Efficient molecular oxygen utilization of micelle-based BiOCl for enhanced in situ H2O2 production induced photocatalytic removal of antibiotics†
Xiaojuan
Bai
 *ab,
Tianqi
Jia
ab,
Haiyan
Li
ab,
Linlong
Guo
ab,
Meipeng
Jian
ab,
Yongwei
Gong
ab,
Junqi
Li
ab,
Zhen
Wei
*ab,
Tianqi
Jia
ab,
Haiyan
Li
ab,
Linlong
Guo
ab,
Meipeng
Jian
ab,
Yongwei
Gong
ab,
Junqi
Li
ab,
Zhen
Wei
 c and
Derek
Hao
*d
c and
Derek
Hao
*d
aBeijing Energy Conservation & Sustainable Urban and Rural Development Provincial and Ministry Co-construction Collaboration Innovation Center, Beijing University of Civil Engineering and Architecture, Beijing, 100044, China. E-mail: baixiaojuan@bucea.edu.cn; heixia.1986@163.com
bKey Laboratory of Urban Stormwater System and Water Environment, Ministry of Education, Beijing University of Civil Engineering and Architecture, Beijing 100044, China
cDepartment of Chemistry, The University of Hong Kong, Hong Kong
dCentre for Catalysis and Clean Energy, Gold Coast Campus, Griffith University, Gold Coast, 4222 Australia. E-mail: haoqiangderek@gmail.com
First published on 1st December 2022
Abstract
Photocatalytic self-Fenton technology is regarded as a promising strategy for the removal of pollutants in wastewater, and the in situ H2O2 production rate is one of the main factors affecting contaminant degradation performance. However, insufficient utilization of molecular oxygen results in poor activity in photocatalytic H2O2 production. Herein, we propose a single-layer BiOCl nanoflower induced by biosurfactant with surface micelles to increase the oxygen adsorption and reduction rate. The DFT results demonstrated that hydrophobic groups (–CH2–) on the surface of micelles increased the O2 adsorption sites and then expanded the O2 adsorption capacity of BiOCl samples, which was also confirmed by the enhancement of the O 1s peak intensity in the XPS spectra. The charge concentrated on the micelle surface can accelerate the reduction of molecular oxygen to reactive oxygen species, thus enhancing H2O2 production to reach 108.6 μM within 60 min in pure water. Simultaneously, single-layer BiOCl increased the utilization rate of H2O2 by decreasing the decomposition of H2O2 itself, resulting in a 16.8-fold increase in sulfamethoxazole degradation efficiency. These results can inspire further developments in the photocatalytic degradation of antibiotics based on in situ H2O2 production.
Environmental significanceThe misuse and improper treatment of antibiotics lead to their discharge into the external environment, such as water and soil, which poses potential threats to ecology and human health. In this work, we construct a BiOCl nanosheet photocatalyst with a self-assembled micelle (SA-BiOCl) via a simple hydrothermal method. More importantly, SA-BiOCl improves in situ H2O2 production by increasing the amount of O2 in solution. The outstanding antibiotic degradation property was attributed to abundant reactive oxygen species, due to the efficient utilization of the in situ-produced H2O2. This study provides a new idea for photocatalytic self-Fenton treatment of antibiotics in wastewater. |
Introduction
Antibiotics are pharmaceuticals and personal care products (PPCPs) that are widely used in industry, agriculture, medicine, and other fields,1–3 and they cause water contamination that can pose a fatal threat to human health and the natural environment.4,5 More importantly, antibiotics with a large π electron conjugation system will form compounds possessing increased toxicity after decomposition, and many traditional methods cannot completely remove them. Among the advanced oxidation processes, there has been excellent performance of photocatalytic self-Fenton techniques with all types of antibiotics.6–8At present, many studies have reported increased H2O2 production in self-Fenton processes by increasing oxygen utilization. For instance, Wu et al.9 used organic semiconductor (DAnTMS) photocatalytic decomposition of water to simultaneously produce H2 and H2O2, and then utilized self-produced oxygen to increase the yield of H2O2. Qian et al.10 regulated reactive oxygen species by constructing electron channels and oxygen adsorption sites, greatly enhancing the efficiency of photogenerated electron transfer to adsorbed oxygen. Furthermore, Chen et al.11 obtained a tubular g-C3N4 sample to construct a solid–liquid–air interface, and the honeycomb-like structure enhanced O2 diffusion, increasing the yield of H2O2 in the channel. Due to the hydroxyl radicals (˙OH) derived from H2O2, which possess strong oxidation capabilities and are important in the decomposition of pollutants, it is crucial to construct photocatalytic systems with high molecular oxygen utilization rates to produce H2O2 for the mineralization of antibiotic wastewater.12–15
Currently, many photocatalytic materials have been used for the photocatalytic oxygen reduction reaction (ORR) to produce H2O2 and degrade antibiotics.16–23 Among the investigated photocatalysts, BiOCl is an important semiconductor with the appropriate energy band structure (3.2–3.7 eV) and particular lamellar structure.24–26 However, there is inadequate surface adsorption sites with pure BiOCl, and its relatively thick lamellae hinder electron transfer, resulting in poor photocatalytic H2O2 production activity.27–30 Therefore, how to realize an increase in the oxygen absorption and enrichment capacity and electron transfer efficiency of BiOCl is a conundrum. Amphiphilic surfactants are self-assembled into micelles that can be used as a template for controlling the size and shape of crystals, and then efficiently promoting electron transfer.31–33 For instance, Yang et al.34 introduced the structural guiding agent polyvinylpyrrolidone to regulate the crystal plane growth of BiOX (X = Cl, Br, I), resulting in a higher electron density and enhanced photocatalytic degradation activity. Surfactants can also expand the spacing between the layered materials during the synthesis process, forming a single atomic layer and weakening the van der Waals interactions between the layers.35 In particular, variable valence metals prevent the rapid decay of the photocurrent and ensure stable visible light-induced photocurrent, and accelerate electron cycling inside the photocatalyst.36 Furthermore, the micelles formed after the biosurfactant is dispersed in water induce the growth of the material, and also bond to the surface of the materials by hydrogen bonds or covalent bonds, forming different surface charge densities and increasing the adsorption properties of non-polar molecules. Surface micelles can also increase the adsorption capacity of oxygen, increasing the ORR rate and enhancing the activation performance of oxygen molecules to promote in situ H2O2 production.37–39 Therefore, biosurfactant-induced single-layer BiOCl with variable valence metals and surface micelles is an interesting strategy for enhancing molecular oxygen utilization.
In this work, we constructed BiOCl nanoflowers with self-assembled micelles (SA-BiOCl) on the surface, and they were induced by saponin powder via a simple hydrothermal method. As a soft template, self-assembled micelles increased the layer spacing between BiOCl during synthesis to form monolayer nanosheets. At the same time, hydrophobic and hydrophilic groups were grafted onto the BiOCl surface to form surface micelles, which provide more reactive sites and increase the adsorption capacity of oxygen. More importantly, the presence of surface micelles and the change of Bi valence during the ORR process improved the separation and mobility of photogenerated carriers, thereby increasing the activation of oxygen molecules for enhancing in situ H2O2 production. The outstanding degradation property was attributed to the abundant ˙OH, which was generated due to the efficient utilization of in situ-produced H2O2. Then, the photocatalytic antibiotic degradation was evaluated under solar illumination, and the enhanced adsorption of oxygen was verified by comparing the yield of H2O2 under He and air conditions. Furthermore, this method can also be expanded and applied to other BiOX (BiOBr, BiOI) systems, which proved its universality. Hence, such nano-photocatalysts modified with biosurfactants increase oxygen adsorption and the electron reduction rate to boost the utilization of molecular oxygen. This is an effective strategy for in situ H2O2 production and provides a novel idea in the field of photocatalytic self-Fenton environmental purification.
Experimental section
2.1 Materials
The reagents used in the experiment are listed in the ESI.†2.2 Synthesis of BiOCl
The synthesis method of BiOCl is described in the ESI.†2.3 Synthesis of SA-BiOCl
First, 2 mmol Bi(NO3)3·5H2O (0.970 g), 2 mmol of NaCl (0.116 g), and 0.1 g saponin powder were dissolved in 70 ml deionized water and 10 ml ethanol in a 100 ml beaker, and then fully stirred for 30 min. Subsequently, the solution was injected into a 150 ml Teflon liner and heated for 18 hours at 160 °C. After the mixed solution was cooled at room temperature, the suspension was washed several times with deionized water and ethanol until the upper solution was clarified. The sample was dried in a vacuum oven at 70 °C, and was named SA-BiOCl. Similarly, the other reaction conditions remained unchanged, and 0.05 g, 0.2 g, and 0.5 g saponin powder was added. These samples were named SA0.05-BiOCl, SA0.2-BiOCl, and SA0.5-BiOCl, respectively.2.4 Photocatalytic H2O2 production
In a beaker, 50 mg of photocatalyst was added to 50 ml of deionized water, and oxygen adsorption equilibrium was achieved by stirring for 30 min in the dark. Then, 1.5 ml of the suspension was removed every 30 min under visible light irradiation, the filtered solution was centrifuged, and the absorbance was measured at 350 nm wavelength by iodometry.2.5 Photocatalytic degradation of pollutants
In a glass bottle, 25 mg catalyst was added to 50 mL contaminant solution. Then, 1.5 ml solution was removed at fixed intervals and tested after filtration (the detailed test methods for the different contaminants are provided in section 4 of the ESI.†).Results and discussion
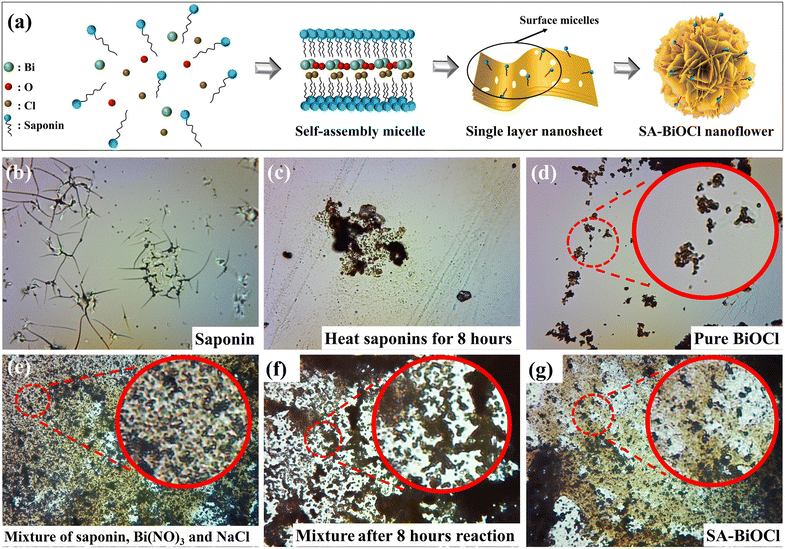
The synthesis of monolayer nanostructures with different morphologies and sizes via the micelles of surfactants is highly attractive, as a variety of micellar structures play the role of the soft template. The SA-BiOCl was fabricated as illustrated in Fig. 1a. Saponin powder contains a variety of surface-active groups. When the charge on the surface of the surfactant is the same as that of the hydrophilic ion part of the solution, the hydrophobic tail of the surfactant is more easily adsorbed on the photocatalyst surface than the ionic part of the molecule due to the repulsion of charge. The surfactant is covered by plentiful oxygen in the hydrophobic endpoint, and is adhered to the surface of the catalyst with the micelle. In addition, a proper amount of saponin powder can inhibit the hydrolysis of Bi3+ and increase the free Bi3+ ions in the solution. Then, Bi3+ has the ability to loosen the structure with negatively charged micelles so that the micelles are more easily bound and remain on the surface of the BiOCl.40To investigate the transformational mode of saponin powder, the morphology of saponin powder before and after the hydrothermal process was observed through an optical microscope. When pure saponin powder is uniformly distributed in solution, it forms micelles (Fig. 1b). Under the action of external energy, the micellar vesicles in saponin powder began to agglomerate and shrink, forming a dense stacked structure (Fig. 1c). Fig. 1d shows that pure BiOCl exists in the form of nanosheets. After adding saponin powder, the adsorption effect of micelles resulted in increased intermolecular forces of Bi, O, and Cl atoms, leading to the accumulation of nanosheets (Fig. 1e). As the reaction progressed, micelles attached to the surface of BiOCl and gradually aggregated (Fig. 1f), which is similar to the transformation of saponin powder during the synthesis of SA-BiOCl. Finally, stable flower-like nanospheres were formed by self-assembly after 18 h (Fig. 1g). Therefore, saponin powder not only provides surface-active sites for O2, but also plays a crucial role in the formation of the SA-BiOCl nanoflower structure.
3.1 Morphology and structure of SA-BiOCl
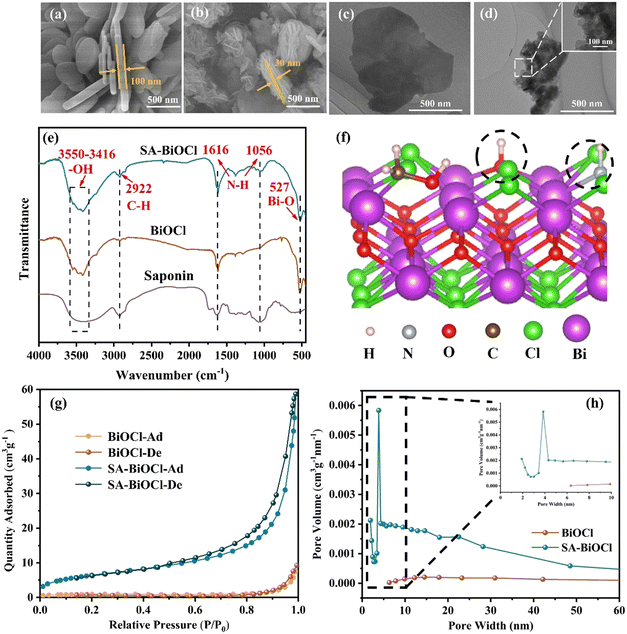
Initially, SEM was used to reveal the microstructure evolution from BiOCl to SA-BiOCl. It was clear that the structure and morphology of SA-BiOCl were significantly different from that of BiOCl. Pure BiOCl exhibits a neat edge and smooth surface nanoplate with a diameter of approximately 1 μm (Fig. 2a). The self-assembled micelles formed by saponin powder could be controlled to adjust the size of the nanosheets, and stacked to assemble a nanoflower structure, with a nanoflower diameter of 300–500 nm (Fig. 2b). Through observing the enlarged TEM images (Fig. 2c and d), we found that the flower spherical structure of clusters was composed of abundant nanosheets, and the thickness of SA-BiOCl was estimated to be 30 nm. This result was also supported by the evolution of saponin powder in the optical microscope image.In the early growth stage, BiOCl crystals were grown along the micellar template into single-layer nanosheets, which was due to the inability of the template to limit the growth of unsealed nanosheets. At this stage, the edges of the unclosed nanosheets grow and extend along their edge planes. Subsequently, the dangling bonds on the surface of BiOCl tend to be saturated, so that the lamellar material is more densely stacked.41,42 Additionally, the densely stacked sheets are bent, which increases the stability of the dangling bonds on the surface. This indicates that saponin powder in a specific concentration range can guide the growth of single-layer BiOCl nanoflowers.
The structure of the surface of SA-BiOCl was examined by Fourier transform infrared (FT-IR) spectroscopy. In the FT-IR spectrum (Fig. 2e), the absorption of SA-BiOCl at 3550–3416 cm−1 is attributed to the –OH stretching frequency caused by intramolecular hydrogen bonding and the N–H stretching vibration peak.43 Due to the deformation vibration of the N–H bond, SA-BiOCl generates corresponding absorption peaks at 1616 cm−1 and 1056 cm−1, which confirms that amino and hydroxyl groups are attached to the surface of SA-BiOCl. The C–H bond tensile vibration induced a peak at 2922 cm−1.44 It was confirmed that hydrophilic and hydrophobic groups covered the surface of SA-BiOCl and formed surface micelles (Fig. 2f). Additionally, the specific surface area of SA-BiOCl was 23.9 m2 g−1 (Table S1†), which is approximately 7 times higher than that of BiOCl (3.3 m2 g−1). The addition of surface-active sites was attributed to the increase in the specific surface area and surface micelles. The peak at 527 cm−1 is a Bi–O stretching vibration peak, which confirms the existence of a Bi–O bond in the samples.45Fig. 2g shows the N2 adsorption/desorption isotherms of BiOCl and SA-BiOCl, which are type IV isotherms, and the obvious hysteresis loops prove the existence of mesoporous structures. Additionally, there were numerous micropores in the surface of SA-BiOCl, with a size of approximately 8 nm (Fig. 2h), and the unique structure and large specific surface area of SA-BiOCl may facilitate the adsorption and photocatalytic activity of the substrate.
The crystal phase and composition of the BiOCl and SA-BiOCl samples were analyzed by X-ray diffraction (XRD) patterns (Fig. 3a). Three sharp characteristic diffraction peaks emerged at 2θ of 25.9°, 32.5°, and 33.4°, corresponding to the (101), (110), and (102) crystal facets of the BiOCl standard structure (JCPDS no. 06-0249), respectively, and confirming that the prepared samples belong to the tetragonal phase.46,47 In the process of BiOCl synthesis, with the addition of saponin powder, the diffraction peak of SA-BiOCl decreases, which was possibly due to the widening of the layer spacing to form single-layer BiOCl. The diffraction peak of SA-BiOCl was weaker and broader than the relative strength of the standard pattern, indicating that saponin powder mainly induces the dominant growth of (110) crystal facets.34 This was also proved by the HRTEM image analysis in Fig. 3b. Pure BiOCl exhibits uniform lattice fringes with a plane spacing of 0.344 and 0.267 nm, which are reflected as the (101) and (102) facets, respectively. As illustrated in Fig. 3c, there are different lattice stripes for SA-BiOCl corresponding to the (101) and (110) facets, respectively, which correspond to the XRD results. Additionally, EDS mapping (Fig. 3d) confirmed that there was a uniform distribution to the elemental composition of SA-BiOCl, and the Bi/Cl atomic ratio approached 1.
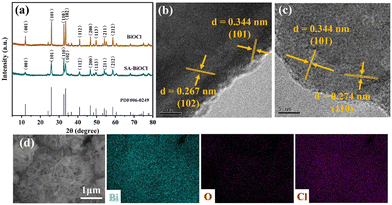 | ||
| Fig. 3 (a) XRD patterns of BiOCl and SA-BiOCl samples. HRTEM images of (b) BiOCl and (c) SA-BiOCl. (d) EDS mapping of SA-BiOCl (Bi, O, Cl). | ||
3.2 The surface micelle increases molecular oxygen adsorption
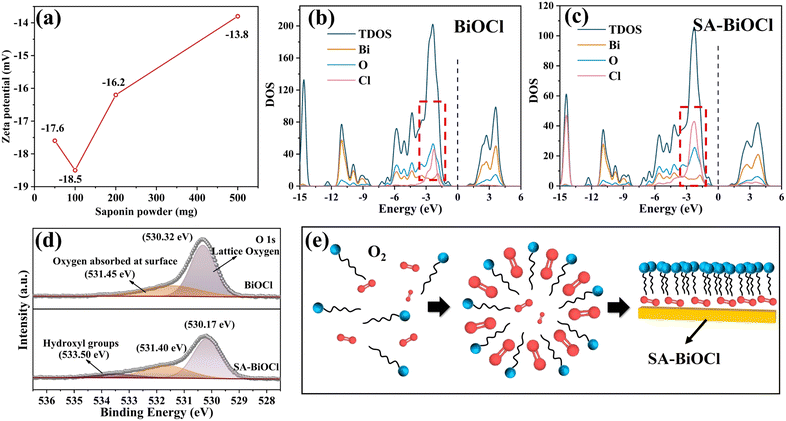
During the SA-BiOCl synthesis process, the stability of self-assembled micelles changed according to various concentrations of saponin powder. Fig. 4a shows that the micelle state was the most stable in the solution with 0.1 g of saponin powder. There are differences in the non-polar forces within the self-assembled micelles with different levels of saponin, resulting in different surface charge densities. The surface polarity changes when there are large quantities of groups on the surface of SA-BiOCl, manifesting an increase in electron density around SA-BiOCl. The density of states (DOS) was analyzed by density functional theory (DFT) calculations. As shown in Fig. 4b and c, the valence band (VB) and conduction band (CB) are composed of a hybrid of the O 2p, Bi 6p, and Cl 3p orbitals. The electronic structures and DOS results of BiOCl and SA-BiOCl reveal that the main difference between the two is the Cl 3p orbital near the Fermi energy level in SA-BiOCl. The introduction of saponin significantly activates the interaction of other atoms with the Cl atom,48 indicating that the introduction of surface micelles increased the amount of electrons around the Fermi energy and facilitated electron transfer.Moreover, the interaction between atoms can be seen in the Raman spectra (Fig. S1†). BiOCl is a tetragonal phase structure with Raman symmetric vibrational modes of A1g, B1g, and Eg. The strongest band existing at 145 cm−1 was assigned to the stretching mode of the internal Bi–Cl bond, while the peak of the other A1g was generated by the stretching mode of the external Bi–Cl bond at 61 cm−1. The peak at 201 cm−1 belongs to the internal Bi–O stretch of Eg, and the weakest peak was assigned to the O atom belonging to Eg/B1g mode. The survey spectrum (Fig. S2†) showed that the composition of BiOCl and SA-BiOCl included Bi, Cl, and O. The Bi 4f spectrum of SA-BiOCl was broadened compared with BiOCl (Fig. S3†), and it can be fitted by two peaks, which are located at approximately 164 and 159 eV. The SA-BiOCl binding energies shifted by 0.2 eV, which may be related to the variation in the surface energy caused by external functional groups and surface micellar structure.34 In addition, a similar change occurred in Cl 2p (Fig. S4†). Moreover, the peaks of the O 1 s at approximately 530 and 531 eV are related to the lattice oxygen and the oxygen absorbed at the surface, respectively (Fig. 4d).49 This was due to the encapsulation of oxygen and attachment to the surface of BiOCl during micellar aggregation (Fig. 4e).
The peak intensity of surface oxygen increases with introduction of the saponin powder, implying that the amount of oxygen absorbed at the surface increased. Also, the O element in the XPS elemental analysis has been increased (Table 1). The new peak appearing at 533.5 eV represents –OH on SA-BiOCl, and this is consistent with our theoretical model.50 Noticeably, the change in the surface energy may cause SA-BiOCl to increase the adsorption of O2, which indicates increased O2 utilization efficiency in the photocatalytic H2O2 production42 that could be proved by the measurement of contact angles and surface free energy (Fig. S5†). The surface free energy of the as-synthesized BiOCl and SA-BiOCl was 93.06 mN m−1 and 92.70 mN m−1, respectively, which indicates that the surface micelles of SA-BiOCl can promote the adsorption of organic molecules.51
| Materials | Elements | ||
|---|---|---|---|
| Bi (atomic%) | O (atomic%) | Cl (atomic%) | |
| BiOCl | 28.39 | 42.69 | 28.92 |
| SA-BiOCl | 26.47 | 46.10 | 27.44 |
To verify the adsorption capacity of the micelles for O2, different samples were dispersed in water, and the content of bubbles was observed under the optical microscope. As shown in Fig. S6a,† the optical microscope image reveals that a large number of micellar vesicles were formed in a solution containing only saponin powder to wrap O2. The bubbles in the solution of SA-BiOCl (Fig. S6b†) were significantly higher than those in pure BiOCl (Fig. S6c†) and BiOCl with saponin powder added (Fig. S6d†), which implies that there is increased adsorption enrichment ability of the surface micelle of SA-BiOCl for O2. To verify the encapsulation and adsorption of micelles, methylene blue (MB) adsorption experiments were carried out. Fig. S7† shows the adsorption of MB at fixed intervals by BiOCl, SA-BiOCl, and BiOCl with saponin powder added in the dark. SA-BiOCl had the most optimal adsorption effect on MB, and there was also increased adsorption of MB by BiOCl material with saponin powder, proving that the hydrophilic group in the micelle plays a promoting role in the adsorption of organic pollutants. Hence, the surface micelle can increase the diffusion of reactants and products between the active sites of SA-BiOCl, thereby promoting photocatalytic activity.
3.3 Optical properties and photoelectrochemical measurements of SA-BiOCl
To further clarify the impact of the surface micelle on the photocatalytic activity of BiOCl, the optical and photoelectrochemical properties were measured. A series of BiOCl samples was evaluated for their light-harvesting ability by UV-vis diffuse reflectance spectroscopy (DRS). Upon increasing the amount of saponin powder, the color of the synthesized samples gradually deepened. Fig. 5a shows that there was a slight redshift in the absorption edge of SA-BiOCl compared with that of BiOCl, which increased the absorption effect of visible light. In addition, there was stronger visible light adsorption for the flower-like material compared with the sheet-like material due to its relatively large specific surface area. This improves the separation efficiency of carriers, thus narrowing the bandgap and increasing the photocatalytic activity.52 However, as the micelles on the BiOCl surface increase, a large number of micelles will stack on the material surface, hindering electron transfer between channels.Based on these results, Tauc plots (ahν)2 = A (hν − Eg) were used to calculate the bandgaps of all BiOCl and SA-BiOCl samples. Fig. 5b shows that the band gaps of BiOCl and SA-BiOCl were 3.51 and 3.43 eV, respectively. Compared with BiOCl, the bandgap of SA-BiOCl was narrower, which indicates that an adjustable bandgap can be realized by surface energy regulation using the biosurfactant modification method. Moreover, there was a substantial increase in the absorption of visible light when the biosurfactant was added to 0.1 g, which can be related to the surface-active groups and the decrease in surface energy.
To further understand the band structure, the position of the CB was determined through the measurement of Mott–Schottky (MS) curves under different frequencies (Fig. S8†). The CBs of BiOCl and SA-BiOCl were −0.59 and −1.22 eV, respectively. After the introduction of saponin powder, the CB of BiOCl was sharply reduced, and the reduction performance of surface O2 was greatly improved. Moreover, the MS curves showed that the slope of the potential curve was positive, with typical n-type semiconductor characteristics. The CB position of SA-BiOCl was approximately −1.22 eV, which indicates that the CB electrons can achieve the reduction of O2 (Fig. 5c). In contrast, pure BiOCl can scarcely reduce O2 to produce ˙O2−.
Furthermore, the photocatalytic activity of semiconductors is mainly related to the migration efficiency of photogenerated electrons. We also measured the photoluminescence (PL), photocurrent response, and electrochemical impedance spectroscopy (EIS) to determine the photoelectrochemical properties. In the PL spectra (Fig. 5d), the main strong emission peak was for pure BiOCl and SA-BiOCl at approximately 622 nm (λexcitation = 250 nm). The intensity for SA-BiOCl was significantly decreased in comparison to that of BiOCl, which indicates that SA-BiOCl has a lower e−–h+ pair recombination rate.53 Therefore, it can be inferred that the appearance of surface micelles and the change in the surface energy of SA-BiOCl inhibit the recombination of photogenerated e− and h+. Obviously, the photocurrent density produced by SA-BiOCl was 3.6 times higher than that produced by BiOCl under visible light irradiation (Fig. 5e), indicating that the electron and hole separation efficiency was higher for SA-BiOCl. At the same time, at the moment of turning on and off the lamp, the photocurrent produced a strong change, indicating that the sample was sensitive to visible light. The Nyquist plot radius of EIS for SA-BiOCl shows a smaller semicircular diameter, indicating that the interfacial charge transfer efficiency of SA-BiOCl was higher than that of BiOCl (Fig. 5f), which is consistent with the photocurrent density. It mainly benefited from the change in surface energy of SA-BiOCl, which accelerated the electron transfer from the interlamination to the surface.
3.4 Performance and mechanism of photocatalytic H2O2 production
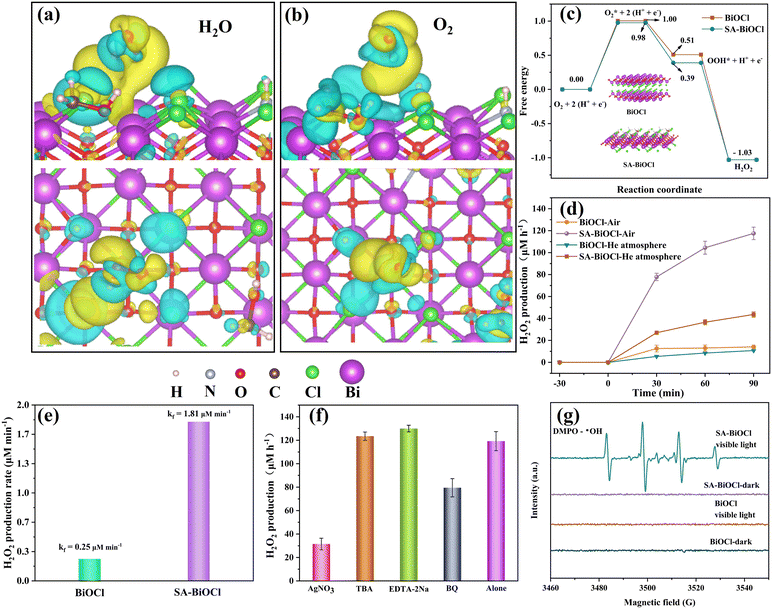
To reveal the effect of the surface micelle on the H2O2 performance in the constructed SA-BiOCl, the structure of SA-BiOCl was further clarified by DFT calculation. The charge density differences (Fig. 6a and b) reflect that the charge transfer of H2O and O2 reacts with external groups on the surface of SA-BiOCl. The yellow region between H2O and O2 for SA-BiOCl mainly appears in the middle of the external functional groups and H2O and O2, indicating the high transfer efficiency of electrons on the surface micelles of SA-BiOCl. More importantly, compared with H2O, when O2 is in contact with surface micelles, the charge is more concentrated near the surface micelles.It can be intuitively seen that the non-polar forces inside the micelles contribute to the transfer of photoproduct charge between SA-BiOCl and O2. In fact, the surface micelles of SA-BiOCl form additional active sites with open channel structures that can effectively promote the full interaction between electrons and O2 in CBs. The above results reveal that SA-BiOCl samples with surface micelles are beneficial for enhancing the O2 utilization efficiency. The entire process of H2O2 production was further studied by DFT calculations, where O2 was initially adsorbed to groups on the surface and reacted with unpaired electrons. Fig. 6c shows that SA-BiOCl requires lower energy in the determination step of ˙O2− and is more likely to generate H2O2 over BiOCl.
The photocatalytic H2O2 production by BiOCl and SA-BiOCl was monitored in pure water (no organic electron donor) under visible light irradiation. Fig. 6d shows that SA-BiOCl displayed efficient properties in photocatalytic H2O2 production under visible light, and the H2O2 concentration reached 121.5 μM within 90 min, which was 7.99 times that of pure BiOCl (15.2 μM). In addition, the H2O2 production of SA-BiOCl was compared with other works in Table 2. The O2 equilibrium condition is widely regarded as a key parameter, due to the photocatalytic generation of H2O2 being the effect of the O2 reduction reaction.54 To illustrate the role of molecular oxygen in H2O2 production, experiments under an air atmosphere and He atmosphere equilibrium conditions were also carried out. In an atmosphere of He, the yields of H2O2 over BiOCl and SA-BiOCl were 10.6 μM and 45.3 μM within 90 min, respectively. Compared with the H2O2 production in the air, there was still a certain amount of H2O2 production in the He atmosphere. This phenomenon is mainly caused by the slow release of O2 encased in micellar vesicles. From Fig. 6e, the formation rate constant (Kf) of SA-BiOCl reached 1.81 μM min−1. Subsequently, the photocatalytic decomposition of H2O2 was evaluated (Fig. S9†). The decomposition rate (Kd) of SA-BiOCl for H2O2 was 1.64 μM min−1, indicating that SA-BiOCl possesses higher in situ H2O2 utilization efficiency. The photocatalyst turns gray after the ORR, which may be due to Bi0 precipitates during the process. Inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis was further performed to explore the leaching of elemental Bi during the photocatalytic reaction (Table 3). The amount of leached Bi in the BiOCl sample was 0.05%, and the leached amount of SA-BiOCl was 1.34%. This phenomenon is due to Bi3+ being reduced to Bi0 during the reaction process, and the construction of the Bi0/BiOCl heterostructure, which accelerates the internal charge migration and conversion efficiency of BiOCl. In addition, Bi0 is continuously oxidized by oxygen to Bi3+ in the ORR process, so that elemental Bi reaches a stable cyclic utilization and conversion equilibrium. This process not only accelerates the utilization efficiency of molecular oxygen, but it also ensures the stability of the material structure.
| Photocatalysts | Conditions | Xe lamp | H2O2 production | Ref. | ||
|---|---|---|---|---|---|---|
| Catalyst | Medium | Time | ||||
| SNGQD/TiO2 | 25 mg | 6 vol% 2-propanol | 1.5 h | 500 W | 82.8 μM | 55 |
| rGO/TiO2/CoPi | 50 mg | O2 atmosphere | 1 h | 300 W | 58 μM | 56 |
| Bi2WO6 | 50 mg | 0.43 mM phenol | 1 h | 300 W | 8 μM | 57 |
| Cv-g-C3N4 | 100 mg | 100 ml H2O | 1 h | 300 W | 92 μM | 58 |
| CdS-GO | 50 mg | 30 ml H2O | 12 h | 300 W | 128 μM | 59 |
| g-C3N4/PDI-BN-rGO | 50 mg | 30 ml H2O | 24 h | 2 kW | 1233 μM | 60 |
| SA-BiOCl | 50 mg | 50 ml H2O | 1 h | 300 W | 108.6 μM | This work |
| Samples | Before reaction (Bi) | After reaction (Bi) |
|---|---|---|
| BiOCl | 0.04% | 0.05% |
| SA-BiOCl | 0.05% | 1.34% |
To further explore the active species that influence H2O2 production, the reactive species scavengers were added over BiOCl and SA-BiOCl to clarify the production path. AgNO3, EDTA-2Na, p-benzoquinone (BQ), and tert-butanol (TBA) were added as the quenchers of e−, h+, ˙O2−, and ˙OH, respectively. As shown in Fig. 6f, EDTA-2Na trapped h+ in solution, reduced the consumption of electrons, and promoted the electron reduction reaction of oxygen. The H2O2 yield significantly decreased after the addition of AgNO3, and there was little effect on the H2O2 production from the consumption of ˙OH by TBA, which proved that H2O2 was produced by the two-electron reduction process rather than by the transformation of ˙OH. The trapping agent used was 5,5-dimethyl-1-pyrroline N-oxide (DMPO) to conduct the electron paramagnetic resonance (EPR) test and verify the active species in H2O2 production. Fig. S10† shows that the strongest ˙O2− signal was from SA-BiOCl under visible light, which proves that ˙O2− exists in large quantities as an intermediate product in the process of H2O2 generation, and provides evidence for the one-electron reduction process. Additionally, ˙O2− is an intermediate in the process of producing H2O2 and has a major role in the degradation of antibiotics. Combined with the above experiments, we found that ˙OH is not the main factor that produces H2O2. Thus, the signal of DMPO – ˙OH indicated that H2O2 decomposed into ˙OH to degrade pollutants (Fig. 6g).
3.5 Photocatalytic pollutant degradation performance
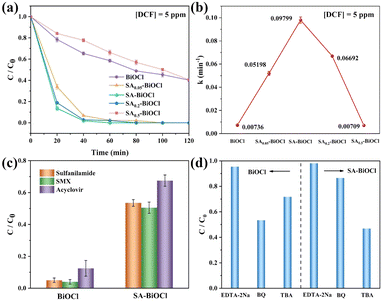
The most optimal photocatalytic performance was obtained from SA-BiOCl (SA0.1-BiOCl) with 0.1 g saponin powder, and this was attributed to the surface micellar increased H2O2 production and utilization efficiency. To this end, we explored the effect of increasing H2O2 production upon the degradation of organic pollutants. As a comparison, we found that the degradation rates of MB by BiOCl and SA-BiOCl were 40.0% and 89.0% within 60 min in pure MB solution, respectively (Fig. S11†). In contrast, the degradation efficiencies of BiOCl and SA-BiOCl reached 65.1% and 100.0%, respectively, when H2O2 was added during the degradation process.To assess the photocatalytic degradation performance of photocatalysts, diclofenac sodium (DCF) was used as a model in this work. As shown in Fig. 7a, the addition of saponin powder significantly impacted the photocatalytic performance of SAx-BiOCl series samples. The degradation rate of BiOCl toward DCF was 60.5% in 120 min under visible light irradiation. In contrast, the photodegradation efficiency of SA-BiOCl was increased to 100%, and exhibited 1.65 times higher photocatalytic activity for DCF than that of pure BiOCl. This was due to an appropriate amount of saponin powder that formed a layered micelle in solution and adsorbed BiOCl on the surface during the hydrothermal process, controlling its growth to smaller diameters. Obviously, the nanoflower structure is the result of anisotropic growth following the Ostwald maturation mechanism. When the BiOCl solution contains a high concentration of saponin powder, spherical micelles will be established to wrap BiOCl, which restricts Bi3+ from entering the interlayer for self-assembly. As a result, excess surfactant acts as a growth inhibitor, limiting crystal growth in all directions and ultimately leading to a decrease in the crystallinity of BiOCl. At the same time, the coating of abundant micelles will enhance the agglomeration effect of BiOCl, increase the particle size, and reduce the surface-active sites of materials, eventually leading to a decrease in the photocatalytic activity.61
Furthermore, the photodegradation of DCF was fitted to the equation: ln(C0/C) = kt,28 and the kinetic rate of DCF degradation on SA-BiOCl (0.0961 min−1) was higher than that of BiOCl (0.00713 min−1), SA0.05-BiOCl (0.05076 min−1), SA0.2-BiOCl (0.06722 min−1), and SA0.5-BiOCl (0.00709 min−1) (Fig. 7b). Apart from DCF, BiOCl and SA-BiOCl were chosen to remove sulfanilamide, sulfamethoxazole (SMX), and acyclovir under visible light irradiation. As shown in Fig. 7c, the degradation rates of SA-BiOCl for sulfanilamide, SMX, and acyclovir were 51.1%, 48.4%, and 65.2% in 120 min, which were 12.8, 16.2, and 3.85 times higher than that of pure BiOCl, respectively. This result is interpreted as the surface free energy reduction caused by the surface micelles attached to SA-BiOCl, whereby organic pollutants are easily adsorbed onto the surface of SA-BiOCl. The increase in H2O2 production and its utilization rate will subsequently promote degradation efficiency. In addition, Fig. S12† shows the degradation results of DCF by SA-BiOCl in four cycles, which illustrates that the removal efficiency is nearly stable.
To explore the degradation mechanism of BiOCl samples, a quenching experiment was conducted. Regarding DCF as the target pollutant, EDTA-2Na, BQ, and TBA were added as quenchers of h+, ˙OH, and ˙O2−, respectively. As shown in Fig. 7d, in the presence of BQ, TBA, and EDTA-2Na, the degradation efficiency of SA-BiOCl on DCF significantly decreased, indicating that the three active species all played a role in DCF degradation. Additionally, the adsorbed molecular oxygen combined with electrons to form ˙O2− and H2O2, which were simultaneously used for antibiotic degradation. Then, the H2O2 is decomposed into ˙OH, which plays a major role in degradation.
Degradation of antibiotics is difficult because of the stable aromatic ring structure. However, direct oxidization by h+ occurs through redox reactions with pollutants. In the process of the electron reduction of O2, h+ plays a role in ring-opening and synergistically degrades pollutants. Therefore, h+ direct oxidation and increased reactive oxygen species synergistically promote the degradation of antibiotics over SA-BiOCl. The antibiotic degradation pathways are shown below:
| Photocatalyst + hv → h+ + e− | (1) |
| h+ + antibiotics → low-molecular weight organic matter | (2) |
| O2 + 2H+ + 2e− ⇌ H2O2 | (3) |
| H2O2 + H+ + e− → ˙OH + H2O | (4) |
| ˙OH + antibiotics → CO2 + H2O | (5) |
It is possible to draw the conclusion that SA-BiOCl has achieved greater photocatalytic performance based on the aforementioned experimental findings. It was difficult to achieve electron–hole separation with pure BiOCl under visible light irradiation because of the large band gap. SA-BiOCl induced by biosurfactants effectively expanded its absorption range of sunlight and reduced the electron–hole recombination. Because of the presence of a mass of micellar vesicles on the SA-BiOCl surface, the photogenerated electrons migrated to the surface of the photocatalyst, and corresponding h+ was created. Then, the accumulated holes in the CB directly degraded the antibiotics through redox reactions. The electronics reduced the surface O2 to produce H2O2, which was then decomposed into ˙OH and used for the oxidation of antibiotics (Fig. 8).
Conclusions
In this work, BiOCl with abundant self-assembled surface micelles was constructed to enhance molecular oxygen activation and H2O2 production, thus increasing the efficiency of antibiotic removal. The presence of surface micelles reduced the surface free energy of SA-BiOCl, which could be beneficial for adsorbing O2 from the air. The surface micelles not only accelerated the charge transfer efficiency on the surface of SA-BiOCl, but they also reduced the Gibbs free energy of the 2e− ORR process, thus promoting 2e− ORR to increase the H2O2 production. Subsequently, H2O2 was converted into ˙OH under the action of SA-BiOCl, which degraded antibiotics in coordination with ˙O2−. Under solar irradiation, the degradation efficiency of pollutants was 12.8 (sulfanilamide), 16.8 (SMX), and 3.85 (acyclovir) times higher than that of pure BiOCl. In brief, this work not only provides novel inspiration for the preparation of photocatalysts with a micellar structure, but it also provides a new method for the removal of antibiotics from wastewater.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (22276011, 21607034, 51978032, 52270086), Beijing Natural Science Foundation (8192011), Science and Technology General Project of Beijing Municipal Education Commission (KM202010016006), the Pyramid Talent Training Project of Beijing University of Civil Engineering and Architecture (JDYC20200313, JDLJ20200301), the Youth Beijing Scholars program, No. 024, Joint Project of the Beijing Municipal Education Commission and Municipal Nature Science Foundation (21JH0024).Notes and references
- Y. Deng, J. Liu, Y. Huang, M. Ma, K. Liu, X. Dou, Z. Wang, S. Qu and Z. Wang, Engineering the Photocatalytic Behaviors of g/C3N4-Based Metal-Free Materials for Degradation of a Representative Antibiotic, Adv. Funct. Mater., 2020, 30, 2002353 CrossRef CAS
.
- S. Das and Y.-H. Ahn, Synthesis and application of CdS nanorods for LED-based photocatalytic degradation of tetracycline antibiotic, Chemosphere, 2021, 291, 132870 CrossRef PubMed
.
- M. H. Abdurahman, A. Z. Abdullah and N. F. Shoparwe, A comprehensive review on sonocatalytic, photocatalytic, and sonophotocatalytic processes for the degradation of antibiotics in water: Synergistic mechanism and degradation pathway, Chem. Eng. J., 2021, 413, 127412 CrossRef CAS
.
- S. Hoang and P.-X. Gao, Nanowire Array Structures for Photocatalytic Energy Conversion and Utilization: A Review of Design Concepts, Assembly and Integration, and Function Enabling, Adv. Energy Mater., 2016, 6, 1600683 CrossRef
.
- D. Hao, C. Liu, X. Xu, M. Kianinia, I. Aharonovich, X. Bai, X. Liu, Z. Chen, W. Wei, G. Jia and B.-J. Ni, Surface defect-abundant one-dimensional graphitic carbon nitride nanorods boost photocatalytic nitrogen fixation, New J. Chem., 2020, 44, 20651–20658 RSC
.
- H.-L. Cao, F.-Y. Cai, K. Yu, Y.-Q. Zhang, J. Lü and R. Cao, Photocatalytic Degradation of Tetracycline Antibiotics over CdS/Nitrogen-Doped–Carbon Composites Derived from in Situ Carbonization of Metal–Organic Frameworks, ACS Sustainable Chem. Eng., 2019, 7, 10847–10854 CrossRef CAS
.
- Y. Yang, G. Zeng, D. Huang, C. Zhang, D. He, C. Zhou, W. Wang, W. Xiong, B. Song, H. Yi, S. Ye and X. Ren, In Situ Grown Single-Atom Cobalt on Polymeric Carbon Nitride with Bidentate Ligand for Efficient Photocatalytic Degradation of Refractory Antibiotics, Small, 2020, 16, 2001634 CrossRef CAS PubMed
.
- H. Mai, D. Chen, Y. Tachibana, H. Suzuki, R. Abe and R. A. Caruso, Developing sustainable, high-performance perovskites in photocatalysis: design strategies and applications, Chem. Soc. Rev., 2021, 50, 13692–13729 RSC
.
- Z. Wu, X. Wang, Y. Li, H. Zhao, J. Wang, H. Huang, Y. Liu and Z. Kang, Converting water impurity in organic solvent into hydrogen and hydrogen peroxide by organic semiconductor photocatalyst, Appl. Catal., B, 2022, 305, 121047 CrossRef CAS
.
- H. Qian, Q. Hou, W. Zhang, Y. Nie, R. Lai, H. Ren, G. Yu, X. Bai, H. Wang and M. Ju, Construction of electron transport channels and oxygen adsorption sites to modulate reactive oxygen species for photocatalytic selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran, Appl. Catal., B, 2022, 319, 121907 CrossRef CAS
.
- L. Chen, S. Li, Z. Yang, C. Chen, C. Chu and B. Chen, Enhanced photocatalytic hydrogen peroxide production at a solid-liquid-air interface via microenvironment engineering, Appl. Catal., B, 2022, 305, 121066 CrossRef CAS
.
- X. Bai, X. Wang, X. Lu, Y. Liang, J. Li, L. Wu, H. Li, Q. Hao, B. J. Ni and C. Wang, Surface defective g-C3N4-xClx with unique spongy structure by polarization effect for enhanced photocatalytic removal of organic pollutants, J. Hazard. Mater., 2020, 398, 122897 CrossRef CAS PubMed
.
- Z. Zhou, K. Li, W. Deng, J. Li, Y. Yan, Y. Li, X. Quan and T. Wang, Nitrogen vacancy mediated exciton dissociation in carbon nitride nanosheets: Enhanced hydroxyl radicals generation for efficient photocatalytic degradation of organic pollutants, J. Hazard. Mater., 2020, 387, 122023 CrossRef CAS PubMed
.
- C.-Y. Wang, X. Zhang, Y.-J. Zhang, J.-J. Chen, G.-X. Huang, J. Jiang, W.-K. Wang and H.-Q. Yu, Direct generation of hydroxyl radicals over bismuth oxybromide nanobelts with tuned band structure for photocatalytic pollutant degradation under visible light irradiation, Appl. Catal., B, 2018, 237, 464–472 CrossRef CAS
.
- D. Hao, Y. Liu, S. Gao, H. Arandiyan, X. Bai, Q. Kong, W. Wei, P. K. Shen and B.-J. Ni, Emerging artificial nitrogen cycle processes through novel electrochemical and photochemical synthesis, Mater. Today, 2021, 46, 212–233 CrossRef CAS
.
- M. Gu, D.-Y. Lee, J. Mun, D. Kim, H.-I. Cho, B. Kim, W. Kim, G. Lee, B.-S. Kim and H.-I. Kim, Solar-to-hydrogen peroxide conversion of photocatalytic carbon dots with anthraquinone: Unveiling the dual role of surface functionalities, Appl. Catal., B, 2022, 312, 121379 CrossRef CAS
.
- Y.-Z. Zhang, C. Liang, H.-P. Feng and W. Liu, Nickel single atoms anchored on ultrathin carbon nitride for selective hydrogen peroxide generation with enhanced photocatalytic activity, Chem. Eng. J., 2022, 446, 137379 CrossRef CAS
.
- H. Hou, X. Zeng and X. Zhang, Production of Hydrogen Peroxide by Photocatalytic Processes, Angew. Chem., Int. Ed., 2020, 59, 17356–17376 CrossRef CAS PubMed
.
- Y. Shiraishi, T. Takii, T. Hagi, S. Mori, Y. Kofuji, Y. Kitagawa, S. Tanaka, S. Ichikawa and T. Hirai, Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion, Nat. Mater., 2019, 18, 985–993 CrossRef CAS PubMed
.
- L. Li, L. Xu, Z. Hu and J. C. Yu, Enhanced Mass Transfer of Oxygen through a Gas–Liquid–Solid Interface for Photocatalytic Hydrogen Peroxide Production, Adv. Funct. Mater., 2021, 31, 2106120 CrossRef CAS
.
- H. Zhang and X. Bai, Photocatalytic production of hydrogen peroxide over Z-scheme Mn3O4/Co9S8 with p-n heterostructure, Appl. Catal., B, 2021, 298, 120516 CrossRef CAS
.
- Y. Sun, L. Han and P. Strasser, A comparative perspective of electrochemical and photochemical approaches for catalytic H2O2 production, Chem. Soc. Rev., 2020, 49, 6605–6631 RSC
.
- S. Fukuzumi, Y.-M. Lee and W. Nam, Solar-Driven Production of Hydrogen Peroxide from Water and Dioxygen, Chem. – Eur. J., 2018, 24, 5016–5031 CrossRef CAS PubMed
.
- Y. Chen, X. Tang, J. Zhong, J. Li, M. Li and T. Zhang, Fabrication of tunable oxygen vacancies on BiOCl modified by spiral carbon fiber for highly efficient photocatalytic detoxification of typical pollutants, Appl. Surf. Sci., 2022, 578, 152122 CrossRef CAS
.
- M. Gao, D. Zhang, X. Pu, X. Shao, H. Li and D. Lv, Combustion Synthesis and Enhancement of BiOCl by Doping Eu3+ for Photodegradation of Organic Dye, J. Am. Ceram. Soc., 2016, 99, 881–887 CrossRef CAS
.
- S. Wang, L. Wang and W. Huang, Bismuth-based photocatalysts for solar energy conversion, J. Mater. Chem. A, 2020, 8, 24307–24352 RSC
.
- S. Chen, D. Huang, M. Cheng, L. Lei, Y. Chen, C. Zhou, R. Deng and B. Li, Surface and interface engineering of two-dimensional bismuth-based photocatalysts for ambient molecule activation, J. Mater. Chem. A, 2021, 9, 196–233 RSC
.
- K. Xu, J. Shen, S. Zhang, D. Xu and X. Chen, Efficient interfacial charge transfer of BiOCl-In2O3 step-scheme heterojunction for boosted photocatalytic degradation of ciprofloxacin, J. Mater. Sci. Technol., 2022, 121, 236–244 CrossRef
.
- C. Yang, Y. He, J. Zhong and J. Li, Photocatalytic performance of rich OVs-BiOCl modified by polyphenylene sulfide, Adv. Powder Technol., 2022, 33, 103427 CrossRef CAS
.
- L. Wang, H. Yin, S. Wang, J. Wang and S. Ai, Ni2+−assisted catalytic one-step synthesis of Bi/BiOCl/Bi2O2CO3 heterojunction with enhanced photocatalytic activity under visible light, Appl. Catal., B, 2022, 305, 121039 CrossRef CAS
.
- T. R. Gordon, M. Cargnello, T. Paik, F. Mangolini, R. T. Weber, P. Fornasiero and C. B. Murray, Nonaqueous Synthesis of TiO2 Nanocrystals Using TiF4 to Engineer Morphology, Oxygen Vacancy Concentration, and Photocatalytic Activity, J. Am. Chem. Soc., 2012, 134, 6751–6761 CrossRef CAS PubMed
.
- X. Xu, Z. Gao, Z. Cui, Y. Liang, Z. Li, S. Zhu, X. Yang and J. Ma, Synthesis of Cu2O Octadecahedron/TiO2 Quantum Dot Heterojunctions with High Visible Light Photocatalytic Activity and High Stability, ACS Appl. Mater. Interfaces, 2016, 8, 91–101 CrossRef CAS PubMed
.
- Y. Liu, L. Wang, H. Feng, X. Ren, J. Ji, F. Bai and H. Fan, Microemulsion-Assisted Self-Assembly and Synthesis of Size-Controlled Porphyrin Nanocrystals with Enhanced Photocatalytic Hydrogen Evolution, Nano Lett., 2019, 19, 2614–2619 CrossRef CAS PubMed
.
- J. Yang, T. Xie, Q. Zhu, J. Wang, L. Xu and C. Liu, Boosting the photocatalytic activity of BiOX under solar light via selective crystal facet growth, J. Mater. Chem. C, 2020, 8, 2579–2588 RSC
.
- Z. Zeng, Z. Yin, X. Huang, H. Li, Q. He, G. Lu, F. Boey and H. Zhang, Single-layer semiconducting nanosheets: high-yield preparation and device fabrication, Angew. Chem., Int. Ed., 2011, 50, 11093–11097 CrossRef CAS PubMed
.
- Y. Fan, Y. Bao, Z. Song, Z. Sun, D. Wang, D. Han and L. Niu, Controllable synthesis of coloured Ag(0)/AgCl with spectral analysis for photocatalysis, RSC Adv., 2018, 8, 24812–24818 RSC
.
- Y. Su, L. Zhang, W. Wang and D. Shao, Internal Electric Field Assisted Photocatalytic Generation of Hydrogen Peroxide over BiOCl with HCOOH, ACS Sustainable Chem. Eng., 2018, 6, 8704–8710 CrossRef CAS
.
- L. Chen, C. Chen, Z. Yang, S. Li, C. Chu and B. Chen, Simultaneously Tuning Band Structure and Oxygen Reduction Pathway toward High-Efficient Photocatalytic Hydrogen Peroxide Production Using Cyano-Rich Graphitic Carbon Nitride, Adv. Funct. Mater., 2021, 31, 2105731 CrossRef CAS
.
- B. O. Burek, J. Timm, D. W. Bahnemann and J. Z. Bloh, Kinetic effects and oxidation pathways of sacrificial electron donors on the example of the photocatalytic reduction of molecular oxygen to hydrogen peroxide over illuminated titanium dioxide, Catal. Today, 2019, 335, 354–364 CrossRef CAS
.
- Z. Chen, N. Mi, C. Li, Y. Teng, Y. Chen and C. Gu, Effects of different variables on photodestruction of perfluorooctanoic acid in self-assembled micelle system, Sci. Total Environ., 2020, 742, 140438 CrossRef CAS PubMed
.
- S. J. Rathi and A. K. Ray, On the existence and stability of single walled SiGe nanotubes, Chem. Phys. Lett., 2008, 466, 79–83 CrossRef CAS
.
- G. Li, Z. Yi, H. Wang, C. Jia and W. Zhang, Factors impacted on anisotropic photocatalytic oxidization activity of ZnO: Surface band bending, surface free energy and surface conductance, Appl. Catal., B, 2014, 158–159, 280–285 CrossRef CAS
.
- H. Shi, X. Wei, J. Zhang, Q. Long, W. Liu, Y. Zhou and Y. Ding, Green Synthesis and Direct Z-Scheme CdSe/BiOCl Heterojunctions for Enhanced Photocatalytic Performance, ChemistrySelect, 2020, 5, 6230–6235 CrossRef CAS
.
- H.-C. Chu, L.-H. Lin, H.-C. Liu, H.-F. Chen, H.-J. Liu and K.-M. Chen, Photocatalytic properties of silicone polyesters using calcium phosphate/titanium
dioxide, J. Appl. Polym. Sci., 2009, 114, 413–419 CrossRef CAS
.
- S. Zhao, Y. Zhang, Y. Zhou, C. Zhang, X. Sheng, J. Fang and M. Zhang, Reactable Polyelectrolyte-Assisted Synthesis of BiOCl with Enhanced Photocatalytic Activity, ACS Sustainable Chem. Eng., 2017, 5, 1416–1424 CrossRef CAS
.
- G. Liu, Y. Chen, X. Liu, X. Wang, M. Liu, C. Gao, G. Wang, Z. Teng, C. Yang and W. Yang, BiOCl microspheres with controllable oxygen vacancies: Synthesis and their enhanced photocatalytic performance, J. Solid State Chem., 2022, 306, 122751 CrossRef CAS
.
- K. Xu, D. Xu, Z. Li, S. Zhang, L. Tong, J. Peng, S. Zhang, J. Shen and X. Chen, Enhanced visible-light photocatalytic degradation of ciprofloxacin hydrochloride by bulk iodine doped BiOCl with rich oxygen vacancy, Appl. Surf. Sci., 2022, 578, 152083 CrossRef CAS
.
- Z. Wei, W. Li, J. Hu, X. Ma and Y. Zhu, Interfacial internal electric field and oxygen vacancies synergistically enhance photocatalytic performance of bismuth oxychloride, J. Hazard. Mater., 2021, 402, 123470 CrossRef CAS PubMed
.
- L. Zhang, W. Wang, D. Jiang, E. Gao and S. Sun, Photoreduction of CO2 on BiOCl nanoplates with the assistance of photoinduced oxygen vacancies, Nano Res., 2015, 8, 821–831 CrossRef CAS
.
- X. Zhao, Y. Xia, H. Li, X. Wang, J. Wei, X. Jiao and D. Chen, Oxygen vacancy dependent photocatalytic CO2 reduction activity in liquid-exfoliated atomically thin BiOCl nanosheets, Appl. Catal., B, 2021, 297, 120426 CrossRef CAS
.
- M. Mehri, S. M. Mousavi-Khoshdel and M. Molaei, First-principle calculations study of pristine, S-, O-, and P-doped g-C3N4 as ORR catalysts for Li-O2 batteries, Chem. Phys. Lett., 2021, 775, 138614 CrossRef CAS
.
- M. Li, Y. Zhang, X. Li, Y. Wang, F. Dong, L. Ye, S. Yu and H. Huang, Nature-Derived Approach to Oxygen and Chlorine Dual-Vacancies for Efficient Photocatalysis and Photoelectrochemistry, ACS Sustainable Chem. Eng., 2018, 6, 2395–2406 CrossRef CAS
.
- M. Li, Y. Zhang, X. Li, S. Yu, X. Du, Y. Guo and H. Huang, In-depth insight into facet-dependent charge movement behaviors and photo-redox catalysis: A case of {001} and {010} facets BiOCl, J. Colloid Interface Sci., 2017, 508, 174–183 CrossRef CAS PubMed
.
- Z. Wei, M. Liu, Z. Zhang, W. Yao, H. Tan and Y. Zhu, Efficient visible-light-driven selective oxygen reduction to hydrogen peroxide by oxygen-enriched graphitic carbon nitride polymers, Energy Environ. Sci., 2018, 11, 2581–2589 RSC
.
- L. Zheng, H. Su, J. Zhang, L. S. Walekar, H. Vafaei Molamahmood, B. Zhou, M. Long and Y. H. Hu, Highly selective photocatalytic production of H2O2 on sulfur and nitrogen co-doped graphene quantum dots tuned TiO2, Appl. Catal., B, 2018, 239, 475–484 CrossRef CAS
.
- G.-H. Moon, W. Kim, A. D. Bokare, N.-E. Sung and W. Choi, Solar production of H2O2 on reduced graphene oxide–TiO2 hybrid photocatalysts consisting of earth-abundant elements only, Energy Environ. Sci., 2014, 7, 4023–4028 RSC
.
- J. Sheng, X. Li and Y. Xu, Generation of H2O2 and OH Radicals on Bi2WO6 for Phenol Degradation under Visible Light, ACS Catal., 2014, 4, 732–737 CrossRef CAS
.
- S. Li, G. Dong, R. Hailili, L. Yang, Y. Li, F. Wang, Y. Zeng and C. Wang, Effective photocatalytic H2O2 production under visible light irradiation at g-C3N4 modulated by carbon vacancies, Appl. Catal.,
B, 2016, 190, 26–35 CrossRef CAS
.
- S. Thakur, T. Kshetri, N. H. Kim and J. H. Lee, Sunlight-driven sustainable production of hydrogen peroxide using a CdS–graphene hybrid photocatalyst, J. Catal., 2017, 345, 78–86 CrossRef CAS
.
- Y. Kofuji, Y. Isobe, Y. Shiraishi, H. Sakamoto, S. Ichikawa, S. Tanaka and T. Hirai, Hydrogen Peroxide Production on a Carbon Nitride–Boron Nitride-Reduced Graphene Oxide Hybrid Photocatalyst under Visible Light, ChemCatChem, 2018, 10, 2070–2077 CrossRef CAS
.
- M. Cai, A. Shui, Y. Wang, H. Xiong, S. Zeng, C. He, J. Qian and B. Du, Enhanced Photocatalytic Properties of Surfactants Modified ZnO Particles Synthesized Directly via Sonochemistry Technique, ChemistrySelect, 2022, 7, e202104016 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2en00933a |
| This journal is © The Royal Society of Chemistry 2023 |