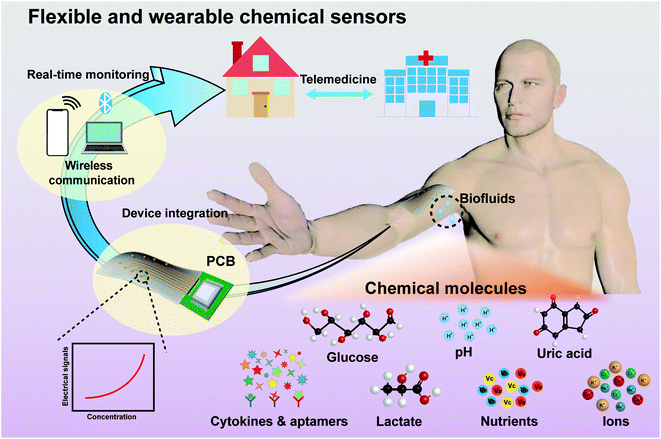
Recent advances in flexible and wearable sensors for monitoring chemical molecules
Hang
Zhao†
ab,
Rui
Su†
c,
Lijun
Teng
b,
Qiong
Tian
b,
Fei
Han
b,
Hanfei
Li
b,
Zhengshuai
Cao
d,
Ruijie
Xie
b,
Guanglin
Li
b,
Xijian
Liu
 *a and
Zhiyuan
Liu
*a and
Zhiyuan
Liu
 *b
*b
aCollege of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, Shanghai 201620, China. E-mail: liuxijian@sues.edu.cn
bNeural Engineering Center, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, PR China. E-mail: zy.liu1@siat.ac.cn
cResearch Center for Human Tissue and Organs Degeneration, Institute of Biomedicine and Biotechnology, Shenzhen Institutes of Advanced Technology Chinese Academy of Sciences, Shenzhen 518055, PR China
dCenter for Opto-Electronic Engineering and Technology, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, PR China
First published on 21st December 2021
Abstract
In recent years, real-time health management has received increasing attention, benefiting from the rapid development of flexible and wearable devices. Conventionally, flexible and wearable devices are used for collecting health data such as electrophysiological signals, blood pressure, heart rate, etc. The monitoring of chemical factors has shown growing significance, providing the basis for the screening, diagnosis, and treatment of many diseases. Nowadays, in order to understand the health status of the human body more comprehensively and accurately, researchers in the community have started putting effort into developing wearable devices for monitoring chemical factors. Progressively, more flexible chemical sensors with wearable real-time health-monitoring functionality have been developed thanks to advances relating to wireless communications and flexible electronics. In this review, we describe the variety of chemical molecules and information that can currently be monitored, including pH levels, glucose, lactate, uric acid, ion levels, cytokines, nutrients, and other biomarkers. This review analyzes the pros and cons of the most advanced wearable chemical sensors in terms of wearability. At the end of this review, we discuss the current challenges and development trends relating to flexible and wearable chemical sensors from the aspects of materials, electrode designs, and soft–hard interface connections.
1. Introduction
Real-time health management is essential for disease prevention. However, it is unrealistic for most people to frequently check their physical condition at a hospital.1,2 Therefore, realizing the real-time monitoring of physical conditions without being restricted by time and place is currently becoming an urgent goal. The emergence of flexible and wearable devices has made real-time health monitoring possible. These devices, which are manufactured based on small, soft, and low-cost materials, have become ideal instruments for realizing real-time healthcare monitoring.3–8 In the past decade, novel flexible electronic devices utilizing unique structural designs and manufacturing methods have shown great potential for many practical applications.9–11 For healthcare monitoring, flexible and wearable devices have been used to detect electrophysiological signals, such as carrying out electromyography (EMG), electrocardiography, (ECG),12–19etc. A previous recent review has summarized emerging flexible and wearable materials and advanced flexible and wearable devices for monitoring physical, electrophysiological, chemical, and biological signals.20Despite the rapid development of flexible and wearable technology, there are currently hardly any commercial flexible and wearable devices for monitoring chemical molecules.21 Biomarkers are molecules that can objectively be measured and used as indicators of normal conditions or disease in the human body.22–25 The levels of these biomarkers (mostly chemical molecules) reflect the health status of the body and provides a basis for the screening, diagnosis, and treatment of diseases.26–28 In the past, due to a lack of equipment and monitoring methods for biomarkers, many studies did not suggest the importance of using biomarkers for real-time and continuous healthcare monitoring. Therefore, the application potential of biomarkers was greatly weakened.29–31 In recent years, with the improvement of wireless communications and flexible and wearable device technology, the monitoring of biomarkers has become possible.32–34 An increasing number of flexible and wearable sensors for biomarkers are being developed. Using flexible and wearable devices for the continuous real-time monitoring of biomarkers can allow for the more in-depth monitoring and diagnosis of personal physical conditions. The Gao and Wang research groups have been working on the development of flexible and wearable devices for biomarkers for many years.35–40 Flexible and wearable devices for the real-time monitoring of biomarkers such as glucose, lactate, ions, and uric acid in some body fluids (sweat and interstitial fluid (ISF)) have been developed, and several review articles have also been published discussing the latest progress relating to these biomarkers.21,41–44 Most relevant reviews mainly describe the electrode materials and performances of sensors, but wearability has rarely been discussed.
Wearable electronics are devices that can be worn or joined to human skin to continuously and closely monitor the activities of an individual without interrupting or limiting the user's motions.3,38,45 The features that affect wearability include the device stretchability, softness, adhesion to skin, breathability, and biocompatibility.46–48 Human skin can sustain large strain (up to at least 15%).49 In order to achieve the stable monitoring of the skin deformation process, ensure consistent contact with the skin, and prevent skin damage, a wearable device needs to have a certain degree of stretchability (more than 15%) and softness and it should exhibit an elastic modulus similar to that of skin (∼100 kPa) to ensure conformal contact with the skin.46 In addition, stable contact between electrodes and the skin mainly depends on strong interfacial adhesion between electrodes and skin. Excellent interface adhesion can effectively improve the signal-to-noise ratio (SNR) and signal acquisition accuracy.50 During long-term monitoring, water vapor and sweat will inevitably appear on the skin. This requires wearable devices to have a certain degree of breathability to avoid skin irritation and electrode failure caused by water vapor and sweat residue.51 Finally, minimizing health and safety issues arising from wearable devices being in contact with the skin is also crucial. As described, the development of biocompatible wearable devices is of great significance.47,52
The integration of flexible and wearable chemical sensors is a complex process. Starting from flexible materials, electrodes for signal monitoring and collection are first designed on flexible materials. These electrodes are key equipment converting chemical signals into measurable analysis signals. The prepared flexible electrodes are combined with a printed circuit board (PCB) to form a complete flexible and wearable device. Pairing with mobile devices through wireless communication technology (Bluetooth or near-field communication (NFC)53–55) can enable real-time data collection, transmission, and analysis. Devices can share results with and receive health guidance from doctors in hospitals remotely in real-time. Flexible and wearable devices set up a bridge between hospitals and homes for advancing telemedicine. Electrochemical analysis can allow in situ detection, high sensitivities, fast responses, and simple detection processes.56 In this review, the discussed chemical sensors are mostly electrochemical sensors. Considering that all the electrochemical sensors use the same sensing mechanism, converting chemical signals into electrical signals, we are inclined to divide this work into sections based on the chemicals or chemical processes being sensed. The emphasis of our review is on the wearability of the chemical sensors (including those for pH levels, glucose, lactate, uric acid, ion levels, cytokines, nutrients, and other biomarkers) (Fig. 1). This review focuses on the wearability of the sensors, including stretchability, softness, adhesion to skin, breathability, and biocompatibility. We also analyze the advantages and disadvantages in terms of practical wearability. At the end of this review, we discuss current challenge and developing trends relating to flexible and wearable chemical sensors from the aspects of materials, electrode designs, and soft–hard interfacial connections.
2. pH sensors
pH sensors are some of the most commonly used sensors, since pH is an important parameter during water quality detection, health monitoring, food processing, and other processes.57–59 Generally, the pH ranges of sweat and blood are 4.5–6.5 and 7.35–7.45, respectively.60,61 Most pH sensors are fabricated based on electrochemical principles. The active materials in these sensors show different electrochemical signals.62–64 Advances in pH sensing technology have focused on improving sensitivity, stability, and biocompatibility through the use of suitable materials and achieving rapid responses over a wide pH range.65Most reported pH sensors operate based on potentiometric methods.66 These sensors consist of a pH-sensitive working electrode and a reference electrode with a stable potential. The sensor operates due to the working electrode material showing different potentials in different pH environments. Glass-based pH electrodes are prime examples, however their application is limited due to the large volume and rigidity.66 In recent years, most reported pH sensors have been presented in thin film form.67 In work by the Santos team, a sensor was assembled from electrodeposited WO3 nanoparticles on a flexible electrode. The fabricated sensor shows sensitivity of −56.7 ± 1.3 mV per pH over a wide pH range of 9 to 5 (Fig. 2a).68 As a typical metal oxide pH sensor, the sensor could be integrated on a flexible substrate, expanding its application to a certain extent, but it lacked good stability and adhesion to the skin. In addition to metal–oxide-based pH sensors, flexible pH monitoring equipment based on polyaniline (PANI) has also been developed in recent years. Rahimi et al. developed a low-cost pH sensor based on PANI for wound monitoring (Fig. 2b).69 PANI was used as a working electrode on a patterned commercial polymer layer.70 The large elastic modulus of the pH sensor array substrate limits the practical application of the sensor. To improve the softness of the substrate, the Li's team integrated a miniature pH sensor on polyimide (PI) consisting of a composite electrode modified with polyaniline.71 The sensor showed good reproducibility and sensitivity but its low stretchability further limited the wearability (Fig. 2c). Oh et al. investigated a stretchable sensor that could detect pH and glucose in sweat based on a polydimethylsiloxane (PDMS) substrate.72 The sensor can work normally under a strain of up to 30%, which enables the sensor to maintain good performance on the surface of the skin. This sensor demonstrated long-term stability for 10 days (Fig. 2d). Zhai et al. prepared a sensing system for the specific detection of pH via modifying an electrode with polyaniline, sodium ionophore X, and a valinomycin-based selective membrane, and this could maintain performance under tensile stress.73 It can be seen that stretchable sensors have received more attention in the wearable field. However, substrate materials with low elastic modulus values and good adhesion to the skin need to be developed.
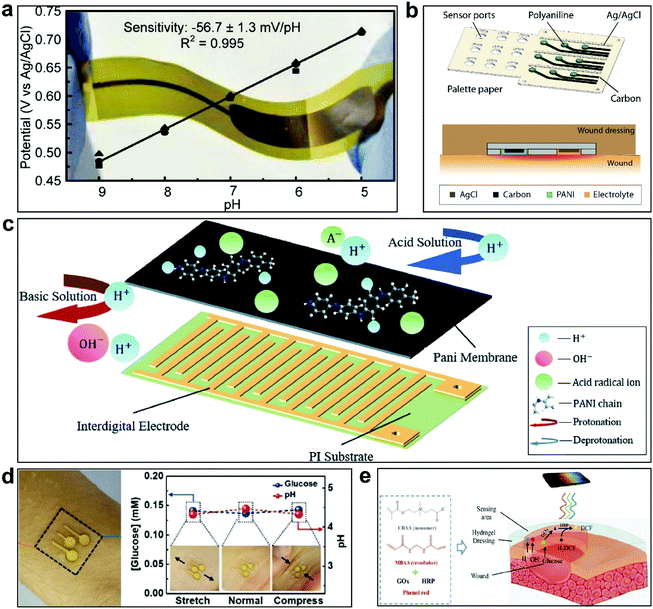 | ||
| Fig. 2 Some pH sensors. (a) A WO3-based flexible pH sensor without softness or stretchablity.68 This figure has been reproduced from ref. 68 with permission from the American Chemical Society; copyright: 2014. (b) A low-cost pH sensor array for wound monitoring.69 This figure has been reproduced from ref. 69 with permission from Elsevier B.V.; copyright: 2016. (c) A flexible pH sensor based on conductive polyaniline membrane.71 The sensor is hard and non-stretchable, limiting its long-term wearability. This figure has been reproduced from ref. 71 with permission from the Royal Society of Chemistry; copyright: 2020. (d) A stretchable and soft electrochemical sweat sensor attached to skin for glucose and pH detection.72 The sensor has good adhesion to the skin and can be directly attached to the skin. In addition, the sensor is able to stretch and compress with the skin due to its softness and stretchability. This figure has been reproduced from ref. 72 with permission from the American Chemical Society; copyright: 2018. (e) A multifunctional pro-healing amphoteric ionic hydrogel for the simultaneous monitoring of pH and glucose for diabetic wound therapy.76 The ionic hydrogel can conformally attach to the skin due to its good adhesion properties and low elastic modulus. This figure has been reproduced from ref. 76 with permission from WILEY-VCH Verlag GmbH & Co. KGaA; copyright: 2019. | ||
In addition to common electrochemical-based pH sensors, there are optics-based pH sensors.74,75 Zhu et al. developed a multifunctional amphoteric ionic hydrogel to monitor wound status in diabetic patients (Fig. 2e).76 In this study, pH was recorded through an image collection and RGB analysis process, as the hydrogel doped with indicator dye shows different colors at different pH values. This study shows a novel path for pH monitoring. However, sensors based on optical signals have a high dependency on the data analysis abilities and their application is only viable in visible environments. The long-term stabilities of these sensors and their applicability in different environments are areas to be advanced in the future.
3. Glucose, lactate, and uric acid sensors
Glucose, lactate, and uric acid are common physiological and chemical molecules in the human body which are directly related to some diseases.77–80 Glucose is the main source of energy for cell activities. At the same time, the presence of too much glucose in the blood is the main cause of diabetes and its many complications. Therefore, maintaining a suitable glucose concentration in the blood is essential for preventing diabetes.81 Lactate is also an important health indicator for organisms, and it is related to the effects of exercise on the body and several diseases, such as heart failure, metabolic disorders, and renal failure.82 Uric acid is generally related to wound healing. The content of uric acid in wound exudate can reflect the status of wound infection. During bacterial infection, uric acid is decomposed under the action of microbial uricase, leading to a significant decrease in its concentration.55 In addition, high uric acid concentrations in body fluids may also cause gout.83 Therefore, the real-time monitoring of glucose, lactate, and uric acid is essential for healthcare. The concentrations of these molecules are important for assessing health.84–86 Typically, glucose levels in blood and sweat are 3.3–6.5 mM (ref. 87) and 0.05–0.1 mM,88 lactate levels are 0.5–1.5 mM and 5–25 mM,89 and uric acid levels are 0.389 ± 0.064 mM and 0.025 ± 0.007 mM,90 respectively. In recent years, chemical sensors for monitoring the above-listed molecules have been developed. In particular, continuous health monitoring based on wearable sensors has been considered to be a promising method for supporting personal healthcare.38,91 Most of these sensors are designed to monitor changes in electrical signals caused by redox reactions, and the concentrations of the corresponding molecules can be obtained based on electrical signals.92,93The content of glucose in the blood is closely related to human health.81,94 Current clinical methods for measuring blood glucose mostly involve blood sampling from the finger. As an invasive method, this undoubtedly causes pain to patients. Therefore, non-invasive glucose monitoring has been widely studied.95–97 The Zahed team developed a glucose sensor for sweat monitoring.98 Graphene electrodes integrated on a PI substrate were modified with glucose oxidase, fabricating a glucose sensor with high sensitivity and stability. However, this substrate does not have enough softness and stretchability to be worn on the body. Hong et al. developed a disposable glucose strip sensor for the sweat and a wearable smart bracelet.99 The disposable sensor strips can be applied to the skin surface, but the smart bracelet is not flexible and the wearing comfort over a long period of time poses challenges (Fig. 3a). The Kim team presented a study using a single wearable skin platform for the non-invasive sampling and analysis of glucose concentrations in sweat and ISF simultaneously (Fig. 3b).96 The substrate material of the sensor is soft and has good adhesion to the skin. The FPCB adopted in this study increases wearability, while the use of a single device to achieve the monitoring of different biofluids provides a reference method for future non-invasive glucose monitoring. Some studies have suggested more accurate monitoring through minimally invasive methods.100–103 The Zhang team developed a glucose sensor with a micro-needle structure.104 Glucose oxidase and a gold nanocoating were used to modify the polymer micro-needles. In addition, methods for monitoring glucose based on optical signals have also been developed.105,106 Li et al. studied a glucose sensor based on chemiluminescence.107 As glucose monitoring is popular in healthcare, designing real-time-monitoring sensors with accurate and reliable results will always be an area of study.
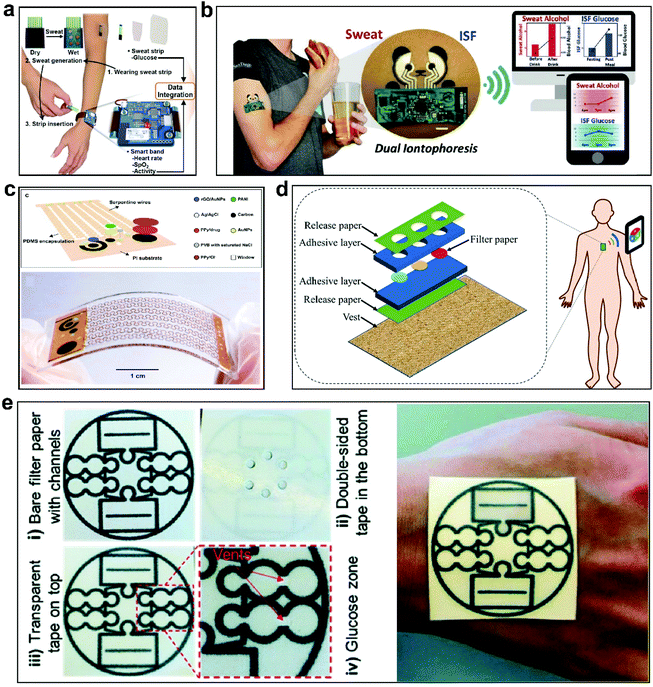 | ||
| Fig. 3 Some glucose, lactate, and uric acid sensors. (a) A disposable sweat-based glucose sensing strip with skin adhesion.99 The sensor can be worn on the wrist, however its large size can make it uncomfortable to wear. This figure has been reproduced from ref. 99 with permission from WILEY-VCH Verlag GmbH & Co. KGaA; copyright: 2018. (b) A soft adhesive glucose sensor for the monitoring of sweat and ISF.96 The sensor is directly attached to the skin with good adhesion. This figure has been reproduced from ref. 96 with permission from WILEY-VCH Verlag GmbH & Co. KGaA; copyright: 2018. (c) A flexible smart dressing without stretchability,117 limiting the wearability. This figure has been reproduced from ref. 117 with permission from WILEY-VCH GmbH, copyright 2021. (d) An optical soft adhesive biosensor for detecting uric acid, glucose, and alcohol in sweat.118 This figure has been reproduced from ref. 118 with permission from Elsevier B.V.; copyright: 2021. (e) A versatile, flexible, and adhesive biosensor for sweat analysis and personalized nutrition assessment.119 The sensor can be attached directly to the skin, however the sensor has a high elastic modulus. This figure has been reproduced from ref. 119 with permission from the Royal Society of Chemistry; copyright: 2019. These sensors achieve certain wearability criteria, such as adhesion to skin, stretchability, and softness. However, these sensors all require improved breathability to improve their long-term wearability. | ||
Sweat is an important method of lactate excretion. In past studies, the relationship between lactate concentration and human health has been widely considered.108 Imani et al. developed a screen-printed three-electrode lactate biosensor for real-time monitoring. The system was integrated on a flexible substrate for monitoring lactate in sweat.29 However, the non-stretchable substrate of the monitoring system limits its wearability. Anastasova et al. designed a sweat sensor with a microfluidic structure. It can simultaneously measure the concentrations of hydrogen, sodium, and lactate in human sweat.109 The sensor is based on a soft polymethyl methacrylate (PMMA) material. Low-elastic-modulus substrate materials can achieve good adhesion with the skin. Wang et al. studied a fabric-based lactic acid sensor. This sensor based on a fabric substrate has breathability and high sensitivity, and the sensitivity can be maintained under high tensile strain of up to 100%.89 Current studies usually monitor lactate in sweat, while a few studies have monitored the lactate content in the blood.37,110 Meanwhile, it is also a challenge to explore standards relating to lactate concentration and physiological health.
The levels of uric acid, as a cause of gout, are very important for the prognosis, diagnosis, and treatment of related diseases.111–113 In recent years, the non-invasive monitoring of uric acid has been researched.39,114,115 The Yang team developed a three-dimensional electrochemical biosensor array for uric acid monitoring.116 The sensor has high sensitivity and stability, but the substrate of the sensor is hard, which does not support wearable applications. The Xu team has developed a multifunctional sensor for wound monitoring integrated on a flexible substrate that can monitor pH, temperature, and uric acid (Fig. 3c).117 The sensor showed good sensitivity and stability, and wearable monitoring was achieved in rats. However, the sensor integrated on a PI substrate is not soft enough, and its applications are limited when facing a large wound area. At present, uric acid monitoring technology has been quite extensively studied but research on its specificity and wearability is still lacking.
Glucose, lactate, and uric acid are common monitoring factors, but their monitoring is not completely independent. The Zhou team has developed a biosensor that can simultaneously detect uric acid, glucose, and alcohol in sweat; the sensor uses optical signals to monitor these chemical factors (Fig. 3d).118 The design of the sensor is novel, and it can convert chemical signals into optical signals. The substrate material is flexible, but it is not soft enough and does not have stretchability. Zhang et al. studied a multifunctional wearable biosensor for monitoring pH, glucose, and lactate in sweat (Fig. 3e).119 The substrate material is non-stretchable and the stability of the sensor presents a challenge. It can be seen that sensor development is trending toward diversification, but the realization of multiple-factor monitoring in one device requires sensors with sufficient specificity and compensation to meet diverse monitoring scenarios.
4. Ion sensors
Sodium, potassium, and chlorine are mainly not only found in extracellular and intracellular fluids but also in human sweat.120,121 Sodium ions and potassium ions play important roles in regulating osmotic pressure. A lack of sodium and potassium ions in sweat may cause dehydration, hyponatremia, hypokalemia, or muscle cramps.122,123 Normally, the content levels of sodium and potassium in sweat are 66.3 ± 46.0 mM and 9.0 ± 4.8 mM, respectively, and the content levels in blood are 140.5 ± 2.2 mM and 4.8 ± 0.8 mM, respectively. The concentrations of chloride ions in blood and sweat are 98.9 ± 6.7 mM and 59.4 ± 30.4 mM, respectively. Typically, the calcium content levels in blood and sweat range from 2.0 to 2.6 mM and from 4 to 60 mM, respectively.87,124 Excessive sodium and chloride content levels may also cause cystic fibrosis (CF).53,122 Calcium is an important part of the skeleton125 and it is also present in biological fluids, such as blood, urine, and sweat.126 An abnormal calcium ion content may cause an acid–base imbalance in the body, liver cirrhosis, or other diseases.126–128 Therefore, the detection of these ions is essential for disease prevention and health management. However, the detection of these ions is mostly carried out in the laboratory.129 These processes are cumbersome and costly, and real-time monitoring and analysis cannot be carried out. Therefore, it is important to develop comfortable, flexible, and wearable devices that can monitor ion concentrations in real time.For the real-time monitoring of ions, corresponding wearable devices have been developed. Schazmann et al.122 fabricated a sodium sensor belt for the collection and analysis of back sweat based on sodium ion-selective electrodes (ISEs), water-impermeable plastic (made via the 3D printing of acrylonitrile butadiene), and sweat-absorbing material (Lycra, used to collect sweat) connected to a potentiometer. Bandodkar et al.130 invented a body surface tattoo potential-based sodium sensor also based on a sodium ISE (Fig. 4a). The adhesion of the tattoo patch to the skin can effectively resist interference from skin deformation on the electrode and realize stable monitoring. The sensor is connected to a Bluetooth wireless transceiver embedded in an armband for the real-time and continuous monitoring of the sodium ion levels in human epidermal sweat. However, the sensor belt has a large volume and poor wearing comfort. The tattoo potential-based sodium sensor has good adhesion to the skin, but the breathability needs to improve. These factors limit the long-term use of the sensors. Wang et al.131 fabricated gold nanodendritic (AuND) arrays in situ on microporous patterned chips via one-step electrodeposition. The AuND electrode exhibits a three-dimensional branched structure, showing significantly high surface area and hydrophobicity. Finally, a wearable sweat belt platform was designed to continuously collect sweat and analyze the concentration of Na+ (Fig. 4b). Bujes-Garrido et al.132 used screen-printed electrodes on Gore-Tex fabric to measure chloride ions. The detection of chloride ions is based on the voltammetric peak potential Nernst shift of ferrocene ethanol in the presence of chloride ions. A Cr/Au electrode array is prepared via photolithography on a PET substrate and then the calcium ISE is prepared via electrochemical deposition (Fig. 4c).127 The logarithmic concentration of calcium ions is proportional to the potential difference between the ISE and the reference electrode. The flexible sensor is connected to a flexible printed circuit board (FPCB), and a Bluetooth connection with a mobile phone realizes signal conversion, processing, and wireless transmission. The ion concentration on the body surface can be monitored in real time via a mobile phone. The above ion sensor can only realize the detection of a single ion, and the sensor has a relatively large volume, high elastic modulus, and poor adhesion with the skin, and it is airtight, limiting its application in the wearable field.
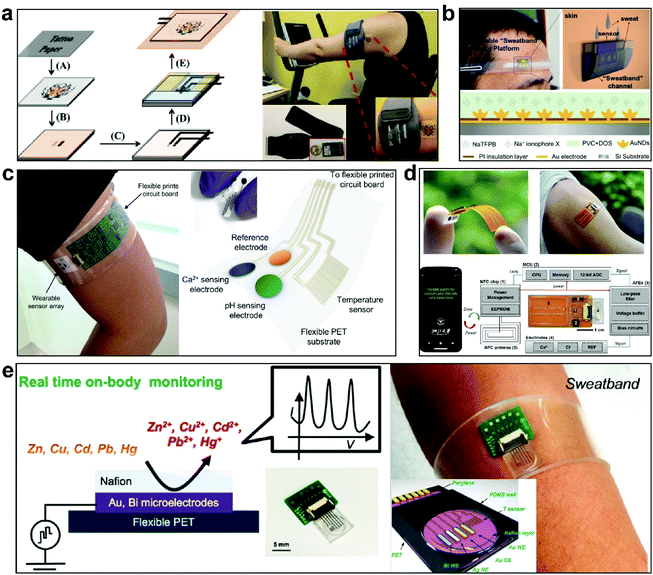 | ||
| Fig. 4 Some ion sensors. (a) An epidermal temporary transfer tattoo potential-based sensor and a miniature wearable wireless transceiver are used to monitor the sodium content in human sweat in real time.130 The tattoo potential-based sodium sensor has the good adhesion with the skin, but the breathability needs to be improved. This figure has been reproduced from ref. 130 with permission from Elsevier B.V.; copyright: 2014. (b) A wearable sweat belt platform was designed to continuously collect and analyze the concentration of Na+ in sweat.131 This figure has been reproduced from ref. 131 with permission from the American Chemical Society; copyright: 2017. (c) A soft multifunctional sensor can monitor pH and calcium ions in sweat.127 This figure has been reproduced from ref. 127 with permission from the American Chemical Society; copyright: 2016. (d) A battery-free and flexible electrochemical patch based on a smartphone, developed to detect calcium and chloride ions in various biological fluid samples in real time.53 This kind of highly flexible paper sensor needs adhesive tape to be fixed to the skin and it needs to be connected to a huge hard device, which cannot be used for a long time. This figure has been reproduced from ref. 53 with permission from Elsevier B.V.; copyright: 2019. (e) A flexible and wearable micro-sensor array based on Au and Bi microelectrodes using electrochemical square wave anodic stripping voltammetry (SWASV) to simultaneously monitor heavy metal ions (Zn, Cd, Pb, Cu, and Hg ions) in human body fluid samples.40 This figure has been reproduced from ref. 40 with permission from the American Chemical Society; copyright: 2016. The sensors shown in (c) and (e) can be made into belts that can be worn directly on the arm or other areas of the human body. However, PET has poor adhesion to the skin and a high elastic modulus, possibly causing errors in monitoring due to the inability to adapt to skin deformation during the wearing process. | ||
Integrating the detection of two or more types of ion in one sensor and improving the wearability have become focuses of next-generation research. Graphene electrodes based on highly flexible paper with integrated ISEs can realize the simultaneous monitoring of sodium ions, calcium ions, potassium ions, and chloride ions.38,133,134 Xu et al.53 integrated an NFC module, an on-site signal processing circuit, and a fully printed stretchable electrode array, allowing the real-time detection of calcium and chloride ions in various biofluids (Fig. 4d). This kind of highly flexible paper needs adhesive tape to be fixed to the skin, and it needs to be connected to a huge hard device, which cannot be used for a long time. Sodium ion and potassium ion sensors and sodium ion and chloride ion sensors have been integrated on a mechanically flexible PET substrate at the same time to realize the simultaneous monitoring of two ions in combination with a FPCB to realize real-time data analysis and transmission. In addition, there are also many heavy metal ions in human body fluids, and these are also closely related to human health. Gao et al.40 fabricated an ion sensor on a PET substrate to selectively measure a variety of heavy metal ions (Zn, Cd, Pb, Cu, and Hg) via anodic stripping voltammetry (Fig. 4e). The concentration of heavy metal ions in sweat can be monitored in real time during exercise via integrating sensors with a FPCB directly on the skin. However, PET has poor adhesion to the skin and this may cause errors in the monitoring results due to the inability to adapt to skin deformation during the wearing process. At present, most developed wearable real-time ion content sensors are based on connections between fabric, paper, or flexible PET film materials and a FPCB that can be used for wireless transmission.38,40,127,133 However, these materials have high elastic modulus values and do not easily adhere to the skin (Table 1). They also have poor stretchability. As a result, such wearable devices cannot avoid detection errors caused by skin and electrode slippage, and long-term wear may cause discomfort. In addition, the numbers of detection electrodes in the sensors are limited and electrode distributions are heterogeneous; this may result in incomplete data and non-comprehensive explanations of problems. In subsequent studies, the use of materials with lower elastic modulus values, better skin adhesion, and higher levels of wear comfort (such as SEBS, PDMS, Ecoflex, etc.) to achieve the integration of high-density electrode arrays for detection over a limited area should be studied.
| Analyte | Analytical technique | Recognition element | Materials and platform | Ref. |
|---|---|---|---|---|
| Glucose | Electrochemical: amperometry | Lactate oxidase (LOX) | PET film | 38 |
| Lactate | Electrochemical: potentiometry | Glucose oxidase (GOX) | FPCB | |
| Potassium ions | Sodium-ionophore-based ISE | |||
| Sodium ions | Potassium-ionophore-based ISE | |||
| Glucose | Electrochemical: amperometry | GOX | PET film | 99 |
| PCB | ||||
| Glucose | Electrochemical: amperometry | GOX | PI film | 98 |
| pH | Electrochemical: potentiometry | PANI | ||
| Glucose | Optical: colorimetry | GOX | Double-sided tape substrates | 118 |
| Uric acid | Uricase | |||
| Alcohol | Alcohol dehydrogenase | |||
| Lactate | Electrochemical: amperometry | LOX | PMMA film | 109 |
| Sodium ions | Electrochemical: potentiometry | Sodium-ionophore-based ISE | ||
| pH | IrOx | |||
| Blood pressure | Ultrasonic analysis | Piezoelectric transducers | SEBS film | 37 |
| Heart rate | Electrochemical: amperometry | GOX | ||
| Glucose | Differential pulse voltammetry | LOX | ||
| Lactate | Alcohol oxidase | |||
| Caffeine | ||||
| Alcohol | ||||
| Glucose | Optical: colorimetry | GOX | Filter paper | 119 |
| Lactate | LOX | |||
| pH | pH indicator solution | |||
| Uric acid | Differential pulse voltammetry Electrochemical: potentiometry | Uricase | PDMS film | 117 |
| pH | PANI | PI film | ||
| Temperature | Temperature sensor chip | FPCB | ||
| Glucose | Electrochemical: amperometry | GOX | PI film | 89 |
| Sodium ions | Electrochemical: potentiometry | Sodium ionophore |
5. Cytokine sensors
Cytokines are mainly small molecular proteins with a wide range of biological activities that are synthesized and secreted by a variety of immune cells (T cells, B cells, macrophages, etc.).135,136 Cytokines have powerful biological functions. For example, interleukin (IL) plays an important regulatory role in immune regulation, hematopoiesis, and inflammation.137–139 Some tumor necrosis factors (TNF-α, TNF-β) can kill virus-infected target cells or tumor cells.56,140 Transforming growth factor-β (TGF-β) can stimulate fibroblasts and osteoblasts to promote the repair of wounded tissue.141 The average concentrations of IL-1, TNF-α, and TGF-β are 17.8, 0.767, and 0.114 ng mL−1, respectively, in wound fluid.142 As common biomarkers, cytokines are closely related to the regulation of the immune system.135 Therefore, the detection of cytokines has been the focus of extensive research in recent years, and the realization of the real-time, accurate, and highly specific detection of cytokines is a target for clinical diagnosis and prevention.The enzyme-linked immunosorbent assay (ELISA) is a traditional and standard cytokine detection method.139,143 The ELISA method is based on specific binding between an enzyme-labeled molecule and an antibody or antigen molecule on a solid carrier. After the substrate solution is added dropwise, the substrate is oxidized under the action of the enzyme to undergo a color-change reaction to determine whether a corresponding immune response is present. However, the analysis of this method is cumbersome and time-consuming.135
Another method for detecting cytokines is electrochemical analysis, which is based on the relationship between a change in impedance or capacitance and the concentration of the measured substance or the CV curve during voltammetry. Aptamers or antibodies that can specifically bind to the detected molecules are immobilized on a substrate, such as a silicon wafer, glass, or a PCB.138,140,144,145 When the detected molecule is bound to the electrode surface, the capacitance decreases as the thickness of the biomolecular film on the electrode surface increases due to the low dielectric constant of the proteins. Alternatively, the electrode impedance varies with the concentration of the measured molecule. However, protein molecules other than the target molecules may also non-specifically bind to the aptamer or antibody, resulting in changes in capacitance or impedance and a reduction in accuracy. Although single or multiple cytokines can be detected in microarray and multiplex modes on hard and rigid substrates, the flexible and wearable real-time monitoring of cytokines for convenient healthcare monitoring is limited. Wang et al.146 developed an aptamer graphene-Nafion field effect transistor (GNFET) sensor for sensitive and continuous cytokine detection (Fig. 5a). The Cr/Au metal electrode of the sensor is prepared using photolithography and electron beam deposition technology to transfer graphene to the electrode. The combination of IFN-γ and the aptamer at different test molecule concentrations leads to a change in graphene charge due to electrostatic induction, resulting in detectable saturation source leakage currents of different magnitudes. In addition, a flexible and wearable sensor was fabricated on ultra-thin PET (6 μm), initially showing high flexibility and robustness for the real-time analysis of human sweat. Although this sensor shows excellent performance in the detection of cytokines, it cannot achieve good levels of wearability. Later, a flexible microfluidic multi-channel sensing platform was studied.141 This sensor was mainly used to analyze the state of wounds, including the collection and real-time monitoring of wound exudate. The real-time monitoring of cytokines (TNF-α, IL-6, IL-8, and TGF-β1) depended on the integration of the sensor, a portable wireless analyzer, and an attached application. The sensor used the dressing SU-8 as a substrate. At the same time, an advanced wireless transmission and data processing system is required to achieve the application of this real-time flexible and wearable device in health management. Although the detection of cytokines has been going on for decades, the development of highly specific, stable, and flexible wearable chemical sensors for the long-term real-time monitoring of cytokines is still in its infancy, requiring continuous exploration.
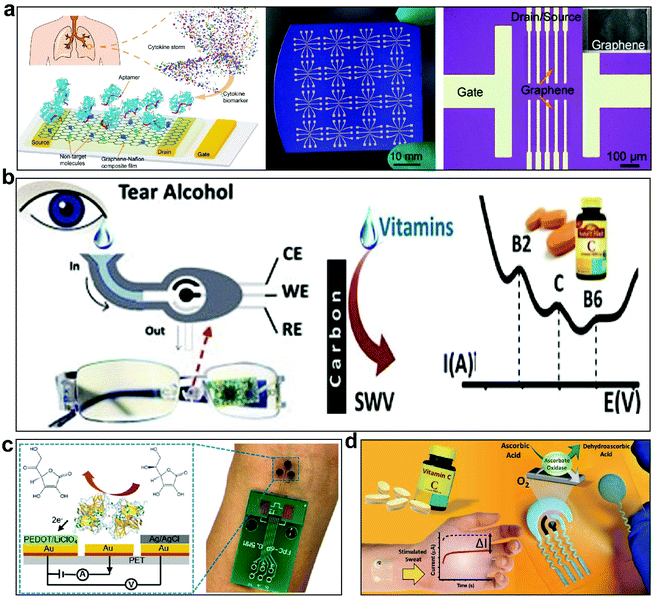 | ||
| Fig. 5 Some cytokine and nutrient sensors. (a) A flexible and regenerative aptamer field-effect transistor biosensor composed of graphene-Nafion composite film used to detect cytokines (IFN-γ, an inflammation and cancer biomarker) in undiluted human biofluid samples.146 Although this sensor shows excellent performance in the detection of cytokines, it cannot achieve good wearability. This figure has been reproduced from ref. 146 with permission from Wiley-VCH GmbH; copyright: 2020. (b) A wearable tear-based bioelectronic platform that integrates a microfluidic electrochemical detector into the nose pad of glasses for the non-invasive monitoring of key tear biomarkers (ethanol, glucose, and vitamins).35 This figure has been reproduced from ref. 35 with permission from Elsevier B.V.; copyright: 2019. (c) A flexible wearable sensor that selectively measures the concentration of vitamin C in biological fluid samples such as sweat, urine, and blood.158 PET is non-stretchable, airtight, and hard with a high elastic modulus. Although it achieves basic wearability, the monitoring process results in a series of problems, such as uncomfortableness during wearing and bringing motion artifacts. This figure has been reproduced from ref. 158 with permission from Wiley-VCH GmbH; copyright: 2020. (d) A disposable, non-invasive, flexible, and printable wearable electrochemical sensor for the intake and dynamic monitoring of vitamin C in epidermal sweat.36 The high-quality adhesion of flexible PU to the skin reduces the impact of skin deformation on the sensor. However, the breathability needs to be resolved for long-term wear. This figure has been reproduced from ref. 36 with permission from American Chemical Society; copyright: 2020. | ||
6. Nutrient sensors
Nutritional balance has a long-term impact on physical and mental health.147,148 A lack of nutrition will affect the developmental potential of children and cause risks such as stunted growth or poor cognitive development.149,150 Thus, preventing early malnutrition is necessary for physical development and growth. Second, monitoring nutrient levels can provide a basis for achieving a healthy diet, weight control, and the prevention of obesity and obesity-related diseases.151,152 For example, vitamins are essential nutrients for the normal growth of the human body. A lack of vitamins can result in many diseases. Detection methods for vitamins mainly include microbial analysis and chromatographic analysis.153–156 These analysis procedures are costly and sample preparation can be complicated and time-consuming. Vinu Mohan et al.157 fabricated a sensor with screen-printed electrodes on a PET substrate. The sample was dropped on the electrode surface for analysis. The results showed that cyclic square wave voltammetry (SWV) can produce obvious electrochemical signals from multiple vitamins (vitamins B12, B1, B2, B6 and C), which is the first proof of the feasibility of using voltammetry to identify multiple vitamins at the same time; this can provide a basis for subsequent related research. However, wearable equipment for real-time nutrient detection is still in the primary stage. Sempionatto et al.35 installed a PET-based sensor on the nose pad of glasses and used screen-printed electrodes and cyclic SWV to detect the multivitamin content in tears (Fig. 5b). This is the first example of the non-invasive monitoring of vitamins and fills a gap relating to the use of flexible and wearable devices for real-time vitamin monitoring. Zhao et al.158 integrated a wearable sensor on PET that can monitor vitamin C in sweat, urine, and blood (Fig. 5c). The sensor realizes the detection of vitamin C from the μM to the mM level through a surface-modified sensor with Au NDs, a conductive polymer, and a protective film layer. At present, most sensors for monitoring nutrients still employ PET as the substrate. As mentioned earlier, PET is non-stretchable, airtight, and hard with a high elastic modulus. Although it can support basic wearability, related monitoring processes will face a series of problems, such as uncomfortableness during wearing and bringing motion artifacts. Later, Sempionatto et al.36 used stretchable silver ink to prepare snake-shaped wires on a flexible polyurethane (PU) substrate (Fig. 5d). The IP electrode and reference electrode were printed with Ag/AgCl ink, the working electrode was printed with Gwent graphite ink, and the wires were finally packaged with Ecoflex. The detection of vitamin C is determined via a redox reaction with ascorbate oxidase on the electrode surface using voltammetry. The high-quality adhesion of flexible PU to the skin reduces the impact of skin deformation on the sensor, which is a major advancement in the area of flexible and wearable devices for nutrient detection. However, this field still faces huge challenges, such as achieving high-efficiency body fluid collection and avoiding the non-specific binding of other substances and aptamers.7. Other biomarker sensors
Some other biomarkers are also important physiologically for human health monitoring.34,159 For example, biomarkers can be used to monitor infection, and some biomarkers can also play a role in regulating the body.22,160 Their monitoring is very important, but limitations relating to detection methods and specificity control during testing have hampered research in this field. The plasma levodopa level is associated with Parkinson's disease progression. Moon et al. developed a finger-based sensing system to detect the pharmacokinetic profile of levodopa (Fig. 6a).161 The system offers the possibility of the personalized treatment of Parkinson's disease in a non-invasive manner. Although this device achieves touch-based sweat sensing to detect levodopa, it does not achieve wearability due to the special detection method. For wound monitoring, infections caused by bacterial breeding can cause changes in many physiological signals.32 The specific monitoring of some biomarkers can be used to analyze bacterial species for targeted treatment. Goud et al. developed a solid contact potential sensor based on flexible printed textiles for the selective detection of fluoride anions liberated upon the biocatalytic hydrolysis of fluorine-containing G-type nerve agents (Fig. 6b).34 The sensor is highly reversible and repeatable, and it can show strong mechanical elasticity under large mechanical strain. Based on the stretchability and breathability of flexible printed textiles and their potential for integration into clothing, this study achieves preliminary wearability, showing possibilities for future wearable monitoring research. The Vargas team developed a dual electrochemical immunosensor chip that can monitor insulin and cortisol simultaneously (Fig. 6c).162 This device uses 0.76 mm-thick PETG (glycol-modified PET) as the substrate. However, PETG is non-stretchable and hard with a high elastic modulus, and it cannot achieve wearability. The Tang team developed a non-invasive molecularly imprinted electrochemical sensor that detects cortisol in sweat in a touch-based manner (Fig. 6d).163 Different from previous work,161 they developed a stretchable epidermal patch based on SEBS which can adhere to skin. It can be seen from past studies that the monitoring of biomarkers often requires specific markers to match them. Converting chemical signals into stable electrical signals is a crucial issue in this field. At the same time, improving sensor integration and creating wearable designs are important challenges facing customized healthcare in the future.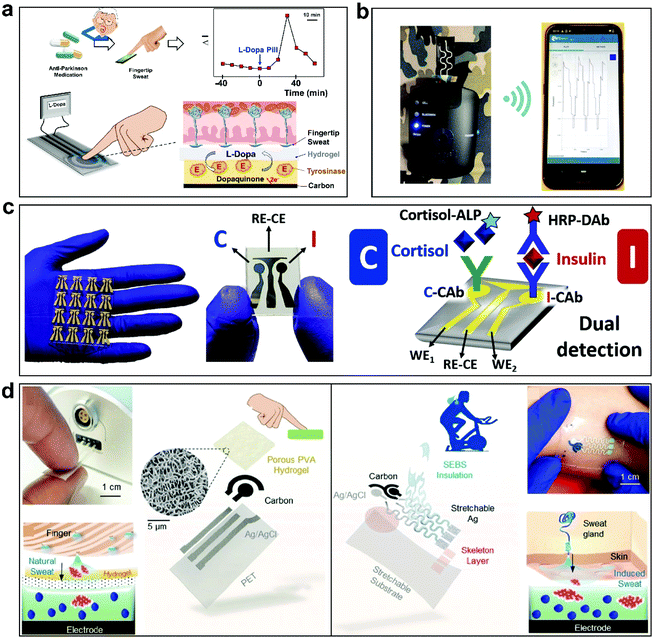 | ||
| Fig. 6 Some sensors for other biomarkers. (a) The non-invasive sweat-based tracking of levodopa pharmacokinetic profiles following oral tablet administration.161 This approach does not achieve wearability due to the special detection method used. This figure has been reproduced from ref. 161 with permission from Wiley-VCH GmbH; copyright: 2021. (b) A textile-based wearable solid-contact flexible fluoride sensor for the bio-detection of G-type nerve agents.34 Based on the stretchability and breathability of flexible printed textiles and their potential for integration into clothing, this study achieves preliminary wearability. This figure has been reproduced from ref. 34 with permission from Elsevier B.V.; copyright: 2021. (c) Simultaneous cortisol/insulin microchip-based detection using dual enzyme tagging.162 This device uses 0.76 mm-thick PETG (glycol-modified PET) as the substrate. However, PETG is non-stretchable and hard with a high elastic modulus and it does not achieve wearability. This figure has been reproduced from ref. 162 with permission from Elsevier B.V.; copyright: 2020. (d) A stretchable stress-free cortisol sensor based on touch.163 The stretchable epidermal patch based on SEBS can adhere to skin. This figure has been reproduced from ref. 163 with permission from Wiley-VCH GmbH; copyright: 2021. | ||
8. Conclusions and outlook
In this review, we summarized the latest developments in the field of flexible and wearable chemical sensors for continuous chemical-signal monitoring on the surface of the body. These flexible and wearable chemical sensors can non-invasively monitor pH levels, glucose, lactate, uric acid, ion levels, cytokines, nutrients, and other biomarkers (including insulin, cortisol, chlorocyanin, fluoride, etc.) in sweat, tears, and ISF. Different from conventional laboratory analytical instruments and most flexible and wearable electrophysiological sensors, novel flexible and wearable chemical sensors show great advantages in terms of the real-time, fast, convenient, and stable detection of different biomarkers at the body surface. The advantages of flexible wearable chemical sensors are mainly related to achieving personalized healthcare at the molecular level. Benefiting from these innovative sensors, it is possible for users to obtain their dynamic health status at any time. In addition, there are some other important small-molecule chemicals, such as proteins, genetic material, and hormones. There is a relatively small amount of related research on flexible wearable sensors for these chemicals, and related research should receive extensive attention from researchers. New technology for achieving the real-time monitoring of biomarkers with extremely low content levels in body fluids should be explored for next-generation flexible and wearable sensing devices.Although great progress has been made in terms of the development of flexible and wearable chemical sensors and real-time health monitoring, some challenges and technical gaps still remain that prevent the widespread use and commercialization of these sensors (Table 2). Since the field of flexible and wearable electronics is a cross-discipline subject, solving the current challenges and overcoming technological gaps in this field will require joint effort from researchers from different disciplines, covering materials science, chemistry, biology, electronics, communications, etc. In terms of materials, soft materials are the basis of flexible and wearable devices. The choice of material should meet the requirements of wearability (stretchability, softness, adhesion to the skin, breathability, and biocompatibility). In order to adapt to the deformation of skin, the material needs to have stretchability. Electrodes can be designed with special structures to improve the stretchable conductivity, such as with serpentine or microcrack structures. In order to ensure conformal attachment to the skin, the substrate material should have a Young's modulus similar to the skin (∼100 kPa) and good adhesion to the skin, adopting approaches such as flexible film or tattoo structures. In order to improve the breathability of devices, fabric-based or porous materials can be selected as substrate materials. For biocompatibility, we need to choose biocompatible materials, for example, a suitable conductive material is biocompatible gold. In addition, capacitive electrodes can be designed to avoid direct contact between the electrode and the skin to improve biocompatibility. Nevertheless, there are few materials that meet all these requirements at the same time, and the exploration of materials still needs to be advanced. In addition, a current working mechanism for chemical molecule detection is based on a change in electrical information caused by the specific binding between the molecule of interest and an enzyme or aptamer modified on the electrode surface. However, this specific binding is not absolute and the non-specific binding of other molecules is usually included. Also, enzymes and aptamers are consumed during the detection process, and it is challenging to achieve the replenishment of these active materials. In addition, making the sensor insensitive to external environmental changes (such as temperature and humidity) remains an urgent problem.
| Field | Challenges |
|---|---|
| Chemical molecules | The real-time monitoring of biomarkers with extremely low content levels in body fluid samples (such as proteins, hormones, DNA, and RNA). |
| Materials | The adhesion and conformity of the material to the skin. |
| Electrode design | The non-specific binding of other molecules to enzymes or aptamers. |
| The regeneration and long-term repeated use of electrodes. | |
| The adaptability of the electrode to changes in the external environment (such as temperature and humidity). | |
| Data transmission | The long-term wear comfort and volume reduction of PCBs. |
| The development of more advanced wireless communications technology that is not limited by energy supply and transmission distance. | |
| Hard–soft interface | The exploration of stable and seamless connections between soft and hard devices to improve the quality of data collection and transmission. |
| Outlook | In order to make flexible and wearable sensors more intelligent, sensors with high-density electrode arrays incorporating AI technology should be combined to achieve more accurate algorithmic predictions based on the analysis of large amounts of data. |
For flexible and wearable sensing devices, in addition to sensors being based on flexible and stretchable materials with electrode integration, the PCB used for data transmission and wireless communication with mobile devices is also crucial. At present, data transmission via Bluetooth and NFC has been widely used in flexible and wearable devices. However, some inherent characteristics of Bluetooth and NFC limit the application scope of these devices. For example, Bluetooth requires huge power consumption while NFC-based data transmission is extremely short-range (1–2 cm). In addition, current PCBs are usually rigid. Although flexibility has been improved via using PI-based FPCBs, the high Young's modulus (2.5 GPa) of PI still cannot meet the requirements for long-term and comfortable wear. Moreover, considering the quality of data collection and transmission, the stable integration of flexible electrodes and PCBs is also crucial. Due to a mismatch in Young's modulus values and delamination between the flexible electrode and the rigid circuit, shifting between the two parts can happen easily. Achieving seamless and stable connections between soft and hard circuits still needs to be explored.
Flexible wearable chemical sensors can be used to establish a relationship between molecular concentrations and output signals, but it is difficult to obtain clear correspondence in a complex environment. It is necessary to introduce new technology to evaluate this complex correspondence precisely. In recent years, artificial intelligence (AI) and deep learning technologies have developed rapidly. In order to make flexible and wearable sensors more intelligent, it may be necessary to combine sensors and AI technology to achieve more accurate algorithmic predictions. More accurate and reliable results can be obtained via the analysis of large amounts of data. With the continuous development of flexible electronics and wireless communications technology, flexible and wearable sensing devices will become more widely used in healthcare, meaning that a large amount of private data will be generated. Data security and privacy issues need to be resolved. Although there are still many challenges when preparing stable wearable chemical sensors, we firmly believe that flexible and wearable chemical sensors could completely change traditional healthcare models in the near future.
Author contributions
H. Zhao and R. Su summarized the literature and wrote the original draft. L. Teng and Q. Tian reviewed the work and modified the language. F. Han commented on the figures in the original draft and modified the language. H. Li, Z, Cao, and R. Xie helped correct the original draft. G. Li, X. Liu, and Z. Liu corrected the original draft and supervised this work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the SIAT Innovative Program for Excellent Young Researcher (2021002227) and Guangdong Basic and Applied Basic Research Foundation (2020A1515110205).References
- S. C. Mukhopadhyay, IEEE Sens. J., 2015, 15, 1321–1330 Search PubMed
.
- S. Patel, H. Park, P. Bonato, L. Chan and M. A. Rodgers, J. Neuroeng. Rehabilitation, 2012, 9, 1–17 CrossRef PubMed
.
- D. H. Kim, N. Lu, R. Ma, Y. S. Kim, R. H. Kim, S. Wang, J. Wu, S. M. Won, H. Tao, A. Islam, K. J. Yu, T. I. Kim, R. Chowdhury, M. Ying, L. Xu, M. Li, H. J. Chung, H. Keum, M. McCormick, P. Liu, Y. W. Zhang, F. G. Omenetto, Y. Huang, T. Coleman and J. A. Rogers, Science, 2011, 333, 838–843 CrossRef CAS PubMed
.
- M. L. Hammock, A. Chortos, B. C. Tee, J. B. Tok and Z. Bao, Adv. Mater., 2013, 25, 5997–6038 CrossRef CAS PubMed
.
- M. Kaltenbrunner, T. Sekitani, J. Reeder, T. Yokota, K. Kuribara, T. Tokuhara, M. Drack, R. Schwodiauer, I. Graz, S. Bauer-Gogonea, S. Bauer and T. Someya, Nature, 2013, 499, 458–463 CrossRef CAS PubMed
.
- Y. Liu, M. Pharr and G. A. Salvatore, ACS Nano, 2017, 11, 9614–9635 CrossRef CAS PubMed
.
- Y. Gu, T. Zhang, H. Chen, F. Wang, Y. Pu, C. Gao and S. Li, Nanoscale Res. Lett., 2019, 14, 263 CrossRef PubMed
.
- E. K. Lee, M. K. Kim and C. H. Lee, Annu. Rev. Biomed. Eng., 2019, 21, 299–323 CrossRef CAS PubMed
.
- S. Hong, S. Lee and D.-H. Kim, Proc. IEEE, 2019, 107, 2185–2197 CAS
.
- J. A. Rogers, T. Someya and Y. Huang, Science, 2010, 327, 1603–1607 CrossRef CAS PubMed
.
- S. Choi, H. Lee, R. Ghaffari, T. Hyeon and D. H. Kim, Adv. Mater., 2016, 28, 4203–4218 CrossRef CAS PubMed
.
- H. Wu, F. Chen, C. You, Y. Zhang, B. Sun and Q. Zhu, Small, 2020, 16, 2001805 CrossRef CAS PubMed
.
- H. Yang, S. Ji, I. Chaturvedi, H. Xia, T. Wang, G. Chen, L. Pan, C. Wan, D. Qi, Y.-S. Ong and X. Chen, ACS Mater. Lett., 2020, 2, 478–484 CrossRef CAS
.
- H. Lee, S. Lee, W. Lee, T. Yokota, K. Fukuda and T. Someya, Adv. Funct. Mater., 2019, 29, 1906982 CrossRef CAS
.
- Z. Li, W. Guo, Y. Huang, K. Zhu, H. Yi and H. Wu, Carbon, 2020, 164, 164–170 CrossRef CAS
.
- C. Wang, X. Li, H. Hu, L. Zhang, Z. Huang, M. Lin, Z. Zhang, Z. Yin, B. Huang, H. Gong, S. Bhaskaran, Y. Gu, M. Makihata, Y. Guo, Y. Lei, Y. Chen, C. Wang, Y. Li, T. Zhang, Z. Chen, A. P. Pisano, L. Zhang, Q. Zhou and S. Xu, Nat. Biomed. Eng., 2018, 2, 687–695 CrossRef PubMed
.
- Q. Wu, Y. Qiao, R. Guo, S. Naveed, T. Hirtz, X. Li, Y. Fu, Y. Wei, G. Deng, Y. Yang, X. Wu and T. L. Ren, ACS Nano, 2020, 14, 10104–10114 CrossRef CAS PubMed
.
- S. P. Lee, G. Ha, D. E. Wright, Y. Ma, E. Sen-Gupta, N. R. Haubrich, P. C. Branche, W. Li, G. L. Huppert, M. Johnson, H. B. Mutlu, K. Li, N. Sheth, J. A. Wright Jr., Y. Huang, M. Mansour, J. A. Rogers and R. Ghaffari, NPJ Digit. Med., 2018, 1, 2 CrossRef PubMed
.
- T. Xie, L. Zhang, Y. Wang, Y. Wang and X. Wang, Ceram. Int., 2019, 45, 2516–2520 CrossRef CAS
.
- Q. Lyu, S. Gong, J. Yin, J. M. Dyson and W. Cheng, Adv. Healthcare Mater., 2021, 10, 2100577 CrossRef CAS PubMed
.
- Y. Yang and W. Gao, Chem. Soc. Rev., 2019, 48, 1465–1491 RSC
.
- G. Tegl, D. Schiffer, E. Sigl, A. Heinzle and G. M. Guebitz, Appl. Microbiol. Biotechnol., 2015, 99, 4595–4614 CrossRef CAS PubMed
.
- Y. Y. Broza, X. Zhou, M. Yuan, D. Qu, Y. Zheng, R. Vishinkin, M. Khatib, W. Wu and H. Haick, Chem. Rev., 2019, 119, 11761–11817 CrossRef CAS PubMed
.
- P. T. J. Scheepers and J. Cocker, TrAC, Trends Anal. Chem., 2019, 113, 116–123 CrossRef CAS
.
- S. M. Park, T. J. Ge, D. D. Won, J. K. Lee and J. C. Liao, Nat. Rev. Gastroenterol. Hepatol., 2021, 18, 521–522 CrossRef PubMed
.
- I. S. Kucherenko, O. O. Soldatkin, D. Y. Kucherenko, O. V. Soldatkina and S. V. Dzyadevych, Nanoscale Adv., 2019, 1, 4560–4577 RSC
.
- Y. Yu, H. Y. Y. Nyein, W. Gao and A. Javey, Adv. Mater., 2020, 32, 1902083 CrossRef CAS PubMed
.
- Z. Jin, L. Yang, S. Shi, T. Wang, G. Duan, X. Liu and Y. Li, Adv. Funct. Mater., 2021, 31, 2103391 CrossRef CAS
.
- S. Imani, A. J. Bandodkar, A. M. Mohan, R. Kumar, S. Yu, J. Wang and P. P. Mercier, Nat. Commun., 2016, 7, 11650 CrossRef CAS PubMed
.
- H. Karimi-Maleh, Y. Orooji, F. Karimi, M. Alizadeh, M. Baghayeri, J. Rouhi, S. Tajik, H. Beitollahi, S. Agarwal, V. K. Gupta, S. Rajendran, A. Ayati, L. Fu, A. L. Sanati, B. Tanhaei, F. Sen, M. Shabani-Nooshabadi, P. N. Asrami and A. Al-Othman, Biosens. Bioelectron., 2021, 184, 113252 CrossRef CAS PubMed
.
- G. S. Perera, T. Ahmed, L. Heiss, S. Walia, M. Bhaskaran and S. Sriram, Small, 2021, 17, 2005582 CrossRef CAS PubMed
.
- Y. Yang, Y. Y. Yu, Y. Z. Wang, C. L. Zhang, J. X. Wang, Z. Fang, H. Lv, J. J. Zhong and Y. C. Yong, Biosens. Bioelectron., 2017, 98, 338–344 CrossRef CAS PubMed
.
- Z. Wang, Z. Hao, S. Yu, C. G. De Moraes, L. H. Suh, X. Zhao and Q. Lin, Adv. Funct. Mater., 2019, 29, 1905202 CrossRef CAS PubMed
.
- K. Y. Goud, S. S. Sandhu, H. Teymourian, L. Yin, N. Tostado, F. M. Raushel, S. P. Harvey, L. C. Moores and J. Wang, Biosens. Bioelectron., 2021, 182, 113172 CrossRef CAS PubMed
.
- J. R. Sempionatto, L. C. Brazaca, L. Garcia-Carmona, G. Bolat, A. S. Campbell, A. Martin, G. Tang, R. Shah, R. K. Mishra, J. Kim, V. Zucolotto, A. Escarpa and J. Wang, Biosens. Bioelectron., 2019, 137, 161–170 CrossRef CAS PubMed
.
- J. R. Sempionatto, A. A. Khorshed, A. Ahmed, A. N. De Loyola e Silva, A. Barfidokht, L. Yin, K. Y. Goud, M. A. Mohamed, E. Bailey, J. May, C. Aebischer, C. Chatelle and J. Wang, ACS Sens., 2020, 5, 1804–1813 CrossRef CAS PubMed
.
- J. R. Sempionatto, M. Lin, L. Yin, E. De la Paz, K. Pei, T. Sonsa-Ard, A. N. de Loyola Silva, A. A. Khorshed, F. Zhang, N. Tostado, S. Xu and J. Wang, Nat. Biomed. Eng., 2021, 5, 737–748 CrossRef CAS PubMed
.
- W. Gao, S. Emaminejad, H. Y. Y. Nyein, S. Challa, K. Chen, A. Peck, H. M. Fahad, H. Ota, H. Shiraki, D. Kiriya, D. H. Lien, G. A. Brooks, R. W. Davis and A. Javey, Nature, 2016, 529, 509–514 CrossRef CAS PubMed
.
- Y. Yang, Y. Song, X. Bo, J. Min, O. S. Pak, L. Zhu, M. Wang, J. Tu, A. Kogan, H. Zhang, T. K. Hsiai, Z. Li and W. Gao, Nat. Biotechnol., 2020, 38, 217–224 CrossRef CAS PubMed
.
- W. Gao, H. Y. Y. Nyein, Z. Shahpar, H. M. Fahad, K. Chen, S. Emaminejad, Y. Gao, L.-C. Tai, H. Ota, E. Wu, J. Bullock, Y. Zeng, D.-H. Lien and A. Javey, ACS Sens., 2016, 1, 866–874 CrossRef CAS
.
- A. J. Bandodkar, I. Jeerapan and J. Wang, ACS Sens., 2016, 1, 464–482 CrossRef CAS
.
- W. Gao, H. Ota, D. Kiriya, K. Takei and A. Javey, Acc. Chem. Res., 2019, 52, 523–533 CrossRef CAS PubMed
.
- J. Kim, A. S. Campbell, B. E. de Avila and J. Wang, Nat. Biotechnol., 2019, 37, 389–406 CrossRef CAS PubMed
.
- L. Yin, J. Lv and J. Wang, Adv. Mater. Technol., 2020, 5, 2000694 CrossRef
.
- D. J. Lipomi, M. Vosgueritchian, B. C. Tee, S. L. Hellstrom, J. A. Lee, C. H. Fox and Z. Bao, Nat. Nanotechnol., 2011, 6, 788–792 CrossRef CAS PubMed
.
- H. Wu, G. Yang, K. Zhu, S. Liu, W. Guo, Z. Jiang and Z. Li, Adv. Sci., 2021, 8, 2001938 CrossRef CAS PubMed
.
- J. Visser, F. P. Melchels, J. E. Jeon, E. M. van Bussel, L. S. Kimpton, H. M. Byrne, W. J. Dhert, P. D. Dalton, D. W. Hutmacher and J. Malda, Nat. Commun., 2015, 6, 6933 CrossRef CAS PubMed
.
- S. Hong, S. Lee and D.-H. Kim, Proc. IEEE, 2019, 107, 2185–2197 CAS
.
- B. Yu, S. Y. Kang, A. Akthakul, N. Ramadurai, M. Pilkenton, A. Patel, A. Nashat, D. G. Anderson, F. H. Sakamoto, B. A. Gilchrest, R. R. Anderson and R. Langer, Nat. Mater., 2016, 15, 911–918 CrossRef CAS PubMed
.
- W. Zeng, L. Shu, Q. Li, S. Chen, F. Wang and X. M. Tao, Adv. Mater., 2014, 26, 5310–5336 CrossRef CAS PubMed
.
- A. Miyamoto, S. Lee, N. F. Cooray, S. Lee, M. Mori, N. Matsuhisa, H. Jin, L. Yoda, T. Yokota, A. Itoh, M. Sekino, H. Kawasaki, T. Ebihara, M. Amagai and T. Someya, Nat. Nanotechnol., 2017, 12, 907–913 CrossRef CAS PubMed
.
- J. Rivnay, R. M. Owens and G. G. Malliaras, Chem. Mater., 2013, 26, 679–685 CrossRef
.
- G. Xu, C. Cheng, W. Yuan, Z. Liu, L. Zhu, X. Li, Y. Lu, Z. Chen, J. Liu, Z. Cui, J. Liu, H. Men and Q. Liu, Sens. Actuators, B, 2019, 297, 126743 CrossRef CAS
.
- J. Kim, S. Imani, W. R. de Araujo, J. Warchall, G. Valdes-Ramirez, T. R. Paixao, P. P. Mercier and J. Wang, Biosens. Bioelectron., 2015, 74, 1061–1068 CrossRef CAS PubMed
.
- P. Kassal, J. Kim, R. Kumar, W. R. de Araujo, I. M. Steinberg, M. D. Steinberg and J. Wang, Electrochem. Commun., 2015, 56, 6–10 CrossRef CAS
.
- H. Filik and A. A. Avan, Talanta, 2020, 211, 120758 CrossRef CAS PubMed
.
- F. Vivaldi, D. Santalucia, N. Poma, A. Bonini, P. Salvo, L. Del Noce, B. Melai, A. Kirchhain, V. Kolivoška, R. Sokolová, M. Hromadová and F. Di Francesco, Sens. Actuators, B, 2020, 322, 128650 CrossRef CAS
.
- M. Sharifuzzaman, A. Chhetry, M. A. Zahed, S. H. Yoon, C. I. Park, S. Zhang, S. Chandra Barman, S. Sharma, H. Yoon and J. Y. Park, Biosens. Bioelectron., 2020, 169, 112637 CrossRef CAS PubMed
.
- A. U. Alam, Y. Qin, S. Nambiar, J. T. W. Yeow, M. M. R. Howlader, N.-X. Hu and M. J. Deen, Prog. Mater. Sci., 2018, 96, 174–216 CrossRef
.
- W. P. Nikolajek and H. M. Emrich, Klin. Wschr., 1976, 54, 287–288 CrossRef CAS PubMed
.
- S. R. Corrie, J. W. Coffey, J. Islam, K. A. Markey and M. A. Kendall, Analyst, 2015, 140, 4350–4364 RSC
.
- J. H. Yoon, S. M. Kim, H. J. Park, Y. K. Kim, D. X. Oh, H. W. Cho, K. G. Lee, S. Y. Hwang, J. Park and B. G. Choi, Biosens. Bioelectron., 2020, 150, 111946 CrossRef CAS PubMed
.
- M. Zea, A. Moya, M. Fritsch, E. Ramon, R. Villa and G. Gabriel, ACS Appl. Mater. Interfaces, 2019, 11, 15160–15169 CrossRef CAS PubMed
.
- J. H. Yoon, S. B. Hong, S. O. Yun, S. J. Lee, T. J. Lee, K. G. Lee and B. G. Choi, J. Colloid Interface Sci., 2017, 490, 53–58 CrossRef CAS PubMed
.
- S. Glab, A. Hulanicki, G. Edwall and F. Ingman, Crit. Rev. Anal. Chem., 1989, 21, 29–47 CrossRef CAS PubMed
.
- L. Manjakkal, D. Szwagierczak and R. Dahiya, Prog. Mater. Sci., 2020, 109, 100635 CrossRef CAS
.
- R. Rahimi, M. Ochoa, A. Tamayol, S. Khalili, A. Khademhosseini and B. Ziaie, ACS Appl. Mater. Interfaces, 2017, 9, 9015–9023 CrossRef CAS PubMed
.
- L. Santos, J. P. Neto, A. Crespo, D. Nunes, N. Costa, I. M. Fonseca, P. Barquinha, L. Pereira, J. Silva, R. Martins and E. Fortunato, ACS Appl. Mater. Interfaces, 2014, 6, 12226–12234 CrossRef CAS PubMed
.
- R. Rahimi, M. Ochoa, T. Parupudi, X. Zhao, I. K. Yazdi, M. R. Dokmeci, A. Tamayol, A. Khademhosseini and B. Ziaie, Sens. Actuators, B, 2016, 229, 609–617 CrossRef CAS
.
- H. J. Park, J. H. Yoon, K. G. Lee and B. G. Choi, Nano Convergence, 2019, 6, 9 CrossRef PubMed
.
- Y. Li, Y. Mao, C. Xiao, X. Xu and X. Li, RSC Adv., 2020, 10, 21–28 RSC
.
- S. Y. Oh, S. Y. Hong, Y. R. Jeong, J. Yun, H. Park, S. W. Jin, G. Lee, J. H. Oh, H. Lee, S. S. Lee and J. S. Ha, ACS Appl. Mater. Interfaces, 2018, 10, 13729–13740 CrossRef CAS PubMed
.
- Q. Zhai, L. W. Yap, R. Wang, S. Gong, Z. Guo, Y. Liu, Q. Lyu, J. Wang, G. P. Simon and W. Cheng, Anal. Chem., 2020, 92, 4647–4655 CrossRef CAS PubMed
.
- D. Wencel, A. Kaworek, T. Abel, V. Efremov, A. Bradford, D. Carthy, G. Coady, R. C. N. McMorrow and C. McDonagh, Small, 2018, 14, 1803627 CrossRef PubMed
.
- T. J. Sørensen, M. Rosenberg, C. G. Frankær and B. W. Laursen, Adv. Mater. Technol., 2019, 4, 1800561 Search PubMed
.
- Y. Zhu, J. Zhang, J. Song, J. Yang, Z. Du, W. Zhao, H. Guo, C. Wen, Q. Li, X. Sui and L. Zhang, Adv. Funct. Mater., 2019, 30, 1905493 CrossRef
.
- H. Lee, Y. J. Hong, S. Baik, T. Hyeon and D. H. Kim, Adv. Healthcare Mater., 2018, 7, 1701150 CrossRef PubMed
.
- P. Mandpe, B. Prabhakar, H. Gupta and P. Shende, Sens. Rev., 2020, 40, 497–511 CrossRef
.
- P. J. Derbyshire, H. Barr, F. Davis and S. P. Higson, J. Physiol. Sci., 2012, 62, 429–440 CrossRef CAS PubMed
.
- M. L. Fernandez, Z. Upton and G. K. Shooter, Curr. Rheumatol. Rep., 2014, 16, 396 CrossRef PubMed
.
- J. Kim, A. S. Campbell and J. Wang, Talanta, 2018, 177, 163–170 CrossRef CAS PubMed
.
- F. Alam, S. RoyChoudhury, A. H. Jalal, Y. Umasankar, S. Forouzanfar, N. Akter, S. Bhansali and N. Pala, Biosens. Bioelectron., 2018, 117, 818–829 CrossRef CAS PubMed
.
- Z. Zhou, T. Shu, Y. Sun, H. Si, P. Peng, L. Su and X. Zhang, Biosens. Bioelectron., 2021, 192, 113530 CrossRef CAS PubMed
.
- C. W. Bae, P. T. Toi, B. Y. Kim, W. I. Lee, H. B. Lee, A. Hanif, E. H. Lee and N. E. Lee, ACS Appl. Mater. Interfaces, 2019, 11, 14567–14575 CrossRef CAS PubMed
.
- H. Teymourian, A. Barfidokht and J. Wang, Chem. Soc. Rev., 2020, 49, 7671–7709 RSC
.
- K. L. Wolkowicz, E. M. Aiello, E. Vargas, H. Teymourian, F. Tehrani, J. Wang, J. E. Pinsker, F. J. Doyle 3rd, M. E. Patti, L. M. Laffel and E. Dassau, Bioeng. Transl. Med., 2021, 6, 10201 Search PubMed
.
- N. M. Farandos, A. K. Yetisen, M. J. Monteiro, C. R. Lowe and S. H. Yun, Adv. Healthcare Mater., 2015, 4, 792–810 CrossRef CAS PubMed
.
- Y. Gao, Y. Huang, J. Chen, Y. Liu, Y. Xu and X. Ning, Anal. Chem., 2021, 93, 10593–10600 CrossRef CAS PubMed
.
- R. Wang, Q. Zhai, T. An, S. Gong and W. Cheng, Talanta, 2021, 222, 121484 CrossRef CAS PubMed
.
- C. T. Huang, M. L. Chen, L. L. Huang and I. F. Mao, Chin. J. Physiol., 2002, 45, 109–116 CAS
.
- R. El Ridi and H. Tallima, J. Adv. Res., 2017, 8, 487–493 CrossRef CAS PubMed
.
- K. Shim, W.-C. Lee, M.-S. Park, M. Shahabuddin, Y. Yamauchi, M. S. A. Hossain, Y.-B. Shim and J. H. Kim, Sens. Actuators, B, 2019, 278, 88–96 CrossRef CAS
.
- F. Mazzara, B. Patella, G. Aiello, A. O'Riordan, C. Torino, A. Vilasi and R. Inguanta, Electrochim. Acta, 2021, 388, 138652 CrossRef CAS
.
- Y. Zhang, J. Li, M. Wu, Z. Guo, D. Tan, X. Zhou, Y. Li, S. Liu, L. Xue and Y. Lei, ACS Appl. Bio Mater., 2020, 3, 8640–8649 CrossRef CAS PubMed
.
- J. Moyer, D. Wilson, I. Finkelshtein, B. Wong and R. Potts, Diabetes Technol. Ther., 2012, 14, 398–402 CrossRef CAS PubMed
.
- J. Kim, J. R. Sempionatto, S. Imani, M. C. Hartel, A. Barfidokht, G. Tang, A. S. Campbell, P. P. Mercier and J. Wang, Adv. Sci., 2018, 5, 1800880 CrossRef PubMed
.
- J. R. Sempionatto, J. M. Moon and J. Wang, ACS Sens., 2021, 6, 1875–1883 CrossRef CAS PubMed
.
- M. A. Zahed, S. C. Barman, P. S. Das, M. Sharifuzzaman, H. S. Yoon, S. H. Yoon and J. Y. Park, Biosens. Bioelectron., 2020, 160, 112220 CrossRef CAS PubMed
.
- Y. J. Hong, H. Lee, J. Kim, M. Lee, H. J. Choi, T. Hyeon and D.-H. Kim, Adv. Funct. Mater., 2018, 28, 1805754 CrossRef
.
- J. Yu, Y. Zhang, Y. Ye, R. DiSanto, W. Sun, D. Ranson, F. S. Ligler, J. B. Buse and Z. Gu, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 8260–8265 CrossRef CAS PubMed
.
- W. L. Hsu, C. Y. Huang, Y. P. Hsu, T. L. Hwang, S. H. Chang, H. Y. J. Wang, L. Y. Feng, S. J. Tzou, K. C. Wei and H. W. Yang, Chem. Eng. J., 2020, 398, 125536 CrossRef CAS
.
- K. Takeuchi, N. Takama, R. Kinoshita, T. Okitsu and B. Kim, Biomed. Microdevices, 2020, 22, 79 CrossRef CAS PubMed
.
- Y. Zeng, J. Wang, Z. Wang, G. Chen, J. Yu, S. Li, Q. Li, H. Li, D. Wen, Z. Gu and Z. Gu, Nano Today, 2020, 35, 100984 CrossRef CAS
.
- B. L. Zhang, Y. Yang, Z. Q. Zhao and X. D. Guo, Electrochim. Acta, 2020, 358, 136917 CrossRef CAS
.
- I. Lee, N. Loew, W. Tsugawa, K. Ikebukuro and K. Sode, Biosens. Bioelectron., 2019, 124–125, 216–223 CrossRef CAS PubMed
.
- M. Adeel, M. M. Rahman, I. Caligiuri, V. Canzonieri, F. Rizzolio and S. Daniele, Biosens. Bioelectron., 2020, 165, 112331 CrossRef CAS PubMed
.
- H. Li, C. Liu, D. Wang and C. Zhang, Biosens. Bioelectron., 2017, 91, 268–275 CrossRef CAS PubMed
.
- J. M. Green, R. C. Pritchett, T. R. Crews, J. R. McLester Jr. and D. C. Tucker, Eur. J. Appl. Physiol., 2004, 91, 1–6 CrossRef CAS PubMed
.
- S. Anastasova, B. Crewther, P. Bembnowicz, V. Curto, H. M. Ip, B. Rosa and G. Z. Yang, Biosens. Bioelectron., 2017, 93, 139–145 CrossRef CAS PubMed
.
- B. Gil, S. Anastasova and G. Z. Yang, Sensors, 2019, 19, 1616 CrossRef CAS PubMed
.
- J. Guo, Anal. Chem., 2016, 88, 11986–11989 CrossRef CAS PubMed
.
- N. Cai, L. Tan, Y. Li, T. Xia, T. Hu and X. Su, Anal. Chim. Acta, 2017, 965, 96–102 CrossRef CAS PubMed
.
- Z. Ament, M. B. Bevers, Z. Wolcott, W. T. Kimberly and A. Acharjee, Transl. Stroke Res., 2021, 12, 293–302 CrossRef CAS PubMed
.
- K. Hayat, A. Munawar, A. Zulfiqar, M. H. Akhtar, H. B. Ahmad, Z. Shafiq, M. Akram, A. S. Saleemi and N. Akhtar, ACS Appl. Mater. Interfaces, 2020, 12, 47320–47329 CrossRef CAS PubMed
.
- X. Wei, M. Zhu, J. Li, L. Liu, J. Yu, Z. Li and B. Ding, Nano Energy, 2021, 85, 106031 CrossRef CAS
.
- M. Yang, H. Wang, P. Liu and J. Cheng, Biosens. Bioelectron., 2021, 179, 113082 CrossRef CAS PubMed
.
- G. Xu, Y. Lu, C. Cheng, X. Li, J. Xu, Z. Liu, J. Liu, G. Liu, Z. Shi, Z. Chen, F. Zhang, Y. Jia, D. Xu, W. Yuan, Z. Cui, S. S. Low and Q. Liu, Adv. Funct. Mater., 2021, 31, 2100852 CrossRef CAS
.
- Z. Zhou, T. Shu, Y. Sun, H. Si, P. Peng, L. Su and X. Zhang, Biosens. Bioelectron., 2021, 192, 113530 CrossRef CAS PubMed
.
- Z. Zhang, M. Azizi, M. Lee, P. Davidowsky, P. Lawrence and A. Abbaspourrad, Lab Chip, 2019, 19, 3448–3460 RSC
.
- M. Chung, G. Fortunato and N. Radacsi, J. R. Soc., Interface, 2019, 16, 20190217 CrossRef CAS PubMed
.
- T. Kaya, G. Liu, J. Ho, K. Yelamarthi, K. Miller, J. Edwards and A. Stannard, Electroanalysis, 2019, 31, 411–421 CrossRef CAS
.
- B. Schazmann, D. Morris, C. Slater, S. Beirne, C. Fay, R. Reuveny, N. Moyna and D. Diamond, Anal. Methods, 2010, 2, 342 RSC
.
- J. Xiao, G. Zhang, R. Xu, H. Chen, H. Wang, G. Tian, B. Wang, C. Yang, G. Bai, Z. Zhang, H. Yang, K. Zhong, D. Zou and Z. Wu, Biomaterials, 2019, 216, 119254 CrossRef CAS PubMed
.
- W. Dang, L. Manjakkal, W. T. Navaraj, L. Lorenzelli, V. Vinciguerra and R. Dahiya, Biosens. Bioelectron., 2018, 107, 192–202 CrossRef CAS PubMed
.
- G. S. Baird, Clin. Chim. Acta, 2011, 412, 696–701 CrossRef CAS PubMed
.
- W. G. Robertson, R. W. Marshall and G. N. Bowers, CRC Crit. Rev. Clin. Lab. Sci., 1981, 15, 85–125 CrossRef CAS PubMed
.
- H. Y. Nyein, W. Gao, Z. Shahpar, S. Emaminejad, S. Challa, K. Chen, H. M. Fahad, L. C. Tai, H. Ota, R. W. Davis and A. Javey, ACS Nano, 2016, 10, 7216–7224 CrossRef CAS PubMed
.
- S. T. Keene, D. Fogarty, R. Cooke, C. D. Casadevall, A. Salleo and O. Parlak, Adv. Healthcare Mater., 2019, 8, 1901321 CrossRef CAS PubMed
.
- D. H. Choi, J. S. Kim, G. R. Cutting and P. C. Searson, Anal. Chem., 2016, 88, 12241–12247 CrossRef CAS PubMed
.
- A. J. Bandodkar, D. Molinnus, O. Mirza, T. Guinovart, J. R. Windmiller, G. Valdes-Ramirez, F. J. Andrade, M. J. Schoning and J. Wang, Biosens. Bioelectron., 2014, 54, 603–609 CrossRef CAS PubMed
.
- S. Wang, Y. Wu, Y. Gu, T. Li, H. Luo, L. H. Li, Y. Bai, L. Li, L. Liu, Y. Cao, H. Ding and T. Zhang, Anal. Chem., 2017, 89, 10224–10231 CrossRef CAS PubMed
.
- J. Bujes-Garrido and M. J. Arcos-Martínez, Sens. Actuators, B, 2017, 240, 224–228 CrossRef CAS
.
- S. Emaminejad, W. Gao, E. Wu, Z. A. Davies, H. Yin Yin Nyein, S. Challa, S. P. Ryan, H. M. Fahad, K. Chen, Z. Shahpar, S. Talebi, C. Milla, A. Javey and R. W. Davis, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 4625–4630 CrossRef CAS PubMed
.
- J. H. Yoon, S. M. Kim, Y. Eom, J. M. Koo, H. W. Cho, T. J. Lee, K. G. Lee, H. J. Park, Y. K. Kim, H. J. Yoo, S. Y. Hwang, J. Park and B. G. Choi, ACS Appl. Mater. Interfaces, 2019, 11, 46165–46175 CrossRef CAS PubMed
.
- G. Liu, M. Qi, M. R. Hutchinson, G. Yang and E. M. Goldys, Biosens. Bioelectron., 2016, 79, 810–821 CrossRef CAS PubMed
.
- J. A. Stenken and A. J. Poschenrieder, Anal. Chim. Acta, 2015, 853, 95–115 CrossRef CAS PubMed
.
- S. Wang, S. Ota, B. Guo, J. Ryu, C. Rhodes, Y. Xiong, S. Kalim, L. Zeng, Y. Chen, M. A. Teitell and X. Zhang, Nano Lett., 2011, 11, 3431–3434 CrossRef CAS PubMed
.
- K. Bhavsar, A. Fairchild, E. Alonas, D. K. Bishop, J. T. La Belle, J. Sweeney, T. L. Alford and L. Joshi, Biosens. Bioelectron., 2009, 25, 506–509 CrossRef CAS PubMed
.
- M. Singh, J. Truong, W. B. Reeves and J. I. Hahm, Sensors, 2017, 17, 428 CrossRef PubMed
.
- Y. Liu, T. Kwa and A. Revzin, Biomaterials, 2012, 33, 7347–7355 CrossRef CAS PubMed
.
- Y. Gao, D. T. Nguyen, T. Yeo, S. B. Lim, W. X. Tan, L. E. Madden, L. Jin, J. Y. K. Long, F. A. B. Aloweni, Y. J. A. Liew, M. L. L. Tan, S. Y. Ang, S. D. O. Maniya, I. Abdelwahab, K. P. Loh, C.-H. Chen, D. L. Becker, D. Leavesley, J. S. Ho and C. T. Lim, Sci. Adv., 2021, 7, 9614 CrossRef PubMed
.
- M. S. Gohel, R. A. Windhaber, J. F. Tarlton, M. R. Whyman and K. R. Poskitt, J. Cardiovasc. Surg., 2008, 48, 1272–1277 Search PubMed
.
- E. L. Chiswick, E. Duffy, B. Japp and D. Remick, Methods Mol. Biol., 2012, 844, 15–30 CrossRef CAS PubMed
.
- Y. Song, P. Chen, M. T. Chung, R. Nidetz, Y. Park, Z. Liu, W. McHugh, T. T. Cornell, J. Fu and K. Kurabayashi, Nano Lett., 2017, 17, 2374–2380 CrossRef CAS PubMed
.
- V. A. Pham Ba, Y. M. Han, Y. Cho, T. Kim, B. Y. Lee, J. S. Kim and S. Hong, ACS Appl. Mater. Interfaces, 2018, 10, 17100–17106 CrossRef PubMed
.
- Z. Wang, Z. Hao, X. Wang, C. Huang, Q. Lin, X. Zhao and Y. Pan, Adv. Funct. Mater., 2020, 31, 2005958 CrossRef
.
- J. R. Sempionatto, V. R. Montiel, E. Vargas, H. Teymourian and J. Wang, ACS Sens., 2021, 6, 1745–1760 CrossRef CAS PubMed
.
- S. Lee, B. Srinivasan, S. Vemulapati, S. Mehta and D. Erickson, Lab Chip, 2016, 16, 2408–2417 RSC
.
- A. K. Yousafzai, M. A. Rasheed and Z. A. Bhutta, J. Child. Psychol. Psychiatry., 2013, 54, 367–377 CrossRef PubMed
.
- V. Kumar, A. K. Sinha, H. P. Makkar, G. de Boeck and K. Becker, Crit. Rev. Food Sci. Nutr., 2012, 52, 899–935 CrossRef CAS PubMed
.
- B. A. Swinburn, I. Caterson, J. C. Seidell and W. P. James, Public Health Nutr., 2004, 7, 123–146 CrossRef CAS PubMed
.
- B. M. Popkin and P. Gordon-Larsen, Int. J. Obes. Relat. Metab. Disord., 2004, 28(Suppl 3), S2–S9 CrossRef PubMed
.
- C. J. Blake, Anal. Bioanal. Chem., 2007, 389, 63–76 CrossRef CAS PubMed
.
- P. Jin, L. Xia, Z. Li, N. Che, D. Zou and X. Hu, J. Pharm. Biomed. Anal., 2012, 70, 151–157 CrossRef CAS PubMed
.
- M. M. Phillips, Anal. Bioanal. Chem., 2015, 407, 2965–2974 CrossRef CAS PubMed
.
- A. Gliszczyńska-Świgło and I. Rybicka, Food Anal. Methods, 2014, 8, 139–146 CrossRef
.
- A. M. Mohan, B. Brunetti, A. Bulbarello and J. Wang, Analyst, 2015, 140, 7522–7526 RSC
.
- J. Zhao, H. Y. Y. Nyein, L. Hou, Y. Lin, M. Bariya, C. H. Ahn, W. Ji, Z. Fan and A. Javey, Adv. Mater., 2021, 33, 2006444 CrossRef CAS PubMed
.
- M. S. Brown, B. Ashley and A. Koh, Front. Bioeng. Biotechnol., 2018, 6, 47 CrossRef PubMed
.
- S. Li, P. Renick, J. Senkowsky, A. Nair and L. Tang, Adv. Wound Care, 2021, 10, 317–327 CrossRef PubMed
.
- J. M. Moon, H. Teymourian, E. De la Paz, J. R. Sempionatto, K. Mahato, T. Sonsa-Ard, N. Huang, K. Longardner, I. Litvan and J. Wang, Angew. Chem., Int. Ed., 2021, 60, 19074–19078 CrossRef CAS PubMed
.
- E. Vargas, E. Povedano, S. Krishnan, H. Teymourian, F. Tehrani, S. Campuzano, E. Dassau and J. Wang, Biosens. Bioelectron., 2020, 167, 112512 CrossRef CAS PubMed
.
- W. Tang, L. Yin, J. R. Sempionatto, J. M. Moon, H. Teymourian and J. Wang, Adv. Mater., 2021, 33, 2008465 CrossRef CAS PubMed
.
Footnote |
| † The authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |