Selective chemical disassembly of elastane fibres and polyurethane coatings in textiles†
Martin B.
Johansen‡
 a,
Bjarke S.
Donslund‡
a,
Bjarke S.
Donslund‡
 b,
Martin L.
Henriksen
b,
Martin L.
Henriksen
 c,
Steffan K.
Kristensen
c,
Steffan K.
Kristensen
 *a and
Troels
Skrydstrup
*a and
Troels
Skrydstrup
 *d
*d
aInterdisciplinary Nanoscience Center (iNANO), Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus C, Denmark. E-mail: skk@inano.au.dk
bCarbon Dioxide Activation Center (CADIAC), Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus C, Denmark
cDepartment of Biological and Chemical Engineering, Aarhus University, Aabogade 40, 8200 Aarhus N, Denmark
dCarbon Dioxide Activation Center (CADIAC), Novo Nordisk Foundation CO2 Research Center, Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus C, Denmark. E-mail: ts@chem.au.dk
First published on 23rd November 2023
Abstract
The textile industry is ever-growing but only a few recycling options exist for waste fabrics to keep end-of-use clothing articles within the value chain. Generally, these include fibre-to-fibre strategies for pure textiles such as cotton or polyester. However, most textiles comprise blends of fibres which encumbers both mechanical and chemical recycling processes. For example, the polymer elastane is added to increase stretchability in many textiles, whereas polyurethane coatings and membranes partly make up rain clothes, bags and artificial leather items. Here we report the selective disassembly of these two polymers into base chemicals adapting a solvolysis process with tert-amyl alcohol. We showcase the successful depolymerisation of elastane recovered from the dissolution of fabric blends, and also the direct disassembly of elastane containing- or PU-coated textiles with polyamide fabrics. This work opens up new opportunities for the recycling of certain blended textiles.
Introduction
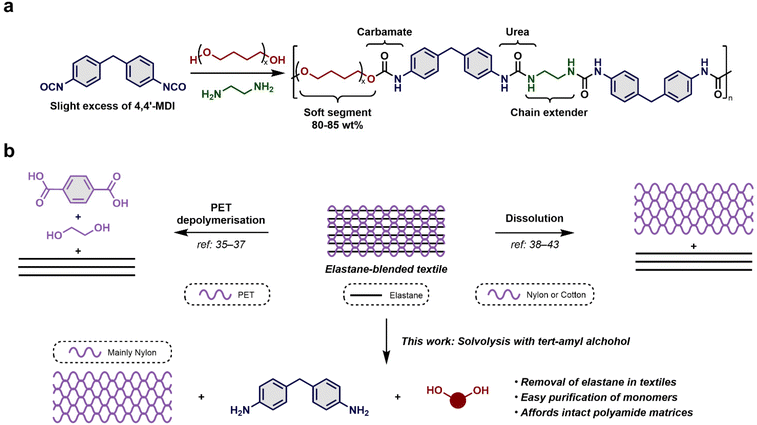
In 2020, the global textile production was estimated to have reached an astonishing 109 million tons. Partially due to a lack of good recycling methods, less than 1% of textiles are recycled in a closed-loop and the majority ends up as waste sent for landfilling or incineration.1,2 Clothing production has doubled in the last 15 years, largely due to the success of fast fashion in which low priced textiles are produced at a rate matching the quick turnarounds in fashion.2,3 The world demand for textile fibres is therefore projected to increase significantly reaching 140 million tons in 2030.4 Polyester, a synthetic fibre, constitutes 52% of the market, whereas the natural fibre cotton covers 24%, leaving the rest to nylon, wool, silk, viscose, elastane etc.1 Chemical and physical recycling initiatives for polyester have already reached commercialisation, while the present recycling of cotton relies on mechanical and physical methods.5–8 Regardless, only 1% of the textiles are kept within a closed recycling loop in which end-of-life textile fibres are converted into new textile fibres for similar quality applications (fibre-to-fibre recycling).2,9 The EU Commission recently published a new strategy stressing that fibre-to-fibre recycling remains a major challenge and recommends increased communication on sustainability to e.g. avoid “downcycling” of food-grade polyethylene terephthalate (PET) bottles into polyester fibres.10,11Input of single component fibres for recycling offers closed-loop possibilities but mixed fibres impede efficient processes for recycling, and especially, recycling of mixed fibres containing elastane is problematic.2,5–11 Elastane (or Spandex – anagram for expands) is a synthetic fibre displaying stretchable properties. It is often used in composite clothing articles in combination with polyester, cotton or nylon, providing high elasticity and durability to the fabric.5 DuPont originally branded the product under the name Lycra, especially for women's undergarment products, while U.S. Rubber named its brand Vyrene.12,13 Although elastane is regularly added to fabrics in quantities below 3%, this polymer is present in 20% of all potentially recyclable textile waste fractions, a number that is projected to increase to 30% in 2030.2,4
Structurally, elastane is composed of a polyurethane-urea block copolymer prepared from a pre-polymer of 4,4′-methylenediisocyanate (MDI) and a macrodiol, usually poly(tetrahydrofuran) (polyTHF). The polymer is cured with a short diamine chain extender, such as ethylenediamine (Scheme 1a).14–17 The polymerisation termination is controlled by the addition of secondary amines such as diethylamine, which fine-tunes the average molecular weight by the formation of a urea end group.16,17 The majority of the polymer (80–85 wt%) is composed of the flexible diol, being referred to as the soft segment. The urea functional groups make up the hard segments and create a pseudo-cross-linked polymer through hydrogen bonding.16 This makes elastane a flexible solid with molecular similarities to flexible polyurethane (PU) solids.
Potential recycling methods for elastane could be inspired by the current strive towards a circular PU economy.18–23 Existing recycling strategies for PU, such as mechanical reuse, have very limited applications for textile waste streams containing elastane, as considerable forces are needed to shred and unravel such elastic materials.11,24 On the other hand, chemical recycling by means of disassembly of the textile polymer to monomers could represent a potential solution.18,25 To date, only a few chemical recycling methods exist for PU deconstruction, including hydrolysis, aminolysis, acidolysis and glycolysis.26–34 However, the scientific literature is scarce when it comes to recycling of textile blends containing elastane, which could be due to issues regarding chemoselectivity or elevated reaction temperature. Nevertheless, a few strategies have been reported. Recently, the selective depolymerisation of a PET-elastane blended textile was revealed to be successful in a CH2Cl2/EtOH solvent system in the presence of KOH or in a THF/ethylene glycol (EG) solvent system with excess KOH to afford the EG and terephthalic acid components, leaving the elastane fibres intact (Scheme 1b, left).35,36 Thus, the remaining elastane could in theory be melted and extruded into new fibres if sufficient fibre quality can be preserved. Adding to this, Wang and co-workers later provided new insights into the alcoholysis of blended fabrics, showcasing the decomposition of elastane in PET glycolysis and showed how initial dissolution strategies could afford elastane, which could be depolymerised into 4,4′-methylenedianiline (4,4′-MDA), polyTHF and 2-imidazolidone by a glycolysis process.37
Dissolution represents a more established method for separating fibre blends, where certain fibres can be selectively solubilised by judicious choice of solvent.38–40 Elastane can accordingly be removed from either nylon or cotton fabrics by applying high-boiling polar amide solvents such as DMF (Scheme 1b, right).41–43 This technology represents a useful operation for the removal of elastane from major fibre components, which can subsequently be spun into new yarns and reused in fabrics. However, the removal of the generally undesirable DMF is energy intensive and the quality of the remaining fibre is often compromised as judged by the degree of depolymerisation and highly dependent on the choice of solvent.41
Our previous endeavours on depolymerisation of PU materials revealed tert-amyl alcohol with catalytic amounts of KOH as an efficient solvolysis medium.44,45 In those studies, we demonstrated the general value of the depolymerisation process for flexible foam-, rigid foam-, flexible solid- and rigid solid polyurethanes. Herein, we disclose our efforts to disassemble elastane by this process initially starting from virgin elastane, moving to elastane recovered by dissolution and finally directly from textile blends to assess the possibility of recovering the main textile component and isolating monomers suitable for repolymerization of elastane (Scheme 1b, bottom). As the solvolysis medium is mainly constituted by a weak nucleophile in the form of a tertiary alcohol, it was hypothesised that the chemoselectivity could leave functional groups of e.g. nylon and cotton unscathed despite elevated reaction temperatures.
Results and discussion
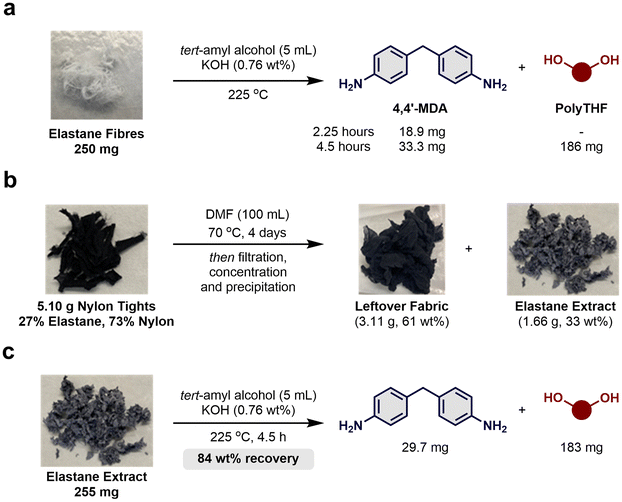
The study was initiated by performing the solvolysis on 250 mg of pure elastane fibre of an unknown composition using tert-amyl alcohol (5 mL) and KOH (1.9 mg) at 225 °C. Resorting to “back-of-the-envelope’ calculations, assuming a polyTHF content of 80–85 wt%, the theoretical yield of 4,4′-MDA from 250 mg elastane was estimated to be 28–37 mg (see ESI-4† for full details and calculations). With a reaction time of 2.25 h in tert-amyl alcohol and catalytic KOH, a GC yield of 18.9 mg of 4,4′-MDA was achieved, meaning that full depolymerisation was not achieved (Scheme 2a). Extension of the reaction time to 4.5 h resulted in increased formation of 4,4′-MDA and polyTHF with isolated yields of 33.3 mg and 186 mg, respectively, as an average of two separate reactions, giving an overall mass recovery of 86 wt%. It was noted that the reaction mixture contained small amounts of precipitate possibly originating from the presence of an anti-sticking agent (potentially magnesium stearate) on the elastane filament, which was not investigated further.46 Neither the diamine chain extender nor the terminating secondary amine was isolated due to the low boiling point (b.p.) of the suspected ethylenediamine (b.p. 117 °C) and diethylamine (b.p. 56 °C).47,48 It is likely that this result represents a full depolymerisation as prolonged reaction times did not increase the yield further.Next, dissolution of elastane from pieces cut from black nylon tights (73% PA, 27% elastane) obtained at a local shop was performed.41–43 From 5.10 g of the tights, 61 wt% of polyamide leftover fabric and 33 wt% of elastane extract were recovered (Scheme 2b). FTIR analysis suggested that the elastane had been effectively removed from the polyamide matrix (see ESI-9† for full details). The elastane extract from the dissolution was subjected to solvolysis using tert-amyl alcohol and a catalytic amount of base (Scheme 2c). After purification, the amine and polyTHF were isolated giving a total mass recovery of 84 wt%. Hence, elastane extracted from a polyamide matrix has been successfully depolymerised using solvolysis to afford its base chemicals. This result offers an opportunity for recycling elastane obtained from dissolution which is too damaged to be remoulded directly.
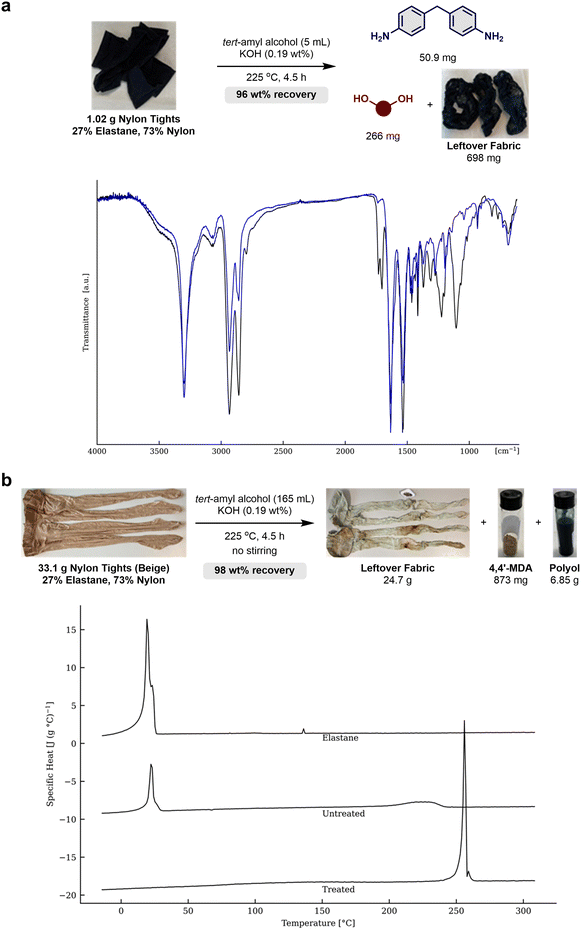
To avoid the need for isolation of elastane from a mixed fabric containing both nylon and elastane, the solvolysis methodology was applied directly on black nylon tights (Scheme 3a, top). Here, the solvolysis process afforded 4,4′-MDA, polyTHF and a leftover fabric with an overall 96% mass recovery. The fabric was investigated via FTIR and the combined transmission spectra of the nylon tights (black) and leftover fabric (blue) were analysed (Scheme 3a, bottom). The polyamide is characterised by N–H stretch at 3298 cm−1, C–H stretch at 2940 cm−1 and 2855 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at 1632 cm−1, N–H stretch at 1533 cm−1, C–N stretch at 1274 cm−1 and C–C stretch at 933 cm−1. Furthermore, elastane is characterised by C–H stretch at 2940 cm−1, 2855 cm−1 and 2799 cm−1, C
O stretch at 1632 cm−1, N–H stretch at 1533 cm−1, C–N stretch at 1274 cm−1 and C–C stretch at 933 cm−1. Furthermore, elastane is characterised by C–H stretch at 2940 cm−1, 2855 cm−1 and 2799 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at 1730 cm−1 and 1708 cm−1, C–O stretch at 1221 cm−1 and C–O–C stretch at 1104 cm−1. A mixture of nylon and elastane is found in the nylon tights, where only nylon is present in the leftover fabric. Hence, the polyamide remained unaltered, while the elastane fibres in the clothing material were depolymerised, and the elastane building blocks were isolated from the polyamide nylon tights.
O stretch at 1730 cm−1 and 1708 cm−1, C–O stretch at 1221 cm−1 and C–O–C stretch at 1104 cm−1. A mixture of nylon and elastane is found in the nylon tights, where only nylon is present in the leftover fabric. Hence, the polyamide remained unaltered, while the elastane fibres in the clothing material were depolymerised, and the elastane building blocks were isolated from the polyamide nylon tights.
The solvolysis process was further tested on two whole pairs of nylon tights (Beige) with a total weight of 33.1 g placed into a 390 mL autoclave (Scheme 4b, top). The stirring bar was omitted as the large fabric size would prevent efficient magnetic stirring. Using 0.19 wt% of KOH in 165 mL of tert-amyl alcohol, the leftover fabric was isolated by filtration and dried in vacuo providing 75 wt% recovered fabric. Here it is worth mentioning that the fabric closest to the bottom of the reactor had several spots of discolouring from overheating, likely due to the lack of stirring and contact of the fabric with the surface of the autoclave. By acid–base extractions, 4,4′-MDA (873 mg) and polyTHF (6.85 g) were separated summing up to a mass recovery of 23 wt% for the elastane components and a combined 98 wt% recovered material. The FTIR spectra of the nylon tights (Beige) before and after solvolysis were similar to those presented in Scheme 3a (see ESI-7† for full details). Differential scanning calorimetry (DSC) (Scheme 4b, bottom) indicates a crystalline peak for pure elastane at 20 °C. The nylon tights (untreated) show both a crystalline peak for elastane at 20 °C and a broad crystalline phase for the polyamide at 225 °C. Finally, the leftover fabric (treated) has no sign of elastane and the polyamide is capable of forming a crystalline phase at 255 °C. This indicates the successful removal of elastane and the polyamide fibre is intact without signs of degradation or contamination as the crystalline phase is uniform and narrow.
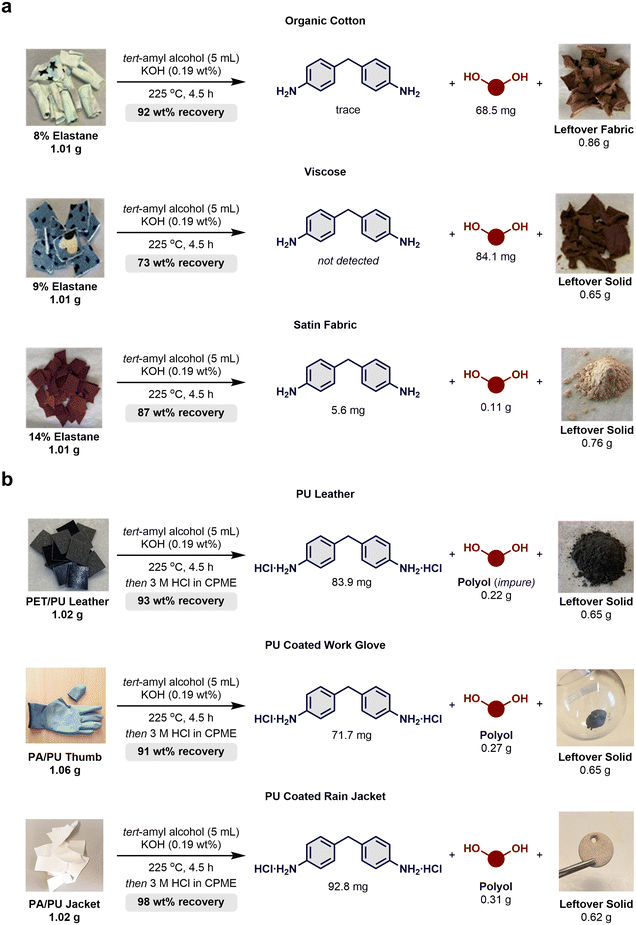
Next, fabrics mainly composed of cotton, viscose and polyester, were tested. The textiles were cut into squares and tested under the solvolysis conditions without any pretreatment. FTIR spectra of the fabrics were recorded before and after the reaction, but it was not possible to firmly conclude on the presence or removal of elastane due to larger overlapping signals (see ESI-10–12† for full details). Starting with organic cotton reported to contain 8 wt% elastane, it was possible to isolate 68.5 mg of polyTHF and discoloured pieces of leftover fabric (Scheme 4a, top). The diamine 4,4′-MDA was detected in trace amounts by 1H NMR analysis of the concentrated reaction mixture but the minute quantities were not isolated. For viscose, a cellulosic material containing 9% of elastane, 4,4′-MDA could not be detected, although 84.1 mg of polyTHF were isolated. Here, most of the discoloured leftover solids were physically fragmented into smaller pieces after solvolysis (Scheme 4a, middle). Next, a satin fabric composed of 86 wt% polyester and 14 wt% elastane was deconstructed into 4,4′-MDA, polyTHF and a polyester residue isolated as a powder (Scheme 4a, bottom). Soluble oligomers of polyester complicated the isolation of 4,4′-MDA and polyTHF, suggesting a lack of chemoselectivity as polyester was partially depolymerised during the solvolysis. To test this hypothesis, a sample of virgin polyester fibre was subjected to the solvolysis conditions affording 5.5 mg (2 wt%) of soluble polyester oligomers (see ESI-13† for full details). Thus, polyester degrades or partly solubilises under the standard solvolysis conditions leading to powdered polyester and soluble oligomers. As such, this showcases the lack of chemoselectivity for the solvolysis process when both carbamates and urea in elastane and esters in PET are present. From these findings, we can hypothesise that the high temperature applied in the solvolysis process may be detrimental to the structural integrity of viscose fibres and in particular polyester terephthalate. While the recovered cotton fabric appeared slightly weakened and brittle, the recovered PA66 from the tights qualitatively retained the properties of the clothing fabric.
With the finding that elastane could be removed directly from certain textiles, our attention moved to PU-coated textiles to explore if the solvolysis protocol could also depolymerise these PUs and offer useful remaining matrices. First, a sample of PU leather was tested. The base layer of the PU leather (rough side) was found to be polyester as deduced by FTIR analysis (see ESI-14† for full details). Although the synthetic leather is marketed as “100% PU,” this only applies to the smooth side of the fabric, while the rough side, at least for this sample, is based on polyester. After solvolysis, 4,4′-MDA could be precipitated by the addition of HCl from the crude mixture, and the polyol was thereby co-isolated with polyester oligomers (Scheme 4b, top). FTIR analysis revealed that elastane was not present in the leftover solids, and we speculate that the remaining black powder was a mixture of polyester and carbon black (see ESI-14† for full details).
Next, a pair of work gloves (brand name: HyFlex, PU coated polyamide) were acquired, and the thumb from one glove was subjected to the solvolysis conditions (Scheme 4b, middle). The leftover solid constituted a melted grey lump pointing to PA6 (m.p. = 218 °C). Following remelting to release the trapped solvent, FTIR analysis confirmed the removal of PU, while characteristic amide peaks were preserved (see ESI-15† for full details). The HCl-salt of 4,4′-MDA was isolated by precipitation in an average of 71.7 mg from two experiments. Besides the polyester polyol, the filtrate contained some silicone oil, potentially used as a surfactant during manufacturing. Nonetheless, a combined 91 wt% was recovered. Finally, a white waterproof workwear jacket (brand name: Flexothane Kleen, PU coated polyamide) was acquired and from the hood, samples were taken and applied to the solvolysis process (Scheme 4b, bottom). Here, the polyamide again appeared to be PA6 as the leftover solid was isolated as a grey crisp disc, which was isolated from the bottom of the reactor (see ESI-16† for full details). The 4,4′-MDA-salt and polyester polyol were recovered totalling a combined mass recovery of 98%. These results suggest that the tested method is applicable to PU leathers and PU coated fabrics, although the presence of polyester complicates purification. For nylon-based materials however, separation was straightforward and depending on the quality, the nylon may be respun to new fibres. To the best of our knowledge, no dissolution technique has been developed for any of these types of materials with the aim of recycling.
Conclusion
In summary, we have reported an approach for the selective depolymerisation of elastane and PU-coating in certain blended fabrics. Solvolysis with tert-amyl alcohol directly on the elastane-containing or PU-coated textile proved successful for polyamide textiles, while the chemical technology showed potential for other polymers such as cotton and viscose, but further optimization is needed to increase the selectivity of the disassembly process. Additionally, this solvolysis process represents an efficient option for the two-step process involving dissolution for fibre separation followed by chemical depolymerisation of elastane and can find use in the case where the quality of the elastane isolated from dissolution is low. As such, this work opens up the possibility of applying solvolysis for the removal and depolymerisation of elastane from fabrics for potential repolymerisation and use in new elastane fibres. Both material streams open up for the possibility of fibre-to-fibre recycling which in conjunction with other innovative methods might lower the degree of dependency on fossil fuels as a starting point for new fabric production as waste fractions can re-enter the value streams. Nonetheless, more data are needed to assess if the solvolysis process using tert-amyl alcohol and a catalytic amount of KOH is viable on scale, in particular with respect to energy consumption.Author contributions
M. B. J., B. S. D., S. K. K., and T. S. came up with the original idea. M. B. J., B. S. D., S. K. K., and T. S. conceptualised the strategy. M. B. J., B. S. D. and S. K. K. designed the experiments. M. B. J., B. S. D., and M. L. H. performed the experiments. M. B. J., B. S. D., M. L. H., S. K. K., and T. S. wrote the manuscript.Data availability
See the ESI† for further details.Conflicts of interest
The authors declare the following competing financial interest(s): Aarhus University is a co-owner of a patent (WO2023194469) covering the tert-amyl alcohol process for deconstruction of polyurethane related to the findings described in this manuscript.Acknowledgements
We deeply appreciate the financial support from the Innovation Fund Denmark (Grant No. 9069-00017B and 0177-00035B), the Carlsberg foundation (Grant No. CF18-1101), the Danish National Research Foundation (Grant No. DNRF118), the Novo Nordisk Foundation CO2 Research Center and Aarhus University. Furthermore, we are grateful to the RePURpose consortium for discussions and valuable input. Pernille Mørup and Lise Ladegaard Lind from Beirholm are acknowledged for providing both the elastane and the PET fibres.References
- A. Scott, C&EN, 2022, 100, 22–28 Search PubMed.
- Ellen MacArthur Foundation A New Textile Economy: Redesigning Fashion's Future. https://ellenmacarthurfoundation.org/a-new-textiles-economy (Accessed 09.08.2023).
- K. Niinimäki, G. Peters, H. Dahlbo, P. Perry, T. Rissanen and A. Gwilt, Nat. Rev. Earth Environ., 2020, 1, 189–200 CrossRef.
- C. Jönsson, R. Wei, A. Biundo, J. Landberg, L. S. Bour, F. Pezzotti, A. Toca, L. M. Jacques, U. T. Bornscheuer and P.-O. Syrén, ChemSusChem, 2021, 14, 4028–4040 CrossRef PubMed.
- P. Harmsen and H. Bos, Textiles For Circular Fashion: Part 1: Fibre Resources and Recycling Options, 2020 Search PubMed.
- P. Harmsen, M. Scheffer and H. Bos, Sustainability, 2021, 13, 9714–9731 CrossRef.
- E. Barnard, J. J. R. Arias and W. Thielemans, Green Chem., 2021, 23, 3765–3789 RSC.
- H. Li, H. A. Aguirre-Villegas, R. D. Allen, X. Bai, C. H. Ben-son, G. T. Beckham, S. L. Bradshaw, J. L. Brown, R. C. Brown, V. S. Cecon, J. B. Curley, G. W. Curtzwiler, S. Dong, S. Gaddameedi, J. E. García, I. Hermans, M. S. Kim, J. Ma, L. O. Mark, M. Mavrikakis, O. O. Olafasakin, T. A. Osswald, K. G. Papanikolaou, H. Radhakrishnan, M. A. Sanchez Castillo, K. L. Sánchez-Rivera, K. N. Tumu, R. C. Van Lehn, K. L. Vorst, M. M. Wright, J. Wu, V. M. Zavala, P. Zhou and G. W. Huber, Green Chem., 2022, 24, 8899–9002 RSC.
- J.-P. Lange, ACS Sustainable Chem. Eng., 2021, 9, 15722–15738 CrossRef CAS.
- European Commission: EU Strategy for Sustainable and Circular Textiles, 2022, https://environment.ec.europa.eu/document/download/74126c90-5cbf-46d0-ab6b-60878644b395_en?filename=COM_2022_141_1_EN_ACT_part1_v8.pdf (Accessed 09.08.2023).
- European Commission: Study on The Technical, Regulatory, Economic and Environmental Effectiveness of Textile Fibres Recycling, 2021, https://www.ecologic.eu/sites/default/files/publication/2022/50030-study-textile-recycling-web.pdf (Accessed 09.08.2023).
- M. Reisch, Chemical & Engineering News, 1999, 77(7), 70 Search PubMed.
- Fast Growth Looms for Spandex Fibers, Chemical & Engineering News, 1962, 40, 34–37 Search PubMed.
- M. F. Sonnenschein and W. Koonce, Polyurethanes, in Encyclopedia of Polymer Science and Technology, John Wiley & Sons, Inc., 2011 Search PubMed.
- M. F. Sonnenschein, Polyurethane Elastomers: Manufacture, Applications, Markets, and Trends, in Polyurethanes: Science, Technology, Markets, and Trends, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, 294–335 Search PubMed.
- K.-H. Wolf, M. Kausch, H. Schröer and M. Schweizer, Fibers, 6. Polyurethane Fibers, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley–VCH Verlag GmbH & Co. KGaA, 2011 Search PubMed.
- J. E. Boliek and S. A. Denney, Fibers, Elastomeric, in Encyclopedia of Polymer Science and Technology, John Wiley & Sons, Inc., 2002 Search PubMed.
- A. Kemona and M. Piotrowska, Polymers, 2020, 12, 1752 CrossRef CAS PubMed.
- N. V. Gama, A. Ferreira and A. Barros-Timmons, Materials, 2018, 11, 1841 CrossRef PubMed.
- D. Simón, A. M. Borreguero, A. de Lucas and J. F. Rodríguez, Waste Manage., 2018, 76, 147–171 CrossRef PubMed.
- J. Datta and P. Kopczyńska, Crit. Rev. Environ. Sci. Technol., 2016, 46, 905–946 CrossRef CAS.
- B. Liu, Z. Westman, K. Richardson, D. Lim, A. L. Stottlemyer, T. Farmer, P. Gillis, V. Vlcek, P. Christopher and M. M. Abu-Omar, ACS Sustainable Chem. Eng., 2023, 11, 6114–6128 CrossRef CAS.
- L. P. Fonseca, A. Duval, E. Luna, M. Ximenis, S. D. Meester, L. Avérous and H. Sardon, Curr. Opin. Green Sustainable Chem., 2023, 41, 100802 CrossRef.
- K. Ragaert, L. Delva and K. Van Geem, Waste Manage., 2017, 69, 24–58 CrossRef CAS PubMed.
- S. C. Kosloski-Oh, Z. A. Wood, Y. Manjarrez, J. P. de los Rios and M. E. Fieser, Mater. Horiz., 2021, 8, 1084–1129 RSC.
- L. R. Mahoney, S. A. Weiner and F. C. Ferris, Environ. Sci. Technol., 1974, 8, 135–139 CrossRef CAS.
- S. Motokucho, A. Yamaguchi, Y. Nakayama, H. Morikawa and H. Nakatani, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2004–2010 CrossRef CAS.
- N. Gama, B. Godinho, G. Marques, R. Silva, A. Barros-Timmons and A. Ferreira, Chem. Eng. J., 2020, 395, 125102 CrossRef CAS.
- N. Gama, B. Godinho, G. Marques, R. Silva, A. Barros-Timmons and A. Ferreira, Polymer, 2021, 219, 123561 CrossRef CAS.
- B. Godinho, N. Gama, A. Barros-Timmons and A. Ferreira, Sustainable Mater. Technol., 2021, 29, e00330 CrossRef CAS.
- M. Grdadolnik, A. Drincic, A. Oreski, O. C. Onder, P. Utrosa, D. Pahovnik and E. Zagar, ACS Sustainable Chem. Eng., 2022, 10, 1323–1332 CrossRef CAS PubMed.
- T. Vanbergen, I. Verlent, J. De Geeter, B. Haelterman, L. Claes and D. de Vos, ChemSusChem, 2020, 13, 3835–3843 CrossRef CAS PubMed.
- P. Zahedifar, L. Pazdur, C. M. L. Vande Velde and P. Billen, Sustainability, 2021, 13, 3583 CrossRef CAS.
- R. Heiran, A. Ghaderian, A. Reghunadhan, F. Sedaghati, S. Thomas and A. H. Haghighi, J. Polym. Res., 2021, 28, 22 CrossRef CAS.
- S. Zhang, W. Xu, R. Du, W. An, X. Liu, S. Xu and Y.-Z. Wang, J. Chem. Eng., 2023, 144032, DOI:10.1016/j.cej.2023.144032.
- S. Zhang, W. Xu, R. Du, X. Zhou, X. Liu, S. Xu and Y.-Z. Wang, Green Chem., 2022, 24, 3284–3292 RSC.
- W.-H. Xu, L. Chen, S. Zhang, R.-C. Du, X. Liu, S. Xu and Y.-Z. Wang, Green Chem., 2023, 25, 245–255 RSC.
- Y.-B. Zhao, X.-D. Lv and H.-G. Ni, Chemosphere, 2018, 209, 707–720 CrossRef CAS PubMed.
- A. Mäurer, M. Schlummer and O. Beck, US8138232B2, 2012.
- A. Mäurer and M. Schlummer, EP2513212B1, 2021.
- J. E. G. Van Dam, J. R. I. Knoop and M. J. A. Van den Oever, WO2020130825A1, 2020.
- C. Gong, K. Zhang, C. Yang, J. Chen, S. Zhang and C. Yi, Text. Res. J., 2021, 91, 18–27 CrossRef CAS.
- Y. Yin, D. Yao, C. Wang and Y. Wang, Text. Res. J., 2014, 84, 16–27 CrossRef.
- M. B. Johansen, B. S. Donslund, S. K. Kristensen, A. T. Lindhardt and T. Skrydstrup, ACS Sustainable Chem. Eng., 2022, 10, 11191–11202 CrossRef CAS.
- M. B. Johansen, B. S. Donslund, E. Larsen, M. B. Olsen, J. A. L. Pedersen, M. Boye, J. K. C. Smedsgård, R. Heck, S. K. Kristensen and T. Skrydstrup, ACS Sustainable Chem. Eng., 2023, 11, 10737–10745 CrossRef CAS.
- R. S. Hanzel and P. J. Sauer, US4296174A, 1981.
- P. Roose, K. Eller, E. Henkes, R. Rossbacher and H. Höke, Amines, Aliphatic, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2015 Search PubMed.
- P. Roose and M. G. Turcotte, Amines, Lower Aliphatic, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., 2016 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc02994h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |