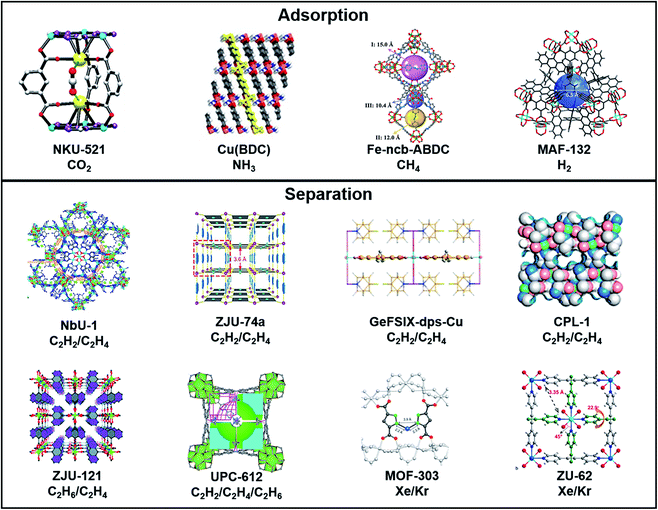
Open Access Article
Open Access ArticleRecent advances in metal–organic frameworks for gas adsorption/separation
Chuanhai
Jiang
,
Xiaokang
Wang
,
Yuguo
Ouyang
,
Kebin
Lu
,
Weifeng
Jiang
,
Huakai
Xu
,
Xiaofei
Wei
,
Zhifei
Wang
,
Fangna
Dai
 * and
Daofeng
Sun
* and
Daofeng
Sun

School of Materials Science and Engineering, College of Science, China University of Petroleum (East China), Qingdao, Shandong 266580, China. E-mail: fndai@upc.edu.cn
First published on 24th March 2022
Abstract
The unique structural advantage of metal–organic frameworks (MOFs) determines the great prospect and developability in gas adsorption and separation. Both ligand design and microporous engineering based on crystal structure are significant lever for coping with new application exploration and requirements. Focusing on the designable pore and modifiable frameworks of MOFs, this review discussed the recent advances in the field of gas adsorption and separation, and analyzed the host–guest interaction, structure–performance relations, and the adsorption/separation mechanism from ligand design, skeleton optimization, metal node regulation, and active sites construction. Based on the function-oriented perspective, we summarized the main research recently, and made an outlook based on the focus of microporous MOFs that require further attention in the structure design and industrial application.
1. Introduction
Metal–organic frameworks (MOFs) are organic–inorganic hybrid crystalline porous materials assembled by organic bridging linker and metal ions/cluster.1 Since the first MOF was reported by Yaghi in 1995,2 numerous MOF materials have been widely researched in the past decades.3 As typical porous materials, the obvious features of MOFs are ultra-high specific surface area, permanent porosity, designable structure, tunable pore size, etc.,4 which have encouraged researchers to explore their application continuously. Compared with other porous solid materials, MOFs can better highlight the controllable continuity from design to structure and performance. At present, the application fields of MOFs contain gas adsorption/separation, luminescence, sensing, catalysis, and drug delivery.5–9 Gas adsorption and separation are considered as one of the earliest and most extensive application for MOFs, and a lot of excellent research works have been reported in the past decades (Fig. 1).10–16 Due to the multifunctional and ever-increasing demands, it is really wise to further focus on exploring structure–performance connection of MOFs by combining diversified strategies.Gas adsorption, such as gas storage or removal, is one of the application to reflect the pore characteristics and advantages for MOFs maximally.17 With the development of science and technology, an urgent issue is that on the one hand, we need to seek ideal materials for storing fuel gas such as H2 and CH4,18 and on the other hand, it is essential to find efficient and economical adsorbents to removal harmful gas such as SO2, NH3, and CO.19–24 MOFs are always promising candidates for gas adsorption due to the precise micropore engineering design. Generally, it is easier to meet the custom-made demands via precise structural adjustments. As the main place, the operation of the pore provides greater potential for binding guest molecules. For different guest molecules, targeted measures are taken to regulate the pore environment.25 For instance, introducing functional sites into the skeleton or pores can increase the binding force between the guest and the frameworks.26 Lewis basic N-sites can enhance the interaction between gas and networks, thus improving the ability of the gas storage.27 Besides, a notable strategy is to produce open metal sites (OMS) by removing coordinated solvent molecules under negative pressure.28
Separation is an indispensable process in the industry, and the significance of gas separation is self-evident. Usually, in the chemical industry field, cryogenic distillation is an effective process for separating gas mixtures.29 However, as an environment- and energy-intensive process, the multiple evaporation–condensation cycles would inevitably cause the waste of energy and resources.30 It is worthy to explore the adsorbents with high adsorption capacity and separation efficiency. MOFs are considered as potential materials for gas separation due to functionalized interface, designable structure, and atom-level control.31 According to the molecular features, a series of measures, such as ligand size adjustment,32 ligand modification,33 secondary building unit (SBU) modification,34 and pore space partition,35 are applied to adjust the pore and network environment. Based on these, identifying and magnifying the slight difference between two or three kinds molecules are the key to optimize the host–guest interactions. In contrast, the traditional solid state absorbents (e.g., zeolite, molecular sieve, and porous carbon) do not show the notable advantages above.36 Generally, the evaluation of the adsorption/separation performance is mainly as follows: the adsorption capacity is a parameter that directly reflects the working capacity, adsorption enthalpy (Qst) represents the enthalpy changes due to the adsorption process, ideal adsorbed solution theory (IAST) is usually the theoretical basis for evaluating the separation potential, and the breakthrough curves obtained from the breakthrough experiments are used to demonstrate the actual separation behavior of mixed gases. Based on the above parameters, we can effectively analyze the adsorption and separation performance of MOFs.
So far, MOF materials have made diversified progress from the original research on the single structure study to the function-oriented construction. Herein, we summarize the relevant studies on MOFs in gas adsorption and separation, and present the issues needed to be focused on. Finally, we look ahead at the prospects in both new MOFs development and its practical application.
2. Gas adsorption and storage
As typical porous materials, MOFs have unique advantage to bring into play gas adsorption performance by material genes editing, post-modification, etc. The pore structure and controllable size of the MOFs decide the potential of high adsorption capacity, and show application prospects in improving the separation effect and reducing the energy consumption. Thus, MOFs are considered as idealized candidates for gas adsorption and storage.37 In order to meet the actual demands, a typical vision is to achieve the selective adsorption of certain guests by diverse strategies. At present, a several MOFs have been proven to be able to achieve gas adsorption including the greenhouse and toxic gases (CO2,21,22 NOx,20 SO2,23,24 NH3,19etc.) and the energy gases (H2, CH4, etc.).182.1 Greenhouse and toxic gas removal
Greenhouse gases such as CO2 are causing global climate warming, which compels the exploration of new materials that can reduce the excess CO2 and relieve the climate problems.38 The combination of gas and adsorbents is based on the force between the guests and the binding site. Thus, Bu et al. explored MOFs with unsaturated alkali metal sites by introducing tetrazole.39 In this work, K+ are installed in the trinuclear Co2+-tetrazole unit in NKU-521 ([Co3K2(TZIA)3(H2O)3](DMA)), the active K+ sites are fully exposed, and show CO2 trapping after removing the coordinated H2O molecules (Fig. 2). In the process of CO2 capture, K+ is considered to promote the combination of gas molecules and skeleton, which increases the isosteric heat of NKU-521a (removing the solvent molecules of NKU-521) by 24%, reaching 41 kJ mol−1.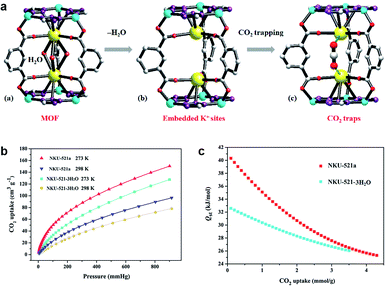 | ||
| Fig. 2 (a) The structure of NKU-521, NKU-521a, and NKU-521a·CO2 (according to the molecular simulation), (b) the CO2 adsorption isotherms, (c) Qst toward CO2 for NKU-521-3H2O and NKU-521a at 273 K and 298 K.39 Reproduced from ref. 39 with permission, copyright John Wiley and Sons, 2019. | ||
Another effective strategy to enhance SO2 absorption and cycles performance was reported by Ilich A. Ibarra.40 MIL-101(Cr) was redesigned with fluorine to promote the ability of SO2 capture, named as MIL-101(Cr)-4F(1%). With the doping of fluorine, the acidity of open Cr(III) sites was significantly enhanced. Compared with MIL-101(Cr), MIL-101(Cr)-4F(1%) shows effective exaltation in SO2 capture (18.4 mmol g−1, 298 K, 1 bar), which attributed is to the difference of the attracting electron. At the same time, MIL-101(Cr)-4F(1%) also exhibited the adsorption–desorption stability of SO2 (about 18.44 mmol g−1, during 50 cycles), which ensures the development of adsorbents with high stability and long cycle life (Fig. 3).
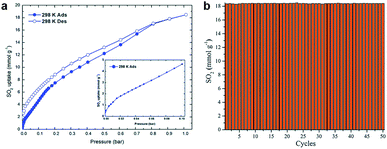 | ||
| Fig. 3 (a) The SO2 adsorption–desorption isotherm of MIL-101(Cr)-4F(1%), illustration: adsorption isotherm enlarged from 0.0–0.1 bar. (b) Adsorption–desorption cycles for 101(Cr)-4F(1%) at 298 K and 1 bar.40 Reproduced from ref. 40 with permission, copyright The Royal Society of Chemistry, 2020. | ||
There exist large sources of NH3 in industry and daily life such as machines, the excrement of animals, and production in industry.41 In order to integrate the adsorption capacity and cycle stability of the adsorbents, especially for corrosive gases. Taking into account the production cost and cycle stability, Li et al. focused on the M(BDC) (M = Cu, Zn, Cd) with structural transformation characteristics to explore a recyclable MOFs-based adsorbent.42 While NH3 touches with M(BDC) (M = Cu, Zn, Cd), the coordination model of the ligands will be transformed by the exchange of NH3 and BDC ligands situated in the opposite position, leading to straight-chain (Cu(BDC)) or folded-chain (Zn(BDC) and Cd(BDC)) (Fig. 4). After transforming, the adsorption capacities of Cu(BDC), Zn(BDC), and Cd(BDC) are improved to 17.2, 14.1, and 7.4 mmol g−1, respectively, realizing the removal of NH3 even in air with a low concentration. Meaningfully, the transformation is proven to be completely reversible via extra activity at 250 °C for 80 minutes, which indicates the potential for recyclable industry application. Besides, Li's group also explored the enlarged preparation of Cu(BDC) and focused on the stability of the adsorbents, laying the groundwork for industrial-scale adsorbent production.43
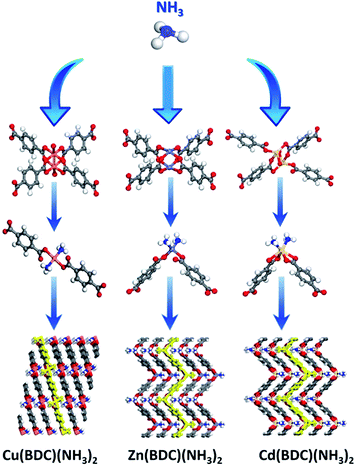 | ||
| Fig. 4 The transform of M(BDC) (M = Cu, Zn, Cd).42 Reproduced from ref. 42 with permission, copyright American Chemical Society, 2020. | ||
2.2 Energy gas adsorption
With the consumption of traditional energy, such as petroleum, coal, and oil shale, people have begun to develop new clear energy sources such as hydrogen and methane to relieve the crisis caused by traditional energy.44 However, current main gas storage and transportation methods are represented by pressurized liquefaction. Usually, it requires high pressure (up to 35–70 MPa at room temperature),45 which compels the exploration of new measures to replace traditional physical storage.At present, in order to enhance the banding density, many strategies usually focus on the design of the metal centers, such as open metal sites,46 coordinatively unsaturated sites,47 and open coordination sites.48 Based on this, Long and co-workers reported V2Cl2.8(btdd), which exposes the V(II) sites to bond with weak π-acids, thus increasing the opportunities of H2 capture.18 Interestingly, this is the first material that exhibits binding enthalpy range between −15 kJ mol−1 and −25 kJ mol−1 (about −21 kJ mol−1), and V(III) dπ and H2 σ* orbital promote bond formation. Apart from improving the density and activity between gaseous substrates and effective metal sites; effective enclosed cages are also applied with gas capture. A typical example about MFM-132 ([Cu3(BTAT)(H2O)3]·9DMF) was reported by Schröder and co-workers.49 Desolvated MFM-132a functionalized by aromatic anthracene indicates that anthracene modification does not reduce the BET (reaching 2466 m2 g−1) or hinder the confirmation of porosity (pore volume = cm3 g−1); instead, it promotes the process of packing H2 into the cages. It has been verified that the binding sites are composed of triangular (Cu2)3(isophthalate)3 and three anthracene panels, forming the closed pockets of 6 Å. Under the same condition, MFM-132a realized the highest density of adsorbed H2 within the pore, reaching 52 g L−1 at 60 bar and 77 K. In the long run, Schröder's works exploit a new direction for gas adsorption by aromatic connection units.
As the simplest hydrocarbon compound, CH4 is considered as a clean energy as it does not produce any harmful gas, only CO2 and H2O. In addition, as the main component of natural gas (NG), developing the methods of efficient CH4 storage is one of the keys to boost the application of NG.50 At present, there are two main methods improving the NG volumetric energy density: Liquefied Natural Gas (LNG) and Compress Natural Gas (CNG). However, neither method is economical or convenient, especially in daily life. In this background, using MOFs adsorbent for gas storage is getting more and more attention. Similar to H2 adsorption discussed above, a similar strategy is applied to CH4 storage. As a comparison to the isoreticular structure, the linkers were extended from BDC to BPDC and ABDC. Three high-connected MOFs, including Fe-ncb-BDC ([Fe3O(LP)3(BDC)1.5]·x(solv)), Fe-ncb-BPDC ([Fe3O(LP)3(BPDC)1.5]·x(solv)), and Fe-ncb-ABDC ([Fe3O(LP)3(ABDC)1.5]·x(solv)) were accurately synthesized.51 Also, they exhibited increasing tendency of adsorption capacity with increasing ligand length to boost CH4 storage effectively. Thus, Fe-ncb-ABDC exhibited the best performance due to the collaboration of confined hierarchical pores and open metal sites (Fig. 5). This is a rare example of CH4 gravimetric and volumetric storage (reaching 0.302/0.37 g g−1, 196/240 cm(STP)3 cm−3, and 298/273 K, respectively) by blend high-connected MOFs, unlike the common low-connected MOFs. There is no doubt that the gravimetric working capacity at 65 bar of Fe-ncb-ABDC exhibits an obvious advantage compared with some benchmark works, such as HUKST-1, MAF-38, USTA-76, NJU-bai43, PCN-14, and MOF-905 (about 0.154, 0.176, 0.201, 0.221, 0.135, and 0.241 g g−1, respectively).52–58 Thus, the high-connected and mixed-linker strategy has proved to improve the CH4 adsorption capacities.
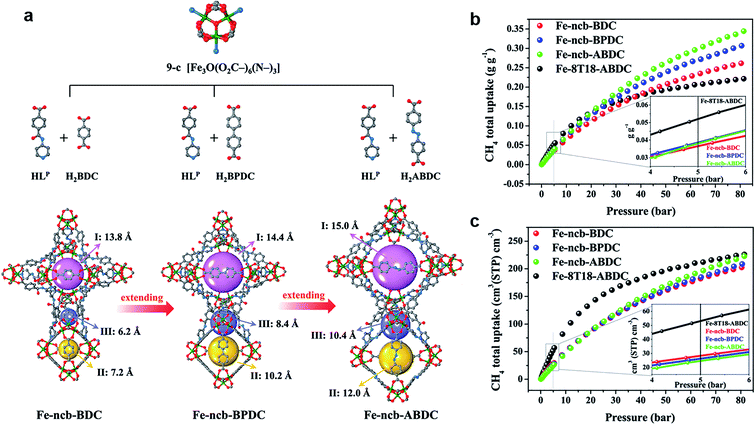 | ||
| Fig. 5 (a) The constructions of Fe-ncb-MOFs. (b) The CH4 gravimetric adsorption isotherms at 298 K. (c) Volumetric adsorption isotherms at 298 K.51 Reproduced from ref. 51 with permission, copyright The American Chemical Society, 2021. | ||
In total, the design strategies taken in the above work are obviously diverse. Introducing unsaturated alkali-metal sites to NKU-521a is an effective way to enhance the binding stability between the host and the guest. MIL-101(Cr) and MFM-132a realize the functionalization of the framework environment by introducing functional groups. Focusing on the connected condition, high-connected mixed-linker Fe-ncb-MOFs result in the collaboration of confined hierarchical pores, further improving the performance for gas adsorption. For M(BDC), the spontaneous reversible transition after adsorption brings a new perspective to the study.
3. Gas separation
The mixed state is widespread and most gases exist as mixtures; thus, gas separation is still a significant process for the industry. However, there exist great challenges for precisely separating similar gas molecules, especially multi-components mixtures with slight differences.59 Generally, cryogenic distillation, liquid adsorbent, and other traditional separation methods require high energy consumption, which greatly increases the industry costs. In contrast, adsorption is a relatively energy-saving and environment-friendly technology, and pressure swing adsorption and temperature swing adsorption are the main technologies for the industrial use of adsorbents. As new porous materials, MOFs have great advantages in gas separation. From the perspective of kinetics or others, many effective strategies, such as microporous engineering,60 size screening,61 and post-synthesis modification,62 are accurately implemented to promote gas separation application. The precise design for MOFs allows that it can play a specific role for different separations by the diverse and designable construction at the molecular scale.3.1 Alkynes/alkenes separation
The separation of alkyne and alkene mixtures is a challenging process. As shown in Table 1, there are many similarities (e.g., close dynamic size, physical performances, and chemical performances), which bring out serious problems in the industry. In order to improve the separation efficiency (especially for separating the trace impurities) and relieve the pressure of the energy, environment, and the chemical industry, MOFs have received more and more attention as a typical adsorbent for separating C2H2/C2H4 and C3H4/C3H6.63,64As we all know, when steam cracking C2H4 to obtain C2H6, C2H2 is generated as a by-product during this process.65 It is universally known that C2H4 is mixed with small amount of C2H2. However, the presence of C2H2 is considered unfriendly due to its explosive properties and poisoning of Ziegler Natta catalysts during polymerization, which is not conducive for the economic industrial production. Thus, a typical truth is to find effective measures for separating the C2H2/C2H4 mixture securely. Based on it, Zhou and co-workers obtained NbU-1 ((NH4){CuII3·[CuIICuI6(OH)6(Ad)6]2}·(H2O)10) to separate C2H2 and C2H4 by accessible and inexpensive materials.66 In this work, NbU-1 showed the obvious separation of C2H2/C2H4 with the proportion of both 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 1
50 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 mixtures (Fig. 6). Importantly, C2H4 purity reaches 99.997% after separating, and the detection limit is lower than 0.003%, which is especially significant for the practical chemical industry application. It is worth noting that the excellent separation performance is contributed by the synergy of the open metal sites from heptanuclear metal cluster and the Lewis adsorption sites from the trigonal channel. Compared with the traditional separation principle, NbU-1 brings into play the function of double Cu(I) sites where it supplies a rich electrical area for the guest molecules to combine into the frameworks. This ideal model exhibits potential in the precise design of MOFs, which broadens the application prospects in chemical separation.
99 mixtures (Fig. 6). Importantly, C2H4 purity reaches 99.997% after separating, and the detection limit is lower than 0.003%, which is especially significant for the practical chemical industry application. It is worth noting that the excellent separation performance is contributed by the synergy of the open metal sites from heptanuclear metal cluster and the Lewis adsorption sites from the trigonal channel. Compared with the traditional separation principle, NbU-1 brings into play the function of double Cu(I) sites where it supplies a rich electrical area for the guest molecules to combine into the frameworks. This ideal model exhibits potential in the precise design of MOFs, which broadens the application prospects in chemical separation.
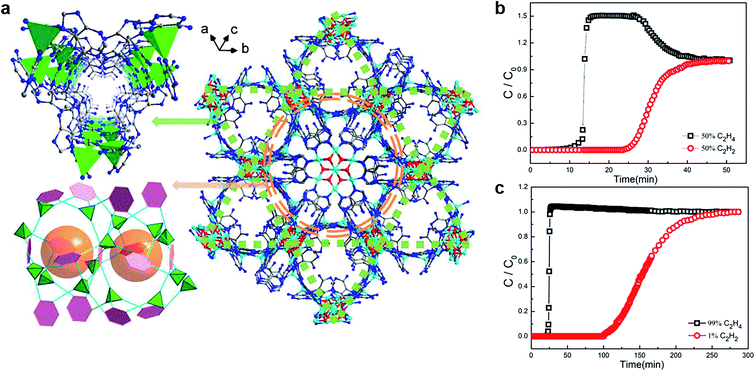 | ||
Fig. 6 (a) The channel and cavities in the 3D structure of NbU-1. (b) and (c) Breakthrough experiment for the mixture of C2H2 and C2H4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1 50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, respectively).66 Reproduced from ref. 66 with permission, copyright The American Chemical Society, 2019. 99, respectively).66 Reproduced from ref. 66 with permission, copyright The American Chemical Society, 2019. | ||
In addition, the channel size of the MOFs is considered as one of the key factors for separating guest molecules. Except for the functional sites, regulating the pore size by the multi-level ligands length is one of the significantly visual ways to select the molecules. Some specific 3D frameworks are considered to be composed of 2D layers and supporting pillar ligand (e.g., pyrazine, bipyridine, and other double connected linker). Some researchers are committed to designing the pore size according to the layer-pillar strategy, i.e., adjusting the layer connector or pillar size. Yang et al. reported a series of MOFs termed as CPL-1 ([Cu2(pzdc)2(pyz)·2H2O]n), CPL-2 ([Cu2(pzdc)2(bpy)·4H2O]n), and CPL-5 ([Cu2(pzdc)2(bpe)·5H2O]n) by introducing pillared ligands (pyz, bpy, and bpe, respectively) as pillars.67 As shown in Fig. 7, with the assistance of different pillar ligands, 4, 9, and 11 Å pores were constructed based on isoreticular chemistry. Since the suitable pores hinder the C2H4 into the skeleton, CPL-1 exhibits the best separation, which is an excellent case of a typical custom channel on MOFs.
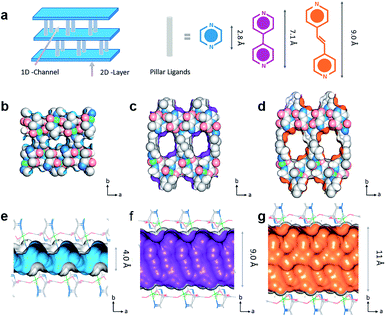 | ||
| Fig. 7 (a) The crystal structure of [Cu2(pzdc)2L]. The pore constructed by three ligands of (b) and (e) CPL-1, (c) and (f) CPL-2, (d) and (g) CPL-5.67 Reproduced from ref. 67 with permission, copyright The American Chemical Society, 2019. | ||
Combining the two strategies of the metal sites and layer-pillar design, Qian et al. reported that Hofmann-type MOFs (ZJU-74a, [Co(pyz)[Ni(CN)4]] exhibited excellent C2H2/C2H4 separation performance, which is attributed to the ultra-strong C2H2 binding force from the [Ni(CN)4]2− units and open metal sites in the sandwich-like structure.68 As shown in Fig. 8, the breakthrough of C2H2 was observed at 140 min, and the C2H4 purity reached 99.9995% after separation. Qian's work offers a new direction to separate high purity C2H4 from the mixture of C2H4/C2H2 by capturing C2H2 precisely. On the one hand, compared with C2H4 adsorption to achieve separation, removing C2H2 is more practical and straightforward for industry application. On the other hand, this is a meaningful development to explore novel thinking under the guidance of precision design strategy.
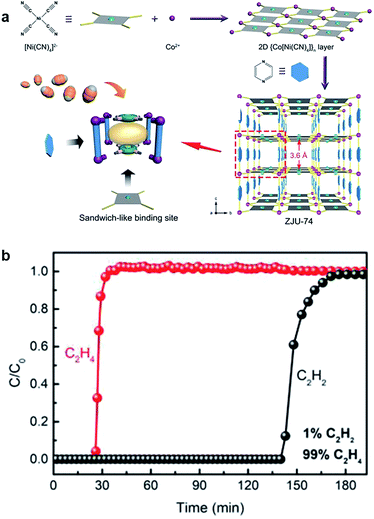 | ||
| Fig. 8 (a) The construction process of ZJU-74a, 3D framework, and sandwich-like binding sites. (b) Experimental column breakthrough curves for a 1/99 C2H2/C2H4 mixture at 298 K and 1 bar.68 Reproduced from ref. 68 with permission, copyright John Wiley and Sons, 2020. | ||
Similarly, the mixed state of C3H4 and C3H6 is also ubiquitous and challenging for the chemical industry. Designing active metal sites is considered as an efficacious strategy for improving the effect of gas separation.65 Yang et al. introduced two adsorption sites into GeFSIX-dps-M (M = Zn and Cu), and the metal in GeFSIX-dps-Zn was replaced completely to obtain GeFSIX-dps-Cu ([Cu(dps)2(GeF6)]).69 Surprisingly, due to effective H-bonding sites in the pore and interlayer space, the tiny connector and fine-tuning in the layer state enhanced the selectivity of C3H4/C3H6 significantly. In this way, alkyne was easier to be allowed into the pore space than the alkene to meet the demands of molecular screening, which was confirmed by both the crystal structure and calculation of C2H4-combined GeFSIX-dps-Cu. In GeFSIX-dps-Cu, site II was combined with C3H4, and it is worth mentioning that the guest-binding behaviour can be observed by the intuitive crystal structure (Fig. 9) even after adsorption, which were also verified by density functional theory (DFT-D).
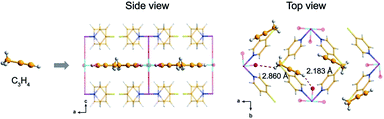 | ||
| Fig. 9 Schematic diagram of GeFSIX-dps-Cu after adsorbing C3H4.69 Reproduced from ref. 69 with permission, copyright John Wiley and Sons, 2020. | ||
3.2 Alkanes/alkenes separation
As one of the most significant raw materials, C2H4 and C3H6 are the key industry chemicals in plastics manufacturing, and they are usually produced by cracking naphtha.70 Generally, “polymer-grade” is the basic requirement for the manufacture of plastics,71 which means that high-purity alkanes need to be obtained economically. However, some by-products such as alkanes are produced during the process of cracking. Therefore, it is really essential to develop effective measures for separating alkanes and alkenes.C2H4 is considered as the core of the petroleum and chemical industry, and the productivity of C2H4 is one of the criteria for judging the industrial level. Adjusting the size and functionalizing the pore environment are the most direct ways to enhance the gas separation performance. Generally, due to the interaction between C2H6 molecules and MOFs skeleton not being strong, most researchers focused on enhancing the interaction between C2H6 and frameworks to get high purity C2H4 in one-step.72 Qian et al. designed the pore environment by introducing the nonpolar naphthalene and anthracene to obtain ZJU-120 (Ni(ndc)(ted)0.5) and ZJU-121 (Ni(adc)(ted)0.5)60 (Fig. 10). In this way, the pore size was limited to 4.4 Å and 3.7 Å for ZJU-120 and ZJU-121, respectively, which is caused by both steric hindrance inside the pore and the significant increase in the density of the nonpolar aromatic system. Compared with Ni(bpc)(ted)0.5 and ZJU-121a, due to the suitable pore size, ZJU-120a can effectively adsorb C2H6 (96 cm3 g−1 at 296 K, 0.5 bar) and exhibit excellent C2H6/C2H4 separation behavior (2.74, IAST selectivity). Controlling the microporous size and polarity by enhancing the C2H6 interaction effectively realized the separation of C2H6/C2H4.
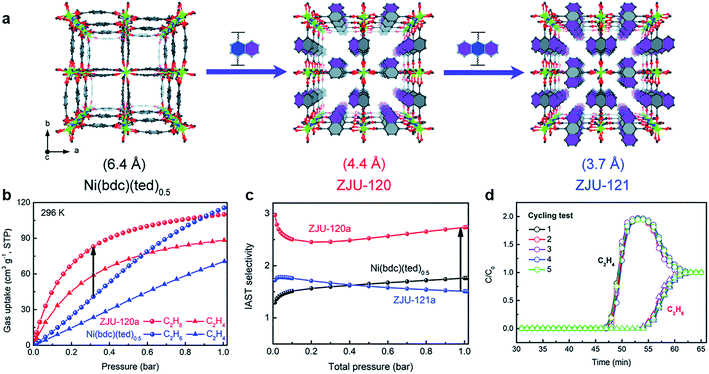 | ||
Fig. 10 (a) Structure of Ni(bdc)(ted)0.5, ZJU-120, and ZJU-121 constructed by introducing different aromatic rings. (b) Gas adsorption isotherms of ZJU-120a and Ni(bdc)(ted)0.5 for C2H6 and C2H4 (196 K). (c) IAST selectivity for C2H6/C2H4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 296 K). (d) Cycling test of ZJU-120 for the C2H6/C2H4 (50 50, 296 K). (d) Cycling test of ZJU-120 for the C2H6/C2H4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 298 K, 1 bar, 1.25 mL min−1).60 Reproduced from ref. 60 with permission, copyright The Royal Society of Chemistry, 2020. 50, 298 K, 1 bar, 1.25 mL min−1).60 Reproduced from ref. 60 with permission, copyright The Royal Society of Chemistry, 2020. | ||
Besides, there are many persuasive examples of functional modification. Ma et al. obtained Ni(TMBDC)(DABCO)0.5 with high C2H6 uptake (up to 2.21 mmol g−1, 0.0625 bar) by controlling the ligand methyl-functionalization, supplying potential advantages for separation.73 Through substituent engineering, He et al. contrasted a series of MOFs modified with different branch chain, including ZJNU-21 ([Cu(L2)]·DMF·CH3OH), ZJNU-22 ([Cu(L3)]·0.5DMF·CH3OH), ZJNU-23 ([Cu(L4)]·DMF), and the maternal structure was named NJU-Bai7. The result also revealed that methyl is a significant functional group to elevate the separation performance.74 Similarly, with methylation, Noro et al. observed the apparent confinement effect to limit the guest into the channel.75 These are representative samples by functionalization to elevate the separation efficiency.
Furthermore, constructing MOFs with excellent separation performance requires finding out the relationships among the structure modules and performing precise design from multiple angles. In 2020, Krishna et al.76 designed 9 kinds of contrastive structure to research the structure–performance relations from three aspects including ligands (e.g., H2BDC and H2DMBDC), pore-partitioning linker (e.g., TPT, TPBz, and TPPy) and metal cluster (e.g., Co2V, Co2Ti, Mg2V, and Mg2Ti) (Fig. 11). These contrasts of the material family indicated that BDC plays a leading role in adsorption, and TPT has a main effect on C2H6/C2H4 separation. For metal center, the highest C2H6 uptake (up to 166.8 cm3 g−1) was achieved by Mg2V-bdc-tpt (Mg2V(OH)(bdc)3tpt); the most excellent C2H2/C2H4 separation performance (with 50/50) was obtained by Co2V-bdc-tpt (Co2V(OH)(bdc)3tpt), and both them could separate C2H4 from the mixture with high purity (up to 99.95%). The influence of three compositions on C2H6/C2H4 separation were systematically discussed in order to reveal the relationships between the separation and the module. The crystal engineering of the linker precisely designs a microporous environment as an achievable method for absorbing and separating instructively.
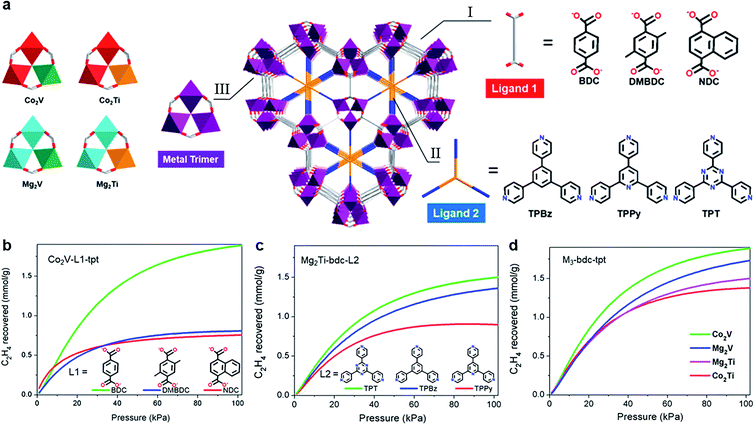 | ||
| Fig. 11 (a) The frameworks composed by three kinds of ligand 1, three kinds of ligand 2, and four kinds of metal cluster. (b) Contradistinction of C2H6/C2H4 (50/50) separation potential of Co2V-L1-tpt, (c) Mg2Ti-bdc-L2, and (d) M3-bdc-tpt.76 Reproduced from ref. 76 with permission, copyright The American Chemical Society, 2020. | ||
Functional modification based on open topological platform promotes the universality study of MOFs. Recently, based on the stable high-valance MOFs, our group reported new pore environment modification by introducing cyclopentadiene cobalt functional group to selectively enhance the interaction between the frameworks and the guest.77 In two typical Zr-MOFs (UPC-612 and UPC-613), isomorphic ligands were used to construct different size cage in which differential guest binding behavior can be precisely achieved. Thus, both UPC-612 and UPC-613 could purify the C2H4/C2H6 or C2H4/C2H2 mixture to obtain polymer grade C2H4. It is worth mentioning that even under 10% content of C2H2 or C2H6, UPC-612 remains the highest separation potential. Furthermore, as a more general state, the ternary mixture of C2H6/C2H4/C2H2 could be separated, abstracting C2H4 in one step. As the first work in one-step C2H4 purification from large proportion ternary mixtures, the spherical force surface formed in the cage modified by cyclopentadiene cobalt serves as strong evidence for precisely controlling the pore environment for one-step purification (Fig. 12).
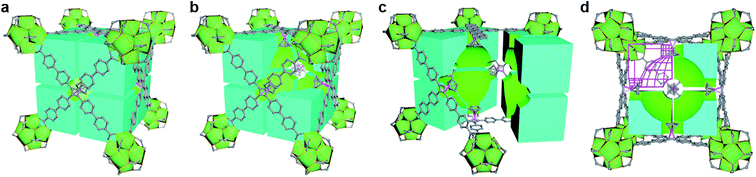 | ||
| Fig. 12 Structural illustration of UPC-612 with cage filling. (a) Cubic cage filling of a basic unit in UPC-612; (b) space division of cubic cage; (c) internal space allocation of UPC-612 after partition; (d) cubic cage filling profile and a sphere like force surface.77 Reproduced from ref. 77 with permission, copyright John Wiley and Sons, 2021. | ||
3.3 Noble gas separation
As the physically and chemically stability gases, noble gases, such as He, Ne, Ar, Kr, and Xe, are widely applied to the medical, electronic component, lighting, and space industry.78 There exist mixtures of noble gases in the off-gases such as used nuclear fuel (UNF) and it is an essential industrial process to separate each component.79–81 However, compared with light hydrocarbon separation, it is a well-known challenge to separate single noble gases component from the mixed state economically and effectively due to the similar physical and chemical properties. Besides, since the actual application environments of noble gas separation is more complex, on the basis of satisfying the working capacity, more research should focus on the material tolerance to complex environments, such as humidity, corrosive, and acid-base environments.Introducing effective site strategy is one of the direct approaches to elevate guest combination behavior. In order to obtain high purity Xe, Luo et al. explored stable MOFs defined ECUT-60 (Co2(bpe)(OLZ4−)) as a potential candidate separating Xe from the off-gases.82 The advantages of both capture load and separation come from the direct restriction from Xe by the van der Waals force. There are three sites that supply the higher binding energy for Xe (−46.05 kJ mol−1, −40.93 kJ mol−1, and −45.53 kJ mol−1, respectively), which indicates the priority opportunity for Xe adsorption (Fig. 13a). Similarly, dual sites of NKMOF-1-Ni reported by Zhao et al. guarantee the separation not only in the mixture of Xe/Kr but also Xe/Ar and Xe/N2, which was investigated by the Qst (about 34.2 kJ mol−1) and the calculation of the Xe potential field.83
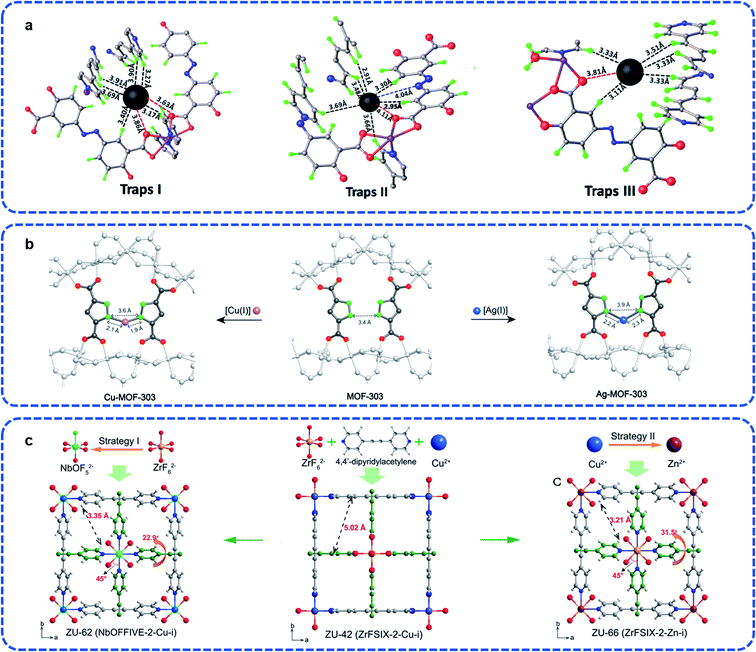 | ||
| Fig. 13 (a) Three adsorption traps in ECUT-60.82 Reproduced from ref. 82 with permission, copyright Elsevier, 2021. (b) The chelated structure of Cu-MOF-303 and Ag-MOF-303 derived from MOF-303.62 Reproduced from ref. 62 with permission, copyright John Wiley and Sons, 2020. (c) The interpenetrated networks of ZU-62, ZU-42, and ZU-66 (viewed along the c-axis).84 Reproduced from ref. 84 with permission, copyright John Wiley and Sons, 2020. | ||
Based on the MOF-303 ([Al(OH)(C5H2O4N2)]), Yaghi et al. reported that the chelation of CuI or AgI on the atom scale can effectively control the pore size to match with Xe, and obtain the derived Cu-MOF-303 and Ag-MOF-303.62 As shown in Fig. 13b, the ligands alternately distributed on both sides of the 1D chain-shaped SBU provide exposed sites for further chelation with metal ions, which reveals the strategy using additional binding sites to match the guest size, and allows the combination of ultrahigh selectivity and uptake. For Ag-MOF-303, it showed unexpected improvement in both Xe adsorption (up to 59 cm−3 cm−3 at 298 K) and Xe/Kr selectivity (about 10.4, v/v = 20/80). From this perspective, it increased to twice as much as that of the original material MOF-303.
In addition to designing the sites directly, the frameworks' self-properties can respond to guest touch. Yang et al. reported interpenetrated ZU-62 (Cu(dpa)2(NbOF5)) with accurate anion pillared pore environment.84 Once the Xe guest gets in touch with the cavity, the size and shape can be adjusted by pillar and pyridine spinning to accommodate the Xe atoms, leading to the expansion of the pore size, which is completely different with Kr atoms. Due to the stronger polarity of the Xe atoms, a significant open pore state was caused by the interaction force between the skeleton and the guests. In total, the purification of Kr directly or the desorption of Xe after adsorption, both methods could obtain high capacity and purity (206 mL g−1, >99.9%; 42 mL g−1, >99.9%; respectively).
In total, by utilizing intuitive size sieving, CPL-1 and MOF-303 can effectively identify the size difference between guest molecules and achieve mixture separation. By designing the binding sites, both NbU-1 and ZJU-74 can achieve efficient and selective adsorption. Modulating host–guest interactions can be achieved through functional group modification (such as ZJU-120a and UPC-612), metal nodes control (such as GeFSIX-dps-Cu), enhancing van der Waals force (such as ECUT-60) and flexible structure transform (such as ZU-62). Further, it is a more meaningful design idea to give full play to the above strategies to form a synergistic effect.
4. Conclusion and outlook
Gas adsorption and separation are necessary scientific topics for industrial activities. The designable and controllable structure of the MOFs implies that further development is imperative. We reviewed recent representative researches of gas storage or removal, light hydrocarbon, and noble gas separation, and analyzed the inspirations and characteristics from ligands/metal nodes design, active combination sites construction, SBU modification, pore division, and other multiple perspectives, providing guidance for designing adsorption/separation-oriented MOFs.At present, MOFs with excellent performance have been widely reported (Table 2). Specifically, pore environment and framework conformation are key to host–guest interactions; thus, how to further sensitively achieve molecules capturing by multi-level crystal engineering is still a key issue needed to be researched thoroughly. In addition, to practically optimize the potential application of the absorbent, the following aspects should be further considered.
| MOFs | Metal | Pore size (Å) | Mixed gas | Q st (kJ mol−1) | Selectivityb | Uptaked | Temp. (K) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Q st values at zero coverage. b Selectivity is calculated by IAST. c The highest Qst values at various surface coverage. d Adsorption uptake obtained from single-component gas adsorption isotherms. e cm3 g−1, at 1 bar. f mmol g−1, at 1 bar. | ||||||||
| NbU-1 | Cu | 4 | C2H2/C2H4 | 38.3 (C2H2), 37.9 (C2H4) | 12.1, C2H2/C2H4 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
81.5e (C2H2) | 273 | 66 |
5.9, C2H2/C2H4 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
53.1e (C2H2) | 298 | ||||||
| CPL-1 | Na | 4 | C2H2/C2H4 | 40.2 (C2H2), 36.8 (C2H4) | 26.75, C2H2/C2H4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
2.07f (C2H2), 0.31f (C2H4) | 298 | 67 |
| CPL-2 | Na | 9 | C2H2/C2H4 | 30.8 (C2H2), 20.3 (C2H4) | 12, C2H2/C2H4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
3.13f (C2H2), 1.86f (C2H4) | 298 | 67 |
| CPL-5 | Na | 11 | C2H2/C2H4 | 31.3 (C2H2), 19.1 (C2H4) | 5.99, C2H2/C2H4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
3.01f (C2H2), 1.84f (C2H4) | 298 | 67 |
| ZJU-74a | Co | 3.9 | C2H2/C2H4 | 45–65 (C2H2)c | 24.2, C2H2/C2H4 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
49e (C2H2) | 296 | 68 |
| GeFSIX-dps-Cu | Cu | — | C2H2/C2H4 | — | 40.1, C2H2/C2H4 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
4.28f (C2H2), 0.16f (C2H4) | 298 | 69 |
| — | C3H4/C3H6 | — | 36.45, C3H4/C3H6 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
3.73f (C3H4), 0.08f (C3H6) | 298 | |||
| ZJU-120a | Ni | 4.4 | C2H6/C2H4 | 27.6 (C2H6) | 2.74, C2H6/C2H4 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
4.91f (C2H6), 3.93f (C2H4) | 296 | 60 |
| ZJU-121a | Ni | 3.7 | C2H6/C2H4 | 47.1 (C2H6), 43.0 (C2H4) | 1.51, C2H6/C2H4 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
3.1f (C2H6), 3.19f (C2H4) | 296 | 60 |
| CPM-733 | Co, V | 7.3 | C2H6/C2H4 | 23.4 (C2H6), 22.5 (C2H4) | 1.75, C2H6/C2H4 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
159.6e (C2H6), 142.7e (C2H4) | 298 | 76 |
| ZJNU-21 | Cu | — | C2H6/C2H4 | 37.36 (C2H6), 33.87 (C2H4) | 1.65, C2H6/C2H4 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
72.7e (C2H6), 71.4e (C2H4) | 298 | 74 |
| ZJNU-22 | Cu | — | C2H6/C2H4 | 37.32 (C2H6), 35.47 (C2H4) | 1.34, C2H6/C2H4 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
65.6e (C2H6), 64.2e (C2H4) | 298 | 74 |
| ZJNU-23 | Cu | — | C2H6/C2H4 | 35.42 (C2H6), 35.11 (C2H4) | 1.23, C2H6/C2H4 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
66.4e (C2H6), 65.6e (C2H4) | 298 | 74 |
| ECUT-60 | Co | 4.5 | Xe/Kr | 30 (Xe), 22.6 (Kr) | 11.36, Xe/Kr = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
4.3f (Xe), 1.45f (Kr) | 298 | 82 |
| NKMOF-1-Ni | Ni | 5.36 | Xe/Kr | 34.2 (Xe), 33.1 (Kr) | 5.2, Xe/Kr = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
2.47f (Xe), 1.86f (Kr) | 273 | 83 |
| Xe/Ar | 34.2 (Xe), 26.1 (Ar) | 41.3, Xe/Ar = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
2.47f (Xe), 0.84f (Ar) | 273 | ||||
| Ag-MOF-303 | Ag, Al | 5.2 | Xe/Kr | 28.2 (Xe) | 10.4, Xe/Kr = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
108e (Xe) | 298 | 62 |
| Cu-MOF-303 | Cu, Al | 5.5 | Xe/Kr | 24.4 (Xe) | 8.2, Xe/Kr = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
91e (Xe) | 298 | 62 |
(i) Taking into account the complex surroundings, stable adsorbent-like high-valence MOFs should receive further attention to adequately address the changeable condition, especially water/acid system, thermal stability, cycle-life, etc.
(ii) Explore environments-friendly synthetic methods, and reduce the organic solvents usage rate to meet the requirements of both clean and scale-up manufacture.
(iii) How to maximally reduce the production cost by exploring more inexpensive raw material and production process.
(iv) Research must also focus more on promoting the production scale, expanding to meet the industrial scenarios.
In conclusion, based on the irreplaceable development potential, more comprehensively precise design strategies will greatly promote the pace of MOFs materials as industrial adsorption/separation carriers (Table 3).
| Abbreviation | Compounds |
|---|---|
| a Different abbreviations for the same molecule. | |
| H3TZIA | 5-(1H-Tetrazol-5-yl)isophthalic acid |
| BDC | 1,4-Dicarboxybenzene |
| H2btdd | Bis(1H-1,2,3-triazolo[4,5-b],[4′,5′-i])dibenzo[1,4]dioxin |
| H6BTAT | 5,5′,5′′-(Benzene-1,3,5-triyltris(anthracene-10,9-diyl))triisophthalic acid |
| BPDC | 4,4′-Biphenyldicarboxylate |
| ABDC | Azobenzene-4,4′-dicarboxylate |
| LP | 4-(Pyridin-4-ylcarbamoyl)benzoate |
| Ad | Adenine |
| pyz | Pyrazine |
| bpy | 4,4′-Bipyridine |
| bpe | trans-1,2-Di(4-pyridyl)-ethylene |
| pyz | Pyrazine |
| dps | 4,4′-Dipyridylsulfide |
| H2ndc | 1,4-Naphthalenedicarboxylic acid |
| DABCO or teda | 1,4-Diazabicyclo[2.2.2]octane |
| H2adc | 9,10-Anthracenedicarboxylic acid |
| TMBDC | 2,3,5,6-Tetramethylterephthalic acid |
| H2L2 (in ZJNU-21) | 5-(5-Methylpyridin-3-yl)isophthalic acid |
| H2L3 (in ZJNU-22) | 5-(5-Methoxypyridin-3-yl)isophthalic acid |
| H2L4 (in ZJNU-23) | 5-(5-Chloropyridin-3-yl)isophthalic acid |
| H2BDC | 1,4-Benzenedicarboxylic acid |
| H2NDC | 1,4-Naphthalenedicarboxylic acid |
| H2DMBDC | 2,5-Dimethylterephthalic acid |
| TPT | 2,4,6-Tri(4-pyridyl)-1,3,5-triazine |
| TPBz | 2,4,6-Tri(4-pyridyl)benzene |
| TPPy | 2,4,6-Tri(4-pyridyl)pyridine |
| bpe | 1,2-Bis(4-pyridyl)ethylene |
| OLZ4− | Olsalazine |
| dpa | 4,4′-Dipyridylacetylene |
Author contributions
Chuanhai Jiang: data collection, validation, conceptualization, writing – original draft. Xiaokang Wang: data collection, visualization, validation. Yuguo Ouyang: data collection, visualization, validation. Kebin Lu: data collection, visualization, validation. Weifeng Jiang: data collection, visualization, validation. Huakai Xu: data collection, visualization, validation. Xiaofei Wei: data collection, visualization, validation. Zhifei Wang: data collection, visualization, validation. Fangna Dai: supervision, visualization, writing & editing, project administration, funding acquisition. Daofeng Sun: supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020KB010); the National Natural Science Foundation of China (21771191); and the Fundamental Research Funds for the Central Universities (19CX05001A).Notes and references
- S. L. James, Chem. Soc. Rev., 2003, 32, 276–288 RSC
.
- O. M. Yaghi, G. Li and H. Li, Nature, 1995, 378, 703–706 CrossRef CAS
.
- L. Jiao, J. Y. R. Seow, W. S. Skinner, Z. U. Wang and H. Jiang, Mater. Today, 2019, 27, 43–68 CrossRef CAS
.
- I. Stassen, N. Burtch, A. Talin, P. Falcaro, M. Allendorf and R. Ameloot, Chem. Soc. Rev., 2017, 46, 3185–3241 RSC
.
- G. L. Yang, X. L. Jiang, H. Xu and B. Zhao, Small, 2021, 17, 2005327 CrossRef CAS PubMed
.
- Z. Xiao, Y. Wang, B. Xu, S. Du, W. Fan, D. Cao, Y. Deng, L. Zhang, L. Wang and D. Sun, Adv. Sci., 2020, 7, 2000065 CrossRef CAS PubMed
.
- Z. Q. Wang, H. Q. Luo, Y. L. Wang, M. Y. Xu, C. T. He and Q. Y. Liu, Inorg. Chem., 2021, 60, 10596–10602 CrossRef CAS PubMed
.
- C. Wang, B. An and W. Lin, ACS Catal., 2018, 9, 130–146 CrossRef
.
- S. Mallakpour, E. Nikkhoo and C. M. Hussain, Coord. Chem. Rev., 2022, 451, 214262 CrossRef CAS
.
- X. Zhang, H. Cui, R. B. Lin, R. Krishna, Z. Y. Zhang, T. Liu, B. Liang and B. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 22514–22520 CrossRef CAS PubMed
.
- H. Cui, Y. Ye, T. Liu, Z. A. Alothman, O. Alduhaish, R. B. Lin and B. Chen, Inorg. Chem., 2020, 59, 17143–17148 CrossRef CAS PubMed
.
- E. Chapman, S. Ullah, H. Wang, L. Feng, K. Wang, H. C. Zhou, J. Li, T. Thonhauser and K. Tan, ACS Appl. Mater. Interfaces, 2021, 13, 43661–43667 CrossRef CAS PubMed
.
- W. Fan, S. Yuan, W. Wang, L. Feng, X. Liu, X. Zhang, X. Wang, Z. Kang, F. Dai, D. Yuan, D. Sun and H. C. Zhou, J. Am. Chem. Soc., 2020, 142, 8728–8737 CrossRef PubMed
.
- R. Lin, S. Xiang, W. Zhou and B. Chen, Chem, 2020, 6, 337–363 CAS
.
- Y. Jiang, T. Hu, L. Yu and Y. Ding, Nanoscale Adv., 2021, 3, 4079–4088 RSC
.
- C. Jiang, X. Wang, K. Lu, W. Jiang, H. Xu, X. Wei, Z. Wang, Y. Ouyang and F. Dai, J. Solid State Chem., 2022, 307, 122881 CrossRef CAS
.
- D. Wu, P. Zhang, G. Yang, L. Hou, W. Zhang, Y. Han, P. Liu and Y. Wang, Coord. Chem. Rev., 2021, 434, 213709 CrossRef CAS
.
- D. E. Jaramillo, H. Z. H. Jiang, H. A. Evans, R. Chakraborty, H. Furukawa, C. M. Brown, M. Head-Gordon and J. R. Long, J. Am. Chem. Soc., 2021, 143, 6248–6256 CrossRef CAS PubMed
.
- T. Yoskamtorn, P. Zhao, X. P. Wu, K. Purchase, F. Orlandi, P. Manuel, J. Taylor, Y. Li, S. Day, L. Ye, C. C. Tang, Y. Zhao and S. C. E. Tsang, J. Am. Chem. Soc., 2021, 143, 3205–3218 CrossRef CAS PubMed
.
- A. M. Wright, C. Sun and M. Dinca, J. Am. Chem. Soc., 2021, 143, 681–686 CrossRef CAS PubMed
.
- J. Perego, C. X. Bezuidenhout, A. Pedrini, S. Bracco, M. Negroni, A. Comotti and P. Sozzani, J. Mater. Chem. A, 2020, 8, 11406–11413 RSC
.
- T. D. Duong, S. A. Sapchenko, I. da Silva, H. G. W. Godfrey, Y. Cheng, L. L. Daemen, P. Manuel, M. D. Frogley, G. Cinque, A. J. Ramirez-Cuesta, S. Yang and M. Schröder, Chem. Sci., 2020, 11, 5339–5346 RSC
.
- P. Brandt, S. H. Xing, J. Liang, G. Kurt, A. Nuhnen, O. Weingart and C. Janiak, ACS Appl. Mater. Interfaces, 2021, 13, 29137–29149 CrossRef CAS PubMed
.
- P. Brandt, A. Nuhnen, M. Lange, J. Mollmer, O. Weingart and C. Janiak, ACS Appl. Mater. Interfaces, 2019, 11, 17350–17358 CrossRef CAS PubMed
.
- X. Zhao, Y. Wang, D. S. Li, X. Bu and P. Feng, Adv. Mater., 2018, 30, 1705189 CrossRef PubMed
.
- X. Y. Li, Z. J. Li, Y. Z. Li, L. Hou, Z. Zhu and Y. Y. Wang, Inorg. Chem., 2018, 57, 12417–12423 CrossRef CAS PubMed
.
- X. Liu, W. Fan, M. Zhang, G. Li, H. Liu, D. Sun, L. Zhao, H. Zhu and W. Guo, Mater. Chem. Front., 2018, 2, 1146–1154 RSC
.
- E. Binaeian, Y. Li and D. Yuan, Chem. Eng. J., 2021, 421, 129655 CrossRef CAS
.
- H. S. Papastathopoulou and W. L. Luyben, Ind. Eng. Chem. Res., 1991, 30, 705–713 CrossRef CAS
.
- L. Li, R. Lin, X. Wang, W. Zhou, L. Jia, J. Li and B. Chen, Chem. Eng. J., 2018, 354, 977–982 CrossRef CAS
.
- S. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schroder, Nat. Chem., 2014, 7, 121–129 CrossRef PubMed
.
- O. Karagiaridi, W. Bury, E. Tylianakis, A. A. Sarjeant, J. T. Hupp and O. K. Farha, Chem. Mater., 2013, 25, 3499–3503 CrossRef CAS
.
- N. Huang, S. Yuan, H. Drake, X. Yang, J. Pang, J. Qin, J. Li, Y. Zhang, Q. Wang, D. Jiang and H. C. Zhou, J. Am. Chem. Soc., 2017, 139, 18590–18597 CrossRef CAS PubMed
.
- W. Fan, X. Wang, X. Liu, B. Xu, X. Zhang, W. Wang, X. Wang, Y. Wang, F. Dai, D. Yuan and D. Sun, ACS Sustainable Chem. Eng., 2018, 7, 2134–2140 CrossRef
.
- Q. G. Zhai, X. Bu, X. Zhao, D. S. Li and P. Feng, Acc. Chem. Res., 2017, 50, 407–417 CrossRef CAS PubMed
.
- H. Wang, D. Luo, E. Velasco, L. Yu and J. Li, J. Mater. Chem. A, 2021, 9, 20874–20896 RSC
.
- M. Ding, R. W. Flaig, H. L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC
.
- W. Liang, P. M. Bhatt, A. Shkurenko, K. Adil, G. Mouchaham, H. Aggarwal, A. Mallick, A. Jamal, Y. Belmabkhout and M. Eddaoudi, Chem, 2019, 5, 950–963 CAS
.
- N. Li, Z. Chang, H. Huang, R. Feng, W. W. He, M. Zhong, D. G. Madden, M. J. Zaworotko and X. H. Bu, Small, 2019, 15, 1900426 CrossRef PubMed
.
- E. Martínez-Ahumada, M. L. Díaz-Ramírez, H. A. Lara-García, D. R. Williams, V. Martis, V. Jancik, E. Lima and I. A. Ibarra, J. Mater. Chem. A, 2020, 8, 11515–11520 RSC
.
- T. Islamoglu, Z. Chen, M. C. Wasson, C. T. Buru, K. O. Kirlikovali, U. Afrin, M. R. Mian and O. K. Farha, Chem. Rev., 2020, 120, 8130–8160 CrossRef CAS PubMed
.
- Y. Chen, Y. Du, P. Liu, J. Yang, L. Li and J. Li, Environ. Sci. Technol., 2020, 54, 3636–3642 CrossRef CAS PubMed
.
- C. Lu, Y. Chen, Y. Wang, Y. Du, J. Yang, L. Li and J. Li, Sep. Purif. Technol., 2022, 282, 120126 CrossRef CAS
.
- T. Asefa, K. Koh and C. W. Yoon, Adv. Energy Mater., 2019, 9, 1901158 CrossRef
.
- U. Eberle, M. Felderhoff and F. Schuth, Angew. Chem., Int. Ed., 2009, 48, 6608–6630 CrossRef CAS PubMed
.
- X. Liang, P. Wang, C. Li, M. Yuan, Q. Shi and J. Dong, Microporous Mesoporous Mater., 2021, 320, 111109 CrossRef CAS
.
- C. Jia, F. G. Cirujano, B. Bueken, B. Claes, D. Jonckheere, K. M. Van Geem and D. De Vos, ChemSusChem, 2019, 12, 1256–1266 CrossRef CAS PubMed
.
- C. Wang, Y. H. Luo, X. T. He, D. L. Hong, J. Y. Wang, F. H. Chen, C. Chen and B. W. Sun, Inorg. Chem., 2019, 58, 3058–3064 CrossRef CAS PubMed
.
- Y. Yan, I. da Silva, A. J. Blake, A. Dailly, P. Manuel, S. Yang and M. Schröder, Inorg. Chem., 2018, 57, 12050–12055 CrossRef CAS PubMed
.
- X. Wu, L. Peng, S. Xiang and W. Cai, Phys. Chem. Chem. Phys., 2018, 20, 30150–30158 RSC
.
- Z. H. Zhang, H. Fang, D. X. Xue and J. Bai, ACS Appl. Mater. Interfaces, 2021, 13, 44956–44963 CrossRef CAS PubMed
.
- Y. Peng, V. Krungleviciute, I. Eryazici, J. T. Hupp, O. K. Farha and T. Yildirim, J. Am. Chem. Soc., 2013, 135, 11887–11894 CrossRef CAS PubMed
.
- J. Jiang, H. Furukawa, Y. B. Zhang and O. M. Yaghi, J. Am. Chem. Soc., 2016, 138, 10244–10251 CrossRef CAS PubMed
.
- M. Zhang, W. Zhou, T. Pham, K. A. Forrest, W. Liu, Y. He, H. Wu, T. Yildirim, B. Chen, B. Space, Y. Pan, M. J. Zaworotko and J. Bai, Angew. Chem., Int. Ed., 2017, 56, 11426–11430 CrossRef CAS PubMed
.
- B. Li, H. M. Wen, H. Wang, H. Wu, M. Tyagi, T. Yildirim, W. Zhou and B. Chen, J. Am. Chem. Soc., 2014, 136, 6207–6210 CrossRef CAS PubMed
.
- J. Lin, C. He, Y. Liu, P. Liao, D. Zhou, J. Zhang and X. Chen, Angew. Chem., Int. Ed., 2016, 55, 4674–4678 CrossRef CAS PubMed
.
- B. Li, H. Wen, H. Wang, H. Wu, T. Yildirim, W. Zhou and B. Chen, Energy Environ. Sci., 2015, 8, 2504–2511 RSC
.
- S. Ma, D. Sun, J. Simmons, C. Collier, D. Yuan and H. Zhou, J. Am. Chem. Soc., 2007, 130, 1012–1016 CrossRef PubMed
.
- J. Wang, Y. Zhang, Y. Su, X. Liu, P. Zhang, R. B. Lin, S. Chen, Q. Deng, Z. Zeng, S. Deng and B. Chen, Nat. Commun., 2022, 13, 200 CrossRef CAS PubMed
.
- J. Pei, J. Wang, K. Shao, Y. Yang, Y. Cui, H. Wu, W. Zhou, B. Li and G. Qian, J. Mater. Chem. A, 2020, 8, 3613–3620 RSC
.
- L. Li, H. M. Wen, C. He, R. B. Lin, R. Krishna, H. Wu, W. Zhou, J. Li, B. Li and B. Chen, Angew. Chem., Int. Ed., 2018, 57, 15183–15188 CrossRef CAS PubMed
.
- H. Wang, Z. Shi, J. Yang, T. Sun, B. Rungtaweevoranit, H. Lyu, Y. B. Zhang and O. M. Yaghi, Angew. Chem., Int. Ed., 2021, 60, 3417–3421 CrossRef CAS PubMed
.
- B. Li, X. Cui, D. O'Nolan, H. M. Wen, M. Jiang, R. Krishna, H. Wu, R. B. Lin, Y. S. Chen, D. Yuan, H. Xing, W. Zhou, Q. Ren, G. Qian, M. J. Zaworotko and B. Chen, Adv. Mater., 2017, 29, 1704210 CrossRef PubMed
.
- H. Wen, L. Li, R. Lin, B. Li, B. Hu, W. Zhou, J. Hu and B. Chen, J. Mater. Chem. A, 2018, 6, 6931–6937 RSC
.
- Q. Qian, X. Gu, J. Pei, H. Wen, H. Wu, W. Zhou, B. Li and G. Qian, J. Mater. Chem. A, 2021, 9, 9248–9255 RSC
.
- J. Li, L. Jiang, S. Chen, A. Kirchon, B. Li, Y. Li and H. C. Zhou, J. Am. Chem. Soc., 2019, 141, 3807–3811 CrossRef CAS PubMed
.
- F. Zheng, L. Guo, B. Gao, L. Li, Z. Zhang, Q. Yang, Y. Yang, B. Su, Q. Ren and Z. Bao, ACS Appl. Mater. Interfaces, 2019, 11, 28197–28204 CrossRef CAS PubMed
.
- J. Pei, K. Shao, J. X. Wang, H. M. Wen, Y. Yang, Y. Cui, R. Krishna, B. Li and G. Qian, Adv. Mater., 2020, 32, 1908275 CrossRef CAS PubMed
.
- T. Ke, Q. Wang, J. Shen, J. Zhou, Z. Bao, Q. Yang and Q. Ren, Angew. Chem., Int. Ed., 2020, 59, 12725–12730 CrossRef CAS PubMed
.
- S. Chu, Y. Cui and N. Liu, Nat. Mater., 2016, 16, 16–22 CrossRef PubMed
.
- H. Wang, X. Dong, V. Colombo, Q. Wang, Y. Liu, W. Liu, X. L. Wang, X. Y. Huang, D. M. Proserpio, A. Sironi, Y. Han and J. Li, Adv. Mater., 2018, 30, 1805088 CrossRef PubMed
.
- D. Lv, P. Zhou, J. Xu, S. Tu, F. Xu, J. Yan, H. Xi, W. Yuan, Q. Fu, X. Chen and Q. Xia, Chem. Eng. J., 2022, 431, 133208 CrossRef CAS
.
- X. Wang, Z. Niu, A. M. Al-Enizi, A. Nafady, Y. Wu, B. Aguila, G. Verma, L. Wojtas, Y. Chen, Z. Li and S. Ma, J. Mater. Chem. A, 2019, 7, 13585–13590 RSC
.
- P. Zhou, L. Yue, X. Wang, L. Fan, D. L. Chen and Y. He, ACS Appl. Mater. Interfaces, 2021, 13, 54059–54068 CrossRef CAS PubMed
.
- A. Schneemann, Y. Jing, J. D. Evans, T. Toyao, Y. Hijikata, Y. Kamiya, K. I. Shimizu, N. C. Burtch and S. I. Noro, Dalton Trans., 2021, 50, 10423–10435 RSC
.
- H. Yang, Y. Wang, R. Krishna, X. Jia, Y. Wang, A. N. Hong, C. Dang, H. E. Castillo, X. Bu and P. Feng, J. Am. Chem. Soc., 2020, 142, 2222–2227 CrossRef CAS PubMed
.
- Y. Wang, C. Hao, W. Fan, M. Fu, X. Wang, Z. Wang, L. Zhu, Y. Li, X. Lu, F. Dai, Z. Kang, R. Wang, W. Guo, S. Hu and D. Sun, Angew. Chem., Int. Ed., 2021, 60, 11350–11358 CrossRef CAS PubMed
.
- K. B. Idrees, Z. Chen, X. Zhang, M. R. Mian, R. J. Drout, T. Islamoglu and O. K. Farha, Chem. Mater., 2020, 32, 3776–3782 CrossRef CAS
.
- A. P. Ladshaw, A. I. Wiechert, A. K. Welty, K. L. Lyon, J. D. Law, R. T. Jubin, C. Tsouris and S. Yiacoumi, Chem. Eng. J., 2019, 375, 122073 CrossRef CAS
.
- X. Xiong, G. Chen, S. Xiao, Y. Ouyang, H. Li and Q. Wang, J. Phys. Chem. C, 2020, 124, 14603–14612 CrossRef CAS
.
- J. Qian, G. Chen, S. Xiao, H. Li, Y. Ouyang and Q. Wang, RSC Adv., 2020, 10, 17195–17204 RSC
.
- H. Zhang, Y. Fan, R. Krishna, X. Feng, L. Wang and F. Luo, Sci. Bull., 2021, 66, 1073–1079 CrossRef CAS
.
- T. Wang, Y. L. Peng, E. Lin, Z. Niu, P. Li, S. Ma, P. Zhao, Y. Chen, P. Cheng and Z. Zhang, Inorg. Chem., 2020, 59, 4868–4873 CrossRef CAS PubMed
.
- Q. Wang, T. Ke, L. Yang, Z. Zhang, X. Cui, Z. Bao, Q. Ren, Q. Yang and H. Xing, Angew. Chem., Int. Ed., 2020, 59, 3423–3428 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2022 |