Active therapy based on the byproducts of micro/nanomotors
Haiying
Liang
 a,
Fei
Peng
a,
Fei
Peng
 b and
Yingfeng
Tu
b and
Yingfeng
Tu
 *a
*a
aNMPA Key Laboratory for Research and Evaluation of Drug Metabolism & Guangdong Provincial Key Laboratory of New Drug Screening, School of Pharmaceutical Sciences, Southern Medical University, Guangzhou 510515, China. E-mail: tuyingfeng1@smu.edu.cn
bSchool of Materials Science and Engineering, Sun Yat-Sen University, Guangzhou 510275, China
First published on 1st December 2022
Abstract
Different from traditional colloidal particles based on Brownian motion, micro/nanomotors are micro/nanoscale devices capable of performing complex tasks in liquid media via transforming various energy sources into mechanical motion or actuation. Such unique self-propulsion endows motors with fantastic capabilities to access and enter the deep layer of targeted diseased tissue, which in turn breaks through the limitation of the poor permeability of traditional pharmaceutical preparations, thus providing giant prospects for active therapy. It is noteworthy that recently several studies, which utilized the byproducts generated in situ by micro/nanomotors to achieve active therapy, in a truly green zero-waste manner, have been carried out. In this minireview, we highlight the recent efforts with respect to active therapy based on the byproducts of micro/nanomotors, expecting to motivate readers to expand the practical biomedical application scope of micro/nanomotors in a broader horizon. Accompanied by ever booming enthusiasm and persevering exploration, micro/nanomotors are on their way to revolutionize conventional fields.
1. Introduction
For quite a long time, scientists have been obsessed with the desire to invent miniaturized machines that are capable of navigating spontaneously toward the targeted locations in biological media and subsequently completing predesignated missions. The bold hypothesis of a miniaturized machine could be traced back to a legendary speech “There's plenty of room at the bottom” in 1959, given by Richard Feynman, a Nobel laureate in physics.1 Shortly thereafter, the imagination came true on the big screen at first. The film “Fantastic voyage” elaborated a story that surgeons sailed in blood by means of a microsubmarine to dissolve the clot and then escaped through eyes, released in 1966.2 The film bears the ardent thirst to apply micro/nanorobots to the diagnosis and treatment of diseases, which impels the development of machines with similar fascinating properties, especially at the micro/nanoscale suitable for clinical applications. The past few decades have witnessed giant breakthroughs in nanotechnology and the embryonic form of miniaturized machines, giving the credit to the unremitting endeavors of researchers. In 2004, the first example of micro/nanomotors, contrived by Sen, Mallouk and Crespi,3 came into existence, which has set off an upsurge in research in the emerging field of micro/nanomotors since then. Subsequently, micro/nanomotors, fabricated with multifarious structures, propelled by manifold mechanisms and applied to diverse applications, have sprung up constantly. In 2016, the Nobel Prize in chemistry was awarded jointly to Jean-Pierre Sauvage, Fraser Stoddart and Ben Feringa, in recognition of their pioneering contributions to the “design and synthesis of molecular machines”,4 which has significantly impulsed the unprecedented development of micro/nanomotors.Micro/nanomotors, also known as micro/nanomachines, micro/nanorobots, and micro/nanoswimmers, are a range of miniaturized devices which have the ability to walk with ease. They are competent to convert chemical fuels5 or external energies6 into mechanical movement or actuation and subsequently perform sophisticated tasks, breaking through the bottleneck of traditional therapeutic platforms. By virtue of their miniaturized size, distinctive automatic navigation, strong cargo towing and effective penetrating capability, micro/nanomotors have displayed limitless potential to revolutionize various research areas, particularly the biomedical field, such as drug delivery,7–10 precise surgery,11–13 sensing,14–18 and imaging.19–22
In the past few years, accompanied by the vigorous advances of micro/nanomotors, a multitude of reviews with regard to motors from different emphasis points have sprung up. It is worth noting that these reviews often focus on the fabrication techniques, propulsion mechanisms, motion behaviors and applications of micro/nanomotors,23–30 which reflects that researchers tend to pay great attention to perfect structures, striking motility capacities and conspicuous application outcomes of motors. On the other hand, it also implies from the side that it is easy to disregard the byproducts produced or generated during the motion process of micro/nanomotors, and what's worse, treat them as waste. However, in comparison to simply taking motors as active drug carriers to deliver therapeutic molecules to the prescribed destinations, it is better to directly use the in situ byproducts formed in response to the microenvironment or external stimuli for active therapy. Therefore, it is time to summarize the current progress in active therapy using micro/nanomotors based on byproducts, which have not been reviewed yet. For one thing, it is of great benefit to remind researchers to set up the consciousness and concept of turning waste into treasure, so as to find out smarter strategies that are in line with practical clinical applications, and for another, it is conducive to laying the foundation for further clinical applications of micro/nanomotors in the future.
2. Active therapy based on the byproducts of micro/nanomotors
Based on the characteristics of byproducts, two classifications can be divided, chemical byproducts and physical byproducts, respectively (Fig. 1). Chemical byproducts and physical byproducts of motors share a mutual feature, that is they actually possess the ability to exert therapeutic effects, thus making them especially suitable for active therapy, which will be expounded in the following sections.2.1. Chemical byproducts
Chemical byproducts are routinely the results of the spontaneous chemical reactions between motors and substrates existing and readily available in the microenvironment, which are divided into hydrogen, nitric oxide, and ammonia in this minireview. In this section, we summarize the recent developments in active therapy by micro/nanomotors based on the chemical byproducts in detail.It is universally acknowledged that active metals could generate H2 bubbles through spontaneous redox reactions with water or acid. Inspired by this, active metal-based motors have been designed for the realization of H2 therapy. Magnesium (Mg)-based micromotors, which are dependent on the Mg–water reaction, are typical representatives of active metal-based motors.34 Our group prepared a biocompatible and biodegradable Mg-based Janus micromotor by asymmetrically wrapping a poly(lactic-co-glycolic acid) (PLGA) layer on the outer surface of Mg microspheres, for the active H2 therapy of acute ischemic stroke (Fig. 2A).35 When immersed in an artificial cerebrospinal fluid, the motor was capable of travelling forward with a speed of 51.1 μm s−1. The continuous thrust supplied by H2 bubbles which escaped from the small opening of the micromotor was utilized as an active pump to enhance the permeation of H2 into deep brain tissue, which in turn dramatically heightened the therapeutic outcome. Apart from providing a driving power source, H2 also acted as a therapeutic component to regulate the inflammatory microenvironment. In the presence of a single therapeutic agent H2, both the intracerebral inflammatory factors and the brain infarct volume of rats were distinctly attenuated, which convincingly showed distinguished neuroprotection and ischemia stroke remedy effects.
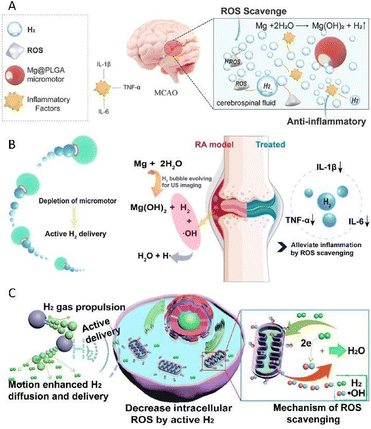 | ||
| Fig. 2 (A) Mg@PLGA micromotor as an active therapy platform for acute ischemic stroke via selectively scavenging ROS and alleviating oxidative stress. This figure has been reproduced from ref. 35 with permission from the John Wiley and Sons, copyright 2021. (B) Mg–HA micromotors capable of regulating the inflammatory microenvironment for the active therapy of rheumatoid arthritis. This figure has been adapted from ref. 36 with permission from the American Chemical Society, copyright 2021. (C) Mg-based micromotors which could disrupt the redox homoeostasis to induce the apoptosis of cancer cells. This figure has been reprinted from ref. 37 with permission from the Elsevier, copyright 2020. | ||
On this basis, our group also designed a magnesium–hyaluronic acid (Mg–HA) micromotor, for the active therapy of rheumatoid arthritis (Fig. 2B).36 Real-time dynamic and accurate monitoring in vivo was realized under the guidance of ultrasound due to the continuous generation of hyper-echo H2 bubbles, which is of great benefit to pave the way to devise next-generation multifunctional smart motors. Furthermore, prominent therapeutic outcomes, involving the down-regulation of inflammatory cytokines and the recovery of joint destruction, were obtained using collagen-induced arthritis rat models. It is worth mentioning that in this system, H2 served as not only a driving force and therapeutic component, but also an ultrasonic imaging agent. The advent of such an integrated platform with multiple functions significantly strengthens the confidence in putting micro/nanomotor technology into real-world clinical practice.
In addition to employing H2 solely to achieve active therapy, embellishing with other functional ingredients may bring about superior efficacy owing to the synergistic effect. Our group developed a kind of Mg-based micromotor by asymmetrically capping Mg microparticles with a PLGA layer, where the anticancer drug doxorubicin (DOX) was loaded.37 Thanks to the selective antioxidant effect of H2, the produced H2 could be used to scavenge cytotoxic oxygen radicals which abundantly exist in the cancer microenvironment, thus breaking the redox homoeostasis and subsequently resulting in the apoptosis of cancer cells (Fig. 2C). Additionally, with the assistance of H2 diffusion, the sensitivity of tumor cells to DOX was remarkably promoted, which also played a crucial role in realizing active hydrogen-chemotherapy synergistic anticancer therapy, thus endowing it with stupendous prospects for biomedical application.
Overall, taking advantage of H2 produced in situ from Mg-based micromotors for active H2 therapy is a considerably promising strategy. First and foremost, in clear contrast to H2 micro/nanocarriers relying on passive diffusion, Mg-based micromotors that rely on the bubble recoil mechanism show astonishing motion performance at low Reynold numbers in biologically relevant media, thus endowing them with possibilities of further penetrating deeper into tissue, which in turn facilitates a better curative outcome. Besides, due to the ingenious construction of a small opening, which serves as the interface for the Mg–water reaction, the H2 release rate of motors is slower than that of hydrogen-rich saline as well as hydrogen-rich water, making it more suitable for practical application. Furthermore, the propellants of Mg-based micromotors, including Mg and water, have great biocompatibility. For instance, Mg acts as an essential trace element in living organisms without harm to humans, and more importantly, Mg-based alloys as biodegradable implants have been successfully applied to cardiovascular and orthopedic diseases.38 Equally, water is ubiquitous and available in organisms, regarded as an ideal safe fuel for powering micro/nanomotors.
Despite this, there are several obstacles that need to be circumvented for further clinical practice. Due to the violent reaction with water, it is hard to achieve controllable sustained release of H2 and maintain an effective therapeutic concentration at the damaged sites. Therefore, more efforts shall be devoted to prolonging the lifetime of Mg-based micromotors. In fact, besides the Mg–water reaction, among active metals, Al is able to reduce water to H2, while Zn can also spontaneously react with an acid to produce H2. Certainly, Al-based motors39 and Zn-based motors40,41 also have been devised, but reports in relation to these two types of motors are rarely involved in the use of H2 for therapy, while most tend to merely treat H2 as a driving power source.
Since NO plays multiple roles in physiological and pathophysiological functions, great attention has been paid to the extension of NO to biomedical applications. However, owing to the instability of NO, it is impractical to exploit NO directly. To this end, considerable efforts have been devoted to developing compounds allowing the generation of NO in vitro or in vivo, that is NO donors. Until now, several types of NO donors, including organic nitrates, organic nitrites, metal–NO complexes, and L-arginine, have been found.46,47 Interestingly, intracellular NO is almost exclusively produced via the biochemical reaction of L-arginine, catalyzed by nitric oxide synthase (NOS) and oxygen.48 Enlightened by such a miraculous biochemical reaction within the human body, NO-driven motors which take L-arginine as fuel for the formation of NO have been synthesized lately.
In 2019, Shen and Mao's team reported a type of NO-driven nanomotor, composed of medically used fluorescent hyperbranched polyamide (HPAM) and L-arginine (Fig. 3A).49 By catalyzing the decomposition of L-arginine, the produced NO was employed not only as a continuous thrust but also as a therapeutic drug to pick up the predescribed task, that is to fight cancer. Moreover, the incorporation of HPAM with conspicuous fluorescence properties allowed monitoring the motion behaviors as well as the uptake of motors, and in 2021, the same group proposed an exosome-loaded microneedle array modified with NO-driven nanomotors for promoting Achilles tendinopathy healing (Fig. 3B).50 When exposed to the microenvironment of Achilles tendinopathy, triggered by endogenous reactive oxygen species and NOS generated in high quantity as a result of inflammation reactions and postinjury stress, L-arginine was found to be converted into NO as the driving force. Benefiting from the ability of autonomous movement, the limitations of targeting and penetration were overcome. As a result, the delivery efficiency of exosomes was improved markedly, thus facilitating the massive accumulation of exosomes at the injury sites accordingly. With the synergistic effect derived from NO and exosomes, a better healing outcome, including alleviating inflammation, accelerating the proliferation of tendon cells and inhibiting extracellular matrix degradation, was observed in both cells and rats.
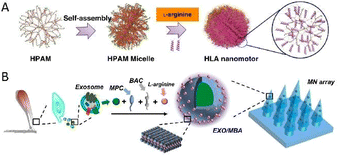 | ||
| Fig. 3 (A) Fabrication process of the NO-driven nanomotor composed of HPAM and L-arginine. This figure has been reproduced from ref. 49 with permission from the Springer Nature, copyright 2019. (B) Manufacturing procedure of exosome-loaded microneedle arrays modified by NO-driven nanomotors. This figure has been adapted from ref. 50 with permission from the American Chemical Society, copyright 2021. | ||
In analogy to Mg-based micromotors, NO-driven motors manifest an eminent athletic performance in the physiological environment by virtue of the biocatalytic conversion of L-arginine into NO. Both the reactant and the products are of benefit to organisms without leaving any harmful residue,49 displaying a high reference value for practical biomedical applications, nevertheless, having quite a few advantages, but simultaneously having certain challenges. As the formation of NO requires to be catalyzed with the assistance of endogenous NOS and oxygen, the locomotion and therapeutic efficacy are susceptible to the expression and activity of NOS at the diseased sites. What's worse, unlike other diseases, the request for cancer treatment is particularly stringent. Only by supplying high levels of NO can the ideal anticancer effect be attained, while low concentrations of NO are conducive to tumor growth,51,52 which runs counter to the original purpose. In order to address the issue, there is an intense demand for increasing the load of L-arginine to make sure that the production of NO exceeds the lowest threshold that can exert an anticancer effect. The problems mentioned above should be taken into account when considering for the advent of more intelligent and controllable micro/nanomotors.
Urease, the first enzyme to be purified and crystallized in 1926,55 is a nickel-containing enzyme found in plants, algae, fungi and several microorganisms.56 Urea, the natural substrate of urease, is ubiquitous in organisms. Urease is capable of catalyzing the hydrolysis of urea into ammonia and carbon dioxide.57 Inspired by such a biochemical reaction within the human body, the idea of applying urease to construct urease-propelled motors arises spontaneously. By decomposing endogenous urea into ammonia and carbon dioxide, urease-propelled motors could not only achieve self-propulsion due to the generation of a concentration gradient, but also realize in situ anticancer therapy owing to the ammonia toxicity.
Sánchez's group reported a urease-propelled nanomotor, consisting of a solid silica core and a mesoporous silica shell, in which the space for the payload of urease and DOX was furnished amply.58 In the presence of the substrate fuel urea, the self-propulsion of nanomotors could be activated by the biocatalytic conversion of the unbalanced distribution of urease. Benefiting from the increased diffusion, the release of DOX from nanomotors increased fourfold in comparison to their passive counterparts (Fig. 4A). Due to the synergistic active therapy of improved DOX release and toxic ammonia, a more effective anticancer activity than a single drug treatment was exhibited.
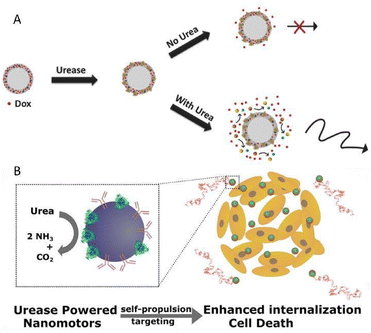 | ||
| Fig. 4 (A) Enhanced DOX release provoked by self-propulsion in the presence of urea. This figure has been reproduced from ref. 58 with permission from the John Wiley and Sons, copyright 2017. (B) Urease-powered nanomotors as in situ ammonia generators and active therapy platforms for 3D bladder cancer spheroid treatment. This figure has been adapted from ref. 59 with permission from the American Chemical Society, copyright 2018. | ||
On this basis, the same group later devised a species of urease-powered nanomotors, based on mesoporous silica nanoparticles coupled with anti-FGFR3 antibodies for the active therapy of bladder cancer (Fig. 4B).59 With the assistance of anti-FGFR3 antibodies whose receptors are over-expressed in bladder cancer cells, motors displayed a more favorable self-propulsion towards cancer cells. As a consequence, the internalization efficiency of the motor system was almost 14 times as high as that of passive particles without antibodies. Meanwhile, the cooperative active therapy brought about extraordinary application potential for bladder cancer therapy, which originated from not only the cytotoxic effect of ammonia, the byproduct of the biocatalysis, but also the therapeutic effect of anti-FGFR3, which has been proved to be able to inhibit cell proliferation and growth. Although the studies on the motor systems shown above only stay at the cellular level without further animal model investigation, there is no doubt that these preliminary studies provide valuable guidance for further exploration, which is worth recognition.
As one type of enzyme-driven motor, no matter what advantages enzyme-driven motors have, urease-propelled motors also have advantages, including good biocompatibility, high specificity and catalytic efficiency of enzymes, together with mild enzymatic reaction conditions.60 Such inherent advantages have endowed urease-propelled motors with infinite application potential in vivo. What's more, since the activity of urease is certain, as long as the substrate urea is supplied steadily, the motor will be able to keep moving for a long time. Interestingly, urea is readily available in vivo, especially in the urinary bladder, where its concentration is up to 300 mM.61 This characteristic also determines that urease-driven motors are more suitable for bladder cancer treatment, with narrow application scope. Every coin has two sides. Given that ammonia is toxic to all cells without selectivity, while taking advantage of toxic ammonia to eliminate cancer cells, the impact on surrounding normal cells remains to be explored on the way to real clinical translation. To this end, it is of great necessity to lock the motors at a specific lesion site in order to minimize systemic side effects.
2.2. Physical byproducts
Physical byproducts generally refer to the physical phenomena occurring during the motion process. Such physical byproducts are mainly classified as self-generated heat and self-generated electric field in this minireview. In fact, in addition to these two physical byproducts, other physical byproducts, such as mechanical force and pressure generated by the dynamic performance of micro/nanomotors, have been discovered recently. However, how to utilize the generated mechanical force and pressure during the motion of micro/nanomotors to achieve active therapy remains to be further explored.He's group developed a gold nanoshell-functionalized polymer multilayer rocket in the shape of a conical cylinder (Fig. 5A).68 In the absence of chemical fuels, the propulsion of rocket under near-infrared (NIR) laser irradiation was attributed to the gold nanoshells with strong plasma resonance absorption in the NIR region, leading to asymmetric local heat distributions on the rocket, which in turn gave rise to a thermophoretic force to push the rocket to move forward in pure water. More importantly, the efficient movement of rocket could also be observed in a cell culture medium with an enhanced viscosity and biofouling effect, indicating the feasibility of further application in vivo. Once the rocket was attached to HeLa cells, the photothermal toxicity of the rocket allowed the apoptosis of the targeted cell in vitro.
 | ||
| Fig. 5 (A) Synthetic procedure of gold nanoshell-functionalized polymer multilayer rockets. This figure has been reproduced from ref. 68 with permission from the John Wiley and Sons, copyright 2015. (B) Biomimetic mem@C@SiO2@DOX nanomotors with self-thermophoretic propulsion enhanced photothermal and chemotherapy under NIR light irradiation. This figure has been reprinted from ref. 69 with permission from the John Wiley and Sons, copyright 2020. (C) Modification of a Janus capsule micromotor with an erythrocyte membrane coating. This figure has been adapted from ref. 72 with permission from the American Chemical Society, copyright 2018. | ||
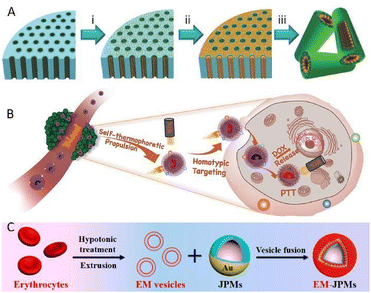
Zhang's team prepared a biomimetic mem@C@SiO2@DOX nanomotor for enhanced synergistic photothermal and chemotherapy of breast cancer (Fig. 5B).69 Thanks to the clever construction of an asymmetric carbon semi-yolk core with a distinguished photothermal effect, the motion control over the motor system via NIR exposure was realized. Additionally, the decoration of a cancer cell membrane contributed to reducing the resistance of viscosity and bioadhesion during the motion process in biological media, thus giving rise to a remarkable elevation of self-thermophoretic propulsion, which was almost twice faster than that without membrane camouflage. What's more, the combination of effective propulsion and homogenous targeting ability resulted in the improvement of cellular uptake efficiency, as validated experimentally. More importantly, by integrating PTT with chemotherapy which stemmed from the anticancer drug DOX loaded on an outer spiky shell, a superior therapeutic effect was demonstrated with over 91% MCF-7 cell growth inhibition rate, which provides precious experience for the clinical practical application of bionic micro/nanomotors for active therapy.
In addition to killing cancer cells, studies have suggested that hyperthermia could also promote thrombus dissolution.70,71 Van Hest et al. developed a photothermal thrombolytic platform based on a Janus capsule micromotor cloaked with an erythrocyte membrane (Fig. 5C).72 The capsule structure consisting of heparin and chitosan was established via a template-assisted layer-by-layer technique, followed by spraying of a gold shell onto one side. NIR laser irradiation to the half-coated gold shell with a photothermal conversion capability enabled the formation of a local thermal gradient surrounding the motor, which in turn generated a thermophoretic force to actuate the motor. What's more, the local temperature elevated apparently, thus facilitating the ablation of thrombus. Besides, such laser-induced localized heating triggered the disruption of the structural integrity of the capsule, bringing about the release of heparin, which in turn accelerated blood clot lysis even further.
Compared with traditional PTT, in which therapeutic components in general collide randomly without control once entering the body, the motion of light-driven motors could be modulated conveniently via tuning the intensity of the input light source,73 which is in line with the development trend of the intelligent era. However, it is also difficult to completely avoid the damage to adjacent normal tissue induced by overheating and massive heat transfer during PTT, which needs to be considered prior to applying PTT in complex realistic scenarios on a large scale.
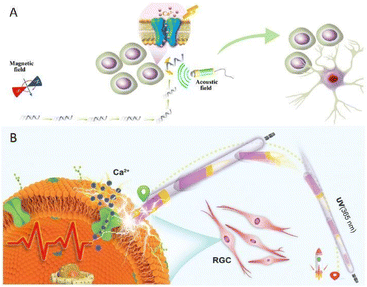
Capitalizing on electrical stimulation to induce cell differentiation was also displayed by our group in 2020. We fabricated a helical-shaped S.platensis@Fe3O4@tBaTiO3 micromotor, which could be precisely maneuvered under the guidance of a rotational magnetic field.76 With the aid of an external ultrasonic field, the introduced piezoelectric BaTiO3 was able to convert the ultrasonic input into electrical stimulation relying on the piezoelectric effect, which in turn induced the differentiation of the targeted neural stem-like cell (Fig. 6A). However, it is regretful that the resulting electrical signal was obtained by introducing an extra ultrasonic field and piezoelectric nanoparticles into the motor system.
 | ||
| Fig. 6 (A) A helical-shaped S.platensis@Fe3O4@tBaTiO3 micromotor to induce the differentiation of the targeted neural stem-like cell. This figure has been reproduced from ref. 76 with permission from the John Wiley and Sons, copyright 2020. (B) Asymmetric TiO2–Au nanowires capable of actively seeking the targeted neuronal retinal ganglion cell and triggering cell activation under UV irradiation. This figure has been reprinted from ref. 78 with permission from the John Wiley and Sons, copyright 2020. | ||
Continuous explorations over the past decade have confirmed that light-induced self-electrophoresis is one of the propulsion mechanisms to power light-driven micro/nanomotors. Briefly, it refers to the movement of the charged motors in a self-generated electric field. In detail, once excited with light irradiation, the photoactive materials upon the motors will undergo a series of photochemical reactions. Among this process, the marvelous design of the Janus structure permits the asymmetric distribution of the light-induced redox ion products around the charged motors, and then a potential difference is formed accordingly, which in turn gives rise to a local self-generated electric field. As a result, charged motors will respond to the self-generated electric field and then move.64,77 It is not hard to see that the as-described motors will generate a physical byproduct, that is a self-generated electric field, during the motion process. For this, the idea of making the maximal use of the self-generated electric field so as to stimulate cells in situ emerges as the times require.
Enlightened by the above idea, our group carried out a preliminary exploration by devising a light-powered motor based on an asymmetric TiO2–Au nanowire in the same year.78 Upon exposure to ultraviolet (UV) irradiation with a low intensity, the motor could be steered in pure water by a self-generated local electric field induced by photochemical water splitting. It is noteworthy that through tuning the UV light orientation on demand, the nanowires were capable of travelling along a predetermined pathway with high precision. Once in contact with the targeted neuronal retinal ganglion cells, the inherent photoelectricity produced during the motion process would act as a stimulus to trigger cell activation (Fig. 6B), rather than an engine for motion, which provides an alternative minimum invasive platform for the active therapy of ophthalmic diseases. In comparison to surgical implantation, of which the invasiveness is an irrefutable flaw restricting their clinical practice, these motor platforms are more appropriate candidates for noninvasive therapy. However, the tissue penetration limitation of light remains a paramount challenge that must be overcome in order to ensure the utility for further clinical practice. It must be admitted that although great progress has been made in vitro, there is still a long way from clinical application, which requires more endeavors to shrink it.
3. Conclusions
On reviewing the development of micro/nanomotors, from the birth of the first motor in 2004 to the present, it could be found that at first we merely focused on how to make particles at the micro/nanoscale swim in liquid media, but later such motion phenomena could not meet our needs. To this end, in-depth studies on why they could move, namely propulsion mechanisms, have been carried out, and in recent years, the practical significance of these micro/nanoswimmers, that is their applications, has also been extensively studied. In biomedicine, how to maximize the efficacy of a certain number of particles is a key factor. In recent years, attention has been paid to the use of motor byproducts, which involves the direct use of the byproducts generated in situ from motors to achieve active therapy than letting motors bring therapeutic components to the designated position. Particularly, with the outstanding motility performance and effective penetrating capability of motors, a superior outcome can be achieved. This is the aim of this minireview, hoping to give readers some enlightenment. Science is endless; in addition to the byproducts highlighted in this minireview, we do believe that there are still many byproducts that are produced or generated during the motion process of micro/nanomotors waiting to be discovered. To this end, great importance should be given to the substances produced or generated during the motion process of micro/nanomotors and, more importantly, to further explore their application potential in combination with expertise and previous reports. Hopefully, more strategies with significant clinical value will be found in the future.The pronounced efforts over the last decade have witnessed the rapid advances in micro/nanomotors in biomedical applications, from test tubes to the cellular level, and to animal models. Despite developing at a very fast pace, the actual biomedical applications of these miniaturized devices are still in the infancy stage. On one hand, as the complexity of organisms poses a problem, it remains a continuing challenge to fully realize the controllability and accuracy over motor systems in vivo. On the other hand, having a command of the path of the motors after mission completion and the long-term impact on the body are indispensable key steps before real-world clinical application. To this end, the materials employed to engineer motors shall be at least biodegradable and biocompatible. Undoubtedly, there is still a long way to narrow the gap between micro/nanomotors and realistic clinical practice, but with closer cooperation with nanomedicine, micro/nano engineering and material chemistry, we do believe that the initial proof-of-concept research studies will be translated into real-world biomedical applications and address clinical issues in the near future. We are keenly looking forward to a new era in which micro/nanomotor-based approaches are expected to become a significant modality within active therapy, truly realizing the envision of the Fantastic Voyage.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22175083), and the High-Level University Construction Project of Southern Medical University (G622280009).References
- Nat. Mater., 2009, 8, 771–771.
- E. G. Jordá, Clin. Transl. Oncol., 2009, 11, 561–563 CrossRef
.
- W. F. Paxton, K. C. Kistler, C. C. Olmeda, A. Sen, S. K. St Angelo, Y. Y. Cao, T. E. Mallouk, P. E. Lammert and V. H. Crespi, J. Am. Chem. Soc., 2004, 126, 13424–13431 CrossRef CAS PubMed
.
- V. Richards, Nat. Chem., 2016, 8, 1090–1090 CrossRef CAS PubMed
.
- S. Sánchez, L. Soler and J. Katuri, Angew. Chem., Int. Ed., 2015, 54, 1414–1444 CrossRef
.
- T. L. Xu, W. Gao, L. P. Xu, X. J. Zhang and S. T. Wang, Adv. Mater., 2017, 29, 27 Search PubMed
.
- X. L. Wei, M. Beltrán-Gastélum, E. Karshalev, B. E. F. de Ávila, J. R. Zhou, D. N. Ran, P. Angsantikul, R. H. Fang, J. Wang and L. F. Zhang, Nano Lett., 2019, 19, 1914–1921 CrossRef CAS
.
- M. F. Zhou, T. Hou, J. X. Li, S. S. Yu, Z. J. Xu, M. Yin, J. Wang and X. L. Wang, ACS Nano, 2019, 13, 1324–1332 CAS
.
- E. Karshalev, B. E. F. de Ávila, M. Beltrán-Gastélum, P. Angsantikul, S. S. Tang, R. Mundaca-Uribe, F. Y. Zhang, J. Zhao, L. F. Zhang and J. Wang, ACS Nano, 2018, 12, 8397–8405 CrossRef CAS PubMed
.
- B. E. F. de Ávila, P. Angsantikul, J. X. Li, M. A. Lopez-Ramirez, D. E. Ramírez-Herrera, S. Thamphiwatana, C. R. Chen, J. Delezuk, R. Samakapiruk, V. Ramez, L. F. Zhang and J. Wang, Nat. Commun., 2017, 8, 9 CrossRef PubMed
.
- S. K. Srivastava, M. Medina-Sánchez, B. Koch and O. G. Schmidt, Adv. Mater., 2016, 28, 832–837 CrossRef CAS
.
- E. Gultepe, J. S. Randhawa, S. Kadam, S. Yamanaka, F. M. Selaru, E. J. Shin, A. N. Kalloo and D. H. Gracias, Adv. Mater., 2013, 25, 514–519 CrossRef CAS PubMed
.
- W. Xi, A. A. Solovev, A. N. Ananth, D. H. Gracias, S. Sánchez and O. G. Schmidt, Nanoscale, 2013, 5, 1294–1297 RSC
.
- Y. Wang, X. X. Liu, C. Chen, Y. D. Chen, Y. Li, H. Ye, B. Wang, H. Y. Chen, J. H. Guo and X. Ma, ACS Nano, 2022, 16, 180–191 CrossRef CAS
.
- Y. Wang, Y. H. Liu, Y. Li, D. D. Xu, X. Pan, Y. D. Chen, D. K. Zhou, B. Wang, H. H. Feng and X. Ma, Research, 2020, 2020, 13 Search PubMed
.
- J. Wang, Biosens. Bioelectron., 2016, 76, 234–242 CrossRef CAS
.
- B. E. F. de Ávila, A. Martín, F. Soto, M. A. Lopez-Ramirez, S. Campuzano, G. M. Vásquez-Machado, W. W. Gao, L. F. Zhang and J. Wang, ACS Nano, 2015, 9, 6756–6764 CrossRef
.
- J. Wu, S. Balasubramanian, D. Kagan, K. M. Manesh, S. Campuzano and J. Wang, Nat. Commun., 2010, 1, 6 CrossRef PubMed
.
- Q. Q. Li, L. T. Liu, H. Q. Huo, L. C. Su, Y. Wu, H. X. Lin, X. G. Ge, J. Mu, X. Zhang, L. T. Zheng and J. B. Song, ACS Nano, 2022, 16, 7947–7960 CrossRef CAS PubMed
.
- A. Aziz, M. Medina-Sánchez, J. Claussen and O. G. Schmidt, Nano Lett., 2019, 19, 6612–6620 CrossRef CAS
.
- X. H. Yan, Q. Zhou, M. Vincent, Y. Deng, J. F. Yu, J. B. Xu, T. T. Xu, T. Tang, L. M. Bian, Y. X. J. Wang, K. Kostarelos and L. Zhang, Sci. Robot., 2017, 2, 14 Search PubMed
.
- E. S. Olson, J. Orozco, Z. Wu, C. D. Malone, B. Yi, W. Gao, M. Eghtedari, J. Wang and R. F. Mattrey, Biomaterials, 2013, 34, 8918–8924 CrossRef CAS PubMed
.
- H. Li, F. Peng, X. Yan, C. Mao, X. Ma, D. A. Wilson, Q. He and Y. F. Tu, Acta Pharm. Sin. B, 2022 DOI:10.1016/j.apsb.2022.10.010
.
- J. F. Ou, K. Liu, J. M. Jiang, D. A. Wilson, L. Liu, F. Wang, S. H. Wang, Y. F. Tu and F. Peng, Small, 2020, 16, 16 Search PubMed
.
- K. Villa and M. Pumera, Chem. Soc. Rev., 2019, 48, 4966–4978 RSC
.
- M. Luo, Y. Z. Feng, T. W. Wang and J. G. Guan, Adv. Funct. Mater., 2018, 28, 23 Search PubMed
.
- Y. F. Tu, F. Peng and D. A. Wilson, Adv. Mater., 2017, 29, 20 Search PubMed
.
- F. Peng, Y. F. Tu and D. A. Wilson, Chem. Soc. Rev., 2017, 46, 5289–5310 RSC
.
- H. Wang and M. Pumera, Chem. Rev., 2015, 115, 8704–8735 CrossRef CAS
.
- M. Guix, C. C. Mayorga-Martinez and A. Merkoçi, Chem. Rev., 2014, 114, 6285–6322 CrossRef CAS PubMed
.
- M. L. Yang, Y. M. Dong, Q. N. He, P. Zhu, Q. Zhuang, J. Shen, X. Y. Zhang and M. Y. Zhao, Oxid. Med. Cell. Longevity, 2020, 2020, 17 Search PubMed
.
- M. Dole, F. R. Wilson and W. P. Fife, Science, 1975, 190, 152–154 CrossRef CAS PubMed
.
- I. Ohsawa, M. Ishikawa, K. Takahashi, M. Watanabe, K. Nishimaki, K. Yamagata, K. Katsura, Y. Katayama, S. Asoh and S. Ohta, Nat. Med., 2007, 13, 688–694 CrossRef CAS PubMed
.
- C. R. Chen, E. Karshalev, J. G. Guan and J. Wang, Small, 2018, 14, 10 Search PubMed
.
- S. H. Wang, K. Liu, Q. Zhou, C. Xu, J. B. Gao, Z. Wang, F. Wang, B. Chen, Y. C. Ye, J. F. Ou, J. M. Jiang, D. A. Wilson, S. W. Liu, F. Peng and Y. F. Tu, Adv. Funct. Mater., 2021, 31, 13 Search PubMed
.
- C. Xu, S. H. Wang, H. Wang, K. Liu, S. Y. Zhang, B. Chen, H. Liu, F. Tong, F. Peng, Y. F. Tu and Y. J. Li, Nano Lett., 2021, 21, 1982–1991 CrossRef CAS PubMed
.
- K. Liu, J. F. Ou, S. H. Wang, J. B. Gao, L. Liu, Y. C. Ye, D. A. Wilson, Y. R. Hu, F. Peng and Y. F. Tu, Appl. Mater. Today, 2020, 20, 9 Search PubMed
.
- Y. J. Chen, Z. G. Xu, C. Smith and J. Sankar, Acta Biomater., 2014, 10, 4561–4573 CrossRef CAS
.
- W. Gao, A. Pei and J. Wang, ACS Nano, 2012, 6, 8432–8438 CrossRef CAS PubMed
.
- W. Gao, A. Uygun and J. Wang, J. Am. Chem. Soc., 2012, 134, 897–900 CrossRef CAS
.
- Z. H. Lin, C. Y. Gao, D. L. Wang and Q. He, Angew. Chem., Int. Ed., 2021, 60, 8750–8754 CrossRef CAS
.
- G. Yetik-Anacak and J. D. Catravas, Vasc. Pharmacol., 2006, 45, 268–276 CrossRef CAS
.
- D. E. Koshland, Jr., Science, 1992, 258, 1861 CrossRef
.
- O. Smith, Nat. Med., 1998, 4, 1215 CrossRef CAS
.
- Y. Q. Yang, Z. J. Huang and L. L. Li, Nanoscale, 2021, 13, 444–459 RSC
.
- P. G. Wang, M. Xian, X. Tang, X. Wu, Z. Wen, T. Cai and A. J. Janczuk, Chem. Rev., 2002, 102, 1091–1134 CrossRef CAS
.
- K. Zhang, H. X. Xu, X. Q. Jia, Y. Chen, M. Ma, L. P. Sun and H. R. Chen, ACS Nano, 2016, 10, 10816–10828 CrossRef CAS
.
- M. A. Marletta, A. R. Hurshman and K. M. Rusche, Curr. Opin. Chem. Biol., 1998, 2, 656–663 CrossRef CAS
.
- M. M. Wan, H. Chen, Q. Wang, Q. Niu, P. Xu, Y. Q. Yu, T. Y. Zhu, C. Mao and J. Shen, Nat. Commun., 2019, 10, 11 CrossRef PubMed
.
- A. L. Liu, Q. Wang, Z. N. Zhao, R. Wu, M. C. Wang, J. W. Li, K. Y. Sun, Z. Y. Sun, Z. Y. Lv, J. Xu, H. M. Jiang, M. M. Wan, D. Q. Shi and C. Mao, ACS Nano, 2021, 15, 13339–13350 CrossRef CAS PubMed
.
- J. Szefel, A. Danielak and W. J. Kruszewski, Adv. Med. Sci., 2019, 64, 104–110 CrossRef
.
- W. P. Fan, N. Lu, P. Huang, Y. Liu, Z. Yang, S. Wang, G. C. Yu, Y. J. Liu, J. K. Hu, Q. J. He, J. L. Qu, T. F. Wang and X. Y. Chen, Angew. Chem., Int. Ed., 2017, 56, 1229–1233 CrossRef CAS PubMed
.
- F. Salvatore and V. Bocchini, Nature, 1961, 191, 705–706 CrossRef CAS PubMed
.
- O. Trédan, C. M. Galmarini, K. Patel and I. F. Tannock, J. Natl. Cancer Inst., 2007, 99, 1441–1454 CrossRef
.
- J. B. Sumner, J. Biol. Chem., 1926, 69, 435–441 CrossRef CAS
.
- M. J. Maroney and S. Ciurli, Chem. Rev., 2014, 114, 4206–4228 CrossRef CAS PubMed
.
- K. Kappaun, A. R. Piovesan, C. R. Carlini and R. Ligabue-Braun, J. Adv. Res., 2018, 13, 3–17 CrossRef CAS PubMed
.
- A. C. Hortelão, T. Patiño, A. Perez-Jiménez, A. Blanco and S. Sánchez, Adv. Funct. Mater., 2018, 28, 10 CrossRef
.
- A. C. Hortelão, R. Carrascosa, N. Murillo-Cremaes, T. Patiño and S. Sánchez, ACS Nano, 2019, 13, 429–439 CrossRef
.
- H. Yuan, X. X. Liu, L. Y. Wang and X. Ma, Bioact. Mater., 2021, 6, 1727–1749 CrossRef CAS PubMed
.
- L. Y. Liu, H. P. Mo, S. W. Wei and D. Raftery, Analyst, 2012, 137, 595–600 RSC
.
- X. S. Li, J. F. Lovell, J. Yoon and X. Y. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed
.
- Y. J. Liu, P. Bhattarai, Z. F. Dai and X. Y. Chen, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC
.
- L. L. Xu, F. Z. Mou, H. T. Gong, M. Luo and J. G. Guan, Chem. Soc. Rev., 2017, 46, 6905–6926 RSC
.
- Y. J. Wu, T. Y. Si, J. X. Shao, Z. G. Wu and Q. He, Nano Res., 2016, 9, 3747–3756 CrossRef
.
- H. R. Jiang, N. Yoshinaga and M. Sano, Phys. Rev. Lett., 2010, 105, 4 Search PubMed
.
- M. J. Xuan, R. Mestre, C. Y. Gao, C. Zhou, Q. He and S. Sánchez, Angew. Chem., Int. Ed., 2018, 57, 6838–6842 CrossRef CAS
.
- Z. G. Wu, T. Y. Si, W. Gao, X. K. Lin, J. Wang and Q. He, Small, 2016, 12, 577–582 CrossRef CAS PubMed
.
- M. Y. Zhou, Y. Xing, X. Y. Li, X. Du, T. L. Xu and X. J. Zhang, Small, 2020, 16, 11 Search PubMed
.
- J. Lv, L. J. Zhang, W. Z. Du, G. X. Ling and P. Zhang, J. Controlled Release, 2022, 345, 572–585 CrossRef CAS PubMed
.
- N. Singh, A. Varma, A. Verma, B. N. Maurya and D. Dash, Nano Res., 2016, 9, 2327–2337 CrossRef CAS
.
- J. X. Shao, M. Abdelghani, G. Z. Shen, S. P. Cao, D. S. Williams and J. C. M. van Hest, ACS Nano, 2018, 12, 4877–4885 CrossRef CAS PubMed
.
- M. J. Xuan, Z. G. Wu, J. X. Shao, L. R. Dai, T. Y. Si and Q. He, J. Am. Chem. Soc., 2016, 138, 6492–6497 CrossRef CAS
.
- L. F. Jaffe and R. Nuccitelli, Annu. Rev. Biophys. Bioeng., 1977, 6, 445–476 CrossRef CAS
.
- S. Nag and N. V. Thakor, Med. Biol. Eng. Comput., 2016, 54, 63–76 CrossRef
.
- L. Liu, B. Chen, K. Liu, J. B. Gao, Y. C. Ye, Z. Wang, N. Qin, D. A. Wilson, Y. F. Tu and F. Peng, Adv. Funct. Mater., 2020, 30, 8 Search PubMed
.
- R. F. Dong, Q. L. Zhang, W. Gao, A. Pei and B. Y. Ren, ACS Nano, 2016, 10, 839–844 CrossRef CAS
.
- B. Chen, L. Liu, K. Liu, F. Tong, S. H. Wang, D. M. Fu, J. B. Gao, J. M. Jiang, J. F. Ou, Y. C. Ye, D. A. Wilson, Y. F. Tu and F. Peng, Adv. Funct. Mater., 2021, 31, 11 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |