Green-to-UV photon upconversion enabled by new perovskite nanocrystal-transmitter-emitter combination†
Mio
Koharagi
a,
Naoyuki
Harada
a,
Keisuke
Okumura
a,
Junji
Miyano
a,
Shota
Hisamitsu
a,
Nobuo
Kimizuka
 *a and
Nobuhiro
Yanai
*a and
Nobuhiro
Yanai
 *ab
*ab
aDepartment of Applied Chemistry, Graduate School of Engineering, Center for Molecular Systems (CMS), Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka 819-0395, Japan. E-mail: yanai@mail.cstm.kyushu-u.ac.jp; n-kimi@mail. cstm.kyushu-u.ac.jp
bPRESTO, JST, Honcho 4-1-8, Kawaguchi, Saitama 332-0012, Japan
First published on 23rd November 2021
Abstract
The first example of triplet–triplet annihilation-based photon upconversion (TTA-UC) from green light to ultraviolet (UV) light sensitized by lead halide perovskite nanocrystals is demonstrated. The combination of a new transmitter that extracts triplet energy from perovskite and a UV emitter with a low triplet energy level lengthens the excitation wavelength of perovskite-sensitized upconverted UV emission.
Triplet–triplet annihilation-based photon upconversion (TTA-UC) is attracting attention because of its potential for a variety of applications, from energy to biotechnology.1–15 In the common TTA-UC mechanism, the photo-excited singlet state S1 of molecular sensitizers undergoes intersystem crossing (ISC) to the triplet state T1, which is followed by triplet energy transfer (TET) from the sensitizer to the emitter and inter-emitter TTA. In this conventional scheme, the UC spectral shift from the excitation wavelength to the emission peak is limited by energy loss due to ISC. To circumvent this energy loss, inorganic semiconductor nanocrystals such as quantum dots have recently been employed as the next-generation triplet sensitizers.16–21 Their small singlet–triplet exchange splitting has enabled TTA-UC with large UC spectral shifts.
As a new family of inorganic triplet sensitizers, our group has reported the first example of TTA-UC sensitized by perovskite nanocrystals (PNCs).22 Since then, TTA-UC at various wavelengths has been achieved using perovskite nanocrystals and thin films as sensitizers, and a deeper understanding of energy transfer from perovskites to molecules has been obtained.23–29 The generation of ultraviolet (UV) light using visible light is useful for photocatalytic applications, but so far, perovskite-sensitized TTA-UC has been limited to excitation by blue light with wavelengths below 500 nm.25,26 This is due to the lack of suitable transmitters that can receive triplet energy from green light-absorbing perovskites with small energy loss and emitters with low triplet energy and UV fluorescence.
Here, we report the first example of green-to-UV TTA-UC using PNCs as the triplet sensitizer (Fig. 1). We found that 4-(2-phenyloxazol-5-yl)benzenesulfonate (PPOS)30 with a sulfonate group acts as transmitters that can receive triplet energy from green light-absorbing PNCs. The combination of PNCs-PPOS with the emitter TIPS-Nph, which was recently developed by our group to exhibit low triplet energy (2.12 eV) and good UV fluorescence,31 was found to form a suitable energy cascade for green-to-UV TTA-UC.
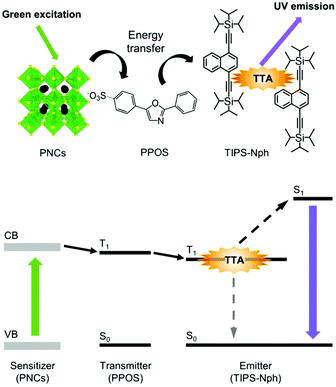 | ||
| Fig. 1 Structures of PNCs, PPOS, and TIPS-Nph, and energy diagram for green-to-UV TTA-UC sensitized by PNCs. | ||
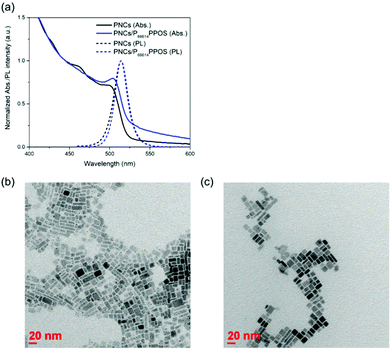
Green light-absorbing CsPbBr3 PNCs were synthesized by the supersaturated recrystallization method following previous studies,32 and purified by centrifugation with ethyl acetate. The purified PNCs showed a photoluminescence peak at 513 nm (2.42 eV, Fig. 2a). As a transmitter, we employed an ionic compound composed of trihexyltetradecylphosphonium cation (P66614) and PPOS anion (P66614PPOS).30 The quaternary phosphonium cation with long alkyl chains is useful for complexing with PNCs by improving the solubility of the transmitter in non-polar solvents. Since sulfonate groups have been reported to bind tightly to Pb ions,33,34 we chose transmitter PPOS with a sulfonate group. To check the triplet energy level, we measured a phosphorescence spectrum of P66614PPOS in toluene at 77 K (Fig. S1, ESI†). The observed phosphorescence peak at 528 nm (2.35 eV) locates between PNCs bandgap (2.42 eV) and TIPS-Nph T1 (2.12 eV),31 confirming the appropriate energy level alignment for the cascade triplet energy transfer.
The surface of PNCs was modified with P66614PPOS by stirring overnight in the presence of P66614PPOS. After the surface modification, the absorption and emission spectra of PNCs showed slight changes in their spectral shapes, suggesting the binding of PPOS to the PNC surface while maintaining the PNC structure (Fig. 2a). The peak position of the PNC emission was not changed by this surface modification. Transmission electron microscopy (TEM) images showed that the size and morphology of PNCs did not change significantly between before and after the modification with P66614PPOS (Fig. 2b and c).
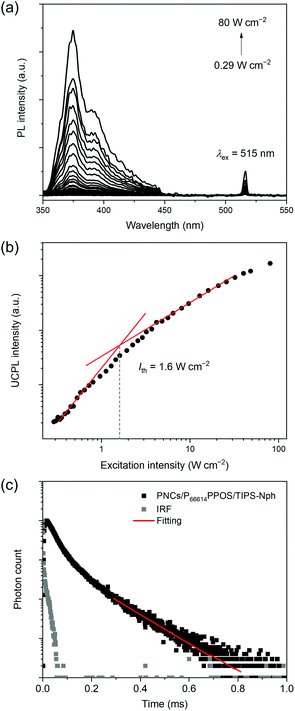
Under excitation by a 515 nm laser, a deaerated toluene solution of PNCs, P66614PPOS (1 mM) and TIPS-Nph (10 mM) showed an upconverted emission at 375 nm with a large UC spectral shift of 0.90 eV (Fig. 3a). The UC emission peak matches well with the TIPS-Nph fluorescence peak. The energy loss of triplet sensitization was minimized by delicate energy level adjustment of PNCs, transmitters, and emitters, and the excitation light to obtain UV emission was successfully extended from previous blue (<500 nm)25,26 to current green (>500 nm).
The UC mechanism based on TTA was verified by the excitation intensity dependence of the UC emission intensity and the UC emission decays. The excitation intensity dependence of the UC emission showed a quadratic to linear transition, which is characteristic of TTA-based UC (Fig. 3b). A threshold excitation intensity Ith of 1.6 W cm−2 was estimated from the intersection between two fitting lines. This Ith value is comparable to the previous examples of PNC-sensitized blue-to-UV TTA-UC (2–5 W cm−2).25,26 The triplet lifetime τT of 0.23 ms was obtained based on the relationship of IUC(t) ∝![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−2t/τT) (Fig. 3c), confirming the mechanism via the long-lived excited triplet state. The TTA-UC efficiency (ηUC, the theoretical maximum is 100%) for the PNCs/P66614PPOS/TIPS-Nph mixed solution was evaluated relative to a standard, Rhodamine 101 in deaerated ethanol (see the ESI†). The ηUC value was determined as 0.014% at Iex = 16 W cm−2. The obtained ηUC value is inferior to those of blue-to-UV TTA-UC sensitized by PNCs (10.2%)26 and using TIPS-Nph emitter (20.5%).31
exp(−2t/τT) (Fig. 3c), confirming the mechanism via the long-lived excited triplet state. The TTA-UC efficiency (ηUC, the theoretical maximum is 100%) for the PNCs/P66614PPOS/TIPS-Nph mixed solution was evaluated relative to a standard, Rhodamine 101 in deaerated ethanol (see the ESI†). The ηUC value was determined as 0.014% at Iex = 16 W cm−2. The obtained ηUC value is inferior to those of blue-to-UV TTA-UC sensitized by PNCs (10.2%)26 and using TIPS-Nph emitter (20.5%).31
In order to understand the low TTA-UC efficiency, we measured the photoluminescence quantum yield (PLQY) of PNCs. PLQY (λex = 445 nm) of the PNCs showed an increase from 42.4% to 78.2% by the PPOS modification, and it was not affected by the further addition of TIPS-Nph (77.2%). The increase in PLQY of PNCs by modifying PPOS is consistent with previous reports in which sulfonate ligands bind strongly to Pb ions at the PNC surface to suppress exciton trapping probability due to bromide vacancies.33,34 The high PLQY of PPOS-modified PNCs indicates the poor energy transfer efficiency, which should be one of the reasons for the low ηUC. Since the efficiency of energy transfer is highly dependent on the orbital overlap between PNCs and PPOS, it is expected that the optimization of the PNC structure and the molecular design of the transmitter would improve the energy transfer efficiency.23,35,36 Another possible reason is the partial quenching of the TIPS-Nph triplet because the triplet lifetime of TIPS-Nph (0.23 ms) was shorter than the reported value (0.88 ms).31 In addition, the large absorption of PNCs in the UV region should induce the reabsorption of upconverted emission.
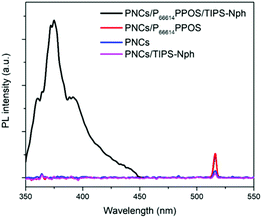
The roles of the transmitter and emitter were further examined by control experiments in the absence of one of them. When the two-component solutions of PNCs/P66614PPOS and PNCs/TIPS-Nph were irradiated with a 515 nm laser, no UC emission was observed (Fig. 4). This suggests that PPOS acts as a transmitter to facilitate the energy transfer from PNCs to TIPS-Nph, and TIPS-Nph does not work as a transmitter. The triplet energy of TIPS-Nph is lower than that of PPOS, which may have partially prevented the back energy transfer and achieved the TTA-UC emission. This was also supported by that a weaker TTA-UC emission intensity was observed when 2,5-diphenyloxazole (PPO) with a higher T1 energy (2.37 eV)31 was used as an emitter instead of TIPS-Nph (PNCs/P66614PPOS/PPO in Fig. S2, ESI†). The essential role of PPOS as the transmitter in the current system was confirmed by the absence of TTA-UC emission for PNCs/PPO and PNCs/PPO/TIPS-Nph (see the ESI† for each concentration), which is different from the case of TTA-UC sensitized by CdS/ZnS.37
In conclusion, we showed PNC-sensitized green-to-UV TTA-UC for the first time. This was achieved by combining a transmitter that can receive triplet energy from green-light-absorbing PNCs and an emitter that has an even lower triplet energy level than the transmitter but fluoresces in the UV region. The advantage of perovskites is that they can absorb light in a wide range of wavelengths, and the absorption wavelength can be easily tuned. If UV emitters with lower triplet energy are developed in the future, it is expected that even longer wavelengths of visible light can be upconverted into UV light, which is very useful for photocatalytic reactions.
Author contributions
N. Y. conceived the project. M. K., N. H., K. O. and N. Y. designed the experiments. M. K. prepared PNCs and UC samples, with the input of K. O. N. H. synthesized TIPS-Nph. J. M. and S. H. prepared P66614PPOS. M. K. and N. H. performed the optical measurements. M. K. and N. Y. wrote the manuscript, with the input of N. H. and N. K. All authors contributed to and have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by JSPS KAKENHI (grant numbers JP20H02713, JP20K21211, JP20H05676, JP21J21739) and the Innovation inspired by Nature Program of Sekisui Chemical Co. Ltd.References
- S. Baluschev, T. Miteva, V. Yakutkin, G. Nelles, A. Yasuda and G. Wegner, Phys. Rev. Lett., 2006, 97, 143903 CrossRef CAS PubMed
.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560 CrossRef CAS
.
- J. Zhao, S. Ji and H. Guo, RSC Adv., 2011, 1, 937 RSC
.
- A. Monguzzi, R. Tubino, S. Hoseinkhani, M. Campione and F. Meinardi, Phys. Chem. Chem. Phys., 2012, 14, 4322 RSC
.
- Y. C. Simon and C. Weder, J. Mater. Chem., 2012, 22, 20817 RSC
.
- J. H. Kim and J. H. Kim, J. Am. Chem. Soc., 2012, 134, 17478 CrossRef CAS PubMed
.
- Q. Liu, B. Yin, T. Yang, Y. Yang, Z. Shen, P. Yao and F. Li, J. Am. Chem. Soc., 2013, 135, 5029 CrossRef CAS PubMed
.
- K. Kamada, Y. Sakagami, T. Mizokuro, Y. Fujiwara, K. Kobayashi, K. Narushima, S. Hirata and M. Vacha, Mater. Horiz., 2017, 4, 83 RSC
.
- S. P. Hill and K. Hanson, J. Am. Chem. Soc., 2017, 139, 10988 CrossRef CAS PubMed
.
- V. Gray, K. Moth-Poulsen, B. Albinsson and M. Abrahamsson, Coord. Chem. Rev., 2018, 362, 54 CrossRef CAS
.
- X. Yang, J. Han, Y. Wang and P. Duan, Chem. Sci., 2019, 10, 172 RSC
.
- A. B. Pun, S. N. Sanders, M. Y. Sfeir, L. M. Campos and D. N. Congreve, Chem. Sci., 2019, 10, 3969 RSC
.
- B. D. Ravetz, A. B. Pun, E. M. Churchill, D. N. Congreve, T. Rovis and L. M. Campos, Nature, 2019, 565, 343 CrossRef CAS PubMed
.
- Y. Sasaki, M. Oshikawa, P. Bharmoria, H. Kouno, A. Hayashi-Takagi, M. Sato, I. Ajioka, N. Yanai and N. Kimizuka, Angew. Chem., Int. Ed., 2019, 58, 17827 CrossRef CAS PubMed
.
- L. Huang, T. Le, K. Huang and G. Han, Nat. Commun., 2021, 12, 1898 CrossRef PubMed
.
- N. J. Thompson, M. W. Wilson, D. N. Congreve, P. R. Brown, J. M. Scherer, T. S. Bischof, M. Wu, N. Geva, M. Welborn, T. V. Voorhis, V. Bulović, M. G. Bawendi and M. A. Baldo, Nat. Mater., 2014, 13, 1039 CrossRef CAS PubMed
.
- Z. Huang, X. Li, M. Mahboub, K. M. Hanson, V. M. Nichols, H. Le, M. L. Tang and C. J. Bardeen, Nano Lett., 2015, 15, 5552 CrossRef CAS PubMed
.
- M. Wu, D. N. Congreve, M. W. B. Wilson, J. Jean, N. Geva, M. Welborn, T. Van Voorhis, V. Bulović, M. G. Bawendi and M. A. Baldo, Nat. Photonics, 2016, 10, 31 CrossRef CAS
.
- C. Mongin, S. Garakyaraghi, N. Razgoniaeva, M. Zamkov and F. N. Castellano, Science, 2016, 351, 369 CrossRef CAS PubMed
.
- N. Yanai and N. Kimizuka, Acc. Chem. Res., 2017, 50, 2487 CrossRef CAS PubMed
.
- V. Gray, J. R. Allardice, Z. Zhang and A. Rao, Chem. Phys. Rev., 2021, 2, 031305 CrossRef
.
- K. Mase, K. Okumura, N. Yanai and N. Kimizuka, Chem. Commun., 2017, 53, 8261 RSC
.
- X. Luo, R. Lai, Y. Li, Y. Han, G. Liang, X. Liu, T. Ding, J. Wang and K. Wu, J. Am. Chem. Soc., 2019, 141, 4186 CrossRef CAS PubMed
.
- S. Wieghold, A. S. Bieber, Z. A. VanOrman, L. Daley, M. Leger, J.-P. Correa-Baena and L. Nienhaus, Matter, 2019, 1, 705 CrossRef CAS
.
- K. Okumura, N. Yanai and N. Kimizuka, Chem. Lett., 2019, 48, 1347 CrossRef CAS
.
- S. He, X. Luo, X. Liu, Y. Li and K. Wu, J. Phys. Chem. Lett., 2019, 10, 5036 CrossRef CAS PubMed
.
- X. Luo, Y. Han, Z. Chen, Y. Li, G. Liang, X. Liu, T. Ding, C. Nie, M. Wang, F. N. Castellano and K. Wu, Nat. Commun., 2020, 11, 28 CrossRef CAS PubMed
.
- Z. A. VanOrman, H. K. Drozdick, S. Wieghold and L. Nienhaus, J. Mater. Chem. C, 2021, 9, 2685 RSC
.
- L. Wang, J. J. Yoo, T.-A. Lin, C. F. Perkinson, Y. Lu, M. A. Baldo and M. G. Bawendi, Adv. Mater., 2021, 33, 2100854 CrossRef CAS PubMed
.
- S. Hisamitsu, J. Miyano, K. Okumura, J. K.-H. Hui, N. Yanai and N. Kimizuka, ChemistryOpen, 2020, 9, 14 CrossRef CAS PubMed
.
- N. Harada, Y. Sasaki, M. Hosoyamada, N. Kimizuka and N. Yanai, Angew. Chem., Int. Ed., 2021, 60, 142 CrossRef CAS PubMed
.
- X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song and H. Zeng, Adv. Funct. Mater., 2016, 26, 2435 CrossRef CAS
.
- D. Yang, X. Li, W. Zhou, S. Zhang, C. Meng, Y. Wu, Y. Wang and H. Zeng, Adv. Mater., 2019, 31, 1900767 CrossRef PubMed
.
- D. P. Nenon, K. Pressler, J. Kang, B. A. Koscher, J. H. Olshansky, W. T. Osowiecki, M. A. Koc, L. Wang and A. P. Alivisatos, J. Am. Chem. Soc., 2018, 140, 17760 CrossRef CAS PubMed
.
- P. Xia, Z. Huang, X. Li, J. J. Romero, V. I. Vullev, G. S. H. Pau and M. L. Tang, Chem. Commun., 2017, 53, 1241 RSC
.
- X. Li, A. Fast, Z. Huang, D. A. Fishman and M. L. Tang, Angew. Chem., Int. Ed., 2017, 56, 5598 CrossRef CAS PubMed
.
- V. Gray, P. Xia, Z. Huang, E. Moses, A. Fast, D. A. Fishman, V. I. Vullev, M. Abrahamsson, K. Moth-Poulsen and M. L. Tang, Chem. Sci., 2017, 8, 5488 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details (Materials, synthesis of PNCs, sample preparation for TTA-UC measurements, characterization, TTA-UC efficiency by the relative method) and phosphorescence and TTA-UC spectra. See DOI: 10.1039/d1nr06588b |
| This journal is © The Royal Society of Chemistry 2021 |