Resonant acoustic mixing (RAM) for efficient mechanoredox catalysis without grinding or impact media†
Farshid
Effaty
 ab,
Lori
Gonnet
ab,
Lori
Gonnet
 b,
Stefan G.
Koenig
b,
Stefan G.
Koenig
 c,
Karthik
Nagapudi
c,
Xavier
Ottenwaelder
c,
Karthik
Nagapudi
c,
Xavier
Ottenwaelder
 *a and
Tomislav
Friščić
*a and
Tomislav
Friščić
 *bd
*bd
aDepartment of Chemistry and Biochemistry, Concordia University, Montreal, Canada. E-mail: dr.x@concordia.ca
bDepartment of Chemistry, McGill University, Montreal, Canada
cGenentech, Inc., One DNA Way, South San Francisco, CA 94080, USA
dSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. E-mail: t.friscic@bham.ac.uk
First published on 12th December 2022
Abstract
Resonant acoustic mixing (RAM) enables mechanoredox catalysis with BaTiO3 as the piezoelectric catalyst on model diazonium coupling reactions. RAM proceeds without formal grinding or impact media, is faster than the analogous ball-milling strategy, and is readily scalable. X-ray diffraction and spectroscopy indicate that reusability of BaTiO3 as a mechanoredox catalyst under ball-milling or RAM might be limited by boration.
Mechanochemical reactions by impact, stress or shear have emerged as powerful approaches for synthesis without bulk solvents.1,2 Mechanochemistry also offers unexpected benefits, such as enhanced control over reaction selectivity, stoichiometry, as well as reactions or products that are difficult to achieve in solution.3 An exciting opportunity in mechanosynthesis, introduced by the Ito group, is the use of a piezoelectric material, such as BaTiO3, to catalyse electron-transfer reactions.4 In such mechanoredox catalysis,5 the mechanical impact from the grinding media (e.g., milling balls) triggers the piezoelectric effect on the catalyst, enabling reaction progress. Grinding media, however, are generally found to complicate mechanochemical reaction design because of unwanted leaching, abrasion, and/or chipping.6 Moreover, the complex motion of such media renders scaling-up difficult, requiring changes to instrument design and/or further trial-and-error screening of reaction parameters such as filling ratios, milling frequency, choice and number of milling balls.7
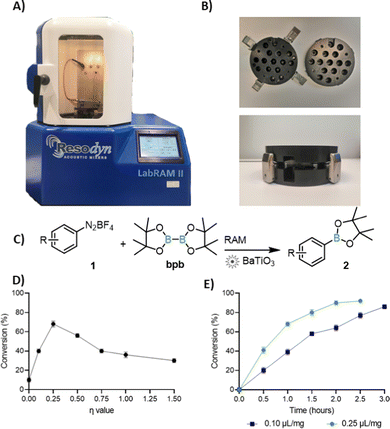
Resonant Acoustic Mixing (RAM, Fig. 1), a technology developed for media-free mixing and blending of sensitive materials, such as explosives,8 can enable mechanosynthesis without either bulk solvent or milling media. So far, RAM has been used for synthesis of cocrystals,8,9 metal–organic frameworks, and for metal-catalysed coupling reactions.10,11 In contrast to ball-milling, RAM reaction design is simplified by the absence of grinding media, and reactions are readily scalable.11
Here we show that, despite the lack of mechanical impact from milling media, RAM is amenable to piezoelectric catalysis. By using commercial cubic BaTiO3 as catalyst, we establish single electron transfer (SET) borylation and arylation of diazonium salts,‡ scalable from ca. 0.1 gram to at least gram amounts (Fig. 1). When the catalyst is reused, a loss of activity is observed, but is smaller than under ball-milling. We tentatively attribute this activity loss to in situ BaTiO3 boration.
Cubic BaTiO3 was obtained from Sigma-Aldrich, with declared particle size <100 nm (BET, ≥99% purity trace metals basis), and was used as-is. As a model reaction we chose the borylation of aryldiazonium salts, with p-chlorobenzenediazonium tetrafluoroborate (1a) and bis(pinacolato)boron (bpb) as test substrates. Because this reaction pioneered mechanoredox catalysis,4 it offers an opportunity to compare RAM and ball-milling protocols. Acoustic mixing was performed using a Resodyn LabRAM II instrument, with samples placed in glass vials mounted on in-house designed holders (Fig. 1A and B).11 RAM of an equimolar mixture (0.3 mmol each) of 1a and bpb at an acceleration of 90g (g = 9.81 m s−2) led to no reaction, as revealed by 1H-NMR analysis in DMSO-d6. Addition of 300 mg (4.3 equivalents) of BaTiO3, however, led to ca. 10% conversion to the anticipated product 2a after 3 h. Similar results were seen when RAM was performed with a small amount of a non-polar liquid additive (ratio of the liquid volume to weight of solid reaction mixture η = 0.25 μL mg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) such as hexanes (17%) or toluene (13%). Acetonitrile (MeCN) as the liquid additive at η = 0.10 μL mg−1 led to 92% NMR conversion to 2a in 3 h, comparable to a 3 hour ball-milling protocol.6
12) such as hexanes (17%) or toluene (13%). Acetonitrile (MeCN) as the liquid additive at η = 0.10 μL mg−1 led to 92% NMR conversion to 2a in 3 h, comparable to a 3 hour ball-milling protocol.6
Screening13 of reaction times at η = 0.25 μL mg−1 indicated that reaction conversion increases for up to 2 h of RAM and then levels off, which is noticeably shorter compared to 3 h reported for ball-milling (Fig. 1E). Attempts to further optimize the reaction through screening η-values between 0 and 1.5 μL mg−1 revealed maximum conversion (ηmax)14 at η = 0.25 μL mg−1 (Fig. 1D). Conversion fell at lower accelerations, and at lower amounts of BaTiO3. Consequently, all work was performed at 90g, using 4.3 equivalents of BaTiO3 (see ESI†). The relevance of BaTiO3 for the reaction was verified by conducting RAM with the same weight amount of Celite, sand, or NaCl. In each case, only trace of reaction was observed by 1H NMR (below 5%).
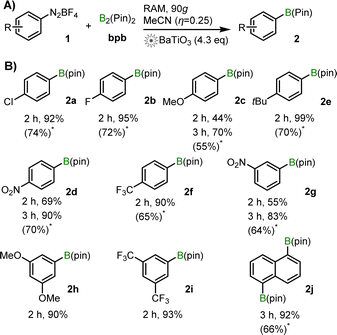
The established RAM conditions were applicable to a range of aryldiazonium salts, with diverse positioning and nature of the aromatic ring substituent. In almost all cases, reactions proceeded with high (>80%) to excellent (>95%) yields within 2 h (Scheme 1). For substrates with unexceptional conversions after 2 h (e.g., 1c, 1d), the yield of product increased significantly upon extending the reaction time to 3 h, e.g., from 69% to 90% for 2d. For substrates 1a–1g, the NMR yields (70–99%) are significantly higher than reported by ball-milling (52–86%, see also ESI†).4
From the examples in Scheme 1, there is no clear trend between the electronic properties of arene and the yield of 2. For example, diazonium salts bearing two meta-substituents, 1h and 1i, both provide more than 90% NMR yields after 2 h of RAM, despite electronically very different groups (–OMe in 1h, –CF3 in 2i). Overall, RAM-based mechanoredox catalysis provides access to a broad range of products with excellent yields, faster than previously reported for ball-milling. Efficiency of RAM opens access to ditopic substrates such as 1j, when using 2.1 equivalents of bpb. Here, 2j was obtained in 92% after 3 h, i.e., 96% yield per each pinacolato boron function (67% isolated yield).
We hypothesize that the RAM reaction mechanism is much the same as via ball-milling, i.e., follows a radical SET mechanism. This is supported by the reaction of 2d in the presence of BaTiO3 and two equivalents of TEMPO radical scavenger (Scheme 2A). After 1 h, the expected4 TEMPO-trapped radical intermediate was obtained in 30% yield, characterised by 1H and 13C NMR, as well as X-ray single-crystal diffraction.
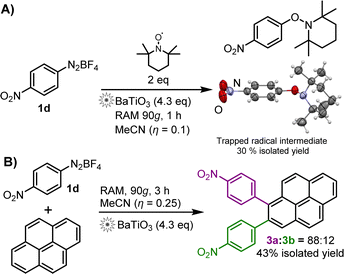 | ||
Scheme 2 (A) Radical-trapping reaction by RAM mechanoredox catalysis, using TEMPO as the radical scavenger. A molecule of the TEMPO-trapped reaction intermediate is shown using a thermal ellipsoid plot (50% probability) based on single crystal X-ray crystal structure analysis (CCDC deposition number 2205247†). (B) Pyrene arylation via BaTiO3-catalysed RAM mechanoredox reaction; the 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b ratio was determined by 1H-NMR spectroscopy in CDCl3 solution. 3b ratio was determined by 1H-NMR spectroscopy in CDCl3 solution. | ||
Application of RAM to mechanoredox catalysis is not limited to borylation, as demonstrated by a successful pyrene arylation (Scheme 2B). RAM of pyrene and 1d proceeded readily over 3 h in the presence of MeCN (η = 0.25 μL mg−1) and BaTiO3 (4.3 equivalents) to produce a 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 mixture of 1- and 2-(4-nitrophenyl)pyrenes (3a, 3b) in 43% overall isolated yield.
12 mixture of 1- and 2-(4-nitrophenyl)pyrenes (3a, 3b) in 43% overall isolated yield.
Because BaTiO3 is present in super-stoichiometric amounts, we also explored the possibility of re-using it. In a typical procedure, with 1a as the model reactant, RAM was conducted over 1 h, and BaTiO3 was separated from the crude reaction mixture by extracting the organic materials with ethyl acetate (EtOAc), followed by centrifugation and drying, before being used in the next reaction cycle. Mass loss of BaTiO3 between cycles ranged between 5 and 10%. Upon several such reactions, the yield of 2a per cycle steadily decreased, from 68% when using pristine BaTiO3 to 11% after the fourth cycle (Table 1).
| Cycle | Ball-milling NMR yield (%) | RAM NMR yield (%) |
|---|---|---|
| a Reactions were performed for 1 h in the presence of MeCN as liquid additive (ball-milling at 30 Hz in a 10 mL zirconia jar and ball; RAM at 90g). 1H NMR yields were obtained by dissolving the crude product in DMSO-d6. | ||
| 1 | 43 | 68 |
| 2 | 32 | 42 |
| 3 | 10 | 21 |
| 4 | 8 | 11 |
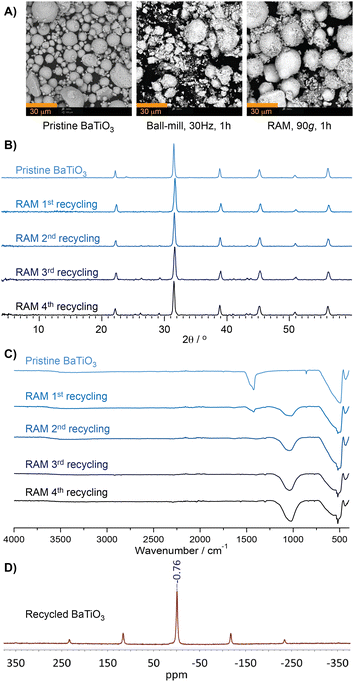
Loss of BaTiO3 activity was also seen in ball-milling reactions (Table 1). A potential cause for the higher yields and improved retention of BaTiO3 activity in RAM might be the gentler nature of acoustic mixing, evident from scanning electron microscopy (SEM) images of BaTiO3 before and after reactions by RAM and by ball milling (Fig. 2A). Commercial BaTiO3 was found to consist of spherical particles, tens of micrometers in size, that were significantly disrupted by ball-milling. By contrast, RAM led to little morphological change, but for a layer of finer material on the larger particles.
Further analysis of BaTiO3 across multiple reaction cycles was carried out by powder X-ray diffraction (PXRD) and Fourier-transform infrared attenuated total reflectance spectroscopy (FTIR-ATR) (Fig. 2B and C, also ESI†).§15 Analysis by PXRD revealed very little broadening of BaTiO3 Bragg reflections after four runs under either RAM or ball-milling conditions, indicating that the loss of reactivity in either protocol is not due to crystal structure disruption. PXRD analysis of materials after the 3rd and 4th cycles revealed the appearance of new, low-intensity X-ray reflections distinct from those of BaTiO3, suggesting the formation of a yet unidentified phase.
Surprisingly, FTIR-ATR spectra revealed the appearance of a new absorption band at 1050 cm−1 immediately after the first cycle. This new band, not seen for pristine BaTiO3, is retained upon extensive washing with EtOAc, suggesting a strongly bound and/or inorganic species arising from the reaction mixture.
Moreover, thermogravimetric analysis (TGA) of recycled BaTiO3, conducted in air up to 900 °C, did not reveal any loss of weight that would have been expected from adsorbed organic materials. Consequently, we speculate that the feature at 1050 cm−1 would be related to a boron species, as the infrared B–O and B–F stretching bands are found around 900–1100 cm−1, depending on the chemical environment.16 In order to verify the presence of boron, a washed sample of the BaTiO3 after one cycle was analysed by 11B solid-state NMR (ssNMR) spectroscopy, which revealed a signal centered at −0.76 pm with respect to F3B⋅O(C2H5)2 (Fig. 2D). The 11B signal exhibited symmetry consistent with a tetrahedral boron species, such as BO4 or BF4.17 No boron signal was detected in pristine BaTiO3 initially used for this reaction (see ESI†). BaTiO3 analysis by X-ray photoelectron spectroscopy (XPS) was largely inconclusive due to the overlap of the B 1s and Ba 4p1/2 regions, but the growth of the peak around 193 eV suggests incorporation of boron (see ESI†).18 Based on spectroscopy, we suggest that the loss of BaTiO3 activity might be due to the incorporation and/or surface adsorption of boron species.¶19
Finally, we explored the potential to scale-up RAM-based mechanoredox reactions. Previous reports indicate that scaling-up RAM conditions can be simple and straightforward.10 Here, the BaTiO3-catalysed reaction between 1a and bpb was immediately adapted from the initial scale of 150 mg reactants to 2 grams, while retaining the same relative amounts of BaTiO3 and MeCN (see ESI†). Such linear scaling-up led to 80% conversion to 1b within 2 h and >99% conversion after 3 h.
In summary, combining mechanoredox catalysis and RAM provides an attractive protocol that simultaneously avoids milling media, enables reactivity driven by the piezoelectric effect, and achieves excellent yields faster than a ball-milling process. While the effectiveness of BaTiO3 as a catalyst drops upon repeated use, the loss of activity is smaller than under ball-milling. This loss appears to be due to inadvertent boration of BaTiO3 by mechanochemical reaction components, which purports the need to develop more robust mechanoredox catalysts.
We thank the support of the Natural Sciences and Engineering Council of Canada (NSERC) Discovery grants RGPIN-2021-03476 (X. O.), RGPIN-2017-06467 (T. F.), NSERC John C. Polanyi Award JCP 562908-2022 (T. F.), the Leverhulme International Professorship (T. F.), Genentech Collaborative Grant (CLL-017956) and Fonds de Recherche du Québec – Nature et Technology (FRQNT) scholarship (F. E.). We thank Drs R. S. Stein and H. M. Titi of McGill Chemistry Characterization (MC2) platform, as well as C. B. Lennox, E. Hamzehpoor (McGill University) and Dr B. G. Fiss (University of Western Ontario).
Conflicts of interest
There are no conflicts to declare.Notes and references
-
(a) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyatt, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC
; (b) M. Pérez-Venegas and E. Juaristi, ACS Sustainable Chem. Eng., 2020, 8, 8881 CrossRef
; (c) T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018 CrossRef PubMed
.
-
(a) O. Galant, G. Cerfeda, A. S. McCalmont, S. L. James, A. Porcheddu, F. Delogu, D. E. Crawford, E. Colacino and S. Spatari, ACS Sustainable Chem. Eng., 2022, 10, 1430 CrossRef CAS
; (b) K. J. Ardila-Fierro and J. G. Hernández, ChemSusChem, 2021, 14, 2145 CrossRef CAS PubMed
; (c) F. Effaty, X. Ottenwaelder and T. Friščić, Curr. Opin. Green Sustainable Chem., 2021, 32, 100524 CrossRef CAS
; (d) W. Pickhardt, S. Grätz and L. Borchardt, Chem. – Eur. J., 2020, 26, 12903 CrossRef CAS PubMed
.
-
(a) J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007 CrossRef PubMed
; (b) F. Cuccu, L. De Luca, F. Delogu, E. Colacino, N. Solin, R. Mocci and A. Porcheddu, ChemSusChem, 2022, 15, e202200362 CrossRef CAS PubMed
; (c) R. F. Koby, T. P. Hanusa and N. D. Schley, J. Am. Chem. Soc., 2018, 140, 15934 CrossRef CAS PubMed
.
- K. Kubota, Y. Pang, A. Miura and H. Ito, Science, 2019, 366, 1500 CrossRef CAS PubMed
.
-
(a) Y. Pang, J. W. Lee, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2020, 59, 22570 CrossRef CAS PubMed
; (b) J. A. Leitch and D. L. Browne, Chem. – Eur. J., 2021, 27, 9721 CrossRef CAS PubMed
; (c) S. M. Zeitler, P. Chakma and M. R. Golder, Chem. Sci., 2022, 13, 4131 RSC
; (d) C. Schumacher, J. G. Hernández and C. Bolm, Angew. Chem., Int. Ed., 2020, 59, 16357 CrossRef CAS PubMed
; (e) M. M. Amer, R. Hommelsheim, C. Schumacher, D. Kong and C. Bolm, Faraday Discuss., 2022 10.1039/D2FD00075J
; (f) J. Jiang, S. Song, J. Guo, J. Zhou and J. Li, Tetrahedron Lett., 2022, 98, 153820 CrossRef CAS
; (g) Z. Ren, Y. Peng, H. He, C. Ding, J. Wang, Z. Wang and Z. Zhang, Chin. J. Chem., 2023, 41, 111 CrossRef
; (h) K. Wang, C. Han, J. Li, J. Qiu, J. Sunarso and S. Liu, Angew. Chem., Int. Ed., 2022, 61, e202110429 CAS
.
-
(a) D. Tan and F. García, Chem. Soc. Rev., 2019, 48, 2274 RSC
; (b) T. Di Nardo and A. Moores, Beilstein J. Org. Chem., 2019, 15, 1217 CrossRef CAS PubMed
.
-
(a) A. Stolle, R. Schmidt and K. Jacob, Faraday Discuss., 2014, 170, 267 RSC
; (b) V. K. Singh, A. Chamberlain-Clay, H. C. Ong, F. León., G. Hum, M. Y. Par, P. Daley-Dee and F. García, ACS Sustainable Chem. Eng., 2021, 9, 1152 CrossRef CAS
.
- S. R. Anderson, D. J. Am Ende, J. S. Salan and P. Samuels, Propellants, Explos., Pyrotech., 2014, 39, 637 CrossRef CAS
.
-
(a) D. J. Am Ende, S. R. Anderson and J. S. Salan, Org. Proc. Dev., 2014, 18, 331 CrossRef CAS
; (b) K. Nagapudi, E. Y. Umanzor and C. Masui, Int. J. Pharm., 2017, 521, 337 CrossRef CAS PubMed
.
- H. M. Titi, J.-L. Do, A. J. Howarth, K. Nagapudi and T. Friščić, Chem. Sci., 2020, 11, 7578 RSC
.
- L. Gonnet, C. B. Lennox, J.-L. Do, I. Malvestiti, S. G. Koenig, K. Nagapudi and T. Friščić, Angew. Chem., Int. Ed., 2022, 61, e202115030 CrossRef CAS PubMed
.
- T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, CrystEngComm, 2009, 11, 418 RSC
.
- P. A. Julien and T. Friščić, Cryst. Growth Des., 2022, 22, 5726 CrossRef CAS
.
- L. Gonnet, T. H. Borchers, C. B. Lennox, J. Vainauskas, Y. Teoh, H. M. Titi, C. J. Barrett, S. G. Koenig, K. Nagapudi and T. Friščíć, Faraday Discuss., 2022 10.1039/D2FD00131D
.
- M. Del Carmen, B. López, G. Fourlaris, B. Rand and F. L. Riley, J. Am. Ceram. Soc., 1999, 82, 1777 CrossRef
.
- D. Peak, G. W. Luther and D. L. Sparks, Geochim. Cosmochim. Acta, 2003, 67, 2551 CrossRef CAS
.
-
K. J. D. MacKenzie and M. E. Smith, Multinuclear solid-state NMR of inorganic materials, Pergamon, Oxford, 2002 Search PubMed
.
- B. R. Strohmeier, Appl. Surf. Sci., 1989, 40, 249 CrossRef CAS
.
-
(a) Y. Cao, P. Zhou, Y. Tu, Z. Liu, B. W. Dong, A. Azad, D. Ma, D. Wang, X. Zhang, Y. Yang, S. Da Jiang, R. Zhu, S. Guo, F. Mo and W. Ma, iScience, 2019, 20, 195 CrossRef CAS PubMed
; (b) A. Sinhamahapatra, P. Pal, A. Tarafdar, H. C. Bajaj and A. B. Panda, ChemCatChem, 2013, 5, 331 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2205247. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc06013b |
| ‡ Diazonium salts should always be handled with care and reactions performed behind a glass shield. |
| § The FTIR-ATR spectrum of pristine BaTiO3 reveals a band at 1425 cm−1, ascribed to small amounts of carbonate.15 Upon mechanoredox catalysis, this band vanishes, and can also be removed by calcination in N2 (see ESI†). |
| ¶ Deactivation of BaTiO3 by boron species would be consistent with a slower loss of catalyst activity reported for mechanoredox arylation reactions with furan.4 |
| This journal is © The Royal Society of Chemistry 2023 |