Encapsulation of lead halide perovskite nanocrystals (NCs) at the single-particle level: strategies and properties
Qixuan
Zhong
,
Muhan
Cao
 * and
Qiao
Zhang
* and
Qiao
Zhang
 *
*
Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University, No. 199 Ren'ai Road, Suzhou 215123, Jiangsu, People's Republic of China. E-mail: mhcao@suda.edu.cn; qiaozhang@suda.edu.cn
First published on 3rd November 2021
Abstract
Lead halide perovskite NCs (APbX3, A = formamidinium (FA), methylammonium (MA) or Cs; X = Cl, Br, I or their mixture) have attracted unprecedented attention due to their excellent photophysical properties and wide application prospects. However, the inherent ionic structure of APbX3 NCs makes them very sensitive to external conditions such as water and oxygen, resulting in poor stability. As a feasible strategy, encapsulation is considered to be effective in improving the stability. In this minireview, we focus on single-particle-level coating, which not only can improve the stability but also maintain the nano effect of the original NCs. This review summarizes the fundamental information on APbX3 NCs and the necessity of single-particle-level coating. Subsequently, a variety of heterostructures at the single-particle level are introduced in detail. Then, their applications are summarized. Moreover, we discuss the challenges and prospects of the single-particle-level heterostructures based on APbX3 NCs.
1. Introduction
Metal halide perovskites were first discovered in 1893,1 but it was not until 2009 that they became a focus of attention, owing to their very first application in photovoltaic devices.2 In 2015, Kovalenko's group3 reported, for the first time, the preparation of all-inorganic lead halide perovskite CsPbX3 NCs (X = Cl, Br, I or their mixture). Their excellent photophysical properties, including high brightness, tunable emission, high color purity (narrow emission peak), high light absorption coefficient, and high defect tolerance, make them promising in optoelectronic fields such as solar cells,4 light-emitting diodes (LEDs),5,6 and so on. For example, CsPbX3-based LEDs have achieved a high external quantum efficiency (EQE) of 12.3% for blue LED,5 22% for green LED,5 and 23% for red LED.7 Considering their outstanding properties and promising performance in devices, APbX3 NCs have been regarded as one of the next-generation optoelectronics candidates.8–11For practical applications, stability is a particularly important parameter. APbX3 NCs are suitable for optoelectronic devices; however, their poor stability is a shortcoming that limits their practical applications. The suboptimal stability of APbX3 NCs is mainly attributed to their inherent ionic properties as well as the capping ligands that are dynamically balanced on the surface.12,13 To enhance durability, a direct and effective method is to prepare highly stable APbX3 NCs encapsulated in an inert shell, such as polymers and oxides.14,15 These shells can significantly improve the stability of APbX3 NCs against environmental stimuli. Depending on the number of APbX3 NCs in one shell, the encapsulation strategies can be divided into multi-particle and single-particle coating. Multi-particle coating has been extensively studied because of the simple synthetic methods. The resulting products usually have a large particle size, which may reach up to several micrometers.16–22 This large size limits the applications of APbX3 NCs at the nanometer scale. Moreover, multi-particle aggregations are not conducive to study the influence of the shell on the photoelectric properties of APbX3 NCs. In contrast, single-particle-level coated APbX3 NCs, in which one APbX3 NC is wrapped by one shell, exhibit significantly enhanced stability with the protection of the inert shell. The single-particle-scale modification can well maintain the properties of APbX3 at the nanoscale, ideal for application in some special fields, such as biological imaging.23 More importantly, the optical and photoelectric properties can be tuned, enabled by passivation of the NC surface.
In this review article, we focus on summarizing the preparations, properties, and applications of encapsulated APbX3 NCs at the single-particle level. The first part introduces the design principle for encapsulated APbX3 NCs. In the second part, APbX3-based heterostructures at the single-particle level are summarized. The third part presents the applications of APbX3-based heterostructures. An outlook from our viewpoint is provided to depict the challenges and opportunities with the single-particle encapsulation of APbX3 NCs in the last part.
2. Design principle of encapsulated APbX3 NCs
2.1 Crystal structure of APbX3 NCs
Taking CsPbX3 as an example, Fig. 1a shows a typical cubic CsPbX3 structure, which is highly symmetric. The Pb ion octahedrally coordinates with six halide (X) ions in a [PbX6]4− configuration, forming a 3D framework perovskite structure through a continuous array of corner-sharing [PbX6]4− octahedrons. CsPbX3 NCs are ionic compounds that can be easily destructed in the presence of polar solvents. The interactions between CsPbX3 and surface ligands present ionic characteristics as well.3,38 The dynamic oleic acid (OA) and oleylamine (OLA) are not tightly bound to the surface of CsPbX3 NCs.39 Instead, proton exchange between coordinated OLA and OA may induce the detachment of ligands (Fig. 1b), resulting in more trap sites and poor optoelectronic performance.39,40 Fully considering the application occasions of CsPbX3 NCs, environmental stimuli, including light, humidity, heat, and oxygen, will cause the collapse of their structure and quenching of optical properties. Therefore, CsPbX3 NCs with high stability and excellent photoelectric properties are urgently required.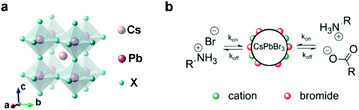 | ||
| Fig. 1 (a) Schematic of the cubic perovskite lattice. (b) Schematic representation of the dynamic surface stabilization by oleylammonium bromide, oleylammonium oleate and OLA. Reproduced with permission from ref. 39. Copyright 2016 American Chemistry Society. | ||
2.2 Single-particle-level coated APbX3 NCs
In recent years, some works have been reported to prepare highly stable core/shell APbX3 NCs. Researchers have developed a variety of strategies for encapsulation of APbX3 NCs with inert materials to enhance their stability against environmental stimuli.13,41–43 The multi-particle coating strategy has been widely reported because of its mild reaction conditions. Amorphous inert materials, including polymers,44–46 silica,22,47 alumina,20etc., are usually selected as shell materials to prepare stable structures. Although the coated perovskites exhibit excellent stability, the obtained samples are usually large and even in bulk form, no longer having the superior properties of nanoscale particles that can be used for important applications, e.g., nanoscale optical research and biological fields. Therefore, it is urgently desired but still challenging to synthesize nanosized encapsulated APbX3 NCs that are normally single-particle coated.Here, we list the difficulties in preparing single-particle coated APbX3 NCs: (1) surface ligand intervention. Most of the reported APbX3 NCs are hydrophobic because their surface is covered by long-alkyl-chain ligands. Since some of the reported shell materials, such as SiO2 and Al2O3, are hydrophilic, it is not easy to grow hydrophilic materials onto the hydrophobic surface directly. (2) Restriction of overgrowth conditions. Some shell materials have to be prepared in the presence of high temperature or polar solvents (such as water). However, unprotected perovskite materials cannot withstand such harsh preparation conditions. (3) Lattice mismatching. To realize the coating of crystalline materials, lattice matching needs to be considered. Due to the particularity of the perovskite structure, it will be better to introduce lattice-matched materials as the shell of APbX3 NCs. Many efforts have been made to overcome these problems to prepare single-particle-coated APbX3 NCs. In the next section, we will summarize some typical cases according to different shell materials.
3. APbX3-based heterostructures
Depending on shell materials, this section is divided into four parts: APbX3/oxide, APbX3/semiconductor, APbX3/metal, and others. The growth mechanism and photophysical properties of different core/shell heterostructures are discussed in this section, and a comparation of the stability before and after encapsulation is listed in Table 1.| Shell materials | Compound | Structure | PL peak [nm] | PLQY (%) | Stability before coating | Stability after coating | Ref. |
|---|---|---|---|---|---|---|---|
| Oxides | CsPbBr3/SiO2 | Janus | 517 | 80 | Remnant PL 17.8% after 7 days water treatment; remnant PL 84% after 6 hours light irradiation; storing in ambient for 1 days | Remnant PL 80% after 7 days water treatment; remnant PL 98% after 6 hours light irradiation; storing in ambient for 4 days | 24 |
| CsPbBr3/ZrO2 | Janus | 514 | 90 | Remnant PL 5% after 8 days water treatment; remnant PL 70% after 3 hours light irradiation | Remnant PL 80% after 8 days water treatment; remnant PL 90% after 3 hours light irradiation | 25 | |
| CsPbBr3/SiO2 | Core/shell | 501 | 90 | Remnant PL 0% after storing in water for 16 min under ultrasonic treatment; storing in ambient <3 days | Remnant PL 112% after storing in water for 40 min under ultrasonic treatment; storing in ambient for 28 days | 26 | |
| CsPbMnX3/SiO2 | Core/shell | 446/607 | 54.7 | Remnant PL 0% after storing in water for 6 days; remnant PL 50% after heating at 100 °C for 1 hour | Remnant PL 90% after storing in water for 6 days; remnant PL 90% after heating at 100 °C for 1 hour | 27 | |
| CsPbI3/SiO2 | Core/shell | 696 | 51.1 | Remnant PL 52–64% after 2 hours water treatment; remnant PL 67.2% after heating at 100 °C; remnant PL 25.8% after 3 hours light irradiation | Remnant PL 95–108% after 2 hours water treatment; remnant PL 85–90% after heating at 100 °C; remnant PL 52.7–62.1% after 3 hours light irradiation | 28 | |
| CsPbI3/ZrO2 | 57.7 | ||||||
| CsPbI3/TaO5 | 59.7 | ||||||
| CsPbBr3/TiO2 | Core/shell | ∼515 | — | Remnant PL 0% after 15 min water treatment; remnant PL 37% after 24 hours light irradiation | Remnant PL 85% after 84 days water treatment; remnant PL 75% after 24 hours light irradiation | 29 | |
| Semiconductors | CsPbBr3/Ag2S | Core/island | 520 | 82 | Remnant PL 0% after 4 days water treatment | Remnant PL 6.35% after 30 days water treatment | 30 |
| CsPbBr3/ZnS | Core/shell | 510 | — | Remnant PL 0% after 2 hours light irradiation | Remnant PL 60% after 48 hours light irradiation | 31 | |
| CsPbBr3/Rb4PbBr6 | Core/shell | 507 | 85 | Remnant PL 0% after 2 hours light irradiation | Remnant PL 90% after 42 hours light irradiation | 32 | |
| CsPbBr3/CdS | Core/shell | 510 | 90 | Remnant PL 65% after 14 hours light irradiation; remnant PL ∼0% after heating at 60 °C for 9 hours | Remnant PL 80% after 14 hours light irradiation; remnant PL 40% after heating at 60 °C for 9 hours | 33 | |
| Metal | CsPbBr3/Pt single atoms | Core/island | 510 | 0.1 | Aggregation after 20 min light irradiation by xenon lamp | Maintain structure after 60 min light irradiation by xenon lamp | 34 |
| Others | CsPbBr3/PbSO4 | Core/shell | 510 | 92 | Remnant PL 44% after 150 min ethanol treatment; remnant PL 50% after 90 min acetone treatment; remnant PL 80% after 300 min light irradiation | Remnant PL 75% after 150 min ethanol treatment; remnant PL 90% after 90 min acetone treatment; remnant PL 105% after 300 min light irradiation | 35 |
| CsPbBr3/ZnSO4 | Core/shell | 515 | 95 | Anion-exchange after 40 min | No anion-exchange after 48 h | 36 | |
| APbX3@PAA-b-PS (A = FA or Cs) | Core/shell | 460–650 | 30–61 | Quenching with added water; remnant PL 18% after 60 min light irradiation | Remnant PL 100% after storing in water for 23 days; remnant PL 92% after 60 min light irradiation | 37 |
3.1 APbX3/oxide
Inert oxides with high stability, such as SiO2 and Al2O3, are regarded as alternative shell materials for protecting CsPbX3 NCs due to their weak light absorption and simple preparation strategies.48 Most of the reported structures are multi-particle coatings, probably due to the uncontrollable reaction conditions and the destruction of APbX3 NCs by polar byproducts. Precisely controlling the growth of oxides on the core is particularly important for achieving single-particle-level coating by oxides. This section introduces successful examples of single-particle oxide-coated APbX3 NCs.In 2018, our group reported CsPbX3/SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 Janus NCs by combining a water-triggered transformation and a sol–gel strategy (Fig. 2a). Cs4PbX6 NCs were synthesized by a hot-injection method, then water was added to the mixture of Cs4PbX6 NCs and tetramethoxysilane (TMOS) under vigorous stirring. In this process, hydrophobic capping ligands (OA and OLA) on the surface of Cs4PbBr6 NCs are removed when CsBr is stripped out from the hexane/water interface. As a result, one side of the NCs contacting water has larger surface energy, so that SiO2 can nucleate and grow on that side of CsPbX3 NCs (Fig. 2b). This method has good universality and can be applied in the preparation of a variety of stable Janus structures. As shown in Fig. 2c, CsPbX3@SiO2 Janus structures with different halide composites were successfully prepared, which exhibit high brightness and stability. By using different oxide precursors, we successfully prepared different oxide-coated CsPbX3 Janus structures, including CsPbBr3/ZrO2
24 Janus NCs by combining a water-triggered transformation and a sol–gel strategy (Fig. 2a). Cs4PbX6 NCs were synthesized by a hot-injection method, then water was added to the mixture of Cs4PbX6 NCs and tetramethoxysilane (TMOS) under vigorous stirring. In this process, hydrophobic capping ligands (OA and OLA) on the surface of Cs4PbBr6 NCs are removed when CsBr is stripped out from the hexane/water interface. As a result, one side of the NCs contacting water has larger surface energy, so that SiO2 can nucleate and grow on that side of CsPbX3 NCs (Fig. 2b). This method has good universality and can be applied in the preparation of a variety of stable Janus structures. As shown in Fig. 2c, CsPbX3@SiO2 Janus structures with different halide composites were successfully prepared, which exhibit high brightness and stability. By using different oxide precursors, we successfully prepared different oxide-coated CsPbX3 Janus structures, including CsPbBr3/ZrO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (Fig. 2d) and CsPbBr3/Ta2O5 (Fig. 2e), by introducing Zr(OC4H9)4 and tantalum(V) ethoxide as the precursor, respectively. With the protection of the oxide shell, the obtained Janus nanoparticles exhibit enhanced stability against water and light (Fig. 2f and g). Due to the small size and colloidal stability of the single-particle coating, for the first time, the coated nanoparticles were spin-coated to form a uniform film that also exhibits high stability. This work confirms the feasibility of coating CsPbX3 on a single-particle scale by effectively removing the ligands on the surface of CsPbX3 NCs and provides a new direction for subsequent research.
25 (Fig. 2d) and CsPbBr3/Ta2O5 (Fig. 2e), by introducing Zr(OC4H9)4 and tantalum(V) ethoxide as the precursor, respectively. With the protection of the oxide shell, the obtained Janus nanoparticles exhibit enhanced stability against water and light (Fig. 2f and g). Due to the small size and colloidal stability of the single-particle coating, for the first time, the coated nanoparticles were spin-coated to form a uniform film that also exhibits high stability. This work confirms the feasibility of coating CsPbX3 on a single-particle scale by effectively removing the ligands on the surface of CsPbX3 NCs and provides a new direction for subsequent research.
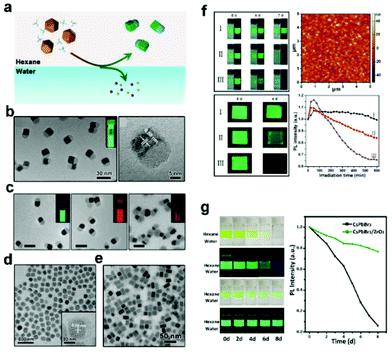 | ||
| Fig. 2 (a) Schematic illustration of the formation of CsPbX3/oxide Janus NCs. TEM images of (b) CsPbBr3/SiO2, (c) CsPbX3/SiO2 (X = Cl/Br or Br/I), (d) CsPbBr3/ZrO2, and (e) CsPbBr3/Ta2O5 Janus NCs. Inset shows the corresponding photographs of the colloidal solutions. (f) High stability of CsPbBr3/SiO2 Janus NCs. Photographs show the stability against water and storage in humid air (40 °C and humidity of 75%) of (I) Janus CsPbBr3/SiO2 and CsPbBr3 obtained from (II) water-triggered and (III) hot-injection methods. Atomic force microscope (AFM) image of CsPbBr3/SiO2 NC thin film. Photostability of different thin films under irradiation of 375 nm UV light. Reproduced with permission from ref. 24. Copyright 2018 American Chemistry Society. (g) High stability of Janus CsPbBr3/ZrO2 NCs. Photographs show the stability against water and the corresponding change of PL intensity. Reproduced with permission from ref. 25. Copyright 2019 American Chemistry Society. | ||
The successful preparation of Janus structures heralds the improved stability of CsPbX3 NCs, which however requires further improvement due to incomplete protection. We shifted our attention to fully coated CsPbX3 NCs at a single-particle level. As an amorphous material, SiO2 has strong plasticity and is easier to fully coat onto most types of nanoparticles.15 However, SiO2 is a hydrophilic material that has a large contact angle (θ) with CsPbX3 NCs, which are hydrophobic. Our group reported a facile one-pot approach to synthesize CsPbBr3/SiO2 core/shell NCs.26 The formation process is shown in Fig. 3a. The precursor solution (CsBr, PbBr2, OA, OLA, and ammonia dissolved in N,N-dimethylformamide) is quickly added into ultradry toluene solution containing TMOS, leading to the precipitation of product. At the very beginning of the reaction, the surface energy of NCs is high, and TMOS rapidly hydrolyzes in the presence of water and nucleates on the surface of CsPbBr3 NCs. It is found that the formation rates, determined by reaction temperature, precursor species, pH value, etc., are critical for the successful preparation of core/shell NCs. Monodisperse, fully coated core/shell CsPbBr3/SiO2 NCs can be realized, in which each nanoparticle has only one CsPbBr3 NC (Fig. 3b). Benefitting from the full protection of the SiO2 shell, the as-prepared core/shell structure can maintain good stability under very harsh ultrasonication treatment in aqueous solution (Fig. 3c). The full coating allows CsPbBr3 NCs to be stably dispersed in aqueous solutions, which indicates potential in biological applications such as fluorescence imaging in vivo. The disadvantage of this method is that precise control of reaction conditions is required.
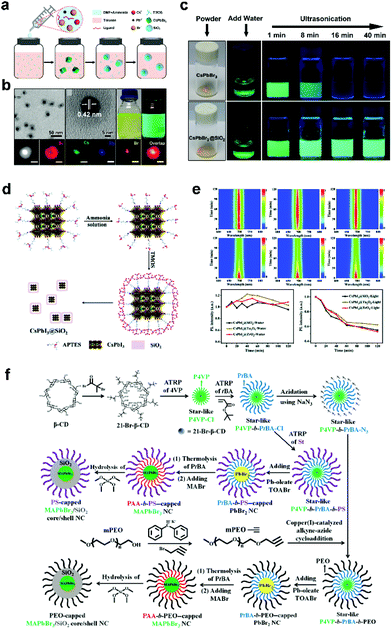 | ||
| Fig. 3 (a) Formation process of CsPbBr3/SiO2 core/shell NCs. (b) TEM and EDS mapping images of CsPbBr3/SiO2 core/shell NCs. (c) High stability of CsPbBr3/SiO2 core/shell NCs against water treatment. Reproduced with permission from ref. 26. Copyright 2018 American Chemistry Society. (d) Synthetic strategy for CsPbI3/SiO2 core/shell NCs using APTES as a linking ligand. (e) High stability of CsPbI3/SiO2 core/shell NCs against water treatment. Reproduced with permission from ref. 28. Copyright 2021 Royal Society of Chemistry. (f) Stepwise representation of the synthetic route to PS-capped MAPbBr3/SiO2 core/shell NCs and PEO-capped MAPbBr3/SiO2 core/shell NCs by exploiting the star-like P4VP-b-PtBA-b-PS and P4VP-b-PtBA-b-PEO as nanoreactors, respectively. CD, cyclodextrin; BMP, 2-bromo-2-methylpropionate; and TOABr, tetraoctylammonium bromide. Reproduced with permission from ref. 49. Copyright 2019 Creative. | ||
In addition to these examples, scientists have devoted many efforts to the preparation of core/shell structured perovskite NCs with different components. Tang et al.27 reported CsPbMnX3/SiO2 (X = Br, Cl) core/shell NCs with two emission peaks synthesized via a facile reverse microemulsion method at room temperature, which can be fabricated into a stable white LED. CsPbX3/Al2O3 structures with tunable thickness were synthesized through a general colloidal atomic layer deposition (c-ALD) method.50 Considering that the hydrophobic ligands on CsPbX3 have poor affinity with hydrophilic oxides, 3-aminopropyl triethoxysilane (APTES) was introduced as a bridging ligand to modify the surface of CsPbX3, so the binding force between the oxides and CsPbX3 is enhanced. After that, CsPbX3/oxide (SiO2, ZrO2, Ta2O5) core/shell28 structures were prepared, as shown in Fig. 3d. All of the core/shell NCs exhibit high stability against water, UV-light irradiation and thermal treatment (Fig. 3e), indicating the effective protection from the oxide shells. Surface engineering is considered an effective strategy to achieve single-particle-level full coating.
Dual-shelled MAPbBr3 NCs coated by polymer and SiO2 were also successfully prepared.49 As shown in Fig. 3f, star-like copolymers, as nanoreactors, were designed to render the growth of core/shell NCs with controlled yet tunable perovskite core diameter, SiO2 shell thickness and surface chemistry. The colloidal stability, chemical composition stability, photostability, and water stability of MAPbBr3 NCs are dramatically improved with the protection of dual shells. This strategy can realize controllable single-particle-level coating through a smart but complex design, and we look forward to the development of a simpler and easier method.
3.2 APbX3/semiconductor
As inert materials, oxides are widely used as shell materials. One disadvantage of oxides is that most of them are insulators, limiting the charge transfer between particles. In contrast, stable semiconductor materials with good carrier transport performance are good candidates. Zheng and coworkers29 reported CsPbBr3/TiO2 core/shell NCs by calcination at 300 °C for 5 h, which allow for efficient photoinduced charge transfer and enhanced photoelectric activity during water treatment. The decoration of CsPbX3 NCs with semiconductors has attracted widespread interest to improve the stability and modify the photoelectric properties.In addition to conventional semiconductor materials, chalcogenide quantum dots (QDs) have been widely introduced to modify APbX3 NCs to regulate their photophysical properties. When semiconductors with different band gaps are compounded, different types of heterostructures can be constructed for different occasions. When applied to perovskites, the biggest challenge here is that the preparation of QDs usually requires high-temperature conditions (often higher than 250 °C) that are not friendly to perovskite NCs. For example, CsPb(Br/I)3/ZnS heterostructure53 with enhanced stability and tunable photoluminescence was prepared by a solution-phase method, in which ZnS QDs are decorated on the surface of CsPb(Br/I)3 NCs. CsPbX3/PbS heterostructures51 were synthesized via in situ growth of PbS using hexamethyldisilathiane as the sulfur precursor. As shown in Fig. 4a and b, CsPbX3/PbS NCs show tunable dual emission feature with the visible and near-infrared (NIR) PL corresponding to CsPbX3 and PbS, respectively. The fluorescence of the complex can be tuned by varying the size of PbS QDs, which is realized by adjusting the reaction time (Fig. 4c and d). CsPbBr3/PbS is a typical type I heterojunction. As shown in Fig. 4e and f, these tiny PbS NCs provide channels for the exciton nonradiative recombination, which can quench the PL. Moreover, results prove that exciton energy effectively transfers from CsPbBr3 to PbS in CsPbBr3/PbS NCs when CsPbBr3 is excited, which may pave the way toward highly efficient QD photovoltaic and optoelectronic devices. Similarly, CsPbBrI2/PbSe54 and CsPbBr3/Ag2S30 heterojunctions were reported. The QDs on CsPbX3 NCs make a great contribution to the improvement of stability and the tuning of optical properties.
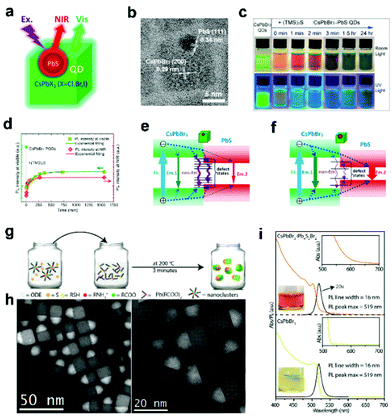 | ||
| Fig. 4 (a) Schematic illustration of CsPbX3/PbS heterostructure. (b) TEM image of CsPbBr3/PbS heterostructure. (c) Photographs of CsPbBr3 NC solution and CsPbBr3/PbS NC reaction solution under daylight and UV light. (d) The intensity evolution of PL peaks in visible and NIR regions, respectively. (e, f) Scheme of the band structure and energy transfer channels of the CsPbBr3/PbS NCs with increasing PbS size, respectively. Reproduced with permission from ref. 51. Copyright 2020 American Chemistry Society. (g) Scheme of the synthesis of CsPbBr3/Pb4S3Br2 heterostructures. (h) STEM images of CsPbBr3/Pb4S3Br2 NCs. (i) Absorbance and PL spectra of CsPbBr3/Pb4S3Br2 heterostructures and CsPbBr3 NCs. Reproduced with permission from ref. 52. Copyright 2021 American Chemistry Society. | ||
Manna's group52 reported an interesting CsPbX3 Janus heterostructure by epitaxial growth of Pb4S3Br2 onto CsPbBr3 NCs at high temperature (Fig. 4g). The sublattice of Pb4S3Br2 is similar to that of CsPbBr3, so that heterostructures can be achieved (Fig. 4h). The overall system is not just the sum of its components but a potentially valuable platform for wave function engineering strategies that are not typically accessible with halide perovskite materials due to compositional alloying. The resulting quasi type II energy level alignment at the heterojunction promotes the rapid splitting of photogenerated excitons, with the photoexcited hole localized in the chalcohalide domain and the electron partially delocalized in the entire nanostructure (Fig. 4i).
Compared to heterostructures obtained from island-like growth, core/shell heterojunctions are highly anticipated. For example, type II CsPbBr3/ZnS core/shell NCs (Fig. 5a)31 were prepared, which exhibit enhanced photoluminescence quantum yield (PLQY) and PL lifetime (Fig. 5b) and improved stability (Fig. 5c). The excellent properties of the core/shell structures lead to their various applications, such as optically pumped LEDs, photodetectors and photocatalysis. Similar CsPbBr3/CdS33,55,56 heterostructures exhibit ultrahigh chemical stability, nonblinking photoluminescence, and high quantum yield due to the reduced electronic traps within the core/shell structure. To further improve the stability, Liu and coworkers57 synthesized a CsPbBr3/nCdS/Al2O3 double-shell structure. The outstanding performance of CsPbBr3/CdS in optoelectronic applications will be introduced in section 4.
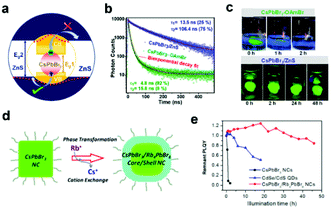 | ||
| Fig. 5 (a) Schematic showing pseudo type II band alignment at the CsPbBr3/ZnS core/shell interface, where the electrons are confined inside the core but the holes are delocalized over both core and shell. (b) Comparison of PL decay shows a huge increment in the PL lifetime of CsPbBr3/ZnS core/shell NCs. (c) Photographs of the films of CsPbBr3/ZnS core/shell NCs and CsPbBr3–OLABr NCs dipped in beakers full of water and excited with UV light. Reproduced with permission from ref. 31. Copyright 2020 American Chemistry Society. (d) Schematic diagram of CsPbBr3/Rb4PbBr6 core/shell NCs. (e) Photostability of the solution under illumination with a 450 nm LED light (175 mW cm−2). Reproduced with permission from ref. 32. Copyright 2018 American Chemistry Society. | ||
Due to the matching of crystal lattices and similarity of elemental composition, some perovskite-like materials are employed as shell materials to achieve full encapsulation at the single-particle level. Core/shell CsPbX3/Cs4PbX6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 structure was first synthesized by a hot-injection method, which opens a new way for the durability enhancement of CsPbX3 NCs. In work by Tian's group,59 amorphous CsPbBrx is introduced as the shell material to encapsulate CsPbBr3 NCs, and the resulting blue-emitting CsPbBr3/CsPbBrx nanodots display high PLQY and improved stability. Li's group32 prepared CsPbBr3 NCs enriched with lead bromide on the surface, and then added excess Rb precursor for overgrowth. As a result, CsPbBr3/Rb4PbBr6 NCs are obtained, in which lead bromide is used as an intermediate transition layer to achieve single-particle coating (Fig. 5d). With the excellent passivation of the surface, CsPbBr3/Rb4PbBr6 core/shell NCs show enhanced PLQY and high stability against photo treatment (Fig. 5e), enabling them to be promising in stable optoelectronic devices. CsPbBr3 can also be introduced as shell material to enhance the properties of FAPbBr3 NCs.60 The disadvantage is that the instability of the ionic structure is not changed.
58 structure was first synthesized by a hot-injection method, which opens a new way for the durability enhancement of CsPbX3 NCs. In work by Tian's group,59 amorphous CsPbBrx is introduced as the shell material to encapsulate CsPbBr3 NCs, and the resulting blue-emitting CsPbBr3/CsPbBrx nanodots display high PLQY and improved stability. Li's group32 prepared CsPbBr3 NCs enriched with lead bromide on the surface, and then added excess Rb precursor for overgrowth. As a result, CsPbBr3/Rb4PbBr6 NCs are obtained, in which lead bromide is used as an intermediate transition layer to achieve single-particle coating (Fig. 5d). With the excellent passivation of the surface, CsPbBr3/Rb4PbBr6 core/shell NCs show enhanced PLQY and high stability against photo treatment (Fig. 5e), enabling them to be promising in stable optoelectronic devices. CsPbBr3 can also be introduced as shell material to enhance the properties of FAPbBr3 NCs.60 The disadvantage is that the instability of the ionic structure is not changed.
3.3 CsPbX3/metal heterostructures
CsPbX3/metal heterostructures have been carefully investigated, in which photoexcited electron–hole pairs are significantly affected by the introduction of metal. Kamat's group63 reported a CsPbBr3/Au hybrid architecture in which Au NCs selectively grow on the corners of CsPbBr3 NCs. This structure is obtained due to facilitating the reduction of AuBr3 by OLA, followed by Au nucleation on the CsPbBr3 surface. Moreover, Sheldon et al.61,64 found that the excess AuBr3 can be partially reduced to produce nonfluorescent Cs2Au2Br6 NCs. During the process, the addition of PbBr2 inhibits the substitution of Au for Pb to produce the final CsPbBr3/Au heterojunction (Fig. 6a–d). The PLQY of CsPbBr3 NCs is decreased when Au nanoparticles are formed on the corners of CsPbBr3 NCs, suggesting the charge separation endowed by the introduction of Au. Inspired by these works, our group62 demonstrated a reversible fluorescence switching of CsPbI3 NCs by depositing and de-depositing metallic Ag nanoparticles on CsPbI3 NCs controlled by light on/off, as shown in Fig. 6e–h. The controllable PL reversibility in the CsPbX3/Ag composite system favors its application in smart fluorescent devices, such as a rewritable platforms. In our very recent work,34 Pt single atoms were successfully deposited on CsPbBr3 NCs through a photoassisted approach, in which the surface is partially oxidized first, followed by the anchoring of single Pt atoms through the formation of Pt–O and Pt–Br bonds. The resulting products can be used as efficient and durable catalysts for photocatalytic semi-hydrogenation of propyne. CsPbX3/metal heterostructures composed of metal and semiconductor characteristics have potential prospects in the fields of optoelectronic devices and catalysis. | ||
| Fig. 6 (a) Schematic illustration of the formation process of CsPbBr3/Au heterostructure and Cs2Au2Br6 NCs. (b) TEM images of CsPbBr3 and CsPbBr3 after gold cation exchange and CsPbBr3 with gold deposition. Reproduced with permission from ref. 61. Copyright 2017 American Chemistry Society. (e) Photographs showing the color change of the CsPbI3/AgI composite system induced by light on/off. TEM images of (f) the original CsPbI3 NCs before irradiation, (g) CsPbI3/Ag NCs, and (h) CsPbI3 NCs after being stored in the dark, respectively. The insets in (f) and (g) are the corresponding HRTEM images of CsPbI3 and CsPbI3/Ag NCs with a scale bar of 5 nm. Reproduced with permission from ref. 62. Copyright 2019 Royal Society of Chemistry. | ||
3.4 Other heterostructures
In addition to the aforementioned heterojunctions, other core/shell structures have been also studied, including CsPbX3/PbSO4,35 CsPbX3/polymer,65 and AuCu/CsPbCl3.66 Our group35 developed a post-treatment method to synthesize a CsPbX3/PbSO4 core/shell structure with high PLQY and stability. In this post-treatment method (Fig. 7a), octylammonium ligand is used to increase the affinity of the sulfate with the surface of CsPbX3 NCs, and then the surface enriched with Pb ions is used to better react with sulfate to produce PbSO4 shells (Fig. 7b and c). The presence of the PbSO4 layer favors the protection of the core CsPbX3 without weakening the charge transfer between CsPbX3 NCs, making them suitable for stable optoelectronic devices. A general approach was demonstrated for fabricating CsPbX3/metal sulfate core/shell structure.36 The resulting core/shell NCs with a controlled shell thickness ranging from 1 to 3 nm exhibit enhanced properties in terms of colloidal stability and PLQY, providing a new opportunity for the controllable preparation of core/shell structures.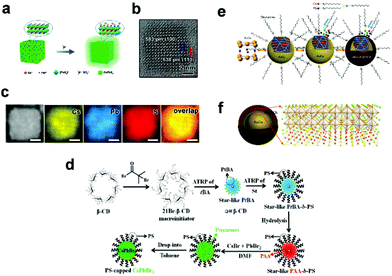 | ||
| Fig. 7 (a) Schematic illustration of the post-treatment method for synthesizing CsPbBr3/PbSO4 core/shell NCs. (b) HRTEM image of one CsPbBr3/PbSO4 core/shell NC. (c) HAADF-STEM image and elemental mapping images showing the elemental distribution of Cs, Pb, and S. The scale bars are 3 nm. Reproduced with permission from ref. 35. Copyright 2021 Wiley-VCH. (d) Stepwise representation of crafting hairy all-inorganic perovskite CsPbBr3 NCs intimately and permanently capped by PS chains via capitalizing on the amphiphilic star-like PAA-b-PS diblock copolymer as a nanoreactor. Reproduced with permission from ref. 65. Copyright 2019 Wiley-VCH. (e) Schematic illustration of the AuCu/CsPbCl3 core/shell NCs’ heteroepitaxial growth process. (f) Schematic of the AuCu/CsPbCl3 core/shell NCs. Reproduced with permission from ref. 66. Copyright 2020 Wiley-VCH. | ||
Lin65 reported an amphiphilic star-like block copolymer nanoreactor strategy to prepare CsPbBr3/polymer NCs with high water and colloidal stabilities (Fig. 7d). The key of this method is the construction of micelles composed of amphiphilic polymers, and the precursor is well dissolved inside the micelles to form CsPbBr3 NCs. The development of this strategy makes it possible for CsPbX3 NCs to be used in biological imaging. APbX3 (A = Cs or FA) NCs encapsulated by amphiphilic polymer (poly(acrylic acid)-block-poly(styrene), PAA-b-PS) micelles were synthesized by a room-temperature reprecipitation method.37 The as-prepared NCs exhibit high stability against polar solvents and high-flux irradiation. The presence of polymers also prevents ion exchange between NCs, so APbX3 can be directly used in white LEDs. The single-particle-level encapsulation of perovskites by organic matter is realized by the in situ synthesis of stable core–shell structure through the confinement of micelles. This process is more demanding on experimental design and operation requirements.
A typical case of core/shell structure is AuCu/CsPbCl3 NCs, in which AuCu NC is capped with CsPbCl3 shell,66 as shown in Fig. 7e. The same ligand OLA and hydrophobic property make it easier to overgrow CsPbCl3 onto the AuCu core. Another significant reason for the core/shell structure is that the CsPbCl3 shell and AuCu core maintain a high degree of lattice match (Fig. 7f).
4. Applications of core/shell structures
The encapsulation can improve the stability and photoelectric performance of bare CsPbX3 NCs, making them more suitable for optoelectronic devices, which have high stability and efficiency requirements. For example, owing to the protection of SiO2 shell and their good film-forming properties (Fig. 3f), CsPbBr3/SiO2 Janus NCs24 have been fabricated into white LEDs (WLEDs), which can well maintain white emission after operation for one hour (Fig. 8a). In sharp contrast, the WLED made of bare CsPbBr3 NCs changed from white to blue within a short period. CsPbX3/semiconductor heterostructure not only improves the stability and passivates the defects of the perovskite, but also makes it more suitable for photoelectric conversion devices, such as LEDs, solar cells, photodetectors, etc. CsPbI3/PbS core/shell NCs67 were fabricated into electroluminescence LEDs using p–i–n structures due to CsPbI3 NC films switching from n-type behavior to nearly ambipolar by PbS capping (Fig. 8b). The red LEDs exhibit dramatically enhanced operation stability, and an EQE of 11.8% can be realized. FAPbBr3/CsPbBr3 core/shell NCs60 with high PLQY and improved stability have been utilized in a green LED, which shows excellent performance with an EQE of 8.1%. Metal–organic framework (MOF)-coated CsPbBr3 NCs have been demonstrated as the emitter for bright and stable LEDs.69 With the protection of MOFs, the as-fabricated LED shows high stability against continuous UV light irradiation, heat, and electrical stress,69 and it is free from ion migration, NC merging, and interface corrosion. The operational lifetime of the resulting LEDs has been greatly extended. This work opens up a new way for the large-scale solution-processed fabrication of perovskite-based LEDs with high brightness and high efficiency.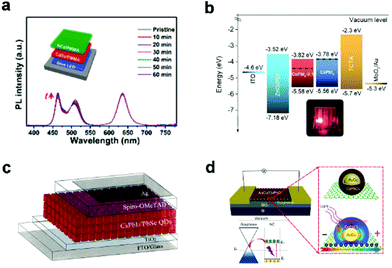 | ||
| Fig. 8 (a) Time-dependent PL spectra of a WLED under continuous current of 1 mA based on CsPbBr3/SiO2 NCs. Inset shows the schematic illustration of the WLED device configuration. Reproduced with permission from ref. 24. Copyright 2018 American Chemistry Society. (b) Device energy level diagram for all functional layers in CsPbI3/PbS core/shell NC-based electroluminescence LEDs. Inset shows the photograph of the working device. Reproduced with permission from ref. 67. Copyright 2018 American Chemistry Society. (c) Schematics of the device architecture of CsPbI3/PbSe NC-based solar cells. Reproduced with permission from ref. 68. Copyright 2018 American Chemistry Society. (d) Schematic of the AuCu/CsPbCl3 NCs nanohybrid photodetector. Reproduced with permission from ref. 66. Copyright 2020 Wiley-VCH. | ||
CsPbBr3 NCs encapsulated in a mesoporous ion-based MOF show significant interfacial charge transfer from CsPbBr3 NCs to MOF, which is ideal for photocatalysis. The composite exhibits excellent and stable catalytic activities in oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) when working as the synergistic photocathode in the photoassisted Li–O2 battery. This work not only provides an efficient photoelectrocatalyst for a photoassisted Li–O2 battery but also highlights the advantage of MOFs in stabilizing perovskites to form synergetic photocatalysts.70 When the perovskite is combined with metals, the excited electrons and holes of the perovskite are separated, which is advantageous for catalysis. For example, our group reported the CsPbBr3/Pt single atom for photocatalytic semi-hydrogenation of propyne.34
With the passivation of PbSe, the CsPbI3/PbSe film shows a lower trap density and prolonged exciton lifetime, which promote carrier separation and collection.68 These improvements result in high-performance CsPbI3/PbSe NC solar cells with a power conversion efficiency of 13.9% and improved stability against moisture (Fig. 8c). CsPbI3/PbTe NCs with enhanced structural stability were synthesized by epitaxial growth strategy. The complex was fabricated as stable WLEDs, demonstrating their potential application value in the field of indoor lighting, such as full-visible-spectrum and plant growth LEDs.71 With the modification of CsPbCl3 shell, the AuCu/CsPbCl3 NCs exhibited enhanced light absorption leading to a remarkably enhanced photo-response in the nanohybrid photodetectors, which is more than 30 times that of the counterpart CsPbCl3 NCs.66 This result illustrates the feasibility of implementing the localized surface plasmon resonance light trapping directly in core/shell NCs for high-performance optoelectronics (Fig. 8d). Biocompatible CsPbBr3/SiO2 core/shell NCs72 with no toxicity, under the protection of SiO2, were used for bio-imaging and drug delivery. This work paves the way for new biomedical applications and processes. Highly stable CsPbBr3/phTEOS-TMOS core/shell NCs have been applied to fluorescence and upconversion imaging in living cells, confirming that the perovskite NCs are suitable for various bioimaging applications.23
5. Conclusion and outlook
In this review, we give a brief overview of the fundamental information for APbX3 NCs and the reasons for their structural instability. The single-particle-level coating structures of various compositions and morphologies are summarized. Based on the excellent performance of the coated APbX3 NCs, their potential applications in optoelectronic devices such as LEDs, solar cells, photodetectors, and catalysis have been introduced. However, there are still some challenges to be considered in the preparation of single-particle-level structures.(1) At present, the shell materials and synthetic methods are monotonous. Most of the shells are oxides and chalcogenides, and the synthetic strategies focus on hot-injection and post-treatment. It is necessary to develop more shell materials and preparation approaches that can be used to improve stability and photoelectronic performance.
(2) Unlike the thickness of the shell layer grown on the surface of metal and semiconductor NCs, which can be easily controlled, it is still very difficult to tune the thickness of the shell layer on APbX3 NCs. The influence of shell thickness on the photoelectric properties of APbX3 NCs is worthy of attention.
(3) The coating of inert materials shows good stability against water treatment. Due to the insufficient compactness of the shell material or the existence of hydrophobic ligands, it is however impossible to obtain long-term dispersion of core/shell structures in aqueous solution, which limits their practical application.
Conflicts of interest
The authors declare no potential conflicts of interest.Acknowledgements
This work is supported by the National Natural Science Foundation of China (51922073), Natural Science Foundation of Jiangsu Province (BK20180097), and Postdoctoral Science Foundation of China (2019M661923). We acknowledge the financial support from the 111 Project, Collaborative Innovation Center of Suzhou Nano Science, Technology (NANO-CIC) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- H. L. Wells, Z. Anorg. Chem., 1893, 3, 195–210 CrossRef.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS.
- A. Swarnkar, A. R. Marshall, E. M. Sanehira, B. D. Chernomordik, D. T. Moore, J. A. Christians, T. Chakrabarti and J. M. Luther, Science, 2016, 354, 92–95 CrossRef CAS PubMed.
- M. Liu, Q. Wan, H. Wang, F. Carulli, X. Sun, W. Zheng, L. Kong, Q. Zhang, C. Zhang, Q. Zhang, S. Brovelli and L. Li, Nat. Photonics, 2021, 15, 379–385 CrossRef CAS.
- J. Song, J. Li, X. Li, L. Xu, Y. Dong and H. Zeng, Adv. Mater., 2015, 27, 7162–7167 CrossRef CAS.
- Y. K. Wang, F. Yuan, Y. Dong, J. Y. Li, A. Johnston, B. Chen, M. I. Saidaminov, C. Zhou, X. Zheng, Y. Hou, K. Bertens, H. Ebe, D. Ma, Z. Deng, S. Yuan, R. Chen, L. K. Sagar, J. Liu, J. Fan, P. Li, X. Li, Y. Gao, M. K. Fung, Z. H. Lu, O. M. Bakr, L. S. Liao and E. H. Sargent, Angew. Chem., 2021, 60, 16164–16170 CrossRef CAS.
- M. Lu, Y. Zhang, S. Wang, J. Guo, W. W. Yu and A. L. Rogach, Adv. Funct. Mater., 2019, 29, 1902008 CrossRef.
- L. N. Quan, B. P. Rand, R. H. Friend, S. G. Mhaisalkar, T. W. Lee and E. H. Sargent, Chem. Rev., 2019, 119, 7444–7477 CrossRef CAS PubMed.
- J. Shamsi, A. S. Urban, M. Imran, L. De Trizio and L. Manna, Chem. Rev., 2019, 119, 3296–3348 CrossRef CAS PubMed.
- A. Dey, J. Ye, A. De, E. Debroye, S. K. Ha, E. Bladt, A. S. Kshirsagar, Z. Wang, J. Yin, Y. Wang, L. N. Quan, F. Yan, M. Gao, X. Li, J. Shamsi, T. Debnath, M. Cao, M. A. Scheel, S. Kumar, J. A. Steele, M. Gerhard, L. Chouhan, K. Xu, X.-G. Wu, Y. Li, Y. Zhang, A. Dutta, C. Han, I. Vincon, A. L. Rogach, A. Nag, A. Samanta, B. A. Korgel, C.-J. Shih, D. R. Gamelin, D. H. Son, H. Zeng, H. Zhong, H. Sun, H. V. Demir, I. G. Scheblykin, I. Mora-Seró, J. K. Stolarczyk, J. Z. Zhang, J. Feldmann, J. Hofkens, J. M. Luther, J. Pérez-Prieto, L. Li, L. Manna, M. I. Bodnarchuk, M. V. Kovalenko, M. B. J. Roeffaers, N. Pradhan, O. F. Mohammed, O. M. Bakr, P. Yang, P. Müller-Buschbaum, P. V. Kamat, Q. Bao, Q. Zhang, R. Krahne, R. E. Galian, S. D. Stranks, S. Bals, V. Biju, W. A. Tisdale, Y. Yan, R. L. Z. Hoye and L. Polavarapu, ACS Nano, 2021, 15, 10775–10981 CrossRef CAS.
- Q. Sun and W.-J. Yin, J. Am. Chem. Soc., 2017, 139, 14905–14908 CrossRef CAS.
- Y. Zhou and Y. Zhao, Energy Environ. Sci., 2019, 12, 1495–1511 RSC.
- P. Reiss, M. Protiere and L. Li, Small, 2009, 5, 154–168 CrossRef CAS PubMed.
- R. Ghosh Chaudhuri and S. Paria, Chem. Rev., 2012, 112, 2373–2433 CrossRef CAS PubMed.
- H. C. Wang, S. Y. Lin, A. C. Tang, B. P. Singh, H. C. Tong, C. Y. Chen, Y. C. Lee, T. L. Tsai and R. S. Liu, Angew. Chem., Int. Ed., 2016, 55, 7924–7929 CrossRef CAS PubMed.
- D. N. Dirin, L. Protesescu, D. Trummer, I. V. Kochetygov, S. Yakunin, F. Krumeich, N. P. Stadie and M. V. Kovalenko, Nano Lett., 2016, 16, 5866–5874 CrossRef CAS PubMed.
- S. Huang, Z. Li, L. Kong, N. Zhu, A. Shan and L. Li, J. Am. Chem. Soc., 2016, 138, 5749–5752 CrossRef CAS PubMed.
- M. Xing, B. Chen, J. Feng, W. Xu, Y. Bai, Y. Zhou, C. Dong, H. Zhong, J. Zhang and Y. Yin, Chem, 2019, 5, 2195–2214 CAS.
- Z. Li, L. Kong, S. Huang and L. Li, Angew. Chem., Int. Ed., 2017, 56, 8134–8138 CrossRef CAS PubMed.
- M. N. An, S. Park, R. Brescia, M. Lutfullin, L. Sinatra, O. M. Bakr, L. De Trizio and L. Manna, ACS Energy Lett., 2021, 6, 900–907 CrossRef CAS.
- L. Xu, J. Chen, J. Song, J. Li, J. Xue, Y. Dong, B. Cai, Q. Shan, B. Han and H. Zeng, ACS Appl. Mater. Interfaces, 2017, 9, 26556–26564 CrossRef CAS.
- P. M. Talianov, O. O. Peltek, M. Masharin, S. Khubezhov, M. A. Baranov, A. Drabavicius, A. S. Timin, L. E. Zelenkov, A. P. Pushkarev, S. V. Makarov and M. V. Zyuzin, J. Phys. Chem. Lett., 2021, 12, 8991–8998 CrossRef CAS PubMed.
- H. Hu, L. Wu, Y. Tan, Q. Zhong, M. Chen, Y. Qiu, D. Yang, B. Sun, Q. Zhang and Y. Yin, J. Am. Chem. Soc., 2018, 140, 406–412 CrossRef CAS.
- H. Liu, Y. Tan, M. Cao, H. Hu, L. Wu, X. Yu, L. Wang, B. Sun and Q. Zhang, ACS Nano, 2019, 13, 5366–5374 CrossRef CAS PubMed.
- Q. Zhong, M. Cao, H. Hu, D. Yang, M. Chen, P. Li, L. Wu and Q. Zhang, ACS Nano, 2018, 12, 8579–8587 CrossRef CAS PubMed.
- X. Tang, W. Chen, Z. Liu, J. Du, Z. Yao, Y. Huang, C. Chen, Z. Yang, T. Shi, W. Hu, Z. Zang, Y. Chen and Y. Leng, Small, 2019, 15, 1900484 CrossRef PubMed.
- B. Tang, X. Zhao, L. J. Ruan, C. Qin, A. Shu and Y. Ma, Nanoscale, 2021, 13, 10600–10607 RSC.
- Z. J. Li, E. Hofman, J. Li, A. H. Davis, C.-H. Tung, L. Z. Wu and W. Zheng, Adv. Funct. Mater., 2017, 28, 1704288 CrossRef.
- K. Fu, Y. He, B. Zhang, X. Gao and G. Zou, J. Electroanal. Chem., 2020, 858, 113835 CrossRef CAS.
- V. K. Ravi, S. Saikia, S. Yadav, V. V. Nawale and A. Nag, ACS Energy Lett., 2020, 5, 1794–1796 CrossRef CAS.
- B. Wang, C. Zhang, S. Huang, Z. Li, L. Kong, L. Jin, J. Wang, K. Wu and L. Li, ACS Appl. Mater. Interfaces, 2018, 10, 23303–23310 CrossRef CAS PubMed.
- X. Tang, J. Yang, S. Li, Z. Liu, Z. Hu, J. Hao, J. Du, Y. Leng, H. Qin, X. Lin, Y. Lin, Y. Tian, M. Zhou and Q. Xiong, Adv. Sci., 2019, 6, 1900412 CrossRef CAS.
- H. Hu, W. Guan, Y. Xu, X. Wang, L. Wu, M. Chen, Q. Zhong, Y. Xu, Y. Li, T.-K. Sham, X. Zhang, L. Wang, M. Cao and Q. Zhang, ACS Nano, 2021, 15, 13129–13139 CrossRef CAS PubMed.
- Q. Zhong, J. Liu, S. Chen, P. Li, J. Chen, W. Guan, Y. Qiu, Y. Xu, M. Cao and Q. Zhang, Adv. Opt. Mater., 2021, 9, 2001763 CrossRef CAS.
- F. Gao, J. Wu, Y. Zhao, T. Song, Z. Deng, P. Wang, Y. Wang and H. Li, Nanoscale, 2021, 13, 10329–10334 RSC.
- M. Imran, B. T. Mai, L. Goldoni, M. Cirignano, H. B. Jalali, F. Di Stasio, T. Pellegrino and L. Manna, ACS Energy Lett., 2021, 6, 2844–2853 CrossRef CAS.
- C. K. MØLler, Nature, 1958, 182, 1436–1436 CrossRef.
- J. De Roo, M. Ibanez, P. Geiregat, G. Nedelcu, W. Walravens, J. Maes, J. C. Martins, I. Van Driessche, M. V. Kovalenko and Z. Hens, ACS Nano, 2016, 10, 2071–2081 CrossRef CAS.
- Q. Zhong, M. Cao, Y. Xu, P. Li, Y. Zhang, H. Hu, D. Yang, Y. Xu, L. Wang, Y. Li, X. Zhang and Q. Zhang, Nano Lett., 2019, 19, 4151–4157 CrossRef CAS.
- Y. Wei, Z. Cheng and J. Lin, Chem. Soc. Rev., 2019, 48, 310–350 RSC.
- D. Yang, X. Li and H. Zeng, Adv. Mater. Interfaces, 2018, 5, 1701662 CrossRef.
- G. H. Ahmed, J. Yin, O. M. Bakr and O. F. Mohammed, ACS Energy Lett., 2021, 6, 1340–1357 CrossRef CAS.
- H. Wu, S. Lin, R. Wang, X. You and Y. Chi, Nanoscale, 2019, 11, 5557–5563 RSC.
- H. Wu, S. Wang, F. Cao, J. Zhou, Q. Wu, H. Wang, X. Li, L. Yin and X. Yang, Chem. Mater., 2019, 31, 1936–1940 CrossRef CAS.
- Y. Wei, X. Deng, Z. Xie, X. Cai, S. Liang, P. A. Ma, Z. Hou, Z. Cheng and J. Lin, Adv. Funct. Mater., 2017, 27, 1703535 CrossRef.
- C. Sun, Y. Zhang, C. Ruan, C. Yin, X. Wang, Y. Wang and W. W. Yu, Adv. Mater., 2016, 28, 10088–10094 CrossRef CAS PubMed.
- Y. Duan, D. Y. Wang and R. D. Costa, Adv. Funct. Mater., 2021, 2104634 CrossRef.
- Y. He, Y. J. Yoon, Y. W. Harn, G. V. Biesold-McGee, S. Liang, C. H. Lin, V. V. Tsukruk, N. Thadhani, Z. Kang and Z. Lin, Sci. Adv., 2019, 5, eaax4424 CrossRef CAS PubMed.
- A. Loiudice, M. Strach, S. Saris, D. Chernyshov and R. Buonsanti, J. Am. Chem. Soc., 2019, 141, 8254–8263 CrossRef CAS PubMed.
- X. Zhang, X. Wu, X. Liu, G. Chen, Y. Wang, J. Bao, X. Xu, X. Liu, Q. Zhang, K. Yu, W. Wei, J. Liu, J. Xu, H. Jiang, P. Wang and X. Wang, J. Am. Chem. Soc., 2020, 142, 4464–4471 CrossRef CAS PubMed.
- M. Imran, L. Peng, A. Pianetti, V. Pinchetti, J. Ramade, J. Zito, F. Di Stasio, J. Buha, S. Toso, J. Song, I. Infante, S. Bals, S. Brovelli and L. Manna, J. Am. Chem. Soc., 2021, 143, 1435–1446 CrossRef CAS PubMed.
- W. Chen, J. Hao, W. Hu, Z. Zang, X. Tang, L. Fang, T. Niu and M. Zhou, Small, 2017, 13, 1604085 CrossRef PubMed.
- J. Zhang, X. Liu, P. Jiang, H. Chen, Y. Wang, J. Ma, R. Zhang, F. Yang, M. Wang, J. Zhang and G. Tu, Nano Energy, 2019, 66, 104142 CrossRef CAS.
- X. Tang, J. Yang, S. Li, W. Chen, Z. Hu and J. Qiu, Front. Chem., 2019, 7, 499 CrossRef CAS PubMed.
- J. Shi, W. Ge, J. Zhu, M. Saruyama and T. Teranishi, ACS Appl. Nano Mater., 2020, 3, 7563–7571 CrossRef CAS.
- X. Liu, X. Zhang, L. Li, J. Xu, S. Yu, X. Gong, J. Zhang and H. Yin, ACS Appl. Mater. Interfaces, 2019, 11, 40923–40931 CrossRef CAS PubMed.
- C. Jia, H. Li, X. Meng and H. Li, Chem. Commun., 2018, 54, 6300–6303 RSC.
- S. Wang, C. Bi, J. Yuan, L. Zhang and J. Tian, ACS Energy Lett., 2017, 3, 245–251 CrossRef.
- C. Zhang, S. Wang, X. Li, M. Yuan, L. Turyanska and X. Yang, Adv. Funct. Mater., 2020, 30, 1910582 CrossRef CAS.
- B. J. Roman, J. Otto, C. Galik, R. Downing and M. Sheldon, Nano Lett., 2017, 17, 5561–5566 CrossRef CAS.
- P. Li, D. Yang, Q. Zhong, Y. Zhang, M. Chen, S. Jiang, J. Chen, M. Cao, Q. Zhang and Y. Yin, Nanoscale, 2019, 11, 3193–3199 RSC.
- S. K. Balakrishnan and P. V. Kamat, ACS Energy Lett., 2017, 2, 88–93 CrossRef CAS.
- F. A. Rodriguez Ortiz, B. J. Roman, J. R. Wen, N. Mireles Villegas, D. F. Dacres and M. T. Sheldon, Nanoscale, 2019, 11, 18109–18115 RSC.
- Y. J. Yoon, Y. Chang, S. Zhang, M. Zhang, S. Pan, Y. He, C. H. Lin, S. Yu, Y. Chen, Z. Wang, Y. Ding, J. Jung, N. Thadhani, V. V. Tsukruk, Z. Kang and Z. Lin, Adv. Mater., 2019, 31, 1901602 CrossRef PubMed.
- M. Gong, M. Alamri, D. Ewing, S. M. Sadeghi and J. Z. Wu, Adv. Mater., 2020, 32, 2002163 CrossRef CAS PubMed.
- X. Zhang, M. Lu, Y. Zhang, H. Wu, X. Shen, W. Zhang, W. Zheng, V. L. Colvin and W. W. Yu, ACS Cent. Sci., 2018, 4, 1352–1359 CrossRef CAS PubMed.
- S. Wang, C. Bi, A. Portniagin, J. Yuan, J. Ning, X. Xiao, X. Zhang, Y. Y. Li, S. V. Kershaw, J. Tian and A. L. Rogach, ACS Energy Lett., 2020, 5, 2401–2410 CrossRef CAS.
- H. Tsai, S. Shrestha, R. A. Vilá, W. Huang, C. Liu, C.-H. Hou, H.-H. Huang, X. Wen, M. Li, G. Wiederrecht, Y. Cui, M. Cotlet, X. Zhang, X. Ma and W. Nie, Nat. Photonics, 2021, 15, 843–849 CrossRef.
- G. Y. Qiao, D. Guan, S. Yuan, H. Rao, X. Chen, J. A. Wang, J. S. Qin, J. J. Xu and J. Yu, J. Am. Chem. Soc., 2021, 143, 14253–14260 CrossRef CAS PubMed.
- L. Zhang, M. Zhu, Y. Sun, J. Zhang, M. Zhang, H. Zhang, F. Zhou, J. Qu and J. Song, Nano Energy, 2021, 90, 106506 CrossRef CAS.
- P. Kumar, M. Patel, C. Park, H. Han, B. Jeong, H. Kang, R. Patel, W. G. Koh and C. Park, J. Mater. Chem. B, 2020, 8, 10337–10345 RSC.
| This journal is © The Royal Society of Chemistry 2021 |