Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ion mobility mass spectrometry and molecular dynamics simulations unravel the conformational stability of zinc metallothionein-2 species†
Manuel David
Peris-Díaz
 *ab,
Alexey
Barkhanskiy
b,
Ellen
Liggett
b,
Perdita
Barran
*ab,
Alexey
Barkhanskiy
b,
Ellen
Liggett
b,
Perdita
Barran
 *b and
Artur
Krężel
*b and
Artur
Krężel
 *a
*a
aDepartment of Chemical Biology, Faculty of Biotechnology, University of Wrocław, F. Joliot-Curie 14a, 50-383 Wrocław, Poland. E-mail: manuel.perisdiaz@uwr.edu.pl; artur.krezel@uwr.edu.pl
bMichael Barber Centre for Collaborative Mass Spectrometry, Manchester Institute of Biotechnology, 131 Princess Street, Manchester, M1 7DN, UK. E-mail: perdita.barran@manchester.ac.uk
First published on 24th March 2023
Abstract
Ion mobility-mass spectrometry (IM-MS) unraveled different conformational stability in Zn4–7-metallothionein-2. We introduced a new molecular dynamics simulation approach that permitted the exploration of all of the conformational space confirming the experimental data, and revealed that not only the Zn–S bonds but also the α–β domain interactions modulate protein unfolding.
Mammalian metallothioneins (MTs) constitute small (∼6–7 kDa) cysteine-rich proteins with a biological role primarily in Zn2+ and Cu+ metabolism.1–3 At least a dozen MT isoforms (MT1–MT4) and multiple subisoforms have been found, which differ in their metal-binding properties, and tissue and cellular localization.4,5 MTs have proved to be highly challenging objects to study using traditional biophysical techniques due to the lack of secondary structures and aromatic amino acids, and the spectroscopic silence of Zn2+.4 To date, only one X-ray structure has been solved for rat hepatic mixed Cd5Zn2MT2 species6 The protein adopts a dumbbell shape with two metal-thiolate clusters named α- and β-domains containing a Cd4Cys11 and Cd1Zn2Cys9 cluster, respectively. Despite the capabilities of Cd2+ and Zn2+ to form an M7MT2 (where M represents a metal ion), there is a divergence of behavior in the thermodynamic and kinetic properties between these two divalent metal ions, which is not without biological consequences.7 The seven Cd2+ ions bind cooperatively in Cd7MT in a domain fashion three to five orders of magnitude tighter than Zn2+.2 In contrast to cadmium, zinc MT2 presents three classes of affinities towards the seven Zn2+ ions. Four Zn2+ are bound with Kd ∼ 10−12 M, another two with Kd ∼ 10−10–10−11 M, while the seventh is weakly bound with Kd of ∼10−8 M.8 As a consequence of this fact, MT2 exists as multiple Zn4–7MT2 species under cellular conditions where the free Zn2+ concentration varies from 10−11 to 10−9 M.9,10 The characterization of isolated MT fractions from several tissues and cell lines supported the role of Zn4–7MT2 species as a zinc buffering system.10–12 The heterogeneity of these species impedes their study by high-resolution structural techniques like cryo-EM, X-ray crystallography, or NMR. Ion mobility-mass spectrometry (IM-MS) has proven well-suited to interrogate heterogeneous protein systems and characterize their conformation and dynamics.13–15 However, the resolution of the IM device may not be enough to separate closely related protein conformations. In some cases, gas-phase activation of protein ions via collisional activation, also referred to as Collision Induced Unfolding (CIU),16 can be used to probe subtle structural differences between similar conformations and study protein ion stability and dynamics. Recently, high-resolution cyclic IM-MS based on traveling wave technology was introduced, allowing for tandem IM workflows.17–19 Despite the structural information that can be derived from IM-MS experiments, it is unable to define protein structure at the atomic level. Recent efforts have focused on the integration of molecular dynamics (MD) with IM-MS, assigning gas-phase structures from in silico methods.20–24 Unfortunately, usually only a few metastable states are explored since biological processes such as protein unfolding or conformational changes are on time scales far beyond those accessible by classical MD simulations. To access other conformational states, such as those sampled during CIU, most of the works have used a thermal unfolding approach. While some reports have shown that thermal unfolding can reproduce many general features observed during a CIU experiment,20,24,25 a recent report has suggested a low reproducibility and lack of conformational sampling.23
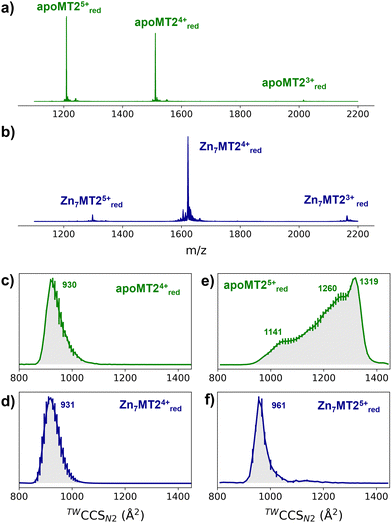
In order to investigate the conformational properties of Zn4–7MT2 species and shed more light on how these species resemble at the microscopic level, IM-MS, CIU, and MD simulations were integrated. The nESI mass spectra of apoMT2 in the presence of tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (termed as “red”) present a charge state distribution (CSD) spanning only three charge states 3 ≤ z ≤ 5, with apoMT25+red and apoMT24+red the most dominant (Fig. 1a). Reconstituted Zn7MT2 protein exhibits a CSD shift toward lower charge state with the Zn7MT24+red ions predominant (Fig. 1b), suggesting that solution-phase conformational changes alter the solvent accessible surface area (SASA). The apoMT24+red and Zn7MT24+red ions displayed a similar collision cross section (CCS) (Fig. 1c and d), hereby not representing the SASA changes.
The CCS for apoMT25+red presents a broad CCS distribution, and upon Zn2+ binding, a single and compact Zn6MT25+red and Zn7MT25+red conformer is observed (Fig. S1, ESI†). IM-MS revealed that 5+ ions undergo a conformational change capturing the CSD shift observed in the mass-to-charge spectrum (Fig. 1e and f). Partially Zn2+-loaded MT2 species were obtained via titration in the presence of TCEP, and native IM-MS under different collisional activation (CA) conditions were recorded (Fig. S2, ESI†). Metal-coupled folding effects can be observed upon Zn2+ binding to apoMT25+red: prior to CA, Zn4–7MT25+red ions populate a CCS ∼ 1000 Å2cf. ∼ 1300 Å2 for apoMT25+red (Fig. S2, ESI†). As the collision energy is increased, the CCS shifts to ∼1150 Å2 in all of the Zn4–7MT2 complexes. Similar results were obtained under 50 or 200 mM ammonium acetate (Fig. S3, ESI†). Unpredictably, fitting the native mass spectra to simulated isotopic distributions revealed the partial retention of protons within the Zn2+ clusters. Lacking a reducing agent during spraying generates signals shifted to lower m/z (Fig. S2, ESI†). Mass spectra simulations estimated the formation of 7–8 disulfides for all Zn4-6MT25+ox and 2 disulfides for Zn7MT25+ox (Table S1, ESI†). As a consequence, while some portion of the ions unfold to ∼1150 Å2, as in the case of reduced complexes, most of the ions are trapped at ∼1000 Å2. To compare the gas-phase stabilities of Zn4–7MT25+ ions, the CCS along the collisional energy assayed were fitted to estimate the CIU50 values to indicate the energy required to activate 50% of the ions to the next conformation.16 A similar CIU50 ∼ 90 eV was calculated for Zn4MT25+red and Zn5MT25+red ions (Fig. S4, ESI†). A gradual increase to CIU50 ∼ 110 eV was then determined for Zn6MT25+red and Zn7MT25+red. Our previous study provided the location of Zn2+ in all Zn4-7MT2 species.26 In Zn4MT2, two Zn2+ are bound in each α- and β-domain forming Zn2Cys6 clusters. The fifth Zn2+ binds to the α-domain forming an αZn3Cys9 cluster. The sixth Zn2+ saturates the α-domain forming an αZn4Cys11 cluster, and the seventh Zn2+ forms the βZn3Cys9 cluster. CIU did not detect structural changes between Zn2Cys6 and Zn3Cys9 clusters but determined elevated structural stability upon the formation of the Zn4Cys11 cluster. Taken together, our results elucidated a plausible structural explanation for why the seventh Zn2+ ion binds with a Kd of ∼10−8 M to MT2 and provided a link between structural and Zn2+ buffering properties.8
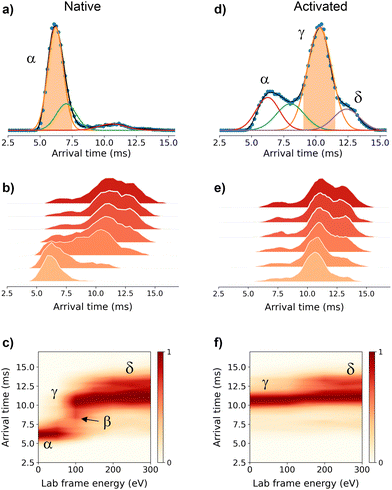
To get further structural insights into the existing conformational families, we employed multistage IM-MS using a cyclic IM-MS instrument. Upon activation of isolated compact conformer α, we observe an unfolding profile that leads largely to a conformation γ, through a β intermediate conformation and a minor extended δ conformation (Fig. 2a–c). The great potential of tandem IM is that it allows not only the examination of the unfolding mechanisms but also the evaluation of the thermodynamic and kinetic stability of the unfolded conformations.18 Isolation of the conformer γ (Fig. 2d) and subsequent CIU activation leads to an unfolding profile with no new features (Fig. 2e). We do not evidence a sign of interconversion to a compact conformation (Fig. 2f). These results suggest that there exists a relatively high transition energy barrier between these states, and propose a plausible irreversible unfolding mechanism that yields a thermodynamically stable conformation that can be of relevant interest under cellular stress conditions.
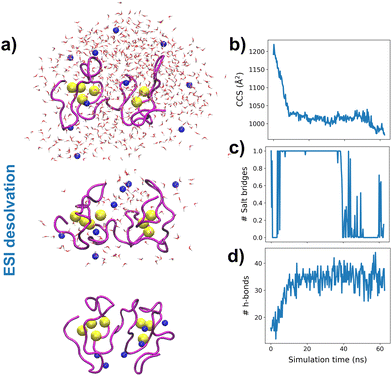
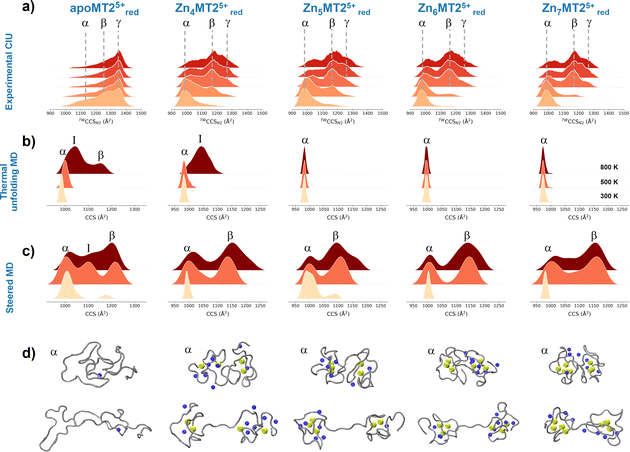
To understand the experimental results from a microscopic point of view, we then performed gas-phase MD simulations (Fig. 3a). We first focused on examining how the Zn4–7MT2 protein complexes are transferred from the solution into the gas-phase (Fig. S5–S10, ESI†). Proteins were placed in aqueous nanodroplets with an excess of charge to 16+ using Na+ as a charge carrier rather than H+. As the simulation evolves, the solvent gradually evaporates, and Na+ ions are ejected until a charge decrease to 5+. Droplet shrinkage is accompanied by a decrease in the CCS from ∼1200 to 1000 Å2 until the structure collapses to a more compact conformation with CCS ∼ 950 Å2 without altering the Zn–S bonds (Fig. S5–S10 and Fig. 3b, ESI†). In this process, salt bridges do not appear to modulate the transition from solution to gas-phase,27 but the collapse of the structure is highlighted by an increased number of h-bonds from ∼20 to ∼40 (Fig. 3c and d). Such results are in excellent agreement with our experimental native IM-MS data (max error < 5%) and shed light on the gas-phase desolvation and protein structure at the atomic level. We then attempted to simulate the CIU of electrosprayed protein complexes. First, a thermal unfolding protocol was performed on representative structures obtained from gas-phase MD desolvation simulations (Fig. 4a and b). All of the protein ions have narrower ΔCCS than the recorded experimental CIU data (Fig. 4a): apoMT25+red has a ΔCCS of 400 Å2vs. ΔCCS 300 Å2 for simulations, and Zn4-7MT25+red has a ΔCCS of 200–250 Å2vs. ΔCCS 70–150 Å2 for simulated proteins. SA identified multiple conformations for apoMT25+, a compact conformation of ∼1000 Å2, and a semi-extended one of ∼1200 Å2, although the extended one with CCS ∼ 1300 Å2 was not sampled (Fig. 4b). Similarly, the compact and semi-extended conformers were present in αZn2βZn2MT25+ and αZn3βZn1MT25+, and once again, the extended conformation was not detected. As the Zn2+ loading increases, the conformational heterogeneity measured as the CCS (ΔCCS) decreases, indicating that Zn2+ promotes protein folding. Consequently, simulations for Zn5MT25+ and higher Zn2+-loaded states only sampled the compact conformations (Fig. 4b). Extending the simulation times from 10 to 100 ns did not influence the conformational sampling. Therefore, thermal unfolding failed to overcome the energetic restraints imposed by the Zn–S bonds and was not able to sample extended conformations obtained upon collisional activation. As the radius of gyration (Rg) correlated well with the CCS values, we used an enhanced sampling algorithm named steered MD (SMD) simulations to accelerate the transitions between different states by using Rg as a collective variable (CV). The force-CCS profiles obtained by SMD simulations clearly distinguished the compact α and extended conformation β and reproduce well their ΔCCS (Fig. 4c and Fig. S11–S17, ESI†). Comparable CCS distributions were obtained when using the end-to-end N-C terminus distance as a CV (Fig. S18–S24, ESI†). We observed that protein unfolding proceeds via destabilizing the interdomain α–β interactions (Fig. 3a). To examine their mechanostability properties, average unfolding forces were calculated from 25 SMD replicates (Fig. S25a and b, ESI†). SMD cannot be quantitatively compared to CIU experiments as the physical principles underlying these approaches are different. However, we found similarities as the low linear correlation between the mean unfolding force and the number of Zn2+ bound to MT2 (Fig. S25b, ESI†). To estimate more accurate protein unfolding, we performed well-tempered metadynamics simulations (Fig. S25c, ESI†). Similar conclusions were obtained as with SMD. Examining the protein conformations, we observed a linear relationship between h-bonds and Zn–S bonds (Fig. S25d, ESI†). Interestingly, neither mean unfolding forces nor estimated free energies were correlated with h-bonds or Zn–S bonds (Fig. S25e, ESI†). These results indicate that protein unfolding is not only controlled by metal–S interactions or h-bonds but also other energetic forces contribute to this process. To date, no single report has characterized the structure and protein conformations for physiologically relevant zinc MT2 species. Here IM-MS aided by MD simulations present a comprehensive structural characterization of these protein complexes. Collectively, our study provides a plausible link between structural and Zn2+ buffering properties and sheds light on the mode of functioning of these small yet critical cellular proteins. In addition, as thermal unfolding has been shown not only here but also in other studies that may lack conformational sampling, we report an alternative MD framework to simulate CIU experiments.
This research was supported by the National Science Centre of Poland (NCN) under the Opus grant no. 2018/31/B/NZ1/00567 (to A.K.), Preludium no. 2018/31/N/ST4/01909 and Etiuda no. 2020/36/T/ST4/00404 (to. M.D.P.D). We acknowledge the support of EPSRC through the strategic equipment award EP/T019328/1, the European Research Council for funding the MS SPIDOC H2020-FETOPEN-1-2016-2017-801406 and Waters Corporation for their continued support of mass spectrometry research within the Michael Barber Centre for Collaborative Mass Spectrometry.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Krężel and W. Maret, Int. J. Mol. Sci., 2017, 18, 1237 CrossRef PubMed.
- M. J. Stillman, Coord. Chem. Rev., 1995, 144, 461–511 CrossRef CAS.
- M. R. Mehlenbacher, et al. , Chem. Sci., 2022, 13, 5289–5304 RSC.
- A. Krężel and W. Maret, Chem. Rev., 2021, 121, 14594–14648 CrossRef PubMed.
- E. Artells, O. Palacios, M. Capdevila and S. Atrian, Metallomics, 2013, 5, 1397–1410 CrossRef CAS PubMed.
- W. Braun, et al. , Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 10124–10128 CrossRef CAS PubMed.
- M. D. Peris-Díaz, et al. , Anal. Chem., 2020, 92, 12950–12958 CrossRef PubMed.
- A. Krężel and W. Maret, J. Am. Chem. Soc., 2007, 129, 10911–10921 CrossRef PubMed.
- A. Krężel and W. Maret, J. Biol. Inorg. Chem., 2006, 11, 1049–1062 CrossRef PubMed.
- Y. Yang, W. Maret and B. L. Vallee, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 5556–5559 CrossRef CAS PubMed.
- A. Krężel and W. Maret, Mol. Med., 2007, 13, 371–375 Search PubMed.
- A. Krężel and W. Maret, Biochem. J., 2007, 402, 551–558 CrossRef PubMed.
- S. M. Bargeen, et al. , Nat. Commun., 2022, 13, 4377 CrossRef PubMed.
- S. C. Wang, et al. , J. Am. Chem. Soc., 2010, 132, 15468–15470 CrossRef CAS PubMed.
- I. Michaelevski, M. Eisenstein and M. Sharon, Anal. Chem., 2010, 82, 9484–9491 CrossRef CAS PubMed.
- S. M. Dixit, D. A. Polasky and B. T. Ruotolo, Curr. Opin. Chem. Biol., 2018, 42, 93–100 CrossRef CAS PubMed.
- K. Giles, et al. , Anal. Chem., 2019, 91, 8564–8573 CrossRef CAS PubMed.
- C. Eldrid, et al. , J. Am. Soc. Mass Spectrom., 2021, 32, 1545–1552 CrossRef CAS PubMed.
- E. Deslignière, et al. , J. Am. Soc. Mass Spectrom., 2021, 32, 2505–2512 CrossRef PubMed.
- R. Beveridge, et al. , J. Am. Chem. Soc., 2019, 141, 4908–4918 CrossRef CAS PubMed.
- E. G. Marklund, et al. , Structure, 2015, 23, 791–799 CrossRef CAS PubMed.
- A. D. Rolland, L. S. Biberic and J. S. Prell, J. Am. Soc. Mass Spectrom., 2022, 33, 369–381 CrossRef CAS PubMed.
- C. Eldrid, et al. , Anal. Chem., 2022, 94, 16113–16121 CrossRef CAS PubMed.
- S. Nash and R. W. Vachet, J. Am. Chem. Soc., 2022, 144, 22128–22139 CrossRef CAS PubMed.
- J. Bellamy-Carter, et al. , Analysis Sensing, 2021, 1, 63–69 CrossRef CAS.
- M. D. Peris-Díaz, et al. , J. Am. Chem. Soc., 2021, 143, 16486–16501 CrossRef PubMed.
- L. Konermann, E. Aliyari and J. H. Lee, J. Phys. Chem. B, 2021, 125, 3803–3814 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc06559b |
| This journal is © The Royal Society of Chemistry 2023 |