A monolithic sponge catalyst for hydrogen generation from sodium borohydride solution for portable fuel cells†
Jifeng
Deng
ab,
Bingxue
Sun
c,
Jinrong
Xu
a,
Yu
Shi
c,
Lei
Xie
c,
Jie
Zheng
 *a and
Xingguo
Li
*a and
Xingguo
Li
 *a
*a
aBeijing National Laboratory for Molecular Science (BNLMS), College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: xgli@pku.edu.cn; zhengjie@pku.edu.cn; Fax: +86-10-62765930; Tel: +86-10-62765930
bAcademy for Advanced Interdisciplinary Studies, Peking University, Beijing 100871, China
cSunan Institute for Molecular Engineering, Peking University, Building 6, Xianshi Road No.88, Changshu Hi-Tech Industrial Development Zone, Jiangsu 215500, China
First published on 21st October 2020
Abstract
A monolithic sponge catalyst composed of amorphous cobalt boride nanoparticles homogeneously deposited on the fibres of a commercial polyvinyl formal (PVFM) sponge is developed for the hydrolysis of NaBH4. The hydrolysis of NaBH4 is confined within the pores of the sponge without visible liquid and the catalyst can be easily recycled after the reaction. A light weight, low cost and easy to use onsite H2 generation system for portable fuel cells is developed using the monolithic sponge catalyst.
Introduction
Proton exchange membrane fuel cells (PEMFCs) are highly promising for both stationary and mobile power source applications due to their high conversion efficiency and zero emission. Hydrogen storage is one of the key enabling technologies for PEMFCs, particularly for mobile applications.1,2 The major research focus in the past two decades has been devoted to reversible hydrogen storage technologies. Unfortunately, it remains highly challenging to achieve high capacity reversible hydrogen storage with mild operation conditions.3 An alternative approach is using single-use hydrogen carriers and recovering the hydrogen carriers from the spent fuel ex situ. This approach is particularly attractive when hydrogen refilling infrastructures are not immediately available, such as outdoor power generators, unmanned aeroplanes or underwater vehicles.4,5Hydrolysis of sodium borohydride (NaBH4) is a well-established technology for on-site hydrogen generation.6 NaBH4 forms very stable aqueous solution with up to 35 wt% at room temperature, which releases H2 in a controllable way when in contact with transition metal catalysts such as Co, Ni, Pt etc.7–9 Using concentrated NaBH4 solution (>20 wt%), a gravimetric hydrogen storage density of 4 wt% can be achieved, which is of practical interest for portable PEMFC applications. In the past two decades, a number of transition metal catalysts have been developed to improve the hydrolysis reactivity.10 Currently, full utilization of the fuel can readily be achieved. The main challenge is to develop light weight, compact H2 generation devices for specific fuel cell applications.
In most prototype H2 generators from the hydrolysis of NaBH4, NaBH4 solution is pumped through a fixed bed reactor in which the catalyst is loaded.5,11 In this case, a pump and an additional battery to power the pump are required, which significantly increases the weight and size of the hydrogen generator. Due to the higher cost of NaBH4 compared to those of other hydrides and reactive metals,12–14 hydrolysis of NaBH4 is best suited for low to medium power fuel cells. In this case, it is necessary to simplify the H2 generation system by minimizing the additional balance-of-plants (BOPs).
In transition metal catalysed NaBH4 hydrolysis, H2 generation occurs at the catalyst–solution interface, which is limited by the surface catalytic sites. Therefore, stable H2 generation can be achieved if the NaBH4 solution and the catalyst are thoroughly mixed. As a result, the catalyst is usually loaded on porous, monolithic substrates in the fixed bed reactor such as Ni-foam, Ti-mesh, carbon materials, honeycomb ceramic and polymers etc.15–20 This strategy also facilitates easy separation of the catalyst and the solution after hydrolysis. However, these conventional porous substrates also increase the total weight of the H2 generation system.
In this work, we report a monolithic, light weight sponge catalyst for controllable hydrogen generation from sodium borohydride solution. The catalyst is composed of Co2B nanoparticles homogeneously coated on the polyvinyl formal (PVFM) sponge. The strong water absorbing ability of the sponge enables a stable hydrogen generation process free of visible liquid within the pores of the sponge. The catalyst can be easily separated from the solution after reaction by simple squeezing. The novel sponge catalyst is highly promising to simplify the NaBH4 based hydrogen generation systems for portable fuel cells.
Experimental
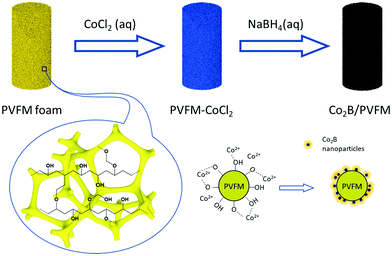
The sponge catalyst is prepared by a two-step impregnation–reduction method, as schematically illustrated in Fig. 1. In a typical experiment, the commercial PVFM sponge is cut into a cylindrical shape with a diameter of 18 mm and a height of 50 mm. The sponge cylinder is immersed in 10 wt% CoCl2 aqueous solution and pressed to adequately absorb the solution. Then the sponge is dried in a vacuum oven at 60 °C for 12 h. Sufficient 5 wt% NaBH4 solution (typically more than 2 times the apparent volume of the sponge) is added to the CoCl2 impregnated sponge dropwise and the sponge is kept in the resulting solution for 2 h until CoCl2 is completely reduced. The sponge is then washed and squeezed with deionized water 3 times to remove the excess solution and the weakly adhered Co–B particles. The catalyst loading (2–30 mg per cm3 sponge) can be controlled by using CoCl2 solution with different concentrations in the impregnation step. To determine the catalyst loading amount, the sponge needs to be dried in a freeze-dryer for 12 h.The samples are characterized by powder X-ray diffraction (XRD, PANalytical X-Pert3 Powder, Cu Kα), field emission scanning electron microscopy (SEM, Hitachi S-4800), field emission high resolution transmission electron microscopy (HRTEM, JEM-2100F) and X-ray photoelectron spectroscopy (XPS, Kratos Analytical Ltd, AXIS Supra, Al Kα). The specific surface area is analyzed by nitrogen adsorption at 77 K (Quantachrome, NOVA2200e). The composition of the catalyst before and after hydrolysis is measured using an inductively coupled plasma-atomic emission spectrometer by dissolving the loaded catalyst in HNO3 (ICP-AES, Leeman Prodigy 7).
In hydrogen generation measurements, the monolithic sponge catalyst is loaded into a flask or a plastic tube containing 10 mL NaBH4 solution of different concentrations. H2 is generated when the NaBH4 solution is absorbed by the sponge. The H2 generation rate is measured using a gas mass flow meter (Beijing Sevenstar Electronic Co., Ltd, D07-1).
Results and discussion
The pristine dry PVFM sponge shows an apparent density of 0.120 g cm−3, and it can absorb about 1 mL water per cm3. The sponge shows interconnected, irregular macropores around 30 μm in diameter woven by fibres several microns in diameter with a smooth surface, as shown by the SEM image (Fig. 2a and b). However, the specific surface area is only 0.447 m2 g−1. The resulting Co–B/PVFM sponge retains the high flexibility of the pristine sponge. The Co–B catalyst particles are strongly adhered to the sponge. No notable detachment of the particles is observed when the sponge is squeezed. The strong adhesion of the Co–B nanoparticles to the PVFM fibres is attributed to the strong interaction of Co2+ with the oxo and hydroxyl groups of PVFM, as schematically illustrated in Fig. 1. After catalyst loading, the apparent density slightly increases to 0.134 g cm−3. The magnified SEM image shows that the fibres become very rough, covered by many nanoparticles. The catalyst loading is 0.014 g cm−3 based on the apparent volume.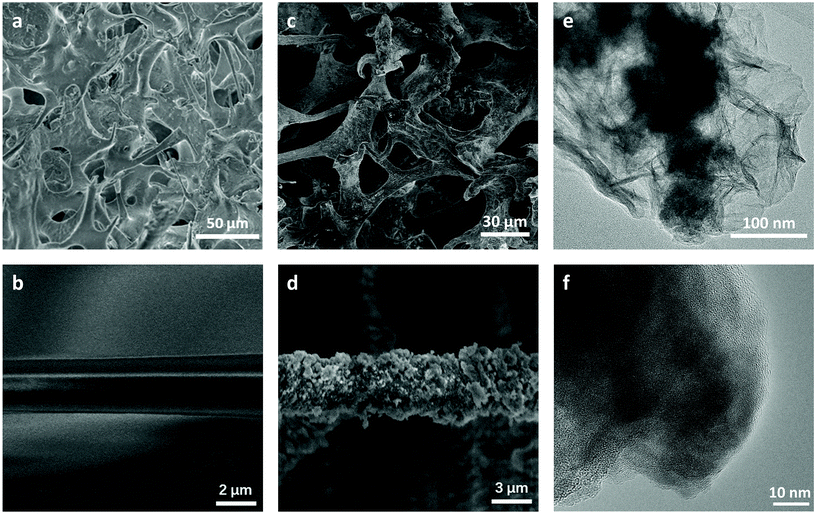 | ||
| Fig. 2 SEM images of the pristine PVFM sponge (a and b) and the Co2B/PVFM sponge (c and d). TEM images of the amorphous Co2B catalyst (e and f). | ||
The TEM image (Fig. 2e and f) suggests that the attached catalyst is composed of nanoparticles wrapped by wrinkled nanosheets. The specific surface area significantly increases to 10.484 m2 g−1, indicating that the loaded catalyst is highly porous. Fig. S1† shows the XRD patterns of the catalyst coated PVFM sponge. There are no diffraction peaks observed, indicating that the catalyst is amorphous, which corresponds to previous studies of other researchers.17,21 ICP-OES measurements show that the Co/B molar ratio is 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, close to that in Co2B. A similar composition has been reported in a previous study on NaBH4 reduction of Co2+ in aqueous solution.22 Thus, the loaded catalyst is amorphous Co2B. Both the composition and morphology are in agreement with previous studies7,17,21,22 on NaBH4 reduction of Co salts in aqueous solution. The XPS spectra of Co 2p and B 1s are shown in Fig. S2b and d.† The peaks at 780.7 eV for Co 2p3/2 and 796.3 eV for Co 2p1/2 correspond to Co2+. The peak at 191.7 eV in the B 1s spectrum is attributed to oxidized B, indicating that the surface of the Co2B particles is oxidized. The XPS results are similar to those of previous studies23–25 on metal boride prepared by similar methods.
1, close to that in Co2B. A similar composition has been reported in a previous study on NaBH4 reduction of Co2+ in aqueous solution.22 Thus, the loaded catalyst is amorphous Co2B. Both the composition and morphology are in agreement with previous studies7,17,21,22 on NaBH4 reduction of Co salts in aqueous solution. The XPS spectra of Co 2p and B 1s are shown in Fig. S2b and d.† The peaks at 780.7 eV for Co 2p3/2 and 796.3 eV for Co 2p1/2 correspond to Co2+. The peak at 191.7 eV in the B 1s spectrum is attributed to oxidized B, indicating that the surface of the Co2B particles is oxidized. The XPS results are similar to those of previous studies23–25 on metal boride prepared by similar methods.
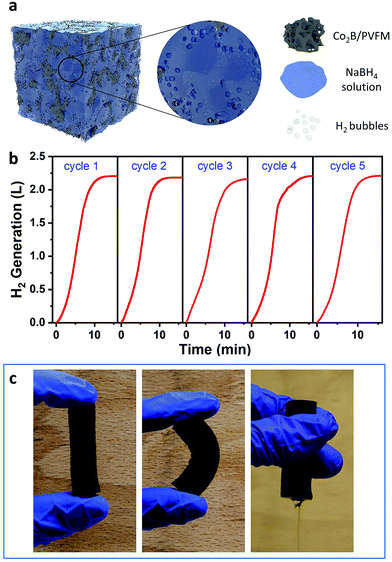
To test the catalytic performance for NaBH4 hydrolysis, the Co2B/PVFM cylinder is loaded into a glass flask containing 10 mL NaBH4–NaOH solution. The hydrolysis reaction is initiated almost instantaneously when the NaBH4 solution is in contact with the Co2B particles. Meanwhile, the NaBH4 solution is rapidly absorbed into the Co2B/PVFM sponge. Therefore, the hydrolysis takes place within the pores of the Co2B coated PVFM sponge, leaving no visible liquid solution, as schematically illustrated in Fig. 3a. H2 can be generated with a stable rate, which is attributed to the good contact of the NaBH4 solution with the Co2B nanoparticles in the pores of the sponge. As shown in Fig. 3b, in cycle 1, more than 2.0 L H2 can be released within 10 min, which is sufficient to power a 20 W fuel cell. After hydrolysis, the remaining solution in the sponge can be easily squeezed out and the sponge can be further washed with clean water for recycling (Fig. 3c). Due to the strong adhesion of the Co2B particles to the sponge, there is no Co2B particle loss during squeezing and washing. The residual NaBO2 in the sponge after washing is negligible, because no Na+ is detected by ICP-OES measurements. The sponge catalyst after washing can be used repeatedly. Benefiting from the complete removal of NaBO2 after each hydrolysis cycle, there is no significant capacity loss in 5 cycles, as shown in Fig. 3b. The easy recycling of the catalyst is a key advantage of the monolithic sponge catalyst compared to catalysts in powder form.
Hydrolysis kinetic studies of H2 generation catalyzed by a monolithic sponge catalyst under different conditions are systematically performed (Fig. S5†). In all kinetic measurements, the PVFM sponge was cut into smaller cylinders (diameter: 18 mm and height: 20 mm) and the excess (more than 30 mL) NaBH4 solution is used to maintain near constant NaBH4 concentration.
As shown in Fig. S5a,† the H2 generation rate increases with the NaOH concentration. As the hydrolysis of BH4− occurs via several BH(4−n)(OH)n− (n = 1, 2 and 3) intermediates, OH− could facilitate the formation of these intermediates on the surfaces of the catalysts.6 Similar effects of OH− have also been reported in the literature.26 The hydrogen generation rate first rises and then falls with the NaBH4 concentration, and reaches the maximum at 5 wt% NaBH4 (Fig. S5b†). According to a previous study, the hydrolysis of NaBH4 catalyzed by Co2B catalysts is a zero-order reaction,7 which should be theoretically independent of the NaBH4 concentration. In the sponge catalyst, the mass transport within the pores of the sponge plays an important role, which is related to the accessibility of the catalytic sites of the reactants (NaBH4 and H2O) and removal of the by-product (NaBO2). The NaBH4 solution could affect the viscosity of the solution and the swelling behaviour of the PVFM sponge,19 which finally result in the complicated dependence of the H2 generation rates on the NaBH4 concentration. Particularly, at higher NaBH4 concentration, the precipitation of sodium metaborate on the catalyst surface is a major factor hindering the H2 generation. Although we could effectively remove NaBO2 after hydrolysis when using 10 wt% NaBH4 solution (Fig. 3b), the reuse of the sponge catalyst is more difficult for more concentrated NaBH4 solution. As shown in Fig. S6,† the activity of the recycled sponge catalyst clearly reduces when 20 wt% NaBH4 solution is used in hydrolysis, which could be attributed to the incomplete removal of the NaBO2 precipitate within the pores. The results of EDS mapping (Fig. S3†) show the different amounts of the residual NaBO2 precipitates after the hydrolysis of 10 wt% and 30 wt% NaBH4 solutions. It is clear that there is more residual NaBO2 adhering to the sponge fiber when more concentrated NaBH4 solution is used in the hydrolysis. As a result, using very concentrated NaBH4 solution is not recommended if the sponge catalyst is intended for reuse.
The effect of the Co2B loading amount is also studied. As shown in Fig. S5c,† increasing the Co2B loading amount from 6.5 to 30 mg cm−3 by using more concentrated CoCl2 solution in impregnation leads to an increase of the hydrogen generation rate per cm3 sponge catalyst from 9.9 to 32.2 mL min−1. However, higher Co2B loading leads to a lower H2 generation rate per gram sponge catalyst. As shown in Fig. S5d,† the highest H2 generation rate at 298 K reaches 1500 mL min−1 per gram catalyst at a concentration of 5 wt% NaBH4, 5% NaOH at 6.5 mg cm−3 Co2B loading, which can be understood from the less efficient exposure of the catalytic sites per gram catalyst at higher Co2B loading. The apparent activation energy (56 kJ mol−1) is calculated from the hydrogen generation rates at different temperatures from 273 to 313 K (Fig. S5e†) using the Arrhenius equation (Fig. S5f†), which is similar to the values reported in previous studies.25
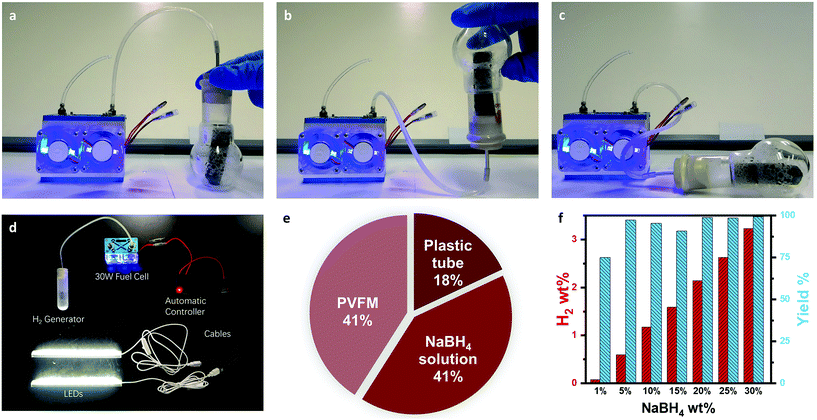
With the monolithic sponge catalyst, the NaBH4 solution is absorbed by the sponge in the hydrogenation process, so that the hydrogen generation system is in the quasi-solid state, i.e. without visible liquid. As a result, there is no restriction of the system orientation as there is no liquid leaking issue. As shown in Fig. 4a–c, the H2 generation device can be placed up straight, horizontally or even upside down. For portable applications, the whole power system may experience significant changes in orientation. This omni-orientation is highly favourable for such applications.
Benefiting from the light weight of the PVMF sponge, the apparent volume of the catalyst monolith can be made larger than that of the NaBH4 solution without a significant increase of the weight. As a result, the NaBH4 solution can be completely absorbed into the PVMF sponge during the hydrolysis. The monolithic sponge catalyst significantly simplifies the H2 generation system. H2 generation can be carried out in a light weight container such as a plastic tube. As shown in Fig. 4d, steady H2 generation can be achieved in a plastic tube with a rubber stopper, which can be used to power a 20 W fuel cell to light up 2 LED tubes. This is the simplest configuration of a hydrogen generation device which minimizes all the possible BOPs. Fig. 4e shows the weight of each component in the H2 generation system. Fig. 4f shows the conversion of NaBH4 and material-based hydrogen storage density when different NaBH4 solutions are used. The weight percentage of the NaBH4 solution is about 40% of the whole system, leading to a material-based hydrogen storage density of 2% when 20% NaBH4 solution is used. The hydrogen storage density can be further improved by using a sponge with lower apparent density. For instance, the commercially available melamine sponge has a very low apparent density of only 6–10 mg cm−3. However, Co2B nanoparticles cannot be directly deposited on the fibres of the commercial melamine sponge.27 Proper surface modification of the melamine sponge is necessary to enhance the adhesion of Co2B nanoparticles to the fibre, which will be highly promising to further improve the effective hydrogen storage density.
The monolithic sponge catalyst has several key advantages compared to conventional porous ceramic supported transition metal catalysts for NaBH4 hydrolysis: (1) steady H2 generation can be achieved in a very simple H2 generation system with minimized BOP; and (2) easy recycling of the catalyst and (3) omni-orientational property are achieved. These unique advantages are particularly promising for small scale H2 generation for low power (<50 W) portable fuel cell applications.
Conclusions
In conclusion, amorphous Co2B nanoparticles can be homogeneously attached to the fibres of the PVFM sponge by simple impregnation–reduction treatment of the commercial PVFM sponge, resulting in a monolithic sponge catalyst for the hydrolysis of NaBH4. The hydrolysis of NaBH4 is confined within the pores of the monolithic sponge without visible liquid, which significantly simplifies the H2 generation system. Steady H2 generation of about 200 mL min−1 can be achieved in a light weight plastic container, resulting in a material-based hydrogen storage density above 2%. There is no restriction on the orientation of the H2 generation unit during operation. The catalyst can be easily recycled after reaction by simply squeezing out the residual solution. Due to these unique advantages, the monolithic sponge catalyst is highly promising to develop high efficiency, easy to operate onsite H2 generation systems for 50 W scale portable fuel cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work is supported by the MOST of China (No. 2018YFB1502104) and NSFC (No. 21771006, 51771002 and 51971004).Notes and references
- U. Eberle, M. Felderhoff and F. Schuth, Chemical and physical solutions for hydrogen storage, Angew. Chem., Int. Ed., 2009, 48, 6608–6630 CrossRef CAS.
- L. Schlapbach and A. Züttel, Hydrogen-storage materials for mobile applications, Nature, 2001, 414, 353–358 CrossRef CAS.
- T. He, P. Pachfule, H. Wu, Q. Xu and P. Chen, Hydrogen carriers, Nat. Rev. Mater., 2016, 1, 16059 CrossRef CAS.
- K. Kim, T. Kim, K. Lee and S. Kwon, Fuel cell system with sodium borohydride as hydrogen source for unmanned aerial vehicles, J. Power Sources, 2011, 196, 9069–9075 CrossRef CAS.
- T. Kim, NaBH4 (sodium borohydride) hydrogen generator with a volume-exchange fuel tank for small unmanned aerial vehicles powered by a PEM (proton exchange membrane) fuel cell, Energy, 2014, 69, 721–727 CrossRef CAS.
- B. H. Liu and Z. P. Li, A review: Hydrogen generation from borohydride hydrolysis reaction, J. Power Sources, 2009, 187, 527–534 CrossRef CAS.
- N. Patel and A. Miotello, Progress in Co-B related catalyst for hydrogen production by hydrolysis of boron-hydrides: A review and the perspectives to substitute noble metals, Int. J. Hydrogen Energy, 2015, 40, 1429–1464 CrossRef CAS.
- H. Cai, P. Lu and J. Dong, Robust nickel-polymer nanocomposite particles for hydrogen generation from sodium borohydride, Fuel, 2016, 166, 297–301 CrossRef CAS.
- C. Salameh, A. Bruma, S. Malo, U. B. Demirci, P. Miele and S. Bernard, Monodisperse platinum nanoparticles supported on highly ordered mesoporous silicon nitride nanoblocks: superior catalytic activity for hydrogen generation from sodium borohydride, RSC Adv., 2015, 5, 58943–58951 RSC.
- H. Sun, J. Meng, L. Jiao, F. Cheng and J. Chen, A review of transition-metal boride/phosphide-based materials for catalytic hydrogen generation from hydrolysis of boron-hydrides, Inorg. Chem. Front., 2018, 5, 760–772 CAS.
- T. H. Oh and S. Kwon, Performance evaluation of hydrogen generation system with electroless-deposited Co–P/Ni foam catalyst for NaBH4 hydrolysis, Int. J. Hydrogen Energy, 2013, 38, 6425–6435 CAS.
- W. Chen, L. Z. Ouyang, J. W. Liu, X. D. Yao, H. Wang, Z. W. Liu and M. Zhu, Hydrolysis and regeneration of sodium borohydride (NaBH4) - A combination of hydrogen production and storage, J. Power Sources, 2017, 359, 400–407 CrossRef CAS.
- L. Ouyang, W. Chen, J. Liu, M. Felderhoff, H. Wang and M. Zhu, Enhancing the Regeneration Process of Consumed NaBH4 for Hydrogen Storage, Adv. Energy Mater., 2017, 7, 1700299 CrossRef.
- Y. Zhu, L. Ouyang, H. Zhong, J. Liu, H. Wang, H. Shao, Z. Huang and M. Zhu, Closing the loop for hydrogen storage: Facile regeneration of NaBH4 from its hydrolytic product, Angew. Chem., 2020, 59, 8623–8629 CrossRef CAS.
- L. Ai, X. Gao and J. Jiang, In situ synthesis of cobalt stabilized on macroscopic biopolymer hydrogel as economical and recyclable catalyst for hydrogen generation from sodium borohydride hydrolysis, J. Power Sources, 2014, 257, 213–220 CrossRef CAS.
- L. Cui, X. Sun, Y. Xu, W. Yang and J. Liu, Cobalt Carbonate Hydroxide Nanowire Array on Ti Mesh: An Efficient and Robust 3D Catalyst for On-Demand Hydrogen Generation from Alkaline NaBH4 Solution, Chem. – Eur. J., 2016, 22, 14831–14835 CrossRef CAS.
- S. Guo, Q. Wu, J. Sun, T. Chen, M. Feng, Q. Wang, Z. Wang, B. Zhao and W. Ding, Highly stable and controllable CoB/Ni-foam catalysts for hydrogen generation from alkaline NaBH4 solution, Int. J. Hydrogen Energy, 2017, 42, 21063–21072 CrossRef CAS.
- D.-W. Zhuang, H.-B. Dai, Y.-J. Zhong, L.-X. Sun and P. Wang, A new reactivation method towards deactivation of honeycomb ceramic monolith supported cobalt-molybdenum-boron catalyst in hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2015, 40, 9373–9381 CrossRef CAS.
- N. Sahiner, Soft and flexible hydrogel templates of different sizes and various functionalities for metal nanoparticle preparation and their use in catalysis, Prog. Polym. Sci., 2013, 38, 1329–1356 CrossRef CAS.
- S. Saha, A. Saha, L.-o. Lepcha, S. Ganguly, D. Banerjee and K. Kargupta, Graphene-Rich G-Co-Ni Nanomatrix: An Optimized Heterogeneous Catalyst for Hydrogen Generation Based on Morphology-Performance Mapping, ChemistrySelect, 2017, 2, 4309–4319 CrossRef CAS.
- M. H. Loghmani, Various Rate Law Orders Through Hydrolysis of Sodium Borohydride Over Co-M-Zr-B (M=Cr, Mo and W) Nano Catalyst, Int. J. Chemoinf. Chem. Eng., 2017, 6, 45–61 CAS.
- L. Wang, M. Zhong, J. Li, X. Zhao, W. Hao and Y. Guo, Highly efficient ferromagnetic Co-B-O catalyst for hydrogen generation, Int. J. Hydrogen Energy, 2018, 43, 17164–17171 CrossRef CAS.
- J. Manna, B. Roy, D. Pareek and P. Sharma, Hydrogen generation from NaBH4 hydrolysis using Co-B/AlPO4 and Co-B/bentonite catalysts, Catal., Struct. React., 2017, 3, 157–164 CrossRef CAS.
- Y. Wang, T. Li, S. Bai, K. Qi, Z. Cao, K. Zhang, S. Wu and D. Wang, Catalytic hydrolysis of sodium borohydride via nanostructured cobalt-boron catalysts, Int. J. Hydrogen Energy, 2016, 41, 276–284 CrossRef CAS.
- N. Patel, G. Guella, A. Kale, A. Miotello, B. Patton, C. Zanchetta, L. Mirenghi and P. Rotolo, Thin films of Co–B prepared by pulsed laser deposition as efficient catalysts in hydrogen producing reactions, Appl. Catal., A, 2007, 323, 18–24 CrossRef CAS.
- Q. Li, W. Yang, F. Li, A. Cui and J. Hong, Preparation of CoB/ZIF-8 supported catalyst by single step reduction and its activity in hydrogen production, Int. J. Hydrogen Energy, 2018, 43, 271–282 CrossRef CAS.
- K.-Y. A. Lin, H.-A. Chang and B.-J. Chen, Multi-functional MOF-derived magnetic carbon sponge, J. Mater. Chem. A, 2016, 4, 13611–13625 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0qi00911c |
| This journal is © the Partner Organisations 2021 |