Open Access Article
Open Access ArticleRecent advances in nanoflowers: compositional and structural diversification for potential applications
Su Jung
Lee
 a,
Hongje
Jang
a,
Hongje
Jang
 *b and
Do Nam
Lee
*b and
Do Nam
Lee
 *a
*a
aIngenium College of Liberal Arts (Chemistry), Kwangwoon University, Seoul 01897, Korea. E-mail: donamlee@hanmail.net
bDepartment of Chemistry, Kwangwoon University, Seoul 01897, Korea. E-mail: hjang@kw.ac.kr
First published on 4th September 2023
Abstract
In recent years, nanoscience and nanotechnology have emerged as promising fields in materials science. Spectroscopic techniques like scanning tunneling microscopy and atomic force microscopy have revolutionized the characterization, manipulation, and size control of nanomaterials, enabling the creation of diverse materials such as fullerenes, graphene, nanotubes, nanofibers, nanorods, nanowires, nanoparticles, nanocones, and nanosheets. Among these nanomaterials, there has been considerable interest in flower-shaped hierarchical 3D nanostructures, known as nanoflowers. These structures offer advantages like a higher surface-to-volume ratio compared to spherical nanoparticles, cost-effectiveness, and environmentally friendly preparation methods. Researchers have explored various applications of 3D nanostructures with unique morphologies derived from different nanoflowers. The nanoflowers are classified as organic, inorganic and hybrid, and the hybrids are a combination thereof, and most research studies of the nanoflowers have been focused on biomedical applications. Intriguingly, among them, inorganic nanoflowers have been studied extensively in various areas, such as electro, photo, and chemical catalysis, sensors, supercapacitors, and batteries, owing to their high catalytic efficiency and optical characteristics, which arise from their composition, crystal structure, and local surface plasmon resonance (LSPR). Despite the significant interest in inorganic nanoflowers, comprehensive reviews on this topic have been scarce until now. This is the first review focusing on inorganic nanoflowers for applications in electro, photo, and chemical catalysts, sensors, supercapacitors, and batteries. Since the early 2000s, more than 350 papers have been published on this topic with many ongoing research projects. This review categorizes the reported inorganic nanoflowers into four groups based on their composition and structure: metal, metal oxide, alloy, and other nanoflowers, including silica, metal–metal oxide, core–shell, doped, coated, nitride, sulfide, phosphide, selenide, and telluride nanoflowers. The review thoroughly discusses the preparation methods, conditions for morphology and size control, mechanisms, characteristics, and potential applications of these nanoflowers, aiming to facilitate future research and promote highly effective and synergistic applications in various fields.
1. Introduction
Dr Richard Feynman introduced the idea and concepts of nanotechnology in his talk titled “There's Plenty of Room at the Bottom” at the American Physical Society's annual meeting in 1959, initiating the exploration of nanoscience and nanotechnology as new materials-science fields.1 In the 1980s, various spectroscopic techniques, such as scanning tunneling microscopy (STM)2–4 and atomic force microscopy (AFM),5,6 were developed for the characterization and tuning of nanomaterials, facilitating the investigation of various types of nanomaterials, such as fullerenes,7,8 graphene,9,10 nanotubes,11,12 nanofibers,13,14 nanorods,15,16 nanowires,17,18 nanoparticles,19,20 nanocones,21,22 and nanosheets.23,24 Recently, the applications of these nanomaterials have expanded to encompass a wide range of commercial fields, including electronics, energy-related fields, cosmetics, and biomedicine.25Among them, flower-shaped hierarchical 3D nanostructures, called nanoflowers,26 have attracted immense attention because of their higher surface-to-volume ratio compared to that of spherical nanoparticles, along with their low cost and eco-friendly preparation methods. Additionally, they enhance the stability of many catalytic reactions by the immobilization of enzymes and proteins.27–31 Numerous publications report the potential utilities of these 3D nanostructures with peculiar morphologies, which are fundamentally based on nanoflowers. As we have explained in the previous paper, nanoflowers can be classified according to their compositions: inorganic, organic, and organic–inorganic hybrid.32 First, inorganic nanoflowers are defined as being composed only of inorganic materials such as metals, metal oxides, alloys, and metalloids.33–36 In addition, as introduced later in this paper, metalloid, carbon, nitride, sulfide, phosphide, selenide, and telluride coated or doped inorganic materials are also defined as inorganic nanoflowers.37–44 Second, nanostructures that include inorganic elements as part of a medium composed of only organic molecules or in which organic molecules are the main components are called organic nanoflowers.45–47 Reported findings have indicated that organic nanoflowers, characterized by an open structure, provide a greater number of accessible active sites and enable easy movement of ions.48 Additionally, the intersecting extended nanopetals within these structures promote the efficient transportation of electrons. These nanoflowers have demonstrated promising outcomes in applications related to energy storage and electrocatalysis.48,49 For example, one-pot synthesis of polyacrylonitrile (PACN) and its copolymer nanostructured particles with various superstructures (flower, pompom, hairy leave, and petal shapes) has been reported. PACN flowers (PACN-F) are prepared with acrylonitrile monomer (ACN) in acetone through free radical polymerization initiated by azobis(isobutyronitrile) (AIBN). Through stabilization and carbonization steps, these PACN flowers are converted to carbon flowers. The morphology is controlled by the incorporation of different co-monomers or by employing various solvents. These carbon flower materials may be applied as promising candidates for various energy, environment, or electronic applications.48 Zheng's group introduced nitrogen, phosphorus, and fluorine doped carbon NFs (NPF@CNFs) by ultrasound-induced polycondensation and pyrolysis. The synthesized NPF@CNF exhibited enhanced activity (E1/2 = 0.85 V (oxygen reduction reaction, ORR), Ej=10 = 1.56 V (oxygen evolution reaction, OER) vs. RHE) and cycling stability (retention above 90% after cycling for 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s) as a bifunctional electrocatalyst toward the ORR and OER during the discharging and charging process of zinc–air batteries, which is even superior to those of commercial Pt/Ir-based catalysts.50 Third, hybrid nanoflowers are described as all components of inorganic nanostructures associated with organic materials.51 Hybrid nanoflowers, which combine biomolecules and inorganic components, have found extensive applications in biosensors, bioassays, biocatalysis, biomedicine, and diagnostics.52–55 This is attributed to their straightforward synthesis, remarkable effectiveness, and ability to improve enzyme stability.52 The fabrication process of hybrid organic–inorganic nanoflowers under mild conditions involves utilizing copper(II) ions as the inorganic element and natural amino acids as the organic element. Asparagine (Asn)-incorporated nanoflowers with diameters in the range of 10–15 μm closely resemble the shape of Dahlia in nature and have hierarchical structures with high surface-to-volume ratios. The nanoflowers have a Brunauer–Emmett–Teller (BET) surface area of 32.0 m2 g−1 and an average pore size of 112.5 nm. The nanoflowers incorporating amino acids exhibit catalytic activity based on a reaction principle like Fenton's reagents. The presence of copper ions enables self-assembly between amino acids and copper phosphate, resulting in intrinsic peroxidase-like activity in the amino acid-incorporated nanoflowers. These amino acid–inorganic hybrid nanoflowers are expected to have significant applications in biosensors, bioanalytical devices, pharmaceuticals, and industrial biocatalysis.56 In particular, inorganic nanoflowers are attracting researchers' attention because of their catalytic efficiency and optical characteristics, depending on the composition, crystal structure, and local surface plasmon resonance (LSPR).32 Thanks to these properties, inorganic nanoflowers are widely used in electro, photo, and chemical catalysts, sensors, supercapacitors and batteries. Moreover, in the synthesis of inorganic nanoflowers, as will be discussed in detail later, the availability of diverse synthesis methods and the ability to regulate reaction conditions make it easier to achieve precise control of form and composition. However, there have been very few reviews of inorganic nanoflowers, and this is the first review of inorganic nanoflowers for catalyst, sensor, supercapacitor, and battery applications.
000 s) as a bifunctional electrocatalyst toward the ORR and OER during the discharging and charging process of zinc–air batteries, which is even superior to those of commercial Pt/Ir-based catalysts.50 Third, hybrid nanoflowers are described as all components of inorganic nanostructures associated with organic materials.51 Hybrid nanoflowers, which combine biomolecules and inorganic components, have found extensive applications in biosensors, bioassays, biocatalysis, biomedicine, and diagnostics.52–55 This is attributed to their straightforward synthesis, remarkable effectiveness, and ability to improve enzyme stability.52 The fabrication process of hybrid organic–inorganic nanoflowers under mild conditions involves utilizing copper(II) ions as the inorganic element and natural amino acids as the organic element. Asparagine (Asn)-incorporated nanoflowers with diameters in the range of 10–15 μm closely resemble the shape of Dahlia in nature and have hierarchical structures with high surface-to-volume ratios. The nanoflowers have a Brunauer–Emmett–Teller (BET) surface area of 32.0 m2 g−1 and an average pore size of 112.5 nm. The nanoflowers incorporating amino acids exhibit catalytic activity based on a reaction principle like Fenton's reagents. The presence of copper ions enables self-assembly between amino acids and copper phosphate, resulting in intrinsic peroxidase-like activity in the amino acid-incorporated nanoflowers. These amino acid–inorganic hybrid nanoflowers are expected to have significant applications in biosensors, bioanalytical devices, pharmaceuticals, and industrial biocatalysis.56 In particular, inorganic nanoflowers are attracting researchers' attention because of their catalytic efficiency and optical characteristics, depending on the composition, crystal structure, and local surface plasmon resonance (LSPR).32 Thanks to these properties, inorganic nanoflowers are widely used in electro, photo, and chemical catalysts, sensors, supercapacitors and batteries. Moreover, in the synthesis of inorganic nanoflowers, as will be discussed in detail later, the availability of diverse synthesis methods and the ability to regulate reaction conditions make it easier to achieve precise control of form and composition. However, there have been very few reviews of inorganic nanoflowers, and this is the first review of inorganic nanoflowers for catalyst, sensor, supercapacitor, and battery applications.
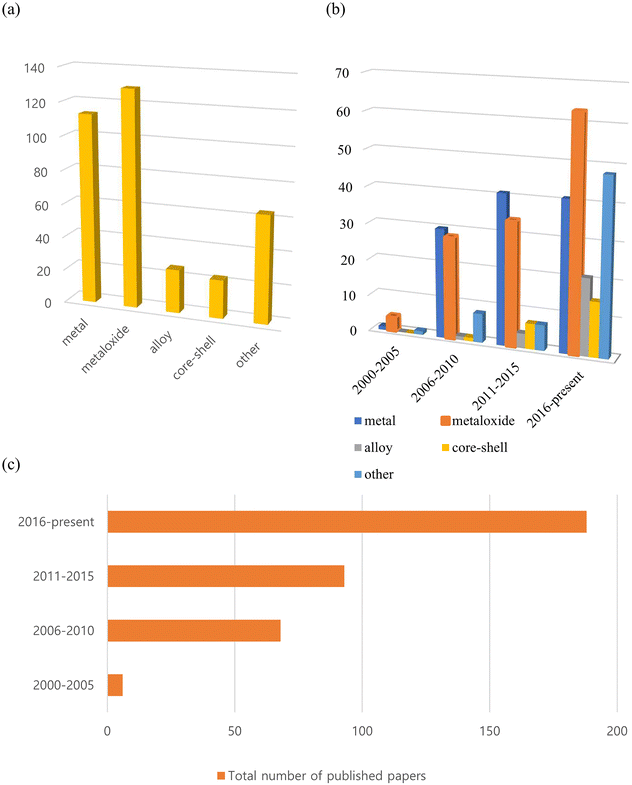
To the best of our knowledge, more than 350 such reports on inorganic nanoflowers have been published since the early 2000s, with an increasing number of studies being published currently. As shown in Fig. 1, many of the published papers are on metal and metal oxide nanoflowers, with the number of papers on metal oxides increasing rapidly. Additionally, numerous studies on alloys, core–shell structures, doped systems, and so on, have been published recently. They investigate the preparation method, morphology, size-control conditions, characteristics, and potential applications of nanoflowers composed of metals, metal oxides, alloys, core–shell, and doped systems. Recent research has primarily focused on optimizing their morphologies and composition ratios for practical applications.
In this review, nanoflowers have been categorized as metal, metal oxide, alloy, and other nanoflowers, depending on their compositions. Synthetic methodologies, experimental parameters' effects on the morphology and size, mechanisms, physical, chemical, and biological properties derived from the physical properties of inorganic materials, synergistic effects among composition ratios, morphologies, and structural effects, with regard to potential applications as photocatalysts,57 electrocatalysts,58 anode materials for lithium-ion storage,59 supercapacitor electrodes,60 and gas sensors61 are primarily considered.
2. Methods for preparing nanoflowers
Continuous research efforts have been made to develop new synthesis methods for the nanoflowers.62–66 According to Kulkarni's book: Nanotechnology: Principles and Practices, nanomaterials are prepared by four types of methods, namely, physical, chemical, biological, and hybrid.67 The preparation of inorganic nanoflowers also uses the same or modified technology as the conventional nanomaterial synthesis technology, as depicted in Fig. 2.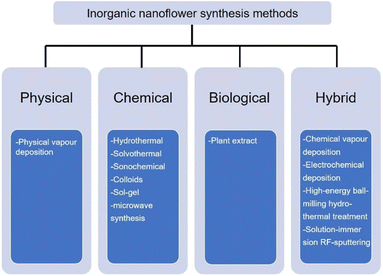 | ||
| Fig. 2 Commonly used synthesis technology of inorganic nanoflowers: physical, chemical, biological, and hybrid. | ||
First, the physical technique is mainly represented by physical vapour deposition in the inorganic nanoflower synthesis. For example, Bi2S3 nanoflowers are prepared on a Si substrate via a simple vapour deposition method.68 The morphology of the Bi2S3 nanoflowers is modulated by tuning the partial pressure of the reactant. The second technology is the chemical synthesis method, such as hydrothermal,69 solvothermal,70 sonochemical,35 colloids,71 sol–gel72 and microwave synthesis.73 TiO2 nanoflowers are synthesized by a hydrothermal method.74 The solvothermal reaction is used to synthesize Pd nanoflowers in the presence of oleic acid.75 IrCo and IrNi nanoflowers are ultrasonicated with IrCl3·xH2O, Co(acac)2 or Ni(acac) and oleylamine and heated in a hot oil bath.35 Hierarchical NiCo2O4 nanoflowers are synthesized using the microwave synthesis method.73 Third, the biological strategy is an environmentally benign and biomimetic approach using natural material extracts, such as Kalanchoe daigremontiana,76Dodonaea angustifolia,77Ocimum sanctum (Tulsi) leaves,78 and Azadirachta indica leaves,79 without utilizing any toxic materials.
The last synthesis technology is the hybrid synthesis strategy that combines physical, chemical, and biological methods: chemical vapour80 and electrochemical81 deposition, high energy ball-milling hydrothermal treatment,60 and the solution-immersion RF-sputtering method.82
As we introduced above, inorganic nanoflower synthesis can utilize various techniques and methods. This can be used differently depending on the material and application of the nanoflower to be synthesized. We will illustrate some commonly used nanoflower synthesis methods with examples for each.
2.1. Physical method
For instance, Yu et al. have described a vapor deposition technique for the synthesis of exquisite Bi2S3 nanoflowers on a silicon substrate.68 The researchers utilize a horizontal tube furnace for the vapor deposition process. Bi2S3 powder and S powder are used as the starting materials. In the furnace tube, a ceramic boat containing Bi2S3 powder is positioned at the center, while sulfur powder is placed between the Bi2S3 and the gas inlet. Silicon wafers are positioned downstream, a few centimeters away from the Bi2S3 powder. The tube is tightly sealed and flushed with high purity argon gas. The temperature is then gradually increased to 650 °C at a heating rate of 8 °C min−1 and maintained at that temperature for 2 hours. Subsequently, the temperature is gradually decreased to room temperature. Throughout the thermal treatment, argon gas is continuously allowed to flow at approximately 100 sccm (standard cubic centimeter per minute) to provide both protection and carrier gas functions. Additionally, they have demonstrated the ability to adjust the shape of the Bi2S3 nanostructures, ranging from flower-like structures to bundles of nanorods, by controlling the partial pressure of the reactants.
There is a need for research aimed at exploring novel methods and improving existing PVD approaches to achieve precise control over the formation and arrangement of nanoflowers. This involves investigating various parameters such as deposition conditions, precursor materials, and substrate properties to optimize PVD processes specifically tailored for nanoflower growth.
2.2. Chemical method
| Nanostructure/morphology | Size | Reaction conditions | Time and temperature in the autoclave | Surfactant or template | Ref. |
|---|---|---|---|---|---|
| TiO2 nanoflowers on a Ti substrate | 360–420 nm | 10 mL H2O2 (37%) + 60 mL NaOH (1 M) | 5 h, 120 ± 2 °C | — | 92 |
| Anatase and brookite TiO2 nanoflowers | — | 7.8 mL tetrabutyl titanate (TBOT) + 1.10 g NaCl + 68 mL aqueous NH3·H2O | 24 h, 180 °C | — | 93 |
| ZnO nanoflowers | 3 μm | 0.549 g, C4H6O4Zn·2H2O (2.5 mmol) + 0.735 g, sodium citrate (2.5 mmol)+ 4 mL NaOH (4.0 M) | 8 h, 120 °C | — | 94 |
| ZnO nanoflowers | 4 μm | (Zn(CH3COOH)2_2H2O) + (1 mM) + NaOH (10 mM) | 8 h, 140 °C | PEG-20000 | 95 |
| Porous NiO nanoflowers | 15.0 (PEG), 16.7 (PVP) nm | 2.9080 g of Ni(NO3)2·6H2O or 2.4884 g of Ni(CH3COO)2·4H2O + HMTA solution (0.5 M) | 6 h, 110 °C | PEG, PVP | 96 |
| Sparse-type and close-type NiO nanoflowers | 5 (CTAB), 2 (SDS) μm | 0.475 g NiCl2·6H2O + 0.300 g urea | 8 h, 160 °C | CTAB, SDS | 97 |
| CuO nanoflowers | 1 μm | 6 mL NH3·H2O (1 M) + 10 mL Cu(NO3)2 (0.01 M) | 6 h, 100 °C | — | 98 |
| Flower-like CuO nanostructures with porous nanosheets on the alumina tube | — | 0.3 M Cu(CH3COO)2·H2O + 25 mL NH3·H2O (17 M) + 1.0 g NaOH | 24 h, 180 °C | — | 99 |
| Nanostructure/morphology | Size | Reaction conditions | Time and temperature in the autoclave | Surfactant or template | Ref. |
|---|---|---|---|---|---|
| TiO2 nanoflowers | 47.56–274.36 nm | NaOH (0.024 M) + 8 mL TiCl3 + 60 mL ethanol | 18 h, 130 °C | — | 34 |
| Porous NiO nanoflowers | — | 0.5 g Ni(ac)2·4H2O + 0.25 g urea + 30 mL ethanol | 3–24 h, 190 °C | — | 100 |
| Zn-doped CuS nanoflowers | 1 μm | 0.25 mmol CuCl + 0.25 mmol Zn(Ac)2·2H2O + 0.75 mmol S powder + 15 mL ethanol | 24 h, 180 °C | — | 101 |
| Mo-doped Bi2Se3 nanoflowers | 100–200 nm | 0.85 mmol Bi(NO3)3·5H2O + 0.15 mmol (NH4)6Mo7O24·4H2O+ 18 mL N,N-dimethylformamide + ammonia + Se powder + Na2SO3 | 20 h, 160 °C | — | 102 |
| Bi2O2.33 nanoflowers | — | 0.11 g NaBiO3 + 20 mL ethanol, + 5.0 mL distilled water + 2.0 mL 1 M HNO3 solution | 3–5 h, 100 °C | PVP | 103 |
For instance, in the first step, 5 mM of Cu(NO3)2·3H2O powder is dissolved in 100 mL of deionized water. In the second step, a 1 mM aqueous solution of hexamethylenetetramine is mixed with 100 mL of deionized water. In the third step, the solutions from steps 1 and 2 are combined and 1 mL of 30% NaOH is added. The resulting solution is continuously stirred for 60 minutes at room temperature. Subsequently, the suspended mixture is transferred into an autoclave and securely sealed. The autoclave is then placed in an oven and maintained at 110 °C for 3 hours. After completing the entire process and allowing the oven to cool to room temperature, the resulting solution is centrifuged and washed with deionized water. Finally, the product, characterized by its black color (CuO nanoflowers), is obtained after drying in a vacuum oven at 60 °C for 2 hours.86
In recent studies, it has been frequently reported that a combination of the hydrothermal method with other synthesis techniques is employed to synthesize nanoflowers with diverse structures. Qu et al. reported a novel approach for the controlled synthesis of three types of 3D fluffy ZnO nanoflowers with distinct nanostructures. This method involves a simple ultrasonic treatment followed by a hydrothermal process.87 One type, denoted as ZnO-0, is directly formed through the hydrothermal reaction without prior ultrasonic treatment, resulting in smooth edges. The other two types, namely ZnO-250 and ZnO-950, are obtained by subjecting the samples to ultrasonic treatment at 250 W and 950 W, respectively, prior to the hydrothermal process, resulting in jagged margins. The experimental findings revealed that increasing the intensity of ultrasonic treatment leads to a decrease in the size, specific surface area, crystallite dimension, intrinsic donor defects, and the signals of reactive radicals in the ZnO nanoflowers.
Here is an overview of the experimental procedure for synthesizing MoSe2 nanoflowers using the colloidal method: in a 100 mL three-neck flask, a mixture of 20 mg (0.1 mmol) of Na2MoO4, 8 mL of oleic acid, and 2 mL of 1-octylamine is prepared and degassed under vacuum at 120 °C for approximately 10 minutes. The reaction vessel is then purged with Ar(g) and slowly heated to around 240 °C at a rate of approximately 5 °C per minute, resulting in the formation of a clear, dark, red-brown solution. Subsequently, 2 mL of the ODE-Se stock solution (0.1 M), which is 1-octadecene and Se solution, is continuously injected at a rate of 0.1 mL min−1 using a syringe pump. After the injection is completed, the solution is aged for an additional 30 minutes at a final temperature of approximately 300 °C. The reaction mixture is then rapidly cooled by removing the flask from the heating mantle. The resulting MoSe2 particles are precipitated by adding 10 mL of toluene and 10 mL of ethanol, followed by centrifugation. The black precipitate is washed three times using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of toluene and ethanol (with centrifugation steps in between washes), and finally suspended in ethanol to form a dark purple colloidal suspension.88
1 mixture of toluene and ethanol (with centrifugation steps in between washes), and finally suspended in ethanol to form a dark purple colloidal suspension.88
For example, 40 mmol of Ni(NO3)2·6H2O is dissolved in 50 mL of distilled water. Subsequently, 5 wt% of the cationic surfactant cetyltrimethyl ammonium bromide (CTAB) is added, and then 3 mL of ammonia is introduced into the solution. The resulting solution is stirred for 10 minutes. The solution, contained within a polypropylene-capped autoclave container, is subjected to microwave irradiation at a power of 300 watts for a duration of 15 minutes. The green precipitates obtained are washed multiple times with distilled water and ethanol to eliminate soluble impurities. Subsequently, the precipitates are dried in a hot oven at 80 °C for 12 hours to obtain Ni(OH)2 samples. The as-prepared powder samples are then subjected to calcination in air at 400 °C for 2 hours to yield NiO nanoflowers.105 The researcher also employed a hydrothermal method using the same amount of precursor, surfactant, and ammonia, with the reaction conducted at 140 °C for 15 minutes. In scanning electron microscopy (SEM) analysis, the microwave treatment process results in the formation of nanostructures that exhibit the same flower-like morphologies but with a higher degree of agglomeration compared to the samples grown through the hydrothermal method. This can be attributed to the indirect heating of molecules through microwave radiation.105
2.3. Biological method
Ag nanoflowers are obtained using natural materials extract: Kalanchoe daigremontiana, as a natural reducing agent.76 The green synthesis of nanospindles of CuO and their subsequent assembly into nanoflowers has been successfully achieved by modifying the solvents used, such as water and ethanol, in combination with the extract obtained from Dodonaea angustifolia.77 Also, CuO nanoflowers are synthesized using eugenol (4-allyl-2-methoxyphenol), which is extracted from the leaves of Ocimum sanctum (Tulsi), serving as a natural reducing agent.78 A simple, environmentally friendly, biomimetic method is employed to fabricate Au/ZnO hybrid nanoflowers using Azadirachta indica (neem) leaf extract as both a reducing agent and a capping agent.79
Upon closer examination of one of these methods, the synthesis of ZnO nanoflowers has been achieved by employing Withania coagulans extract as a reducing agent. For the synthesis of ZnO nanoflowers, a solution is prepared by dissolving 0.2 g of zinc acetate in 50 mL of distilled water. The pH of the solution is adjusted to 12 using a 2 M solution of NaOH. Subsequently, 5 mL of Withania coagulans plant extract is added to the solution, which is then heated at 90 °C for 5 hours with continuous stirring. To obtain the final product, the reaction mixture is subjected to centrifugation at 6000 rpm for 15 minutes. The resulting ZnO nanoflowers are collected and further incubated at 37 °C overnight.108
2.4. Hybrid method
A simple and reproducible technique for producing 3D Pt nanostructures on silicon substrates has been reported at ambient temperature using potentiostatic pulse plating.111 An aqueous solution containing 1 M H2PtCl6 and 1 M H2SO4 is mixed and stirred for 5 hours at room temperature. The Pt catalyst is then electrodeposited onto a flat silicon substrate using potentiostatic pulse plating in a three-electrode cell system with a saturated calomel reference electrode (SCE). The positive potential pulse (+0.05 V) lasted for 5 ms, while the negative potential pulse (−0.02 V) lasted for 1 ms. This bipolar pulse electrodeposition method facilitates the synthesis of 3D Pt nanoflowers on the Si substrate. Following Pt electrodeposition, the sample is washed with deionized (DI) water to remove surface contamination and dried under ambient conditions.
Also, the galvanic replacement method is used to create monomorphic single-crystalline Pt nanoflowers by transforming Te nanowires into a fully-formed Pt nanostructure.112 The template-free ultrasonic electrodeposition method is employed to produce Pt nanoflowers directly on the bare Au electrodes.110 This method is utilized for synthesizing not only Pt nanoflowers but also Ag, Pd, AuPt, and AuAgCu nanoflowers.113–116
3. Experimental parameters' effect
The morphology of nanoflowers plays a crucial role in various applications and scientific studies. The unique and intricate structures of nanoflowers offer distinct advantages: a large surface area-to-volume ratio and enhanced catalytic properties. By understanding the morphology and controlling the growth conditions and parameters, researchers can manipulate the size, shape, and arrangement of nanoflower structures, leading to improved performance in sensing devices, energy storage systems, drug delivery platforms, and more. Here, we aim to introduce how various experimental conditions can influence the size and shape of nanoflowers, using several examples.Porous nanoflower- and nanourchin-like structures are synthesized using the hydrothermal method, employing either hexamethylenetetramine (HMTA) or urea as a precipitant, with or without the addition of surfactants such as poly(ethylene glycol) (PEG) or poly(vinyl pyrrolidone)(PVP).96 Based on the information provided in Table 3, it can be observed that NiO precursors synthesized using HMTA as a precipitant exhibited a morphology resembling nanoflowers, while those prepared with urea as a precipitant displayed a shape similar to that of nanourchins. NiO-HMTA, NiO-HMTA-PEG, NiO-HMTA-PVP, and NiO-urea samples exhibit a high surface area ranging from 31 to 66 m2 g−1. Also, NiO-HMTA-PVP possesses the highest BET surface area and pore volume. As a highly active catalyst, NiO-HMTA-PVP exhibits the highest surface area, highest concentration of oxygen adspecies (Oads), and the best reducibility. It demonstrates superior catalytic performance with a T50% of 253 °C and T90% of 266 °C.96
| Sample code | Precipitating agent | Ni/precipitating agent molar ratio | Surfactant | Crystal phase | Crystallite sizea (nm) | Morphology | BET surface area (m2 g−1) | Average pore size (nm) | Pore volume (cm3 g−1) |
|---|---|---|---|---|---|---|---|---|---|
| a The crystallite sizes are calculated according to Scherrer's equation using the FWHM of the (200) line of the XRD patterns. | |||||||||
| NiO-HMTA | HMTA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | Cubic | 19.1 | Irregular nanoflower-like spheres | 31.5 | 26.4 | 0.202 |
| NiO-HMTA-PEG | HMTA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PEG | Cubic | 15.0 | Nanoflower-like spheres | 56.1 | 26.2 | 0.296 |
| NiO-HMTA-PVP | HMTA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PVP | Cubic | 16.7 | Nanoflower-like spheres | 66.3 | 22.9 | 0.414 |
| NiO-urea | Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | Cubic | 25.2 | Nanourchin-like spheres | 30.5 | 9.6 | 0.103 |
| NiO-bulk | — | — | — | Cubic | 78.6 | — | 5.6 | — | — |
Sparse-type and close-type NiO nanoflowers are successfully synthesized using a hydrothermal process with the assistance of CTAB and sodium dodecyl sulfate (SDS) as surfactants, respectively. The performance of the close-type NiO nanoflowers is found to be superior, and this can be attributed to several factors. Firstly, the close-type nanoflower architecture exhibits a higher degree of porosity and compactness. Additionally, there are more nanojunctions between adjacent nanosheets, creating a well-connected structure. Furthermore, the ultrathin nanosheets assemble abundant micro reaction rooms. Finally, the thickness of the petal structures is smaller than twice the Debye length.97
In another study, in the hydrothermal synthesis, nanorods and nanoneedles assembled NiO nanoflowers are synthesized using different surfactants, namely CTAB and ethylene glycol (EG), respectively. Based on TEA and SEM analysis, it is determined that the nanorods have an average diameter of approximately 900 nm and a length of approximately 6 μm. On the other hand, the nanoneedles exhibit a thicker structure at the roots with sharper emanative ends. Each needle has a length of around 2.5 μm and a diameter of approximately 80 nm at the middle.118
Borbón et al. conducted a hydrothermal study to examine how the reaction time affects the size and morphology of ZnO nanoflowers. They observe that well-defined flower-like structures are formed within 5 minutes of the reaction. Regardless of the reaction time (10, 15, and 30 minutes), the nanoflowers exhibit similar high-density morphologies. The dimensions of the petals in each nanoflower increase as the crystal growth continued over time, with the nanorods becoming wider and larger (Table 4). However, between 10 and 15 minutes, there is no significant change observed. The research also showed a decrease in sheet resistance from 38.5 MΩ sq−1 at 5 minutes to 0.00344 MΩ sq−1 at 30 minutes. The reduction in the resistivity of the samples could be attributed to a decrease in crystal defects and an increase in crystallite size as the reaction time increased, as confirmed by XRD results. This suggests that the electrical resistance can be adjusted by varying the reaction time.119
| Reaction time (min) | Crystallite size (nm) | Petal width (nm) | Petal length (nm) | Single flower length (nm) | Band-gap energy (eV) | Sheet resistance (MΩ sq−1) |
|---|---|---|---|---|---|---|
| 05 | 14.73 | 288 ± 27 | 714 ± 81 | 1523 ± 151 | 3.21 | 38.5000 |
| 10 | 22.22 | 345 ± 45 | 740 ± 90 | 1674 ± 180 | 3.27 | 18.5000 |
| 15 | 24.11 | 327 ± 48 | 772 ± 57 | 1653 ± 154 | 3.10 | 0.01020 |
| 30 | 24.06 | 370 ± 50 | 772 ± 85 | 1785 ± 150 | 3.28 | 0.00344 |
A hydrothermal method is employed to synthesize well-defined and uniformly sized spherical mesoporous nanoflowers. The synthesis process involves using a solvent mixture of cyclohexane and water, with cetylpyridinium bromide (CPB) acting as the template, tetraethyl orthosilicate (TEOS) as the source of inorganic silica, and urea as the hydrolysis additive. The study aims to investigate the effects of solvent composition (Vcyclohexane/Vwater), hydrothermal temperature, and Si to CPB molar ratio on the morphology and structure of the nanoflowers.120
First, various solvent compositions are examined, including higher cyclohexane content (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and lower cyclohexane content (5
1) and lower cyclohexane content (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 1
7, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Results show that a higher cyclohexane content leads to uniform flowers with a loose flower structure, while a relatively lower cyclohexane content results in a more compact structure and uniform-sized flowers. However, changing the ratio to 1
2). Results show that a higher cyclohexane content leads to uniform flowers with a loose flower structure, while a relatively lower cyclohexane content results in a more compact structure and uniform-sized flowers. However, changing the ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or 0 caused the spherical morphology of the nanoflowers to be severely disrupted. Nonetheless, the XRD patterns of the samples still indicate favorable mesoporous characteristics. This suggests that the mesoporous structure is primarily determined by the template, while cyclohexane plays a crucial role in shaping the spherical morphology of the nanoflowers.
3 or 0 caused the spherical morphology of the nanoflowers to be severely disrupted. Nonetheless, the XRD patterns of the samples still indicate favorable mesoporous characteristics. This suggests that the mesoporous structure is primarily determined by the template, while cyclohexane plays a crucial role in shaping the spherical morphology of the nanoflowers.
Second, spherical nanoflowers are synthesized at various hydrothermal temperatures ranging from 80 °C to 140 °C. Interestingly, all four samples, synthesized at 80 °C, 100 °C, 120 °C, and 140 °C, respectively, displayed uniform flower sizes and maintained the fundamental spherical morphology, indicating that temperatures within the investigated range do not significantly affect the basic shape of the nanoflowers. When comparing the samples synthesized at 80 °C and 100 °C, there is no noticeable variation in flower size, but the petals of the latter appeared more elongate and exhibit better overall appearance. At 120 °C, there is a significant change in both flower size and petal shape. The diameter decreases to less than 200 nm, and the nanostructure becomes looser. However, when the temperature is increased to 140 °C, the flower size increased to 400 nm, but the petals do not fully expand.
Third, the synthesis of the samples involves using different molar ratios of TEOS to CPB. The results clearly demonstrate that this ratio has a significant impact on the morphology of the materials. When the TEOS/CPB ratios are 4.37 and 6.02 (sample B and D), the nanoflowers exhibit a diameter of approximately 200 nm. On the other hand, when the ratios are 3.58 and 5.21 (samples A and C), the diameter increased to approximately 500 nm. Additionally, the mesostructures or petal structures of the materials varied noticeably across the different TEOS/CPB ratios in the four samples. For instance, the wrinkled structure observed in the sample with a ratio of 4.37 (sample B) stretched further compared to the sample with a ratio of 6.02 (sample D). Furthermore, the BET measurement results revealed that the surface area of sample B is 501 m2 g−1, which is higher than that of sample D (352 m2 g−1).
ZnO nanoflowers, referred to as ZnO-0, are synthesized through a hydrothermal process. Additional types of ZnO nanoflowers, namely ZnO-250 and ZnO-950, are created by subjecting them to ultrasonic treatments using different ultrasonic devices prior to the hydrothermal procedure. The nanosheets within the ZnO-0 nanoflowers have smooth edges, while the edges of ZnO-250 and ZnO-950 nanoflowers display jagged margins resembling carnations.87 Analysis of the materials yielded the following findings: the average diameter of the ZnO nanoflowers decreases from 2.31 μm to 1.95 μm as the intensity of ultrasonic treatment increased. This reduction in size is also observed in the nanosheets constituting the nanoflowers. Specifically, the average thickness of the nanosheets decreases from 40.32 nm to 18.72 nm, and their average length varied from 350.35 nm to 270.21 nm with higher ultrasonic intensity. As a result, ZnO-950 nanoflowers exhibit the smallest size, primarily attributed to the more pronounced acoustic cavitation induced by the ultrasonic treatment.
Ag nanoflowers (Ag NFs) with hierarchical structures consisting of large buds (250–580 nm) and thin petals (9–22 nm) are synthesized.121 The size of the buds increases significantly with higher concentrations of ascorbic acid (0.1–1 M), as a reducing agent, resulting in the formation of Ag NFs named Ag NF I, II, III, IV, and V. The use of higher concentrations of ascorbic acid leads to a more spontaneous reduction and larger bud size. The specific surface area of the Ag NFs, measured from the BET adsorption isotherm,122,123 also increases with higher concentrations of ascorbic acid (2.08, 3.82, and 5.15 m2 g−1 for Ag NF I, III, and V). However, when the concentration of ascorbic acid is too high (2 M), the flower structure with thin petals cannot be maintained. Changing the reaction time (10 s ∼120 min) does not affect the bud size of Ag NF III (0.3 M of AgNO3 and 0.3 M of ascorbic acid), confirming its spontaneous anisotropic growth. The bud size of Ag NF III is not affected by changes in temperature below 80 °C, but the petal structure of the flower changes when the reaction temperature exceeds 90 °C.
Ag hierarchical assemblies are formed in a solution with the help of small acid molecules, without the use of polymer surfactants or capping agents. As shown in Fig. 3a, irregular Ag particles with Ag+ reduced by ascorbic acid (no extra acid added) are observed, with approximately 1 μm size. A magnified image of the Ag particle shows that it comprises 50–100 nm thick Ag nanosheets (Fig. 3b), with the structure resembling Ag nanoparticles obtained through a similar procedure. The addition of citric acid forms perfect microspheres with a diameter of ∼2 nm composed of numerous close-packed 20 nm thick Ag nanosheets (Fig. 3c and d). In the presence of mandelic acid, flower-like Ag particles are formed from loosely packed 50 nm thick Ag nanosheets (Fig. 3e and f), while using toluene sulfonic acid forms microscale Ag yarn-balls assembled from cross-linked 50 nm thick Ag nanosheets (Fig. 3g and h).124
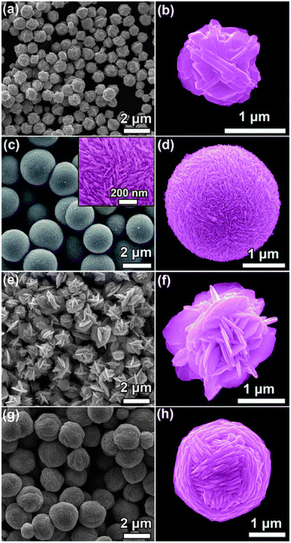 | ||
| Fig. 3 SEM images of Ag structures prepared through a chemical reduction of Ag+ ions (AgNO3) by ascorbic acid as the reducing agent, without using any polymersurfactant or capping agent. Before ascorbic acid is added to initiate the reduction reaction, (a and b) no acid, (c and d) citric acid, (e and f) mandelic acid, and (g and h) toluenesulfonic acid are added to the reaction system to control the assembly of the produced Ag nanostructures. Adapted with permission from ref. 124. Copyright © the Royal Society of Chemistry 2011. | ||
Flower-shaped NiO nanostructures are currently being synthesized using microwave and hydrothermal methods. The hydrothermally reacted sample exhibits cubic phase NiO nanostructures with a reduced particle size of 8 nm, as observed in the XRD patterns. In contrast, the microwave-reacted sample has a larger particle size of 12 nm. The UV band gap curves confirm a blue shift, indicating the quantum confinement effect of the NiO nanostructures, and this is further supported by the photoluminescence spectrum. The SEM analysis of the hydrothermally processed samples reveal an abundance of flowers with minimal agglomeration. These flowers form clusters with a diameter ranging from 1 to 2.5 μm. Conversely, the nanostructures produced through the microwave treatment process exhibit similar flower-like morphologies but show higher levels of agglomeration compared to the hydrothermally grown samples due to indirect heating of molecules caused by microwave radiation.105
The researcher conducts an investigation into the diameter distribution of Pd–Ag nanoflowers, taking into account the composition ratio of Pd to Ag. The Pd1Ag2 nanoflowers obtained in their original state exhibited a narrow size distribution, with an average diameter of approximately 29.27 ± 3.74 nm. This size is significantly smaller than that of Pd1Ag1 (33.20 ± 4.23 nm) and Pd1Ag3 (32.48 ± 4.27 nm).125
Uniform-sized silica nanoflowers with a large accessible surface area are successfully synthesized using a visible light-driven green chemistry route. The synthesis process involves the use of CTAB as a structure-directing agent in combination with various co-solvents. The size, BET surface area, pore volume, and pore diameter of the resulting SiO2 nanoparticles are analyzed and summarized in Table 5. CTAB molecules within a mixture of water, anisole, and hexanol self-assemble into micelles that play a crucial role in providing the necessary template derived from these micellar aggregates. This template subsequently controls the shape and size of the nanoparticles. Therefore, due to the interaction between co-solvents and CTAB, the shape and size of Si nanoparticles can be influenced. As the number of alcohol co-solvents increases from C-3 to C-8, the size of the nanoparticles decreases, while the BET surface area and pore volume increase.126
| Co-solvent | Solvent | Size of NPs (nm) | BET surface area (m2 g−1) | Pore diameter (nm) | Pore volume (cm3 g−1) |
|---|---|---|---|---|---|
| Propanol | Methyl phenyl ether (anisole) | 505 | 567 | 3.26 | 0.564 |
| Butanol | 480 | 580 | 3.27 | 0.603 | |
| Pentanol | 440 | 621 | 3.27 | 0.768 | |
| Hexanol | 385 | 705 | 3.44 | 0.996 | |
| Heptanol | 385 | 702 | 3.45 | 1.015 | |
| Octanol | 380 | 711 | 3.45 | 1.021 |
4. Mechanism
Nanoflowers are complex nanostructures composed of nanoscale petals or branches arranged in a flower-like pattern. They are typically synthesized using various techniques, as introduced in Section 2. Methods for preparing nanoflowers. The specific mechanism of nanoflower formation depends on the materials and fabrication techniques employed. Here, we will provide a general overview of commonly used approaches with representative examples.The first mechanism involves synthesizing surfactant-free, template-free, and seedless nanoflowers. The synthesis of NiO nanoflowers is initiated with the chemical reaction and the subsequent hydrothermal process, leading to the formation of Ni(OH)2 nuclei. The growth process of nanoflowers typically commences with the formation of small nuclei. The chemical reaction can be described as follows:
| CO(NH2)2 + 3H2O → NH4HCO3 + NH3·H2O → OH− + NH4+ | (1) |
| Ni2+ + NH3 + H2O ↔ [Ni(H2O)6−x(NH3)x]2+ | (2) |
| Ni2+ + OH− → Ni(OH)2↓ → NiO | (3) |
Subsequently, the nuclei grow into nanosheets. With an extended hydrothermal time, the surface energy decreases, leading to a gradual self-orientation of thin nanosheets. This self-orientation process ultimately results in the formation of flower-like hierarchical structures composed of Ni(OH)2. Following that, the obtained Ni(OH)2 structures are subjected to calcination at 500 °C. This calcination process transforms the Ni(OH)2 into NiO nanoflowers, characterized by the assembly of smooth nanosheets.127
The following mechanism encompasses the creation of nanoflowers through a process that eliminates the use of surfactants and templates, relying on seed mediation. The Au@CdSe and Ag@CdSe core–shell nanoflowers are synthesized via a one-pot hot-injection method.128 Initially, gold clusters act as heterogeneous nucleation sites for the growth of CdSe nanocrystals. As CdSe clusters with a magic-size, which refers to a specific size of stable CdSe clusters due to their bulk crystalline atomic packing and fully closed outer shell, and zinc blende crystal structure begin to form, they diffuse towards the gold nanocrystals and bind to surface defect sites. Through a multiple injection process involving the addition of more Cd and Se ions, additional CdSe species attach to the CdSe core clusters on the gold surface. These attached species undergo a transformation into wurtzite and adopt a multiple-branched structure, ultimately resulting in the formation of nanoflower shapes. The proposed mechanism is illustrated in Scheme 1. Third, seedless NiO nanoflowers assembled with nanorods or nanoneedles are synthesized using two surfactants: CTAB and EG.118 The synthesis process involves the following steps. In the case of nanorod-assembled NiO nanoflowers, ammonia aqueous is used as an alkaline reagent to release OH− ions. CTAB, a surfactant with a hydrophobic part, interacts with Ni(OH)2 and preferentially absorbs onto the CTA+ heads, causing the Ni(OH)2 nanoparticles to connect and form nanorods. CTAB acts as an adhesive, facilitating the gathering of the nanorods. Finally, driven by the minimum surface energy theory, the nanorods self-assemble into flower-like architecture. For the formation of nanoneedle-assembled hierarchical NiO nanoflowers, Ni2+ and C2O2−4 form a NiC2O4·2H2O polymer type ribbon due to the complexation of Ni+ and C2O2−4. EG, a surfactant with symmetrical structures and functional group-OH, acts as a ligand to Ni and blocks the crystal surface parallel to the [0,1,1] direction. The microstructures formed by NiC2O4·2H2O are connected along the [0,1,1] direction, resulting in needle-like structures. Over time, the NiC2O4·2H2O nanoneedles aggregate and assemble into hierarchical needle-flowers. Finally, NiO nanoflowers are obtained through thermal calcination.
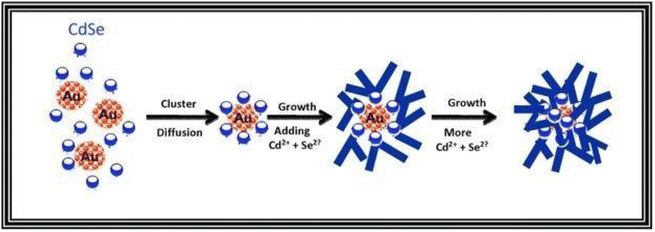 | ||
| Scheme 1 Proposed mechanism for the formation of the Au@CdSe nanoflowers. Reprinted with permission from ref. 128. Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
The growth mechanism of seed mediated flower-like CuO nanostructures utilizing PEG 4000 as a surfactant and template can be explained as follows.129 Initially, the formation of CuCO3 precipitate occurs through the following chemical reaction:
| 2Cu(NO3)2 + NH4HCO3 → 2CuCO3 + 5NO + NO2 + 5H2O | (4) |
Under hydrothermal conditions, CuCO3 undergoes a transformation into CuO through the following reaction:
| CuCO3 → CuO + CO2 | (5) |
The formation of flower-like CuO nanostructures is likely guided by the proposed mechanism depicted in Fig. 4. According to this mechanism, PEG plays a role as a soft template for the development of CuO nanoflowers. In the initial stage, the combination of Cu2+ aqueous solution and PEG leads to the generation of initial nucleation seeds, serving as the starting point for particle growth. PEG, being a nonionic surfactant with a molecular structure of H(–O–CH2–CH2)n–OH, tends to form chain-like structures when dissolved in water. When the nucleation seeds reach a critical size, the PEG chains act as templates by absorbing smaller particles through the end OH bonds for the formation of the CuO flower nanostructure. Subsequently, these absorbed particles aggregate around the larger CuO seeds. Following a 2-hour annealing process at 600 °C, the PEG templates are removed, resulting in the formation of flower-like CuO nanostructures.
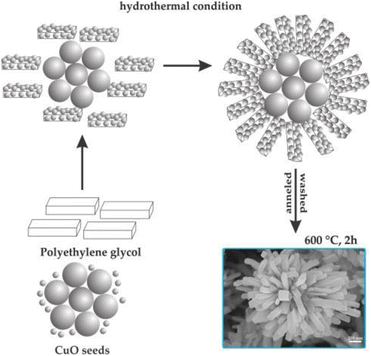 | ||
| Fig. 4 Formation mechanism of flower-like CuO hierarchical nanorods. Reprinted with permission from ref. 129. Copyright © 2014 Elsevier Ltd. All rights reserved. | ||
Finally, the fabrication of hollow-channel Au nanoflowers is achieved through a simple one-step synthesis method, utilizing a bifunctional template composed of an auto-degradable nanofiber.130 This templated synthesis involves three primary steps (Fig. 5). (1) In situ nucleation: the initial formation of primary Au nanocrystals occurs on the template composed of methyl orange–FeCl3 (MO–FeCl3). This is facilitated by strong interactions, including the coordination between Au(III) and azobenzene in MO molecules, as well as the electrostatic interaction between AuCl4− ions and Fe3+. The absorption of AuCl4− onto the MO–FeCl3 nanofiber takes place due to these interactions, achieved through the reduction of HAuCl4 by ascorbic acid (AA). After the introduction of the reducing agent AA, Au nuclei form on the surface of the template, resulting in the generation of primary Au nanocrystals. (2) Anisotropic growth: the primary nanoparticles experience anisotropic growth, resulting in the formation of flower-like structures. This growth process leads to the development of the hollow-channel Au nanoflowers. (3) Auto-degradation of the template: the presence of AA catalyzes the auto-degradation of the template. As a result, the as-formed Au nanoflowers contribute to the degradation process, leading to the dissolution of the nanofiber template.
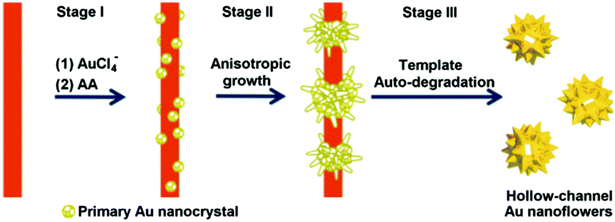 | ||
| Fig. 5 Schematic illustration of the proposed mechanism for the formation of hollow gold nanoflowers: stage (I) Au nucleates on the template surface; stage (II) Au primary crystal undergoes anisotropic growth to form branches; stage (III) the as-formed Au nanoflowers catalyze the degradation of the template in the presence of AA, leaving the open hollow channel and releasing the individual HAuNFs. Adapted with permission from ref. 130. Copyright © The Royal Society of Chemistry 2016. | ||
5. Synthesis methods and characterization of inorganic nanoflowers
5.1. Nanoflowers with metallic composition
In this section, the synthesis methods and characteristics of nanoflowers having a metal constituent, such as Au, Pt, Pd, and Ag, are mainly reviewed. Various shapes, surfaces, densities of corners and edges of nano-petals, particle size distribution, and growth mechanisms are also described.The branched Au137,138 nanomaterials are obtained in the presence of (1-hexadecyl) trimethyl ammonium chloride (CTAC), or gemini amphiphiles, as capping agents.
In addition, the anisotropic Au flower-shaped nanomaterials are synthesized using a surfactant-free biocompatible Good's buffer, such as 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES).131 Synthesis of hollow-channel Au nanoflowers using MO–FeCl3 nanofiber as a bifunctional template is also reported.130
Interestingly, unlike other synthetic strategies, relatively monodisperse Au nanoflowers139 without seeds and other surfactants have been formed using the nontoxic chemical hydroxylamine (NH2OH) without stabilizers or adjustment of the pH environment. First, 50 μL of aqueous HAuCl4 solution (wt 1%) is added to 2.5 mL of deionized water. Subsequently, 10 μL of aqueous NH2OH solution (wt 50%) is injected into the solution with stirring and reacted for an additional minute. An Au nanoflower dispersion is obtained, with Au nanoflowers of approximately 100 nm diameter, with relatively narrow size distribution, based on SEM and high-resolution transmission electron microscopy (HR-TEM) analyses (Fig. 6A–C). These Au nanoflowers can be used as a nitroaromatic pesticide degradation platform, to catalyze the otherwise impossible reduction of pendimethalin by NaBH4. This novel degradation platform could be beneficial for environmental protection, including the remediation of soil contamination and sewage treatment.
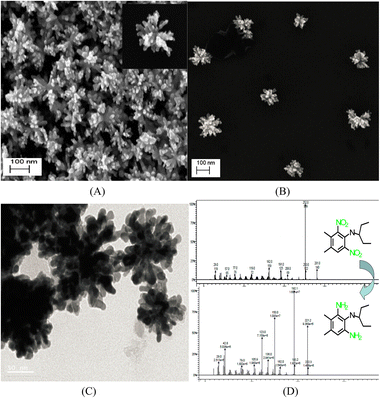 | ||
| Fig. 6 (A and B) SEM and (C) TEM images of gold nanoflowers that are synthesized; mass spectral data of (D) show the process of degradation of pendimethalin (above) to N-(1-ethylpropyl)-2,6-diamino-3,4-xylidine (below). Reprinted with permission from ref. 139. Copyright © 2014, American Chemical Society. All rights reserved. | ||
According to Table 7, although the liquid phase synthesis153 has also been reported for preparing flower-shaped Pt nanomaterials, one of the most widely used methods is electrodeposition, such as potentiostatic111 and galvanic methods,112 as well as sonoelectrodeposition.110
The 3D ultra-fine Pt nanoflowers deposited on the surface of a carbon-coated gas diffusion layer electrode (C-GDL) are also prepared by a one-step electrodeposition method (Scheme 2).154 The morphological features of the Pt nanomaterials are modified from nanospheres to 3D nanoflowers by a difference in current density. The Pt nanoflowers electrodeposited at a current density of −24 mA cm−2 for 15 min exhibit high peak power density of 660 mW cm−2 at 0.6 V in polymer electrolyte fuel cells (PEFCs).
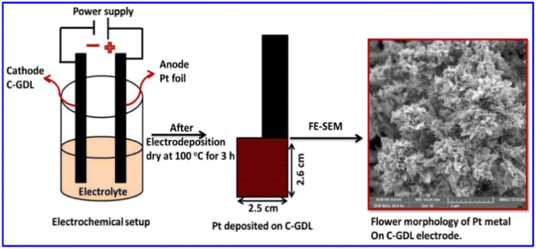 | ||
| Scheme 2 Illustration of Pt metal nanoparticles, directly deposited on carbon-coated GDL, and its surface morphology. Reprinted with permission from ref. 154. Copyright © 2020 Elsevier Inc. All rights reserved. | ||
Zuo et al. used the galvanic replacement reaction of Te nanowires (Te NWs) to synthesize monomorphic single-crystalline Pt dendritic flowers with enriched edge and corner atoms.112 As shown in Scheme 3, after the addition of the as-prepared Te NWs to the CTAB aqueous solution, H2PtCl6 (pH = 7) is injected into the mixture of Te NWs and CTAB to generate a metastable Te@Pt structure. Subsequently, H2PtCl6 (pH = 7) is injected for a second time when the color of the solution turns amber, to form Pt nanoflowers. The Pt nanoflowers exhibit excellent catalytic activity for glycerol electro-oxidation under acidic conditions, with higher mass activity and better structural stability than commercial Pt/C (20% Pt), indicating their potential utility in direct-glycerol fuel cells.
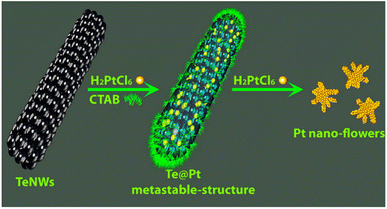 | ||
| Scheme 3 Schematic illustration of the evolution from Te NWs to Pt nanoflowers with well-defined morphologies. Reprinted with permission from ref. 112. Copyright © 2015, American Chemical Society. All rights reserved. | ||
In recent years, Pd nanostructures have attracted immense research attention because of their larger surface-area-to-volume ratio and numerous active centers due to their unique morphology.162–165 The key factors affecting the properties and functions of a material are architecture, crystallographic features, size, shape, and specific crystal planes.166–169 Among the various nanostructures, 3D Pd nanomaterials, with a porous nature, large surface areas, and numerous active centers, have been considered as promising catalytic materials.166,170 This section introduces the fabrication methodologies and applications of Pd nanoflowers.
The Pd nanoflowers have been synthesized by liquid phase synthetic methods171 and electrodeposition172 (Table 8), like the Au and Pt nanomaterials.
For instance, Maniam and Chetty reported the electrodeposition of shape-controlled Pd nanoflowers on an electrochemically activated carbon-black substrate (Vulcan XC-72R) using a potentiostatic technique, with/without different concentrations of PEG-6000 as an additive.172 The PEG polymer surrounding the crystal nuclei hinders crystal growth and generates a steric hindrance effect to reduce particle–particle aggregation.173 Additionally, the concentration of PEG strongly influences particle aggregation. Electrodeposition of Pd with 10−2 mM PEG changes the morphology from spherical to flower-like, forming well-dispersed nanoflowers with 0.2 mM PEG. The PEG acts as a scaffold for the nucleation sites at an optimum concentration (0.2 mM), to form flower-like shapes without any nucleation. In terms of the electrochemical surface area (ESA) and mass-specific current density, the electrodeposited Pd nanoflowers exhibit three- to four-fold enhanced electrocatalytic activities compared to spherical Pd deposits in the oxygen reduction reaction, formic acid oxidation, and CO stripping reaction.
What is noteworthy is the development of green chemistry research. Pd nanoflowers are synthesized using carrageenan as the capping agent, which is a natural macromolecule from the ocean, and L-ascorbic acid as the reducing agent (Fig. 7).171 Experimental data indicate that the critical factor for the synthesis of Pd nanoflowers is the use of carrageenan, while the concentration of L-ascorbic acid affects the length of thorns grown on Pd nanoflowers. To investigate the effect of concentration on the surface morphology of Pd nanoflowers, Pd products are prepared using 20, 40, 60, 120, and 180 mg L-ascorbic acid in synthetic solutions and labeled Pd-AA20, Pd-AA40, Pd-AA60, Pd-AA120, and Pd-AA180, respectively. Pd-AA60(0) indicates the synthesis of Pd-AA60 without carrageenan. As shown in the SEM image (Fig. 8), the average particle size of Pd-AA60 is approximately 280 ± 30 nm, with Pd-AA60 exhibiting the longest and thinnest thorns (approximately 132 ± 15 nm long and 25 ± 7 nm thick). All Pd products using carrageenan exhibit flower-like morphology, except Pd-AA60(0). The electrocatalytic activity of the synthesized Pd nanoflowers toward ethanol oxidation is investigated in an alkaline medium.
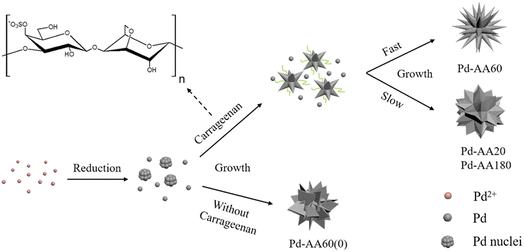 | ||
| Fig. 7 Schematic illustration of the formation mechanism of Pd nanoflowers. Reprinted with permission from ref. 171. Copyright © 2017, American Chemical Society. All rights reserved. | ||
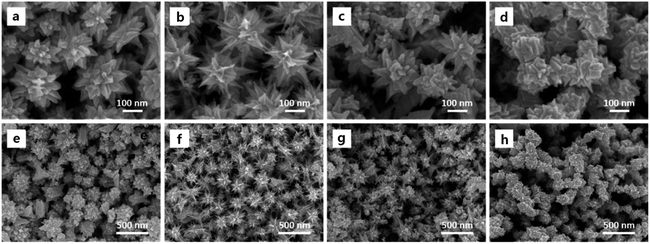 | ||
| Fig. 8 SEM images of Pd-AA20 (a and e), Pd-AA60 (b and f), Pd-AA180 (c and g), and Pd-AA60(0) (d and h). Reprinted with permission from ref. 171. Copyright © 2017, American Chemical Society. All rights reserved. | ||
Pd-AA60, with the longest thorns, exhibits higher catalytic current density (1160 mA mg−1) than commercial Pd/C, the reported Pd/C samples,174 and other prepared Pd products (Pd-AA20, Pd-AA180, and Pd-AA60(0)) with short thorns exhibit better cycle stability, with catalytic activity maintenance of above 96% after 300 cycles.
In this section, since Ag nanoflowers (Table 9) are also synthesized with the aforementioned strategies for manufacturing Au, Pt, and Pd nanoflowers, we would like to introduce some unique generation methods for Ag nanoflowers.
Zhang et al. reported the fabrication of novel self-assembled silver hierarchical structures by incorporating a small amount of acid in conventional solution chemistry, without using any polymer surfactant or capping agent.124 This acid-directed synthesis inhibits the sorption of polymer molecules on metal surfaces, forming Ag particles with coarse morphology, which can be utilized as substrates to detect chemical or biological molecules through SERS. As we explained in Section 3. Experimental parameters' effect, the SEM images in Fig. 3 show the Ag assemblies prepared with the assistance of different acids at low and high concentrations, with uniform-structure Ag particles also being produced, corresponding to the acid incorporated by the acid-directed process. The irregular Ag particles, composed of Ag+ ions reduced by ascorbic acid, have an approximate size of 1 μm. They are made up of Ag nanosheets with a thickness of 50–100 nm. Adding citric acid forms perfect microspheres with a diameter of about 2 nm, which are composed of closely packed Ag nanosheets measuring 20 nm in thickness. In the presence of mandelic acid, flower-like Ag particles are formed, consisting of loosely packed Ag nanosheets with a thickness of 50 nm. Using toluenesulfonic acid results in the formation of microscale Ag yarn-balls assembled by cross-linked Ag nanosheets with a thickness of 50 nm. The obtained Ag particles with rough surfaces are promising SERS platforms for sensing applications, and the fabricated core–shell Ag wires exhibit high SERS sensitivity toward melamine, with a detection sensitivity of 5 ppm.
Long-range ordered Ag nanoflower arrays on patterned wafers are fabricated using a lithographic template-guided method based on the optical interference method and the difference in hydrophilicity between silicon and the photoresist.192 As shown in Fig. 9a, 1D and 2D ordered array templates are obtained for supplying alternate relative hydrophilic and hydrophobic regions, using holographic technology. The photoresist part is more hydrophilic than silicon, as shown by the contact angle of the droplet in Fig. 9b.193 As shown in Fig. 9c, the prepared photolithographic template is immersed in the reaction solution containing AgNO3, PVP, ascorbic acid, and citric acid, to form Ag nanoparticles. Ag seeds are induced on the photoresist surface owing to the difference in hydrophilicity between the photoresist and silicon. Finally, the seeds grow into silver nanoflowers with abundant hotspots.
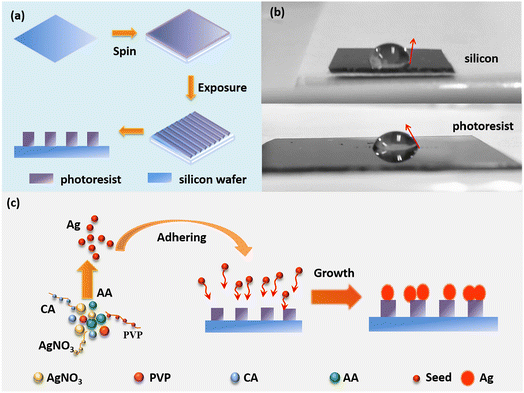 | ||
| Fig. 9 Schematic diagram of fabricating the LROSNA structure. (a) Preparation of lithographic templates. (b) Optical image of the contact angle for hydrophilicity difference between silicon (the top) and photoresist (the bottom). (c) Schematic diagram of preparing the LROSNA structure. Reprinted with permission from ref. 192. Copyright © 2019 Elsevier B.V. All rights reserved. | ||
As SERS substrates, the 1D and 2D pattern structures demonstrate high sensitivity with low detection limits of 5 × 10−10 M and 5 × 10−9 M, respectively, using rhodamine 6G as a probe molecule to investigate the hotspot effect of the nanogaps within/between the Ag nanoflowers.
| Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|
| Fluorescent Au nanoflowers | Seedless, surfactantless room temperature synthesis using 5-hydroxyindole-3-acetic acid | SERS and electrocatalyst | 134/2008 |
| Au nanoflowers | Vesicle-directed generation using gemini amphiphiles | SERS | 137/2010 |
| Au nanoflowers | Melanin assisted seedless, template free synthesis | Catalysis, biochemistry, and Raman fields | 140/2012 |
| Hydrangea flower-like hierarchical Au nanostructures | One-pot, seedless synthesis using ascorbic acid and CTAC | SERS-based sensors | 138/2015 |
| Au nanoflowers with sheet-like petals | Seedless, reduction synthesis using ascorbic acid and silver nitrate | SERS substrates and sensors | 132/2016 |
| Hollow-channel Au and Ag nanoflowers | One step fabrication using an auto-degradable MO–FeCl3 nanofiber as a bifunctional template | Catalysts and SERS probes | 130/2016 |
| Au nanoflowers, nanostars, and nanosnowflakes | Seed-less and surfactant-free approach using catechol as the reducing and structure-inducing agent | Catalyst and photothermal therapy | 136/2019 |
| Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|
| Pt nanoflowers with thin projections (petals) | Stabilizing reagent-free synthesis using NaBH4 | Surface-assisted laser desorption/ionization mass spectrometry of biomolecules | 155/2007 |
| Pt nanoflowers on a Si substrate | Potentiostatic pulse plating method | Electrocatalysts toward methanol and CO oxidation | 111/2009 |
| Porous Pt nanoflowers on clean indium tin oxide (ITO) | One-step and template-free electrodeposition method | Electrocatalysts for the methanol electro-oxidation of direct methanol fuel cells (DMFCs) | 142/2010 |
| Pt nanoflowers on the bare Au electrodes | Template-free ultrasonic electrodeposition method | Nonenzymatic glucose sensors | 110/2012 |
| Pt nanoflowers composed of an ordered array of nanoparticles | Ethanol reduction of H2PtCl6 | Electrocatalysts for DMFCs and catalyst in C–C coupling reaction | 153/2013 |
| 3D Pt nanoflowers on porous silicon | Electroless plating method | Electrocatalysts for monolithic integrated micro DMFCs | 156/2013 |
| Pt nanoflowers | Sonoelectrodeposition method | Electrocatalysts and nonenzymatic sensors | 157/2014 |
| Pt needle-like nanoflowers on a glassy carbon electrode (GCE) substrate | Template-free electrochemically potentiostatic method | Electrocatalysts for methanol oxidation reaction in DMFCs | 158/2015 |
| Pt nanoflowers | Chemical reduction and seed-mediated growth using tri-sodium citrate and ascorbic acid | — | 159/2016 |
| Nanoflower-shaped Pt on GCE | One-step electrochemical deposition method | Sensor for simultaneous detection of lead and cadmium at trace levels | 160/2019 |
| Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|
| Pd porous single-crystalline nanoflowers | Seed mediated growth method using ascorbic acid and CTAB | Electrocatalysts for ethanol electro-oxidation | 174/2014 |
| Porous Pd nanoflowers | Polyol process using Pd(acac)2 and oleylamine | Electrocatalysts for methanol electro-oxidation | 175/2009 |
| Spinous flower-like, cone-like, flower and coral reef-like Pd dendrites on glassy carbon plates | Electrochemical methods using cyclic voltammetry | Electrocatalysts toward formic acid electro-oxidation of microfluidic fuel cell channels | 81/2013 |
| Pd nanoflowers | Green synthesis (seedless chemical reduction method) using HEPES | Electrocatalysts for methanol electro-oxidation in alkaline media | 170/2013 |
| 3D Pd nanoflowers on Ni-YSZ (yttria-stabilized zirconia) anode | Galvanic displacement reaction | Catalysts for direct ethanol solid oxide fuel cells | 114/2016 |
| Pd nanoflowers | Radiolytic reduction of PdII(acac)2 in ethanol under a CO atmosphere | Plasmonic photocatalysts for the Suzuki–Miyaura cross-coupling reaction | 176/2019 |
| Pd nanoflowers on Ti film or on GCE | Electrodeposition process | Electrocatalysts in the reduction of Cr(VI) | 177/2019 |
| Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|
| Rose-, spike-, and snowflake-shaped Ag nanostructures | One-pot reduction method using ascorbic acid, poly(ethylene glycol), sodium acetate, and sodium citrate | SERS matrices | 194/2007 |
| Branched Ag nanoflowers | Biosynthesis using biofriendly molecule-rutin | SERS and antibacterial activities against Pseudomonas aeruginosa, S. Faecalis, and Escherichia coli bacterium | 195/2009 |
| Ag nanoflowers with multiple petals on ITO glass | Double-potentiostatic electrodeposition method | SERS | 196/2012 |
| Ag nanoflowers | Trisodium citrate-assisted biosynthesis using trisodium citrate and Canarium album foliar broths | SERS substrates | 197/2013 |
| Ag nanoflowers with nanogaps | Ice-water bath method using citric acid, and PVP | Broadband scatterers for high performance random lasers | 198/2017 |
| Ag nanoflowers on fluorine-doped tin oxide (FTO) glass (nanorods, dendrites, decahedrons, and icosahedrons are also formed) | Cyclic scanning electrodeposition method | Catalysts of non-enzymatic electrochemical glucose biosensors | 199/2017 |
| 3D and 2D Ag nanoflowers | Seedless green liquid reduction method using AgNO3, ascorbic acid, and sodium citrate | Multiple phase SERS-based molecule detection of food additives and environmental pollutants | 200/2019 |
| Ag nanoflowers | Green synthesis using Kalanchoe daigremontiana extract | Photocatalysts in the dye degradation of methylene blue and antibacterial activities against the Gram-negative bacteria Escherichia coli and the Gram-positive Staphylococcus aureus | 76/2019 |
| Ag nanoflowers with nanoleaves | Electrochemical deposition approach | SERS sensing substrates | 113/2019 |
| Compound | Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|---|
| Ni | Ni nanospheres, nanowires, and nanoflowers | Surfactant-free non-aqueous sol–gel approach | — | 201/2008 |
| Ni nanoflowers | Capping agent assisted and Ag-catalyzed growth | Catalysts | 202/2010 | |
| Co | Co nanoflowers | Solvothermal synthesis using RuCl3·xH2O (35 wt% Ru) and hexadecylamine | Catalysts for hydrogenolysis of glycerol to propylene glycol | 33/2009 |
| Cu | Cu nanoflowers | Capping agent assisted reduction process using oleylamine and CTAB | Catalysts in oxygen reduction reactions | 203/2018 |
5.2. Nanoflowers with metal oxide composition
Here, the unique properties of each metal-oxide material are introduced, and methods and properties of synthesizing nanoflowers using these materials are summarized.Over the past decade, various TiO2 nanostructures have been investigated and used to form nanomaterials, such as nanosheets,218 nanowalls,219 nanorods,220 nanowires,221 nanotubes,222 and nanobelts.223 In particular, TiO2 nanostructures with 3D dimensions, such as flowers and urchins, are promising candidates for many applications because of their properties.224
Our investigation (Table 11) shows that the most commonly used method for synthesizing TiO2 nanoflowers is a hydrothermal method to synthesize nanomaterials by chemical reaction in aqueous media within a pressurized vessel (known as an autoclave).225 Anatase and brookite TiO2 nanoflowers are prepared using tetrabutyl titanate, NaCl and NH3·H2O.93 Hierarchical 3D TiO2 nanoflowers on Ti substrate are processed by the low temperature hydrothermal method.92 TiO2 and reduced TiO2 nanoflowers on FTO glass are fabricated using hybrid technology combining hydrothermal and immersion reduction processes.226
| Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|
| Anatase and brookite TiO2 nanoflowers | Hydrothermal process using tetrabutyl titanate, NaCl and NH3·H2O | Photocatalysts in the photodegradation of methyl orange | 93/2009 |
| Flower-like TiO2 nanostructures | Low-temperature hydrothermal process using Ti powder and HF | Photocatalysts for the degradation of methylene blue dye | 228/2010 |
| Rutile TiO2, stacks of multilayered nanoflowers with hexagonal nanopetals | Acid vapor oxidation method | Catalysis, solar cells, and electronic devices | 229/2011 |
| Hierarchically flower-like TiO2 superstructures | Surfactant-free alcohothermal strategy in a HF–H2O–C2H5OH mixed solution using titanate nanotubes | Photocatalysts for decomposition of acetone in air and methyl orange in aqueous solution under UV illumination | 230/2011 |
| TiO2 nanoflowers | Hydrothermal method using titanium(IV) butoxide and acetic acid | Photoelectrodes for dye-sensitized solar cells (DSSCs), photocatalysts for aqueous methylene-blue photo-oxidation | 231/2011; 232/2020 |
| Rutile-TiO2 nanowires and nanoflowers on FTO glass and glass | Hydrothermal method using titanium(IV) butoxide and HCl | Solar cells, gas sensors, optoelectronics etc. | 233/2015 |
| Rutile/anatase TiO2 heterojunction nanoflowers | Hydrothermal approach using titanium tetrachloride, urea, and CTAB | Photocatalysts for the photodegradation of real-dye waste water | 234/2015 |
| TiO2 nanoflowers on ITO | PVP surfactant assisted liquid phase deposition technique using (NH4)2TiF6, PVP, and boric acid | Photovoltaic materials of the photoelectrochemical cell | 62/2015 |
| TiO2 nanoflowers on TiO2 seed layer coated p-Si substrates | Hydrothermal method using titanium butoxide and HCl | Electron field emission and self-powered ultraviolet photodetector | 235/2016 |
| Hierarchical 3D TiO2 nanoflowers on Ti substrate | Low temperature hydrothermal process using Ti plate and H2O2, and NaOH | Volatile organic compound sensors (acetone, methanol, 2-butanone, toluene and 2-propanol) | 92/2016 |
| Rutile TiO2 nanoflower films on FTO glass | Hydrothermal process using tetrabutyl titanate and HCl | Photoanodes for DSSCs | 236/2017 |
| TiO2 and reduced TiO2 nanoflowers on FTO glass | Hydrothermal process and dipping reduction process with NaBH4 | Photoanodes for superior photoelectrochemical water splitting | 226/2017 |
| Rutile-TiO2 nanoflower thin film on FTO substrate | Hydrothermal method using titanium(IV) isopropoxide and HCl | Solar cells | 69/2020 |
| Rutile- phase TiO2 nanoflowers | Hydrothermal method using titanium(IV) butoxide, HCl, and CTAB | — | 237/2020 |
| 3D TiO2 hierarchical nanoflowers | Template-free solvothermal alcoholysis reaction using titanium trichloride, NaOH, and ethanol | Photocatalysts in the degradation of organic pollutants in wastewater | 34/2021 |
Rutile TiO2 3D nanoflowers are synthesized by the hydrothermal method in a saturated sodium chloride solution74 and characterized by X-ray diffraction (XRD), whereby all the diffraction peaks indicate that the product is pure rutile TiO2. The effects of the solvent on NF synthesis are investigated using FE-SEM. sample 1, synthesized in saturated NaCl solution, consists of partly agglomerating TiO2 nanoflowers, with a diameter of 2–3 μm. The powder (sample 2) prepared by the hydrothermal method in anhydrous ethanol is stacked with uneven nanospheres. Furthermore, using deionized water as the solvent produces nanobulks with irregular shapes (sample 3). Sensors are fabricated using the as-synthesized TiO2 samples and tested, to evaluate their ethanol gas-sensing ability and to investigate the influence of morphology on it. The gas response of the sensor, fabricated using partly agglomerated TiO2 nanoflowers (sample 1) with a large surface area, is higher than that of the others.227
CuO nanoflowers are manufactured by various synthesis strategies: liquid phase synthesis, chemical deposition, hydrothermal method, photo-synthesis, and green synthesis (Table 12).
| Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|
| CuO nanoflowers on Cu plates | Direct reaction between a Cu plate and a KOH solution at room temperature | Field emission for nano-electronic devices | 263/2008 |
| CuO nanoflowers | Hydrothermal method using Cu(NO3)2·3H2O and NH3·H2O | H2O2 sensor | 98/2010 |
| CuO nanoflowers on the glass substrate | Low-temperature chemical bath method using copper nitrate and hexamethylenetetramine | Electrochemical pH sensor | 264/2011 |
| CuO nanoribbons and nanoflowers | Low-temperature, one-pot water bath method using Cu(OAc)2·H2O and NaOH | Supercapacitors | 265/2013 |
| CuO nanoflowers | Solution combustion method using cupric nitrate and glycine | Photocatalysts for the photodegradation of methyl orange | 266/2013 |
| CuO nanoplatelets and nanoflowers | Hydrothermal method | Catalysts for the degradation of methylene blue | 267/2014 |
| Hierarchical 3D-flower-like CuO nanostructure on copper foil | Chemical bath deposition method | Supercapacitors | 268/2015 |
| Hierarchical CuO nanoflowers on the surface of flexible Cu foil | Chemical deposition method | Supercapacitor electrodes | 269/2016 |
| Flower-like CuO nanostructures with porous nanosheets on an alumina tube | Hydrothermal method using Cu(CH3COO)2·H2O, NH3·H2O and NaOH | H2S sensors | 99/2017 |
| CuO nano-flowered surfaces | Submerged photo-synthesis of crystallites | Antibacterial agents against Gram-positive (Staphylococcus aureus) bacteria and Gram-negative (Escherichia coli K12) bacteria | 270/2017 |
| CuO nanoflowers | Low temperature solution process using copper nitrate, HMTA, and NaOH | Adsorbent for the removal of Pb2+ in waste-water treatment | 271/2017 |
| Hierarchical flower-like CuO film on Cu foil | One-step solution route at room temperature using Cu foil, NaOH, and (NH4)2S2O8 | Photocatalysts for the degradation of methylene blue | 272/2017 |
| CuO nanospindles and CuO nanoflowers | Green synthesis process using Dodonaea angustifolia extract | Anti-microbial agents against Gram-negative E. coli and Gram-positive S. aureus | 77/2018 |
| Hierarchical porous CuO/Cu nanoflowers | Alkaline solution oxidation process in a water bath using Cu powder, sodium hydroxide, and ammonium peroxydisulfate | Non-enzymatic H2O2 sensors | 273/2018 |
| Hierarchical CuO nanoflowers | Hydrothermal method using CuSO4·5H2O, CTAB, sodium citrate, and KOH | Non-enzymatic sensors for H2O2 and glucose | 85/2019 |
| Flower-shaped CuO nanostructures | Biosynthesis using Cu(CH3COO)2 and Ocimum sanctum (Tulsi) leaves-extracted eugenol (4-allyl-2-methoxyphenol) | Photocatalysts and antibacterial agents | 78/2020 |
| Co3O4, NiO and CuO nanoflowers | Hydrothermal method | Photodiode, photodetector, sensor, battery and supercapacitor applications | 274/2020 |
| CuO nanoflowers on a glass slide | Wet chemical method using Cu foil, (NH4)2S2O8, and NaOH | Inflammable gas sensors (acetone, ethanol, methanol, isopropyl alcohol, toluene, chloroform and ammonia gas) | 275/2020 |
| CuO nanoflowers | Hydrothermal method | Catalysts for the degradation of methylene blue in the presence of H2O2 in waste water | 286/2021 |
Their applications are also very diverse from hydrazine, H2O2, H2S, and pH sensors to field emission for nano-electronic devices, supercapacitors, photocatalysts and absorbent for waste-water treatment, and antibacterial agents (Table 12).
For example, flower-like CuO nanostructures are synthesized using a wet chemical-assisted hydrothermal reaction,129 with the formation mechanism illustrated in Fig. 4. The first nucleation seeds, formed by a mixed aqueous solution of Cu2+ and PEG, provide the nuclei for particle growth. PEG chains, nonionic surfactants composed of H(–O–CH2–CH2)n–OH, absorb small seeds using the terminal OH bonds as soft templates when the nucleation seeds reach a critical dimension. Subsequently, they aggregate around the large seeds to form CuO flower nanostructures. Finally, the annealing process removes the PEG templates, forming flower-like CuO hierarchical nanorods. Fig. 10 shows FE-SEM images of the CuO nanoflowers obtained through hydrothermal treatment and calcination. Highly uniform nano-sized flowers (petals with 60–100 nm diameter and 500–700 nm length), consisting of many nanorods, are formed. The CuO nanoflowers are coated on SiO2/Si substrates attached to Pt interdigitated electrodes to fabricate volatile gas sensors. The fabricated sensors show high selectivity and sensitivity toward ethanol vapor at approximately 230 °C (the optimized working temperature).
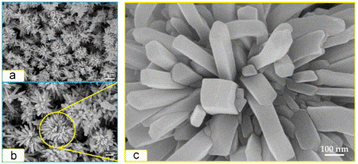 | ||
Fig. 10 FE-SEM images of CuO nanoflowers prepared at 170 °C for 24 h with different magnifications of: (a) 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00×, (b) 200 00×, (b) 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00×, and (c) 1 00×, and (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00×. Reprinted with permission from ref. 129. Copyright © 2014 Elsevier Ltd. All rights reserved. 00×. Reprinted with permission from ref. 129. Copyright © 2014 Elsevier Ltd. All rights reserved. | ||
| Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|
| NiO nanoparticles and nanoflowers | Soft synthesis without organics at low temperature using Ni powder and water | — | 291/2008 |
| NiO with novel flower-like morphology | Calcination in a muffle furnace under a nitrogen atmosphere | Catalysts for CO oxidation | 292/2009 |
| Hierarchical porous NiO nanoflowers | Microwave-assisted fabrication and calcination | Supercapacitor | 106/2010 |
| NiO nanoflowers | Wet chemical process with a subsequent thermal treating process | Electrochemical sensors | 293/2011 |
| NiO nanodiscs and nanoflowers | Hydrothermal synthesis in a non-basic solution and calcination | Electrochemical test and water treatment | 294/2011 |
| NiO nanoflowers | Surfactant-free hydrothermal process and thermal decomposition | Li-ion batteries | 295/2011 |
| Porous NiO nanoflowers and nanourchins | Hydrothermal method using HMTA or urea as the precipitator in the absence or presence of a surfactant (PEG or PVP) | Catalysts for toluene combustion | 96/2012 |
| NiO nanoflowers | Solution plasma process | NO2 gas sensor | 296/2012 |
| Flower shaped NiO nanostructures | Microwave and hydrothermal methods | — | 105/2014 |
| NiO hierarchical bundle-like nanoflowers | Hydrothermal-route assisted EG and subsequent thermal calcination | Gas sensors | 297/2015 |
| NiO nanoflowers | Template-less surfactant-free hydrothermal synthesis using nickel nitrate and HMTA | Photoelectrochemical hydrogen production | 298/2015 |
| 3D hierarchical porous NiO nanoflowers | Solvothermal and post-calcination | Li-ion batteries | 287/2016 |
| 3D flower-like NiO | Surfactant-assisted hydrothermal method | Ethanol gas sensors | 299/2016 |
| Flake–flower NiO architecture | Hydrothermal process and calcination using Ni(NO3)2·6H2O and PVP | Ethanol gas sensors | 300/2016 |
| Rose-like, sphere-like, and plate–flower-like NiO nanostructures | Surfactant-assisted hydrothermal method and calcination using Ni(NO3)2·6H2O, or NiCl2·6H2O, and PVP, or CTAB | Ethanol gas sensors | 301/2017 |
| Nanoneedle-assembled hierarchical and nanosheet-assembled hierarchical NiO nanoflowers | Hydrothermal method and subsequent calcination | Ethanol gas sensors | 302/2017 |
| Sparse-type and close-type NiO nanoflowers | Hydrothermal process assisted by CTAB or SDS | Ethanol gas sensors | 97/2017 |
| Porous NiO nanoflowers | Solvothermal method and calcination using nickel acetate tetrahydrate (Ni(ac)2·4H2O) and urea | Li-ion batteries | 100/2017 |
| 3D flower-like NiO hierarchical structures | Hydrothermal synthesis and calcination using Ni(NO3)2·6H2O, cetyltrimethyl ammonium bromide (CTAB) and ammonia, or NiCl2·6H2O, Na2C2O4, and ethylene glycol (EG) | Acetylene gas sensors | 118/2018 |
| 3D hierarchical flower-like NiO nanostructures with three different morphologies | Template-free and low-cost hydrothermal method and subsequent calcinations with different volumes of ammonia (25%–28% wt%) | Ethanol gas sensors | 127/2020 |
| NiO nanoflowers | Polymer directed hydrothermal method and calcination using Pluronic F-127 block copolymer, NiNO3·6H2O, and urea | Electrostatic adsorption of Congo red dye for wastewater treatment | 303/2021 |
As shown in Scheme 4,286 Pluronic F127 acts as a micellar template for the fabrication of a lamellar structure. Ni(NO3)2·6H2O is ultrasonically dissolved in water and mixed evenly under stirring in a water bath to form a homogeneous suspension. Subsequently, 1,3,5-trimethyl benzene (TMB), which acts as an interface-adjusting agent that avoids piling up of the lamellar structure, is slowly injected into the homogeneous suspension. The hydrophobic poly(phenylene oxide) segment of F127 can interact with TMB molecules through van der Waals interactions, while Ni2+ undergoes oriented attachment and self-assembles with the hydrophilic segment of F127. Addition of concentrated NH3·H2O to the emulsion system promotes crystal growth. An Ni-based hydroxide precursor is generated through the hydrothermal process, and F127 and TMB are removed on decomposition to generate numerous mesopores during the high-temperature sintering process, forming NiO nanoflowers with mesoporous nanosheets. The synthesized samples are labeled NiO–NF-x, where x (0.5, 1, 3, 5, and 7 mL) is the volume of NH3·H2O. The FE-SEM images of the NiO–NF samples in Fig. 11 show the influence of the amount of NH3·H2O on their morphology. The size range of the nanoflowers is 1–2 μm. On increasing the amount of NH3·H2O to 3 mL, flower-like NiO–NF-3.0, composed of mesoporous hexagonal nanosheets, is formed (Fig. 11c). With increasing alkalinity, the stacked nanosheets become thicker (Fig. 11d and e) due to the faster formation of Ni hydroxide facilitated by higher NH3·H2O content. Moreover, the size of the mesopores on the nanosheets remains around 10 nm. Notably, NiO–NF-3.0 exhibits the smallest mesopores (primarily 9.5 nm) and the thinnest nanosheets (approximately 15 nm), indicating a higher number of active sites and promising electrocatalytic performance. In the electrochemical nitrogen reduction reaction, the NiO–NF-3.0 electrode materials exhibit excellent electrochemical properties in 0.1 M Na2SO4.
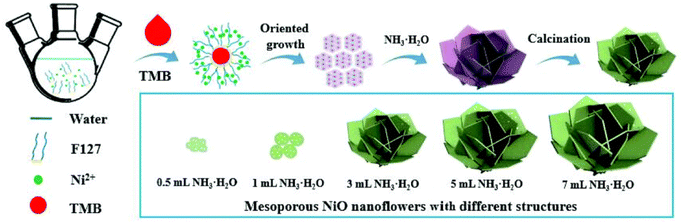 | ||
| Scheme 4 Schematic illustration of the formation mechanism of NiO–NF-0.5, NiO–NF-1.0, NiO–NF-3.0, NiO–NF-5.0, and NiO–NF-7.0. Adapted with permission from ref. 286. Copyright © the Royal Society of Chemistry 2021. | ||
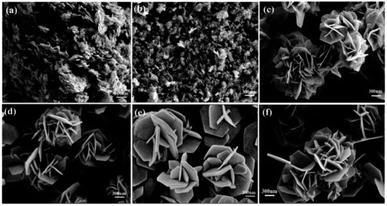 | ||
| Fig. 11 FE-SEM images of (a) NiO–NF-0.5, (b) NiO–NF-1.0, (c) NiO–NF-3.0, (d) NiO–NF-5.0, (e) NiO–NF-7.0, and (f) NiO–NF-3.0 before annealing. Adapted with permission from ref. 286. Copyright © The Royal Society of Chemistry 2021. | ||
Multifunctional nanoflowers composed of partial hydroxide nickel oxide (NiOx) are grown on cotton fabric using a chemical bath deposition technique to develop UV filters and flexible gas/chemical sensors.288 Nickel sulfate hexahydrate and potassium persulfate are mixed at room temperature, followed by the addition of an ammonium hydroxide solution to the prepared precursor solution under vigorous stirring. Pre-treated cotton cellulose samples are dipped in the prepared precursor solutions and kept undisturbed for 96 h, followed by hot-air-oven drying at 80 ± 6 °C for 1 h. At the beginning of the growth process, the Ni dissolved in potassium persulfate is immediately hydrolyzed and reacts with ammonium hydroxide to generate Ni(OH)2 nuclei with an electrostatic driving force, minimizing the surface energy of cotton cellulose, promoting the growth of nanosheets.289,290 A high saturation level facilitates the formation and enhancement of nucleation sites, whereas a low saturation level facilitates crystal growth. On increasing the reaction time, the growth of nanosheets increases through further nucleation and assembly, forming nanostructured NiOx-3D-green-button chrysanthemum flowers on the cotton fabric (Fig. 12). The ultraviolet protection factor (UPF) indicates the number of UV rays (both UV-A and UV-B) blocked by the material.288 The UPF of the nanoflower modified on cotton fabric, measured using a UV transmittance analyzer by an in vitro method according to the AATCC 183:2004 standard, is 2000. According to chemical/gas sensing measurements, the selectivity response of the NiOx-modified fabric is 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 431 toward trimethylamine at room temperature. Additionally, it exhibits good thermal stability and increased hydrophobicity compared to unmodified materials. Owing to these excellent features, the nanostructured cotton fabric can be used as a flame retardant, hydrophobic screen, and protective suit against gas leaks and UV rays.
431 toward trimethylamine at room temperature. Additionally, it exhibits good thermal stability and increased hydrophobicity compared to unmodified materials. Owing to these excellent features, the nanostructured cotton fabric can be used as a flame retardant, hydrophobic screen, and protective suit against gas leaks and UV rays.
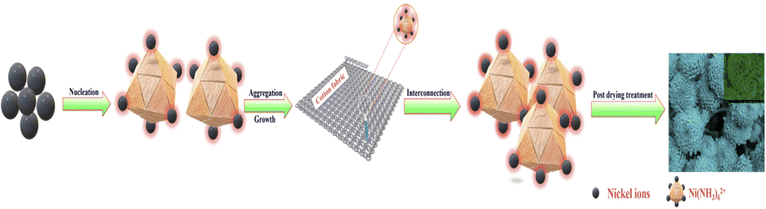 | ||
| Fig. 12 Schematic representation of the growth process of the NiOx on cotton cellulose. Reprinted with permission from ref. 288. Copyright © 2019, American Chemical Society. | ||
| Compound | Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|---|
| SnO2 | Super-hydrophobic 3D SnO2 flowers with nanoporous petals | Controlled shape-preserving thermal oxidation process | — | 304/2004 |
| SnO | Nanoparticle-attached SnO nanoflowers | Free cation-induced decomposition of an intermediate product tin oxide hydroxide (Sn6O4(OH)4) | Anodes for high-capacity lithium-ion rechargeable batteries | 305/2009 |
| α-Fe2O3 (hematite) | Porous α-Fe2O3 flower-like nanostructures | Solvothermal reactions and sequential calcinations | Lithium-ion batteries and photocatalysts | 306/2008 |
| CeO2 | CeO2 nanoflowers with controlled shape (cubic, four-petaled, and starlike) | Rapid thermolysis of (NH4)2Ce(NO3)6 in oleic acid/oleylamine | Catalysts in CO oxidation | 307/2008 |
| MnO2 | Monodisperse 3D manganese oxide nanoflowers | Reduction of potassium permanganate (KMnO4) in deionized water with formamide (HCONH2) at 40 °C | Supercapacitor electrode material | 308/2009 |
| MnO4 | Homogenous manganate nanoflowers | Microwave-assisted hydrothermal synthesis | Nuclear decontamination uses | 117/2019 |
| Bi2O3 | Large-area arrays of 1D nanowires and nanoflowers of Bi2O3 | Oxidative metal vapor phase deposition technique | — | 83/2007 |
| Nonstoichiometric Bi2O2.33 nanoflowers | One-step solvothermal route using NaBiO3 precursor in aqueous solution with PVP | — | 103/2014 | |
| MgO | Magnesium oxide nanoflowers | Chemical precipitation method, sequential calcinations, and surface modification with acacia gum | Adsorbents for the removal of divalent metallic species from synthetic waste water | 309/2015 |
| ZnO | Flower-like ZnO nanostructures | Hydrothermal method in the absence of surfactants or organic solvents | — | 310/2007 |
| ZnO nanoflowers on Si substrate | Seed-layer assisted solution route | Optical, optoelectronic and sensing devices | 311/2010 | |
| ZnO nanoflowers | Solution plasma | — | 312/2011 | |
| Flower-like ZnO-on-ZnO nanorod arrays on a zinc substrate | Low-temperature hydrothermal synthesis | — | 313/2013 | |
| ZnO nanoflowers | Biosynthesis using Bacillus licheniformis MTCC 9555 | Photocatalysts | 314/2014 | |
| Hierarchical ZnO nanoflowers | PEG-20000 assisted hydrothermal synthesis | H2S gas sensors | 95/2015 | |
| Three types of 3D fluffy ZnO nanoflowers | Direct hydrothermal reaction with/without ultrasonic treatment | Photocatalysts | 87/2020 | |
| Single-crystalline ZnO nanoflowers | Hydrothermal method with post-annealing treatment | Ultraviolet photodetectors | 315/2021 | |
| ZnO nanoflowers | Bioinspired synthesis using Withania coagulans extract as a reducing agent | Antibacterial agents and bioethanol production | 108/2021 | |
| CdO | CdO nanoflowers on soda lime glass, fused silica glass, Si and ITO-coated glass | Sol–gel method | Photodetection application | 316/2019 |
Flower-like NiO nanostructures with three different morphologies are synthesized by a modified hydrothermal method by changing the volume of ammonia (25–28% wt%).127 Each product exhibits unique morphology and different gas sensing ability. Ni et al. compared the electrochemical performances of the synthesized CuO nanoflowers with previously reported CuO-based nanomaterials, including nanoflowers prepared under different synthesis conditions, for H2O2 determination and glucose biosensing.85
5.3. Alloy nanoflowers
Bimetallic nanoparticles composed of two different metal elements have recently attracted significant attention as catalysts owing to their alloy effects or synergistic effects, resulting in enhanced catalytic performance compared to that of their corresponding monometallic counterparts.321–325Pd is the most commonly used anode catalyst for formic acid oxidation in direct formic acid fuel cells,326–328 and according to a size-effect study of the electro-oxidation of formic acid, Pd nanoparticles with optimal binding energy show the highest activity.329,330
Hybrid catalysts, such as, PdNi,331 PdCo,332,333 PdCu,334 and PdIr335 exhibit enhanced formic acid oxidation compared to single-component catalysts because of their synergistic effects that can manipulate the binding energy of Pd. A similar effect is expected for bimetallic-alloy nanoflowers.
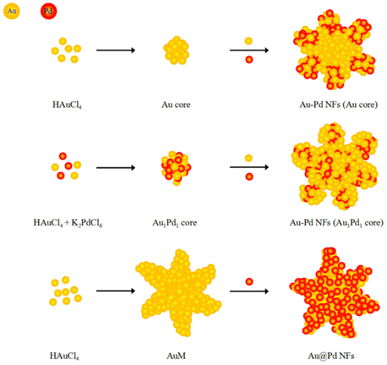 | ||
| Scheme 5 Schematic illustration of the formation of three morphology-controlled Au–Pd nanoflowers (Au–Pd NFs) by seed-mediated growth. Adapted with permission from ref. 336. Published by MDPI. Copyright@ 2017 by Tao Ma et al. | ||
At a low potential of −0.30 V (vs. Pt) and a low temperature of 30 °C, flower-like AuPt alloy nanoparticles are synthesized by the one-step electrochemical reduction method.341 In this method, deep eutectic solvents are used as shape-directing agents and solvents. Through the potentiostatic method, a standard three-electrode cell with a Pt wire counter electrode, a Pt quasi-reference electrode, and a GCE plate (15 mm × 10 mm × 1 mm) working electrode are used for the electrochemical-deposition synthesis of flower-like AuPt alloy nanoparticles in ethaline with 10% water (Fig. 13). Another promising strategy for electro-organic synthesis is mentioned next. The electrode modified with the as-prepared nanomaterials is utilized as the anode in the electrochemical oxidation reaction, using the GCE modified with AuPt nanoflowers, which is directly used to electrooxidize xanthene (XT) to xanthone (XO) with a high yield under a constant low potential (0.80 V vs. Ag/AgCl) at room temperature.
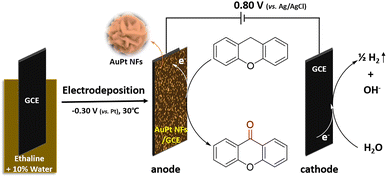 | ||
| Fig. 13 Schematic illustration of the preparation of AuPt nanoflowers (AuPt NFs) and the electrochemical synthesis of xanthone from xanthene. Adapted with permission from ref. 341 Published by Springer Nature. Copyright © 2018, Aoqi Li et al. | ||
Imura et al. reported the preparation of bimetallic Au–Ag nanoflowers by reducing HAuCl4 in a solution containing melamine (as the capping agent) and ascorbic acid (as the reducing agent), which are weakly adsorbed on the surface of the metal.342 Bimetallic Au–Ag nanoflowers are obtained with a clean surface using a support (g-Al2O3)38,343–347 and an extracting method (extracting the surface ligands with water).348,349 A weakly adsorbing capping agent, adequate washing processes to remove capping agents from the metal surface, and a supporting method to inhibit aggregation are vital factors in obtaining surface-clean Au nanoflowers.347 Bimetallic nanoflowers with clean surfaces are applied for the aerobic oxidation of 1-phenylethyl alcohol. Surface-clean Au nanoflowers and spherical Au–Ag nanoparticles of almost the same size and Au/Ag ratio exhibit lower rates for the oxidation of acetophenone than bimetallic nanoflowers.347
A novel atmospheric microplasma induced liquid chemistry (AMILC) method has been applied for the controlled synthesis of 2D and 3D binary Pt3Co nanoflowers by Wang et al.352 As shown in the atmospheric microplasma (AMP) setup in Fig. 14, the electrolyte consists of 0.002 M chloroplatinic acid hydrate (H2PtCl6·xH2O), 0.5 M sodium sulfate (Na2SO4), and variable molar concentrations of cobalt(II) sulfate heptahydrate (CoSO4·7H2O) dissolved in distilled water (40 mL). Bimetallic Pt–Co nanostructures are generated on the cathodic Si substrate, half-inserted in the aqueous electrolyte and separated by at least 3 cm from the anodic capillary.
 | ||
| Fig. 14 Schematic diagram of the atmospheric microplasma-induced liquid chemistry synthesis method. Reprinted with permission from ref. 352. Copyright © 2020 IOP Publishing Ltd. | ||
PtRu alloy nanoflower catalysts are synthesized by controlling their compositions through a solvothermal method and using oleylamine as a coordinating solvent, reductant, and surfactant, through the co-reduction of H2PtCl6·6H2O and RuCl3·xH2O, without additional surfactants, reducing, or structure-directing agents.353 These PtRu nanocatalysts exhibit different morphologies depending on the Pt/Ru ratio. The PtRu nanoflowers composed of nanodendrites exhibit excellent electrocatalytic properties toward methanol electro-oxidation through the normalization of the electrochemically active surface area for direct methanol fuel cells; these properties are derived from the synergetic effect between Pt and Ru and their unique 3D interconnected nanostructures.
Hierarchical nanoporous (hnp) PtCu alloy nanoflowers with different components are fabricated from a PtCuAl precursor alloy by selectively etching Al atoms, with different Pt and Cu ratios, by dissolving the Cu atoms partially.354 In particular, the hnp–Pt35Cu65 sample exhibits superior catalytic activity compared to other PtCu catalysts with different Pt contents. The SEM images (Fig. 15a and b) reveal that each nanoflower consists of a cluster of nanorod-like leaves, approximately 50 nm in width and 100 nm in length. These leaves exhibit uniform interconnected nanopores measuring approximately 5 nm in size, spanning the entire sample. The TEM and HR-TEM images show flower-like structures with bimodal size distributions (Fig. 15c). Pores of various sizes corresponding to the bright region, confirm the formation of hierarchical nanoporous channels, whereas the dark skeleton is attributed to the interconnected nanoscale backbone. The HR-TEM image of hnp–Pt35Cu65 and the ordered lattice fringes are well-resolved in Fig. 15d; the hnp–Pt35Cu65 sample shows good structural stability in the hierarchical nanoporous flower-like architecture. The lattice spacing is determined to be 0.226 nm, indicating that it corresponds to the (1 1 1) crystal plane of the PtCu alloy.
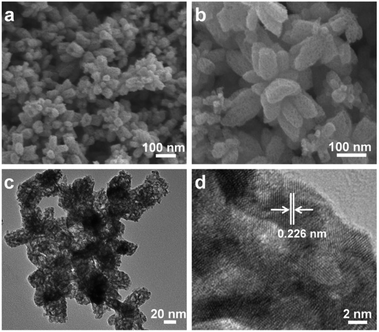 | ||
| Fig. 15 SEM (a and b), TEM (c), and HR-TEM (d) images of the hnp–Pt35Cu65 alloy. Reprinted with permission from ref. 354. Copyright © 2017 Elsevier Inc. | ||
Through a facile wet-chemical method, ultrafine 2D alloyed PdCu nanosheet-constructed 3D flowers are fabricated from a mixture of Na2PdCl4 and CuCl2·2H2O (metal precursors) in DMF (solvent), acetic acid (reducing agent), and W(CO)6 (structural regulator).359 As shown in TEM images, the flower-like Pd2Cu nanoflowers consist of numerous ultrafine nanosheets. The optimal Pd2Cu nanoflowers with a mass/specific activity value of 4714.1 mA mg−1/13.7 mA cm−2 show significantly increased activity toward EGOR, with 4.4 and 6.85-fold increases relative to commercial Pd/C catalysts (1066.8 mA mg−1/2.0 mA cm−2). Pd2Cu nanoflowers exhibit high long-term stability and are a novel class of cost-efficient electrocatalysts that are advantageous for fuel-cell reactions.
An Ir-alloyed ultrathin ternary PdIrCu nanosheet-composed flower (NCF) structure has been synthesized through a one-pot solvothermal reduction in the absence of any surfactant.360 The Cu and Ir atoms together modify the electronic structure of Pd during synthesis, forming an unparalleled ultrathin NCF structure. The PdIrCu/C NCF catalyst exhibits significantly enhanced electrocatalytic activity compared to that of commercial Pd/C for formic acid electro-oxidation. Fig. 16 illustrates the preparation procedures and catalytic mechanism of PdCu and PdIrCu NCF for enhanced formic acid electro-oxidation. Initially, the precursor reactants are transformed into 2D NCFs, which are then cross-stacked into 3D NCFs. The enhanced electrocatalytic performance of PdIrCu/C NCF is attributed to the following: (1) a change in the electronic structure of Pd due to Pd, Ir, and Cu; easy formation of Ir–OH species at a lower potential by the unique surface chemistry of Ir, facilitating the oxidation of COad and its removal from the active sites of catalysts, reducing the poisoning effect; (2) an increase in the specific surface area and formation of more available catalytic active sites by the ultrathin-nanosheet structure of PdIrCu/C; and (3) the enhancement of electroconductivity and promotion of the mass transport process in the electrocatalysis reaction by the unique 3D NCF structure.
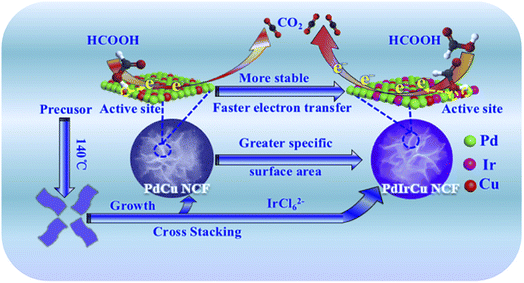 | ||
| Fig. 16 Schematic illustration of the synthesis procedures and enhanced electrocatalytic performance mechanisms of the PdIrCu NCF catalyst. Reprinted with permission from ref. 360. Copyright © 2018, American Chemical Society. | ||
| Compound | Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|---|
| AuPt | AuPd nanoflowers on a polyamidoamine dendrimer-modified surface | Electrodeposition | Electrode materials, electrocatalysts | 115/2006 |
| AuAgCu | Hollow Au–Ag–Cu nanoflowers | Combined seed mediated and galvanic replacement method | Electrocatalysts for direct ethylene glycol fuel cells | 116/2017 |
| Compound | Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|---|
| PtPd | Porous Pt–Pd alloy nanoflowers | One-pot cochemical reduction method in a poly(allylamine hydrochloride) based aqueous solution | Cathode electrocatalyst in direct alcohol fuel cells | 355/2013 |
| Pt3Co | Pt3Co nanoflowers | Solvothermal method without seeds and templates | Electrocatalysts in DMFCs | 70/2014 |
| PtAg | Multi-branched AgPt alloyed dendritic nanoflowers | One-pot successive aqueous coreduction strategy with the assistance of 3-aminopyrazine-2-carboxylic acid (Apzc) as a structure-directing agent | SERS | 356/2017 |
| PtPdAg | Porous ternary Pt–Pd–Ag alloy nanoflowers | Pd-seed mediated co-reduction | Cathode electrocatalyst in advancing fuel cell technology | 357/2017 |
| Compound | Variety of formed nanostructures | Synthesis method | Application | Ref./year |
|---|---|---|---|---|
| NiCo | NiCo nanoflowers | An amphiphilic polymer(Jeffamine)-assisted homogeneous polyol reduction method | Magnetorheological fluids | 361/2014 |
| MoWSe | Mo1−xWxSe2 nanoflowers | Colloidal synthesis | Electrocatalysts for the hydrogen evolution reaction in both acidic and alkaline aqueous solutions | 71/2017 |
| IrCo | IrCo nanoflowers | Ultrasonication with IrCl3·xH2O, Co(acac)2 or Ni(acac) and oleylamine, heated in a hot oil bath | Proton exchange membrane water electrolyzers | 35/2019 |
| IrNi | IrNi nanoflowers |
5.4. Other nanoflowers
Hierarchically structured spherical mesoporous silica nanoflowers (HSMNFs) with well-defined morphologies and uniform sizes are synthesized hydrothermally in a mixture of cyclohexane and water using CPB as the template. TEOS and urea are used as the source of inorganic silica and the hydrolysis additive, respectively.120 The flower, with 200–500 nm size, approximately 10 nm petal thickness, contained 4.0 nm and ca. 40 nm mesopores and macropores, respectively, with up to 502 m2 g−1 surface area. The nanoflower size, structure, and petal thickness strongly depend on the hydrothermal temperature and the molar ratio of Si to CPB; the optimal conditions are 120 °C (hydrothermal temperature) and 4.37 (molar ratio of Si to CPB). This material could be used for catalysis, adsorption, and controlled drug release in medicine because of its hierarchical porous structure, nanoflower morphology, and large surface area.
Dandelion flower-like silica nanoflowers with a uniform size, containing an accessible large surface area, have been synthesized through a visible-light-driven green chemistry method.126 CTAB and urea are dissolved in water, while TEOS is added to a mixture of methyl phenyl ether and hexanol. Subsequently, both the solutions are mixed in a vial by stirring, followed by stirring under visible light (tungsten bulb) for 20 h at 90 °C. After cooling the mixed solution to room temperature, the products are collected and purified by filtration and washing, followed by air-drying and calcination (at 575 °C for 5 h under atmospheric conditions) to remove the structure-directing agent, CTAB. The TEM images indicate that the synthesized silica nanoflowers have a relatively narrow size distribution (with a diameter of 385 ± 5 nm), and the edges of the nanoflower are composed of elongated spikes oriented outwards in three dimensions (Fig. 17D–F).
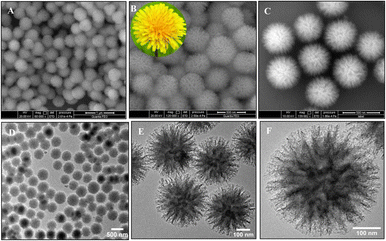 | ||
| Fig. 17 (A–C) FE-SEM micrographs of SiO2 nanomaterials at different magnifications, representing their hierarchical dandelion flower-like morphology. Photograph of a dandelion flower is presented in the inset of Fig. (B). TEM images of (D and E) a bunch of SNF and (F) a single SiO2 nanoflower containing elongated spikes which assemble in a divergent way to form dandelion-like flowery morphology. Reprinted with permission from ref. 126. Copyright © 2019 Elsevier B.V. All rights reserved. | ||
The SiO2 nanoparticles are synthesized by modulating the chain length of alcohols from propanol to octanol, while maintaining the other reaction conditions, to investigate the effect of co-solvents on nanoflower morphology.
The TEM analysis indicates that the morphologies of SiO2 nanoparticles are affected by the alcohol chain length. A noticeable flowery architecture encompassing elongated spikes is formed in higher chain-length alcohol co-solvents, which becomes persistent for hexanol to octanol. Additionally, the specific surface area gradually increases with increasing chain length of the alcohols (propanol to octanol), remaining almost constant beyond hexanol (Table 5). Amine-functionalized SiO2 nanoflowers have been synthesized using (3-aminopropyl)triethoxysilane (SNF-1), N-[3-(trimethoxysilyl) propyl]ethylenediamine (SNF-2), and N1-(3-trimethoxysilyl propyl)diethylenetriamine (SNF-3), respectively, for use as a CO2 adsorbent. Furthermore, chromogenic-functionalized SiO2 nanoflower composites have been fabricated from amine-functionalized nanoflowers for the detection of CO2.
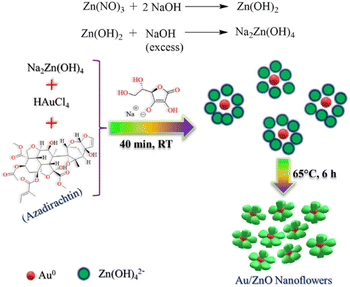 | ||
| Scheme 6 Schematic chart of the fabrication procedure of Au/ZnO hybrid nanoflowers using A. indica (neem) leaf extract. Reprinted with permission from ref. 79. Copyright © 2021, American Chemical Society. | ||
The BET surface area of catalysts toward ZnO, Au, and Au/ZnO hybrid nanoflowers have been measured; the BET surface area of Au/ZnO nanoflowers is 19.23 m2 g−1 for the reduction of 4-nitrophenol to 4-aminophenol, which is wider than that of the others (Table 18). Additionally, the activation energy of the Au/ZnO hybrid nanoflowers (32.46 ± 1.62 kJ mol−1) is lower than that of ZnO (57.75 ± 2.85 kJ mol−1) and Au (48.40 ± 2.41 kJ mol−1). Thus, Au/ZnO hybrid nanoflowers exhibit higher catalytic activity for nitro reduction compared to ZnO and Au. Other metal–metal oxides have been developed as catalysts, electrode materials, and theranostic agents (Table 19).
| System | E a (kJ mol−1) | A (min−1) | ΔS (J mol−1 K−1) | Surface area (m2 g−1) |
|---|---|---|---|---|
| ZnO | 57.75 ± 2.85 | 2.36–2.54 (×108) | 160.76 ± 4.82 | 7.79 |
| Au | 48.40 ± 2.41 | 1.39–1.62 (×107) | 137.2 ± 4.11 | 8.64 |
| Au/ZnO nanoflowers | 32.46 ± 1.62 | 1.15–1.74 (×105) | 98.5 ± 2.94 | 19.23 |
| Compound | Structure | Synthesis method | Application | Ref./year |
|---|---|---|---|---|
| FeTiO3 | Ilmenite FeTiO3 nanoflowers | High-energy ball-milling of ilmenite and subsequent mild hydrothermal treatment in 1 M NaOH aqueous solution | Electrode materials for supercapacitors | 60/2011 |
| FeMnO | Fe0.6 Mn0.4O nanoflowers | High-temperature thermal–decomposition reaction | Theranostic agent with T1–T2 dual-mode MRI for diagnostic purposes, and combined with magnetic hyperthermia for effective therapeutics | 370/2016 |
| NiCo2O4 | NiCo2O4 nanoflowers | Microwave synthesis | Catalysts for the electro-oxidation reaction of methanol in DMFCs | 73/2019 |
| Core | Shell | Structure | Synthesis method | Application | Ref./year |
|---|---|---|---|---|---|
| Au | Pd | Nanoflowers with an Au core and Pd petals | Seed-mediated growth method | Catalysts for Suzuki coupling reaction | 371/2011 |
| Au@Pd core–shell mesoporous nanoflowers | Seed-mediated growth method | Catalysts of methanol oxidation reaction for DMFCs | 372/2021 | ||
| AuPd | Pd | Au–Pd@Pd core–shell nanoflowers | Polyallylamine-assisted water-based synthesis | Electrocatalyst for ethanol electro-oxidation | 373/2015 |
| Au | AgAu | Au@AgAu alloy nanoflowers | Deposition of HAuCl4 on the surface of Au@Ag core–shell nanoparticles | Antibacterial agents | 375/2018 |
| Au | Rh | Au@Rh core–shell nanoflowers | Seeded growth | Electrocatalysts for the hydrogen evolution reaction | 376/2021 |
| Core | Shell | Structure | Synthesis method | Application | Ref./year |
|---|---|---|---|---|---|
| Pd | Pt | Pd-core Pt-shell nanoflowers supported on carbon | Coreduction method | Catalysts for the oxygen reduction reaction of fuel cells | 377/2018 |
| Pt deposited on Pd nanoflowers | Coreduction of Pd and Pt precursors with N,N-dimethylformamide and CTAC as the structure directing agent | Catalysts for methanol oxidation reaction of fuel cells | 378/2020 | ||
| Ag | NiO | NiO layer deposited on Ag nanoflower arrays on a Cu substrate | One-step solution-immersion process and subsequent RF-sputtering method | Anode materials for lithium-ion batteries | 82/2015 |
| Fe3O4 | MnO2 | Fe3O4@MnO2 core–shell nanoflowers | Solvothermal method | Colorimetric detection of phenol in wastewater | 379/2015 |
| MnCo2O4 | Ni(OH)2 | MnCo2O4@Ni(OH)2 multicomponent core–shell nanoflowers on Ni foam | Stepwise hydrothermal process | Supercapacitor electrodes | 380/2016 |
| Co3O4 | NiCo2S4 | Ultrathin NiCo2S4 shell on porous Co3O4 nanoflower array on a Ni-foam substrate | Stepwise hydrothermal process | Supercapacitors | 381/2017 |
| NiCo2O4 | MnO2 | MnO2@NiCo2O4 core–shell nanoflowers on Ni-foam | Hydrothermal process and calcination | Supercapacitors | 382/2021 |
5.4.3.1. Au-core nanoflowers. As mentioned in Section 5.3.1, Au-based Nanoflowers, Au@AuPd (Au–Pd NFs with Au cores, shown in Scheme 5), and Au@Pd (Au@Pd NFs) core–shell nanoflowers are fabricated by a seed-mediated reduction method, whereby Au nanoparticles as seeds induce the growth of a layer of Au and Pd petals and Pd nanoparticles decorated Au nanoflowers as seeds, respectively (Scheme 5).336 The catalytic activities for 4-nitrophenol reduction are in the order of Au@Pd core–shell nanoflowers (Au@Pd NFs) > Au–Pd alloy nanoflowers (Au–Pd NFs (Au1Pd1 core)) > Au@AuPd (Au–Pd NFs (Au core)) core–shell nanoflowers. Thus, the content and reactive surfaces of Pd in the alloys affect their catalytic activities.336
Xu et al. reported the synthesis of Au@Pd core–shell nanoflowers through the reduction of Pd ions by hydroquinone on gold seeds.371 Yin et al. synthesized Au@Pd core–shell mesoporous nanoflowers with Au nanoparticles coated with mesoporous Pd petals, using poly(styrene)-b-poly(ethylene oxide) (PS-b-PEO) as a polymeric micelle-assembled template to induce mesopores in Pd petals by the seed-growth method.372 A facile one-pot synthesis of Au–Pd@Pd core–shell nanoflowers has also been performed in an aqueous polyallylamine solution.373
The Au@CdSe NFs are synthesized via one-pot heterogeneous nucleation and growth. Au nanoparticles are produced by injecting an HAuCl4 solution into an alkyl amine (oleylamine or hexadecylamine) at room temperature, followed by increasing the temperature gradually to 150 °C or 300 °C,128 adding trioctylphosphine oxide to the reaction mixture, and injecting CdO in oleic acid and Se in trioctylphosphine multiple times under stirring. As shown in Scheme 1, the CdSe clusters are adsorbed on the surface defects of the Au core and grow as multiple arms and branches after the injection. Using hexadecylamine instead of oleylamine or increasing the reaction temperature to 300 °C forms larger Au cores, improving the interface between the Au and CdSe domains to produce a core–shell structure. The Ag@CdSe nanoflowers are also prepared using the same synthetic strategy.
The Au@AgPd core–shell nanoflowers are synthesized by a seed-mediated reduction method. First, Au seeds with a narrow size distribution of 13.5 ± 1.0 nm are synthesized by the citrate reduction of HAuCl4·4H2O. Subsequently, AgNO3 and H2PdCl4 are co-reduced by L-ascorbic acid to grow on the surface of the Au seeds using CTAB, which assists the AgPd to bloom on the Au seed.374 The size distribution of Au@AgPd nanoflowers is narrow, with a diameter of 38.4 ± 2.1 nm, branch length of 10.5 ± 1.3 nm, and width of 5.4 ± 0.7 nm. According to inductively coupled plasma mass spectrometry (ICP-MS) analysis, the weight percentages of Au, Ag, and Pd in the Au@AgPd nanoflowers are 14.2%, 40.6%, and 45.3%, respectively, consistent with the ratio of Au/Ag/Pd in the precursors. Compact branches are rarely observed on large Au cores, especially over 60 nm, whereas tiny Au seeds exhibit dense branches on their surface. The Au@AgPd nanoflowers could be applied for the in situ SERS monitoring of the catalytic reaction of 4-nitrothiophenol to 4-aminothiophenol.
5.4.3.2. ZnO-core nanoflowers. Harito et al. reported PbO2-decorated ZnO@TiO2 core–shell nanoflowers. First, a bicarbonate electrolyte produces flower shaped ZnO nanowires on the Zn plate surface by an electrochemical anodizing process317 performed at a cell voltage of 5 V in 30 mM sodium bicarbonate, to produce the optimized uniform ZnO nanoflowers, according to FE-SEM analysis. Second, a layer of TiO2 is coated on the surface of the ZnO nanoflowers via dip coating and calcination to enhance its properties for the photocatalytic degradation of organic dyes. Third, PbO2 is deposited over the ZnO@TiO2 core–shell nanoflowers using anodic electrodeposition, which is applied for the electrochemical oxidation of the dye. These nanoflowers can be used as catalysts for wastewater treatment.
5.4.3.3. Other core–shell nanoflowers. Other core–shell nanoflowers composed of Pd Ag, Fe, Mn, Ni, and Co have been reported for applications in catalysts, anode materials, sensors, and supercapacitors, as shown in Table 21.
5.4.3.4. Conclusion. Core–shell nanoflowers have been investigated in the past decade, with a small number of published papers. The introduced core–shell nanostructures have been fabricated using various modified hydrothermal or solvothermal processes, solution phase synthetic methods, and so on (see Tables 20 and 21). The components of the reported core–shell nanoflowers become increasingly complex from the simple bimetallic Au@Pd371,372 and Pd@Pt377,378 core–shell nanoflowers to the NiCo2O4@MnO2 core–shell nanoflowers.374 The composition ratio of the core–shell nanoflowers is one of the leading factors that affect the shape of the nanomaterial and its catalytic performance. For example, closely spaced and uniformly branched Au@AgPd core–shell nanoflowers are synthesized when the ratio of Pd/Ag is 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, exhibiting high catalytic activity owing to its small size, highly dispersed alloy shell, and defect abundance.374 The size of Au@Rh nanoflowers increases with an increase in the amount of RhCl3 added as the precursor. The Au68Rh32 nanoflowers with high Rh content exhibit high performance in the hydrogen evolution and plasmon-enhanced hydrogen evolution reactions.376
1, exhibiting high catalytic activity owing to its small size, highly dispersed alloy shell, and defect abundance.374 The size of Au@Rh nanoflowers increases with an increase in the amount of RhCl3 added as the precursor. The Au68Rh32 nanoflowers with high Rh content exhibit high performance in the hydrogen evolution and plasmon-enhanced hydrogen evolution reactions.376
| Core | Doping | Structure | Synthesis method | Application | Ref./year |
|---|---|---|---|---|---|
| ZnO | Pt | Pt-doped on ZnO nanoflowers | One-step hydrothermal method | Solar cells and gas sensors | 319/2010 |
| Ag | Ag-doped ZnO nanoflowers on the substrate | Low-temperature hydrothermal process | NO gas sensors | 318/2018 | |
| SnO2 | Zn | Zn-doped SnO2 nanoflowers | Hydrothermal method | Photoanode materials for dye-sensitized solar cells | 384/2011 |
| TiO2 | Ni | Ni-doped rutile TiO2 nanoflowers | Acid treatment of the precursor hydrogen titanate nanowires and immersing the nanowires in an aqueous H2SO4 solution containing NiSO4 | Photocatalysts for wastewater treatment | 37/2016 |
| CeO2 | Fe | Porous Fex-doped Ce1−xO2 with flower-like morphology | Citric acid-assisted hydrothermal process | Catalysts for H2S-selective oxidation | 385/2020 |
| Mo2C | N | N-doped porous molybdenum carbide nanoflowers | Templating method and calcination using Pluronic F127, dopamine hydrochloride, and ammonium molybdate | Acetylcholinesterase (AChE) biosensors for detecting organophosphorus pesticides | 386/2021 |
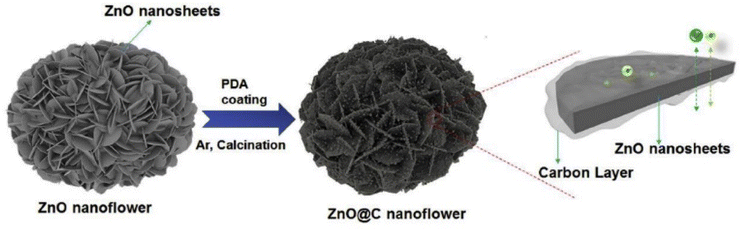 | ||
| Scheme 7 Schematic illustration for the synthesis of ZnO@C nanoflowers. Reprinted with permission from ref. 320. Copyright © 2018 Elsevier B.V. All rights reserved. | ||
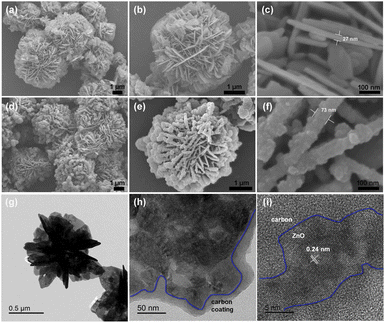 | ||
| Fig. 18 SEM images at different magnifications: (a) low, (b) medium, and (c) high magnification images of pure ZnO nanoflowers; (d) low, (e) medium, and (f) high magnification images of ZnO@C nanoflowers. (g and h) TEM images and (i) HR-TEM image of ZnO@C nanoflowers. Reprinted with permission from ref. 320. Copyright © 2018 Elsevier B.V. All rights reserved. | ||
| Core | Coating | Structure | Synthesis method | Application | Ref./year |
|---|---|---|---|---|---|
| Au | SiO2 | Silica-coated Au nanoflowers on alumina | (1) Melamine assisted surfactant-free synthesis134 | — | 38/2017 |
| (2) Addition of α-Al2O3 (ref. 38 and 347) | |||||
| (3) Silica coating using TEOS387,388 | |||||
| Fe3O4 | C | Fe3O4 nanoflowers coated by a black C layer | One-pot solvothermal route | Biosensors | 39/2020 |
| Compound | Structure | Synthesis method | Application | Ref./year |
|---|---|---|---|---|
| TiN | TiN nanoflower buds | Ammonialization of hydrothermally prepared titanium dioxide nanobuds | Pt-free counter electrodes for DSSC | 40/2021 |
| Bi2S3 | Bi2S3 nanoflowers on Si substrates | Vapor deposition process | Nanoscale photodetectors and optical switches | 60/2008 |
| CdS | CdS and CdSe nanoflowers, branched nanowires, and nanotrees | Solvothermal method using Cd(Ac)2·2H2O and CS(NH2)2 or Na2SeO3 in a mixed solution made of diethylenetriamine and deionized water | Branched CdS nanowires can be applied as photocatalysts for the degradation of acid fuchsin | 57/2006 |
| CdSe | ||||
| VS2 | 1T-VS2 nanoflowers | Hydrothermal method using Na3VO4·12H2O and CH3CSNH2 | Electrocatalysts for the hydrogen evolution reaction | 58/2017 |
| MoS2 | MoS2 nanoflowers | Green hydrothermal synthesis from ammonium molybdenum hydrate and thiourea without using any acids or bases | Photocatalysts for the degradation of methylene blue and crystal violet dyes under natural sunlight | 389/2018 |
| MoS2 nanoflowers | Hydrothermal method | Phase-change materials for thermal energy storage | 390/2021 | |
| MnInS | Mn0.4In1.6S3 nanoflower solid solutions | Hydrothermal method | Photocatalysts | 41/2019 |
| NiCoP | Ni0.7Co0.3P nanoflowers | Hydrothermal–phosphorization method | Electrocatalysts for water splitting in alkaline media | 42/2019 |
| NiCoP | NiCoP nanoflowers or FeCoP nanoflowers on Ni foam | Hydrothermal and low-temperature phosphating method | Supercapacitors | 391/2021 |
| FeCoP | ||||
| CuCoP | Hydrangea-like CuCoPx nanoflowers | Solvothermal and phosphating method | Electrocatalysts for the methanol oxidation reaction | 392/2021 |
| ZnSe | Particle- and flower-shaped ZnSe nanocrystals | Green chemical approach | — | 393/2008 |
| ZnSe rose-like nanoflowers or microspheres on Zn foil | Hydrothermal method | — | 394/2014 | |
| MoSe2 | Colloidal MoSe2 nanoflowers | Colloidal synthesis | — | 88/2015 |
| MoSe2 nanoflowers on carbon cloth | Chemical vapor deposition | Electrocatalysts | 80/2018 | |
| Bi2Se3 | Hierarchical Bi2Se3 nanoflowers | Hot-solution injection method at relatively low temperature (130 °C) | Photocatalysts in the presence of H2O2 | 43/2018 |
| ZnTe | ZnTe nanoflowers, nanodots, and nanorods | High temperature-injection method using zinc stearate as precursors and trioctylphosphine or tributylphosphine as activating agents | — | 44/2007 |
| Core | Doping | Structure | Synthesis method | Application | Ref./year |
|---|---|---|---|---|---|
| CuS | Zn | Zn-doped CuS nanoflowers | Ethanol solvothermal process | Photoelectric and photocatalytic applications | 101/2016 |
| Bi2Se3 | Nd | NdIII doped Bi2Se3 nanoflowers | Hydrothermal synthesis | — | 395/2009 |
| Mo | Mo-doped Bi2Se3 nanoflowers | Solvothermal method | Absorbents for the removal of organic dyes from wastewaters | 102/2013 | |
| MoSe2 | Ru | Ru-doped MoSe2 nanoflowers | Colloidal process | Catalysts of the hydrogen evolution reaction | 89/2019 |
| Co | Homogeneously Co-doped and Co-edge doped MoSe2 nanoflowers | Colloidal synthesis | Catalysts for the hydrogen evolution reaction at the cathode of an electrochemical water splitting cell | 90/2020 | |
| (1) Addition of the dopant atoms to the mixture at the beginning of the reaction | |||||
| (2) Hot injection method |
6. Applications of nanoflowers
Several studies on the properties of nanoflowers have demonstrated promising potential in various applications as catalysts, sensors, supercapacitors, wastewater treatments, chemical reactions, and green energy sources.6.1. Nanoflowers for catalysis
Nanoflowers with highly branched structures exhibit a reasonably large specific surface area and high specific activity (i.e., activity per unit surface area) because of the high densities of the edges, corners, and stepped atoms on their branches.139 Thus, the study of catalysis by nanoflowers is of special interest.The catalytic activities of Au–Pd nanoflowers for the reduction of 4-nitrophenol exhibit the following order: Au@Pd nanoflowers (AuPd alloy) > Au–Pd nanoflowers (Au1Pd1 core) > Au–Pd nanoflowers (Au core). This could be attributed to the different contents and exposed reactive surfaces of Pd in the alloys.336
Bimetallic Au–Ag nanoflower catalysts with a clean surface exhibit higher reaction rates for acetophenone formation than those with Au nanoflowers and spherical nanoparticles of almost the same size and Au/Ag ratio.342 Additionally, the catalytic activities of flower-like NiO for the oxidation of CO292 and Au@Pd core–shell nanoflowers for the Suzuki coupling reaction371 have been studied.
Zhou et al. have reported on the utilization of nanoporous Pt–Cu alloy nanoflower catalysts for the hydrolytic dehydrogenation of NH3BH3, a promising hydrogen storage material.354 The as-prepared hnp–PtCu catalysts exhibit better catalytic performance than pure Pt and Cu catalysts. Also, the hnp–Pt35Cu65 sample, characterized by its stable hierarchical nanoporous flower-like architecture, exhibits superior catalytic performance compared to other PtCu catalysts with different Pt contents. This confirms the significant influence of the Pt-to-Cu ratio on catalytic activity. Table 27 provides the turnover frequency (TOF) values and activation energy (Ea) of all catalysts in the hydrolytic dehydrogenation of NH3BH3. The values for the Pt35Cu65 sample are comparable to or higher than those reported for most Pt-based or other noble metal-based catalysts.
| Catalyst | TOF (mol H2 molM−1 min−1) M = Pt, Ru and Ag | E a (kJ mol−1) | Ref. |
|---|---|---|---|
| a PVP: poly(N-vinyl-2-pyrrolidone), PAMAM:multifunctional poly(amidoamine), RGO: reduced graphene oxide. | |||
| RuCu nanoparticles/graphene | 135 | 30.39 | 396 |
| Pd–Pt@PVP nanoparticles | 125 | 51.7 | 397 |
| hnp–Pt35Cu65 nanoflowers | 108 | 40.5 | 354 |
| Ag0.3Co0.7 nanoparticles/PAMAM | 52.8 | 35.66 | 398 |
Pd–Pt (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanoparticles 1) nanoparticles |
51.9 | — | 399 |
| PdPt cubic nanoparticles | 50.02 | 21.8 | 400 |
| Pt0.65Ni0.35 nanoparticles | 44.3 | 39.0 | 401 |
| PtPd spherical nanoparticles | 22.51 | 57.3 | 400 |
RuCu (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/γ-A12O3 1)/γ-A12O3 |
16.4 | 52 | 402 |
| Cu nanoparticles/RGO | 3.61 | 38.2 | 403 |
Mesoporous NiO nanoflowers consisting of crossed nanosheets, are synthesized using Pluronic F127 as a micellar template (synthesis details can be found in Scheme 4).286 The process of assembling the interface is controlled by adjusting the amount of NH3·H2O, which led to the ability to modify the thickness and dimensions of the mesoporous NiO nanosheets. These nanoflowers are utilized for electrochemical nitrogen reduction reaction (NRR), a sustainable method for NH3 synthesis that offers a viable alternative to the industrial energy and Haber–Bosch process under ambient conditions.406,407 NRR measurements demonstrate that NiO–NF-3.0, having the smallest mesopores and the thinnest nanosheets in all NiO–NF samples, exhibits excellent NRR performance, with an NH3 yield of 16.16 μg h−1 mg−1cat. and faradaic efficiency (FE) of 9.17% at −0.4 V vs. reversible hydrogen electrode in 0.1 M Na2SO4. X-ray photoelectron spectroscopy (XPS) confirms the presence of Ni2+ and Ni3+ in all NiO–NF samples, with NiO–NF-3.0 displaying the highest Ni3+ content, correlating with its superior electrochemical NRR performance. This can be attributed to the electropositivity promotion of NiO-NFs by Ni3+, facilitating the adsorption of N2 gas and enhancing the electrocatalytic properties. The NRR performances of the five different NiO–NF electrodes follow the order: NiO–NF-3.0 > NiO–NF-5.0 > NiO–NF-7.0 > NiO–NF-1.0 > NiO–NF-0.5. Interestingly, the abundance of Ni3+ in NiO-NFs also follows the same order, indicating that the activity in the NRR is influenced by the abundance of Ni3+, enabling easier nitrogen adsorption compared to Ni2+. These improved NRR performances are comparable to or even surpass those of reported catalysts containing noble metals, transition metal oxides, and NiO composite materials. Table 28 provides a summary of NRR performances for various recently reported catalysts, and the prepared NiO–NF-3.0 demonstrates comparable or superior performance to most of these catalysts.
| Catalyst | Electrolyte | Yield (μg h−1 mg−1cat.) | FE (%) | Ref. |
|---|---|---|---|---|
| Pd nanoparticles | 0.1 M PBS | 4.5 | 8.2% | 408 |
| Pd–Ag–S nanosponges | 0.1 M Na2SO4 | 9.73 | 18.41% | 409 |
| Mn3O4 nanocubes | 0.1 M Na2SO4 | 11.6 | 3% | 410 |
| Fe2O3 nanorods | 0.1 M Na2SO4 | 15.9 | 0.94% | 411 |
| NiO nanodots on graphene | 0.1 M Na2SO4 | 18.6 | 7.8% | 412 |
| N-C@NiO nanosheets on graphite paper | 0.1 M HCl | 14.022 | 30.43% | 413 |
| NiO nanoflowers | 0.1 M Na2SO4 | 16.16 | 9.17% | 286 |
The photocatalytic activities of TiO2 nanoflowers (TNF-T-t, T = calcination temperature, t = time of calcination) for the photo-oxidation of aqueous methylene blue under UV irradiation have been evaluated. The TNF-T-t (T = 400–800 °C, t = 1–5 h) photocatalysts show remarkable activity for the photo-oxidation at pH 6 under UV excitation (365 nm), with activities in the following order: TNF-700-1 > TNF-600-1 > TNF-500-1 > TNF-400-1∼P25 TiO2 (the commercially available photocatalyst) TNF-800-1. Excessive rutile formation by prolonged calcination of the TNFs at 700 °C is detrimental to their dye-degradation performance, as it reduces the photocatalyst surface area and inhibits OH˙ formation.220 The MoS2 nanoflowers exhibit almost 100% degradation of methylene blue and crystal violet under natural sun light.415
Three types of fluffy ZnO nanoflowers with different nanostructures: one with smooth edges (ZnO-0 generated via a hydrothermal process without ultrasonic treatment) and two with different jagged margins (ZnO-250 and ZnO-950 produced via ultrasonic treatment at 250 W and 950 W, respectively), have been evaluated as photocatalysts for the degradation of methyl orange. ZnO-0 exhibits the highest photocatalytic activity with a kinetic constant of 0.0478 min−1.324 The photocatalytic behavior of Bi2Se3 nanoflowers has been explored by degrading rhodamine B in the presence of H2O2, whereby the degradation rate of rhodamine B increases up to 93% after 2 h of visible-light irradiation.43 The Ni-free rutile TiO2 nanoflowers and Ni-doped rutile TiO2 nanoflowers exhibit two and four times the photocatalytic activity of Degussa P25 TiO2 powder, respectively, for the photodegradation of rhodamine B in water under Xe lamp illumination. This could be attributed to their higher specific surface areas (115 and 118 m2 g−1 for Ni-free rutile TiO2 nanoflowers and Ni-doped rutile TiO2 nanoflowers, respectively) than Degussa P25 TiO2 powder (50 m2 g−1), and Ni-doping that favors light harvesting and charge separation.37
Plasmonic Pd nanoflowers exhibit high photocatalytic activity for the Suzuki–Miyaura cross-coupling reactions under visible light irradiation.156 Hierarchical flower-like TiO2 superstructures show significantly higher photocatalytic activity than commercial Degussa P25 and tabular-shaped anatase TiO2, due to a hierarchically porous structure, exposed {001} facets, and increased light harvesting ability.218
6.2. Sensors
Studies on the detection of industrially produced volatile organic compounds and toxic gases have attracted considerable attention because they cause environmental pollution, which seriously affects human safety. Semiconducting nanomaterials with large surface-area-to-volume ratios are potential candidates for gas-sensing applications.A CuO nanoflower-based gas-sensing device has been reported to exhibit a very strong response for acetone vapor (250–2250 ppm concentration). The stability of the sensing device is maintained with a very small change (3.3% change in response) on repeating measurements for one month.275 A hierarchical 3D TiO2 nanoflower sensor exhibits high selectivity toward acetone at 60 °C with response magnitudes of 3.45% and 66.58% corresponding to response times of 33 s and 19 s and recovery times of 11 s and 37 s for 1 ppm and 700 ppm acetone, respectively.92 Additionally, N-doped porous Mo2C nanoflowers have been used to fabricate acetylcholinesterase biosensors for detecting organophosphorus pesticides.386
The NiO nanoflowers show excellent responses to NO2 (among various gases, such as, H2, NO2, CO, toluene, NH3, and H2S) at 150 °C and 100 ppm due to the enhanced interaction between NO2 and NiO owing to their mesoporous flower structure and large surface area.379 The 530 nm-light-assisted Au@MoS2 gas sensor utilizing the localized surface plasmon resonance (LSPR) effect of Au nanoparticles has been developed to detect NO2 gas with a ppb-level detection limit and robust resistance to humidity interference.61 By utilizing 530 nm light irradiation, the gas sensor can achieve a limit of detection as low as 10 ppb for NO2, without requiring a specific operating temperature. Additionally, it demonstrates robust resistance to humidity, maintaining steady response values with only a slight drift of 6.5% across a wide range of humidity variations (10–90% relative humidity) because the illumination of light can decelerate the adsorption of H2O molecules while facilitating the desorption of H2O molecules.414 The effective utilization of the LSPR enhances the absorption of visible light by MoS2, leading to an increased surface carrier concentration. Table 29 provides a comparison of the gas-sensitive properties between the Au–MoS2 gas sensor assisted by 530 nm light and other previously reported NO2 sensors that utilize visible-light assistance. The prepared nanoflowers exhibit superior performance in terms of response value, detection limit, and recovery time when compared to other gas sensing materials.
| Gas-sensing material | NO2 (ppm) | Response value | Detection limit (ppb) | Recovery time | Excitation source | Ref. |
|---|---|---|---|---|---|---|
| ZnO–Ag nanoparticles | 5 | 4.5 | 500 | No | 430 nm LED | 415 |
| CdS nanoflakes | 5 | 89% | 20 | 113 s | 530 nm LED | 416 |
| WO3 | 0.16 | 2.9 | 160 | 18.3 min | 480 nm LED | 417 |
| CdSe quantum dots/ZnO | 1 | 20 | 200 | >60 min | 535 nm LED | 418 |
| Au nanoparticles-ZnO nanorods | 1 | 1.093 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2500 s | 495 nm LED | 419 |
| Black NiO | 0.372 | 31.04% | 57 | No | 480 nm LED | 420 |
| WO3 nanofibers | 0.4 | 1.49 | 160 | >60 min | 430 nm LED | 421 |
| Au–MoS2 nanoflowers | 1 | 8.1 | 10 | 27 s | 530 nm LED | 61 |
6.3. Supercapacitors
The widespread applications of supercapacitors (also called electrochemical capacitors or ultracapacitors) in transportable electronics, uninterruptible power supplies, hybrid electric vehicles, and renewable energy sources have received significant attention from researchers owing to their high power densities, long cycle life, low cost, and fast charge–discharge rates compared to those of lithium-ion batteries and conventional capacitors.422,423 Transition metal oxides with nanostructures exhibit immense potential as electrodes for supercapacitors because of their excellent pseudocapacitive behavior and relatively high electrical conductivity.424–426Electrochemical tests of CuO nanoflowers fabricated on the surface of flexible Cu foil exhibit a high specific capacitance of 284.5 F g−1 at a current density of 0.5 mA cm−2 and high electrode cyclability (with a 20% loss in capacitance over 1000 cycles).261 Among the three different morphologies of NiO nanomaterials (particle, honeycomb, and 3D nanoflower), NiO nanoflowers show the highest specific capacitance (364 F g−1 at 1 A g−1), rate capability (64% capacitance retention from 1 A g−1 to 10 A g−1), and cycling stability.427 The Co3O4@NiCo2S4 core–shell nanoflower array show excellent electrochemical performance: 1234 F g−1 specific capacitance at a current density of 1 A g−1, with a maximum energy density of 168 W h kg−1, and approximately 11% loss of initial specific capacitance after 5000 cycles, owing to the unique configuration and strong synergistic effect of the porous Co3O4 core and the ultrathin NiCo2S4 shell.381
Nanoflower-like MCoP (M = Ni and Fe) composites on Ni foam, denoted as NF@MCoP, are synthesized through hydrothermal and low-temperature phosphating methods. NF@MCoP provides a larger electroactive area, shorter electron transfer distance, and faster electron conductivity due to its unique 3D structure, nanoflower morphology, and the conductive substrate, and exhibits enhanced electrochemical performance.391 Optimization of the feeding molar ratio of M/Co, the amount of dimethyl sulfoxide (DMSO), and the hydrothermal time of Co precursors is carried out to improve the electrochemical capacitive performance of as-prepared NF@MCoP electrodes. The samples with a M/Co feeding molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 exhibits a higher specific capacitance compared to the other samples (1
2 exhibits a higher specific capacitance compared to the other samples (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, and 1.5
1.5, and 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The sample synthesized using 8 mL of DMSO demonstrates the highest specific capacitance, which aligns well with the cyclic voltammetry result. Extending the hydrothermal time to 6 hours results in a decrease in the electrochemical performance of nanoflower-like MCoP composites on Ni foam. The nanoflower-like composites show remarkable energy density, power density, and long-term cycling stability. The area-specific capacitance values of NF@FeCoP and NF@NiCoP composites are measured to be 6.2 and 6.9 F cm−2, respectively.
1). The sample synthesized using 8 mL of DMSO demonstrates the highest specific capacitance, which aligns well with the cyclic voltammetry result. Extending the hydrothermal time to 6 hours results in a decrease in the electrochemical performance of nanoflower-like MCoP composites on Ni foam. The nanoflower-like composites show remarkable energy density, power density, and long-term cycling stability. The area-specific capacitance values of NF@FeCoP and NF@NiCoP composites are measured to be 6.2 and 6.9 F cm−2, respectively.
According to chronopotentiometry and cyclic voltammetry investigations, the hybrid battery–supercapacitor (HBS) devices display excellent capacitive performance, with 48.1 W h kg−1 at 2400 W kg−1 for NF@FeCoP and 33.3 W h kg−1 at 3746 W kg−1 for NF@NiCoP, along with high retention rates of 88% and 89% after 5000 cycles for NF@FeCoP and NF@NiCoP, respectively. The excellent performance of the NF@MCoP nanostructure can be attributed to several factors, including the highly reactive surface and short diffusion path provided by the nanoflower structure, the significant influence of DMSO on the morphology of NF@MCoP, and the low series resistance resulting from direct deposition on Ni foam.
6.4. Li-ion battery
Due to its high voltage, high capacity, long cycle life, and excellent safety performance,428,429 the lithium-ion battery is an efficient energy storage device that has gained widespread use in portable electronics and is increasingly becoming the primary power source for electric vehicles.280,430 To enhance the rate at which lithium ions intercalate and deintercalate in electrode materials, as well as to improve the capacity, cyclability, and safety, there has been significant focus on nano-sized electrode materials in recent years. Particularly, extensive research has been conducted on constructing 3D hierarchical nanostructures.305,431–433 SnO nanoflowers have the potential to serve as effective anode materials in lithium-ion rechargeable batteries, exhibiting a high capacity of approximately 800 mA h g−1, which is close to the theoretical value of 875 mA h g−1.305 In addition, the reversible capacities of metal oxide materials with 3D hierarchical nanostructures have been found to be significantly high when used as anode electrode materials in Li-ion batteries. This is primarily due to their large surface areas.434,435 Hence, it is crucial to have precise control over the morphology during the preparation of the nanoflowers to ensure their effectiveness in applications related to lithium-ion rechargeable batteries.Cao et al. studied the electrochemical properties of NiO electrodes in a lithium-ion battery. They found that the initial discharge capacity of the NiO nanoflowers is approximately 1300 mA h g−1.295
The high surface area (71.9 m2 g−1) of the porous flower-like α-Fe2O3 nanostructures, as determined by the BET test, significantly enhances its performance in Li-ion battery applications.306 Increasing the surface area or porosity of the hematite crystals improves the lithium intercalation performance.306 In Fig. 19, the charge/discharge curves of the flower-like α-Fe2O3 nanostructures are compared for the first, tenth, and twentieth cycles. The initial discharge capacity is 974.43 mA h g−1. Even after 10 and 30 cycles, the discharge capacities remain higher than that of graphite, with values of 705.5 and 548.47 mA h g−1, respectively. These flower-like nanostructures exhibit superior cycling performance compared to the previously reported α-Fe2O3 hollow spindles and microspheres (1313 and 1298 mA h g−1) due to their higher surface area.436
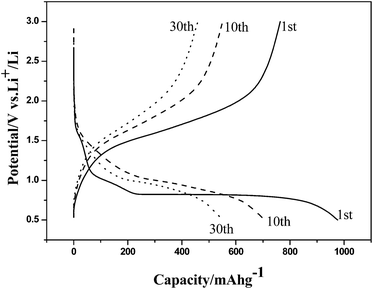 | ||
| Fig. 19 The first (solid line), 10th (dashed line), and 30th (dotted line) charge–discharge curves of flower-like α-Fe2O3 nanostructures at a current density of 0.2 mA cm−2. Reprinted with permission from ref. 306. Copyright © 2008, American Chemical Society. All rights reserved. | ||
Interestingly, the electrochemical properties of porous NiO nanoflowers are compared to those of similar NiO nanostructures (Table 30). The NiO nanoflower electrode demonstrates a favorable and consistent discharge capacity. Also, at a high current rate of 1C, the electrode exhibited an initial discharge capacity of 671.2 mA h g−1 and maintained a stable capacity of 306.8 mA h g−1 even after 80 cycles with a high coulombic efficiency of 98%. The produced NiO nanoflowers demonstrated promising outcomes as anode materials for Li-ion batteries, attributed to their high surface area and short diffusion length.100
| Sample | Current density (mA g−1) | Potential range (V) | Initial capacity (mA h g−1) | Capacity retention (mA h g−1) | Ref. |
|---|---|---|---|---|---|
| NiO nanotubes | 25 | 0.01–3.0 | 610 | 200 after 200 cycles | 285 |
| NiO microspheres | 50 | 0.01–3.0 | 1570 | 80 after 30 cycles | 437 |
| Hollow NiO microspheres | 100 | 0.02–3.0 | 1100 | 560 after 50 cycles | 438 |
| NiO nanospheres | 100 | 0.01–3.0 | 1200 | 518 after 60 cycles | 439 |
| NiO–Ni nanocomposite | 286 | 0.02–3 | 503 | 420 after 100 cycles | 440 |
| Three-dimensional porous NiO | 200 | 0.05–3.0 | 800 | 520 after 30 cycles | 441 |
| Flower-like NiO microspheres | 50 | 0.01–3.0 | 1104 | 105 after 30 cycles | 442 |
| Urchin-like NiO microspheres | 50 | 0.01–3.0 | 1295 | 134 after 50 cycles | 442 |
| Electrospun NiO nanofibers | 100 | 0.005–3.0 | 1280 | 583 after 100 cycles | 443 |
| Porous NiO nanoparticles | 15 | 0.01–3.0 | 900 | 380 after 100 cycles | 444 |
| Hierarchical NiO nano/microspheres | 100 | 0.01–3.0 | 1165 | 720 after 100 cycles | 445 |
| Hierarchical NiO microspheres | 700 | 0.005–3.0 | 1452 | 598 after 100 cycles | 446 |
| Hollow NiO nanotubes | 200 | 0.01–3.0 | 1072 | 600 after 100 cycles | 447 |
| NiO nanoflowers | 100 | 0.01–3.0 | 1936.9 | 552 after 50 cycles | 100 |
7. Conclusion and future perspectives
Research related to flower-like hierarchical nanomaterials has developed considerably, with the exploration of synthetic technologies, growth mechanisms, particle size distributions, morphologies, chemical, physical, and optical properties to enhance catalytic, energy-related, and biomedical applications. Numerous types of inorganic-based nanoflowers have been synthesized through physical, chemical, biological, and hybrid methods: physical vapour deposition, solvothermal and hydrothermal processes, chemical vapour deposition, sol–gel, plant extract (green or bio synthesis), electrochemical deposition, galvanic displacement, microwave-assisted synthesis, high-energy ball-milling hydrothermal treatment, and the solution-immersion RF-sputtering method. In the last two decades, research related to flower-shaped hierarchical nanomaterials has progressed rapidly, and the number of related publications has gradually increased owing to their merits, such as high surface-to-volume ratio, strong adsorption capacity, high loading efficiency, and excellent catalytic activity.Research on alloy and core–shell nanoflowers has particularly gained prominence, and flower-shaped nanostructure alloys and metal/metal oxide core–shell structures have been investigated for potential applications in the photo- and electro-catalysis of water splitting and fuel cells, owing to their composition and synergistic effects.
The modification of synthetic pathways, not by using surfactants, templates, or stabilizing agents, but rather by promoting reactions at lower temperatures and for shorter times has led to the development of simple, cost-effective, and eco-friendly synthetic routes for industrial-scale applications. Additionally, numerous studies have been conducted by controlling the reaction conditions (including the type and amount of reagents (precursors, reducing agents, surfactants, template agents), reaction time, temperature, and ratio of metals) to optimize the morphology and size distribution for specific applications. Numerous unique functionalities and potential applications have been reported.
Future research could focus on the development of uniform and monodisperse nanoflowers with high electrochemical, optical, and physical properties, suitable for various applications. Additionally, research on alloy, core–shell, and surface-treated nanoflowers is expected to expand multi-metallic compositions with modified structures. Furthermore, future studies on nanomaterials will potentially encompass the combination of multiple nanostructured materials, such as CoS nanoflowers wrapped in reduced graphene oxides,448 nitrogen-doped graphene-encapsulated Ni–Cu alloy nanoflowers,449 Pt nanoflower monolayers on single-walled carbon nanotube membranes,450 and amorphous carbon nanotube–nickel oxide nanoflower hybrids.451
Author contributions
Conceptualization, H. J. and D. N. L.; methodology, S. J. L., H. J. and D. N. L.; validation, S. J. L. and H. J.; formal analysis, S. J. L.; investigation, S. J. L.; data curation, S. J. L. and D. N. L.; writing—original draft preparation, S. J. L.; writing—review and editing, H. J. and D. N. L.; supervision, D. N. L.; funding acquisition, S. J. L., H. J., and D. N. L. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (grants 2021R1A2C1004285 and 2019R1A2C2086770), by the Nano Material Technology Development Program through the NRF funded by the Ministry of Science, ICT, and Future Planning (2009-0082580), and by a Center for Women in Science, Engineering and Technology (WISET) grant funded by the Ministry of Science and ICT (MSIT) under the program for returners into R&D.Notes and references
- R. P. Feynman, Eng. Sci., 1960, 23, 22–36 Search PubMed . https://resolver.caltech.edu/CaltechES:23.5.1960Bottom.
- G. Binnig and H. Rohrer, US Pat., US4343993A, 1982 Search PubMed.
- G. Binnig, H. Rohrer, C. Gerber and E. Weibel, Appl. Phys. Lett., 1982, 40, 178, DOI:10.1063/1.92999.
- G. Binnig, H. Rohrer, C. Gerber and E. Weibel, Phys. Rev. Lett., 1982, 49, 57, DOI:10.1103/PhysRevLett.49.57.
- G. Binnig, C. F. Quate and C. Gerber, Phys. Rev. Lett., 1986, 56, 930, DOI:10.1103/PhysRevLett.56.930.
- G. Binnig, US Pat., 4724318A, 1990 Search PubMed.
- X. Fan, N. Soin, H. Li, H. Li, X. Xia and J. Geng, Energy Environ. Mater., 2020, 3, 469–491, DOI:10.1002/eem2.12071.
- M. Gaboardi, N. S. Amade, M. Aramini, C. Milanese, G. Magnani, S. Sanna, M. Ricco and D. Pontiroli, Carbon, 2017, 120, 77–82, DOI:10.1016/j.carbon.2017.05.025.
- T. Tang, T. Zhang, W. Li, X. Huang, X. Wang, H. Qiu and Y. Hou, Nanoscale, 2019, 11, 7440–7446, 10.1039/C8NR09495K.
- G. Liu, W. Jin and N. Xu, Chem. Soc. Rev., 2015, 44, 5016–5030, 10.1039/C4CS00423J.
- Y. Xiang, W. Zhu, W. Guo, F. Lou, B. Deng, D. Liu, Z. Xie, J. Li, D. Qu and H. Tang, J. Alloys Compd., 2017, 719, 347–352, DOI:10.1016/j.jallcom.2017.05.217.
- P. Nikolaev, M. J. Bronikowski, R. K. Bradley, F. Rohmund, D. T. Colbert, K. A. Smith and R. E. Smalley, Chem. Phys. Lett., 1999, 313, 91–97, DOI:10.1016/S0009-2614(99)01029-5.
- M. C. Wu, A. Sápi, A. Avila, M. Szabó, J. Hiltunen, M. Huuhtanen, G. Tóth, A. Kukovecz, Z. Kónya, R. Keiski, W. F. Su, H. Jantunen and K. Kordás, Nano Res., 2011, 4, 360–369, DOI:10.1007/s12274-010-0090-9.
- D. H. Nam, T. H. Kim, K. S. Hong and H. S. Kwon, ACS Nano, 2014, 8(11), 11824–11835, DOI:10.1021/nn505536t.
- Y. Li and W. Shen, Chem. Soc. Rev., 2014, 43, 1543–1574, 10.1039/C3CS60296F.
- C. Morita-Imura, T. Kobayashi, Y. Imura, T. Kawai and H. Shindo, RSC Adv., 2015, 5, 75889–75894, 10.1039/C5RA17369H.
- R. Takahata, S. Yamazoe, K. Koyasu and T. Tsukuda, J. Am. Chem. Soc., 2014, 136, 8489–8491, DOI:10.1021/ja503558c.
- S. M. Alia, S. Shulda, C. Ngo, S. Pylypenko and B. S. Pivovar, ACS Catal., 2018, 8, 2111–2120, DOI:10.1021/acscatal.7b03787.
- R. Kumar, A. Kumar, N. Verma, V. Khopkar, R. Philip and B. Sahoo, ACS Appl. Nano Mater., 2020, 3, 8618–8631, DOI:10.1021/acsanm.0c01284.
- M. Hirasawa, T. Orii and T. Seto, Appl. Phys. Lett., 2006, 88, 093119, DOI:10.1063/1.2182018.
- K. Shoyama and F. Würthner, J. Am. Chem. Soc., 2019, 141, 13008–13012, DOI:10.1021/jacs.9b06617.
- R. Bhuvaneswari, V. Nagarajan and R. Chandiramouli, J. Electron Spectrosc. Relat. Phenom., 2018, 227, 15–22, DOI:10.1016/j.elspec.2018.06.002.
- B. Jiang, Y. Guo, J. Kim, A. E. Whitten, K. Wood, K. Kani, A. E. Rowan, J. Henzie and Y. Yamauchi, J. Am. Chem. Soc., 2018, 140, 12434–12441, DOI:10.1021/jacs.8b05206.
- Y. T. Tsai, S. J. Chang, I. T. Tang, Y. J. Hsiao and L. W. Ji, IEEE Sens. J., 2018, 18, 5559–5565, DOI:10.1109/JSEN.2018.2830508.
- S. Sharifi, S. Behzadi, S. Laurent, M. L. Forrest, P. Stroeve and M. Mahmoudi, Chem. Soc. Rev., 2012, 41, 2323–2343, 10.1039/c1cs15188f.
- Y. Li, H. Wu and Z. Su, Coord. Chem. Rev., 2020, 416, 213342, DOI:10.1016/j.ccr.2020.213342.
- P. Shende, P. Kasture and R. S. Gaud, Artif. Cells, Nanomed., Biotechnol., 2018, 46(S1), 413–422, DOI:10.1080/21691401.2018.1428812.
- A. Guerrero-Martínez, S. Barbosa, I. Pastoriza-Santos and L. M. Liz-Marzán, Curr. Opin. Colloid Interface Sci., 2011, 16, 118–127, DOI:10.1016/j.cocis.2010.12.007.
- M. A. Mahmoud, R. Narayanan and M. A. El-Sayed, Acc. Chem. Res., 2013, 46, 1795–1805, DOI:10.1021/ar3002359.
- M. A. Mahmoud, B. Garlyyev and M. A. El-Sayed, J. Phys. Chem. Lett., 2014, 5, 4088–4094, DOI:10.1021/jz502071v.
- N. Soetan, H. F. Zarick, C. Banks, J. A. Webb, G. Libson, A. Coppola and R. Bardhan, J. Phys. Chem. C, 2016, 120, 10320–10327, DOI:10.1021/acs.jpcc.6b01238.
- S. J. Lee, H. Jang and D. N. Lee, Pharmaceutics, 2022, 14, 1887, DOI:10.3390/pharmaceutics14091887.
- Q. Liu, X. Guo, Y. Li and W. Shen, J. Phys. Chem. C, 2009, 113, 3436–3441, DOI:10.1021/jp8081744.
- K. Seal, H. Chaudhuri, S. Basu, M. K. Mandal and S. Pal, Arabian J. Sci. Eng., 2021, 46, 6315–6331, DOI:10.1007/s13369-020-04988-4.
- F. Lv, W. Zhang, W. Yang, J. Feng, K. Wang, J. Zhou, P. Zhou and S. Guo, Small Methods, 2020, 4, 1900129, DOI:10.1002/smtd.201900129.
- K. Gwon, J. D. Park, S. Lee, I. Han, J. S. Yu and D. N. Lee, J. Ind. Eng. Chem., 2021, 99, 264–270, DOI:10.1016/j.jiec.2021.04.038.
- L.-L. Lai, W. Wen and J.-M. Wu, RSC Adv., 2016, 6, 25511–25518, 10.1039/C6RA01752E.
- Y. Imura, S. Koizumi, R. Akiyama, C. Morita-Imura and T. Kawai, Langmuir, 2017, 33, 4313–4318, DOI:10.1021/acs.langmuir.7b00974.
- A. Moyano, E. Serrano-Pertierra, M. Salvador, J. C. Martínez-García, Y. Piñeiro, S. Yañez-Vilar, M. Gónzalez-Gómez, J. Rivas, M. Rivas and M. C. Blanco-López, Biosensors, 2020, 10, 80, DOI:10.3390/bios10080080.
- S. Gnanasekar and A. N. Grace, ACS Appl. Nano Mater., 2021, 4, 8251–8261, DOI:10.1021/acsanm.1c01447.
- H. Liang, B. Guo, J. Huang, T. Feng, W. Wang, B. Dong and L. Cao, ACS Appl. Nano Mater., 2019, 2, 5245–5253, DOI:10.1021/acsanm.9b01102.
- K. Dai, X. Gao, L. Yin, Y. Feng, X. Zhou, Y. Zhao and B. Zhang, Appl. Surf. Sci., 2019, 494, 22–28, DOI:10.1016/j.apsusc.2019.07.160.
- D. Li, M. Fang, C. Jiang, H. Lin, C. Luo, R. Qi, R. Huang and H. Peng, J. Nanopart. Res., 2018, 20, 228, DOI:10.1007/s11051-018-4342-z.
- S. H. Lee, Y. J. Kim and J. Park, Chem. Mater., 2007, 19, 4670–4675, DOI:10.1021/cm0711360.
- J. B. Lee, S. Peng, D. Yang, Y. H. Roh, H. Funabashi, N. Park, E. J. Rice, L. Chen, R. Long, M. Wu and D. Luo, Nat. Nanotechnol., 2012, 7, 816–820, DOI:10.1038/nnano.2012.211.
- Y. Xiao, M. Zhang, F. X. Wang and G. B. Pan, CrystEngComm, 2012, 14, 1933–1935, 10.1039/C2CE06401D.
- S. Karan and B. Mallik, J. Phys. Chem. C, 2008, 112, 2436–2447, DOI:10.1021/jp709780a.
- S. Chen, D. M. Koshy, Y. Tsao, R. Pfattner, X. Yan, D. Feng and Z. Bao, J. Am. Chem. Soc., 2018, 140(32), 10297–10304, DOI:10.1021/jacs.8b05825.
- Z. Xu, X. Zhuang, C. Yang, J. Cao, Z. Yao, Y. Tang, J. Jiang, D. Wu and X. Feng, Adv. Mater., 2016, 28, 1981–1987, DOI:10.1002/adma.201505131.
- Y. Zheng, S. Chen, K. A. I. Zhang, J. Zhu, J. Xu, C. Zhang and T. Liu, ACS Appl. Mater. Interfaces, 2021, 13(11), 13328–13337, DOI:10.1021/acsami.1c01348.
- D. Shcharbin, I. Halets-Bui, V. Abashkin, V. Dzmitruk, S. Loznikova, M. Odabası, Ö. Acet, B. Önal, N. Özdemir, N. Shcharbina and M. Bryszewska, Colloids Surf., B, 2019, 182, 110354, DOI:10.1016/j.colsurfb.2019.110354.
- S. W. Lee, S. A. Cheon, M. I. Kim and T. J. Park, J. Nanobiotechnol., 2015, 13, 54, DOI:10.1186/s12951-015-0118-0.
- J. Cui and S. Jia, Coord. Chem. Rev., 2017, 352, 249–263, DOI:10.1016/j.ccr.2017.09.008.
- D. Shcharbin, I. Halets-Bui, V. Abashkin, V. Dzmitruk, S. Loznikova, M. Odabaşı, Ö. Acet, B. Önal, N. Özdemir, N. Shcharbina and M. Bryszewska, Colloids Surf., B, 2019, 182, 110354, DOI:10.1016/j.colsurfb.2019.110354.
- K. H. Kim, J.-M. Jeong, S. J. Lee, B. G. Choi and K. G. Lee, J. Colloid Interface Sci., 2016, 484, 44–50, DOI:10.1016/j.jcis.2016.08.059.
- Z.-F. Wu, Z. Wang, Y. Zhang, Y.-L. Ma, C.-Y. He, H. Li, L. Chen, Q.-S. Huo, L. Wang and Z.-Q. Li, Sci. Rep., 2016, 6, 22412, DOI:10.1038/srep22412.
- W. T. Yao, S. H. Yu, S. J. Liu, J. P. Chen, X. M. Liu and F. Q. Li, J. Phys. Chem. B, 2006, 110(24), 11704–11710, DOI:10.1021/jp060164n.
- Y. Qu, M. Shao, Y. Shao, M. Yang, J. Xu, C. T. Kwok, X. Shi, Z. Lu and H. Pan, J. Mater. Chem. A, 2017, 5, 15080–15086, 10.1039/C7TA03172F.
- T. P. Nguyen and I. T. Kim, Nanomaterials, 2020, 10, 1336, DOI:10.3390/nano10071336.
- T. Tao, A. M. Glushenkov, H. Liu, Z. Liu, X. J. Dai, H. Chen, S. P. Ringer and Y. Chen, J. Phys. Chem. C, 2011, 115, 17297–17302, DOI:10.1021/jp203345s.
- P. Chen, J. Hu, M. Yin, W. Bai, X. Chen and Y. Zhang, ACS Appl. Nano Mater., 2021, 4, 5981–5991, DOI:10.1021/acsanm.1c00847.
- M. Y. A. Rahman, A. A. Umar, L. Roza and A. A. Salleh, J. Exp. Nanosci., 2015, 10, 925–936, DOI:10.1080/17458080.2014.933494.
- Y. Jiang, X.-J. Wu, Q. Li, J. Li and D. Xu, Nanotechnology, 2011, 22, 385601, DOI:10.1088/0957-4484/22/38/385601.
- L. Lu, K. Ai and Y. Ozaki, Langmuir, 2008, 24, 1058–1063, DOI:10.1021/acssuschemeng.7b03425.
- L. Storozhuk, M. O. Besenhard, S. Mourdikoudis, A. P. LaGrow, M. R. Lees, L. D. Tung, A. Gavriilidis and N. T. K. Thanh, ACS Appl. Mater. Interfaces, 2021, 13, 45870–45880, DOI:10.1021/acsami.1c12323.
- A. Narayanaswamy, H. Xu, N. Pradhan, M. Kim and X. Peng, J. Am. Chem. Soc., 2006, 128, 10310–10319, DOI:10.1021/ja0627601.
- S. K. Kulkarni, Nanotechnology: Principles and Practices, Springer, Berlin/Heidelberg, Germany, 2015, pp. 55–133, DOI:10.1007/978-3-319-09171-6.
- X. Yu and C. Cao, Cryst. Growth Des., 2008, 8, 3951–3955, DOI:10.1021/cg701001m.
- S. B. Wategaonkar, R. P. Pawar, V. G. Parale, K. S. Pakhare, B. M. Sargar and R. K. Mane, Macromol. Symp., 2020, 393, 2000040, DOI:10.1002/masy.202000040.
- J. N. Zheng, L. L. He, C. Chen, A. J. Wang, K. F. Ma and J. J. Feng, J. Power Sources, 2014, 268, 744–751, DOI:10.1016/j.jpowsour.2014.06.109.
- O. E. Meiron, V. Kuraganti, I. Hod, R. Bar-Ziv and M. Bar-Sadan, Nanoscale, 2017, 9, 13998–14005, 10.1039/C7NR04922F.
- A. Nagar, A. Kumar, S. Parveen, A. Kumar, H. Dhasmana, S. Husain, A. Verma and V. K. Jain, Mater. Today, 2020, 32, 402–406, DOI:10.1016/j.matpr.2020.02.087.
- A. Y. Faid and H. Ismail, ChemistrySelect, 2019, 4, 7896–7903, DOI:10.1002/slct.201901580.
- X. Gao, Y. Li, W. Zeng, C. Zhang and Y. Wei, J. Mater. Sci.: Mater. Electron., 2017, 28, 18781–18786, DOI:10.1007/s10854-017-7827-0.
- B. Zhang, H. Peng, L. Yang, H. Li, H. Nan, Z. Liang, H. Song, H. Su, C. Li and S. Liao, J. Mater. Chem. A, 2015, 3, 973–977, 10.1039/C4TA04989F.
- G. A. Molina, R. Esparza, J. L. Lopez-Miranda, A. R. Hernandez-Martinez, B. L. Espana-Sanchez, E. A. Elizalde-Pena and M. Estevez, Colloids Surf., B, 2019, 180, 141–149, DOI:10.1016/j.colsurfb.2019.04.044.
- S. Andra, R. Ramoorthy and M. Muthalagu, Res. Express, 2018, 5, 065043, DOI:10.1088/2053-1591/aabb61.
- H. Siddiqui, M. S. Qureshi and F. Z. Haque, Nano-Micro Lett., 2020, 12, 29, DOI:10.1007/s40820-019-0357-y.
- R. Biswas, B. Banerjee, M. Saha, I. Ahmed, S. Mete, R. A. Patil, Y. R. Ma and K. K. Haldar, J. Phys. Chem. C, 2021, 125(12), 6619–6631, DOI:10.1021/acs.jpcc.0c10149.
- N. Masurkar, N. K. Thangavel and L. M. R. Arava, ACS Appl. Mater. Interfaces, 2018, 10, 27771–27779, DOI:10.1021/acsami.8b07489.
- N. Arjona, M. Guerra-Balcazar, F. M. Cuevaz-Muniz, L. Alvarez-Contreras, J. Ledesma-Garcia and L. G. Arriaga, RSC Adv., 2013, 3, 15727–15733, 10.1039/C3RA41681J.
- W. Zhao, N. Du, H. Zhang and D. Yang, J. Power Sources, 2015, 285, 131–136, DOI:10.1016/j.jpowsour.2015.03.088.
- L. Kumari, J. H. Lin and Y. R. Ma, Nanotechnology, 2007, 18, 295605, DOI:10.1088/0957-4484/18/29/295605.
- J.-H. Lee, Sens. Actuators, B, 2009, 140(1), 319–336, DOI:10.1016/j.snb.2009.04.026.
- Y. Ni, Z. Sun, Z. Zeng, F. Liu and J. Qin, New J. Chem., 2019, 43, 18629, 10.1039/C9NJ04236A.
- M. A. Khan, N. Nayan, M. K. S. Ahmad, S. C. Fhong, M. Tahir, R. A. Mohamed Ali and M. S. Mohamed Ali, Molecules, 2021, 26, 2700, DOI:10.3390/molecules26092700.
- Y. Qu, R. Huang, W. Qi, M. Shi, R. Su and Z. He, Catal. Today, 2020, 355, 397–407, DOI:10.1016/j.cattod.2019.07.056.
- S. Sun, S. Feng, M. Terrones and R. E. Schaak, Chem. Mater., 2015, 27, 3167–3175, DOI:10.1021/acs.chemmater.5b01129.
- K. Vasu, O. E. Meiron, A. N. Enyashin, R. Bar-Ziv and M. Bar-Sadan, J. Phys. Chem. C, 2019, 123, 1987–1994, DOI:10.1021/acs.jpcc.8b11712.
- O. Zimron, T. Zilberman, S. R. Kadam, S. Ghosh, S. L. Kolatker, A. Neyman, R. Bar-Ziv and M. Bar-Sadan, Isr. J. Chem., 2020, 60, 624, DOI:10.1002/ijch.201900162.
- D. Li, J. Lao, C. Jiang, C. Luo, R. Qi, H. Lin, R. Huang, G. I. N. Waterhouse and H. Peng, Int. J. Hydrogen Energy, 2019, 44, 30876–30884, DOI:10.1016/j.ijhydene.2019.10.041.
- B. Bhowmik, V. Manjuladevi, R. K. Gupta and P. Bhattacharyya, IEEE Sens. J., 2016, 16, 3488–3495, DOI:10.1109/JSEN.2016.2530827.
- B. Zhao, F. Chen, Q. Huang and J. Zhang, Chem. Commun., 2009, 5115–5117, 10.1039/B909883F.
- Y. Xu, J. Jin, X. Li, Y. Han, H. Meng, T. Wang and X. Zhang, Mater. Res. Bull., 2016, 76, 235–239, DOI:10.1016/j.materresbull.2015.11.062.
- W. Guo, X. Li, H. Qin and Z. Wang, Phys. E, 2015, 73, 163–168, DOI:10.1016/j.physe.2015.05.006.
- G. Bai, H. Dai, J. Deng, Y. Liu and K. Ji, Catal. Commun., 2012, 27, 148–153, DOI:10.1016/j.catcom.2012.07.008.
- R. Miao, W. Zeng and Q. Gao, Mater. Lett., 2017, 186, 175–177, DOI:10.1016/j.matlet.2016.09.127.
- A. Gu, G. Wang, X. Zhang and B. Fang, Bull. Mater. Sci., 2010, 33, 17–20, DOI:10.1007/s12034-010-0002-3.
- Z. Li, J. Wang, N. Wang, S. Yan, W. Liu, Y. Q. Fu and Z. Wang, J. Alloys Compd., 2017, 725, 1136–1143, DOI:10.1016/j.jallcom.2017.07.218.
- Y. B. Mollamahale, Z. Liu, Y. Zhen, Z. Q. Tian, D. Hosseini, L. Chen and P. K. Shen, Int. J. Hydrogen Energy, 2017, 42, 7202–7211, DOI:10.1016/j.ijhydene.2016.05.193.
- P. Wang, Y. Gao, P. Li, X. Zhang, H. Niu and Z. Zheng, ACS Appl. Mater. Interfaces, 2016, 8, 15820–15827, DOI:10.1021/acsami.6b04378.
- M. Zhong, X. Meng, F. Wu, J. Li and Y. Fang, Nanoscale Res. Lett., 2013, 8, 451, DOI:10.1186/1556-276X-8-451.
- H. Guan, X. Zhang and Y. Xie, J. Phys. Chem. C, 2014, 118, 27170–27174, DOI:10.1021/jp509045d.
- I. Bilecka and M. Niederberger, Nanoscale, 2010, 2, 1358–1374, 10.1039/B9NR00377K.
- G. A. Babu, G. Ravi, M. Navaneethan, M. Arivanandhan and Y. Hayakawa, J. Mater. Sci.: Mater. Electron., 2014, 25, 5231–5240, DOI:10.1007/s10854-014-2293-4.
- Y. Ren and L. Gao, J. Am. Ceram. Soc., 2010, 93, 3560–3564, DOI:10.1111/j.1551-2916.2010.04090.x.
- V. Makarov, A. Love, O. Sinitsyna, S. Makarova, I. Yaminsky, M. Taliansky and N. Kalinina, Acta Naturae, 2014, 6, 35–44 CrossRef CAS PubMed.
- M. Hasana, M. Altaf, A. Zafar, S. G. Hassan, Z. Ali, G. Mustafa, T. Munawar, M. S. Saif, T. Tariq, F. Iqbal, M. Q. Khan, A. Mahmood, N. Mahmood and X. Shu, Mater. Sci. Eng., C, 2021, 119, 111280, DOI:10.1016/j.msec.2020.111280.
- H. Zhang, W. Zhou, Y. Du, P. Yang and C. Wang, Electrochem. Commun., 2010, 12, 882–885, DOI:10.1016/j.elecom.2010.04.011.
- M. Q. Guo, H. S. Hong, X. N. Tang, H. D. Fang and X. H. Xu, Electrochim. Acta, 2012, 63, 1–8, DOI:10.1016/j.electacta.2011.11.114.
- J. N. Tiwari, F. M. Pan and K. L. Lin, New J. Chem., 2009, 33, 1482–1485, 10.1039/B901534P.
- Y. Zuo, L. Wu, K. Cai, T. Li, W. Yin, D. Li, N. Li, J. Liu and H. Han, ACS Appl. Mater. Interfaces, 2015, 7, 17725–17730, DOI:10.1021/acsami.5b03826.
- J. Bi, Mater. Lett., 2019, 236, 398–402, DOI:10.1016/j.matlet.2018.10.138.
- L. I. Sun, L. L. Liu, L. H. Luo, Y. F. Wu, J. J. Shi, L. Cheng, X. Xu and Y. M. Guo, J. Fuel Chem. Technol., 2016, 44, 607–612, DOI:10.1016/S1872-5813(16)30027-5.
- L. Qian and X. Yang, J. Phys. Chem. B, 2006, 110, 16672–16678, DOI:10.1021/jp063302h.
- H. Xu, B. Yan, K. Zhang, J. Wang, S. Li, C. Wang, Z. Xiong, Y. Shiraishi, Y. Du and P. Yang, ACS Sustainable Chem. Eng., 2017, 5, 10490–10498, DOI:10.1021/acssuschemeng.7b02491.
- D. Alby, F. Salles, A. Geneste, B. Prélot, J. Zajac and C. Charnay, J. Hazard. Mater., 2019, 368, 661–669, DOI:10.1016/j.jhazmat.2019.01.064.
- H. Zhang, W. G. Chen, Y. Q. Li, L. F. Jin, F. Cui and Z. H. Song, Front. Chem., 2018, 6, 472, DOI:10.3389/fchem.2018.00472.
- S. Borbón, S. Lugo and I. López, Mater. Sci. Semicond., 2019, 91, 310–315, DOI:10.1016/j.mssp.2018.12.001.
- H. Yang, S. Liao, C. Huang, L. Du, P. Chen, P. Huang, Z. Fu and Y. Li, Appl. Surf. Sci., 2014, 314, 7–14, DOI:10.1016/j.apsusc.2014.06.128.
- M. A. C., F. K. P., S. Singh and S. Baik, Sci. Rep., 2016, 6, 34894, DOI:10.1038/srep34894.
- C. Yang, X. Cui, Z. Zhang, S. W. Chiang, W. Lin, H. Duan, J. Li, F. Kang and C.-P. Wong, Nat. Commun., 2015, 6, 8150, DOI:10.1038/ncomms9150.
- R. Ma, B. Kang, S. Cho, M. Choi and S. Baik, ACS Nano, 2015, 9, 10876–10886, DOI:10.1021/acsnano.5b03864.
- B. Zhang, P. Xu, X. Xie, H. Wei, Z. Li, N. H. Mack, X. Han, H. Xu and H. L. Wang, J. Mater. Chem., 2011, 21, 2495–2501, 10.1039/C0JM02837A.
- H. Xu, B. Yan, K. Zhang, J. Wang, S. Li, C. Wang, Z. Xiong, Y. Shiraishi and Y. Du, ChemElectroChem, 2017, 4, 2527–2534, DOI:10.1002/celc.201700611.
- S. Das, A. Samanta and S. Jana, Chem. Eng. J., 2019, 374, 1118–1126, DOI:10.1016/j.cej.2019.05.114.
- S. Cao, T. Han and L. Peng, J. Mater. Sci.: Mater. Electron., 2020, 31, 17291–17296, DOI:10.1007/s10854-020-04283-w.
- K. M. AbouZeid, M. B. Mohamed and M. S. El-Shall, Small, 2011, 7, 3299–3307, DOI:10.1002/smll.201100688.
- N. H. Hung, N. D. Thanh, N. H. Lam, N. D. Dien, N. D. Chien and D. D. Vuong, Mater. Sci. Semicond. Process., 2014, 26, 18–24, DOI:10.1016/j.mssp.2014.03.052.
- S. Ye, F. Benz, M. C. Wheeler, J. Oram, J. J. Baumberg, O. Cespedes, H. K. Christenson, P. L. Coletta, L. J. C. Jeuken, A. F. Markham, K. Critchley and S. D. Evans, Nanoscale, 2016, 8, 14932–14942, 10.1039/C6NR04045D.
- J. Xie, Q. Zhang, J. Y. Lee and D. I. C. Wang, ACS Nano, 2008, 2, 2473–2480, DOI:10.1021/nn800442q.
- C. Y. Song, B. Y. Yang, W. Q. Chen, Y. X. Dou, Y. J. Yang, N. Zhou and L. H. Wang, J. Mater. Chem. B, 2016, 4, 7112–7118, 10.1039/C6TB01046F.
- B. K. Jena and C. R. Raj, Langmuir, 2007, 23, 4064–4070, DOI:10.1021/la063243z.
- B. K. Jena and C. R. Raj, Chem. Mater., 2008, 20, 3546–3548, DOI:10.1021/cm7019608.
- S. Li, L. Zhang, T. Wang, L. Li, C. Wang and Z. Su, Chem. Commun., 2015, 51, 14338–14341, 10.1039/C5CC05676D.
- H. Ma, Z. Liu, Y. Wei and L. Jiang, Colloids Surf., A, 2019, 582, 123889, DOI:10.1016/j.colsurfa.2019.123889.
- L. Zhong, X. Zhai, X. Zhu, P. Yao and M. Liu, Langmuir, 2010, 26, 5876–5881, DOI:10.1021/la903809k.
- C. Y. Song, N. Zhou, B. Y. Yang, Y. J. Yang and L. H. Wang, Nanoscale, 2015, 7, 17004–17011, 10.1039/C5NR04827C.
- K. Mao, Y. Chen, Z. Wu, X. Zhou, A. Shen and J. Hu, J. Agric. Food Chem., 2014, 62, 10638–10645, DOI:10.1021/jf5034015.
- A. J. Wang, Y. F. Li, M. Wen, G. Yang and H. Y. Wang, New J. Chem., 2012, 36, 2286–2291, 10.1039/C2NJ40380C.
- Y. Wang, N. Shah and G. P. Huffman, Energy Fuels, 2004, 18, 1429–1433, DOI:10.1021/ef049959o.
- A. Fukuoka, J. Kimura, T. Oshio, Y. Sakamoto and M. Ichikawa, J. Am. Chem. Soc., 2007, 129, 10120–10125, DOI:10.1021/ja0703123.
- S. H. Joo, J. Y. Park, C. K. Tsung, Y. Yamada, P. D. Yang and G. A. Somorjai, Nat. Mater., 2009, 8, 126–131, DOI:10.1038/nmat2329.
- J. H. Yuan, K. Wang and X. H. Xia, Adv. Funct. Mater., 2005, 15, 803–809, DOI:10.1002/adfm.200400321.
- J. Chen, B. Lim, E. P. Lee and Y. Xia, Nano Today, 2009, 4, 81–95, DOI:10.1016/j.nantod.2008.09.002.
- G. A. Somorjai, Chemistry in Two Dimensions: Surfaces, Cornell University Press, Ithaca, NY, 1981 Search PubMed.
- C. Wang, H. Daimon, T. Onodera, T. Koda and S. A. Sun, Angew. Chem., Int. Ed. Engl., 2008, 47, 3588–3591, DOI:10.1002/anie.200800073.
- K. M. Bratlie, H. Lee, K. Komvopoulos, P. Yang and G. A. Somorjai, Nano Lett., 2007, 7, 3097–3101, DOI:10.1021/nl0716000.
- N. V. Long, C. M. Thi, M. Nogami and M. Ohtaki, New J. Chem., 2012, 36, 1320–1334, 10.1039/C2NJ40027H.
- J. Zhang, H. Yang, J. Fang and S. Zou, Nano Lett., 2010, 10, 638–644, DOI:10.1021/nl903717z.
- Z. W. Chen, M. Waje, W. Z. Li and Y. S. Yan, Angew. Chem., Int. Ed. Engl., 2007, 46, 4060–4063, DOI:10.1002/anie.200700894.
- S. H. Sun, F. Jaouen and J. P. Dodelet, Adv. Mater., 2008, 20, 3900–3904, DOI:10.1002/adma.200800491.
- A. Dandapat, A. Mitra, P. K. Gautam and G. A. De, Nanomater. Nanotechnol., 2013, 3, 11, DOI:10.5772/56868.
- P. Dhanasekaran, K. Lokesh, P. K. Ojha, A. K. Sahu, S. D. Bhat and D. Kalpana, J. Colloid Interface Sci., 2020, 572, 198–206, DOI:10.1016/j.jcis.2020.03.078.
- H. Kawasaki, T. Yonezawa, T. Watanabe and R. Arakawa, J. Phys. Chem. C, 2007, 111, 16278–16283, DOI:10.1021/jp075159d.
- M. Wang, X. Wang, J. Li and L. Liu, J. Mater. Chem. A, 2013, 1, 8127, 10.1039/c3ta11997a.
- H. Heli, N. Sattarahmady, R. D. Vais and A. R. Mehdizadeh, Sens. Actuators, B, 2014, 192, 310–316, DOI:10.1016/j.snb.2013.10.124.
- R. Ojani, E. Hasheminejad and J. B. Raoof, Energy, 2015, 90, 1122–1131, DOI:10.1016/j.energy.2015.06.061.
- H. M. Ngo, N. D. Lai and I. Ledoux-Rak, Nanoscale, 2016, 8, 3489–3495, 10.1039/C5NR07571H.
- T. L. Nguyen, V. H. Cao, T. H. Y. Pham and T. G. Le, J. Chem., 2019, 2019, 6235479, DOI:10.1155/2019/6235479.
- E. Antolini, Energy Environ. Sci., 2009, 2, 915–931, 10.1039/B820837A.
- A. R. Siamaki, A. E. R. S. Khder, V. Abdelsayed, M. S. El-Shall and B. F. Gupton, J. Catal., 2011, 279, 1–11, DOI:10.1016/j.jcat.2010.12.003.
- X. Q. Huang and N. F. Zheng, J. Am. Chem. Soc., 2009, 131, 4602–4603, DOI:10.1021/ja9009343.
- H. Bai, M. Han, Y. Du, J. Bao and Z. Dai, Chem. Commun., 2010, 46, 1739–1741, 10.1039/B921004K.
- X. Huang, S. Tang, X. Mu, Y. Dai, G. Chen, Z. Zhou, F. Ruan, Z. Yang and N. Zheng, Nat. Nanotechnol., 2011, 6, 28–32, DOI:10.1038/nnano.2010.235.
- S. Guo and E. Wang, Nano Today, 2011, 6, 240–264, DOI:10.1016/j.nantod.2011.04.007.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed. Engl., 2008, 48, 60–103, DOI:10.1002/anie.200802248.
- X. Lu, M. Rycenga, S. E. Skrabalak, B. Wiley and Y. Xia, Annu. Rev. Phys. Chem., 2009, 60, 167–192, DOI:10.1146/annurev.physchem.040808.090434.
- H. Zhang, M. Jin, Y. Xiong, B. Lim and Y. Xia, Acc. Chem. Res., 2013, 46, 1783–1794, DOI:10.1021/ar300209w.
- A. Wang, F. F. Li, J. N. Zheng, H. X. Xi, Z. Y. Meng and J. J. Feng, RSC Adv., 2013, 3, 10355–10362, 10.1039/C3RA40556G.
- N. Ma, X. Liu, Z. Yang, G. Tai, Y. Yin, S. Liu, H. Li, P. Guo and X. S. Zhao, ACS Sustainable Chem. Eng., 2018, 6, 1133–1140, DOI:10.1021/acssuschemeng.7b03425.
- K. K. Maniam and R. Chetty, Fuel Cells, 2013, 13, 1196–1204, DOI:10.1002/fuce.201200162.
- W. Guo, G. S. Luo and Y. J. Wang, J. Colloid Interface Sci., 2004, 271, 400–406, DOI:10.1016/j.jcis.2003.08.056.
- K. Qi, Q. Y. Wang, W. T. Zheng, W. Zhang and X. Q. Cui, Nanoscale, 2014, 6, 15090–15097, 10.1039/C4NR05761A.
- Z. Yin, H. Zheng, D. Ma and X. Bao, J. Phys. Chem. C, 2009, 113, 1001–1005, DOI:10.1021/jp807456j.
- I. Sarhid, I. Abdellah, C. Martini, V. Huc, D. Dragoe, P. Beaunier, I. Lampre and H. Remita, New J. Chem., 2019, 43, 4349–4355, 10.1039/C8NJ06370B.
- M. del C. Aguirre, J. Appl. Electrochem., 2019, 49, 795–809, DOI:10.1007/s10800-019-01323-0.
- R. Jin, Y. C. Cao, E. Hao, G. S. Metraux, G. C. Schatz and C. A. Mirkin, Nature, 2003, 425, 487–490, DOI:10.1038/nature02020.
- C. Yang, X. Ciu, Z. Zhang, S. W. Chiang, W. Lin, H. Duan, J. Li, F. Kang and C. P. Wong, Nat. Commun., 2015, 6, 8150, DOI:10.1038/ncomms9150.
- B. Wiley, Y. Sun and Y. Xia, Acc. Chem. Res., 2007, 40, 1067–1076, DOI:10.1021/ar7000974.
- L. Zhang, Y. Zhao, Z. Lin, F. Gu, S. P. Lau, L. Li and Y. Chai, Nanoscale, 2015, 7, 13420–13426, 10.1039/C5NR02611C.
- X. Liu, Z. Liu, S. Hao and W. Chu, Mater. Lett., 2012, 80, 66–68, DOI:10.1016/j.matlet.2012.04.031.
- P. J. Rivero, A. Urrutia, J. Goicoechea, Y. Rodríguez, J. M. Corres, F. J. Arregui and I. R. Matías, J. Appl. Polym. Sci., 2012, 126, 1228–1235, DOI:10.1002/app.36886.
- J. Li, D. Zhang, J. B. Guo and J. Wei, Chin. J. Chem. Phys., 2014, 27, 718–724, DOI:10.1063/1674-0068/27/06/718-724.
- L. M. Chen and Y. N. Liu, J. Raman Spectrosc., 2012, 43, 986–991, DOI:10.1002/jrs.3137.
- P. Manivasagan, J. Venkatesan, K. Sivakumar and S. K. Kim, Crit. Rev. Microbiol., 2016, 42, 209–221, DOI:10.3109/1040841X.2014.917069.
- S. Preciado-Flores, D. A. Wheeler, T. M. Tran, Z. Tanaka, C. Jiang, M. Barboza-Flores, F. Qian, Y. Li, B. Chen and J. Z. Zhang, Chem. Commun., 2011, 47, 4129–4131, 10.1039/C0CC05517D.
- T. Jiang, B. Wang, L. Zhang and J. Zhou, J. Alloys Compd., 2015, 632, 140–146, DOI:10.1016/j.jallcom.2015.01.164.
- M. S. Bootharaju, V. M. Burlakov, T. M. Besong, C. P. Joshi, L. G. AbdulHalim, D. M. Black, R. L. Whetten, A. Goriely and O. M. Bakr, Chem. Mater., 2015, 27, 4289–4297, DOI:10.1021/acs.chemmater.5b00650.
- H. Zheng, D. Ni, Z. Yu, P. Liang and H. Chen, Sens. Actuators, B, 2016, 231, 423–430, DOI:10.1016/j.snb.2016.03.045.
- C. R. Rekha, V. U. Nayar and K. G. Gopchandran, J. Sci.: Adv. Mater. Devices, 2018, 3(2), 196–205, DOI:10.1016/j.jsamd.2018.03.003.
- D. Wu, M. Hu, Y. Zhang, J. Zhou and Z. Wang, Appl. Surf. Sci., 2020, 505, 144520, DOI:10.1016/j.apsusc.2019.144520.
- H. B. Eral, D. J. C. M. ‘t Mannetje and J. M. Oh, Colloid Polym. Sci., 2013, 291, 247–260, DOI:10.1007/s00396-012-2796-6.
- Z. Yang, T. C. Chiu and H. T. Chang, Open Nanosci. J., 2007, 1, 5–12, DOI:10.2174/1874140100701010005.
- B. K. Jena, B. K. Mishra and S. Bohidar, J. Phys. Chem. C, 2009, 113, 14753–14758, DOI:10.1021/jp904689f.
- J. Bian, Z. Li, Z. Chen, X. Zhang, Q. Li, S. Jiang, J. He and G. Han, Electrochim. Acta, 2012, 67, 12–17, DOI:10.1016/j.electacta.2012.01.073.
- L. Wu, W. Wu, X. Jing, J. Huang, D. Sun, T. Odoom-Wubah, H. Liu, H. Wang and Q. Li, Ind. Eng. Chem. Res., 2013, 52, 5085–5094, DOI:10.1021/ie303518z.
- Q. Chang, X. Shi, X. Liu, J. Tong, D. Liu and Z. Wang, Nanophotonics, 2017, 6, 1151–1160, DOI:10.1515/nanoph-2017-0010.
- Y. H. Chang, C. Liu, S. Rouvimov, T. Luo and S. P. Feng, Chem. Commun., 2017, 53, 6752–6755, 10.1039/C7CC02738A.
- Y. Tian, H. Liu, Y. Chen, C. Zhou, Y. Jiang, C. Gu, T. Jiang and J. Zhou, Sens. Actuators, B, 2019, 301, 127142, DOI:10.1016/j.snb.2019.127142.
- F. L. Jia, L. Z. Zhang, X. Y. Shang and Y. Yang, Adv. Mater., 2008, 20, 1050–1054, DOI:10.1002/adma.200702159.
- S. Senapati, S. K. Srivastava, S. B. Singh and K. Biswas, Cryst. Growth Des., 2010, 10, 4068–4075, DOI:10.1021/cg100740e.
- Z. Li, Z. Ma, Y. Wen, Y. Ren, Z. Wei, X. Xing, H. Sun, Y. W. Zhang and W. Song, ACS Appl. Mater. Interfaces, 2018, 10, 26233–26240, DOI:10.1021/acsami.8b06722.
- Y. L. Liao, W. X. Que, Q. Y. Jia, Y. C. He, J. Zhang and P. Zhong, J. Mater. Chem., 2012, 22, 7937–7944, 10.1039/C2JM16628C.
- Y. Wang, Y. He, Q. Lai and M. Fan, J. Environ. Sci., 2014, 26, 2139–2177, DOI:10.1016/j.jes.2014.09.023.
- Z. Zhang, C. Wang, R. Zakaria and J. Y. Ying, J. Phys. Chem. B, 1998, 102, 10871–10878, DOI:10.1021/jp982948.
- M. Y. Guo, M. K. Fung, F. Fang, X. Y. Chen, A. M. C. Ng, A. B. Djurišić and W. K. Chan, J. Alloys Compd., 2011, 509, 1328–1332, DOI:10.1016/j.jallcom.2010.10.028.
- X. Wang, S. Zhang, Y. Xie, H. Wang, H. Yu, Y. Shen, Z. Li, S. Zhang and F. Peng, Int. J. Hydrogen Energy, 2016, 41, 20192–20197, DOI:10.1016/j.ijhydene.2016.09.029.
- O. K. Varghese, M. Paulose and C. A. Grimes, Nat. Nanotechnol., 2009, 4, 592–597, DOI:10.1038/nnano.2009.226.
- M. Ferroni, M. Carotta, V. Guidi, G. Martinelli, F. Ronconi, M. Sacerdoti and E. Traversa, Sens. Actuators, B, 2001, 77, 163–166, DOI:10.1016/S0925-4005(01)00688-8.
- M. Paulose, O. K. Varghese, G. K. Mor, C. A. Grimes and K. G. Ong, Nanotechnology, 2006, 17, 398–402, DOI:10.1088/0957-4484/17/2/009.
- H. Tokudome, Y. Yamada, S. Sonezaki, H. Ishikawa, M. Bekki and K. Kanehira, Appl. Phys. Lett., 2005, 87, 213901, DOI:10.1063/1.2135392.
- Y. G. Guo, Y. S. Hu and J. Maier, Chem. Commun., 2006, 2783–2785, 10.1039/B605090E.
- M. Minella, D. Versaci, S. Casino, F. Di Lupo, C. Minero, A. Battiato, N. Penazzi and S. Bodoardo, Electrochim. Acta, 2017, 230, 132–140, DOI:10.1016/j.electacta.2017.01.190.
- D. Y. Liang, C. Cui, H. H. Hu, Y. P. Wang, S. Xu, B. Ying, P. G. Li, B. Q. Lu and H. L. Shen, J. Alloys Compd., 2014, 582, 236–240, DOI:10.1016/j.jallcom.2013.08.062.
- S. Jafari, B. Mahyad, H. Hashemzadeh, S. Janfaza, T. Gholikhani and L. Tayebi, Int. J. Nanomed., 2020, 15, 3447–3470, DOI:10.2147/IJN.S249441.
- J. Roy, J. Ind. Eng. Chem., 2022, 106, 1–19, DOI:10.1016/j.jiec.2021.10.024.
- M. A. Lara, M. J. Sayagués, J. A. Navío and M. C. Hidalgo, J. Mater. Sci., 2018, 53, 435–446, DOI:10.1007/s10853-017-1515-6.
- D. Dahlan, S. K. Md Saad, A. U. Berli, A. Bajili and A. A. Umar, Phys. E, 2017, 91, 185–189, DOI:10.1016/j.physe.2017.05.003.
- H. Z. Yao, W. Y. Fu, L. Liu, X. Li, D. Ding, P. Y. Su, S. Feng and H. B. Yang, J. Alloys Compd., 2016, 680, 206–211, DOI:10.1016/j.jallcom.2016.04.133.
- B. Babu, K. Mallikarjuna, C. V. Reddy and J. Park, Mater. Lett., 2016, 176, 265–269, DOI:10.1016/j.matlet.2016.04.146.
- J. Y. Su, L. Zhu, P. Geng and G. H. Chen, J. Hazard. Mater., 2016, 316, 159–168, DOI:10.1016/j.jhazmat.2016.05.004.
- D. P. Kumar, N. L. Reddy, M. Karthikeyan, N. Chinnaiah, V. Bramhaiah, V. D. Kumari and M. V. Shankar, J. Colloid Interface Sci., 2016, 477, 201–208, DOI:10.1016/j.jcis.2016.05.014.
- G. Liu, J. Y. Liao, A. Duan, Z. Zhang, M. Fowler and A. Yu, J. Mater. Chem. A, 2013, 1, 12255–12262, 10.1039/C3TA12329D.
- G. Huang, C.-H. Lu and H.-H. Yang, Novel Nanomaterials for Biomedical, Environmental and Energy Applications, ed. X. Wang and X. Chen, Elsevier, Amsterdam, 1st edn, 2019, chapter 3, pp. 89–109, DOI:10.1016/B978-0-12-814497-8.00003-5.
- W. Dong, H. Li, J. Xi, J. Mu, Y. Huang, Z. Ji and X. Wu, J. Alloys Compd., 2017, 724, 280–286, DOI:10.1016/j.jallcom.2017.06.246.
- L. Huang, T. M. Liu, H. J. Zhang, W. W. Guo and W. Zeng, J. Mater. Sci.: Mater. Electron., 2012, 23, 2024–2029, DOI:10.1007/s10854-012-0697-6.
- M. Liu, L. Piao, W. Lu, S. Ju, L. Zhao, C. Zhou, H. Li and W. Wang, Nanoscale, 2010, 2, 1115–1117, 10.1039/C0NR00050G.
- X. Yang, C. Jin, C. Liang, D. Chen, M. Wu and J. C. Yu, Chem. Commun., 2011, 47, 1184–1186, 10.1039/C0CC04216A.
- Q. Xiang and J. Yu, Chin. J. Catal., 2011, 32, 525–531, DOI:10.1016/S1872-2067(10)60186-6.
- J. Y. Liao, B. X. Lei, D. B. Kuang and C. Y. Su, Energy Environ. Sci., 2011, 4, 4079–4085, 10.1039/C1EE01574E.
- J. Harris, R. Silk, M. Smith, Y. Dong, W. T. Chen and G. I. N. Waterhouse, ACS Omega, 2020, 5, 18919–18934, DOI:10.1021/acsomega.0c02142.
- A. Q. D. Faisal, J. Mater. Sci.: Mater. Electron., 2015, 26, 317–321, DOI:10.1007/s10854-014-2402-4.
- H. Xu, G. Li, G. Zhu, K. Zhu and S. Jin, Catal. Commun., 2015, 62, 52–56, DOI:10.1016/j.catcom.2015.01.001.
- Z. Song, H. Zhou, P. Tao, B. Wang, J. Mei, H. Wang, S. Wen, Z. Song and G. Fang, Mater. Lett., 2016, 180, 179–183, DOI:10.1016/j.matlet.2016.05.178.
- J. Ma, W. Ren, J. Zhao and H. Yang, J. Alloys Compd., 2017, 692, 1004–1009, DOI:10.1016/j.jallcom.2016.09.134.
- S. Shamsudin, M. K. Ahmad, N. Nafarizal, C. F. Soon, R. A. Rahim, D. A. Alakendram, M. Shimomura and K. Murakami, Int. J. Integr. Eng., 2020, 12, 197–205 Search PubMed.
- H. Siddiqui, M. S. Qureshi and F. Z. Haque, Optik, 2016, 127, 2740–2747, DOI:10.1016/j.ijleo.2015.11.220.
- R. M. Mohamed, F. A. Harraz and A. Shawky, Ceram. Int., 2014, 40, 2127–2133, DOI:10.1016/j.ceramint.2013.07.129.
- R. Al-Gaashani, S. Radiman, N. Tabet and A. R. Daud, J. Alloys Compd., 2011, 509, 8761–8769, DOI:10.1016/j.jallcom.2011.06.056.
- X. Zhao, P. Wang, Z. Yan and N. Ren, Opt. Mater., 2015, 42, 544–547, DOI:10.1016/j.optmat.2014.12.032.
- M. Yin, C. K. Wu, Y. B. Lou, C. Burda, J. T. Koberstein, Y. M. Zhu and S. O'Brien, J. Am. Chem. Soc., 2005, 127, 9506–9511, DOI:10.1021/ja050006u.
- K. Zhou, R. Wang, B. Xu and Y. Li, Nanotechnology, 2006, 17, 3939–3943, DOI:10.1088/0957-4484/17/15/055.
- Y. Xu, D. Chen and X. J. Jiao, J. Phys. Chem. B, 2005, 109, 13561–13566, DOI:10.1021/jp051577b.
- J. Ramirez-Ortiz, T. Ogura, J. Medina-Valtierra, S. E. Acosta-Ortiz, P. Bosch, J. A. D. I. Reyes and V. H. Lara, Appl. Surf. Sci., 2001, 174, 177–184, DOI:10.1016/S0169-4332(00)00822-9.
- M. L. Zhong, D. C. Zeng, Z. W. Liu, H. Y. Yu, X. C. Zhong and W. Q. Qiu, Acta Mater., 2010, 58, 5926–5932, DOI:10.1016/j.actamat.2010.07.008.
- C. L. Hsu, J. Y. Tsai and T. J. Hsueh, Sens. Actuators, B, 2016, 224, 95–102, DOI:10.1016/j.snb.2015.10.018.
- M. Wan, D. Jin, R. Feng, L. Si, M. Gao and L. Yue, Inorg. Chem. Commun., 2011, 14, 38–41, DOI:10.1016/j.inoche.2010.09.025.
- A. Ananth, S. Dharaneedharan, M. S. Heo and Y. S. Mok, Chem. Eng. J., 2015, 262, 179–188, DOI:10.1016/j.cej.2014.09.083.
- Udayabhanu, P. C. Nethravati, M. A. P. Kumar, D. Suresh, K. Lingaraju, H. Rajanaika, H. Nagabhushana and S. C. Sharma, Mater. Sci. Semicond. Process., 2015, 33, 81–88, DOI:10.1016/j.mssp.2015.01.034.
- R. C. Wang, S. N. Lin and J. Y. Liu, J. Alloys Compd., 2017, 696, 79–85, DOI:10.1016/j.jallcom.2016.11.214.
- X. Liu, J. Chen, P. Liu, H. Zhang, G. Li, T. An and H. Zhao, Appl. Catal., A, 2016, 521, 34–41, DOI:10.1016/j.apcata.2015.10.005.
- D. Das, B. C. Nath, P. Phukon and S. K. Dolui, Colloids Surf., B, 2013, 101, 430–433, DOI:10.1016/j.colsurfb.2012.07.002.
- T. Andana, M. Piumetti, S. Bensaid, L. Veyre, C. Thieuleux, N. Russo, D. Fino, E. A. Quadrelli and R. Pirone, Appl. Catal., B, 2017, 216, 41–58, DOI:10.1016/j.apcatb.2017.05.061.
- R. Dang, X. Jia, X. Liu, H. Ma, H. Gao and G. Wang, Nano Energy, 2017, 33, 427–435, DOI:10.1016/j.nanoen.2017.01.024.
- X. Wang, X. Wu, L. Yuan, K. Huang and S. Feng, Mater. Des., 2017, 113, 297–304, DOI:10.1016/j.matdes.2016.10.029.
- A. P. M. Udayan and S. N. Sawant, J. Phys. Chem. Solids, 2021, 150, 109883, DOI:10.1016/j.jpcs.2020.109883.
- M. Fang, R. Zheng, Y. Wu, D. Yue, X. Qian, Y. Zhao and Z. Bian, Environ. Sci.: Nano, 2019, 6, 105–114, 10.1039/C8EN00930A.
- L. Zhang, Y. C. Zhu, Y. Y. Liang, W. W. Zhao, J. J. Xu and H. Y. Chen, Anal. Chem., 2018, 90, 5439–5444, DOI:10.1021/acs.analchem.8b00742.
- L. Xu, Q. Yang, X. Liu, J. Liu and X. Suna, RSC Adv., 2014, 4, 1449–1455, 10.1039/C3RA45598J.
- S. Anantharaj, H. Sugime and S. Noda, ACS Appl. Mater. Interfaces, 2020, 12, 27327–27338, DOI:10.1021/acsami.0c08979.
- C. X. Wang, W. Zeng, T. F. Li and Y. Q. Li, Mater. Technol., 2014, 30, 205–212, DOI:10.1179/1753555714Y.0000000243.
- L. Yu, G. Zhang, Y. Wu, X. Bai and D. Guo, J. Cryst. Growth, 2008, 310, 3125–3130, DOI:10.1016/j.jcrysgro.2008.03.026.
- S. Zaman, M. H. Asif, A. Zainelabdin, G. Amin, O. Nur and M. Willander, J. Electroanal. Chem., 2011, 662, 421–425, DOI:10.1016/j.jelechem.2011.09.015.
- B. Heng, C. Qing, D. Sun, B. Wang, H. Wang and Y. Tang, RSC Adv., 2013, 3, 15719–15726, 10.1039/C3RA42869A.
- M. Umadevi and A. Jegatha Christy, Spectrochim. Acta, Part A, 2013, 109, 133–137, DOI:10.1016/j.saa.2013.02.028.
- J. Liao, H. Li, X. Zhang and D. Xiao, Micro Nano Lett., 2014, 9, 432–436, DOI:10.1049/mnl.2014.0199.
- S. K. Shinde, D. P. Dubal, G. S. Ghodakec and V. J. Fulari, RSC Adv., 2015, 5, 4443–4447, 10.1039/C4RA11164H.
- J. Ye, Z. Li, Z. Dai, Z. Zhang, M. Guo and X. Wang, J. Electron. Mater., 2016, 45, 4237–4245, DOI:10.1007/s11664-016-4587-1.
- F. Nishino, M. Jeem, L. Zhang, K. Okamoto, S. Okabe and S. Watanabe, Sci. Rep., 2017, 7, 1063, DOI:10.1038/s41598-017-01194-5.
- G. Bhanjana, N. Dilbaghi, K. H. Kim and S. Kumar, J. Mol. Liq., 2017, 244, 506–511, DOI:10.1016/j.molliq.2017.09.034.
- F. Gao, L. Zhu, H. Li and H. Xie, Mater. Res. Bull., 2017, 93, 342–351, DOI:10.1016/j.materresbull.2017.05.033.
- R. Yuan, H. Li, X. Yin, L. Zhang and J. Lu, J. Mater. Sci. Technol., 2018, 34, 1692–1698, DOI:10.1016/j.jmst.2017.11.030.
- N. K. Yetim, N. Aslan, A. Sarıoğlu, N. Sarı and M. M. Koç, J. Mater. Sci.: Mater. Electron., 2020, 31, 12238–12248, DOI:10.1007/s10854-020-03769-x.
- S. Ganguly, R. Jha, P. K. Guha and C. Jacob, J. Electron. Mater., 2020, 49, 5070–5076, DOI:10.1007/s11664-020-08246-z.
- B. Miao, W. Zeng, L. Y. Lin and S. Xu, Phys. E, 2013, 52, 40–45, DOI:10.1016/j.physe.2013.03.006.
- X. Yan, X. Tong, J. Wang, C. Gong, M. Zhang and L. Liang, J. Alloys Compd., 2013, 556, 56–61, DOI:10.1016/j.jallcom.2012.12.124.
- X. Li, W. Liu, J. Ma, Y. Wen and Z. Wu, Appl. Catal., B, 2015, 179, 239–248, DOI:10.1016/j.apcatb.2015.05.034.
- Z. Skoufa, E. Heracleous and A. A. Lemonidou, J. Catal., 2015, 322, 118–129, DOI:10.1016/j.jcat.2014.11.014.
- Y. J. Mai, J. P. Tu, X. H. Xia, C. D. Gu and X. L. Wang, J. Power Sources, 2011, 196, 6388–6393, DOI:10.1016/j.jpowsour.2011.03.089.
- S. G. Hwang, G. O. K. Kim, S. R. Yun and K. S. Ryu, Electrochim. Acta, 2012, 78, 406–411, DOI:10.1016/j.electacta.2012.06.031.
- X. Wang, L. Li, Y. Zhang, S. Wang, Z. Zhang, L. Fei and Y. Qian, Cryst. Growth Des., 2006, 6, 2163–2165, DOI:10.1021/cg060156w.
- D. Su, H. S. Kim, W. S. Kim and G. Wang, Chem.–Eur. J., 2012, 18, 8224–8229, DOI:10.1002/chem.201200086.
- B. Varghese, M. V. Reddy, Z. Yanwu, C. S. Lit, T. C. Hoong, G. V. Subba Rao, B. V. R. Chowdari, A. T. S. Wee, C. T. Lim and C. H. Sow, Chem. Mater., 2008, 20, 3360–3367, DOI:10.1021/cm703512k.
- S. A. Needham, G. X. Wang and H. K. Liu, J. Power Sources, 2006, 159, 254–257, DOI:10.1016/j.jpowsour.2006.04.025.
- M. Zhou, W. Xiong, H. Li, D. Zhang and Y. Lv, Dalton Trans., 2021, 50, 5835–5844, 10.1039/D1DT00213A.
- Y. Pang, J. Zhang, D. Chen and X. Jiao, RSC Adv., 2016, 6, 30395, 10.1039/C5RA27715A.
- D. K. Subbiah, K. Babu, A. Das and J. B. B. R. Rayappan, ACS Appl. Mater. Interfaces, 2019, 11, 20045–20055, DOI:10.1021/acsami.9b04682.
- M. Tian, X. Hu, L. Qu, S. Zhu, Y. Sun and G. Han, Carbon, 2016, 96, 1166–1174, DOI:10.1016/j.carbon.2015.10.080.
- L. Wang and H. Yu, Springer Briefs in Molecular Science, Springer Singapore, Singapore, 2018, DOI:10.1007/978-981-10-6062-5.
- M. A. Shah, Nanoscale Res. Lett., 2008, 3, 255–259, DOI:10.1007/s11671-008-9147-z.
- B. Zhao, X. K. Ke, J. H. Bao, C. L. Wang, L. Dong, Y. W. Chen and H. L. Chen, J. Phys. Chem. C, 2009, 113, 14440–14447, DOI:10.1021/jp904186k.
- C. Xia, X. Yanjun and W. Ning, Sens. Actuators, B, 2011, 153, 434–438, DOI:10.1016/j.snb.2010.11.011.
- X. Wang, L. Wan, T. Yu, Y. Zhou, J. Guan, Z. Yu, Z. Li and Z. Zou, Mater. Chem. Phys., 2011, 126, 494–499, DOI:10.1016/j.matchemphys.2011.01.040.
- F. Cao, F. Zhang, R. Deng, W. Hu, D. Liu, S. Song and H. Zhang, CrystEngComm, 2011, 13, 4903, 10.1039/C1CE05237C.
- P. Wu, J. H. Sun, Y. Y. Huang, G. F. Gu and D. G. Tong, Mater. Lett., 2012, 82, 191–194, DOI:10.1016/j.matlet.2012.05.087.
- S. Cao, W. Zeng, H. Long, J. Gong, Z. Zhu and L. Chen, Mater. Lett., 2015, 161, 275–277, DOI:10.1016/j.matlet.2015.08.129.
- A. Qurashi, Z. Zhang, M. Asif and T. Yamazaki, Energy, 2015, 40, 15801–15805, DOI:10.1016/j.ijhydene.2015.07.114.
- J. Wang, W. Zeng and Z. Wang, Ceram. Int., 2016, 42, 4567–4573, DOI:10.1016/j.ceramint.2015.11.150.
- R. Miao and W. Zeng, Mater. Lett., 2016, 171, 200–203, DOI:10.1016/j.matlet.2016.02.052.
- S. Liu, W. Zeng and T. Chen, Phys. E, 2017, 85, 13–18, DOI:10.1016/j.physe.2016.08.016.
- Y. Zhang and W. Zeng, Mater. Lett., 2017, 195, 217–219, DOI:10.1016/j.matlet.2017.02.124.
- S. Munkaila, J. Bentley, K. Schimmel, T. Ahamad, S. M. Alshehri and B. S. Bastakoti, J. Mol. Liq., 2021, 324, 114676, DOI:10.1016/j.molliq.2020.114676.
- A. Chen, X. Peng, K. Koczkur and B. Miller, Chem. Commun., 2004, 17, 1964–1965, 10.1039/B407313D.
- J. Ning, Q. Dai, T. Jiang, K. Men, D. Liu, N. Xiao, C. Li, D. Li, B. Liu, B. Zou, G. Zou and W. W. Yu, Langmuir, 2009, 25, 1818–1821, DOI:10.1021/la8037473.
- S. Zeng, K. Tang, T. Li, Z. Liang, D. Wang, Y. Wang, Y. Qi and W. Zhou, J. Phys. Chem. C, 2008, 112, 4836–4843, DOI:10.1021/jp0768773.
- H. P. Zhou, Y. W. Zhang, H. X. Mai, X. Sun, Q. Liu, W. G. Song and C. H. Yan, Chem.–Eur. J., 2008, 14, 3380–3390, DOI:10.1002/chem.200701348.
- J. Ni, W. Lu, L. Zhang, B. Yue, X. Shang and Y. Lv, J. Phys. Chem. C, 2009, 113, 54–60, DOI:10.1021/jp806454r.
- V. Srivastava, Y. C. Sharma and M. Sillanpaa, Ceram. Int., 2015, 41, 6702–6709, DOI:10.1016/j.ceramint.2015.01.112.
- L. Jiang, G. Li, Q. Ji and H. Peng, Mater. Lett., 2007, 61, 1964–1967, DOI:10.1016/j.matlet.2006.07.167.
- Q. Li, H. Sun, M. Luo, W. Weng, K. Cheng, C. Song, P. Du, G. Shen and G. Han, J. Alloys Compd., 2010, 503, 514–518, DOI:10.1016/j.jallcom.2010.05.043.
- G. Saito, S. Hosokai and T. Akiyama, Mater. Chem. Phys., 2011, 130, 79–83, DOI:10.1016/j.matchemphys.2011.05.084.
- R. Shi, P. Yang, X. Dong, Q. Ma and A. Zhang, Appl. Surf. Sci., 2013, 264, 162–170, DOI:10.1016/j.apsusc.2012.09.164.
- R. M. Tripathi, A. S. Bhadwal, R. K. Gupta, P. Singh, A. Shrivastav and B. R. Shrivastav, J. Photochem. Photobiol., B, 2014, 141, 288–295, DOI:10.1016/j.jphotobiol.2014.10.001.
- H. Wu, Z. Yuan, B. Wang, F. Nie, J. He, X. Wang and L. Liu, Opt. Mater., 2021, 122, 111683, DOI:10.1016/j.optmat.2021.111683.
- J. K. Rajput, T. K. Pathak, H. C. Swart and L. P. Purohit, Phys. Status Solidi A, 2019, 216, 1900093, DOI:10.1002/pssa.201900093.
- C. Harito, S. Z. J. Zaidi, D. V. Bavykin, A. S. Martins, B. Yuliarto, F. C. Walsh and C. P. de Leon, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2020, 11, 035018, DOI:10.1088/2043-6254/abb238.
- Y. T. Tsai, S. J. Chang, L. W. Ji, Y. J. Hsiao, I. T. Tang, H. Y. Lu and Y. L. Chu, ACS Omega, 2018, 3, 13798–13807, DOI:10.1021/acsomega.8b01882.
- X. Y. Xue, Z. H. Chen, L. L. Xing, C. H. Ma, Y. J. Chen and T. H. Wang, J. Phys. Chem. C, 2010, 114, 18607–18611, DOI:10.1021/jp1070067.
- H. Liu, L. Shi, D. Li, J. Yu, H. M. Zhang, S. Ullah, B. Yang, C. Li, C. Zhu and J. Xu, J. Power Sources, 2018, 387, 64–71, DOI:10.1016/j.jpowsour.2018.03.047.
- W. Yu, M. D. Porosoff and J. G. Chen, Chem. Rev., 2012, 112, 5780–5817, DOI:10.1021/cr300096b.
- J. Wang and H. Gu, Molecules, 2015, 20, 17070–17092, DOI:10.3390/molecules200917070.
- S. De, J. Zhang, R. Luque and N. Yan, Energy Environ. Sci., 2016, 9, 3314–3347, 10.1039/C6EE02002J.
- K. D. Gilroy, A. Ruditskiy, H. C. Peng, D. Qin and Y. Xia, Chem. Rev., 2016, 116, 10414–10472, DOI:10.1021/acs.chemrev.6b00211.
- Y. Yan, J. S. Du, K. D. Gilroy, D. Yang, Y. Xia and H. Zhang, Adv. Mater., 2017, 29, 1605997, DOI:10.1002/adma.201605997.
- C. Rice, S. Ha, R. I. Masel and A. Wieckowski, J. Power Sources, 2003, 115, 229, DOI:10.1016/S0378-7753(03)00026-0.
- Y. M. Zhu, Z. Khan and R. I. Masel, J. Power Sources, 2005, 139, 15–20, DOI:10.1016/j.jpowsour.2004.06.054.
- X. W. Yu and P. G. Pickup, J. Power Sources, 2008, 182, 124–132, DOI:10.1016/j.jpowsour.2008.03.075.
- W. P. Zhou, A. Lewera, R. Larsen, R. I. Masel, P. S. Bagus and A. Wieckowski, J. Phys. Chem. B, 2006, 110, 13393, DOI:10.1021/jp061690h.
- W. J. Zhou and J. Y. Lee, J. Phys. Chem. C, 2008, 112, 3789, DOI:10.1021/jp077068m.
- C. Y. Du, M. Chen, W. G. Wang, G. P. Yin and P. F. Shi, Electrochem. Commun., 2010, 12, 843, DOI:10.1016/j.elecom.2010.03.046.
- D. Morales-Acosta, J. Ledesma-Garcia, L. A. Godinez, H. G. Rodriguez, L. Alvarez-Contreras and L. G. Arriaga, J. Power Sources, 2010, 195, 461, DOI:10.1016/j.jpowsour.2009.08.014.
- V. Mazumder, M. F. Chi, M. N. Mankin, Y. Liu, O. Metin, D. H. Sun, K. L. More and S. H. Sun, Nano Lett., 2012, 12, 1102, DOI:10.1021/nl2045588.
- C. X. Xu, Y. Q. Liu, J. P. Wang, H. R. Geng and H. J. Qiu, J. Power Sources, 2012, 199, 124, DOI:10.1016/j.jpowsour.2011.10.075.
- X. Wang, Y. Tang, Y. Gao and T. H. Lu, J. Power Sources, 2008, 175, 784–788, DOI:10.1016/j.jpowsour.2007.10.011.
- T. Ma, F. Liang, R. Chen, S. Liu and H. Zhang, Nanomaterials, 2017, 7, 239, DOI:10.3390/nano7090239.
- T. Ma, W. Yang, S. Liu, H. Zhang and F. A. Liang, Catalysts, 2017, 7, 38, DOI:10.3390/catal7020038.
- Y. W. Lee, M. Kim, Y. Kim, S. W. Kang, J. H. Lee and S. W. Han, J. Phys. Chem. C, 2010, 114, 7689–7693, DOI:10.1021/jp9119588.
- Y. Han, X. Yang, Y. Liu, Q. Ai, S. Liu, C. Sun and F. Liang, Sci. Rep., 2016, 6, 22239, DOI:10.1038/srep22239.
- R. S. Downing, P. J. Kunkeler and H. V. Bekkum, Catal. Today, 1997, 37, 121–136, DOI:10.1016/S0920-5861(97)00005-9.
- A. Li, W. Duan, J. Liu, K. Zhuo, Y. Chen and J. Wang, Sci. Rep., 2018, 8, 13141, DOI:10.1038/s41598-018-31402-9.
- Y. Imura, R. Akiyama, S. Furukawa, R. Kan, C. Morita-Imura, T. Komatsu and T. Kawai, Chem.–Asian J., 2019, 14, 547–552, DOI:10.1002/asia.201801711.
- S. Yamazoe, K. Koyasu and T. Tsukuda, Acc. Chem. Res., 2014, 47, 816–824, DOI:10.1021/ar400209a.
- Z. Cao, H. Chen, S. Zhu, W. Zhang, X. Wu, G. Shan, U. Ziener and D. Qi, Langmuir, 2015, 31, 4341–4350, DOI:10.1021/acs.langmuir.5b00437.
- Z. Cao, H. Chen, S. Zhu, Z. Chen, C. Xu, D. Qi and U. Ziener, Colloids Surf., A, 2016, 489, 223–233, DOI:10.1016/j.colsurfa.2015.11.001.
- C. M. Imura, T. Mori, Y. Imura and T. Kawai, New J. Chem., 2016, 40, 7048–7052, DOI:10.1021/acsomega.1c01895.
- Y. Imura, S. Furukawa, K. Ozawa, C. Morita-Imura, T. Kawai and T. Komatsu, RSC Adv., 2016, 6, 17222–17227, 10.1039/C5RA27146K.
- J. A. Lopez-Sanchez, N. Dimitratos, C. Hammond, G. L. Brett, L. Kesavan, S. White, P. Miedziak, R. Tiruvalam, R. L. Jenkins, A. F. Carley, D. Knight, C. J. Kiely and G. J. Hutchings, Nat. Chem., 2011, 3, 551–556, DOI:10.1038/nchem.1066.
- Z. Niu and Y. Li, Chem. Mater., 2014, 26, 72–83, DOI:10.1021/cm4022479.
- L. Zhang, X. F. Zhang, X. L. Chen, A. J. Wang, D. M. Han, Z. G. Wang and J. J. Feng, J. Colloid Interface Sci., 2019, 536, 556–562, DOI:10.1016/j.jcis.2018.10.080.
- G. D. Rodrigues, L. R. de Lemos, L. H. M. da Silva and M. C. H. da Silva, J. Chromatogr. A, 2013, 1279, 13–19, DOI:10.1016/j.chroma.2013.01.003.
- Y. Wang, B. Ouyang, B. Zhang, Y. Boluo, Y. Huang, R. V. Ramanujan, K. K. Ostrikov and R. S. Rawat, J. Phys. D: Appl. Phys., 2020, 53, 225201, DOI:10.1088/1361-6463/ab7797.
- M. Li, H. Zheng, G. Han, Y. Xiao and Y. Li, Catal. Commun., 2017, 92, 95–99, DOI:10.1016/j.catcom.2017.01.014.
- Q. Zhou, L. Qi, H. Yang and C. Xu, J. Colloid Interface Sci., 2018, 513, 258–265, DOI:10.1016/j.jcis.2017.11.040.
- G. Fu, K. Wu, J. Lin, Y. Tang, Y. Chen, Y. Zhou and T. Lu, J. Phys. Chem. C, 2013, 117, 9826–9834, DOI:10.1021/jp400502y.
- S. S. Chen, X. X. Lin, A. J. Wang, H. Huang and J. J. Feng, Sens. Actuators, B, 2017, 248, 214–222, DOI:10.1016/j.snb.2017.03.129.
- A. Chalgin, F. Shi, F. Li, Q. Xiang, W. Chen, C. Song, P. Tao, W. Shang, T. Deng and J. Wu, CrystEngComm, 2017, 19, 6964–6971, 10.1039/C7CE01721A.
- H. Xu, B. Yan, K. Zhang, C. Wang, J. Zhong, S. Li, Y. Du and P. Yang, Colloids Surf., A, 2017, 522, 335–345, DOI:10.1016/j.colsurfa.2017.03.015.
- X. Guo, H. Shang, J. Guo, H. Xu and Y. Du, Appl. Surf. Sci., 2019, 481, 1532–1537, DOI:10.1016/j.apsusc.2019.03.234.
- H. M. An, Z. L. Zhao, L. Y. Zhang, Y. Chen, Y. Y. Chang and C. M. Li, ACS Appl. Mater. Interfaces, 2018, 10, 41293–41298, DOI:10.1021/acsami.8b13361.
- I. Arief and P. K. Mukhopadhyay, Phys. B, 2014, 448, 73–76, DOI:10.1016/j.physb.2014.04.068.
- H. J. Kim, S. J. Lee, S. Y. Park, J. H. Jung and J. S. Kim, Adv. Mater., 2008, 20, 3229–3234, DOI:10.1002/adma.200800246.
- F. Torney, B. G. Trewyn, V. S. Y. Lin and K. Wang, Nat. Nanotechnol., 2007, 2, 295–300, DOI:10.1038/nnano.2007.108.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036, DOI:10.1021/ja974025i.
- W. Pan, J. Ye, G. Ning, Y. Lin and J. Wang, Mater. Res. Bull., 2009, 44, 280–283, DOI:10.1016/j.materresbull.2008.06.006.
- J. S. Kang, J. Lim, W. Y. Rho, J. Kim, D. S. Moon, J. Jeong, D. Jung, J. W. Choi, J. K. Lee and Y. E. Sung, Sci. Rep., 2016, 6, 30829, DOI:10.1038/srep30829.
- J. E. Lee, N. Lee, H. Kim, J. Kim, S. H. Choi, J. H. Kim, T. Kim, I. C. Song, S. P. Park, W. K. Moon and T. Hyeon, J. Am. Chem. Soc., 2010, 132, 552–557, DOI:10.1021/ja905793q.
- N. K. Mal, M. Fujiwara and Y. Tanaka, Nature, 2003, 421, 350–353, DOI:10.1038/nature01362.
- L. Wang, X. Liu, Y. Jiang, P. Liu, L. Zhou, L. Ma, Y. He, H. Li and J. Gao, Catalysts, 2019, 9(12), 1026, DOI:10.3390/catal9121026.
- X. L. Liu, C. T. Ng, P. Chandrasekharan, H. T. Yang, L. Y. Zhao, E. Peng, Y. B. Lv, W. Xiao, J. Fang, J. B. Yi, H. Zhang, K. H. Chuang, B. H. Bay, J. Ding and H. M. Fan, Adv. Healthcare Mater., 2016, 5, 2092–2104, DOI:10.1002/adhm.201600357.
- J. Xu, A. R. Wilson, A. R. Rathmell, J. Howe, M. Chi and B. J. Wiley, ACS Nano, 2011, 5, 6119–6127, DOI:10.1021/nn201161m.
- S. Yin, Z. Wang, S. Liu, S. Jiao, W. Tian, Y. Xu, X. Li, L. Wang and H. Wang, Nanoscale, 2021, 13, 3208–3213, 10.1039/D0NR08758K.
- X. Qiu, Y. Dai, Y. Tang, T. Lu, S. Wei and Y. Chen, J. Power Sources, 2015, 278, 430–435, 10.1039/C8CC02816H.
- Y. Lai, L. Dong, R. Liu, S. Lu, Z. He, W. Shan, F. Geng, Y. Cai and J. Liu, Chin. Chem. Lett., 2020, 31, 2437–2441, DOI:10.1016/j.cclet.2020.04.050.
- W. Yan, L. Yang, H. Wang, J. Zhang and W. Shen, Nanoscale, 2018, 10, 15661–15668, 10.1039/C8NR04196B.
- M. P. D. S. Rodrigues, A. H. B. Dourado, L. D. O. Cutolo, L. S. Parreira, T. V. Alves, T. J. A. Slater, S. J. Haigh, P. H. C. Camargo and S. I. C. de Torresi, ACS Catal., 2021, 11, 13543–13555, DOI:10.1021/acscatal.1c02938.
- A. T. N. Nguyen and J. H. Shim, Appl. Surf. Sci., 2018, 458, 910–916, DOI:10.1016/j.apsusc.2018.07.161.
- Y. Ren, X. Yang, L. Li, C. Li, X. Zhang, Z. Lu and X. Yu, Catal. Lett., 2020, 150, 3415–3423, DOI:10.1007/s10562-020-03242-8.
- Y. Xiong, S. Chen, F. Ye, L. Su, C. Zhang, S. Shen and S. Zhao, Anal. Methods, 2015, 7, 1300–1306, 10.1039/C4AY02687J.
- Y. Zhao, L. Hu, S. Zhao and L. Wu, Adv. Funct. Mater., 2016, 26, 4085–4093, DOI:10.1002/adfm.201600494.
- M. Wang and X. Zhang, ChemistrySelect, 2017, 2, 9537–9545, DOI:10.1002/slct.201700746.
- N. Zhang, C. Xu, H. Wang, J. Zhang, Y. Liu and Y. Fang, J. Mater. Sci.: Mater. Electron., 2021, 32, 1787–1799, DOI:10.1007/s10854-020-04947-7.
- Y. Yang, T. Liu, L. Zhang, S. Zhao, W. Zeng, S. Hussain, C. Deng, H. Pan and X. Peng, J. Mater. Sci.: Mater. Electron., 2016, 27, 6202–6207, DOI:10.1007/s10854-016-4550-1.
- X. Dou, D. Sabba, N. Mathews, L. H. Wong, Y. M. Lam and S. Mhaisalkar, Chem. Mater., 2011, 23, 3938–3945, DOI:10.1021/cm201366z.
- X. Zheng, Y. Li, Y. Zheng, L. Shen, Y. Xiao, Y. Cao, Y. Zhang, C. Au and L. Jiang, ACS Catal., 2020, 10, 3968–3983, DOI:10.1021/acscatal.9b05486.
- L. Zhou, X. Zhou, C. Zhao, Y. Liu, Y. Li, L. Ma, Y. He, Y. Jiang and J. Gao, Microchem. J., 2021, 165, 106169, DOI:10.1016/j.microc.2021.106169.
- Y. Imura, S. Hojo, C. Morita and T. Kawai, Langmuir, 2014, 30, 1888–1892, DOI:10.1021/la403681w.
- Y. Lu, Y. Yin, Z.-Y. Li and Y. Xia, Nano Lett., 2002, 2, 785–788, DOI:10.1021/nl025598i.
- H. K. Sadhanala, S. Senapati, K. V. Harika, K. K. Nanda and A. Gedanken, New J. Chem., 2018, 42, 14318–14324, 10.1039/C8NJ01731J.
- S. Santhosh, M. Sathish, S. Iyer, S. Kalluri and A. Madhavan, Mater. Lett., 2021, 302, 130343, DOI:10.1016/j.matlet.2021.130343.
- X. Sun, P. Yang, S. Wang, C. Jin, M. Ren and H. Xing, Langmuir, 2021, 37, 10403–10412, DOI:10.1021/acs.langmuir.1c00737.
- F. Zhao, H. Yang, K. Qin, G. Cui and Q. Liu, J. Taiwan Inst. Chem. Eng., 2021, 126, 244–251, DOI:10.1016/j.jtice.2021.06.054.
- Q. Dai, N. Xiao, J. Ning, C. Li, D. Li, B. Zou, W. W. Yu, S. Kan, H. Chen, B. Liu and G. Zou, J. Phys. Chem. C, 2008, 112, 7567–7571, DOI:10.1021/jp7120559.
- Q. Zeng, S. Xue, S. Wu, K. Gan, L. Xu, J. Han, W. Zhou and R. Zou, Ceram. Int., 2014, 40, 2847–2852, DOI:10.1016/j.ceramint.2013.10.029.
- A. Alemi, A. Babalou, M. Dolatyari, A. Klein and G. Meyer, Z. Anorg. Allg. Chem., 2009, 635, 2053–2057, DOI:10.1002/zaac.200900028.
- N. Cao, K. Hu, W. Luo and G. Z. Cheng, J. Alloys Compd., 2014, 590, 241–246, DOI:10.1016/j.jallcom.2013.12.134.
- M. Rakap, J. Power Sources, 2015, 276, 320–327, DOI:10.1016/j.jpowsour.2014.11.146.
- D. D. Ke, Y. Li, J. Wang, L. Zhang, J. D. Wang, X. Zhao, S. Yang and S. Han, Int. J. Hydrogen Energy, 2016, 41, 2564–2574, DOI:10.1016/j.ijhydene.2015.11.142.
- Z. Zhang, Y. Jiang, M. Chi, Z. Yang, C. Wang and X. Lu, RSC Adv., 2015, 5, 94456–94461, 10.1039/C5RA18698F.
- A. J. Amali, K. Aranishi, T. Uchida and Q. Xu, Part. Part. Syst. Charact., 2013, 30, 888–892, DOI:10.1002/ppsc.201300100.
- X. J. Yang, F. Y. Cheng, J. Liang, Z. L. Tao and J. Chen, Int. J. Hydrogen Energy, 2009, 34, 8785–8791, DOI:10.1016/j.ijhydene.2009.08.075.
- G. P. Rachiero, U. B. Demirci and P. Miele, Int. J. Hydrogen Energy, 2011, 36, 7051–7065, DOI:10.1016/j.ijhydene.2011.03.009.
- Y. Yang, Z.-H. Lu, Y. Hu, Z. Zhang, W. Shi, X. Chen and T. Wang, RSC Adv., 2014, 4, 13749–13752, 10.1039/C3RA47023G.
- H. Ahmed, P. Adebayo, M. Ahmed and A. I. Atbab, J. Energy Technol. Policy, 2023, 13, 1, DOI:10.7176/JETP/13-1-07.
- A. Morozan, B. Jousselme and S. Palacin, Energy Environ. Sci., 2011, 4, 1238–1254, 10.1039/C0EE00601G.
- S. Zhang, C. Zhao, Y. Liu, W. Li, J. Wang, G. Wang, Y. Zhang, H. Zhang and H. Zhao, Chem. Commun., 2019, 55, 2952–2955, 10.1039/C9CC00123A.
- T. L. Frölicher, E. M. Fischer and N. Gruber, Nature, 2018, 560, 360–364, DOI:10.1038/s41586-018-0383-9.
- J. Wang, L. Yu, L. Hu, G. Chen, H. Xin and X. Feng, Nat. Commun., 2018, 9, 1795, DOI:10.1038/s41467-018-04213-9.
- H. Wang, S. Liu, H. Zhang, S. Yin, Y. Xu, X. Li, Z. Wang and L. Wang, Nanoscale, 2020, 12, 13507–13512, 10.1039/D0NR02884C.
- X. Wu, L. Xia, Y. Wang, W. Lu, Q. Liu, X. Shi and X. Sun, Small, 2018, 14, 1803111, DOI:10.1002/smll.201803111.
- X. Xiang, Z. Wang, X. Shi, M. Fan and X. Sun, ChemCatChem, 2018, 10, 4530–4535, DOI:10.1002/cctc.201801208.
- K. Chu, Y. Liu, J. Wang and H. Zhang, ACS Appl. Energy Mater., 2019, 2, 2288–2295, DOI:10.1021/acsaem.9b00102.
- Y. Chen, B. Wu, B. Sun, N. Wang, W. Hu and S. Komarneni, ACS Sustainable Chem. Eng., 2019, 7, 18874–18883, DOI:10.1021/acssuschemeng.9b04024.
- M. Reddeppa, B.-G. Park, G. Murali, S. H. Choi, N. D. Chinh, D. Kim, W. Yang and M.-D. Kim, Sens. Actuators, B, 2020, 308, 127700, DOI:10.1016/j.snb.2020.127700.
- Q. Zhang, G. Xie, M. Xu, Y. Su, H. Tai, H. Du and Y. Jiang, Sens. Actuators, B, 2018, 259, 269–281, DOI:10.1016/j.snb.2017.12.052.
- H.-Y. Li, J.-W. Yoon, C.-S. Lee, K. Lim, J.-W. Yoon and J.-H. Lee, Sens. Actuators, B, 2018, 255, 2963–2970, DOI:10.1016/j.snb.2017.09.118.
- C. Zhang, A. Boudiba, P. De Marco, R. Snyders, M.-G. Olivier and M. Debliquy, Sens. Actuators, B, 2013, 181, 395–401, DOI:10.1016/j.snb.2013.01.082.
- A. S. Chizhov, M. N. umyantseva, R. B. Vasiliev, D. G. Filatova, K. A. Drozdov, I. V. Krylov, A. M. Abakumov and A. M. Gaskov, Sens. Actuators, B, 2014, 205, 305–312, DOI:10.1016/j.snb.2014.08.091.
- C. Chen, Q. Zhang, G. Xie, M. Yao, H. Pan, H. Du, H. Tai, X. Du and Y. Su, Mater. Res. Express, 2020, 7, 015924, DOI:10.1088/2053-1591/ab6b64.
- X. Geng, D. Lahem, C. Zhang, C.-J. Li, M.-G. Olivier and M. Debliquy, Ceram. Int., 2019, 45, 4253–4261, DOI:10.1016/j.ceramint.2018.11.097.
- L. Giancaterini, S. M. Emamjomeh, A. De Marcellis, E. Palange, A. Resmini, U. Anselmi-Tamburini and C. Cantalini, Sens. Actuators, B, 2016, 229, 387–395, DOI:10.1016/j.snb.2016.02.007.
- S. Boukhalfa, K. Evanoff and G. Yushin, Energy Environ. Sci., 2012, 5, 6872, 10.1039/C2EE21110F.
- X. F. Sun, Y. L. Xu, J. Wang and S. C. Mao, Int. J. Electrochem. Sci., 2012, 7, 3205–3214 CrossRef CAS.
- P. Lin, Q. J. She, B. L. Hong, X. A. J. Liu, Y. N. Shi, Z. Shi, M. S. Zheng and Q. F. Dong, J. Electrochem. Soc., 2010, 157, A818, DOI:10.1149/1.3425624.
- X. Zhang, W. Shi, J. Zhu, W. Zhao, J. Ma, S. Mhaisalkar, T. L. Maria, Y. Yang, H. Zhang, H. H. Hng and Q. Yan, Nano Res., 2010, 3, 643–652, DOI:10.1007/s12274-010-0024-6.
- T. Zhu, J. S. Chen and X. W. Lou, J. Mater. Chem., 2010, 20, 7015–7020, 10.1039/C0JM00867B.
- Y. Yang, Y. Liang, Z. Zhang, Y. Zhang, H. Wu and Z. Hu, J. Alloys Compd., 2016, 658, 621–628, DOI:10.1016/j.jallcom.2015.10.253.
- F. Y. Cheng, Z. L. Tao, J. Liang and J. Chen, Chem. Mater., 2008, 20, 667–681, DOI:10.1021/cm702091q.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935, DOI:10.1126/science.12127.
- L. Gu, W. Xie, S. Bai, B. Liu, S. Xue, Q. Li and D. He, Appl. Surf. Sci., 2016, 368, 298–302, DOI:10.1016/j.apsusc.2016.01.270.
- Y. Li, B. Tan and Y. Wu, Nano Lett., 2008, 8, 265–270, DOI:10.1021/nl0725906.
- F. Cheng, Z. Tao, J. Liang and J. Chen, Chem. Mater., 2008, 20, 667–681, DOI:10.1021/cm702091q.
- L. Chun, X. Wu, X. Lou and Y. Zhang, Electrochim. Acta, 2010, 55, 3089–3092, DOI:10.1016/j.electacta.2010.01.016.
- S. K. Cheah, E. Perre, M. Rooth, M. Fondell, A. Hårsta, L. Nyholm, M. Boman, T. Gustafsson, J. Lu, P. Simon and K. Edstr€om, Nano Lett., 2009, 9(9), 3230–3233, DOI:10.1021/nl9014843.
- C. M. Doherty, R. A. Caruso, B. M. Smarsly, P. Adelhelm and C. J. Drummond, Chem. Mater., 2009, 21, 5300–5306, DOI:10.1021/cm9024167.
- S. Y. Zeng, K. B. Tang, T. W. Li, Z. H. Liang, D. Wang, Y. K. Wang and W. W. Zhou, J. Phys. Chem. C, 2007, 111, 10217–10225, DOI:10.1021/jp0719661.
- L. Liu, Y. Li, S. Yuan, M. Ge, M. Ren, C. Sun and Z. Zhou, J. Phys. Chem. C, 2010, 114, 251–255, DOI:10.1021/jp909014w.
- X. H. Huang, J. P. Tu, C. Q. Zhang and F. Zhou, Electrochim. Acta, 2010, 55, 8981–8985, DOI:10.1016/j.electacta.2010.08.039.
- G. Zhang, Y. Chen, B. Qu, L. Hu, L. Mei, D. Lei, Q. Li, L. Chen and Q. Li, Electrochim. Acta, 2012, 80, 140–147, DOI:10.1016/j.electacta.2012.06.107.
- X. Li, A. Dhanabalan and C. Wang, J. Power Sources, 2011, 196, 9625–9630, DOI:10.1016/j.jpowsour.2011.06.097.
- C. Wang, D. Wang, Q. Wang and H. Chen, J. Power Sources, 2010, 195, 7432–7437, DOI:10.1016/j.jpowsour.2010.04.090.
- J. H. Pan, Q. Huang, Z. Y. Koh, C. Jin, D. Neo, X. Z. Wang and Q. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 6292–6299, DOI:10.1021/am401330g.
- V. Aravindan, P. S. Kumar, J. Sundaramurthy, W. C. Ling, S. Ramakrishna and S. Madhavi, J. Power Sources, 2013, 227, 284–290, DOI:10.1016/j.jpowsour.2012.11.050.
- F. Zhang, D. Jiang and X. Zhang, Nano-Struct. Nano-Objects, 2016, 5, 1–6, DOI:10.1016/j.nanoso.2015.12.002.
- Q. Wang, Y.-F. Xu, G.-L. Xu, H. Su, S.-Y. Shen, T.-T. Tu and L. Huang, J. Alloys Compd., 2015, 648, 59–66, DOI:10.1016/j.jallcom.2015.06.221.
- P. Lv, H. Zhao, Z. Zeng, C. Gao, X. Liu and T. Zhang, Appl. Surf. Sci., 2015, 329, 301–305, DOI:10.1016/j.apsusc.2014.12.170.
- L. Liu, Y. Guo, Y. Wang, X. Yang, S. Wang and H. Guo, Electrochim. Acta, 2013, 114, 42–47, DOI:10.1016/j.electacta.2013.09.152.
- Y. Zhao, Q. Pang, Y. Meng, Y. Gao, C. Wang, B. Liu, Y. Wei, F. Du and G. Chen, Chem.–Eur. J., 2017, 23, 13150–13157, DOI:10.1002/chem.201702399.
- B. Liu, H. Q. Peng, J. Cheng, K. Zhang, D. Chen, D. Shen, S. Wu, T. Jiao, X. Kong, Q. Gao, S. Bu, C. S. Lee and W. Zhang, Small, 2019, 15, 1901545, DOI:10.1002/smll.201901545.
- L. Su, W. Jia, L. Zhang, C. Beacham, H. Zhang and Y. Lei, J. Phys. Chem. C, 2010, 114, 18121–18125, DOI:10.1021/jp107636r.
- D. Banerjee, U. K. Ghorai, N. S. Das, B. Das, S. Thakur and K. K. Chattopadhyay, ACS Omega, 2018, 3, 6311–6320, DOI:10.1021/acsomega.8b00798.
| This journal is © The Royal Society of Chemistry 2023 |