined by Chen et al.27 and Ryabukhin et al.,28 respectively. The nail samples were immersed in a 1 wt% solution of the surfactant Triton X-100 and placed in an ultrasonic bath for 20 min. After this treatment, the nails were rinsed five times with Milli-Q water and then dried overnight at 60 °C in an oven. The hair samples were rinsed sequentially with acetone (25 ml, 10 min sonication), then with deionized water three times (25 ml, 10 min sonication each time) and finally with acetone (25 ml, 10 min sonication), discarding the wash solution between each step. After washing, the hair samples were dried overnight at 60 °C in a drying oven.
After cleaning, 10–100 mg of nail or hair samples were accurately weighed into acid-cleaned polypropylene tubes. 1 ml of concentrated HNO3 (69% HNO3, BDH) was added to each sample, and the tubes were capped and allowed to sit for 48 h at room temperature. The resultant solution was diluted with 4 ml of deionized water and then filtered (0.4 μm) into a fresh acid-cleaned polypropylene tube. The resultant solution samples were analyzed by AAS.
3 Results and discussion
Data on the stored arsenic content in humans, obtained via hair and nail composition analysis, are shown in Table 1. The data showed that the arsenic concentration ranged from 0.11 μg g−1 to 2.68 μg g−1 which can be classified to be high levels in individuals according to other studies.29 The arsenic levels in the hair and nail samples are positively associated with the groundwater arsenic concentrations in the area. Although the data shown in the Table 1 are scattered, as expected in any physiological dataset, the general tendency shows a positive correlation between the average nail and hair arsenic concentrations found in people and the arsenic concentration in the water they drink. The average nail and hair arsenic concentrations (see Fig. S1, ESI†) in people drinking water containing higher arsenic concentrations tend to be larger than those in people consuming water with lower arsenic concentrations, indicating more severe health implications for those individuals with access to poorer water quality and emphasizing the need for appropriate treatment.| Samples kind/region | μg As g−1 sample | As concentration in the area (ppb) |
|---|---|---|
| Hair/Ticapampa | 2.68 | 850 |
| Hair/Ticapampa | 1.34 | 850 |
| Nail/Ticapampa | 0.12 | 850 |
| Nail/Ticapampa | 2.46 | 850 |
| Hair/Tacna | 0.27 | 321 |
| Nail/Tacna | 0.46 | 321 |
| Hair/Inclan | 0.11 | 298 |
| Nail/Inclan | 1.36 | 298 |
| Nail/Inclan | 1.12 | 298 |
| Nail/Tacna | 0.84 | 22 |
| Nail/Tacna | 0.57 | 22 |
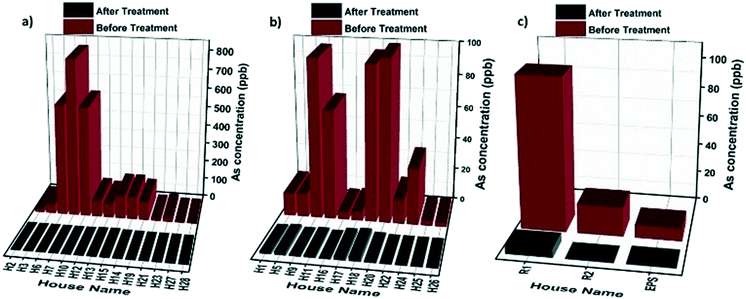
The filtration units were evaluated for their arsenic removal efficiencies before and after filtration in April 2019 in different regions using the type I household (14 studied households), type II household (12 studied households), and type III community (3 studied communities) filtration units. As we can observe from Fig. 3, in all the regions using different types of filtration setups (types I–III), the arsenic concentrations in the filtrated water dropped below the 10 ppb WHO threshold.
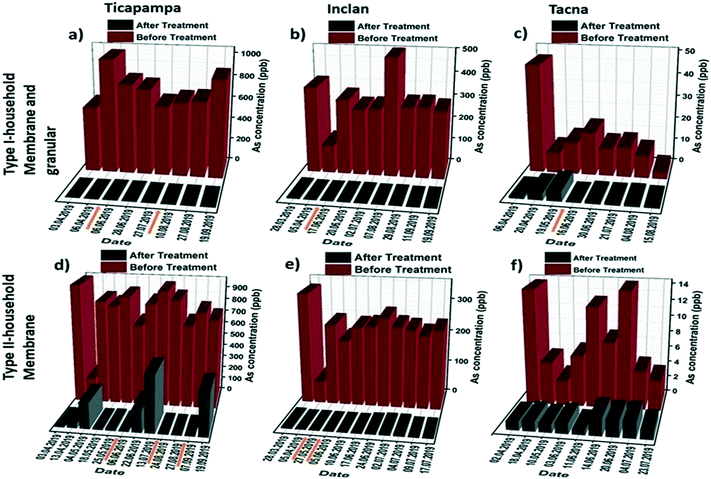
Due to the relatively high arsenic content of the raw groundwater in some regions within the studied area, a two-stage filtration approach combining the granular media and hybrid membrane was used to realize the optimum arsenic removal performance. Fig. 4a–c show the results for the arsenic removal performance of the type I household filtration units in three households in different regions. The results show that residual arsenic levels below the WHO guideline limit of 10 ppb were achieved by all the filtration systems of the installed type I unit. Even though the installed filtration units were not exhausted with the high arsenic levels, the hybrid membranes were blocked after a few days due to the high turbidity of the water and fouling phenomena. For that reason, we only changed the filter membranes in the type I household filtration units (not the granular media). In Ticapampa (see Fig. 4a) the membranes were replaced two times, while in Inclan and Tacna (see Fig. 4b and c) the membranes were replaced once during the six month period. According to the obtained results, even under extreme conditions the type I household filtration units can reduce the concentration of arsenic in water to values that meet the requirements set by the WHO on drinking water during the period of the field study. The performance of the type 1 units is also shown in Fig. S2 (see the ESI†) for the Moquegua and Chiclayo regions. As can be observed, there is no need to change the membrane during the field study in these regions because of the low turbidity of their water. The performance of the type II household filtration units in different regions is shown in Fig. 4d–f, including the concentrations of arsenic in raw water and the values measured after passing through the tested filtration units by gravitational pressure. Arsenic concentrations in the effluents of the type II household filtration units dropped significantly below 10 ppb during the 6 month period in the low and high-level arsenic-contaminated regions of Inclan with two replacements and Tacna with one replacement, turning tap water into drinkable water. The efficiency of arsenic removal reaches up to 99.9%. However, the average arsenic concentration in the Ticapampa region is significantly higher than those in Tacna and Inclan, which leads to the increased effluent arsenic concentrations after a few weeks for the type II household installation filtration unit. The decreasing fractional removal with an increasing arsenic concentration in the Ticapampa area shown in Fig. 4a is consistent with a mechanism where arsenic removal is limited by the availability of adsorption sites and the filters need to be changed regularly. In the case of low and medium contamination regions, even though the hybrid membranes are not saturated with high arsenic levels, the hybrid membranes in the type II household filtration units were blocked after a few days due to the high turbidity of the water and fouling phenomena. To prevent the fouling phenomena and also the blocking of the membrane by other particles, sand filters were installed as pre-treatment for the household filtration units, and stone–sand filters were installed as pre-treatment for the community filtration units, starting from August 2019.
To evaluate the arsenic removal performance for large-scale water facilities, type III community filtration units were installed using the granular media for the large-scale community-based filtration in EPS Tacna as a low contamination region, as well as the Inclan and Moquegua regions as the high contamination regions. The water was passed through the filtration units via gravitational pressure at a flow rate of approximately 0.1 L s−1, operating intermittently for 7 months up to a total volume of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 litres.
000 litres.
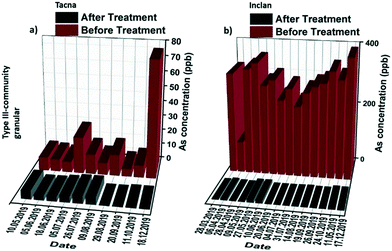
The concentrations of arsenic remaining in water after treatment for the Inclan and Tacna communities are shown in Fig. 5, where during the entire span of the study, the quality of water is improved meeting the WHO standards, i.e., well below the 10 ppb threshold. Even in the case of high contamination regions in Inclan, using 20 kg of the granular media, the arsenic concentration of water from an average 300 ppb concentration dropped to below 10 ppb during the entire study period. In the case of the EPS Tacna and Torata installation units, the water passed through the media continuously for 7 months, and the entire treated volume of approximately 1815 m3 was purified by bringing the arsenic level below 10 ppb. The results for the community systems demonstrated that high efficiency, long-term performance and high arsenic removal capacity can be achieved with the type III community filtration unit in both high and low arsenic contamination areas, with essentially no maintenance and additional operating costs.
Based on the above results obtained for the households and small communities, a large-scale study was implemented in an existing water treatment plant in Yacango. Yacango village relies for water supply on a spring water reservoir with an arsenic concentration of 20 ppb. In this large-scale water treatment plant, granular materials were used for 5 months. The daily filtrated volume using 680 kg of the granular materials was 147 m3, with a flow rate of ∼6 m3 h−1. The granular adsorbent always yielded an effluent arsenic concentration of <10 ppb in the first 5 months of operation. A total of 37 million liters of water was treated until July 2020, yielding the operating capacity under these specific conditions, that is, 1 kg of materials could filter 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 liters of contaminated water. Due to the saturation of the granular media arsenic removal efficiency after 5 months of adsorbent operation (see Fig. 6), the materials were regenerated in July 2020. The granular adsorbents were regenerated using NaOH (1 M) and could subsequently be brought back to service following a short rinsing with distilled water. As can be observed in Fig. 6, the regenerated adsorbent shows a renewed high performance for arsenic removal, extending the operating lifetime of the adsorbent.
000 liters of contaminated water. Due to the saturation of the granular media arsenic removal efficiency after 5 months of adsorbent operation (see Fig. 6), the materials were regenerated in July 2020. The granular adsorbents were regenerated using NaOH (1 M) and could subsequently be brought back to service following a short rinsing with distilled water. As can be observed in Fig. 6, the regenerated adsorbent shows a renewed high performance for arsenic removal, extending the operating lifetime of the adsorbent.
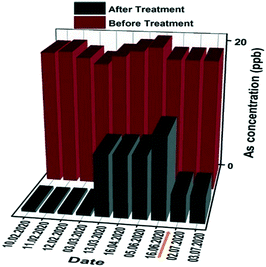 | ||
| Fig. 6 Arsenic concentration before and after filtration through the large-scale filtration plant in Yacango. The brown arrow indicates the first onsite regeneration event. | ||
4 Conclusions
The goal of this study was to investigate the arsenic removal efficiency of protein-based granular materials and protein-based hybrid membranes at the household and community levels for a broad range of groundwater compositions in Peru and assess their potential for arsenic removal in such a broad context.Hair and nail samples collected from volunteers in all the regions under screening featured high arsenic content, indicating a strong correlation between arsenic exposure in groundwater and its biological accumulation and highlighting the urgent need for viable solutions.
The type II filtration unit (only membranes) was effective for arsenic removal at medium and low contamination concentrations. However, this kind of filtration unit was not sufficient for removing the required amounts of arsenic in Ticapampa tap water, where extremely contaminated water levels were observed; in such cases, the type I filtration unit (combined granular media and membranes) was still capable of virtually removing the effluent arsenic and bringing back water to drinking quality according to the WHO standards. The type III community filtration unit (only granular) in Inclan and Tacna operated on tap water with 300 μg L−1 and 40 μg L−1 arsenic contents and featured complete arsenic removal over 7 months of steady-state operation, again bringing back water to drinking quality.
Taken together, the results from this field study show that the water purification technology based on milk protein nanofibril–carbon hybrids is a promising technology for tackling the problem of arsenic groundwater contamination at minimal operating costs and infrastructure requirements, for the benefits of a targeted population of 15 million people worldwide according to WHO estimations.
Conflicts of interest
RM, SB and AR are the inventors of the two related patents (EP2921216A1 and US 63065824) filed on behalf of ETH Zurich and BluAct Technologies GmbH.Acknowledgements
The authors acknowledge major support from Herberth Pacheco de la Jara, SABAVIDA, Marc-André Bünzli, Martin Jaggi, Rosa Maria Guzman Alcayhuaman, and Carlos Enrique Muñoz from the Swiss Agency for Development and Cooperation (SDC). SDC kindly supported the project financially. We thank Christophe Zeder (ETHZ) for his assistance during AAS measurements.References
- S. Bordoloi, S. K. Nath, S. Gogoi and R. K. Dutta, Arsenic and iron removal from groundwater by oxidation–coagulation at optimized pH: Laboratory and field studies, J. Hazard. Mater., 2013, 260, 618–626 CrossRef CAS PubMed.
- C. M. George, L. Sima, M. H. J. Arias, J. Mihalic, L. Z. Cabrera, D. Danz, W. Checkleya and R. H. Gilman, Arsenic exposure in drinking water: an unrecognized health threat in Peru, Bull. W. H. O., 2014, 92(8), 565–572 CrossRef PubMed.
- World Health Organization, Arsenic in drinking water, 2001, http://www.who.int/inf-fs/en/fact210.html Search PubMed.
- M. Berg, H. C. Tran, T. C. N. Guyen, H. V. P. Ham, R. Schertenleib and W. R. Giger, Arsenic Contamination of Groundwater and Drinking Water in Vietnam: A Human Health Threat, Environ. Sci. Technol., 2001, 35, 2621–2626 CrossRef CAS PubMed.
- A. H. Khan, S. B. Rasul, A. Munir, M. Habibuddowla, M. Alauddin, S. S. Newaz and A. Hussan, Appraisal of a simple arsenic removal method for groundwater of Bangladesh, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2000, 35(7), 1021–1041 CrossRef.
- J. Beth, C. Poschenrieder, M. Llugany, J. BarceGby, P. Tumea, F. J. Tobias, J. L. Barranzuelac and E. R. Vksquez, Arsenic and heavy metal contamination of soil and vegetation around a copper mine in Northern Peru, Sci. Total Environ., 1997, 203, 83–91 CrossRef.
- A. Bebbington and M. Williams, Water and Mining Conflicts in Peru, Mt. Res. Dev., 2008, 28(3), 190–195 CrossRef.
- A. van Geen, C. Bravo, V. Gil, S. Sherpa and D. Jack, Lead exposure from soil in Peruvian mining towns: a national assessment supported by two contrasting examples, Bull. W. H. O., 2012, 90, 878–886 CrossRef PubMed.
- Metales Pesados y contaminación Ambiental, Retos para la Prevención y control, MINSA, 2016, http://www.dge.gob.pe/portal/docs/renace/JornadaCientifica/viernes23/vigilanciametalesperu.pdf.
- D. Mondal, R. Periche, B. Tineo, L. A. Bermejo, M. Mahmudur Rahman, A. Sidique, M. D. A. Rahman, J. L. Solis and G. J. F. Cruz, Arsenic in Peruvian rice cultivated in the major rice growing region of Tumbes river basin, Chemosphere, 2020, 241, 125070 CrossRef CAS PubMed.
- J. Bundschuh, M. I. Litter, F. Parvez, G. Román-Ross, H. B. Nicolli, J. S. Jean, C.-W. Liu, D. López, M. A. Armienta, L. R. G. Guilherme, A. Gomez Cuevas, L. Cornejo, L. Cumbal and R. Toujaguez, One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries, Sci. Total Environ., 2012, 429, 2–35 CrossRef CAS PubMed.
- D. Lakshmanan, D. A. Clifford and G. Samanta, Comparative study of arsenic removal by iron using electrocoagulation and chemical coagulation, Water Res., 2010, 44, 5641–5652 CrossRef CAS PubMed.
- Q. Yang, C. H. Lau and Q. Ge, Novel Ionic Grafts that Enhance Arsenic Removal via Forward Osmosis, ACS Appl. Mater. Interfaces, 2019, 11, 17828–17835 CrossRef CAS PubMed.
- A. A. L. S. Duarte, S. J. A. Cardoso and A. J. Alçada, Emerging and innovative techniques for arsenic removal applied to a small water supply system, Sustainability, 2009, 1, 1288–1304 CrossRef CAS.
- O. X. Leupin and S. J. Hug, Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron, Water Res., 2005, 39, 1729–1740 CrossRef CAS PubMed.
- W. H. Ficklin, Separation of arsenic (III) and arsenic (V) in ground waters by ion-exchage, Talanta, 1983, 30, 371–373 CrossRef CAS PubMed.
- S. Bolisetty and R. Mezzenga, Amyloid–carbon hybrid membranes for universal water purification, Nat. Nanotechnol., 2016, 11, 365–371 CrossRef CAS PubMed.
- S. Bolisetty, N. Reinhold, C. Zeder, M. N. Orozco and R. Mezzenga, Efficient purification of arsenic-contaminated water using amyloid–carbon hybrid membranes, Chem. Commun., 2017, 53, 5714–5717 RSC.
- Q. I. Zhang, S. Bolisetty, Y. Cao, S. Handschin, J. Adamcik, Q. Peng and R. Mezzenga, Selective and Efficient Removal of Fluoride from Water: In Situ Engineered Amyloid Fibril/ZrO2 Hybrid Membranes, Angew. Chem., Int. Ed., 2019, 58, 6012–6016 CrossRef CAS PubMed.
- S. Bolisetty, M. Peydayesh and R. Mezzenga, Sustainable technologies for water purification from heavy metals: review and analysis, Chem. Soc. Rev., 2019, 48, 463–487 RSC.
- S. Bolisetty, N. M. Coray, A. Palika, G. A. Prenosilc and R. Mezzenga, Amyloid hybrid membranes for removal of clinical and nuclear radioactive, Environ. Sci.: Water Res. Technol., 2020, 6, 3249–3254 RSC.
- M. Peydayesh, S. Bolisetty, T. Mohammadi, R. Mezzenga, M. Peydayesh, S. Bolisetty, T. Mohammadi and R. Mezzenga, Assessing the binding performance of amyloid–carbon membranes toward heavy metal ions, Langmuir, 2019, 35, 4161–4170 CrossRef CAS PubMed.
- A. Palika, A. Rahimi, S. Bolisetty, S. Handschin, P. Fischer and R. Mezzenga, Amyloid Hybrid Membranes for Bacterial & Genetic Material Removal from Water and their Anti-fouling properties, Nanoscale Adv., 2020, 2, 4665–4670 RSC.
- A. Palika, A. Armanious and A. Rahimi, et al., An antiviral trap made of protein nanofibrils and iron oxyhydroxide nanoparticles oxyhydroxide nanoparticles, Nat. Nanotechnol., 2021, 16, 918–925 CrossRef CAS PubMed.
- S. Bolisetty and R. Mezzenga, Composite material used in filter for water treatment and recovery of metals, comprises amyloid fibrils and activated carbon in intimate contact, and optionally support material, EP2921216-A1, WO2015140074-A1, 2015.
- A. Palika, A. Rahimi, S. Bolisetty and R. Mezzenga, Antiviral compositions and method of killing virus, US63065824, 2020.
- K. L. B. Chen, C. J. Amarasiriwardena and D. C. Christian, Determination of Total Arsenic Concentrations in Nails by Inductively Coupled Plasma Mass Spectrometry, Biol. Trace Elem. Res., 1997, 67, 109–125 CrossRef PubMed.
- Y. S. Ryabukhin, Activation analysis of hair as an indicator of contamination of man by environmental trace element pollutants. IAEA Report, IAEA/RL/50, Vienna, 1978 Search PubMed.
- A. G. Gaulta, H. A. L. Rowlanda, J. M. Charnocka, R. A. Wogeliusa, I. Gomez-Morillac, S. Vongd, M. Lengd, S. Samrethd, M. L. Sampsond and D. A. Polya, Arsenic in hair and nails of individuals exposed to arsenic-rich groundwaters in Kandal province, Cambodia, Sci. Total Environ., 2008, 393, 168–176 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ew00456e |
| This journal is © The Royal Society of Chemistry 2021 |