Low-temperature resistant gel polymer electrolytes for zinc–air batteries†
Jiao
Wu
ab,
Yuchao
Wang
a,
Danni
Deng
b,
Yu
Bai
a,
Mengjie
Liu
a,
Xin
Zhao
a,
Xiang
Xiong
a and
Yongpeng
Lei
 *a
*a
aState Key Laboratory of Powder Metallurgy, Central South University, Changsha, Hunan 410083, China. E-mail: leiyongpeng@csu.edu.cn; lypkd@163.com
bSchool of Material Science and Engineering, Central South University of Forestry and Technology, Changsha, Hunan 410004, China
First published on 13th June 2022
Abstract
The rapid development of wearable devices has put forward high requirements for stable, solid-state, flexible and even stretchable energy storage systems. Owing to their high specific energy density and volumetric energy density, metal–air batteries especially high-safety zinc–air batteries (ZABs), have attracted widespread attention. However, limited by the reduced ionic conductivity of electrolyte and the sluggish kinetics of oxygen reduction/evolution reactions at the air cathode during discharge/charge processes below 0 °C, the performances of ZABs severely deteriorate. Rationally designed gel polymer electrolytes (GPEs) not only offer superior mechanical performance but also provide ZABs with accelerated ion transport to boost electrochemical performance at low temperatures. Herein, the types of GPEs towards electrochemical energy systems are first summarized. And then, the research toolbox for GPEs and assembled ZABs is put forward. Next, the design strategies for low-temperature tolerant GPEs in ZABs are highlighted, such as introduction of organic solvents, alkalization of hydrogel electrolytes, construction of double-network electrolytes, etc. Finally, current challenges and perspectives are proposed. This review provides up-to-date insights on the rational design of GPEs for ZABs, which can be expanded to other metal–air batteries, metal–sulfur batteries, metal-ion batteries and so on.
 Jiao Wu | Jiao Wu received her Bachelor’s degree from Dalian University (2020). She is currently pursuing her Master’s degree. |
1. Introduction
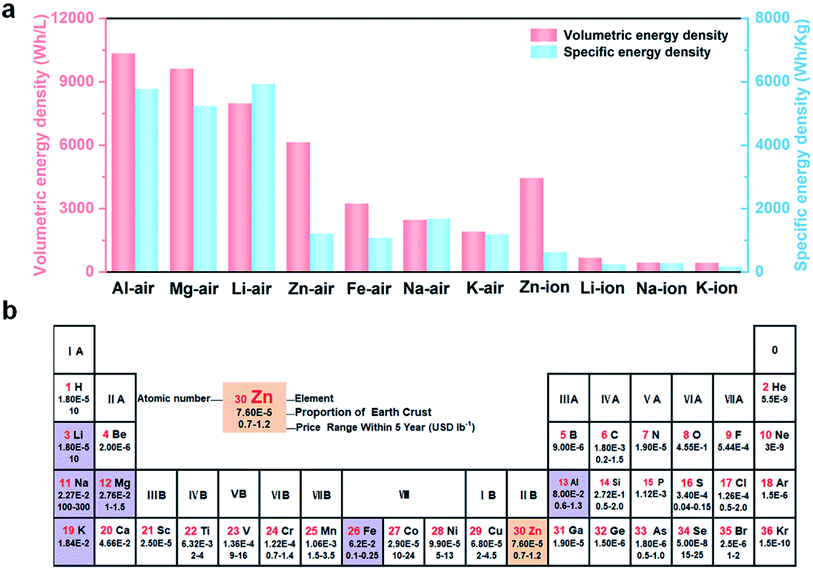
The research studies on (quasi-) solid-state batteries used in flexible electronics have received great interest because of their portability, wearability, safety, etc.1–3 At the same time, the demand for rechargeable batteries with high energy density under harsh conditions is increasing.4 In particular, high latitudes/altitudes and other alpine regions place urgent requirements on batteries with low-temperature resistance.5,6 Owing to their significantly higher energy density than metal-ion batteries, metal–air batteries have attracted considerable attention as next-generation energy conversion/storage systems.7,8 The theoretical specific energy density and volumetric energy density of typical metal–air batteries and metal-ion batteries are shown in Fig. 1a.9,10Obviously, metal–air batteries with Mg, Al and Li anodes display higher theoretical energy densities than others. However, the high reactivity and low reduction potential of Mg/Al could result in rapid self-discharge and low coulombic efficiency of the corresponding metal–air batteries.11 In addition, Li metal has a lower abundance in the Earth’s crust and higher cost compared to other metal anode materials (Fig. 1b).12 And it is easily damaged when exposed to air and electrolyte solutions, leading to potential safety hazards.13 It's worth noting that metal Zn displays the intrinsic advantages of relatively high energy density, low cost, high safety (aqueous and non-flammable), etc., which endow Zn–air batteries (ZABs) with broad prospects in advanced electronic devices.14
The timeline for the development of ZABs is shown in Fig. 2.15–25 Since the emergence of ZABs, batteries have been continuously developed towards flexibility and low-temperature resistance. ZABs consist of a zinc anode, electrolyte and a porous air electrode with catalysts (Fig. 3a). Liquid electrolytes can be divided into alkaline, non-alkaline (neutral and acidic-alkaline dual electrolytes) and ionic liquid systems according to the pH and ion types.26–29 In contrast to alkaline electrolytes, non-alkaline electrolytes face sluggish oxygen catalysis kinetics and low reactant concentrations while ionic liquids display relatively low conductivity and high cost, greatly limiting their applications in ZABs.30 Therefore, most studies on ZABs have been carried out in alkaline electrolytes.31,32 During the discharge process, the oxygen at the three-phase boundary among electrolyte, catalyst and air is reduced to hydroxide ions (OH−): O2 + 2H2O + 4e− → 4OH− (oxygen reduction reaction, ORR). O2 in air significantly reduces the cost, size and weight of the batteries. The generated OH− ions migrate to the zinc electrode to form zincate ions (Zn(OH)2−4), which are further decomposed into insoluble zinc oxide (ZnO): Zn + 4OH− → Zn(OH)2−4 + 2e−, Zn(OH)2−4 → ZnO + H2O + 2OH−.33 The whole discharge reaction is 2Zn + O2 → 2ZnO. The charge process of ZABs is the opposite of the electrochemical reactions described above, and the oxygen evolution reaction (OER) occurs at the air electrode. At present, KOH solutions are widely used because of their high ionic conductivity (550 mS cm−1 for 35 wt% KOH solvent at 25 °C), low viscosity (2.2339 mPa s) and large oxygen diffusion coefficient, offering outstanding electrochemical kinetics and mass transfer of batteries. At the same time, the salt solution of zinc is added into alkaline electrolyte to supply zinc ions and promote dissolution equilibrium.34
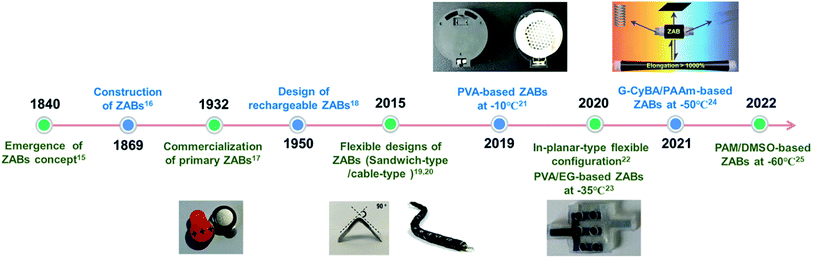 | ||
| Fig. 2 Timeline for the development of ZABs.15–25 | ||
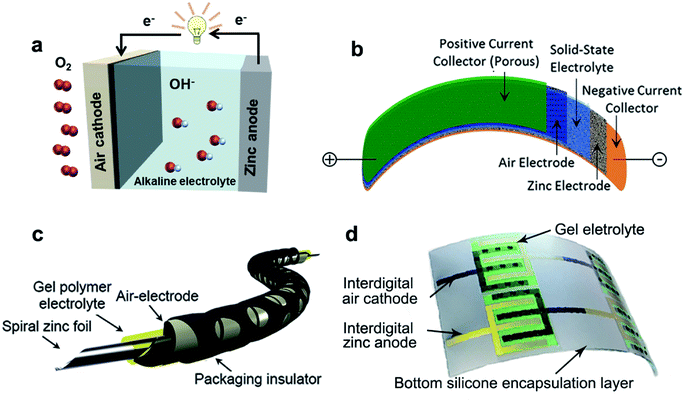 | ||
| Fig. 3 Illustration of various configurations of ZABs: (a) liquid-type and (b) sandwich-type. Reproduced with permission from ref. 10. Copyright 2017, Wiley. (c) Cable-type. Reprinted with permission from ref. 20. Copyright 2015, Wiley. (d) In-plane-type. Reproduced with permission from ref. 22. Copyright 2020, Elsevier. | ||
Up to now, the reported flexible ZABs are mainly based on three designs: sandwich-type,19 cable-type20 and in-plane-type (Fig. 3b–d).22 In these battery configurations, to achieve ion conduction while separating the cathode/anode, some single-phase polymer backbones with alkaline (OH−) functional groups (alkaline anion exchange membranes, AAEMs), alkaline electrolyte impregnated membranes (cellulose membranes or filter papers) and gel polymer electrolytes (GPEs) were developed.35–37 However, the poor water retention properties of those membranes cause a rapid drop in ionic conductivity and an apparent attenuation in battery performance during long-term operation.38 By contrast, GPEs possess a cross-linked network with a three-dimensional skeleton structure, which could hold a large amount of solvents to maintain a high ionic conductivity and provide great mechanical properties. Replacing liquid electrolytes with GPEs can not only meet the pursuit of portability and wearability, but also solve the problems of solvent volatilization and leakage to some extent.39,40 In fact, GPEs play important roles as both electrolyte for ion migration and as a separator between the positive and negative electrodes, which significantly influence the power output, rate characteristics and cycle life of ZABs.41,42
Although there have been important breakthroughs in flexible ZABs at room temperature, their performance at low temperatures may be their Achilles' heel.43 The freezing of GPEs causes a decrease in conductivity and an increment in internal resistance.44 Modulating the solvated structure of electrolyte solutions has been proven to be a way to effectively improve the low-temperature tolerance of batteries.45,46 However, GPEs with saturated alkaline electrolytes are far from meeting the requirements (high current density, long cycle, etc.) for extremely low-temperature scenarios (≤−40 °C).47–49 In addition, there is a lack of in-depth guidance for constructing low-temperature resistant GPEs.50–52 In this review, the advantages, structures and working principles of ZABs are first introduced. Next, the commonly used polymers for advanced battery systems, especially ZABs are presented. And their characterization methods are systematically summarized in a research toolbox. Then, the design strategies (introduction of organic solvents, alkalization of hydrogel electrolytes and construction of double network (DN) hydrogel electrolytes) of low-temperature resistant GPEs towards ZABs are concluded in detail. The output performance, stability and mechanical properties of the assembled ZABs are also involved. Finally, the current challenges and prospects are presented.
2. The types and characterization methods of GPEs
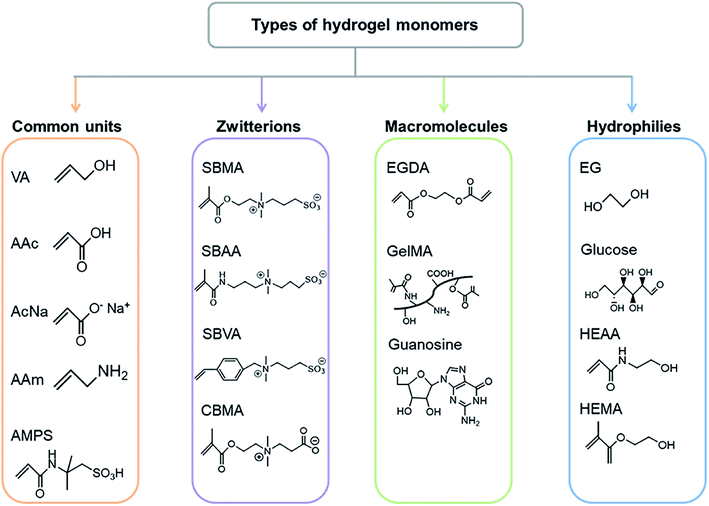
In general, the ideal GPEs for ZABs should present the following properties: (i) High ionic conductivity. (ii) Excellent ionic accessibility and compatibility at the electrode/electrolyte interface. (iii) High chemical and electrochemical stability. (iv) Weak electrode corrosion. (v) Good thermal and mechanical stabilities under various climatic conditions. (vi) Easy packing and assembly. (vii) Low cost and environmental friendliness.53 In practice, it is difficult for electrolytes to satisfy all requirements. To meet the applications in ZABs, the balance between different properties needs to be considered. Because of the great compatibility of the etheroxy group with various compounds, polyoxyethylene (PEO) was first studied as a solid polymer and used in all-solid-state electronic devices.54 However, problems including low water absorption, poor interfacial properties and sharply decreased conductivity at high salt concentrations led to its gradual replacement by highly absorbent polymers, such as polyvinyl alcohol (PVA), polyacrylic acid (PAA), polyacrylamide (PAM), etc. To date, a variety of GPEs with specific characteristics have been applied to different battery systems (Table S1†). And the different types of monomers are summarized in Fig. 4. These GPEs contain abundant hydrophilic groups (–OH, –COOH, –SO3, and –NH2), which will facilitate fast polymerization and the accommodation of solvents.55 To further improve the electrical conductivity, mass transfer capacity and flexibility of GPEs, strategies such as the introduction of additives and cross-linking are widely applied. What's more, complex functionality, e.g., suppressing dendrites, frost resistance and self-healing could also be achieved. Therefore, the adjustable structural components of GPEs endow them with potential in flexible battery devices. The intrinsic properties of GPEs were clearly related to the output performance of assembled batteries. Considering the diversity and complexity of polymer monomers and additives, only three of the most commonly used polymers in ZABs are selected for introduction here.PVA is a typical water-soluble polymer containing a large number of hydroxyl groups, which makes it easy to form macromolecular network structures through chemical cross-linking (Fig. 5a).56 Therefore, this commonly used polymer has properties such as excellent chemical stability, durability, non-toxicity and a simple preparation process. The PVA-KOH-based GPEs are suitable for ZABs due to the above-mentioned advantages and their adaptability to KOH.57–59 In 2000, Lewandowski and partners60 prepared alkaline PVA GPE for the first time and investigated its electrochemical properties, which showed conductivities of 1 to 10 mS cm−1. Cao et al.61 fabricated PVA-KOH-based flexible electrolytes and assembled foldable ZABs, which exhibited a maximum power density of 28 mW cm−2. After 30 cycles of repeated bending, the voltage overpotential (difference of charge–discharge potential) only increased to 30 mV. However, the tightly connected networks in PVA made it less absorptive to liquid electrolytes, which caused low ionic conductivity.62 Through cross-linking,63 heat treatment64 and compounding with other materials,65 the mechanical properties and absorption capacity of PVA can be enhanced.
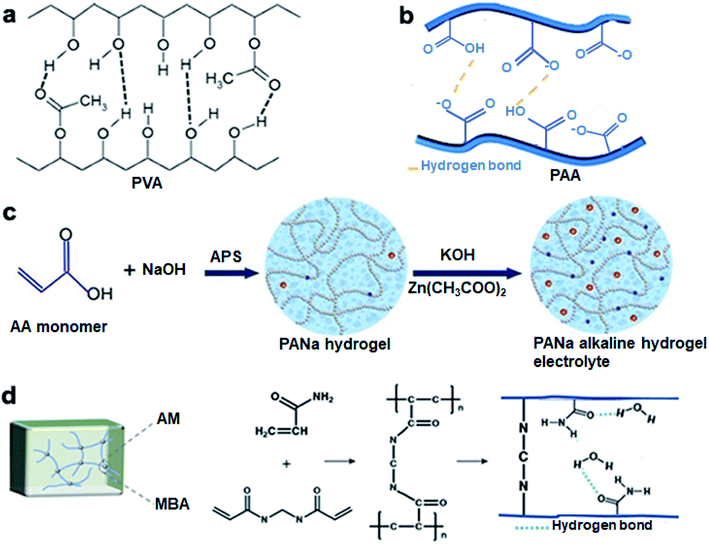 | ||
| Fig. 5 Structures of (a) PVA and (b) PAA. Reproduced with permission from ref. 56. Copyright 2019, Elsevier. Reproduced with permission from ref. 66. Copyright 2020, Elsevier. The polymerization processes of (c) PANa and (d) PAM. Reproduced with permission from ref. 69. Copyright 2018, Elsevier. Reproduced with permission from ref. 72. Copyright 2022, Elsevier. | ||
PAA could form hydrogen bonds with lots of H2O molecules on account of the existence of carboxylic acid groups, thus presenting strong water absorption and storage properties (Fig. 5b).66 Compared to PVA, PAA demonstrates higher ionic conductivity and better mechanical properties.67 PAA-KOH-based GPE was prepared by Iwakura et al.68 in 2001. The strong alkali absorption capacity of PAA enables it to have high conductivity (600 mS cm−1), similar to that of liquid electrolytes. However, the high water content also makes the PAA brittle, resulting in poor cycling performance of batteries. In view of this, the polyacrylic acid hydrogel electrolyte (sodium hydroxide neutralization in PAA, called PANa) was synthesized by Zhi and companions (Fig. 5c).69 PANa has the following advantages: high chemical stability in strong alkaline electrolytes, super absorbence caused by the concentration difference of ionic groups inside and outside the hydrogel network, and robust mechanical properties even in high-concentration saturated solutions.70,71 These strengths enable PANa-based ZABs to exhibit unprecedented ultra-long cycling stability (up to 800 cycles at 2 mA cm−2). Meanwhile, due to the strong interactions between PANa and the zinc electrode, the stable electrolyte/electrode interface was formed and the formation of zinc dendrites was mitigated.
PAM could easily form macromolecular networks by adding cross-linkers and initiators, of which the amide groups tend to form hydrogen bonds with water (Fig. 5d).72 Owing to its sufficient water absorption capacity and excellent deformation ability, PAM is widely used in various battery systems.73 In 2018, through in situ polymerization, Zhi et al.74 prepared PAM alkaline hydrogel for ZABs. The hydrogen bond network in PAM could dissipate the externally applied energy. Even compressed to 54% of the deformation, the output power of PAM-based ZABs did not decrease obviously. During the 220 charge/discharge cycles, the discharge performance had almost no attenuation.
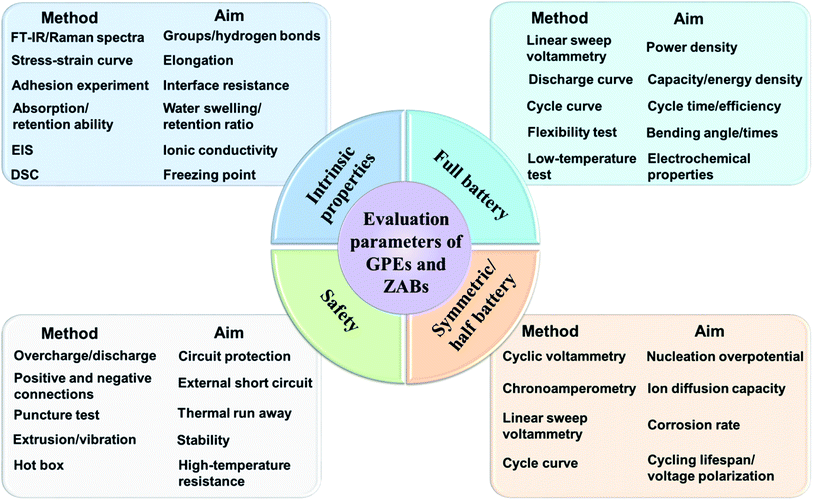
To study the physicochemical and mechanical properties of modified GPEs, the commonly used characterization methods are put forward and generalized in a toolbox (Fig. 6). For the intrinsic properties of GPEs, the paths of polymerization and cross-linking could be characterized using Fourier transform infrared (FT-IR) spectra and Raman spectra. Meanwhile, aiming at the applications in flexible ZABs, the mechanical properties, adhesion properties and water absorption/retention capacity of GPEs are fundamentally important parameters. Furthermore, under low-temperature conditions, the ion transport capacity (ionic conductivity) and the freezing point of GPEs can be estimated by electrochemical impedance spectroscopy (EIS) and differential scanning calorimetry (DSC), respectively. To verify the feasibility of GPEs in flexible ZABs, electrochemical tests are necessary to obtain various indicators of assembled batteries, such as: (i) Output performance, including power density, specific capacity and energy density. (ii) Cycle performance, including charge/discharge time and energy conversion efficiency. (iii) Flexibility performance, e.g. charge/discharge cycle at different bending angles or stress. (iv) Low temperature adaptability, performing those electrochemical tests at low temperatures. In addition, Zn‖Zn symmetric cells and half reactions are generally used to assess the stability of the electrolyte/zinc interface. Such a simplified setup facilitates the detection of electrode polarization and corrosion. As another key issue, battery safety also needs to be considered, especially for wearable devices. Artificial over-charge/discharge, short-circuit, puncture experiments etc. deserve focus.
3. Design strategies for low-temperature tolerant GPEs in ZABs
The excellent water storage capacity provides the liquid-like properties of GPEs. However, the freezing of the water molecules in GPEs leads to the collapse of the internal network structure and loss of flexibility. At the same time, the decreased water solubility at low temperatures contributes to salt precipitates and a reduced ionic conductivity of the GPEs. Hence, the frost and low ionic conductivity of GPEs at low temperature are the main reasons for the degradation of battery performance.75 On the one hand, the freezing GPE could not sufficiently come into contact with the catalytic active sites, which results in large interfacial resistance, high overpotential and low current during battery operation.76 On the other hand, the low ionic conductivity slows down the reaction kinetics, leading to severe polarizations and capacity degradation.77 Considering the formation of ice crystals is an ordered arrangement of free H2O molecules linked by hydrogen bonds, the suitability of GPEs for low temperatures needs to overcome the bonding between solvent molecules. The following three strategies have been applied: (1) The introduction of organic solvents, such as dimethyl sulfoxide (DMSO), ethylene glycol (EG), glycerol (Gly), etc., to break the hydrogen bonds between free H2O molecules. (2) The alkalization of hydrogels to enhance the binding energy between hydrogel networks and H2O molecules to inhibit the presence of free water. (3) The design of DN GPEs to add hydrophilic backbones and reduce the freezing point. Freeze-resistant and compatible GPEs enable battery systems to adapt to extreme conditions.3.1 Introduction of organic solvents
The low-temperature resistance of electrolytes is mainly related to overcoming the bonding between free H2O molecules, i.e. breaking the hydrogen bonds. Many small molecule solvents containing hydrogen bonded acceptors or donors perform the function of lowering the freezing point, such as various alcohols and especially DMSO with a high donor number.78,79 These organic solvents could serve as good media and form hydrogen bonds with free H2O molecules, thus limiting the activity of water. Besides, several studies have demonstrated that the introduction of organic solvents into electrolytes could inhibit the passivation of Zn surfaces and promote uniform Zn dissolution/deposition.80–82Inspired by these, we25 introduced DMSO into PAM and obtained the low-temperature resistant PAM organohydrogel electrolyte. As shown in the inset of Fig. 7a, Zn2+ is surrounded by a stable solvation structure in the water-based electrolyte. The high dielectric constant of DMSO (47.2) indicates that the addition of DMSO can easily replace H2O within the solvent sheath of Zn2+, thus reconfiguring the solvent structure. As a result, the “O” atoms in DMSO could combine with the “H” atoms in the H2O molecules to form stronger hydrogen bonds, breaking the order of H2O molecules at low temperatures and dramatically decreasing the freezing point. The DSC curve in Fig. 7a shows that the PAM organohydrogel had excellent frost resistance with a freezing point below −70 °C.
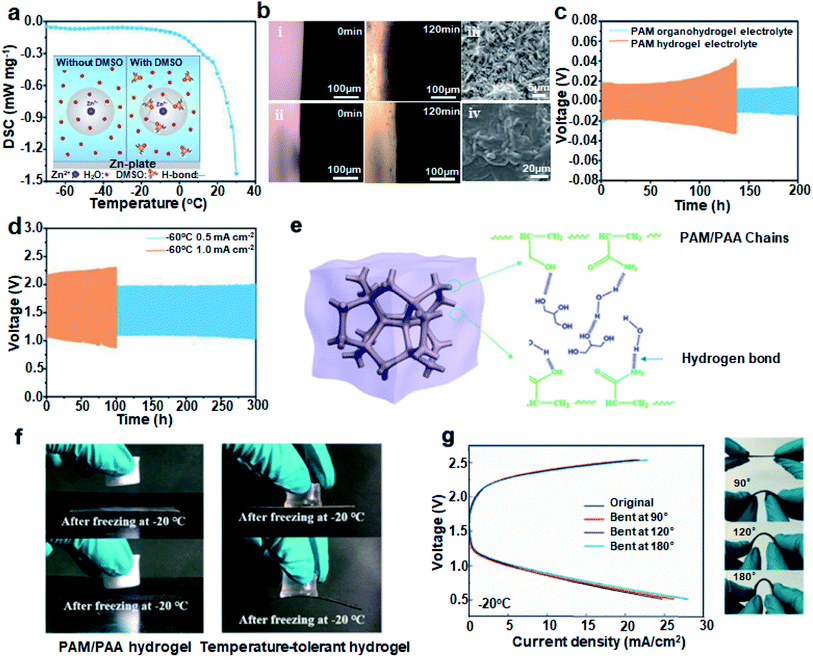 | ||
| Fig. 7 (a) DSC curve of PAM organohydrogel electrolyte. The inset shows the schematic diagram of the effect of DMSO. (b) The in situ optical visualizations of the interface during charge/discharge at 5 mA cm−2 in (i) 6 M KOH + 0.2 M Zn(Ac)2 and (ii) 6 M KOH + 0.2 M Zn(Ac)2 + 2 M DMSO and SEM images of Zn plates after cycling with (iii) PAM hydrogels and (iv) PAM organohydrogels as electrolytes, respectively. (c) Cycling performance of symmetric Zn‖Zn batteries at 2 mA cm−2. (d) Charge/discharge curves of ZABs at 0.5 and 1 mA cm−2 at −60 °C. (e) Schematic diagram of PAM/PAA with Gly. (f) Adhesion of the PAM/PAA hydrogels and the temperature-tolerant hydrogels to zinc flakes and carbon cloth. (g) Bending tests and charge/discharge polarization curves at −20 °C. Reproduced with permission from ref. 90. Copyright 2020, American Chemical Society. | ||
During the electrodeposition process, Zn2+ ions form tight ion pairs ([Zn(H2O)6]2+) with free H2O molecules. The large numbers of active H2O molecules at the electrolyte/anode interface are easily decomposed because the equilibrium potential of H2O/H2 is higher than that of Zn2+/Zn, leading to the generation of zinc dendrites and the occurrence of various side reactions (hydrogen evolution reaction and corrosion).83,84 The addition of organic solvent molecules in place of active water to modulate the solvation conformation around Zn2+ to [Zn(H2O)x]2+ (x < 6) can effectively reduce the solvation energy, which inhibits dendrites/side reactions and increases the reversibility of the Zn ions.85In situ optical visualization revealed that the zinc/electrolyte interface with the addition of DMSO to liquid electrolyte remained smooth after charge/discharge cycles, while the interface without DMSO was uneven (photos of (i) and (ii) in Fig. 7b). This means that side reactions such as hydrogen evolution and corrosion have occurred at the zinc anode, causing the interface to change. The scanning electron microscopy (SEM) images are also recorded to show that PAM hydrogel based batteries generate dense zinc dendrites after cycling, while the PAM organohydrogel based batteries have dendrite-free interfaces ((iii) and (iv) in Fig. 7b). Owing to the stable, dendrite-free interface and antifreeze organohydrogel, the long stripping/plating cycle of a Zn‖Zn symmetric cell exceeds 200 h (Fig. 7c). Also, the PAM-based full ZABs with the addition of DMSO exhibited record-breaking cycle stability at −60 °C. The charge/discharge life was up to 300 h at a current density of 0.5 mA cm−2 with a capacity retention of 90% at −60 °C (Fig. 7d). Li et al.86 also added DMSO to construct DN poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS)/PAM, which exhibited high mechanical strength and good ionic conductivity. Moreover, the introduction of DMSO could change the path of the OER in ZABs and reduce the charge voltage to improve the energy efficiency. These advantages bring out the extensive applications of DMSO in antifreeze electrolytes.
The layer structure of montmorillonite (MMT) could promote ionic conductivity by creating a directional conductive channel, which has been demonstrated to enhance the stability of hydrogels.87 We88 prepared PAM/MMT organohydrogel electrolytes in a mixed solution of DMSO and H2O. The assembled PAM/MMT-based ZABs provided a maximum power density of 30 mW cm−2 at −40 °C, while maintaining an 86% specific capacity corresponding to ZABs at room temperature. This work provides a feasible way to improve the electrical conductivity of GPEs and the electrochemical properties of ZABs.
EG and Gly are also commonly used organic solvents with abundant hydroxyl groups. The strong hydrogen bond interactions between EG and H2O molecules significantly weaken the hydrogen bond formation within the H2O molecules, resulting in a low freezing point.89 Xu et al.90 introduced Gly into PAM/PAA hydrogels to investigate its effect on freezing behavior, adhesion and mechanical properties. As seen in Fig. 7e, the terminal groups on the PAM/PAA chains formed a large number of hydrogen bonds with Gly and H2O molecules, which widened the operating temperature range to −20 to 70 °C. The Gly-free PAM/PAA hydrogels were frozen at −20 °C, while the temperature-tolerant hydrogels were almost unchanged. They remained tightly attached to the zinc anode and air electrode, reducing the interfacial internal resistance under bending (Fig. 7f). The discharge polarization curves of ZABs at different bending angles basically overlapped, which showed a high interfacial stability (Fig. 7g). In addition, Xi and partners23 assembled the ZABs using PVA/EG that exhibited an open-circuit voltage of 1.25 V at −30 °C. The PVA/EG GPEs displayed frost resistance at −30 °C and high water retention at 70 °C. The usage of small-amount but efficient electrolyte additives greatly satisfies the requirements for low-temperature GPEs.
The introduction of small molecule organic additives containing hydrogen bond acceptors or donors is known to reduce the freezing point, but implies the partial sacrifice of ionic conductivity.91 Moreover, the organic solvent molecules may impair the ORR/OER kinetics of ZABs, i.e. the reaction path changed from the expected oxygen catalysis reaction to an alcohol oxidation reaction after the addition of Gly solvent.92 And the occurrence of side reactions greatly slows down the discharge rate of ZABs. Therefore, developing GPEs with high ionic conductivity and suppressing side reactions needs to be considered.
3.2 Alkalization of hydrogel electrolytes
The key factor affecting the freezing resistance of hydrogels is the polarity of their terminal groups. The more polar terminal groups in hydrogels, the stronger the interactions between hydrogels and H2O molecules. The abundant polar groups help to anchor the free H2O molecules in hydrogels, and this strong interfacial interaction could disrupt the ordered structure of H2O molecules and inhibit the formation of ice crystals.93 Alkalization of hydrogel electrolytes provides an effective strategy to enhance their polarity, which endows the electrolytes with both high ionic conductivity and excellent low-temperature resistance.Thus, Chen et al.94 proposed a carboxyl-alkalified PAA (A-PAA) electrolyte, and this strategy introduced a strong electrostatic attraction to generate more polarized terminal groups in hydrogels. Compared to the interactions between PAA and H2O molecules (PAA–W), the interaction energy between A-PAA and H2O molecules ((A-PAA)–W) significantly increased from −12.92 to −16.96 kcal mol−1, which was also almost three times greater than that between two H2O molecules (W–W) of −5.75 kcal mol−1 (Fig. 8a). As can be seen in Fig. 8b, the strong interactions with H2O molecules made A-PAA unsusceptible to loss of water at room temperature and lowered the freezing point below −20 °C. In the following work, they combined the interface engineering of a stereoscopic electrode to greatly extend the operating temperature domain (−30 to 80 °C) of A-PAA-based batteries, with the high ionic conductivity of A-PAA (90 mS cm−1) at −30 °C (Fig. 8c).95 The ultra-high ionic conductivity of A-PAA hydrogels was further confirmed by the abundant interconnected pores that could adsorb large amounts of electrolytes and promote free diffusion of ions (Fig. 8d). The electrostatic potential maps of the different polymer backbones also verified the stronger attraction of A-PAA to H2O molecules (Fig. 8e). Even at −30 °C, the power density of A-PAA-based ZABs reaches 63.6 mW cm−2. And the energy density is 789 W h Kg−1, which is 88.6% of that at room temperature. When performing charge/discharge cycles, it can continuously operate for up to 500 cycles (90 h). Benefiting from the good frost resistance and mechanical properties of A-PAA, the assembled ZABs maintained charge/discharge stability under bending, twisting, folding and cutting at −30 °C (Fig. 8f). Under this extreme condition, the ZABs could even charge the phone (Fig. 8g). These studies help in deeply understanding the interactions between hydrogel end groups and H2O molecules, illustrating reasonable guidance for the exploration of freeze-tolerance mechanisms and the selection of suitable hydrogel matrices.
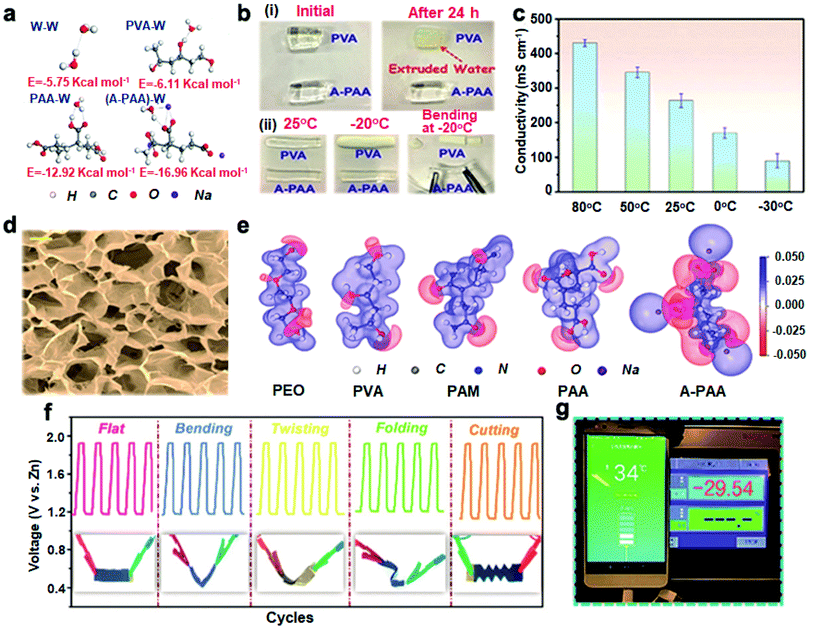 | ||
| Fig. 8 (a) The interaction energy of W–W, PVA–W, PAA–W and (A-PAA)–W. (b) The comparison of the (i) water retention and (ii) frost resistance of the two hydrogels. Reproduced with permission from ref. 94. Copyright 2020, Wiley. (c) Ionic conductivity of KOH-filled A-PAA at different temperatures. (d) SEM image of the freeze-dried A-PAA hydrogels. Scale bar: 10 μm. (e) Electrostatic potential maps of H2O molecules and different polymers. (f) Cycle curves of flexible ZABs under various mechanical deformations at −30 °C. (g) Photo of A-PAA-based ZAB charged phones at −30 °C. Reproduced with permission from ref. 95. Copyright 2021, the Royal Society of Chemistry. | ||
3.3 Construction of DN electrolytes
In addition to overcoming the mechanical bottleneck of single network (SN) hydrogels, the DN hydrogels consisting of two intertwined cross-linked networks also introduce more hydrophilic backbones to enhance the interactions between hydrogels and H2O molecules. The reversible toughening mechanism of DN hydrogel is based on the sacrificial bonds (and/or non-covalent bonds) and the ordered unfolding of the hydrogel network structure. And the increase of polar end groups simultaneously enhances the ability to bind with H2O molecules, lowering the freezing point.96 Thus, the construction of DN hydrogels provides attractive properties for various applications, such as toughness, mechanical strength, low-temperature resistance, etc.For the needs of wide-temperature tolerance and high stretchability, the small molecule-based supramolecular-polymer (SP) DN hydrogel electrolytes consisting of guanosine-cyclohexylboronic acid (G-CyBA) networks interpenetrated with polyacrylamide (PAAm) networks were designed by Liu et al.24 During the polymerization process, the G-CyBA monomers formed a stable hydrogen-bonded g-quadruplex structure through self-assembly. Then, π–π stacking of g-quadruplex formed the g-quadruplex nanowires with a highly ordered and right-handed helical superstructure (Fig. 9a). The apparent upfield shift of the B signal in the 11B nuclear magnetic resonance spectra demonstrated the generation of G-CyBA borate complexes, which reveal the hierarchical assembly process of small molecule-based supramolecular polymer hydrogels (Fig. 9b). DSC curves showed that 6 M KOH-filled G-CyBA/PAAm SP DN hydrogels exhibited the melting peak of ice crystals at a lower temperature (−55.5 °C) compared to 6 M KOH-filled PAAm SN hydrogels (−43.3 °C) and 6 M KOH (−36.3 °C) (Fig. 9c). Simultaneously, the interaction energy between SP-DN (G-CyBA)4–K+/PAAm and H2O molecules was significantly reduced to −26.32 kcal mol−1 with respect to the SN of PAAm (−3.02 kcal mol−1) and the supramolecular (G-CyBA)4-K+ (−13.11 kcal mol−1). Hence, SP-DN hydrogels presented excellent anti-freezing and anti-drying properties in the temperature range of −80 to 100 °C without impairing conductivity. As shown in Fig. 9d, the 6 M KOH-filled SP DN hydrogels provided an ionic conductivity of 300.1, 252.2 and 63.2 mS cm−1 at −40, −50 and −80 °C, respectively. They could also retain an elongation of over 1000% in a wide temperature range from −80 to 100 °C (Fig. 9e). Correspondingly, the assembled stretchable fibrous ZABs presented a power density of 107.6 mW cm−2 (Fig. 9f) and a charge/discharge cycle (2 mA cm−2) over 48 h at −40 °C. In the in situ constant current discharge/charge test (2 mA cm−2) ZABs immersed in water maintained stability for 2 h, which revealed their environmental stability (Fig. 9g). In conclusion, the DN design of the hydrogel electrolytes with wide-temperature tolerance and flexibility offers options for environmental adaptability-required energy storage devices.
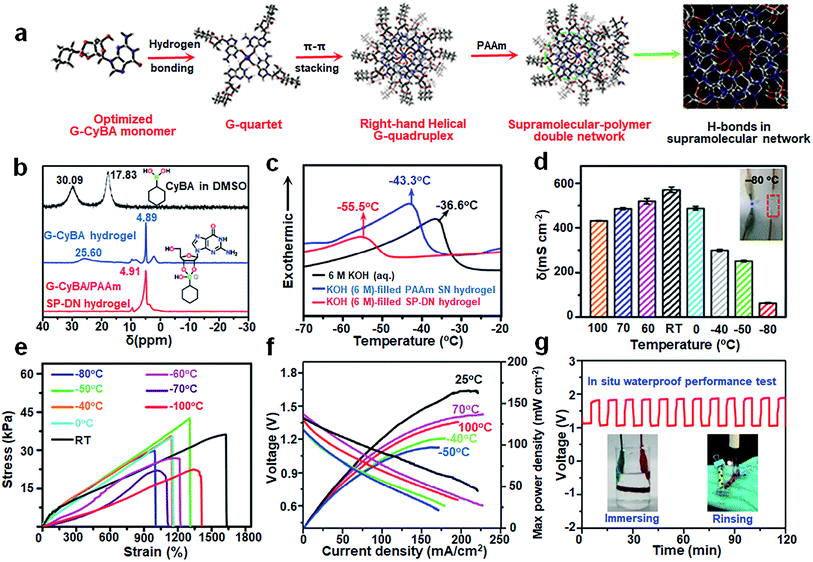 | ||
| Fig. 9 (a) The assembly mechanism of the G-CyBA/PAAm SP-DN hydrogels. (b) 11B nuclear magnetic resonance spectra (c) DSC curves of 6 M KOH filled G-CyBA/PAAm SP-DN hydrogel, 6 M KOH filled PAAm SN hydrogel and 6 M KOH. (d) Ionic conductivity and (e) tensile stress–strain curves of the SP-DN hydrogels at different temperatures. (f) Discharge curves and the corresponding power densities of the ZABs at various temperatures. (g) In situ waterproof performance test. Reproduced with permission from ref. 24. Copyright 2021, Elsevier. | ||
In order to efficiently combine superior mechanical properties, good ionic conductivity and antifreeze properties, Zheng et al.97 prepared an alkaline DN hydrogel electrolyte (PAMPS-K/MC) interpenetrated by PAMPS and modified cellulose (MC) (Fig. 10a). From the FT-IR spectra of hydrogels before and after thermal polymerization, the vanishing of the characteristic peaks of AMPS at 1613, 961 and 832 cm−1 proves the covalent cross-linking of the PAMPS-K networks with MC (Fig. 10b). The generated hydrogen bonds will strongly enhance the mechanical strength and flexibility of DN hydrogels. The PAMPS-K/MC immersed in highly concentrated KOH solution displayed good freezing resistance, maintaining an ionic conductivity of 18.1 mS cm−1 at −20 °C. The specific capacities of the assembled DN hydrogel-based ZABs were essentially unabated when the temperature decreased from room temperature to 0 and −20 °C (Fig. 10c). Miao et al.98 introduced cellulose into PAM to obtain a highly flexible DN electrolyte (PAMC). The PAMC could be easily stretched to 7 times its length without breaking and the water retention rate was still 72% after 144 h at room temperature. When PAMC was applied to ZABs at −20 °C, a maximum power density of 35.8 mW cm−2 and a stable cycle of 3.5 h were demonstrated. Notably, the absorption of high-concentration alkaline electrolyte and the synergistic interactions between polar groups played an important role in lowering the freezing point of DN GPEs.
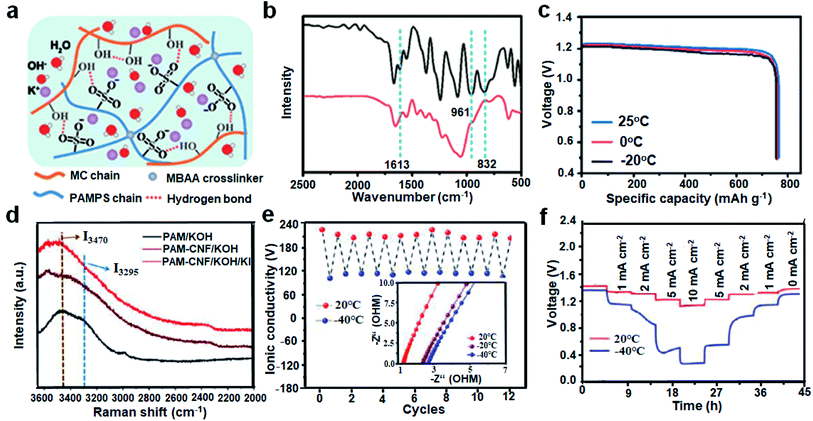 | ||
| Fig. 10 (a) Schematic diagram of the PAMPS-K/MC hydrogels. (b) FT-IR spectra of the freeze-dried PAMPS/MC hydrogels (red) and the mixture of AMPS/MC (black). (c) Specific capacity of the ZABs at different temperatures. Reproduced with permission from ref. 97. Copyright 2021, American Chemical Society. (d) Raman spectra. (e) Ionic conductivity at 20 and −40 °C. (f) Rate performance. Reproduced with permission from ref. 101. Copyright 2021, Elsevier. | ||
Electrolyte additives could play a considerable role in modulating the charge/discharge behavior of ZABs.99 For instance, Cheng and colleagues100 suggested that the KI-containing ZABs undergoing the IO3−/I− mechanism had a much lower thermodynamic barrier than the batteries with the O2/OH− reaction happening at the air cathode, benefitting the reduction of charge voltage. Thus, Wei et al.101 reported a DN hydrogel electrolytes consisting of PAM and cellulose nanofibers with KOH/KI solution (PAM-CNF/KOH/KI). The lowest I3295/I3470 (strong O–H in tetrahedra/weak O–H in incomplete tetrahedra) of 0.82 towards PAM-CNF/KOH/KI indicated that the ion–water interactions significantly weakened the hydrogen bonds between H2O molecules (Fig. 10d). Due to the formed hydrogen bonds between polymer–water and the disrupted hydrogen bonding structure of free H2O molecules by ionic hydration, the freezing point of GPEs was significantly reduced. The electrical conductivity of hydrogels remained stable during repeated cooling–heating–cooling cycles, implying their excellent stability to temperature changes (Fig. 10e). Therefore, PAM-CNF/KOH/KI-based ZABs exhibited great electrochemical performances even at −40 °C (Fig. 10f). The maximum power density of 10 mW cm−2 and a stable discharge platform at 10 mA cm−2 were presented. The above research studies illustrated the significance of the DN electrolyte modification in reducing freezing temperature and improving mechanical properties, which provided the reference for exploring suitable polymer systems in cold environments.
4. Conclusions and outlook
This review summarizes the up-to-date advances in low-temperature resistant GPEs in ZABs. As the ‘‘blood’’ of flexible ZABs, multifunctional GPEs significantly affect battery performance through the modulation of mass transfer and interfacial behavior. The above-mentioned three strategies provide mainstream design directions for the development of low-temperature resistant GPEs. Their pros and cons are summarized in Fig. 11a. In order to meet the demand for low-temperature and flexible application scenarios, the continuous optimization of cost, ionic conductivity, flexibility, temperature adaptability, and environmental friendliness should be carried out. Based on antifreeze GPEs, the performances of ZABs at low temperatures are concluded in Fig. 11b and Table 1. The decreased current densities during charge/discharge at low temperatures were observed, indicating slow cycling processes and weak rate performances. Furthermore, the power density, specific capacity and cycle life of GPE based ZABs at low temperatures were severely degraded compared to those at room temperatures. Hence, the ion transport, low-temperature tolerance, and the GPEs/electrodes interface stability should be paid attention. In addition, to evaluate the low-temperature performance of GPEs and the corresponding ZABs, the detailed characterization and in-depth mechanism of antifreezing electrolytes are needed to reveal the structure–performance relationships. The systematic and comprehensive testing criteria should also be established. Based on the above discussions, some challenges and perspectives are put forward, hoping to push the development of low-temperature ZABs out of the stage of laboratory exploration (Fig. 12).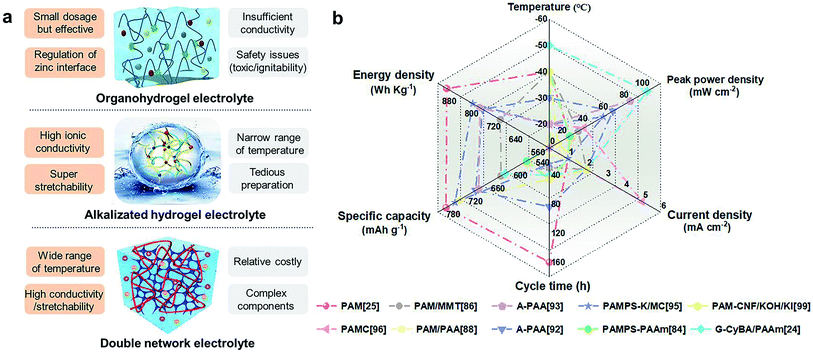 | ||
| Fig. 11 (a) The comparison of the three design strategies for low-temperature resistant GPEs. Orange for pros and grey for cons. Reproduced with permission from ref. 92. Copyright 2020, Wiley. Reproduced with permission from ref. 24. Copyright 2021, Elsevier. (b) The performance of previously reported ZABs with low-temperature resistant hydrogels. | ||
| Design strategy | GPEs' performance | ZABs' performance | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Types | Elongation at 25 °C | Water retention at 25 °C | T (°C) | Ionic conductivity (mS cm−1) | Maximum power density (mW cm−2) | Specific capacity (mAh g−1) | Energy density (W h kg−1) | Cycle time (h)@current density (mA cm−2) | ||
| Addition of organic solvent | PAM (DMSO) | 620% at 0.04 Mpa | 87.2% at 168 h | −60 | 0.087 | — | — | — | 300@0.5 | 25 |
| 100@1.0 | ||||||||||
| −40 | 0.4 | 21.9 | 778.4 | 918.5 | 160@1.0 | |||||
| PAM/MMT (DMSO) | — | — | −20 | 80.7 | 81 | — | — | — | 88 | |
| −40 | 27.1 | 30 | 631 | 725 | 29@2.0 | |||||
| PAMPS-PAAm (DMSO) | 370% at 220 kpa | 64.2% at 40 h | −40 | 17.2 | 21.8 | 562 | 523.4 | 45@1 | 86 | |
| PVA (EG) | — | — | — | — | — | — | — | 23 | ||
| PAM/PAA (Gly) | 500% at 110 kpa | 19.1% at 48 h | −20 | 3.6 | 8.2 | 506.2 | 556.8 | 10@1 | 90 | |
| Alkalization | A-PAA | 550% at 68 kpa | — | −30 | 90 | 63.6 | 699 | 789 | 83.3@2.0 | 94 |
| 600% at 80 kpa | 60% at 240 h | −20 | 199 | 80.5 | 691 | 798 | — | 95 | ||
| Dual-network | G-CyBA/PAAm | 1650% at 37 kpa | 99% at 12 h | −50 | 252.2 | 97 | 620 | — | 40@2 | 24 |
| PAMC | 700% | 72% at 144 h | −20 | 96.3 | 35.8 | — | — | 3.5@5 | 98 | |
| PAM-CNF/KOH/KI | 319% at 16.3 kpa | 86% at 12 h | −40 | 223 | 10 | 743 | — | 45@2 | 101 | |
| PAMPS-K/MC | 140% at 45 kpa | — | −20 | 18.1 | 54.2 | 754.2 | 824.6 | 24@1 | 97 | |
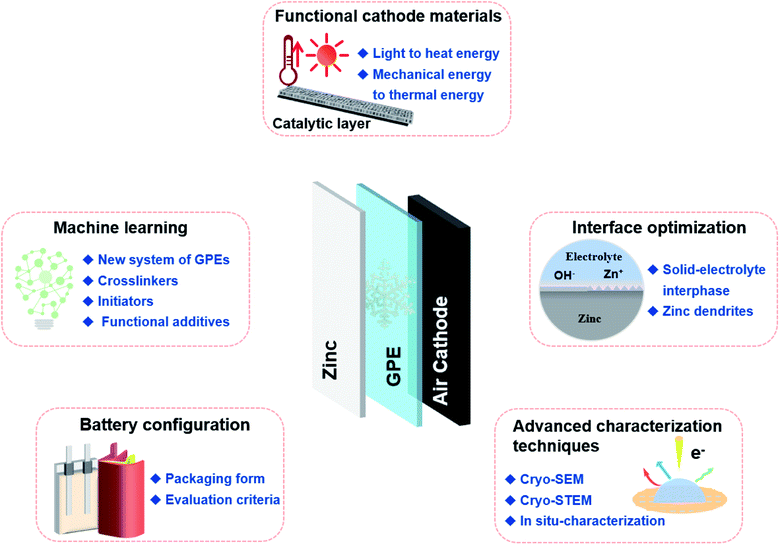
(1) New GPEs systems and various functional material additives for low-temperature applications remain to be developed. At present, the variety of monomers suitable for GPEs is limited. There is much room to investigate initiators and cross-linkers in the polymerization process to improve the physicochemical properties of GPEs. At the same time, additives can be added to increase the electrical conductivity and promote the decomposition/deposition of zinc to inhibit the growth of zinc dendrites. Of course, proper modification of the electrolyte should ensure a good match at GPEs/electrode interfaces to achieve long-term stability. It is worth noting that with the rapid development of machine learning-enhanced high-throughput experiments,102 a large number of potential candidate compounds and inorganic fillers can be screened from the materials database to achieve GPEs with coupled low-temperature resistance and high conductivity. The successful combination of simulation and experiment will promote the research of GPEs with low cost, high performance and a wide-temperature domain.
(2) The interfacial film between GPEs and the zinc anode is critical to low-temperature performance. Since different GPEs produce specific interfaces with the zinc anode, the specific reaction properties need to be studied systematically. Meanwhile, the growth of zinc dendrites on the anode surface will seriously damage the output performance and service life of batteries. In addition to electrolyte modification, zinc dendrites could be inhibited to some extent by designing a protective coating, which is beneficial to eliminate the “tip effect” and improve the cycling stability.103 Further in-depth research should be carried out to reveal the failure mechanism of ZABs at low temperature.
(3) Although the oxygen catalysis performance of air electrodes is insensitive to low temperature, functional modification of air electrodes is expected to improve the interfacial characteristics and ion transport behavior of adjacent GPEs. For example, the additives in the catalyst layer with the ability to convert light energy to thermal energy will increase the temperature inside the ZABs, thereby enhancing low-temperature tolerance.104 Considering the possible mechanical deformation of flexible ZABs during operation, the conversion of mechanical energy to thermal energy is another way to improve low-temperature resistance. Notably, the excellent conductivity and gas transport capability should be maintained when air electrodes are functionally modified.105
(4) In order to better explain the mechanism of attenuated battery performance, it is necessary to explore more reliable and advanced characterization techniques to analyze ion diffusion and complex interface evolution at low temperature, such as cryo-SEM, cryo-STEM, etc.106 Furthermore, the non-destructive and highly quantitative in situ detection methods are expected to reveal the electrochemical reactions at low temperature or under stress and strain conditions. However, the currently existing flexible battery structures pose great challenges to the construction of in situ characterization devices. The coupling of battery, environment, stress application devices and detection systems, etc., needs to be comprehensively considered.
(5) The configuration of flexible ZABs needs to be further optimized. At present, the configuration of ZABs is basically the traditional battery template. However, wearable devices require high bending, warping and even stretching performance. And the packaging forms of flexible ZABs should also suit harsh environments (desiccation, high humidity, high temperatures, high pressure, etc.). Among them, the integrated micro-batteries with self-standing electrodes was noted for they are well-suited to wearable devices.107 Furthermore, standardized criteria and tests should be established not only for mechanical properties of GPEs and electrodes (such as bending times, compressive ability, tensile properties, etc.), but also for the flexibility of ZABs.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the State Key Laboratory of Powder Metallurgy, Central South University.References
- H. Z. Dou, M. Xu, Y. Zheng, Z. Q. Li, G. B. Wen, Z. Zhang, L. X. Yang, Q. Y. Ma, A. P. Yu, D. Luo, X. Wang and Z. W. Chen, Adv. Mater., 2022, 34, 2110585 CrossRef CAS PubMed.
- S. Wang, J. Xu, W. Wang, G. J. N. Wang, R. Rastak, F. Molina-Lopez, J. W. Chung, S. Niu, V. R. Feig, J. Lopez, T. Lei, S. K. Kwon, Y. Kim, A. M. Foudeh, A. Ehrlich, A. Gasperini, Y. Yun, B. Murmann, J. B. H. Tok and Z. Bao, Nature, 2018, 555, 83–88 CrossRef CAS PubMed.
- S. Y. Zhao, D. W. Xia, M. H. Li, D. Y. Cheng, K. L. Wang, Y. S. Meng, Z. Chen and J. Bae, ACS Appl. Mater. Interfaces, 2021, 13, 12033–12041 CrossRef CAS PubMed.
- Y. C. Wang, L. Xu, L. S. Zhan, P. Y. Yang, S. H. Tang, M. J. Liu, X. Zhao, Y. Xiong, Z. Y. Chen and Y. P. Lei, Nano Energy, 2022, 92, 106780 CrossRef CAS.
- M. F. Chen, W. J. Zhou, A. R. Wang, A. X. Huang, J. Z. Chen, J. L. Xu and C. P. Wong, J. Mater. Chem. A, 2020, 8, 6828–6841 RSC.
- Y. X. Zuo, Y. Yu, C. C. Zuo, C. L. Ning, H. Liu, Z. Q. Gu, Q. Q. Cao and C. M. Shen, Energies, 2019, 12, 612 CrossRef CAS.
- Y. Ma, A. Sumboja, W. Zang, S. Yin, S. Wang, S. J. Pennycook, Z. K. Kou, Z. L. Liu, X. Li and J. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 1988–1995 CrossRef CAS PubMed.
- B. Sun, K. Kretschmer, X. Xie, P. Munroe, Z. Peng and G. Wang, Adv. Mater., 2017, 29, 1606816 CrossRef PubMed.
- H. S. Hirsh, Y. X. Li, D. H. S. Tan, M. H. Zhang, E. Y. Zhao and Y. S. Meng, Adv. Energy Mater., 2020, 10, 2001274 CrossRef CAS.
- J. Fu, Z. P. Cano, M. G. Park, A. P. Yu, M. Fowler and Z. W. Chen, Adv. Mater., 2017, 29, 1604685 CrossRef PubMed.
- M. A. Rahman, X. Wang and C. Wen, J. Electrochem. Soc., 2013, 160, A1759 CrossRef CAS.
- F. X. Wu and G. Yushin, Energy Environ. Sci., 2017, 10, 435–459 RSC.
- J. S. Lee, S. T. Kim, R. Cao, N. S. Choi, M. Liu, K. T. Lee and J. Cho, Adv. Energy Mater., 2011, 1, 2 CrossRef.
- M. F. Sanad, A. R. P. Santiago, S. A. Tolba, M. A. Ahsan, O. F. Delgado, M. S. Adly, E. M. Hashem, M. M. Abodouh, M. S. El-Shall, S. T. Sreenivasan, N. K. Allam and L. Echegoyen, J. Am. Chem. Soc., 2021, 143, 4064–4073 CrossRef CAS PubMed.
- A. Smee, Philos. Mag., 1840, 16, 315–321 Search PubMed.
- L. Maiche, French Pat, 1878, 127069 Search PubMed.
- P. Gu, M. B. Zheng, Q. X. Zhao, X. Xiao, H. G. Xue and H. Pang, J. Mater. Chem. A, 2017, 5, 7651–7666 RSC.
- A. R. Mainar, L. C. Colmenares, J. A. Blázquez and I. Urdampilleta, Int. J. Energy Res., 2018, 42, 903–918 CrossRef.
- J. Fu, D. U. Lee, F. M. Hassan, L. Yang, Z. Y. Bai, M. G. Park and Z. W. Chen, Adv. Mater., 2015, 27, 5617–5622 CrossRef CAS PubMed.
- J. Park, M. Park, G. Nam, J. S. Lee and J. Cho, Adv. Mater., 2015, 27, 1396–1401 CrossRef CAS PubMed.
- L. An, B. L. Huang, Y. Zhang, R. Wang, N. Zhang, T. Y. Dai, P. X. Xi and C. H. Yan, Angew. Chem., Int. Ed., 2019, 58, 9459–9463 CrossRef CAS PubMed.
- K. Tang, C. Z. Yuan, Y. Xiong, H. B. Hu and M. Z. Wu, Appl. Catal., B, 2020, 260, 118209 CrossRef CAS.
- J. Yin, J. Jin, H. B. Liu, B. L. Huang, M. Lu, J. Y. Li, H. W. Liu, H. Zhang, Y. Peng, P. X. Xi and C. H. Yan, Adv. Mater., 2020, 32, 2001651 CrossRef CAS PubMed.
- C. N. Gu, X. Q. Xie, Y. J. Liang, J. J. Li, H. Wang, K. F. Wang, J. P. Liu, M. K. Wang, Y. F. Zhang, M. X. Li, H. J. Kong and C. S. Liu, Energy Environ. Sci., 2021, 14, 4451–4462 RSC.
- Q. C. Wang, Q. G. Feng, Y. P. Lei, S. H. Tang, L. Xu, Y. Xiong, G. Z. Fang, Y. C. Wang, P. Y. Yang, J. J. Liu, W. Liu and X. Xiong, Nat. Commun., 2022 DOI:10.1038/s41467-022-31383-4.
- J. W. Hu, X. B. Cai, J. Wu, C. C. Xin, J. Y. Guo, Z. R. Liu, J. Z. Wei, X. S. Cheng, C. Hao, H. P. Dong, G. F. Zhang, N. Wang, Y. P. Lei, W. Liu and Y. T. Shi, Chem. Eng. J., 2022, 430, 133105 CrossRef CAS.
- W. Sun, F. Wang, B. Zhang, M. Y. Zhang, V. Küpers, X. Ji, C. Theile, P. Bieker, K. Xu, C. S. Wang and M. Winter, Science, 2021, 371, 46–51 CrossRef CAS PubMed.
- L. J. Li and A. Manthiram, Adv. Energy Mater., 2016, 6, 1502054 CrossRef.
- M. Kar, B. Winther-Jensen, M. Armand, T. J. Simons, O. Winther-Jensen, M. Forsyth and D. R. MacFarlane, Electrochim. Acta, 2016, 188, 461–471 CrossRef CAS.
- X. R. Liu, X. Y. Fan, B. Liu, J. Ding, Y. D. Deng, X. P. Han, C. Zhong and W. B. Hu, Adv. Mater., 2021, 33, 2006461 CrossRef CAS PubMed.
- X. L. Xu, S. Wang, S. Q. Guo, K. S. Hui, J. M. Ma, D. A. Dinh, K. N. Hui, H. Wang, L. P. Zhang and G. W. Zhou, Adv. Powder Mater., 2022, 1, 100027 CrossRef.
- J. Sun, N. K. Guo, T. S. Song, Y. R. Hao, J. W. Sun, H. Xue and Q. Wang, Adv. Powder Mater., 2022, 1, 100023 CrossRef.
- P. Tan, B. Chen and H. R. Xu, Energy Environ. Sci., 2017, 10, 2056–2080 RSC.
- L. T. Ma, S. M. Chen, D. H. Wang, Q. Yang, F. N. Mo, G. J. Liang, N. Li, H. Y. Zhang, J. A. Zapien and C. Y. Zhi, Adv. Energy Mater., 2019, 9, 1803046 CrossRef.
- J. Fu, J. Zhang, X. Song, H. Zarrin, X. Tian, J. Qiao, L. Rasen, K. Li and Z. Chen, Energy Environ. Sci., 2016, 9, 663–670 RSC.
- J. Fu, F. M. Hassan, C. Zhong, J. Lu, H. Liu, A. Yu and Z. Chen, Adv. Mater., 2017, 29, 1702526 CrossRef PubMed.
- Y. Y. Zuo, K. L. Wang, M. H. Wei, S. Y. Zhao, P. F. Zhang and P. C. Pei, Cell Rep. Phys. Sci., 2022, 3, 100687 CrossRef CAS.
- M. J. Wu, G. X. Zhang, L. Du, D. C. Yang, H. M. Yang and S. H. Sun, Small Methods, 2021, 5, 2000868 CrossRef CAS PubMed.
- X. Gong, Q. Yang, C. Zhi and P. S. Lee, Adv. Energy Mater., 2021, 11, 2003308 CrossRef CAS.
- T. Liu, J. Mou, Z. Wu, C. Lv, J. Huang and M. Liu, Adv. Funct. Mater., 2020, 30, 2003407 CrossRef CAS.
- Z. Cao, H. Hu, M. Wu, K. Tang and T. Jiang, J. Mater. Chem. A, 2019, 7, 17581–17593 RSC.
- H. Miao, B. Chen, S. Li, X. Wu, Q. Wang, C. Zhang, Z. Sun and H. Li, J. Power Sources, 2020, 450, 227653 CrossRef CAS.
- M. Z. Chen, Y. Y. Zhang, G. C. Xing, S.-L. Chou and Y. X. Tang, Energy Environ. Sci., 2021, 14, 3323–3351 RSC.
- S. H. Li, H. Y. Pan, Y. T. Wang and J. Q. Sun, J. Mater. Chem. A, 2020, 8, 3667–3675 RSC.
- Z. X. Liu, X. B. Luo, L. P. Qin, G. Z. Fang and S. Q. Liang, Adv. Powder Mater., 2022, 1, 100011 CrossRef.
- C. X. Zhao, J. N. Liu, N. Yao, J. Wang, D. Ren, X. Chen, B. Q. Li and Q. Zhang, Angew. Chem., Int. Ed., 2021, 60, 15281–15285 CrossRef CAS PubMed.
- Z. Chen, X. L. Li, D. H. Wang, Q. Yang, L. T. Ma, Z. D. Huang, G. J. Liang, A. Chen, Y. Guo, B. B. Dong, X. Y. Huang, C. Yang and C. Y. Zhi, Energy Environ. Sci., 2021, 14, 3492–3501 RSC.
- C. Lu and X. Chen, Nano Lett., 2020, 20, 1907–1914 CrossRef CAS PubMed.
- X. T. Jin, L. Song, C. L. Dai, H. Y. Ma, Y. K. Xiao, X. Q. Zhang, Y. Y. Han, X. Y. Li, J. T. Zhang, Y. Zhao, Z. P. Zhang, L. Duan and L. T. Qu, Energy Storage Mater., 2022, 44, 517–526 CrossRef.
- F. Mo, G. Liang, Q. Meng, Z. Liu, H. Li, J. Fan and C. Zhi, Energy Environ. Sci., 2019, 12, 706–715 RSC.
- F. Mo, G. Liang, D. Wang, Z. Tang, H. Li and C. Zhi, EcoMat, 2019, 1, e12008 CrossRef CAS.
- X. J. Sui, H. S. Guo, P. G. Chen, Y. N. Zhu, C. Y. Wen, Y. H. Gao, J. Yang, X. Y. Zhang and L. Zhang, Adv. Funct. Mater., 2020, 30, 1907986 CrossRef CAS.
- M. F. Chen, J. Z. Chen, W. J. Zhou, X. Han, Y. G. Yao and C. P. Wong, Adv. Mater., 2021, 33, 2007559 CrossRef CAS PubMed.
- J. Mindemark, M. J. Lacey, T. Bowden and D. Brandell, Prog. Polym. Sci., 2018, 81, 114–143 CrossRef CAS.
- Y. T. Wei, Y. C. Shi, Y. Chen, C. H. Xiao and S. J. Ding, J. Mater. Chem. A, 2021, 9, 4415–4453 RSC.
- F. Santos, J. P. Tafur, J. Abad and A. J. F. Romero, J. Electroanal. Chem., 2019, 850, 113380 CrossRef CAS.
- Y. H. Ye, Y. F. Zhang, Y. Chen, X. S. Han and F. Jiang, Adv. Funct. Mater., 2020, 30, 2003430 CrossRef CAS.
- Q. F. Rong, W. W. Lei, J. Huang and M. J. Liu, Adv. Energy Mater., 2018, 8, 1801967 CrossRef.
- G. W. Shao, R. Yu, X. Zhang, X. Chen, F. L. He, X. Zhao, N. L. Chen, M. D. Ye and X. Y. Liu, Adv. Funct. Mater., 2020, 30, 2003153 CrossRef CAS.
- A. Lewandowski, K. Skorupska and J. Malinska, Ionics, 2000, 133, 265–271 CrossRef CAS.
- L. Yang, L. Shi, D. Wang, Y. L. Lv and D. P. Cao, Nano Energy, 2018, 50, 691–698 CrossRef CAS.
- Q. S. Nian, T. J. Sun, S. Liu, H. H. Du, X. D. Ren and Z. L. Tao, Chem. Eng. J., 2021, 423, 130253 CrossRef CAS.
- H. Y. Lu, J. S. Hu, L. T. Wang, J. Z. Li, X. Ma, Z. C. Zhu, H. Q. Li, Y. J. Zhao, Y. J. Li, J. X. Zhao and B. G. Xu, Adv. Funct. Mater., 2022, 32, 2112540 CrossRef CAS.
- H. W. Kim, J. M. Lim, H. J. Lee, S. W. Eom, Y. T. Hong and S.-Y. Lee, J. Mater. Chem. A, 2016, 4, 3711–3720 RSC.
- Y. Zhong, Z. Pan, X. Wang, J. Yang, Y. Qiu, S. Xu, Y. Lu, Q. Huang and W. Li, Adv. Sci., 2019, 6, 1802243 CrossRef PubMed.
- C. X. Zheng, K. Y. Lu, Y. Lu, S. L. Zhu, Y. Y. Yue, X. W. Xu, C. T. Mei, H. N. Xiao, Q. L. Wu and J. Q. Han, Carbohydr. Polym., 2020, 250, 116905 CrossRef CAS PubMed.
- Q. Wang, H. Miao, S. Sun, Y. Xue and Z. Liu, Chem.–Eur. J., 2018, 24, 14816–14823 CrossRef CAS PubMed.
- C. Iwakura, N. Furukawa, T. Ohnishi, K. Sakamoto, S. Nohara and H. Inoue, Electrochemistry, 2001, 69, 659–663 CrossRef CAS.
- Y. Huang, Z. Li, Z. X. Pei, Z. X. Liu, H. F. Li, M. S. Zhu, J. Fan, Q. B. Dai, M. D. Zhang, L. M. Dai and C. Y. Zhi, Adv. Energy Mater., 2018, 8, 1802288 CrossRef.
- H. Wang, J. Liu, J. Q. Wang, M. M. Hu, Y. P. Feng, P. P. Wang, Y. Y. Wang, N. Y. Nie, J. H. Zhang, H. Chen, Q. H. Yuan, J. W. Wu and Y. Huang, ACS Appl. Mater. Interfaces, 2019, 11, 49–55 CrossRef CAS PubMed.
- L. Sartore, S. Pandini, F. Baldi, F. Bignotti and L. D. Landro, J. Appl. Polym. Sci., 2017, 134, 45655 CrossRef.
- T. T. Wei, Y. K. Ren, Z. Q. Li, X. X. Zhang, D. H. Ji and L. H. Hu, Chem. Eng. J., 2022, 434, 134646 CrossRef CAS.
- J. Zhang, L. D Zeng, Z. W. Qiao, J. Wang, X. C. Jiang, Y. S. Zhang and H. H. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 30247–30258 CrossRef CAS PubMed.
- L. Ma, S. Chen, Z. Pei, Y. Huang, G. Liang, F. Mo, Q. Yang, J. Su, Y. Gao, J. A. Zapien and C. Zhi, ACS Nano, 2018, 12, 1949–1958 CrossRef CAS PubMed.
- X. F. Zhang, X. F. Ma and T. Hou, Angew. Chem., Int. Ed., 2019, 58, 7366–7370 CrossRef CAS PubMed.
- Z. G. Zhao, K. J. Zhang, Y. X. Liu, J. J. Zhou and M. J. Liu, Adv. Mater., 2017, 29, 1701695 CrossRef PubMed.
- W. X. Shang, W. T. Yu, Y. Y. Ma, Y. He, Z. X. Zhao, M. Ni, H. Zhao and P. Tan, Adv. Mater. Interfaces, 2021, 8, 2101256 CrossRef CAS.
- Z. F. Wang, H. F. Li, Z. J. Tang, Z. X. Liu, Z. H. Ruan, L. T. Ma, Q. Yang, D. H. Wang and C. Y. Zhi, Adv. Funct. Mater., 2018, 28, 1804560 CrossRef.
- S. Y. Zhao, Y. Y. Zuo, T. Liu, S. Zhai, Y. W. Dai, Z. J. Guo, Y. Wang, Q. J. He, L. C. Xia, C. Y. Zhi, J. Bae, K. L. Wang and M. Ni, Adv. Energy Mater., 2021, 11, 2101749 CrossRef CAS.
- S. W. Huang, L. Hou, T. Y. Li, Y. C. Jiao and P. Y. Wu, Adv. Mater., 2022, 34, 2110140 CrossRef CAS PubMed.
- B. Y. Zhang, L. P. Qin, Y. Fang, Y. Z. Chai, X. S. Xie, B. G. Lu, S. Q. Liang and J. Zhou, Sci. Bull., 2022, 67, 955–962 CrossRef CAS.
- L. H. Li, H. Chen, E. He, L. Wang, T. T. Ye, J. Lu, Y. D. Jiao, J. C. Wang, R. Gao, H. S. Peng and Y. Zhang, Angew. Chem., Int. Ed., 2021, 60, 15317–15322 CrossRef CAS PubMed.
- L. Chen, Y. P. Wang, X. Zhao, Y. C. Wang, Q. Li, Q. C. Wang, Y. G. Tang and Y. P. Lei, J. Mater. Sci. Technol., 2022, 110, 128–135 CrossRef.
- Q. Li, Y. C. Wang, J. Zeng, Q. M. Wu, Q. C. Wang, L. Sun, L. Xu, T. Ye, X. Zhao, L. Chen, Z. Y. Chen, L. M. Chen and Y. P. Lei, Chinese Chem. Lett., 2021, 32, 3355–3358 CrossRef CAS.
- F. Q. Cao, B. H. Wu, T. Y. Li, S. T. Sun, Y. C. Jiao and P. Y. Wu, Nano Res., 2022, 15, 2030–2039 CrossRef CAS.
- D. Q. Jiang, H. Y. Wang, S. Wu, X. Y. Sun and J. Li, Small Methods, 2021, 6, 2101043 CrossRef PubMed.
- Y. Ma, L. B. Li, G. X. Gao, X. Y. Yang and Y. You, Electrochim. Acta, 2016, 187, 535–542 CrossRef CAS.
- T. T. Cui, Y. P. Wang, T. Ye, J. Wu, Z. Q. Chen, J. Li, Y. P. Lei, D. S. Wang and Y. D. Li, Angew. Chem., Int. Ed., 2022, 61, e202115219 CAS.
- E. Cevik, S. T. Gunday, A. Bozkurt, R. Amine and K. Amine, J. Power Sources, 2020, 474, 228544 CrossRef CAS.
- R. Chen, X. B. Xu, S. Y. Peng, J. M. Chen, D. F. Yu, C. H. Xiao, Y. L. Li, Y. T. Chen, X. F. Hu, M. J. Liu, H. Yang, I. Wyman and X. Wu, ACS Sustainable Chem. Eng., 2020, 8, 11501–11511 CrossRef CAS.
- Z. Z. Liu, J. M. Zhang, J. Liu, Y. J. Long, L. M. Fang, Q. W. Wang and T. Liu, J. Mater. Chem. A, 2020, 8, 6219–6228 RSC.
- L. Han, K. Liu, M. Wang, K. Wang, L. Fang, H. Chen, J. Zhou and X. Lu, Adv. Funct. Mater., 2018, 28, 1704195 CrossRef.
- Z. Lin, C. Qiang and X. Kun, Prog. Chem., 2014, 26, 1032–1038 Search PubMed.
- Z. X. Pei, Z. W. Yuan, C. J. Wang, S. L. Zhao, J. Y. Fei, L. Wei, J. S. Chen, C. Wang, Ro. J. Qi, Z. W. Liu and Y. Chen, Angew. Chem., Int. Ed., 2020, 59, 4793–4799 CrossRef CAS PubMed.
- Z. X. Pei, L. Y. Ding, C. Wang, Q. Q. Meng, Z. W. Yuan, Z. Zhou, S. L. Zhao and Y. Chen, Energy Environ. Sci., 2021, 14, 4926–4935 RSC.
- J. Cong, Z. W. Fan, S. S. Pan, J. Tian, W. Z. Lian, S. Li, S. J. Wang, D. C. Zheng, C. G. Miao, W. P. Ding, T. L. Sun and T. Z. Luo, ACS Appl. Mater. Interfaces, 2021, 13, 34942–34953 CrossRef CAS PubMed.
- N. Sun, F. Lu, Y. Yu, L. Su, X. P. Gao and L. Q. Zheng, ACS Appl. Mater. Interfaces, 2020, 12, 11778–11788 CrossRef CAS PubMed.
- B. Chen, H. Miao, M. M. Yin, R. G. Hu, L. Xia, C. F. Zhang and J. L. Yuan, Chem. Eng. J., 2021, 417, 129179 CrossRef CAS.
- J. Cao, D. D. Zhang, R. Chanajaree, Y. L. Yue, Z. Y. Zeng, X. Y. Zhang and J. Q. Qin, Adv. Powder Mater., 2022, 1, 100007 CrossRef.
- Z. H. Song, J. Ding, B. Liu, X. R. Liu, X. P. Han, Y. D. Deng, W. B. Hu and C. Zhong, Adv. Mater., 2020, 32, 1908127 CrossRef CAS PubMed.
- Y. N. Zhang, H. L. Qin, M. Alfred, H. Z. Ke, Y. B. Cai, Q. Q. Wang, F. L. Huang, B. Liu, P. F. Lv and Q. F. Wei, Energy Storage Mater., 2021, 42, 88–96 CrossRef.
- H. Y. Jing, P. Zhu, X. B. Zheng, Z. D. Zhang, D. S. Wang and Y. D. Li, Adv. Powder Mater., 2022, 1, 100013 CrossRef.
- J. X. Zheng, Z. H. Huang, Y. Zeng, W. Q. Liu, B. B. Wei, Z. B. Qi, Z. H. Wang, C. Xia and H. F. Liang, Nano Lett., 2022, 22, 1017–1023 CrossRef CAS PubMed.
- H. C. Song, S. Wang, X. Y. Song, J. Wang, K. Z. Jiang, S. H. Huang, M. Han, J. Xu, P. He, K. J. Chen and H. S. Zhou, Energy Environ. Sci., 2020, 13, 1205–1211 RSC.
- Y. C. Wang, Y. Liu, W. Liu, J. Wu, Q. Li, Q. G. Feng, Z. Y. Chen, X. Xiong, D. S. Wang and Y. P. Lei, Energy Environ. Sci., 2020, 13, 4609–4624 RSC.
- Z. W. Zhang, Y. Z. Li, R. Xu, W. J. Zhou, Y. B. Li, S. T. Oyakhire, Y. C. Wu, J. W. Xu, H. S. Wang, Z. Yu, D. T. Boyle, W. Huang, Y. Ye, H. Chen, J. Wan, Z. N. Bao, W. Chiu and Y. Cui, Science, 2022, 375, 66–70 CrossRef CAS PubMed.
- Y. P. Lei, Q. C. Wang, S. J. Peng, S. Ramakrishna, D. Zhang and K. C. Zhou, Adv. Energy Mater., 2020, 10, 1902115 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ta02381d |
| This journal is © The Royal Society of Chemistry 2022 |