Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
From sugars to FDCA: a techno-economic assessment using a design concept based on solvent selection and carbon dioxide emissions
Amir
Al Ghatta
 *a,
James D. E. T.
Wilton-Ely
*a,
James D. E. T.
Wilton-Ely
 *b and
Jason P.
Hallett
*b and
Jason P.
Hallett
 *a
*a
aDepartment of Chemical Engineering, Imperial College, South Kensington Campus, London SW7 2AZ, UK. E-mail: a.al-ghatta16@imperial.ac.uk; j.hallett@imperial.ac.uk
bDepartment of Chemistry, Imperial College, Molecular Sciences Research Hub, White City Campus, London W12 0BZ, UK. E-mail: j.wilton-ely@imperial.ac.uk
First published on 18th January 2021
Abstract
The synthesis of the molecule 2,5-furandicarboxylic acid (FDCA) from sugars is key to unlocking the potential for the replacement of the oil derivative PET (polyethylene terephthalate) by polyethylene furanoate (PEF). Although much research and investment has been dedicated to the synthesis of FDCA, there remains limited commercial activity in this area due to the challenges related to the stability and isolation of the FDCA precursor, 5-hydroxymethylfurfural (HMF). High yields of HMF can be obtained from fructose at high loadings in water–organic solvent mixtures (methyl isobutyl ketone, MIBK; γ-valerolactone, GVL), dimethyl sulfoxide (DMSO) or ionic liquids. Each of these approaches suffers from various drawbacks in terms of catalyst development, product separation and environmental impact. It is therefore necessary to understand which of these processes has the potential for scale-up, while ensuring low environmental impact and a competitive selling price. In this study, a process simulation (rather than a life cycle assessment) was performed to evaluate the associated emissions and selling price of FDCA based on its production using different solvents. It was determined that the cost and CO2 emissions associated with the isolation of HMF undermine the economic and environmental viability of the transformation of sugars to FDCA. In contrast, a two-step, one-pot reaction represents an ideal solution to reduce both cost and environmental impact, making FDCA competitive with terephthalic acid (the corresponding precursor for PET). The choice of solvent and the process were then evaluated and ranked based on safety, CO2 emissions, selling price and state of development though a scoring methodology. A system based on a water/GVL mixture is closer to commercial applicability but the process is limited by extensive formation of humins, which reduces the overall yield of the process, increasing the minimum selling price of FDCA. Using DMSO or ionic liquids minimises emissions and leads to the lowest cost of FDCA but further study is needed to improve the oxidation step. This investigation analyses the possible routes to FDCA from sugars based on the current literature, placing the emphasis on process economics but also considering the CO2 emissions from processing the sugars.
1. Introduction
The processing of renewable feedstocks as substitutes for oil-derived products is one of the main challenges associated with attempts to decrease CO2 emissions and move towards a sustainable economy. Sugars represent the main feedstock for the production of chemicals from biomass and are capable of substituting a large variety of oil derivatives.1–4 The versatile platform chemical, 5-hydroxymethylfurfural (HMF) is one of the key intermediates derived from sugar dehydration that could potentially replace many monomeric building blocks used for different polymers.5,6 FDCA represents the most valuable of these since it is the main monomer used for the synthesis of polyethylene furanoate (PEF), which is widely considered a viable replacement for polyethylene terephthalate (PET) for food and beverage packaging (Fig. 1).7 In contrast to other substitutes for petrochemically-derived plastics, PEF has superior barrier properties, making it an improved option for carbonated drinks and better able to protect the contents from aerial oxidation.7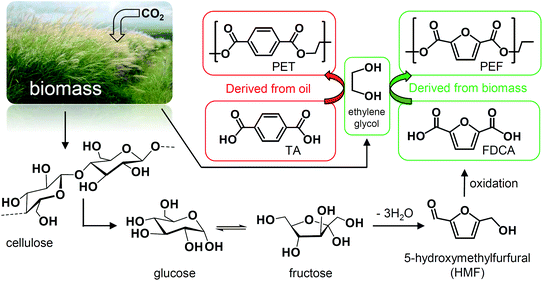 | ||
| Fig. 1 Production of PEF using FDCA generated from sugars as a pathway to substitute the terephthalic acid (TA) used to make PET. | ||
PET production has been optimised through the development of efficient polymerisation technologies and improvements in the synthesis of the monomer terephthalic acid (TA) in high purity and yield from p-xylene.8,9 These efforts have decreased the price of PET remarkably and allowed it to become the first commercialised polyester worldwide. Currently the demand of PET is expected to increase by around 5.6% per year with a current worldwide production of over 73 million tons per year,10,11 and construction of further chemical plants is planned. Recently, Indorama has taken over the construction of the former Mossi & Ghisolfi PET plant to produce 1.2 million tons per year in Corpus Christi (US). This will make it the largest PET plant worldwide and will represent a new milestone in the development of this technology since it will be double the capacity of current PET plants.12,13 In contrast to the well-established status of PET, PEF struggles to attain commercial applicability due to its high cost of production compared to PET, even if the demand for green products (especially in the plastics field) is increasing through consumer choice and environmental legislation.14 Due to the large scale on which PET is produced, the CO2 emissions associated with its lifecycle are extremely high. It has been estimated that if only 20% of the carbon content in PET plastics were substituted with biorenewable carbon, it would lead to a saving of 40 million barrels of oil.15,16 Fozer and co-workers recently performed a life cycle assessment for the production of terephthalic acid (TA) through different biorenewable routes and compared them with traditional routes. This revealed that if p-xylene is substituted with the more sustainable p-cymene, CO2 emissions related to the synthesis of TA could be reduced by a factor of 40.17 However a technoeconomic assessment is needed in order to evaluate the feasibility of this process on a large scale since current biorenewable routes are characterized by significant inefficiencies.
FDCA represents a very promising substitute for TA in the synthesis of PEF but the production cost currently renders it uncompetitive. The reason for this can be traced to the high cost of producing FDCA from sugars, which is adversely impacted by the options available to synthesize the intermediate HMF. The isolation of HMF has proved challenging due to the instability of this molecule and its high affinity with the reaction media, which make solvent extraction or distillation unfeasible.18,19 Moreover, HMF undergoes decomposition even at room temperature, which makes storage on a large scale expensive due to the need for refrigeration.20,21 However, there is still great interest in replacing PET with PEF due to its superior barrier proprieties (e.g., towards O2 and CO2) and the importance of exploiting renewable feedstocks in place of petrochemically-derived precursors to help reduce CO2 emissions.22 Patel and co-workers have estimated that the greenhouse gas (GHG) emissions associated with PET production can be reduced by more than half by substituting terephthalic acid with FDCA.23 This aspect, combined with the lower price of fructose compared to p-xylene, has the potential to deliver a process where both environmental impact and profitability are improved. This is important when considering that the production of TA from p-xylene is characterized by very low margins and is strongly affected by the price volatility of the two compounds. However, the synthesis of PEF and FDCA still needs further development in order to make PEF commercially viable. Various companies are trying to develop a large-scale process to commercialise FDCA on a bulk scale. In 2014, Avabiochem built a pilot plant in Switzerland to produce 20 tons of HMF per year from fructose using a biphasic system based on water and organic solvent. However, the high-purity HMF needed for the synthesis of FDCA is still not economically viable, limiting the scope for speciality chemicals and R&D purposes.24 Avantium has patented the YXY technology designed to produce FDCA from sugars. In this process the sugars are converted to 5-alkoxymethylfurfural with a Lewis or Brønsted acid in a mixture of water and alcohol, bypassing the drawbacks associated with the isolation and stability of HMF.25 The ether is then oxidised to FDCA using the Amoco Mid-Century process, which proved to be more efficient due to the greater stability of the ether derivative compared to HMF.26,27 The main challenge in this process lies in the recyclability of the system, which is limited by the formation of side products, such as humins, which lead to higher purification costs.26
While many catalytic systems have been developed for the efficient synthesis of HMF, most rely on the use of fructose, as direct synthesis of HMF from feedstocks closer to biomass, such as glucose and cellulose, still require major improvements.28–30 Over 80% HMF yield can be obtained from fructose at high substrate loadings (essential for favourable economics) through acid-catalysed dehydration in a biphasic system comprising water and a hydrophobic organic solvent. Various organic solvents proved to be extremely efficient for the extraction of HMF from saturated salt solutions, showing a partition coefficient higher than 1 for many alcohol- and ketone-based organic solvents.31–33 The advantages of using these systems lie in the facile separation of HMF from the water phase compared to other reaction media. For this purpose, methyl isobutyl ketone (MIBK) has proven to be the most suitable solvent, with a good partition coefficient and relatively low boiling point compared to other solvents, such as butanol, which also proved to be suitable for this purpose.34–36 While this system is capable of reaching a high yield of HMF, disadvantages still exist, such as the need for large amounts of organic solvent and harsh reaction conditions (over 150 °C). Dimethyl sulfoxide (DMSO) and ionic liquids have proven to be more favourable reaction media compared to water since higher yields can be achieved at high fructose loadings. Near quantitative yields at high fructose loadings can be achieved using a Brønsted acid in DMSO and ionic liquids37–39 and it is generally accepted that these media behave as both a catalyst and solvent.40–42 DMSO generally requires longer reaction times and higher temperatures, while in ionic liquids high selectivity and over 90% yield can be achieved in the absence of a catalyst.43–46 When catalysts such as Amberlyst 70 and heteropolyacids are used, they can deliver yields close to 100% in short reaction times.47–50 However, the separation of HMF from these solvents is challenging and expensive due to the strong affinity of HMF with the reaction media and the high boiling point of the solvent. This requires vacuum distillation at low pressure or addition of a co-solvent combined with an extraction stage.51–53 Extraction from DMSO was achieved by Gajula and co-workers by diluting the reaction media with water and extracting the HMF with a hydrophobic organic solvent, exploiting the high affinity between water and DMSO. However, this approach suffers from severe drawbacks since it does not guarantee high purity HMF due to the partitioning of DMSO between the phases. It also requires large amounts of organic solvents followed by addition of large volumes of water, which compromises both solvent regeneration and process energy requirements.54 It has been found that HMF can be separated efficiently from hydrophobic, non-coordinating ionic liquids using water, while the partition coefficient is heavily compromised if hydrogen bonding acceptors are present.55 However, these systems are affected by leaching of the ionic liquid into the water phase, which raises issues related to the toxicity and cost of these solvents. While separation is facilitated by the use of ionic liquids with non-coordinating anions, these media lead to substantially lower HMF yields compared to ionic liquids with coordinating anions.19 Therefore, the efficiency of HMF synthesis in DMSO and ionic liquids cannot be usefully exploited due to separation issues. In contrast, FDCA has more favourable physical properties, which can aid the separation from these solvents. For example, the low solubility of FDCA in water can be exploited to precipitate this compound from the reaction mixture. At room temperature, around 45% water composition (in DMSO) decreased the solubility of FDCA to less than 5%, while another study showed that, when using ionic liquids, the amount of water needed is even less (40% for [bmim]Cl and 20% for [bmim]Br; bmim = 1-butyl-3-methylimidazolium) to achieve the same low FDCA solubility.54,55 Therefore, a two-step, one-pot reaction to synthesise FDCA from sugars is needed for these solvents in order to overcome the separation issues. Recently, Dumesic and co-workers established this concept in a GVL–water system in which fructose was dehydrated to HMF in 70% yield and then converted to FDCA in quantitative yield using a Pt/C catalyst. The authors exploited the poor solubility of FDCA at low temperature to separate the product in high purity.56 The same approach has been tried for DMSO and ionic liquids but to date the studies have failed to achieve high FDCA yields from sugars and so further investigation is needed. Liu and co-workers achieved 65% FDCA yield from fructose in DMSO/water mixtures,57 while a heteropolyacid has recently been used to convert glucose and fructose directly to FDCA, albeit in low yield.58 However, these results all required extensive dilution, making product separation impractical, leading to the yields being reported only as non-isolated HPLC yields. The system reported by Dumesic is the only example in which recyclability of solvent and catalyst is combined with FDCA separation. However, this approach is limited by the low yield of fructose dehydration, which has been reported to be inefficient in water/GVL mixtures by other researchers.59
Extensive research has been directed towards the development of catalysts that can maximise the oxidation of HMF to FDCA. Oxidation in pure water as a solvent is already well established and a wide range of catalysts has been reported to achieve quantitative yields under base free conditions, as summarised in various reviews.60 In contrast, catalyst development for this oxidation reaction in DMSO and in ionic liquids has proved to be much more challenging with researchers struggling to achieve the same efficiency as reported in water.61–63
Despite the high level of research activity in this area, it is still not clear which solvent system can guarantee the best process economics with minimum emissions or can be defined as the “greenest” approach. With the prospect that catalyst development will deliver high yields and selectivity for the dehydration and oxidation steps, it is clearly important to ascertain for which solvent system these catalysts should be designed. This would ensure the best economic model for selling FDCA at a competitive price while guaranteeing low CO2 emissions with minimum environmental impact. Indeed, the processing of renewable feedstocks requires that the transformation is low in carbon emissions to avoid undermining the main environmental benefits derived from replacing an oil-based feedstock. For the integrated, high yield production of FDCA from sugars, it appears that water/MIBK, GVL, DMSO and ionic liquids are the most promising solvents to achieve such high yields of FDCA from sugars since they can allow the processing of sugars at high loadings, which is essential for an efficient process design.
In the biphasic water/MIBK system, isolation of HMF is needed in order to proceed to the second oxidation step that uses water as the solvent in a well-established reaction. Alternatively, DMSO and ionic liquids do not require HMF separation as FDCA will precipitate from the solvent on addition of water and cooling. In this context, efficient solvent regeneration is fundamental to limit both the energy expenditure and CO2 emissions and so the energy cost of water removal should be completely or partially compensated by the heat of reaction.
1.1. Framework and objectives
Simulations were performed for different processes to form FDCA from HMF and these were evaluated based on the estimated minimum selling price of FDCA and the CO2 emissions associated with the process. On account of the high cost of all solvents used, the processes were designed so that maximum solvent recovery was achieved. In the course of the process design, the energy to recover and regenerate the solvent was calculated based on CO2 emissions from the generation of steam in a furnace using natural gas as the fuel. Refrigeration cycles were implemented in the process design to estimate the CO2 emissions and their contribution included in the capital and operating costs of the plant. The annual capital and utility costs will ultimately define the cost of the final product. In the case of DMSO and ionic liquids, the initial water content in the solvent was investigated for its impact on the process economics. The cost of isolating HMF was also evaluated to show the benefits of producing FDCA directly, without passing through the prohibitive procedure to isolate HMF. Scenarios based on the use of ionic liquids and DMSO were evaluated with various water contents. For the conversion of HMF to FDCA, at least 20% water content was specified as this is needed in the oxidation reaction to favour the formation of the geminal diols needed for the aldehyde oxidation step.64 For the dehydration reaction, the highest yield and substrate loadings reported in the literature were used for the different processes. For the purposes of the study, complete selectivity for FDCA was assumed in the oxidation step but this is clearly an area in which improvement is needed; this aspect was therefore further evaluated by comparing the results reported in the literature. For the water/GVL system, the yield and process conditions reported by Dumesic and co-workers56 were used since they have already been optimised extensively.Following the process simulation, the aim was to evaluate each process based on the minimum selling price of FDCA, CO2 emissions, solvent cost, safety and the state of development through a scoring method which was used to assign a number between 1 and 3, according to the criteria specified in the methodology.
2. Methodology and strategy
2.1. Parameters for simulation
Aspen Plus v9 was used for process simulation with the integrated Aspen Economics package for estimation of operating and annual costs for a chemical plant processing 300 kg h−1 of fructose.Different thermodynamic models were chosen for each flowsheet. For HMF partitioning between water and MIBK at different salt concentrations, non-random two-liquid model (NRTL) parameters were imported from the study by de Haan and co-workers.65 This model describes in detail and high accuracy the effect of partitioning HMF from water into MIBK through the addition of NaCl, taking into account the salting out effect. An ionic liquid/water equilibrium was simulated using the IULAM database, which has proved to be accurate for the simulation of biphasic systems involving gas/vapour phases and ionic liquids.66 For water/DMSO or GVL solvents, the NRTL database was used.
Crystallisation of the compounds was simulated through a separator unit in Aspen according to the literature conditions54–56 needed to achieve full separation with the enthalpy of crystallisation for HMF and FDCA taken from the literature to be 19.8 kJ mol−1 (NIST67) and 55.1 kJ mol−1,68 respectively. Utilities costs for steam, electricity, and waste water treatment (WWT) were estimated according the guidelines reported by Ulrich and Vasudevan69 for petrochemical plants in the USA, which are based on the utility prices according to the Marshall and Swift (M&S) inflation index and the cost of energy. For waste water treatment (WWT), three different approaches were considered based on the quality of the water to be treated. Table 1 summarises these costs.
| Item | Price ($) |
|---|---|
| a Price obtained from Alibaba Group Holding Limited. b Price obtained from independent Commodity Intelligence Services (ICIS). | |
| Cost of fuel (natural gas)70 | 2.63 per GJ |
| Fructose | 0.6 per kg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Glucose | 0.3 per kg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| Unbleached cellulose | 0.1 per kg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| Oxygen56 | 40 per ton |
| Low pressure steam (3 bar) | 0.116 per kg |
| High pressure steam (10 bar) | 0.120 per kg |
| WWT primary | 0.031 per kg |
| WWT secondary | 0.184 per kg |
| WWT tertiary | 0.574 per kg |
| Electricity | 0.0824 per kW h |
| Cooling water71 | 0.05572 per m3 |
| Marshal and Swift (M&S) | 500 |
The cost of treating the waste water streams was classified based on the treatment method. Distilled water only needs primary filtration, while secondary filtration is required for water that has been in contact with an organic phase or has been used as the reaction medium involving compounds which are biodegradable. Finally, tertiary treatment is used if the streams need chemical processing.
Distillation columns were optimized by first approximation using the short-cut distillation column module (DSTWU) and then re-optimized with RadFrac to obtain the desired purities at minimum boiler heat demand by varying the feed stage position. For multiple effect evaporators, the cost was approximated based on the combination of a heat exchanger and the vessel. Capital costs were calculated based on the installation costs calculated in Aspen Economics and annualized for a period of 10 years. Reactors for dehydration and oxidation were simulated with a stoichiometric reactor. Heats of reaction reported by Aspen were found to be in agreement with the experimental literature with an error of 10%.72
Each flowsheet was optimized through heat integration using pinch point techniques and the costs of heat exchangers were evaluated through the Aspen Energy Analyser. The final energy input required was treated as being supplied by steam generated from methane combustion with an efficiency of 80% and CO2 emissions were calculated accordingly.
2.2. Methodology
The processes being considered will have different configurations based on the processing units required for solvent regeneration. The yield and reaction conditions of the dehydration steps were taken from the literature. Since a large variety of studies are reported, it was considered that maximum yield and selectivity would be achieved for those reactions tested at high loading (30%), providing yields of more than 80%, while all other processes were employed using reported conditions. The discrepancy between the conditions chosen and the ones reported in the literature is discussed in the section on state of development and evaluated using a scoring methodology. Table 2 presents a summary (with references) of the operating conditions and separation strategies. Various processes under different conditions were analysed for the dehydration of fructose. Two different scenarios were analysed for the water–MIBK solvent system to compare the cost of isolation of HMF and the production of FDCA directly in the plant.| Substrate | Solvent | Dehydration | Literature reference | Product separation | Solvent regeneration |
|---|---|---|---|---|---|
| Fructose | Water–MIBK (HMF isolation) | 30% loading | 31–33 and 74 | Antisolvent (hexane) | Multiple effect evaporator, distillation column |
| 150 °C | |||||
| 99% yield | |||||
| Fructose | Water–MIBK (FDCA synthesis) | 30% loading | 32 and 33 | Acidification at room temperature | Multiple effect evaporator, extractor |
| 150 °C | |||||
| 99% yield | |||||
| Fructose | Water–GVL | 15% loading | 56 and 59 | Cooling at 10 °C | Filtration, flash evaporation |
| 180 °C | |||||
| 70% yield | |||||
| Fructose | DMSO | 30% loading | 37 and 75 | Antisolvent (water) | Distillation column |
| 150 °C | |||||
| 99% yield | |||||
| Fructose | [bmim]Cl/Br | 30% loading | 38, 39, 45, 47–50 and 76 | Antisolvent (water) | Multiple effect evaporator, flash evaporation |
| 80–140 °C | |||||
| 99% yield | |||||
| Glucose | [bmim]Cl | 10% loading | 43, 73 and 77 | Antisolvent (water) | Multiple effect evaporator |
| 120 °C | |||||
| 70% yield | |||||
| Cellulose | [bmim]Cl | 10% loading | 77–80 | Antisolvent (water) | Multiple effect evaporator |
| 120 °C | |||||
| 70% yield |
For the isolation of HMF, a multiple effect evaporator was used due to the high boiling point of HMF (116 °C, 1 mbar) and its instability at high temperature. Multiple effect evaporators minimise the energy input by integrating the energy required between two adjacent stages. While this approach is very efficient for concentration of solutions, the complete removal of solvent requires extreme conditions which would lead to excessive use of vacuum. Therefore, a hydrophobic solvent such as hexane is needed to precipitate HMF as a solid, followed by regeneration of the MIBK/hexane mixture by distillation. In other cases, where FDCA is synthesised without HMF isolation, FDCA is precipitated from the solvent by addition of water and cooling to 5 °C. The amount of water required will be discussed in the process description. In the case of ionic liquids, glucose and cellulose are also included in the analysis as potentially cheaper feedstocks since higher yields of HMF can be obtained in these solvents due to the high solvating ability of the ionic liquids towards cellulose and the favourable effect of the anions on the catalytic activity.30,73
For all the processes, the same conditions were considered for the oxidation step, since most studies of this reaction are performed between 120–130 °C at pressures between 3 and 10 bar.57,58,81–84 In order to use units at temperatures lower than 20 °C, vapour absorption and vapour compression cycles were implemented in the process design and costs related to these units and their use were included in the overall plant cost. It has been reported that the choice of catalyst can impact the capital cost of a plant.85 However, the large variety of catalysts reported and the extensive research required for catalyst development in each of the solvents investigated, led to this aspect being excluded in order to yield a fair comparison between the processes. The process evaluation was performed using the scoring methodology reported in Table 3. The carbon dioxide emissions and the minimum selling price (MSP) were calculated based on the results obtained from the process simulation using Aspen Plus. The CO2 emissions were estimated based on the combustion of methane to satisfy the energy demand of the plant, while the minimum selling price of FDCA was calculated based on the annualized utility, feedstock and capital cost. The solvent systems selected have high associated costs and, in a real plant, fresh solvent would need to be integrated into the process periodically (affecting the process economics). Therefore, solvent cost was another factor that was considered in the assessment. The processes were further evaluated based on their state of development and safety, the methodology for this aspect will be discussed in the related paragraph. The criteria used for the evaluation of the process, which are based on the results obtained from the simulations, are reported in Table 3.
| Score | CO2 emissions (kg per ton) | MSP ($ per kg) | Solvent cost ($ per kg) |
|---|---|---|---|
| 1 | >200 | >0.9 | >4 |
| 2 | 100–200 | 0.7–0.9 | 2–4 |
| 3 | <200 | <0.7 | <2 |
2.3. Modelling of refrigeration cycles
Refrigeration is needed for multiple units in the separation sections. Cooling is required for the precipitation of FDCA and condensation of vapours at low pressure in the multiple effect evaporators. In order to estimate both the price and emissions associated with refrigeration, it was assumed that refrigeration is achieved in the plant by ammonia vapour compression and LiBr/water cycles according to the degree of cooling needed and the excess energy available in the plant. In the cases where no excess steam was available, or the degree of cooling was below 0 °C, vapour compression was assumed. When the unit required cooling at temperatures above 1 °C, LiBr vapour absorption was used whenever excess steam from the reaction section was available. Fig. 2 shows the configuration of the two cycles which were implemented in the simulation. The ammonia cycle was simulated using the thermodynamic model ENRTL-RK, which proved to be a very reliable model for ammonia.86 The compressor will increase the ammonia pressure to 12 bar so that the boiling point is high enough to be condensed with cooling water (boiling point of ammonia at 12 bar is 40 °C). The high-pressure liquid ammonia is then expanded so that the boiling point decreases to the required value. In the evaporator the refrigerant will absorb heat via vaporisation.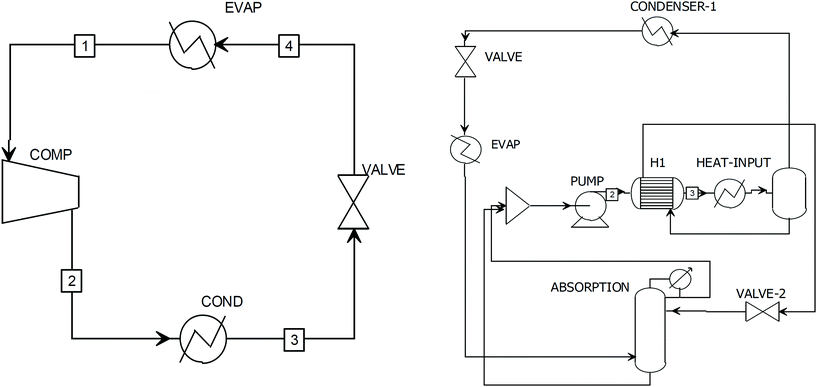 | ||
| Fig. 2 Refrigeration cycles to supply cold streams in the system simulated in Aspen Plus showing the ammonia refrigeration cycle (left) and vapour absorption using water/lithium bromide (right). | ||
Vapour absorption was simulated using the thermodynamic model ELECNRTL with LiBr dissociation in water simulated with the ELEC wizard function in Aspen.87 The simulation was conducted with the same parameters reported by Somers and co-workers87 to generate chilled water at 1 °C. Flow rates of the refrigerant fluid were adjusted using a design specification analysis in Aspen Plus to satisfy the cold utility requirements and maintain the vapour temperature after flash evaporation at 89 °C, which is the optimum condition reported in the literature. The cycle works at two different pressures, 68 mbar and 6.8 mbar. At high pressure the LiBr solution (concentration: 57.4% by mass) is pumped to the heating unit where process steam is recovered prior to heat integration with H1 (Fig. 2, right). The vapour phase separated in the flash evaporator is condensed at high pressure followed by reduction of the pressure through the valve to produce a cold stream to supply the units. The vapours are then recovered in an absorption column equipped with a condenser with the concentrated liquid phase solution coming from the separator. The solution is then recycled to repeat the cycle.
2.4. Energy integration
Heat integration was performed by considering a minimum ΔT of 5 °C. It was assumed that heat from cooling the reactor is used to generate saturated steam at a pressure corresponding to 5 °C less than the operating temperature of the reactor. As a priority, the reactor energy is first integrated for solvent regeneration and interstream integrated using the Aspen Energy Analyser through a pinch point methodology. The remaining heat requirements are supplied by excess energy produced in the reactor or integration with steam supplied by an external combined heat and power plant (CHP) at low or medium pressure according to the operating temperature of the unit. If the heat requirement for all utilities can be satisfied, and excess steam is available, this will be supplied as input for the vapour absorption cycle to generate cold streams to satisfy units operating at temperatures less than 20 °C, if needed.2.5. Estimation of minimum selling price and CO2 emission
The minimum selling price of FDCA is estimated from the operating cost of the plant for utility usage and capital cost. This is estimated by equipment sizing and cost estimation using Aspen Economics with annualisation over 10 years. The minimum selling price is estimated by dividing the total operating cost by the productivity. Carbon dioxide emissions were estimated based on the heat and electricity requirements to generate steam and run the compressors in the plant and the refrigeration units. Steam supplied externally was assumed to be generated by natural gas combustion in a boiler with an efficiency of 80% and the associated CO2 emissions were calculated accordingly. For electricity, compressors were considered as operating at 45% efficiency with CO2 emissions of 450 kg MW−1 h−1.882.6. Abbreviations used to refer to the different processes
For convenience, each process is described by an abbreviation using the format SUBSTRATE–SOLVENT–WATERCONTENT–PRODUCT, as shown in Table 4. For example, F-3-20-FDCA refers to a process using fructose (F) as the substrate with DMSO (3) as the solvent and a water content of 20% to produce FDCA.| Substrate | Solvent | Water content | Product |
|---|---|---|---|
| F = fructose | 1 = water–MIBK | Expressed in % and valid only for DMSO, [bmim]Cl and [bmim]Br | HMF, FDCA |
| G = glucose | 2 = GVL–water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
||
| C = cellulose | 3 = DMSO | ||
| 4 = [bmim]Cl | |||
| 5 = [bmim]Br |
3. Isolation of HMF through a water–MIBK biphasic system
The production of HMF in high yield requires the tuning of multiple parameters for an optimum output. While the acid dehydration reaction is not efficient in pure water, it is very efficient in a biphasic system with the addition of NaCl. The role of the salt is to stabilize HMF and improve the partition coefficient through the salting out effect.89 By moving HMF from the water into the organic phase, the selectivity can be increased remarkably, reaching over 80%. Operating conditions with a water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MIBK ratio of 1
MIBK ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 at 150 °C were employed and these are typical of conditions reported previously to guarantee high selectivity.18–20,60 It was calculated that 15% NaCl loading in the water phase will guarantee complete separation of HMF into the MIBK phase without the need for any further extraction unit. Complete selectivity will be assumed, even if improvements in process conditions are still needed.
7 at 150 °C were employed and these are typical of conditions reported previously to guarantee high selectivity.18–20,60 It was calculated that 15% NaCl loading in the water phase will guarantee complete separation of HMF into the MIBK phase without the need for any further extraction unit. Complete selectivity will be assumed, even if improvements in process conditions are still needed.
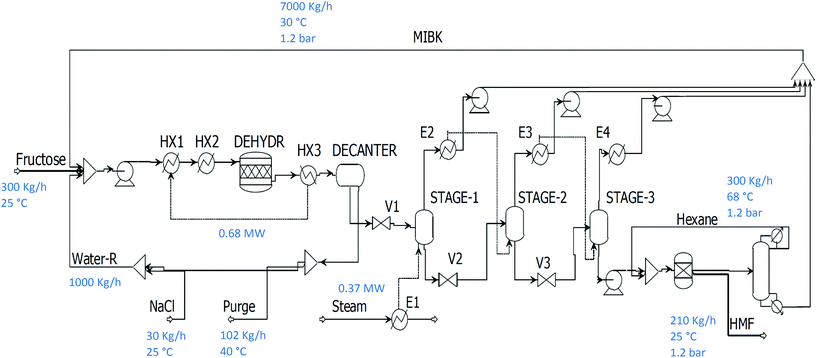
The process is reported in Fig. 3. The reactor operates at 20 bar to avoid any vaporisation of the liquid phase. The reaction mixture is then quenched at 40 °C to favour separation and avoid HMF degradation at high temperature and acidic conditions. The water phase is recycled after being decanted and partially purged to avoid accumulation of water produced during the dehydration. The MIBK phase containing all the HMF is sent to the multiple effect evaporator operating at 1, 0.1 and 0.001 bar. The purpose of the multiple effect evaporator is to concentrate HMF from the organic solvent phase while avoiding HMF evaporation from excessive pressure reduction and heating. It was calculated that a pressure of 1 mbar is the minimum achievable to keep HMF in the liquid phase. The outlet stream from the final stage consists of concentrated HMF at 50% composition (by mass) with the remainder being the residual solvent. Conventional air drying is unsuitable due to the high boiling point and flammability of this solvent. Therefore, it was decided to proceed by adding a hydrophobic, apolar organic solvent to favour precipitation and give high purity. Hexane has been widely used in the literature as an effective antisolvent for this purpose.19 A distillation column operating at 1.2 bar is used to separate hexane from MIBK, followed by recycling of the hexane in the crystalliser, while the MIBK streams are collected and recycled to the feed.
4. Synthesis of FDCA after dissolution of HMF in water
The following configuration exploits the well-known approach for the oxidation of HMF under base-free conditions in water. HMF can be obtained in the water phase using the same process described in the previous paragraph by modifying the separation section. In this case, the process benefits from the fact that full MIBK removal is not necessary. This is because the HMF can be transferred into the water phase using an absorption column from the partially concentrated HMF/MIBK mixture produced by the multiple effect evaporator. In this way, the pressure at the last stage of the multiple effect evaporator can be lower and save refrigeration costs in the exchanger E4 (Fig. 3).The amount of water used to dissolve the HMF needs to produce an HMF concentration under 0.1 M in order to avoid catalyst deactivation in the oxidation unit through premature precipitation of FDCA from the water phase. Separation is then achieved by addition of HCl to obtain the FDCA product by precipitation. Fig. 4 shows the modified separation section used to produce FDCA.
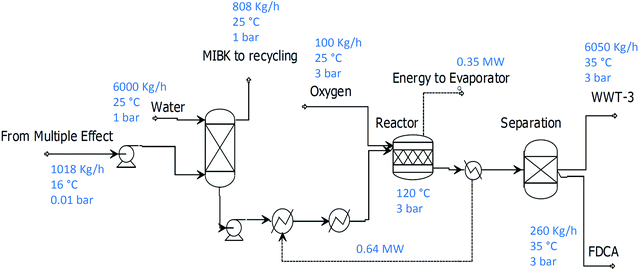 | ||
| Fig. 4 Process flowsheet to produce FDCA by transfer of HMF to the water phase from MIBK, followed by oxidation. | ||
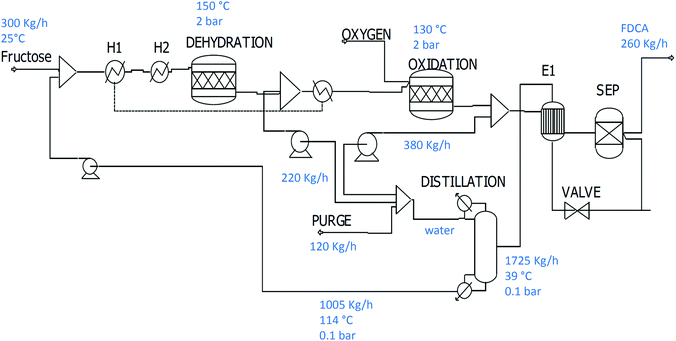
5. Synthesis of FDCA using water–GVL mixtures
This system was simulated using the same parameters reported by Dumesic and co-workers.56 Fructose is obtained at 70% yield using an acid catalyst with any side products separated by activated carbon. The HMF stream is then sent to the oxidation step where full conversion and high yield is achieved (Fig. 5). Product separation is then achieved by cooling and filtration. In order to regenerate the solvent, water produced in the reaction mixture needs to be removed. Through simulation, it has been estimated that a flash evaporator (rather than a distillation) is more viable since the amount of GVL lost through pressure reduction is not large (0.2%) due to the high boiling point of GVL. Moreover, its biodegradability renders this compound harmless to the environment after waste water treatment. The flash evaporator operates at 0.1 bar and the vapours that are condensed are sent to a secondary waste water treatment (WWT-2).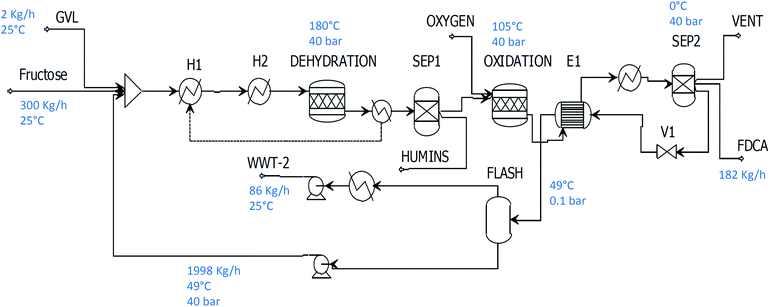 | ||
| Fig. 5 The two stage water/GVL process for the synthesis of FDCA from fructose without product isolation. | ||
The CO2 emissions and FDCA selling price will be influenced also by the nature and capacity of the adsorbent used for the removal of humins. These (carbon-based) adsorbents are generally burned once saturation has been achieved due to the difficulties in their regeneration on account of their high affinity for organic molecules like humins. The carbon footprint for this process would be lower if bio-derived adsorbents were to be used in place of petrochemically-derived adsorbents, though such bio-based materials are currently substantially more expensive. This aspect is not included in the present study since it is currently difficult to assess.
6. Synthesis of FDCA using DMSO
For acid catalysed reactions, dimethyl sulfoxide (DMSO) has been shown to be a good solvent, capable of delivering high selectivity, although high temperatures are required. As reported in the literature, the dehydration reaction is very sensitive to water content,75,90 affecting the kinetics of the reaction. However, a higher tolerance to water would be highly desirable in order to reduce the energy cost during solvent regeneration due to the high boiling point of DMSO (196 °C at 1 atm). Vacuum will need to be applied to reduce the boiling point but this inevitably increases the heat of vaporisation. In this study, the process (Fig. 6) was evaluated at three different solvent compositions, at water contents of 0, 10 and 20%. FDCA was separated by addition of water to arrive at a mass composition of 45%54 and then cooled at 5 °C. Solvent regeneration was performed using a distillation column operating under vacuum prior to heat recovery of the feed.7. Synthesis of FDCA using ionic liquids
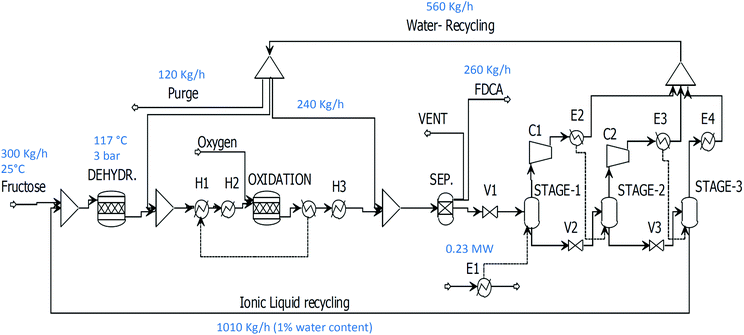
Ionic liquids such as [bmim]Cl and [bmim]Br have proven to be excellent solvents for the high-yield dehydration of sugars to HMF at high loadings under mild conditions across a wide range of temperatures (80–140 °C).38,39,45,47–49,73,76 Moreover, the dehydration proved to be efficient even at high water content and low temperatures.38 The ultimate product, FDCA, can be separated from ionic liquids by water addition. When [bmim]Cl is used as the solvent, a composition of 40% is needed to achieve 5% solubility55 and complete recovery can be achieved by cooling to 5 °C. In the case of [bmim]Br, a smaller amount of water is needed for precipitation. Already at 25% mass composition, the FDCA solubility falls below 5% at room temperature.Regeneration of the ionic liquid solvent is performed in a multiple effect evaporator at different pressures according to the level of water needed in the dehydration step. In this case, compressors need to be used between the stages (Fig. 7) to enhance the heat recovery and guarantee that the minimum ΔT is maintained (5 °C). The heat required to evaporate the water from the ionic liquid is supplied in E1 at 115 °C which is compensated by the heat of reaction produced in the oxidation.
The flowsheet with the configuration of the multiple effect evaporator is shown in Fig. 7 and can be modified according to the water content required in the dehydration step. If 10 or 20% water content is required when [bmim]Cl is used as solvent, only one compressor in STAGE-2 (C2) will be required. When [bmim]Br is used at 20% water content, only one flash evaporator is needed for regeneration and the recovery of FDCA will also be more facile at lower water content. The different process parameters for the evaluation of the drying section are reported in Table 5. The final pressure in STAGE-3 will determine whether refrigeration is needed. The advantages of working with a higher water content lie in the possibility of lower heating requirements and the ability to use a (cheaper) refrigerant suitable for higher temperatures. This could be implemented using vapour absorption produced by the excess heat generated in the plant instead of more expensive vapour compression. For all processes using ionic liquids and fructose as a substrate, the heat of reaction will easily satisfy the requirements for the exchanger in E1. This will not be the case when glucose or cellulose is used, due to the dilute conditions and lower yield in the dehydration step. These factors decrease the available heat in the oxidation step due to the greater water volume that needs to be evaporated as a result of using dilute glucose or cellulose solutions.
| Ionic liquid | Substrate | W in (%) | W out (%) | P 1 (bar) | C 1 (bar) | P 2 (bar) | C 2 (bar) | P 3 (bar) | Heat surplus (%) | E4 (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters are water concentration (Win) by mass fraction, water concentration (Wout) to be achieved. Pi is pressure in STAGE-1 and C1 is the pressure outlet following compression in STAGE-1. Heat surplus refers to the energy available in the plant after integration in the MEE. E4 is the boiling temperature in the condenser. | ||||||||||
| [bmim]Cl | Fructose | 40 | 0 | 0.5 | 2 | 0.01 | 0.8 | 0.001 | 33 | −23 |
| [bmim]Cl | Fructose | 40 | 10 | 0.5 | — | 0.05 | 0.8 | 0.010 | 41 | 7 |
| [bmim]Cl | Fructose | 40 | 20 | 0.8 | — | 0.10 | 0.6 | 0.010 | 45 | 7 |
| [bmim]Br | Fructose | 25 | 20 | — | — | — | — | 0.080 | 58 | 13 |
| [bmim]Cl | Glucose | 40 | 20 | 0.1 | — | 0.01 | 1.0 | 0.001 | 0 | −23 |
| [bmim]Cl | Cellulose | 40 | 20 | 0.1 | — | 0.01 | 1.0 | 0.001 | 0 | −23 |
8. Results: process evaluation
According to the simulations performed for the different processes, the packages and utilities reported in Table 6 are required for each process, contributing to different capital costs and CO2 emissions.| Process | LP steam | HP steam | VAC | VCC | Compressor |
|---|---|---|---|---|---|
| CO2 emissions are associated with steam, vapour compression and compressors. VAC is the vapour absorption cycle, VCC is the vapour compression cycle. Process abbreviations described in section 2.6 and Table 4. | |||||
| F-1-HMF | X | X | X | ||
| F-1-FDCA | X | X | |||
| F-2-FDCA | X | X | |||
| F-3-0-FDCA | X | X | X | ||
| F-3-10-FDCA | X | X | X | ||
| F-3-20-FDCA | X | X | |||
| F-4-0-FDCA | X | X | X | ||
| F-4-10-FDCA | X | X | |||
| F-4-20-FDCA | X | X | |||
| F-5-20-FDCA | |||||
| G-4-20-FDCA | X | X | |||
| C-4-FDCA | X | X | |||
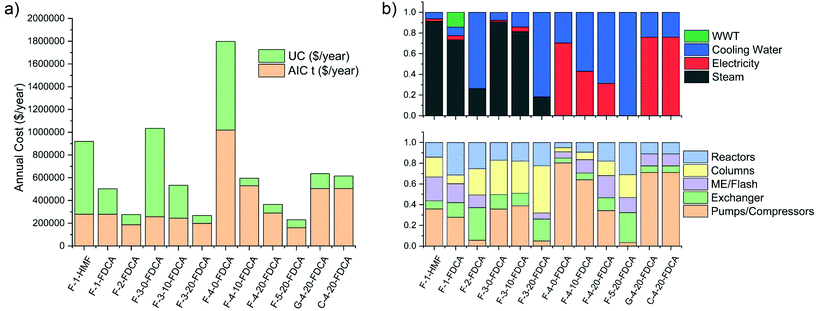
The contribution to the annual operating cost of the different plants is reported in Fig. 8 with the respective contributions from operating and annualized installation costs.
According to the results from the simulations with Aspen Plus, the process to isolate HMF (F-1-HMF) is compromised by its high heat demand, which results in excessive steam usage in the multiple effect evaporators and distillation column. This is in line with the analysis conducted by Dumesic and co-workers, in which HMF was isolated from a mixture of acetone and water at a cost of 1.7$ per kg.91 The process suffers from the major drawback that no heat is produced in the plant since the only reaction is the dehydration of fructose to HMF, which releases no energy for integration. The high utility consumption is due to the low and medium pressure steam with refrigeration needed to condense MIBK at low pressure. Major improvements in the process can be achieved if isolation of HMF is avoided to produce FDCA (F-1-FDCA), since the heat of reaction can be used to partially compensate for the evaporator requirements. In this case, the process requires increased water consumption, resulting in a higher cost, for the extraction of HMF so as to perform the oxidation under dilute conditions. Since separation of HMF is performed at low pH, this stream is not recyclable and will need to be treated in the waste water treatment (WWT) facility. In contrast, the GVL–water (F-2-FDCA) system proved to be much more economical, even if higher flowrates are needed in the system due to the high dilution conditions (Table 2), as no addition of an antisolvent is needed for HMF separation. Solvent regeneration can be achieved simply by flash evaporation, reducing both capital costs and utility expenses. Heats of reaction can satisfy most of the energy requirements for solvent regeneration and, since no excessive refrigeration is needed, the vapour absorption cycle (VAC) will be sufficient to satisfy the demand for cooling, avoiding the need to install compressor units with a high electricity consumption.
In the case of DMSO and [bmim]Cl, the operating costs are closely related to the water content, which directly impacts the utility costs, in the case of DMSO, and capital costs when ionic liquids are used. For DMSO, the major contribution derives from the steam consumption due to the high boiling point of the solvent. For ionic liquids, capital costs are the major contributors due to the need for vapour compressors in the multiple effect evaporator, which is the most expensive process section. In the scenario employing 20% water content (F-3-20-FDCA), these costs are drastically reduced since the oxidation step can fully satisfy the heating and cooling requirements with the vapour absorption cycle (VAC). If a dry ionic liquid is needed (F-4-0-FDCA), the process can largely satisfy the heat requirements, but major expenditures derive from the need for a refrigerant at low temperature, which requires both the installation of a vapour absorption and compression package. For higher water contents (F-4-10, F-4-20), milder conditions are required for solvent regeneration, decreasing the capital cost and refrigerant costs drastically. A more favourable scenario arises when [bmim]Br is used as the solvent (F-5-20), since the much lower solubility of FDCA in this medium avoids the need to employ a multiple effect evaporator or add water. In this case, a simple flash evaporator is needed to regenerate the solvent with no compressors or vapour compression cycle (VCC) required.
The utilization of glucose or cellulose (G-4, C-4) as feedstocks leads to higher capital costs due to the more dilute process conditions required by these substrates. This leads to the need for larger amounts of water to be evaporated with less energy available from the oxidation step due the lower dehydration yield. In this case, lower pressures are needed in the evaporator to achieve the separation but this leads to higher refrigeration costs associated with the compressor in the VCC.
The CO2 emissions and minimum product selling price (MSP) for the different processes are reported in Fig. 9. The main sources of the CO2 emissions are the steam required to regenerate the solvents, the heating of the feed to reach the dehydration temperature and the electricity consumption. Isolation of HMF results in both the highest price and the highest CO2 emissions mainly due to the greater use of steam by the utilities in the plant. Therefore, reduced emissions will be associated with processes that can satisfy the energy required for solvent regeneration through the heat of reaction. In the case of [bmim]Cl (F-4), the heat of reaction easily compensates for the evaporator requirements, but electricity is needed to run the compressors in the inter-stage and refrigeration cycles. It is evident that the processes which can tolerate high water contents are the most economically and environmentally favourable, since the high cost of operating the multiple effect evaporators is greatly reduced when the system is able to tolerate high water content. In the GVL–water process (process F-2), the minimum product selling price (MSP) is high due to the low yielding dehydration of fructose to HMF, which decreases the overall efficiency of the plant. This is the case even when taking into consideration the low capital and utility costs and the excess energy available in the plant. In this case, further valorisation of the side products is needed to make the process more techno-economically efficient. A process which uses [bmim]Br as solvent (F-5) at high water content seems to be ideal, achieving an effective balance between minimising carbon emissions and achieving a low MSP. High yields can be achieved in the dehydration step with no requirement to add water as an antisolvent due to the low solubility of FDCA, which avoids the need to install costly VCC or MEE units.
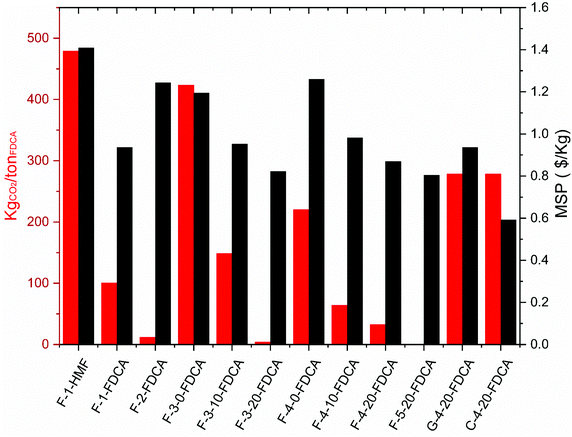 | ||
| Fig. 9 CO2 emissions and minimum selling price (MSP) of product evaluated through different processes. | ||
The use of glucose or cellulose (processes G-4 and C-4) as feedstocks does not offer any clear benefit in terms of carbon emissions since less energy is available to regenerate the solvent and there will be higher water consumption due to the dilute conditions needed to treat these feedstocks. Harsher conditions are then required in the evaporators, as well as integration of a vapour compression cycle (VCC), both of which contribute to higher emissions and higher MSP. However, when inexpensive cellulose is used as a feedstock, the MSP can be improved remarkably and make the process more economically competitive.
An assessment of the CO2 emissions associated with FDCA production has been performed by Patel and co-workers, which estimated a value between 590–970 kgCO2 per tonFDCA using the Amoco Mid-Century oxidation process.23
In processing all feedstocks, the solvent cost need to be considered as they are actually higher than the cost of the feedstock and product. Ionic liquids and GVL are not yet produced on a large scale but estimates are available from the literature. Methyl isobutyl ketone (MIBK) is the cheapest solvent option with a price range between $1.2–1.4 per kilogram, while GVL is estimated to be $2.33 per kilogram.92 Dimethyl sulfoxide (DMSO) is sold at prices between $2–3 per kg while ionic liquids cost around $7 per kg. Table 7 takes these prices into account alongside the evaluation of the processes based on the scoring method mentioned in Section 2.2.
| Process | MSP | CO2 emissions | Solvent cost | Overall |
|---|---|---|---|---|
| F-1-HMF | 1 | 1 | 3 | 5 |
| F-1-FDCA | 1 | 2 | 3 | 6 |
| F-2-FDCA | 1 | 3 | 2 | 6 |
| F-3-0-FDCA | 1 | 1 | 2 | 4 |
| F-3-10-FDCA | 1 | 1 | 2 | 4 |
| F-3-20-FDCA | 2 | 3 | 2 | 7 |
| F-4-0-FDCA | 1 | 1 | 1 | 3 |
| F-4-10-FDCA | 1 | 3 | 1 | 5 |
| F-4-20-FDCA | 2 | 3 | 1 | 6 |
| F-5-20-FDCA | 2 | 3 | 1 | 6 |
| G-4-20-FDCA | 2 | 1 | 1 | 4 |
| C-4-20-FDCA | 3 | 1 | 1 | 5 |
9. Discussion of the state of the art and future development
The process evaluations demonstrate that the solvent and feedstock used have a significant impact on the process economics of the plant. The difficulties in isolating HMF are translated into high costs for the final product and high CO2 emissions. These aspects can be drastically reduced by partially concentrating HMF in the MIBK phase and transferring it into the water phase to perform the oxidation and exploit the heat of this reaction for solvent regeneration. The greatest economy can be achieved using cellulose as the starting material, since the low cost of this feedstock broadly compensates for the dilute conditions needed and the high energy required to regenerate the solvent. In terms of carbon footprint, emissions can be reduced substantially using organic solvent processes based on GVL and DMSO (F-2, F-3-20) which can tolerate high water content. The simulation suggests that a very effective process can be achieved when [bmim]Br (F-5-20) is used as solvent, resulting in minimum emissions and the lowest MSP when fructose is used as the feedstock. These results suggest that there is still a significant need for development to improve yields in order to achieve these optimal values. However, the various processes need different levels of improvement in terms of catalyst development to ensure high yields and maximise the economics of the process. In this context, the dehydration reaction needs less development than the oxidation reaction. Table 8 reports the current state of development for each reaction. This was classified using three levels according to where improvements need to be achieved and whether the process has been demonstrated on a lab scale with isolation of the final product. A score of 3 is equivalent to a fully proven reaction where no further improvements are necessary, while 2 indicates that the reaction is established but needs improvements and 1 denotes a reaction that is currently just a proof of concept.| Process | Dehydration | Oxidation | Separation | Overall |
|---|---|---|---|---|
| Scoring: 3 = fully proven reaction where no further improvements are necessary; 2 = established reaction needing improvements; 1 = reaction that currently exists as a proof of concept. | ||||
| F-1-HMF | 3 | 3 | 3 | 9 |
| F-1-FDCA | 3 | 3 | 3 | 9 |
| F-2-FDCA | 3 | 3 | 3 | 9 |
| F-3-0-FDCA | 3 | 1 | 2 | 6 |
| F-3-10-FDCA | 3 | 1 | 2 | 6 |
| F-3-20-FDCA | 2 | 2 | 2 | 6 |
| F-4-0-FDCA | 3 | 1 | 2 | 6 |
| F-4-10-FDCA | 3 | 1 | 2 | 6 |
| F-4-20-FDCA | 3 | 1 | 2 | 6 |
| F-5-20-FDCA | 3 | 1 | 2 | 6 |
| G-4-20-FDCA | 3 | 1 | 1 | 5 |
| C-4-20-FDCA | 2 | 1 | 1 | 4 |
The steps involving water–MIBK mixtures have already been explored and few improvements are likely to be achieved in terms of reaction conditions since many catalysts have been demonstrated to achieve high yields in this medium. In contrast, the processes using DMSO or ionic liquids face significant challenges in terms of achieving efficient oxidation. So far, few examples have been reported and these systems operate under conditions that are not techno-economically feasible. For example, it was reported that a two-step reaction from fructose to FDCA in DMSO with a water content of 30% is possible, but the conditions used are too dilute to perform the product separation and only HPLC yields are reported.57,81,82 Moreover, this process requires dry DMSO for the dehydration phase and water had to be added subsequently in order to perform the oxidation. Shimizu and co-workers observed that the water generated during the dehydration has a negative impact, leading to over-dehydration and other degradation products.75 In ionic liquids, many different catalysts have been developed for the efficient dehydration using homogeneous and heterogeneous catalysts, which can give near quantitative yields of HMF.38,39,45,47–49,73,76 However, only [bmim]Cl has been explored for the oxidation reaction with Chen and co-workers58 demonstrating that FDCA can be produced from sugars in a one-pot procedure using a polyoxovanadate catalyst. However, the yields from this reaction were found to be low (<50%) and the conditions were too dilute for feasible application. For the same reason, the separation of FDCA after reaction still needs to be demonstrated even though reports have recently appeared, which suggest a potential separation methodology.54,55 However, oversaturation due to FDCA precipitation and the influence of byproducts on the thermodynamics of crystallisation must also be taken into consideration. Glucose dehydration is typically performed using chromium-based catalysts,77 which could also have a negative effect on the separation. Similarly, when unbleached cellulose is used as the feedstock, lignin impurities can impact negatively on the crystallization and could inhibit the dehydration reaction to some extent. To overcome these issues, further studies need to be performed, for example on the compatibility of the Lewis acid catalysts used for the glucose and cellulose dehydration with the catalyst systems needed for the oxidation reaction. Several tin-based systems proved to be efficient for glucose dehydration in [emim]Br (emim = 1-ethyl-3-methylimidazolium), reaching yields greater than 70% at substrate loadings higher than 10%.93,94 Further development is needed to deliver catalysts that can achieve high-yield cellulose conversion to HMF under conditions which favour scale up. So far, only mixtures of Lewis acids95–98 or a two-step process (hydrolysis and dehydration)80,99 appear to give a sufficiently high yield.
The processes which have been proven at lab scale and are closer to scale up are those using organic solvents, such as MIBK (F-1) and GVL (F-2). The former gives a higher yield of reaction and a reduced minimum selling price (MSP) while the latter has lower emissions but a higher MSP due to the inefficiency of the dehydration reaction. However, as reported in Fig. 8, the costs associated with plant installation are much lower, which is an important factor to consider for investment purposes. Accordingly, the scoring for the dehydration, oxidation and separation sections are shown in Table 8. The process F-1-HMF is assigned an arbitrary value of 3 for the (non-existent) oxidation stage as the aim of the process is solely the production of HMF.
10. Solvent safety considerations
The solvent is the main component in the transformation of sugars into FDCA since it influences the formation of side products100 and impacts the technoeconomic feasibility of the process. For safety reasons, flammability, toxicity, stability and biodegradability need to be considered. If flammable solvents are used, major safety issues arise for the chemical plant since the solvent will present a fire and explosion hazard, especially as the oxidation reactions considered here are performed with oxygen at high pressure, which can enhance the explosion hazard. Toxicity is another important factor which needs to be considered and is related to the route of exposure or harmfulness to aquatic life. The volatility of the organic solvent makes the confinement of spillages and their recovery more difficult due to the greater dispersion of vapours compared to liquids, enhancing the issues related to toxicity and flammability. This will result in further costs to ensure the health and safety of personnel and the working environment, as well as the additional cost of installing a flare system in the plant. MIBK is a low boiling point, flammable organic solvent with high toxicity to humans through inhalation,101 although it does not represent an issue in WWT since it is highly biodegradable. Control measures must be in place to minimise the emission of this solvent to the atmosphere. In the processes F-1-HMF and F-1-FDCA, the organic solvent is used as an extracting medium to maximise HMF yield. Since the solvent is not used as a reaction medium, stability issues related to this compound do not need to be considered. In the biphasic system formed, the MIBK phase contains only HMF, while the acid remains in the water phase and so the likelihood of degradation is drastically reduced.In the case of F-2, γ-valerolactone (GVL) is used as the phase for reaction, therefore issues related to stability of this solvent need to be considered. It has been reported that GVL reacts with water under acidic conditions at temperatures higher than 100 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 102 to form 2-hydroxyvaleric acid (2-HVA). However, the nature of the equilibrium for this reaction makes this degradation a minor issue and the solvent system can be considered stable for the dehydration step. Further studies need to be performed to validate GVL stability during the oxidation step. GVL is a high boiling point solvent, therefore the risk of exposure to such an organic solvent is minimal. However, its flammability represents a danger since the oxidation is performed with GVL as a component in the reaction mixture.
102 to form 2-hydroxyvaleric acid (2-HVA). However, the nature of the equilibrium for this reaction makes this degradation a minor issue and the solvent system can be considered stable for the dehydration step. Further studies need to be performed to validate GVL stability during the oxidation step. GVL is a high boiling point solvent, therefore the risk of exposure to such an organic solvent is minimal. However, its flammability represents a danger since the oxidation is performed with GVL as a component in the reaction mixture.
Ionic liquids and DMSO are non-flammable and non-volatile solvents, therefore the oxidation can be performed safely with minimal risk. It has been demonstrated, however, that ionic liquids such as [bmim]Cl exhibit substantial toxicity to aquatic life and to humans when swallowed. However, their negligible vapour pressure makes them very safe to handle in an industrial plant and easy to recover due to their high thermal and chemical stability. In contrast, DMSO has low toxicity to aquatic life and humans on account of its biodegradability but it undergoes decomposition under acidic or basic conditions at high temperatures103,104 and to methyl sulfide and dimethyl sulfide under oxidative conditions.105,106Table 9 summarises these properties using a scoring technique with the aim of evaluating the safety considerations when using these solvents.
| Solvent | Flammability | Toxicity | Volatility | Stability | Biodegradability | Overall safety |
|---|---|---|---|---|---|---|
| Scoring on the basis of 1 being the least favourable and 3 being the most favourable. | ||||||
| MIBK | 1 | 1 | 1 | 3 | 3 | 9 |
| GVL | 2 | 2 | 2 | 2 | 3 | 11 |
| DMSO | 3 | 3 | 2 | 1 | 3 | 12 |
| Ionic liquids | 3 | 1 | 3 | 3 | 1 | 11 |
11. Overall evaluation of the processes
Fig. 10 shows a summary of the different evaluations performed to determine process considerations, state of development and safety. According to this analysis, the organic solvent GVL system (F-2) results in the most favourable process largely due to the advanced state of the development and safety. The DMSO (F-3-20) and ionic liquid (F-5-20) processes still need substantial research and development but they have great potential to enable a successful, economical, safe and efficient process. In particular, the high cost and moderate toxicity associated with ionic liquids requires a strong justification for their use, which the current state of development does not yet deliver. Dimethyl sulfoxide (F-3-20) proved to be the most promising solvent thanks to its relatively environmentally friendly properties but there is currently a lack of evidence for the ability of this solvent to achieve high yields for both dehydration and oxidation reactions. Processes using the organic solvent MIBK are more developed but they are compromised in terms of process performance and safety due to the high selling price and substantial associated CO2 emissions. In Fig. 10b, these considerations are summarised according to the degree of development of each process evaluated as a percentage of maximum score. The processes with GVL and MIBK lie on the far left of the graph, indicating an advanced state of development. At the bottom right of Fig. 10b are shown the processes with low scores for process economics and development, which correspond to those based on fructose with low water content and those using glucose or cellulose as a feedstock. On the top right are the most promising processes where further research and development is needed.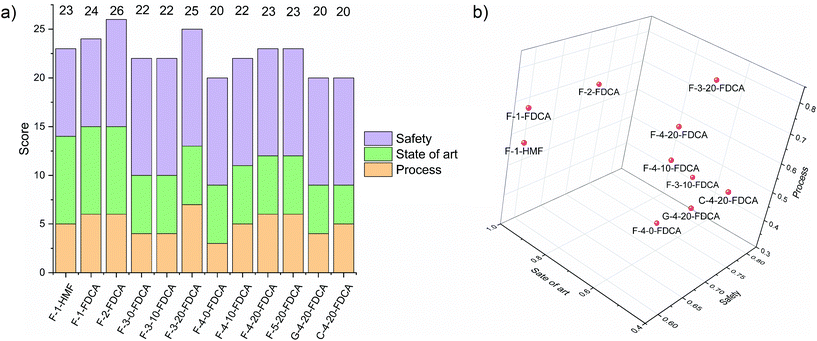 | ||
| Fig. 10 Final score results for the different processes showing (a) evaluation of each process based on the sum of all scores and (b) each category evaluated based on degree of advancement. | ||
12. Conclusions
The dehydration of sugars to HMF followed by subsequent oxidation to FDCA has experienced huge interest but, as yet, no commercial activity. Research has mostly been focused on the use of different solvents which ensure high dehydration yields, each of which displays different advantages and disadvantages. From our analysis, the isolation of HMF is techno-economically unfeasible due to the high cost associated with the heat demand. By avoiding HMF isolation and performing the oxidation in situ, the synthesis of FDCA becomes economically more viable. The necessary balance between heat and refrigeration requirements indicates that, for DMSO and ionic liquids, the water content is an important parameter necessary to achieve an acceptable selling price and minimise CO2 emissions. The system based on the use of GVL as the solvent has a high selling price due to the limited yield in the dehydration step but it represents the best compromise between process evaluation, state of development and safety. Dimethyl sulfoxide (DMSO) is a promising alternative but it requires more development to make the dehydration step tolerant to high water content and high fructose concentration. In the same way, ionic liquids can deliver good process economics but their high cost and toxicity still needs to be offset by further improvements in the oxidation step, which has not yet developed beyond proof of concept work. The use of MIBK to produce FDCA is well established but has limitations due to the use of a volatile flammable organic solvent with a large carbon footprint for the process. The use of a cheaper feedstock such as glucose does not bring any economic advantage to the process due to the low yield of dehydration to HMF and the dilute conditions needed. In contrast, the use of cellulose could reduce the process costs substantially, but further development is needed before it can be used directly as a feedstock in such processes.Conflicts of interest
There are no conflict of interest to declare.Acknowledgements
The Imperial College President's PhD Scholarship programme is gratefully acknowledged for funding (A. A. G.). The same author wishes to thank the EPSRC for the award of an EPSRC Doctoral Prize Fellowship. We acknowledge the contribution of L. Fan, who conducted a preliminary assessment ahead of the study.References
- S. Takkellapati, T. Li and M. A. Gonzalez, Clean Technol. Environ. Policy, 2018, 20, 1615–1630 CrossRef CAS.
- K. Wilson and A. F. Lee, in Advances in Biorefineries: Biomass and Waste Supply Chain Exploitation, Elsevier Ltd., 2014, pp. 624–658 Search PubMed.
- G. Li, W. Liu, C. Ye, X. Li and C.-L. Si, Int. J. Polym. Sci., 2018, 4, 1–21 Search PubMed.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick Jr., J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS.
- J. Tomaszewska, D. Bieliński, M. Binczarski, J. Berlowska, P. Dziugan, J. Piotrowski, A. Stanishevsky and I. A. Witońska, RSC Adv., 2018, 8, 3161–3177 RSC.
- J. Deng, X. Liu, C. Li, Y. Jiang and J. Zhu, RSC Adv., 2015, 5, 15930–15939 RSC.
- J. G. Rosenboom, D. K. Hohl, P. Fleckenstein, G. Storti and M. Morbidelli, Nat. Commun., 2018, 9, 1–7 CrossRef CAS.
- D. I. Collias, A. M. Harris, V. Nagpal, I. W. Cottrell and M. W. Schultheis, Ind. Biotechnol., 2014, 10, 91–105 CrossRef CAS.
- L. N. Ji, Appl. Mech. Mater., 2013, 312, 406–410 Search PubMed.
- Polyethylene Terephthalate Market Size | Industry Report, 2019–2025, https://www.grandviewresearch.com/industry-analysis/polyethylene-terephthalate-market (accessed 18 October 2020).
- PET global production 2020 | Statista, https://www.statista.com/statistics/650191/global-polyethylene-terephthalate-production-outlook (accessed 18 October 2020).
- Acquisition of former M&G US PET plant completed | ICIS, https://www.icis.com/explore/resources/news/2019/01/02/10301262/acquisition-of-former-mg-us-pet-plant-completed (accessed 18 October 2020).
- PET production capacity of plants in Europe | Statista, https://www.statista.com/statistics/952727/pet-production-capacity-of-plants-in-europe/, (accessed 18 October 2020).
- E4tech, Re-Cord and Wur, From the Sugar Platform to biofuels and biochemicals, 2015 Search PubMed.
- Naturally good? Searching for new bio-based raw materials for industry, https://www.basf.com/global/en/media/magazine/archive/issue-6/naturally-good-searching-for-new-bio-based-raw-materials-for-industry.html (accessed 18 October 2020).
- S. Madival, R. Auras, S. P. Singh and R. Narayan, J. Cleaner Prod., 2009, 17, 1183–1194 CrossRef CAS.
- M. Volanti, D. Cespi, F. Passarini, E. Neri, F. Cavani, P. Mizsey and D. Fozer, Green Chem., 2019, 21, 885–896 RSC.
- M. Dashtban, A. Gilbert and P. Fatehi, RSC Adv., 2014, 4, 2037–2050 RSC.
- A. Al Ghatta, J. D. E. T. Wilton-Ely and J. P. Hallett, ChemSusChem, 2019, 12, 4452–4460 CrossRef.
- K. I. Galkin and V. P. Ananikov, ChemSusChem, 2019, 12, 185–189 CrossRef CAS.
- K. I. Galkin, E. A. Krivodaeva, L. V. Romashov, S. S. Zalesskiy, V. V. Kachala, J. V. Burykina and V. P. Ananikov, Angew. Chem., Int. Ed., 2016, 55, 8338–8342 CrossRef CAS.
- M. B. Banella, J. Bonucci, M. Vannini, P. Marchese, C. Lorenzetti and A. Celli, Ind. Eng. Chem. Res., 2019, 58, 8955–8962 CrossRef CAS.
- A. J. J. E. Eerhart, A. P. C. Faaij and M. K. Patel, Energy Environ. Sci., 2012, 5, 6407–6422 RSC.
- T. Kläusli, Green Process. Synth., 2014, 3, 235–236 Search PubMed.
- G. J. M. Gruter and F. Dautzenberg, EP pat, 1834950A1, 2006 Search PubMed.
- J. C. van der Waal, E. Mazoyer, H. J. Baars and G.-J. M. Gruter, in Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications and Academic Perspectives, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2016, pp. 311–329 Search PubMed.
- E. Mazoyer, A. S. V. D. S. Dias, B. McKay, H. J. Baars, V. P. C. Vreeken, G. J. M. Gruter and D. L. Sikkenga, US patent, 9643945B2, 2013 Search PubMed.
- H. Wang, C. Zhu, D. Li, Q. Liu, J. Tan, C. Wang, C. Cai and L. Ma, Renewable Sustainable Energy Rev., 2019, 103, 227–247 CrossRef CAS.
- T. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548–572 RSC.
- R.-J. J. Van Putten, J. C. van der Waal, E. De Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS.
- T. Tuercke, S. Panic and S. Loebbecke, Chem. Eng. Technol., 2009, 32, 1815–1822 CrossRef CAS.
- Y. Román-Leshkov and J. A. Dumesic, Top. Catal., 2009, 52, 297–303 CrossRef.
- Y. Roman-Leshkov, J. N. Chheda and J. a Dumesic, Science, 2006, 312, 1933–1937 CrossRef CAS.
- V. V. Ordomsky, J. Van Der Schaaf, J. C. Schouten and T. A. Nijhuis, J. Catal., 2012, 287, 68–75 CrossRef CAS.
- G. Tsilomelekis, T. R. Josephson, V. Nikolakis and S. Caratzoulas, ChemSusChem, 2014, 7, 117–126 CrossRef CAS.
- C. Moreau, R. Durand, S. Razigade, J. Duhamet, P. Faugeras, P. Rivalier, R. Pierre and G. Avignon, Appl. Catal., A, 1996, 145, 211–224 CrossRef CAS.
- T. Thananatthanachon and T. B. Rauchfuss, ChemSusChem, 2010, 3, 1139–1141 CrossRef CAS.
- C. Li, Z. K. Zhao, A. Wang, M. Zheng and T. Zhang, Carbohydr. Res., 2010, 345, 1846–1850 CrossRef CAS.
- F. Liu, J. Barrault, K. De Oliveira Vigier and F. Jérôme, ChemSusChem, 2012, 5, 1223–1226 CrossRef CAS.
- L.-K. K. Ren, L.-F. F. Zhu, T. Qi, J.-Q. Q. Tang, H.-Q. Q. Yang and C.-W. W. Hu, ACS Catal., 2017, 7, 2199–2212 CrossRef CAS.
- J. J. Li, J. J. Li, D. Zhang and C. Liu, J. Phys. Chem. B, 2015, 119, 13398–13406 CrossRef CAS.
- C. Shi, Y. Zhao, J. Xin, J. Wang, X. Lu, X. Zhang and S. Zhang, Chem. Commun., 2012, 48, 4103–4105 RSC.
- S. Eminov, A. Brandt, J. D. E. T. Wilton-Ely and J. P. Hallett, PLoS One, 2016, 11, 1–15 CrossRef.
- S. Eminov, J. D. E. T. Wilton-Ely and J. P. Hallett, ACS Sustainable Chem. Eng., 2014, 2, 978–981 CrossRef CAS.
- Y. N. Li, J. Q. Wang, L. N. He, Z. Z. Yang, A. H. Liu, B. Yu and C. R. Luan, Green Chem., 2012, 14, 2752–2758 RSC.
- M. E. Zakrzewska, E. Bogel-łukasik and R. Bogel-łukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS.
- M. Benoit, Y. Brissonnet, E. Guélou, K. De Oliveira Vigier, J. Barrault and F. Jérôme, ChemSusChem, 2010, 3, 1304–1309 CrossRef CAS.
- Y. Xiao and Y. F. Song, Appl. Catal., A, 2014, 484, 74–78 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. M. Aida and R. L. L. Smith, ChemSusChem, 2009, 2, 944–946 CrossRef CAS.
- E. A. Khokhlova, V. V. Kachala, V. P. Ananikov and N. D. Zelinsky, Russ. Chem. Bull., 2013, 62, 829–0834 Search PubMed.
- Z. Wei, Y. Liu, D. Thushara and Q. Ren, Green Chem., 2012, 14, 1220–1226 RSC.
- C. Shi, J. Xin, X. Liu, X. Lu and S. Zhang, ACS Sustainable Chem. Eng., 2016, 4, 557–563 CrossRef CAS.
- X. Sun, Z. Liu, Z. Xue, Y. Zhang and T. Mu, Green Chem., 2015, 17, 2719–2722 RSC.
- S. Gajula, K. Inthumathi, S. R. Arumugam and K. Srinivasan, ACS Sustainable Chem. Eng., 2017, 5, 5373–5381 CrossRef CAS.
- A. Al Ghatta, J. D. E. T. Wilton-Ely and J. P. Hallett, ACS Sustainable Chem. Eng., 2019, 7, 16483–16492 CrossRef.
- A. Hussain Motagamwala, W. Won, C. Sener, D. M. Alonso, C. T. Maravelias and J. A. Dumesic, Sci. Adv., 2018, 4, 1–8 Search PubMed.
- H. Liu, X. Cao, T. Wang, J. Wei, X. Tang, X. Zeng, Y. Sun, T. Lei, S. Liu and L. Lin, J. Ind. Eng. Chem., 2019, 77, 209–214 CrossRef CAS.
- R. Chen, J. Xin, D. Yan, H. Dong, X. Lu and S. Zhang, ChemSusChem, 2019, 12, 2715–2724 CrossRef CAS.
- L. Qi, Y. F. Mui, S. W. Lo, M. Y. Lui, G. R. Akien and I. T. Horváth, ACS Catal., 2014, 4, 1470–1477 CrossRef CAS.
- M. Sajid, X. Zhao and D. Liu, Green Chem., 2018, 20, 5427–5453 RSC.
- D. Yan, J. Xin, Q. Zhao, K. Gao, X. Lu, G. Wang and S. Zhang, Catal. Sci. Technol., 2018, 8, 164–175 RSC.
- Z. Yang, W. Qi, R. Su and Z. He, Energy Fuels, 2016, 31, 533–541 CrossRef.
- T. Stahlberg, E. Eyjolfsdottir, Y. Y. Gorbanev, I. Sdaba and A. Riisager, Catal. Lett., 2012, 142, 1089–1097 CrossRef CAS.
- A. Al Ghatta, J. D. E. T. Wilton-Ely and J. P. Hallett, ACS Sustainable Chem. Eng., 2020, 8, 2462–2471 CrossRef CAS.
- S. Altway, S. C. Pujar and A. B. de Haan, Fluid Phase Equilib., 2018, 475, 100–110 CrossRef CAS.
- V. R. Ferro, C. Moya, D. Moreno, R. Santiago, J. De Riva, G. Pedrosa, M. Larriba, I. Diaz and J. Palomar, Ind. Eng. Chem. Res., 2018, 57, 980–989 CrossRef CAS.
- Chemical Properties of 2-Furancarboxaldehyde, 5-(hydroxymethyl)-(CAS 67-47-0), https://www.chemeo.com/cid/27-602-3/2-Furancarboxaldehyde%2C5-%28hydroxymethyl%29-#ref-nist-webbook (accessed 4 November 2020).
- H. Ban, T. Pan, Y. Cheng, L. Wang and X. Li, J. Chem. Eng. Data, 2018, 63, 1987–1993 CrossRef CAS.
- G. D. Ulrich and P. T. Vasudevan, Chem. Eng., 2006, 6, 66–69 Search PubMed.
- Natural gas prices in the United States and Europe from 1980 to 2030 (in U.S. dollars per million British thermal units), https://www.statista.com/statistics/252791/natural-gas-prices (accessed 4 November 2020).
- Cooling Water Prices | Utilities Cost Database | Intratec.us, https://www.intratec.us/chemical-markets/cooling-water-price (accessed 9 March 2020).
- S. P. Verevkin, V. N. Emel'yanenko, E. N. Stepurko, R. V. Ralys, D. H. Zaitsau and A. Stark, Ind. Eng. Chem. Res., 2009, 48, 10087–10093 CrossRef CAS.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS.
- H. Xia, S. Xu, X. Yan and S. Zuo, Fuel Process. Technol., 2016, 152, 140–146 CrossRef CAS.
- K. Shimizu, R. Uozumi and A. Satsuma, Catal. Commun., 2009, 10, 1849–1853 CrossRef CAS.
- C. Moreau, A. Finiels and L. Vanoye, J. Mol. Catal. A: Chem., 2006, 253, 165–169 CrossRef CAS.
- C. Li, Z. Zhang and Z. K. Zhao, Tetrahedron Lett., 2009, 50, 5403–5405 CrossRef CAS.
- S. Eminov, P. Filippousi, A. Brandt, J. Wilton-Ely and J. Hallett, Inorganics, 2016, 4, 1–15 CrossRef.
- B. Kim, J. Jeong, D. Lee, S. Kim, H.-J. Yoon, Y.-S. Lee and J. K. Cho, Green Chem., 2011, 13, 1503 RSC.
- Y. Zhang, H. Du, X. Qian and E. Y. X. Chen, Energy Fuels, 2010, 24, 2410–2417 CrossRef CAS.
- S. Wang, Z. Zhang and B. Liu, ACS Sustainable Chem. Eng., 2015, 3, 406–412 CrossRef CAS.
- G. Chen, L. Wu, H. Fan and B. G. Li, Ind. Eng. Chem. Res., 2018, 57, 16172–16181 CrossRef CAS.
- G. Yi, S. P. Teong and Y. Zhang, Green Chem., 2016, 18, 979–983 RSC.
- D. K. Mishra, H. J. Lee, J. Kim, H. S. Lee, J. K. Cho, Y. W. Suh, Y. Yi and Y. J. Kim, Green Chem., 2017, 19, 1619–1623 RSC.
- C. Triebl, V. Nikolakis and M. Ierapetritou, Comput. Chem. Eng., 2013, 52, 26–34 CrossRef CAS.
- R. Mansouri, I. Boukholda, M. Bourouis and A. Bellagi, Energy, 2015, 93, 2374–2383 CrossRef CAS.
- C. Somers, A. Mortazavi, Y. Hwang, R. Radermacher, P. Rodgers and S. Al-Hashimi, Appl. Energy, 2011, 88, 4197–4205 CrossRef CAS.
- E. & I. S. Department for Business, 2018 Government GHG Conversion Factors for Company Reporting, 2018 Search PubMed.
- S. Mohammad, C. Held, E. Altuntepe, T. Köse and G. Sadowski, J. Phys. Chem. B, 2016, 120, 3797–3808 CrossRef CAS.
- K. Dong, J. Zhang, W. Luo, L. Su and Z. Huang, Chem. Eng. J., 2018, 334, 1055–1064 CrossRef CAS.
- A. H. Motagamwala, K. Huang, C. T. Maravelias and J. A. Dumesic, Energy Environ. Sci., 2019, 12, 2212–2222 RSC.
- J. Byun and J. Han, Energy, 2019, 175, 546–553 CrossRef CAS.
- Q. Xu, Z. Zhu, Y. Tian, J. Den, J. Shi and Y. Fu, BioResources, 2014, 9, 303–315 CAS.
- M. Zhang, K. Su, H. Song, Z. Li and B. Cheng, Catal. Commun., 2015, 69, 76–80 CrossRef CAS.
- L. Zhou, R. Liang, Z. Ma, T. Wu and Y. Wu, Bioresour. Technol., 2013, 129, 450–455 CrossRef CAS.
- Y. Zhang, J. Pan, M. Gan, H. Ou, Y. Yan, W. Shi and L. Yu, RSC Adv., 2014, 4, 11664–11672 RSC.
- Y. Su, M. H. Brown, G. Li, X. Zhou, J. E. Amonette, L. J. Fulton, D. M. Camaioni and Z. C. Zhang, Appl. Catal., A, 2011, 391, 436–442 CrossRef CAS.
- Y. Su, M. H. Brown, X. Huang, X. Zhou, J. E. Amonette and Z. C. Zhang, Appl. Catal., A, 2009, 2, 117–122 CrossRef.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Cellulose, 2011, 18, 1327–1333 CrossRef CAS.
- A. Al Ghatta, X. Zhou, G. Casarano, J. D. E. T. Wilton-Ely and J. P. Hallett, ACS Sustainable Chem. Eng., 2021 DOI:10.1021/acssuschemeng.0c07963.
- M. D. Stout, R. A. Herbert, G. E. Kissling, F. Suarez, J. H. Roycroft, R. S. Chhabra and J. R. Bucher, Toxicology, 2008, 244, 209–219 CrossRef CAS.
- C. Yuet Yan Wong, A. Wing-Tat Choi, M. Y. Lui, B. Fridrich, A. K. Horváth, L. T. Mika and I. T. Horváth, Struct. Chem., 2016, 28, 1–7 Search PubMed.
- Z. Wang, S. M. Richter, B. D. Gates and T. A. Grieme, Org. Process Res. Dev., 2012, 16, 1994–2000 CrossRef CAS.
- T. Santosusso and D. Swern, J. Org. Chem., 1976, 41, 2762–2768 CrossRef CAS.
- G. A. Halliday, R. J. Young and V. V. Grushin, Org. Lett., 2003, 5, 2003–2005 CrossRef CAS.
- X. Xiang, L. He, Y. Yang, B. Guo, D. Tong and C. Hu, Catal. Lett., 2011, 141, 735–741 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |