Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amphiphilic dendrimers against antibiotic resistance: light at the end of the tunnel?
Christina
Galanakou
 ,
Dinesh
Dhumal
,
Dinesh
Dhumal
 * and
Ling
Peng
* and
Ling
Peng
 *
*
Centre Interdisciplinaire de Nanoscience de Marseille, CINaM, UMR 7325, Aix Marseille University, CNRS, Parc Scientifique et Technologique de Luminy, Marseille, 13288, France. E-mail: dhumaldinesh212@gmail.com; ling.peng@univ-amu.fr
First published on 22nd February 2023
Abstract
With the alarming and prevailing antimicrobial resistance (AMR) comes an urgent need for novel antimicrobial agents that are not only effective and robust but also do not induce resistance development. Amphiphilic dendrimers are emerging as a promising new paradigm to combat bacterial AMR. They can mimic antimicrobial peptides to produce potent antibacterial activity yet with a low likelihood of generating resistance. In addition, they are stable against enzymatic degradation thanks to their unique dendritic architecture. Importantly, these amphiphilic dendrimers are composed of distinct hydrophobic and hydrophilic entities bearing dendritic structures, which can be precisely designed and synthesized to optimize the hydrophobic–hydrophilic balance yielding potent antibacterial activity while minimizing adverse effects and drug resistance. In this short review, we present the challenges and current state of research in developing amphiphilic dendrimers as new antibiotic substitutes. We start with a brief overview of the advantages and opportunities associated with using amphiphilic dendrimers to combat bacterial AMR. We then outline the specific considerations and the mechanisms underlying the antibacterial activity of amphiphilic dendrimers. We focus on the importance of the amphiphilic nature of a dendrimer that balances hydrophobicity and hydrophilicity via gauging the hydrophobic entity and the dendrimer generation, branching unit, terminal group and charge to allow high antibacterial potency and selectivity while minimizing toxicity. Finally, we present the future challenges and perspectives for amphiphilic dendrimers as antibacterial candidates for combating AMR.
Antimicrobial resistance (AMR) is an urgent global health crisis. In 2019, an estimated 4.95 million deaths were associated with bacterial AMR, a figure likely to exceed 10 million per year by 2050, surpassing cancer-related deaths.1,2 In addition, AMR not only affects humans but also animals and the environment.3,4 The emergence of AMR is a combined product of bacterial evolution alongside immense selective pressure caused by the inappropriate use of antibiotics.5 Importantly, multidrug-resistant bacteria frequently accumulate genes that contribute to the development of resistance against multiple antibiotics, leaving clinicians with limited or no therapeutic choices. Pathogens of particular importance include Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species that are collectively described by the acronym “ESKAPE”.6,7 These pathogens are related to high mortality risk infections and can evade the biocidal activity of both conventional and new-generation antibiotics. Therefore, novel antibacterial agents are urgently required to fight against bacterial AMR.
The development of new antibiotics has, however, halted in recent decades, largely because of difficulties related to scientific, regulatory, and economic concerns.8–10 Most antimicrobial agents used today were discovered in the 1950s, and even the latest “novel” antibacterial drug class, the lipopeptides such as vancomycin, has been available for routine use since the end of the 20th century.11 Alternative strategies to combat AMR include the use of antibiotics in combination with other antibiotics or adjuvants and the use of antibacterial therapies that utilize bacteriophages, photodynamic treatments, antibodies, antimicrobial peptides (AMPs), nucleic acid therapeutics, and nanoparticles.12–19
Among these different approaches, AMPs are particularly appealing because they possess unique properties that enable a broad spectrum of antibacterial activity while not generating resistance.14,19 AMPs are amphiphilic, combining cationic surface charges and hydrophobic components. Their primary mechanism of action relies on the electrostatic interactions with the negatively charged bacterial surface through their positively charged amino acid residues. This is followed by subsequent penetration of their hydrophobic entity into the bacteria cell membrane to cause membrane disruption, and hence cell killing and bacterial death.12,20 As the primary interaction of AMPs with bacterial membrane is not mediated through a specific target, the development of antimicrobial resistance is highly unlikely and inconsistent. In addition to their primary mechanism of action, AMPs may also exert antibacterial activity via immunomodulatory mechanisms that neither harm host cells nor induce drug resistance.
Despite these promising features, AMPs have not yet progressed towards their clinical use. This is mainly due to their peptide composition leaving them open to inherent proteolytic instability and short half-lives with elusive antimicrobial potencies in vivo.19 Efforts made to overcome these disadvantages and impart higher antibacterial potency, have led to AMP mimics offering improved efficacy and stability as well as less adverse effects as potential antibiotic substitutes. AMP mimics21 include peptides bearing D-enantiomeric or β-amino acids, peptoids, and polymeric antimicrobials, and a unique family of compounds called dendrimers which are of particular current interest.22 Dendrimers are composed of a core, repetitive branching units and terminal groups (Fig. 1A), and have a precise chemical structure, radial dendritic architecture, and multivalent cooperativity confined within a small volume.23 Notably, the dendritic structural feature creates steric hindrance and impedes access to enzyme active sites, hence enhancing proteolytic stability.
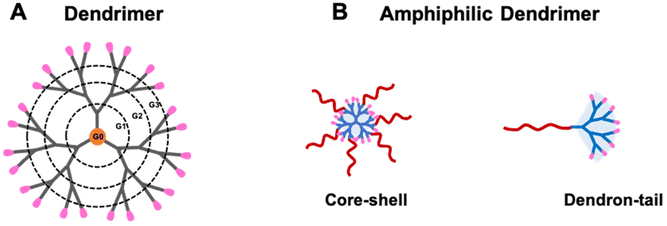 | ||
| Fig. 1 Cartoon illustrations of (A) a dendrimer and (B) amphiphilic dendrimers. (A) The dendrimer structure is composed of a central core, repeat branching units forming consecutive levels or generations (G1, G2 and G3) and terminal groups on the surface (shown in pink). (B) Amphiphilic dendrimers of core–shell and dendron-tail structures composed of distinct hydrophobic (red) and hydrophilic (blue) entities. Reproduced from ref. 30 with permission from ACS Publications, copyright 2023. | ||
Multiple attempts have been made to develop peptide dendrimers as potential mimics of natural and synthetic AMPs based on their close chemical and structural relationship.21,24 However, while peptide dendrimers do demonstrate a greater stability than linear peptides, they still suffer from enzymatic degradation. Poly(amidoamine) (PAMAM) dendrimers, conceptualized in the later 70s as peptide mimics23,25 have gained increasingly more interest as antibacterial candidates thanks to their high biocompatibility and resilience to proteolytic degradation.26 High-generation PAMAM dendrimers are highly effective against various bacteria but also exhibit high cytotoxicity even at low concentrations.27–29
To overcome these limitations, small amphiphilic dendrimers have been conceived and synthesized as novel antibacterial candidates with the aim of further enhancing chemical and enzymatic stability and thereby improving antibacterial potency while reducing eventual toxicity. Amphiphilic dendrimers are composed of distinct hydrophobic and hydrophilic entities, and can be precisely designed and synthesized with core–shell or dendron-tail structure (Fig. 1B).30,31 Specifically, amphiphilic dendrimers offer the inherent antimicrobial activity of detergent-like amphiphiles as well as the chemical and structural stability of dendrimers. In addition, their amphipathic nature enables these dendrimers to self-assemble into nanostructures, thus further improving their proteolytic stability and simultaneously allowing their accumulation at the infection site via the enhanced permeability and retention (EPR) effect.32 The EPR effect refers to the specific enrichment of macromolecules or nanosized particles within tumor or inflammation lesions owing to the leaky vasculature and dysfunctional lymphatic drainage found specifically at these locations.33,34 Although the EPR effect has been widely exploited for nanotechnology-based cancer treatment,35,36 it was first discovered during studies of inflammation involving a bacterial infection where vascular permeability was found to be increased.37 Similarly, infected tissue displays dysfunctional lymphatic drainage resulting from increased interstitial pressure and tissue destruction. Consequently, nanosystems are expected to exploit the EPR effect to preferentially deliver antibacterial agents to sites of infection, thereby improving therapeutic efficacy and reducing adverse effects32,38 such as that achieved with nanotechnology-based cancer therapy.
In this short review, we first highlight the advantages and opportunities associated with amphiphilic dendrimers as antibacterial candidates to treat AMR using representative examples. We then outline the specific considerations and the mechanisms underlying the antibacterial activity of amphiphilic dendrimers. Importantly, the amphiphilicity and the hydrophobic–hydrophilic balance play critical roles in yielding amphiphilic dendrimers with high potency and selectivity towards resistant bacteria while minimizing cytotoxicity to host cells. We conclude by presenting our view of the future challenges and perspectives for amphiphilic dendrimers as antibacterial candidates.
Amphiphilicity imparts and promotes antibacterial activity
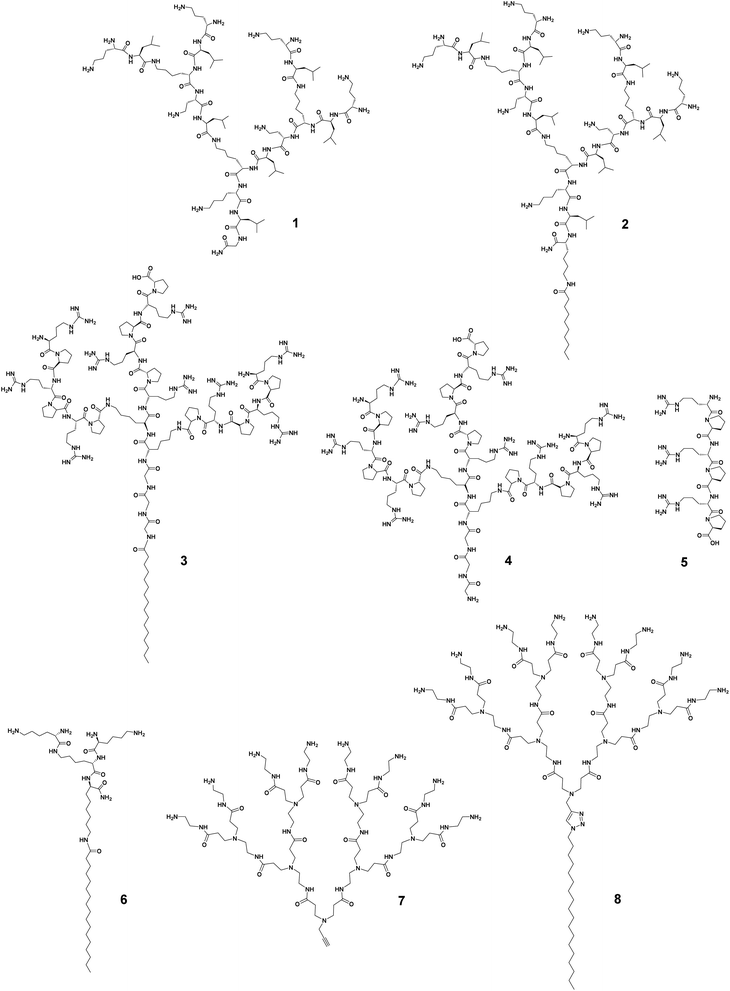
The antibacterial activity has been studied in many peptide dendrimers with a view to creating AMP mimics.22 Prof. Jean-Louis Reymond and colleagues have worked intensively in this regard. Recently, they reported that imparting amphiphilicity to peptide dendrimers via the conjugation of a lipidic chain considerably increased the antibacterial activity against various drug-resistant bacteria.39 Specifically, the peptide dendrimer of the second-generation composed of the repeating unit lysine–leucine, 1 (Fig. 2), was active against only P. aeruginosa. After conjugating a small C10 hydrophobic fatty acid chain to 1, the resulting amphiphilic dendrimer 2 (Fig. 2) was highly potent against both Gram-negative and -positive bacteria as well as multidrug-resistant bacteria (Table 1).39 This study highlights the importance of imparting amphiphilicity to the dendrimer in order to improve antibacterial activity, permitted in this example by introducing a hydrophobic chain.| Dendrimer structure variation | Dendrimer number | Hydrophobic chain | Generation | Surface functionality | MIC [μg mL−1] | Hemolysis (HC50) [μg mL−1] | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | MRSA | E. coli | P. aeruginosa | |||||||
| a “—”: not available. | ||||||||||
| Hydrophobic tail | 1 | No chain | 2 | Primary amine | —a | —a | >85 | >64 | >1700 | 39 |
| 2 | C10 | 2 | Primary amine | 64 | —a | 1 | 3 | 650 | ||
| 3 | C16 | 2 | Guanidine | 11.85 | 11.85 | 2.96 | 11.85 | 265 | 40 | |
| 4 | No chain | 2 | Guanidine | >384.86 | >384.86 | 21.8 | 87.21 | —a | ||
| Hydrophobic tail length | 13 | C6 | 2 | Primary amine | >500 | >500 | >500 | —a | >500 | 42 |
| 14 | C12 | 2 | Primary amine | 15 | 15 | 60 | —a | >500 | ||
| 15 | C18 | 2 | Primary amine | 5 | 8 | 10 | —a | 23 | ||
| Generation dependence | 9 | C14 | 3 | Primary amine | 3.9 | —a | 7.8 | —a | 10 | 43 |
| 10 | C14 | 2 | Primary amine | 1.95 | —a | 3.9 | —a | 63 | ||
| 11 | C14 | 1 | Primary amine | 3.9 | —a | 3.9 | —a | >5000 | ||
| Terminal functionality | 8 | C18 | 3 | Primary amine | 6 | 6 | 6 | 6 | 194 | 44 |
| 18 | C18 | 3 | Tertiary amine | 63 | 42 | 6 | 65 | 214 | ||
| 19 | C18 | 3 | Guanidine | 200 | >200 | 200 | 100 | 159 | ||
| 20 | C18 | 3 | Carboxylic acid | 100 | 75 | 100 | >200 | >400 | ||
Recently, Lai et al. reported similar findings.40 They synthesized amphiphilic peptide dendrimers composed of a C16-alkyl chain attached to the peptide dendron with arginine–proline repeating motifs. Remarkably, the amphiphilic dendrimer 3 (Fig. 2) had a minimum inhibitory concentration (MIC) over 60-fold lower (geometric mean MIC of 2.2 μg mL−1) than that of the corresponding peptide dendrimer 4 (138 μg mL−1), whereas the linear peptide homolog 5 showed no activity at all (Table 1). These data in comparison to linear peptides support the importance of the dendritic structure and its unique multivalence for higher antibacterial activity, which can be further increased upon imparting amphiphilicity to the peptide dendrimer. The amphiphilic feature of 3 also enabled the dendrimers to self-assemble into nanostructures for more potent antibacterial activity.40 A similar observation was reported by Gide et al., where nanostructures formed by the amphiphilic lipidated polylysine dendrimer 6 showed broad spectrum activity against both Gram-negative and -positive bacteria, including multidrug-resistant strains as well as biofilms.41 Collectively, these studies demonstrate that imparting amphiphilicity can effectively promote and enhance the antibacterial and antibiofilm activities of conventional peptide dendrimers, allowing greater potency and a broader spectrum.
Potent antibacterial activity shown by high-generation cationic PAMAM dendrimers demonstrated the interest of positively charged dendrimer terminals able to interact with negatively charged bacterial surface.45 Unfortunately, however, high-generation PAMAM dendrimers are not only toxic but also difficult to synthesize in a defect-free form, whereas low-generation PAMAM dendrimers, though not toxic, lack antibacterial activity.46 The addition of a hydrophobic alkyl chain (C18) to the small PAMAM dendron 7 generated the amphiphilic dendrimer 8 (Fig. 2) that was highly effective against Gram-negative and -positive bacteria as well as drug-resistant strains with MIC values of 6.0 μg mL−1 (Table 1), whereas 7 without amphiphilicity showed no notable antibacterial activity at all.44 Importantly, the amphiphilic dendrimer 8 retained activity against methicillin-resistant Staphylococcus aureus (MRSA) biofilm. Altogether, these exemplary studies strongly support the importance and impact of amphiphilicity to transform dendrimers from non-active or weakly active to highly active candidates against antibiotic-resistant bacteria.
Fine-tuning amphiphilicity to balance antibacterial activity and toxicity
The potent antimicrobial efficacy shown by some amphiphilic dendrimers can unfortunately be associated with high toxicity. This is not surprising since many AMP-mimicking amphiphilic dendrimers exert their activity by first interacting with and then disrupting the bacterial membrane. A similar mechanism can occur towards eukaryotic cell membranes, thereby causing cytotoxicity. The trade-off between the desired antibacterial activity and undesired cytotoxicity is primarily and critically modulated by the hydrophilic–hydrophobic balance of the amphiphilic dendrimers and needs careful gauging to maximize the activity while minimizing the toxicity. This hydrophilic–hydrophobic balance is influenced by several structural characteristics of the amphiphilic dendrimers, such as the nature of the hydrophobic entity, dendrimer scaffold, surface functionality, charge density, as well as the number and size of the terminal groups. Minor changes to these factors can significantly tilt this balance. Consequently, substantial efforts have been dedicated to finding an optimum hydrophilic–hydrophobic balance and charge density that avoids cytotoxicity without compromising the antibacterial activity.The cytotoxic and hemolytic potential of amphiphilic dendrimers can be critically influenced by dendrimer generation and the nature of the exponentially increasing terminal units. Chen et al. studied amphiphilic polyester dendrimers as antibacterial candidates.43 These dendrimers are composed of alkyl chains and polyester dendrons based on 2,2-bis(methylol)propionic acid (bis-MPA). Notably, the prime candidate 9 carrying a C14 alkyl chain (Fig. 3) exhibited highly potent antibacterial activity (MIC of 4.0–8.0 μg mL−1) without any notable cytotoxicity or hemolytic activity, even at concentrations >5000 μg mL−1. However, the first-generation dendrimer 11 (Fig. 3) was highly hemolytic (HC50 10–63 μg mL−1), while the second-generation dendrimer 10 (Fig. 3) was moderately hemolytic (HC50 2500 μg mL−1), although they both showed antibacterial activity (Table 1). This could be explained by the overall hydrophobicity of the antimicrobial candidate, the higher generation dendrimer having more dendrimer terminals bearing positive charges hence lowering its hydrophobicity and thereby also its toxicity.43 This study demonstrated the critical nature of an optimum amphiphilic balance in the molecular structure of the dendrimer in order to achieve negligible toxicity while retaining activity against a broad spectrum of bacteria.
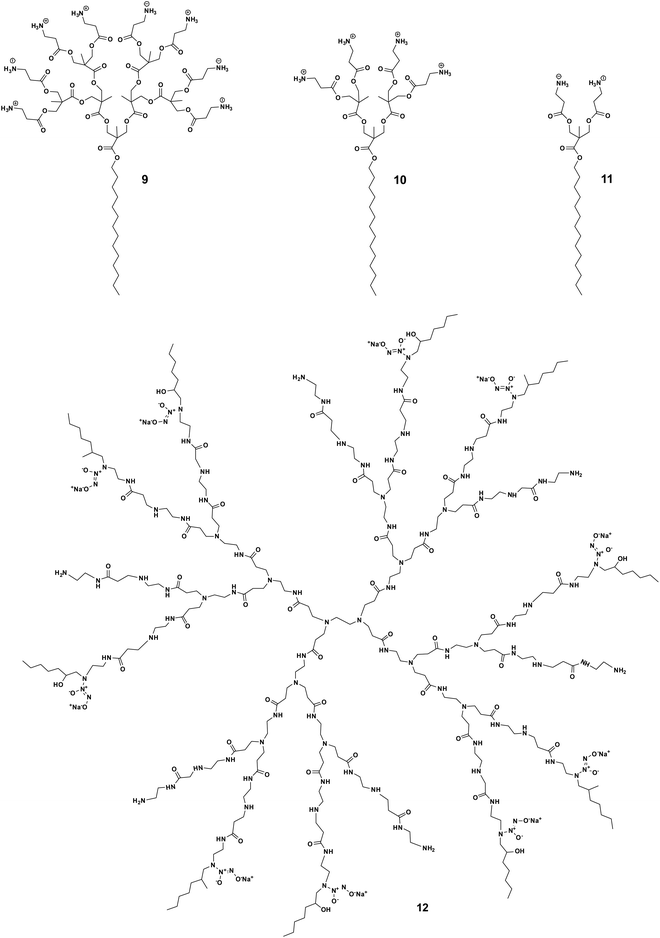 | ||
| Fig. 3 Impact of dendron generation on the trade-off between activity and toxicity shown by the amphiphilic dendrimers 9, 10, 11 and 12. | ||
Another exemplary study was made with the core–shell amphiphilic dendrimers synthesized by Worley et al.47 These dendrimers are PAMAM dendrimers functionalized with a nitric oxide donor and a hexyl aliphatic chain as the dendrimer terminal. Notably, the toxicity of these dendrimers increased with the generation number: the generation 4 dendrimer exhibited greater toxicity than the generation 1 dendrimer (IC50 = 75 and 1150 μg mL−1, respectively). This is understandable considering the exponentially increased number of hydrophobic terminals with increasing dendrimer generation, translating to high toxicity. Interestingly, the generation 3 dendrimer 12 (Fig. 3) was the only generation capable of eradicating both Gram-negative and -positive biofilms at nontoxic concentrations (10–50 μg mL−1) well below the IC50 values (450 μg mL−1). These results again indicate that fine tuning of the hydrophobic–hydrophilic balance between dendrimer generation and functional group density with amphiphilic characteristics can effectively maximize antibacterial action while minimizing toxicity to mammalian cells.
For a given dendrimer generation number, the increase in the hydrophobic tail length can increase the antibacterial activity as well as the toxicity of amphiphilic dendrimers. Guo et al. synthesized amphiphilic dendrimers 13–15 (Fig. 4) based on a PAMAM dendron with varying lengths of alkyl chains.42 As the hydrophobic chain length increased from C6 to C18, the hemolytic activity increased along with antibacterial activity. For example, the amphiphilic dendrimer 15 was more active and also more hemolytic than 13 and 14 (Table 1). A similar trend in hemolytic activity was observed for amphiphilic peptide dendrimers.39 The elongation of the fatty acid chain from C6 to C24 in the peptide dendrimer 2 led to a gradual increase in hemolysis, while the antibacterial activity increased until a plateau was reached.39
 | ||
| Fig. 4 Impact of hydrophobic chain length and number on the balance between antibacterial activity and toxicity as demonstrated by the amphiphilic dendrimers 13, 14, 15, 16 and 17. | ||
In a rare and surprising example, Meyers et al. reported that the carboxylic acid-terminated amphiphilic dendrimer 16 connected to a single aliphatic chain (Fig. 4) was more toxic to HUVEC cells than the amphiphilic dendrimer 17 carrying double aliphatic chains (Fig. 4).48 The selectivity index of the double chain dendrimer 17 was improved as this had a lower MIC against tested Gram-positive bacteria in comparison with that of the single chain dendrimer 16.48 Currently, only saturated alkyl chains and their effect on activity and toxicity have been studied; knowledge on the effect of unsaturated fatty acids and other types of lipid chains as the hydrophobic part of amphiphilic dendrimers is lacking.
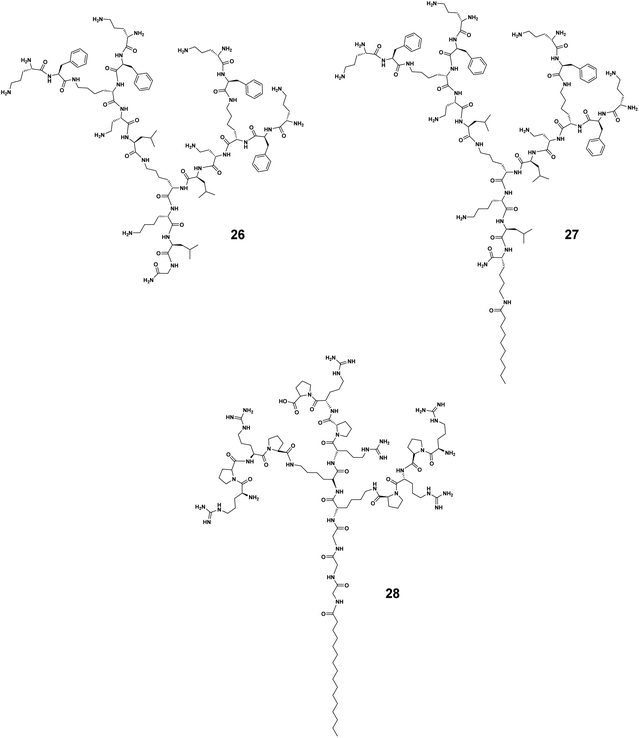
The hydrophobic–hydrophilic balance of the amphiphilic dendrimers can be additionally altered by the terminal functionality, charge, and charge density, as well as the steric size, hence impacting the trade-off between activity and toxicity. We have recently studied the antibacterial activity of amphiphilic PAMAM dendrimers composed of a long C18 alkyl chain and a PAMAM dendron bearing one of the following terminals: primary amine (8 in Fig. 2), tertiary amine (18, Fig. 5), guanidine (19, Fig. 5), or carboxylic acid (20, Fig. 5).44 The primary amine dendrimer 8 was the most active dendrimer against both Gram-negative and -positive bacteria as well as drug-resistant bacteria (Table 1). The tertiary amine dendrimer 18 showed similar activity against E. coli as 8 but had a better safety profile, probably due to the more dispersed cationic charge over the larger tertiary amine terminal when compared with that of the primary amine terminal. It should be mentioned that the carboxylate-terminated dendrimer 20 failed to show any antibacterial activity but was also neither toxic nor hemolytic, as supported and confirmed by results from other groups.42,49
 | ||
| Fig. 5 Impact of terminal functionalities on the activity and toxicity of the amphiphilic dendrimers 18, 19, 20, 21, 22, 23, 24 and 25. | ||
Surprisingly, the guanidine-terminated dendrimer 19 was inactive against all bacteria tested.44 This finding correlate with the results previously reported with guanidine molecular umbrella 21 (Fig. 5).43 In addition, 19 was found to be toxic, which can be attributed to the guanidine terminals that can strongly interact with the eukaryotic cell membrane. These results contradict those previously published supporting arginine-rich moieties as a useful means to generate antibacterial candidates.50 For example, the amphiphilic carbosilane dendrimer 22 bearing guanidine terminals was very active against E. coli and S. aureus with an MIC value of 2.0 μM, which was similar to that of the amine-terminating dendrimer analogue 23.51 The antibacterial activity of the arginine-terminated dendrimer 22 is reminiscent of some highly potent AMPs, such as indolicidin and tritrpticin, which are rich in arginine. Nevertheless, this is just one consideration as these peptides are also rich in tryptophan, which imparts essential and balancing hydrophobicity to these peptides.52 We therefore suggest that the carbosilane dendritic scaffold may provide adequate hydrophobicity to these dendrimers balancing the effects of their highly cationic terminals.51
Another aspect to be considered while designing more selective antimicrobial peptide dendrimers is the incorporation of hydrophobic amino acids at the peptide dendrimer terminals. Sowińska et al. designed two groups of polylysine peptide dendrimers using orthogonally substituted lysine as a core, and the dendrimer terminals were either substituted with lysine or tryptophan with 4 or 8 positive charges, respectively.53 The dendrimers carrying four positive charges such as 24 (Fig. 5) had a broad spectrum of activity with MIC values of 15–31 μM, whereas those with tryptophan terminals were 4–8 times more active. As an example, the dendrimer 25 (Fig. 5) bearing a C12 alkyl chain as a core and tryptophan terminals, showed marked antibacterial activity against multidrug-resistant clinical isolates of different bacteria strains with a mean MIC value of 7.0 μM. Unfortunately, dendrimer 25 (Fig. 5) was also highly cytotoxic at 20 μM. Apparently, the dodecyl chain in combination with the tryptophan amino acid residues conferred significant hydrophobicity to the dendrimer and rendered it cytotoxic. Nonetheless, this study also highlighted the importance of the terminal group hydrophobicity and its impact on amphiphilicity, and thereby on the complex activity–toxicity relationship.53
Amphiphilic dendrimers have increased stability
As linear peptides, many AMPs can be easily degraded by various enzymes and therefore show poor stability and low bioavailability as drug candidates. Thanks to their dendritic structural feature, dendrimeric peptides or peptide dendrimers are different, showing considerable resistance to proteolytic degradation. The dendritic architecture forms a steric hurdle for enzymes, hindering their access to active sites for hydrolyzation of these peptides. Amphiphilic dendrimers show even greater resilience towards enzymatic degradation by virtue of their self-assembly into nanostructures, thus prolonging their circulation time. As such, amphiphilic dendrimers often stably retain their antibacterial activity in the presence of sera, salts, and enzymes.For example, the peptide dendrimer 26 had good antibacterial activity but was not stable in the presence of serum (Fig. 6).39 Addition of a C10-alkyl chain to 26 yielded the amphiphilic dendrimer 27 that allowed an increase in serum half-life from 6 to 20 h (for 26 and 27, respectively). This is in line with literature reports that lipidation can be used to prolong the circulation of peptides or nucleic acids via binding to serum proteins.54 Further studies on 27 showed extremely promising antibacterial efficacy in animal models in which the peptide dendrimers lacking a hydrophobic chain had failed. These results indicate that imparting amphiphilicity to peptide dendrimers is a valuable strategy to enhance both the serum stability and antibacterial activity, and may determine the clinical translation of these dendrimer candidates.
In 2019, Wang J. et al. identified that the arginine–proline (RP) sequence prevented the enzymatic degradation of AMPs in the presence of trypsin and chymotrypsin, but that the activity of these peptides was hampered in the presence of salts.55 They further integrated the RP sequence into amphiphilic peptide dendrimers to capitalize on the dendritic structure and improve the stability.40 The authors identified the two most potent amphiphilic peptide dendrimers, 3 and 28 (Fig. 6), bearing a hexadecanoid acid tail (C16) with a peptide dendron generation of 3 and 2, respectively. Both dendrimers were active with respective mean geometric MIC values of 2.2 and 2.9 μM against nine different bacterial strains. Importantly, they both remained bactericidal in the presence of either monovalent or divalent cations, even after 48 h of serum incubation. When exposed to high salt concentrations (150 mM NaCl or 2.0 mM CaCl2), the MIC value of 3 against E. coli was only slightly increased to 3.0 or 2.0 μM, respectively. A similar trend was observed for 28, the MIC of which was increased by 3.5- and 2.5-fold under the same conditions, respectively. Furthermore, the MIC values against E. coli were slightly increased 0.5-fold for 3 and 1-fold for 28 in the presence of 8.0 μg mL−1 of trypsin and chymotrypsin. Collectively, these results demonstrate that amphiphilic dendrimers exhibit considerable resilience and stability against enzyme degradation, serum sensitivity, and high salt concentration destabilization.40
Amphiphilic dendrimers mainly act on bacterial membrane
Encouraged by the antibacterial performance of amphiphilic dendrimers, studies on their mechanisms have been actively pursued. Current results suggest that many amphiphilic dendrimers mediate membrane depolarization and disruption, representing the primary mechanism responsible for their antibacterial activity.39,40,43,44,56 Specifically, the cationic terminals of the amphiphilic dendrimers interact with the negatively charged bacteria membrane to allow the insertion of the hydrophobic entity which causes membrane disruption, depolarization, and subsequent cell lysis. Although amphiphilic dendrimers can disrupt both Gram-negative and -positive bacterial membranes, they usually show higher potency against Gram-negative bacteria. This is because the outer membrane of Gram-negative bacteria differs from that of Gram-positive bacteria by an extra outer layer comprising negatively charged lipopolysaccharides (LPS). The amphiphilic dendrimers being positively charged preferably interact with the negatively charged LPS layer via electrostatic adsorption.One study on the amphiphilic peptide dendrimers 27 demonstrated the importance of the hydrophobic chain added to the peptide dendrimer for crossing the LPS layer of the bacteria.39 For example, the peptide dendrimer 1 and its peptide dendrimer analogs were all inactive against P. aeruginosa wild-type strains but showed activity against P. aeruginosa mutants lacking LPS. In contrast, the amphiphilic dendrimer 27 was active against all strains regardless of LPS composition of the bacterial outer membrane. These results highlighted the importance of the alkyl chain in allowing the amphiphilic dendrimers to cross the bacterial membrane and thereby express their antibacterial activity. This is in line with a similar reported effect of the N-terminal fatty acid chain in the structure of polymyxin B.57 Furthermore, time-dependent bacteria-killing experiments showed a rapid bactericidal effect against P. aeruginosa within 30 minutes upon addition of 27.39 Such rapid killing kinetics often implies a membrane-disruptive mechanism of action.
To further demonstrate that membrane disruption is indeed the primary mechanism underlying the antibacterial activity of the amphiphilic dendrimers, we performed a series of experimental studies alongside computer modeling on the amphiphilic PAMAM dendrimer 8.44 We first examined the action of 8 on both Gram-negative and -positive bacteria using a live/dead cell staining assay with the fluorescent dyes SYTO9 and propidium iodide (PI). SYTO9 is a universal dye that crosses intact membranes and stains nucleic acids of all live cells green, whereas PI can only cross compromised membranes, emitting a red fluorescence once bound to nucleic acids. Both Gram-negative bacteria, such as E. coli, and Gram-positive bacteria, such as MRSA, displayed a prominent red fluorescence upon treatment with 8 implying a mechanism of action associated with membrane disruption. This was confirmed by scanning electron microscopy studies that revealed obvious membrane deformities such as blebbing and release of intracellular content at the cell surface of bacteria treated with 8. Further membrane permeabilization and depolarization assays confirmed that 8 interacted with the bacterial membrane, causing significant outer membrane permeation in E. coli and rapid inner membrane depolarization in both E. coli and MRSA.
In addition, the self-assembling properties of 8 played an additional crucial role in the mechanism of action, inducing the adsorption and accumulation of 8 on the bacterial membrane via electrostatic interaction, leading to a high local concentration of 8 at the bacterial surface. The enriched 8 on the bacterial surface then formed nanomicelles via self-assembly for more effective and stronger binding with the bacterial membrane via cooperative multivalent binding. Once bound on the bacterial surface, these dendrimer nanomicelles coercively interacted with and inserted into the bacterial membrane with additional hydrophobic interactions, producing profound membrane disruption and hence potent antibacterial activity (Fig. 7).44
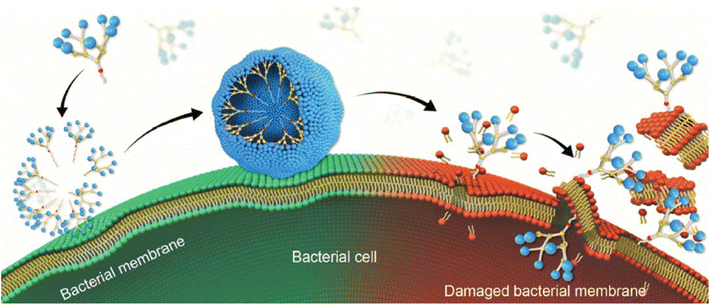 | ||
| Fig. 7 Cartoon illustration of the mechanism underlying the antibacterial activity of the amphiphilic dendrimer 8via membrane adsorption, self-assembly, interaction, insertion, disintegration and disruption. Reproduced from ref. 44 with permission from Royal Society of Chemistry. | ||
Similarly, Lai et al. demonstrated that their amphiphilic peptide dendrimers self-assembled into nanoparticles.40 These nanoparticles not only interacted with the bacterial membrane to exert a killing effect but also involved other mechanisms that addressed intracellular targets (see the section below). In addition, Gide et al. came to the same conclusion using the amphiphilic dendrimer 6 (Fig. 2), which formed nanoparticles to interact with and compromise the bacterial membrane to exert its antibacterial activity.41 These different studies provide support for the important contribution of the self-assembling of the amphiphilic dendrimers to create localized high concentrations on the bacterial surface membrane for strong binding and subsequent membrane disruption, ultimately inducing lysis and killing of the bacteria.
Amphiphilic dendrimers also act on intracellular targets
Although membrane disruption is the primary mechanism employed by amphiphilic dendrimers to exert their antibacterial activity, recent studies report that amphiphilic dendrimers may interact with intracellular targets.40 For example, Lai et al. used continuous-scanning fluorescence imaging to demonstrate that FITC-labeled 3 localized within bacteria. Further transcriptome studies revealed the downregulation in expression of essential genes involved in the synthesis of peptidoglycan and ribosome subunits upon treatment with 3.40 In addition, the negatively impacted genes were involved in pathways associated with basic metabolic processes, including cellular carbohydrate metabolism as well as glycolysis/gluconeogenesis and oxidative phosphorylation. Therefore, 3 exerted antibacterial activity through a multimodal mechanism primarily involving membrane integrity disruption and affected bacterial energy production and metabolism (Fig. 8).40 The finding of amphiphilic dendrimers interacting with multiple targets is encouraging with regards the desired prevention of target bacteria developing resistance.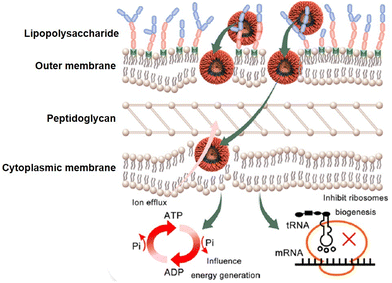 | ||
| Fig. 8 Multimodal mechanism for the antibacterial activity of the amphiphilic peptide dendrimer 3. Reproduced from ref. 40 with permission from ACS Publications, copyright 2023. | ||
Amphiphilic dendrimers prevent bacteria from acquiring drug resistance
Acquired drug resistance occurs when bacteria gain the ability to resist the activity of antibacterial agents by evolutionary processes. Recent studies demonstrate that amphiphilic dendrimers can circumvent acquired bacterial resistance. For example, Lai et al. studied the acquired resistance of E. coli to the amphiphilic dendrimer 3 (MIC of 1.0 μM) at sub-MICs for up to 30 days.40 No notable acquired resistance was observed against 3, whereas E. coli quickly generated effective resistance upon treatment with sub-MICs of ciprofloxacin after 6 days. Notably, the genes encoding cationic antimicrobial peptide resistance that are primarily involved in bacterial membrane synthesis were significantly upregulated in E. coli upon treatment with 3. This upregulation may have decreased the affinity for 3 by increasing the positive charge on the outer bacterial membrane as well as by altering the metabolism of amino acids and sugars within the inner membrane. However, despite upregulation of resistance genes, the multimodal mechanism of 3 ensured a maintained strong bactericidal effect. Notably, following treatment of E. coli with 3, 1479 genes exhibited significant differential expression (out of a total of 5130 genes), that was upregulated in 809 genes and downregulated in 670 genes. These differentially expressed genes were mostly associated with ribosomal function, valine, leucine, and isoleucine biosynthesis, and C5-branched dibasic acid metabolism. These results indicate that 3 acted differently from most antibiotics, which have only a very limited number of targets, and that the actions of 3 are more likely to cause comprehensive changes to multiple functions and structural features of bacteria. Therefore, 3 may have crossed the bacterial membrane after initially damaging it to generate a comprehensive response involving DNA transcription and translation, protein synthesis, energy production, transmembrane transport, and other processes in E. coli.Guo et al. also reported the lack of drug resistance induced in either S. aureus or E. coli to amphiphilic dendrimer.42 In particular, the MIC value of their reported amphiphile remained unchanged despite repeat exposure of both S. aureus and E. coli at sub-MIC levels. These results further support the encouraging findings of the inherent difficultly for bacteria to develop resistance to cationic amphiphilic dendrimers which target not only bacterial membrane but also internal biosynthetic and metabolic processes.
Conclusion and future perspectives
The alarming situation of AMR in bacteria imposes the rapid development of novel antibacterial agents that are effective and stable while inducing no toxicity or resistance development. Amphiphilic dendrimers are emerging as a promising solution to combat bacterial AMR. They are composed of distinct hydrophobic and hydrophilic entities bearing dendritic structures, which can be precisely designed and synthesized. The primary mechanism underlying their antibacterial activity involves the binding of their positively charged entities with the negatively charged bacterial surface via multivalent electrostatic interactions. This is then followed by penetration of their hydrophobic entity into the bacterial cell membrane resulting in membrane disruption and thereby cell death.44 Additional modes of action of the amphiphilic dendrimers have been reported that involve intracellular targets, such as synthesis of membranes, amino acids and nucleic acids.40 The potent antibacterial activity achieved by amphiphilic dendrimers appears to involve multimodal mechanisms of action that effectively kill bacteria including multidrug-resistant species.Notably, the hydrophilic–hydrophobic balance of the amphiphilic dendrimers is crucial to ensure high potency against a broad spectrum of bacteria and to avoid adverse effects on host cells, especially red blood cells. The trade-off between antibacterial activity and toxicity needs careful consideration when designing amphiphilic dendrimers as antibacterial candidates. The precise choice of appropriate dendrimer structure, generation and surface functionalities, as well as hydrophobic entities can impact the level of selectivity that is achieved. Although amphiphilic dendrimers encompass core–shell and dendron-tail structures30,31 (Fig. 1B), the latter have a more straightforward design allowing a more precise synthesis in accordance with the desired balance between hydrophilicity and hydrophobicity. As such dendron-tail structures are the most studied for their antibacterial activity and in particular to overcome antibiotics resistance.
To date, most studies on antibacterial activity involve amphiphilic peptide dendrimers, PAMAM dendrimers and polyester dendrimers. Many other dendrimers with different scaffolds have been evaluated for use as antibacterial agents in recent years, including carbosilane dendrimers,58–60 phosphorus dendrimers61 and glycodendrimers dendrimers62etc. More investigations are now needed on the antibacterial activity of amphiphilic dendrimers with these different dendrimer scaffolds for combatting AMR.
In parallel, more efforts should be applied to preclinical investigations of potent amphiphilic dendrimers in different animal models of infectious diseases to thoroughly assess their robustness and pave the way towards their clinical application. It should be mentioned that knowledge on amphiphilic dendrimer-AMP mimics that exert antibacterial activity via immunomodulatory mechanisms is still lacking, and further studies in this regard are required to accelerate the clinical translation of amphiphilic dendrimers as effective and potent antibacterial candidates. In addition, focus should be given to the development of narrow-spectrum antibiotics, as the use of broad-spectrum antibiotics not only generates drug resistance but also harms beneficial microbial communities inhabiting humans. Narrowing the spectrum of antibacterial agents now appears to represent a feasible perspective for precision antibacterial therapies to combat AMR.
It is to note that amphiphilic dendrimers can self-assemble into nanostructures that can accommodate drug molecules31,63,64 for combination therapy and simultaneously harness the advantage of the nanotechnology-based drug delivery for specific accumulation at the site of infection and inflammation. Targeted drug delivery can be readily achieved through either passive targeting via the enhanced permeability and retention effect or active targeting via conjugation of ligands, antibodies or other bacterial cell-specific targeting moieties, enabling effective treatments while reducing adverse effects and toxicities.
Finally, combatting bacterial AMR needs a One Health approach as this is a critical global problem affecting human and animal life as well as the environment.4 The first step in line with this approach involves improving regulations and policies aimed at halting the excessive use of antimicrobials in agriculture and animal husbandry. Alongside this, antibiotic stewardship programs within the clinical setting group together specialists dedicated to optimizing use of antibiotic therapy in humans. Finally, a community-based surveillance of the migration of individual and animals infected with resistant bacteria would help limit the spread of infections.65 Such combined efforts should help reduce bacterial infections and enable progress to be made in the fight against antibiotic resistance.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the French National Research Agency under the framework of the ERA-NET EURONANOMED European Research projects ‘TARBRAINFECT’ (LP) and ‘antineuropatho’ (LP), EU H2020 Research and Innovation program NMBP “SAFE-N-MEDTECH” (2019–2023) (grant agreement no. 814607, LP), AMIDEX d'Aix-Marseille Université (LP), Erasmus+ Traineeship program (CG), ED250 Aix-Marseille Université PhD fellowship in Chemical Sciences (CG). We thank Dr Leo Lee and Mr Nian Zhang for their critical comments.References
- C. J. Murray, K. S. Ikuta and F. Sharara,
et al., Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis, Lancet, 2022, 399(10325), 629–655, DOI:10.1016/S0140-6736(21)02724-0
.
- J. O'Neill, Tackling Drug-Resistant Infections Globally: Final Report and Recommendations, Review on Antimicrobial Resistance, 2016.
- D. G. J. Larsson and C. F. Flach, Antibiotic resistance in the environment, Nat. Rev. Microbiol., 2022, 20(5), 257–269, DOI:10.1038/s41579-021-00649-x
.
- T. R. Walsh, A one-health approach to antimicrobial resistance, Nat. Microbiol., 2018, 3(8), 854–855, DOI:10.1038/s41564-018-0208-5
.
- J. M. Blair, M. A. Webber, A. J. Baylay, D. O. Ogbolu and L. J. Piddock, Molecular mechanisms of antibiotic resistance, Nat. Rev. Microbiol., 2015, 13(1), 42–51, DOI:10.1038/nrmicro3380
.
- D. M. P. de Oliveira, B. M. Forde and T. J. Kidd,
et al., Antimicrobial Resistance in ESKAPE Pathogens, Clin. Microbiol. Rev., 2020, 33(3), e00181-19, DOI:10.1128/CMR
.
- M. S. Mulani, E. E. Kamble, S. N. Kumkar, M. S. Tawre and K. R. Pardesi, Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review, Front. Microbiol., 2019, 10, 539, DOI:10.3389/fmicb.2019.00539
.
- C. Årdal, M. Balasegaram and R. Laxminarayan,
et al., Antibiotic development—economic, regulatory and societal challenges, Nat. Rev. Microbiol., 2020, 18(5), 267–274, DOI:10.1038/s41579-019-0293-3
.
- U. Theuretzbacher, K. Outterson, A. Engel and A. Karlén, The global preclinical antibacterial pipeline, Nat. Rev. Microbiol., 2020, 18(5), 275–285, DOI:10.1038/s41579-019-0288-0
.
- M. Miethke, M. Pieroni and T. Weber,
et al., Towards the sustainable discovery and development of new antibiotics, Nat. Rev. Chem., 2021, 5(10), 726–749, DOI:10.1038/s41570-021-00313-1
.
- E. D. Brown and G. D. Wright, Antibacterial drug discovery in the resistance era, Nature, 2016, 529(7586), 336–343, DOI:10.1038/nature17042
.
- C. D. Fjell, J. A. Hiss, R. E. W. Hancock and G. Schneider, Designing antimicrobial peptides: Form follows function, Nat. Rev. Drug Discovery, 2012, 11(1), 37–51, DOI:10.1038/nrd3591
.
- T. J. Hall,
et al., A call for action to the biomaterial community to tackle antimicrobial resistance, Biomater. Sci., 2020, 8, 4951–4974, 10.1039/D0BM01160F
.
- R. E. W. Hancock and H. G. Sahl, Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies, Nat. Biotechnol., 2006, 24(12), 1551–1557, DOI:10.1038/nbt1267
.
- M. Iubatti, I. M. Gabas and L. M. Cavaco,
et al., Antisense Peptide Nucleic Acid-Diaminobutanoic Acid Dendron Conjugates with SbmA-Independent Antimicrobial Activity against Gram-Negative Bacteria, ACS Infect. Dis., 2022, 8(5), 1098–1106, DOI:10.1021/acsinfecdis.2c00089
.
- A. R. Kirtane, M. Verma, P. Karandikar, J. Furin, R. Langer and G. Traverso, Nanotechnology approaches for global infectious diseases, Nat. Nanotechnol., 2021, 16(4), 369–384, DOI:10.1038/s41565-021-00866-8
.
- S. J. Lam, N. M. O'Brien-Simpson and N. Pantarat,
et al., Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers, Nat. Microbiol., 2016, 16162, DOI:10.1038/nmicrobiol.2016.162
.
- J. M. V. Makabenta, A. Nabawy, C. H. Li, S. Schmidt-Malan, R. Patel and V. M. Rotello, Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections, Nat. Rev. Microbiol., 2021, 19(1), 23–36, DOI:10.1038/s41579-020-0420-1
.
- N. Mookherjee, M. A. Anderson, H. P. Haagsman and D. J. Davidson, Antimicrobial host defence peptides: functions and clinical potential, Nat.
Rev. Drug Discovery, 2020, 19(5), 311–332, DOI:10.1038/s41573-019-0058-8
.
- M. Melo, R. Ferre and M. A. R. B. Castanho, Antimicrobial peptides: linking partition, activity and high membrane-bound concentrations, Nat. Rev. Microbiol., 2009, 7, 245–250 CrossRef CAS PubMed
.
- B. H. Gan, J. Gaynord, S. M. Rowe, T. Deingruber and D. R. Spring, The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions, Chem. Soc. Rev., 2021, 50(13), 7820–7880, 10.1039/d0cs00729c
.
-
M. Malkoch and S. G. Gallego, Dendrimer Chemistry: Synthetic Approaches Towards Complex Architectures, Royal Society of Chemistry, 2020. 10.1039/9781788012904
.
- D. A. Tomalia, H. Baker and J. Dewald,
et al., A New Class of Polymers: Starburst-Dendritic Macromolecules, Polym. J., 1985, 17, 117–132 CrossRef CAS
.
- Z. Liu, A. W. Young and P. Hu,
et al., Tuning the membrane selectivity of antimicrobial peptides by using multivalent design, ChemBioChem, 2007, 8(17), 2063–2065, DOI:10.1002/cbic.200700502
.
- D. A. Tomalia, A. M. Naylor and W. A. Goddard, Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter, Angew. Chem., Int. Ed. Engl., 1990, 29(2), 138–175, DOI:10.1002/anie.199001381
.
-
Z. Lyu, L. Ding, D. Dhumal, A. Y. T. Huang and L. Peng, Poly(amidoamine) (PAMAM) Dendrimers: Synthesis and Biological Applications, Dendrimer Chemistry: Synthetic Approaches Towards Complex Architectures, Royal Society of Chemistry, 2020, ch. 4, pp. 253–255. 10.1039/9781788012904-00085
.
- A. Castonguay, E. Ladd, T. G. M. van de Ven and A. Kakkar, Dendrimers as bactericides, New J. Chem., 2012, 36(2), 199–204, 10.1039/c1nj20481e
.
- J. Lazniewska, K. Milowska and T. Gabryelak, Dendrimers-revolutionary drugs for infectious diseases, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2012, 4(5), 469–491, DOI:10.1002/wnan.118
.
- M. A. Mintzer, E. L. Dane, G. A. O'Toole and M. W. Grinstaff, Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases, Mol. Pharmaceutics, 2012, 9(3), 342–354, DOI:10.1021/mp2005033
.
- J. Chen, D. Zhu, X. Liu and L. Peng, Amphiphilic Dendrimer Vectors for RNA Delivery: State-of-the-Art and Future Perspective, Acc. Mater. Res., 2022, 3(5), 484–497, DOI:10.1021/accountsmr.1c00272
.
- Z. Lyu, L. Ding, A. Tintaru and L. Peng, Self-Assembling Supramolecular Dendrimers for Biomedical Applications: Lessons Learned from Poly(amidoamine) Dendrimers, Acc. Chem. Res., 2020, 53(12), 2936–2949, DOI:10.1021/acs.accounts.0c00589
.
- E. A. Azzopardi, E. L. Ferguson and D. W. Thomas, The enhanced permeability retention effect: A new paradigm for drug targeting in infection, J. Antimicrob. Chemother., 2013, 68(2), 257–274, DOI:10.1093/jac/dks379
.
- H. Maeda, The 35th anniversary of the discovery of EPR effect: A new wave of nanomedicines for tumor-targeted drug delivery-personal remarks and future prospects, J. Pers. Med., 2021, 11(3), 229, DOI:10.3390/jpm11030229
.
- Y. Matsumura and H. Maeda, A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs, Cancer Res., 1986, 46, 6387–6392 CAS
.
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review, J. Controlled Release, 2000, 65(1–2), 271–284 CrossRef CAS PubMed
.
- Y. Shi, R. van der Meel, X. Chen and T. Lammers, The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy, Theranostics, 2020, 10(17), 7921–7924, DOI:10.7150/thno.49577
.
- H. Maeda, Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective
drug targeting, Proc. Jpn. Acad., Ser. B, 2012, 88(3), 53–71 CrossRef CAS PubMed
.
- Y. Liu, D. Sun and Q. Fan,
et al., The enhanced permeability and retention effect based nanomedicine at the site of injury, Nano Res., 2020, 13(2), 564–569, DOI:10.1007/s12274-020-2655-6
.
- T. N. Siriwardena, M. Stach and R. He,
et al., Lipidated Peptide Dendrimers Killing Multidrug-Resistant Bacteria, J. Am. Chem. Soc., 2018, 140(1), 423–432, DOI:10.1021/jacs.7b11037
.
- Z. Lai, Q. Jian and G. Li,
et al., Self-Assembling Peptide Dendron Nanoparticles with High Stability and a Multimodal Antimicrobial Mechanism of Action, ACS Nano, 2021, 15(10), 15824–15840, DOI:10.1021/acsnano.1c03301
.
- M. Gide, A. Nimmagadda and M. Su,
et al., Nano-Sized Lipidated Dendrimers as Potent and Broad-Spectrum Antibacterial Agents, Macromol. Rapid Commun., 2018, 39(24), 1800622, DOI:10.1002/marc.201800622
.
- W. Guo, Y. Wang and P. Wan,
et al., Cationic amphiphilic dendrons with effective antibacterial performance, J. Mater. Chem. B, 2022, 10(3), 456–467, 10.1039/d1tb02037d
.
- A. Chen, A. Karanastasis and K. R. Casey,
et al., Cationic Molecular Umbrellas as Antibacterial Agents with Remarkable Cell-Type Selectivity, ACS Appl. Mater. Interfaces, 2020, 12(19), 21270–21282, DOI:10.1021/acsami.9b19076
.
- D. Dhumal, B. Maron and E. Malach,
et al., Dynamic self-assembling supramolecular dendrimer nanosystems as potent antibacterial candidates against drug-resistant bacteria and biofilms, Nanoscale, 2022, 14, 9286–9296, 10.1039/d2nr02305a
.
- M. Gholami, R. Mohammadi and M. Arzanlou,
et al., In vitro antibacterial activity of poly(amidoamine)-G7 dendrimer, BMC Infect. Dis., 2017, 395, DOI:10.1186/s12879-017-2513-7
.
- A. M. Holmes, J. R. Heylings, K. W. Wan and G. P. Moss, Antimicrobial efficacy and mechanism of action of poly(amidoamine) (PAMAM) dendrimers against opportunistic pathogens, Int. J. Antimicrob. Agents, 2019, 53(4), 500–507, DOI:10.1016/j.ijantimicag.2018.12.012
.
- B. V. Worley, K. M. Schilly and M. H. Schoenfisch, Anti-biofilm efficacy of dual-action nitric oxide-releasing alkyl chain modified poly(amidoamine) dendrimers, Mol. Pharmaceutics, 2015, 12(5), 1573–1583, DOI:10.1021/acs.molpharmaceut.5b00006
.
- S. R. Meyers, F. S. Juhn, A. P. Griset, N. R. Luman and M. W. Grinstaff, Anionic amphiphilic dendrimers as antibacterial agents, J. Am. Chem. Soc., 2008, 130(44), 14444–14445, DOI:10.1021/ja806912a
.
- J. Pan, L. Guo, L. Ouyang, D. Yin and Y. Zhao, Synthesis, antibacterial activity and cytotoxicity of novel Janus peptide dendrimers, Synlett, 2012, 1937–1940, DOI:10.1055/s-0031-1290403
.
- C. J. C. Edwards-Gayle, G. Barrett and S. Roy,
et al., Selective Antibacterial Activity and Lipid Membrane Interactions of Arginine-Rich Amphiphilic Peptides, ACS Appl. Bio Mater., 2020, 3(2), 1165–1175, DOI:10.1021/acsabm.9b00894
.
- I. Heredero-Bermejo, J. M. Hernández-Ros and L. Sánchez-García,
et al., Ammonium and guanidine carbosilane dendrimers and dendrons as microbicides, Eur. Polym. J., 2018, 101, 159–168, DOI:10.1016/j.eurpolymj.2018.02.025
.
- D. I. Chan, E. J. Prenner and H. J. Vogel, Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action, Biochim. Biophys. Acta, Biomembr., 2006, 1758(9), 1184–1202, DOI:10.1016/j.bbamem.2006.04.006
.
- M. Sowińska, A. Laskowska and A. Guśpiel,
et al., Bioinspired Amphiphilic Peptide Dendrimers as Specific and Effective Compounds against Drug Resistant Clinical Isolates of E. coli, Bioconjugate Chem., 2018, 29(11), 3571–3585, DOI:10.1021/acs.bioconjchem.8b00544
.
- F. Kratz, Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles, J. Controlled Release, 2008, 132(3), 171–183, DOI:10.1016/j.jconrel.2008.05.010
.
- J. Wang, J. Song and Z. Yang,
et al., Antimicrobial Peptides with High Proteolytic Resistance for Combating Gram-Negative Bacteria, J. Med. Chem., 2019, 62(5), 2286–2304, DOI:10.1021/acs.jmedchem.8b01348
.
- R. Kannan, P. Prabakaran and R. Basu,
et al., Mechanistic study on the antibacterial activity of self-assembled poly(aryl ether)-based amphiphilic dendrimers, ACS Appl. Bio Mater., 2019, 2(8), 3212–3224, DOI:10.1021/acsabm.9b00140
.
- T. Velkov, P. E. Thompson, R. L. Nation and J. Li, Structure-activity relationships of polymyxin antibiotics, J. Med. Chem., 2010, 53(5), 1898–1916, DOI:10.1021/jm900999h
.
- F. J. de la Mata, R. Gómez, J. Cano, J. Sánchez-Nieves, P. Ortega and S. G. Gallego, Carbosilane dendritic nanostructures, highly versatile platforms for pharmaceutical applications, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2022, 23, e1871, DOI:10.1002/wnan.1871
.
- E. Fuentes-Paniagua, J. M. Hernández-Ros and M. Sánchez-Milla,
et al., Carbosilane cationic dendrimers synthesized by thiol-ene click chemistry and their use as antibacterial agents, RSC Adv., 2014, 4(3), 1256–1265, 10.1039/c3ra45408h
.
- S. Quintana-Sanchez, N. Gómez-Casanova and J. Sánchez-Nieves,
et al., The Antibacterial Effect of PEGylated Carbosilane Dendrimers on P. aeruginosa Alone and in Combination with Phage-Derived Endolysin, Int. J. Mol. Sci., 2022, 23(3), 1873, DOI:10.3390/ijms23031873
.
- S. Mignani, J. Bignon, X. Shi and J. P. Majoral, First-in-Class Phosphorus Dendritic Framework, a Wide Surface Functional Group Palette Bringing Noteworthy Anti-Cancer and Anti-Tuberculosis Activities: What Lessons to Learn?, Molecules, 2021, 26(12), 3708, DOI:10.3390/molecules26123708
.
- P. Hoyos, A. Perona, O. Juanes, Á. Rumbero and M. J. Hernáiz, Synthesis of Glycodendrimers with Antiviral and Antibacterial Activity, Eur. J. Chem., 2021, 27(28), 7593–7624, DOI:10.1002/chem.202005065
.
- T. Wei, C. Chen and J. Liu,
et al., Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(10), 2978–2983, DOI:10.1073/pnas.1418494112
.
- Y. Jiang, Z. Lyu and B. Ralahy,
et al., Dendrimer nanosystems for adaptive tumor-assisted drug delivery via extracellular vesicle hijacking, Proc. Natl. Acad. Sci. U. S. A., 2023, 120(7), e2215308120, DOI:10.1073/pnas.2215308120
.
- D. A. Goff and T. M. File, The Evolving Role of Antimicrobial Stewardship in Management of Multidrug Resistant Infections, Infect. Dis. Clin. North Am., 2016, 30(2), 539–551, DOI:10.1016/j.idc.2016.02.012
.
| This journal is © The Royal Society of Chemistry 2023 |