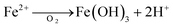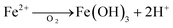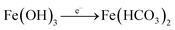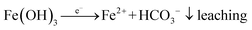Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal distribution in first flush in highway runoff of one of the busiest motorway junctions in the UK†
Julia
Zakharova
 *a,
Hamid
Pouran
*a,
Hamid
Pouran
 a and
Andrew
Wheatley
b
a and
Andrew
Wheatley
b
aFaculty of Science and Engineering, University of Wolverhampton, Wolverhampton, UK. E-mail: J.Zakharova@wlv.ac.uk
bSchool of Architecture, Building and Civil Engineering, Loughborough University, Loughborough, UK
First published on 12th May 2023
Abstract
Although the ‘first flush’ phenomenon has been extensively studied, there is still a niche remaining for a further contribution to this topic. The work reported in this paper addresses the challenges connected with the first flush from junction 24 of the M1 motorway in the UK. The event monitoring indicated that such factors as ADWP, rainfall intensity plus the catchment cleanliness and the loss of roughness, acting in combination, are the key factors in determining the presence of pollutants in the first flush. In addition, this study has also helped us to better understand the mechanism of iron release due to the presence of anaerobic and aerobic conditions – it showed the greatest proportion of its mass (73.6%), compared to other pollutants, in the first 30% of the runoff volume, which would suggest that the local conditions of the catchment can confound such a simple theory as that of pollutant dilution. The unexpectedly high presence of dissolved iron could be attributed to dissolved organic carbon, humic substances and anaerobic microbial activity.
Water impactChemicals washed away by highway runoff and accumulated in the water-treatment lagoon could undergo chemical changes, with potential direct or indirect negative impacts for the local ecosystems. For example, an increase in the dissolution of Fe3+ to Fe2+ could potentially reduce the uptakes of other essential chemicals needed for the growth of plants located in the vicinity. |
Introduction
The first flush phenomenon in stormwater discharge has been a hot topic for many years and has resulted in some polar opinions, i.e. on the part of those who have observed it and those who have not and hence ignore it. Why then has a first flush of pollutants been noted in a number of rainfall events, whereas in others a clear first flush has not been apparent? There are many factors which affect this phenomenon. The two main groups are: climatic factors and factors that are characteristic of the catchment area. Moreover, even when these factors are taken into consideration, the results might be confused or distorted on account of the particular combination of factors. One simple example is that under the same conditions (involving the same rainfall event and the same catchment area) different pollutants might behave differently. Why is the first flush so important and why does it give us such hot debates? The answer is very simple but sometimes not sufficiently obvious: it is important to know the treatment volume of the runoff. In other words, if the ‘first flush’ does exist then only the ‘dirtiest’ portion of the water – and sometime it will be a minor part of the total volume1 – might be delivered to the treatment stage, which will significantly reduce the cost of storm water treatment facilities (please note that here we are talking about a separate sewerage system). Sansalone and Cristina2 found that during a first flush there is a disproportionately high pollutant mass during the rising limb of the runoff hydrograph. This finding is consistent with our statement above regarding stormwater treatment facilities. Furthermore, it is important to couple the first flush phenomenon with the annual average daily traffic volume (AADT) and the most recent study conducted by Revitt et al.3 put forward an innovative approach to the prediction of pollutant concentrations in highway runoff.There is ambiguity in the published data on the effects of storm intensity and ADWP and more results are needed. Deletic (cited in ref. 4) found no ‘first flush’ effect, whereas Mosley and Peake,5 Prestes et al.6 and Lindfors et al.7 provided evidence of a ‘first flush’ of pollutants. A further complication is that, in the majority of cases ‘first flush’ has been linked only to suspended solids. Little is known about metals and other pollutants in connection with the first flush. One of the few studies conducted by Sansalone et al.8 reports on a number of metals (Cd, Zn, Cu and Pb) – dissolved and particulate-bound – which for some events showed a pronounced first flush but for other events showed a weak first flush. We do not want to underestimate the quality of that study, however to enhance its fullness it would be useful to analyse the reason why some of the metals were responsible for a pronounced first flush, whereas some of them were not. Hence, it can be seen that despite the fact that the first flush phenomenon has been studied to a good extent, there is a niche for a further contribution to this topic, which constitutes the aim of this study and the associated research questions.
The aim of this paper is to study the distribution of the pollutant load vs. the volume in stormwater influents. It is based on the example of two captured rainfall events which have the same rainfall characteristics but show completely different outcomes in terms of the pollutants' performance during the ‘first flush’ from the M1 (J24) treatment lagoon. Such a study should lead to a better understanding of the first flush phenomenon and of it variability in terms of different pollutants, including organic and metals. This study provides some data and conclusions in answer to the following questions:
1. What is the pollutant mass distribution vs. the volume?
2. Which pollutants are responsible for the pronounced first flush and why?
3. Which parameters have an impact on the first flush?
Methodology
Sampling site
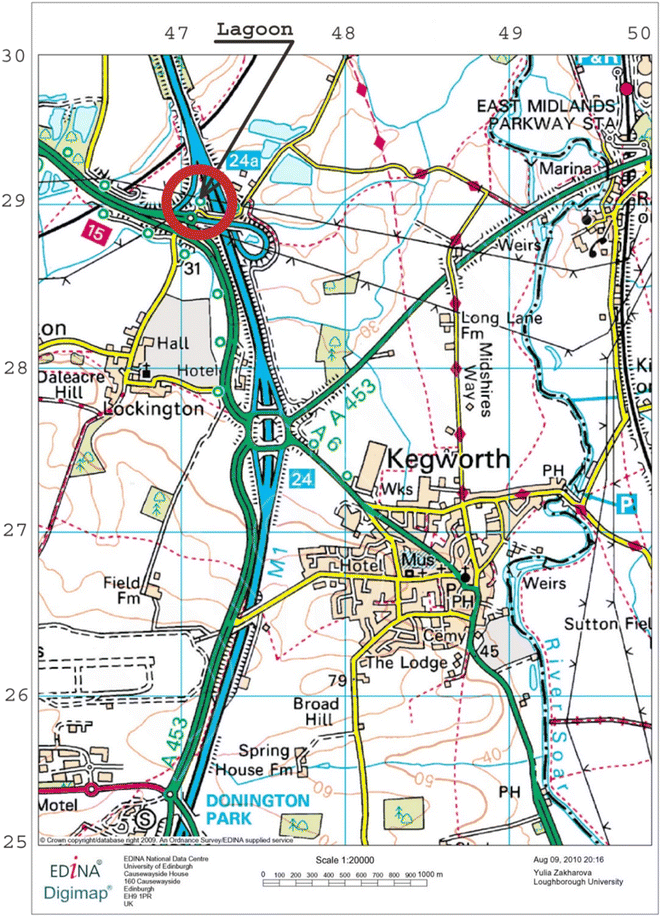
The test site is the junction between the M1/M42/A50 (J24) at Kegworth, (Fig. 1) which was rebuilt in 1996 and includes an interceptor and a SuDS lagoon (Fig. 2a). Peak traffic flows are 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 vehicles an hour. Those who wish to obtain more information on the test site, i.e. the conditions of runoff formation for the catchment area, may refer to the paper by Zakharova et al.9 During rainfall the runoff flows along the ditch adjacent to the motorway which links up with the drainage from the A50 slipway and then passes into an oil, silt interceptor before a SuDS lagoon. More details about the oil interceptor and its performance during rainfall events have been presented in Zakharova et al.10 It drains an impermeable area of around 3000 m2. The volume of the lagoon is 2000 m3 with an average depth of 0.9 m. The rainfall events were observed over a period of two months (September and December).
000 vehicles an hour. Those who wish to obtain more information on the test site, i.e. the conditions of runoff formation for the catchment area, may refer to the paper by Zakharova et al.9 During rainfall the runoff flows along the ditch adjacent to the motorway which links up with the drainage from the A50 slipway and then passes into an oil, silt interceptor before a SuDS lagoon. More details about the oil interceptor and its performance during rainfall events have been presented in Zakharova et al.10 It drains an impermeable area of around 3000 m2. The volume of the lagoon is 2000 m3 with an average depth of 0.9 m. The rainfall events were observed over a period of two months (September and December).
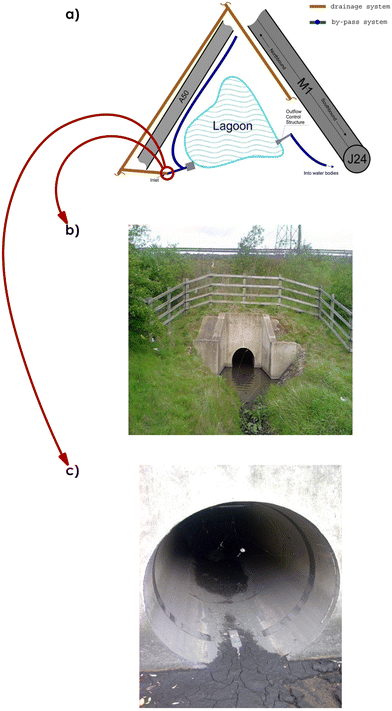 | ||
| Fig. 2 Inlet: (a) schematic view of the SuDS lagoon with the inlet (circled); (b) view of the inlet when it is filled with water; (c) view of the inlet with the flow meter during dry weather. | ||
Flow measurement
To measure the discharge from the motorway catchment into the inlet (Fig. 2b), a “STARFLOW” Ultrasonic Doppler Instrument (Starflow Model 6526B) was used. The instrument measures water velocity, depth and temperature integrated into a single unit. Water velocity is measured acoustically by recording the Doppler shift from particles and air bubbles carried in the water. Water depth is measured by a pressure transducer which records the hydrostatic pressure of the water above the instrument. Temperature is measured in order to refine the acoustic recordings, which are affected by the temperature. The STARFLOW was installed in the inlet pipe with a diameter of 1000 mm (Fig. 2c) near the downstream end so as to maximise non-turbulent flow conditions and it was positioned with the sensor pointing upstream. The data from the flow measurements was averaged every 15 minutes during runoff events with a data logger.Sample collection and preparation for analysis
During observed rainfall events grab samples were taken from the inlet. Pre-washed 1 L polyethylene bottles, pre-soaked in 10% HNO3, were used. Before sampling, each bottle was rinsed twice with the sample source. Subsamples for the analysis of metals (100 mL aliquots) were immediately acidified with concentrated HNO3 (5 ml l−1). For dissolved metal analysis, a similar 100 mL sample was filtered under vacuum through a millipore 0.45 μm pre-acid-washed membrane filter and stored in acid-washed plastic bottles. The filtered samples were similarly acidified with concentrated HNO3 to pH < 2. Samples for other analyses (TSS, TOC and hardness) were stored at 4 °C until analysis had been performed within 24 h. More detail about the measurements of these parameters as well as the calibration of the instrument can be found in the ESI† of the paper Zakharova et al.9Sample analysis
Total metals were analysed by the aqua regia method11 using microwave digestion, performed in a CEM Mars Xpress microwave, and 30 mL of sample was used which had been acidified with 2 mL of concentrated HNO3 and 5 mL of concentrated HCl. When the samples were cooled, they were filtered through Whatman No. 1 paper and diluted with distilled deionised water to 50 mL in volumetric flasks. For quality assurance purposes, blanks and internal standards were included. Total and dissolved metals were measured with an ICP-OES analyser (Thermo Jarrell Ash Atom Scan 16). Whilst the ICP-OES's detection limit for each of the tested elements is a function of wavelength, for both of the determinants considered the limit of detection was 0.002 mg L−1. For more information on the calibration of the instrument as well as quality control for the chemical analysis, please see the ESI† of the paper Zakharova et al.9Results and discussion
Hydrograph characteristic
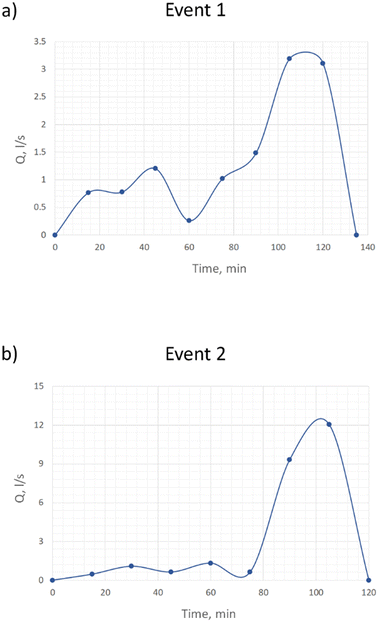
Fig. 3 shows hydrographs – graphs of the flowrates at different moments in time – for two captured rainfall events. The shape of the hydrograph depends on many factors, the two main ones being the characteristic of the rainfall and the catchment relief. From these figures we can see that the rain intensity at the beginning of the rainfall event was low in comparison with that at the end of the event, where for both rains one can see the increasing limb of the hydrograph. The increase in flowrates means that more water was participating in the flow, which was why the flowrates on the ‘rising’ limb did increase in accordance with the exponential relationship. We can see that at some point both hydrographs showed their maximum value – the time of the flow concentration. We can also see that the flowrate in both cases rapidly, almost sharply, decreased to zero, which could be explained by the small size of the catchment area – it is less than 10 ha. Al Mamun et al.12 contribute to our thoughts on the relationship between the size of the catchment and the hydrograph's shape. They go further by discussing the way in which the shape of the catchment has an influence on the runoff hydrograph, which in turn influences the nature of the pollution generated from the catchment area and its first flush characteristics.Pollutant mass distribution vs. volume
It is known that the variation in the pollutant mass flowrates during rainfall events can be described by means of two curves: the hydrograph Q(t) and the pollutograph C(t) for each pollutant considered, where Q is the flowrate in L s−1 or m3 s−1 and C is the concentration in mg L−1.13In this case, to enable us to compare the pollutant mass flow rate curves for two storm events, we have presented a dimensionless representation of the two events. This representation has been produced by drawing the curve that gives the variation in the cumulative pollutant mass divided by the total pollutant mass in relation to the cumulative volume divided by the total volume. The following relationships have been used, bearing in mind that Q and C vary linearly between two measurements:
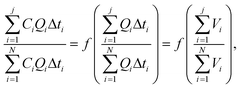 | (1) |
The most recent use of such M(V) curves was published by Bertrand-Krajewski et al.13
The drawings of the M(V) curves for the two events are shown in Fig. 4 and 5. In both cases the numbers indicate the order of drawing each point of the M(V) curve from both the hydrograph and the pollutograph. The example is given for the dissolved iron, Fedis, the element behaviour of which will be further described thoroughly due to the unexpectedly surprising results that were obtained. For other pollutants Fig. 6 has been created where only the final results such as those for the cumulative pollutant mass fractions and cumulative runoff volume can be seen for two events. For a more detailed analysis concerning all other pollutants, please see our additional material on the basis of which a mass balance of the pollutants in the first 30% of the runoff volume has been summarised in Table 1. Our data coincide very well with those obtained by Lind et al.,14 who indicated that in their case 50–60% of the total mass of metals was transported within the first 30% of runoff.
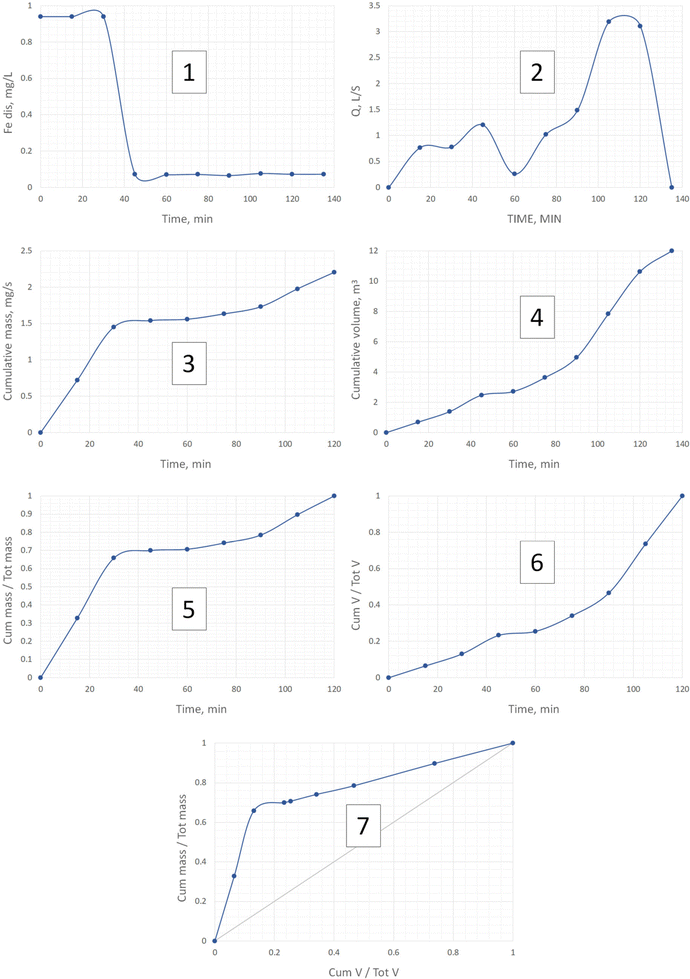 | ||
| Fig. 4 Example of the drawing of one M(V) curve for Fedis for event 1. The numbers 1–7 indicate the order of drawing each point of the M(V) curve from both the hydrograph and the pollutograph. | ||
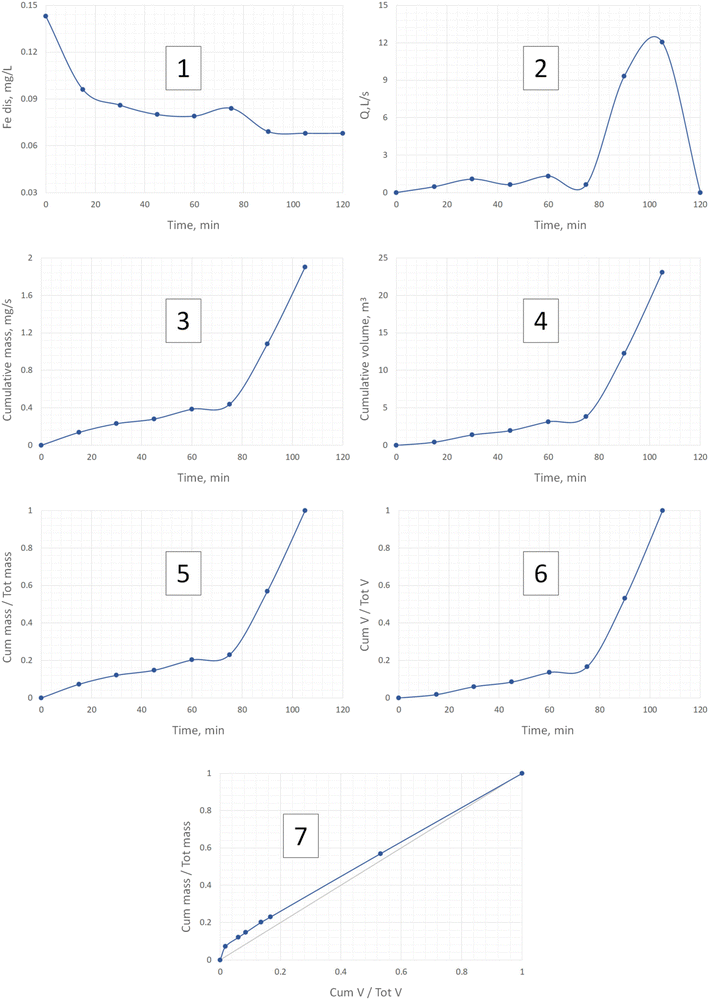 | ||
| Fig. 5 Example of the drawing of one M(V) curve for Fedis for event 2. The numbers 1–7 indicate the order of drawing each point of the M(V) curve from both the hydrograph and the pollutograph. | ||
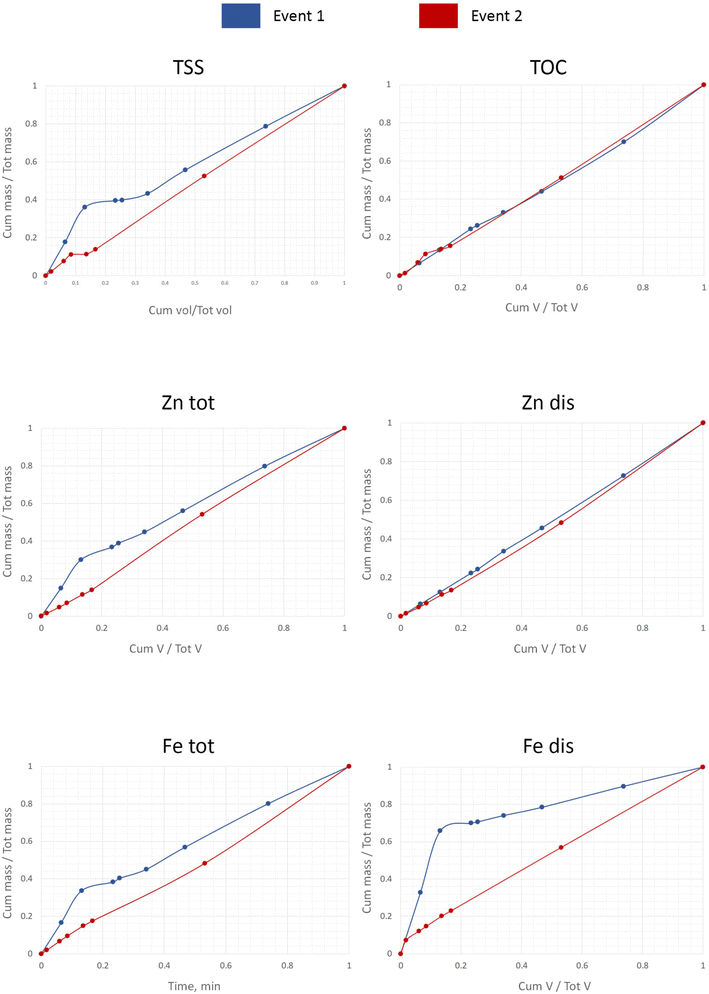 | ||
| Fig. 6 First flush graphical analysis of the ratio runoff mass fraction and volume fraction for two rainfall events. | ||
| Pollutants | Pollutant mass, % | |
|---|---|---|
| Event 1 (peak flow 3.2 L s−1) | Event 2 (peak flow 12.1 L s−1) | |
| TSS | 39.7 | 19 |
| TOC | 29.5 | 17.4 |
| Fetot | 45.3 | 17.3 |
| Fedis | 73.6 | 19.5 |
| Zntot | 43.3 | 14.8 |
| Zndis | 28.8 | 14.1 |
Wanielista and Yousef (cited in Davis and McCuen4), on the basis of their data, suggest that the ‘first flush’ may be defined as occurring when 50% of the mass is present in the first 25% of the volume. Bertrand-Krajewski et al.,13 on the other hand, suggested a definition of the ‘first flush’ as being when 80% of the mass pollutants occur in the first 30% of runoff volume.
Perera et al.15 have suggested a completely different insight into the first flush phenomenon. They introduced a parameter which defines the point where the first flush ends. Thus, if the fraction of the pollutant load discharge is greater than the fraction of runoff discharged during the same time interval, then under those circumstances the first flush is said to exist. Perera et al.15 found that the first flush runoff varies over the initial 30–50% of the runoff volume and therefore, at the minimum, the first 30% of the runoff should be considered as critical.
Cristina and Sansalone16 state that high-runoff volume events will typically exhibit a continuous flush which, according to their study, transported 80% of the total particle-number density in the first 60% of the storm's duration.
Tables 2 and 3 represent the changing mass of the pollutants (multiplication of the concentrations to the flow rate) with time. One peculiar feature of this data is as follows. Event 1 showed a high mass pollutant level at the beginning of the rainfall and a subsequent sharp decrease in the pollutants due to dilution, indicating that the ADWP plays a crucial role in rainfall analysis. By contrast, event 2 had a much lower level of mass pollutants compared to event 1. As was previously discussed, these two rains showed their maximum flow rates towards the end of the event, so they are comparable from the view point of hydrographic pattern. Nevertheless, by looking at Fig. 6 and Table 2 one can see that event 1 displayed the ‘first flush’ for most of the pollutants, unlike event 2. In other words, the first flush phenomenon is clearly apparent for a number of pollutants in the case of event 1 and barely visible for all pollutants for event 2. More surprisingly, Fedis showed the greatest contribution to the ‘first flush’ (73.6%) for event 1, as traditionally it was thought that the first flush phenomenon was linked with solids and particulate-bound metals.8
| Time, min | Q, L s−1 | TSS | TOC | Fetot | Fedis | Zntot | Zndis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg L−1 | mg s−1 | mg L−1 | mg s−1 | mg L−1 | mg s−1 | mg L−1 | mg s−1 | mg L−1 | mg s−1 | mg L−1 | mg s−1 | ||
| Q – flow rate; Cum – cumulative; Tot – total; V – volume. | |||||||||||||
| 0 | 0 | 119.5 | 0 | 7.78 | 0 | 3.76 | 0 | 0.94 | 0 | 0.273 | 0 | 0.025 | 0 |
| 15 | 0.768 | 119.5 | 91.776 | 7.78 | 5.975 | 3.76 | 2.887 | 0.94 | 0.722 | 0.273 | 0.21 | 0.025 | 0.019 |
| 30 | 0.778 | 119.5 | 92.971 | 7.78 | 6.053 | 3.76 | 2.925 | 0.94 | 0.731 | 0.273 | 0.212 | 0.025 | 0.019 |
| 45 | 1.203 | 15 | 18.045 | 8.31 | 9.997 | 0.659 | 0.793 | 0.073 | 0.088 | 0.079 | 0.095 | 0.025 | 0.03 |
| 60 | 0.26 | 6 | 1.56 | 6.4 | 1.664 | 1.395 | 0.363 | 0.07 | 0.018 | 0.111 | 0.029 | 0.024 | 0.006 |
| 75 | 1.022 | 17 | 17.374 | 5.85 | 5.979 | 0.782 | 0.799 | 0.072 | 0.074 | 0.081 | 0.083 | 0.028 | 0.029 |
| 90 | 1.485 | 43 | 63.855 | 6.72 | 9.979 | 1.371 | 2.036 | 0.066 | 0.098 | 0.105 | 0.156 | 0.025 | 0.037 |
| 105 | 3.191 | 37 | 118.07 | 7.33 | 23.39 | 1.251 | 3.992 | 0.077 | 0.246 | 0.105 | 0.335 | 0.026 | 0.083 |
| 120 | 3.108 | 35 | 108.78 | 8.66 | 26.92 | 1.106 | 3.437 | 0.073 | 0.227 | 0.091 | 0.283 | 0.027 | 0.084 |
| 135 | 0 | 35 | 0 | 8.66 | 0 | 1.106 | 0 | 0.073 | 0 | 0.091 | 0 | 0.027 | 0 |
| Time, min | Q, L s−1 | TSS | TOC | Fetot | Fedis | Zntot | Zndis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg L−1 | mg s−1 | mg L−1 | mg s−1 | mg L−1 | mg s−1 | mg L−1 | mg s−1 | mg L−1 | mg s−1 | mg L−1 | mg s−1 | ||
| Q – flow rate; Cum – cumulative; Tot – total; V – volume. | |||||||||||||
| 0 | 0 | 14 | 0 | 3.32 | 0 | 0.726 | 0 | 0.143 | 0 | 0.081 | 0 | 0.04 | 0 |
| 15 | 0.464 | 11 | 5.104 | 3.98 | 1.847 | 0.719 | 0.334 | 0.096 | 0.045 | 0.068 | 0.032 | 0.038 | 0.018 |
| 30 | 1.077 | 11.5 | 12.386 | 7.57 | 8.153 | 0.712 | 0.767 | 0.086 | 0.093 | 0.073 | 0.079 | 0.039 | 0.042 |
| 45 | 0.631 | 12.5 | 7.888 | 10.31 | 6.506 | 0.728 | 0.459 | 0.08 | 0.05 | 0.084 | 0.053 | 0.044 | 0.028 |
| 60 | 1.323 | 9.5 | 0.662 | 2.79 | 3.691 | 0.661 | 0.875 | 0.079 | 0.105 | 0.08 | 0.106 | 0.044 | 0.058 |
| 75 | 0.636 | 9 | 5.724 | 3.51 | 2.233 | 0.692 | 0.440 | 0.084 | 0.053 | 0.092 | 0.059 | 0.046 | 0.029 |
| 90 | 9.335 | 9.5 | 88.683 | 5.53 | 51.623 | 0.632 | 5.9 | 0.069 | 0.644 | 0.102 | 0.952 | 0.049 | 0.457 |
| 105 | 12.044 | 9 | 108.396 | 5.84 | 70.337 | 0.7 | 8.431 | 0.068 | 0.819 | 0.09 | 1.084 | 0.056 | 0.674 |
| 120 | 0 | 9 | 0 | 5.84 | 0 | 0.7 | 0 | 0.068 | 0 | 0.09 | 0 | 0.056 | 0 |
For event 1, the accumulation of sediments was observed in the inlet and drains. During dry weather, because of evaporation, the TSS concentration increases as well as that of total metals, so this explains the behaviour of the curves for TSS, Fetot and Zntot. However, more complicated processes of equilibrium and re-solubilisation may take place for iron in particular, which is more soluble anaerobically. This could explain the enhancement of the first flush effect for Fedis following ADWP. The accumulation of the sediment suggests that the local characteristics of the catchment play an important role in the first flush formation. We will elaborate on this message further.
Rainfall event 1 showed the ‘first flush’ with more than 40% of Fetot, Zntot and TSS discharging within the first 30% of the runoff volume (see Table 2). The Fedis showed the greatest proportion of its mass (73.6%) in the first 30% of the runoff volume. Other dissolved components (TOC and Zndis) did not demonstrate these first flush characteristics.
These two sets of data (Fig. 6) confirm that TSS and associated metals behave differently compared to dissolved pollutants. From this figure one can see that rainfall event 2 created linear concentrations vs. flow with no indication of ‘first flush’ for all pollutants, apart from TOC.
Why, then, in our case did event 1 show such a pronounced first flush for some pollutants? The explanation of this particular case lies in a number of factors which all came together and created this scenario for some pollutants.
First of all, the previous rainfall events resulted in the transport of erodible deposits to the inlet (Fig. 2a). The above-mentioned observation could be coupled together with the so-called degree of ‘catchment cleanliness’ described by Al Mamun et al.12 This is supposed to be a catchment that is not subject to any regular cleaning at all, suggesting that there are favourable conditions for the first flush.
Secondly, at the time of sampling, sample point one was not dry and contained the most concentrated water due to evaporation. This relates to another definition of the catchment, as given by Al Mamun et al.12 They introduced the concept of the relative ‘roughness’ of the catchment. Rough catchments are not susceptible to experiencing the first flush. However, it seems that in our case the roughness was lost in event 1 but was clearly present in event 2, where the first flush was not apparent.
Thirdly, although the rainfall event did not have a high intensity at the beginning of the event, it produced just enough water in order to turn the rain into the runoff. The fourth factor was that the ADWP created favourable conditions for Fedis, in particular, in order to show a pronounced first flush. This phenomenon has been discussed below.
Potential chemical mechanism to explain high concentrations of Fedis
Fig. 7 shows two sample points (the Inlet and the lagoon itself, see Fig. 2a), where we can see the increase in Fedis with the decrease in hardness. More to the point, since Fe is known to be a poorly dissolved metal, our observation that the dissolved part of it comprised around 50% of the total iron before and after event 1 was evidence of its extremely unusual behaviour. In other words, we can see that the release of Fedis in the lagoon was coupled with a decrease in hardness. Zndis, on the contrary did not follow that trend.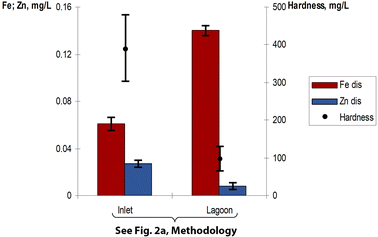 | ||
| Fig. 7 Metals and water hardness profile during dry weather Fedis is 0.14 mg L−1 in the lagoon, comprising almost 50% of its total form (Fetot is 0.29 mg L−1). | ||
Iron speciation in natural waters is quite complex and it has received a great deal of attention from a number of researchers.17–26 The element's chemistry is dominated by extensive hydrolysis but also organic complexation as well as redox transformations. Furthermore, another possible reason of iron release could be alteration of aerobic and anaerobic conditions.
In our case, it was observed that during the sampling campaign there was a fluctuation of water levels in the inlet because of dry and wet weather periods. It is suggested that in this case this could result in alternating aerobic and anaerobic conditions and therefore in iron reduction and oxidation (ferrolysis) in particular, which was reflected in the measured hardness.
Further explanation of iron release mechanism, as one of the possible hypothesis, can be seen by presenting eqn (2) and (3). These equations provided are two examples of reactions that can occur in both aerobic and anaerobic conditions.
During wet periods, Fe (OH)3 undergoes reduction, with organic matter supplying the electrons:
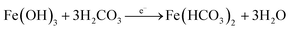 | (2) |
Once the surface drains, aerobic conditions prevail again, oxygen is in excess, and Fe2+ re-oxidises and generates acidity:
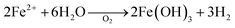 | (3) |
Such conditions whereby Fedis is released during dry weather give rise to high concentrations of Fe, resulting in the ‘first flush’. For example, Garcia-Balboa (cited in Eisele and Gabby)21 found that bacteria growing in the absence of oxygen, i.e. anaerobic organisms, often ferment organic acids. Consequently, a broad range of microorganisms, both bacteria and fungi, could be effective in promoting iron dissolution.18,19
However, there is a number of previous studies which have shown that the increase in dissolved iron may also be linked to Fe-redox cycling, either under reducing conditions in the riverbed or under the influence of light and dissolved organic carbon (DOC).22 Another study conducted by Gaffney et al.23 showed that organic carbon might be a predominant control factor in iron mobility.
There have been a number of studies about the behaviour of iron and its speciation in coastal waters. The crucial role affecting the solubility of iron might be fulfilled by the anions and cations which are abundantly present in seawater.24 Batchelli et al.25 confirmed that the presence of iron in coastal waters is strongly but reversibly bound to humic substances and that it therefore might be available for complexation by siderophore-type ligands released by microorganisms. To complement this, Matsunaga et al.24 found that fulvic acid makes the iron bioavailable. In other words, the natural organic ligands control the speciation of iron and thus its bioavailability in natural waters.
Krachler et al.,26 while investigating peat bogs, found that the peat was able to produce strong chelate ligands (humic and fulvic acids) which enhance the weathering rates of iron-silicate minerals and greatly increase the solubility of iron in river water. They also concluded that peatland-draining rivers are important sources of dissolved iron for the ocean margins.
To summarise these findings, in our case all three aspects discussed above (anaerobic activity, dissolved organic carbon, humic acids and) could promote iron dissolution, which in this instance affected 50% of the total iron. We even believe that a synergistic effect could be present, i.e. the combination of two or more factors could take place in this case.18 More to the point, this analysis should be coupled with an awareness of the sampling point conditions, which would shed more light on the catchment characteristics.
Conclusions
The dimensionless M(V) curves indicating the distribution of pollutant mass vs. its volume in stormwater discharges have been used to compare a number of pollutant discharges from two rainfall events at the M1, junction 24. These M(V) curves appear to be variable.The results obtained in this study show that prolonged rainfall events dilute pollutant concentrations but they also show that the local conditions of the catchments can confound these simple results. Pollutants were increased by dry weather (ADWP) not only as a result of evaporation but also due to their re-solubilisation from the sediments.
In this study we distinguished a pronounced first flush phenomenon (event 1) at the M1 (J24), in a relatively small catchment area (of less than 10 ha) for such pollutants as TSS, Fetot. These pollutants showed more than 40% of their mass in the first 30% of the runoff volume. The Fedis showed 73.6% of its mass in the first 30% of the runoff volume. A weaker but still visible first flush was observed for such pollutants as organics (TOC) and Zn in its total and dissolved forms.
This paper suggests that not only the size of the catchment but also its cleanliness and roughness can affect the observation of the first flush. These two parameters have to be coupled with the ADWP and rainfall intensity, which was why the first flush phenomenon was not observed or was extremely weak during event 2.
This research suggest a potential chemical mechanism for generating high concentrations of Fedis. Some fluctuations of the water level in the inlet provoked alternating aerobic and anaerobic conditions, resulting in iron reduction and oxidation in particular. It is apparent that the quantity of pollutants could have increased not only as a result of evaporation but also due to their re-solubilisation from the sediment. This suggests that the local conditions of the catchment can confound simple and traditional expectations of pollutant behaviour. Although it is known that Fe2+ is not a toxic metal in the concentrations presented in this study, it is tremendously important to understand its behaviour as it may compete with other cations, such as Ca, Mg and Zn, and thereby affect the nutrient or chemical availability.
Author contributions
Dr Julia Zakharova: conceptualisation; investigation; writing original draft. Dr Hamid Pouran: writing original draft; visualisation. Prof Andrew Wheatley: funding acquisition; resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Loughborough University. The authors wish to thank Mr Geoff Russel and Mrs Jayshree Bhuptani for assistance with laboratory and field support. The authors appreciate the valuable comments of reviewers. The authors are also immensely grateful to Mr David Chapman and Mr Dmitry Zakharov for their support and help, without which the publication of this paper would not have been possible.References
- L. Vezzaro, M. L. Christensen, C. Thirsing, M. Grum and P. S. Mikkelsen, Water quality-based real time control of integrated urban drainage systems: a preliminary study from Copenhagen, Denmark, Procedia Eng., 2014, 70, 1707–1716, DOI:10.1016/j.proeng.2014.02.188.
- J. J. Sansalone and C. M. Cristina, First flush concepts for dissolved solids in small impervious watersheds, J. Environ. Eng., 2004, 130, 1301–1314 CrossRef CAS.
- D. M. Revitt, J. B. Ellis, N. Gilbert, J. Bryden and L. Lundy, Development and application of an innovative approach to predicting pollutant concentrations in highway runoff, Sci. Total Environ., 2022, 825, 1–10, DOI:10.1016/j.scitotenv.2022.153815.
- A. Davis and R. McCuen, Stormwater Management for Smart Growth, Springer Science and Business Media Inc., USA, 2005 Search PubMed.
- L. M. Mosley and B. M. Peake, Partitioning of metals (Fe, Pb, Cu, Zn) in urban run-off from the Kaikorai Valley, Dunedin, New Zealand, N. Z. J. Mar. Freshwater Res., 2001, 35, 615–624 CrossRef CAS.
- E. Prestes, V. Anjous, F. Sodre and M. Grassi, Copper, lead and cadmium loads and behaviour in urban storm water runoff in Curitiba, Brazil, J. Braz. Chem. Soc., 2006, 17(1), 53–60 CrossRef CAS.
- S. Lindfors, H. Osterlund, L. Lundy and M. Viklander, Evaluation of measured dissolved and bio-met predicted bioavailable Cu, Ni and Zn concentrations in runoff from three urban catchments, J. Environ. Manage., 2021, 287 DOI:10.1016/j.jenvman.2021.112263.
- J. J. Sansalone and S. G. Buchberger, Partitioning and first flush of metals in urban roadway storm water, J. Environ. Eng., 1997, 2, 134–143 CrossRef.
- J. Zakharova, H. Pouran, J. Bridgeman, A. Wheatley and M. Arif, Understanding metal concentration and speciation in motorway runoff, Environ. Technol., 2020, 1–31, DOI:10.1080/09593330.2020.1850874.
- J. Zakharova, H. J. Pouran, A. Wheatley and M. Arif, Assessment of oil-interceptor performance for solid removal in highway runoff, Environ. Technol., 2021 DOI:10.1080/09593330.2021.1968040.
- APHA, Standard methods for the examination of water and wastewater, American Public Health Association, Washington, DC, 21st edn, 2005 Search PubMed.
- A. Al Mamun, S. Shams and M. Nuruzzaman, Review of uncertainty of the first-flush phenomenon in diffuse pollution control, Appl. Water Sci., 2020, 10(53) DOI:10.1007/s13201-019-1127-1.
- J. Bertrand-Krajewski, G. Chebbo and A. Saget, Distribution of pollutant mass vs. volume in stormwater discharges and the first flush phenomenon, Water Res., 1998, 32, 2341–2356 CrossRef CAS.
- B. B. Lind, M. Backstrom and E. Geisler, First flush effect on metals and anions in stormwater runoff from roads in mid-Sweden, in Urban transport vii:urban transport and the environment for the 21st century, WIT Press, Southampton, 2001, pp. 497–508 Search PubMed.
- T. Perera, J. McGree, P. Egodawatta, K. B. S. N. Jinadasa and A. Goonetilleke, New conceptualisation of first flush phenomena in urban catchments, J. Environ. Manage., 2021, 251, 1–8 Search PubMed.
- C. M. Cristina and J. J. Sansalone, ‘First flush’ power law and particle separation diagrams for urban storm-water suspended particulates, J. Environ. Eng., 2003, 4, 298–307 CrossRef.
- H. M. Pouran, Bacterial Cell-Mineral Interface, its impact on Biofilm Formation and Bioremediation, in Handbook of Environmental Materials Management, Springer International Publishing, Cham, 2018, pp. 1–15 Search PubMed.
- H. M. Pouran, Engineered Nanomaterials in the Environment, their Potential Fate and Behaviour and Emerging Techniques to Measure them, in Handbook of Environmental Materials Management, ed. C. Hussain, Cham, 2018 Search PubMed.
- H. M. Pouran, S. A. Banwart and M. Romero-Gonzalez, Characterising the Cell Surface Properties of Hydrocarbon-Degrading bacterial Strains; A case study, in Handbook of Environmental Materials Management, ed. C. Hussain, Cham, 2018 Search PubMed.
- H. Pouran, R. Colodrero, S. Wu, G. Hix, J. Zakharova and H. Zhang, Assessment of ATR-FTIR spectroscopy with multivariate analysis to investigate the binding mechanisms of Ag and TiO2 nanoparticles to chelex-100 or metsorb for the DGT technique, J. Anal. Methods Chem., 2020, 12, 959–969, DOI:10.1038/c9ay02458a.
- T. C. Eisele and K. L. Gabby, Review of reductive leaching of iron by anaerobic bacteria, Miner. Process. Extr. Metall. Rev., 2014, 35, 75–105, DOI:10.1080/08827508.2012.703627.
- L. Sigg, H. Xue, D. Kistler and R. Schonenberger, Size Fractionation (dissolved, colloidal and particulate) of trace metals in the Thur river, Switzerland, Aquat. Geochem., 2000, 6, 413–434 CrossRef CAS.
- J. W. Gaffney, K. N. White and S. Boult, Oxidation state and size of Fe controlled by organic matter in natural waters, Environ. Sci. Technol., 2008, 42, 3575–3581 CrossRef CAS PubMed.
- K. Matsunaga, J. Nishioka, K. Kuma, K. Toya and Y. Suzuki, Riverine input of bioavailable iron supporting phytoplankton growth in Kesennuma bay (Japan), Water Res., 1998, 32(11), 3436–3442 CrossRef CAS.
- S. Batchelli, F. L. L. Muller, K. Chang and C. Lee, Evidence for strong but dynamic iron – humic colloidal associations in humic-rich coastal waters, Environ. Sci. Technol., 2010, 44, 8485–8490 CrossRef CAS PubMed.
- R. Krachler, R. F. Krachler, F. Kammer, A. Suphandag, F. Jirsa, S. Ayromlou, T. Hofmann and B. K. Keppler, Relevance of peat draining rivers for the riverine input of dissolved iron into ocean, Sci. Total Environ., 2020, 408, 2402–2408 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ew00919f |
| This journal is © The Royal Society of Chemistry 2023 |