Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impact of fuel cells on hydrogen energy pathways in a sustainable energy economy†
Jonathan G.
Love
 *ab,
Anthony P.
O'Mullane
*ab,
Anthony P.
O'Mullane
 abd,
Fanny A.
Boulaire
ce and
Ian D. R.
Mackinnon
abd,
Fanny A.
Boulaire
ce and
Ian D. R.
Mackinnon
 ae
ae
aCentre for Clean Energy Technology and Practices, Queensland University of Technology (QUT), Brisbane, QLD 4001, Australia
bSchool of Chemistry and Physics, Queensland University of Technology (QUT), Brisbane, QLD 4001, Australia
cCSIRO Land & Water, Brisbane, QLD 4001, Australia
dCentre for Materials Science, Queensland University of Technology (QUT), Brisbane, QLD 4001, Australia
eSchool of Earth and Atmospheric Science, Queensland University of Technology (QUT), Brisbane, QLD 4001, Australia. E-mail: jonathan.love@qut.edu.au
First published on 21st July 2022
Abstract
The drive to decarbonise the electricity and transport industries when transitioning from a fossil fuel economy to a hydrogen economy requires careful consideration of techno-environmental implications. National and regional strategies for adopting hydrogen energy highlight an overarching objective to use hydrogen for electrification that requires a concomitant transition to fuel cell technologies. We therefore examine the impact of emergent fuel cell technologies on the sustainability of various hydrogen energy pathways. Using a technology neutral framework, we show that hydrogen derived from fossil fuels for use in fuel cells (i.e., blue hydrogen), is techno-environmentally unviable in a future economy. We propose that a narrative focused on a sustainable energy economy, rather than a hydrogen economy, shifts the debate to meet the requirements of national and regional strategies with key implications for the energy industry and policy maker.
Introduction
The internationally agreed limitations on greenhouse gas (GHG) emissions to achieve climate neutrality1 and net zero emissions2 by 2050 have generated a resurgent interest in hydrogen as a clean energy carrier. This renewed interest reinstates a transition to a hydrogen economy onto both political and economic agendas. New national strategy papers and roadmaps3–11 highlight the opportunities for large-scale domestic and international export/import markets for hydrogen as well as beneficial contributions to achieving the United Nations (UN) sustainability development goals (SDG's)12 and emissions reduction targets.1 The global annual production13 of hydrogen reached 70 million tonnes (MT) in 2019, and is expected to grow to 528 MT year−1 according to the IEA's Net Zero Emissions (NZE) scenario14 with 102 MT year−1 predicted to be used in stationary Fuel Cells (FCs).The NZE scenario predicts14 that 63% of hydrogen demand by 2050 will be renewable “green” hydrogen and 37% from fossil fuel (FF) with Carbon Capture Utilisation or Sequestration (CCUS) (“blue” hydrogen) of which a large percentage is predicted to be used in fuel cells to produce clean energy. A recent analysis15 of 2050 scenarios indicates a higher hydrogen demand of 800 MT than IEA's NZE scenario and a likely higher green hydrogen to blue hydrogen ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 based on cost competitiveness. However, the question of whether blue hydrogen should be part of the hydrogen energy transition is a hot topic in the European Union (EU)16 and in particular, Australia.17,18 Recently the UK has established a twin track blue and green hydrogen strategy.19
20 based on cost competitiveness. However, the question of whether blue hydrogen should be part of the hydrogen energy transition is a hot topic in the European Union (EU)16 and in particular, Australia.17,18 Recently the UK has established a twin track blue and green hydrogen strategy.19
An analysis20 of global electrical utilities concluded that there is significant electricity sector inertia via carbon lock-in of coal and natural gas (NG) fuels that will be embedded in the energy system for many decades. The FF industry is now focusing business plans on blue hydrogen21 that expand FF interests into the growing hydrogen energy industry.22 While this focus may minimize supply disruption(s) during the transition of the global energy industry, there is a potential risk of even higher carbon lock-in for blue hydrogen via use of coal and NG fuels.
A recent analysis23 based on a 20 year Global Warming Potential (GWP) for methane, indicated that the climate impact of blue hydrogen from NG may be greater than burning NG directly for the specific scenario that was analysed. More importantly, other analyses17,24–26 conclude that hydrogen derived from FFs has a high economic risk that will put downward pressure on the growth of blue hydrogen production. Modelling of the global energy system concluded that FF production will need to decrease by 3% per year to limit temperature increases below 1.5 °C with nearly 60% of NG and 90% of coal needing to remain unextracted.27
While all published hydrogen strategies, roadmaps and predictions of hydrogen growth are top-down analyses, technology roadmaps for FCs28 are too broad to provide more accurate predictions for the growth of hydrogen from different origins for use in FCs. In this work, this knowledge gap is addressed by a bottom-up analysis of FCs in a technology neutral framework that addresses how different FCs such as Proton Exchange Membrane Fuel Cell (PEMFC) and Solid Oxide Fuel Cell (SOFC) impact the sustainability outcomes of different hydrogen energy pathways (HEPs). Three key metrics were analysed for each HEP: the amount of primary energy, the mass of fossil fuel consumed and mass of direct CO2 that must be abated for carbon neutrality.
This analysis determined that key differences between stationary PEMFC and SOFC technologies create an interdependence between the energy source and the available FC technology at the final point of electricity generation. Further, the impact of fugitive emissions is discussed for the analysed HEPs because of the much higher Global Warming Potential (GWP) of methane.
This analysis within the energy-environment nexus suggests that a sustainable energy economy should be the ultimate objective rather than a hydrogen economy alone due to the potential to entrench unwanted HEPs with poor sustainability outcomes. We propose a technology neutral sustainable energy framework to evaluate these possible future economies. With this framework, we determine that blue hydrogen use in PEMFCs for stationary electricity generation has poor environmental outcomes. This determination is based on the level of direct CO2 emissions and fossil fuel consumption, compared with direct conversion of fossil fuels to electricity via SOFCs: an approach that does not first require conversion to hydrogen. This outcome points to the conclusion that stationary PEMFCs will trend more strongly towards using green hydrogen than blue hydrogen. Furthermore, we propose that these alternative energy pathways will impact the 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 green to blue hydrogen ratio predicted by the IEA for the NZE scenario by 2050.
37 green to blue hydrogen ratio predicted by the IEA for the NZE scenario by 2050.
Hydrogen energy and fuel cell transition
Japan is one of the largest consumers of NG and imports 77 MT per year (2019) with 39% from Australia, 28% from South East Asia, 18% from the Middle East with the remaining 15% from Russia, USA and other exporters.29 About 50 MT of NG is used in Japan's power industry to generate 323 TW h of electricity.29 Japan has announced interest in the commercial supply of clean hydrogen for use in FCs to transition away from FF imports while limiting a return to nuclear power to meet international climate obligations. An active FC development and implementation program also aims to progress Japan's hydrogen and FC transition.4,30 Other countries3,5–10 have also announced interest in clean hydrogen, with several countries, such as Australia,3 Norway11 and Saudi Arabia ramping up their renewable energy production to be part of a future hydrogen energy export market.Hydrogen is already an important chemical that is widely used through an established global industry for its generation, distribution and use. Most of today's hydrogen is produced by steam reforming of natural gas (NG) (“grey” hydrogen) and to a lesser degree by coal gasification (“black” or “brown” hydrogen for bituminous and lignite, respectively) without CCUS. We note the introduction of technology alternatives that may reduce GHGs such as CO2 and CH4 when used via a FF pathway. Examples of such alternative technologies include autothermal reforming,31–34 pyrolysis35–37 and plasma processing.38 Each of these technologies have the potential for lower GHG emissions from the production of H2 using FF sources. However, for this work we focus on existing, large-scale processes for H2 production using FF sources such as Steam Reforming (SR) and Coal Gasification (CG) for which there are well-defined input/output and efficiency parameters.
Given the IEA's recent projections for hydrogen demand, the current commensurate carbon dioxide (CO2) annual emissions of ∼685 MT per annum from FF derived hydrogen could rise to over 5000 MT by 2050 if the established practice continues.39 Most of the hydrogen produced today is used across many chemical and physical industry sectors. For example, 55% of global hydrogen is used by the oil refining and agricultural industries.13 The hydrogen industry is now transitioning to include energy use applications and, in particular, to convert it to electrical energy via FCs for stationary and mobile power applications.14 In the context of electricity as the end use, many HEPs can be defined where FCs convert either natural gas or hydrogen sustainably, when combined with CCUS. Recently published hydrogen roadmaps and strategies show that a number of countries and regions3–11 have significant aspirations for new large-scale applications of hydrogen as an energy carrier with a focus on electricity generation for both stationary power and transportation. These roadmaps describe a range of transition scenarios including production of hydrogen using FF with no CCUS and the production of clean hydrogen from renewable energy. We designate the hydrogen energy and FC transition in terms of hydrogen production and utilization as shown in Table 1. This table illustrates that the emerging hydrogen energy industry (HEI) is distinct from the established hydrogen industry in that FCs and their flexibility of performance are major influencing factors not evident in the latter category. Our analysis includes the interdependence of hydrogen use in FCs and the hydrogen production process.
| Transition sector | Established industry | Emerging energy industry |
|---|---|---|
| Hydrogen production industry | Hydrogen produced from fossil fuels with CO2 emitted to the atmosphere: “grey”, “black” and “brown” hydrogen from NG, bituminous coal, and lignite coal respectively | Hydrogen produced from renewable and sustainable methods: “green”, “clean” and sustainable hydrogen |
| Hydrogen use industry | Hydrogen used predominantly in chemical processing and physical industries | Hydrogen used predominantly as an energy carrier in FCs for stationary and mobile power applications |
Methods
Analysis of hydrogen energy pathways
In a hydrogen energy and FC transition, we consider the available HEPs and provide an analysis of the many options available using a technology neutral framework. The focus of this work is on the transition to clean electricity in stationary power applications as a primary goal for hydrogen energy use, while considering combined heat and power where relevant. A similar analysis can be applied to mobile applications but is not presented here.This work focusses on the techno-environmental aspects of the available HEPs, omitting consideration of their economics. We note that questions on the veracity of economic data40 for different energy forms may benefit from more reliable quantification via this “bottom up” approach to technology choices. In order to provide the technical basis for future economic analyses, this work focusses on the important techno-environmental aspects given the rapid requirement for a NZE scenario rather than assessing over-arching, or high-level, financial considerations and questions of subsidies.
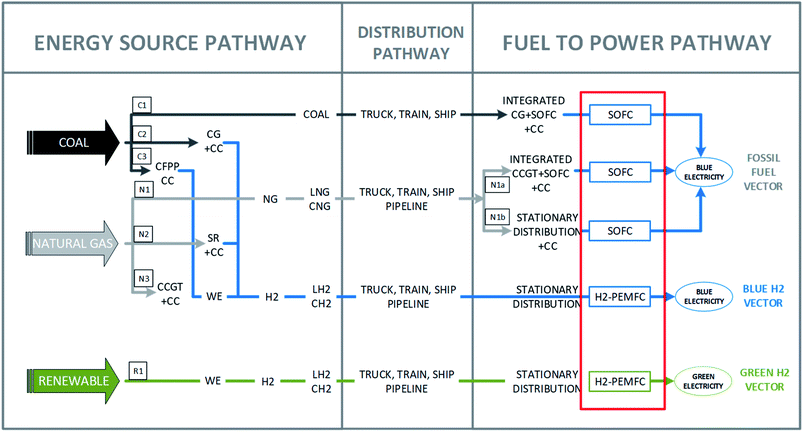
Here, the clean HEPs considered in a technology neutral framework for the emerging HEI are shown in Fig. 1. The primary source of energy proceeds via an Energy Source (ES) pathway and is transferred via a Fuel Distribution (FD) pathway for use as electricity in stationary and mobile power applications via a Fuel to Power pathway (F2P). The ES and F2P pathways are at different locations for hydrogen export applications. Both the ES and the F2P pathways can also be in proximate locations for domestic applications where the fuel is used as a long-term energy storage medium.
Clean hydrogen has been defined41 as both renewable (“green”) hydrogen and “blue” hydrogen that is “produced with minimal emissions, so it can include non-renewable sourced electricity paired with offsets or carbon capture and storage (CCS) to mitigate carbon emissions from its production”. We expand this definition to carbon capture, utilisation and storage (CCUS).
There are two hydrogen energy vectors: “blue” and “green” hydrogen that are “energy-rich substances that facilitates the translocation and/or storage of energy with the intention of using it at a distance in time and/or space from the primary production site”42 and identified in the schematic in Fig. 1. Further detail is provided in ESI Fig. S1† including the different emission species: direct CO2, fugitive methane and hydrogen. Electricity generated from blue and green hydrogen are also identified in a similar manner. HEPs involve various energy conversion processes such as (i) fossil fuel to hydrogen (ii) fossil fuel to power (iii) power to hydrogen and (iv) hydrogen to power. This work focusses on the performance of specific FCs for the F2P pathway as highlighted in Fig. 1 and on the impact of FC performance on the sustainability of hydrogen production methods in the ES pathway.
The FC that is expected to be predominantly used in the emerging HEI is the hydrogen PEMFC (H2-PEMFC) due to current commercial advantages. The alternative FCs that are analysed in this work operate at higher temperatures and encompass both Molten Carbonate Fuel Cells (MCFC) and SOFC. Although a similar analysis applies to MCFC, this work will focus on SOFCs as a comparator to H2-PEMFCs.
Comparisons of different FCs are widely reported28,43–46 with further information provided in the results section and ESI Fig. S2, S3 and ESI Note 1.† Two important performance characteristics for H2-PEMFCs and SOFCs are compared in this work because they determine key sustainability outcomes of HEPs. Firstly, H2-PEMFCs must use high purity hydrogen47,48 whereas SOFCs can operate on a flexible range of fuels including natural gas, coal gasification product, synthetic fuels and biofuels as well as lower purity hydrogen.46 Secondly, as described in the results section, SOFCs have an inherent advantage due to higher electrical efficiency than H2-PEMFCs and provide higher overall/system efficiencies for combined heat and power (CHP) applications in distributed generation.
Key metrics
This method focusses on three metrics (ESI Table S1†) for each available HEP in a technology neutral framework that delivers the same electrical energy (Eu) at an end use location. Metric 1 is the amount of primary energy (EES,j) required at the start of the Energy Source pathway for jth Hydrogen Energy Pathway. This metric is analysed because each hydrogen energy pathway needs different amounts of primary energy due to the different aggregate efficiencies (Π(εj)) of the unit processes in the pathway to deliver the same final hydrogen demand and electrical energy (Eu). Metric 2 is the corresponding amount of fossil fuel (M(FF)ES,j) that is consumed to deliver the final amount of electricity (Eu). This metric is analysed to highlight the consumption of a finite amount of an essential natural resource. Metric 3 is the mass of direct CO2 (M(CO2)j) that must be fully abated for jth HEP. This metric is analysed because each HEP has different environmental risk and cost outcomes associated with direct CO2 emissions and its abatement.The final electricity used at the end of the HEPs (Eu) is derived [eqn (1)] from the predicted hydrogen demand per year in 2050 for stationary power applications (MH2), set for the application as MH2 = 102 MT year−1 to achieve NZE targets.14 This quantity of hydrogen will produce Eu = 1969 TW he of electrical energy per year based on the higher heating value (HHV) of hydrogen (HHVH2 = 39.4 × 10−6 TW h T−1) and using the HHV efficiency for H2-PEMFC (εH2-PEMFC = 49%) (see below). For reference, in 2018 the amount of electricity consumed in the global electricity system was 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315 TW he with electricity consumption increasing at about 900 TW he every year.49
315 TW he with electricity consumption increasing at about 900 TW he every year.49
| Eu = MH2 × HHVH2 × εH2-PEMFC | (1) |
The first metric is the amount of primary energy (EES) required at the start of the ES pathway for HEP (j) and is given in [eqn (2)].
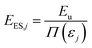 | (2) |
The second metric is the mass of fossil fuel (M(FF)ES,j) relating to the FF energy EES,j given by [eqn (3)] where HHVFF is the HHV for the FF required for HEP(j).
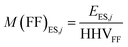 | (3) |
The third metric relates to waste products such as CO2 or GHG equivalent(s) from the HEPs that are key performance indicators for the global energy industry and highlighted in ESI Fig. S1.† This metric is an indicator of commercial risk and therefore, of cost to an emerging industry as well as a risk in achieving the UN SDG's and NZE targets.
CO2 emissions during materials mining, processing and transport of energy assets have not been analysed. A lifecycle analysis (LCA) for the energy systems predicted in 2050 is beyond the scope of this work but will be important to undertake in the future.
Both hydrogen and methane may also enter the atmosphere through fugitive emissions or deliberate venting for safety reasons. In the case of methane, the probability of fugitive emissions occurring from FF50–52 and NG use23,53–57 with negative consequences to the environment58 amplifies the risk associated with direct NG use and for NG to blue hydrogen pathways, particularly as distribution and downstream networks age.55,56 Methane emissions from coal mines59 is also a concern60 and have a similar negative impact on the blue hydrogen pathway from coal. The formation of nitrous oxide (N2O) in some pathways is also a significant risk.61
We have not quantified the equivalent CO2 emissions from methane, hydrogen and nitrous oxide in each HEP in our work due to (i) the dearth of reliable industry data on the percent emission rates, (ii) the speculative nature of emerging energy conversion technologies implemented at industrial scale, (iii) technology, operations and policy improvements anticipated to abate methane and N2O emissions and (iv) different views on GWP values for short lived climate pollutants.62 This is a topic of significant importance needing careful detailed evidence-based analysis using reliable industry data to ensure the future energy system is more sustainable than todays. Although detailed analysis of fugitive emissions is beyond the scope of this work, the impact of methane, nitrous oxide and hydrogen emissions are addressed in the discussion section below.
To simplify our analysis, we quantify only the amount of CO2 directly emitted that must be fully abated with each HEP (M(CO2)HEP,j) [eqn (4)] to achieve NZE targets.
| M(CO2)j = 3.67 × M(FF)ES,j × fC,FF | (4) |
Heat lost to the environment during energy conversion pathways is not shown in Fig. 1. Distributed stationary FCs in close proximity to a user's heat demand may provide heat as a complementary product for end users. However, this work focusses on electrification outcomes and does not analyse any subsequent heat use that favours SOFCs over PEMFCs.
ESI Table S2† shows the analysis outcomes for a number of HEPs shown in Fig. 1. A selection of these results are described further.
Results
PEMFC operating with blue hydrogen
The use of blue hydrogen from NG to generate clean electricity in H2-PEMFCs for stationary applications (HEP,j = N2 in Fig. 1) includes two sequential processes where significant energy is lost along with the energy lost during the storage and transport of hydrogen. The aggregate efficiency (HHV) for the N2 HEP (Π(εN2) in [eqn (2)]) includes the efficiency of SR (εSR), H2-PEMFC (εH2-PEMFC) and hydrogen storage and transport (εS&T,H2) as shown below [eqn (5)].| Π(εN2) = εSR × εH2-PEMFC × εS&T,H2 | (5) |
The first significant energy loss is the steam reforming pathway of NG to produce blue hydrogen, where the efficiency factor εSR is between 67 and 76% (HHV) based on industry white papers39 for conventional to modern plants. In this analysis, we choose εSR = 76%, for modern plants. Natural gas typically contains more than 90% methane (n = 1) with smaller quantities of n = 2 (ethane), n = 3 (propane), n = 4 (n-butane, iso-butane) and other hydrocarbons. The steam reforming39 of these compounds is shown in [eqn (6)] with additional hydrogen arising from water, followed by the water gas shift reaction [eqn (7)]. For blue hydrogen, the CO2 that is normally dispersed to the atmosphere is captured and either utilized or sequestered.
| CnH2n+2 + nH2O ⇌ nCO + (2n + 1)H2 | (6) |
| nCO + nH2O ⇌ nCO2 + nH2 | (7) |
There is a large energy loss via the electrochemical energy conversion in the F2P pathway using H2-PEMFC where blue hydrogen is converted to electricity. The reported efficiency for H2-PEMFC systems is over a wide range. For example, values range between 32 and 49% HHV (ref. 28) and 30–33% HHV (ref. 46) due to (i) the use of higher or lower heating values of hydrogen, (ii) the use of efficiency at maximum rated power or at the peak efficiency point at a derated power level,63 (iii) the manufacturer and their specific FC system solution, (iv) the size of the FC system where higher efficiencies are expected with increased power ratings from lower percentage parasitic power consumption, (v) the use of initial efficiency for new FCs versus aggregate efficiency over the FC lifetime and (vi) the use of DC stack efficiency rather than grid connected exported power from a complete FC system inclusive of internal parasitic power consumption.
A typical installation for H2-PEMFC systems is their use in distributed stationary power systems and virtual power plants.28,30 In this analysis, we choose εH2-PEMFC = 49% HHV corresponding to the peak efficiency at the beginning of life for PowerCell Sweden AB's commercial product: PowerCellution Power Generation System 100 kW system63 with specified efficiency of 58% LHV. A rationale for why H2-PEMFCs can have a peak efficiency of 49% HHV is given below.
Ultimately, storage and transport of hydrogen carriers will have a substantial impact on the cost and rate of growth of the emerging HEI due to the need for additional, or new, infrastructure. Storage and transport of hydrogen will have an energy efficiency penalty compared to established FF storage and transport, depending on the type of hydrogen carrier (e.g. liquid hydrogen, compressed hydrogen, ammonia, methylcyclohexane, other liquid organic hydrogen carriers (LOHCs), or metal hydrides). Therefore, to simplify our analysis, an assumption of a storage and transport efficiency εS&T,NG of 87.3% (i.e., 12.7% energy loss) for NG is used (ESI Note S2†).
For hydrogen, it is assumed that liquid hydrogen is the dominant form with higher energy loss from liquefaction (15% compared to 9% for LNG) and higher boil-off gas losses (8% compared to 1.4% for LNG) during an 8 day shipping route (ESI Note S2†). Losses from regasification for both LH2 and LNG were estimated from the heat of evaporation (ESI Note S2†). The storage and transport efficiency for hydrogen of εS&T,H2 = 74.4% (i.e., 25.6% energy loss) is used in this work. Further detailed analysis of storage and transport options are outside the scope of this work but would be of significant benefit to the community.
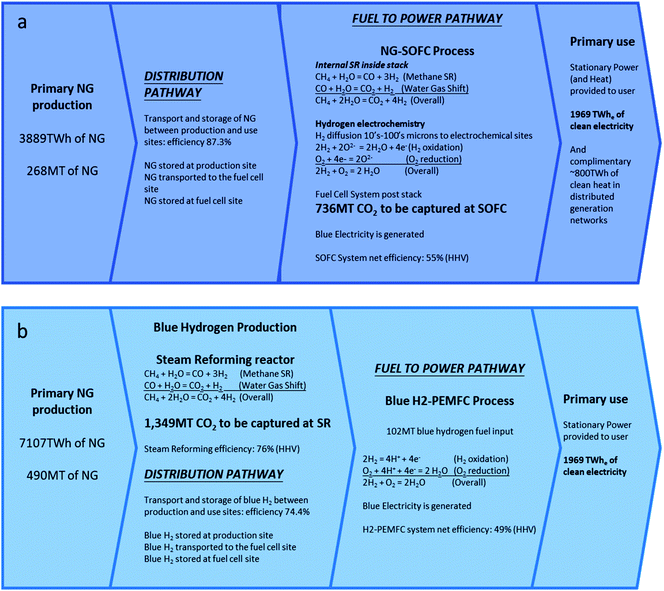
Outcomes for the three metrics aligned to this HEP (N2 in Fig. 1) that starts with NG at the ES Pathway, producing blue hydrogen from SR and ending with 1969 TW he electricity delivered to the user with an H2-PEMFC is:
• EES,N2 = 7107 TW hNG year−1 [eqn (2)].
• M(FF)ES,N2 = 490 MT year−1 of NG [eqn (3)].
• M(CO2)N2 = 1349 MT year−1, [eqn (4)].i.e., the total abatement of CO2 required per year at the SR plant using CCUS to achieve zero emissions.
The corresponding analysis for the HEP that uses coal (pathway C2) imposes twice the techno-economic and environmental risk for a CCUS pathway compared to using NG for the same electrification outcome (ESI Note S3†).
Efficiency of fuel cells
FC efficiency is a major factor for the outcomes from this work. FCs electrochemically convert chemical energy within a fuel to electrical energy and useable heat. The overall electrochemical reaction in a PEMFC is shown in [eqn (10)] while the electrochemical half reactions, hydrogen oxidation reaction (HOR) and oxygen reduction reaction (ORR) for the anode and cathode are shown in [eqn (8) and (9)], respectively.Anode half reaction HOR
| 2H2 → 4H+ + 4e− | (8) |
Cathode half reaction ORR
| O2 + 4H+ + 4e− → 2H2O | (9) |
Overall reaction
| 2H2 + O2 → 2H2O | (10) |
The key equation for the net system electrical efficiency64 for any FC system (εFCSystem) for any fuel can be described by [eqn (11)].
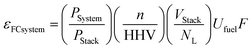 | (11) |
The term (n/HHV) is recognised as the moles of electrons generated from the fuel per unit energy content of the fuel (moles of electrons per unit fuel energy) and termed the Electrons Per Fuel Energy (EPFE) factor. The EPFE factor is unique to the specific fuel composition used in the FC and provided in ESI Table S3.† This value is not related to the type nor technology of FC. For FCs operating with hydrogen, the EPFE is 8.279 moles electrons per MJ of hydrogen.
The term (VStack/NL) is the average layer voltage (Vlayer,av) in the FC stack which is defined by [eqn (12)].
| Vlayer,av = OCV − Id × Rlayer,av | (12) |
| Vmin,Pmax2 − OCV × Vmin,Pmax + Pd,max × Rlayer,av = 0 | (13) |
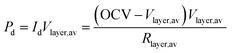 | (14) |
There is a trade-off between power density [eqn (14)] and efficiency [eqn (11)]. For example, operating at higher layer voltages for higher efficiency produces a lower power density, and vice versa. That is, operating at lower voltages (down to Vmin,Pmax) for higher power density produces a lower efficiency. The tradeoff is essentially between capital equipment cost related to power density that defines the number of layers and how much material per kW power output is required in light of the operating fuel cost over the lifetime of the FC. These key parameters determine the efficiency of the FC in the F2P pathway and applies to all types of FCs.
The lifetime degradation of the FC stack performance, caused by materials changes that lead to higher average layer specific area resistance, is factored into the average layer voltage at the start of operation. On balance, it is generally considered that a starting average layer voltage of around 0.7 V provides a reasonable balance between high-power density and high efficiency requirements but may range between 0.6 and 0.8 V.
The fuel utilization Ufuel depends on practical outcomes related to the FC technology (e.g., materials, design, manufacturing methods) as well as selection of operating conditions. For example, the stack cannot operate at 100% utilisation of fuel; yet fuel utilisation is a practical upper limit defined by the product manufacturer. This upper limit is based on the cell and stack technology, materials, design and operating conditions.
With the aim to maximise stack efficiency (εFCStack) and to minimise fuel gas cost, the optimum design of a FC control system ensures operation at a constant maximum allowable fuel utilisation Ufuel,max. This maximum fuel utilisation is defined by the product manufacturer at the highest practical level that does not cause performance degradation of the FC stack and consequently shortens product lifetimes. The FC control system achieves this by adjusting the controllable set point of the fuel flow rate for the measured present value of current drawn by the stack (i.e., a dynamic variable). The different FC technologies and manufacturing constraints on stack assembly define the maximum allowable fuel utilisation for a given product. PEMFCs are operated to ensure no fuel starvation65,66 with fuel utilization typically43 no more than 95%.
The above section describes the efficiency of DC electricity generation from the stack (εFCStack) flowing to the external circuit inside the FC system [eqn (15)].
| εFCStack = (n/HHV) × (VStack/NL) × Ufuel × F | (15) |
The FC system uses a fraction of this electricity to operate balance of plant components such as electronic circuits, pumps, actuated valves and conversion of DC power from the stack to an external power connection. The latter may be an AC or a DC power connection and will typically be at a higher voltage than the stack voltage. Hence, this configuration requires a voltage step-up converter. Electricity consumed within the FC system by the balance of plant decreases gross DC efficiency of the FC stack (εFCStack) by a parastic power loss factor related to the ratio of power exported to the external circuit (Psystem) to the gross power generated by the stack (Pstack). The net export efficiency of the FC system (εFCSystem) is described in [eqn (16)]. Typical system power losses are not generally reported but are expected67,68 to be of the order of 20% of the gross DC power from the stack for smaller distributed H2-PEMFC systems depending on system configuration and power rating.
 | (16) |
In summary, an HHV efficiency of the H2-PEMFC, εH2-PEMFC = 49% used in our work can be derived from the following key parameters (i) EPFE of 8.279 moles electrons per MJ of hydrogen, (ii) average stack layer of 0.8 V, (iii) fuel utilisation of 0.95, (iv) parasitic power consumption at 20% of the stack power with a ratio of system power to stack power of 80%. Some H2-PEMFCs will have lower efficiency if operated at a lower average cell voltage, at lower utilization or if they consume more parasitic power in the system. In general, this efficiency is for initial operating conditions. However, as degradation of stack materials occurs, the stack voltage will decrease and therefore efficiency decreases with continued use. The cause(s) of degradation rates and effects on lifetimes are complex mechanisms in FCs and are not considered in this model. With increased deployment of FCs in the field aligned with ongoing asset monitoring, reliable rates of degradation for specific FC types will add to our understanding of long-term performance degradation.
Alternate pathways
In a technology neutral framework that also considers either utilization or sequestration of captured carbon, there exists another pathway for FF suppliers that is commonly overlooked in the analysis of HEPs. That pathway is the use of SOFCs and MCFCs for energy pathways N1b (for NG) and C1 (for coal), as in Fig. 1. Because MCFCs have similar outcomes to SOFCs, we have focused on SOFC for clarity of discussion. Unlike H2-PEMFCs that require high purity hydrogen to operate,47,48 SOFCs can operate on a flexible range of fuels. Low temperature FCs such as H2-PEMFCs are readily poisoned by low levels of CO69,70 that restrict applications to the use of pure hydrogen47,48 fuels (>99.97%). Although NG can be used in PEMFC systems71 with 100% external reforming and shift reactors that reduce CO to required levels, they have lower electrical efficiencies and are presently not commercially attractive.In comparison, a SOFC is not poisoned by CO due primarily to the catalytic nature of the fuel electrode and to a higher operating temperature of the SOFC stack (>650 °C). This higher operating temperature enables CO to electrochemically react with oxygen anions64 [eqn (18)] or to proceed via a WGS reaction [eqn (7)] and subsequent electrochemical oxidation of hydrogen [eqn (19)].
Cathode Half Reaction ORR
| O2 + 4e− → 2O2− | (17) |
Anode half reaction CO OR
| 2CO + 2O2− → 2CO2 + 4e− | (18) |
Anode half reaction HOR
| 2H2 + 2O2− → 2H2O + 4e− | (19) |
In addition, provision of sufficient steam ensures that carbon formation is not thermodynamically favorable.72 Unlike PEMFCs that are limited to pure hydrogen as a fuel, SOFCs are fuel flexible and can directly convert lower purity hydrogen fuel along with a variety of gaseous fuels such as NG, gasified coal and many others including biofuels and renewable or no/low carbon synthetic fuels as hydrogen carriers (e.g., renewable methane, methanol, ethanol, ammonia). As an example, ESI Fig. S3† shows a schematic representation of the SOFC system for operation of SOFCs with NG along with relevant chemical and electrochemical reactions.
The efficiency of SOFCs has been reported to be 45–60% LHV46 and commercial SOFC systems operating on NG have specifications for electrical efficiency (εSOFC) of 53–65% (LHV) that is 48–58% (HHV)73 for a 300 kW system. A rationale for the higher efficiency for NG-SOFCs (59% HHV) compared to H2-PEMFCs (48% HHV) can be seen from above and [eqn (11)–(16)] which remain relevant. Nevertheless, key factors for NG-SOFC and H2-PEMFC systems are different with an example summarized in Table 2.
| Factor | H2-PEMFC | NG-SOFC |
|---|---|---|
| Parasitic power loss | 0.8 | 0.91 |
| EPFE (moles electrons per Joule) | 8.28 × 10−6 | 8.89 × 10−6 |
| Average stack layer, V | 0.8 | 0.825 |
| Fuel utilisation | 0.95 | 0.9 |
| ε FCSystem (HHV) | 49% | 58% |
A major difference relates to improved parasitic losses for SOFC from the endothermic steam reforming of methane that takes place inside the stack. These losses reduce the cooling demand from the air flow and reduces the air blower power demand.74 A peak electrical efficiency εSOFC of 58% (HHV) is used in this work for NG operated SOFCs.
In this case (N1b in Fig. 1), NG is the transportable and stored energy form with the same efficiency εS&T,NG of 87.3% as used above. Outcomes for the three metrics relevant to this HEP (N1b in Fig. 1) that starts with NG at ES pathway, producing 1969 TW he electricity delivered to an end user via SOFC in the F2P pathway is:
• EES,N1b = 3899 TW hNG year−1 [eqn (2)].
• M(FF)ES,N1b = 268 MT year−1 of NG [eqn (3)].
• M(CO2)N1b =736 MT year−1 [eqn (4)], i.e., the total abatement of CO2 required at the SOFC in the F2P pathway using CCU to achieve zero emissions.
This pathway (N1b) is the most sustainable outcome for all FF use cases considered (ESI Table S2†). In comparison to the blue hydrogen pathway N2, this pathway N1b requires only 60% of NG supply and has 40% less CO2 emissions for abatement.
A similar analysis for SOFCs integrated with coal fired power plants75,76 is provided in ESI Table S2.† In general, SOFCs have been used with carbon-based fuels such as syngas and NG, although SOFCs are 100% compatible with hydrogen of a lower purity requirement than H2-PEMFCs46 albeit with a reduced electrical efficiency. This compatibility provides an additional option for SOFCs that enables reduced risk of stranded SOFC assets as compositions of available H2-NG gas blends become more renewable over time and with an eventual full transition to 100% hydrogen.
Blue hydrogen from water electrolysis and FF-based mains power
Energy pathways using mains electricity generated from coal and NG plants (NEPs C3 and N3, respectively) to generate blue hydrogen utilises a water electrolysis process. The global weighted average generating efficiency77 for these FF power plants in 2016 used in this work are: 35.5% (HHV) for coal (εPP,Coal) and 44.3% (HHV) for natural gas (εPP,NG). We assume that there is 95% power distribution efficiency78 (εPDL) for mains power applications and that the efficiency for rectification of AC to DC conversion is incorporated within the electrolyser efficiency value.We recognize that the efficiency of electrolysers depends on numerous factors, similar to that of FCs described above, including variations between manufacturers, electrolyser configurations, locations, scale of the electrolyser and each electrolyser type (e.g. alkaline, proton exchange membrane (PEM), anion exchange membrane (AEM) and solid oxide (SOEC)) have different efficiency ranges.28 This work focuses on the PEM electrolyser cells (PEMEC) and uses an electrolyser efficiency13εPEMEC = 65% (HHV) that equates to 60.6 kW he electricity consumption per kg hydrogen produced. We also note that water sustainability may trigger an efficiency penalty when treated seawater and/or wastewater sources are used instead of potable drinking water. This potential penalty is not included in this analysis.
Equations [eqn (2)–(4)] were used with Π(εj) including the efficiencies εPDL, εPEMEC, εH2-PEMFC and εS&T,H2 for both HEPs C3 and N3 with coal εPP-COAL and NG εPP-NG fired power plants, respectively. Outcomes for the three metrics relevant to HEP (C3 in Fig. 1) that starts with black coal producing blue hydrogen from water electrolysis and ending with the same 1969 TW he year−1 electricity delivered to the user using H2-PEMFC is: 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 641 TW hCoal year−1 or 3499 MT year−1 of black coal with a total abatement of CO2 at 8437 MT year−1 required at the coal fired power plant using CCUS to achieve zero emissions. The outcome is improved slightly for NG fired power plants (HEP N3 in Fig. 1) with 19
641 TW hCoal year−1 or 3499 MT year−1 of black coal with a total abatement of CO2 at 8437 MT year−1 required at the coal fired power plant using CCUS to achieve zero emissions. The outcome is improved slightly for NG fired power plants (HEP N3 in Fig. 1) with 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746 TW hNG year−1 or 1363 MT year−1 of NG and a total abatement of CO2 of 3751 MT year−1 required at the gas fired power plant using CCUS to achieve zero emissions.
746 TW hNG year−1 or 1363 MT year−1 of NG and a total abatement of CO2 of 3751 MT year−1 required at the gas fired power plant using CCUS to achieve zero emissions.
Clearly, HEPs that use national electricity grids with high proportions of FFs as primary ESs to produce hydrogen from water electrolysis show poor sustainability outcomes (ESI Table S2†).
Green hydrogen from water electrolysis and renewable power
Green hydrogen is produced from water electrolysis when using 100% renewable electricity from solar, wind, hydro, geothermal and tidal energies and this relates to HEP R1 in Fig. 1. In recent times, due to a significant increase in solar and wind power installations connected to a mains network, excess electricity has been generated during some periods of the day. This excess electricity is dispatched at much lower price points and may make a renewable HEP (R1) economically attractive when hydrogen is produced from grid balancing activities.79,80The amount of renewable electricity for pathway R1 with H2-PEMFC was determined using [eqn (1)–(4)] with Π(εj) that includes the efficiency of electricity conversion from renewable power to the electrolyser (εEC) taking into account energy losses from power conversion, transmission, storage, auxiliary plant items and power used at periods where there is no hydrogen production.81 Recent analysis of a renewable hydrogen production pilot plant showed81 this efficiency εEC = 83%, although this has yet to be validated by independent review. Efficiencies for a PEM electrolyser (εPEMEC), the H2-PEMFC (εH2-PEMFC) and hydrogen storage and transport (εS&T,H2) are also included in Π(εj) [eqn (1)–(4)].
The outcomes for the three metrics based on this HEP (R1 in Fig. 1) that starts with renewable power generation, producing green hydrogen from water electrolysis and ending with the same 1969 TW he year−1 electricity delivered to the user using H2-PEMFC is: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 012 TW hre year−1 of renewable electricity. Most importantly, this pathway utilises no FF's and has no requirement for CCUS. These outcomes are further improved when solid oxide electrolyser cells (SOECs) are used due to a higher efficiency13 (ESI Table S2†).
012 TW hre year−1 of renewable electricity. Most importantly, this pathway utilises no FF's and has no requirement for CCUS. These outcomes are further improved when solid oxide electrolyser cells (SOECs) are used due to a higher efficiency13 (ESI Table S2†).
Results summary
Selected outcomes from this analysis are summarised in Fig. 2 and 3 and Table 3. A more complete set of outcomes for all HEPs shown in Fig. 1 is provided in ESI Table S2.† The select outcomes described here relate to (i) NG as the primary ES and alternate pathways via use in H2-PEMFCs (pathway N2) and direct use of NG in SOFCs (pathway N1b), (ii) coal to blue hydrogen and use in H2-PEMFCs (pathway C2), (iii) FF fired power stations and production of blue hydrogen from water electrolysis using mains electricity and use in H2-PEMFCs (pathways C3 and N3 for coal and NG fuelled power plants (pathways C3 and N3 for coal and NG fuelled power plants, respectively) and (iv) the renewable HEPs pathway R1 and use of green hydrogen in H2-PEMFC.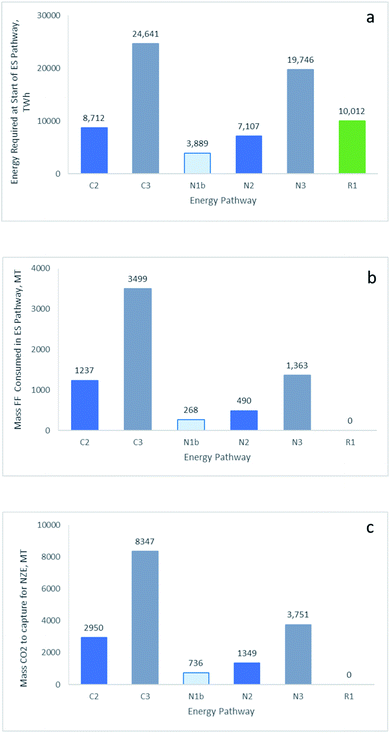 | ||
| Fig. 2 Comparison of outcomes for select energy pathways shown in Fig. 1. (a) Energy required at the start of the ES pathway to deliver 1969 TW he at the end of the F2P pathway. (b) Corresponding mass of FF required and (c) mass of CO2 to be captured. Legend: direct use of NG in SOFC (N1b); via blue H2 – used in H2-PEMFC from coal (C2) and NG (N2); mains power electrolysis – using coal (C3) and NG (N3) to produce mains power for water electrolysis and production of blue H2 used in H2-PEMFC; via green H2 – using renewable power for water electrolysis and production of green hydrogen used in H2-PEMFC. | ||
| Performance indicator | Description | Pathway N2 blue H2-PEMFC | Pathway N1b NG-SOFC | Pathway R1 green H2-PEMFC |
|---|---|---|---|---|
| Primary NG energy consumption | Energy of primary NG used to produce 1969 TW he of electricity for use | 7107 TW h | 3899 TW h | 0 TW h |
| Primary production of NG | Mass of NG to produce 1969 TW he of electricity for use | 490 MT | 268 MT | 0 T |
| Solar installed | Installed solar infrastructure required with 5 kW h/kW solar incidence to produce 1969 TW he of electricity for use | 0 GW | 0 GW | 6.1 GW |
| Level of abatement for generation | Mass of CO2 to be abated per 1969 TW he electricity produced for use | 1349 MT | 0 T | 0 T |
| Level of abatement for use | Mass of CO2 to be abated per 1969 TW he electricity produced for use | 0 T | 736 MT | 0 T |
The amount of electricity produced from the F2P pathway that is used to compare HEPs was based on a supply of 102 MT per annum of hydrogen by 2050 as predicted by the IEA in the NZE scenario14 for stationary power applications. We use the efficiency for a H2-PEMFC at the higher end of the current known range (49% HHV; refer to methods section) and the utilization of 102 MT per annum of hydrogen equates to a supply of 1969 TW he electricity per annum. Our analysis compares each HEP for the primary energy required at the start of the ES pathway to meet the end user's electricity demand of 1969 TW he at the end of the F2P pathway (Fig. 2a). The amount of FF required (Fig. 2b) and the risk to sustainability based on the amount of CO2 that needs to be abated per annum (Fig. 2c) are also determined for each pathway.
Fig. 2 shows that: (i) HEPs involving the use of FF powered mains electricity to power water electrolysis for production of blue hydrogen and use in H2-PEMFCs (pathways C3 and N3 for coal and NG, respectively) provides the least viable outcome for the amount of FF consumed and for risk in the amount of CO2 to be captured. This result has significant implications for a roll out of electrolysers that are powered by national electricity networks with a high FF load; (ii) NG HEPs for blue hydrogen use in H2-PEMFCs (pathway N2) is substantially more sustainable than using coal to produce blue hydrogen (pathway C2); (iii) the renewable energy HEP (pathway R1) has the best sustainable outcome in terms of CO2 emissions. However, this scenario shows a high energy source demand in the form of renewable electricity that puts pressure on the sustainability of material supply for installation of new renewable power infrastructure; and (iv) the direct use of NG in SOFCs (pathway N1b) is a more sustainable pathway for NG suppliers than producing blue hydrogen from NG and use in H2-PEMFCs (pathway N2).
This final point has significant implications for the NG industry with aspirations to supply blue hydrogen. Fig. 3 highlights a selective comparison for outcome (iv) above, with NG as the primary energy source and explicit pathways for distribution and use. A similar comparison can be made with coal as the primary energy source as described in the methods section.
Converting NG to blue hydrogen at a primary supplier location in the ES pathway for end use in a H2-PEMFC in the F2P pathway (pathway N2), will significantly increase the depletion rate of natural gas reserves and imposes a higher economic and environmental risk for carbon abatement processes compared to the alternative pathway of using natural gas directly in SOFCs (pathway N1b). This comparison highlights a key existential issue for NG producers as suppliers of blue hydrogen for use in H2-PEMFCs in electricity supply networks.
A responsible and sustainable pathway that involves direct use of NG in SOFCs is available with a substantially lower carbon footprint, especially if the infrastructure for NG supply is in place. The more sustainable energy pathway (i.e., N1b in Fig. 1) shifts the location for a carbon capture (CC) process to an end user site along with a shift in technology selection to SOFC equipped with CCU capability. We recognize that CC is not a common inclusion in the Steam Reforming (SR) plants of today, nor in SOFCs. Nevertheless, this common requirement impacts both pathways. Thus, both pathways will require high CC efficacies to become sustainable. Utilization of carbon and/or CO2 will be required in SOFC locations where geological sequestration is not possible. An analysis of the respective CC facilities required for this end use is beyond the scope of this work. We note that currently, CC capability has lower efficacy than is preferred82,83 and recent analysis23 suggests that the SR process to produce blue hydrogen including CC may be an unviable pathway past a 20 year horizon.
Table 3 summarises the outcomes depicted in Fig. 3 and compares these with a renewables pathway (R1) shown in Fig. 1 that provides the best and most sustainable outcomes. Table 3 provides an example for solar installation as a use case due to the significant growth of solar installations in recent times. During the early part of our global energy transition, the consumption of FF's to process materials, manufacture and build infrastructure for renewable HEPs and transport of green hydrogen, may be acceptable to enable this transition. However, such a transitionary phase in the global energy transformation is not analysed in this work. Nevertheless, we conclude from this study that from today, building 100% renewable powered industries such as mining, processing, manufacturing and distribution for a renewable HEP is a viable sustainability target.
Discussion
A sustainable energy economy
This work analyses the impact of FC technology performance on HEPs in a technology neutral framework to achieve the UN SDGs12 and, in particular, Goal 12; “Responsible Consumption and Production”. We interpret this goal to mean the “responsible consumption of primary natural energy sources and responsible production of clean electricity”. This interpretation implies that effective assessment metrics are not only financial (e.g., the cost per kilogram of hydrogen) but include other critical factors explicit in related SDGs (e.g., “Industry Innovation and Infrastructure”; “Climate Action”; “Good Health and Wellbeing”).Our analysis shows that, while distinct, the ES pathway, where energy is sourced, and F2P pathway, where energy is used, influence sustainability outcomes because they are inherently linked together and impact sustainability outcomes.
An important outcome of this analysis is that the type of FC available for the F2P pathway where hydrogen is used impacts the energy source and processes required to achieve a sustainable outcome. Table 3 clearly shows for H2-PEMFC (or SOFC without CCU), green hydrogen is the responsible use case. Table 3 and Fig. 3 clearly shows that the use of blue hydrogen in H2-PEMFCs (or SOFCs) is problematic because direct use of NG in SOFC with integrated CCU is a more responsible and sustainable choice because of lower CO2 abatement requirements and lower NG consumption. The results point to a supplier – consumer interrelationship (Table 4) to meet the SDGs that may invoke domestic, regional and/or international hydrogen agreements on supply and demand policy.
| Energy generation primary ES | Energy use fuel cell technology |
|---|---|
| Coal | Coal gasification plants equipped with SOFC and CCU |
| Natural gas | Distributed NG-SOFC-CCU plants with heat utilization in the local proximity |
| Biofuels and carbon neutral synthetic fuels | Distributed SOFC-CCU plants with heat utilization in the local proximity |
| Blue hydrogen | Not for use in stationary fuel cells |
| Green hydrogen | For use in fuel cells |
This analysis highlights that a technology neutral approach to hydrogen strategies combined with continued reference to a transition of the FF economy to a hydrogen economy or a hydrogen society may result in worse environmental outcomes than intended, based on direct and indirect CO2 emissions and fossil fuel consumption. We propose an alternative narrative that emphasizes a transition to a sustainable energy economy rather than to a hydrogen economy alone that could entrench poor sustainability outcomes such as carbon lock-in similar to conventional electricity generation.20 To this end, we invite further analysis and systematic evaluation of sustainable energy systems and their integration into a revised global economy. In keeping with this narrative, we believe that determination of embedded carbon intensity in hydrogen energy vectors should be emphasized in order to better distinguish sustainable energy outcomes from the notional colour schemes prevalent today (ESI Table S4†). Criteria for establishing new energy infrastructure within a sustainable energy economy are shown in Table 5.
| Hydrogen economy | Sustainable energy economy |
|---|---|
| H2 energy carrier dominant with profit and loss based on hydrogen supply and use | Most sustainable energy pathways dominant with profit and loss based on sustainable outcomes |
| Hydrogen focus potentially overlooks more sustainable renewable electricity pathways | Hybrid hydrogen and electron energy carriers; establish which HEP user is most sustainable |
| H2 generation from both FF and RE in “clean” hydrogen energy pathways compete with each other; thus, encouraging more FF plants. Potential for established grey H2 to continue and for further carbon lock-in | RE dominant over FF; RE systems are more sustainable |
| Further FF use restricted to eliminate carbon lock in and FF plants with no CCUS or CCUS with low efficacy are phased out | |
| Efficiency analysis of competing pathways potentially overlooked as hydrogen production and use dominates profit demand | Most efficient energy pathway dominates and is incentivized |
| Blue H2 will be used in FCs creating further carbon lock-in | Blue H2 use in FCs is not a viable energy pathway and is disallowed |
| Sustainable sourcing of materials is not considered | Considers most sustainable materials, sourcing, processing, recycling, waste impacts |
| Energy and carbon footprints are not considered | Minimises energy footprint and eliminates carbon footprint in manufacture and distribution |
| Emissions GWP impact of methane, NOx and H2 on climate chemistry not included | Includes emissions GWP impact of methane, NOx and H2 and considers regulations, measurement and control |
We propose the following:
(i) that the energy source pathway is a clean energy pathway with low/no long-term carbon emissions. In this context, CCUS efficiency will need to exceed 90%;
(ii) that the efficiency from the primary ES to end use as electricity at consumer sites is maximized;
(iii) the interdependence of fuel cell technology, energy use and primary ES for sustainable outcomes is considered and
(iv) materials used to build the infrastructure assets are sustainably sourced, with net low energy demand, environmental impact and recycling pathways.
This work has addressed criteria (i), (ii) and (iii). Analyses of criteria (iv) and other use cases are noted for future work within a suitable framework. The framework introduced here enables analysis of other energy pathways to deliver best outcomes for a sustainable energy economy.
Impact of fugitive emissions
The impact of fugitive emissions highlighted earlier, although outside the scope of this work, is of importance to the sustainability of each HEP. To place a sustainable energy economy into context, we discuss below the broad range of emissions relevant to the pursuit of blue and/or green hydrogen component(s) to future energy systems. The impact of all GHG emissions is not quantitatively evaluated in the model enumerated above in order to simplify our assessment of the impact of fuel cells on hydrogen energy vectors. Nevertheless, we present a framework within which other GHGs (e.g., N2O, NOx, NH3) may be considered as industry relevant data become available. We then describe criteria for a sustainable energy economy that may enable considered discussion of unintended policy initiatives as well as the capacity to meet UN SDGs into the future.A recent analysis23 determined that the climate impact of blue hydrogen produced from the conventional established method of steam reforming (SR) of NG is greater than using NG directly in combustion devices. This analysis is based on a 20 year GWP for methane and used a range of values in a sensitivity analysis for methane emission levels across the NG supply chain from well-head to SR reactor between 1.53% to 4.3%. In this study, an additional 25% NG consumption for electricity generation was estimated for the CC process across a range of 85–90% effectiveness.
Outcomes from this recent analysis23 may change with reliable values for (i) the use of thermal recovery for CC processes that can reduce the NG required, (ii) best industry emissions practice for NG distribution from well to the SR plant, (iii) other methods of producing hydrogen from NG such as autothermal reforming and pyrolysis and (iv) when longer GWP time frames are considered. However, their analysis23 raises an interesting economic question: what additional imposition is incurred on the levelized cost of hydrogen when achieving (i) the stringent methane emissions (<1.5%) required across the well-to-reactor NG supply chain including the concomitant cost of increased monitoring and maintenance, and (ii) the very high carbon capture rates (>90%) inclusive of long-term capture retention required to make blue hydrogen environmentally viable? A recent levelized cost analysis84 concluded that there is a trade-off between cost and carbon capture efficiencies.
The question of time frame for the impact of emissions on the environment may require alignment to the 2050 ambitions for achieving net zero emissions aimed at limiting the global temperature increase to below 1.5 °C. In other words, short lived GHG's such as methane are likely to have an amplified effect on these ambitions. We recognise that methane emissions are not only emitted by the NG industry and that significant methane emissions arise from the coal industry.52,59,60 A similar analysis between direct use of coal versus hydrogen from coal pathways including impact of methane emissions from coal mines and additional energy required for CC from CG processes may have analogous outcomes to our analysis of NG pathways.
Hydrogen is a light gas with a significantly higher diffusion coefficient in air85 (0.756 cm2 s−1 at 1 atm and 20 °C) than methane (0.210 cm2 s−1 at 1 atm and 20 °C). Hydrogen gas emissions in the HEI may be higher than methane from NG operations if not tightly regulated. Hydrogen emissions can occur via deliberate venting from electrolyser and fuel cell operations when they change operating states and during periodic purging but also due to safety protocols, maintenance events, flaring, tankage transfers, under-utilised gas in power generation and fugitive emissions.
There has been limited analysis of climate impact from hydrogen emissions. Many media and industry reports state or imply that hydrogen is a zero emissions fuel.3 However, the IPCC reports86 that hydrogen is an indirect GHG with a GWP over 100 years of 5.8 due to chemical effects in the troposphere. For example, hydrogen depletion of OH radicals, may reduce their effectiveness as chemical cleaning agents of the atmosphere. Depletion of these radicals causes an increase in the levels of harmful GHGs such as short-lived methane.86
Although further analysis is required on this important issue, one interpretation of the IPCC emissions report58 is that the growth of the HEI with its concomitant hydrogen emissions will need to match a decrease in methane emissions from NG and coal operations to counteract potential harmful reactions in the troposphere related to OH radical chemistry. This additional complexity would further reduce the environmental sustainability of a blue hydrogen pathway. An analysis of hydrogen's impact to the climate concluded87,88 that for every 1% of hydrogen emitted from the hydrogen industry it will have a 0.6% impact on the climate compared to the current FF industry. This raises the question; how much hydrogen will be emitted to the atmosphere from the emerging HEI at industrial scale and what impact will this have on global warming?
The use of FF's, hydrogen and hydrogen carriers such as ammonia can also lead to nitrous oxide (N2O) and NOx (nitric oxide, NO and nitrogen dioxide, NO2) production and emissions. The GWP of N2O is very high at 298 (100 year timeframe)58 whereas for NOx the GWP has significant uncertainty86 but is a harmful pollutant in urban environments with a significant impact on human health.89 Methods to avoid N2O and NOx formation and regulate emission levels in the FF and HEI will be required to achieve NZE targets. The impact of N2O and NOx in the HEPs analysed here is not in the scope of this work.
Our study focusses on the environmental impact from direct CO2 emissions that should be abated during use of FF's and of blue hydrogen in fuel cells in comparison to that for green hydrogen. Although not included for reasons stated above, the contribution from short lived GHGs may also be incorporated in the following way. For example, for a particular HEP, the sum of the equivalent CO2 emissions per unit electrical energy from the FC at the user's location can be defined as in [eqn (20)].
The equivalent CO2 emissions for a given HEP is the sum of contributions from: direct CO2 emissions, methane, N2O and hydrogen emissions.
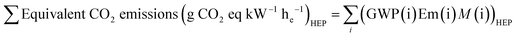 | (20) |
| Em(CO2)CC = Em(CO2) × (1 − effectiveness of CCUS) | (21) |
We have not quantified the equivalent CO2 emissions from each HEP due to the dearth of reliable industry data90,91 on the percent emission rates and speculative nature of future outcomes from CCUS and the implementation of new energy conversion technologies. There is an expectation that emissions are below prescribed safety levels and can be substantially reduced through increased monitoring, more reliable systems90 and with policy decisions informed from life-cycle assessments.91 Rather, we choose to limit our analysis only to direct CO2 emissions. However, the equivalent CO2 emission level from methane, nitrous oxide and hydrogen is sufficiently important that a careful, detailed evidence-based analysis using reliable data is required to ensure that future energy systems are more sustainable than today's systems. To encourage further analysis, we provide an example in ESI Note S4† to display the workings described above. Associated plots for three example scenarios (ESI Note S4†) show that the trend in outcomes between HEPs shown in Fig. 2 remain the same for the range of values investigated for CC effectiveness and emission rates. However, the example shown in ESI Note S4† uses the same values for each factor across all HEPs whereas, in practice they will differ, and thus, the relative impacts between HEPs may change.
Conclusions
Using a technology neutral framework, we compare stationary PEMFC and SOFC performance for environmental sustainability when powered from a range of energy generation pathways. This comparison is made for an expected production of 1969 TW he of clean electricity per annum as projected for the IEA NZE guideline for hydrogen energy demand in 2050, using three metrics: (1) the amount of primary energy required at the start of the ES pathway, (2) the mass of fossil fuel relating to the FF energy, and (3) the amount of CO2 that needs to be abated for net zero emissions from the HEPs.HEPs where blue hydrogen is produced, from water electrolysis using mains electricity generated from FF, for use in PEMFCs provides the most unsustainable environmental outcomes. This combination of energy pathways utilizes 1363 MT per annum of NG and produces 3750 MT per annum of CO2 that needs to be abated by CC. Electrolysers powered from a national electricity network with a high FF basis are unlikely to meet the United Nations SDGs without substantial effort and expenditure to implement CCUS. However, national or regional networks with a high percentage of renewable energy will trend towards green H2 production outcomes.
For blue hydrogen use in a PEMFC, SR of NG is a more responsible choice than coal gasification with 490 MT of NG required and 1350 MT of CO2 to be abated compared to 1237 MT of coal and 2950 MT CO2 to be abated from coal gasification. However, the direct use of NG in an SOFC is a more efficient and sustainable pathway with a substantially lower 268 MT of NG required and 736 MT of CO2 to be abated.
A net zero emissions outcome to meet UN SDG guidelines will require abatement or capture of the majority, if not all, of the CO2 and consideration of methane, nitrous oxide and hydrogen emissions23,58 at additional cost that requires further analysis. However, the above conclusion highlights that it is problematic for NG producers to become suppliers of blue hydrogen for use in PEMFCs in the electricity supply networks when the more responsible sustainable pathway of direct NG use in SOFC's is available, especially for locations where an NG supply infrastructure is already in place.
Finally, renewable energy HEPs have the best sustainability outcome. Green H2 production for use in a PEMFC requires 6.1 GW of solar energy to produce 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 012 TW he of renewable electricity with no requirement for CC. However, this pathway requires a high renewable electrical energy demand due to modest conversion rates for solar efficiency and water electrolysis. Current solar power and water electrolysis efficiencies place pressure on the sustainability of material supply for renewables infrastructure. This impost on sustainability suggests that further technology advances on conversion efficiency will greatly enhance the viability of this pathway to net zero emissions.
012 TW he of renewable electricity with no requirement for CC. However, this pathway requires a high renewable electrical energy demand due to modest conversion rates for solar efficiency and water electrolysis. Current solar power and water electrolysis efficiencies place pressure on the sustainability of material supply for renewables infrastructure. This impost on sustainability suggests that further technology advances on conversion efficiency will greatly enhance the viability of this pathway to net zero emissions.
There is a major difference between SOFCs, that can convert carbonaceous fuels to both heat and power at high efficiency, and PEMFCs, that are restricted to high purity hydrogen conversion to power at lower efficiency. This produces very different outcomes when considering the best use of fuel creating an interdependence between the primary ES pathway and the available FC technology in the F2P pathway. This interdependency significantly impacts sustainability and climate change outcomes. We recommend consideration of these issues in future analyses for policy deliberation and with consumer regulatory frameworks for clean electricity and transport. We also recommend that a sustainable energy economy is the ultimate objective rather than a hydrogen economy. These recommendations will significantly increase the share of current predictions for future hydrogen demand in favour of renewable hydrogen.
The implementation of a sustainable energy economy framework shows that the FF industry is therefore best advised to pivot business plans toward renewable hydrogen for stationary FC applications. The next best option is for the FF industry to pursue direct conversion of NG to electricity at high efficiencies using SOFC technology equipped with CCU. However, the FF industry will need to achieve close to total elimination of CO2 emissions as well as methane and N2O emissions. The use of technologies that enable a progressive transition to blends of NG and renewable hydrogen that evolve to accept higher proportions of renewable input over time may be one strategy to ensure no lock-in of unsustainable FF assets for future decades.
Author contributions
Jonathan Love: Conceptualisation, Analysis, Investigation, Methodology, Writing – original draft, Visualization, Writing – review & editing. Fanny Boulaire: Investigation, Writing – review & editing. Anthony O'Mullane: Funding acquisition, Writing – review & editing. Ian Mackinnon: Funding acquisition, Writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding from ARENA, industry and university partners as part of an Australian Government Research and Development Program – Renewable Hydrogen for Export (Contract No. 2018/RND012). The views expressed herein are not necessarily the views of the Australian Government, and the Australian Government does not accept responsibility for any information or advice contained herein.References
- United Nations, Paris Agreement,Paris, 2016 Search PubMed.
- International Energy Agency World Energy Outlook 2020, Paris, 2020 Search PubMed.
- Commonwealth of Australia, Australia's National Hydrogen Strategy, 2019 Search PubMed.
- Hydrogen and Fuel Cell Strategy Council of Japan, The Strategic Road Map for Hydrogen and Fuel Cells, 2019 Search PubMed.
- Ministry of Trade, Industry and Energy, Hydrogen Economy Roadmap Korea, 2019 Search PubMed.
- X. Meng, A. Gu, X. Wu, L. Zhou, J. Zhou, B. Liu and Z. Mao, Int. J. Hydrogen Energy, 2021, 46, 28887–28899, DOI:10.1016/j.ijhydene.2020.11.049.
- Federal Ministry for Economic Affairs and Energy, Germany, The National Hydrogen Strategy, 2020 Search PubMed.
- World Nuclear Association, Fukushima Daiichi Accident, https://www.world-nuclear.org/information-library/safety-and-security/safety-of-plants/fukushima-daiichi-accident.aspx, accessed April, 2021 Search PubMed.
- J. Chatzimarkakis, C. Levoyannis, A. van Wijk and F. Wouters, Hydrogen Act. Towards the Creation of the European Hydrogen Economy, 2021 Search PubMed.
- The US Department of Energy, Hydrogen Program Plan, https://www.hydrogen.energy.gov/roadmaps_vision.html, accessed August, 2021 Search PubMed.
- The Norwegian Government's hydrogen strategy, Towards a low emission society, https://www.regjeringen.no/en/dokumenter/the-norwegian-governments-hydrogen-strategy/id2704860/, (accessed July, 2022) Search PubMed.
- United Nations, Transforming our world: the 2030 Agenda for Sustainable Development, A/RES/70/1, 2015 Search PubMed.
- International Energy Agency, The Future of Hydrogen, IEA, Paris, 2019 Search PubMed.
- International Energy Agency, Net Zero by 2050 A Roadmap for the Global Energy Sector, IEA Special Report, 2021 Search PubMed.
- Energy Transitions Commission, Making the Hydrogen Economy Possible – Accelerating Clean Hydrogen in an Electrified Economy. Version 1.2, 2021 Search PubMed.
- J. Parnell, Europe's Green Hydrogen Revolution Is Turning Blue. With the EU on the cusp of announcing its long-term hydrogen strategy, a huge question remains: Should blue hydrogen be excluded?, https://www.greentechmedia.com/articles/read/europes-green-hydrogen-revolution-is-turning-blue, accessed August, 2021 Search PubMed.
- T. Longden, F. J. Beck and F. Jotzo, Australia is at a crossroads in the global hydrogen race – and one path looks riskyhttps://theconversation.com/australia-is-at-a-crossroads-in-the-global-hydrogen-race-and-one-path-looks-risky-157864, accessed August, 2021 Search PubMed.
- A. Finkel, Getting to Zero. Australia's Energy Transition, https://www.quarterlyessay.com.au/essay/2021/03/getting-to-zero, accessed March, 2021 Search PubMed.
- UK Hydrogen Strategy (accessible HTML version), https://www.gov.uk/government/publications/uk-hydrogen-strategy/uk-hydrogen-strategy-accessible-html-version, accessed September, 2021 Search PubMed.
- G. Alova, Nat. Energy, 2020, 5, 920–927, DOI:10.1038/s41560-020-00686-5.
- P. Koutsogeorgas, Z. Ripecky, The future of big oil in the hydrogen economy, Master's project, Duke University, Retrieved from https://hdl.handle.net/10161/22708) Search PubMed.
- Energy Information Australia, Natural Gas and Hydrogen, https://energyinformationaustralia.com.au/wp-content/uploads/2019/10/Natural-Gas-and-Hydrogen-Landscape-Final-2019.pdf, accessed September, 2021 Search PubMed.
- R. W. Howarth and M. Z. Jacobson, Energy Sci. Eng., 2021, 1–12, DOI:10.1002/ese3.956.
- T. Longden, F. Jotzo, M. Prasad and R. Andrews, CCEP Working Paper 20-07, ZCEAP Working Paper ZCWP03-20, August 2020, The Australian National University, 2020 Search PubMed.
- T. Longden, F. J. Beck, F. Jotzo, R. Andrews and M. Prasad, CCEP Working Paper 21-03, ZCEAP Working Paper ZCWP02-21, March 2021, The Australian National University, 2021 Search PubMed.
- T. Longden, F.J. Beck, F. Jotzo, R. Andrews and M. Prasad, Appl. Energy, 2022, 306, 118145, DOI:10.1016/j.apenergy.2021.118145.
- D. Welsby, J. Price, S. Pye and P. Ekins, Nature, 2021, 597, 230–234, DOI:10.1038/s41586-021-03821-8.
- International Energy Agency, Technology Roadmap - Hydrogen and Fuel Cellshttps://www.iea.org/reports/technology-roadmap-hydrogen-and-fuel-cells, accessed June, 2021.
- EIA, U.S. Energy Information Analysis, Country Analysis Executive Summary: Japan (Last Updated: October 2020), https://www.eia.gov/international/content/analysis/countries_long/Japan/japan.pdf Search PubMed.
- N. Behling, M. C. Williams and S. Managi, Econ. Anal. Pol., 2015, 48, 204–221, DOI:10.1016/j.eap.2015.10.002.
- R. Carapellucci and L. Giordano, J. Power Sources, 2020, 469, 228391, DOI:10.1016/j.jpowsour.2020.228391.
- J. Guo, Z. Hou and X. Zheng, Chin. J. Catal., 2010, 31, 1115–1121, DOI:10.1016/S1872-2067(10)60109-X.
- T. F. Liu, H. Temur and G. Veser, Chem. Eng. Technol., 2009, 32, 1358–1366, DOI:10.1002/ceat.200900203.
- Y. Yan, H. Li, L. Li, L. Zhang and J. Zhang, Chem. Eng. Process., 2018, 125, 311–317, DOI:10.1016/j.cep.2018.01.010.
- B. Parkinson, J. W. Matthews, T. B. McConnaughy, D. C. Upham and E. W. McFarland, Chem. Eng. Technol., 2017, 40, 1022–1030, DOI:10.1002/ceat.201600414.
- D. C. Toncu, G. Toncu and S. Soleimani, presented in Part at the IOP Conference Series: Material Science and Engineering, Modern Technologies in Industrial Engineering (ModTech2015), Mamaia, Romania, 2015, DOI:10.1088/1757-899X/95/1/012026.
- T. Li, C. Rehmet, Y. Cheng, Y. Jin and Y. Cheng, Plasma Chem. Plasma Process., 2017, 37, 1033–1049, DOI:10.1007/s11090-017-9806-x.
- J. O. Pacheco-Sotelo, R. Valdivia-Barrientos and M. Pacheco-Pacheco, Green Technologies to Improve the Environment on Earth, 2019, 21 DOI:10.5772/intechopen.82097.
- D. Bonaquist, Analysis of CO2 Emissions, Reductions, and Capture for Large-Scale Hydrogen Reduction Plants, A White Paper, 2010 Search PubMed.
- A. Dorr and T. Seba, Rethinking Energy the Great Stranding: How Inaccurate Mainstream LCOE Estimates Are Creating a Trillion-Dollar Bubble in Conventional Energy Assets 2021 Search PubMed.
- Australian and Global Hydrogen Demand Growth Scenario Analysis, 2019 Search PubMed.
- Z. Abdin, A. Zafaranloo, A. Rafiee, W. Merida, W. Lipiski and K. R. Khalilpour, Renew. Sustain. Energy Rev., 2020, 120, DOI:10.1016/j.rser.2019.109620.
- N. Sazali, W. N. W. Salleh, A. S. Jamaludin and M. N. Mhd Razali, Membranes, 2020, 10, 99, DOI:10.3390/membranes10050099.
- US Department of Energy, Hydrogen and Fuel Cell Technologies Office. Comparison of Fuel Cell Technologies, https://www.energy.gov/eere/fuelcells/comparison-fuel-cell-technologies, accessed July, 2021 Search PubMed.
- S. Mekhilef, R. Saidur and A. Safari, Renew. Sustain. Energy Rev., 2012, 16, 981–989, DOI:10.1016/j.rser.2011.09.020.
- I. Staffell, D. Scamman, A. V. Abad, P. Balcombe, P. E. Dodds, P. Ekins, N. Shahd and K. R. Warda, Energy Environ. Sci., 2019, 12, 463–491, 10.1039/c8ee01157e.
- International Organization for Standardization, ISO 14687:2019(en) Hydrogen fuel quality — Product specification, https://www.iso.org/obp/ui/#iso:std:iso:14687:ed-1:v1:en, accessed August, 2021 Search PubMed.
- US Department of Energy, Hydrogen Fuel Quality Specifications for Polymer Electrolyte Fuel Cells in Road Vehicles, https://www.energy.gov/eere/fuelcells/downloads/hydrogen-fuel-quality-specifications-polymer-electrolyte-fuel-cells-road Search PubMed.
- International Energy Agency, Electricity Information: Overviewhttps://www.iea.org/reports/electricity-information-overview, accessed July, 2021.
- S. Schwietzke, O. A. Sherwood, L. M. P. Bruhwiler, J. B. Miller, G. Etiope, E. J. Dlugokencky, S. E. Michel, V. A. Arling, B. H. Vaughn, J. W. C. White and P. P. Tans, Nature, 2016, 538, 88–91, DOI:10.1038/nature19797.
- B. Hmiel, V. V. Petrenko, M. N. Dyonisius, C. Buizert, A. M. Smith, P. F. Place, C. Harth, R. Beaudette, Q. Hua, B. Yang, I. Vimont, S. E. Michel, J. P. Severinghaus, D. Etheridge, T. Bromley, J. Schmitt, X. Faïn, R. F. Weiss and E. Dlugokencky, Nature, 2020, 578, 409–412, DOI:10.1038/s41586-020-1991-8.
- Intergovernmental Panel on Climate Change (IPCC), Chapter 4 Fugitive Emissions, Volume 2 Energy, 2006 Search PubMed.
- P. Balcombe, K. Anderson, J. Speirs, N. Brandon and A. Hawkes, ACS Sustainable Chem. Eng., 2017, 5, 3–20, DOI:10.1021/acssuschemeng.6b00144.
- X. Ren, D. L. Hall, T. Vinciguerra, S. E. Benish, P. R. Stratton, D. Ahn, J. R. Hansford, M. D. Cohen, S. Sahu, H. He, C. Grimes, J. D. Fuentes, P. B. Shepson, R. J. Salawitch, S. H. Ehrman and R. Dickerson, J. Geophys. Res. Atmos., 2019, 124, 1862–1878, DOI:10.1029/2018JD029690.
- G. Plant, E. A. Kort, C. Floerchinger, A. Gvakharia, I. Vimont and C. Sweeney, Geophys. Res. Lett., 2019, 64, 8500–8507, DOI:10.1029/2019GL082635.
- A. Waxman, A. Khomaini, B. Leibowicz and S. Olmstead, Environ. Res. Lett., 2020, 15, 014004, DOI:10.1088/1748-9326/ab5e6f.
- D. J. Zimmerle, L. L. Williams, T. L. Vaughn, C. Quinn, R. Subramanian, G. P. Duggan, B. Willson, J. D. Opsomer, A. J. Marchese, D. M. Martinez and A. L. Robinson, Environ. Sci. Technol., 2015, 49, 9374–9383, DOI:10.1021/acs.est.5b01669.
- IPCC, Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. V. Masson-Delmotte, P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu and B. Zhou, Cambridge University Press, In Press, 2021 Search PubMed.
- N. Kholod, M. Evans, R. C. Pilcher, V. Roshchanka, F. Ruiz, M. Coté and R. Collings, J. Clean. Prod., 2020, 256, 120489, DOI:10.1016/j.jclepro.2020.120489.
- United States Environmental Protection Agency, Frequent Questions About Coal Mine Methane, https://www.epa.gov/cmop/frequent-questions, accessed 30th August, 2021 Search PubMed.
- P. Sun, B. Young, A. Elgowainy, Z. Lu, M. Wang, B. Morell and a. T. Hawkins, Environ. Sci. Technol., 2019, 53, 7103–7113, DOI:10.1021/acs.est.8b06197.
- M. R. Allen, K. P. Shine, J. S. Fuglestvedt, R. J. Millar, M. Cain, D. J. Frame and A. H. Macey, NPJ Clim. Atmos. Sci., 2018, 1(16) DOI:10.1038/s41612-018-0026-8.
- PowerCell Sweden AB, https://powercellution.com/power-generation-system-100, accessed 30th August, 2021.
- A. Dicks and A. J. R. David, Fuel Cell Systems Explained, John Wiley & Sons Ltd, 3rd edn, 2018 Search PubMed.
- W. R. W. Daud, R. E. Rosli, E. H. Majlan, S. A. A. Hamid, R. Mohamed and T. Husaini, Renewable Energy, 2017, 113, 620–638, DOI:10.1016/j.renene.2017.06.027.
- Z. Liu, L. Yang, Z. Mao, W. Zhuge, Y. Zhang and L. Wang, J. Power Sources, 2006, 157, 166–176, DOI:10.1016/j.jpowsour.2005.08.006.
- L. Zhao, J. Brouwer, S. James, J. Siegler, E. Peterson, A. Kansal and J. Liu, Int. J. Hydrogen Energy, 2017, 42, 10158–10174, DOI:10.1016/j.ijhydene.2017.03.004.
- L. Zhao, J. Brouwer, S. James, J. Siegler, E. Peterson, A. Kansal and J. Liu, ASME 2014, 12th International Conference on Fuel Cell Science, Engineering and Technology, Boston, Massachusetts, USA, 2014 Search PubMed.
- J. Baschuk and X. Li, Int. J. Energy Res., 2001, 25, 695–713, DOI:10.1002/er.713.
- V. F. Valdés-López, T. Mason, P. R. Shearing and D. J. L. Brett, Prog. Energy Combust. Sci., 2020, 79, 100842, DOI:10.1016/j.pecs.2020.100842.
- G. Gwak, M. Kim, D. Kim, M. Faizan, K. Oh, J. Lee, J. Choi, N. Lee, K. Lim and H. Ju, Energies, 2019, 12, 905, DOI:10.3390/en12050905.
- P. V́agner, R. Kod́yma and K. Bouzeka, Sustain. Energy Fuels, 2019, 3, 2076, 10.1039/c9se00030e.
- Bloom Energy, Energy Server 5 300kW Product Datasheethttps://www.bloomenergy.com/wp-content/uploads/es5-300kw-datasheet-2019.pdf, accessed June, 2021 Search PubMed.
- M. Powell, K. Meinhardt, V. Sprenkle, L. Chick and G. McVay, J. Power Sources, 2012, 205, 377–384, DOI:10.1016/j.jpowsour.2012.01.098.
- S. Chen, N. Lior and W. Xiang, Appl. Energy, 2015, 146, 298–312, DOI:10.1016/j.apenergy.2015.01.100.
- H. Ghezel-Ayagh, Integrated Coal Gasification Solid Oxide Fuel Cell Systems, Washington, DC, 2010 Search PubMed.
- S. Nierop and S. Humperdinck, ECOFYS Final Report. International Comparison of Fossil Power Efficiency and CO2 Intensity, 2018 Search PubMed.
- EIA, US Energy Information Adminstration, How much electricity is lost in electricity transmission and distribution in the United States? Last updated: May 14, 2021, https://www.eia.gov/tools/faqs/faq.php?id=105%26t=3, accessed June, 2021 Search PubMed.
- B. Guinot, F. Montignac, B. Champel and D. Vannucci, Int. J. Hydrogen Energy, 2015, 40, 8778–8787, DOI:10.1016/j.ijhydene.2015.05.033.
- A. E. Samani, A. D’Amicis, J. D. M. DeKooning, D. Bozalakov, P. Silva and L. Vandevelde, IET Renew. Power Gener., 2020, 14, 3070–3078, DOI:10.1049/iet-rpg.2020.0453.
- S. S. Mohammadshahi, F. A. Boulaire, J. G. Love, S. A. Gorji and I. D. R. Mackinnon, Int. J. Hydrogen Energy, 2022, 47(2), 782–808, DOI:10.1016/j.ijhydene.2021.10.072.
- International Energy Agency, About CCUS, https://www.iea.org/reports/about-ccus, accessed August, 2021 Search PubMed.
- D. Y. C. Leung, G. Caramanna and M. M. Maroto-Valer, Renew. Sustain. Energy Rev., 2014, 39, 426–443, DOI:10.1016/j.rser.2014.07.093.
- B. Parkinson, P. Balcombe, J. F. Speirs, A. D. Hawkes and K. Hellgardt, Energy Environ. Sci., 2019, 12, 19–40, 10.1039/C8EE02079E.
- Diffusion in Gases, in CRC Handbook of Chemistry and Physics, (Internet Version 2021), ed. John Rumble, CRC Press/Taylor & Francis, Boca Raton, FL, 102nd edn, 2021 Search PubMed.
- P. Forster, V. Ramaswamy, P. Artaxo, T. Berntsen, R. Betts, D. W. Fahey, J. Haywood, J. Lean, D. C. Lowe, G. Myhre, J. Nganga, R. Prinn, G. Raga, M. Schulz and R. Van Dorland, Changes in Atmospheric Constituents and in Radiative Forcing, Cambridge, United Kingdom and New York, NY, USA, 2007 Search PubMed.
- R. G. Derwent, W. J. Collins, C. E. Johnson and D. S. Stevenson, Clim. Change, 2001, 49, 463–487, DOI:10.1023/A:1010648913655.
- R. Derwent, P. Simmonds, S. O’Doherty, A. Manning, W. Collins and D. Stevenson, Int. J. Nucl. Hydrogen Prod. Appl., 2006, 1, 57–67, DOI:10.1504/IJNHPA.2006.009869.
- T. Boningari and P. G. Smirniotis, Curr. Opin. Chem. Eng., 2016, 13, 133–141, DOI:10.1016/j.coche.2016.09.004.
- R. A. Alvarez, D. Zavala-Araiza, D. R. Lyon, D. T. Allen, Z. R. Barkley, A. R. Brandt, K. J. Davis, S. C. Herndon, D. J. Jacob, A. Karion, E. A. Kort, B. K. Lamb, T. Lauvaux, J. D. Maasakkers, A. J. Marchese, M. Omara, S. W. Pacala, J. Peischl, A. L. Robinson, P. B. Shepson, C. Sweeney, A. Townsend-Small, S. C. Wofsy and S. P. Hamburg, Science, 2018, 361, 186–188, DOI:10.1126/science.aar7204.
- S. A. Roman-White, J. A. Littlefield, K. G. Fleury, D. T. Allen, P. Balcombe, K. E. Konschnik, J. Ewing, G. B. Ross and F. George, ACS Sustainable Chem. Eng., 2021, 9, 10857–10867, DOI:10.1021/acssuschemeng.1c03307.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2se00923d |
| This journal is © The Royal Society of Chemistry 2022 |