Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reconsidering terms for mechanisms of polymer growth: the “step-growth” and “chain-growth” dilemma
Chin Han
Chan
 a,
Jiun-Tai
Chen
b,
Wesley S.
Farrell
a,
Jiun-Tai
Chen
b,
Wesley S.
Farrell
 c,
Christopher M.
Fellows
de,
Daniel J.
Keddie
c,
Christopher M.
Fellows
de,
Daniel J.
Keddie
 f,
Christine K.
Luscombe
f,
Christine K.
Luscombe
 g,
John B.
Matson
g,
John B.
Matson
 *h,
Jan
Merna
i,
Graeme
Moad
*h,
Jan
Merna
i,
Graeme
Moad
 j,
Gregory T.
Russell
j,
Gregory T.
Russell
 k,
Patrick
Théato
k,
Patrick
Théato
 *l,
Paul D.
Topham
*l,
Paul D.
Topham
 m and
Lydia
Sosa Vargas
m and
Lydia
Sosa Vargas
 n
n
aFaculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
bNational Yang Ming Chiao Tung University, Department of Applied Chemistry, Hsinchu, 30010, Taiwan
cUnited States Naval Academy, Chemistry Department, Annapolis, Maryland 21402, USA
dThe University of New England, Armidale, NSW 2351, Australia
eDesalination Technologies Research Institute, Saline Water Conversion Corporation, Al Jubail 31951, Kingdom of Saudi Arabia
fSchool of Sciences, University of Wolverhampton, Wolverhampton, WV1 1LY, UK
gOkinawa Institute of Science and Technology (OIST), 1919-1 Tancha, Onna-son, Okinawa 904-0495, Japan
hVirginia Tech, Department of Chemistry and Macromolecules Innovation Institute, Blacksburg, Virginia 24061, USA. E-mail: jbmatson@vt.edu
iUniversity of Chemistry and Technology Prague, Department of Polymers 166 28, Prague 6, Czech Republic
jCSIRO Manufacturing, Clayton, Victoria 3168, Australia
kSchool of Physical and Chemical Sciences, University of Canterbury, Private Bag 4800, Christchurch 8140, New Zealand
lKarlsruhe Institute of Technology, Institute for Chemical Technology and Polymer Chemistry, Engesser Str. 18, D-76131 Karlsruhe, Germany. E-mail: patrick.theato@kit.edu
mAston Institute of Materials Research, School of Engineering and Applied Science, Aston University, Birmingham, B4 7ET, UK
nSorbonne Université, Équipe Chimie des Polymères, Paris, 75005, France
First published on 5th April 2022
Abstract
The terms “step-growth polymerization” and “chain-growth polymerization” are used widely in both written and oral communications to describe the two main mechanisms of polymer growth. As members of the Subcommittee on Polymer Terminology (SPT) in the Polymer Division of the International Union of Pure and Applied Chemistry (IUPAC), we are concerned that these terms are confusing because they do not describe the fundamental differences in the growth of polymers by these methods. For example, both polymerization methods are comprised of a series of steps, and both produce polymer chains. In an effort to recommend comprehensive terms, a 1994 IUPAC Recommendation from the then version of SPT suggested polycondensation and polyaddition as terms for the two variants of “step-growth polymerization”, and similarly chain polymerization and condensative chain polymerization for the two variants of “chain-growth polymerization”. However, these terms also have shortcomings. Adding to the confusion, we have identified a wide variety of other terms that are used in textbooks for describing these basic methods of synthesizing polymers from monomers. Beyond these issues with “step-growth” and “chain-growth”, synthesis of polymers one monomer unit at a time presents a related dilemma in that this synthetic strategy is wholly encompassed by neither of the traditional growth mechanisms. One component of the mission of IUPAC is to develop tools for the clear communication of chemical knowledge around the world, of which recommending definitions for terms is an important element. Here we do not endorse specific terms or recommend new ones; instead, we aim to convey our concerns with the basic terms typically used for classifying methods of polymer synthesis, and in this context we welcome dialogue from the broader polymer community in a bid to resolve these issues.
Introduction
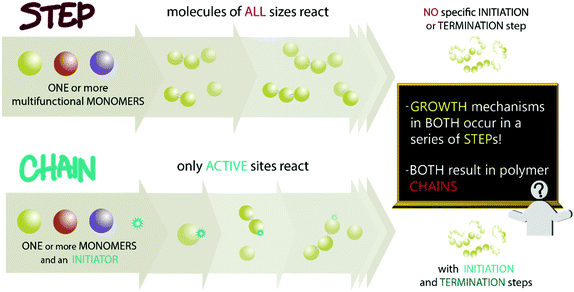
Nearly all polymers that are chemically synthesized from monomers can be grouped into two classes based on their mechanism of polymer growth. The terms “step-growth” and “chain-growth” are currently used widely by the polymer chemistry community to classify these mechanisms of polymer growth.1 In brief, “step-growth” typically refers to polymers that are synthesized from one (or more) type(s) of multifunctional monomer(s) where at least bifunctionality is required, with growth occurring between monomers, oligomers, or polymers of any length. An example is the synthesis of linear polyamides from diamines and dicarboxylic acids. “Chain-growth” generally describes polymers that increase in molar mass by a chain reaction process of monomers adding to polymeric active sites; active sites are typically created through inclusion of an external initiator in the polymerization reaction. An example is the synthesis of polystyrene from styrene and a radical initiator. The potential confusion created from these traditional terms is immediately apparent: both growth mechanisms require a series of (elementary) steps, and both produce polymer chains. Also, it is a tautology to say “-growth polymerization” because there cannot be polymerization without growth. Fig. 1 shows these two mechanisms of growth graphically for the synthesis of linear polymers, highlighting elements of our concerns with the terms “step-growth” and “chain-growth”.Recommending consistent and logical terminology to the global chemistry community is one goal of the International Union of Pure and Applied Chemistry (IUPAC). The IUPAC Subcommittee on Polymer Terminology (SPT), a body that dates back to 1952 in one form or another (referred to here as SPT, even when we technically mean an earlier version of this subcommittee with a different name),2 seeks to provide guidance and recommendations on issues of terminology and nomenclature related to polymers. This goal is carried out mostly through publications recommending definitions of terms and systems of nomenclature that can be applied and understood globally.
As current members of and contributors to SPT, in this discussion we seek to notify the community of our concerns with terms used to describe mechanisms of polymer growth in the scientific literature and in textbooks. A messy situation currently exists where a wide variety of terminology is used, which is obviously undesirable for such a fundamental matter. Common terms include “step-growth” and “chain-growth”, but also many others detailed below, including some terms proposed by this subcommittee that fail to meet our standard of clear and self-consistent terminology.
A 1974 document from this subcommittee defined the terms “addition polymerization” (polymerization by a repeated addition process) and “condensation polymerization” (polymerization by a repeated condensation process).3 In a 1994 Recommendations document from SPT, it was recognized that “addition polymerization” and “condensation polymerization” only distinguish between polymerizations in which a small-molecule by-product (a condensate) is produced and those where one is not.4 In other words, these terms do not identify a mechanism of polymer growth. Thus, the terms polyaddition and polycondensation were recommended for polymerizations in which the growth of polymer chains proceeds by addition reactions or condensation reactions between molecules of all degrees of polymerization (i.e., “step-growth”), usually in a non-chain reaction. The terms chain polymerization and condensative chain polymerization were recommended as terms for polymers made in a reaction where monomers react only with active polymer chains via a chain reaction pathway (i.e., “chain-growth”). This is summarized in Fig. 2, reproduced from the 1994 document, which deprecated use of the terms “chain-growth” and “step-growth”.
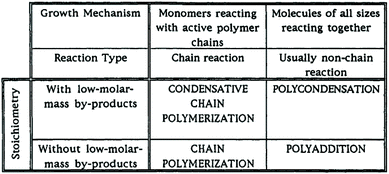 | ||
| Fig. 2 Recommendations on polymerization terminology made by SPT in 1994.4 | ||
While the four terms suggested in 1994 are more comprehensive and without doubt superior to preceding terminology, there have been four issues with these terms from the outset:
(1) The terms polycondensation and polyaddition sound very similar to the historical terms “condensation polymerization” and “addition polymerization”, proposed by Carothers nearly a century ago.5 Carothers’ terms represented the first attempt at terminology in the present context, and these terms are still widely used today (see below), even though their shortcomings became evident almost immediately; for example, polyurethanes, first made in 1937 in a “step-growth” manner,6 involve no condensate in their preparation. This similarity between polyaddition and “addition polymerization” has created significant confusion because addition is employed quite differently in these two sets of terms, having migrated from meaning “chain-growth” under Carothers to “step-growth” under IUPAC.
(2) Furthermore, polyaddition is itself a questionable term. IUPAC has defined the term addition reaction to be a chemical reaction of two or more reacting molecular entities, resulting in a single reaction product containing all atoms of all components with formation of two chemical bonds and a net reduction in bond multiplicity in at least one of the reactants.7 Thus a chain polymerization (as defined in 1994) is a series of addition reactions, while polyaddition has a limited definition that excludes chain polymerization.
(3) In the 1994 document,4chain polymerization was implicitly proposed as a term covering two situations: (i) a generic term encompassing all polymerizations that proceed via “chain growth”-type growth mechanisms, i.e., a chain reaction in which the growth of a polymer chain proceeds exclusively by reaction(s) between monomer(s) and reactive site(s) on the polymer chain with regeneration of the reactive site(s) at the end of each growth step, and (ii) a specific subclass of chain polymerizations in which there is no condensate, which is the case in most chain polymerizations. The function of the term chain polymerization as both a generic term and a specific term has created some confusion. In contrast, in the case of “step-growth”-type growth mechanisms, no generic term was proposed, and filling this void is probably one reason why the term “step-growth” is still widely used. In internal SPT discussions we have used “non-chain polymerization” to cover polymerizations that proceed in a “step-growth” manner, but this is not ideal in that it defines these polymerizations by what they are not rather than providing a definition that alludes to their common characteristics.
(4) Another problem of a similar nature is that all forms of polymerization generate polymer chains, but the term chain polymerization might be taken to imply that only such polymerizations do so, and that polyaddition and polycondensation do not. The issue here is that the word “chain” has multiple meanings (in this case macromolecule and chain reaction, respectively). As stated above, in the current IUPAC definition the chain in chain polymerization refers to polymerization occurring through a chain reaction process; this may not be immediately obvious. This overlap in meaning creates ambiguity and therefore uncertainty, especially for non-native speakers of English. The current terminology also causes problems when considering reactions such as the polymerization of a dithiol and an α,ω-diene (a thiol–ene polymerization): the polymer forms in a “step-growth” type growth mechanism, but in a radical chain reaction process.
In addition to the specific points listed above, there are additional issues with the 1994 terms that have arisen in the intervening quarter century due to developments in polymer synthesis. For example, there are new polymerization growth mechanisms that fall outside the two traditional categories, and are therefore not covered by any current terminology, recommended by IUPAC or otherwise (detailed below). There are also methods to synthesize polymers from monomers that have been known for decades that do not fall cleanly into either of these traditional categories.
In view of the above situation, it is not surprising that the 1994 terms have not been widely adopted by the polymer community. In fact, we have found no textbooks that employ all four terms recommended in the 1994 document (see below). For this reason, many of us find ourselves using the terms “step-growth” and “chain-growth” in our publications and classes, even though IUPAC has never endorsed these terms, and despite the confusion this situation causes to students learning polymer chemistry. Here, we attempt to outline the dilemmas caused by the terms “step-growth” and “chain-growth” and their various synonyms and subclasses.
This contribution is not a recommendation of terms we think should be used; rather, we simply aim to make clear to the community why we think all present terminology is problematic. No set of terms is perfect, but we believe there must be a better system than the current options. Ideally, we would like to avoid the many shortfalls mentioned above, at the same time employing descriptive accuracy and finding simplicity that will be attractive to our diverse community. That this can be achieved is evidenced, for example, by the widespread replacement of the illogical term ‘polydispersity index’ by the more logical ‘dispersity’ in the decade since publication of IUPAC Recommendations (by SPT) on this matter in 2009.8
Discussion
Historical development of terms
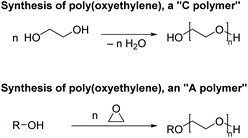
Although Staudinger attempted to classify different types of polymerization processes,9 it was Wallace H. Carothers who first recognized the mechanistic distinction that would eventually lead to the “step-growth”/“chain-growth” classifications. Carothers was dealing with terms for polymers prepared by the two mechanisms in a pioneering period when even the definition of the general term “polymer” was still under discussion.10 At that time some definitions of polymer stated that a polymer and its monomer must have the same atoms in the same proportions.9,11 When introducing his theory of the preparation of high-molar-mass polymers by reaction of monomers bearing functional groups, Carothers proposed the terms “addition or A polymers” for polymers in which the repeat unit has the same molecular formula as the monomer, and “condensation or C polymers” where the repeat unit is different from the monomer(s). Further, he defined two types of mechanisms of polymer growth: (i) “addition polymerizations” leading to A polymers, and (ii) “condensation polymerizations” leading to C polymers. The problem with these terms would soon become clear—by first classifying polymer structures as “A” or “C”, then deriving the terms “addition polymerization” and “condensation polymerization” for their growth mechanisms, a direct correspondence between polymer structure and mechanism of polymer growth was created. This system does not effectively capture polymers that can be synthesized in different ways. For example, poly(oxyethylene) is a C polymer when synthesized by polycondensation of ethane-1,2-diol, but an A polymer if it is prepared by ring-opening polymerization of oxirane (Fig. 3).Soon after Carothers there was the discovery by Otto Bayer at I. G. Farbenindustrie of the formation of polyurethanes from the reaction of diols and diisocyanates.6,12 This polymerization proceeds like a condensation polymerization, but no low-molar-mass by-product is released, meaning that technically it is an “addition polymerization” according to the terminology of Carothers. In view of this difficulty, Bayer named his process a “polyaddition”. This led to confusion because of the similarity of the terms “addition polymerization” and “polyaddition”.13
Despite these difficulties, the Carothers terminology was adopted for a long time, as reflected by it being the basis of the 1974 IUPAC recommendation,3 as already mentioned. Of greatest significance was this terminology being used in Flory's influential 1953 textbook.14
Flory continued to use the terms “addition polymers” and “condensation polymers”, as well as “addition polymerization” and “condensation polymerization”, but he was well aware of inescapable problems with them. For example, he wrote: “Whether or not the structural unit differs in composition from the monomer from which it is derived is of no particular significance. The principal justification for the differentiation between condensation and addition polymers (and polymerizations) lies in the marked contrast between the processes by which they are formed”. And elsewhere: “The original Carothers distinction between addition and condensation polymers, if applied quite literally, oftentimes fails to serve the desired purpose”. Flory also recognized the problem posed by polyurethanes and the like, writing (his italics) “a polymerization process which proceeds by a reaction between pairs of functional groups with the formation of a type of interunit functional group not present in the monomer(s) will be regarded as a condensation polymerization”. In a polymerization sense this is reasonable, but in a broader chemistry sense it is not, because polyurethane formation does not involve “condensation” as it is generally understood by all chemists. [IUPAC defines the term condensation reaction as a (usually stepwise) reaction in which two or more reactants (or remote reactive sites within the same molecular entity) yield a single main product with accompanying formation of water or of some other small molecule, e.g. ammonia, ethanol, acetic acid, hydrogen sulfide.15]
Flory supported his recommendation as follows: “It is thus appropriate to broaden the definition of condensation polymers … to include also those polymers which on chemical degradation (e.g., by hydrolysis) yield monomeric end products differing in composition from the structural units”. Flory does this because he wants to avoid “the confusion which would arise” if the one polymer were to be categorized in different ways depending on how it was made, e.g., the poly(oxyethylene) example above. This means that, for example, nylon-6 made by ring-opening polymerization of ε-caprolactam, a chain-polymerization process, would still be termed a condensation polymer by Flory. He recognized the incongruity of this, giving the example of poly(lactic acid), but nevertheless still felt compelled to have a classification primarily based on polymer structure rather than mechanism.
The mechanism of chain polymerization was elucidated by Norrish and Brookman in 1939.16 According to a search of the Chemical Abstracts Service database using SciFinder™, the term chain polymerization (not “chain-growth polymerization”) was first introduced to refer to these reactions by Hoshino and Iwakura in 1947.17 It is not clear when the term “step(-growth)” first appeared, but Elias writes that it was in response to the problems in the polymer-based classification of Carothers:13 “It turned out later that the true distinguishing factor was … the growth steps. …Organic chemists therefore started to refer to ‘condensation polymerizations’ as ‘step-growth polymerizations’ since the reaction products could be easily isolated and reacted again after several ‘steps’ whereas those of the known ‘addition polymerizations’ could not”. In other words, the slow rate of “step-growth” polymerizations usually allows for them to be easily stopped and their intermediates isolated, similar to “steps” in preparative synthesis of low-molar-mass organic compounds. Therefore, this use has nothing to do with steps in the mechanistic sense, a fact not widely appreciated today. In this sense this term is “an unlucky choice of words”.13
Unlucky or not, the term “step-growth” has stuck, becoming part of the dominant classification system of “step-growth polymerization” and “chain-growth polymerization”. According to a SciFinder™ search, these terms do not appear in the abstracts of papers in the chemical literature before their use in a well-known 1967 textbook by Robert Lenz,18 although in the textbook the author makes no claim to be introducing a new terminology.
Terms used in current textbooks
Perhaps because of the problems with all existing terms, and despite the 1994 classification system proposed by SPT, terminology in textbooks continues to vary widely. It is largely pointless to suggest terminology if it is not adopted. We therefore investigated what terms people actually use for basic classification of polymerization reactions. We examined the terms used in approximately 40 textbooks, including multiple editions of some. This allowed us to gauge the influence of the definitions recommended by SPT in 1994 over time. We present our findings in two categories.First, Table 1 gives terms used in a selection of textbooks on general chemistry and organic chemistry (as indicated in the Category column). The selection is not intended to be comprehensive but represents a range of textbooks that we use in our classes across the world. A careful look at these textbooks reveals that despite most of these books being published in the last decade, the terms recommended in 1994 are completely absent. Some authors discuss polymerization but do not categorize by specific types of polymerization methods, perhaps in part due to confusion over which terminology to use. In terms of book categories, it is evident that general chemistry textbooks almost all retain the 1930s “addition/condensation polymerization” classification. With organic chemistry textbooks there is a strong preference for “chain-growth” and “step-growth”.
| Author(s) | Year (edition) | Category | Terminology employed |
|---|---|---|---|
| a The relevant chapters from the first edition were removed for the second edition. They remain available as an electronic resource from the publisher. | |||
| Blackman et al.19 | 2012 (2nd) | General | Addition or chain-growth, condensation or step-growth |
| Burrows et al.20 | 2017 (3rd) | General | Addition polymerization, condensation polymerization |
| Chang21 | 2007 (7th) | General | Addition reactions, condensation reactions |
| Housecroft & Constable22 | 2010 (4th) | General | Addition polymerization |
| Kotz et al.23 | 2018(10th) | General | Addition polymers, condensation polymers |
| Mahaffy et al.24 | 2014 (2nd) | General | Addition polymers, condensation polymers |
| McMurry et al.25 | 2015 (7th) | General | None |
| Bruice26 | 2014 (7th) | Organic | Chain-growth polymerization, step-growth polymerization |
| Bruice27 | 2016 (8th) | Organic | Chain-growth polymerization, step-growth polymerization |
| Carey & Sundberg28,29 | 1990 (3rd) | Organic | None |
| Clayden et al.30 | 2001 (1st) | Organic | Polymerizations by carbonyl substitution reactions, polymerization by electrophilic aromatic substitution, polymerization by the SN2 reaction, polymerization by nucleophilic attack on isocyanates, polymerization of alkenes |
| Clayden et al.31 | 2012 (2nd) | Organic | Nonea |
| Karty32 | 2014 | Organic | Chain-growth polymerization, step-growth polymerization |
| Okuyama & Maskill33 | 2013 (1st) | Organic | None |
| Vollhardt & Schore34 | 2014 (7th) | Organic | None |
Overall, it is clear that in undergraduate chemistry textbooks there exists a somewhat chaotic situation regarding classifications: there is no dominant terminology for basic mechanisms of polymer growth, and IUPAC-recommended terminology is absent. This is unlikely to be because it is rejected, but almost certainly because it is not known. This situation is undesirable in several ways. First, it propagates the use of logically flawed terminology. Second, it means that students are confronted by different terms for the same thing, depending upon the textbook used. Finally, how should translators of books into languages other than English deal with a non-uniform situation like this?
It is reasonable to expect more uniform usage of terminology from authors of textbooks on polymer science. Here we examined a selection of these with respect to which basic polymerization terms are used. Our survey is presented in Table 2, again organized by author last name. Rather than attempting to categorize polymer science books, we instead have given their titles, and these cover a full spectrum from synthetic chemistry to engineering and processing.
| Author(s) | Year (edition) | Title | Terminology employed |
|---|---|---|---|
| Carraher39 | 2017 (4th) | Introduction to polymer chemistry | Addition polymerization, step-reaction polymerization |
| Carraher40 | 2017(10th) | Polymer chemistry | Addition polymerization, step-reaction polymerization |
| Cowie41 | 1991 (2nd) | Polymers: chemistry and physics of modern materials | Addition polymerization, step-growth polymerization; both together referred to as “chain growth mechanism” |
| Cowie42 | 2007 (3rd) | Polymers: chemistry and physics of modern materials | Addition polymerization, step-growth polymerization; “chain growth” used to mean both forms together and addition individually |
| Dotson et al.43 | 1995 (1st) | Polymerization process modeling | Chainwise, stepwise |
| Elias35 | 1997 (1st) | An introduction to polymer science | Chain-growth polymerization, step-growth polymerization; polyelimination, chain polymerization, polycondensation, polyaddition |
| Elias13 | 2005 (2nd) | Macromolecules, vol. 1 – structure and properties | Chain-growth polymerization, step-growth polymerization; polyelimination, chain polymerization, polycondensation, polyaddition |
| Hiemenz & Lodge44 | 2007 (2nd) | Polymer chemistry | Addition polymers/chain-growth polymerization, condensation polymers/step-growth polymerization |
| Koltzenburg et al.45 | 2017 (1st) | Polymer chemistry | Chain-growth polymerization, step-growth polymerization |
| Nicholson46 | 2017 (5th) | The chemistry of polymers | Chain polymerization, step polymerization |
| Novak47 | 1995 (1st) | Organic polymer chemistry: a primer | Chain-growth, step-growth (said to be synonymous with condensation) |
| Odian48 | 1991 (3rd) | Principles of polymerization | Chain, step (shortenings of chain-reaction, step-reaction) |
| Odian38 | 2004 (4th) | Principles of polymerization | Chain polymerization, step polymerization |
| Painter & Coleman49 | 1994 (1st) | Fundamentals of polymer science | Chain/addition polymerization, step-growth polymerization |
| Painter & Coleman50 | 2008 (1st) | Essentials of polymer science and engineering | Chain/addition polymerization, step-growth polymerization |
| Ravve51 | 2012 (3rd) | Principles of polymer chemistry | Chain-growth polymerization, step-growth polymerization |
| Rudin52 | 1982 (1st) | The elements of polymer science and engineering | Chain-growth polymerization, step-growth polymerization (polycondensation sometimes instead) |
| Rudin & Choi53 | 2012 (3rd) | The elements of polymer science and engineering | Chain-growth polymerization, step-growth polymerization (polycondensation sometimes instead) |
| Stevens54 | 1999 (3rd) | Polymer chemistry: an introduction | Chain-reaction condensation, chain-reaction polymerization, step-reaction polymerization, step-reaction addition |
| Walton & Lorimer55 | 2000 (1st) | Polymers | Chain polymerization, step-growth polymers |
| Young & Lovell36 | 1991 (2nd) | Introduction to polymers | Chain polymerization, step polymerization; polycondensation, polyaddition |
| Young & Lovell37 | 2011 (3rd) | Introduction to polymers | Chain polymerization, step polymerization; polycondensation, polyaddition |
The first and overwhelming conclusion from Table 2 is that there is no consensus among polymer science textbook writers regarding which terms should be used, nor are there any preferred sets of terms. Invariably the word “step” is used, but in a variety of different ways: “step-growth”, “stepwise”, “step-reaction” or just plain “step”. Sometimes it is partnered with the same variant of “chain” (e.g. “chainwise” with “stepwise”), but often it is paired with “addition”.
A few authors discuss the 1994 recommendations from SPT. For example, Elias includes a discussion of IUPAC-recommended terms, and he adopts all the terms of Fig. 2 aside from condensative chain polymerization, which he spurns on the grounds that “it is illogical to label one subclass with an adjective (condensative chain polymerization) but not the other (chain polymerization)”. We agree with Elias on this point, noted above as issue (3) in the introduction. Instead, Elias proposes “polyelimination”,13,35 which nicely complements polycondensation and polyaddition in a linguistic sense, but seems flawed in several ways: (1) such processes do not meet the usual definition of an elimination reaction;15 (2) “polyelimination” sounds like a degradation process, and indeed the different process of side-chain elimination from polymers is sometimes called polyelimination;13 (3) a polycondensation could for the same reason be termed a polyelimination. It is telling that Elias explains how the term “step-growth polymerization” is deeply defective, and yet he used it throughout his 1997 textbook due to lack of a better alternative, but then dropped it in his 2005 textbook, opting for the IUPAC-recommended terms polyaddition and polycondensation instead. Indeed, Elias long ago recognized the terminology dilemma we discuss here.
Young and Lovell also hint at IUPAC influence in two ways: (1) they write of a “modern preference” to use chain and step without “growth”, implying that this is a matter of taste; (2) they introduce the step-polymerization subclasses of polycondensation and polyaddition, in accordance with Fig. 2.36,37 However, they make no mention of IUPAC recommendations, and indeed their usage predates the 1994 recommendations.
Finally, Odian suggests in his latest edition, published in 2004,38 that the 1994 recommendations from this subcommittee suggested “polycondensation” as a replacement for all types of “step polymerization”, neglecting to mention the recommended use of polyaddition in polymerizations that follow “step-growth” kinetics but lack a condensate (e.g., polyurethane synthesis). He uses the IUPAC-recommended term chain polymerization but does not mention condensative chain polymerization.
Several authors in Table 2 use “polycondensation” as a synonym for “step(-growth) polymerization”, and thereby fail to acknowledge that such polymerizations need not involve condensation. Ironically, nearly all textbooks do the one thing that SPT neglected to do in 1994—provide generic terms. The problem is that they have all used some combination of the old, flawed terms. We strive to remedy this problem.
Problems arising from translations of “step-growth” and “chain-growth” into other languages
The official language of IUPAC is English, so we focus here on terminology in English. However, it is worth noting that the terms “step-growth” and “chain-growth” present additional problems for non-native English speakers. While difficulties and ambiguities arise with translations of many technical terms, we find that a clear and precise definition in English tends to reduce problems in translation. In contrast, the lack of clarity and precision in “step-growth” and “chain-growth” in English appears to become further magnified when translated into other languages.For example, in German “step” is translated as “Stufe”, which can also mean “stair”, although in the context of a reaction step the term “Schritt” is used. The Czech language has the same translation, where “step” is translated as “stupeň” but “krok” is used for a reaction step. A related problem arises in Spanish, where the word “step” has been translated into “etapas” (stages). The word “etapas” implies a sequential evolution and can result in thinking it is related to a chain polymerisation reaction. Problems with the word “chain” arise in other languages such as French, where “enchainment” is used to describe the concept of polymerisation (enchaînement = linkage). This linkage process implies a sequence of steps (étapes), meaning steps or stages, leading to confusion when the two basic polymerization mechanisms are discussed.
Similar problems arise in languages outside of the Indo-European family of languages. In Chinese, both the meanings of “step by step” ( ) and “stair-like” (
) and “stair-like” (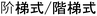 ) are used in the translation of “step-growth”. For “chain-growth”, both the meanings of “long chain” (
) are used in the translation of “step-growth”. For “chain-growth”, both the meanings of “long chain” (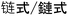 ) and “a sequence of reactions” (
) and “a sequence of reactions” (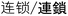 ) are used in the translation. Even for the same meaning, different Chinese characters are also used, making the situation even more complicated. In Japanese, step-growth translates directly as step-growth (
) are used in the translation. Even for the same meaning, different Chinese characters are also used, making the situation even more complicated. In Japanese, step-growth translates directly as step-growth ( , dankai-seichou or
, dankai-seichou or 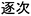 , chikuji), and chain-growth translates as sequential (
, chikuji), and chain-growth translates as sequential (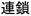 , rensa). In the three related languages of Malay, Brunei, and Indonesian, “step-growth polymerization” is “pempolimeran/pempolimerisasi langkah”, where “langkah” translates as “footwork while walking.” “Chain polymerization” is “pempolimeran/pempolimerisasi rantai” where “rantai” means chain of strands. In other languages such as Thai, the English terms are often used but spelled phonetically, perhaps avoiding some of the confusion generated in other languages when translating directly.
, rensa). In the three related languages of Malay, Brunei, and Indonesian, “step-growth polymerization” is “pempolimeran/pempolimerisasi langkah”, where “langkah” translates as “footwork while walking.” “Chain polymerization” is “pempolimeran/pempolimerisasi rantai” where “rantai” means chain of strands. In other languages such as Thai, the English terms are often used but spelled phonetically, perhaps avoiding some of the confusion generated in other languages when translating directly.
This brief analysis shows how the currently dominant terminology can lead to various problems when translated into different languages. However, we stress that the problem here is fundamentally one of consistent use of logically sensible, English-language terms. We anticipate that more sensible terms in English could avoid some of the problems that arise in other languages.
Polymerizations that lie outside of traditional growth mechanisms
There are some reactions that unambiguously generate macromolecules but cannot be fitted into any of the four classes of Fig. 2. Equally, there are other polymerization processes that can be fit into this categorization only by unnatural extension of the meaning of terms inconsistent with their IUPAC definitions. Thus, although IUPAC defines polymerization as the process of converting a monomer or a mixture of monomers into a polymer, there are situations where, under the current terminology of Fig. 2, monomers can be converted into polymer without the process formally being in a class of polymerization. This clearly warrants some consideration.The first situation, reactions that produce polymers but do not fit into any class in Fig. 2, arises from iterative processes. For example, a laboratory solid-phase peptide synthesis (SPPS) unambiguously generates a macromolecule through reaction at a specific site on a macromolecule, with generation of low-molar-mass by-products, but by a repetitive series of coupling and deprotection reactions.56 No chain reactions occur, so SPPS cannot be labelled a chain polymerization, but neither is it a polycondensation because reactions do not occur between molecules of all degrees of polymerization. A related example is reversible addition–fragmentation chain transfer single-unit monomer insertion (RAFT SUMI), where a chain reaction takes place between propagating species and monomers without generation of low-molar-mass by-products to add a single monomer unit to an existing oligomer or polymer.57 However, it cannot be labelled a chain polymerization because no single RAFT SUMI reaction step leads to formation of a macromolecule. Rather, much like SPPS, RAFT SUMI is a sequence of separately conducted (chain) reactions, each of which appends a single monomer unit to the polymer chain. Analogous problems in terminology arise in dendrimers, which are also synthesized by iterative processes.58
The second situation, polymerizations which require extending the definitions in Fig. 2, arises because condensation and addition, as defined by the IUPAC recommendations of 1994,15 do not exhaust the possible ways of generating chemical bonds. If addition is defined as consistent with chemical intuition as a reaction giving a net reduction in bond multiplicity in at least one of the reactants, it is clear that there could also be polymerization reactions in which bond multiplicity remains constant59 or increases.60 Polymerization can also occur via colligation, the generation of a bond by the combination of two radicals, or coordination, where the two electrons in a newly formed bond come from only one of the precursor molecules. An example of polymerization by colligation is Gilch polymerization and related reactions,61,62 which proceed predominantly through the reaction of biradicals. Gilch polymerization may proceed to a large degree by reactions between molecules of any degree of polymerisation in which bonds are formed by radical combination.63
In these two situations, the current terminology can only be made to fit by disregarding part of the definition (interpreting chain reaction so broadly as to make it meaningless) or interpreting it in a different way in polymer chemistry than in physical organic chemistry (using a truncated form of the IUPAC definition of addition that omits the stipulation on bond multiplicity).
Moving forward
In 2019, IUPAC approved a project with the goal of recommending a solution to the terminology problems discussed here. Many of the authors on this contribution are members of the task group for this project.64 Specifically, we seek to provide an umbrella term that captures the current IUPAC endorsed terms of polycondensation and polyaddition, which our analysis here indicates could be a reason for the ongoing terminology problem. We also aim to provide a similar structure, including an umbrella term, for chain polymerizations that encompasses those with and without condensates. Finally, we will suggest terms for reactions that generate polymers but currently cannot be classified using any of the existing polymerization terms. We welcome input from the community on this matter.Conclusion
Here we have outlined our concerns with the terms “step-growth” and “chain-growth”, which remain in use, along with several related terms, despite their flaws and the deprecation of their use by IUPAC in 1994. An analysis of terms used historically and in current textbooks was particularly illuminating: despite a clear understanding for many decades of the two types of basic mechanisms of polymer growth, we as a community still have not agreed on terms to describe these two cases. The present use of similar-sounding terms with different meanings adds to the confusion, and this lack of clear and logical terminology causes problems in translating the terms from English into other languages. Furthermore, there are examples of reactions or processes in the field of polymer synthesis that produce polymers, but there are no terms that describe these polymerizations. As a group of polymer scientists, we are working to find a solution. We welcome input from the community as we attempt to remedy these dilemmas. Please let us know your thoughts by emailing us at polymer.terminology@iupac.org. Comments will be read until the end of 2022.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge IUPAC for support of this work through project 2019-027-1-400. We thank the members of SPT for helpful discussions and critical feedback in the preparation of this manuscript.Notes and references
- Here we stylize current IUPAC terms, used as intended in IUPAC documents, in italics; we place non-IUPAC terms in quotes.
- R. Jones, Chem. Int., 2017, 39, 4–6 Search PubMed
.
- IUPAC Comm. on Macromolecular Nomenclature, Pure Appl. Chem., 1974, 40, 477–492 CrossRef
.
- I. Mita, R. F. T. Stepto and U. W. Suter, Pure Appl. Chem., 1994, 66, 2483–2486 CAS
.
- W. H. Carothers, Chem. Rev., 1931, 8, 353–426 CrossRef CAS
.
- I. G. Farbenindustrie AG, Patent, 728981, 1937 Search PubMed
.
-
IUPAC. Compendium of Chemical Terminology, ed. A. D. McNaught and A. Wilkinson, Blackwell Scientific Publications, Oxford, 2nd edn (the “Gold Book”), 1997, DOI:10.1351/goldbook.A00133
.
- F. T. S. Robert, Pure Appl. Chem., 2009, 81, 351–353 CrossRef
.
- H. Staudinger, Ber. Dtsch. Chem. Ges. A/B, 1920, 53, 1073–1085 CrossRef
.
- W. H. Carothers, J. Am. Chem. Soc., 1929, 51, 2548–2559 CrossRef CAS
.
-
K. Hess, Die Chemie der Zellulose und ihrer Begleiter, Akademischen Verlagsgesellschaft m. b. H., Leipzig, 1928 Search PubMed
.
- O. Bayer, Angew. Chem., 1947, A59, 257–272 CrossRef CAS
.
-
H.-G. Elias, Macromolecules: Volume 1: Chemical Structures and Syntheses, Wiley, 2005 Search PubMed
.
-
P. J. Flory, Principles of polymer chemistry, Cornell University Press, Ithaca, N.Y., 1953 Search PubMed
.
- P. Muller, Pure Appl. Chem., 1994, 66, 1077–1184 CrossRef
.
- R. G. W. Norrish and E. Brookman, Proc. R. Soc. London, Ser. A, 1939, 171, 147–171 CAS
.
- T. Hoshino and Y. Iwakura, Proc. Jpn. Acad., 1947, 23, 9–11 CrossRef CAS
.
-
R. W. Lenz, Organic Chemisty of Synthetic High Polymers, John Wiley & Sons, New York, 1st edn, 1967 Search PubMed
.
-
A. Blackman, S. Bottle, S. Schmid, M. Mocerino and U. Wille, Chemistry, John Wiley & Sons, 2nd edn, 2012 Search PubMed
.
-
A. Burrows, J. Holman, A. Parsons, G. Pilling and G. Price, Chemistry3: Introducing Inorganic, Organic and Physical Chemistry, Oxford University Press, 3rd edn, 2017 Search PubMed
.
-
R. Chang, Chemistry, McGraw-Hill Higher Education, 9th edn, 2007 Search PubMed
.
-
C. E. Housecroft and E. C. Constable, Chemistry: An Introduction to Organic, Inorganic and Physical Chemistry, Prentice Hall, 4th edn, 2010 Search PubMed
.
-
J. C. Kotz, P. M. Treichel, J. Townsend and D. Treichel, Chemistry & Chemical Reactivity, Cengage Learning, 10th edn, 2018 Search PubMed
.
-
P. G. Mahaffy, J. C. Kotz, J. McMurry, R. B. Bucat, R. Tasker, P. Treichel and G. C. Weaver, Chemistry: Human Activity, Chemical Reactivity, Nelson Education Limited, 2nd edn, 2014 Search PubMed
.
-
J. E. McMurry, R. C. Fay and J. K. Robinson, Chemistry, Pearson Education, 7th edn, 2015 Search PubMed
.
-
P. Y. Bruice, Organic Chemistry, Pearson, 7th edn, 2014 Search PubMed
.
-
P. Y. Bruice, Organic Chemistry, Pearson Education, 8th edn, 2016 Search PubMed
.
-
F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry: Part A: Structure and Mechanisms, Springer US, 3 edn, 1990 Search PubMed
.
-
F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry: Part B: Reactions and Synthesis, Springer US, 3 edn, 1990 Search PubMed
.
-
J. Clayden, N. Greeves, S. Warren and P. Wothers, Organic Chemistry, Oxford University Press, 1st edn, 2001 Search PubMed
.
-
J. Clayden, N. Greeves and S. Warren, Organic Chemistry, OUP, Oxford, 2012 Search PubMed
.
-
J. Karty, Organic Chemistry: Principles and Mechanisms, International Student Edition, W. W. Norton, Incorporated, 2014 Search PubMed
.
-
T. Okuyama and H. Maskill, Organic Chemistry: A Mechanistic Approach, OUP Oxford, 1st edn, 2013 Search PubMed
.
-
K. P. C. Vollhardt and N. E. Schore, Organic Chemistry: Structure and Function, W. H. Freeman, 7th edn, 2014 Search PubMed
.
-
H.-G. Elias, An Introduction to Polymer Science, Wiley, 1997 Search PubMed
.
-
R. J. Young and P. A. Lovell, Introduction to Polymers, CRC Press, Boca Raton, London, 2nd edn, 1991 Search PubMed
.
-
R. J. Young and P. A. Lovell, Introduction to Polymers, CRC Press, London, 3rd edn, 2011 Search PubMed
.
-
G. Odian, Principles of Polymerization, Wiley, 4th edn, 2004 Search PubMed
.
-
C. E. Carraher, Introduction to Polymer Chemistry, CRC Press, 4th edn, 2017 Search PubMed
.
-
C. E. Carraher, Carraher's Polymer Chemistry, CRC Press, 10th edn, 2017 Search PubMed
.
-
J. M. G. Cowie, Polymers: Chemistry and Physics of Modern Materials, Taylor & Francis, 2nd edn, 1991 Search PubMed
.
-
J. M. G. Cowie and V. Arrighi, Polymers: Chemistry and Physics of Modern Materials, CRC Press, 3rd edn, 2007 Search PubMed
.
-
N. A. Dotson, R. Galvan, R. L. Laurence and M. Tirrell, Polymerization Process Modeling, Wiley, 1995 Search PubMed
.
-
P. C. Hiemenz and T. P. Lodge, Polymer Chemistry, CRC Press, 2nd edn, 2007 Search PubMed
.
-
S. Koltzenburg, M. Maskos, O. Nuyken, K. Hughes, R. Mülhaupt and K. Matyjaszewski, Polymer Chemistry, Springer Berlin Heidelberg, 2017 Search PubMed
.
-
J. Nicholson, The Chemistry of Polymers, Royal Society of Chemistry, 2017 Search PubMed
.
-
B. M. Novak, Organic Polymer Chemistry: A Primer, Saunders College Publishing, Fort Worth, 1995 Search PubMed
.
-
G. Odian, Principles of Polymerization, Wiley, 3rd edn, 1991 Search PubMed
.
-
P. C. Painter and M. M. Coleman, Fundamentals of Polymer Science: An Introductory Text, Technomic Pub. Co., Lancaster, Pa., 1st edn, 1994 Search PubMed
.
-
P. C. Painter and M. M. Coleman, Essentials of Polymer Science and Engineering, DEStech Publications, Incorporated, 2008 Search PubMed
.
-
A. Ravve, Principles of Polymer Chemistry, Springer New York, 3rd edn, 2012 Search PubMed
.
-
A. Rudin, The Elements of Polymer Science and Engineering, Academic Press, San Diego, 1st edn, 1982 Search PubMed
.
-
A. Rudin and P. Choi, The Elements of Polymer Science and Engineering, Elsevier Science, 3rd edn, 2012 Search PubMed
.
-
M. P. Stevens, Polymer Chemistry : An Introduction, Oxford University Press, New York, Oxford, 3rd edn, 1999 Search PubMed
.
-
D. J. Walton and J. P. Lorimer, Polymers, Oxford University Press, 2000 Search PubMed
.
- R. B. Merrifield, J. Am. Chem. Soc., 1963, 85, 2149–2154 CrossRef CAS
.
- S. Houshyar, D. J. Keddie, G. Moad, R. J. Mulder, S. Saubern and J. Tsanaktsidis, Polym. Chem., 2012, 3, 1879–1889 RSC
.
- A. Fradet, J. Chen, K.-H. Hellwich, K. Horie, J. Kahovec, W. Mormann, R. F. T. Stepto, J. Vohlídal and E. S. Wilks, Pure Appl. Chem., 2019, 91, 523–561 CrossRef CAS
.
- H. Nishida, H. Morikawa, T. Nakahara, T. Ogata, K. Kusumoto and T. Endo, Polymer, 2005, 46, 2531–2540 CrossRef CAS
.
- N. Zaquen, J. Vandenbergh, M. Schneider-Baumann, L. Lutsen, D. Vanderzande and T. Junkers, Polymers, 2015, 7, 418–452 CrossRef CAS
.
- H. G. Gilch and W. L. Wheelwright, J. Polym. Sci., Part A-1: Polym. Chem., 1966, 4, 1337–1349 CrossRef CAS
.
- W. F. Gorham, J. Polym. Sci., Part A-1: Polym. Chem., 1966, 4, 3027–3039 CrossRef CAS
.
- A.-K. Schönbein, M. Wagner, P. W. M. Blom and J. J. Michels, Macromolecules, 2017, 50, 4952–4961 CrossRef
.
- IUPAC, Project Details: Basic Classification and Definitions of Polymerization Reactions, https://iupac.org/projects/project-details/?project_nr=2019-027-1-400.
| This journal is © The Royal Society of Chemistry 2022 |